In Silico and Chromatographic Methods for Analysis of Biotransformation of Prospective Neuroprotective Pyrrole-Based Hydrazone in Isolated Rat Hepatocytes
Abstract
1. Introduction
2. Results and Discussion
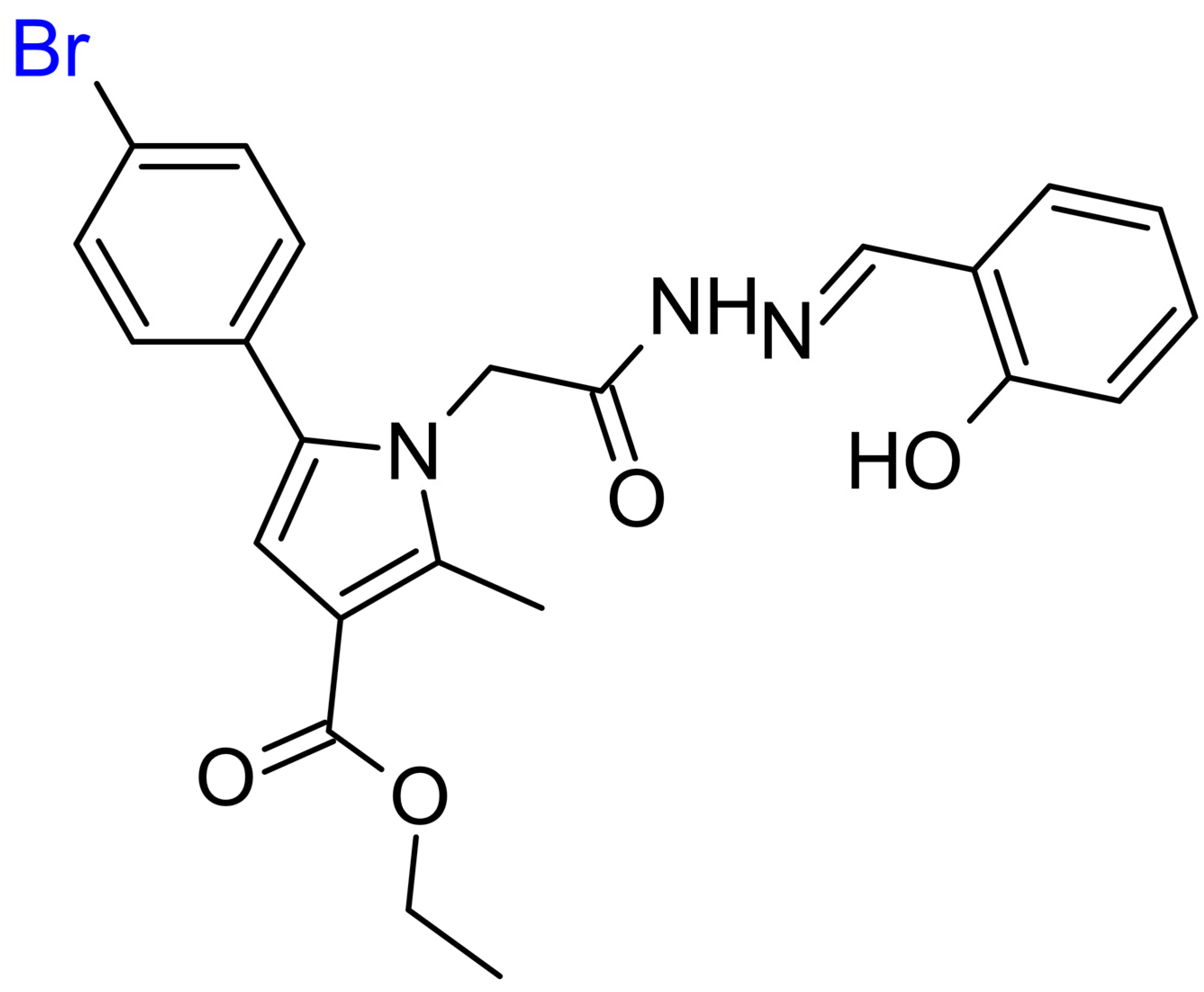
2.1. Selection of the Target Hydrazone 11b
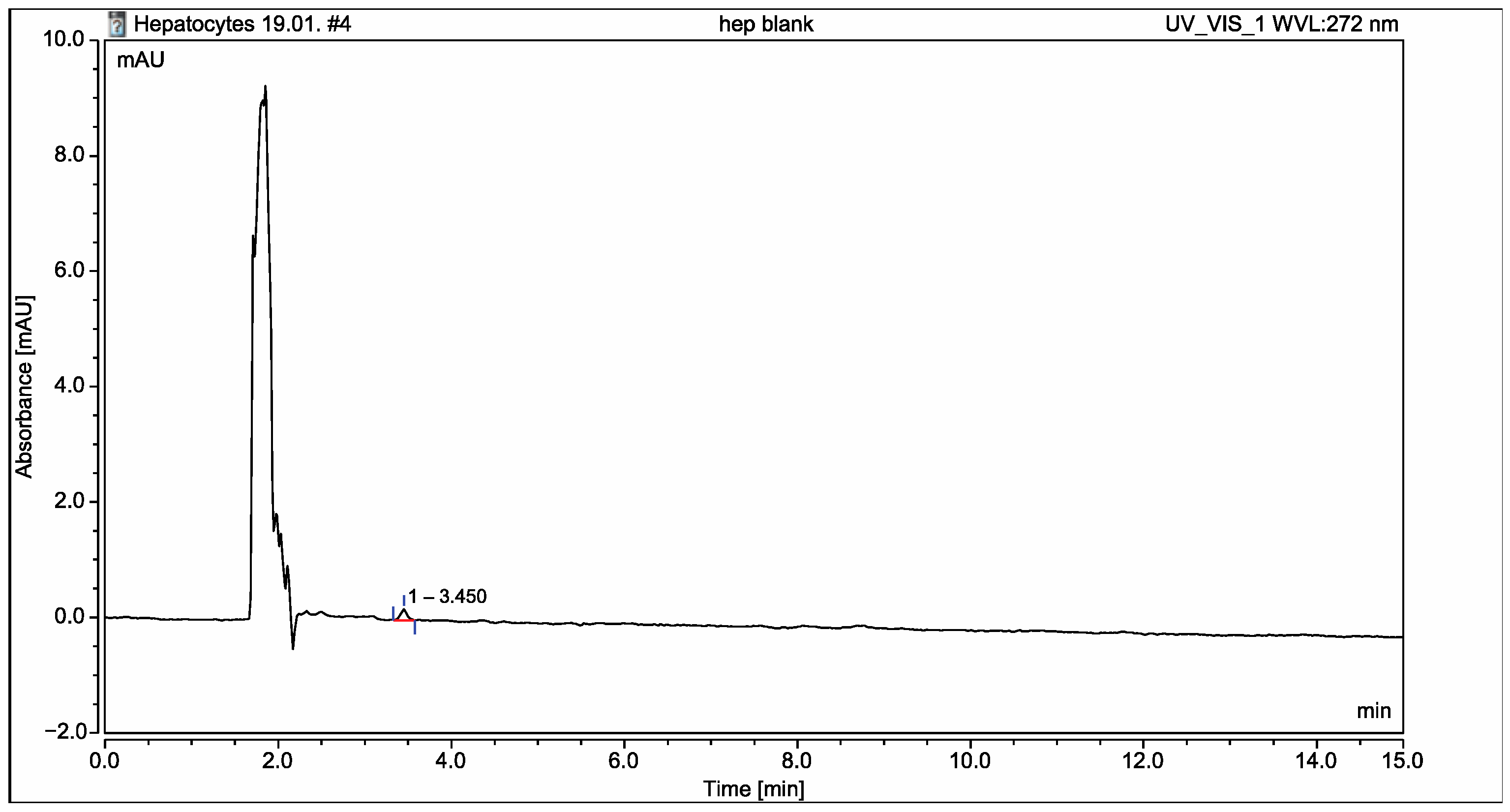
2.2. Application of the Developed C18 HPLC Method for Identification of the Possible Metabolites in Isolated Rat Hepatocytes
2.3. Development of HILIC Method
2.4. Development of Phenyl–hexyl HPLC Method
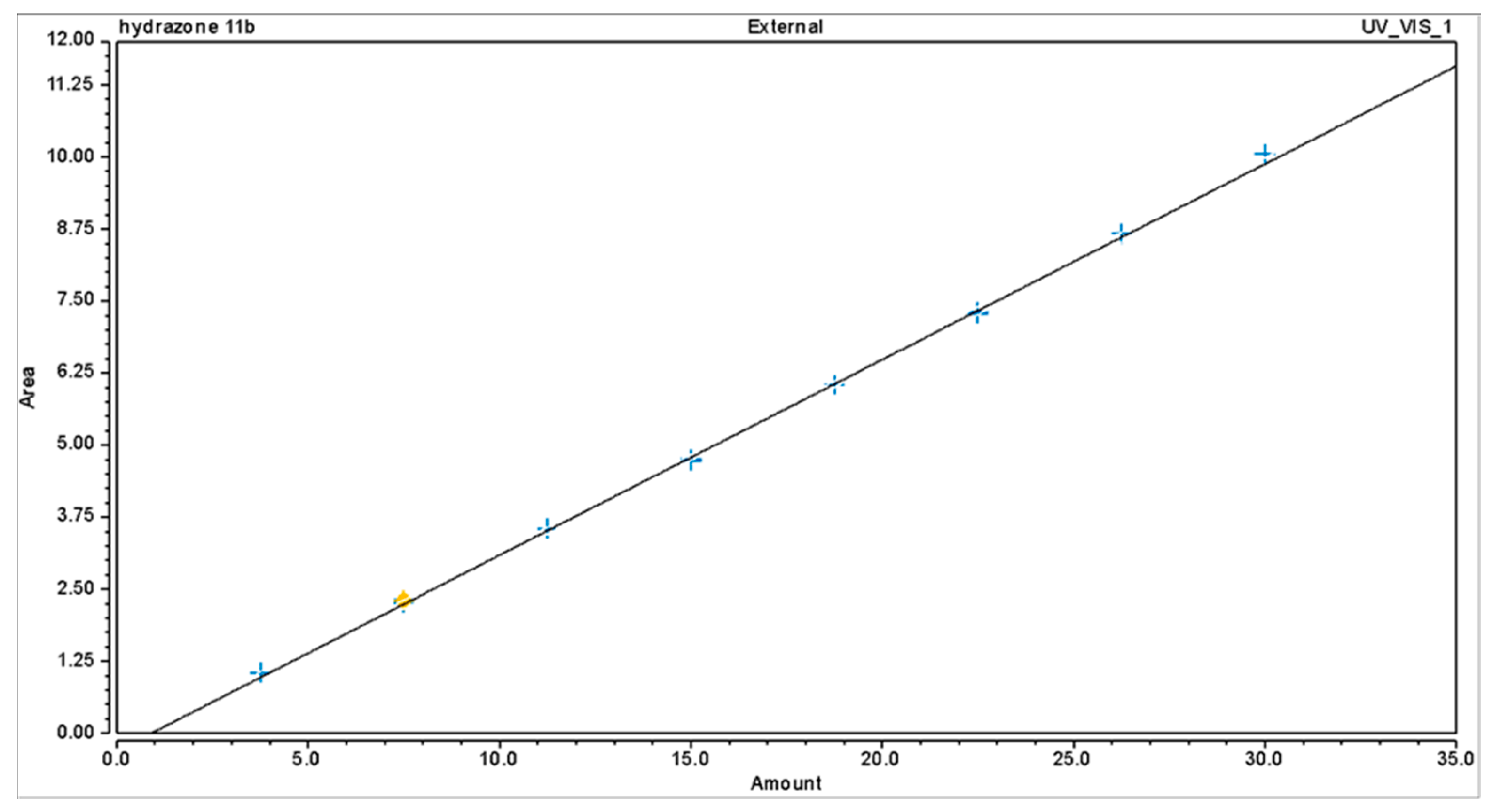
2.5. Method Validation
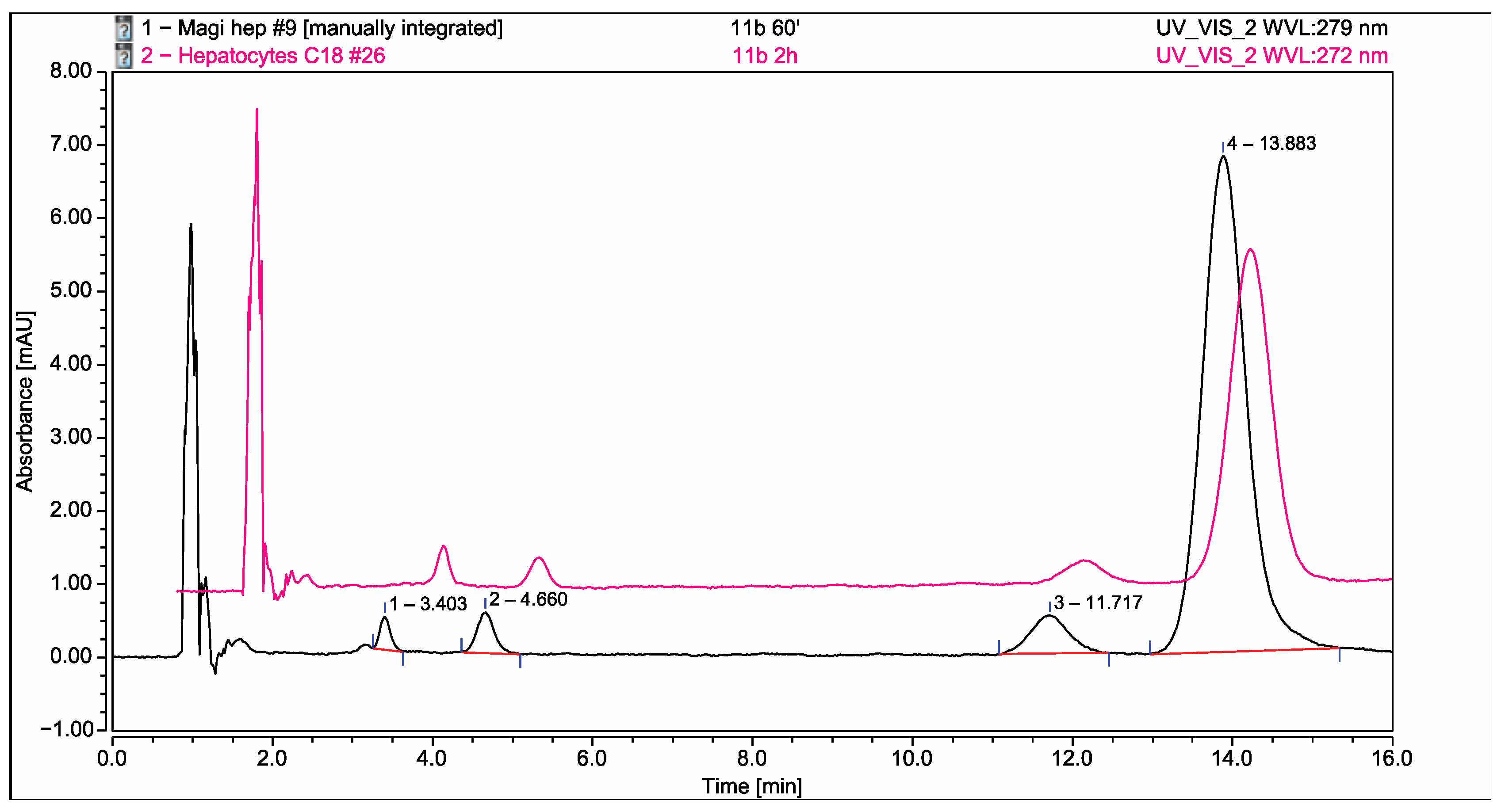
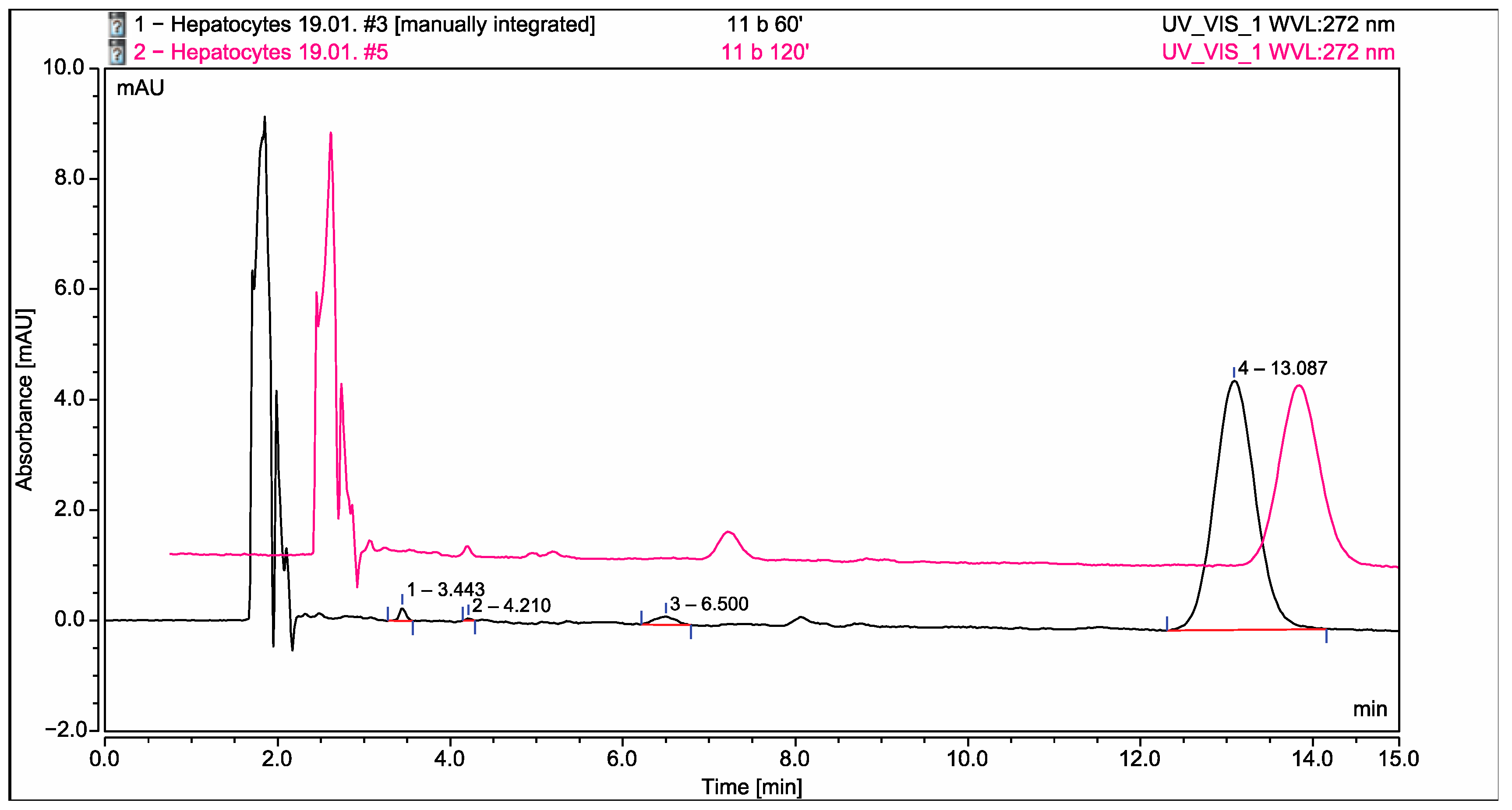
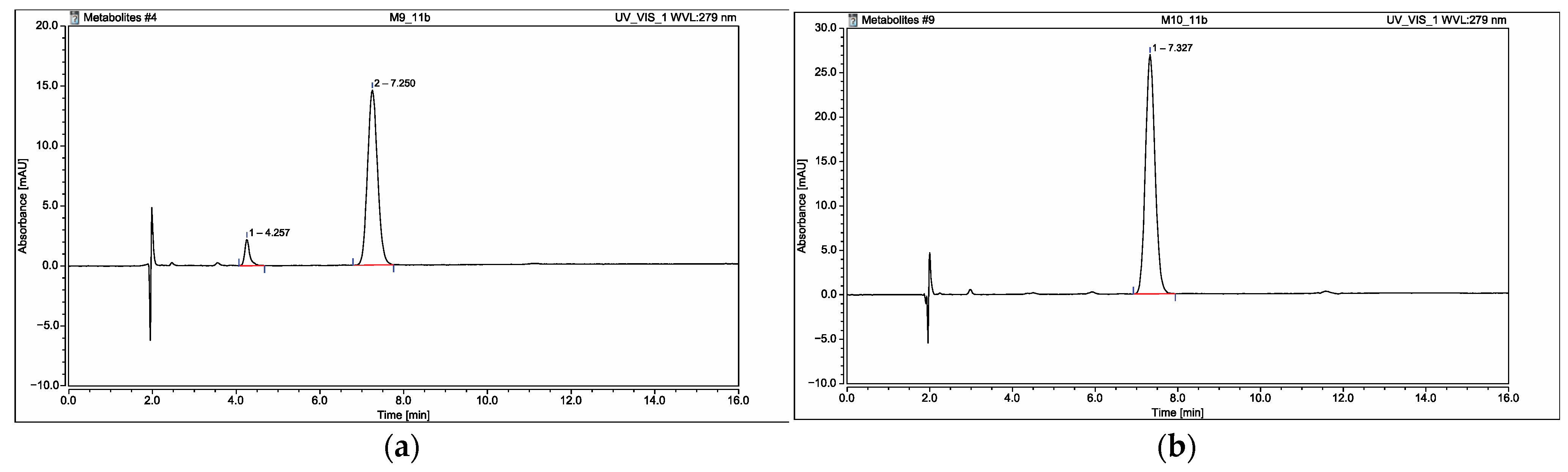
2.6. Application of the Developed and Validated Method for Identification of the Possible Metabolites in Isolated Rat Hepatocytes
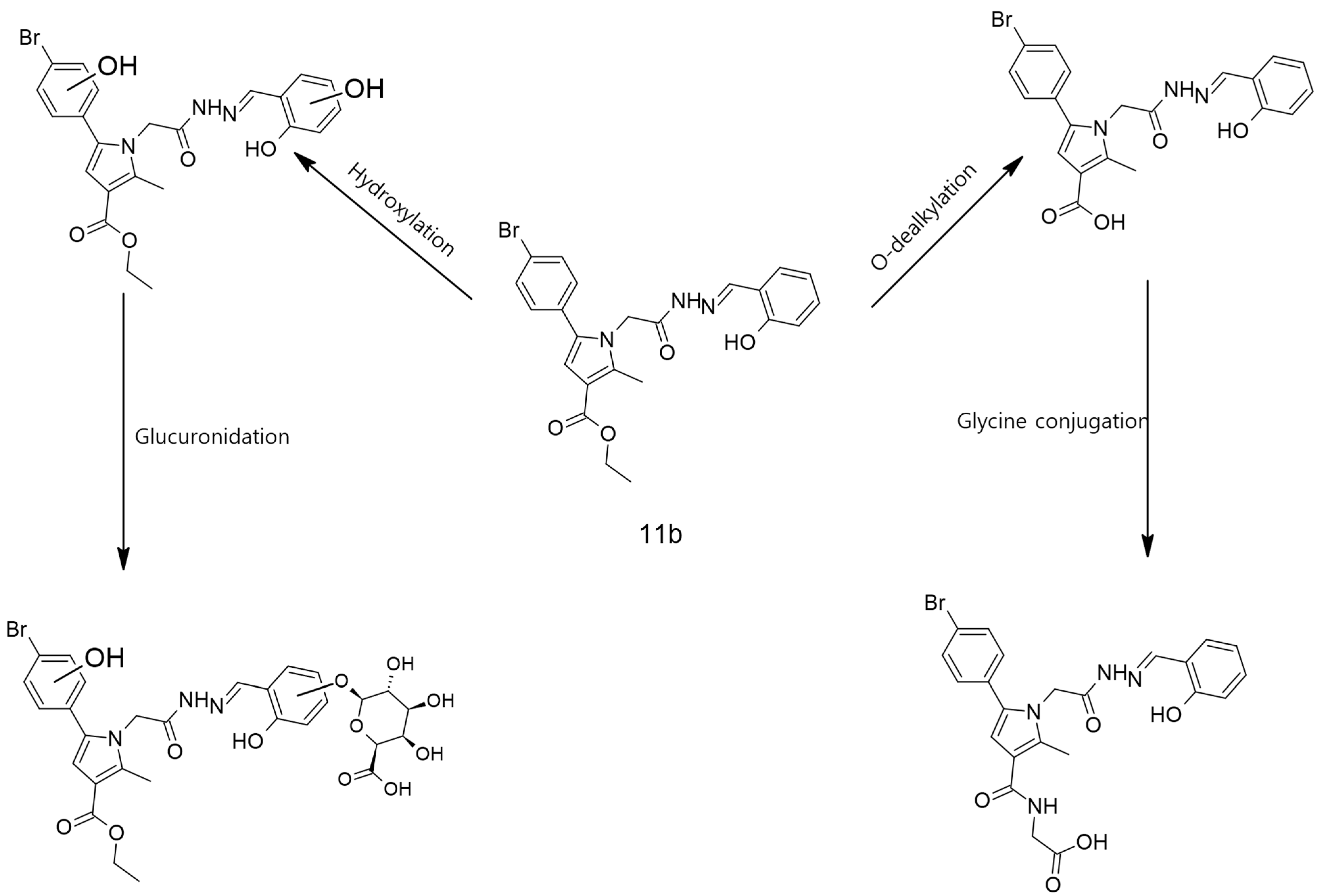
2.7. Prediction of the Possible Metabolites’ Structures via BioTranformation 3.0
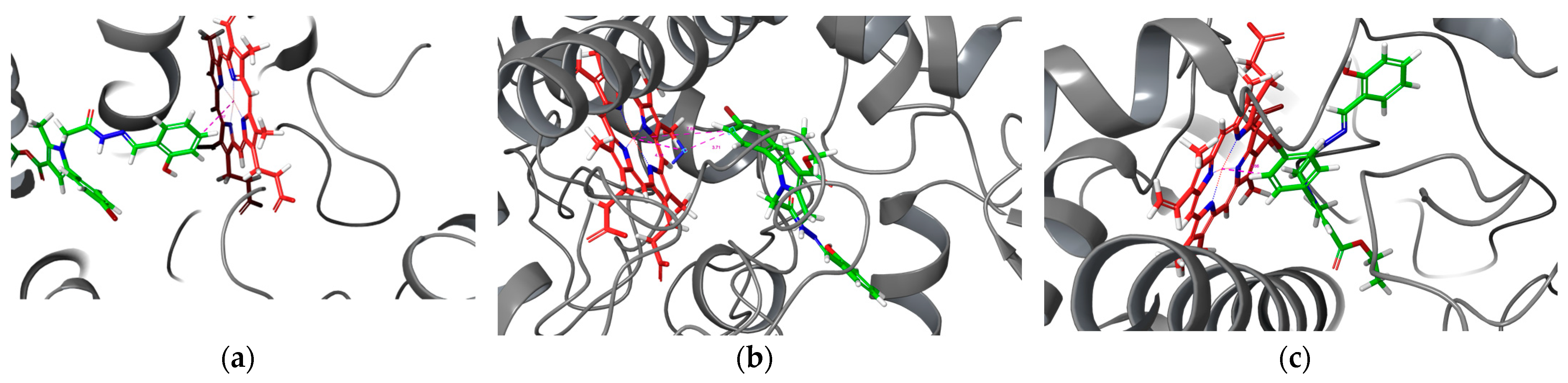
2.8. Application of the Docking Approach
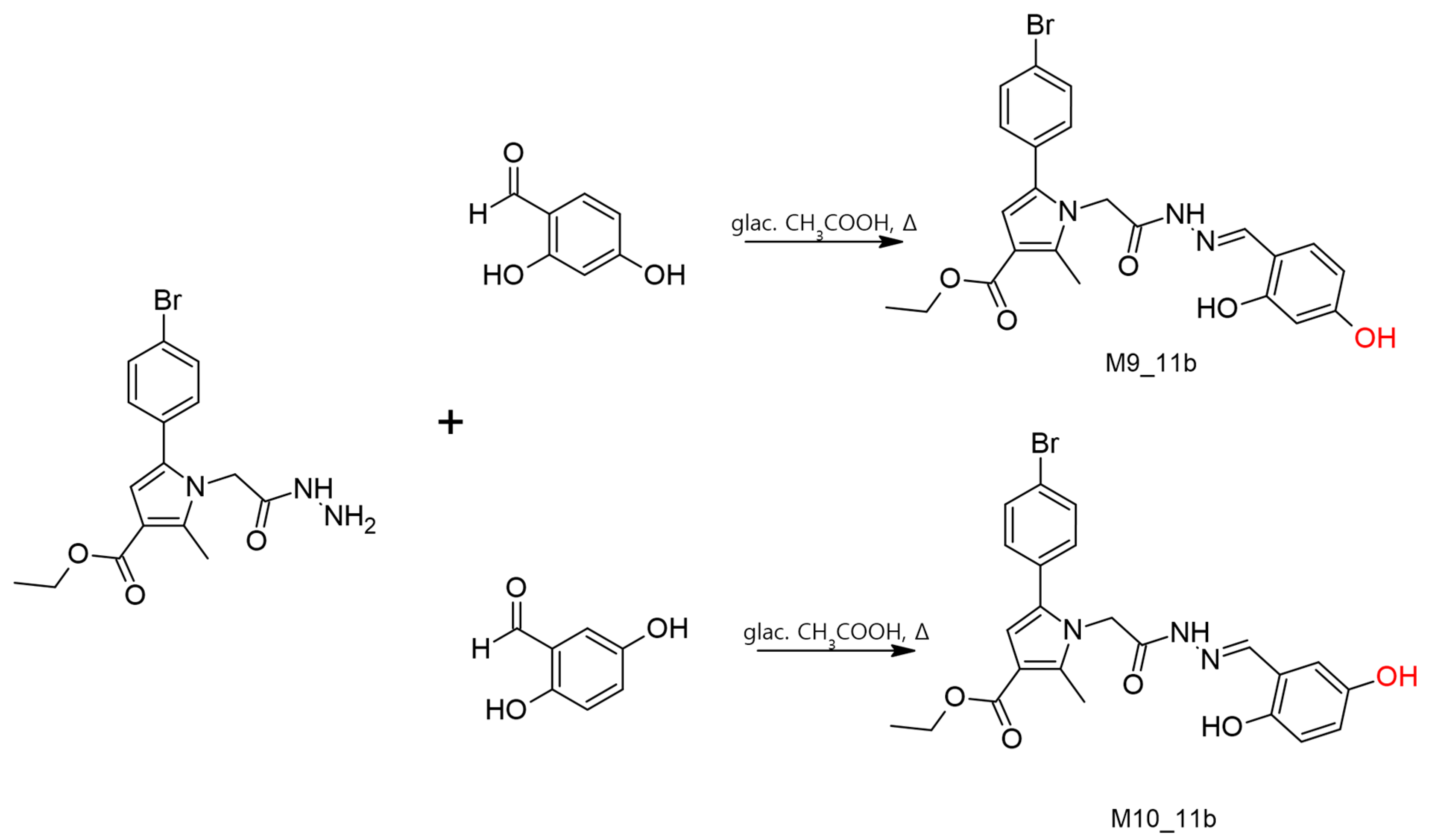
2.9. Conventional Synthesis of the Tentative Metabolites and Their Full Chemical Characterization
2.10. Application of LC-MS Method for Verification of the Obtained Results
3. Materials and Methods
3.1. Formatting of Mathematical Components
3.2. Chromatographic Parameters
3.3. Preparation of the Working and Sample Solutions
3.4. Isolated Rat Hepatocytes
3.5. In Silico Approaches
3.5.1. BioTransformer 3.0
3.5.2. Molecular Docking
- Selection and Preparation of Proteins
- Ligands preparation
- Docking protocol
3.6. Synthesis of the Suggested Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rollas, S.; Küçükgüzel, S.G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef]
- Sah, P.P.; Peoples, S.A. Isonicotinyl hydrazones as antitubercular agents and derivatives for idendification of aldehydes and ketones. J. Am. Pharm. Assoc. 1954, 43, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Bavin, E.M.; Drain, D.J.; Seiler, M.; Seymour, E.D. Some further studies on tuberculostatic compounds. J. Pharm. Pharmacol. 1954, 4, 844–855. [Google Scholar] [CrossRef]
- Lapin, I.P.; Samsonova, M.L. Sravnenie farmakologicheskoĭ aktivnosti i toksichnosti antidepressantov nialamida, fenelzina i iproniazida [Comparison of pharmacological activity and toxicity of the antidepressants nialamide, phenelzine and iproniazide]. Farmakol Toksikol. 1969, 32, 526–530. [Google Scholar] [PubMed]
- Das, S.; Nahar, L.; Nath, R.; Nath, D.; Sarker, S.S.; Talukdar, A.D. Chapter Six—Neuroprotective natural products. Annu. Rep. Med. Chem. 2020, 55, 179–206. [Google Scholar]
- Rehman, M.U.; Wali, A.F.; Ahmad, A.; Shakeel, S.; Rasool, S.; Ali, R.; Rashid, S.M.; Madkhali, H.; Ganaie, M.A.; Khan, R. Neuroprotective Strategies for Neurological Disorders by Natural Products: An update. Curr. Neuropharmacol. 2019, 17, 247–267. [Google Scholar] [CrossRef]
- Clarke, C.J.; Haselden, J.N. Metabolic profiling as a tool for understanding mechanisms of toxicity. Toxicol. Pathol. 2008, 36, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Jadav, T.; Sahu, A.K.; Kalia, K.; Sengupta, P. An Exploration of Advancement in Analytical Methodology for Quantification of Anticancer Drugs in Biomatrices. Anal. Sci. 2019, 35, 719–732. [Google Scholar] [CrossRef]
- Zeki, Ö.C.; Eylem, C.C.; Reçber, T.; Kır, S.; Nemutlu, E. Integration of GC-MS and LC-MS for untargeted metabolomics profiling. J. Pharm. Biomed. Anal. 2020, 190, 113509. [Google Scholar] [CrossRef] [PubMed]
- Zaitsu, K.; Katagi, M.; Kamata, H.T.; Kamata, T.; Shima, N.; Miki, A.; Tsuchihashi, H.; Mori, Y. Determination of the metabolites of the new designer drugs bk-MBDB and bk-MDEA in human urine. Forensic Sci. Int. 2009, 188, 131–139. [Google Scholar] [CrossRef]
- Xiao, J.F.; Zhou, B.; Ressom, H.W. Metabolite identification and quantitation in LC-MS/MS-based metabolomics. Trends Anal. Chem. 2012, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kondeva-Burdina, M.; Mateev, E.; Angelov, B.; Tzankova, V.; Georgieva, M. In Silico Evaluation and In Vitro Determination of Neuroprotective and MAO-B Inhibitory Effects of Pyrrole-Based Hydrazones: A Therapeutic Approach to Parkinson’s Disease. Molecules 2022, 27, 8485. [Google Scholar] [CrossRef] [PubMed]
- Tzankova, D.; Kuteva, H.; Mateev, E.; Stefanova, D.; Dzhemadan, A.; Yordanov, Y.; Mateeva, A.; Tzankova, V.; Kondeva-Burdina, M.; Zlatkov, A.; et al. Synthesis, DFT Study, and In Vitro Evaluation of Antioxidant Properties and Cytotoxic and Cytoprotective Effects of New Hydrazones on SH-SY5Y Neuroblastoma Cell Lines. Pharmaceuticals 2023, 16, 1198. [Google Scholar] [CrossRef] [PubMed]
- Mateev, E.; Angelov, B.; Kondeva-Burdina, M.; Valkova, I.; Georgieva, M.; Zlatkov, A. Design, synthesis, biological evaluation and molecular docking of pyrrole-based compounds as antioxidant and MAO-B inhibitory agents. Farmacia 2022, 70, 344–354. [Google Scholar] [CrossRef]
- Rai, M.; Paudel, N.; Sakhrie, M.; Gemmati, D.; Khan, I.A.; Tisato, V.; Kanase, A.; Schulz, A.; Singh, A.V. Perspective on Quantitative Structure–Toxicity Relationship (QSTR) Models to Predict Hepatic Biotransformation of Xenobiotics. Livers 2023, 3, 448–462. [Google Scholar] [CrossRef]
- Hlengwa, N.; Masilela, C.; Mtambo, T.R.; Sithole, S.; Naidoo, S.; Machaba, K.E.; Shabalala, S.C.; Ntamo, Y.; Dludla, P.V.; Milase, R.N. In Vitro Hepatic Models to Assess Herb–Drug Interactions: Approaches and Challenges. Pharmaceuticals 2023, 16, 409. [Google Scholar] [CrossRef] [PubMed]
- Mateeva, A.; Kondeva-Burdina, M.; Nedialkov, P.; Peikova, L.; Georgieva, M. Development of Hyphenated Techniques and Network Identification Approaches for Biotransformational Evaluation of Promising Antitubercular N-pyrrolyl hydrazide-hydrazone in Isolated Rat Hepatocytes. Chromatographia 2023, 86, 497–505. [Google Scholar] [CrossRef]
- Dejaegher, B.; Vander Heyden, Y. HILIC methods in pharmaceutical analysis. J. Sep. Sci. 2010, 33, 698–715. [Google Scholar] [CrossRef] [PubMed]
- Bieke, D.; Liu, Q.; Cai, J.; Nichols, R.G.; Tian, Y.; Zhang, J.; Smith, P.B.; Wang, Y.; Yan, C.; Patterson, A.D. A Quantitative HILIC-MS/MS Assay of the Metabolic Response of Huh-7 Cells Exposed to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. Metabolites 2019, 9, 118. [Google Scholar] [CrossRef]
- Virgiliou, C.; Gika, H.G.; Theodoridis, G.A. HILIC-MS/MS Multi-Targeted Method for Metabolomics Applications. Methods Mol. Biol. 2018, 1738, 65–81. [Google Scholar] [CrossRef]
- Cai, X.; Zou, L.; Dong, J.; Zhao, L.; Wang, Y.; Xu, Q.; Xue, X.; Zhang, X.; Liang, X. Analysis of highly polar metabolites in human plasma by ultra-performance hydrophilic interaction liquid chromatography coupled with quadrupole-time of flight mass spectrometry. Anal. Chim. Acta 2009, 650, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Noga, S. Hydrophilic interaction liquid chromatography (HILIC)--a powerful separation technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Petruczynik, A.; Wróblewski, K.; Waksmundzka-Hajnos, M. Comparison of chromatographic conditions for analysis of selected psychotropic drugs in human serum. J. Chromatogr. Sci. 2015, 53, 394–400. [Google Scholar] [CrossRef][Green Version]
- Studzińska, S.; Bocian, S.; Siecińska, L.; Buszewski, B. Application of phenyl-based stationary phases for the study of retention and separation of oligonucleotides. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1060, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Hawkins, B.A.; Du, J.J.; Groundwater, P.W.; Hibbs, D.E.; Lai, F. A Guide to In Silico Drug Design. Pharmaceutics 2022, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.; Srinivas, K. Modern drug discovery process: An in silico approach. J. Bioinform. Seq. Analysis. 2011, 2, 89–94. [Google Scholar]
- Han, Y.; Zhang, J.; Hu, C.Q.; Zhang, X.; Ma, B.; Zhang, P. In silico ADME and Toxicity Prediction of Ceftazidime and Its Impurities. Front. Pharmacol. 2019, 10, 434. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, Y.; Li, L.; Shen, X.; Chen, G.; Wang, X.; Liang, X.; Tan, M.; Huang, Z. Computational Approaches in Preclinical Studies on Drug Discovery and Development. Front. Chem. 2020, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Tyzack, J.D.; Kirchmair, J. Computational methods and tools to predict cytochrome P450 metabolism for drug discovery. Chem. Biol. Drug Des. 2019, 93, 377–386. [Google Scholar] [CrossRef]
- Yim, S.K.; Kim, K.; Chun, S.; Oh, T.; Jung, W.; Jung, K.; Yun, C.H. Screening of Human CYP1A2 and CYP3A4 Inhibitors from Seaweed In Silico and In Vitro. Mar. Drugs 2020, 18, 603. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.; Kim, D.; Jung, H.; Pan, J.; Chi, Y.; Ahn, T.; Yun, C. Oxidation of human cytochrome P450 1A2 substrates by Bacillus megaterium cytochrome P450 BM3. J. Mol. Catal. B Enzym. 2010, 63, 179–187. [Google Scholar] [CrossRef]
- Angelov, B.; Mateev, E.; Georgieva, M.; Tzankova, V.; Kondeva-Burdina, M. In vitro effects and in silico analysis of newly synthetized pyrrole derivatives on the activity of different isoforms of Cytochrome P450: CYP1A2, CYP2D6 and CYP3A4. Pharmacia 2022, 69, 1013–1017. [Google Scholar] [CrossRef]
- Bijev, A.; Georgieva, M. Pyrrole-based hydrazones synthesized and evaluated in vitro as potential tuberculostatics. Lett. Drug Des. Discov. 2010, 7, 430–437. [Google Scholar] [CrossRef]
- Theodoridis, G.; Gika, H.; Wilson, I. LC-MS-based methodology for global metabolite profiling in metabonomics/metabolomics. TrAC Trends Anal. Chem. 2008, 27, 251–260. [Google Scholar] [CrossRef]
- Fau, D.; Berson, A.; Eugene, D.; Fromenty, B.; Fisch, C.; Pessayre, D. Mechanism for the hepatotoxicity of the antiandrogen, nilutamide. Evidence suggesting that redox cycling of this nitroaromatic drug leads to oxidative stress in isolated hepatocytes. J. Pharmacol. Exp. Ther. 1992, 263, 69–77. [Google Scholar]
- Sansen, S.; Yano, J.K.; Reynald, R.L.; Schoch, G.A.; Griffin, K.J.; Stout, C.D.; Johnson, E.F. Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. J. Biol. Chem. 2007, 282, 14348–14355. [Google Scholar] [CrossRef]
- Schoch, G.A.; Yano, J.K.; Sansen, S.; Dansette, P.M.; Stout, C.D.; Johnson, E.F. Determinants of cytochrome P450 2C8 substrate binding: Structures of complexes with montelukast, troglitazone, felodipine, and 9-cis-retinoic acid. J. Biol. Chem. 2008, 283, 17227–17237. [Google Scholar] [CrossRef]
- Wang, A.; Stout, C.D.; Zhang, Q.; Johnson, E.F. Contributions of ionic interactions and protein dynamics to cytochrome P450 2D6 (CYP2D6) substrate and inhibitor binding. J. Biol. Chem. 2015, 290, 5092–5104. [Google Scholar] [CrossRef]
- Ekroos, M.; Sjögren, T. Structural basis for ligand promiscuity in cytochrome P450 3A4. Proc. Natl. Acad. Sci. USA 2006, 103, 13682–13687. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Hu, T.; Or, P.M.; Wong, J.; Kwan, Y.W.; Wan, D.C.; Hoi, P.M.; Lai, P.B.; Yeung, J.H. Enzyme kinetic and molecular docking studies for the inhibitions of miltirone on major human cytochrome P450 isozymes. Phytomedicine 2013, 20, 367–374. [Google Scholar] [CrossRef]

| Mobile Phase | Ratio | Ret. Time of 11b (Mins) |
|---|---|---|
| AN: Phosphate buffer pH 3.5: CH3OH | 5:35:60 | >25 |
| AN: Phosphate buffer pH 3.5: CH3OH | 10:35:55 | 23.166 |
| AN: Phosphate buffer pH 3.5: CH3OH | 35:35:30 | 9.843 |
| AN: Phosphate buffer pH 3.5: CH3OH | 17:30:53 | 13.093 |
| Sample | Level (%) | Spike, μM | Recovery, μM | Recovery (%) | ±SD | ±RSD, (%) |
|---|---|---|---|---|---|---|
| Hydrazone 11b | 50% | 7.5 | 7.59 | 101.14 | 0.9948 | 0.9947 |
| 7.44 | 99.27 | |||||
| 7.47 | 99.62 | |||||
| 100% | 15 | 15.05 | 100.30 | 0.1516 | 0.1513 | |
| 15.00 | 100.01 | |||||
| 15.03 | 100.21 | |||||
| 150% | 22.5 | 22.49 | 99.94 | 0.1853 | 0.1853 | |
| 22.55 | 100.21 | |||||
| 22.47 | 99.85 |
| Compound | CYP1A2 (PDB: 2HI4) | CYP2C8 (PDB: 2VN0) | CYP2C9 (PDB: 5W0C) | CYP3A4 (PDB: 2V0M) | ||||
|---|---|---|---|---|---|---|---|---|
| IFD | MM/GBSA | IFD | MM/GBSA | IFD | MM/GBSA | IFD | MM/GBSA | |
| 11b | n/a | n/a | −9.46 | −51.95 | −6.87 | −45.64 | −8.08 | −51.15 |
| Alpha-naphthoflavone | −11.241 | −89.435 | n/d | n/d | n/d | n/d | n/d | n/d |
| Troglitazone | n/d | n/d | −9.317 | −58.83 | n/d | n/d | n/d | n/d |
| *9W6 | n/d | n/d | n/d | n/d | −9.02 | −58.36 | n/d | n/d |
| Ketoconazole | n/d | n/d | n/d | n/d | n/d | n/d | −12.194 | −53.54 |
| Compound | tR (Min) | Chemical Formula | Theoretical [M + H]+ | MS Fragmentation |
|---|---|---|---|---|
| 11b | 15.91 | C23H22BrN3O4 | 484.086638 | 484.0863 (99.53), 486.0836 (100), 438.0439 (32.42) |
| M9_11b | 13.28 | C23H22BrN3O5 | 500.081552 | 500.0815 (99.32), 502.0789 (100), 454.0370 (34.08), 107.0496 (17.92) |
| M10_11b | 13.41 | C23H22BrN3O5 | 500.081552 | 500.0817 (99.66), 502.0789 (100), 454.0392 (15.86), 107.0495 (11.26) |
| HPLC Method | Stationary Phase | Mobile Phase | Chromatographic Parameters |
|---|---|---|---|
| C18 | Purospher® STAR RP-18 (4.6 × 125 mm, particles size 5 μm) | acetonitrile: phosphate buffer pH 3.5: methanol in ratio 35/35/30 (v/v/v) | 1 mL/min, 25 °C, 20 μL, 272 and 279 nm. |
| HILIC | Acclaim® Mixed-Mode HILIC (4.6 × 150 mm, particles size 5 μm) | Not obtained | 1 mL/min, 25 °C, 20 μL, 272 and 279 nm. |
| Phenyl–hexyl | XSelect® CSH Phenyl–Hexyl (4.6 × 150 mm, particles size 5 μm) | acetonitrile: phosphate buffer pH 3.5: methanol—17:30:53 (v/v/v) | 1mL/min, 25 °C, 20 μL, 272 and 279 nm. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateeva, A.; Kondeva-Burdina, M.; Mateev, E.; Nedialkov, P.; Lyubomirova, K.; Peikova, L.; Georgieva, M.; Zlatkov, A. In Silico and Chromatographic Methods for Analysis of Biotransformation of Prospective Neuroprotective Pyrrole-Based Hydrazone in Isolated Rat Hepatocytes. Molecules 2024, 29, 1474. https://doi.org/10.3390/molecules29071474
Mateeva A, Kondeva-Burdina M, Mateev E, Nedialkov P, Lyubomirova K, Peikova L, Georgieva M, Zlatkov A. In Silico and Chromatographic Methods for Analysis of Biotransformation of Prospective Neuroprotective Pyrrole-Based Hydrazone in Isolated Rat Hepatocytes. Molecules. 2024; 29(7):1474. https://doi.org/10.3390/molecules29071474
Chicago/Turabian StyleMateeva, Alexandrina, Magdalena Kondeva-Burdina, Emilio Mateev, Paraskev Nedialkov, Karolina Lyubomirova, Lily Peikova, Maya Georgieva, and Alexander Zlatkov. 2024. "In Silico and Chromatographic Methods for Analysis of Biotransformation of Prospective Neuroprotective Pyrrole-Based Hydrazone in Isolated Rat Hepatocytes" Molecules 29, no. 7: 1474. https://doi.org/10.3390/molecules29071474
APA StyleMateeva, A., Kondeva-Burdina, M., Mateev, E., Nedialkov, P., Lyubomirova, K., Peikova, L., Georgieva, M., & Zlatkov, A. (2024). In Silico and Chromatographic Methods for Analysis of Biotransformation of Prospective Neuroprotective Pyrrole-Based Hydrazone in Isolated Rat Hepatocytes. Molecules, 29(7), 1474. https://doi.org/10.3390/molecules29071474








