Acylated Inulin as a Potential Shale Hydration Inhibitor in Water Based Drilling Fluids for Wellbore Stabilization
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Inhibition Evaluation
2.2.1. Shale Cuttings Hot-Rolling Dispersion Test
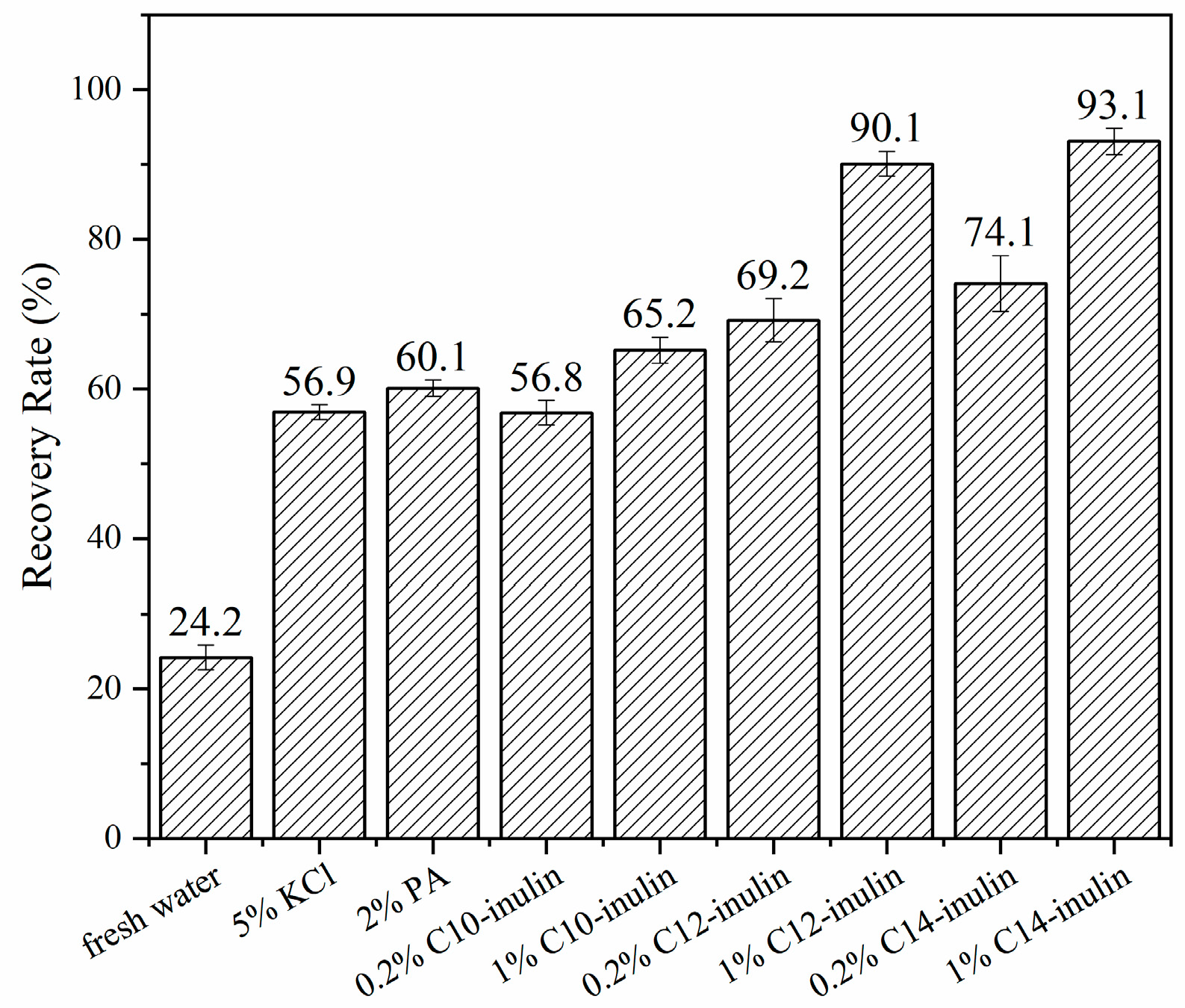
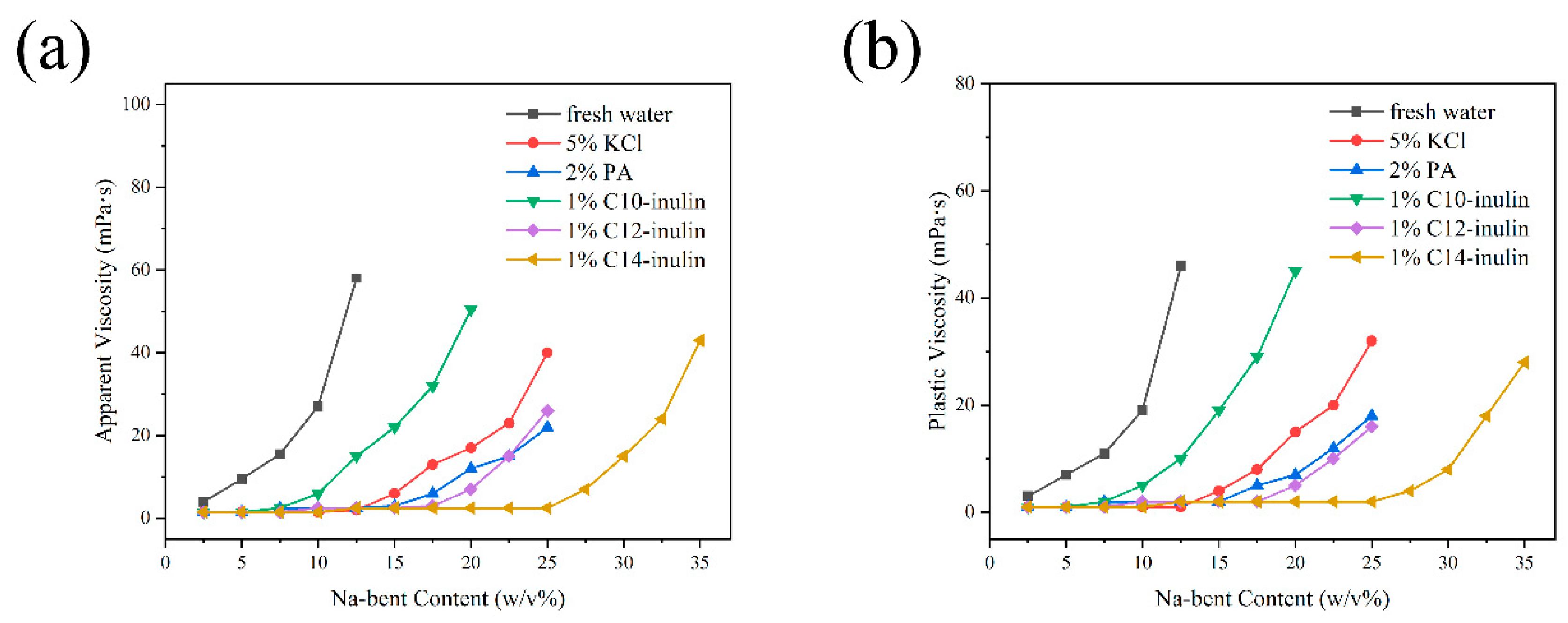
2.2.2. Na-Bent Hydration Test
2.2.3. Capillary Suction Time Test
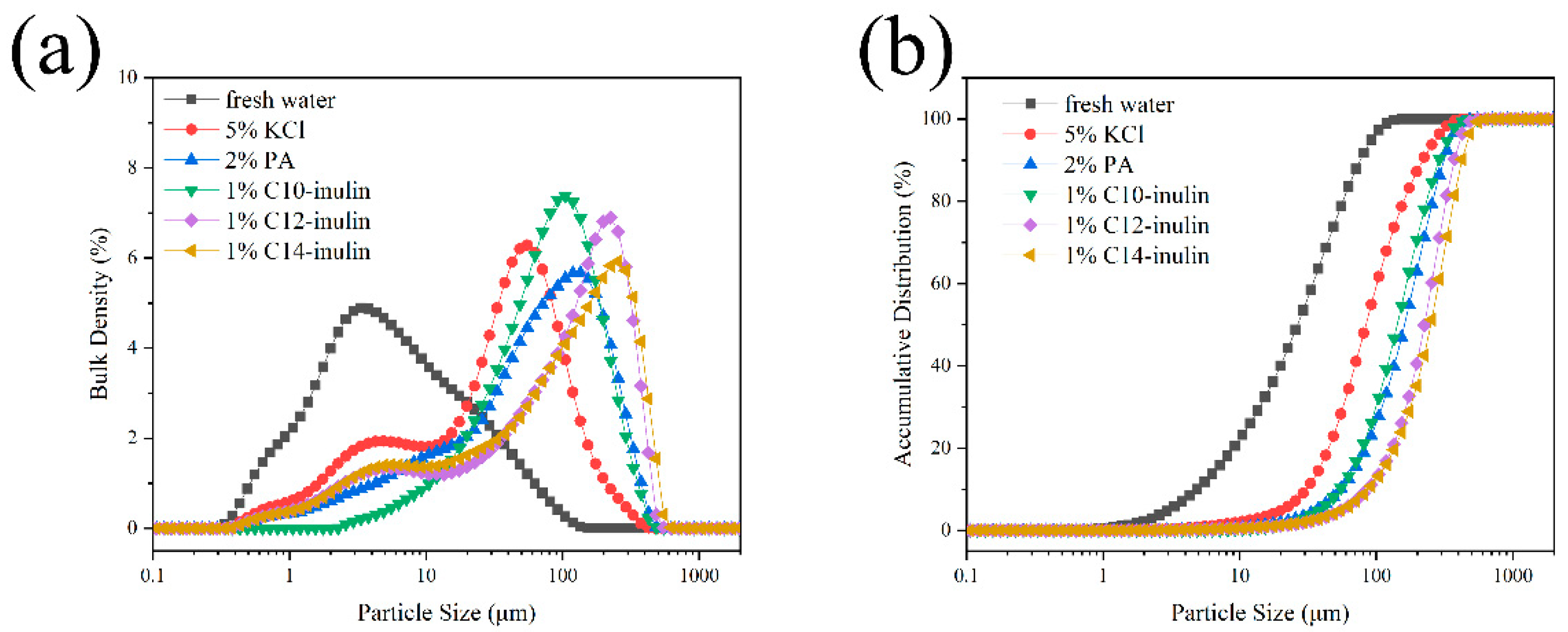
2.2.4. Particle Size Distribution
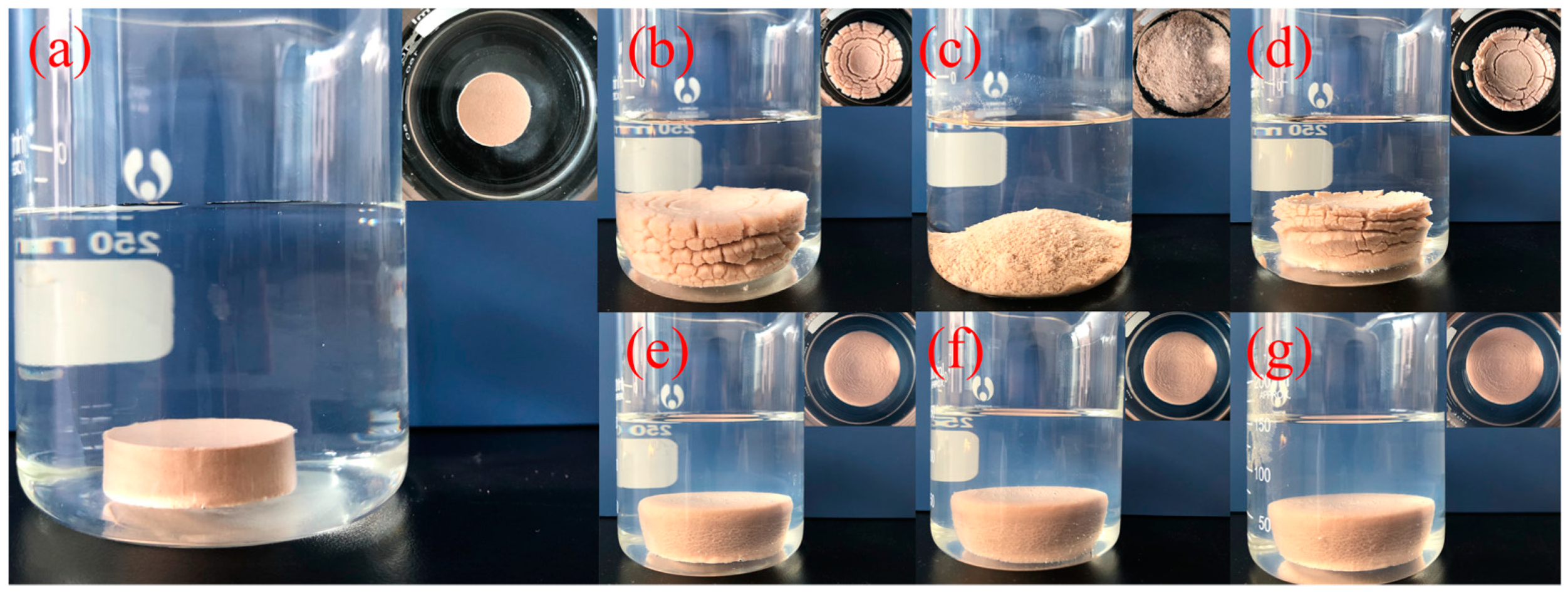
2.2.5. Immersion Test
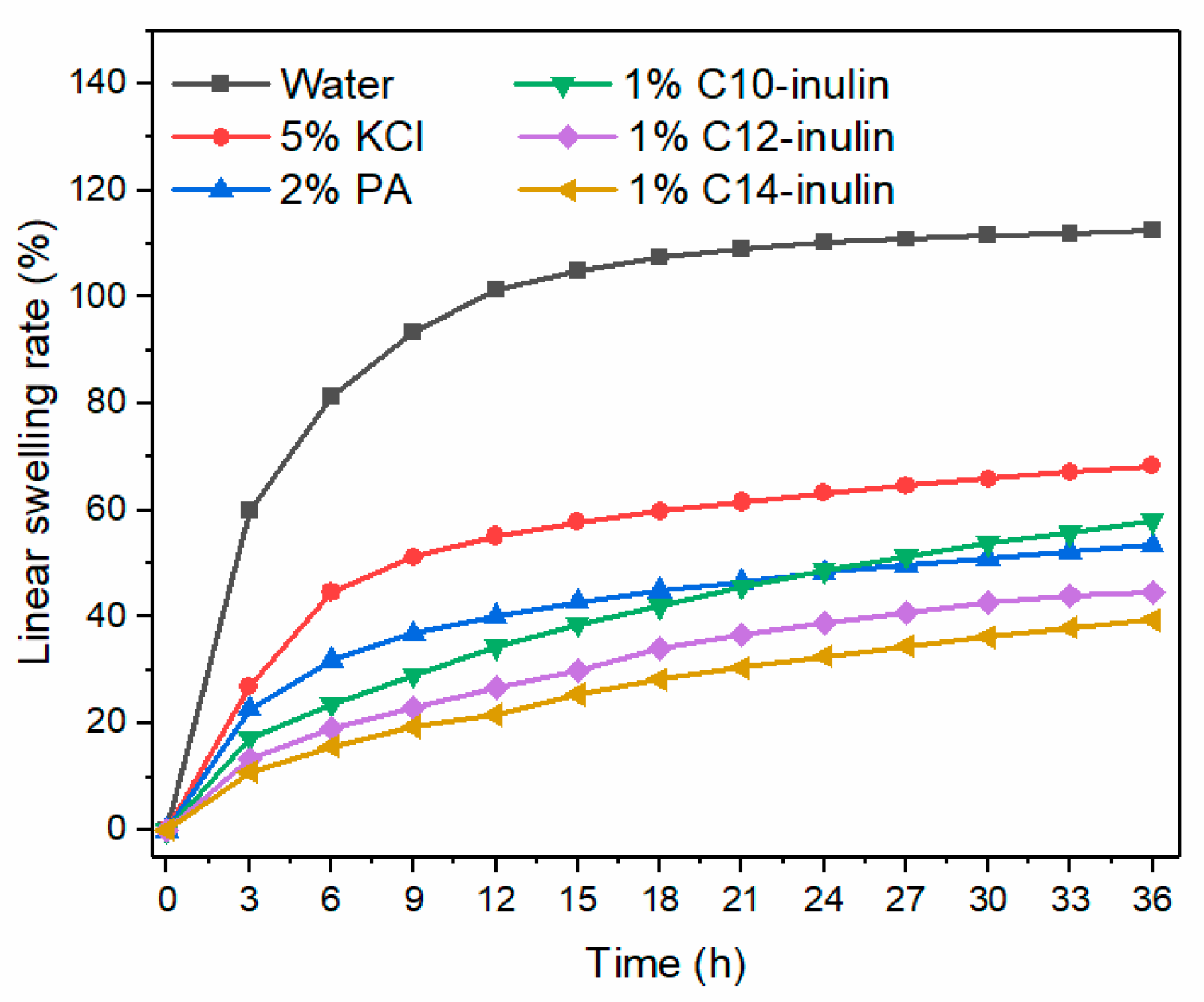
2.2.6. Linear Swelling Test
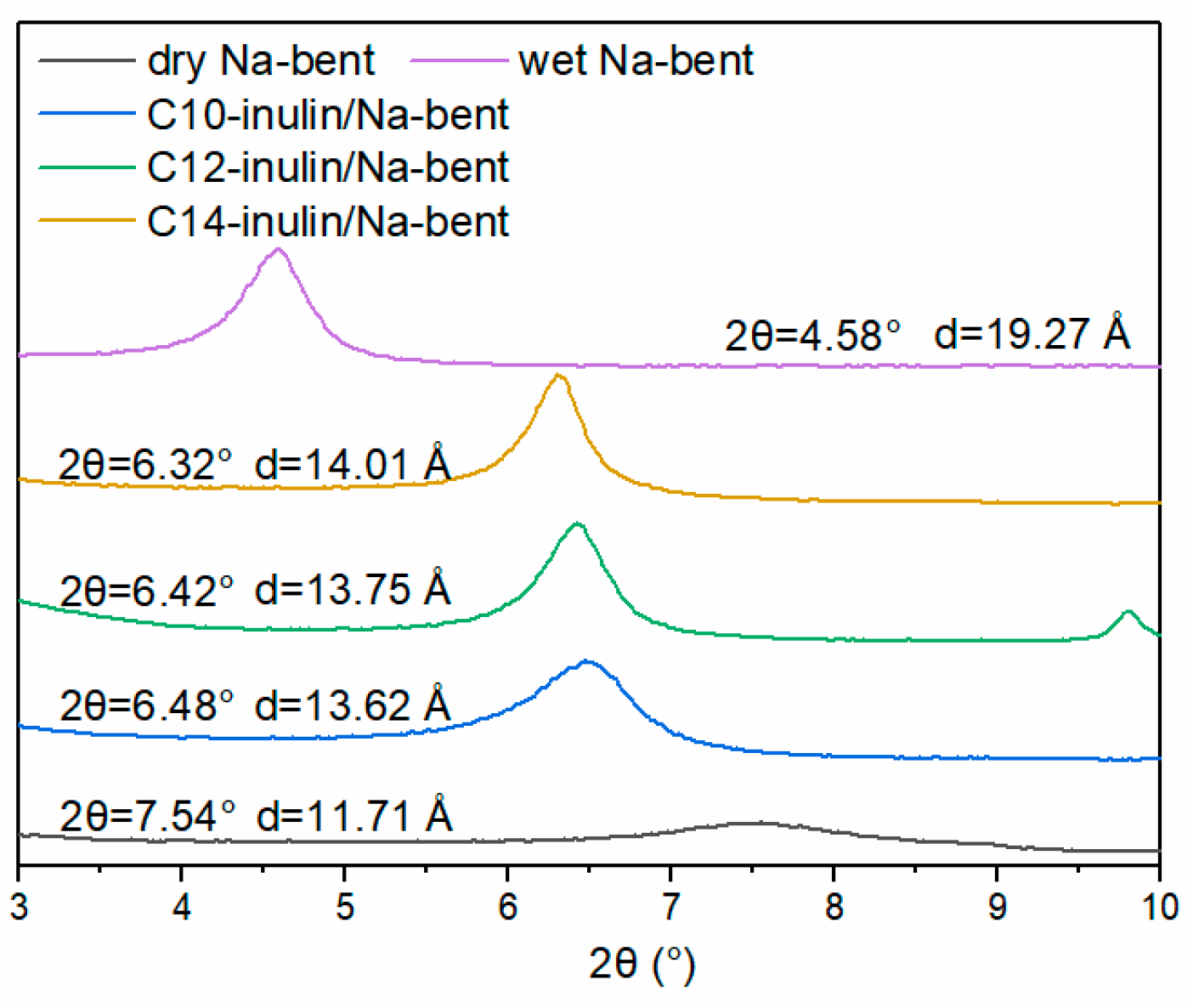
2.2.7. XRD Analysis
2.3. Inhibitive Mechanism Investigation
2.3.1. Static Surface Tension Measurement
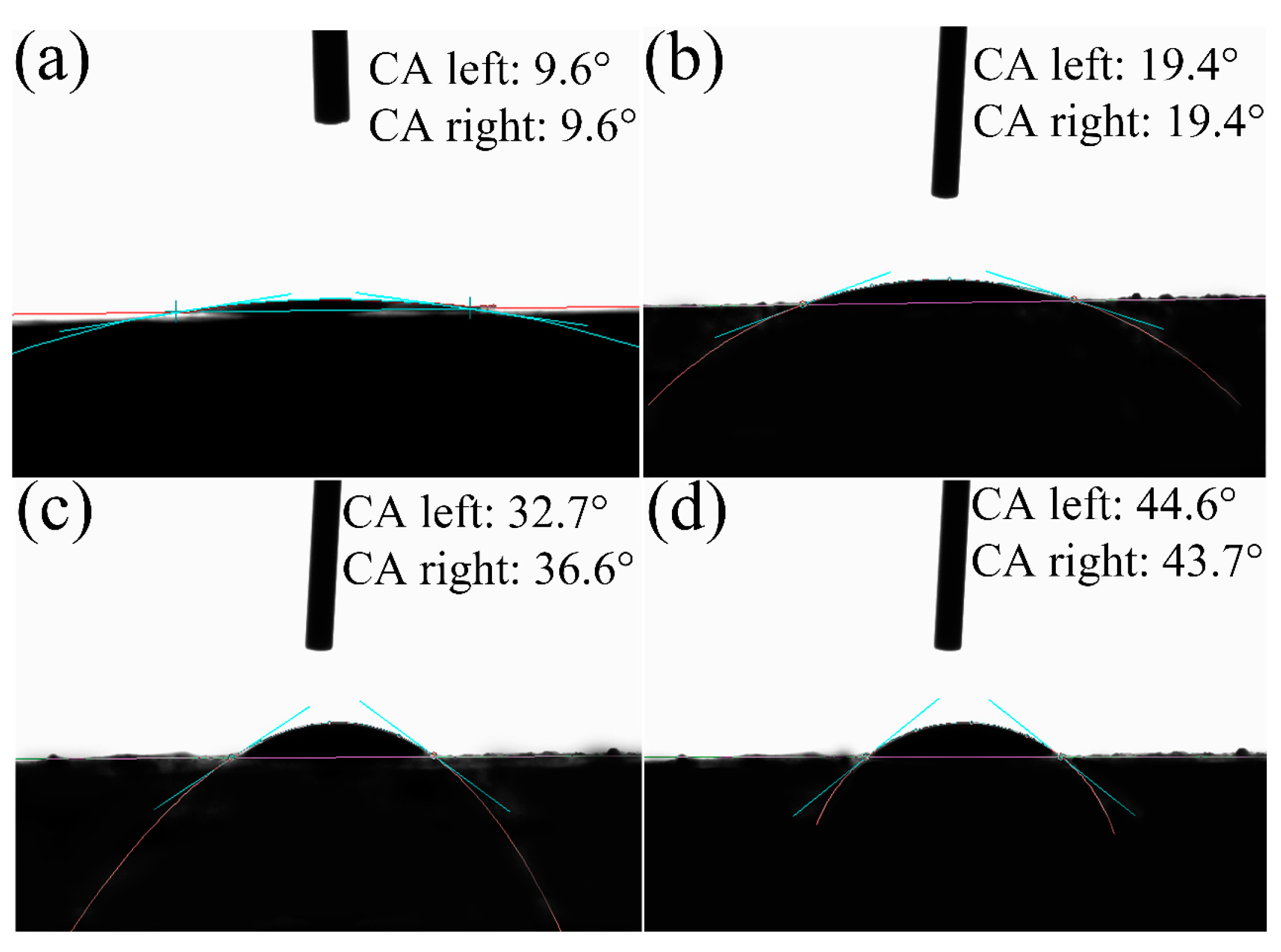
2.3.2. Contact Angle Measurement
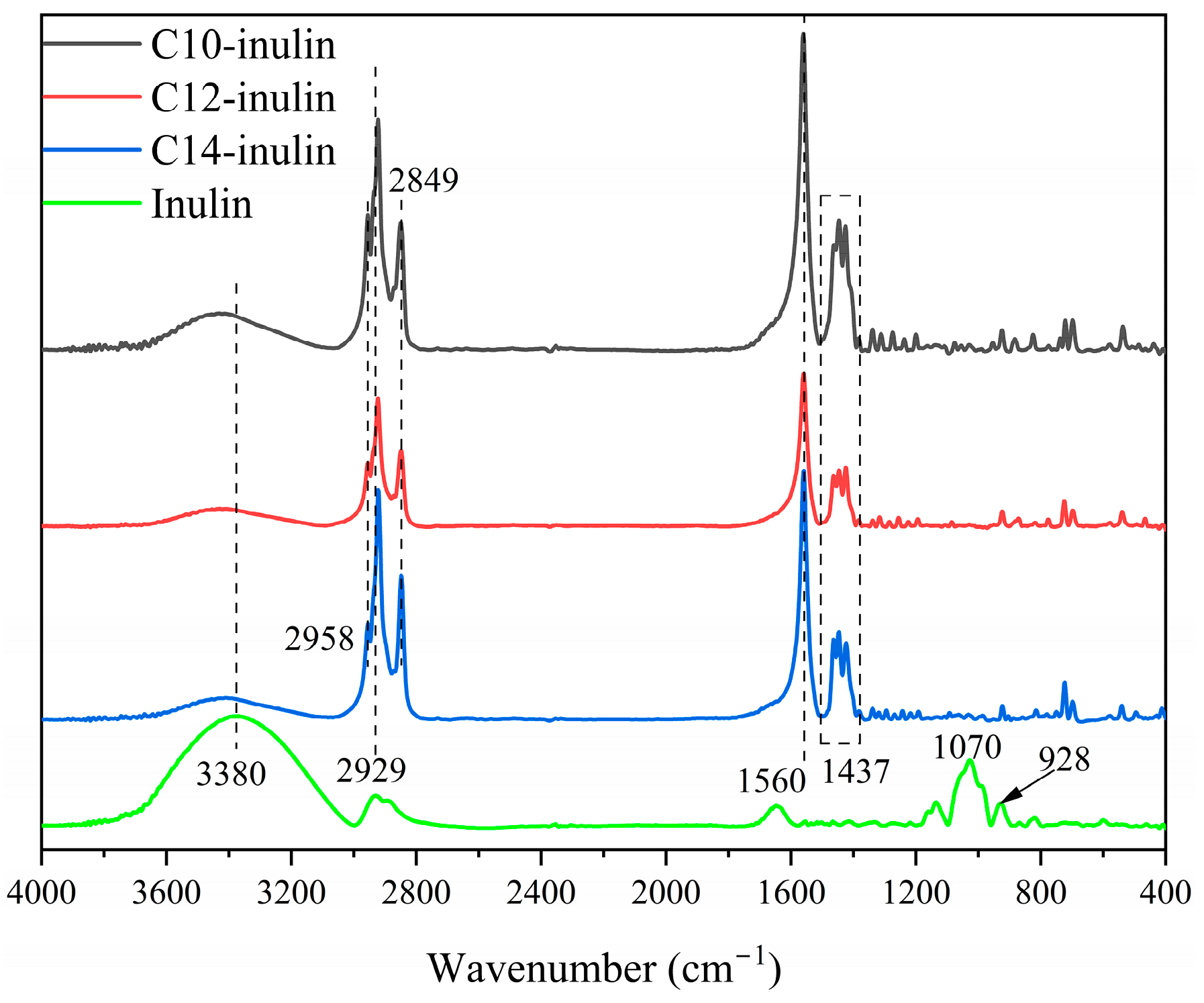
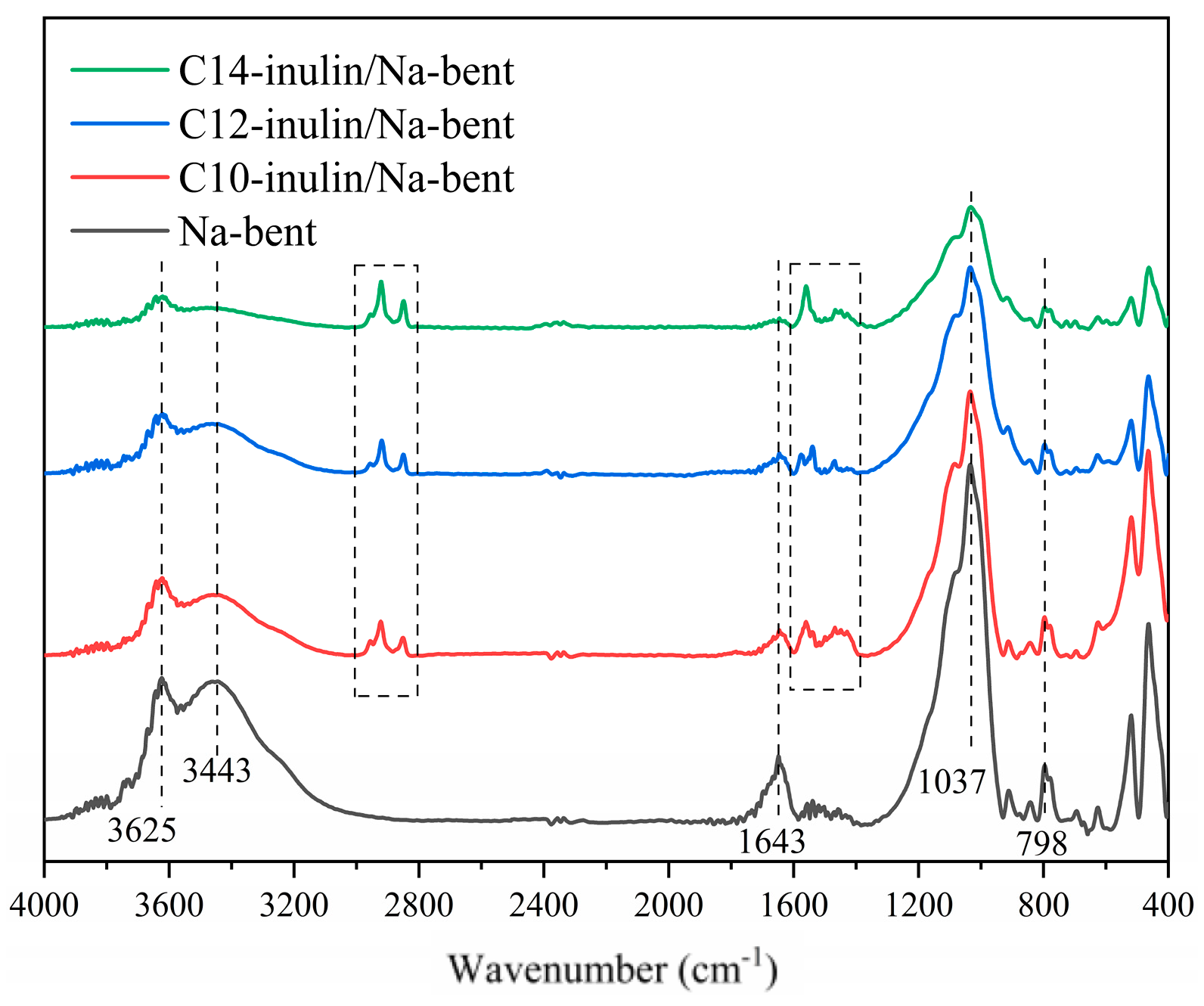
2.3.3. FTIR Analysis
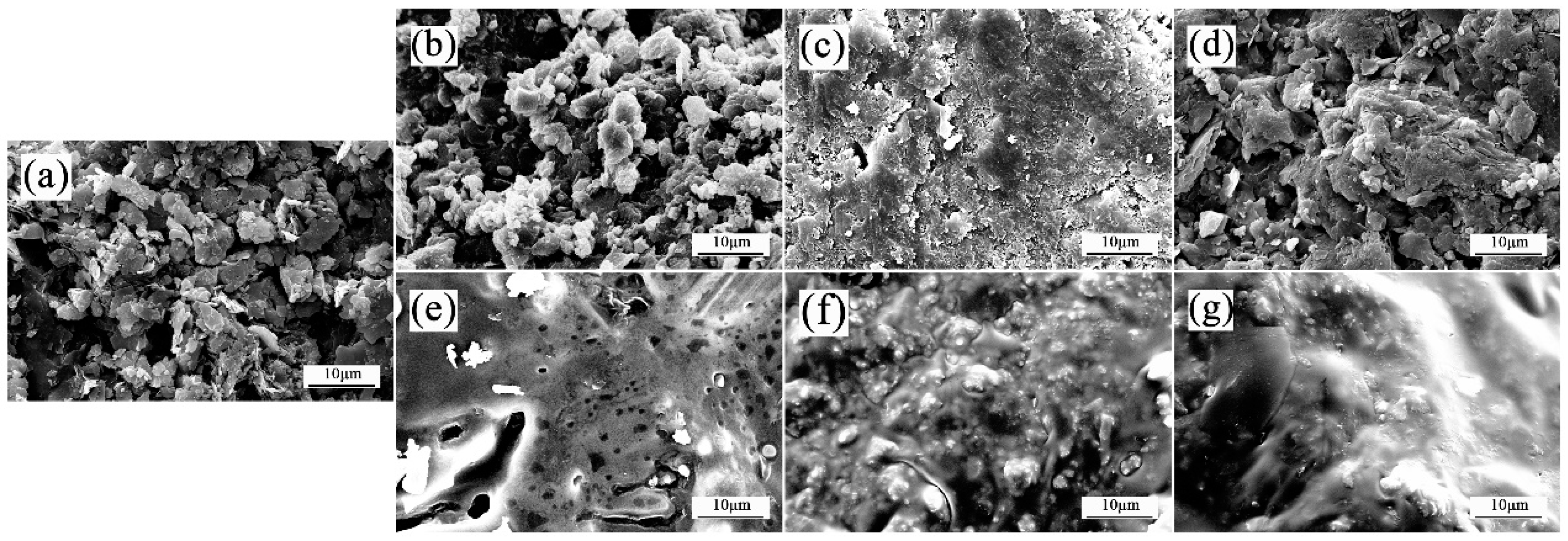
2.3.4. Morphology
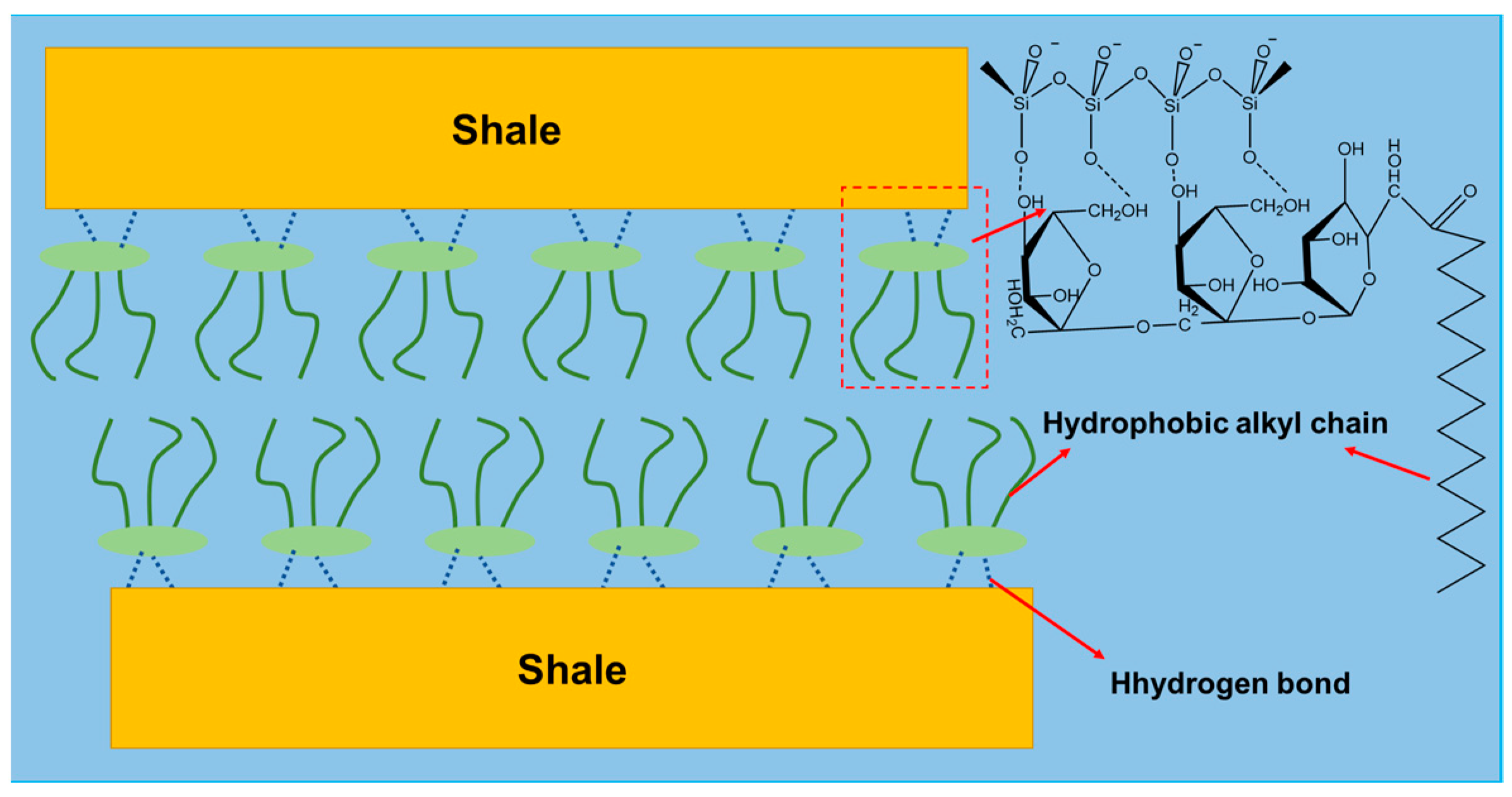
2.3.5. Probable Inhibition Mechanism
2.3.6. Environmental Aspects
3. Materials and Methods
3.1. Materials
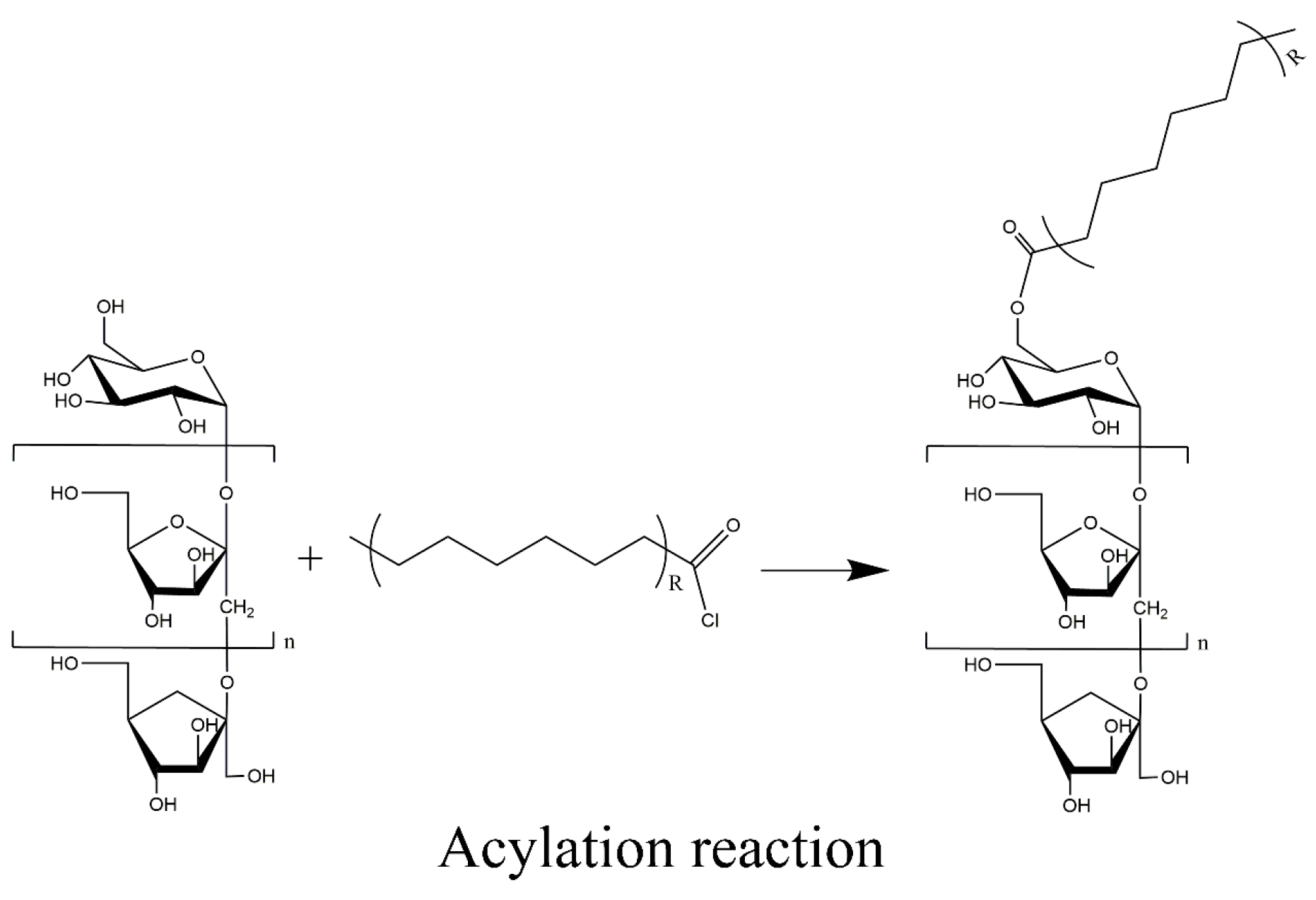
3.2. Acylated Inulin Preparation
3.3. Inhibition Property Evaluation
3.3.1. Shale Cuttings Hot-Rolling Dispersion Test
3.3.2. Na-Bent Hydration Test
3.3.3. Capillary Suction Time Test
3.3.4. Immersion Test
3.3.5. Linear Swelling Test
3.3.6. Ecotoxicological Test
3.3.7. Biodegradability Test
3.4. Characterization Methods
3.4.1. Fourier-Transform–Infrared (FT-IR) Spectroscopy
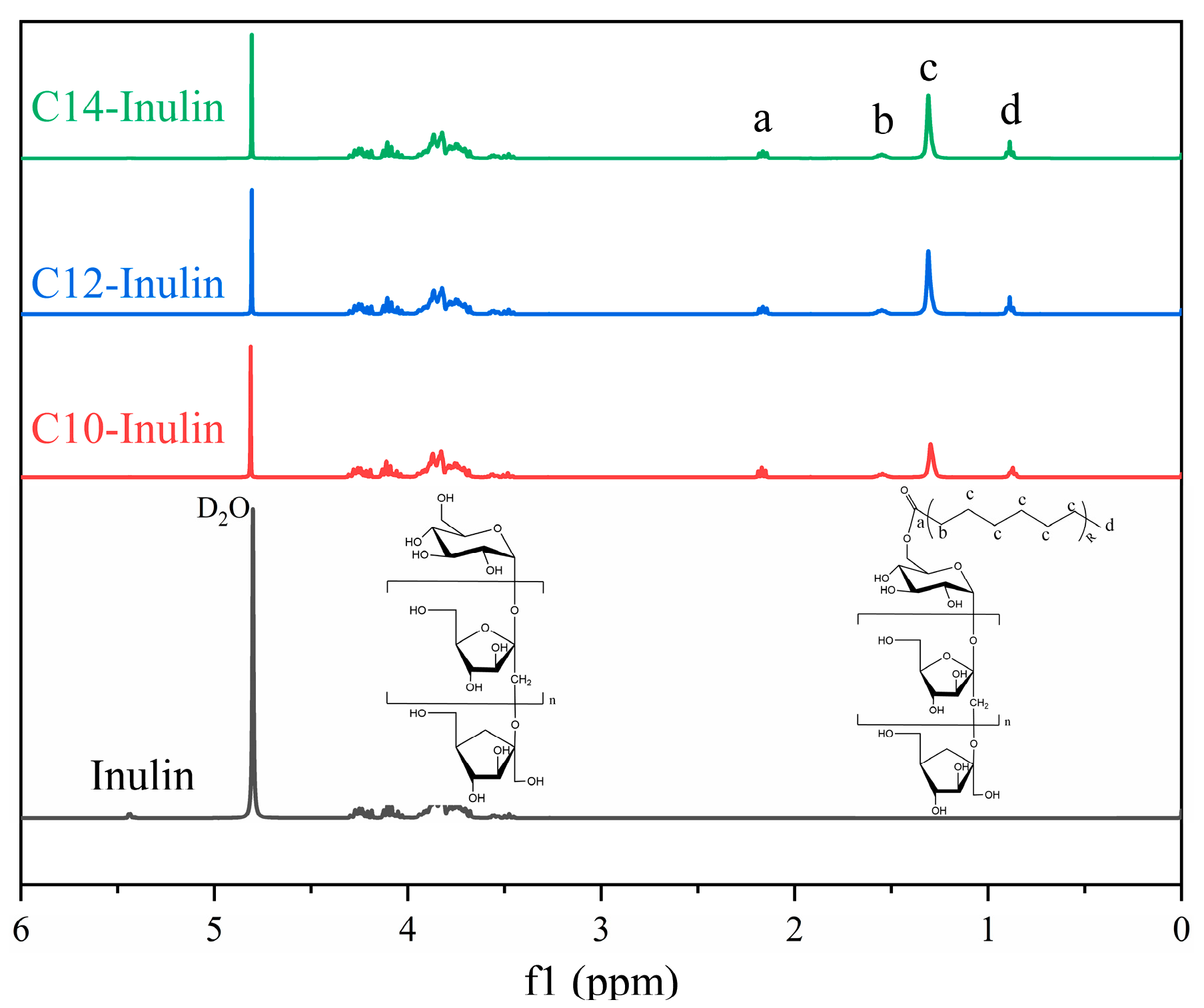
3.4.2. 1H NMR Nuclear Magnetic Resonance (NMR)
3.4.3. Scanning Electron Microscopy (SEM)
3.4.4. X-ray Diffraction (XRD)
3.4.5. Particle Size Distribution
3.4.6. Static Surface Tension Measurement
3.4.7. Contact Angle Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| OBDFs | oil-based drilling fluids |
| WBDFs | water-based drilling fluids |
| SHIs | shale hydration inhibitors |
| SBSs | sugar-based surfactants |
| C10 | decanoyl chloride |
| C12 | lauroyl chloride |
| C14 | myristoyl chloride |
| XRD | X-ray diffraction |
| SEM | scanning electron microscopy |
| KCl | potassium chloride |
| PA | poly (ester amine) |
| Na-bent | sodium-based bentonite |
| AV | apparent viscosity |
| PV | plastic viscosity |
| CST | capillary suction time |
| CA | contact angle |
| COD | chemical oxygen demand |
| BOD | biochemical oxygen demand |
| EC50 | concentration for 50% of maximal effect |
References
- Wilson, M.; Wilson, L. Clay mineralogy and shale instability: An alternative conceptual analysis. Clay Miner. 2014, 49, 127–145. [Google Scholar] [CrossRef]
- Lin, H.; Sun, X.; Yuan, Y.; Lai, X.; Qu, H.; Luo, C. Experimental investigation on the dynamic volume changes of varied-size pores during shale hydration. J. Nat. Gas Sci. Eng. 2022, 101, 104506. [Google Scholar] [CrossRef]
- Diaz-Perez, A.; Cortes-Monroy, I.; Roegiers, J. The role of water/clay interaction in the shale characterization. J. Pet. Sci. Eng. 2007, 58, 83–98. [Google Scholar] [CrossRef]
- Tan, P.; Jin, Y.; Han, K.; Hou, B.; Chen, M.; Guo, X.; Gao, J. Analysis of hydraulic fracture initiation and vertical propagation behavior in laminated shale formation. Fuel 2017, 206, 482–493. [Google Scholar] [CrossRef]
- Gholami, R.; Elochukwu, H.; Fakhari, N.; Sarmadivaleh, M. A review on borehole instability in active shale formations: Interactions, mechanisms and inhibitors. Earth-Sci. Rev. 2018, 177, 2–13. [Google Scholar] [CrossRef]
- Zeynali, M.E. Mechanical and physico-chemical aspects of wellbore stability during drilling operations. J. Pet. Sci. Eng. 2012, 82, 120–124. [Google Scholar] [CrossRef]
- Ding, Y.; Luo, P.; Liu, X.; Liang, L. Wellbore stability model for horizontal wells in shale formations with multiple planes of weakness. J. Nat. Gas Sci. Eng. 2018, 52, 334–347. [Google Scholar] [CrossRef]
- Barati, P.; Shahbazi, K.; Kamari, M.; Aghajafari, A. Shale hydration inhibition characteristics and mechanism of a new amine-based additive in water-based drilling fluids. Petroleum 2017, 3, 476–482. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Khan, K.; Abdulraheem, A.; Al-Majed, A.; Awal, M. Analysis of wellbore instability in vertical, directional, and horizontal wells using field data. J. Pet. Sci. Eng. 2007, 55, 83–92. [Google Scholar] [CrossRef]
- Jingyuan, M.; Boru, X.; Yuxiu, A. Advanced developments in low-toxic and environmentally friendly shale inhibitor: A review. J. Pet. Sci. Eng. 2022, 208, 109578. [Google Scholar] [CrossRef]
- Bunger, A.P.; Sarout, J.; Kear, J.; Piane, C.D.; Detournay, E.; Josh, M.; Dewhurst, D.N. Experimental chemoporoelastic characterization of shale using millimeter-scale specimens. J. Pet. Sci. Eng. 2014, 118, 40–51. [Google Scholar] [CrossRef]
- Shivhare, S.; Kuru, E. A study of the pore-blocking ability and formation damage characteristics of oil-based colloidal gas aphron drilling fluids. J. Pet. Sci. Eng. 2014, 122, 257–265. [Google Scholar] [CrossRef]
- Agency, E.P. European Waste Catalogue and Hazardous Waste List; Environmental Protection Agency Ireland: Wexford, Ireland, 2002. [Google Scholar]
- Liu, X.; Zeng, W.; Liang, L.; Xiong, J. Experimental study on hydration damage mechanism of shale from the Longmaxi Formation in southern Sichuan Basin, China. Petroleum 2016, 2, 54–60. [Google Scholar] [CrossRef]
- Quainoo, A.K.; Bavoh, C.B.; Duartey, K.O.; Alhassan, D. Clay swelling inhibition mechanism based on inhibitor-water interaction: A COSMO-RS molecular simulation approach. Upstream Oil Gas Technol. 2022, 9, 100080. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Z.; Qu, L.; Peng, X.; Ma, R. Nanoparticles and polymers complexes as a harmless shale plugging inhibitor for ocean water-based drilling fluids. Ocean Eng. 2023, 286, 115563. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, L.; Yu, P.; Chen, Z. Experimental study on the application of an ionic liquid as a shale inhibitor and inhibitive mechanism. Appl. Clay Sci. 2017, 150, 267–274. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, G.; Shi, Y.; Yang, X. Application of ionic liquid and polymeric ionic liquid as shale hydration inhibitors. Energy Fuels 2017, 31, 4308–4317. [Google Scholar] [CrossRef]
- Bai, X.; Wang, H.; Luo, Y.; Zheng, X.; Zhang, X.; Zhou, S.; Pu, X. The structure and application of amine-terminated hyperbranched polymer shale inhibitor for water-based drilling fluid. J. Appl. Polym. Sci. 2017, 134, 45466. [Google Scholar] [CrossRef]
- Jiang, G.; Qi, Y.; An, Y.; Huang, X.; Ren, Y. Polyethyleneimine as shale inhibitor in drilling fluid. Appl. Clay Sci. 2016, 127–128, 70–77. [Google Scholar]
- Qu, Y.; Lai, X.; Zou, L.; Su, Y. Polyoxyalkyleneamine as shale inhibitor in water-based drilling fluids. Appl. Clay Sci. 2009, 3, 265–268. [Google Scholar] [CrossRef]
- Zhong, H.; Qiu, Z.; Tang, Z.; Zhang, X.; Xu, J.; Huang, W. Study of 4,4′-methylenebis-cyclohexanamine as a high temperature-resistant shale inhibitor. J. Mater. Sci. 2016, 51, 7585–7597. [Google Scholar] [CrossRef]
- Huang, X.; Dai, Z.; Zhang, C.; Lv, K.; Meng, X.; Wang, R.; Lalji, S.M.; Khan, M.A. Improving the performance of a polyamine shale inhibitor by introducing a hydrophobic group in oil and gas development. Fuel 2024, 355, 129435. [Google Scholar] [CrossRef]
- Lv, K.; Liu, J.; Jin, J.; Sun, J.; Huang, X.; Liu, J.; Guo, X.; Hou, Q.; Zhao, J.; Liu, K.; et al. Synthesis of a novel cationic hydrophobic shale inhibitor with preferable wellbore stability. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128274. [Google Scholar] [CrossRef]
- Jia, H.; Huang, P.; Wang, Q.; Han, Y.; Wang, S.; Zhang, F.; Pan, W.; Lv, K. Investigation of inhibition mechanism of three deep eutectic solvents as potential shale inhibitors in water-based drilling fluids. Fuel 2019, 244, 403–411. [Google Scholar] [CrossRef]
- Medved, I.; Gaurina-Međimurec, N.; Pašić, B.; Mijić, P. Green Approach in Water-Based Drilling Mud Design to Increase Wellbore Stability. Appl. Sci. 2022, 12, 5348. [Google Scholar] [CrossRef]
- Moslemizadeh, A.; Aghdam, S.K.-y.; Shahbazi, K.; Zendehboudi, S. A triterpenoid saponin as an environmental friendly and biodegradable clay swelling inhibitor. J. Mol. Liq. 2017, 247, 269–280. [Google Scholar] [CrossRef]
- Shadizadeh, S.R.; Moslemizadeh, A.; Dezaki, A.S. A novel nonionic surfactant for inhibiting shale hydration. Appl. Clay Sci. 2015, 118, 74–86. [Google Scholar] [CrossRef]
- Han, L.; Ratcliffe, I.; Williams, P. Synthesis, characterisation and physicochemical properties of hydrophobically modified inulin using long-chain fatty acyl chlorides. Carbohydr. Polym. 2017, 178, 141–146. [Google Scholar] [CrossRef]
- Evans, M.; Han, L.; Ratcliffe, I.; Williams, P. Synthesis, characterisation and properties of novel biosurfactants based on hydrophobically-modified inulins. Gums Stabilisers Food Ind. 2016, 18, 123. [Google Scholar]
- Morros, J.; Levecke, B.; Infante, M.R. Chemical hydrophobic modification of inulin in aqueous media: Synthesis of β-hydroxyalkyl ethers of inulin. Carbohydr. Polym. 2010, 81, 681–686. [Google Scholar] [CrossRef]
- Muhammad, U.; Zhang, C.; Prasanna, J.P.; Arshad, M.; Li, X.; Muhammad, B.; Junaid, H.; Shabbir, A. Potential applications of hydrophobically modified inulin as an active ingredient in functional foods and drugs—A review. Carbohydr. Polym. 2021, 252, 117176. [Google Scholar]
- Jain, R.; Mahto, V.; Sharma, V. Evaluation of polyacrylamide-grafted-polyethylene glycol/silica nanocomposite as potential additive in water based drilling mud for reactive shale formation. J. Nat. Gas Sci. Eng. 2015, 26, 526–537. [Google Scholar] [CrossRef]
- Fares, M.M.; Khanfar, M. Inulin and poly (acrylic acid) grafted inulin for dissolution enhancement and preliminary controlled release of poorly water-soluble Irbesartan drug. Int. J. Pharm. 2011, 410, 206–211. [Google Scholar] [CrossRef]
- Kokubun, S.; Ratcliffe, I.; Williams, P.A. Synthesis, characterization and self-assembly of biosurfactants based on hydrophobically modified inulins. Biomacromolecules 2013, 14, 2830–2836. [Google Scholar] [CrossRef]
- Namazi, H.; Fathi, F.; Dadkhah, A. Hydrophobically modified starch using long-chain fatty acids for preparation of nanosized starch particles. Sci. Iranica. 2011, 18, 439–445. [Google Scholar] [CrossRef][Green Version]
- Barclay, T.; Ginic-Markovic, M.; Johnston, M.R.; Cooper, P.D.; Petrovsky, N. Analysis of the hydrolysis of inulin using real time 1H NMR spectroscopy. Carbohydr. Res. 2012, 352, 117–125. [Google Scholar] [CrossRef]
- Cerantola, S.; Kervarec, N.; Pichon, R.; Magné, C.; Bessieres, M.; Deslandes, E. NMR characterisation of inulin-type fructooligosaccharides as the major water-soluble carbohydrates from Matricaria maritima (L.). Carbohydr. Res. 2004, 339, 2445–2449. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Tan, W.; Wang, G.; Dong, F.; Li, Q.; Guo, Z. Antioxidant activity of inulin derivatives with quaternary ammonium. Starch 2017, 69, 1700046. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, W.; Li, Q.; Dong, F.; Gu, G.; Guo, Z. Synthesis of inulin derivatives with quaternary phosphonium salts and their antifungal activity. Int. J. Biol. Macromol. 2018, 113, 1273–1278. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, Z.; Huang, W.; Wang, M. Mechanism and method for controlling low-temperature rheology of water-based drilling fluids in deepwater drilling. J. Pet. Sci. Eng. 2017, 154, 405–416. [Google Scholar] [CrossRef]
- An, Y.; Yu, P. A strong inhibition of polyethyleneimine as shale inhibitor in drilling fluid. J. Pet. Sci. Eng. 2018, 161, 1–8. [Google Scholar] [CrossRef]
- Peng, B.; Luo, P.; Guo, W.; Yuan, Q. Structure-property relationship of polyetheramines as clay-swelling inhibitors in water-based drilling fluids. J. Appl. Polym. Sci. 2013, 129, 1074–1079. [Google Scholar] [CrossRef]
- ISO 10414-1; Recommended Practice for Field Testing Water-Based Drilling Fluids. API Recommended Practice 13B-1; American Petroleum Institute: Washington, DC, USA, 2003.


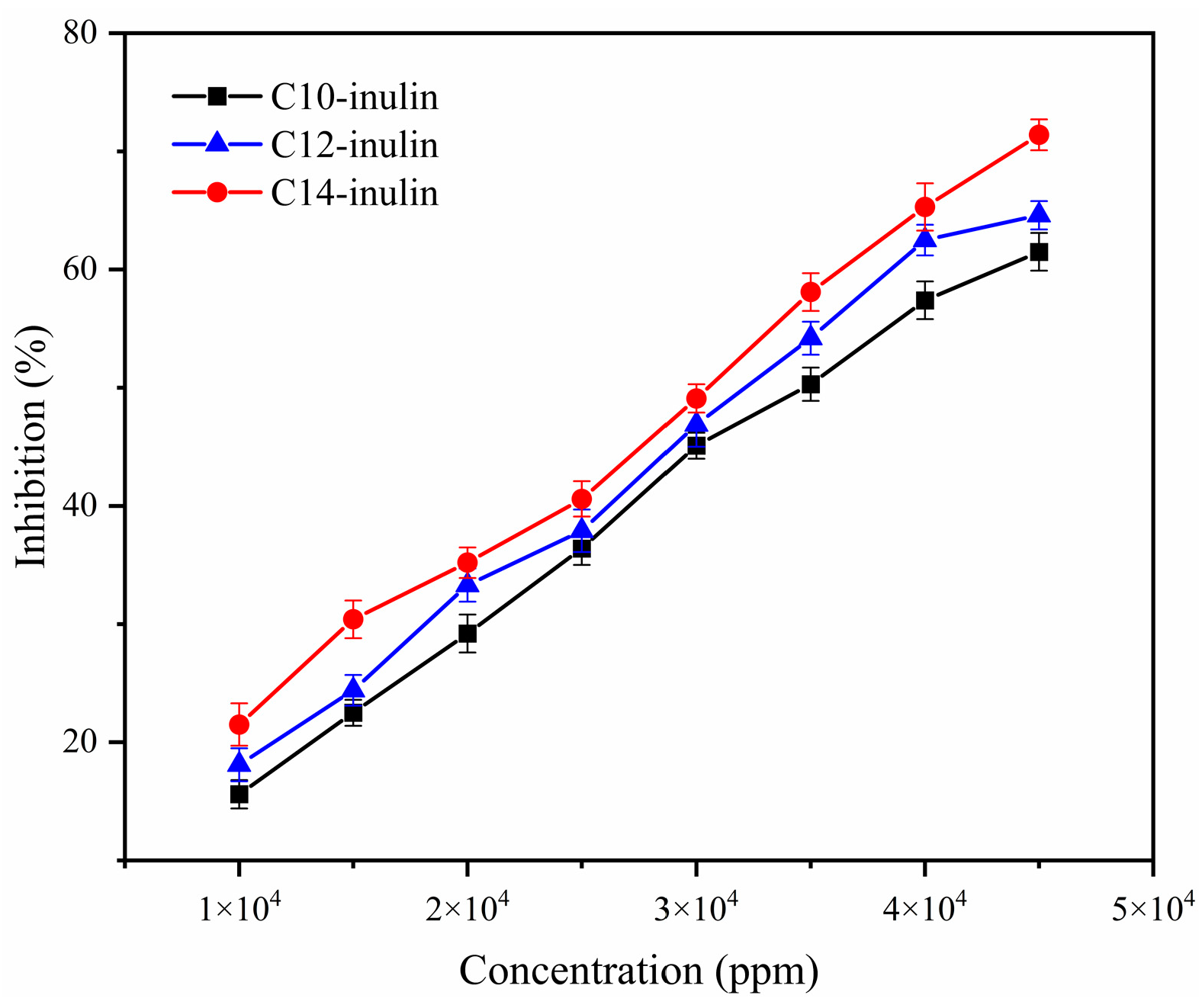

| Linear Regression Equation | R2 | EC50 (ppm) | |
|---|---|---|---|
| C10-inulin | y = 0.0014x + 2.5726 | 0.9954 | 33,876.7143 |
| C12-inulin | y = 0.0014x + 4.2571 | 0.9918 | 32,673.5 |
| C14-inulin | y = 0.0014x + 7.0988 | 0.9947 | 30,643.7143 |
| Measured Values | Standard Value | Classification | |||
|---|---|---|---|---|---|
| C10-Inulin | C12-Inulin | C14-Inulin | |||
| BOD5 (ppm) | 15.4 | 15.7 | 15.9 | <20 | qualified |
| COD (ppm) | 49.6 | 56.1 | 58.9 | <100 | qualified |
| BOD5/COD | 0.31 | 0.28 | 0.27 | 0.10 | biodegradable |
| Mineral Composition | Content (%) | Component of Clay Mineral | Relative Content (%) |
|---|---|---|---|
| Quartz | 41.3 | Kaolinite | 1.9 |
| Potassium feldspar | 2.1 | Illite | 29.9 |
| Sodium feldspar | 3.8 | Chlorite | 16.1 |
| Calcite | 6.9 | Illite/Smectite mixed layer | 52.1 |
| Dolomite | 4.2 | ||
| Clay mineral | 41.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, K.; Shen, H.; Sun, J.; Huang, X.; Du, H. Acylated Inulin as a Potential Shale Hydration Inhibitor in Water Based Drilling Fluids for Wellbore Stabilization. Molecules 2024, 29, 1456. https://doi.org/10.3390/molecules29071456
Lv K, Shen H, Sun J, Huang X, Du H. Acylated Inulin as a Potential Shale Hydration Inhibitor in Water Based Drilling Fluids for Wellbore Stabilization. Molecules. 2024; 29(7):1456. https://doi.org/10.3390/molecules29071456
Chicago/Turabian StyleLv, Kaihe, Haokun Shen, Jinsheng Sun, Xianbin Huang, and Hongyan Du. 2024. "Acylated Inulin as a Potential Shale Hydration Inhibitor in Water Based Drilling Fluids for Wellbore Stabilization" Molecules 29, no. 7: 1456. https://doi.org/10.3390/molecules29071456
APA StyleLv, K., Shen, H., Sun, J., Huang, X., & Du, H. (2024). Acylated Inulin as a Potential Shale Hydration Inhibitor in Water Based Drilling Fluids for Wellbore Stabilization. Molecules, 29(7), 1456. https://doi.org/10.3390/molecules29071456







