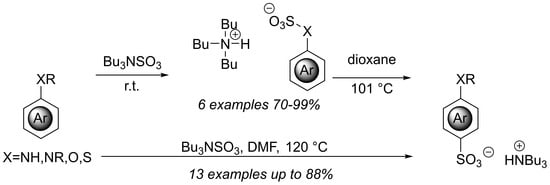
Rearrangement of Arylsulfamates and Sulfates to Para-Sulfonyl Anilines and Phenols
Abstract
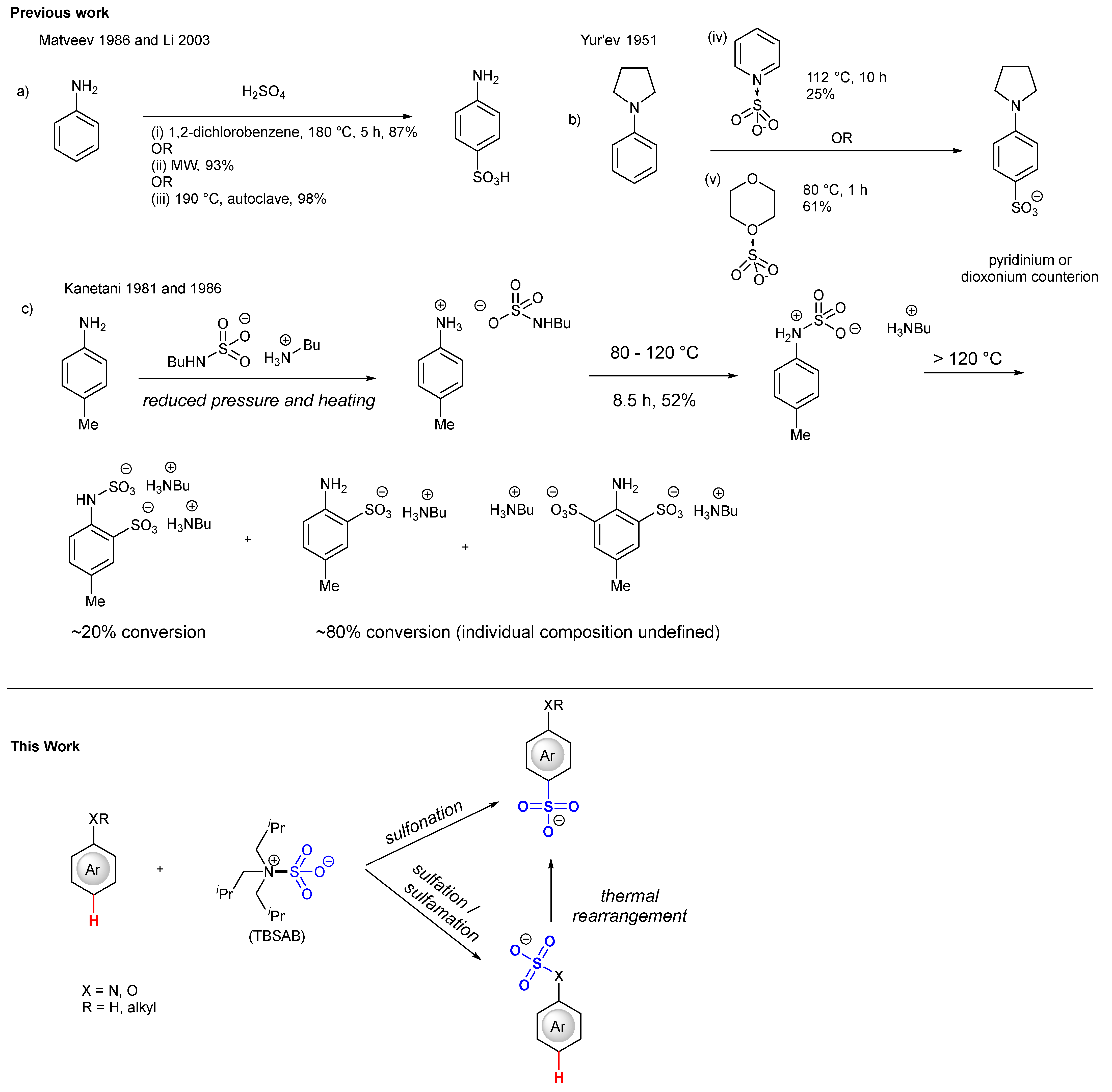
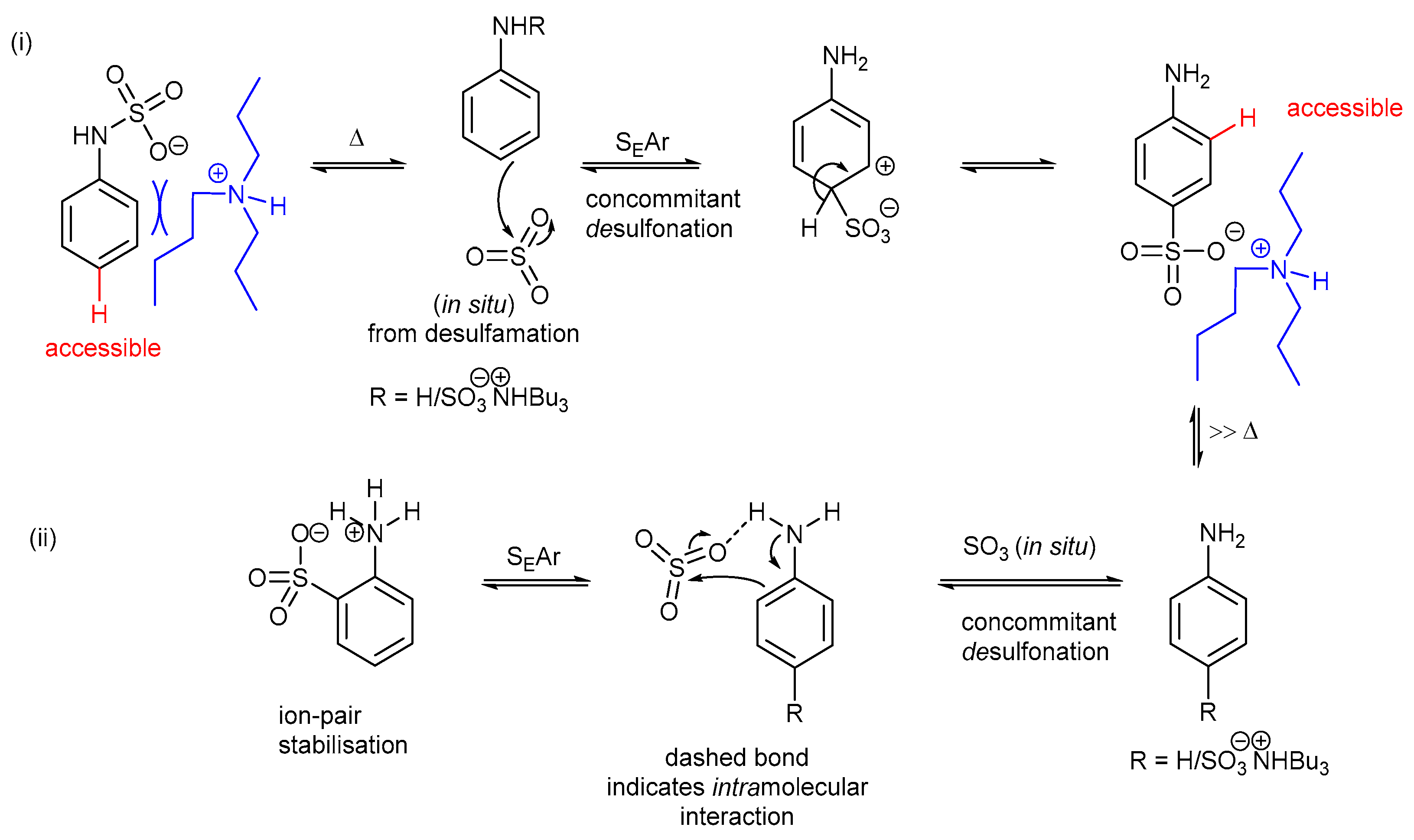
1. Introduction
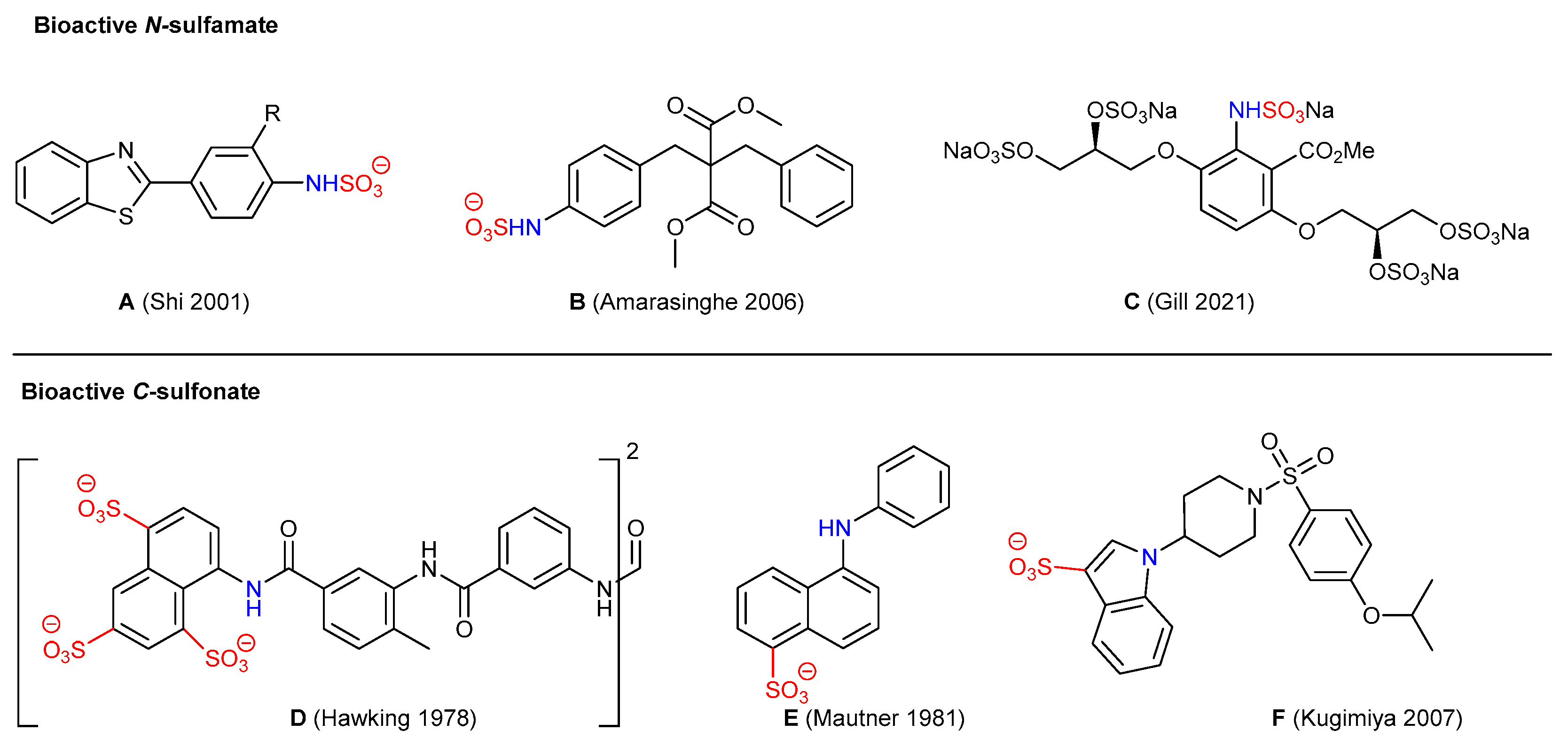
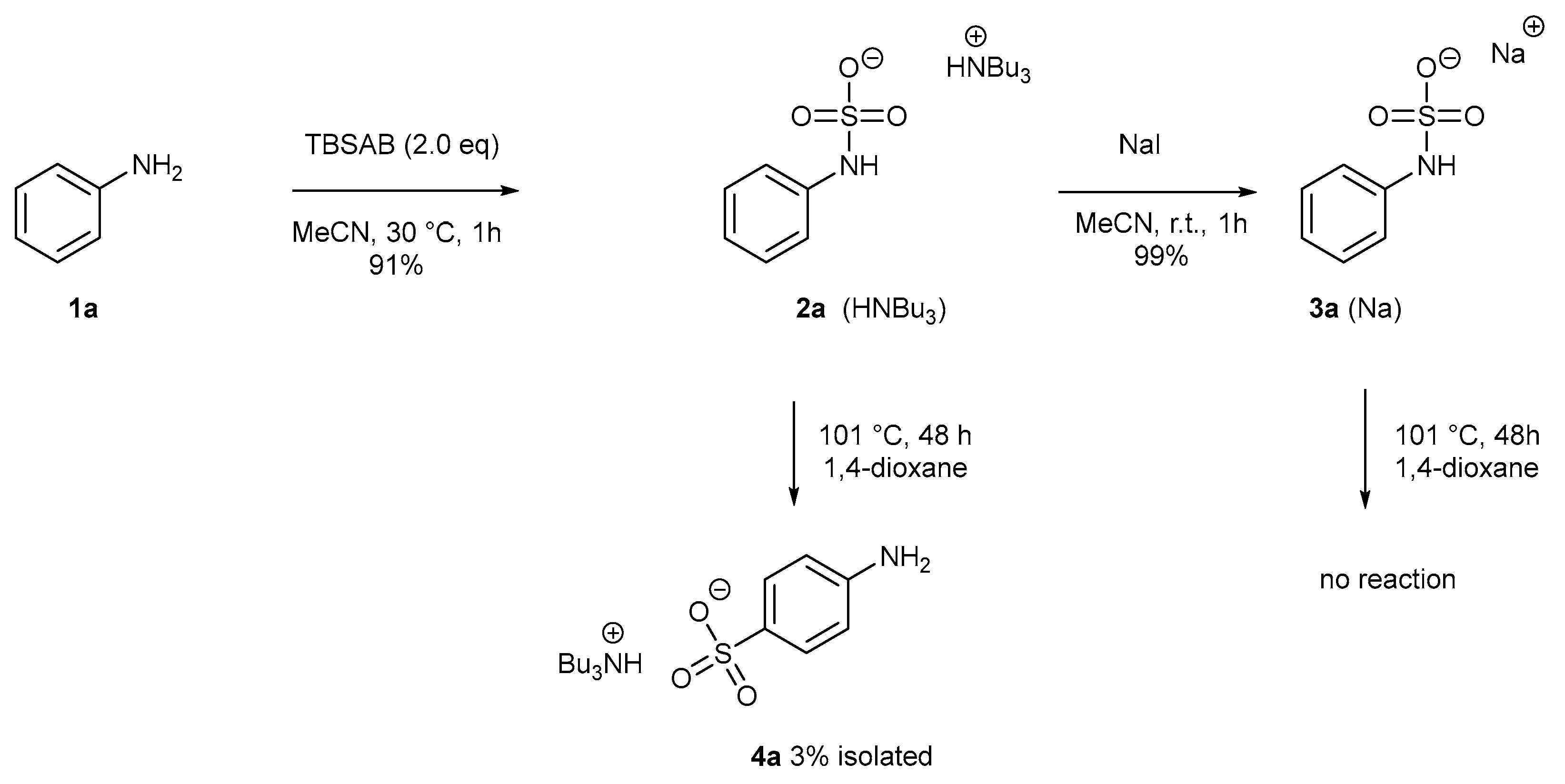
2. Results and Discussion
3. Control Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, D.-F.; Bradshaw, T.D.; Chua, M.-S.; Westwell, A.D.; Stevens, M.F.G. Antitumour Benzothiazoles. Part 15:1 The Synthesis and Physico-Chemical Properties of 2-(4-Aminophenyl)benzothiazole Sulfamate Salt Derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, K.K.D.; Evdokimov, A.G.; Xu, K.; Clark, C.M.; Maier, M.B.; Srivastava, A.; Colson, A.E.; Gerwe, G.S.; Stake, G.E.; Howard, B.W.; et al. Design and synthesis of potent, non-peptidic inhibitors of HPTPb. Bioorg. Med. Chem. Lett. 2006, 16, 4252–4256. [Google Scholar] [CrossRef]
- Gill, D.M.; Povinelli, A.P.R.; Zazeri, G.; Shamir, S.A.; Mahmoud, A.M.; Wilkinson, F.L.; Alexander, M.Y.; Cornelio, M.L.; Jones, A.M. The modulatory role of sulfated and non-sulfated small molecule heparan sulfate-glycomimetics in endothelial dysfunction: Absolute structural clarification, molecular docking and simulated dynamics, SAR analyses and ADME studies. RSC Med. Chem. 2021, 12, 779–790. [Google Scholar] [CrossRef]
- Sidgwick, G.P.; Weston, R.; Mahmoud, A.M.; Schiro, A.; Serracino-Inglott, F.; Tandel, S.M.; Skeoch, S.; Bruce, I.N.; Jones, A.M.; Alexander, M.Y.; et al. Novel Glycomimetics Protect against Glycated Low-Density Lipoprotein-Induced Vascular Calcification In Vitro via Attenuation of the RAGE/ERK/CREB Pathway. Cells 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Jones, A.M.; Sidgwick, G.; Arafat, A.M.; Wilkinson, F.L.; Alexander, M.Y. Small molecule glycomimetics inhibit vascular calcification via c-Met/Notch3/HES1 signalling. Cell. Phys. Biochem. 2019, 53, 323–336. [Google Scholar]
- Hawking, F. Suramin: With special reference to onchocerciasis. Adv. Pharmacol. Chemother. 1978, 15, 289–322. [Google Scholar]
- Mautner, H.G.; Merrill, R.E.; Currier, S.F.; Harvey, G. Interaction of aromatic dyes with the coenzyme A binding site of choline acetyltransferase. J. Med. Chem. 1981, 24, 1534–1537. [Google Scholar] [CrossRef]
- Kugimiya, A.; Tachibana, Y. Indolecarboxylic Acid Derivative Having PGD2 Receptor Antagonistic Activity. WO/2007/029629 A1, 27 March 2007. [Google Scholar]
- Benedetti, A.M.; Gill, D.M.; Tsang, C.W.; Jones, A.M. Chemical methods for N- and O-sulfation of small molecules, amino acids and peptides. ChemBioChem 2020, 21, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, J.A.; Benedetti, A.M.; Jones, A.M. A Novel Exchange Method to Access Sulfated Molecules. Sci. Rep. 2020, 10, 16559. [Google Scholar] [CrossRef]
- Blackburn, J.M.; Short, M.A.; Castanheiro, T.; Ayer, S.K.; Muellers, T.D.; Roizen, J.L. Synthesis of N-Substituted Sulfamate Esters from Sulfamic Acid Salts by Activation with Triphenylphosphine Ditriflate. Org. Lett. 2017, 19, 6012–6015. [Google Scholar] [CrossRef]
- Kanetani, F.; Okada, E.; Negoro, K. Insertion of Sulfur Trioxide into the N-Si Bond of Anilinotrimethylsilane. An Improved Method for the Preparation of Free Phenylamidosulfuric acid. Bull. Chem. Soc. Jpn. 1986, 59, 2517–2520. [Google Scholar] [CrossRef]
- Qingdao University of Science and Technology. Preparation of Amido Sulfonate Derivative Using Sulfur Trioxide. CN114605295 A, 10 June 2022.
- Mihai, M.T.; Williams, B.D.; Phipps, R.J. Para-Selective C-H Borylation of Common Arene Building Blocks Enabled by Ion-Pairing with a Bulky Countercation. J. Am. Chem. Soc. 2019, 141, 15477–15482. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.E.; Morrill, C.; Phipps, R.J. Regioselective Radical Arene Amination for the Concise Synthesis of ortho-Phenylenediamines. J. Am. Chem. Soc. 2021, 143, 9355–9360. [Google Scholar] [CrossRef] [PubMed]
- Matveev, L.G.; Chalykh, S.N.; Okhterova, I.A.; Nazarova, N.E.; Chalykh, E.A.; Gradov, V.A. Synthesis and Properties of Sulfate Salts of Para-Substituted Aromatic-Amines. J. Appl. Chem. USSR 1985, 58, 770–774. [Google Scholar]
- Li, H.-Z.; Xiao, L.-W.; Li, H.-Y.; Wang, K.-F.; Li, X. A Study on the Sulfonation of Aromatic Amines with Sulfuric Acid under Microwave Irradiation. J. Chem. Res. 2003, 2003, 493–494. [Google Scholar] [CrossRef]
- Yur’ev, Y.K.; Arbatskii, A.V. Nitrosation and sulfonation of 1-phenylpyrrolidine. Vestnik Moskovskogo Universiteta 1951, 6, 97–102. [Google Scholar]
- Kanetani, F.; Yamaguchi, H. Studies of Reactions of Amines with Sulfur Trioxide. VI. Thermal Reactions of Anilinium, Dimethylanilinium, and Trimethylanilinium Salts of Butylamidosulfuric acid. Bull. Chem. Soc. Jpn. 1981, 54, 3048–3058. [Google Scholar] [CrossRef]
- Kanetani, F. Preparation of Arylimidobis(sulfates). Bull. Chem. Soc. Jpn. 1986, 59, 952–954. [Google Scholar] [CrossRef]
- Koleva, G.; Galabov, B.; Kong, J.; Schaefer, H.F., III; von R. Schleyer, P. Electrophilic Aromatic Sulfonation with SO3: Concerted or Classic SEAr mechanism? J. Am. Chem. Soc. 2011, 133, 19094–19101. [Google Scholar] [CrossRef]
- Moors, S.L.C.; Deraet, X.; Assche, G.V.; Geerlings, P.; De Proft, F. Aromatic sulfonation with sulfur trioxide: Mechanism and kinetic model. Chem. Sci. 2017, 8, 680–688. [Google Scholar] [CrossRef]
- Morley, J.O.; Roberts, D.W. Molecular Modeling Studies on Aromatic Sulfonation. 1. Intermediates Formed in the Sulfonation of Toluene. J. Org. Chem. 1997, 62, 7358–7363. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.O.; Roberts, D.W.; Watson, S.P. Experimental and molecular modelling studies on aromatic sulfonation. J. Chem. Soc. Perkin Trans. II 2002, 2, 538–544. [Google Scholar] [CrossRef]
- Galabov, B.; Nalbantova, D.; von R. Schleyer, P.; Schaefer, H.F., III. Electrophilic Aromatic Substitution: New Insights into an Old Class of Reactions. Acc. Chem. Res. 2016, 49, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Bochkareva, T.P.; Passat, B.V.; Popov, K.R.; Platonova, N.V.; Koval’cuk, T.I. Sulfonation of substituted azoles with sulfur trioxide in dichloroethane. Khimiya Geterotsiklicheskikh Soedin. 1987, 23, 1084–1089. [Google Scholar] [CrossRef]
- Lally, J.M.; Spillane, W.J. The Photochemistry of Phenylsulphamic Acid: Photorearrangement and Photodegradation. J. Chem. Soc. Chem. Commun. 1987, 1, 8–9. [Google Scholar] [CrossRef]
- Benson, G.A.; Spillane, W.J. Sulfamic Acids and Its N-Substituted Derivatives. Chem. Rev. 1980, 80, 151–186. [Google Scholar] [CrossRef]
- Maarsen, P.K.; Cerfontain, H. Aromatic Sulphonation. Part 56. The Rearrangment of Phenylsulphamic Acid to Aniliniumsulphonic Acids in Concentrated Sulphuric Acid: Evidence for an Intermolecular Reaction Pathway. J. Chem. Soc. Perkin Trans. II 1977, 2, 921–928. [Google Scholar] [CrossRef]
- Newcomer, R.; McKee, J.; Zanger, M. Triflic acid-catalyzed rearrangement of unalkylated benzene sulfonamides. Synth. Commun. 2016, 46, 949–955. [Google Scholar] [CrossRef]
- Kanetani, F.; Yamaguchi, H. Studies of Reactions of Amines with Sulfur Trioxide. V. Transsulfonation of Amine Salts of Some N-Substituted Amidosulfuric Acids. Bull. Chem. Soc. Jpn. 1978, 51, 3039–3046. [Google Scholar] [CrossRef]
- Hopkins, A.; Day, R.A.; Williams, A. Sulfate Group Transfer between Nitrogen and Oxygen: Evidence Consistent with an Open “Exploded” Transition State. J. Am. Chem. Soc. 1983, 105, 6062–6070. [Google Scholar] [CrossRef]
- Gilbert, E.E. The Reactions of Sulfur Trioxide, and of its Adducts, with organic compounds. J. Am. Chem. Soc. 1962, 62, 549–589. [Google Scholar] [CrossRef]
- Tyrer, D. Sulfonation of Hydrocarbons. U.S. Patent 1210725, 2 January 1917. [Google Scholar]
- Roeges, N. A simple preparation of sulfanilic acid. J. Chem. Educ. 1968, 45, 274. [Google Scholar] [CrossRef]
- Bamberger, E.; Hindermann, E. Umlagerung der Phenylsulfaminsäure. Chem. Ber. 1897, 30, 654. [Google Scholar] [CrossRef]
- Illuminati, G. A Reinvestigation of the Role of Phenylsulfamic Acid in the Formation of Aminobenzenesulfonic Acids. J. Am. Chem. Soc. 1956, 78, 2603–2606. [Google Scholar] [CrossRef]
- Spillane, W.J.; Scott, F.L. Radiosulphur studies on the rearrangement of phenylsulphamic acid to sulphanilic acid. Tetrahedron Lett. 1967, 8, 1251–1253. [Google Scholar] [CrossRef]
- Spillane, W.J.; Scott, F.L. The Rearrangement of Phenylsulphamic Acid to Sulphanilic Acid in the Presence of [35S] Sulphuric Acid. J. Chem. Soc. B 1968, 779–781. [Google Scholar] [CrossRef]
- Jones, A.M. Tributylsulfoammonium Betaine. The Encyclopaedia of Reagents for Organic Synthesis (e-EROS). 2021. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/047084289X.RN02393 (accessed on 4 March 2024).
- Gill, D.M.; Male, L.; Jones, A.M. Sulfation made simple: A strategy for synthesising sulfated molecules. Chem. Commun. 2019, 55, 4319–4322. [Google Scholar] [CrossRef] [PubMed]
- Montero Bastidas, J.R.; Oleskey, T.J.; Miller, S.L.; Smith, M.R., III; Maleczka, R.E., Jr. Para-Selective, Iridium-Catalyzed C−H Borylations of Sulfated Phenols, Benzyl Alcohols, and Anilines Directed by Ion-Pair Electrostatic Interactions. J. Am. Chem. Soc. 2019, 141, 15483–15487. [Google Scholar] [CrossRef]
- Prinsen, A.J.; Koeberg-Telder, A.; Cerfontain, H. Sulphonation of polymetylbenzenesulphonic acids. Evidence for a buttressing effect. Tetrahedron 1970, 26, 1953–1960. [Google Scholar] [CrossRef]
- Alexander, E.R. Mechanism of the Sulfonation of Aromatic Amines. II. Sulfonation at Elevated Temperatures with Sulfuric Acid. J. Am. Chem. Soc. 1947, 69, 1599–1602. [Google Scholar] [CrossRef]
- Pereira, M.R.R.C.; Ribeiro, A.F.G.; Silva, A.M.S.; Silva, V.L.M. Ohmic heating-assisted regioselective sulfonation of aniline: Synthesis of sulfanilic acid. New J. Chem. 2022, 46, 20481–20489. [Google Scholar] [CrossRef]
- Morrill, C.; Gillespie, J.E.; Phipps, R.J. An Aminative Rearrangement of O-(Arenesulfonyl)hydroxylamines: Facile Access to ortho-Sulfonyl Anilines. Angew. Chem. Int. Ed. 2022, 61, e202204025, Angew. Chem. 2022, 134, e202204025. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jones, A.M. A Sulfonative Rearrangement of N-Aryl Sulfamates to para-Sulfonyl Anilines. ChemRxiv 2022. [Google Scholar] [CrossRef]
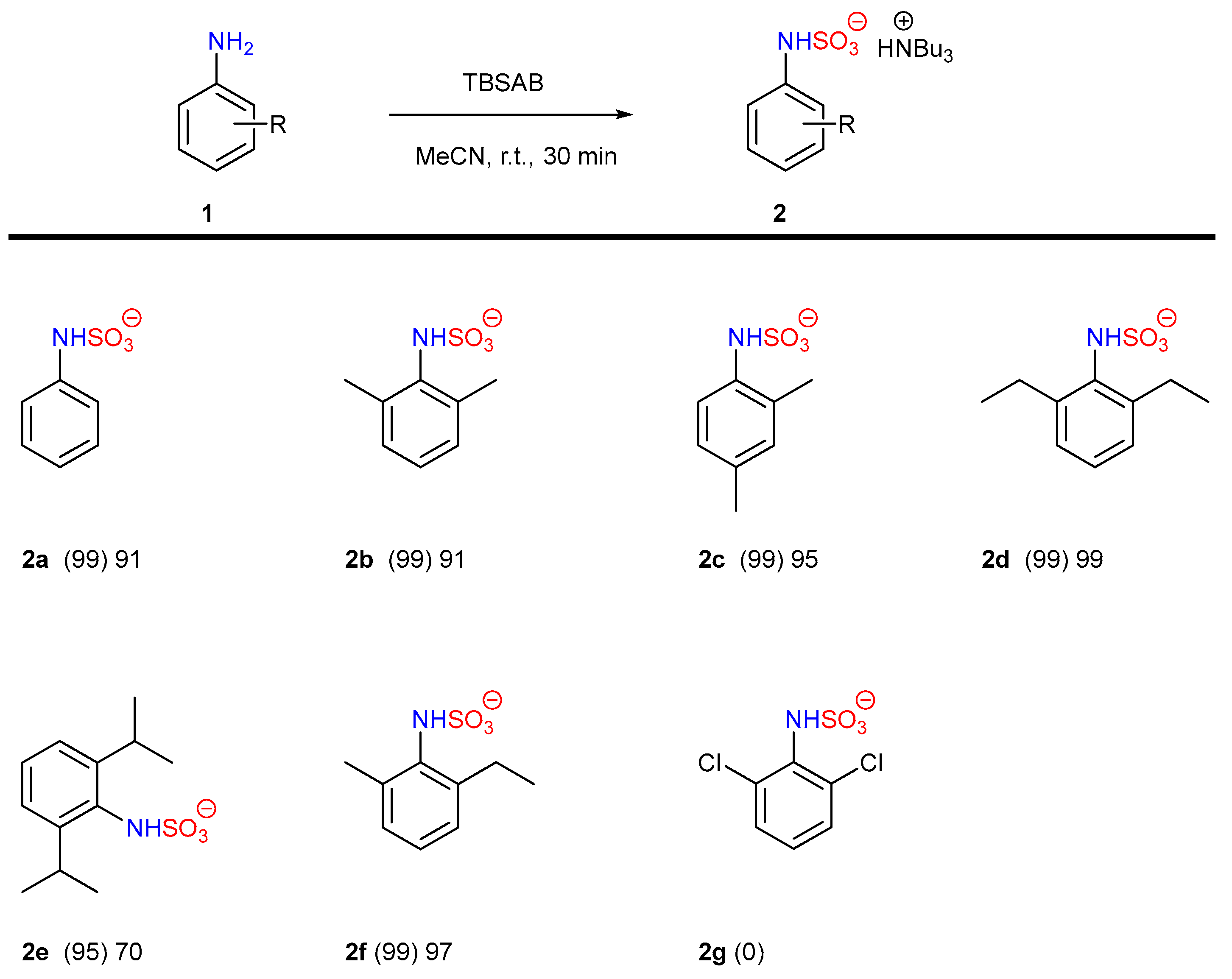
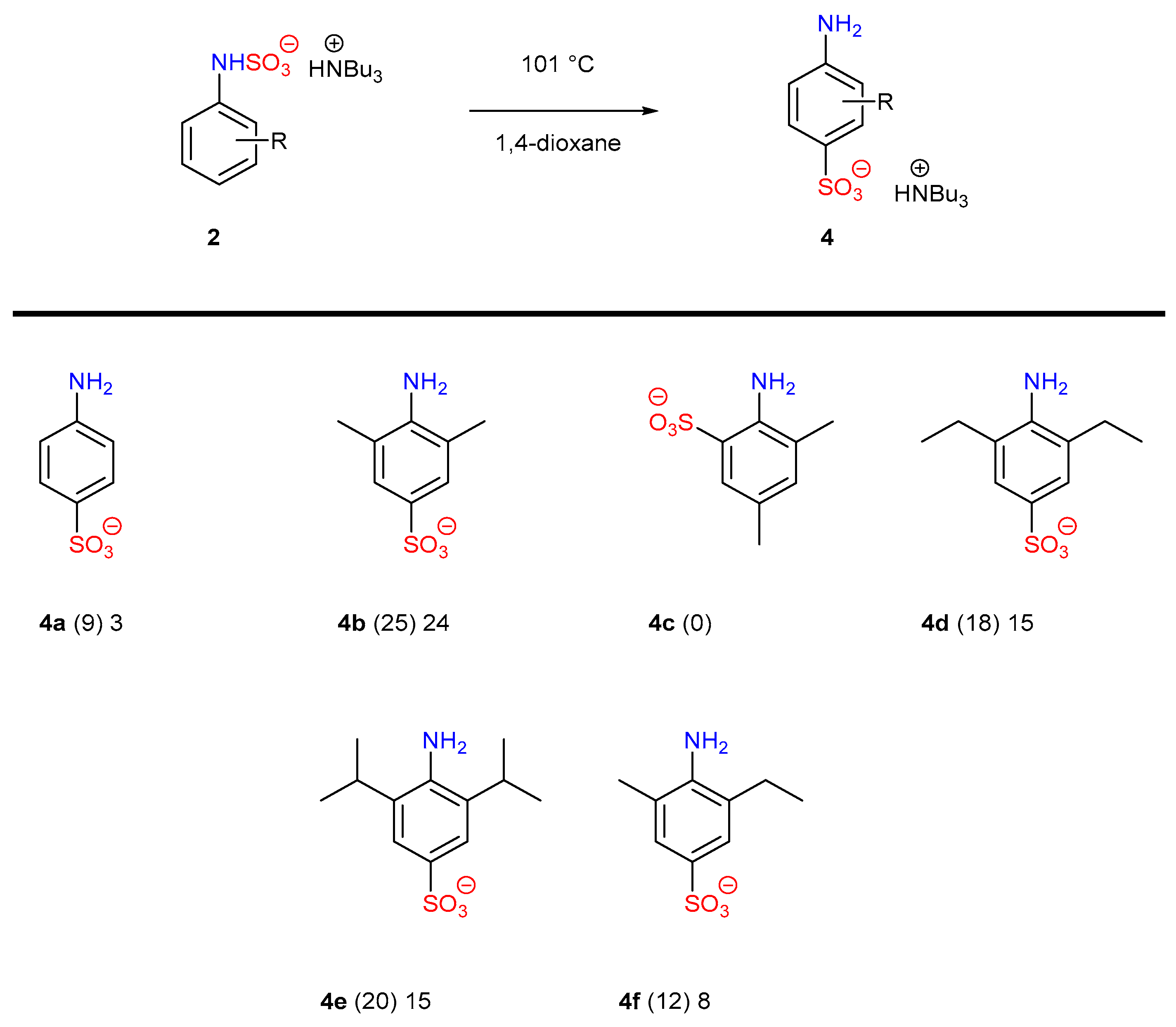
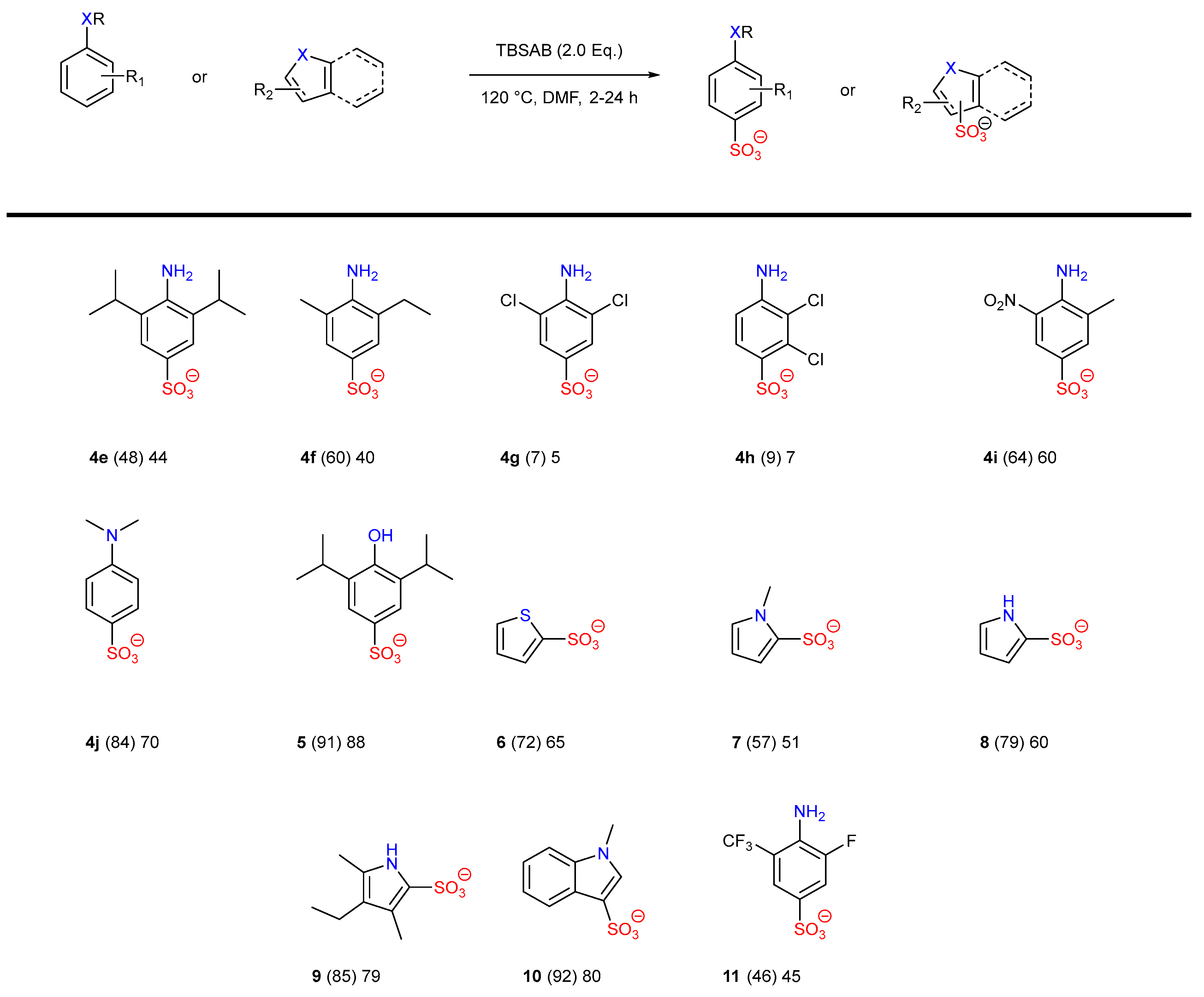

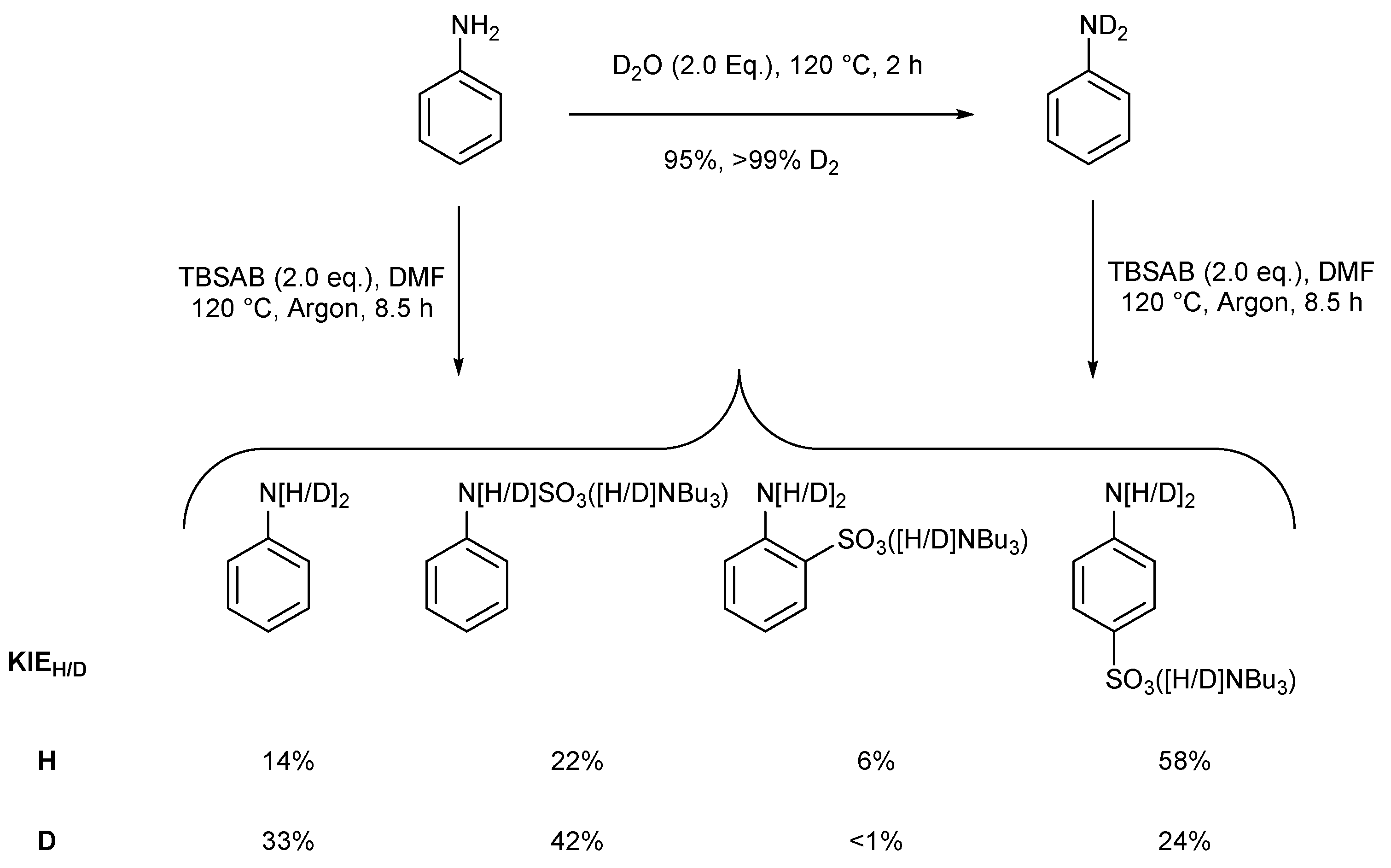
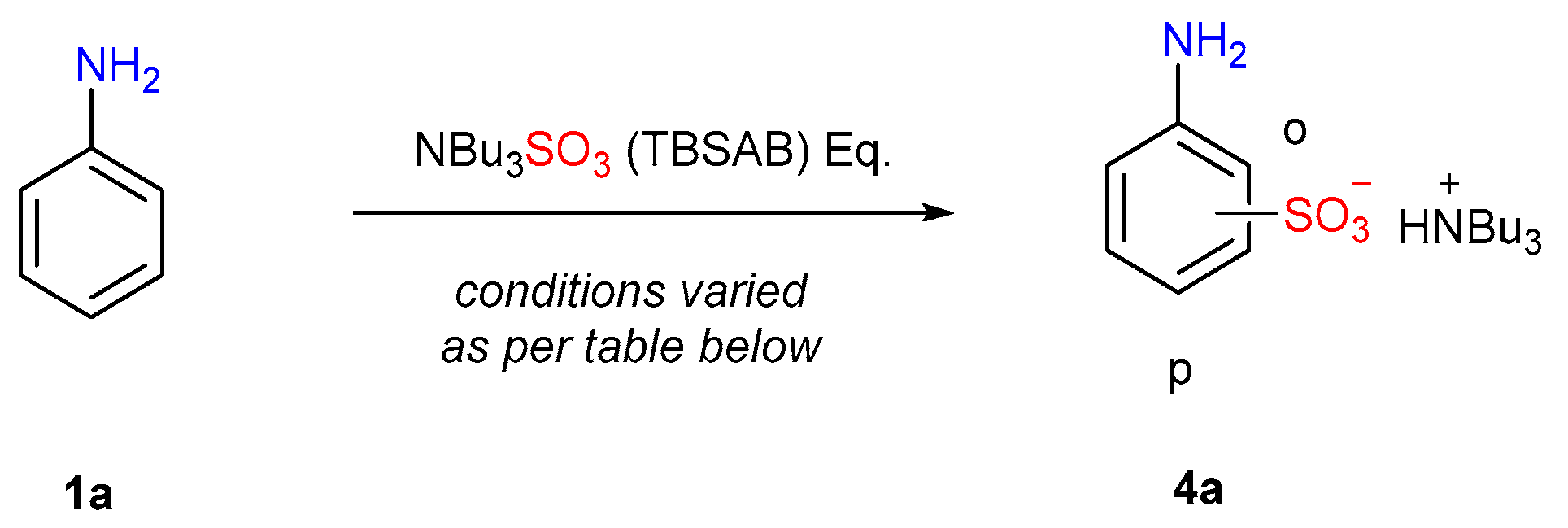
| Entry | Eq. | T (°C) | Atmosphere | Solvent | p-4a at t = 2 h (%) a | p-4a at t = 24 h (%) a | Selectivity p:o a |
|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 101 | Ar | 1,4-Dioxane | 6 | 7 | - |
| 2 | 1.0 | 101 | Ar | 1,4-Dioxane | 1 | 6 | - |
| 3 | 1.5 | 101 | Ar | 1,4-Dioxane | 7 | 10 | - |
| 4 | 2 | 101 | Ar | 1,4-Dioxane | 4 | 12 | - |
| 5 | 4 | 101 | Ar | 1,4-Dioxane | 4 | 9 | - |
| 6 | 2 | 101 | air | 1,4-Dioxane | 3 | 11 | - |
| 7 b | 2 | 80 | Ar | 1,4-Dioxane | - | - | - |
| 8 | 2 | 100 | Ar | Formic Acid | - | - | - |
| 9 | 2 | 100 | Ar | Butan-2-ol | - | - | - |
| 10 | 2 | 80 | Ar | DMF | - | - | - |
| 11 | 2 | 100 | Ar | DMF | 4 | 13 | - |
| 12 | 2 | 120 | Ar | DMF | 32 | 58 | >10:1 |
| 13 | 2 | 140 | Ar | DMF | 30 | 49 | 5:1 |
| 14 | 2 | 120 | Ar | DMSO | 25 | 48 | 8:1 |
| 15 | 2 | 140 | Ar | DMSO | 15 | 35 | 2:1 |
| 16 | 2 | 160 | Ar | DMSO | - | - | - |
| 17 | 2 | 180 | Ar | DMSO | - | - | - |
| 18 | 2 | 120 | Ar | 1,2-dichlorobenzene | 27 | 52 | >10:1 |
| 19 | 2 | 140 | Ar | 1,2-dichlorobenzene | 20 | 44 | 4:1 |
| 20 c | 2 | 160 | Ar | 1,2-dichlorobenzene | - | - | - |
| 21 c | 2 | 180 | Ar | 1,2-dichlorobenzene | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Jones, A.M. Rearrangement of Arylsulfamates and Sulfates to Para-Sulfonyl Anilines and Phenols. Molecules 2024, 29, 1445. https://doi.org/10.3390/molecules29071445
Zhou Y, Jones AM. Rearrangement of Arylsulfamates and Sulfates to Para-Sulfonyl Anilines and Phenols. Molecules. 2024; 29(7):1445. https://doi.org/10.3390/molecules29071445
Chicago/Turabian StyleZhou, Yifei, and Alan M. Jones. 2024. "Rearrangement of Arylsulfamates and Sulfates to Para-Sulfonyl Anilines and Phenols" Molecules 29, no. 7: 1445. https://doi.org/10.3390/molecules29071445
APA StyleZhou, Y., & Jones, A. M. (2024). Rearrangement of Arylsulfamates and Sulfates to Para-Sulfonyl Anilines and Phenols. Molecules, 29(7), 1445. https://doi.org/10.3390/molecules29071445