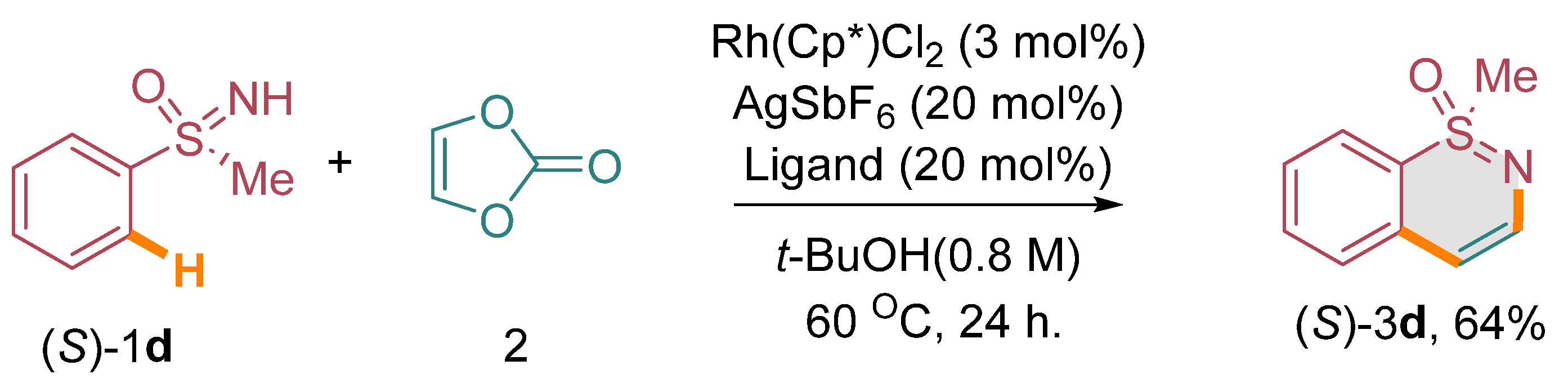Abstract
In this study, we report the synthesis of unsubstituted 1,2-benzothiazines through a redox-neutral Rh(III)-catalyzed C–H activation and [4+2]-annulation of S–aryl sulfoximines with vinylene carbonate. Notably, the introduction of an N-protected amino acid ligand significantly enhances the reaction rate. The key aspect of this redox-neutral process is the utilization of vinylene carbonate as an oxidizing acetylene surrogate and an efficient vinylene transfer agent. This vinylene carbonate enables the cyclization with the sulfoximine motifs, successfully forming a diverse array of 1,2-benzothiazine derivatives in moderate to good yields. Importantly, this study highlights the potential of Rh(III)-catalyzed C–H activation and [4+2]-annulation reactions for the synthesis of optically pure 1,2-benzothiazines with high enantiomeric purity.
1. Introduction
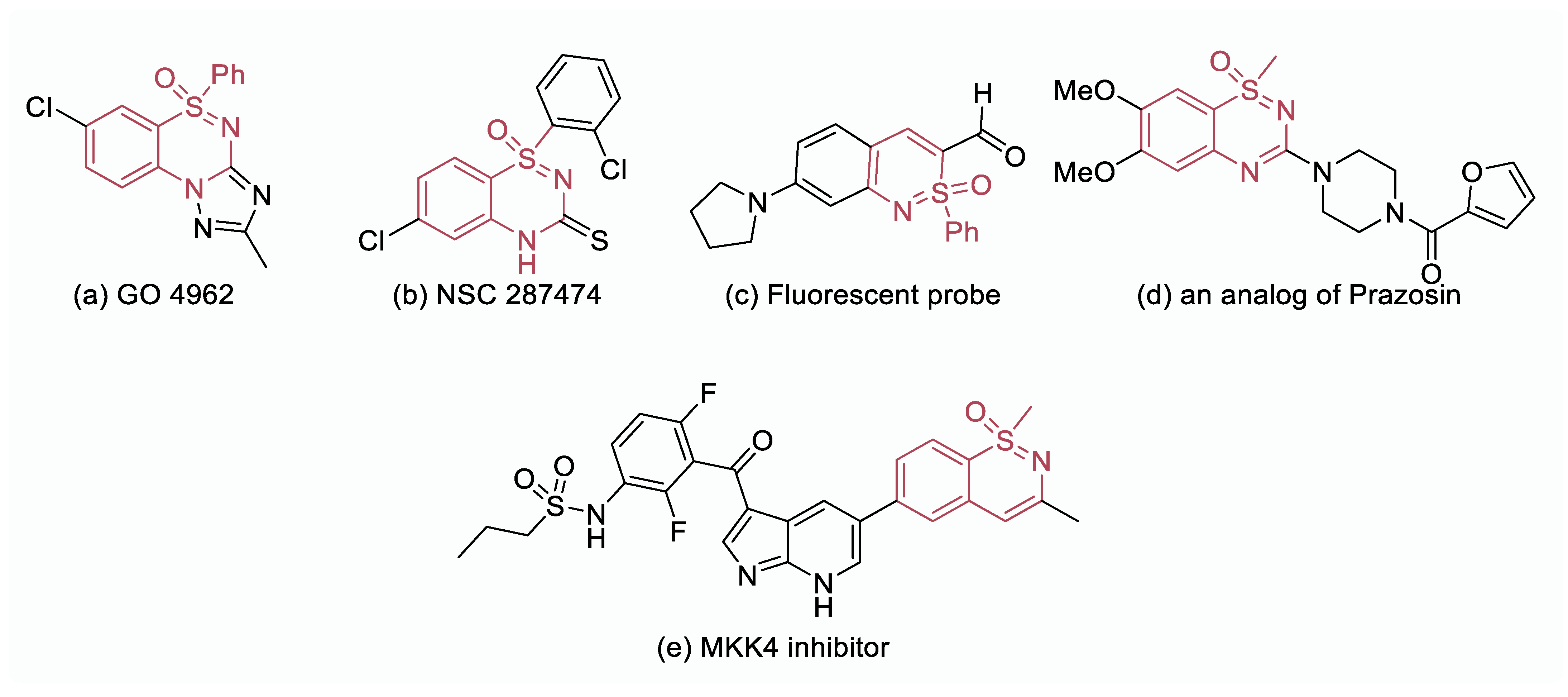
In recent years, the field of medicinal chemistry has experienced a remarkable expansion, particularly in the exploration of sulfur stereogenic complex scaffolds, which possess unique physicochemical properties. One prominent class of such scaffolds is S(VI)-stereogenic sulfoximines, which have attracted considerable attention due to their versatility and adaptability in synthesizing innovative molecular structures. By incorporating aryl/alkyl groups into sulfoximine frameworks, the complexity and chemical diversity of compounds can be significantly enhanced. This is evident from the wide range of biologically active compounds derived from sulfoximines and benzothiazine moieties [1,2,3,4]. An excellent example of the pharmacological potential of sulfoximines and benzothiazines is the compound GO4962 (Figure 1a), which acts as a partial agonist for benzodiazepine receptors, exhibiting anxiolytic and anticonvulsive properties [5]. Another noteworthy compound, NSC 287474 (Figure 1b), has emerged as a promising inhibitor of reverse transcriptase, offering protection to lymphocytes against HIV [6,7]. Furthermore, a fluorescent probe for the detection of Cu(II) has been developed based on sulfoximine and benzothiazine molecular entities (Figure 1c) [8]. Interestingly, analogs of prazosin, an antihypertensive agent, have also been synthesized using sulfoximines, demonstrating their potential in the development of therapeutic agents (Figure 1d) [9]. Additionally, a sulfoximine-based compound has been identified as an inhibitor of mitogen-activated protein kinase 4 (MKK4) (Figure 1e), showcasing the diverse pharmacological properties that can be achieved through the manipulation of sulfoximines and benzothiazine molecular entities. Overall, the rich pharmacological properties and wide-ranging applications of sulfoximines and benzothiazines make them valuable building blocks for the synthesis of innovative and biologically relevant compounds in medicinal chemistry.
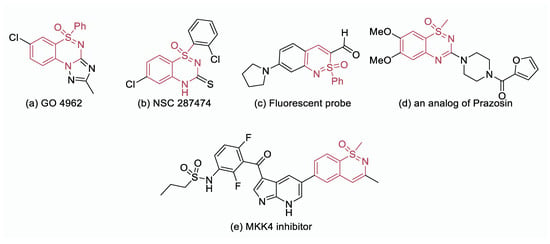
Figure 1.
Biologically active compounds containing sulfoximine scaffold.
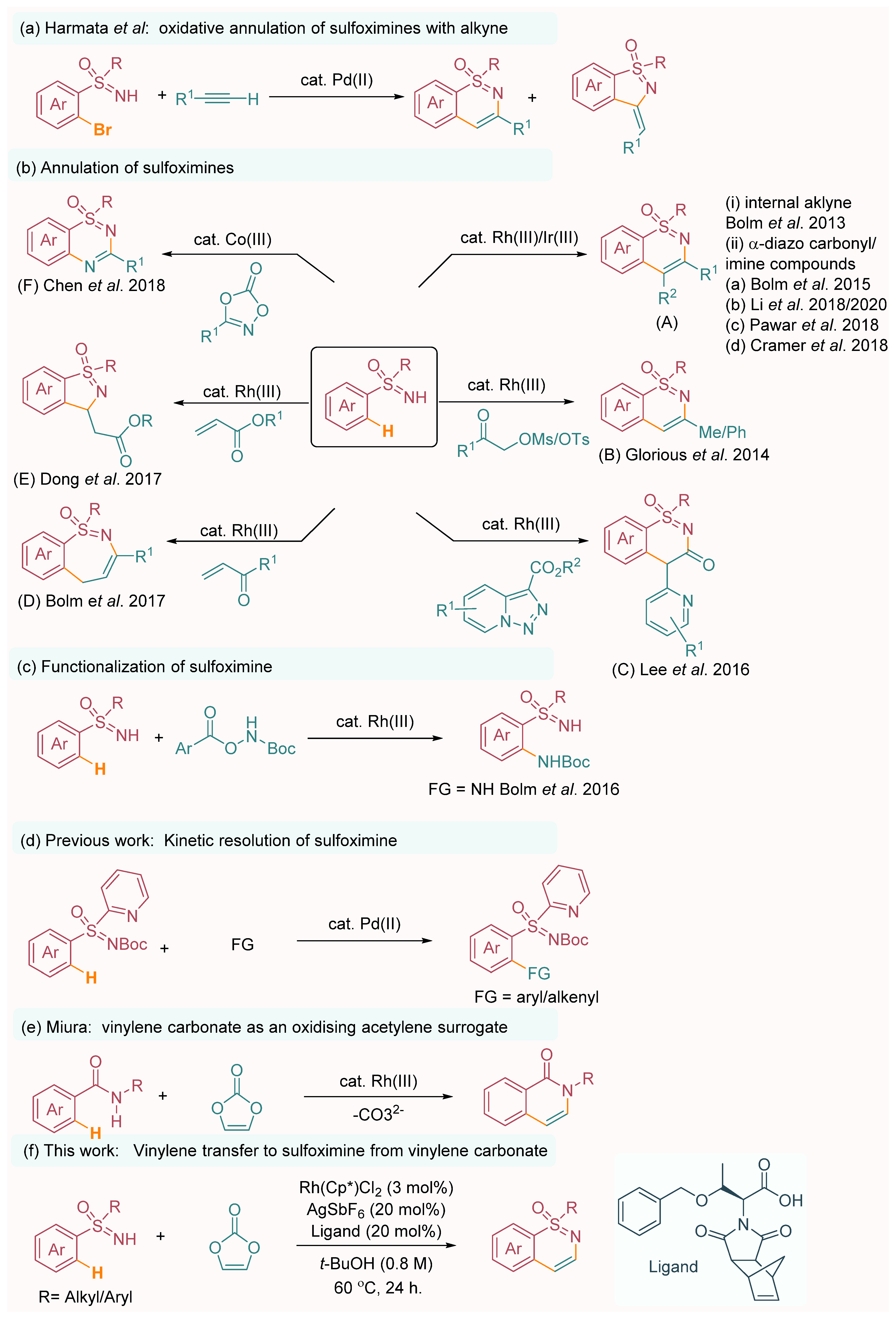
Recently, sulfoximines have also emerged as leading precursors for a wide range of chemical transformations, enabling the generation of complex sulfur-bearing scaffolds. One particularly notable innovation in this field is the palladium-catalyzed synthesis of 1,2-benzothiazines, which was introduced by Harmata and coworkers (Scheme 1a) [10]. This method utilizes terminal alkynes as the preferred coupling partners, offering a novel approach to accessing diverse benzothiazine derivatives. In 2013, Bolm’s research group demonstrated the rhodium-catalyzed C–H activation/cyclization of sulfoximines with alkynes, resulting in the construction of heterocyclic cores bearing sulfoximine functionalities (Scheme 1b-A-i) [11]. Building upon this work, two years later, the same group reported another significant advancement with a rhodium-catalyzed directed migratory carbene insertion of conventional NH-sulfoximines into aromatic C–H bonds. Subsequent regioselective dehydrative ring closures facilitated the synthesis of aryl-fused sulfoximines (Scheme 1b-A-ii(a)) [12]. In 2014, Glorius et al. successfully employed α-MsO/TsO ketones as C(sp3)-based electrophiles and oxidized alkyne equivalents in Rh(III)-catalyzed redox-neutral annulations to desired 3-methyl/aryl 1,2-benzothiazines (Scheme 1b-B) [13]. In 2016, Lee’s research group made a significant contribution by reporting the synthesis of 1,2-benzothiazine skeletons derived from NH-sulfoximine through the use of a Rh(III) catalyst in the presence of heterocyclic coupling partners (Scheme 1b-C) [14]. In 2017, the Bolm group made a significant contribution by reporting the synthesis of 1,2-benzothiazepine utilizing α,β-unsaturated carbonyl compounds, thereby showcasing the effectiveness of the Rh(III) catalyst in facilitating the formation of 1,2-benzothiazepine (Scheme 1b-D) [15]. Similarly, the Dong group also made noteworthy progress by introducing a method for synthesizing 1,2-benzoisothiazoles using α, β-unsaturated esters under the influence of the Rh(III) catalyst (Scheme 1b-E) [16]. In 2017, the Chen group made a significant contribution by reporting a Co(III)-catalyzed C–H activation/double C–N bond formation reaction (Scheme 1b-F) [17]. This reaction involved the utilization of free NH-sulfoximines and 1,4,2-dioxazol-5-ones as substrates, and it was conducted under the influence of microwave irradiation to construct structurally orchestrated thiadiazine 1-oxide derivatives.
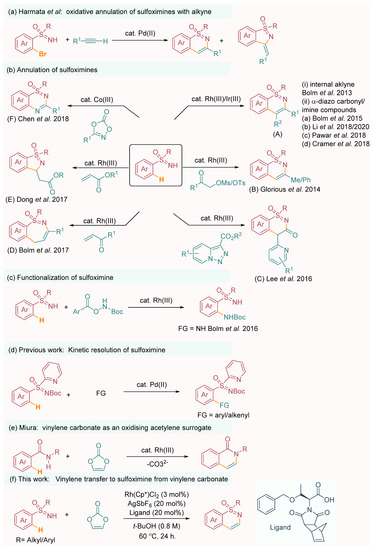
Scheme 1.
Synthetic methods for annulation of sulfoximines. (a) Harmata’s work [10]; (b)-A-(i) Bolm’s work [11]; (b)-A-(ii-a)-Bolm’s work [12]; (b)-A-(ii-b)-Li’s work [18]; (b)-A-(ii-c)-Pawar’s work [19]; (b)-A-(ii-d)-Cramer’s work [20,21]; (b)-B-Glorious work [13]; (b)-C-Lee’s work [14]; (b)-D-Bolm’s work [15]; (b)-E-Dong’s work [16]; (b)-F-Chen’s work [17]; (c)-Bolm’s work [22]; (d)-Previous work [23]; (e)-Miura’s work [24]; (f)-This work.
The Pawar group made an important contribution by reporting the utilization of the Ir(III) catalyst for the synthesis of 1,2-benzothiazines through C–H activation of sulfoximine with α-diazo carbonyl compounds (Scheme 1b-A-ii(c)) [19]. In addition, the Bolm group presents a novel approach for the synthesis of heterocyclic thiadiazine 1-oxide by employing Rh(III) catalysis to facilitate the amination of sulfoximine. The amidating agent utilized in this process is tert-butyl (2,4,6-trichlorobenzoyl)oxycarbamate (Scheme 1c) [22]. One notable advantage of sulfoximine is its sulfur atom, which possesses four coordination centers, making it a prochiral or chiral entity. Recognizing this unique feature, several research groups have embarked on developing asymmetric C–H activation methods specifically targeting sulfoximines. A significant breakthrough in this area was reported by Li and co-workers, who described an unprecedented enantiodivergent [4+2] annulative coupling of sulfoximines with α-diazocarbonyl compounds. This groundbreaking approach relied on the use of a chiral Rh(III) catalyst to facilitate a desymmetrizing C–H activation process (Scheme 1b-A-ii(b)) [18]. Notably, Cramer and his research group made significant contributions to this field. Cramer’s work focused on the development of a chiral rhodium catalyst-assisted strategy for the synthesis of chiral 1,2-benzothiazines. By utilizing α-diazo carbonyl compounds as reactants and sulfoximines as starting materials, the team successfully achieved the formation of structurally diverse 1,2-benzothiazine derivatives. One key aspect of their approach was the implementation of desymmetrization [20] and kinetic resolution [21] processes (Scheme 1b-A-ii(d)), which enabled the generation of enantioenriched products. To achieve high levels of enantioselectivity, Cramer employed a chiral rhodium catalyst in combination with an N-diprotected chiral amino acid as an additive. The chiral catalyst played a crucial role in selectively activating the C–H bond of the sulfoximine, while the chiral additive facilitated the efficient differentiation of the two enantiomeric sulfoximine substrates, leading to enantioenriched 1,2-benzothiazine products. The Shi group recently reported the enantioselective annulation of sulfoximines using chiral carboxylic acids as a chiral source. This study employed Ir(III) [25] and Ru(III) [26,27] catalysts to achieve the desired enantioselectivity. Building on this research, our group made a significant contribution by reporting a palladium-catalyzed enantioselective arylation of sulfoximines (Scheme 1d) [23]. In our study, we utilized BOC-protected amino acids as a chiral source, resulting in high levels of enantioselectivity. More recently, Liu’s group addressed another challenging aspect of sulfoximine chemistry by developing a Rh(III)-catalyzed C–H annulation and cyclization strategy. Fused isochromeno-1,2-benzothiazines were successfully synthesized by employing 4-diazoisochroman-3-imines as coupling partners with sulfoximines [28]. In parallel to these advancements, significant progress has been made in the field of C–H activation, enabling the construction of various C–C, C–N, C–O, C–S, and C–X bonds [19,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. Despite these remarkable achievements, the non-substituted vinylene annulation of S-aryl sulfoximines remains a formidable challenge. The main hurdle lies in the requirement for a suitable acetylene surrogate that can effectively produce vinylene-fused compounds, which adds complexity to the reaction design and necessitates the development of innovative synthetic approaches. The utilization of acetylene itself as a reactant poses challenges due to its gaseous nature, which requires specialized equipment and introduces safety hazards into the process [43,44,45]. Despite attempts to address this limitation, the most commonly used acetylene surrogate, bis(trimethylsilyl)acetylene, has shown limited reactivity and stability, resulting in restricted product formation [46]. To overcome these obstacles, alternative acetylene surrogates have been explored, such as vinyl acetate [47,48,49] and alpha-halo acetaldehyde [22]. However, the outcomes of these reactions have been moderate in terms of product yields. In a more recent development, Miura and colleagues described a rhodium-catalyzed annulative coupling reaction employing vinylene carbonate as an acetylene equivalent (Scheme 1e) [24]. The process successfully synthesized unsubstituted 1,2-benzothiazines, achieving a yield of 30%. This strategy offers a promising resolution to the inherent difficulties encountered in acetylene-based reactions.
Vinylene carbonate serves as an efficient and practical acetylene surrogate, offering improved reactivity and stability. This advancement opens new possibilities for the synthesis of vinylene-fused compounds in a more controlled and efficient manner. Taking inspiration from previous discoveries in the field and drawing on our group’s extensive research on sulfoximine-assisted annulation and annulative functionalizations [50,51,52,53,54,55,56,57], we focused our attention on investigating the annulation of NH-sulfoximines with vinylene carbonate as a means to construct 1,2-benzothiazine derivatives. An intriguing aspect of vinylene carbonate is its dual role as both an acetylene surrogate and an oxidizing agent; it can be used for a redox-neutral process [24,58,59,60,61,62,63,64,65,66,67,68], which enhances the catalyst turnover and contributes to the overall efficiency of the reaction.
2. Results and Discussion
In this article, we present the successful implementation of a Rh(III)-catalyzed redox-neutral [4+2]-C–H annulation, utilizing sulfoximines and vinylene carbonate (Scheme 1f). This strategy enables the formation of unsubstituted 1,2-benzothiazines with moderate to good yields. By utilizing vinylene carbonate as a versatile acetylene surrogate and incorporating Rh(III) catalysis, we demonstrate the practicality and effectiveness of this approach for the synthesis of complex sulfur-containing heterocycles. The developed methodology expands the synthetic toolbox for accessing 1,2-benzothiazine derivatives and holds promise for the construction of diverse sulfur-bearing scaffolds in future applications.
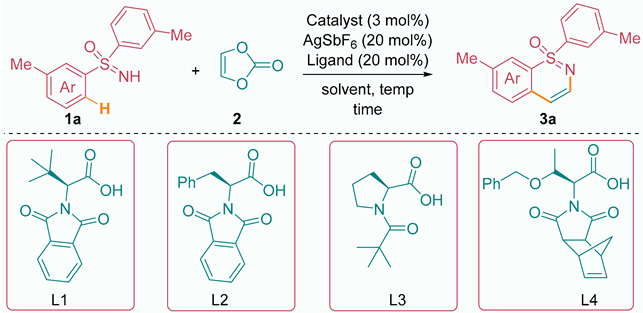
In this study, our objective was to investigate the potential use of sulfoximine (1a) and vinylene carbonate (2) as a model substrate to evaluate the effectiveness of a rhodium complex catalyst in combination with various additives, ligands, and solvents. To begin with, we employed a catalytic system consisting of 3.0 mol% [Cp*RhCl2]2, 20 mol% AgSbF6, and 20 mol% L1 as a ligand, with trifluoroethanol (TFE) serving as the solvent. The reaction was conducted at a temperature of 80 °C. Under these conditions, we successfully obtained the desired product 3a with a yield of 30%. This result is documented in Table 1, entry 1, which summarizes the experimental details and outcomes of our investigation. By employing the specified catalytic system, we were able to demonstrate the efficacy of the rhodium complex catalyst in promoting the desired transformation and generating the target product. However, it was observed that at 80 °C, the sulfoximine substrate underwent cleavage to form sulfoxide as an undesired byproduct. To overcome this issue and promote smoother reaction progress, the decision was made to lower the reaction temperature to 60 °C. With the identical catalytic conditions employed in entry 1, we proceeded to screen various solvents at the reduced temperature of 60 °C. This screening process, detailed in Table 1, entries 2 to 6, allowed us to identify the most effective solvent. Among the tested solvents, t-BuOH exhibited superior performance, providing the desired product 3a with a yield of 55% (entry 6). Subsequently, our attention turned to the investigation of different ligands, as documented in Table 1, entries 7 to 9, using the catalytic system established in entry 6. Through this screening process, we aimed to identify a suitable ligand that would further enhance the yield of product 3a. Ultimately, ligand L4 proved to be the most suitable, delivering the desired compound with a yield of 75% (entry 9). These results highlight the significance of ligand selection in the context of the catalytic system, as it played a crucial role in promoting the formation of the target product and achieving higher yields.

Table 1.
Optimization of reaction conditions a.
Our experimental findings provide compelling evidence for the crucial role of the ligand in facilitating the C–H activation process through the chelation-assisted C–H metalation–deprotonation (CMD) pathway. Notably, in the absence of a suitable ligand, as demonstrated in entry 10, the reaction failed to proceed, underscoring the indispensability of the ligand in promoting the desired transformation. In addition to exploring the impact of the ligand, we also aimed to investigate the influence of the catalyst on the reaction outcome. However, our attempts to employ 3 mol% of Co(III) and Ir(III) complexes, as documented in Table 1 (entries 11 and 12), were met with limited success, as the desired product was not obtained. Nevertheless, our exploration of alternative catalysts led us to evaluate the performance of [Ru(p-cymene)Cl2]2 at a catalyst loading of 3 mol%. Encouragingly, this catalyst enabled the formation of product 3a with a yield of 55%, as shown in Table 1, entry 13. This result highlights the potential of this ruthenium-based catalyst in the present reaction system, albeit with a moderate yield. Furthermore, we investigated the reaction in the presence of AcOH (acetic acid) and pivalic acid under the catalytic conditions shown in entry 9 without a ligand. Although these acids had a significant impact on the CMD process [69,70,71], the reaction led to a diminished product yield of 23% and 39%, (see the supplementary material) respectively (entries 14 and 15). Although the exact reason for the reaction outcome in the presence of amino acid ligand L4 is unknown, we believe that the ligand bulkiness assists in promoting the reactivity.
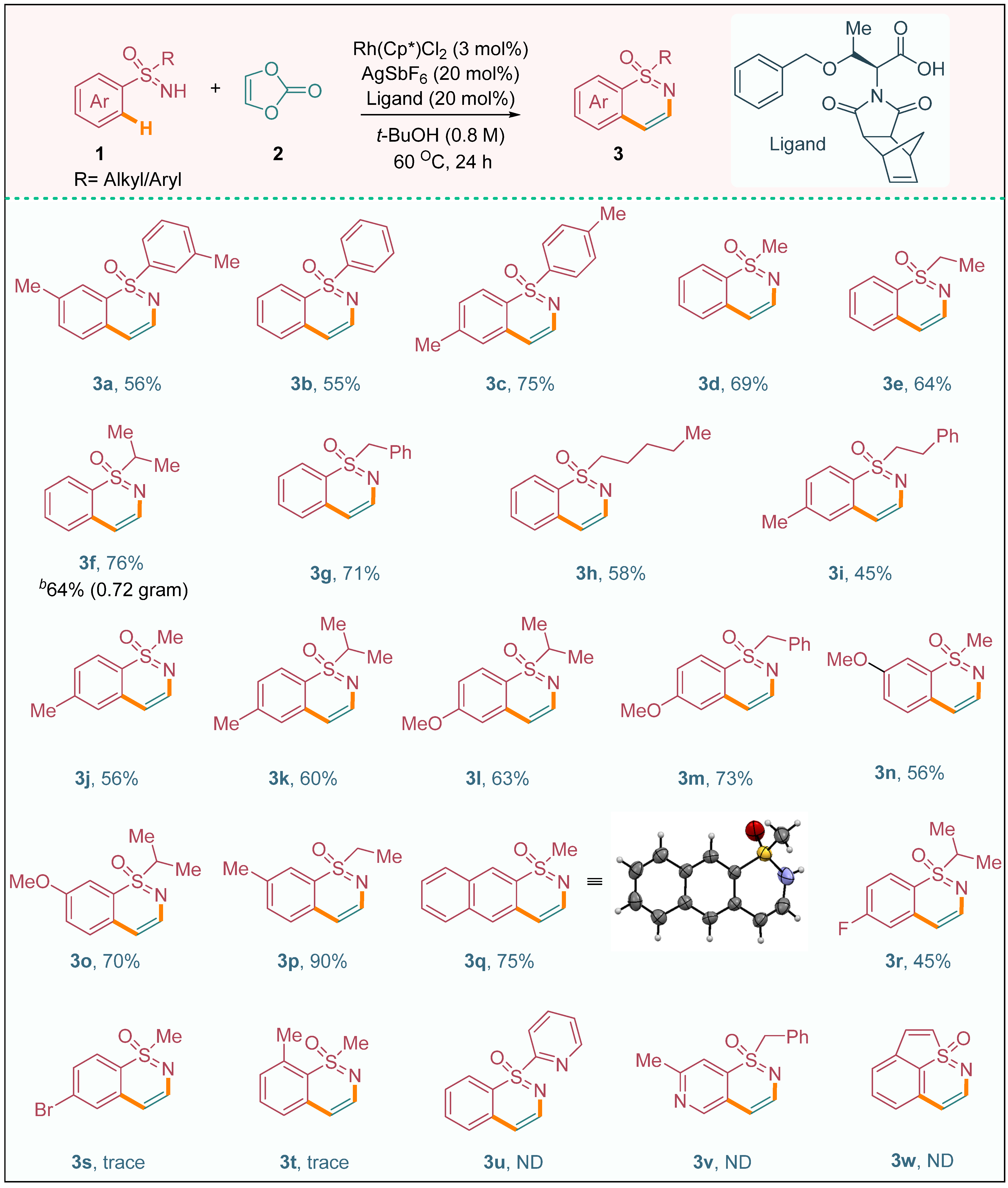
These observations emphasize the significance of both the ligand and the catalyst in orchestrating the C–H activation process and achieving efficient conversion of the starting materials into the desired product. Following an extensive series of optimization endeavors, we proceeded to explore the applicability of various sulfoximine derivatives in the reaction system, as depicted in Scheme 2. Specifically, we investigated the reaction of di-aryl sulfoximines featuring donating groups (1a and 1c) or no substitution (1b) with vinylene carbonate (2) to assess their potential as viable substrates. Gratifyingly, these reactions resulted in the formation of the desired products 3a, 3b, and 3c, exhibiting moderate to good yields. The successful annulation of methyl phenyl sulfoximine (1d) with vinylene carbonate (2) proceeded smoothly, leading to the formation of the annulation product 3d at a satisfactory yield of 69%. This result highlights the efficiency and effectiveness of our reaction system in transforming methyl phenyl sulfoximine into the desired 1,2-benzothiazine derivative.
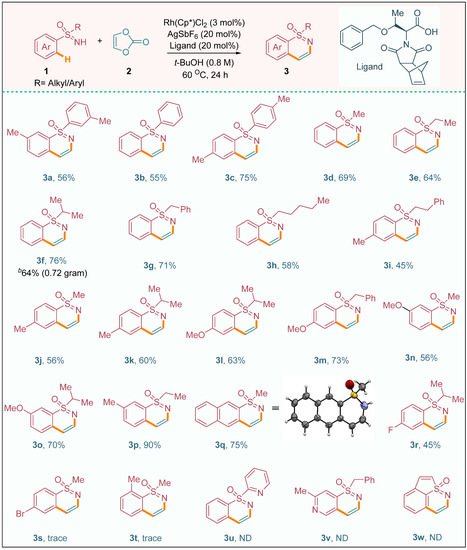
Scheme 2.
The substrate scope of sulfoximines a. a Reaction conditions: 1a (0.3 mmol), 2 (3.0 equiv), [Rh(Cp*)Cl2]2 (3.0 mol%), and AgSbF6 (20 mol%) in t–BuOH (2.4 mL) under nitrogen at 60 °C for 24 h. b Gram scale. ND = not determined.
In a similar fashion, phenyl alkyl/benzyl sulfoximines (1e–i) exhibited excellent reactivity and performance, affording the desired 1,2-benzothiazine derivatives 3e–i with moderate to good yields. These findings indicate the broad substrate scope and versatility of our methodology, as it enables the construction of diverse 1,2-benzothiazine derivatives from various phenyl alkyl/benzyl sulfoximines. The reliable and consistent formation of the desired products further establishes the potential of sulfoximines as valuable building blocks for the synthesis of 1,2-benzothiazine derivatives. These findings not only expand the synthetic possibilities but also provide valuable insights into the reactivity patterns and synthetic utility of different sulfoximine derivatives in this annulation process. The successful implementation of our methodology extended to the annulation of aryl-alkylsulfoximines 1j–m, which featured para-donating group substitutions on the aryl motifs and different alkyl/benzyl moieties. This transformation provided a convenient route for accessing the corresponding 1,2-benzothiazine derivatives 3j (56% yield), 3k (60% yield), 3l (63% yield), and 3m (73% yield). Notably, the annulation occurred selectively at the sterically less hindered C–H bond of the meta-substituted aryl moiety in the sulfoximine, resulting in the formation of products 3n (56% yield) and 3o (70% yield). Furthermore, we successfully synthesized the ethyl meta-tolyl sulfoximine-derived product 3p, achieving an impressive yield of 90%. Additionally, the annulation of β-naphthyl methyl sulfoximine (1q) with 2 yielded the desired product 3q in a high yield of 75%. The structures of the synthesized compounds were further confirmed by X-ray diffraction analysis (see the supplementary material), providing unequivocal evidence for the formation of product 3q. These results highlight the robustness and versatility of our methodology, enabling the efficient synthesis of diverse 1,2-benzothiazine derivatives from a range of aryl-alkyl sulfoximines with different substituents. The high yields obtained in these transformations demonstrate the effectiveness of our approach and open up new possibilities for the synthesis of structurally diverse and pharmaceutically relevant 1,2-benzothiazine derivatives. In contrast to the successful annulation reactions observed with other sulfoximines, the presence of an electron-withdrawing group on the aryl moiety in sulfoximine 1r significantly affected the reactivity, resulting in a low yield of product 3r. The electron-withdrawing nature of the substituent likely diminished the reactivity of the C–H bond, leading to a less favorable chelation-assisted C–H metalation–deprotonation (CMD) process and subsequently hindering the formation of the desired product. Regrettably, the aryl-sulfoximines bearing para-bromo substitution (1s) and ortho-methyl substitution (1t) did not participate in the annulation reaction. This lack of reactivity can be attributed to a combination of electronic and steric factors that impede the CMD process. The presence of a para-bromo substituent likely has a strong electron-withdrawing effect, further diminishing the reactivity of the C–H bond. Similarly, the ortho-methyl substitution introduces steric hindrance, making it difficult for the catalyst to access and activate the C–H bond effectively. Likewise, heterocyclic sulfoximines exert a significant influence on reactivity. In particular, the compounds 2-pyridyl phenyl sulfoximine (3u) and benzyl(imino)(2-methylpyridin-4-yl)-λ6-sulfanone (3v) are distinct; they do not participate in the annulation process due to the strong binding affinity of nitrogen to the metal catalyst. Moreover, the non-involvement of benzothiophene sulfoximine (3w) is attributed to the lack of planarity between the nitrogen atom of the sulfoximine and the arene C–H bonds, thereby exerting a discernible impact on reactivity. These findings, therefore, warrant further investigation.
Moreover, the observations emphasize the importance of the electronic and steric properties of the sulfoximine substrates in governing the success of the annulation reaction. It is intriguing to note that the incorporation of alkyl groups into the sulfoximine motifs, including methyl (1d), ethyl (1e), isopropyl (1f, 1k, 1l, and 1o), and benzyl (1g and 1m), resulted in highly efficient annulation reactions, and no cleavage of the starting materials was observed. These alkyl-substituted sulfoximines exhibited excellent reactivity and demonstrated remarkable effectiveness in yielding the desired products.
Moreover, the success of the annulation reaction was not limited to small-scale reactions but extended to gram-scale synthesis as well. For instance, NH-sulfoximine 1f was subjected to the annulation process, and a substantial quantity of product 3f (0.75 g) was obtained with an impressive yield of 64%. Overall, the introduction of alkyl groups on the sulfoximine moieties significantly enhanced the efficiency and applicability of the annulation reaction, enabling the synthesis of diverse compounds in satisfactory yields while maintaining the integrity of the starting materials.
The incorporation of chiral sulfoximines plays a pivotal role in the molecular design of pharmaceutical compounds due to their significant presence and potential therapeutic applications. Consequently, the development of efficient synthetic methodologies for the construction of chiral sulfoximine derivatives has garnered considerable attention within the scientific community. The ability to access diverse chiral sulfoximine scaffolds through synthetic approaches holds great significance in drug discovery and medicinal chemistry. These chiral motifs can impart unique stereochemical properties to the target molecules, influencing their biological activity, selectivity, and pharmacokinetic profiles. Therefore, the exploration and advancement of synthetic methods to access chiral sulfoximines are of paramount importance for the development of novel pharmaceutical agents. The pursuit of efficient and versatile synthetic strategies for chiral sulfoximine synthesis not only addresses the growing demand for enantiopure compounds but also enables the exploration of structure–activity relationships, identification of new drug targets, and optimization of therapeutic properties. Consequently, the field of chiral sulfoximine synthesis continues to attract significant research interest and holds immense promise for the discovery and development of pharmaceutical compounds with enhanced efficacy and reduced side effects.
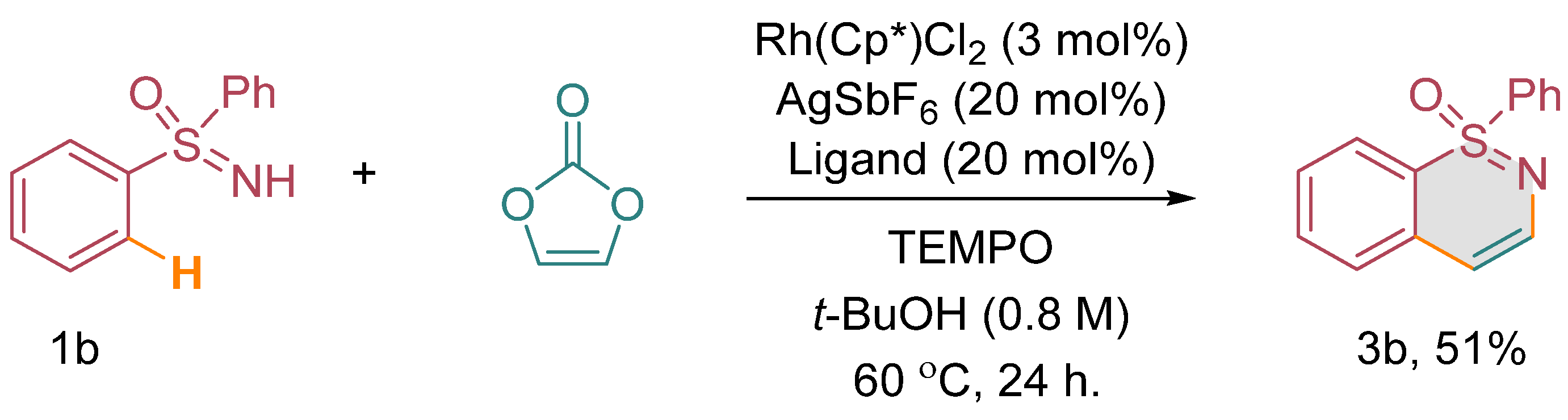
Considering the limitations in accessing optically active 1,2-benzothiazine derivatives through conventional methods, we conceived the idea of investigating the annulation of enantiopure sulfoximines with vinylene carbonate under carefully optimized catalytic conditions. Our objective was to achieve the synthesis of these valuable compounds with preserved stereochemistry, which would otherwise be challenging to obtain using conventional synthetic approaches. To demonstrate the feasibility of this approach, we focused on the annulation of chiral methyl phenyl sulfoximine, specifically the (S)-enantiomer, designated as (S)-1d, with vinylene carbonate (2). Our expectation was to obtain the corresponding optically active 1,2-benzothiazine derivative, namely (S)-3d [(Scheme 3) (see the supplementary material)]. It was crucial for us to confirm that the chiral sulfoximine’s stereointegrity would be maintained throughout the annulation process. Our experimental results affirmed the successful conversion of (S)-1d to (S)-3d through the annulation reaction. Notably, the stereogenic centers of the chiral sulfoximine remained unchanged, indicating the retention of its stereochemical integrity during the annulation process. Furthermore, to investigate the involvement of radicals in the reaction, we performed a radical quenching experiment using diphenyl sulfoxime 1b, vinylene carbonate, and TEMPO as a radical quencher under optimized catalytic conditions. Surprisingly, we obtained a 51% yield of the annulated product 3b, indicating that the reaction was not quenched by radicals (Scheme 4).
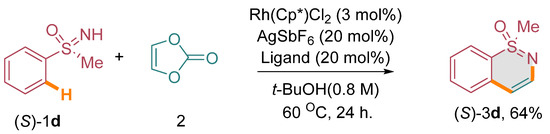
Scheme 3.
Annulation with enantiopure sulfoximines.
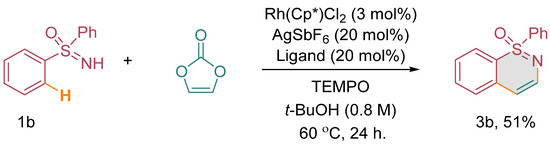
Scheme 4.
Radical quenching experiment.
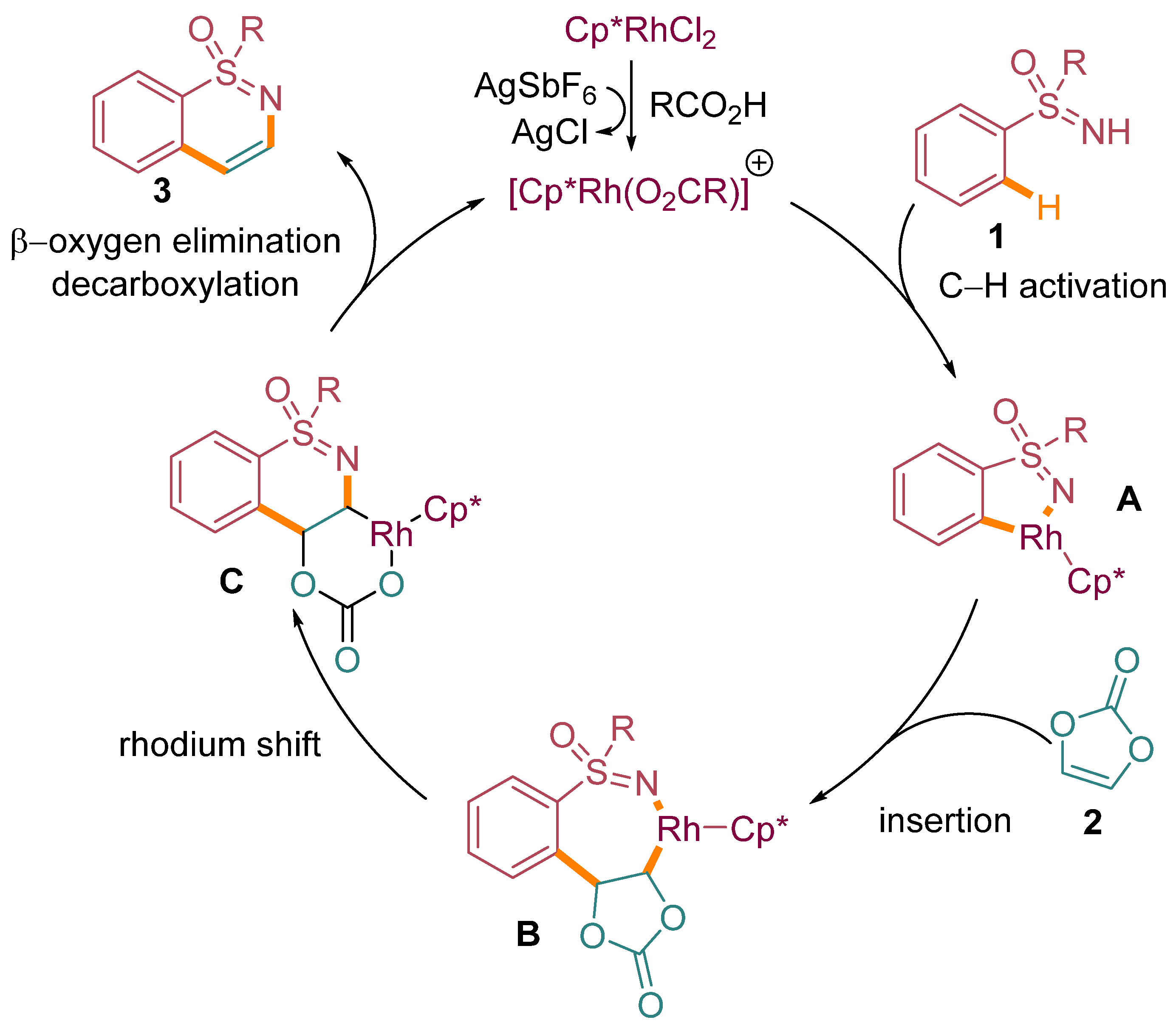
A plausible mechanistic pathway, supported by previous studies [24], has been proposed to elucidate the key steps involved in the annulation process. As illustrated in Scheme 5, the reaction initiation involves the formation of a five-membered rhodacycle intermediate A. This intermediate is formed through the coordination of the cationic rhodium catalyst with the nitrogen atom of the sulfoximine moiety in compound 1. Importantly, this coordination is facilitated by the carboxylate-assisted metalation–deprotonation (CMD) process, which involves the activation of the ortho-C–H bond of the arene moiety in compound 1.
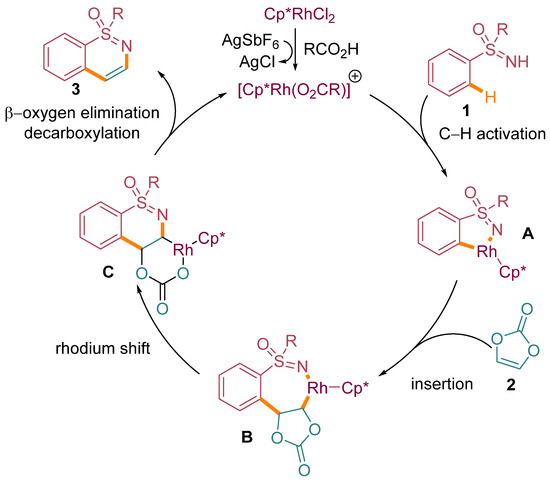
Scheme 5.
Proposed mechanism for the annulation of sulfoximines.
The N-protected amino acid ligand further enhances this ortho-C–H bond activation. Following the formation of intermediate A, migratory insertion occurs, where vinylene carbonate 2 reacts with the rhodium center, leading to the formation of a seven-membered rhodium complex, denoted as intermediate B. This step involves the migration of the rhodium atom from the five-membered ring to the carbonate moiety to give intermediate C. The overall process is redox-neutral, preserving the oxidation state of the rhodium catalyst. Notably, this step is followed by a β-oxygen elimination process, resulting in the closure of the catalytic cycle and the liberation of the final product. The reaction represented in Scheme 5 visualizes the sequential transformations and intermediates along this proposed mechanistic pathway.
3. Materials and Methods
3.1. General Information
All reagents and solvents were purchased from commercial sources, including BLD Pharmatech Ltd. headquartered in Shanghai, China., TCI Chemicals India Pvt Ltd., Turkapally, Hyderabad, India., Spectrochem Pvt. Ltd., Dabholkar Wadi, Mumbai, India., Avra Synthesis Pvt Ltd., Sai Enclave Habsiguda, Hyderabad, India., Jinay Pharmaceuticals Pvt Ltd., Thane, Mumbai, India., Sigma Aldrich Chemicals Pvt Ltd., Bangalore, India., and Finar Chemicals Pvt Ltd., Ahmedabad, Gujarat, India., and were used without further purification. Analytical thin-layer chromatography (TLC) was performed on HSGF 254 plates with a thickness of 0.15–0.2 mm. All products were characterized by their NMR and MS spectra. 1H and 13C nuclear magnetic resonance spectra (NMR) were acquired on a Bruker 400 MHz or 500 MHz NMR spectrometer, and chemical shifts were reported in parts per million (ppm) downfield from tetramethylsilane. The coupling constants (J) were indicated in Hz, and proton coupling patterns were described as singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m), doublet of doublets (dd), and broad (br). High-resolution mass spectra (HRMS) data were measured on an Agilent G6520 Q-TOF instrument (Palo Alto, CA, USA) with electrospray ionization (ESI).
3.2. General Procedure for the Synthesis of Substrates 1a–1w
A solution of sulfide (1.0 mmol) in MeOH (7.0 mL) was added to (NH4)2CO3 (1.2 mmol). Subsequently, PhI(OAc)2 (2.2 mmol) was slowly added, and the solution was stirred at room temperature. After complete consumption of the starting material, the solvents were removed under reduced pressure, and the crude product was purified by flash column chromatography using an eluent of EtOAc/hexane from 10:1 to 1:1 to yield the desired product 1.
3.3. General Procedure for the Synthesis of Compounds 3a–3w
Sulfoximine 1 (0.3 mmol), vinylene carbonate (0.6 mmol), [Cp*RhCl2]2 (3.0 mol%; 0.009 mmol), and AgSbF6 (20 mol%; 0.06 mmol) were added to a 15 mL screw-capped vial under nitrogen. Tertiary butanol was added under nitrogen, and the resulting mixture was stirred at 60 °C for 24 h. Upon completion of the reaction, the solvents were evaporated under reduced pressure, and the residue was purified by silica gel chromatography using an eluent of EtOAc/hexane from 18:1 to 10:3 to yield the desired product 3.
3.4. General Procedure for the Synthesis of Ligands L1, L2, and L4
To prepare the title compound, a mixture of a-amino acid (1.0 equiv.) and its corresponding anhydride (1.0 equiv.) was heated at 120 °C for 4 h. After cooling to room temperature, 50 mL of CHCl3 and 50 mL of aqueous 1 N HCl were added to the reaction mixture. The aqueous layer was extracted with CHCl3, and the combined organic layer was washed with brine and dried over Na2SO4. The solvent was then removed under reduced pressure to yield the title compound.
3.5. Characterization of the Products
7-Methyl-1-(m-tolyl)benzo[e][1,2]thiazine 1-oxide (3a): Yellow gummy liquid (45 mg, 56% yield); 1H NMR (500 MHz, CDCl3) δ 7.82–7.75 (m, 1H), 7.75–7.70 (m, 1H), 7.52–7.42 (m, 2H), 7.33 (dd, J = 8.2, 1.7 Hz, 1H), 7.28 (d, J = 8.2 Hz, 1H), 7.24 (d, J = 6.9 Hz, 1H), 7.12–7.08 (m, 1H), 6.21 (dd, J = 6.9, 0.8 Hz, 1H), 2.45 (s, 3H), 2.30 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 139.52, 139.14, 137.00, 136.15, 134.64, 133.97, 133.29, 129.57, 128.93, 126.46, 126.23, 124.35, 120.42, 101.74, 21.33, 21.25. HRMS (ESI): Calculated for C16H16NOS [M + H]+: 270.0948, found: 270.0950.
1-Phenylbenzo[e][1,2]thiazine 1-oxide (3b): Yellow gummy liquid (40 mg, 55% yield); 1H NMR (500 MHz, CDCl3) δ 7.96–7.92 (m, 2H), 7.70–7.63 (m, 1H), 7.62–7.56 (m, 2H), 7.52–7.48 (m, 1H), 7.36 (dd, J = 8.1, 1.2 Hz, 1H), 7.33–7.29 (m, 2H), 7.28–7.25 (m, 1H), 6.24 (dd, J = 6.9, 0.8 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 140.24, 138.53, 135.90, 133.49, 132.12, 129.13, 128.99, 126.47, 126.14, 125.13, 120.22, 101.52. HRMS (ESI): Calculated for C14H12NOS [M + H]+: 242.0635, found: 242.0639.
6-Methyl-1-(p-tolyl)benzo[e][1,2]thiazine 1-oxide (3c): Yellow solid (70 mg, 75% yield); m.p.: 140–141 °C; 1H NMR (500 MHz, CDCl3) δ 7.79 (d, J = 8.4 Hz, 2H), 7.38–7.33 (dd, J = 8.5, 0.8 Hz, 2H), 7.28–7.23 (m, 1H), 7.20 (d, J = 8.3 Hz, 1H), 7.14–7.11 (t, J = 1.0 Hz 1H), 7.05 (dd, J = 8.2, 1.7 Hz, 1H), 6.13 (dd, J = 6.9, 0.8 Hz, 1H), 2.44 (s, 3H), 2.38 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 144.60, 142.82, 137.96, 137.07, 135.82, 129.64, 129.05, 127.88, 125.81, 125.03, 118.23, 101.39, 21.67, 21.55. HRMS (ESI): Calculated for C16H16NOS [M + H]+: 270.0948, found: 270.0948.
1-Methyl-1λ4-benzo[e][1,2]thiazine 1-oxide (3d): Yellow solid (38 mg, 69% yield); m.p.: 134–135 °C; 1H NMR (500 MHz, CDCl3) δ 7.77 (d, J = 8.0 Hz, 1H), 7.58–7.53 (m, 1H), 7.40 (td, J = 7.7, 1.2 Hz, 1H), 7.31 (dd, J = 8.0, 1.2 Hz, 1H), 7.06 (d, J = 6.9 Hz, 1H), 6.07 (dd, J = 6.8, 0.8 Hz 1H), 3.56 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 137.87, 135.84, 132.77, 126.62, 126.45, 123.58, 119.39, 101.63, 45.33. HRMS (ESI): Calculated for C9H10NOS [M + H]+: 180.0478, found: 180.0480.
1-Ethyl-1λ4-benzo[e][1,2]thiazine 1-oxide (3e): Yellow gummy liquid (37 mg, 64% yield); 1H NMR (500 MHz, CDCl3) δ 7.72 (d, J = 8.1 Hz, 1H), 7.58–7.54 (m, 1H), 7.41–7.37 (m, 1H), 7.32 (dd, J = 8.0, 1.2 Hz, 1H), 7.12 (d, J = 6.9 Hz, 1H), 6.01 (dd, J = 7.0, 0.8 Hz, 1H), 3.81–3.60 (m, 2H), 1.23 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 137.79, 137.02, 133.12, 126.70, 126.64, 124.04, 116.52, 101.15, 51.30, 8.58. HRMS (ESI): Calculated for C10H11NOSNa [M + Na]+: 216.0454, found: 216.0454.
1-Isopropyl-1λ4-benzo[e][1,2]thiazine 1-oxide (3f): Yellow gummy liquid (47 mg, 76% yield); 1H NMR (500 MHz, CDCl3) δ 7.75–7.66 (m, 1H), 7.56–7.52 (m, 1H), 7.38–7.33 (m, 1H), 7.27 (d, J = 5.6 Hz, 1H), 7.10 (d, J = 6.9 Hz, 1H), 5.92 (dd, J = 6.9, 0.8 Hz, 1H), 3.88–3.82 (m, 1H), 1.50 (d, J = 6.9 Hz, 3H), 1.18 (d, J = 6.7 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 138.90, 137.79, 133.01, 126.34, 126.23, 124.52, 115.57, 100.45, 58.23, 17.16, 13.52. HRMS (ESI): Calculated for C11H14NOS [M + H]+: 208.0791, found: 208.0790.
1-Benzyl-1λ4-benzo[e][1,2]thiazine 1-oxide (3g): Yellow solid (55 mg, 71% yield); m.p.: 166–167 °C; 1H NMR (500 MHz, CDCl3) δ 7.60 (d, J = 8.0 Hz, 1H), 7.51–7.46 (m, 1H), 7.33–7.27 (m, 2H), 7.24–7.20 (m, 2H), 7.17 (dt, J = 6.9, 1.5 Hz, 2H), 7.14 (dd, J = 8.1, 1.1 Hz, 1H), 6.98 (d, J = 6.9 Hz, 1H), 5.75 (d, J = 6.9 Hz, 1H), 4.83–4.62 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 139.53, 137.95, 133.05, 131.12, 128.76, 128.70, 128.54, 128.42, 127.82, 126.00, 125.87, 125.25, 116.23, 100.46, 64.66. HRMS (ESI): Calculated for C15H14NOS [M + H]+: 256.0791, found: 256.0796.
1-Pentyl-1λ4-benzo[e][1,2]thiazine 1-oxide (3h): Yellow gummy liquid (41 mg, 58% yield); 1H NMR (500 MHz, CDCl3) δ 7.72 (dd, J = 8.0, 1.2 Hz, 1H), 7.58–7.54 (m, 1H), 7.42–7.38 (m, 1H), 7.34–7.30 (m, 1H), 7.11 (d, J = 6.9 Hz, 1H), 6.02 (dd, J = 7.0, 0.8 Hz, 1H), 3.78–3.57 (m, 2H), 1.82–1.43 (m, 2H), 1.42–1.17 (m, 4H), 0.84 (t, J = 7.2 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 137.53, 136.69, 133.06, 126.67, 126.62, 123.99, 117.28, 101.19, 56.66, 29.99, 23.52, 22.02, 13.65. HRMS (ESI): Calculated for C13H18NOS [M + H]+: 236.1104, found: 236.1103.
6-Methyl-1-phenethyl-1λ4-benzo[e][1,2]thiazine 1-oxide (3i): Yellow gummy liquid (39 mg, 45% yield); 1H NMR (500 MHz, CDCl3) δ 7.58 (d, J = 8.2 Hz, 1H), 7.27–7.16 (m, 4H), 7.16–7.09 (m, 4H), 6.02 (d, J = 7.0 Hz, 1H), 4.07–3.95 (m, 2H), 3.13–2.76 (m, 2H), 2.44 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 144.49, 136.66, 136.33, 128.82, 128.42, 126.99, 126.62, 124.10, 114.94, 101.69, 57.84, 30.12, 21.80. HRMS (ESI): Calculated for C17H18NOS [M + H]+: 284.1104, found: 284.1108.
1,6-Dimethyl-1λ4-benzo[e][1,2]thiazine 1-oxide (3j): Yellow gummy liquid (32 mg, 56% yield); 1H NMR (500 MHz, CDCl3) δ 7.68 (d, J = 8.2 Hz, 1H), 7.25–7.22 (m, 1H), 7.11 (s, 1H), 7.03 (d, J = 6.9 Hz, 1H), 6.01 (dd, J = 7.0, 0.8 Hz, 1H), 3.57 (s, 3H), 2.43 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 143.92, 136.95, 135.80, 128.20, 128.19, 126.36, 126.34, 123.66, 117.21, 101.78, 45.38, 45.37, 21.75. HRMS (ESI): Calculated for C10H12NOS [M + H]+: 194.0635, found: 194.0632.
1-Isopropyl-6-methyl-1λ4-benzo[e][1,2]thiazine 1-oxide (3k): Yellow gummy liquid (40 mg, 60% yield); 1H NMR (500 MHz, CDCl3) δ 7.61 (d, J = 8.2 Hz, 1H), 7.19 (dd, J = 8.2, 1.6 Hz, 1H), 7.08 (d, J = 6.9 Hz, 2H), 5.86 (dd, J = 6.9, 0.8 Hz, 1H), 3.85 (p, J = 6.8 Hz, 1H) 3.88–3.82 (m, 1H), 2.41 (s, 3H), 1.49 (d, J = 6.9 Hz, 3H), 1.20 (d, J = 6.7 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 144.01, 138.50, 137.88, 127.82, 126.11, 124.62, 113.14, 100.45, 58.62, 21.74, 17.10, 13.71. HRMS (ESI): Calculated for C12H16NOS [M + H]+: 222.0948, found: 222.0950.
1-Isopropyl-6-methoxy-1λ4-benzo[e][1,2]thiazine 1-oxide (3l): Yellow gummy liquid (45 mg, 63% yield); 1H NMR (500 MHz, CDCl3) δ 7.66–7.59 (dd, J = 9.0, 0.5 Hz, 1H), 7.08 (d, J = 6.9 Hz, 1H), 6.92 (dd, J = 8.9, 2.5 Hz, 1H), 6.64 (d, J = 2.5 Hz, 1H), 5.84 (dd, J = 6.9, 0.7 Hz, 1H), 3.87 (s, 3H), 3.82–3.75 (m, J = 6.8 Hz, 1H), 1.46 (d, J = 6.9 Hz, 3H), 1.19 (d, J = 6.8 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 163.05, 140.32, 139.46, 139.41, 126.81, 115.59, 108.11, 107.41, 100.43, 100.41, 59.06, 55.53, 16.97, 13.76. HRMS (ESI): Calculated for C12H15NO2SNa [M + Na]+: 260.0716, found: 260.0717.
1-Benzyl-6-methoxy-1λ4-benzo[e][1,2]thiazine 1-oxide (3m): Yellow gummy liquid (63 mg, 73% yield); 1H NMR (500 MHz, CDCl3) δ 7.46 (dd, J = 8.9, 0.7 Hz, 1H), 7.29–7.25 (m, 1H), 7.24–7.20 (m, 2H), 7.21–7.11 (m, 2H), 6.95 (d, J = 6.9 Hz, 1H), 6.84 (dd, J = 8.9, 2.5 Hz, 1H), 6.49 (d, J = 2.5 Hz, 1H), 5.64 (dd, J = 7.0, 0.8 Hz, 1H), 4.72–4.54 (m, 2H), 3.83 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 162.92, 140.42, 140.26, 131.08, 128.67, 128.37, 128.05, 127.38, 115.10, 108.98, 106.93, 100.30, 65.08, 55.41. HRMS (ESI): Calculated for C16H16NO2S [M + H]+: 286.0897, found: 286.0896.
7-Methoxy-1-methyl-1λ4-benzo[e][1,2]thiazine 1-oxide (3n): Yellow solid (35 mg, 56% yield); m.p.: 141–142 °C; 1H NMR (500 MHz, CDCl3) δ 7.28–7.25 (m, 1H), 7.23–7.15 (m, 2H), 6.93 (dd, J = 7.0, 2.0 Hz, 1H), 6.06–6.00 (m, 1H), 3.88 (d, J = 0.9 Hz, 3H), 3.61 (d, J = 1.9 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 158.36, 134.46, 129.35, 128.29, 122.29, 119.89, 105.28, 101.68, 55.82, 45.12. HRMS (ESI): Calculated for C10H11NO2SNa [M + Na]+: 232.0403, found: 232.0403.
1-Isopropyl-7-methoxy-1λ4-benzo[e][1,2]thiazine 1-oxide (3o): Yellow gummy liquid (50 mg, 70% yield); 1H NMR (500 MHz, CDCl3) δ 7.22 (d, J = 8.7 Hz, 1H), 7.17 (dd, J = 8.8, 2.6 Hz, 1H), 7.13 (d, J = 2.5 Hz, 1H), 6.99 (d, J = 6.9 Hz, 1H), 5.88 (dd, J = 6.9, 0.8 Hz, 1H), 3.91–3.87 (m, 1H), 3.85 (s, 3H), 1.50 (d, J = 6.9 Hz, 3H), 1.19 (d, J = 6.7 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 158.08, 135.99, 131.61, 127.99, 122.41, 115.94, 106.15, 100.29, 58.22, 55.75, 17.27, 13.55. HRMS (ESI): Calculated for C12H16NO2S [M + H]+: 238.0897, found: 238.0894.
1-Ethyl-7-methyl-1λ4-benzo[e][1,2]thiazine 1-oxide (3p): Yellow gummy liquid (56 mg, 90% yield); 1H NMR (500 MHz, CDCl3) δ 7.49 (s, 1H), 7.38 (dd, J = 8.3, 1.8 Hz, 1H), 7.21 (d, J = 8.1 Hz, 1H), 7.06 (d, J = 6.9 Hz, 1H), 5.96 (d, J = 6.9 Hz, 1H), 3.97–3.48 (m, 2H), 2.42 (s, 3H), 1.20 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 137.35, 136.83, 134.77, 134.43, 126.45, 123.33, 116.29, 100.73, 51.31, 21.27, 8.54. HRMS (ESI): Calculated for C11H14NOS [M + H]+: 208.0791, found: 208.0794
1-Methyl-1λ4-naphtho [2,3-e][1,2]thiazine 1-oxide (3q): Yellow crystalline solid (51 mg, 75% yield); m.p.: 172–173 °C; 1H NMR (500 MHz, CDCl3) δ 8.43 (s, 1H), 7.94 (dd, J = 8.4, 1.1 Hz, 1H), 7.87 (d, J = 8.4 Hz, 1H), 7.75 (s, 1H), 7.62–7.58 (m, 1H), 7.52–7.48 (m, 1H), 6.96 (d, J = 7.1 Hz, 1H), 6.22 (d, J = 7.1 Hz, 1H), 3.62 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 135.94, 135.64, 131.71, 131.16, 129.13, 128.90, 127.84, 126.23, 125.23, 123.91, 121.87, 102.36, 45.35. HRMS (ESI): Calculated for C13H12NOS [M + H]+: 230.0635, found: 230.0637
6-Fluoro-1-isopropyl-1λ4-benzo[e][1,2]thiazine 1-oxide (3r): Yellow gummy liquid (30 mg, 45% yield); 1H NMR (500 MHz, CDCl3) δ 7.77–7.67 (m, 1H), 7.13 (dd, J = 6.9, 0.8 Hz, 1H), 7.06 (ddd, J = 8.8, 8.1, 2.5 Hz, 1H), 6.91 (dd, J = 9.8, 2.5 Hz, 1H), 5.86 (dd, J = 6.9, 0.8 Hz, 1H), 3.8–3.74 (m, 1H), 1.48 (d, J = 6.8 Hz, 3H), 1.18 (d, J = 6.7 Hz, 3H). 19F NMR (471 MHz, CDCl3) δ -104.43. 13C NMR (101 MHz, CDCl3) δ 141.74, 127.95, 127.85, 114.70, 114.46, 111.11, 110.89, 99.91, 59.05, 16.92, 13.68. HRMS (ESI): Calculated for C11H13FNOS [M + H]+: 226.0697, found: 226.0696.
4. Conclusions
In summary, we have presented a demonstration of the Rhodium(III)-catalyzed, redox-neutral [4+2]-C–H annulation of sulfoximine with the application of vinylene carbonate as an acetylene equivalent. Through this innovative approach, it is possible to achieve the direct synthesis of unsubstituted aryl-fused sulfoximine heterocycles. The reaction exhibits a broad scope, making a wide array of 1,2-benzothiazine derivatives in moderate to good yields.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules28135014/s1. The optimization details and the spectra of the representative compounds.
Author Contributions
K.K. and S.S. contributed equally. Concept was designed by K.K., S.S. and A.K.S. Experiments and investigation were conducted by K.K. and S.S.; formal analysis and data curation were conducted by K.K. and S.S.; writing, review and editing were conducted by A.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
We thank SERB-India (Grant No. CRG-2019-1802) and the University of Hyderabad UoH-IoE, UOH/IOE/RC1-20-006 for financial support. S.S. and K.K. thank CSIR-UGC, India, for fellowships. We thank Mahesh for solving the X-ray crystal data.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This manuscript is dedicated to Castern Bolm for his outstanding contributions to the development novel synthetic methods on sulfoximines.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Sirvent, J.A.; Lücking, U. Novel Pieces for the Emerging Picture of Sulfoximines in Drug Discovery: Synthesis and Evaluation of Sulfoximine Analogues of Marketed Drugs and Advanced Clinical Candidates. ChemMedChem 2017, 12, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Lücking, U. Neglected Sulfur(vi) Pharmacophores in Drug Discovery: Exploration of Novel Chemical Space by the Interplay of Drug Design and Method Development. Org. Chem. Front. 2019, 6, 1319–1324. [Google Scholar] [CrossRef]
- Lücking, U. Sulfoximines: A Neglected Opportunity in Medicinal Chemistry. Angew. Chem. Int. Ed. 2013, 52, 9399–9408. [Google Scholar] [CrossRef] [PubMed]
- Frings, M.; Bolm, C.; Blum, A.; Gnamm, C. Sulfoximines from a Medicinal Chemist’s Perspective: Physicochemical and In Vitro Parameters Relevant for Drug Discovery. Eur. J. Med. Chem. 2017, 126, 225–245. [Google Scholar] [CrossRef]
- Bartoszyk, G.D.; Dooley, D.J.; Barth, H.; Hartenstein, J.; Satzinger, G. Stereoselective Pharmacological Effects and Benzodiazepine Receptor Affinity of the Enantiomers of Gö 4962. J. Pharm. Pharmacol. 1987, 39, 407–408. [Google Scholar] [CrossRef]
- De Clercq, E. The Role of Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) in the Therapy of HIV-1 Infection. Antivir. Res. 1998, 38, 153–179. [Google Scholar] [CrossRef]
- Buckheit, R.W.J.; Fliakas-Boltz, V.; Decker, W.D.; Roberson, J.L.; Pyle, C.A.; White, E.L.; Bowdon, B.J.; McMahon, J.B.; Boyd, M.R.; Bader, J.P. Biological and Biochemical Anti-HIV Activity of the Benzothiadiazine Class of Nonnucleoside Reverse Transcriptase Inhibitors. Antivir. Res. 1994, 25, 43–56. [Google Scholar] [CrossRef]
- Yongpruksa, N.; Pandey, S.; Baker, G.A.; Harmata, M. Benzothiazines in Organic Synthesis. Synthesis of Fluorescent 7-Amino-2,1-Benzothiazines. Org. Biomol. Chem. 2011, 9, 7979–7982. [Google Scholar] [CrossRef]
- Dillard, R.D.; Yen, T.T.; Stark, P.; Pavey, D.E. Synthesis and Blood Pressure Lowering Activity of 3-(Substituted-Amino)-1,2,4-Benzothiadiazine 1-Oxide Derivatives. J. Med. Chem. 1980, 23, 717–722. [Google Scholar] [CrossRef]
- Harmata, M.; Rayanil, K.; Gomes, M.G.; Zheng, P.; Calkins, N.L.; Kim, S.Y.; Fan, Y.; Bumbu, V.; Lee, D.R.; Wacharasindhu, S.; et al. 1, 2-Benzothiazines from sulfoximines and allyl methyl carbonate by rhodium-catalyzed cross-coupling and oxidative cyclization. Org. Lett. 2005, 7, 143–145. [Google Scholar] [CrossRef]
- Dong, W.; Wang, L.; Parthasarathy, K.; Pan, F.; Bolm, C. Rhodium-Catalyzed Oxidative Annulation of Sulfoximines and Alkynes as an Approach to 1,2-Benzothiazines. Angew. Chem. Int. Ed. 2013, 52, 11573–11576. [Google Scholar] [CrossRef]
- Cheng, Y.; Bolm, C. Regioselective Syntheses of 1,2-Benzothiazines by Rhodium-Catalyzed Annulation Reactions. Angew. Chem. Int. Ed. 2015, 54, 12349–12352. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; de Azambuja, F.; Glorius, F. α-MsO/TsO/Cl Ketones as Oxidized Alkyne Equivalents: Redox-Neutral Rhodium(III)-Catalyzed C−H Activation for the Synthesis of N-Heterocycles. Angew. Chem. Int. Ed. 2014, 53, 2754–2758. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.H.; Son, J.-Y.; Kim, J.E.; Lee, P.H. ChemInform Abstract: Synthesis of 1,2-Benzothiazines by a Rhodium-Catalyzed Domino C—H Activation/Cyclization/Elimination Process from S-Aryl Sulfoximines and Pyridotriazoles. ChemInform 2016, 47, 3498–3501. [Google Scholar] [CrossRef]
- Wen, J.; Cheng, H.; Raabe, G.; Bolm, C. Rhodium-Catalyzed [4 + 3] Annulations of Sulfoximines with α,β-Unsaturated Ketones Leading to 1,2-Benzothiazepine 1-Oxides. Org. Lett. 2017, 19, 6020–6023. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, L. Rhodium-Catalyzed Benzoisothiazole Synthesis by Tandem Annulation Reactions of Sulfoximines and Activated Olefins. Org. Biomol. Chem. 2017, 15, 9983–9986. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Y.; Wang, T.; Huang, Q.; Wang, Z.; Chen, Z. Microwave-Assisted Cp*CoIII-Catalyzed C–H Activation/Double C–N Bond Formation Reactions to Thiadiazine 1-Oxides. Org. Lett. 2017, 19, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Wan, B.; Li, X. Enantiodivergent Desymmetrization in the Rhodium(III)-Catalyzed Annulation of Sulfoximines with Diazo Compounds. Angew. Chem. Int. Ed. 2018, 57, 15534–15538. [Google Scholar] [CrossRef]
- Aher, Y.N.; Lade, D.M.; Pawar, A.B. Cp*Ir(Iii)-Catalyzed C–H/N–H Functionalization of Sulfoximines for the Synthesis of 1,2-Benzothiazines at Room Temperature. Chem. Commun. 2018, 54, 6288–6291. [Google Scholar] [CrossRef]
- Sun, Y.; Cramer, N. Enantioselective Synthesis of Chiral-at-Sulfur 1,2-Benzothiazines by CpxRhIII-Catalyzed C−H Functionalization of Sulfoximines. Angew. Chem. Int. Ed. 2018, 57, 15539–15543. [Google Scholar] [CrossRef] [PubMed]
- Brauns, M.; Cramer, N. Efficient Kinetic Resolution of Sulfur-Stereogenic Sulfoximines by Exploiting CpXRhIII-Catalyzed C−H Functionalization. Angew. Chem. Int. Ed. 2019, 58, 8902–8906. [Google Scholar] [CrossRef]
- Huang, J.-R.; Bolm, C. Microwave-Assisted Synthesis of Heterocycles by Rhodium(III)-Catalyzed Annulation of N-Methoxyamides with α-Chloroaldehydes. Angew. Chem. Int. Ed. 2017, 56, 15921–15925. [Google Scholar] [CrossRef]
- Mukherjee, K.; Grimblat, N.; Sau, S.; Ghosh, K.; Shankar, M.; Gandon, V.; Sahoo, A.K. Kinetic Resolution of Sulfur-Stereogenic Sulfoximines by Pd(Ii)–MPAA Catalyzed C–H Arylation and Olefination. Chem. Sci. 2021, 12, 14863–14870. [Google Scholar] [CrossRef]
- Ghosh, K.; Nishii, Y.; Miura, M. Rhodium-Catalyzed Annulative Coupling Using Vinylene Carbonate as an Oxidizing Acetylene Surrogate. ACS Catal. 2019, 9, 11455–11460. [Google Scholar] [CrossRef]
- Li, J.-Y.; Xie, P.-P.; Zhou, T.; Qian, P.-F.; Zhou, Y.-B.; Li, H.-C.; Hong, X.; Shi, B.-F. Ir(III)-Catalyzed Asymmetric C–H Activation/Annulation of Sulfoximines Assisted by the Hydrogen-Bonding Interaction. ACS Catal. 2022, 12, 9083–9091. [Google Scholar] [CrossRef]
- Zhou, T.; Qian, P.-F.; Li, J.-Y.; Zhou, Y.-B.; Li, H.-C.; Chen, H.-Y.; Shi, B.-F. Efficient Synthesis of Sulfur-Stereogenic Sulfoximines via Ru(II)-Catalyzed Enantioselective C–H Functionalization Enabled by Chiral Carboxylic Acid. J. Am. Chem. Soc. 2021, 143, 6810–6816. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.-F.; Zhou, T.; Li, J.-Y.; Zhou, Y.-B.; Shi, B.-F. Ru(II)/Chiral Carboxylic Acid-Catalyzed Asymmetric [4 + 3] Annulation of Sulfoximines with α,β-Unsaturated Ketones. ACS Catal. 2022, 12, 13876–13883. [Google Scholar] [CrossRef]
- Wang, B.; Han, X.; Li, J.; Li, C.; Liu, H. Molecules Activation and Intramolecular Annulation for the Synthesis of Fused Isochromeno-1, 2-Benzothiazines Activation and Intramolecular Annulation for the Scaffolds Under. Molecules 2020, 25, 2515–2532. [Google Scholar] [CrossRef]
- Alberico, D.; Scott, M.E.; Lautens, M. Aryl−Aryl Bond Formation by Transition-Metal-Catalyzed Direct Arylation. Chem. Rev. 2007, 107, 174–238. [Google Scholar] [CrossRef]
- Chen, X.; Engle, K.M.; Wang, D.-H.; Yu, J.-Q. ChemInform Abstract: Palladium(II)-Catalyzed C—H Activation/C—C Cross-Coupling Reactions: Versatility and Practicality. ChemInform 2009, 40, 5094–5115. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, M.A.; Shen, Q.; Hartwig, J.F. A General and Long-Lived Catalyst for the Palladium-Catalyzed Coupling of Aryl Halides with Thiols. J. Am. Chem. Soc. 2006, 128, 2180–2181. [Google Scholar] [CrossRef] [PubMed]
- Platon, M.; Wijaya, N.; Rampazzi, V.; Cui, L.; Rousselin, Y.; Saeys, M.; Hierso, J.-C. Thioetherification of Chloroheteroarenes: A Binuclear Catalyst Promotes Wide Scope and High Functional-Group Tolerance. Chem.—Eur. J. 2014, 20, 12584–12594. [Google Scholar] [CrossRef] [PubMed]
- Guilbaud, J.; Labonde, M.; Selmi, A.; Kammoun, M.; Cattey, H.; Pirio, N.; Roger, J.; Hierso, J.-C. Palladium-Catalyzed Heteroaryl Thioethers Synthesis Overcoming Palladium Dithiolate Resting States Inertness: Practical Road to Sulfones and NH-Sulfoximines. Catal. Commun. 2018, 111, 52–58. [Google Scholar] [CrossRef]
- Ghosh, P.; Ganguly, B.; Das, S. N−H and C−H Functionalization of Sulfoximine: Recent Advancement and Prospects. Asian J. Org. Chem. 2020, 9, 2035–2082. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, J.Y.; Kwak, J.; Chang, S. ChemInform Abstract: Recent Advances in the Transition Metal-Catalyzed Twofold Oxidative C—H Bond Activation Strategy for C—C and C—N Bond Formation. ChemInform 2012, 43, 5068–5083. [Google Scholar] [CrossRef]
- Colby, D.A.; Tsai, A.S.; Bergman, R.G.; Ellman, J.A. Rhodium Catalyzed Chelation-Assisted C–H Bond Functionalization Reactions. Acc. Chem. Res. 2012, 45, 814–825. [Google Scholar] [CrossRef]
- Song, G.; Wang, F.; Li, X. ChemInform Abstract: C—C, C—O and C—N Bond Formation via Rhodium(III)-Catalyzed Oxidative C—H Activation. ChemInform 2012, 43, 3651–3678. [Google Scholar] [CrossRef]
- Rouquet, G.; Chatani, N. Catalytic Functionalization of C(sp2)−H and C(sp3)−H Bonds by Using Bidentate Directing Groups. Angew. Chem. Int. Ed. 2013, 52, 11726–11743. [Google Scholar] [CrossRef]
- Wencel-Delord, J.; Glorius, F. ChemInform Abstract: C—H Bond Activation Enables the Rapid Construction and Late-Stage Diversification of Functional Molecules. ChemInform 2013, 44, 369–375. [Google Scholar] [CrossRef]
- Song, G.; Li, X. Substrate Activation Strategies in Rhodium(III)-Catalyzed Selective Functionalization of Arenes. Acc. Chem. Res. 2015, 48, 1007–1020. [Google Scholar] [CrossRef]
- Hummel, J.R.; Boerth, J.A.; Ellman, J.A. Transition-Metal-Catalyzed C–H Bond Addition to Carbonyls, Imines, and Related Polarized π Bonds. Chem. Rev. 2017, 117, 9163–9227. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Qiu, D.; Wang, J. Transition-Metal-Catalyzed Cross-Couplings through Carbene Migratory Insertion. Chem. Rev. 2017, 117, 13810–13889. [Google Scholar] [CrossRef] [PubMed]
- Voronin, V.V.; Ledovskaya, M.S.; Bogachenkov, A.S.; Rodygin, K.S.; Ananikov, V.P. Acetylene in Organic Synthesis: Recent Progress and New Uses. Molecules 2018, 23, 2442. [Google Scholar] [CrossRef]
- Trotuş, I.-T.; Zimmermann, T.; Schüth, F. Catalytic Reactions of Acetylene: A Feedstock for the Chemical Industry Revisited. Chem. Rev. 2014, 114, 1761–1782. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, D.; Liu, Y.; Zeng, L.; Lei, A. Cobalt-Catalyzed Electrooxidative C-H/N-H [4 + 2] Annulation with Ethylene or Ethyne. Nat. Commun. 2018, 9, 798. [Google Scholar] [CrossRef]
- Reus, C.; Liu, N.-W.; Bolte, M.; Lerner, H.-W.; Wagner, M. Synthesis of Bromo-, Boryl-, and Stannyl-Functionalized 1,2-Bis(Trimethylsilyl)Benzenes via Diels–Alder or C–H Activation Reactions. J. Org. Chem. 2012, 77, 3518–3523. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.-J.; Han, T.; Ruan, W.; Wen, T.-B. Rh(III)-Catalyzed Oxidative Coupling of Benzoic Acids with Geminal-Substituted Vinyl Acetates: Synthesis of 3-Substituted Isocoumarins. J. Org. Chem. 2015, 80, 620–627. [Google Scholar] [CrossRef]
- Chu, H.; Sun, S.; Yu, J.-T.; Cheng, J. Rh-Catalyzed Sequential Oxidative C–H Activation/Annulation with Geminal-Substituted Vinyl Acetates to Access Isoquinolines. Chem. Commun. 2015, 51, 13327–13329. [Google Scholar] [CrossRef]
- Webb, N.J.; Marsden, S.P.; Raw, S.A. Rhodium(III)-Catalyzed C–H Activation/Annulation with Vinyl Esters as an Acetylene Equivalent. Org. Lett. 2014, 16, 4718–4721. [Google Scholar] [CrossRef]
- Shankar, M.; Rit, R.K.; Sau, S.; Mukherjee, K.; Gandon, V.; Sahoo, A.K. Double Annulation of Ortho- and Peri-C–H Bonds of Fused (Hetero)Arenes to Unusual Oxepino-Pyridines. Chem. Sci. 2020, 11, 10770–10777. [Google Scholar] [CrossRef]
- Shankar, M.; Guntreddi, T.; Ramesh, E.; Sahoo, A.K. Transformable Sulfoximine Assisted One-Pot Double Annulation of Vinylic C–H Bonds with Unactivated Alkynes. Org. Lett. 2017, 19, 5665–5668. [Google Scholar] [CrossRef] [PubMed]
- Shankar, M.; Saha, A.; Sau, S.; Ghosh, A.; Gandon, V.; Sahoo, A.K. Harnessing Sulfur and Nitrogen in the Cobalt(Iii)-Catalyzed Unsymmetrical Double Annulation of Thioamides: Probing the Origin of Chemo- and Regio-Selectivity. Chem. Sci. 2021, 12, 6393–6405. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Shankar, M.; Sau, S.; Sahoo, A.K. Multiple Annulations of Inert C(Sp2)–H Bonds with Alkynes. Chem. Commun. 2022, 58, 4561–4587. [Google Scholar] [CrossRef]
- Mukherjee, K.; Shankar, M.; Ghosh, K.; Sahoo, A.K. An Orchestrated Unsymmetrical Annulation Episode of C(Sp2)–H Bonds with Alkynes and Quinones: Access to Spiro-Isoquinolones. Org. Lett. 2018, 20, 1914–1918. [Google Scholar] [CrossRef]
- Ghosh, K.; Shankar, M.; Rit, R.K.; Dubey, G.; Bharatam, P.V.; Sahoo, A.K. Sulfoximine-Assisted One-Pot Unsymmetrical Multiple Annulation of Arenes: A Combined Experimental and Computational Study. J. Org. Chem. 2018, 83, 9667–9681. [Google Scholar] [CrossRef] [PubMed]
- Guntreddi, T.; Shankar, M.; Kommu, N.; Sahoo, A.K. Construction of Pyranoisoquinolines via Ru(II)-Catalyzed Unsymmetrical Double Annulation of N-Methoxybenzamides with Unactivated Alkynes. J. Org. Chem. 2019, 84, 13033–13044. [Google Scholar] [CrossRef]
- Shankar, M.; Saha, A.; Ghosh, A.; Sau, S.; Sahoo, A.K. Sulfur and Nitrogen Modulated One-Pot Double Annulation of Arenes. J. Org. Chem. 2021, 86, 14942–14955. [Google Scholar] [CrossRef]
- Mihara, G.; Ghosh, K.; Nishii, Y.; Miura, M. Concise Synthesis of Isocoumarins through Rh-Catalyzed Direct Vinylene Annulation: Scope and Mechanistic Insight. Org. Lett. 2020, 22, 5706–5711. [Google Scholar] [CrossRef]
- Ghosh, K.; Nishii, Y.; Miura, M. Oxidative C–H/C–H Annulation of Imidazopyridines and Indazoles through Rhodium-Catalyzed Vinylene Transfer. Org. Lett. 2020, 22, 3547–3550. [Google Scholar] [CrossRef]
- Kitano, J.; Nishii, Y.; Miura, M. Selective Synthesis of C4-Functionalized Benzofurans by Rhodium-Catalyzed Vinylene Transfer: Computational Study on the Cyclopentadienyl Ligand. Org. Lett. 2022, 24, 5679–5683. [Google Scholar] [CrossRef]
- Kim, S.; Choi, S.B.; Kang, J.Y.; An, W.; Lee, S.H.; Oh, H.; Ghosh, P.; Mishra, N.K.; Kim, I.S. Synthesis of Cinnolines via Rh(III)-Catalyzed Annulation of N-Aryl Heterocycles with Vinylene Carbonate. Asian J. Org. Chem. 2021, 10, 3005–3014. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.; Li, J.; Lai, R.; Guan, M.; Wu, Y.H. Ru(II)-Catalyzed C–H Activation Reaction between 2-Phenyl quinazolinone and Vinylene Carbonate. Synlett 2021, 32, 1963–1968. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Li, B.; Tan, Y.; Zhao, H.; Li, Z.; Zhang, C.; Ma, W. Ruthenium-Catalyzed Vinylene Carbonate Annulation by C−H/N−H Functionalizations: Step-Economical Access to Indoles. Adv. Synth. Catal. 2022, 364, 838–844. [Google Scholar] [CrossRef]
- Liu, M.; Yan, K.; Wen, J.; Liu, W.; Wang, M.; Wang, L.; Wang, X. Synthesis of Substituted 1-Hydroxy-2-Naphthaldehydes by Rhodium-Catalyzed C−H Bond Activation and Vinylene Transfer of Enaminones with Vinylene Carbonate. Adv. Synth. Catal. 2022, 364, 512–517. [Google Scholar] [CrossRef]
- Li, X.; Huang, T.; Song, Y.; Qi, Y.; Li, L.; Li, Y.; Xiao, Q.; Zhang, Y. Co(III)-Catalyzed Annulative Vinylene Transfer via C–H Activation: Three-Step Total Synthesis of 8-Oxopseudopalmatine and Oxopalmatine. Org. Lett. 2020, 22, 5925–5930. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Nan, J.; Yin, J.; Huang, G.; Ren, X.; Ma, Y. Rhodium-Catalyzed Dehydrogenative Annulation of N-Arylmethanimines with Vinylene Carbonate for Synthesizing Quinolines. Org. Lett. 2021, 23, 8527–8532. [Google Scholar] [CrossRef]
- Nan, J.; Yin, J.; Gong, X.; Hu, Y.; Ma, Y. Rhodium-Catalyzed C–H Annulation of Free Anilines with Vinylene Carbonate as a Bifunctional Synthon. Org. Lett. 2021, 23, 8910–8915. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Li, Y.; Li, Y.; Sun, Y.; Xia, C.; Li, Y. Manganese-Catalyzed [4 + 2] Annulation of N–H Amidines with Vinylene Carbonate via C–H Activation. J. Org. Chem. 2021, 86, 18204–18210. [Google Scholar] [CrossRef]
- Huang, J.; Liu, F.; Du, F.; Zeng, L.; Chen, Z. Cp∗Rh/Ag Catalyzed C–H Activation/Cyclization Sequences of NH-Sulfoximines to Fused Aza-Polyheterocycles under Gentle Conditions. Green Synth. Catal. 2023, 4, 160–168. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Qi, Z.; Qi, X.; Li, X.; Lan, Y. The Mechanism of N–O Bond Cleavage in Rhodium-Catalyzed C—H Bond Functionalization of Quinoline N-Oxides with Alkynes: A Computational Study. Chem. Eur. J. 2015, 21, 10131–10137. [Google Scholar] [CrossRef]
- Dateer, R.B.; Chang, S. Selective Cyclization of Arylnitrones to Indolines under External Oxidant-Free Conditions: Dual Role of Rh(III) Catalyst in the C–H Activation and Oxygen Atom Transfer. J. Am. Chem. Soc. 2015, 137, 4908–4911. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).