Antibacterial Activities and Underlying Mechanisms of the Compound SYAUP-491 against Xanthomonas oryzae pv. oryzae
Abstract
1. Introduction
2. Results and Discussion
2.1. Antibacterial Activity In Vitro
2.2. Antibacterial Activity In Vivo
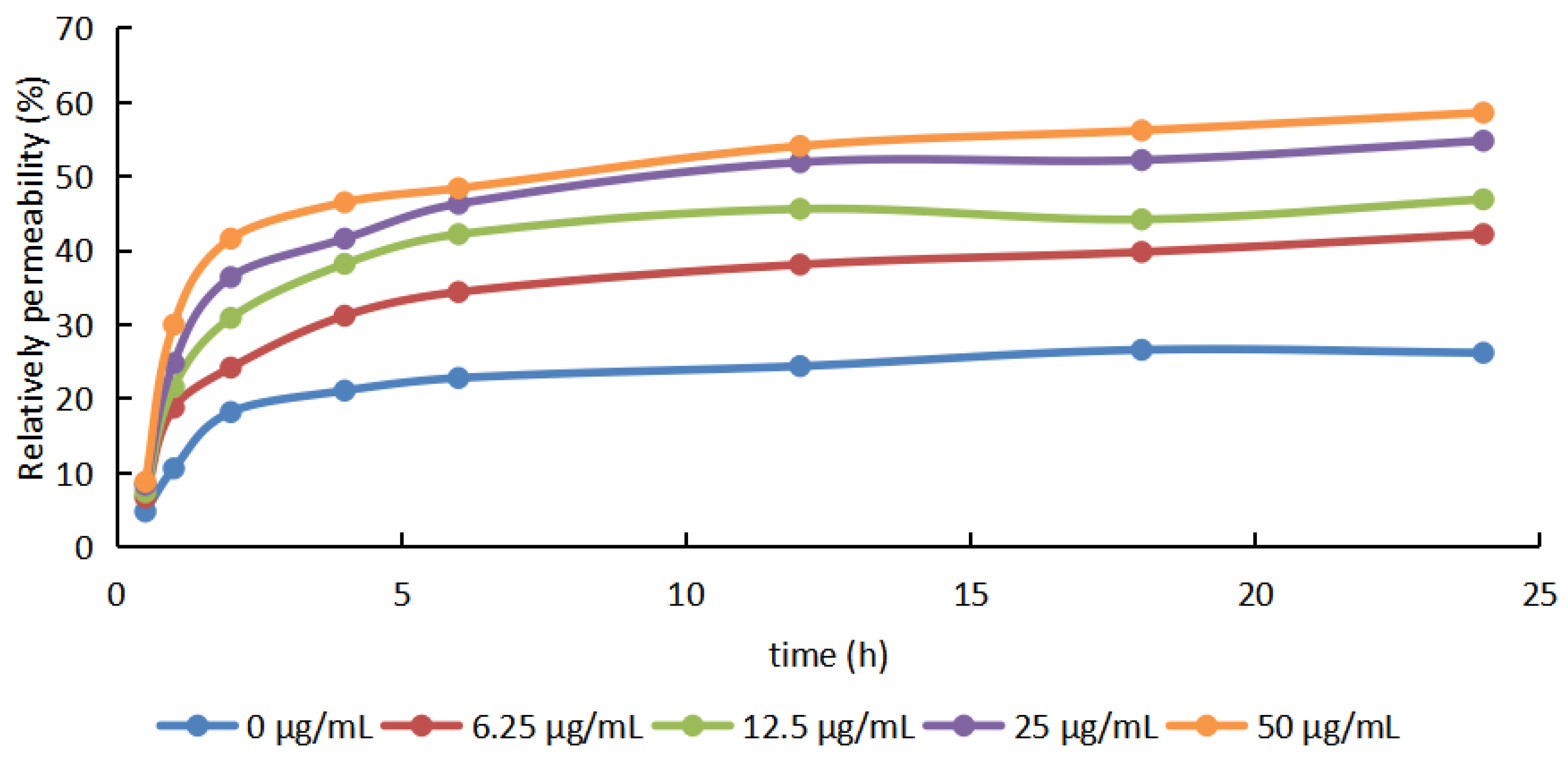
2.3. Membrane Permeability
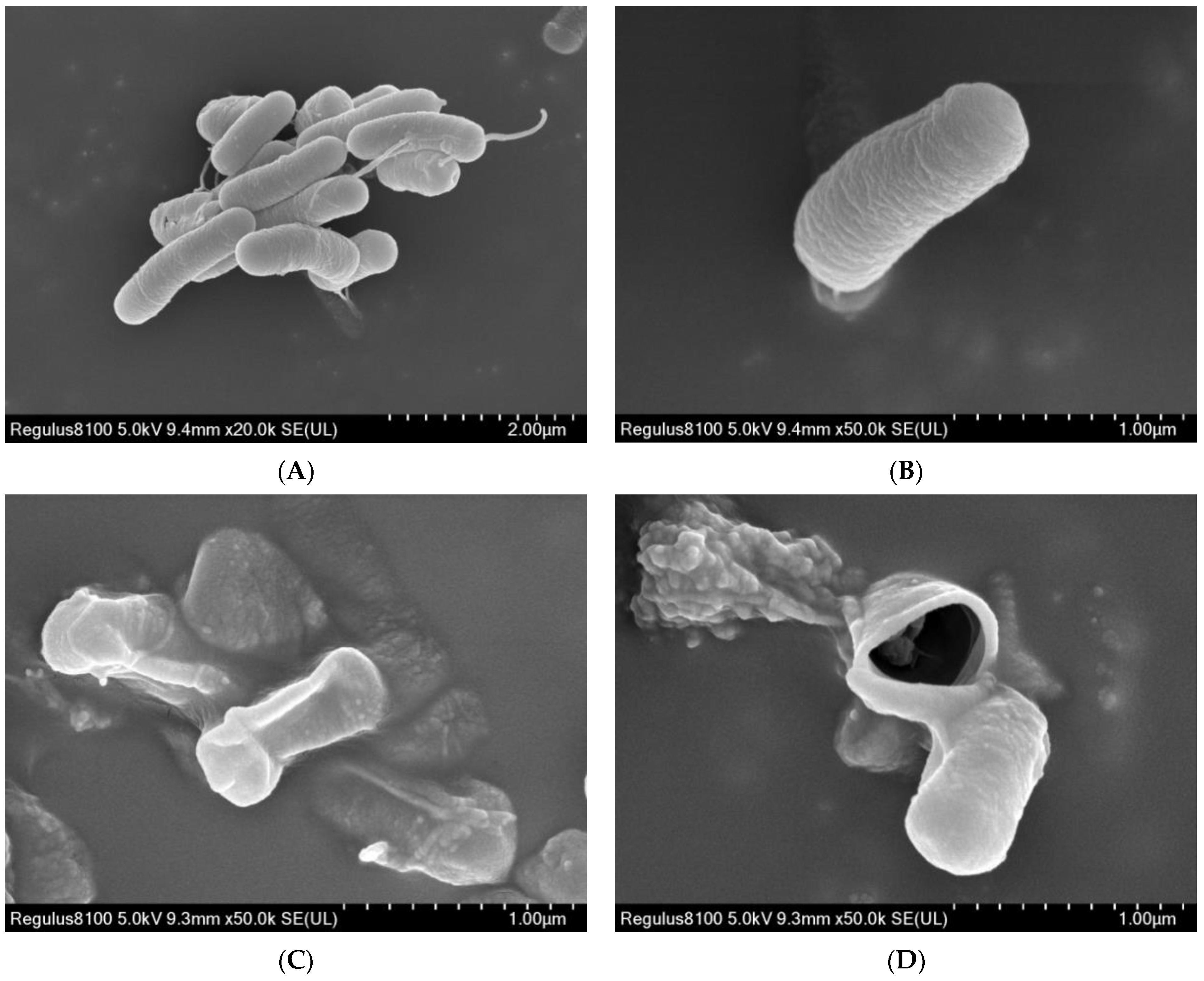
2.4. Morphological Changes
2.5. Analysis of Proteomic Results
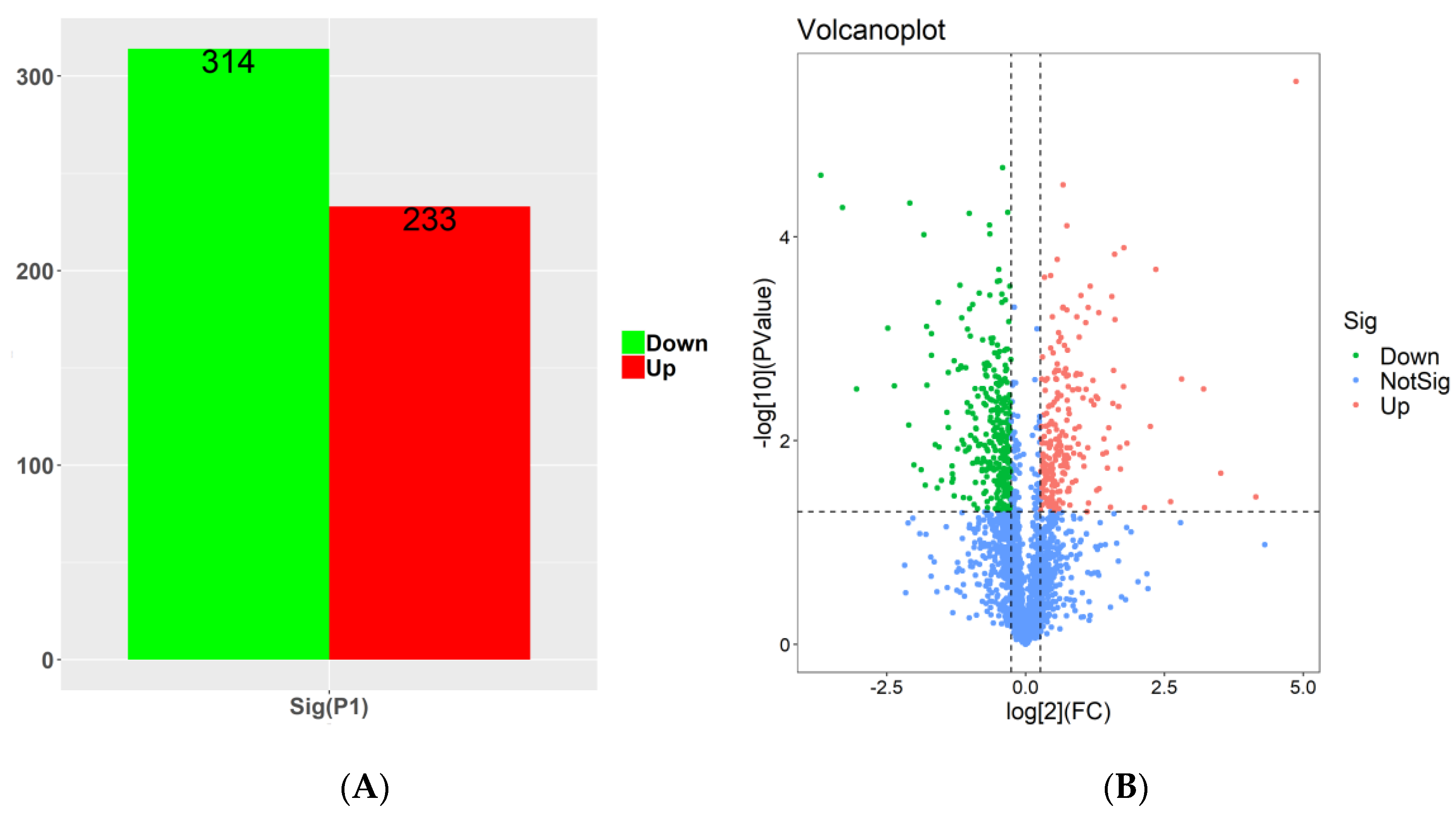
2.5.1. Global Analysis
2.5.2. Clusters of Orthologous Groups (COG) Functions
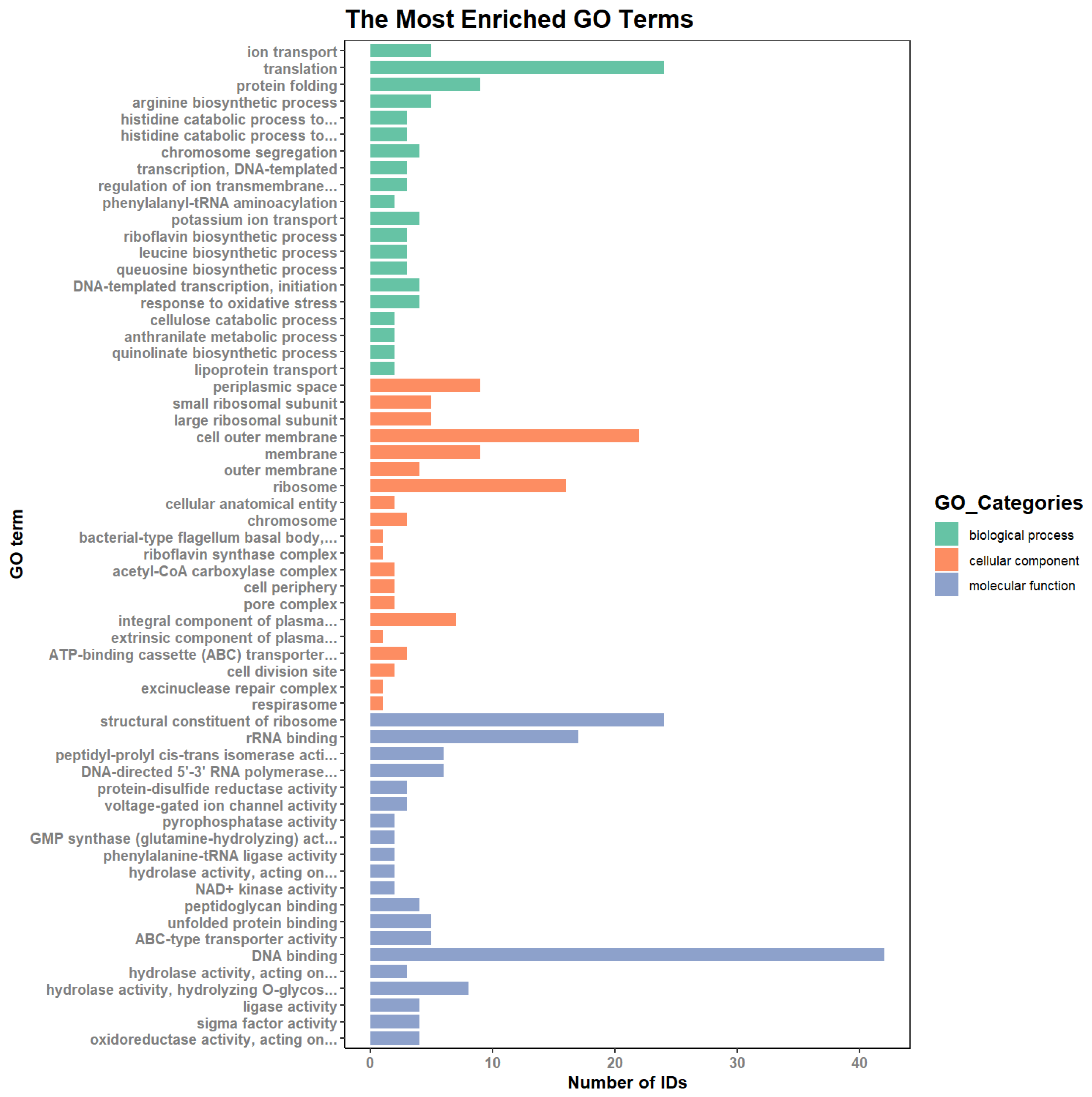
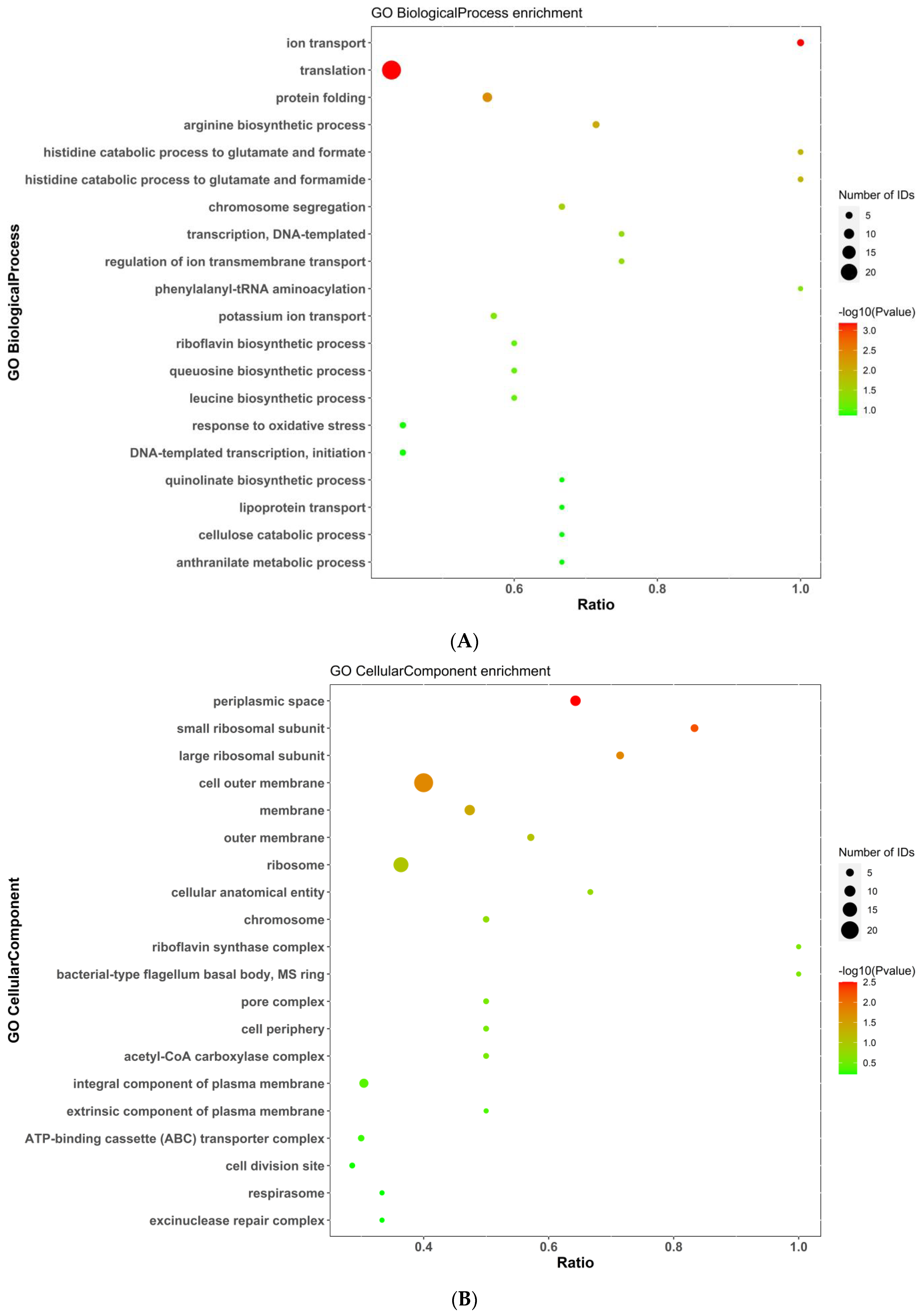
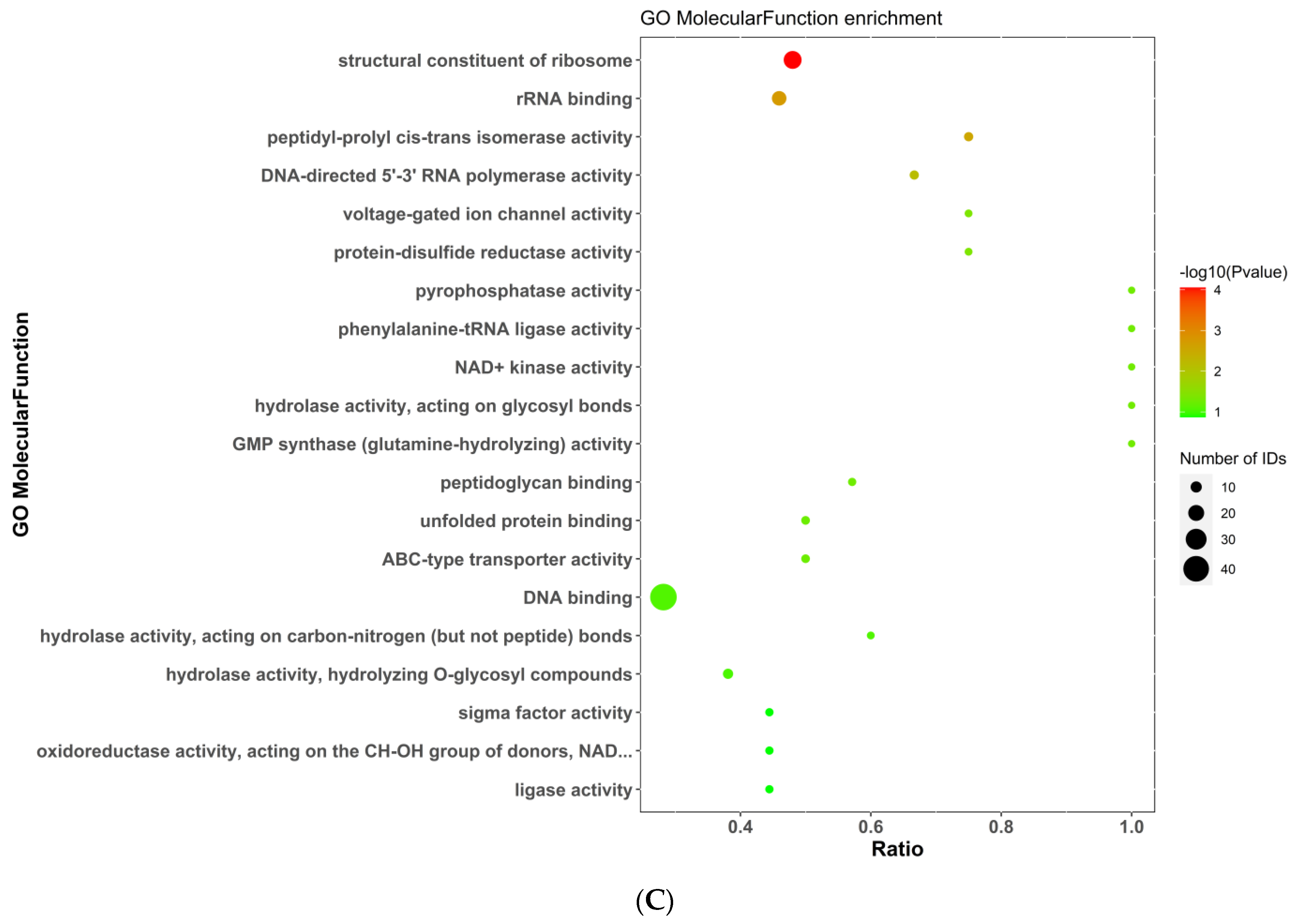
2.5.3. Gene Ontology (GO) Analysis
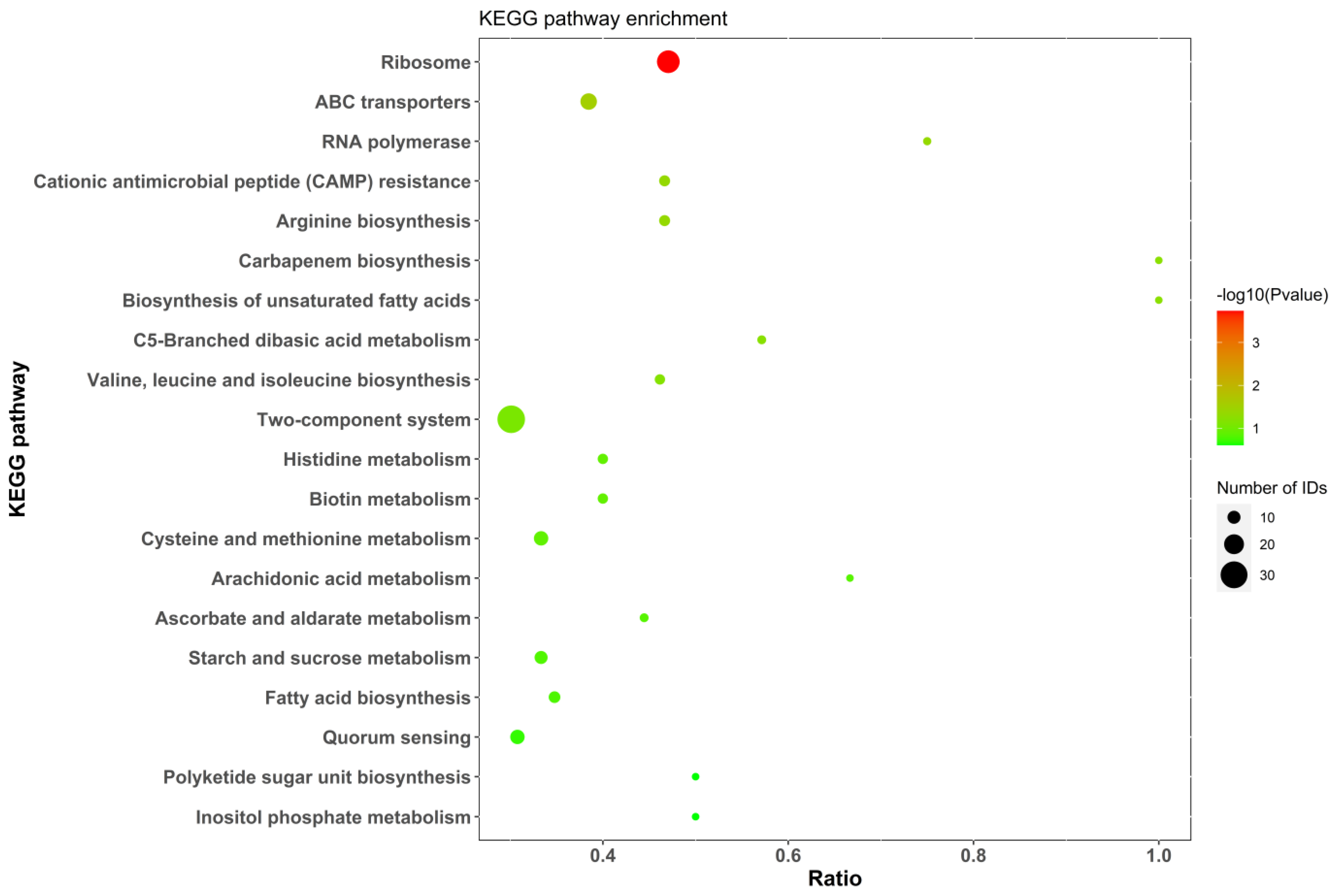
2.5.4. KEGG Analysis
3. Materials and Methods
3.1. Materials and Instrument
3.2. Antibacterial Activity In Vitro
3.2.1. Turbidimetry Test
3.2.2. Inhibition Zone Test Using Filter Paper
3.3. Antibacterial Activity In Vivo
3.4. Membrane Permeability
3.5. Morphological Changes
3.6. Proteomic Analysis of the Effects of Compound SYAUP-491 on Xoo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Liu, J.; Triplett, L.; Leach, J.E.; Wang, G.L. Novel insights into rice innate immunity against bacterial and fungal pathogens. Annu. Rev. Phytopathol. 2014, 52, 213–241. [Google Scholar] [CrossRef] [PubMed]
- Adamu, A.; Ahmad, K.; Siddiqui, Y.; Ismail, I.S.; Asib, N.; Bashir Kutawa, A.; Adzmi, F.; Ismail, M.R.; Berahim, Z. Ginger essential oils-loaded nanoemulsions: Potential strategy to manage bacterial leaf blight disease and enhanced rice yield. Molecules 2021, 26, 3902. [Google Scholar] [CrossRef] [PubMed]
- Bangratz, M.; Wonni, I.; Kini, K.; Sondo, M.; Brugidou, C.; Béna, G.; Gnacko, F.; Barro, M.; Koebnik, R.; Silué, D.; et al. Design of a new multiplex PCR assay for rice pathogenic bacteria detection and its application to infer disease incidence and detect co-infection in rice fields in Burkina Faso. PLoS ONE 2020, 15, e0232115. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Long, Q.; Zhao, Y.; Wu, Y.; Ge, S.; Wang, P.; Yang, C.G.; Chi, Y.; Song, B.; Yang, S. Sulfone-Based Probes Unraveled Dihydrolipoamide S-Succinyltransferase as an Unprecedented Target in Phytopathogens. J. Agric. Food Chem. 2019, 67, 6962–6969. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Wang Ca Zhao, K. Rice Routes of Countering Xanthomonas oryzae. Int. J. Mol. Sci. 2018, 19, 3008. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Shaaban, M.A.; Refaat, H.M.; Heiba, H.I.; Ibrahim, S.S. Anticancer and radiosensitizing evaluation of some new pyranothiazole-Schiff bases bearing the biologically active sulfonamide moiety. Eur. J. Med. Chem. 2012, 53, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Bornaghi, L.F.; Innocenti, A.; Vullo, D.; Charman, S.A.; Supuran, C.T.; Poulsen, S.A. Sulfonamide linked neoglycoconjugates--a new class of inhibitors for cancer-associated carbonic anhydrases. J. Med. Chem. 2010, 53, 2913–2926. [Google Scholar] [CrossRef] [PubMed]
- Hain, E.; Adejumo, H.; Anger, B.; Orenstein, J.; Blaney, L. Advances in antimicrobial activity analysis of fluoroquinolone, macrolide, sulfonamide, and tetracycline antibiotics for environmental applications through improved bacteria selection. J. Hazard. Mater. 2021, 415, 125686. [Google Scholar] [CrossRef]
- Zhang, H.Z.; He, S.C.; Peng, Y.J.; Zhang, H.J.; Gopala, L.; Tangadanchu, V.K.R.; Gan, L.L.; Zhou, C.H. Design, synthesis and antimicrobial evaluation of novel benzimidazole-incorporated sulfonamide analogues. Eur. J. Med. Chem. 2017, 136, 165–183. [Google Scholar] [CrossRef]
- Lumangtad, L.A.; Claeys, E.; Hamal, S.; Intasiri, A.; Basrai, C.; Yen-Pon, E.; Beenfeldt, D.; Vermeire, K.; Bell, T.W. Syntheses and anti-HIV and human cluster of differentiation 4 (CD4) down-modulating potencies of pyridine-fused cyclotriazadisulfonamide (CADA) compounds. Bioorganic Med. Chem. 2020, 28, 115816. [Google Scholar] [CrossRef]
- Doerge, D.R.; Decker, C.J. Inhibition of peroxidase-catalyzed reactions by arylamines: Mechanism for the anti-thyroid action of sulfamethazine. Chem. Res. Toxicol. 1994, 7, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, R.; Anjaneyulu, K.; Couturier, E.; Malaisse, W.J. Opposite effects of hypoglycemic and hyperglycemic sulfonamides upon ionophore-mediated calcium transport. Biochem. Pharmacol. 1980, 29, 1879–1882. [Google Scholar] [CrossRef]
- Humphries, P.S.; Bersot, R.; Kincaid, J.; Mabery, E.; McCluskie, K.; Park, T.; Renner, T.; Riegler, E.; Steinfeld, T.; Turtle, E.D.; et al. Carbazole-containing sulfonamides and sulfamides: Discovery of cryptochrome modulators as antidiabetic agents. Bioorganic Med. Chem. Lett. 2016, 26, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.R.; Camí, G.E.; Liu-González, M.; Vega, D.R.; Vullo, D.; Juárez, A.; Pedregosa, J.C.; Supuran, C.T. Salts of 5-amino-2-sulfonamide-1,3,4-thiadiazole, a structural and analog of acetazolamide, show interesting carbonic anhydrase inhibitory properties, diuretic, and anticonvulsant action. J. Enzyme Inhib. Med. Chem. 2016, 31, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Khadikar, P.V.; Supuran, C.T. Topological modeling of lipophilicity, diuretic activity, and carbonic inhibition activity of benzene sulfonamides: A molecular connectivity approach. Bioorganic Med. Chem. Lett. 2014, 14, 5661–5666. [Google Scholar] [CrossRef] [PubMed]
- De Liguoro, M.; Fioretto, B.; Poltronieri, C.; Gallina, G. The toxicity of sulfamethazine to Daphnia magna and its additivity to other veterinary sulfonamides and trimethoprim. Chemosphere 2009, 75, 1519–1524. [Google Scholar] [CrossRef]
- Kang, J.G.; Hur, J.H.; Choi, S.J.; Choi, G.J.; Cho, K.Y.; Ten, L.N.; Park, K.H.; Kang, K.Y. Antifungal activities of N-arylbenzenesulfonamides against phytopathogens and control efficacy on wheat leaf rust and cabbage club root diseases. Biosci. Biotechnol. Biochem. 2002, 66, 2677–2682. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, S.G.; Wan, Y.Q.; Wen, Y.; Zhang, W.H. Novel Coumarin 7-Carboxamide/Sulfonamide Derivatives as Potential Fungicidal Agents: Design, Synthesis, and Biological Evaluation. Molecules 2022, 27, 6904. [Google Scholar] [CrossRef]
- Jabusch, T.W.; Tjeerdema, R.S. Partitioning of penoxsulam, a new sulfonamide herbicide. J. Agric. Food Chem. 2005, 53, 7179–7183. [Google Scholar] [CrossRef]
- Brazier-Hicks, M.; Howell, A.; Cohn, J.; Hawkes, T.; Hall, G.; Mcindoe, E.; Edwards, R. Chemically induced herbicide tolerance in rice by the safener metcamifen is associated with a phased stress response. J. Exp. Bot. 2020, 71, 411–421. [Google Scholar] [CrossRef]
- Li, X.; Cui, Z.; Chen, X.; Wu, D.; Qi, Z.; Ji, M. Synthesis of 2-acyloxycyclohexylsulfonamides and evaluation on their fungicidal activity. Int. J. Mol. Sci. 2013, 14, 22544–22557. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, X.; Wang, M.; Qin, P.; Qi, Z.; Ji, M.; Liu, X.; Babu, P.V.; Li, X.; Cui, Z.N. Design, synthesis and fungicidal activity of novel 2-substituted aminocycloalkylsulfonamides. Bioorganic Med. Chem. Lett. 2017, 27, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Meng, S.; Xie, Y.; Yang, Y.; Zhang, Y.; He, L.; Wang, K.; Qi, Z.; Ji, M.; Qin, P.; et al. Synthesis, Fungicidal Activity and SAR of 2-Thiazolamide/Pyrazolamide-Cyclohexylsulfonamides against Botrytis cinerea. Molecules 2019, 24, 2607. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, S.; Xiao, L.; Wan, Y.; He, L.; Wang, K.; Qi, Z.; Li, X. Synthesis and biological activity of novel hydantoin cyclohexyl sulfonamide derivatives as potential antimicrobial agents in agriculture. Pest. Manag. Sci. 2022, 78, 1438–1447. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, X.; Chen, X.; Wang, D.; Yu, X.; Jiang, W. HPLC-MS/MS analysis of zinc-thiazole residues in foods of plant origin by a modified derivatization-QueChERS method. Food Chem. 2022, 386, 132752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, H.; Li, X.; Shi, H.; Wei, F.; Zhu, G. Baseline sensitivity of natural populations and resistance of mutants of Xanthomonas oryzae pv. oryzae to a novel bactericide, zinc thiazole. Plant Pathol. 2013, 62, 1378–1383. [Google Scholar] [CrossRef]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002, 40, 443–465. [Google Scholar] [CrossRef]
- Rezzonico, F.; Stockwell, V.O.; Duffy, B. Plant agricultural streptomycin formulations do not carry antibiotic resistance genes. Antimicrob. Agents Chemother. 2009, 53, 3173–3177. [Google Scholar] [CrossRef]
- Vidaver, A.K. Uses of antimicrobials in plant agriculture. Clin. Infect. Dis. 2002, 34, S107–S110. [Google Scholar] [CrossRef]
- Azevedo-Barbosa, H.; Dias, D.F.; Franco, L.L.; Hawkes, J.A.; Carvalho, D.T. From Antibacterial to Antitumour Agents: A Brief Review on The Chemical and Medicinal Aspects of Sulfonamides. Mini Rev. Med. Chem. 2020, 20, 2052–2066. [Google Scholar] [CrossRef]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.Q.; Liu, J.L.; Sun, B.X.; Liu, J. The bactericide research of high throughput screening. Agrochemicals 2015, 54, 678–680. (In Chinese) [Google Scholar]
- Ali, S.M.; Khan, A.A.; Ahmed, I.; Musaddiq, M.; Ahmed, K.S.; Polasa, H.; Rao, L.V.; Habibullah, C.M.; Sechi, L.A.; Ahmed, N. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 20. [Google Scholar] [CrossRef]
- Li, P.; Hu, D.; Xie, D.; Chen, J.; Jin, L.; Song, B. Design, Synthesis, and Evaluation of New Sulfone Derivatives Containing a 1,3,4-Oxadiazole Moiety as Active Antibacterial Agents. J. Agric. Food Chem. 2018, 66, 3093–3100. [Google Scholar] [CrossRef]
- Yi, C.; Chen, J.; Wei, C.; Wu, S.; Wang, S.; Hu, D.; Song, B. α-Haloacetophenone and analogues as potential antibacterial agents and nematicides. Bioorganic Med. Chem. Lett. 2020, 30, 126814. [Google Scholar] [CrossRef]
- Zhou, J.; Tao, Q.Q.; Wang, P.Y.; Shao, W.B.; Wu, Z.B.; Li, Z.; Yang, S. Antimicrobial evaluation and action mechanism of pyridinium-decorated 1,4-pentadien-3-one derivatives. Bioorganic Med. Chem. Lett. 2018, 28, 1742–1746. [Google Scholar] [CrossRef] [PubMed]
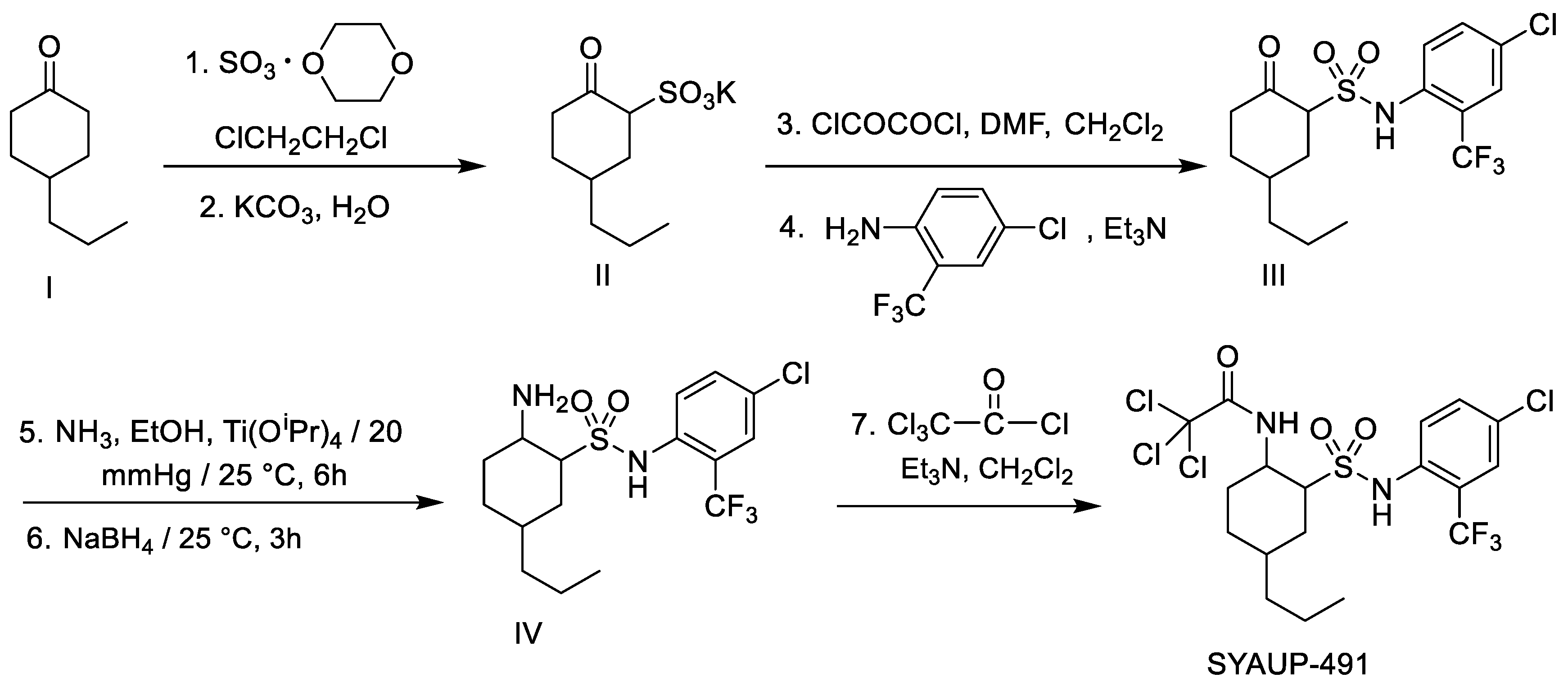



| Molecular Weight and Formula | State | Melting Point (°C) | Yield (%) | 1H NMR (CDCl3, δ) | IR (ν, cm−1) | HRMS (z/e) |
|---|---|---|---|---|---|---|
| 544.23 C18H21Cl4F3N2O3S | White crystal | 123–125 | 98.5 | 0.85 (t, J = 7.1 Hz, 3H, CH3), 2.34–1.18 (m, 11H, C6H12), 3.83 (t, J = 10.3 Hz, 1H, CH-N), 4.17 (td, J = 7.1, 3.7 Hz, 1H, CH-SO2), 7.55–7.82 (m, 3H, Ph-H), 8.67 (d, J = 8.4 Hz, 1H, CO-NH), 9.80 (s, 1H, SO2-NH) | 3435, 3336, 2958, 2873, 1689 | 543.0047 (M + H) |
| Compound | Inhibition Rate (%) | Regression Equation | EC50 (μg/mL) | |
|---|---|---|---|---|
| 100 (μg/mL) | 25 (μg/mL) | |||
| SYAUP-491 | 98.5 ± 1.2 a | 81.2 ± 1.6 b | y = 1.3193x + 3.8884 | 6.96 |
| Streptomycin sulfate | 100 a | 95.2 ± 3.0 a | y = 1.9398x + 3.8441 | 3.94 |
| Sulfadiazine | 36.6 ± 4.0 d | 18.9 ± 5.7 d | — | — |
| Sulfisoxazole | 38.1 ± 2.7 d | 22.3 ± 2.9 d | — | — |
| Thiodiazole copper | 29.7 ± 4.9 e | 11.7 ± 3.2 e | — | — |
| Zhongshengmycin | 19.6 ± 5.6 f | 6.9 ± 4.2 e | — | — |
| Zinc thiazole | 57.2 ± 4.1 b | 35.0 ± 2.7 c | y = 0.5639x + 3.9052 | 87.43 |
| Cu(OH)2 | 47.9 ± 4.7 c | 36.2 ± 4.5 c | y = 0.4967x + 3.9014 | 102.92 |
| Compound | Mean IZD (mm) |
|---|---|
| SYAUP-491 | 16.8 ± 1.1 b |
| Streptomycin sulfate | 19.9 ± 0.3 a |
| Cu(OH)2 | 9.9 ± 0.5 c |
| Zinc thiazole | 7.6 ± 0.6 d |
| CK | — |
| Compound | Morbidity (%) | Protective Activity (%) | Curative Activity (%) |
|---|---|---|---|
| SYAUP-491 | 100 | 32.2 ± 3.3 b | 74.1 ± 6.3 a |
| Streptomycin sulfate | 100 | 50.1 ± 3.4 a | 38.8 ± 2.8 b |
| Zinc thiazole | 100 | 30.4 ± 6.6 b | 71.5 ± 7.5 a |
| CK | 100 | / | / |
| Name | Total Spectra | Matched Spectrum | Peptide | Identified Protein |
|---|---|---|---|---|
| Total | 560,926 | 307,012 | 30,951 | 2252 |
| ID | Accession | Gene Name | Description | KO | FC(SY_CK) | p-Value (SY_CK) |
|---|---|---|---|---|---|---|
| RS3_XANOR | Q5GWU0 | rpsC | 30S ribosomal protein S3 | xoo:XOO3577 | 0.742866 | 0.047873 |
| RL21_XANOR | Q5H2E7 | rplU | 50S ribosomal protein L21 | xoo:XOO1620 | 0.8138 | 0.047358 |
| RS18_XANOR | Q5H052 | rpsR | 30S ribosomal protein S18 | xoo:XOO2415 | 0.624458 | 0.012651 |
| RS5_XANOR | Q5GWV1 | rpsE | 30S ribosomal protein S5 | xoo:XOO3566 | 0.820401 | 0.021934 |
| RL15_XANOR | Q5GWV4 | rplO | 50S ribosomal protein L15 | xoo:XOO3563 | 0.677525 | 0.009118 |
| RL17_XANOR | Q5GWW0 | rplQ | 50S ribosomal protein L17 | xoo:XOO3557 | 0.733943 | 0.002582 |
| RL14_XANOR | Q5GWU4 | rplN | 50S ribosomal protein L14 | xoo:XOO3573 | 0.789138 | 0.001995 |
| RS17_XANOR | Q5GWU3 | rpsQ | 30S ribosomal protein S17 | xoo:XOO3574 | 0.781231 | 0.032046 |
| RS21_XANOR | Q05I90 | rpsU | 30S ribosomal protein S21 | xoo:XOO4929 | 0.607631 | 0.031546 |
| RL4_XANOR | Q5GWT5 | rplD | 50S ribosomal protein L4 | xoo:XOO3582 | 0.7409 | 0.0078 |
| RS8_XANOR | Q05HS7 | rpsH | 30S ribosomal protein S8 | xoo:XOO4896 | 0.692477 | 0.002344 |
| RS4_XANOR | Q5GWV8 | rpsD | 30S ribosomal protein S4 | xoo:XOO3559 | 0.720856 | 0.00134 |
| RS14_XANOR | Q5GWU8 | rpsN | 30S ribosomal protein S14 | xoo:XOO3569 | 0.711797 | 0.031258 |
| RS12_XANOR | Q5GWS8 | rpsL | 30S ribosomal protein S12 | xoo:XOO3589 | 0.493916 | 0.01166 |
| RS13_XANOR | Q5GWV6 | rpsM | 30S ribosomal protein S13 | xoo:XOO3561 | 0.670199 | 0.007928 |
| RS2_XANOR | Q5H1E0 | rpsB | 30S ribosomal protein S2 | xoo:XOO1977 | 0.649435 | 0.001001 |
| RL27_XANOR | Q5H2E6 | rpmA | 50S ribosomal protein L27 | xoo:XOO1621 | 0.639016 | 0.000094 |
| RL22_XANOR | Q5GWT9 | rplV | 50S ribosomal protein L22 | xoo:XOO3578 | 0.701991 | 0.00116 |
| RS19_XANOR | Q5GWT8 | rpsS | 30S ribosomal protein S19 | xoo:XOO3579 | 0.731434 | 0.014835 |
| RL2_XANOR | Q5GWT7 | rplB | 50S ribosomal protein L2 | xoo:XOO3580 | 0.635694 | 0.018403 |
| RL32_XANOR | Q05I49 | rpmF | 50S ribosomal protein L32 | xoo:XOO4732 | 0.701572 | 0.036503 |
| RL29_XANOR | Q5GWU2 | rpmC | 50S ribosomal protein L29 | xoo:XOO3575 | 0.446445 | 0.001933 |
| RL34_XANOR | Q05HP6 | rpmH | 50S ribosomal protein L34 | xoo:XOO4957 | 0.121112 | 0.003118 |
| RL33_XANOR | Q5GU11 | rpmG | 50S ribosomal protein L33 | xoo:XOO4558 | 0.101754 | 0.000052 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Wang, Y.; Liu, H.; Liu, W.; Zhang, X.; An, M.; Yu, M.; Wu, Y.; Li, X.; Wang, J. Antibacterial Activities and Underlying Mechanisms of the Compound SYAUP-491 against Xanthomonas oryzae pv. oryzae. Molecules 2024, 29, 1413. https://doi.org/10.3390/molecules29061413
Li L, Wang Y, Liu H, Liu W, Zhang X, An M, Yu M, Wu Y, Li X, Wang J. Antibacterial Activities and Underlying Mechanisms of the Compound SYAUP-491 against Xanthomonas oryzae pv. oryzae. Molecules. 2024; 29(6):1413. https://doi.org/10.3390/molecules29061413
Chicago/Turabian StyleLi, Lina, Yuxin Wang, He Liu, Wei Liu, Xinchen Zhang, Mengnan An, Miao Yu, Yuanhua Wu, Xinghai Li, and Jianzhong Wang. 2024. "Antibacterial Activities and Underlying Mechanisms of the Compound SYAUP-491 against Xanthomonas oryzae pv. oryzae" Molecules 29, no. 6: 1413. https://doi.org/10.3390/molecules29061413
APA StyleLi, L., Wang, Y., Liu, H., Liu, W., Zhang, X., An, M., Yu, M., Wu, Y., Li, X., & Wang, J. (2024). Antibacterial Activities and Underlying Mechanisms of the Compound SYAUP-491 against Xanthomonas oryzae pv. oryzae. Molecules, 29(6), 1413. https://doi.org/10.3390/molecules29061413






