A Study on the Adsorption of Rhodamine B onto Adsorbents Prepared from Low-Carbon Fossils: Kinetic, Isotherm, and Thermodynamic Analyses
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Activated Carbons
- LA—precursor (low-rank coal Labin) activated with sodium hydroxide at 600 °C in a nitrogen atmosphere for 45 min (weight ratio of activator:precursor, 2:1);
- SA—precursor (low-rank coal Spitsbergen) activated with sodium hydroxide at 600 °C in a nitrogen atmosphere for 45 min (weight ratio of activator:precursor, 2:1)
2.2. Adsorption/Desorption Study
3. Materials and Methods
3.1. Materials
3.2. Preparation of Activated Carbons
3.3. Activated Carbon Characterization
3.4. Adsorption Study
3.5. Desorption Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, S.; Jiao, Y.; Zou, J.; Zheng, Z.; Zhu, G.; Deng, R.; Wang, C.; Peng, Y.; Wang, J. Adsorption of Sb(III) from Solution by Immobilized Microcystis aeruginosa Microspheres Loaded with Magnetic Nano-Fe3O4. Water 2024, 16, 681. [Google Scholar] [CrossRef]
- Azam, K.; Shezad, N.; Shafiq, I.; Akhter, P.; Akhtar, F.; Jamil, F.; Shafique, S.; Park, Y.-K.; Hussain, M. A Review on Activated Carbon Modifications for the Treatment of Wastewater Containing Anionic Dyes. Chemosphere 2022, 306, 135566. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Zhou, L.; Liu, Z.; Heng, J.Y.Y.; Chen, W. Biomass-Derived Activated Carbons for the Removal of Pharmaceutical Mircopollutants from Wastewater: A Review. Sep. Purif. Technol. 2020, 253, 117536. [Google Scholar] [CrossRef]
- Blachnio, M.; Derylo-Marczewska, A.; Charmas, B.; Zienkiewicz-Strzalka, M.; Bogatyrov, V.; Galaburda, M. Activated Carbon from Agricultural Wastes for Adsorption of Organic Pollutants. Molecules 2020, 25, 5105. [Google Scholar] [CrossRef] [PubMed]
- Lara-Ibeas, I.; Megías-Sayago, C.; Louis, B.; Le Calvé, S. Adsorptive removal of gaseous formaldehyde at realistic concentrations. J. Environ. Chem. Eng. 2020, 8, 103986. [Google Scholar] [CrossRef]
- de Castro, C.S.; Viau, L.N.; Andrade, J.T.; Mendonçac, T.A.P.; Gonçalvesc, M. Mesoporous activated carbon from polyethyleneterephthalate (PET) waste: Pollutant adsorption in aqueous solution. New J. Chem. 2018, 42, 14612–14619. [Google Scholar] [CrossRef]
- Jedynak, K.; Charmas, B. Application of Activated Carbons Obtained from Polymer Waste for the Adsorption of Dyes from Aqueous Solutions. Materials 2024, 17, 748. [Google Scholar] [CrossRef]
- Saxena, M.; Sharma, N.; Saxena, R. Highly efficient and rapid removal of a toxic dye: Adsorption kinetics, isotherm, and mechanism studies on functionalized multiwalled carbon nanotubes. Surf. Interfaces 2020, 21, 100639. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.; Azhar, Q.M.; Kumar, P.S.; Yusuf, A.A.; Al-Buriahi, A.K.; Mohamed, R.M.S.R.; Al-shaibani, M.M. Sustainable approaches for removing Rhodamine B dye using agriculturalwaste adsorbents: A review. Chemosphere 2022, 287, 132080. [Google Scholar] [CrossRef]
- Bhat, A.H.; Chishti, H.T.N. Adsorption of rhodamine-B by polypyrrole Sn (IV) tungstophosphate nanocomposite cation exchanger: Kinetic-cum-thermodynamic investigations. Sep. Sci. Technol. 2022, 58, 287–301. [Google Scholar] [CrossRef]
- Sundararajan, M.; Sailaja, V.; John Kennedy, L.; Judith Vijaya, J. Photocatalytic Degradation of Rhodamine B under Visible Light Using Nanostructured Zinc Doped Cobalt Ferrite: Kinetics and Mechanism. Ceram. Int. 2017, 43, 540–548. [Google Scholar] [CrossRef]
- Wang, Q.; He, D.; Li, C.; Sun, Z.; Mu, J. Honeycomb-like cork activated carbon modified with carbon dots for high-efficient adsorption of Pb(II) and rhodamine B. Ind. Crops Prod. 2023, 196, 116485. [Google Scholar] [CrossRef]
- Al-sareji, O.J.; Grmasha, R.A.; Meiczinger, M.; Al-Juboori, R.A.; Somogyi, V.; Hashim, K.S. A Sustainable Banana Peel Activated Carbon for Removing Pharmaceutical Pollutants from Different Waters: Production, Characterization, and Application. Materials 2024, 17, 1032. [Google Scholar] [CrossRef]
- Pevida, C.; Plaza, M.G.; Arias, B.; Fermoso, J.; Rubiera, F.; Pis, J.J. Surface Modification of Activated Carbons for CO2 Capture. Appl. Surf. Sci. 2008, 254, 7165–7172. [Google Scholar] [CrossRef]
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, L.; Liu, L.; et al. Microwave assisted preparation of activated carbon from biomass. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Bazan-Wozniak, A.; Nosal-Wiercińska, A.; Yilmaz, S.; Pietrzak, R. Low-rank coals as precursors of effective carbonaceous adsorbents for the removal of Rhodamine B. J. Mol. Liq. 2023, 389, 122949. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.-E.; Baek, S.-H.; Choi, U.; Bae, H.-J. Preparation of Activated Carbon from Korean Anthracite: Simultaneous Control of Ash Reduction and Pore Development. Processes 2023, 11, 2877. [Google Scholar] [CrossRef]
- Xing, B.-L.; Guo, H.; Chen, L.-J.; Chen, Z.-F.; Zhang, C.-X.; Huang, G.-X.; Xie, W.; Yu, J.-L. Lignite-derived high surface area mesoporous activated carbons for electrochemical capacitors. Fuel Process. Technol. 2015, 138, 734–742. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, L.; Zhang, F.; Yu, C.; Wei, Z. Multicarboxylic hyperbranched polyglycerol modified SBA-15 for the adsorption of cationic dyes and copper ions from aqueous media. Appl. Surf. Sci. 2012, 258, 5291–5298. [Google Scholar] [CrossRef]
- Wawrzyniak, A.; Wiśniewska, M.; Nowicki, P. Carbon Adsorbents Obtained from Pistachio Nut Shells Used as Potential Ingredients of Drinking Water Filters. Molecules 2023, 28, 4497. [Google Scholar] [CrossRef]
- Pereira, M.F.R.; Soares, S.F.; Órfão, J.J.M.; Figueiredo, J.L. Adsorption of dyes on activated carbons: Influence of surface chemical groups. Carbon 2003, 41, 811. [Google Scholar] [CrossRef]
- Chwastowski, J.; Guzik, M.; Bednarz, S.; Staroń, P. Upcycling Waste Streams from a Biorefinery Process—A Case Study on Cadmium and Lead Biosorption by Two Types of Biopolymer Post-Extraction Biomass. Molecules 2023, 28, 6345. [Google Scholar] [CrossRef] [PubMed]
- Kerrou, M.; Bouslamti, N.; Raada, A.; Elanssari, A.; Mrani, D.; Slimani, M.S. The Use of Sugarcane Bagasse to Remove the Organic Dyes from Wastewater. Int. J. Anal. Chem. 2021, 2021, 5570806. [Google Scholar] [CrossRef] [PubMed]
- Emrooz, H.B.M.; Maleki, M.; Rashidi, A.; Shokouhimehr, M. Adsorption mechanism of a cationic dye on a biomass-derived microand mesoporous carbon: Structural, kinetic, and equilibrium insight. Biomass Convers. Biorefin. 2021, 11, 943–954. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, N.; Giri, B.S.; Chowdhary, P.; Chaturvedi, P. Removal of methylene blue dye using rice husk, cow dung and sludge biochar: Characterization, application, and kinetic studies. Bioresour. Technol. 2020, 306, 123202. [Google Scholar] [CrossRef] [PubMed]
- Jabar, J.M.; Odusote, Y.A. Utilization of prepared activated biochar from water lily (Nymphaea lotus) stem for adsorption of malachite green dye from aqueous solution. Biomass Convers. Biorefin. 2021, 14, 5999–6010. [Google Scholar] [CrossRef]
- Ma, M.; Chen, Y.; Zhao, X.; Tan, F.; Wang, Y.; Cao, Y.; Cai, W. Effective removal of cation dyes from aqueous solution using robust cellulose sponge. J. Saudi Chem. Soc. 2020, 24, 915–924. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Hosseini, S.S.; Akbari, A.; Ramavandi, B. Hydroxyapatite biomaterial production from chicken (femur and beak) and fishbone waste through a chemical less method for Cd2+ removal from shipbuilding wastewater. J. Hazard. Mater. 2021, 413, 125428. [Google Scholar] [CrossRef]
- Zghal, S.; Jedidi, I.; Cretin, M.; Cerneaux, S.; Abdelmouleh, M. Adsorptive Removal of Rhodamine B Dye Using Carbon Graphite/CNT Composites as Adsorbents: Kinetics, Isotherms and Thermodynamic Study. Materials 2023, 16, 1015. [Google Scholar] [CrossRef]
- Hossain, M.H.; Alam, M.S. Adsorption kinetics of Rhodamine-B on used black tea leaves. Iran. J. Environ. Health Sci. Eng. 2012, 9, 2. [Google Scholar] [CrossRef]
- Cheng, Z.-L.; Li, Y.-X.; Liu, Z. Novel adsorption materials based on graphene oxide/Beta zeolite composite materials and their adsorption performance for rhodamine B. J. Alloys Compd. 2017, 708, 255–263. [Google Scholar] [CrossRef]
- Xiao, W.; Garba, Z.M.; Sun, S.; Lawan, I.; Wang, L.; Lin, M.; Yuan, Z. Preparation and evaluation of an effective activated carbon from white sugar for the adsorption of rhodamine B dye. J. Clean. Prod. 2020, 253, 119989. [Google Scholar] [CrossRef]
- Hayeeye, F.; Sattar, M.; Chinpa, W.; Sirichote, O. Kinetics and thermodynamics of Rhodamine B adsorption by gelatin/activated carbon composite beads. Colloids Surf. A Physicochem. Eng. 2017, 513, 259–266. [Google Scholar] [CrossRef]
- Paluch, D.; Bazan-Wozniak, A.; Wolski, R.; Nosal-Wiercińska, A.; Pietrzak, R. Removal of Methyl Red from Aqueous Solution Using Biochar Derived from Fennel Seeds. Molecules 2023, 28, 7786. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Li, H. High surface area activated carbon derived from chitin for efficient adsorption of Crystal Violet. Diam. Relat. Mater. 2021, 118, 108516. [Google Scholar] [CrossRef]
- Lesaoana, M.; Pakade, V.E.; Chimuka, L. Crosslinker-less surface-imprinted Macadamia derived activated carbons for trace Cr(III) removal from aqueous solution. Environ. Technol. Innov. 2019, 14, 100336. [Google Scholar] [CrossRef]
- Chandra Joshi, N.; Singh, A. Adsorptive Performances and Characterisations of Biologically Synthesised Zinc Oxide Based Nanosorbent (ZOBN). Groundw. Sustain. Dev. 2020, 10, 100325. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U. The Use of Rapeseed Husks to Remove Acidic and Basic Dyes from Aquatic Solutions. Appl. Sci. 2024, 14, 1174. [Google Scholar] [CrossRef]
- Basu, A.; Ali, S.S.; Hossain, S.S.; Asif, M. A Review of the Dynamic Mathematical Modeling of Heavy Metal Removal with the Biosorption Process. Processes 2022, 10, 1154. [Google Scholar] [CrossRef]
- Hutson, N.D.; Yang, R.T. Theoretical Basis for the Dubinin-Radushkevitch (D-R) Adsorption Isotherm Equation. Adsorption 1997, 3, 189–195. [Google Scholar] [CrossRef]
- Jayasuriya, D.M.N.H.; Nadarajah, K. Understanding Association between Methylene Blue Dye and Biosorbent: Palmyrah Sprout Casing in Adsorption Process in Aqueous Phase. Water Sci. Eng. 2023, 16, 154–164. [Google Scholar] [CrossRef]
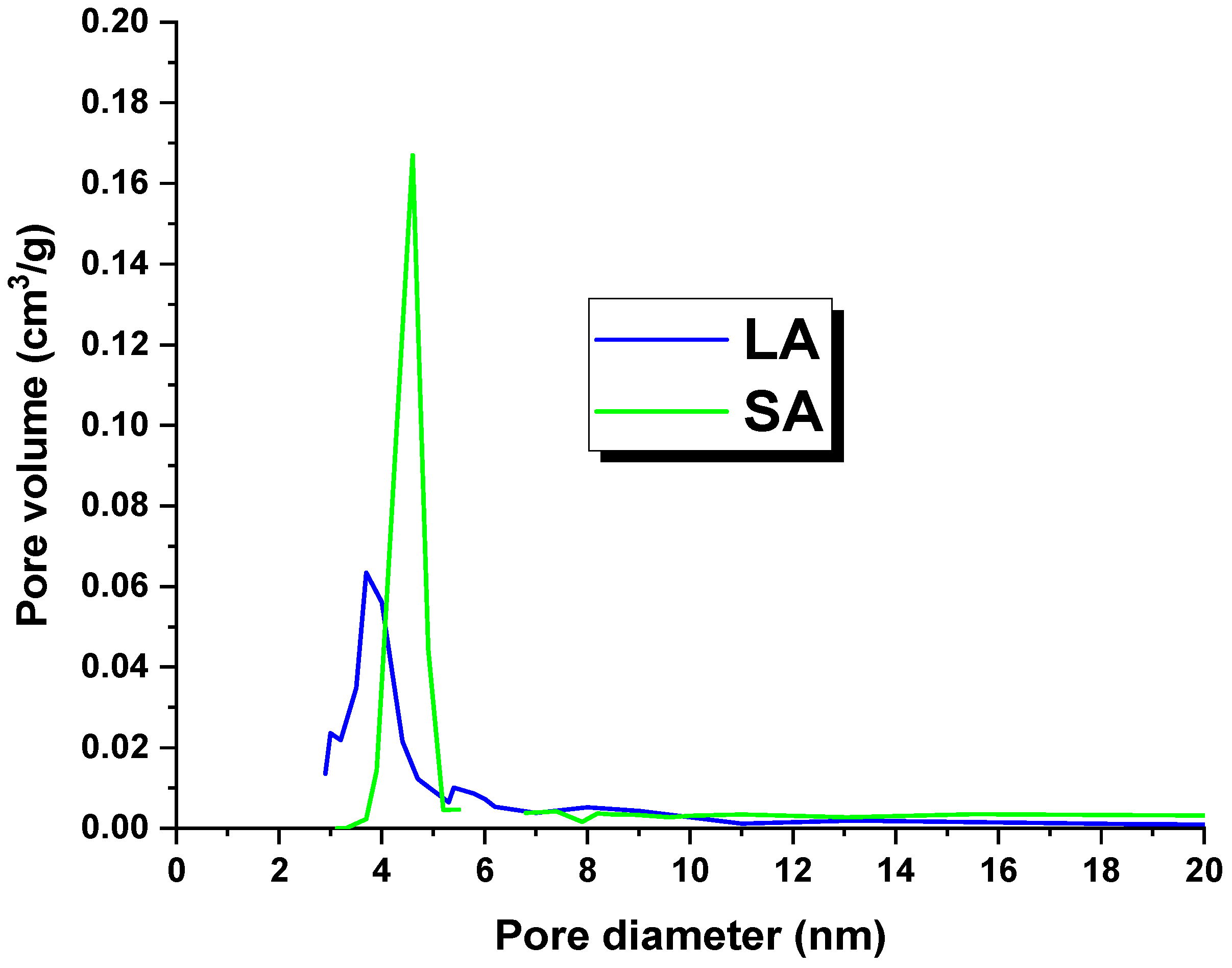


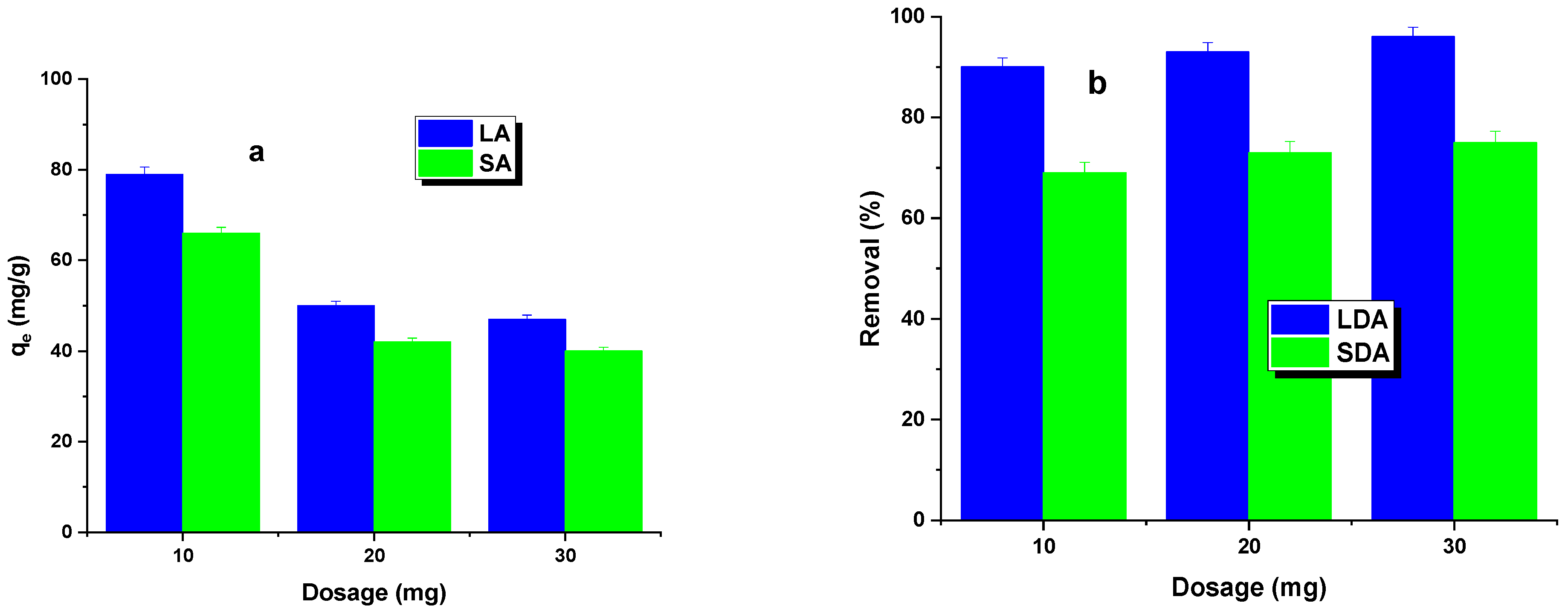


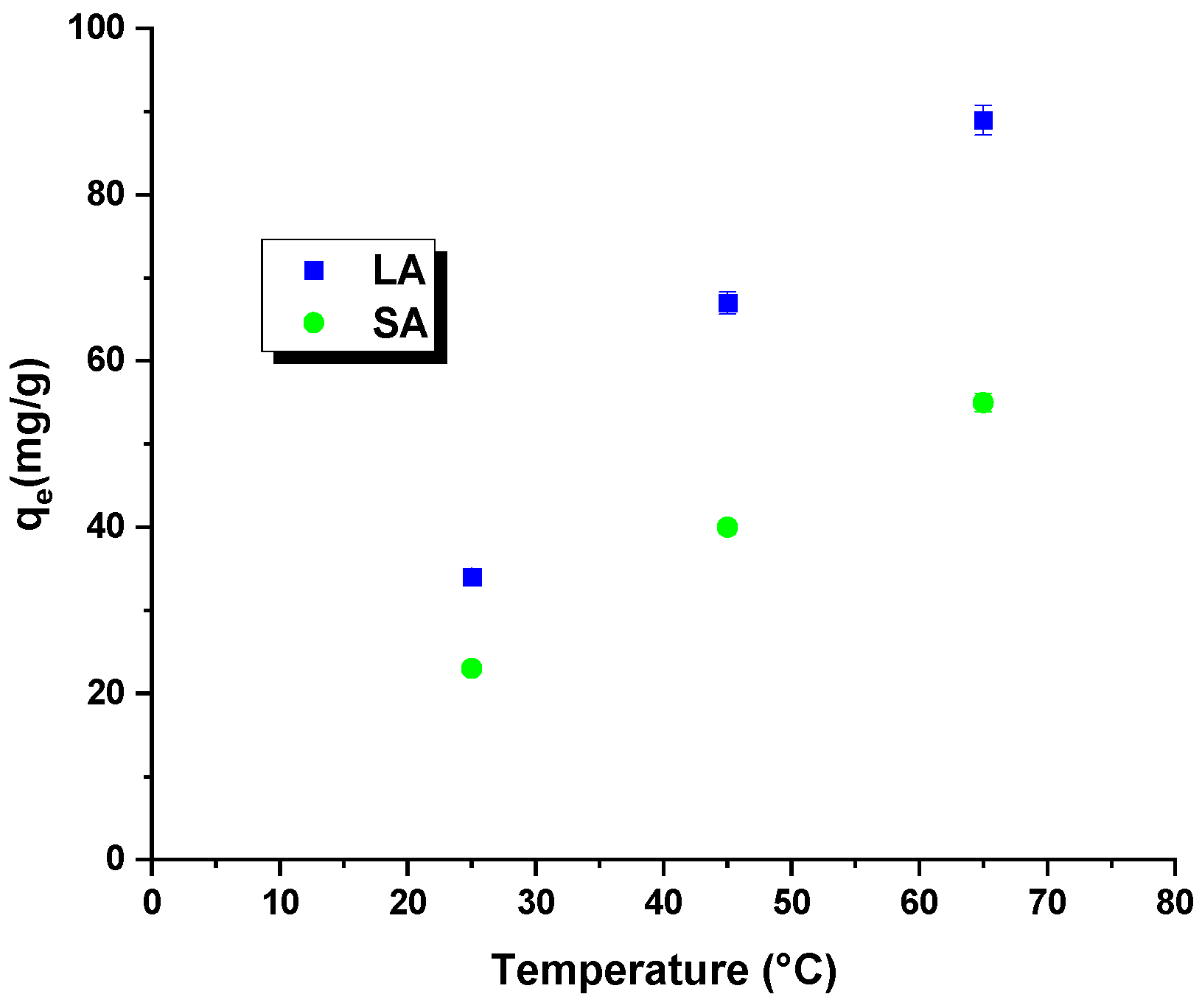
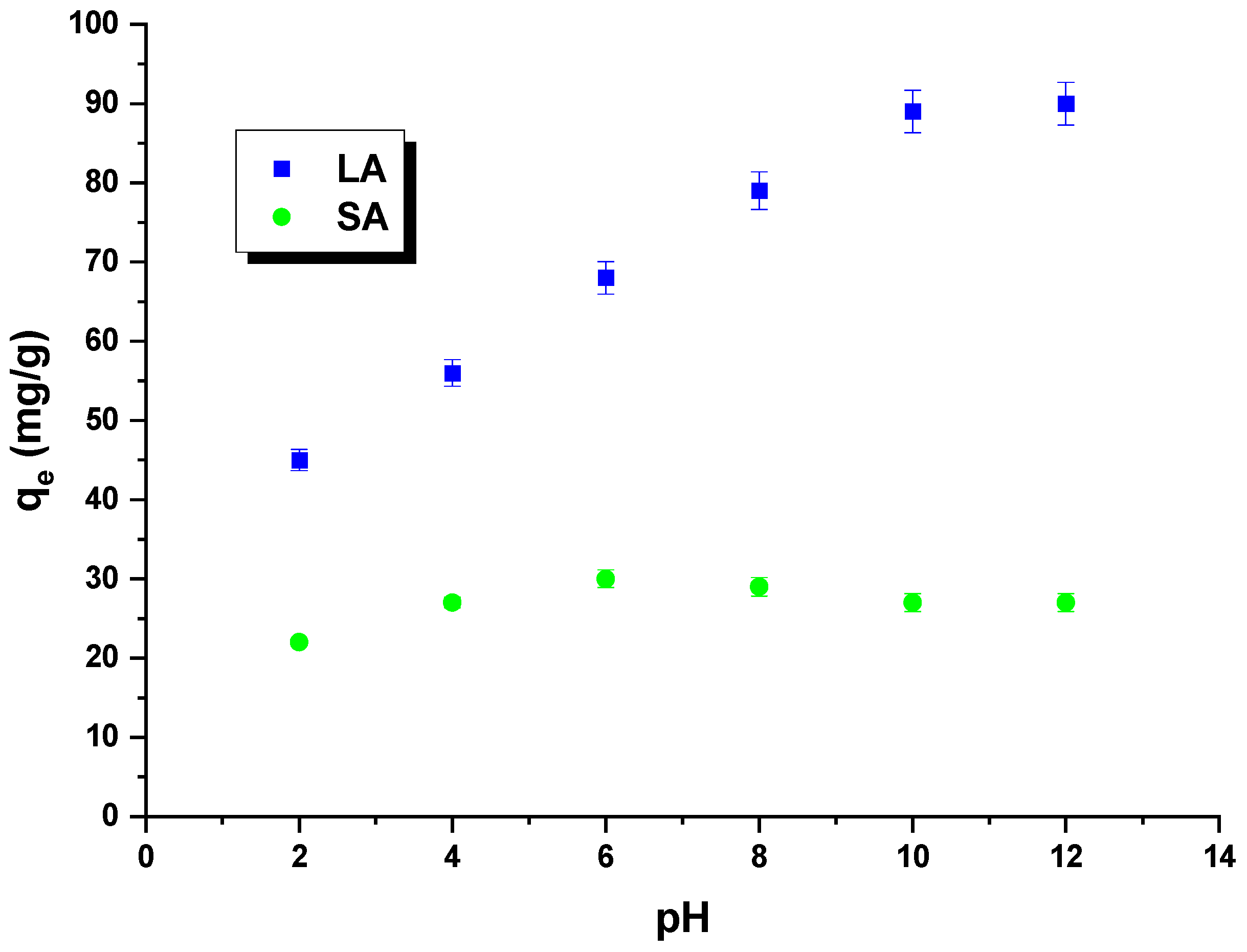
| Sample | Cdaf | Hdaf | Ndaf | Sdaf | Odaf * | Ash |
|---|---|---|---|---|---|---|
| LA | 74.6 | 1.8 | 1.0 | 6.2 | 16.4 | 9.6 |
| SA | 49.4 | 1.0 | 0.3 | 0.6 | 48.7 | 43.4 |
| Sample | Surface Area * (m2/g) | Micropore Area (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|---|
| LA | 520 | 198 | 0.40 | 0.14 | 3.05 |
| SA | 138 | 57 | 0.42 | 0.03 | 12.32 |
| Sample | Acidic Groups * (mmol/g) | Basic Groups * (mmol/g) | pHpzc * |
|---|---|---|---|
| LA | 1.01 | 0.00 | 7.4 |
| SA | 0.00 | 1.30 | 6.0 |
| Models | Parameters | Rhodamine B | |
|---|---|---|---|
| LA | SA | ||
| qt (mg/g) | 60 | 35 | |
| pseudo-first-order | R2 | 0.897 | 0.789 |
| k1 (1/min) | 8.67 × 10−3 | 2.41 × 10−2 | |
| qe,cal (mg/g) | 16 | 10 | |
| pseudo-second-order | R2 | 0.999 | 0.999 |
| k2 (g/mg × min) | 1.44 × 10−3 | 1.08 × 10−3 | |
| qe,cal (mg/g) | 59 | 34 | |
| intraparticle diffusion | R2 | 0.667 | 0.567 |
| kIPD (mg/g × min1/2) | 2.989 | 2.545 | |
| X (mg/g) | 29 | 19 | |
| Isotherms | Parameters | Rhodamine B | |
|---|---|---|---|
| LA | SA | ||
| qe [mg/g] | 117 | 61 | |
| Langmuir | R2 | 0.991 | 0.987 |
| qmax [mg/g] | 119 | 63 | |
| KL (L/mg) | 0.071 | 0.052 | |
| Freundlich | R2 | 0.902 | 0.876 |
| KF (mg/g(L/mg)1/n) | 112.33 | 34.67 | |
| 1/n | 0.189 | 0.345 | |
| Temkin | R2 | 0.508 | 0.604 |
| B (J/mol) | 67.98 | 123.89 | |
| AT (L/mg) | 15.45 | 5.23 | |
| Dubinin–Radushkevich | R2 | 0.899 | 0.845 |
| qmax (mg/g) | 110 | 57 | |
| E (kJ/mol) | 3.897 | 1.678 | |
| Sample | Temperature [°C] | ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (J/mol × K) |
|---|---|---|---|---|
| LA | 25 | −9.89 | 42.56 | 167.22 |
| 45 | −10.89 | |||
| 65 | −12.67 | |||
| SA | 25 | −4.56 | 30.45 | 111.23 |
| 45 | −8.89 | |||
| 65 | −10.89 |
| Sample | H2O | KOH | HCl | CH3COOH |
|---|---|---|---|---|
| LA | 56 | 41 | 82 | 13 |
| SA | 43 | 32 | 79 | 7 |
| Adsorbent | Adsorption Capacity (mg/g) | References |
|---|---|---|
| Adsorbent from black tea leaves | 53.2 | [30] |
| Graphene oxide/beta zeolite composite | 64.47 | [31] |
| Activated carbon for white sugar | 123.46 | [32] |
| Gelatin/activated carbon composite beads | 256.41 | [33] |
| LA | 119 | This study |
| SA | 63 | This study |
| LabinKOH | 175 | [16] |
| SpitsbergenKOH | 82 | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazan-Wozniak, A.; Jędrzejczak, A.; Wolski, R.; Kaczmarek, S.; Nosal-Wiercińska, A.; Cielecka-Piontek, J.; Yagmur-Kabas, S.; Pietrzak, R. A Study on the Adsorption of Rhodamine B onto Adsorbents Prepared from Low-Carbon Fossils: Kinetic, Isotherm, and Thermodynamic Analyses. Molecules 2024, 29, 1412. https://doi.org/10.3390/molecules29061412
Bazan-Wozniak A, Jędrzejczak A, Wolski R, Kaczmarek S, Nosal-Wiercińska A, Cielecka-Piontek J, Yagmur-Kabas S, Pietrzak R. A Study on the Adsorption of Rhodamine B onto Adsorbents Prepared from Low-Carbon Fossils: Kinetic, Isotherm, and Thermodynamic Analyses. Molecules. 2024; 29(6):1412. https://doi.org/10.3390/molecules29061412
Chicago/Turabian StyleBazan-Wozniak, Aleksandra, Aleksandra Jędrzejczak, Robert Wolski, Sławomir Kaczmarek, Agnieszka Nosal-Wiercińska, Judyta Cielecka-Piontek, Sultan Yagmur-Kabas, and Robert Pietrzak. 2024. "A Study on the Adsorption of Rhodamine B onto Adsorbents Prepared from Low-Carbon Fossils: Kinetic, Isotherm, and Thermodynamic Analyses" Molecules 29, no. 6: 1412. https://doi.org/10.3390/molecules29061412
APA StyleBazan-Wozniak, A., Jędrzejczak, A., Wolski, R., Kaczmarek, S., Nosal-Wiercińska, A., Cielecka-Piontek, J., Yagmur-Kabas, S., & Pietrzak, R. (2024). A Study on the Adsorption of Rhodamine B onto Adsorbents Prepared from Low-Carbon Fossils: Kinetic, Isotherm, and Thermodynamic Analyses. Molecules, 29(6), 1412. https://doi.org/10.3390/molecules29061412








