Design, Synthesis and Bioactivity Evaluation of Heterocycle-Containing Mono- and Bisphosphonic Acid Compounds
Abstract
1. Introduction
2. Results and Discussion
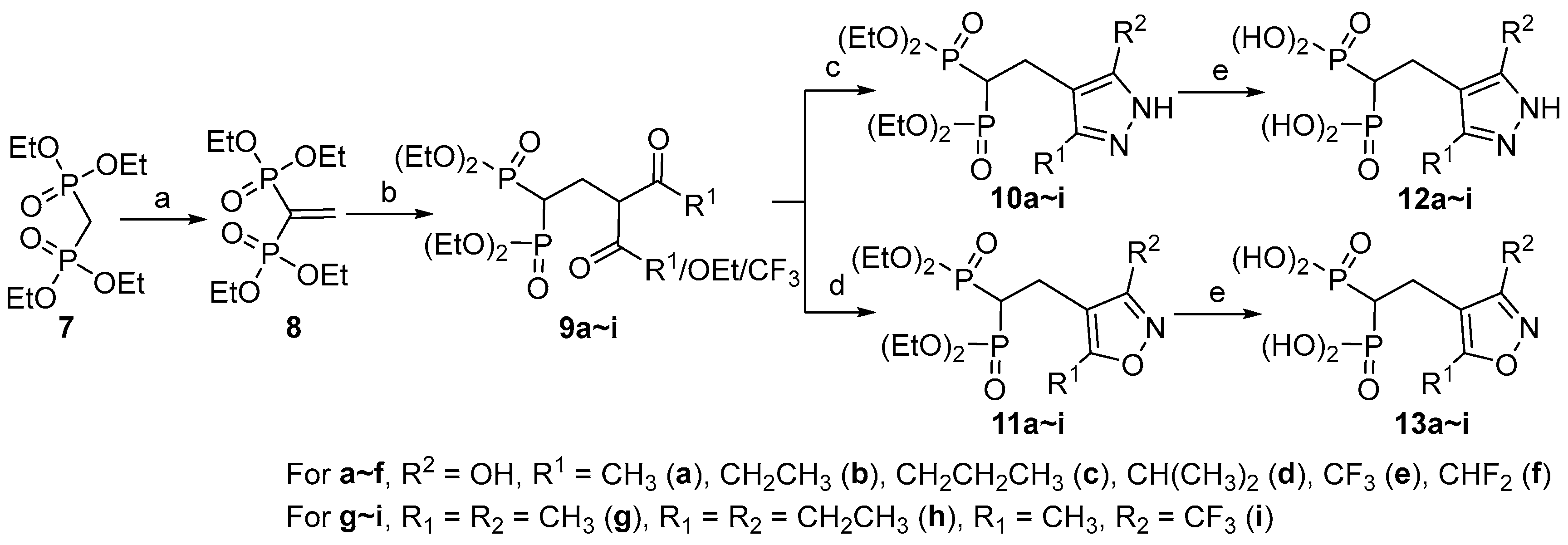
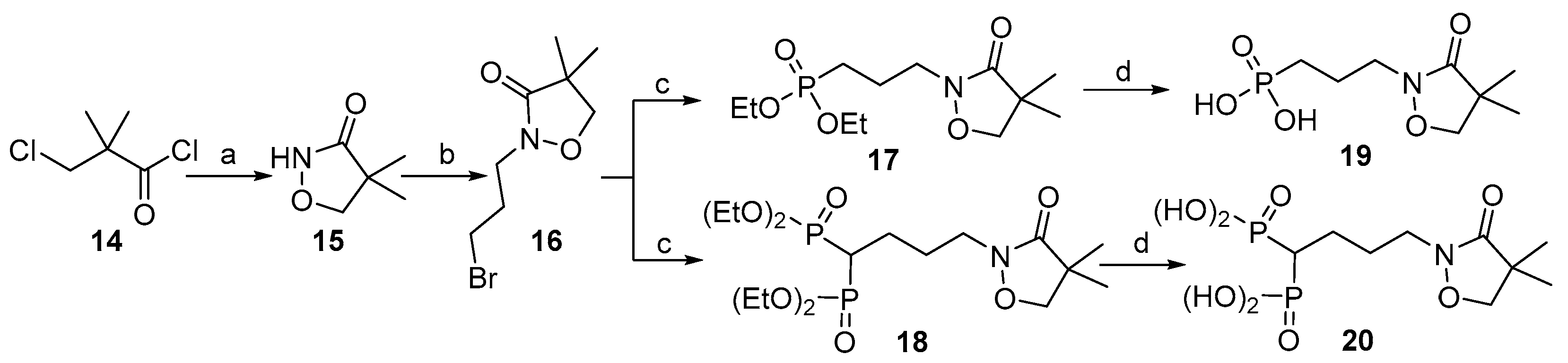
2.1. Chemistry
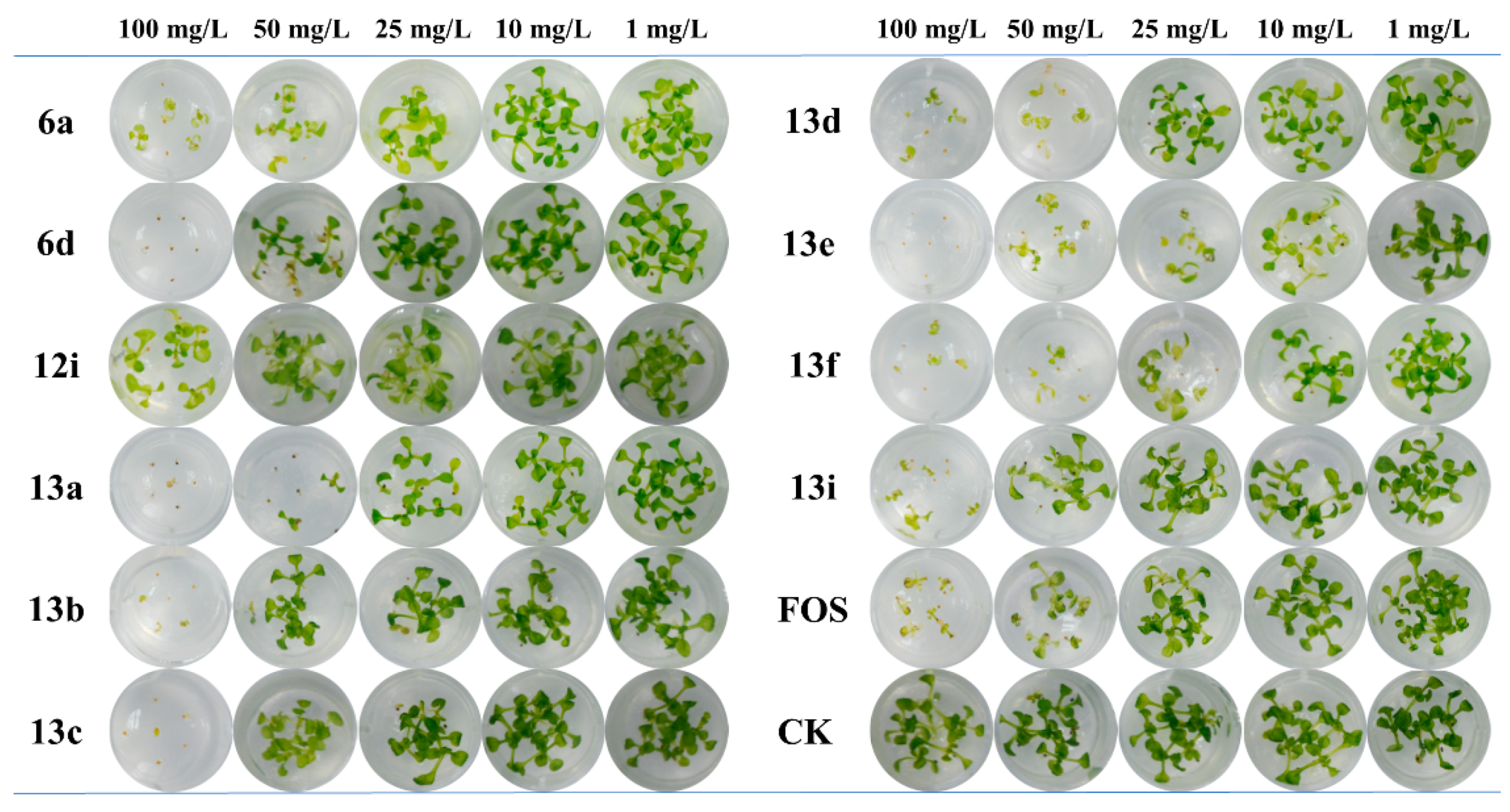
2.2. Arabidopsis Growth Inhibitory Activity
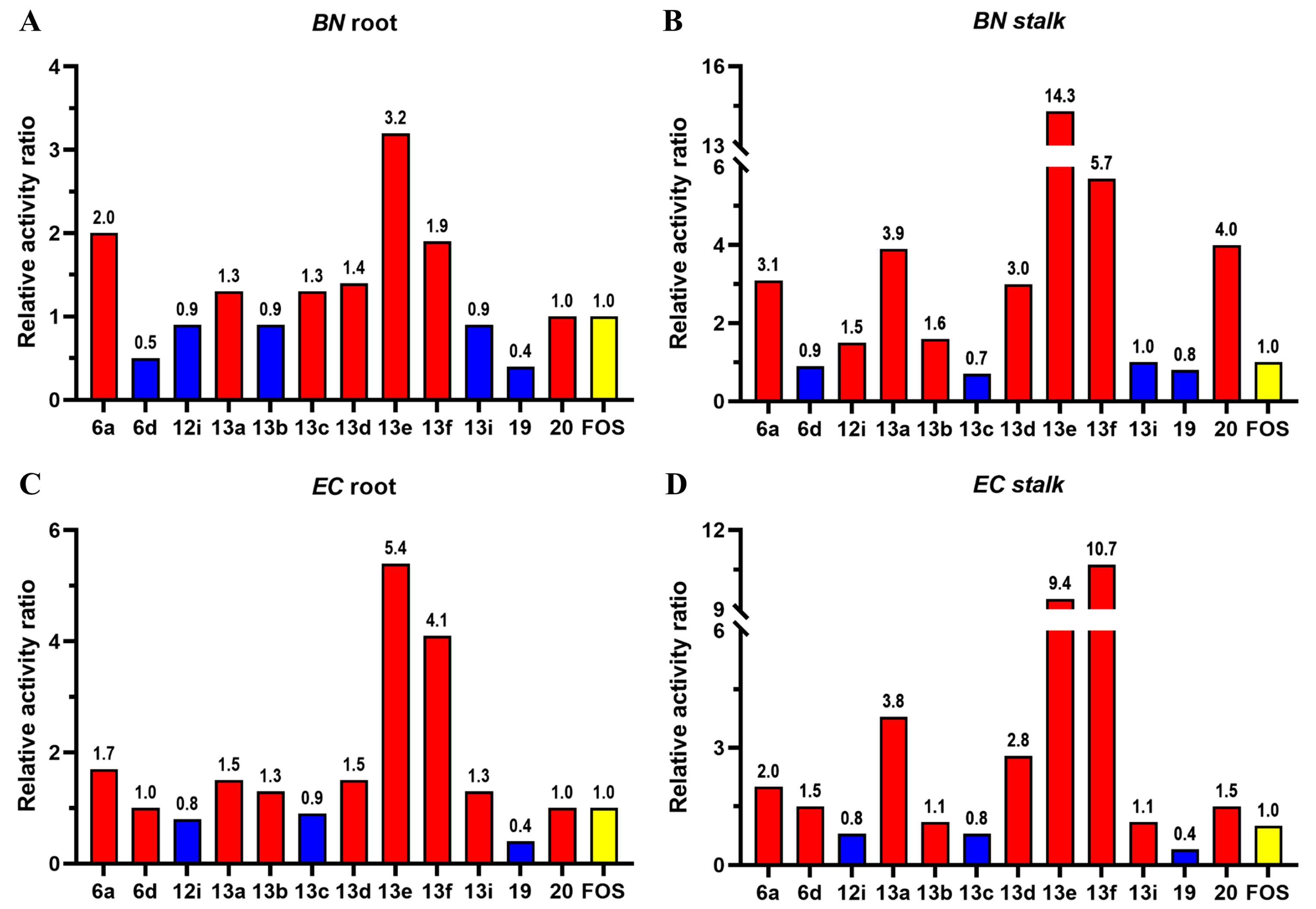
2.3. Pre-Emergence Herbicidal Activity
2.4. Post-Emergence Herbicidal Activity
2.5. DXR Inhibitory Activity
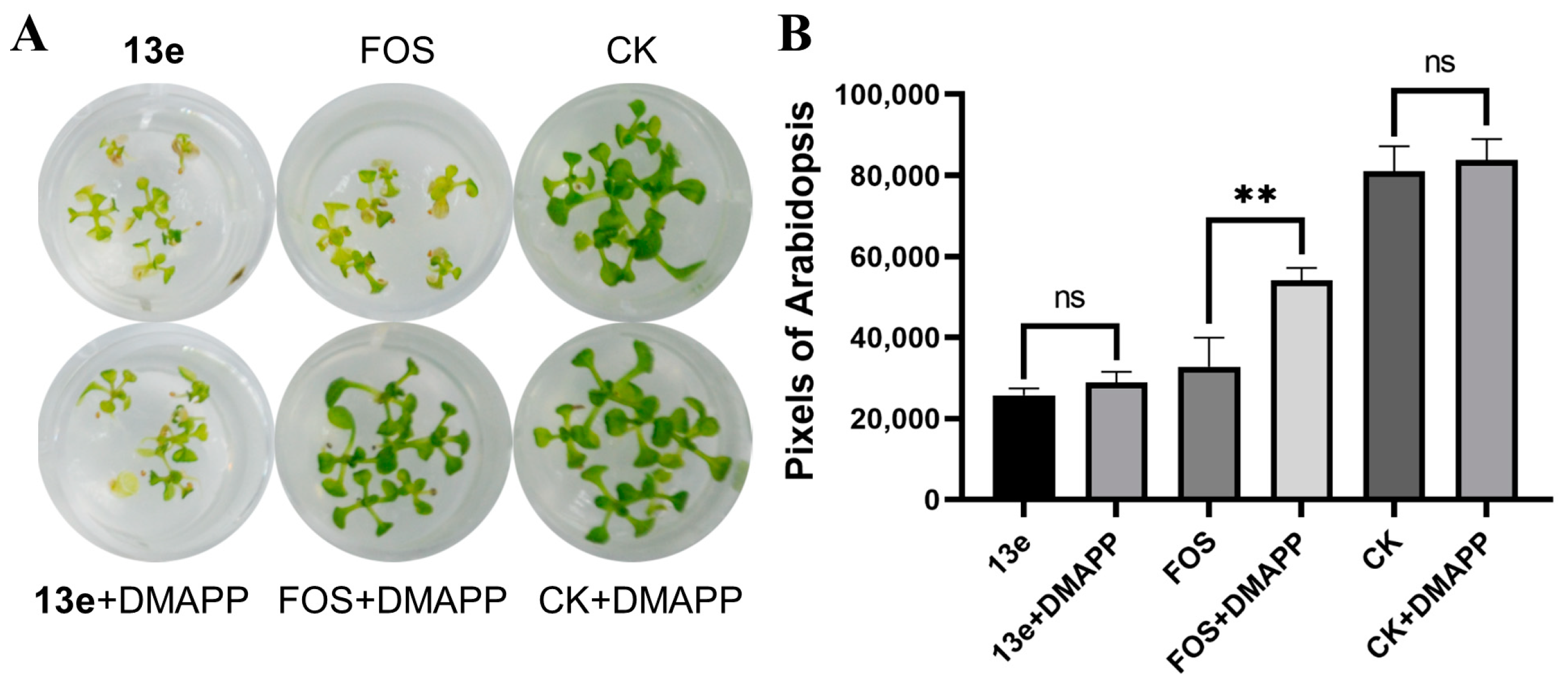
2.6. DMAPP Rescue and Molecule Docking
2.7. Discussion on Structure–Activity Relationships
3. Materials and Methods
3.1. Instruments and Reagents
3.2. Synthesis
3.3. Biological Assays
3.3.1. Arabidopsis Growth Inhibition Assay
3.3.2. Pre-Emergence Herbicidal Inhibition Assay
3.3.3. Post-Emergence Herbicidal Inhibition Assay
3.3.4. DXR Enzyme Inhibition Assay
3.3.5. DMAPP Rescue
3.4. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frank, A.; Groll, M. The methylerythritol phosphate pathway to isoprenoids. Chem. Rev. 2017, 117, 5675–5703. [Google Scholar] [CrossRef]
- Allamand, A.; Piechowiak, T.; Lièvremont, D.; Rohmer, M.; Grosdemange-Billiard, C. The multifaceted MEP pathway: Towards new therapeutic perspectives. Molecules 2023, 28, 1403. [Google Scholar] [CrossRef] [PubMed]
- Chatzivasileiou, A.O.; Ward, V.; Edgar, S.M.; Stephanopoulos, G. Two-step pathway for isoprenoid synthesis. Proc. Natl. Acad. Sci. USA 2018, 116, 506–511. [Google Scholar] [CrossRef]
- Pu, X.; Dong, X.; Li, Q.; Chen, Z.; Liu, L. An Update on the function and regulation of methylerythritol phosphate and mevalonate pathways and their evolutionary dynamics. J. Integr. Plant Biol. 2021, 63, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Lipko, A.; Pączkowski, C.; Perez-Fons, L.; Fraser, P.D.; Kania, M.; Hoffman-Sommer, M.; Danikiewicz, W.; Rohmer, M.; Poznanski, J.; Swiezewska, E. Divergent contribution of the MVA and MEP pathways to the formation of polyprenols and dolichols in Arabidopsis. Biochem. J. 2023, 480, 495–520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chang, W.C.; Xiao, Y.; Liu, H.W.; Liu, P. Methylerythritol phosphate pathway of isoprenoid biosynthesis. Annu. Rev. Biochem. 2013, 82, 497–530. [Google Scholar] [CrossRef]
- Masini, T.; Kroezen, B.S.; Hirsch, A.K. Druggability of the enzymes of the non-mevalonate-pathway. Drug Discov. Today 2013, 18, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Yajima, S.; Hara, K.; Iino, D.; Sasaki, Y.; Kuzuyama, T.; Ohsawa, K.; Seto, H. Structure of 1-deoxy-D-xylulose 5-phosphate reductoisomerase in a quaternary complex with a magnesium ion, NADPH and the antimalarial drug fosmidomycin. Acta Crystallogr. F. 2007, 63, 466–470. [Google Scholar] [CrossRef]
- Wu, X.; Bu, M.; Yang, Z.; Ping, H.; Song, C.; Duan, J.; Zhang, A. Design and synthesis of fosmidomycin analogs containing aza-linkers and their biological activity evaluation. Pest Manag. Sci. 2023. [Google Scholar] [CrossRef] [PubMed]
- Possell, M.; Ryan, A.; Vickers, C.E.; Mullineaux, P.M.; Hewitt, C.N. Effects of fosmidomycin on plant photosynthesis as measured by gas exchange and chlorophyll fluorescence. Photosynth. Res. 2010, 104, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Okuhara, M.; Kuroda, Y.; Goto, T.; Okamoto, M.; Terano, H.; Kohsaka, M.; Aoki, H.; Imanaka, H. Studies on new phosphonic acid antibiotics. III. Isolation and characterization of FR-31564, FR-32863 and FR-33289. J. Antibiot. 1980, 33, 24–28. [Google Scholar] [CrossRef]
- Okuhara, M.; Kuroda, Y.; Goto, T.; Okamoto, M.; Terano, H.; Kohsaka, M.; Aoki, H.; Imanaka, H. Studies on new phosphonic acid antibiotics. I. FR-900098, isolation and characterization. J. Antibiot. 1980, 33, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Masini, T.; Hirsch, A.K.H. Development of inhibitors of the 2C-Methyl-D-erythritol 4-Phosphate (MEP) pathway enzymes as potential anti-infective agents. J. Med. Chem. 2014, 57, 9740–9763. [Google Scholar]
- Kesharwani, S.; Sundriyal, S. Non-hydroxamate inhibitors of 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR): A critical review and future perspective. Eur. J. Med. Chem. 2021, 213, 113055. [Google Scholar] [CrossRef]
- Knak, T.; Abdullaziz, M.A.; Höfmann, S.; Alves Avelar, L.A.; Klein, S.; Martin, M.; Fischer, M.; Tanaka, N.; Kurz, T. Over 40 years of fosmidomycin drug research: A comprehensive review and future opportunities. Pharmaceuticals 2022, 15, 1553. [Google Scholar]
- Shen, S.; Kozikowski, A.P. Why hydroxamates may not Be the best histone deacetylase inhibitors-what some may have forgotten or would rather forget? ChemMedChem 2015, 11, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hermant, P.; Bosc, D.; Piveteau, C.; Gealageas, R.; Lam, B.; Ronco, C.; Roignant, M.; Tolojanahary, H.; Jean, L.; Renard, P.Y.; et al. Controlling plasma stability of hydroxamic acids: A MedChem toolbox. J. Med. Chem. 2017, 60, 9067–9089. [Google Scholar] [CrossRef]
- McClure, J.J.; Li, X.; Chou, C.J. Advances and challenges of HDAC inhibitors in cancer therapeutics. Adv. Cancer Res. 2018, 138, 183–211. [Google Scholar] [PubMed]
- Cohen, S.M. A bioinorganic approach to fragment-based drug discovery targeting metalloenzymes. Acc. Chem. Res. 2017, 50, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Adamek, R.N.; Dick, B.L.; Credille, C.V.; Morrison, C.N.; Cohen, S.M. Targeting metalloenzymes for therapeutic intervention. Chem. Rev. 2018, 119, 1323–1455. [Google Scholar]
- Andaloussi, M.; Lindh, M.; Björkelid, C.; Suresh, S.; Wieckowska, A.; Iyer, H.; Karlén, A.; Larhed, M. Substitution of the phosphonic acid and hydroxamic acid functionalities of the DXR inhibitor FR900098: An attempt to improve the activity against Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 2011, 21, 5403–5407. [Google Scholar] [CrossRef]
- Deng, L.; Endo, K.; Kato, M.; Cheng, G.; Yajima, S.; Song, Y. Structures of 1-deoxy-D-xylulose-5-phosphate reductoisomerase/lipophilic phosphonate complexes. ACS Med. Chem. Lett. 2010, 2, 165–170. [Google Scholar] [CrossRef]
- Yajima, S.; Hara, K.; Sanders, J.M.; Yin, F.; Ohsawa, K.; Wiesner, J.; Jomaa, H.; Oldfield, E. Crystallographic structures of two bisphosphonate: 1-deoxyxylulose-5-phosphate reductoisomerase complexes. J. Am. Chem. Soc. 2004, 126, 10824–10825. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Sundriyal, S.; Rubio, V.; Shi, Z.Z.; Song, Y. Coordination chemistry based approach to lipophilic inhibitors of 1-deoxy-D-xylulose-5-phosphate reductoisomerase. J. Med. Chem. 2009, 52, 6539–6542. [Google Scholar] [CrossRef] [PubMed]
- Lamberth, C. Heterocyclic chemistry in crop protection. Pest. Manag. Sci. 2013, 69, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Schwender, J.; Zeidler, J.; Lichtenthaler, H.K. Properties and inhibition of the first two enzymes of the non-mevalonate pathway of isoprenoid biosynthesis. Biochem. Soc. T. 2000, 28, 792–793. [Google Scholar] [CrossRef]
- Li, G.; Su, Y.; Yan, Y.H.; Peng, J.Y.; Dai, Q.Q.; Ning, X.L.; Zhu, C.L.; Fu, C.; McDonough, M.A.; Schofield, C.J.; et al. MeLAD: An integrated resource for metalloenzyme-ligand associations. Bioinformatics 2019, 36, 904–909. [Google Scholar] [CrossRef]
- Padmaja, A.; Reddy, G.S.; Venkata Nagendra Mohan, A.; Padmavathi, V. Michael adducts-source for biologically potent heterocycles. Chem. Pharm. Bul. 2008, 56, 647–653. [Google Scholar] [CrossRef]
- Bulman Page, P.C.; Moore, J.P.; Mansfield, I.; McKenzie, M.J.; Bowler, W.B.; Gallagher, J.A. Synthesis of bone-targeted oestrogenic compounds for the inhibition of bone resorption. Tetrahedron 2001, 57, 1837–1847. [Google Scholar] [CrossRef]
- Chang, J.H. Herbicidal 3-Isoxazolidinones and Hydroxamic Acids. US 4405357, 20 September 1983. [Google Scholar]
- Adeyemi, C.M.; Faridoon; Isaacs, M.; Mnkandhla, D.; Hoppe, H.C.; Krause, R.W.M.; Kaye, P.T. Synthesis and antimalarial activity of N-benzylated (N-arylcarbamoyl)alkyl-phosphonic acid derivatives. Bioorgan. Med. Chem. 2016, 24, 6131–6138. [Google Scholar] [CrossRef] [PubMed]
- Chofor, R.; Risseeuw, M.; Pouyez, J.; Johny, C.; Wouters, J.; Dowd, C.; Couch, R.; Van Calenbergh, S. Synthetic fosmidomycin analogues with altered chelating moieties do not inhibit 1-deoxy-D-xylulose 5-phosphate reductoisomerase or Plasmodium falciparum growth in vitro. Molecules 2014, 19, 2571–2587. [Google Scholar] [CrossRef] [PubMed]
- Enders, D.; Wahl, H.; Papadopoulos, K. Asymmetric Michael additions via SAMP/RAMP hydrazones enantioselective synthesis of 2-substituted 4-oxophosphonates. Liebigs Ann. 1995, 7, 1177–1184. [Google Scholar] [CrossRef]
- Dutta, S.; Malla, R.K.; Bandyopadhyay, S.; Spilling, C.D.; Dupureur, C.M. Synthesis and kinetic analysis of some phosphonate analogs of cyclophostin as inhibitors of human acetylcholinesterase. Bioorgan. Med. Chem. 2010, 18, 2265–2274. [Google Scholar] [CrossRef][Green Version]
- Lolli, M.L.; Lazzarato, L.; Di Stilo, A.; Fruttero, R.; Gasco, A. Michael addition of Grignard reagents to tetraethyl ethenylidenebisphosphonate. J. Organomet. Chem. 2002, 650, 77–83. [Google Scholar] [CrossRef]
- Zare, A.; Hasaninejad, A.; Parhami, A.; Zare, A.R.M.; Khalafi Nezhad, A. Microwave-assisted michael addition of amides to alpha, beta-unsaturated esters under solvent-free conditions. Pol. J. Chem. 2008, 82, 1059–1066. [Google Scholar]
- Sauret-Güeto, S.; Botella-Pavía, P.; Flores-Pérez, U.; Martínez-García, J.F.; San Román, C.; León, P.; Boronat, A.; Rodríguez-Concepción, M. Plastid cues posttranscriptionally regulate the accumulation of key enzymes of the methylerythritol phosphate pathway in Arabidopsis. Plant Physiol. 2006, 141, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Wang, X.; Duan, L.; Duan, J.; Li, W.; Zhang, A. Synthesis and evaluation of halogenated 5-(2-Hydroxyphenyl)pyrazoles as pseudilin analogues targeting the enzyme IspD in the methylerythritol phosphate pathway. J. Agric. Food Chem. 2020, 68, 3071–3078. [Google Scholar] [CrossRef]
- Wang, Y.E.; Yang, D.; Huo, J.; Chen, L.; Kang, Z.; Mao, J.; Zhang, J. Design, synthesis, and herbicidal activity of thioether containing 1,2,4-triazole schiff bases as transketolase inhibitors. J. Agric. Food Chem. 2021, 69, 11773–11780. [Google Scholar] [CrossRef]
- Kuntz, L.; Trisch, D.; Grosdemange-Billiard, C.; Hemmerlin, A.; Willem, A.; Bach, T.; Rohmer, M. Isoprenoid biosynthesis as a target for antibacterial and antiparasitic drugs: Phosphonohydroxamic acids as inhibitors of deoxyxylulose phosphate reducto-isomerase. Biochem J. 2005, 386, 127–135. [Google Scholar] [CrossRef]
- Wu, X.; Ping, H.; Song, C.; Duan, J.; Zhang, A. Optimization synthesis of phosphorous-containing natural products fosmidomycin and FR900098. Phosphorus Sulfur Silicon Relat. Elem. 2023, 198, 446–452. [Google Scholar] [CrossRef]
- Corral, M.G.; Leroux, J.; Stubbs, K.A.; Mylne, J.S. Herbicidal properties of antimalarial drugs. Sci. Rep. 2017, 7, 45871. [Google Scholar] [CrossRef] [PubMed]

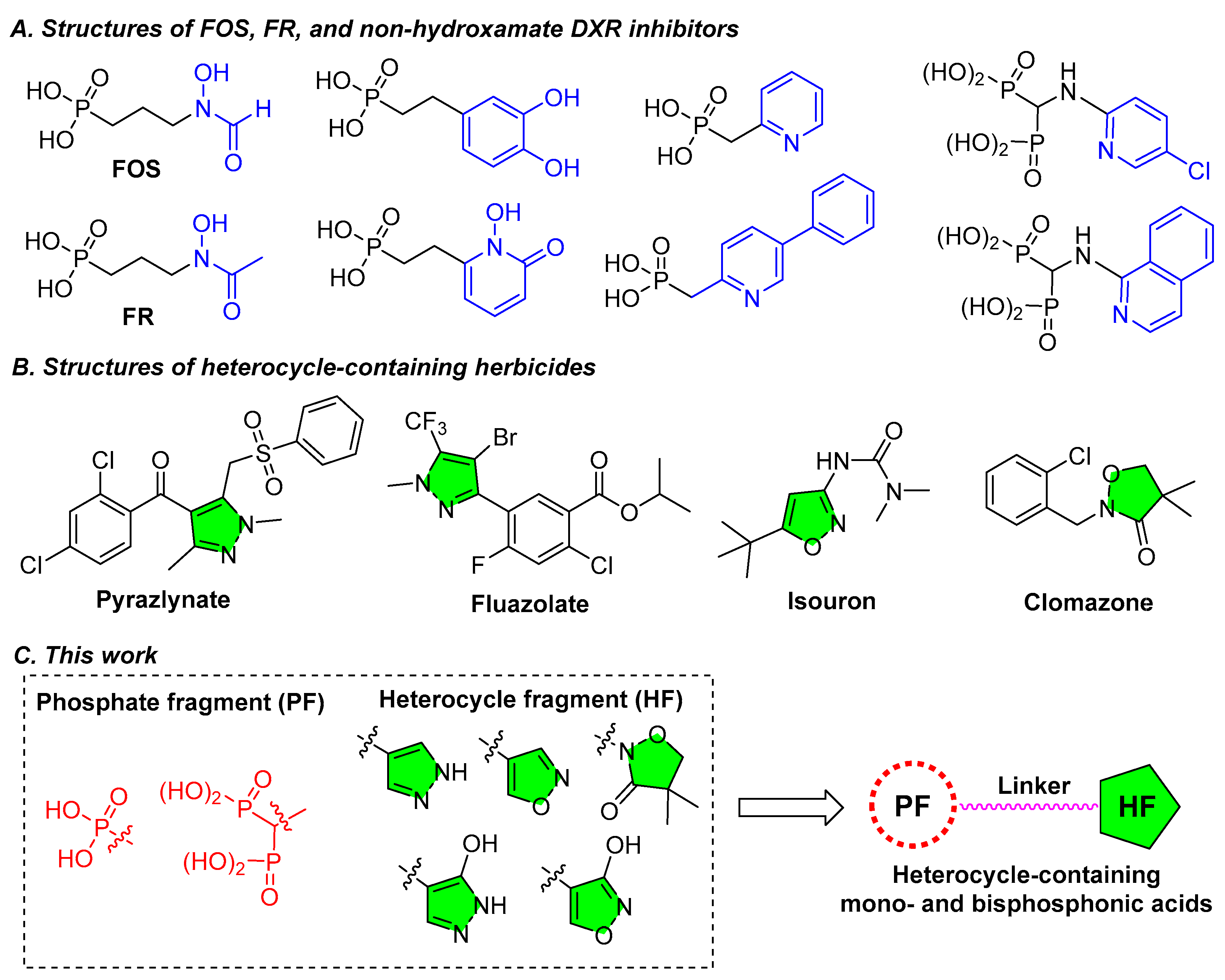


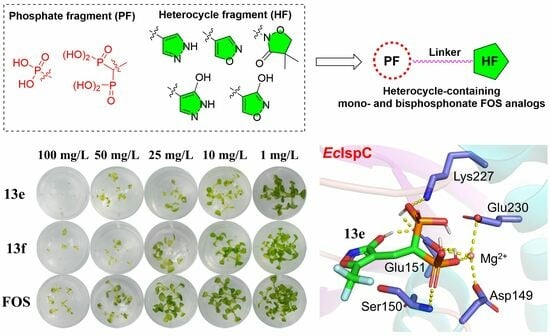
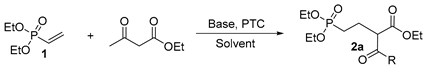 | |||||
| Entry | Base | PTC | Solvent | Temp (°C) | Yield (%) b |
| 1 | t-BuOK | -- | THF | r.t. | 60 |
| 2 | NaH | -- | THF | r.t. | 45 |
| 3 | NaOEt | -- | EtOH | r.t. | 40 |
| 4 | t-BuOK | -- | THF | 66 | 62 |
| 5 | DBU | -- | THF | r.t. | 50 |
| 6 | DBN | -- | THF | r.t. | 53 |
| 7 | Cs2CO3 | TBAI | CH3CN | 80 | 80 |
| 8 | Cs2CO3 | TEBAC | CH3CN | 80 | 85 |
| 9 | K2CO3 | TBAI | CH3CN | 80 | 83 |
| 10 | K2CO3 | TEBAC | CH3CN | 80 | 90 |
| Comp | EC50 (mg/L) a | Comp | EC50 (mg/L) | Comp | EC50 (mg/L) |
|---|---|---|---|---|---|
| 5a | >100 | 6f | >100 | 13b | 37.7 ± 2.8 |
| 5b | >100 | 12a | >100 | 13c | 48.4 ± 5.3 |
| 5c | >100 | 12b | >100 | 13d | 23.1 ± 1.9 |
| 5d | >100 | 12c | >100 | 13e | 7.8 ± 1.2 |
| 5e | >100 | 12d | >100 | 13f | 8.7 ± 1.3 |
| 5f | >100 | 12e | >100 | 13g | >100 |
| 6a | 28.6 ± 3.9 | 12f | >100 | 13h | >100 |
| 6b | >100 | 12g | >100 | 13i | 40.7 ± 2.9 |
| 6c | >100 | 12h | >100 | 19 | >100 |
| 6d | 45.7 ± 4.8 | 12i | 88.3 ± 4.3 | 20 | >100 |
| 6e | >100 | 13a | 21.6 ± 3.8 | FOS | 27.5 ± 3.1 |
| Comp | EC50 (mg/L) | |||
|---|---|---|---|---|
| BN | EC | |||
| Root | Stalk | Root | Stalk | |
| 6a | 17.3 | 10.5 | 24.0 | 18.9 |
| 6d | 67.5 | 38.2 | 40.1 | 25.7 |
| 12i | 36.6 | 21.7 | 53.1 | 47.7 |
| 13a | 26.9 | 8.5 | 27.1 | 10.2 |
| 13b | 38.8 | 20.2 | 29.9 | 35.3 |
| 13c | 27.7 | 46.9 | 43.9 | 45.8 |
| 13d | 25.2 | 11.0 | 27.2 | 13.5 |
| 13e | 10.7 | 2.3 | 7.4 | 4.1 |
| 13f | 17.9 | 5.8 | 9.7 | 3.6 |
| 13i | 40.5 | 33.5 | 32.1 | 34.6 |
| 19 | 79.3 | 39.2 | >100 | 88.0 |
| 20 | 34.6 | 8.2 | 40.0 | 25.2 |
| FOS | 34.7 | 32.9 | 40.2 | 38.4 |
| Comp | Visual Evaluation b | Loss of Weight (%) | ||
|---|---|---|---|---|
| AR | EC | AR | EC | |
| 6a | + | + | 35.6 | 30.8 |
| 13a | ++ | + | 52.3 | 35.8 |
| 13d | ++ | + | 45.7 | 34.9 |
| 13e | +++ | ++ | 70.3 | 53.5 |
| 13f | +++ | ++ | 61.8 | 48.1 |
| 20 | + | + | 23.6 | 22.9 |
| FOS | + | + | 31.5 | 27.4 |
| Comp | InR (%) a | Comp | InR (%) | Comp | InR (%) |
|---|---|---|---|---|---|
| 5a | 11.9 | 6f | 11.7 | 13b | 27.4 |
| 5b | 12.9 | 12a | 13.7 | 13c | 28.4 |
| 5c | 6.1 | 12b | 21.9 | 13d | 14.0 |
| 5d | 17.7 | 12c | 32.3 | 13e | 54.9 |
| 5e | 23.0 | 12d | 12.4 | 13f | 24.4 |
| 5f | 32.4 | 12e | 22.8 | 13g | 7.3 |
| 6a | 15.8 | 12f | 6.1 | 13h | 10.3 |
| 6b | 19.5 | 12g | 11.6 | 13i | 30.3 |
| 6c | 7.5 | 12h | 2.3 | 19 | 22.5 |
| 6d | 17.3 | 12i | 27.1 | 20 | 29.5 |
| 6e | 12.1 | 13a | 60.2 | FOS | 98.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Yang, Z.; Bu, M.; Duan, J.; Zhang, A. Design, Synthesis and Bioactivity Evaluation of Heterocycle-Containing Mono- and Bisphosphonic Acid Compounds. Molecules 2023, 28, 7509. https://doi.org/10.3390/molecules28227509
Wu X, Yang Z, Bu M, Duan J, Zhang A. Design, Synthesis and Bioactivity Evaluation of Heterocycle-Containing Mono- and Bisphosphonic Acid Compounds. Molecules. 2023; 28(22):7509. https://doi.org/10.3390/molecules28227509
Chicago/Turabian StyleWu, Xin, Zili Yang, Mengwei Bu, Jiang Duan, and Aidong Zhang. 2023. "Design, Synthesis and Bioactivity Evaluation of Heterocycle-Containing Mono- and Bisphosphonic Acid Compounds" Molecules 28, no. 22: 7509. https://doi.org/10.3390/molecules28227509
APA StyleWu, X., Yang, Z., Bu, M., Duan, J., & Zhang, A. (2023). Design, Synthesis and Bioactivity Evaluation of Heterocycle-Containing Mono- and Bisphosphonic Acid Compounds. Molecules, 28(22), 7509. https://doi.org/10.3390/molecules28227509