Abstract
In this research, with an aim to develop novel pyrazole oxime ether derivatives possessing potential biological activity, thirty-two pyrazole oxime ethers, including a substituted pyridine ring, have been synthesized and structurally identified through 1H NMR, 13C NMR, and HRMS. Bioassay data indicated that most of these compounds owned strong insecticidal properties against Mythimna separata, Tetranychus cinnabarinus, Plutella xylostella, and Aphis medicaginis at a dosage of 500 μg/mL, and some title compounds were active towards Nilaparvata lugens at 500 μg/mL. Furthermore, some of the designed compounds had potent insecticidal effects against M. separata, T. cinnabarinus, or A. medicaginis at 100 μg/mL, with the mortalities of compounds 8a, 8c, 8d, 8e, 8f, 8g, 8o, 8s, 8v, 8x, and 8z against A. medicaginis, in particular, all reaching 100%. Even when the dosage was lowered to 20 μg/mL, compound 8s also expressed 50% insecticidal activity against M. separata, and compounds 8a, 8e, 8f, 8o, 8v, and 8x displayed more than 60% inhibition rates against A. medicaginis. The current results provided a significant basis for the rational design of biologically active pyrazole oxime ethers in future.
1. Introduction
In recent years, compounds containing N-heterocycles are found to have wide application in pesticidal and medicinal fields [1,2,3,4,5]. As an important N-heterocycle, pyridine-based derivatives have drawn extensive interest owing to their diversified bioactivities, including insecticidal [6,7,8], fungicidal [9], herbicidal [10], antibacterial [11], and anticancer properties [12,13]. For example, Chlorfluazuron, Pyridalyl, Chlorantraniliprole, and Cyantraniliprole (Figure 1), commercial insecticides with a substituted pyridyl framework, demonstrated wonderful insecticidal properties against some important lepidopterous pests such as Plutella xylostella, Mythimna separata (Walker), and so on [14,15]. More recently, agrochemical scientists are focusing on the research of the structural modification of pyridine-based pesticides, and a number of bioactive compounds containing a pyridyl ring were reported [16,17,18,19], among which Tetrachloraniliprole and Cyclaniliprole were the typical commercial pesticides [20]. Therefore, the wide spread use of pyridine compounds as a skeleton in the field of crop protection establishes the pyridyl unit as a vital structural class.
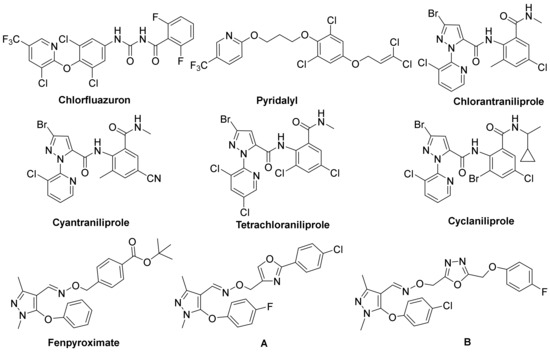
Figure 1.
Structures of Chlorfluazuron, Pyridalyl, Chlorantraniliprole, Cyantraniliprole, Tetrachloraniliprole, Cyclaniliprole, Fenpyroximate and compounds A–B.
In addition, pyrazole oxime ethers are also significant N-heterocyclic compounds, which act as an active pharmacophore during the development of new agrochemicals and drugs [21,22]. Many pyrazole oxime ether derivatives are reported to display various pesticidal and pharmacological activities involving fungicidal [23], insecticidal [24], anti-TMV [25], and antiproliferative effects [26]. For instance, Fenpyroximate (Figure 1) was an outstanding acaricide invented by Nihon Nohyaku in 1991. In the past few decades, Fenpyroximate has been extensively applied in protecting certain kinds of crops because of its efficient insecticidal activities towards agricultural mites like Polyphagotarsonemus latus (Banks), Tetranychus urticae (Koch), and so on [27]. Thereafter, many studies about the structural derivation of Fenpyroximate were performed. Recently, Wang et al. found that oxazole-containing pyrazole oxime ether derivative A (Figure 1) had a broad spectrum of insecticidal and fungicidal properties [28]. Sun et al. reported pyrazole oxime ether B (Figure 1) with an aryloxy-linked 1,3,4-oxadiazole moiety displaying satisfactory insecticidal effects [29]. This greatly promoted the development of bioactive pyrazole oxime ether molecules.
Inspired by the aforementioned reports, we hypothesized that the introduction of prominent pyridine pharmacophore into a pyrazole oxime ether unit might result in some novel potentially insecticidal agents. Herein, we describe the design and preparation of a series of new pyrazole oxime ether derivatives carrying pyridyl moiety (Figure 2). Furthermore, the insecticidal activities of the newly obtained pyrazole oxime ethers were systematically assessed and discussed.
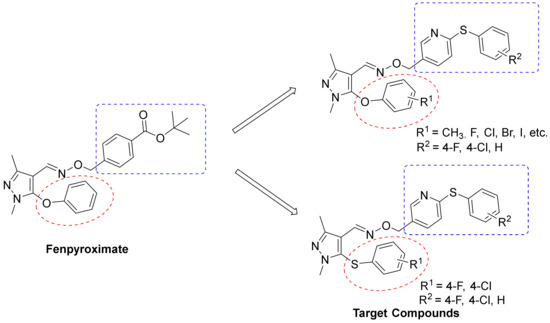
Figure 2.
Design of the target compounds.
2. Results and Discussion
2.1. Chemistry
The synthetic pathways for the title compounds 8a–z and 11a–f are illustrated in Scheme 1 and Scheme 2, respectively. The crucial intermediates 3 were efficiently synthesized through a two-step process, selecting 5-chloro-1,3-dimethyl-1H-pyrazole-4-carbaldehyde (1) as the starting material. The reaction of intermediate 1 with diverse substituted phenols or substituted thiophenols was conducted under alkaline conditions to produce compounds 2 or 9a,b smoothly. Next, intermediates 2 or 9a,b were reacted with hydroxylamine hydrochloride, utilizing potassium hydroxide as the base to afford the key intermediates 3 or 10a,b conveniently. The important synthons 5-chloromethyl-2-(substituted phenylthio)pyridine (7a–c) were prepared from commercially available methyl 6-chloronicotinate (4). Compound 4 underwent an aromatic nucleophilic substitution with substituted thiophenols, choosing potassium carbonate as the deacid reagent and DMF as the solvent to generate intermediates 5a–c in good yields. The further reduction reaction with LiAlH4 obtained compounds 6a–c successfully. Compounds 6a–c were then converted into the significant intermediates 7a–c by treatment with thionyl chloride. Subsequently, compounds 7a–c were treated with the important intermediates 3 or 10a,b in N and N-dimethylformamide, utilizing potassium carbonate as the acid scavenger to obtain the desired compounds 8a–z and 11a–f in satisfactory yields. Additionally, the structures of the targeted compounds 8a–z and 11a–f were effectively determined by 1H NMR, 13C NMR and HRMS.
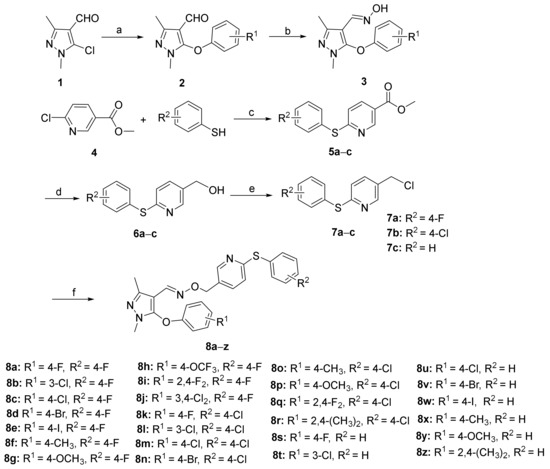
Scheme 1.
Synthesis of the designed compounds 8a–z. Reagents and conditions: (a) substituted phenol, KOH, DMF, 50 °C, 3–4 h, 110 °C, 5–8 h, 49–73%; (b) NH2OH·HCl, KOH, CH3OH, reflux, 3–4 h, 55–69%; (c) substituted thiophenol, K2CO3, DMF, 55 °C, 3–4 h, 110 °C, 5–6 h, 66–71%; (d) LiAlH4, THF, 0 °C, 2–3 h, 33–42%; (e) SOCl2, CH2Cl2, 0 °C, 2–3 h, 71–78%; (f) compound 3, K2CO3, DMF, 55 °C, 4–6 h, 43–73%.
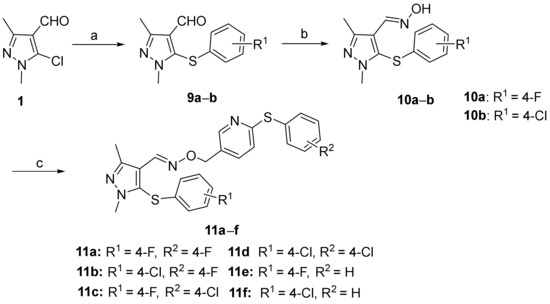
Scheme 2.
Synthesis of the designed compounds 11a–f. Reagents and conditions: (a) substituted thiophenol, KOH, DMF, H2O, 55 °C, 2–3 h, 100 °C, 6–8 h, 47–53%; (b) NH2OH·HCl, KOH, CH3OH, r.t., 10 min, reflux, 3 h, 62–65%; (c) compounds 7a–c, K2CO3, DMF, 55 °C, 5–6 h, 42–74%.
2.2. Biological Activities
The designed compounds 8a–z and 11a–f were screened for insecticidal activities towards Mythimna separata, Tetranychus cinnabarinus, Plutella xylostella, Aphis medicaginis and Nilaparvata lugens, and Tolfenpyrad, Fenpyroximate, Pyridalyl, Imidacloprid, and Abamectin were selected as reference drugs, respectively. As depicted in Table 1, most of the desired compounds had satisfactory insecticidal properties against M. separata at the dosage of 500 μg/mL, and the larvicidal effects of compounds 8a–f, 8h, 8j, 8l, 8n and 8s–z against M. separata were all 100%, respectively, which were comparable to that of Tolfenpyrad (100%). At 100 μg/mL, some targeted compounds also demonstrated interesting insecticidal activities against M. separata, for example, compounds 8a, 8b, 8d, 8n, 8s, 8t, 8u, 8v, 8w, 8x, and 8z owned 100%, 70%, 100%, 60%, 100%, 60%, 70%, 100%, 50%, 60%, and 50% inhibition rates, which were equal to or higher than Tolfenpyrad (50%). Even when the dosage reached 20 μg/mL, compound 8s still possessed 50% insecticidal effect towards M. separata, which was better than Tolfenpyrad (40%). Among the aryloxy derivatives, when R2 was 4-fluoro, the substituents (R1) on the phenyl ring were 4-fluoro (8a) and 4-bromo (8d), and it was more helpful to improve insecticidal properties towards M. separata at 100 μg/mL than other substituents; when R2 was 4-chloro, compound 8n (R1 = 4-Br) had better insecticidal activity against M. separata than compounds 8k–m and 8o–r at 100 μg/mL; and when R2 was H, the substituents (R1) on the phenyl ring were 4-fluoro (8s), 4-chloro (8u) and 4-bromo (8v), and it was more favorable to increase larvicidal activities towards M. separata at 100 μg/mL than other substituents. Among the arylthio derivatives, the mortalities of compounds 11d (R1 = 4-Cl, R2 = 4-Cl) and 11e (R1 = 4-F, R2 = H) against M. Separata were 80% and 70% at 500 μg/mL, which were much higher than those of 11a–c and 11f. From the list illustrated in Table 1, we found that most title compounds displayed wonderful inhibitory effects towards T. cinnabarinus and P. xylostella at 500 μg/mL, and compounds 8a, 8c, 8i, 8k, and 8m demonstrated 80% to 100% inhibition rates towards T. cinnabarinus at the dosage of 100 μg/mL, which were near to that of Fenpyroximate. Table 2 showed that most of the prepared compounds possessed excellent insecticidal properties against A. medicaginis at 500 μg/mL, and some of them still had 80% to 100% mortalities against A. medicaginis at a lower dosage of 100 μg/mL. Even at 20 μg/mL, some compounds were active towards A. medicaginis, with the inhibition rates of compounds 8a, 8c–f, 8o, 8v, 8x, and 8z, especially, being 50% to 70%. From the structure–insecticidal activity data (Table 2), we can see that at a dosage of 500 μg/mL, the aryloxy derivatives 8a-z exhibited much better insecticidal effects against A. medicaginis than corresponding arylthio analogues 11a-f. Among the aryloxy derivatives, when R2 is 4-fluoro, the substituents (R1) on the phenyl ring were 4-fluoro (8a), 4-chloro (8c) and 4-methyl (8f), it was more beneficial to ameliorate inhibitory activity towards A. medicaginis at 20 μg/mL than corresponding analogues 8k (R1 = 4-F, R2 = 4-Cl), 8s (R1 = 4-F, R2 = H), 8m (R1 = 4-Cl, R2 = 4-Cl), 8u (R1 = 4-Cl, R2 = H), 8o (R1 = 4-CH3, R2 = 4-Cl), and 8x (R1 = 4-CH3, R2 = H). Additionally, some of these compounds represented perfect inhibition effects against N. lugens at 500 μg/mL, for instance, compounds 8a, 8c–i, 8s, 8u, 8w, 8x and 8z held 100%, 100%, 100%, 100%, 100%, 100%, 100%, 80%, 100%, 100%, 90%, 100%, and 80% mortalities against N. lugens, which were equal to that of Abamectin. The data provided in Table 1 and Table 2 also indicated that compounds 8a (R1 = 4-F, R2 = 4-F), 8c (R1 = 4-Cl, R2 = 4-F), 8d (R1 = 4-Br, R2 = 4-F), 8s (R1 = 4-F, R2 = H), 8v (R1 = 4-Br, R2 = H), 8x (R1 = 4-CH3, R2 = H), and 8z (R1 = 2,4-(CH3)2, R2 = H) showed extensive insecticidal properties towards the tested pests, such as M. separata, T. cinnabarinus, P. xylostella, A. medicaginis, and N. lugens, which might be chosen as potential insecticidal agent candidates for deeper structural optimization and bioactivity research. All the aforementioned data suggested that the biological activity spectra of pyrazole oxime ether derivatives were vitally improved through importing the significant pyridine unit.

Table 1.
Insecticidal activities of title compounds 8a–z and 11a–f (mortality, %).

Table 2.
Insecticidal activities of target compounds 8a–z and 11a–f (mortality, %).
3. Materials and Methods
3.1. Chemistry
All chemical agents were chemically pure and treated by standard methods. 1H NMR, and 13C NMR spectra were screened through a Bruker AV400 spectrometer (Bruker, Billerica, MA, USA) in the solvent of CDCl3, and TMS was used as the internal reference. High-resolution mass spectra (HRMS) were collected by a Waters Xevo G2-XS Q-TOF mass spectrometer (Waters, Milford, CT, USA). The melting point was tested through an X-4 binocular microscope melting point detector (Beijing Tech Instrument Co., Beijing, China) and uncorrected. Intermediates 1–3 were prepared by reported procedures [30].
3.1.1. General Approach to Preparation of 5a–c
To a well-stirred solution of substituted thiophenol (55 mmol) in DMF (80 mL), potassium carbonate was added (110 mmol, 15.18 g) at room temperature, and the mixture was kept at 55 °C for another 3–4 h. Next, compound 4 (36 mmol, 6.18 g) was added thereto. The reaction mixture was heated to 110 °C for 5–6 h. After cooling, the solution was poured into ice water (100 mL). The generating precipitates were filtrated to obtain intermediates 5a–c.
Methyl 6-(4-fluorophenylthio)nicotinate (5a): colorless solid, yield 66%, m.p. 102–104 °C; 1H NMR (400 MHz, CDCl3) δ 8.96 (s, 1H, Py-H), 8.00 (d, J = 8.40 Hz, 1H, Py-H), 7.66–7.54 (m, 2H, Ar-H), 7.15 (t, J = 8.60 Hz, 2H, Ar-H), 6.85 (d, J = 8.40 Hz, 1H, Py-H), 3.89 (s, 3H, OCH3); 13C NMR (101 MHz, CDCl3) δ 167.18, 165.61, 162.51, 150.81, 137.72, 131.21, 124.64, 122.10, 119.61, 117.30, 52.33. HRMS calcd for C13H11FNO2S [M + H]+ 264.0495, found 246.0499.
Methyl 6-(4-chlorophenylthio)nicotinate (5b): colorless solid, yield 71%, m.p. 97–99 °C; 1H NMR (400 MHz, CDCl3) δ 8.97 (s, 1H, Py-H), 8.02 (d, J = 8.50 Hz, 1H, Py-H), 7.53 (m, 2H, Ar-H), 7.15 (t, J = 8.60 Hz, 2H, Ar-H), 6.85 (d, J = 8.40 Hz, 1H, Py-H), 3.89 (s, 3H, OCH3); 13C NMR (101 MHz, CDCl3) δ 166.53, 165.57, 150.87, 137.43, 136.69, 136.23, 130.12, 127.92, 122.27, 119.93, 52.63. HRMS calcd for C13H11ClNO2S [M + H]+ 280.0199, found 280.0203.
Methyl 6-(phenylthio)nicotinate (5c): colorless solid, yield 69%, m.p. 72–74 °C; 1H NMR (400 MHz, CDCl3) δ 8.98 (d, J = 2.20 Hz, 1H, Py-H), 8.00–7.98 (m, 1H, Py-H), 7.63–7.59 (m, 2H, Ar-H), 7.51–7.40 (m, 3H, Ar-H), 6.84 (d, J = 8.50 Hz, 1H, Py-H), 3.89 (s, 3H, OCH3); 13C NMR (101 MHz, CDCl3) δ 167.69, 165.67, 150.76, 137.36, 135.53, 129.97, 129.91, 129.40, 121.93, 119.74, 52.32. HRMS calcd for C13H12NO2S [M + H]+ 246.0589, found 246.0590.
3.1.2. General Approach to Preparation of 6a–c
To a cold (0 °C) solution of compound 5 (30 mmol) in THF (50 mL), LiAlH4 (60 mmol, 2.28 g) was added in portions, and the mixture was kept at 0 °C for another 2–3 h. When the reaction was achieved, ice water (2 mL) was added dropwise slowly thereto. After filtration, the filter liquor was evaporated under reduced pressure to provide a residue, which was further purified through silica gel column chromatography, selecting a mixture of ethyl acetate/petroleum ether (1:2, v/v) as the eluent to give intermediates 6a–c.
[6-(4-Fluorophenylthio)pyridin-3-yl]methanol (6a): yellow oil, yield 37%; 1H NMR (400 MHz, CDCl3) δ 8.24 (s, 1H, Py-H), 7.60–7.40 (m, 3H, Py-H and Ar-H), 7.07 (t, J = 8.70 Hz, 2H, Ar-H), 6.77 (d, J = 8.20 Hz, 1H, Py-H), 4.55 (s, 2H, CH2); 13C NMR (101 MHz, CDCl3) δ 164.61, 162.12, 159.95, 148.02, 137.18, 136.34, 133.47, 125.97, 121.08, 117.07, 61.54. HRMS calcd for C12H11FNOS [M + H]+ 236.0545, found 236.0550.
[6-(4-Chlorophenylthio)pyridin-3-yl]methanol (6b): yellow oil, yield 42%; 1H NMR (400 MHz, CDCl3) δ 8.30 (d, J = 2.30 Hz, 1H, Py-H), 7.50 (d, J = 8.30 Hz, 1H, Py-H), 7.42 (d, J = 8.50 Hz, 2H, Ar-H), 7.33 (d, J = 8.50 Hz, 2H, Ar-H), 6.87 (d, J = 8.20 Hz, 1H, Py-H), 5.44 (s, 1H, OH), 4.57 (s, 2H, CH2); 13C NMR (101 MHz, CDCl3) δ 159.00, 147.80, 136.68, 135.85, 135.49, 133.93, 129.96, 121.91, 61.59. HRMS calcd for C12H11ClNOS [M + H]+ 252.0250, found 252.0255.
[6-(Phenylthio)pyridin-3-yl]methanol (6c): yellow oil, yield 33%; 1H NMR (400 MHz, CDCl3) δ 8.28 (d, J = 2.20 Hz, 1H, Py-H), 7.53–7.51 (m, 2H, Py-H and Ar-H), 7.48–7.45 (m, 1H, Ar-H), 7.43–7.34 (m, 3H, Ar-H), 6.83 (d, J = 8.20 Hz, 1H, Py-H), 4.57 (s, 2H, CH2), 3.74 (s, 1H, OH); 13C NMR (101 MHz, CDCl3) δ 160.26, 148.11, 136.29, 134.75, 133.27, 131.00, 129.75, 129.18, 121.52, 61.81.HRMS calcd for C12H12NOS [M + H]+ 218.0640, found 218.0643.
3.1.3. General Approach to Preparation of 7a–c
To a cold (0 °C) solution of compound 6 (30 mmol) in CH2Cl2 (60 mL), thionyl chloride was added dropwise (60 mmol, 7.14 g), and then the mixture was stirred at 0 °C for an additional 2–3 h. After the reaction was achieved, 20 mL of water was added slowly thereto. The resulting solution was extracted with CH2Cl2 (3 × 30 mL). The collected extracts were dried on anhydrous sodium sulfate, filtered, and condensed to obtain the key intermediates 7a–c.
5-Chloromethyl-2-(4-fluorophenylthio)pyridine (7a): yellow oil, yield 73%; 1H NMR (400 MHz, CDCl3) δ 8.40 (s, 1H, Py-H), 7.64–7.54 (m, 2H, Ar-H), 7.52 (d, J = 8.30 Hz, 1H, Py-H), 7.14 (t, J = 8.60 Hz, 2H, Ar-H), 6.86 (d, J = 8.30 Hz, 1H, Py-H), 4.52 (s, 2H, CH2); 13C NMR (101 MHz, CDCl3) δ 164.79, 162.30, 149.24, 137.55, 129.54, 125.48, 120.80, 117.13, 42.94. HRMS calcd for C12H10ClFNS [M + H]+ 254.0207, found 254.0212.
5-Chloromethyl-2-(4-chlorophenylthio)pyridine (7b): yellow oil, yield 78%; 1H NMR (400 MHz, CDCl3) δ 8.39 (s, 1H, Py-H), 7.59–7.45 (m, 3H, Py-H and Ar-H), 7.37 (d, J = 8.40 Hz, 2H, Ar-H), 6.91 (d, J = 8.30 Hz, 1H, Py-H), 4.50 (s, 2H, CH2); 13C NMR (101 MHz, CDCl3) δ 161.03, 149.37, 137.20, 136.28, 135.64, 129.93, 121.28, 42.94. HRMS calcd for C12H10Cl2NS [M + H]+ 269.9911, found 269.9915.
5-Chloromethyl-2-(phenylthio)pyridine (7c): colorless oil, yield 71%; 1H NMR (400 MHz, CDCl3) δ 8.38 (d, J = 2.40 Hz, 1H, Py-H), 7.58–7.56 (m, 2H, Py-H and Ar-H), 7.47–7.40 (m, 4H, Ar-H), 6.84 (d, J = 8.30 Hz, 1H, Py-H), 4.49 (s, 2H, CH2); 13C NMR (101 MHz, CDCl3) δ 162.08, 149.26, 137.13, 135.16, 130.44, 129.81, 129.45, 129.43, 121.04, 43.06. HRMS calcd for C12H11ClNS [M + H]+ 236.0301, found 236.0305.
3.1.4. General Approach to Preparation of Target Compounds 8a–z
To a solution of compound 3 (11 mmol), potassium carbonate (20 mmol, 2.76 g) in DMF (30 mL), compound 7 (10 mmol) was added, and then the mixture was stirred at 55 °C for 4–6 h. After the reaction was completed, 30 mL of water was added thereto. Next, the mixture was extracted with CH2Cl2 (3 × 20 mL). The gathered extracts were further dried on anhydrous sodium sulfate, filtered, and concentrated to generate the crude product, which was then separated by silica gel column chromatography, choosing a mixture of ethyl acetate/petroleum ether (1:4, v/v) as the eluent to afford the targeted compounds 8a–z successfully, and the corresponding spectral data of compounds 8a–z are illustrated below. 1H NMR and 13C NMR spectra are presented in Supplementary Materials.
5-(4-Fluorophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-fluorophenylthio)pyridin-3-yl]methyl}oxime (8a): yellow oil, yield 61%; 1H NMR (400 MHz, CDCl3) δ 8.29 (d, J = 2.30 Hz, 1H, Py-H), 7.71 (s, 1H, CH=N), 7.53–7.50 (m, 2H, Ar-H), 7.33–7.31 (m, 1H, Py-H), 7.06 (t, J = 8.60 Hz, 2H, Ar-H), 6.94–6.90 (m, 2H, Ar-H), 6.80–6.73 (m, 3H, Py-H and Ar-H), 4.85 (s, 2H, CH2), 3.55 (s, 3H, CH3), 2.28 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 164.59, 162.11, 160.83, 159.86, 157.45, 152.57, 149.74, 147.64, 146.92, 140.98, 137.36, 129.58, 125.96, 120.50, 116.95, 116.59, 116.36, 99.83, 72.81, 34.18, 14.62. HRMS calcd for C24H21F2N4O2S [M + H]+ 467.1353, found 467.1352.
5-(3-Chlorophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-fluorophenylthio)-pyridin-3-yl]methyl}oxime (8b): yellow oil, yield 62%; 1H NMR (400 MHz, CDCl3) δ 8.30 (d, J = 2.30 Hz, 1H, Py-H), 7.74 (s, 1H, CH=N), 7.56–7.53 (m, 2H,Ar-H), 7.33–7.31 (m, 1H, Py-H), 7.18 (t, J = 8.20 Hz, 1H, Ar-H), 7.09 (t, J = 8.60 Hz, 2H, Ar-H), 7.04–7.01 (m, 1H, Ar-H), 6.84 (s, 1H,Py-H), 6.77–6.72 (m, 2H, Ar-H), 4.86 (s, 2H, CH2), 3.57 (s, 3H, CH3), 2.30 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 164.64, 162.15, 160.85, 157.07, 149.69, 147.05, 146.78, 140.83, 137.31, 135.39, 130.80, 129.56, 125.99, 123.94, 120.56, 116.99, 116.78, 115.82, 113.53, 100.14, 72.87, 34.28, 14.54. HRMS calcd for C24H21ClFN4O2S [M + H]+ 483.1058, found 483.1062.
5-(4-Chlorophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-fluorophenylthio)-pyridin-3-yl]methyl}oxime (8c): yellow oil, yield 59%; 1H NMR (400 MHz, CDCl3) δ 8.34 (d, J = 2.20 Hz, 1H, Py-H), 7.75 (s, 1H, CH=N), 7.60–7.56 (m, 2H,Ar-H), 7.35–7.33 (m, 1H, Py-H), 7.27–7.23 (m, 2H, Ar-H), 7.13 (t, J = 8.60 Hz, 2H, Ar-H), 6.81–6.79 (m, 3H, Py-H and Ar-H), 4.89 (s, 2H, CH2), 3.59 (s, 3H, CH3), 2.32 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 164.71, 162.22, 160.90, 155.17, 149.62, 147.07, 140.95, 137.41, 129.93, 129.61, 128.75, 125.89, 120.65, 117.06, 116.84, 116.57, 100.01, 72.88, 34.27, 14.59. HRMS calcd for C24H21ClFN4O2S [M + H]+ 483.1058, found 483.1062.
5-(4-Bromophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-fluorophenylthio)-pyridin-3-yl]methyl}oxime (8d): yellow oil, yield 66%; 1H NMR (400 MHz, CDCl3) δ 8.33 (d, J = 2.20 Hz, 1H, Py-H), 7.74 (s, 1H, CH=N), 7.58–7.54 (m, 2H, Ar-H), 7.37–7.32 (m, 3H, Ar-H and Py-H), 7.11 (t, J = 8.60 Hz, 2H, Ar-H), 6.80 (d, J = 8.20 Hz, 1H, Py-H), 6.74 (d, J = 9.00 Hz, 2H, Ar-H), 4.88 (s, 2H, CH2), 3.58 (s, 3H, CH3), 2.31 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 164.66, 162.18, 160.90, 155.74, 149.76, 147.08, 140.89, 137.33, 132.88, 129.57, 126.02, 120.64, 117.04, 116.79, 116.14, 100.03, 72.92, 34.27, 14.58. HRMS calcd for C24H21BrFN4O2S [M + H]+ 527.0553, found 527.0558.
5-(4-Iodophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-fluorophenylthio)-pyridin-3-yl]methyl}oxime (8e): yellow oil, yield 53%; 1H NMR (400 MHz, CDCl3) δ 8.34 (d, J = 2.30 Hz, 1H, Py-H), 7.73 (s, 1H, CH=N), 7.57 (d, J = 2.90 Hz, 2H, Ar-H), 7.56 (d, J = 2.40 Hz, 2H, Ar-H), 7.34 (d, J = 8.20 Hz, 1H, Py-H), 7.11 (t, J = 8.50 Hz, 2H, Ar-H), 6.81 (d, J = 8.30 Hz, 1H, Py-H), 6.63 (d, J = 8.40 Hz, 2H, Ar-H), 4.87 (s, 2H, CH2), 3.57 (s, 3H, CH3), 2.31 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 164.65, 162.17, 160.88, 156.60, 149.79, 147.04, 140.87, 138.85, 137.36, 129.56, 126.04, 120.68, 117.51, 117.02, 116.80, 100.06, 86.47, 72.93, 34.27, 14.61. HRMS calcd for C24H21FIN4O2S [M + H]+ 575.0414, found 575.0417.
5-(4-Methylphenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-fluorophenylthio)- pyridin-3-yl]methyl}oxime (8f): yellow oil, yield 52%; 1H NMR (400 MHz, CDCl3) δ 8.34 (d, J = 2.30 Hz, 1H, Py-H), 7.74 (s, 1H, CH=N), 7.57–7.54 (m, 2H,Ar-H), 7.38–7.35 (m, 1H, Py-H), 7.11–7.05 (m, 4H, Ar-H), 6.80–6.73 (m, 3H, Py-H and Ar-H), 4.90 (s, 2H, CH2), 3.57 (s, 3H, CH3), 2.32 (s, 3H, CH3), 2.28 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 164.64, 162.15, 160.76, 154.68, 149.81, 148.14, 146.82, 141.35, 137.31, 133.19, 130.40, 129.71, 126.12, 120.63, 116.97, 116.76, 115.08, 99.90, 72.83, 34.20, 20.58, 14.88. HRMS calcd for C25H24FN4O2S [M + H]+ 463.1604, found 463.1606.
5-(4-Methoxyphenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-fluorophenyl- thio) pyridin-3-yl]methyl}oxime (8g): yellow oil, yield 68%; 1H NMR (400 MHz, CDCl3) δ 8.33 (d, J = 2.30 Hz, 1H, Py-H), 7.71 (s, 1H, CH=N), 7.56–7.52 (m, 2H, Ar-H), 7.38–7.35 (m, 1H, Py-H), 7.09 (t, J = 8.60 Hz, 2H, Ar-H), 6.78 (s, 5H, Py-H and Ar-H), 4.89 (s, 2H, CH2), 3.73 (s, 3H, OCH3), 3.56 (s, 3H, CH3), 2.29 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 164.61, 162.13, 160.80, 155.74, 150.59, 149.84, 148.47, 146.82, 141.34, 137.33, 129.67, 126.05, 120.58, 116.98, 116.76, 116.31, 114.90, 99.64, 72.82, 55.67, 34.20, 14.91. HRMS calcd for C25H24FN4O3S [M + H]+ 479.1553, found 479.1559.
5-(4-Trifluoromethoxyphenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-fluoro-phenylthio) pyridin-3-yl]methyl}oxime (8h): yellow oil, yield 54%; 1H NMR (400 MHz, CDCl3) δ 8.33 (d, J = 2.20 Hz, 1H, Py-H), 7.75 (s, 1H, CH=N), 7.57–7.54 (m, 2H, Ar-H), 7.36–7.33 (m, 1H, Py-H), 7.15–7.10 (m, 4H, Ar-H), 6.87 (d, J = 9.10 Hz, 2H, Ar-H), 6.79 (d, J = 8.20 Hz, 1H, Py-H), 4.86 (s, 2H, CH2), 3.59 (s, 3H, CH3), 2.31 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 164.66, 162.18, 160.96, 154.91, 149.79, 147.08, 144.74, 140.83, 137.27, 129.49, 125.97, 122.87, 121.69, 120.56, 119.14, 116.98, 116.77, 116.31, 100.03, 72.91, 34.27, 14.56. HRMS calcd for C25H21F4N4O3S [M + H]+ 533.1270, found 533.1274.
5-(2,4-Difluorophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-fluorophenyl-thio) pyridin-3-yl]methyl}oxime (8i): yellow oil, yield 64%; 1H NMR (400 MHz, CDCl3) δ 8.30 (d, J = 2.30 Hz, 1H, Py-H), 7.72 (s, 1H, CH=N), 7.56–7.53 (m, 2H, Ar-H), 7.36–7.33 (m, 1H, Py-H), 7.09 (t, J = 8.60 Hz, 2H, Ar-H), 6.91–6.86 (m, 1H, Ar-H), 6.77 (d, J = 8.30 Hz, 1H, Py-H), 6.71–6.68 (m, 2H, Ar-H), 4.86 (s, 2H, CH2), 3.62 (s, 3H, CH3), 2.27 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 164.63, 162.14, 160.90, 159.44, 156.99, 153.04, 150.54, 149.64, 147.05, 140.66, 137.39, 137.14, 129.54, 125.96, 120.51, 116.98, 116.76, 111.20, 105.81, 99.43, 72.84, 34.21, 14.37. HRMS calcd for C24H20F3N4O2S [M + H]+ 485.1259, found 485.1262.
5-(3,4-Dichlorophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-fluorophenylthio) pyridin-3-yl]methyl}oxime (8j): yellow oil, yield 61%; 1H NMR (400 MHz, CDCl3) δ 8.32 (d, J = 2.20 Hz, 1H, Py-H), 7.75 (s, 1H, CH=N), 7.57–7.54 (m, 2H, Ar-H), 7.33–7.29 (m, 2H, Py-H and Ar-H), 7.10 (t, J = 8.60 Hz, 2H, Ar-H), 6.96 (d, J = 2.90 Hz, 1H, Ar-H), 6.79 (d, J = 8.20 Hz, 1H, Py-H), 6.74–6.71 (m, 1H, Ar-H), 4.87 (s, 2H, CH2), 3.58 (s, 3H, CH3), 2.31 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 164.65, 162.17, 160.95, 155.32, 149.71, 147.18, 146.44, 140.63, 137.29, 133.59, 131.22, 129.49, 127.30, 126.00, 120.57, 117.43, 116.98, 116.76, 114.93, 100.14, 72.96, 34.31, 14.37. HRMS calcd for C24H20Cl2FN4O2S [M + H]+ 517.0668, found 517.0673.
5-(4-Fluorophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-chlorophenylthio)-pyridin-3-yl]methyl}oxime (8k): yellow oil, yield 57%; 1H NMR (400 MHz, CDCl3) δ 8.33 (d, J = 2.20 Hz, 1H, Py-H), 7.73 (s, 1H, CH=N), 7.47 (d, J = 8.40 Hz, 2H, Ar-H), 7.38–7.34 (m, 3H, Py-H and Ar-H), 6.97–6.92 (m, 2H, Ar-H), 6.82–6.78 (m, 3H, Py-H and Ar-H), 4.88 (s, 2H, CH2), 3.57 (s, 3H, CH3), 2.30 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 160.09, 157.49, 152.59, 149.85, 147.69, 146.98, 141.05, 137.33, 136.08, 135.38, 129.83, 129.50, 121.11, 116.62, 116.55, 116.47, 116.39, 99.83, 72.82, 34.24, 14.69. HRMS calcd for C24H21ClFN4O2S [M + H]+ 483.1058, found 483.1062.
5-(3-Chlorophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-chlorophenylthio)-pyridin-3-yl]methyl}oxime (8l): yellow oil, yield 53%; 1H NMR (400 MHz, CDCl3) δ 8.30 (d, J = 2.30 Hz, 1H, Py-H), 7.74 (s, 1H, CH=N), 7.47 (d, J = 8.50 Hz, 2H, Ar-H), 7.36–7.32 (m, 3H, Py-H and Ar-H), 7.18 (t, J = 8.20 Hz, 1H, Ar-H), 7.02 (d, J = 8.80 Hz, 1H, Ar-H), 6.85–6.82 (m, 3H, Py-H and Ar-H), 4.87 (s, 2H, CH2), 3.56 (s, 3H, CH3), 2.30 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 160.03, 157.07, 149.81, 147.03, 146.78, 140.85, 137.29, 136.07, 135.39, 130.81, 129.81, 123.95, 121.12, 115.84, 113.53, 100.14, 72.85, 34.29, 14.55. HRMS calcd for C24H21Cl2N4O2S [M + H]+ 499.0762, found 499.0766.
5-(4-Chlorophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-chlorophenylthio)-pyridin-3-yl]methyl}oxime (8m): yellow oil, yield 48%; 1H NMR (400 MHz, CDCl3) δ 8.32 (d, J = 2.30 Hz, 1H, Py-H), 7.73 (s, 1H, CH=N), 7.46 (d, J = 8.50 Hz, 2H, Ar-H), 7.35–7.31 (m, 3H, Py-H and Ar-H), 7.20 (d, J = 9.00 Hz, 2H, Ar-H), 6.84 (d, J = 8.30 Hz, 1H, Py-H), 6.77 (d, J = 9.00 Hz, 2H, Ar-H), 4.86 (s, 2H, CH2), 3.55 (s, 3H, CH3), 2.29 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 160.08, 155.16, 149.87, 147.13, 146.99, 140.91, 137.33, 136.06, 135.34, 129.92, 129.86, 129.81, 129.54, 128.71, 121.13, 116.58, 100.00, 72.86, 34.26, 14.61. HRMS calcd for C24H21Cl2N4O2S [M + H]+ 499.0762, found 499.0768.
5-(4-Bromophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-chlorophenylthio)-pyridin-3-yl]methyl}oxime (8n): yellow oil, yield 48%; 1H NMR (400 MHz, CDCl3) δ 8.31 (d, J = 2.30 Hz, 1H, Py-H), 7.72 (s, 1H, CH=N), 7.45 (d, J = 8.20 Hz, 2H, Ar-H), 7.35–7.31 (m, 5H, Py-H and Ar-H), 6.85 (d, J = 8.20 Hz, 1H, Py-H), 6.72 (d, J = 8.50 Hz, 2H, Ar-H), 4.86 (s, 2H, CH2), 3.55 (s, 3H, CH3), 2.29 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 160.02, 155.72, 149.89, 147.03, 146.97, 140.88, 137.29, 136.02, 135.30, 132.87, 129.87, 129.79, 129.60, 121.17, 117.05, 116.13, 100.02, 72.87, 34.27, 14.60. HRMS calcd for C24H21BrClN4O2S [M + H]+ 543.0257, found 543.0256.
5-(4-Methylphenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-chlorophenylthio)pyridin-3-yl]methyl}oxime (8o): yellow oil, yield 73%; 1H NMR (400 MHz, CDCl3) δ 8.35 (d, J = 2.30 Hz, 1H, Py-H), 7.74 (s, 1H, CH=N), 7.48 (d, J = 8.10 Hz, 2H, Ar-H), 7.36 (t, J = 8.20 Hz, 3H, Py-H and Ar-H), 7.06 (d, J = 8.10 Hz, 2H, Ar-H), 6.86 (d, J = 8.20 Hz, 1H, Py-H), 6.74 (d, J = 8.20 Hz, 2H, Ar-H), 4.91 (s, 2H, CH2), 3.57 (s, 3H, CH3), 2.32 (s, 3H, CH3), 2.28 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 159.96, 154.67, 149.87, 148.13, 146.82, 141.39, 137.45, 136.00, 135.35, 133.20, 130.41, 130.04, 129.82, 129.63, 121.22, 115.07, 99.90, 72.80, 34.22, 20.60, 14.90. HRMS calcd for C25H24ClN4O2S [M + H]+ 479.1308, found 479.1313.
5-(4-Methoxyphenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-chlorophenyl-thio)pyridin-3-yl]methyl}oxime (8p): yellow oil, yield 47%; 1H NMR (400 MHz, CDCl3) δ 8.33 (d, J = 2.30 Hz, 1H, Py-H), 7.71 (s, 1H, CH=N), 7.46 (d, J = 8.50 Hz, 2H, Ar-H), 7.39–7.32 (m, 3H, Py-H and Ar-H), 6.84 (d, J = 8.20 Hz, 1H, Py-H), 6.77 (s, 4H, Ar-H), 4.89 (s, 2H, CH2), 3.72 (s, 3H, CH3), 3.55 (s, 3H, OCH3), 2.30 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 159.96, 155.73, 150.58, 149.92, 148.46, 146.79, 141.36, 137.40, 136.00, 135.29, 130.00, 129.80, 129.61, 121.16, 116.31, 114.89, 99.62, 72.78, 55.66, 34.21, 14.92. HRMS calcd for C25H24ClN4O3S [M + H]+ 495.1258, found 495.1262.
5-(2,4-Difluorophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-chlorophenyl-thio)pyridin-3-yl]methyl}oxime (8q): yellow oil, yield 61%; 1H NMR (400 MHz, CDCl3) δ 8.32 (d, J = 2.20 Hz, 1H, Py-H), 7.73 (s, 1H, CH=N), 7.49 (d, J = 8.40 Hz, 2H, Ar-H), 7.36 (d, J = 8.40 Hz, 3H, Py-H and Ar-H), 6.92–6.84 (m, 2H, Py-H and Ar-H), 6.71–6.69 (m, 2H, Ar-H), 4.87 (s, 2H, CH2), 3.63 (s, 3H, CH3), 2.28 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 160.14, 159.46, 157.02, 153.05, 150.55, 149.76, 147.08, 140.71, 137.19, 136.09, 135.39, 129.83, 129.51, 121.10, 117.34, 111.17, 110.98, 105.84, 105.36, 99.43, 72.84, 34.25, 14.42. HRMS calcd for C24H20ClF2N4O2S [M + H]+ 501.0964, found 501.0968.
5-(2,4-Dimethylphenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-chlorophenyl-thio)pyridin-3-yl]methyl}oxime (8r): yellow oil, yield 48%; 1H NMR (400 MHz, CDCl3) δ 8.34 (d, J = 2.20 Hz, 1H, Py-H), 7.67 (s, 1H, CH=N), 7.47 (d, J = 8.50 Hz, 2H, Ar-H), 7.36–7.34 (m, 3H, Py-H and Ar-H), 6.99 (s, 1H, Ar-H), 6.85–6.81 (m, 2H, Py-H and Ar-H), 6.38 (d, J = 8.30 Hz, 1H, Ar-H), 4.89 (s, 2H, CH2), 3.56 (s, 3H, CH3), 2.32 (s, 3H, CH3), 2.30 (s, 3H, CH3), 2.23 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 159.96, 152.87, 149.87, 148.54, 146.82, 141.40, 137.39, 136.02, 135.33, 133.05, 132.22, 130.05, 129.81, 129.63, 127.51, 126.19, 121.18, 113.33, 99.51, 72.76, 34.11, 20.58, 16.04, 14.93. HRMS calcd for C26H26ClN4O2S [M + H]+ 493.1465, found 493.1470.
5-(4-Fluorophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(phenylthio)pyridin-3-yl]methyl}oxime (8s): yellow oil, yield 46%; 1H NMR (400 MHz, CDCl3) δ 8.33 (d, J = 2.20 Hz, 1H, Py-H), 7.72 (s, 1H, CH=N), 7.56–7.54 (m, 2H, Ar-H), 7.39–7.35 (m, 4H, Py-H and Ar-H), 6.96–6.92 (m, 2H, Ar-H), 6.81–6.77 (m, 3H, Py-H and Ar-H), 4.87 (s, 2H, CH2), 3.56 (s, 3H, CH3), 2.30 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 161.16, 159.90, 157.49, 152.59, 149.75, 147.69, 146.98, 141.00, 137.28, 134.99, 130.90, 129.69, 129.47, 129.20, 120.88, 116.63, 116.39, 99.87, 72.90, 34.23, 14.69. HRMS calcd for C24H22FN4O2S [M + H]+ 449.1447, found 449.1452.
5-(3-Chlorophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(phenylthio)pyridin-3-yl]methyl}oxime (8t): yellow oil, yield 53%; 1H NMR (400 MHz, CDCl3) δ 8.32 (d, J = 2.30 Hz, 1H, Py-H), 7.75 (s, 1H, CH=N), 7.58–7.55 (m, 2H, Ar-H), 7.40 (t, J = 2.80 Hz, 3H, Py-H and Ar-H), 7.32–7.29 (m, 1H, Ar-H), 7.18 (t, J = 8.20 Hz, 1H, Ar-H), 7.02 (d, J = 8.90 Hz, 1H, Ar-H), 6.85 (t, J = 2.20 Hz, 1H, Py-H), 6.81–6.69 (m, 2H, Ar-H), 4.87 (s, 2H, CH2), 3.57 (s, 3H, CH3), 2.31 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 161.14, 157.07, 149.69, 147.07, 146.79, 140.82, 137.24, 135.40, 135.01, 130.94, 130.82, 129.69, 129.45, 129.19, 123.96, 120.89, 115.83, 113.52, 100.17, 72.93, 34.29, 14.54. HRMS calcd for C24H22ClN4O2S [M + H]+ 465.1152, found 465.1155.
5-(4-Chlorophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(phenylthio)pyridin-3-yl]methyl}oxime (8u): yellow oil, yield 43%; 1H NMR (400 MHz, CDCl3) δ 8.31 (d, J = 2.30 Hz, 1H, Py-H), 7.74 (s, 1H, CH=N), 7.56–7.54 (m, 2H, Ar-H), 7.39–7.38 (m, 3H, Py-H and Ar-H), 7.31–7.28 (m, 1H, Ar-H), 7.17 (t, J = 8.20 Hz, 1H, Ar-H), 7.01 (d, J = 8.00 Hz, 1H, Ar-H), 6.84 (s, 1H, Py-H), 6.81–6.64 (m, 2H, Ar-H), 4.86 (s, 2H, CH2), 3.56 (s, 3H, CH3), 2.31 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 161.11, 157.07, 149.67, 147.04, 146.77, 140.81, 137.24, 135.39, 135.00, 130.92, 130.82, 129.69, 129.46, 129.19, 123.95, 120.88, 115.83, 113.52, 100.16, 72.91, 34.29, 14.55. HRMS calcd for C24H22ClN4O2S [M + H]+ 465.1152, found 465.1154.
5-(4-Bromophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(phenylthio)pyridin-3-yl]methyl}oxime (8v): yellow oil, yield 51%; 1H NMR (400 MHz, CDCl3) δ 8.35 (d, J = 2.30 Hz, 1H, Py-H), 7.74 (s, 1H, CH=N), 7.57 (d, J = 6.20 Hz, 2H, Ar-H), 7.40–7.30 (m, 6H, Py-H and Ar-H), 6.82 (d, J = 8.30 Hz, 1H, Py-H), 6.74 (d, J = 8.40 Hz, 2H, Ar-H), 4.87 (s, 2H, CH2), 3.57 (s, 3H, CH3), 2.31 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 161.15, 155.74, 149.75, 147.08, 147.04, 140.86, 137.30, 134.96, 132.89, 130.98, 129.69, 129.46, 129.17, 120.97, 117.05, 116.15, 100.05, 72.96, 34.27, 14.61. HRMS calcd for C24H22BrN4O2S [M + H]+ 509.0647, found 509.0650.
5-(4-Iodophenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(phenylthio)pyridin-3-yl]methyl}oxime (8w): yellow oil, yield 64%; 1H NMR (400 MHz, CDCl3) δ 8.36 (d, J = 2.30 Hz, 1H, Py-H), 7.74 (s, 1H, CH=N), 7.59–7.56 (m, 4H, Ar-H), 7.42–7.40 (m, 3H, Py-H and Ar-H), 7.34–7.31 (m, 1H, Ar-H), 6.84 (d, J = 8.20 Hz, 1H, Py-H), 6.63 (d, J = 8.80 Hz, 2H, Ar-H), 4.88 (s, 2H, CH2), 3.57 (s, 3H, CH3), 2.31 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 161.15, 156.60, 149.79, 147.05, 147.00, 140.85, 138.85, 137.31, 134.96, 131.01, 129.70, 129.45, 129.17, 121.02, 117.51, 100.08, 86.47, 72.99, 34.28, 14.62. HRMS calcd for C24H22IN4O2S [M + H]+ 557.0508, found 557.0511.
5-(4-Methylphenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(phenylthio)pyridin-3-yl]methyl}oxime (8x): yellow oil, yield 55%; 1H NMR (400 MHz, CDCl3) δ 8.35 (d, J = 2.20 Hz, 1H, Py-H), 7.74 (s, 1H, CH=N), 7.58–7.55 (m, 2H, Ar-H), 7.40–7.33 (m, 4H, Py-H and Ar-H), 7.06 (d, J = 8.30 Hz, 2H, Ar-H), 6.86–6.67 (m, 3H, Py-H and Ar-H), 4.90 (s, 2H, CH2), 3.56 (s, 3H, CH3), 2.32 (s, 3H, CH3), 2.27 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 161.03, 154.68, 149.80, 148.12, 146.82, 141.32, 137.33, 134.91, 133.19, 131.09, 130.41, 129.66, 129.61, 129.12, 120.96, 115.08, 99.92, 72.88, 34.21, 20.60, 14.88. HRMS calcd for C25H25N4O2S [M + H]+ 445.1698, found 445.1702.
5-(4-Methoxyphenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(phenylthio)- pyridin-3-yl]methyl}oxime (8y): yellow oil, yield 45%; 1H NMR (400 MHz, CDCl3) δ 8.35 (s, 1H, Py-H), 7.72 (s, 1H, CH=N), 7.57–7.55 (m, 2H, Ar-H), 7.40–7.35 (m, 4H, Py-H and Ar-H), 6.80 (s, 5H, Py-H and Ar-H), 4.90 (s, 2H, CH2), 3.74 (s, 3H, CH3), 3.57 (s, 3H, OCH3), 2.31 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 161.05, 155.76, 150.61, 149.80, 148.49, 146.85, 141.33, 137.33, 134.91, 131.04, 129.66, 129.60, 129.13, 120.97, 116.32, 114.91, 99.66, 72.88, 55.69, 14.89. HRMS calcd for C25H25N4O3S [M + H]+ 461.1647, found 461.1652.
5-(2,4-Dimethylphenoxy)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(phenylthio)- pyridin-3-yl]methyl}oxime (8z): yellow oil, yield 49%; 1H NMR (400 MHz, CDCl3) δ 8.35 (d, J = 2.20 Hz, 1H, Py-H), 7.67 (s, 1H, CH=N), 7.59–7.56 (m, 2H, Ar-H), 7.42–7.40 (m, 3H, Py-H and Ar-H), 7.34–7.32 (m, 1H, Ar-H), 7.00 (s, 1H, Ar-H), 6.84–6.79 (m, 2H, Py-H and Ar-H), 6.38 (d, J = 8.30 Hz, 1H, Ar-H), 4.90 (s, 2H, CH2), 3.57 (s, 3H, CH3), 2.31 (d, J = 7.50 Hz, 6H, 2 × CH3), 2.24 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 161.07, 152.87, 149.73, 148.54, 146.87, 141.38, 137.36, 134.96, 133.05, 132.21, 131.00, 129.70, 129.62, 129.18, 127.49, 126.20, 120.94, 113.31, 99.52, 72.84, 34.11, 20.57, 16.03, 14.92. HRMS calcd for C26H27N4O2S [M + H]+ 459.1855, found 459.1858.
3.1.5. General Approach to Preparation of 9a,b
To a mixture of potassium hydroxide (36 mmol, 2.02 g), water (2 mL) and DMF (50 mL), a solution of substituted thiophenol (30 mmol) in DMF (10 mL) was added dropwise at room temperature, and the mixture was stirred at 55 °C for 2–3 h. Then, compound 1 (20 mmol, 3.17 g) was added slowly thereto, and the reaction mixture was heated to 100 °C for 6–8 h. The reaction system was cooled and poured into ice water (150 mL). The resulting mixture was extracted with ethyl acetate (3 × 60 mL). The combined ethyl acetate layer was dried on anhydrous magnesium sulfate, filtered, and removed in vacuum to form intermediates 9a,b, which were directly used in the following step.
3.1.6. General Approach to Preparation of 10a,b
To a mixture of hydroxylamine hydrochloride (15 mmol) and CH3OH (40 mL), potassium hydroxide (20 mmol, 1.12 g) was added in portions, and the resulting solution was stirred at room temperature for 10 min. Next, compound 9 (10 mmol) was added slowly thereto, and the reaction system was refluxed for 3 h. After cooling, the mixture was dumped into ice water (100 mL), and the resulting solid was filtered to give intermediates 10a,b.
5-(4-Fluorophenylthio)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde oxime (10a): colorless solid, yield 62%, m.p. 182–184 °C; 1H NMR (400 MHz, CDCl3) δ 8.22 (s, 1H, OH), 7.97 (s, 1H, CH=N), 7.09–6.91 (m, 4H, Ar-H), 3.80 (s, 3H, CH3), 2.44 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 163.06, 160.60, 147.44, 144.15, 131.94, 129.45, 129.37, 129.25, 117.33, 116.62, 36.62, 14.48. HRMS calcd for C12H13FN3OS [M + H]+ 266.0763, found 266.0769.
5-(4-Chlorophenylthio)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde oxime (10b): colorless solid, yield 65%, m.p. 210–212 °C; 1H NMR (400 MHz, CDCl3) δ 8.18 (s, 1H, OH), 7.52 (s, 1H, CH=N), 7.23 (d, J = 8.50 Hz, 2H, Ar-H), 6.93 (d, J = 8.60 Hz, 2H, Ar-H), 3.79 (s, 3H, CH3), 2.44 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 147.60, 144.19, 131.89, 129.99, 129.66, 128.11, 117.57, 36.63, 14.51. HRMS calcd for C12H13ClN3OS, [M + H]+ 282.0468, found 282.0472.
3.1.7. General Approach to Preparation of Target Compounds 11a–f
To a mixture of potassium carbonate (20 mmol, 2.76 g), compound 10 (11 mmol) in DMF (30 mL), compound 7 (10 mmol) was added, and the generating solution was heated to 55 °C for 5–6 h. Subsequently, 30 mL of water was added slowly thereto. The formative mixture was extracted through dichloromethane (3 × 20 mL). The collected extracts were then dried on anhydrous sodium sulfate, filtered, and removed under reduced pressure to obtain the crude product, which was purified through silica gel column chromatography eluting with ethyl acetate/petroleum ether (1:4, v/v) to obtain the designed compounds 11a-f smoothly, and the spectral data of compounds 11a-f are depicted below. 1H NMR and 13CNMR spectra are listed in Supplementary Materials.
5-(4-Fluorophenthio)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-fluorophenylthio)pyridin-3-yl]methyl}oxime (11a): yellow oil, yield 64%; 1H NMR (400 MHz, CDCl3) δ 8.44 (d, J = 2.30 Hz, 1H, Py-H), 8.14 (s, 1H, CH=N), 7.59–7.56 (m, 2H, Ar-H), 7.52–7.49 (m, 1H, Py-H), 7.12 (t, J = 8.60 Hz, 2H, Ar-H), 7.00–6.92 (m, 4H, Ar-H), 6.84 (d, J = 8.20 Hz, 1H, Py-H), 5.04 (s, 2H, CH2), 3.76 (s, 3H, CH3), 2.39 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 164.68, 163.00, 162.19, 161.04, 160.54, 149.93, 147.60, 143.39, 137.37, 131.85, 129.57, 129.26, 126.00, 120.64, 117.19, 117.02, 116.80, 116.59, 73.10, 36.63, 14.70. HRMS calcd for C24H21F2N4OS2 [M + H]+ 483.1125, found 483.1129.
5-(4-Chlorophenthio)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-fluorophenylthio)pyridin-3-yl]methyl}oxime (11b): yellow oil, yield 59%; 1H NMR (400 MHz, CDCl3) δ 8.43 (s, 1H, Py-H), 8.10 (s, 1H, CH=N), 7.58–7.54 (m, 2H, Ar-H), 7.50–7.48 (m, 1H, Py-H), 7.19 (d, J = 8.40 Hz, 2H, Ar-H), 7.11 (t, J = 8.50 Hz, 2H, Ar-H), 6.88 (d, J = 8.30 Hz, 2H, Ar-H), 6.83 (d, J = 8.20 Hz, 1H, Py-H), 5.03 (s, 2H, CH2), 3.76 (s, 3H, CH3), 2.40 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 164.65, 162.17, 161.02, 149.96, 147.68, 143.22, 137.35, 133.03, 132.64, 130.89, 129.62, 128.07, 126.03, 120.64, 117.44, 117.00, 116.79, 73.12, 36.63, 14.70. HRMS calcd for C24H21ClFN4OS2 [M + H]+ 499.0829, found 499.0833.
5-(4-Fluorophenthio)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-chlorophenylthio)pyridin-3-yl]methyl}oxime (11c): yellow oil, yield 42%; 1H NMR (400 MHz, CDCl3) δ 8.45 (d, J = 2.30 Hz, 1H, Py-H), 8.14 (s, 1H, CH=N), 7.53–7.48 (m, 3H, Py-H and Ar-H), 7.37 (d, J = 8.50 Hz, 2H, Ar-H), 7.00–6.90 (m, 5H, Py-H and Ar-H), 5.04 (s, 2H, CH2), 3.76 (s, 3H, CH3), 2.39 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 163.00, 160.54, 160.23, 150.04, 147.59, 143.40, 137.46, 136.05, 135.40, 131.86, 129.89, 129.84, 129.55, 129.35, 129.27, 121.21, 117.17, 116.81, 116.59, 73.08, 36.63, 14.70. HRMS calcd for C24H21ClFN4OS2 [M + H]+ 499.0829, found 499.0834.
5-(4-Chlorophenthio)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(4-chlorophenylthio)pyridin-3-yl]methyl}oxime (11d): yellow oil, yield 55%; 1H NMR (400 MHz, CDCl3) δ 8.44 (d, J = 2.30 Hz, 1H, Py-H), 8.10 (s, 1H, CH=N), 7.52–7.48 (m, 3H, Py-H and Ar-H), 7.36 (d, J = 8.50 Hz, 2H, Ar-H), 7.19 (d, J = 8.60 Hz, 2H, Ar-H), 6.89 (t, J = 8.80 Hz, 3H, Py-H and Ar-H), 5.04 (s, 2H, CH2), 3.76 (s, 3H, CH3), 2.40 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 160.23, 150.04, 147.68, 143.25, 137.45, 136.04, 135.39, 133.02, 132.65, 131.08, 130.90, 129.97, 129.85, 129.84, 129.63, 129.56, 128.07, 121.21, 117.43, 73.10, 36.63, 14.71. HRMS calcd for C24H21Cl2N4OS2 [M + H]+ 515.0534, found 515.0536.
5-(4-Fluorophenthio)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(phenylthio)pyridin-3-yl]methyl}oxime (11e): yellow oil, yield 65%; 1H NMR (400 MHz, CDCl3) δ 8.45 (d, J = 2.30 Hz, 1H, Py-H), 8.14 (s, 1H, CH=N), 7.58–7.56 (m, 2H, Ar-H), 7.50–7.47 (m, 1H, Py-H), 7.41–7.39 (m, 3H, Ar-H), 7.00–6.91 (m, 4H, Ar-H), 6.85 (d, J = 8.30 Hz, 1H, Py-H), 5.04 (s, 2H, CH2), 3.76 (s, 3H, CH3), 2.39 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 162.99, 161.29, 160.53, 149.93, 147.59, 143.35, 137.40, 134.96, 131.83, 130.96, 129.69, 129.47, 129.35, 129.27, 129.18, 120.96, 117.21, 116.80, 116.58, 73.15, 36.62, 14.71. HRMS calcd for C24H22FN4OS2 [M + H]+ 465.1219, found 465.1221.
5-(4-Chlorophenthio)-1,3-dimethyl-1H-pyrazole-4-carbaldehyde O-{[6-(phenylthio)pyridin-3-yl]methyl}oxime (11f): yellow oil, yield 74%; 1H NMR (400 MHz, CDCl3) δ 8.45 (d, J = 2.30 Hz, 1H, Py-H), 8.10 (s, 1H, CH=N), 7.58–7.55 (m, 2H, Ar-H), 7.49–7.46 (m 1H, Py-H), 7.40–7.39 (m, 3H, Ar-H), 7.18 (d, J = 8.60 Hz, 2H, Ar-H), 6.89–6.84 (m, 3H, Py-H and Ar-H), 5.03 (s, 2H, CH2), 3.75 (s, 3H, CH3), 2.40 (s, 3H, CH3); 13C NMR (101 MHz, CDCl3) δ 161.28, 149.90, 147.68, 143.20, 137.41, 134.96, 133.04, 132.63, 130.95, 130.88, 129.69, 129.63, 129.45, 129.19, 128.08, 120.98, 117.46, 73.16, 36.63, 14.72. HRMS calcd for C24H22ClN4OS2 [M + H]+ 481.0924, found 481.0928.
3.2. Biological Assay
3.2.1. Insecticidal Activities against Mythimna separata and Plutella xylostella
The larvicidal properties of compounds 8a–z and 11a–f against Mythimna separata and Plutella xylostella were determined by a leaf-dipping method [31]. Firstly, the tested sample was dissolved in the solvent of DMF including 1% Tween-80 emulsifier, and diluted to the demanded dosages through water. Some corn leaves were fully immersed in the generated solutions for 3 s and then air-dried. Subsequently, the treated leaves were released into a culture plate containing filter paper, into which third-instar M. separata and second-instar P. xylostella larvae were inoculated. The above culture plate was put into a room for normal cultivation at 25 °C. Mortality was evaluated 48 h after treatment. The larvae who had no response to the touch of a writing brush were considered dead. Each assay was performed three times. Tolfenpyrad and Pyridalyl, as the controls, were studied under equal conditions.
3.2.2. Insecticidal Activities against Tetranychus cinnabarinus, Aphis medicaginis and Nilaparvata lugens
The insecticidal activities of compounds 8a–z and 11a–f towards Tetranychus cinnabarinus, Aphis medicaginis, and Nilaparvata lugens were researched with a spray approach [32]. Through the use of a Potter spray tower, broad bean leaves owning T. cinnabarinus or A. medicaginis, and oryza sativa seedlings possessing N. lugens, respectively, were sprayed with solutions of the examined compounds. After that, the above broad bean leaves or oryza sativa seedlings were placed in a room for general cultivation at 25 °C. Mortality was counted 48 h after spray treatment. Each experiment was executed three times. Fenpyroximate, Imidacloprid, and Abamectin were separately chosen as corresponding controls and investigated under similar conditions.
4. Conclusions
In conclusion, a series of novel pyridyl-containing pyrazole oxime ethers were synthesized and assessed for the insecticidal activities against five crop pests. The results displayed that the majority of the designed compounds exhibited favorable insecticidal properties towards M. separata, T. cinnabarinus, P. xylostella, and A. medicaginis at a dosage of 500 μg/mL. Some of the desired compounds demonstrated moderate to good insecticidal effects against M. separata and T. cinnabarinus when the dosage was reduced to 100 μg/mL, and some derivatives expressed satisfactory inhibitory activities against A. medicaginis at dosages of 100 μg/mL and 20 μg/mL, compounds 8a, 8c–f, 8o, 8v, 8x, and 8z, especially, still had 50% to 70% of their insecticidal activity against A. medicaginis at 20 μg/mL. In addition, a few derivatives also possessed wonderful insecticidal properties towards N. lugens at 500 μg/mL. Interestingly, compounds 8a, 8c, 8d, 8s, 8v, 8x, and 8z indicated broad-spectrum insecticidal properties towards all five pests, and they could be selected as promising insecticidal lead compounds for deeper structural modification and biological activity studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29122767/s1, Supplementary Materials (1H NMR and 13C NMR spectra of the target compounds 8a–z and 11a–f).
Author Contributions
H.D. designed the experiment; J.H., B.Z., X.W., Q.C., X.J., T.K., L.Y., Y.Z., R.C. and Y.X. accomplished the experiment and analyzed the data; H.D. wrote this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (No. 22177057).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zeun, R.; Scalliet, G.; Oostendorp, M. Biological activity of sedaxane-a novel broad-spectrum fungicide for seed treatment. Pest Manag. Sci. 2013, 69, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.B.; Sharkawi, S.M.Z.; El-Daly, M. Design, synthesis and novel isoindoline hybrids as COX-2 inhibitors: Anti-inflammatory, analgesic activities and docking study. Bioorg. Chem. 2018, 80, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, X.G.; Zhang, Q.; Fan, J.P.; Liu, L.; Liu, M.Z.; Zhang, H.J.; Li, J.; Guo, Y. Iodine-mediated oxidative cyclization for one pot synthesis of new 8-hydroxyquinaldine derivatives containing a N-phenylpyrazole as pesticidal agents. Bioorg. Med. Chem. Lett. 2018, 280, 3376–3380. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Subedi, L.; Lim, D.; Jung, J.K.; Kim, S.Y.; Seo, S.Y. Synthesis and anti-neuroinflammatory activity of N-heterocyclic analogs based on natural biphenyl-neolignanhonokiol. Bioorg. Med. Chem. Lett. 2019, 29, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.Y.; Zhao, B.; Fan, Z.J.; Yu, B.; Zhang, N.L.; Li, Z.M.; Zhu, Y.L.; Zhou, J.H.; Kalinina, T.A.; Glukhareva, T.V. Synthesisandbiologicalactivity of novel succinate dehydrogenase inhibitor derivatives as potent fungicide candidates. J. Agric. Food Chem. 2019, 67, 13185–13194. [Google Scholar] [CrossRef] [PubMed]
- Kagabu, S.; Murase, Y.; Imai, R.; Ito, N.; Nishimura, K. Effect of substituents at the 5-position of the pyridine ring of imidacloprid on insecticidal activity against Periplaneta americana. Pest Manag. Sci. 2007, 63, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Zhuang, Y.Y.; Wu, N.B.; Feng, Y.; Cheng, J.G.; Li, Z.; Chen, J.; Yuan, J.; Xu, X.Y. Synthesisandbiologicalevaluation of nitromethylene neonicotinoids based on enhanced conjugation. J. Agric. Food Chem. 2013, 61, 10858–10863. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.X.; Guo, S.X.; He, F.; Wang, H.Y.; Xu, F.Z.; Dai, A.L.; Zhang, R.F.; Wu, J. Novel anthranilic amide derivatives bearing the chiral thioether and trifluoromethylpyridine: Synthesis and bioactivity. Bioorg. Med. Chem. Lett. 2020, 30, 126902. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.T.; Du, X.H. Design, synthesis, and fungicidal activity of novel N-substituted piperazine-containing phenylpyridines against cucumber downy mildew. Pest Manag. Sci. 2022, 78, 1806–1814. [Google Scholar] [CrossRef]
- Cai, Z.F.; Zhang, W.L.; Cao, Y.Y.; Du, X.H. Synthesis and herbicidal activities of 2-phenylpyridinecompounds containing alkenylmoieties. J. Heterocycl. Chem. 2022, 59, 1247–1252. [Google Scholar] [CrossRef]
- Guo, S.X.; He, F.; He, F.; Dai, A.L.; Zhang, R.F.; Chen, S.H.; Wu, J. Synthesis and biological activities of novel trifluoromethylpyridine amide derivatives containing sulfur moieties. RSC Adv. 2020, 10, 35658–35670. [Google Scholar] [CrossRef] [PubMed]
- Mansour, B.; Bayoumi, W.A.; El-Sayed, M.A.; Abouzeid, L.A.; Massoud, M.A.M. In vitro cytotoxicity and docking study of novel symmetric and asymmetric dihydropyridines and pyridines as EGFR tyrosine kinase inhibitors. Chem. Biol. Drug Des. 2022, 100, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Muhammad, Z.A.; Abdel-aziz, M.R.; Abdel-aziz, H.M.; Gaber, H.M.; Elaasser, M.M. One-pot synthesis of new thiadiazolyl-pyridines as anticancer and antioxidant agents. J. Heterocycl. Chem. 2018, 55, 530–536. [Google Scholar] [CrossRef]
- Wang, B.L.; Zhu, H.W.; Li, Z.M.; Wang, L.Z.; Zhang, X.; Xiong, L.X.; Song, H.B. Synthesis, biological evaluation and SAR analysis of novel poly-heterocyclic compounds conaintingpyridylpyrazole group. Pest Manag. Sci. 2018, 74, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.Z.; Wang, Y.Y.; Luo, D.X.; Yu, G.; Guo, S.X.; Fu, H.; Zhao, Y.H.; Wu, J. Design, synthesis and insecticidal activity and 3D-QSR study for novel trifluoromethyl pyridine derivatives containing an 1,3,4-oxadiazole moiety. RSC Adv. 2018, 8, 6306–6314. [Google Scholar] [CrossRef] [PubMed]
- Khallaf, A.; Wang, P.; Zhuo, S.P.; Zhu, H.J.; Liu, H. Synthesis, insecticidal activities, and structure-activity relationships of 1,3,4-oxadiazole-ring-containing pyridylpyrazole-4-carboxamidesas novel insecticides of the anthranilic diamidefamily. J. Heterocycl. Chem. 2021, 58, 2189–2202. [Google Scholar] [CrossRef]
- Liu, Z.J.; Song, R.J.; Zhang, D.S.; Wu, R.; Liu, T.; Wu, Z.X.; Song, B.A. New synthetic method and insecticidal activities of novel imidazopyridine mesoionic derivatives containing an ester group. J. Agric. Food Chem. 2022, 70, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Song, R.J.; Zhang, D.S.; Wu, R.; Liu, T.; Wu, Z.X.; Zhang, J.; Hu, D.Y. Synthesis, and insecticidal activity, and mode of action of novel imidazopyridine mesoionic derivatives containing an amido group. Pest Manag. Sci. 2022, 78, 4983–4993. [Google Scholar] [CrossRef]
- Lan, S.L.; Xiao, T.; Guan, S.F.; Liu, A.P.; Long, C.Y.; Zhong, F.J.; Huang, Z.C.; Liu, X.P.; Zhang, Z.; Liu, W.D. Design, synthesis, and insecticidal activity of pyrimidinamine derivatives containing 2-pyridinyloxy moiety. J. Heterocycl. Chem. 2023, 60, 1058–1069. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, P.W.; Zhao, J.H.; Zhang, H.Y.; Wang, X.Y.; Li, L.S.; Xiong, L.X.; Yang, N.; Li, Y.X.; Yuchi, Z.G.; et al. Design, synthesis and biological activity of diamide compounds based on 3-substituent of the pyrazole ring. Pest Manag. Sci. 2022, 78, 2022–2033. [Google Scholar] [CrossRef]
- Lv, X.H.; Li, Q.S.; Ren, Q.S.; Ren, Z.L.; Chu, M.J.; Sun, J.; Zhang, X.; Xing, M.; Zhu, H.L.; Cao, H.Q. (E)-1,3-Diphenyl-1H-pyrazole derivatives containing O-benzyl oxime moiety as potential immunosuppressive agents: Design, synthesis, molecular docking and biological evaluation. Eur. J. Med. Chem. 2016, 108, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, K.S.; Hassan, H.A.; Abdel-Aziz, S.A.; Marzouk, A.A.; Shams, R.; Osawa, K.; Abdel-Aziz, M.; Konno, H. Development and assessment of 1,5-diarylpyrazole/oxime hybrids targeting EGTR and JNK-2 as antiproliferative agents: A comprehensive study through synthesis, molecular docking, and evaluation. Molecules 2023, 28, 6521. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Chen, J.; Li, G.; Ge, S.S.; Shi, Y.J.; Fang, Y.; Ling, Y. Design, synthesis, and bioactivities of novel oxadiazole-substitutedpyrazole oximes. Bioorg. Med. Chem. Lett. 2017, 27, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zheng, D.D.; Miao, H.Y.; Qian, C.; Dai, H.; Liang, K.; Zhou, B.B.; Shi, Y.J.; Xun, X.; Wang, Y. Synthesis and biological activities of novel pyrazole oxime derivatives containingbenzotriazolyl moiety. Chin. J. Org. Chem. 2020, 40, 4315–4321. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, N.; Zhou, Q.; Zhou, Y.X.; Gong, C.Y.; Zhang, Y.Y.; Xue, W. Synthesis and biological activities of novel chalcone derivatives containing pyrazole oxime ethers. Fitoterapia. 2023, 166, 105458. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Chen, S.; Zhu, P.; Huang, M.L.; Gao, W.J.; Zhu, R.; Qiang, J.Q.; Peng, Y.F.; Zhang, Y.A.; Dai, H.; et al. Design, synthesis, and biological evaluation of novel thiazolyl substituted bis-pyrazole oxime derivatives with potent antitumor activities by selectively inducing apoptosis and ROS in cancer cells. Med. Chem. 2019, 15, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Motoba, K.; Nishizawa, H.; Suzuki, T.; Hamaguchi, H.; Uchida, M.; Funayama, S. Species-specific detoxification metabolism of Fenpyroximate, a potent acaricide. Pestic. Biochem. Physiol. 2000, 67, 73–84. [Google Scholar] [CrossRef]
- Wang, S.L.; Shi, Y.J.; He, H.B.; Li, Y.; Li, Y.; Dai, H. Synthesis and bioactivity of novel pyrazole oxime derivatives containing oxazole ring. Chin. Chem. Lett. 2015, 26, 672–674. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Zhang, Z.Z.; Qian, Y.; Luo, G.C.; Zhou, B.B.; Miao, L.S.; Chen, Y.D.; Dai, H.; Xu, B.L.; et al. Synthesis and bioactivities of novel pyrazole oxime derivatives containing 1,3,4-oxadiazole group. Chin. J. Org. Chem. 2023, 43, 1584–1590. [Google Scholar] [CrossRef]
- Dai, H.; Yao, W.; Fang, Y.; Sun, S.Y.; Shi, Y.J.; Chen, J.; Jiang, G.Q.; Shi, J. Design, synthesis and bioactivities of novel isoxazole-containing pyrazole oxime derivatives. Molecules 2017, 22, 2000. [Google Scholar] [CrossRef]
- Yao, Y.Y.; Ren, C.L.; Chen, L.; Zhong, L.K.; Xu, T.M.; Tan, C.C. Synthesis and insecticidal activity of 3-ethyl sulfone pyridine substituted aryl triazole compounds. Chin. J. Org. Chem. 2021, 41, 2055–2062. [Google Scholar] [CrossRef]
- Xu, R.B.; Xia, R.; Luo, M.; Xu, X.Y.; Cheng, J.G.; Shao, X.S.; Li, Z. Design, synthesis, crystal structures, and insecticidal activities of eight-membered azabridge neonicotinoid analogues. J. Agric. Food Chem. 2014, 62, 381–390. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).