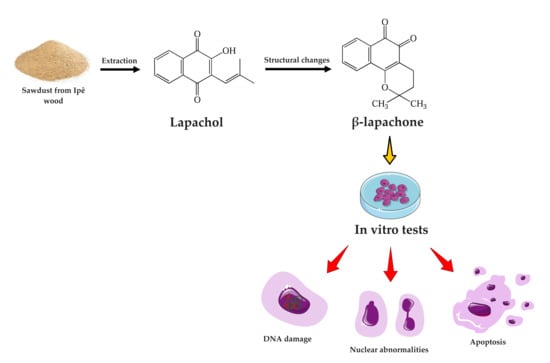
Anticancer Potential and Safety Profile of β-Lapachone In Vitro
Abstract
1. Introduction
2. Results
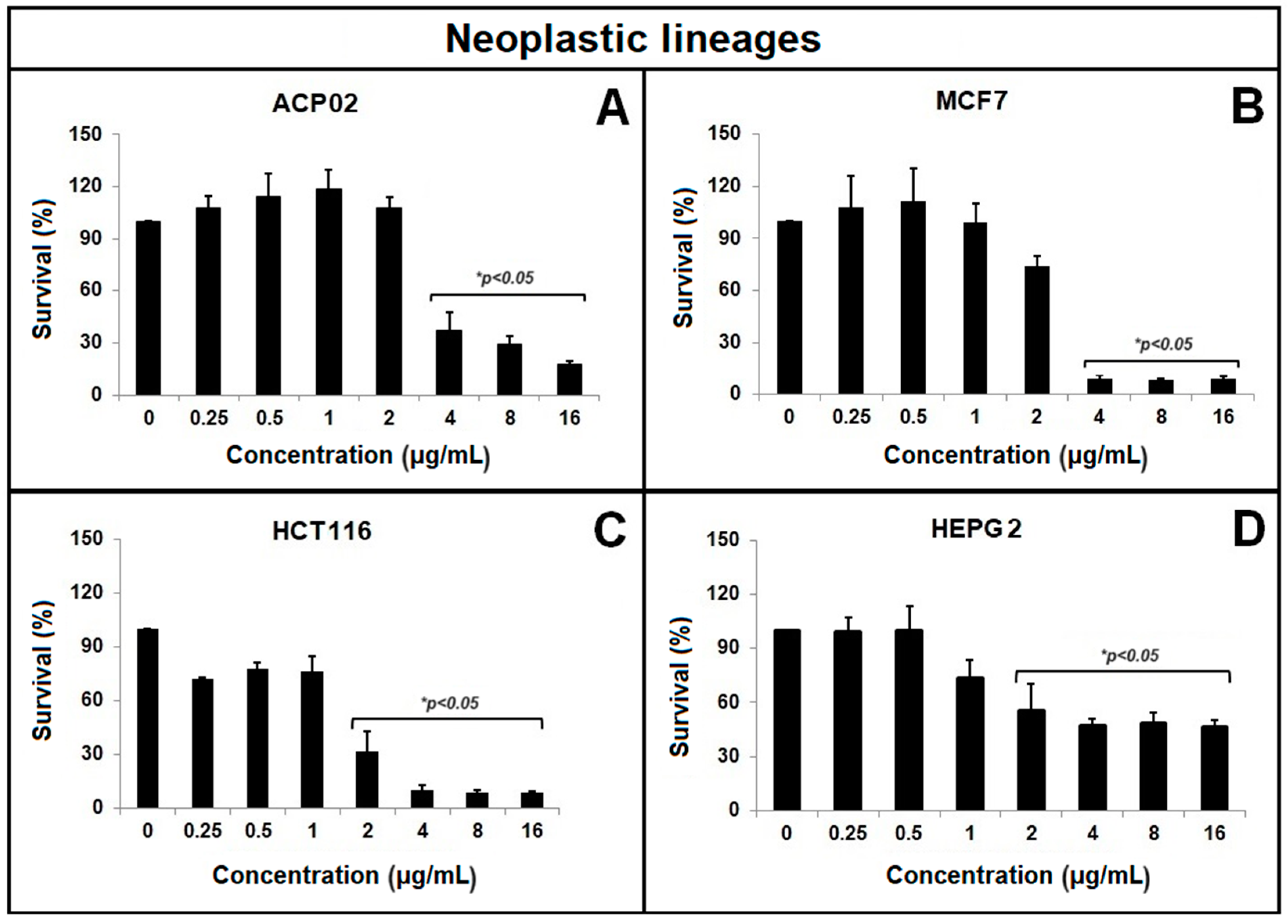
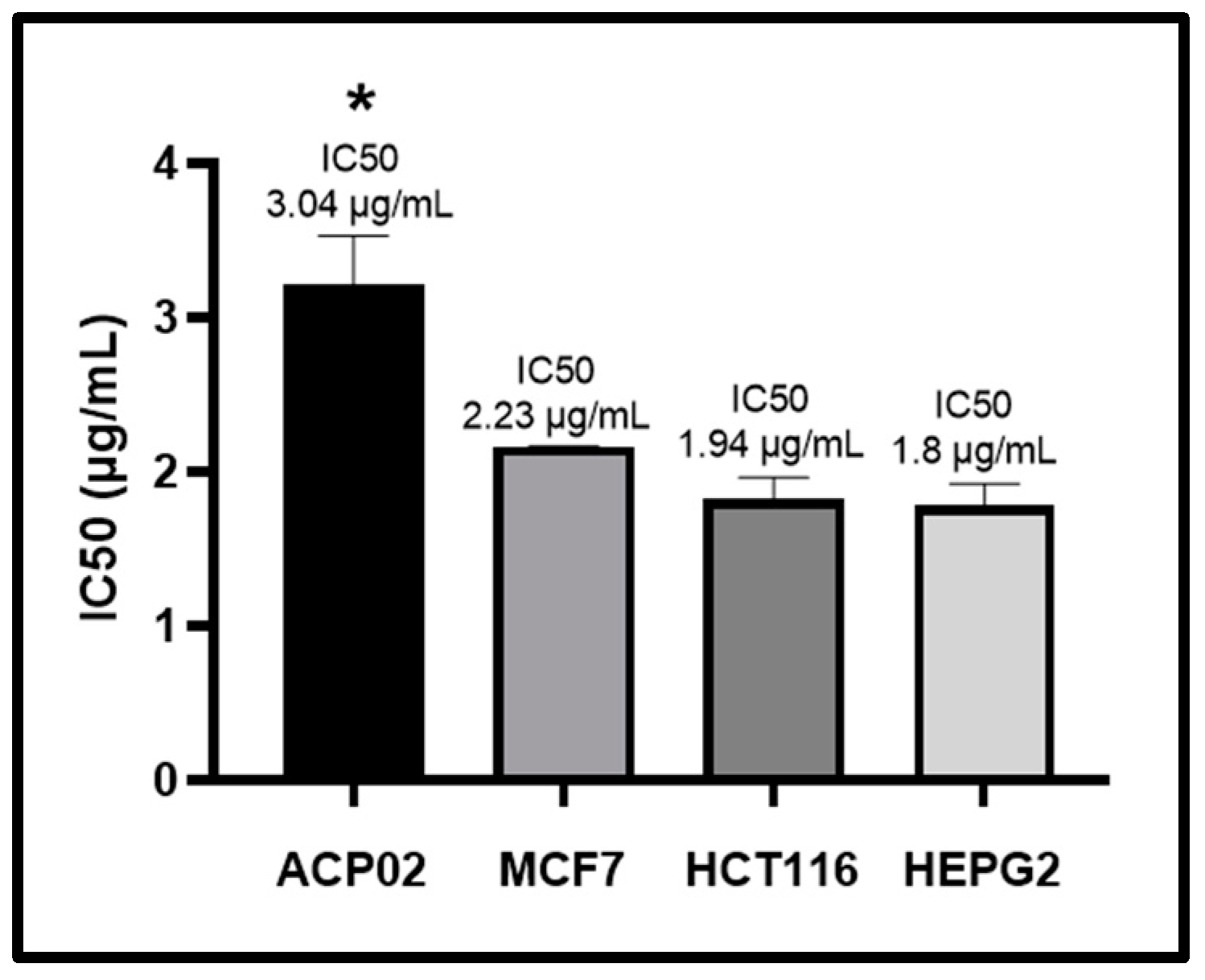
2.1. MTT Test
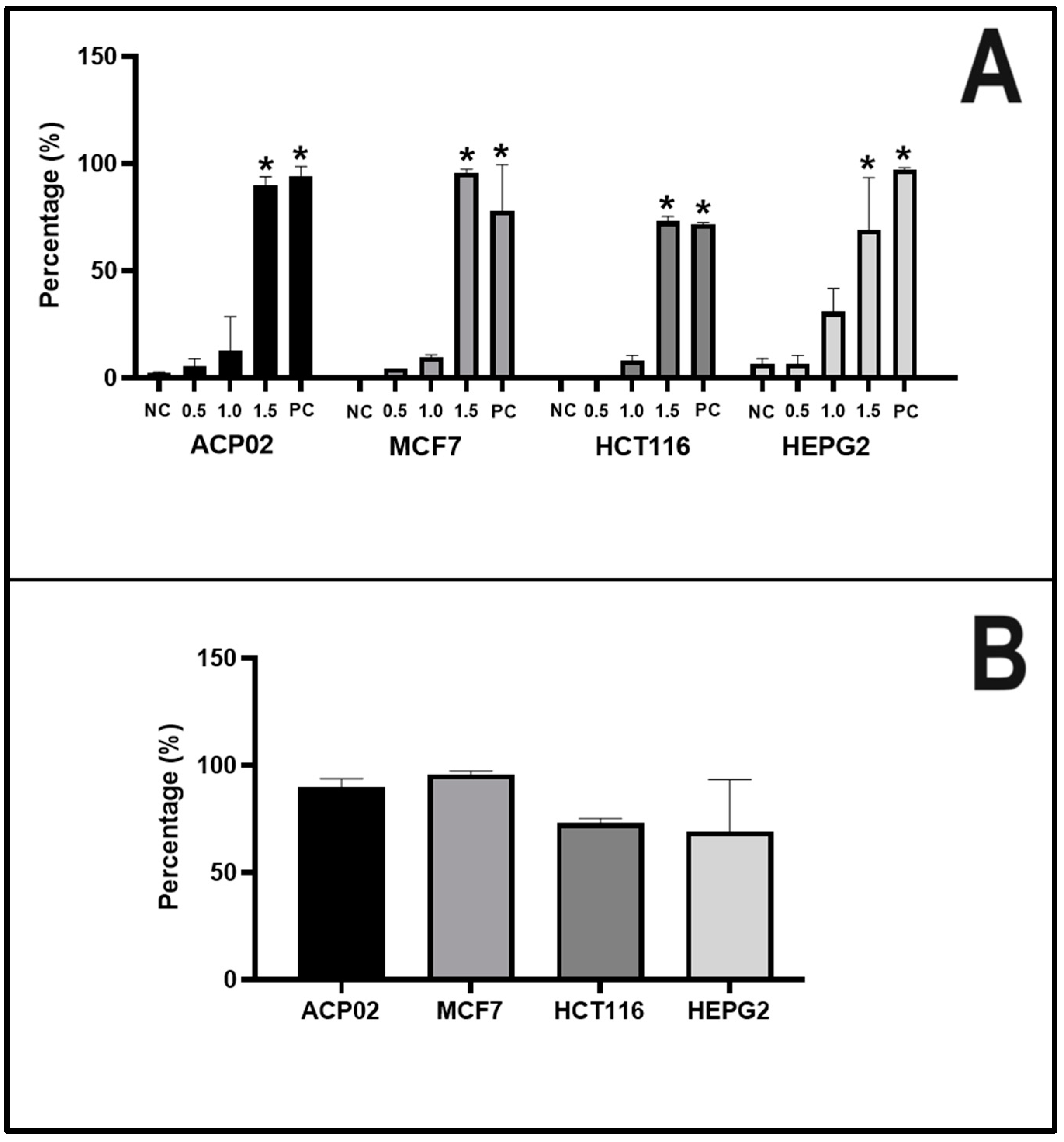
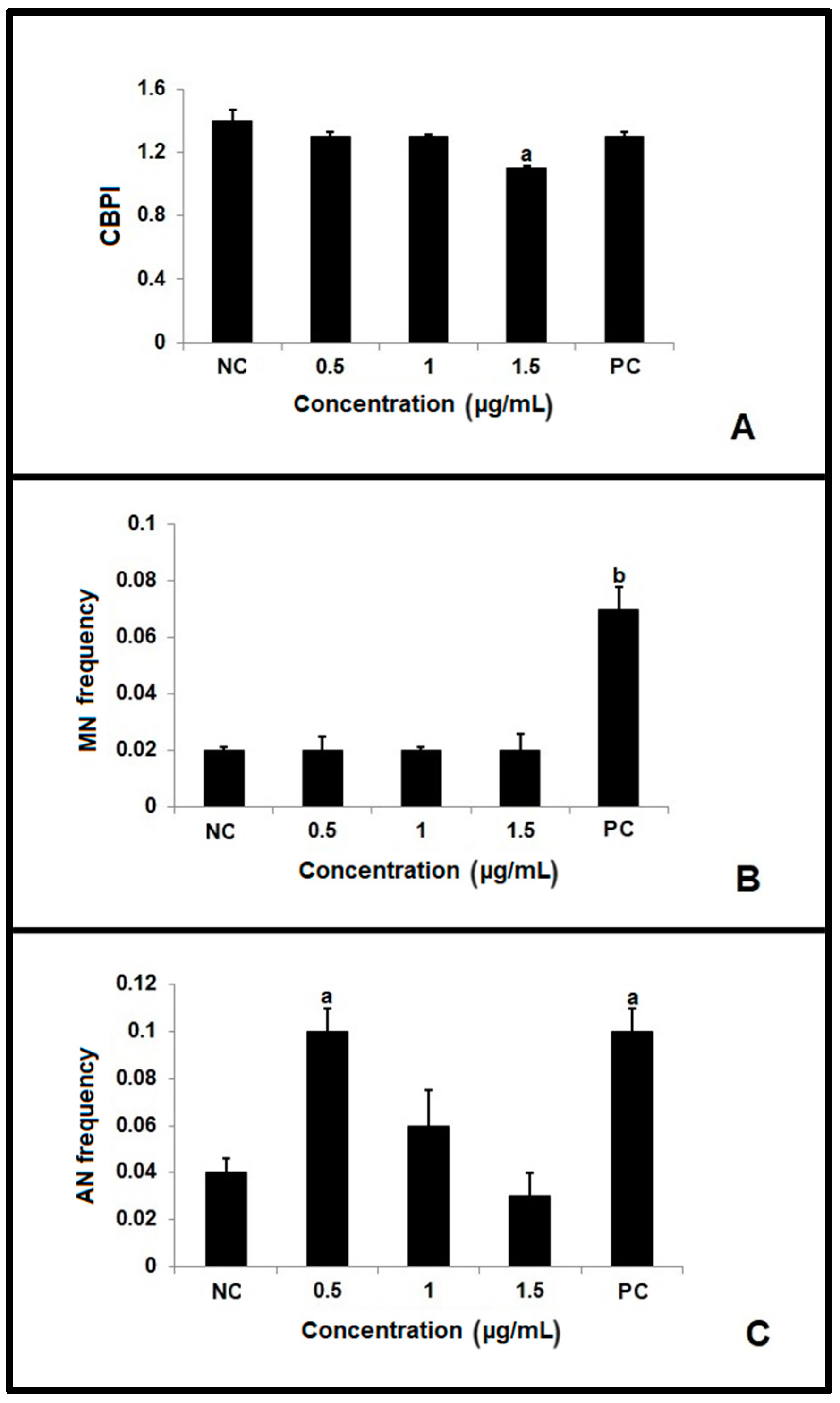
2.2. Apoptotic Index
2.3. Comet Assay
2.4. Micronucleus Test
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Extraction and Characterization of β-Lapachone
4.2. Cell Lines and Growing Conditions
4.3. Cell Viability
4.3.1. MTT Test
4.3.2. Apoptotic Index
4.4. Genotoxicity Assays
4.4.1. Comet Assay
4.4.2. Micronucleus Test
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandey, M.; Debnath, M.; Gupta, S.; Chikara, S.K. Phytomedicine: An ancient approach turning into future potential source of therapeutics. J. Pharmacogn. Phytother. 2011, 3, 27–37. [Google Scholar]
- Stankovic, M.S.; Radic, Z.S.; Blanco-Salas, J.; Vázquez-Pardo, F.M.; Ruiz-Téllez, T. Screening of selected species from Spanish flora as a source of bioactive substances. Ind. Crops Prod. 2017, 95, 493–501. [Google Scholar] [CrossRef]
- Aleebrahim-Dehkordy, E.; Rafieian-Kopaei, M.; Amini-Khoei, H.; Abbasi, S. In Vitro Evaluation of Antioxidant Activity and Antibacterial Effects and Measurement of Total Phenolic and Flavonoid Contents of Quercus brantii L. Fruit Extract. J. Diet. Suppl. 2019, 16, 408–416. [Google Scholar] [CrossRef]
- Da Costa, D.C.F.; Rangel, L.P.; Martins-Dinis, M.M.D.C.; Ferretti, G.D.S.; Ferreira, V.F.; Silva, J.L. Anticancer Potential of Resveratrol, β-Lapachone and Their Analogues. Molecules 2020, 25, 2–22. [Google Scholar]
- Pradeep, K.S.; Mallya, P.; Jain, V. Naphthoquinones in the Treatment of Cancer. Pharm. J. Pharm. Sci. Res. 2020, 12, 587–590. [Google Scholar]
- Wuerzberger, S.M.; Pink, J.J.; Planchon, S.M.; Byers, K.L.; Bornmann, W.G.; Boothman, D.A. Induction of apoptosis in MCF-7: WS8 breast cancer cells by β-Lapachone. Cancer Res. 1998, 58, 1876–1885. [Google Scholar]
- Dong, Y.; Chin, S.F.; Blanco, E.; Bey, E.A.; Kabbani, W.; Xie, X.J.; Bornmann, W.G.; Boothman, D.A.; Gao, J. Intratumoral delivery of β-lapachone via polymer implants for prostate cancer therapy. Clin. Cancer Res. 2009, 15, 131–139. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.J.; Yu, D.; Pardee, A.B. Potent Induction of Apoptosis by β-Lapachone in Human Multiple Myeloma Cell Lines and Patient Cells. Mol. Med. 2000, 6, 1008–1015. [Google Scholar] [CrossRef]
- Gupta, D.; Podar, K.; Tai, Y.T.; Lin, B.; Hideshima, T.; Akiyama, M.; LeBlanc, R.; Catley, L.; Mitsiades, N.; Mitsiades, C.; et al. β-lapachone, a novel plant product, overcomes drug resistance in human multiple myeloma cells. Exp. Hematol. 2002, 30, 711–720. [Google Scholar] [CrossRef]
- Park, E.J.; Min, K.J.; Lee, T.J.; Yoo, Y.H.; Kim, Y.S.; Kwon, T.K. β-Lapachone induces programmed necrosis through the RIP1-PARP-AIF-dependent pathway in human hepatocellular carcinoma SK-Hep1 cells. Cell Death Dis. 2014, 5, e1230. [Google Scholar] [CrossRef]
- Ough, M.; Lewis, A.; Bey, E.A.; Gao, J.; Ritchie, J.M.; Bornmann, W.; Boothman, D.A.; Oberley, L.W.; Cullen, J.J. Efficacy of beta-lapachone in pancreatic câncer treatment: Exploiting the novel, therapeutic target NQO1. Cancer Biol. Ther. 2005, 4, 95–102. [Google Scholar] [CrossRef]
- Silvers, M.A.; Deja, S.; Singh, N.; Egnatchik, R.A.; Sudderth, J.; Luo, X.; Beg, M.S.; Burgess, S.C.; DeBerardinis, R.J.; Boothman, D.A.; et al. The NQO1 bioactivatable drug, β-lapachone, alters the redox state of NQO1 pancreaztic cancer cells, causing perturbation in central carbon metabolism. J. Biol. Chem. 2017, 292, 18203–18216. [Google Scholar] [CrossRef]
- Chau, Y.P.; Shiah, S.G.; Don, M.J.; Kuo, M.L. Involvement of hydrogen peroxide in topoisomerase inhibitor β-lapachone-induced apoptosis and differentiation in human leukemia cells. Free Radic. Biol. Med. 1998, 24, 660–670. [Google Scholar] [CrossRef]
- Da Silva, J.; Erdtmann, B.; Henriques, J.A.P. Genética Toxicológica, 1st ed.; Alcancer: Porto Alegre, Brasil, 2003; 424p. [Google Scholar]
- Schauer, M.; Peiper, M.; Theisen, J.; Knoefel, W. Prognostic factors in patients with diffuse type gastric cancer (linitis plastica) after operative treatment. Eur. J. Med. Res. 2011, 16, 29–33. [Google Scholar] [CrossRef]
- Dias, R.B.; De Araújo, T.B.S.; De Freitas, R.D.; Rodrigues, A.C.B.C.; Sousa, L.P.; Salesb, C.B.S.; Valverdea, L.F.; Soaresa, M.B.P.; Dos Reisa, M.G.; Coletta, R.D.; et al. β-Lapachone and its iodine derivatives cause cell cycle arrest at G2/M phase and reactive oxygen species-mediated apoptosis in human oral squamous cell carcinoma cells. Free Radic. Biol. Med. 2018, 126, 87–100. [Google Scholar] [CrossRef]
- Boik, J. Natural Compounds in Cancer Therapy; Oregon Medical Press: Princeton, MI, USA, 2001. [Google Scholar]
- Gong, Q.; Hu, J.; Wang, P.; Li, X.; Zhang, X. A comprehensive review on β-lapachone: Mechanisms, structural modifications, and therapeutic potentials. Eur. J. Med. Chem. 2021, 210, 112962. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.T.; Cheong, J.; Choi, Y.H. β-Lapachone-induced apoptosis is associated with activation of caspase-3 and inactivation of NF-jB in human colon cancer HCT-116 cells. Anti-Cancer Drugs 2003, 14, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Lien, Y.C.; Kung, H.N.; Lu, K.S.; Jeng, C.J.; Chau, Y.P. Involvement of endoplasmic reticulum stress and activation of MAP kinases in ß-lapachone-induced human prostate cancer cell apoptosis. Histol. Histopathol. 2008, 23, 1299–1308. [Google Scholar] [PubMed]
- Yu, H.Y.; Kim, S.O.; Jin, C.Y.; Kim, G.Y.; Kim, W.J.; Yoo, Y.H.; Choi, Y.H. β-lapachone-Induced Apoptosis of Human Gastric Carcinoma AGS Cells Is Caspase-Dependent and Regulated by the PI3K/ Akt Pathway. Biomol. Ther. 2014, 22, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Choi, K.S.; Kwon, T.K. β-Lapachone-induced reactive oxygen species (ROS) generation mediates autophagic cell death in glioma U87 MG cells. Chem. Biol. Interact. 2011, 189, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Pardee, A.B. β-lapachone induces cell cycle arrest and apoptosis in human colon cancer cells. Mol. Med. 1999, 5, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Bey, E.A.; Bentle, M.S.; Reinicke, K.E.; Dong, Y.; Yang, C.R.; Girard, L.; Minna, J.D.; Bornmann, W.G.; Gao, J.; Boothman, D.A. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by β-lapachone. Proc. Natl. Acad. Sci. USA 2007, 104, 11832–11837. [Google Scholar] [CrossRef] [PubMed]
- Bey, E.A.; Reinicke, K.E.; Srougi, M.C.; Varnes, M.; Anderson, V.E.; Pink, J.J.; Li, L.S.; Patel, M.; Cao, L.; Moore, Z.; et al. Catalase abrogates β-lapachone-induced PARP1 hyperactivation-directed programmed necrosis in NQO1-positive breast cancers. Mol. Cancer Ther. 2013, 12, 2110–2120. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, M. Apoptotic DNA fragmentation and tissue homeostasis. Trends Cell Biol. 2002, 12, 84–89. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 5th ed.; Garland Science: New York, NY, USA, 2008; 1616p. [Google Scholar]
- Fulda, S. Targeting apoptosis for anticancer therapy. Semin. Cancer Biol. 2015, 31, 84–88. [Google Scholar] [CrossRef]
- Porfírio-Dias, C.L.; Melo, K.M.; Bastos, C.E.M.C.; Ferreira, T.A.A.; Azevedo, L.F.C.; Salgado, H.L.; Santos, A.S.; Rissino, J.D.; Nagamachi, C.Y.; Pieczarka, J.C. Andiroba oil (Carapa guianensis Aubl) shows cytotoxicity but no mutagenicity in the ACP02 gastric cancer cell line. J. Appl. Toxicol. 2020, 40, 1060–1066. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Park, S.J.; Yoon, S.M.; Jung, J.; Woo, H.N.; Yi, S.L.; Song, S.Y.; Park, H.J.; Kim, C.; Lee, J.S.; et al. Systemic delivery and preclinical evaluation of Au nanoparticle containing β-lapachone for radiosensitization. J. Control Release 2009, 139, 239–245. [Google Scholar] [CrossRef]
- Park, C.; Youn, H.; Kim, H.; Noh, T.; Kook, Y.H.; Oh, E.T.; Park, H.J.; Kim, C. Cyclodextrin-covered gold nanoparticles for targeted delivery of an anti-cancer drug. J. Mater. Chem. 2009, 19, 2310–2315. [Google Scholar] [CrossRef]
- Blanco, E.; Bey, E.A.; Khemtong, C.; Yang, S.G.; Setti-Guthi, J.; Chen, H.; Kessinger, C.W.; Carnevale, K.A.; Bornmann, W.G.; Boothman, D.A.; et al. β-Lapachone Micellar Nanotherapeutics for Non–Small Cell Lung Cancer Therapy. Cancer Res. 2010, 70, 3896–3904. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Cho, J.Y.; Yoon, S.H.; Jang, I.; Yu, K. Pharmacokinetics and tolerability of MB12066, a beta-lapachone derivative targeting NA D(P)H: Quinone oxidoreductase 1: Two independent, double-blind, placebo-controlled, combined single and multiple ascending dose first-in-human clinical trials. Drug Des. Devel Ther. 2017, 11, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Gerber, D.E.; Beg, M.S.; Fattah, F.; Frankel, A.E.; Fatunde, O.; Arriaga, Y.; Dowell, J.E.; Bisen, A.; Leff, R.D.; Meek, C.C.; et al. Phase 1 study of ARQ 761, a β-lapachone analogue that promotes NQO1-mediated programmed cancer cell necrosis. Br. J. Cancer 2018, 119, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T.; Surralles, J.; Crott, J.W.; Parry, J.; Norppa, H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 2011, 26, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Koppers, A.J. Apoptosis and DNA damage in human spermatozoa. Asian J. Androl. 2011, 13, 36–42. [Google Scholar] [CrossRef]
- Ross, W.P. DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer. 2016, 16, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Villamil, S.F.; Stoppani, A.O.M.; Dubin, M. Redox Cycling of β-Lapachone and Structural Analogues in Microsomal and Cytosol Liver Preparations. Meth. Enzymol. 2004, 378, 67–87. [Google Scholar]
- Alcântara, D.D.; Ribeiro, H.F.; Cardoso, P.C.; Araújo, T.M.; Burbano, R.R.; Guimarães, A.C.; Khayat, A.S.; De Oliveira Bahia, M. In vitro evaluation of the cytotoxic and genotoxic effects of artemether, an antimalarial drug, in a gastric cancer cell line (PG100). J. Appl. Toxicol. 2013, 33, 151–156. [Google Scholar] [CrossRef]
- Calandrini de Azevedo, L.F.; Ferreira, T.A.A.; Melo, K.M.; Porfírio Dias, C.L.; Bastos, C.E.M.C.; Santos, S.F.; Santos, A.S.; Nagamachi, C.Y.; Pieczarka, J.C. Aqueous ethanol extract of Libidibia ferrea (Mart. Ex Tul) L.P. Queiroz (juca) exhibits antioxidant and migration-inhibiting activity in human gastric adenocarcinoma (ACP02) cells. PLoS ONE 2020, 15, e0257134. [Google Scholar] [CrossRef]
- Melo, K.M.; Grisolia, C.K.; Pieczarka, J.C.; De Souza, L.R.; De Souza Filho, J.; Nagamachi, C.Y. FISH in micronucleus test demonstrates aneugenic action of rotenone in a common freshwater fish species, Nile tilapia (Oreochromis niloticus). Mutagenesis 2014, 29, 215–219. [Google Scholar] [CrossRef]
- Ribeiro, L.R.; Salvadori, D.M.F.; Marques, E.K. Mutagenese Ambiental, 1st ed.; Editora Ulbra: Canoas, Brasil, 2003; 355p. [Google Scholar]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1998, 175, 184–191. [Google Scholar] [CrossRef]
- Collins, A.R.; Ma, A.G.; Duthie, S.J. The kinetics of repair and oxidative DNA damage (Strand breaks and oxidized pyrimidines) in human cells. Mutat. Res. 1995, 336, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. The cytokinesis-block micronucleus technique: A detailed description of the method and its application to genotoxicity studies in human populations. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1993, 285, 35–44. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, K.M.M.; Calandrini de Azevedo, L.F.; Rissino, J.D.; Vale, V.V.; Costa, E.V.S.; Dolabela, M.F.; Nagamachi, C.Y.; Pieczarka, J.C. Anticancer Potential and Safety Profile of β-Lapachone In Vitro. Molecules 2024, 29, 1395. https://doi.org/10.3390/molecules29061395
Lima KMM, Calandrini de Azevedo LF, Rissino JD, Vale VV, Costa EVS, Dolabela MF, Nagamachi CY, Pieczarka JC. Anticancer Potential and Safety Profile of β-Lapachone In Vitro. Molecules. 2024; 29(6):1395. https://doi.org/10.3390/molecules29061395
Chicago/Turabian StyleLima, Karina Motta Melo, Luana França Calandrini de Azevedo, Jorge Dores Rissino, Valdicley Vieira Vale, Erica Vanessa Souza Costa, Maria Fani Dolabela, Cleusa Yoshiko Nagamachi, and Julio Cesar Pieczarka. 2024. "Anticancer Potential and Safety Profile of β-Lapachone In Vitro" Molecules 29, no. 6: 1395. https://doi.org/10.3390/molecules29061395
APA StyleLima, K. M. M., Calandrini de Azevedo, L. F., Rissino, J. D., Vale, V. V., Costa, E. V. S., Dolabela, M. F., Nagamachi, C. Y., & Pieczarka, J. C. (2024). Anticancer Potential and Safety Profile of β-Lapachone In Vitro. Molecules, 29(6), 1395. https://doi.org/10.3390/molecules29061395