Graphene Materials from Coke-like Wastes as Proactive Support of Nickel–Iron Electro-Catalysts for Water Splitting
Abstract
1. Introduction
2. Results
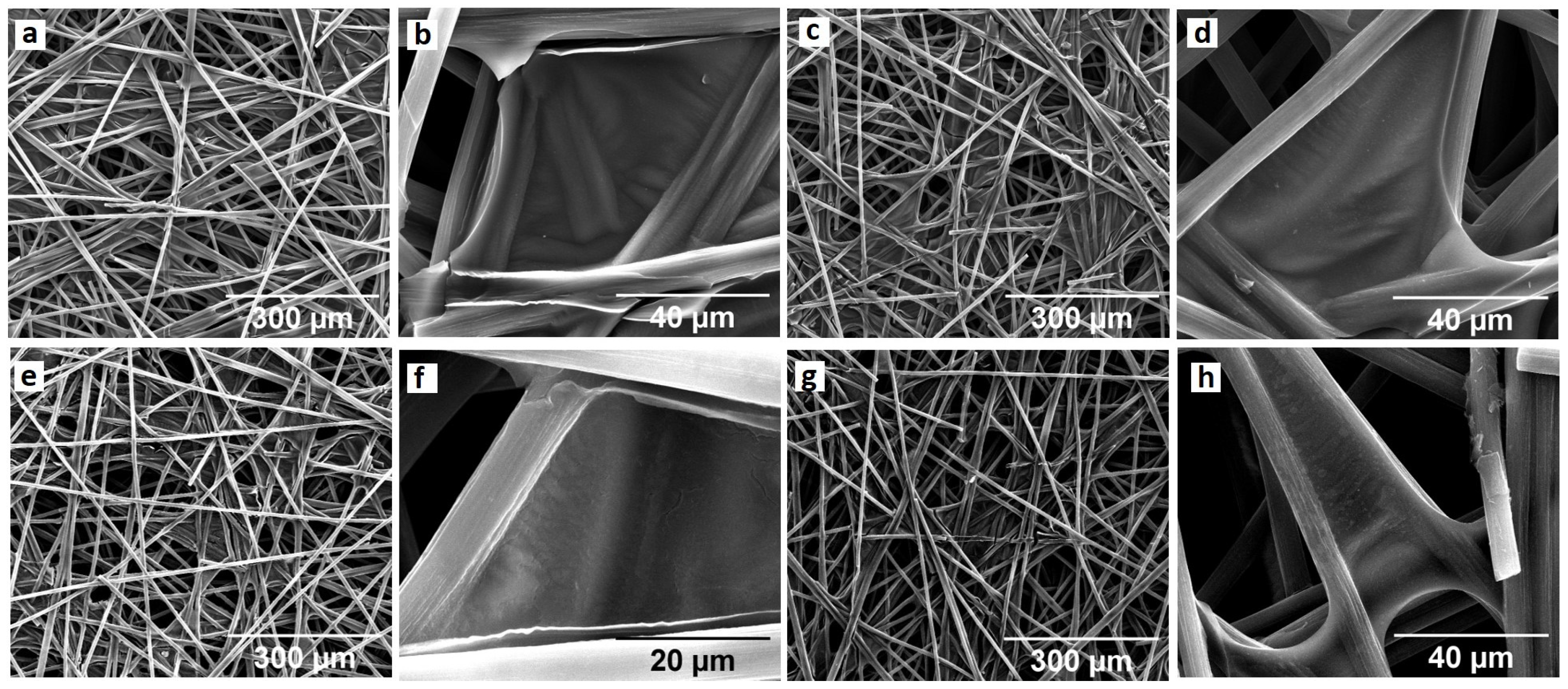
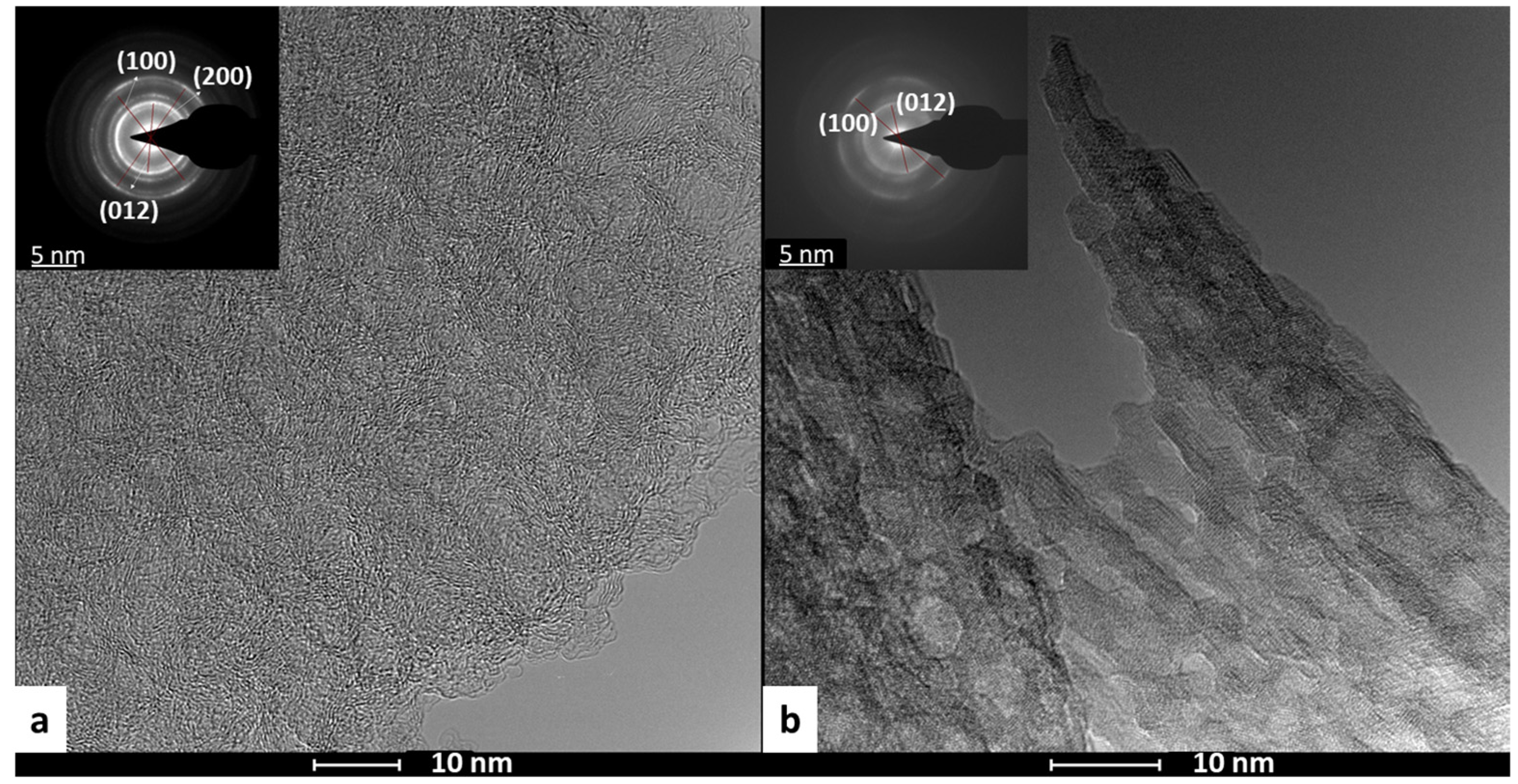
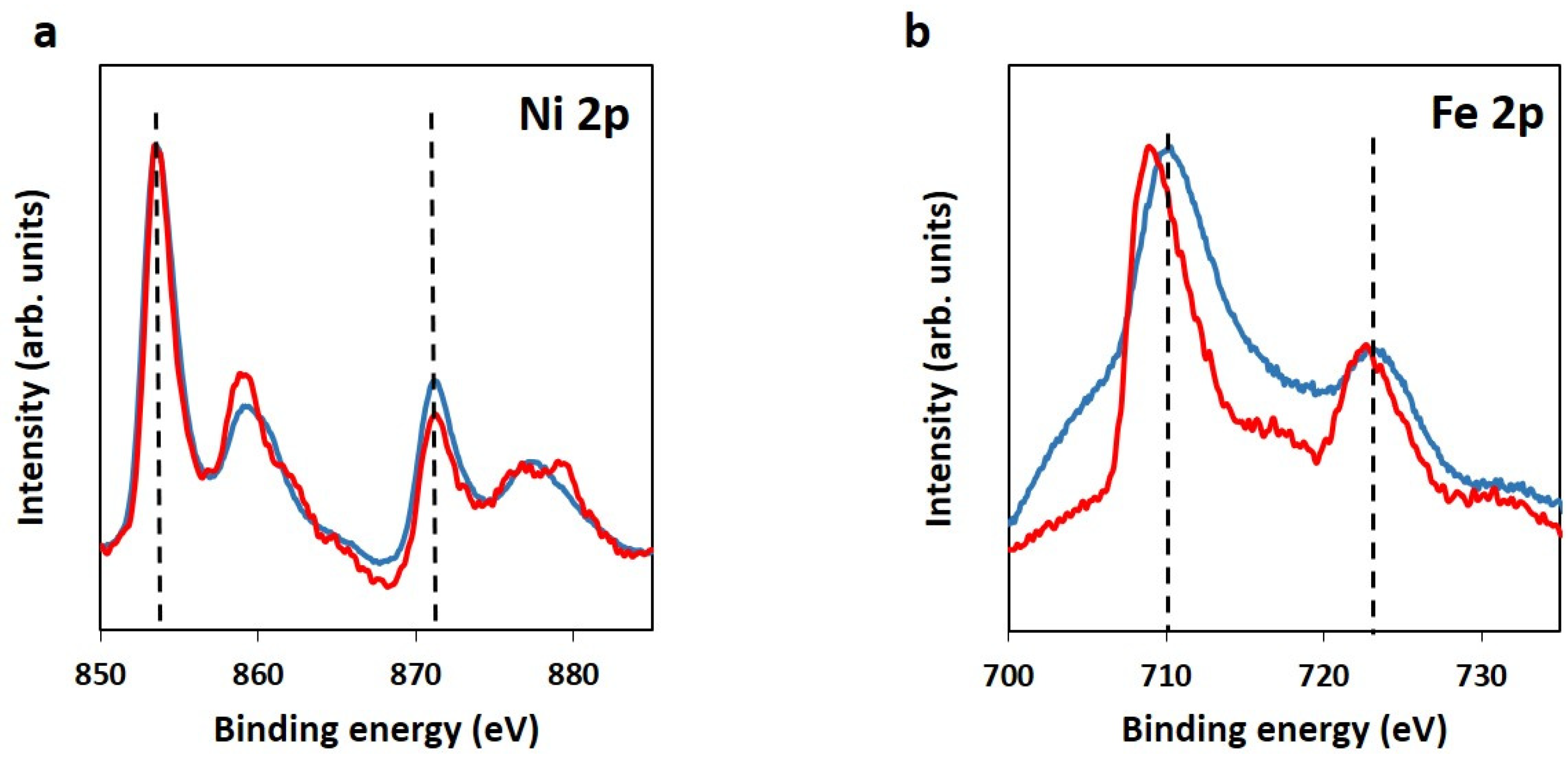
2.1. Preparation of Hybrid Graphene/NiFe 3D Electrodes from GO from a Coke-like Wastes
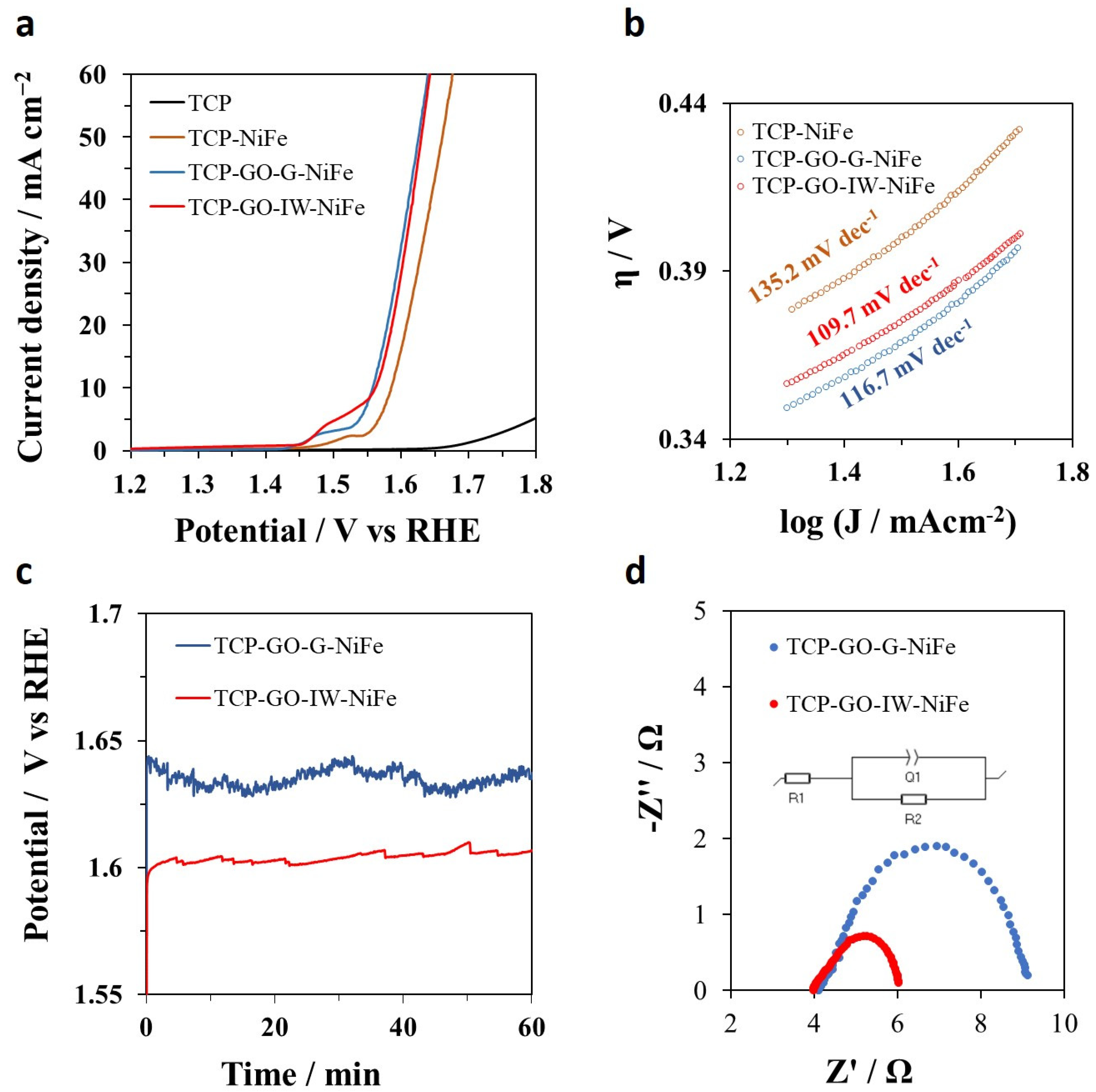
2.2. Electrocatalytic OER Activity of the as-Prepared 3D TCP/Graphene/NiFe Electrodes
3. Experimental Section
3.1. Raw Materials and Graphene Oxide Preparation
3.2. Preparation of Electrodes Containing Graphene/Ni-Fe Hybrid Catalysts
3.3. Electrochemical Measurements
3.4. Scientific Equipment—Characterization of Supports and Hybrid Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nathan, S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef]
- Jalil, A.A.; Hamid, M.Y.S.; Jusoh, N.W.S.; Hassan, N.S. Sustainable energy for better social, environmental, and economic returns. Environ. Sci. Pollut. Res. 2023, 30, 116876–116877. [Google Scholar] [CrossRef]
- Alfani, D.; Binotti, M.; Macchi, E.; Silva, P.; Astolfis, M. CO2 power plants for waste heat recovery: Design optimization and part-load operation strategies. Appl. Therm. Eng. 2021, 195, 117013. [Google Scholar] [CrossRef]
- Huang, H.; Liu, M.; Li, X.; Guo, X.; Wang, T.; Li, S.; Lei, H. Numerical simulation and visualization study of a new tapered-slope serpentine flow field in proton exchange membrane fuel cell. Energy. 2022, 246, 123406. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of Electrocatalysts for Oxygen- and Hy-drogen-Involving Energy Conversion Reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, P.; Shao, Q.; Huang, X. Recent Progress in Advanced Electrocatalyst Design for Acidic Oxygen Evolution Reaction. Adv. Mater. 2021, 33, 2004243. [Google Scholar] [CrossRef] [PubMed]
- deKrafft, K.E.; Wang, C.; Xie, Z.; Su, X.; Hinds, B.J.; Lin, W. Electrochemical water oxidation with carbon-grafted iridium complexes. ACS Appl. Mater. Interfaces 2012, 4, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xu, X.; Tang, T.; Zhong, Y.; Shao, Z. Perovskite-Based Electrocatalysts for Cost-Effective Ultrahigh-Current-Density Water Splitting in Anion Exchange Membrane Electrolyzer Cell. Small Methods 2022, 6, 2201099. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, X.; Kim, H.; Shao, Z.; Jung, W. Advanced electrocatalysts with unusual active sites for electrochemical water splitting. InfoMat. 2024, 6, e12494. [Google Scholar] [CrossRef]
- Dionigi, F.; Zeng, Z.; Sinev, I.; Mezdorf, T.; Deshpande, S.; Bernal Lopez, M.; Kunze, S.; Zegkinoglou, I.; Sarodnik, H.; Fan, D.; et al. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. Nat. Commun. 2020, 11, 2522. [Google Scholar] [CrossRef]
- Doyle, R.L.; Godwin, I.J.; Brandon, M.P.; Lyons, M.E.G. Redox and electrochemical water splitting catalytic properties of hydrated metal oxide modified electrodes. Phys. Chem. Chem. Phys. 2013, 15, 13737–13783. [Google Scholar] [CrossRef]
- Javaid, S.; Xu, X.; Chen, W.; Chen, J.; Hsu, H.; Wang, S.; Yang, X.; Li, Y.; Shao, Z.; Jones, F.; et al. Ni2+/Co2+ doped Au-Fe7S8 nanoplatelets with exceptionally high oxygen evolution reaction activity. Nano Energy 2021, 89, 106463. [Google Scholar] [CrossRef]
- Gong, M.; Dai, H. A mini review on NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Wang, Q.; Shang, L.; Shi, R.; Zhang, X.; Zhao, Y.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; Zhang, T. NiFe layered double hydroxide nanoparticles on Co,N-codoped carbon nanoframes as efficient bifunctional catalysts for rechargeable Zinc-Air batteries. Adv. Energy Mater. 2017, 7, 1700467. [Google Scholar] [CrossRef]
- Meng, C.; Ling, T.; Ma, T.-Y.; Wang, H.; Hu, Z.; Zhou, Y.; Mao, J.; Du, X.-W.; Jaroniec, M.; Qiao, S.-Z. Atomically and Electronically Couples Pt and CoO Hybrid nanocatalysts for Enhanced Electrocatalytic Performance. Adv. Mater. 2017, 29, 1604607. [Google Scholar] [CrossRef]
- Tahir, M.; Mahmood, N.; Zhu, J.; Mahmood, A.; Butt, F.K.; Rizwan, S.; Aslam, I.; Tanveer, M.; Idrees, F.; Shakir, I.; et al. One Dimensional Graphitic Carbon Nitrides as effective Metal-Free Oxygen Reduction Catalysts. Sci. Rep. 2015, 5, 12389. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Li, Y.; Wang, H.; Liang, Y.; Wu, J.Z.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; Dai, H. An Advanced Ni-Fe Layered Double Hydroxide Electrocatalyst for Water Oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z. Hydrogen evolution: Guiding principles. Nat. Energy 2016, 1, 16155. [Google Scholar] [CrossRef]
- Tahir, M.; Pan, L.; Idrees, F.; Zhang, X.; Wang, L.; Zou, J.; Wang, Z.L. Electrocatalytic oxygen evolution reaction for energy conversion and storage: A comprehensive review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Jia, Y.; Yao, X. Defects in Carbon-Based Materials for Electrocatalysis. Acc. Chem. Res. 2023, 56, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Li, N.; Bao, L.; Zhang, P.; Lu, X. Intrinsic carbon structural imperfections for enhancing energy conversion electrocatalysts. Chem. Eng. J. 2023, 466, 143060. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, C.; Du, D.; Lin, Y. Robust noble metal-based electrocatalysts for oxygen evolution reaction. Chem. Soc. Rev. 2019, 48, 3181–3192. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, F.; Liu, Y.; Ma, N.; Wang, Z.; Zhang, X. Environment-friendly method to produce graphene that employs vitamin C and amino acid. Chem. Mater. 2010, 22, 2213–2218. [Google Scholar] [CrossRef]
- Coleman, J. Liquid exfoliation of defect-free graphene. Acc. Chem. Res. 2013, 46, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Eom, S.H.; Chung, J.S.; Hur, S.H. Large-scale production of high-quality reduced graphene oxide. Chem. Eng. J. 2013, 233, 297–304. [Google Scholar] [CrossRef]
- Sierra, U.; Álvarez, P.; Blanco, C.; Granda, M.; Santamaría, R.; Menéndez, R. New alternatives to graphite for producing Graphene materials. Carbon 2015, 193, 812–818. [Google Scholar] [CrossRef]
- Fernández-García, L.; Alvarez, P.; Pérez-Mas, A.; Blanco, C.; Santamaría, R.; Menéndez, R.; Granda, M. Peculiarities of the production of graphene oxides with controlled properties from industrial coal liquids. Fuel 2017, 203, 253–260. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, P.; Granda, M.; Blanco, C.; Santamaría, R.; Romasanta, L.J.; Verdejo, R.; López-Manchado, M.A.; Menéndez, R. Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie methods. Carbon 2013, 65, 156–164. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, P.; Santamaría, R.; Granda, M.; Gutiérrez, M.D.; Rodríguez-Reinoso, F.; Menéndez, R. Critical temperatures in the synthesis of graphene-like materials by thermal exfoliation-reduction of graphite oxide. Carbon 2013, 52, 476–485. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, P.; Santamaría, R.; Granda Ares, P.; Rodríguez, F. The effect of the parent graphite on the structure of graphene oxide. Carbon 2012, 50, 275–282. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, G.; Zhang, F. Multiple roles of graphene in heterogeneous catalysis. Chem. Soc. Rev. 2015, 44, 3023–3035. [Google Scholar] [CrossRef] [PubMed]
- Axet, M.R.; Durand, J.; Gouygou, M.; Serp, P. Surface coordination chemistry on graphene and two-dimensional carbon materials for well-defined single atom supported catalysts. Adv. Organomet. Chem. 2019, 71, 53–174. [Google Scholar] [CrossRef]
- Wala, A.; Algozeeb, P.; Savas, E.; Luong, D.X.; Chen, W.; Kittrell, C.; Bhat, M.; Shahsavari, R.; Tour, J.M. Flash Graphene from Plastic Waste. ACS Nano 2020, 14, 1559515604. [Google Scholar] [CrossRef]
- Luong, D.X.; Bets, K.V.; Algozeeb, W.A.; Stanford, M.G.; Kittrell, C.; Chen, W.; Salvatierra, R.V.; Ren, M.; McHugh, E.A.; Advincula, P.A.; et al. Gram-Scale Bottom-up Flash Graphene Synthesis. Nature 2020, 577, 647–651. [Google Scholar] [CrossRef]
- Wang, M.; Wang, H.; Gu, Y.; Zhu, M.; Kumar, M.; Liang, J.; Tie, Z.; Ma, J.; Jin, Z. In Situ Generation of Flash Graphene Supported Spherical Bismuth Nanoparticles in Less than 200 ms for Highly Selective Carbon Dioxide Electroreduction. ACS Mater. Lett. 2024, 6, 100–108. [Google Scholar] [CrossRef]
- González-Ingelmo, M.; Álvarez, P.; Granda, M.; Rocha, V.G.; González, Z.; Sierra, U.; Sánchez-Page, B.; Jiménez, M.V.; Pérez Torrente, J.J.; Blasco, J.; et al. On the study of the preparation of graphene-anchored NHC-iridium catalysts from a coke-like waste with application in water splitting. Appl. Surf. Sci. 2024, 655, 159556. [Google Scholar] [CrossRef]
- Granda, M.; Blanco, C.; Álvarez, p.; Patrick, J.W.; Menéndez, R. Chemicals from coal coking. Chem. Rev. 2014, 114, 1608–1636. [Google Scholar] [CrossRef]
- Fortgang, P.; Tite, T.; Barnier, V.; Zehani, N.; Maddi, C.; Lagarde, F.; Loir, A.; Jaffrezic-Renault, N.; Donnet, C.; Garrelie, F.; et al. Robust Electrografting on self-organized 3D graphene electrodes. ACS Appl. Mater. Interfaces 2016, 8, 1424–1433. [Google Scholar] [CrossRef]
- González-Ingelmo, M.; Granda, M.; Ruiz, B.; Fuente, E.; Sierra, E.; Rocha, V.G.; González, Z.; Álvarez, P.; Menéndez, R. Proactive Effect of Algae-Based Graphene Support on the Oxygen Evolution Reaction Electrocatalytic Activity of NiFe. Materials 2023, 16, 7641. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel–iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sasikumar, M.; Arulraj, A.; Rajasudha, V.; Murugadoss, G.; Kumar, M.R.; Peera, S.G.; Mangalaraja, R.V. NiFe Layered Double Hydroxide Electrocatalyst Prepared via an Electrochemical Deposition Method for the Oxygen Evolution Reaction. Catalysts 2022, 12, 1470. [Google Scholar] [CrossRef]
- Gultom, N.S.; Abdullah, H.; Hsu, C.; Kuo, D. Activating nickel iron layer double hydroxide for alkaline hydrogen evolution reaction and overall water splitting by electrodepositing nickel hydroxide. Chem. Eng. J. 2021, 419, 129608. [Google Scholar] [CrossRef]
- Cai, Z.; Zhou, D.; Wang, M.; Bak, S.M.; Wu, Y.; Wu, Z.; Sun, X. Introducing Fe2+ into nickel–iron layered double hydroxide: Local structure modulated water oxidation activity. Angew. Chem. 2018, 130, 9536–9540. [Google Scholar] [CrossRef]
- Jia, D.; Gao, H.Y.; Xing, L.W.; Chen, X.; Dong, W.J.; Huang, X.B.; Wang, G. 3D Self-Supported Porous NiO@NiMoO4 Core–Shell Nanosheets for Highly Efficient Oxygen Evolution Reaction. Inorg. Chem. 2019, 58, 6758–6764. [Google Scholar] [CrossRef]
- Klaus, S.; Cai, Y.; Louie, M.W.; Trotochaud, L.; Bell, A.T. Effects of Fe Electrolyte Impurities on Ni(OH)2/NiOOH Structure and Oxygen Evolution Activity. J. Phys. Chem. C. 2015, 119, 7243–7254. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zhao, D.D.; Bao, S.J.; Zhou, W.H.; Li, H.L. Preparation of hexagonal nanoporous nickel hydroxide film and its application for electrochemical capacitor. Electrochem. Commun. 2007, 9, 869–874. [Google Scholar] [CrossRef]
- Seah, M.P.; Briggs, D. (Eds.) Practical Surface Analysis: Auger and X-ray Photoelectron Spectroscopy, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1990; Volume 1, p. 657. [Google Scholar]
- Elgrabli, D.; Floriani, M.; Abella-Gallart, S.; Meunier, L.; Gamez, C.; Delalain, P.; Rogerieux, F.; Boczkowski, J.; Lacroix, G. Biodistribution and clearance of instilled carbon nanotubes in rat lung. Part. Fibre Toxicol. 2008, 5, 20–33. [Google Scholar] [CrossRef]
| Sample | XRD | Elemental Analysis | Raman | XPS | |||
|---|---|---|---|---|---|---|---|
| d002 (nm) a | Lc (nm) b | n c | C (Wt.%) | Id/Ig | Csp2 (%) | Csp3 (%) | |
| GO-G | 0.898 | 9 | 10 | 47.5 | 0.95 | 43.5 | 5.6 |
| GO-IW | 0.796 | 11.6 | 14 | 49.64 | 1.07 | 33.5 | 7.8 |
| Sample | Ni (wt.%) | Fe (wt. %) | Ratio Ni/Fe |
|---|---|---|---|
| TCP-GO-G-NiFe | 84.3 | 15.7 | 5.3 |
| TCP-GO-IW-NiFe | 87.2 | 12.8 | 6.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Ingelmo, M.; Rocha, V.G.; González, Z.; Sierra, U.; Barriga, E.D.; Álvarez, P. Graphene Materials from Coke-like Wastes as Proactive Support of Nickel–Iron Electro-Catalysts for Water Splitting. Molecules 2024, 29, 1391. https://doi.org/10.3390/molecules29061391
González-Ingelmo M, Rocha VG, González Z, Sierra U, Barriga ED, Álvarez P. Graphene Materials from Coke-like Wastes as Proactive Support of Nickel–Iron Electro-Catalysts for Water Splitting. Molecules. 2024; 29(6):1391. https://doi.org/10.3390/molecules29061391
Chicago/Turabian StyleGonzález-Ingelmo, María, Victoria G. Rocha, Zoraida González, Uriel Sierra, Enrique Diaz Barriga, and Patricia Álvarez. 2024. "Graphene Materials from Coke-like Wastes as Proactive Support of Nickel–Iron Electro-Catalysts for Water Splitting" Molecules 29, no. 6: 1391. https://doi.org/10.3390/molecules29061391
APA StyleGonzález-Ingelmo, M., Rocha, V. G., González, Z., Sierra, U., Barriga, E. D., & Álvarez, P. (2024). Graphene Materials from Coke-like Wastes as Proactive Support of Nickel–Iron Electro-Catalysts for Water Splitting. Molecules, 29(6), 1391. https://doi.org/10.3390/molecules29061391








