Abstract
Magnetic nanoparticles (MNPs), either paramagnetic or superparamagnetic depending on their composition and size, have been thoroughly studied as magnetic resonance imaging (MRI) contrast agents using in vitro and in vivo biomedical preclinical studies, while some are clinically used. Their magnetic properties responsible in some cases for high magnetization values, together with large surface area-to-volume ratios and the possibility of surface functionalization, have been used in MRI-based diagnostic and theranostics applications. MNPs are usually used as positive (T1) or negative (T2) MRI contrast agents, causing brightening or darkening of selected regions in MRI images, respectively. This review focusses on recent developments and optimization of MNPs containing Gd, Mn, Fe and other lanthanide ions which may function as dual-mode T1–T2 MRI contrast agents (DMCAs). They induce positive or negative contrast in the same MRI scanner upon changing its operational mode between T1-weighted and T2-weighted pulse sequences. The type of contrast they induce depends critically on their r2/r1 relaxivity ratio, which for DMCAs should be in the 2–10 range of values. After briefly discussing the basic principles of paramagnetic relaxation in MNPs, in this review, the basic strategies for the rational design of DMCAs are presented and typical examples are discussed, including in vivo preclinical applications: (1) the use of NPs with a single type of contrast material, Gd- or Mn-based NPs or superparamagnetic NPs with appropriate size and magnetization to provide T2 and T1 contrast; and (2) inclusion of both types of T1 and T2 contrast materials in the same nanoplatform by changing their relative positions.
1. Introduction
Magnetic resonance imaging (MRI) is one of the most prominent clinical imaging modalities resulting from its many favorable characteristics. These include non-invasiveness, use of low-energy radiofrequency radiation, in contrast to invasive X-ray computed tomography (CT), positron-emission tomography (PET) and single-photon emission computed tomography (SPECT), which use damaging high-energy ionizing radiation, providing tomographic images with large penetration depth of any area of the body, in contrast to optical imaging (OI), outstanding spatial resolution (50–100 μm) and remarkable soft tissue contrast (see Table 1 for a comparison of some properties of imaging modalities for clinical applications). The contrast in the MRI images is generated by differences in intensities of 1H NMR resonances, most frequently of water protons, with an important contribution of lipids in some cases. These intensities are governed by several parameters, including the local tissue 1H concentrations, diffusion and flow of water molecules, and most importantly by their intrinsic differences in proton spin–lattice or longitudinal relaxation times (T1) and spin–spin or transverse relaxation times (T2) [1,2]. However, the sensitivity of MRI is relatively low, due to the small population difference between the two proton spin energy states in the presence of a magnetic field, making it difficult to detect small lesions and limiting the time resolution of the technique. In recent decades, there has been a trend toward clinical MRI equipment operating at higher magnetic field strengths (B0), which improved its contrast-to-noise ratios and spatial resolution. Presently, medical diagnostic MRI is typically performed at B0 = 0.5–3 T, and research equipment can operate at B0 values up to 11 T.
MRI contrast agents (CAs) are often employed to enhance the contrast between normal and disease tissues by significantly decreasing their T1 and T2 values [3,4,5,6,7]. This acceleration of the spin relaxation of water protons, in the region where they accumulate relative to surrounding tissues, results from oscillating magnetic fields produced by metal ions present in the CAs, allowing sensitive MRI detection of that region. Their efficacy is measured by their relaxivity, r1 or r2, defined as the paramagnetic enhancement of the water proton relaxation rates, normalized to a 1 mM metal ion concentration [3,4,5].

Table 1.
Comparison of some typical properties of imaging modalities for clinical applications [8,9].
Table 1.
Comparison of some typical properties of imaging modalities for clinical applications [8,9].
| Technique | Resolution | Penetration Depth | Sensitivity |
|---|---|---|---|
| MRI | 50–100 μm | No limit | 10−4–10−5 mM |
| PET | 1–2 mm | No limit | pM |
| SPECT | 1–2 mm | No limit | nM |
| OI | 2–5 mm | <2 cm | <<nM |
| CT | 50–200 μm | No limit | 0.1 mM a |
a For an iodine-containing CA.
A class of MRI CAs contains strongly paramagnetic metal ions, such as Gd3+, Mn2+ and Fe3+, encapsulated by a strongly binding chelating ligands, or in the form of paramagnetic nanoparticles (MNPs). These CAs increase the longitudinal relaxation rates of water protons significantly more than their transverse relaxation rates, giving rise to bright spots in T1-weighted (T1w) MRI images and are called positive or T1 CAs [3,4,5,6,7,10]. Another class of CAs, containing paramagnetic metal ions, such as Tb3+, Dy3+ and Ho3+, in the form of paramagnetic chelates or NPs, or as superparamagnetic NPs, such as iron-oxide NPs (SPIONs), induce protons in their vicinity to preferably undergo spin–spin relaxation, originating negative (or dark) contrast in T2-weighted MRI images and are called negative or T2 CAs. In addition to r1 and r2, the r2/r1 ratio (always ≥1, as T2 ≤ T1) represents another important factor in identifying the class of MRI CAs. This ratio should be close to one for a T1 CA (positive contrast), while it is large for a T2 CA [10,11,12,13].
Nowadays, approximately 40% of the MRI exams are carried out after administration of a CA. The materials used as CAs for clinical studies must meet the following requirements: (1) high relaxivity; (2) high water solubility or colloidal stability; (3) low osmolality to avoid pain from osmotic shock and other adverse effects upon injection; (4) low toxicity, which requires both high thermodynamic and kinetic stability (no transmetallation by endogenous metal ions such as Zn2+) in the case of metal chelates, and no leaching of toxic free metal ions in vivo from NPs; (5) rapid excretion, ideally not much longer than the MRI exam time; (6) a biodistribution with high specificity for the area of interest.
Various MRI contrast agents have been investigated and developed to achieve this goal. Gd-based CAs (GBCAs) include macrocyclic chelates of open-chain DTPA-type (DTPA = diethylenetriaminepentaacetic acid) or macrocyclic DOTA-type (DOTA = 2,2′,2″,2′″-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl) tetraacetic acid) derivatives, such as Magnevist® (Bayer Schering Pharma AG, Berlin, Germany), Dotarem® (Guerbet, Paris, France), Omniscan® (GE Healthcare, Chicago, IL, USA) and ProHance® (Bracco, Milan, Italy) [7], which are clinically used as T1 MRI CAs. These molecular agents have low r1 and r2 values and short blood circulation times due to their efficient renal excretion, and thus large amounts of injection doses are needed (typically 0.1 mmol/kg body weight) to achieve detectable contrast levels. This can increase the risk of toxicity due to potential release of free Gd3+ ions in the body [14]. This has occurred in some cases to patients with chronic kidney disease, which developed nephrogenic systemic fibrosis (NSF), characterized by skin thickening and hyperpigmentation and extracutaneous fibrosis [15,16,17]. In addition, recent studies indicated that clinically developed GBCAs could be deposited in the brain after repeated use, and could cause neurotoxicity, although this has not been proven [18,19]. In both cases, tissue deposits of linear GBCAs are much higher than those of macrocyclic GBCAs.
Biocompatible dextran-coated superparamagnetic iron oxide nanoparticles (SPIONs) Feridex® (Bayer HealthCare Pharmaceuticals, Wayne, NJ, USA), Sinerem®(Guerbet, Paris, France), and Resovist®(Bayer Schering Pharma AG, Berlin, Germany), have been approved by the Food and Drug Administration (FDA), USA, for clinical trials in liver (Feridex® and Resovist®) and lymph nodes (Sinerem®) MRI, as well as Lumirem® (Guerbet, Paris, France) for gastrointestinal imaging. However, Feridex® and Sinerem® were withdrawn from the market, mostly for commercial reasons. Nowadays, only Resovist® is used for liver imaging in some countries [7,20].
Magnetic NP (MNP)-based MRI CAs have many advantages relative to metal chelates, including larger magnetic moments and longer blood circulation times, which cause higher image contrast. They can also be used as multifunctional nanoplatforms for multimodal imaging, therapy and drug delivery (theranostics) after surface functionalization. [21]. MNPs are composed of two parts: a magnetic core that enhances MRI and a surface-coating ligand layer responsible for colloidal stability and minimizes toxicity. This review focusses on recent developments and optimization and in vivo applications of MNPs containing Gd, Mn, Fe and other lanthanide ions which may function as dual-mode T1–T2 MRI contrast agents (DMCAs). As the kind of contrast provided by MNPs depends fundamentally on their r2/r1 ratios, the description of the principles for rational design of DMCAs starts by a brief summary of the basic theory of paramagnetic relaxation in MNPs. Then, as their r2/r1 ratios are critically dependent on the MNPs composition, size and surface-coating, the different ways in which these properties can be modulated to design T1 or T2 single-mode MRI CAs or T1–T2 DMCAs are illustrated using selected examples. As the MNPs composition is a very important factor, a particular attention is given to systems using a single type of contrast material (e.g., Gd- or Mn-based T1 contrast materials or superparamagnetic T2 contrast materials) or both types of contrast materials in the same nanoplatform.
2. Basic Principles of Paramagnetic Relaxation in Small Complexes and Nanoparticles
The observed longitudinal proton relaxation rate () of a small paramagnetic complex in aqueous solution is the sum of a paramagnetic and a diamagnetic term, where the first is proportional to the concentration of the paramagnetic ion and the second is the contribution of the water solvent:
The observed relaxivity (in mM−1 s−1 units) is the sum of the inner (r1is) and outer sphere (r1os) terms:
The same equations apply to R2 and r2. These contributions are evaluated using the dominant dipole–dipole relaxation mechanism, which is modeled by the Solomon–Bloembergen–Morgan (SBM) theory for r1is, and the Freed theory for r1os [3,4,5,6,22]. For r1, the main parameters determining the IS contribution are the number of water molecules in the first coordination sphere of the metal ion (q), and the three processes responsible for the time fluctuation of the nucleus–electron interactions, which are the inner-sphere water residence time (τM = kex−1, where kex is the water exchange rate), the molecular reorientational correlation time (τR) and the electron spin relaxation time (T1e) of the metal ion, which is frequency dependent. The OS contribution is determined by the diffusion correlation time (τD) and the distance of closest approach (a) of the water molecules freely diffusing near the complex.
The r1 and r2 values of paramagnetic NPs also have in principle IS and OS contributions. The IS contribution results from the exchange of the water protons directly coordinated to the metal ions at the NPs surface with bulk water, as those located below the surface have a negligible effect. The IS r1 depends on the hydration number of the surface ions and the NP’s surface-to-volume ratio [23]. In the case of Gd3+ ions, they affect the bound water proton relaxation mainly through the dipolar mechanism, which can be modeled by the SBM equations. When other paramagnetic Ln3+ ions with high magnetic moments and very short T1e values (e.g., Tb3+, Dy3+, Ho3+) (Table 2) are present, a Curie term is also present [24,25,26].

Table 2.
Main chemical and physical characteristics of paramagnetic ions used in MRI CAs.
However, for surface-coated NPs, the OS contribution dominates the T1 and T2 relaxation, as the water protons are indirectly in contact with the paramagnetic metal ions in a NP due to the surface coating. It results from the diffusion of water molecules in the fluctuating magnetic field inhomogeneities created in their vicinity by the magnetized NPs, and does not contain the Curie contribution. The r1 value is approximately proportional to the square of the spin magnetic moment (μs2 = S(S + 1)ħ2 for transition metal ions and μs2 = 4S(S + 1) + L(L + 1)ħ2 for Ln3+ ions, where S is the spin quantum number and L is the orbital quantum number) multiplied by the number (N) of Ln3+ ions in a NP which can interact with a water proton, as given by Equation (3):
r1 ∝ Nμs2
Transverse relaxation of water protons is induced by fluctuations of local magnetic fields generated by the NPs. Thus, r2 is proportional to the square of the total magnetic moment (μ) of the NP, as given by Equation (4):
r2 ∝ μ2
In the case of superparamagnetic NPs, the OS contribution is also dominant. However, for a colloidal dispersion in the presence of a magnetic field, the return of their magnetization to equilibrium is determined by two different processes [11]: (a) the Néel relaxation, describing the return of the magnetization of each of the NPs to equilibrium after a perturbation that tilts that magnetization away from the direction of its easy axis; its relaxation time (τN) defines the fluctuations that arise from jumps of the magnetization between different easy directions; (b) Brownian relaxation, defined by the relaxation time τB, which characterizes the viscous rotation of the particle. The global magnetic relaxation rate of the colloid is the sum of the Néel (τN−1) and Brownian (τB−1) relaxation rates, τ−1 = τN−1 + τB−1, where τ is the global magnetic relaxation time. In these systems, r1 and r2 can be described by Freed’s model for paramagnetic systems, using the diffusion correlation time (τD) and considering the electron spin longitudinal relaxation time τS1 as equal to the Néel relaxation time τN.
The magnetization of the superparamagnetic iron oxide NPs reaches its saturation value MS at B0 ≤ 0.5 T, which is at the lower limit for commonly used clinical MRI scanners. Therefore, their R2 is in practice usually independent of B0. The r1 value is governed by the volume fraction of the superparamagnetic particles (υ), the diffusion correlation time (τD = d2/4D, where d is the diameter of the particle and D is the diffusion coefficient), and the magnetization of the NP (MS) at the B0 value of the clinical MRI scanner. A discussion of the transverse relaxivity of spherical superparamagnetic NPs can be found in the literature [11,27,28,29].
Superparamagnetic NPs are the T2 CAs most commonly used in MRI, usually with a very high r2 (typically 60–400 s−1mM−1), in particular the SPIONs. This can hamper the interpretation of T2w images due to the difficulty in distinguishing the CA-induced darkening from partial-volume artifacts, motion artifacts, and tissue inhomogeneities [30].
3. Magnetic Nanoparticles as T1–T2 Dual-Mode MRI Contrast Agents
3.1. NPs for T1 or T2 Single-Mode MRI Contrast
The type of contrast provided by MNPs as MRI CAs depends on their r1 and r2 values, as well as their r2/r1 ratios, which are dependent on their composition, size and surface-coating [31]. The ideal T1 MRI CA should have high r1 values and r2/r1 ratios close to 1.0. The most important contribution to the r1 values comes from the metal ions present on the MNP surface, which interact directly with nearby water proton spins by an IS mechanism. The most efficient paramagnetic ions are Gd3+ (S = 7/2), Mn2+ (S = 5/2), and Fe3+ (S = 5/2) (S is the total spin quantum number) due to their high number of unpaired electrons, high magnetic moments and long T1e values (Table 2) [3,4,5,6,22].
Several inorganic Gd3+-containing NPs with the largest possible Gd3+ densities [32], such as Gd2O3 [33,34], Gd2O2S [35], Gd-carbonates [36], GdF3 [37,38], and GdPO4 [39,40], have been proposed as potential T1 MRI CAs. Small (<10 nm diameter) Gd2O3 NPs have been the most intensively investigated, stabilized by coating with D-glucuronic acid [41], polyvinylpyrrolidone (PVP) [42], or polyethylene glycol (PEG) [43]. Paramagnetic NPs incorporating Gd3+ into the particle core or shell in a core/shell construct with large magnetization values have been proposed as T1 MRI CAs. An example of this approach is NaYF4:Yb, Er@NaGdF4 core/shell NPs (20−40 nm diameter), with Gd3+ positioned only on the outer shell, where it can interact directly with water protons and promote T1w image contrast [44].
Many small (<10 nm diameter) Mn-containing NPs, including MnO (Mn2+), Mn2O3 (Mn3+) and MnO2 (Mn4+), have also been studied as T1 MRI CAs [45,46,47,48]. Their r1 values depend strongly on their geometry and morphology, as their interfaces with water critically influence their contrast effects. Some typical examples are zwitterionic dopamine sulfonate (ZDS)-coated ultrasmall MnO NPs (USMnO@ZDS) [49], PEG-AS1411 aptamer-coated MnO (AS1411-PEG-MnO) NPs [50] and ligand-free Mn3O4 NPs [51]. Non-oxide Mn2+-containing materials, such as other inorganic (i.e., KMnF3, MnWO4), Mn2+-chelate-based, hybrid organic/inorganic and Mn2+-based layered double hydroxide (Mn-LDH) NPs have also been exploited as T1 MRI probes.
The use of high spin Fe3+ ions as T1 MRI probes within nanostructures for in vitro studies or preclinical investigations has been recently reviewed [52], including examples such as amphiphilic polymer-based NPs like Fe3+-chelated poly(lactic-co-glycolic) acid (PLGA) NPs, NPs containing polyphenolic Fe3+-binding units such as Fe3+-loaded synthetic melanin nanoparticles (SMNPs) and bimetallic (Gd, Fe)-phenolate coordination polymer (CN) NPs. In several of these systems, although the Fe3+ ion is not hydrated (q = 0) and therefore the IS (q ≥ 1) contribution to relaxivity is not present, the reported r1 values are higher than those expected for the OS (q = 0) contribution alone, which suggests the presence of a significant contribution from the water molecules of the second hydration sphere (SS).
The ideal T2 MRI CAs should have high r2 values and high (≥10) r2/r1 ratios to induce predominantly T2 proton spin relaxation, leading to a decrease in signal intensity in T2w or T2w* MRI images [31]. The systems that fulfil these conditions are SPIONs and paramagnetic lanthanide (Ln3+)-based NPs (Ln = Tb, Dy and Ho), specially at high magnetic fields (B0).
As discussed in Section 2, SPIONs are the T2 CAs most commonly used in MRI, having a very high r2 (usually 60–400 s−1mM−1). Examples are Resovist® (a carboxydextran-coated SPION), octapod NPs with higher r2 values than spherical NPs, highlighting the importance of size and morphology optimization to obtain NPs with high r2 [53], ultrasmall SPIONs (USPIONs) with a scaffold of bovine serum albumin (BSA) [54], SPIONs conjugated with PEG of molecular weight (MW) in the 600–8000 amu range [55] and ferrimagnetic iron oxide nanocubes (FIONs) encapsulated in PEG phospholipids to become water-dispersible (WFIONs) [56]. The WFIONs have an extremely high r2 value of 761 mM−1.s−1 at 3 T.
Paramagnetic NPs containing Tb3+, Dy3+ or Ho3+ have high r2 values due to their high magnetic moments and very short (<1 ps) electronic relaxation times (Table 2) [24]. Therefore, they can be alternatives to SPIONs as efficient T2 and T2* CAs [13,57,58,59,60], in particular at high MRI fields (B0 > 3 T). This is because r2 generally increases with B0, but the contribution from the Curie spin relaxation mechanism increases with B02. The maximum r2 values are obtained at slower inner sphere water exchange (τM = 0.1–10 μs) than for r1 (τM = 1–100 ns) [61]. Also, in contrast to iron oxide particles, Ln3+-containing NPs show no saturation of the magnetization, even at magnetic field strengths as high as 30 T [62].
The highest payload of Ln3+ ions per particle at a particular site can be delivered by inorganic Ln3+-containing NPs, including Ln2O3, LnF3, and NaLnF4. NPs with a diameter of 50–100 nm contain approximately 106 Ln3+ ions each and their magnetic properties make them good candidates for T2w and T2w* MRI CAs [63]. Dy3+-based nanomaterials are the most studied, as they show the largest T2 relaxation effects [23]. Moreover, the T2 effects for Dy3+-based nanomaterials, such as β-NaDyF4, are two orders of magnitude higher than for clinically approved iron oxide nanomaterials under the increasingly used very high field magnets (7 T and higher). This arises due to the Curie spin relaxation mechanism of the Dy3+-based systems [64]. However, coating of the particles is necessary to avoid leaching of free Ln3+.
3.2. NPs as T1–T2 DMCAs
While conventional MRI CAs respond only in a single imaging mode, either T1 or T2, NPs with multimodal capabilities provide complementary diagnostic information. However, when hybrid imaging systems, such as PET/CT or PET/MRI scanners are not available, a combination of two different imaging devices must be used separately, which is an inconvenient, time-consuming and expensive procedure [65,66,67,68,69]. The development of MRI T1–T2 dual-mode CAs in a single nanoplatform is an attractive solution to overcome ambiguities in conventional MRI diagnostics, especially when the biological targets are small, as well as the image matching difficulties caused by relocating the imaging object, and by the discrepancies resulting from different depth penetrations and spatial/time resolutions of multiple imaging strategies [69]. In fact, T1–T2 dual-modal MRI images can be easily acquired by changing the parameters of the pulse sequences in the operational mode of the same MRI scanner.
Dual-modal T1–T2 MRI CAs (DMCAs) should have both high r1 and r2 values, with r2/r1 ratios (~2–10) between those of ideal T1 and T2 MRI CAs. If Gd-based NPs are to be used as DMCAs, their r2/r1 ratios should be increased from their usual values of ~1, while the use of SPION-based DMCAs requires a decrease in their r2/r1 ratios from their usual very high values. Mn-based NPs are useful as DMCAs because of their suitable r2/r1 ratios.
The approaches proposed to design DMCAs comprise: (1) use of NPs with a single type of contrast material, Gd- or Mn-based NPs or superparamagnetic NPs with appropriate size and magnetization to provide T2 and T1 contrast; or (2) include both T1 and T2 contrast materials in the same NP. Both have advantages and disadvantages [31,69,70,71,72].
3.2.1. DMCAs Based on a Single Type of Contrast Material
- NPs based on a typical T1 agent
This strategy is based on the clustering of a T1 contrast material within non-magnetic porous matrices to obtain enhanced r1 and specially r2 values. The T1 contrast material includes Gd-based complexes (e.g., Gd (DOTA)) or NPs (e.g., Gd2O3) [73,74,75,76], Mn-based NPs (e.g., MnO, MnOx) or Mn2+ ions [77,78,79], mixed Gd/Dy NPs [80,81], and Fe3+ cations [82,83,84] entrapped in porous matrices, such as polymers (e.g., poly(amidoamine) (PAMAM) dendrimers, poly-(α,β)-DL-aspartic acid (PASA), mesoporous poly dopamine (MPDA)), porous silica, proteins (e.g., bovine serum albumin (BSA), hydrogels (e.g., hyaluronic acid (HA)/CH (chitosan)) or organic frameworks (e.g., NCP and CP3 coordination polymers)) (Table 3). This entrapment of metal chelates and NPs by serum proteins is likely to occur by opsonization [76]. Some DMCAs have also theranostic properties, by including drugs such as doxorubicin (DOX) in the matrices [78,79,83,84]. The ideal range of r2/r1 ratio can be approached by increasing r2 of water protons through restricting water diffusion around the complex through clustering [73]. Another possibility is to decrease r1 by restraining the metal ion coordination and chemical exchange of surrounding water molecules through geometrical confinement.

Table 3.
Summary of basic properties of the examples of DMCA NPs based on a typical T1 contrast agent discussed in this section. Some of the systems in this Table have been used as theranostic agents. * Not available.
A few of the systems summarized in Table 3 are discussed here in more detail. Marasini et al. reported a Gd-based T1–T2 DMCA consisting of Gd2O3 NPs (2 nm diameter) coated with the hydrophilic polymer PASA using the one-pot polyol method. The NPs had r1 = 19.1 mM−1.s−1 and r2 = 53.7 mM−1.s−1 (r2/r1 = 2.8) at 3 T. After i.v. injection of Gd2O3@PASA NPs into the mice tail vain, T1 and T2 contrasts were observed in the T1w and T2w MRI images of the mouse livers at 3 T, respectively (Figure 1) [74].
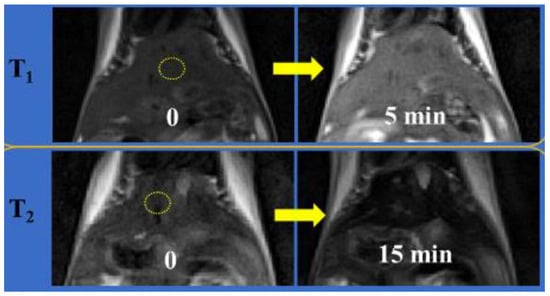
Figure 1.
T1 and T2 MR images of mice liver before (labeled as “0”) and 5 and 15 min after intravenous injection of an aqueous solution of PASA-coated Gd2O3 nanoparticles into mice tails, respectively (Nmice = 2 for each modal imaging). The echo time = 10 (or 37) ms, repetition time = 385 (or 1620) ms, pixel bandwidth = 299 (or 197) Hz, number of acquisitions = 8 (or 4), echo train length = 3 (or 13), flip angle = 120° (or 120°), slice thickness = 1.0 (or 1.0) mm, and slice gap = 1.1 (or 1.1) mm were used for T1 (or T2) MR image measurements. Reproduced from [74].
A Mn-based T1–T2 DMCA was reported consisting of dual mesoporous silica spheres loaded with a MnO nanocluster (diameter < 2 nm) (Mn-DMSS) with a hydrodynamic diameter in the 100–200 nm range (Figure 2), with r1 = 10.1 mM−1.s−1 and r2 = 169.7 mM−1.s−1 (r2/r1 = 16.8) at 3 T. In vivo MRI experiments on Sprague Dawley (SD) rats at 3 T showed a 29% signal enhancement in the liver in T1w images and a 28% signal decrease in T2w images upon injection of Mn-DMSS NPs (Figure 2) [77].
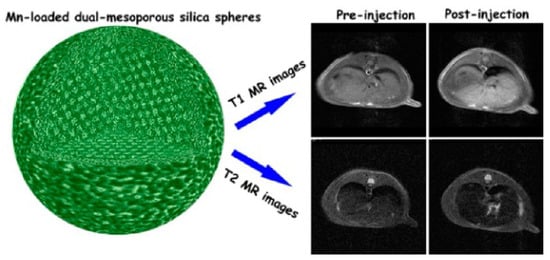
Figure 2.
(Left): a model structure of Mn-DMSS NPs; (Right): Simultaneous T1 and T2 imaging of a SD rat liver on a 3.0 T MRI scanner: In vivo T1-weighted (top) and T2-weighted (bottom) MR images. Reproduced with permission from [77]. Copyright from the American Chemical Society.
A Gd/Dy-based novel PEGylated Dy-doped NaGdF4 nanoprobe (NaGdF4:Dy @DSPE-PEG2000) was developed as a T1/T2-weighted MRI/CT imaging nanoplatform, with r1 = 5.17 mM−1.s−1 and r2 = 10.64 mM−1.s−1 (r2/r1 = 2.4) at 9.4 T and strong X-ray attenuation properties (44.70 HU L g−1) in vitro. T1w/T2w MRI/CT imaging in vivo of mice injected with the nanoprobe led to significant contrast enhancement of liver, spleen and kidneys at 24 h post injection. This multifunctional nanoprobe had low cytotoxicity and was completely excreted from the injected mice [81].
Finally, a Fe-based dual-mode T1–T2 MRI DMCA was reported consisting of a one-pot synthesized nanostructured coordination polymer containing Fe3+ (Fe-NCP) functionalized with BSA. These NPs, with a hydrodynamic diameter of 97 nm and a ζ potential of −31.2 mV, had high colloidal stability, low cytotoxicity and good relaxivities, r1 = 5.3 mM−1.s−1 and r2 = 10.9 mM−1.s−1 (r2/r1 = 2.1) at 7.0 T. Their T1–T2 MRI contrast was verified in in vitro phantoms, ex vivo C57BL/6J mice and in vivo GL261 glioblastoma tumor-bearing mice. The vivo MRI of Fe−NCPs showed high T1 and T2 contrast in the tumor in a very short period of time and were safe for the mouse. Their large long-term uptake in the spleen was related to their rapid clearance by the mononuclear phagocytic system (MPS) due to the NPs size being bigger than 40 nm [83].
Table 3 shows that not all the systems described reach the low r2/r1 values recommended for efficient DMCAs, illustrated by the second and fourth examples discussed above [77,83].
- 2.
- NPs based on a typical T2 agent: USPIONs and FeOx
This strategy is based on using superparamagnetic NPs with appropriate size and magnetization to provide T2 and T1 contrast simultaneously. Although SPIONs with a core diameter less than 10 nm can produce positive contrast in T1w images at low concentrations, their high T2 effects (high r2/r1) resulting from their high magnetic moment limit their application as T1–T2 DMCAs. However, the magnetic moment of Fe3O4 NPs is strongly dependent on their size and decreases rapidly as their size decreases due to the reduction in the volume magnetic anisotropy and spin disorder (canting) at their surface, which suppresses the T2 effect and therefore maximizes the T1 contrast effect, controlling their relaxivities [11]. Therefore, the appropriate ultra-small-sized Fe3O4 NPs (USPIONs) are potential candidates for T1–T2 DMCAs.
Some examples of such systems are summarized in Table 4, where the USPION core was coated with hydrophilic polymers, e.g., poly(methacrylic acid) (PMAA)-polytrimethylene terephthalate (PMAA-PTTM) [85], poly(acrylic acid) (PAA) [86,87] and PEG [88], or silica [89], to prevent the aggregation of the NPs and to ensure a small particle size. The core was usually spherical, but in some cases nanoplates [89] or nanocubes [90,91] were used. Some of the systems summarized in Table 4 are now discussed in more detail.
Li et al. reported monodispersed water-soluble and biocompatible USPIONs (3.3 nm average diameter) grafted with thiol functionalized (PMAA)-(PTTM) using a high-temperature co precipitation method. These NPs, with r1 = 8.3 and r2 = 35.1 s−1.mM−1 (r2/r1 = 4.2) at 4.7 T, showed in vitro and in vivo potential as T1–T2 DMCAs upon i.v. injection in mice, as positive and negative contrasts were observed in the T1w and T2w MRI images of liver and kidneys, respectively [85]. Miao et al. developed PAA-coated USPIONs (5.1 nm core diameter and 41.35 nm average hydrodynamic size) with r1 = 10.52 and r2 = 38.97 s−1.mM−1 (r2/r1 = 3.70) at 1.41 T. The NPs had in vitro and in vivo potential as T1–T2 DMCAs, as illustrated by T1w and T2w MRI images at 3 T, with positive contrast observed in the rabbit vasculature, while the rabbit popliteal lymph node exhibited negative contrast [87]. Wang et al. reported USPION-PEG (P-UDIOC) NPs with extremely small core size (2.3 nm) and a compact hydrophilic PEG surface and with r1 = 1.37 and r2 = 7.53 s−1.mM−1 (r2/r1 = 5.5) at the ultrahigh field (UHF) of 7.0 T. These UHF-tailored T1–T2 DMCAs showed dual enhanced T1–T2 contrast at 7 T and enabled a clear visualization of microvasculature as small as ≈140 μm in diameter under UHF MRI (Figure 3), extending the detection limit of 7 T MRI angiography (MRA) [88].
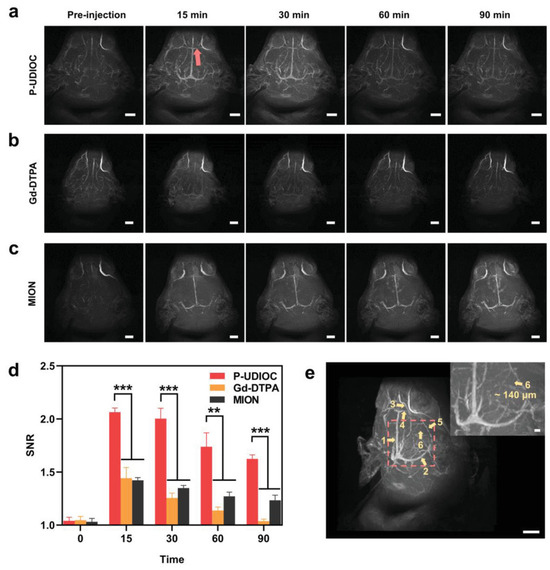
Figure 3.
Vascular imaging performance of P-UDIOC-enhanced UHF MRI. (a) P-UDIOC, (b) Gd-DTPA and (c) MION enhanced MRA maximum intensity projection images of the rat brain (scale bar = 5 mm). (d) Quantitative analysis of vascular imaging performance by calculating SNR values of inferior cerebral vein indicated by the red arrow in a before and after i.v. injection of P-UDIOC, Gd-DTPA, and MION (n = 3). Statistical analysis was performed using a Student’s t-test, with *** indicating p < 0.001, ** indicating p < 0.01. (e) 3D volume image of the rat brain at 15 min post-injection of P-UDIOC, showing high spatial resolution of the vasculature (scale bar = 5 mm). The yellow arrows and labeled numbers in the image show the vascular details including 1, superior sagittal sinus; 2, transverse sinus; 3, anterior cerebral artery; 4, inferior cerebral vein; 5, basal vein; 6, middle meningeal artery. The inset image in (e) is an enlargement of the region indicated by the red dashed square (scale bar = 500 μm). Reproduced from [88].
An example of a system with a non-spherical core consisting of Fe3O4 crystal nanoplates was reported by Zhou et. al. [89] These superparamagnetic magnetite nanoplates had (111) exposed facets which much contributed to their T1 and T2 contrast effects. The main contribution to r1 of the magnetic nanoplates is the chemical exchange with bulk water (given by the exchange lifetime, τM) of the water molecules bound at the inner-sphere of the Fe3+ ions on the highly exposed particle surface iron-rich Fe3O4 (111) surfaces, according to the dominant IS model [11]. The r2 values are dominated by the OS mechanism, which accounts for the effect of fluctuations of the local magnetic field inhomogeneities induced by the tumbling NPs on the protons of outer-sphere water molecules diffusing nearby. According to Freed’s theory, the r2 value is proportional to the square of the magnetization (Ms2), which relates to the intrinsic superparamagnetism of the nanoplates and determines the strength of the local magnetic field inhomogeneities, as well as to the square of the effective radius of the magnetic core (R2), which determines the field perturbation areas for the outer-sphere protons. The rapid random flipping of the anisotropic nanoplates, when represented by an equivalent simulated sphere, generate a larger area of local field inhomogeneity compared with nanospheres under the same applied magnetic field. The balance of T1 and T2 contrasts was attained by controlling the structure and surface features of the nanoplates, including morphology (nanoplates vs. spheres in IOP-4.8@stPE) and surface coating (e.g., IOP-4.8 vs. IOP-4.8@SiO2 and IOP-4.8@stPE), with a large decrease in r1 due to blocking of the exposure of the facets causing the disappearance of the IS contribution and corresponding decrease in r2/r1, leading to change from DMCAs to T2 agents. The decrease in the nanoplate thickness decreased the r2/r1 value, as IOP-8.8 (r2/r1 ~ 8.18) is T2-dominated, while the IOP-4.8 (r2/r1 ~ 4.22) is a T1–T2 DMCA [89].
Table 4 shows that not all the systems described reach the low r2/r1 values recommended for efficient DMCAs [90,91].
- 3.
- Triggered aggregation change in ESIONs
The design of T1–T2 DMCAs can also be based on a different mechanism, based on the change in the aggregation state of extremely small-sized iron oxide nanoparticles (ESIONs) in the size range of 1.5–4 nm with relatively high r1 values, triggered by an external signal, such as pH change, a redox reaction or laser light. The aggregation of ESIONPs can generate T2 contrast effects due to the enhancement of magnetic field inhomogeneity and magnetic coupling between Fe centers. Some examples of such systems are summarized in Table 4. An example is based on pH-sensitive hydrazine functionalized ESIONPs forming assemblies (IONAs) cross-linked by small-molecular aldehyde derivative ligands. The dynamic formation and cleavage of hydrazone (–C=N–N–) (f) linkages in neutral and acidic environments, respectively, is the base for the reversible response of the nanoassemblies to pH variations. At pH 7.5, IONAs are stable and with high r2/r1 = 34.2, while at acidic pH 5.5, such as in the acidic tumor microenvironment (TME), the hydrazone bonds are cleaved and the IONAs are disassembled into hydrophilic dispersed ESIONs, with a r2/r1 = 4.1. The change in this relaxivity ration results from an increase in the number of second sphere water molecules (qSS) and a large decrease in the magnetization (Ms) of the dispersed ESIONs relative to the IONAs, which affect their r1 and r2 values, respectively [92]. Another example is based on ESIONs linked with the targeting ligand folic acid (FA) binding arthritis-associated macrophage cells, and the light responsive diazirine (DA) through a PEG spacer (Fe3O4-PEG-(DA)-FA). These nanoparticles can form nanocomposites (NCs) upon laser irradiation to have tunable r1 and r2 values upon variation in the laser irradiation time. The change in the r2/r1 value from 2.36 in the NPs to 18.8 in the NCs led to the use of the designed Fe3O4-PEG-(DA)-FA NPs as T1 CAs in in vivo MRI of an arthritis mouse model without lasers and enhanced T1–T2 DMCAs in the arthritis inflamed region under laser irradiation due to the formation of NCs that accumulated within the arthritis region and their limited intravasation back to the blood circulation [93].
Again, Table 4 shows that not all the systems described reach the low r2/r1 values recommended for efficient DMCAs: while the first one does [92], the second one reaches quite high r2/r1 values [93]. Besides this, in some cases, T1/T2 triggering effects, such as pH decrease or reduction by GSH in the TME, led to final r2/r1 values below or above the range where T1–T2 DMCAs operate, forming instead T1/T2 switching MRI CAs [94,95,96,97,98,99,100,101]. This illustrates how the control of the switching mechanism at the molecular level is difficult.

Table 4.
Summary of basic properties of the examples of NPs based on a typical T2 ((U)SPION)) contrast agent discussed in this section.
Table 4.
Summary of basic properties of the examples of NPs based on a typical T2 ((U)SPION)) contrast agent discussed in this section.
| NPs Components | r1 (mM−1.s−1) | r2 (mM−1.s−1) | r2/r1 | Bo (T) | dH (nm); ζ (mV) | In Vitro/In Vivo Model for MRI | Ref. |
|---|---|---|---|---|---|---|---|
| USPION-PMAA-PTTM | 8.3 | 35.1 | 4.2 | 4.7 | 3.3 (core); -* | In vitro phantoms /In vivo mice | [85] |
| USPION-PAA | 8.20 | 16.67 | 2.03 | 7.0 | 1.7 (core): -* | In vivo Kunming mice | [86] |
| 6.15 | 28.62 | 4.65 | 4.6 (core); -* | ||||
| USPION-PAA | 10.52 | 38.97 | 3.7 | 3.0 | 41.3; -14.7 | In vivo rabbit | [87] |
| USPION-PEG | 1.37 | 7.53 | 5.5 | 7.0 | 2.3 (core); dH ≈12; -* | In vivo rat brain angiography (MRA) | [88] |
| Fe3O4 nanoplates: | 0.5 | In vitro phantoms | [89] | ||||
| IOP-4.8 | 43.18 | 182.2 | 4.22 | 4.8 thickness | |||
| IOP-4.8@SiO2 | 2.0 | 118.73 | 59.3 | 5.6 thickness | |||
| IOP-4.8@stPE spheres | 3.59 | 338.9 | 94.4 | 90; -* | |||
| Fe3O4 nanocubes | 5.23 | 89.68 | 17.1 | 3.0 | 27.8; -* | In vivo SD rats | [90] |
| ND-PEG-tNCIO | 31.8 | 790.6 | 24.7 | 50; −61 | In vitro phantoms | [91] | |
| IONA → ESIONP dispersed pH 7.4 → 5.5. | 3.2 → 108.0 | 5.1 → 22.3 | 34.2 → 4.1 | 3.0 | 80 | In vitro A549 cells In vivo tumor mice | [92] |
| Fe3O4-PEG-(DA)-FA → NCs (Laser) | 3.83 → 1.60 | 9.04 → 31.6 | 2.36 → 18.8 | * | * | In vivo arthritis mouse model | [93] |
* Not available.
3.2.2. DMCAs including Both T1 and T2 Contrast Materials in the Same Nanoparticle
The strategy of including both T1 and T2 contrast materials in the same nanoplatform has used several different designs to obtain hybrid nanostructures: (a) NPs integrating a superparamagnetic T2-contrasting material inside a paramagnetic T1 material (e.g., a Gd3+ complex); (b) doping a superparamagnetic NP with paramagnetic T1 contrast materials inside them; (c) T1 and T2 contrast materials connected side-by-side to form hybrid nano-oligomers (DB-HNT). In all these designs, with both contrast materials in the same nanoplatform, the magnetic fields generated by each CA disturb the relaxation process of the other. However, as typical paramagnetic T1 CAs have low r1 values compared to the r2 values of superparamagnetic T2 agents due to their relative magnetic moments, their effect on the T1 relaxation of the T1 contrast material is much larger than the opposite. The T2 agent generates a strong magnetic field induced by the external Bo, which is dependent on 1/r3 (r = distance from the T2 agent), which affects the electronic spin relaxation time (T1e) of the paramagnetic T1 agent depending on their relative locations. The resulting effects of this magnetic coupling depends on the separation distance (d) between T1 and T2 agents [102] and also on their relative positions within the NP (Figure 4) [103].
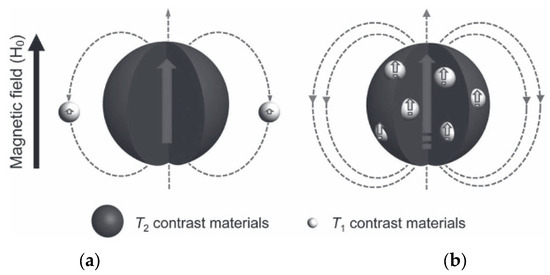
Figure 4.
Two spin phenomena between T2 and T1 contrast materials with different locations. (a) Left—the local magnetic field intensity of T1 contrast materials is reduced when located outside of the T2 contrast material. (b) Right—The local magnetic field strengths of T1 and T2 contrast materials are enhanced simultaneously when T1 contrast materials are located inside the T2 contrast materials. Reproduced from [103].
- NPs with a T2 material inside a paramagnetic T1 material
For systems consisting of a superparamagnetic core within a paramagnetic shell, the strong magnetic field from the core opposes the magnetic field created by the shell and reduces it (Figure 4, left), strongly quenching its r1 value. Their magnetic interaction is proportional to the inverse sixth power of their separation distance (d−6), as shown by a study where the distance-dependent magnetic resonance tuning (MRET) strategy for tuning the r1 value of a T1 agents was introduced [102]. A series of spherical NPs was designed, consisting of three components, where the paramagnetic enhancer (Gd-DOTA) was separated from the superparamagnetic quencher (a 12 nm Zn0.4Fe2.6O4 NP) by controlling the thickness of a SiO2 separating layer decreasing from 18 to 2 nm (Zn0.4Fe2.6O4@SiO2@ Gd-DOTA). A decrease in r1 was consistently observed as the separation distance between the T1 and T2 agents decreased. The T1 MRI signal was quenched when the d value between the enhancer and the quencher decreased and r1 decreased from 1.58 to 0.13 mM−1.s−1 (3 T), which was a consequence of the increase in the T1e value of the T1 agent [102].
The strategy of including a layer of increasing thickness to increase the T2 core-T1 shell distance, and thus modulating their magnetic coupling, was pursued using not only inorganic porous materials like SiO2, but also micellar structures incorporating organic block copolymers and inorganic porous materials as possible frameworks [104]. The basic properties of several examples from the literature are summarized in Table 5. These involve the formation of spherical or cubic core–shell NPs integrating a superparamagnetic T2-contrasting core (e.g., Fe3O4, MnFe2O4) inside a paramagnetic T1 shell (e.g., Gd2O3, Gd2O(CO3)2, MnO) [105,106,107,108,109,110,111,112], or conjugating the superparamagnetic core with a paramagnetic Gd3+ or Mn2+ complex at its surface [113,114,115,116,117]. Generally, a sharp decrease in the magnetic coupling effect upon increasing the core–shell separation was verified experimentally by including a silica shell of increasing thickness between the two materials [105,106,107], or by increasing the distance between the core and the layer of pendant paramagnetic complexes through longer spacer groups [102,113]. Here, we will discuss only a few of the examples in more detail.
For instance, a core–shell-type T1–T2 DMCA agent has been described, where the T1 contrast material, a Gd2O(CO3)2 layer (1.5 nm thickness) was located on the shell in order to be in direct contact with water molecules, to obtain for high T1 contrast; the superparamagnetic T2 contrast material, MnFe2O4 (15 nm size) was located at the core, from where it could induce a long-range magnetic field for the relaxation of water molecules. The two materials were separated by a SiO2 layer of increasing (4, 8, 12, 16 and 20 nm) thickness (MnFe2O4@SiO2@Gd2O(CO3)2 NPs). As the SiO2 layer became thicker, the magnetic coupling decreased, the T1 quenching was reduced and r1 increased (from 2.0 to 32.5 mM−1.s−1) while the r2 decrease was weaker (332 to 213 mM−1.s−1). When the thickness of the SiO2 layer was equal of larger than 16 nm, the r2/r1 values decreased from 160 to 6.5, and the NPs became T1–T2 DMCAs, as both T1 and T2 effects became larger than the effects of the individual single-mode contrasts (Figure 5) [105].
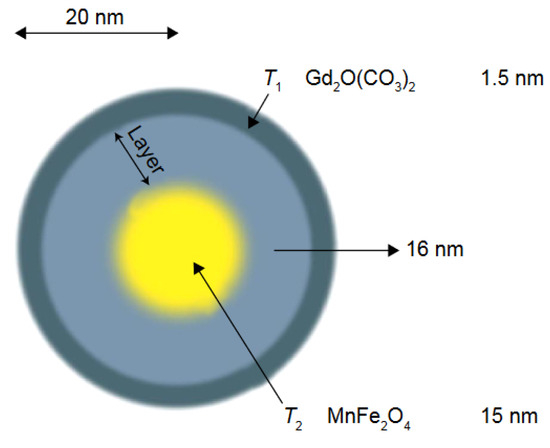
Figure 5.
Schematic image of the core–shell-type dual-mode nanoparticle [MnFe2O4@SiO2@Gd2(CO3)2]. The T1 contrast material is positioned on the shell to have direct contact with the water for high T1 contrast effects, and the superparamagnetic contrast material is located at the core, inducing a long-range magnetic field for the relaxation of water. Reproduced from [31].
A smart nanotheranostic system for early diagnosis and therapy of cancer was developed, consisting of camptothecin (CPT)-loaded mesoporous silica nanoparticles (MSN) capped with manganese oxide (MnOx)-coated SPIONs (MnOx-SPION@MSN@CPT NPs). The acid, oxidative stress and redox (GSH) response of MnOx regulated the CPT drug release from the MSN channels, while the high magnetization of the surface SPIONs achieved high r2 values (102.2 mM−1.s−1). At the same time, degradation of the MnOx shell caused release of Mn2+ in the TME, enhancing r1 (2 → 13.6 mM−1.s−1). The efficacy of this MRI responsive theranostic T1–T2 DMCA was confirmed in vitro on pancreatic cancer cells and in vivo on tumor-bearing mice (Figure 6) [111].
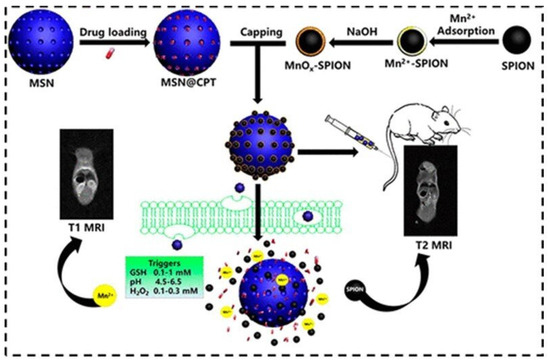
Figure 6.
Schematic illustration for the synthesis of MnOx-SPION capped MSN and controlled drug release in response to the tumor microenvironment. In vivo T2 and T1 MRI images of a mouse upon injection of a MnOx-SPION@MSN solution through tail vein. Reproduced with permission from [111]. Copyright from the American Chemical Society.
Some examples of systems conjugating the coating of SPIONs with a paramagnetic Gd3+ or Mn2+ complex will now be discussed briefly (Table 5) [113,114,115,116,117]. Up to now, the highest relaxivities for T1–T2 DMCAs are r1 = 31.6 mM−1.s−1and r2 = 836.7 mM−1.s−1 (at 1.47 T) which was obtained by Long et al. for Fe3O4@ALA-GdDOTA [113]. Superparamagnetic silica-coated iron oxide core–shell nanoparticles (Fe3O4@SiO2) conjugated at the surface to Gd-DTPA and the arginine-glycine-aspartic acid (RGD) peptide as a targeting ligand (Fe3O4@SiO2(Gd-DTPA)-RGD) NPs were synthetized. The NPs with a 21 nm diameter were water-dispersible, stable, and biocompatible, and with r1 = 4.2 mM−1.s−1 and r2 = 17.4 mM−1.s−1 (r2/r1 = 4.1) at the Gd/Fe molar ratio of 0.3:1. In vitro MRI experiments with U87MG and MCF- tumor cells over-expressing the high-affinity αvβ3 integrin showed targeted T1w positive and T2w negative contrast when loaded with the NPs (Figure 7) as well as in in vivo U87MG tumor mice injected with the NPs, proving their potential as targeted T1–T2 DMCAs [117].
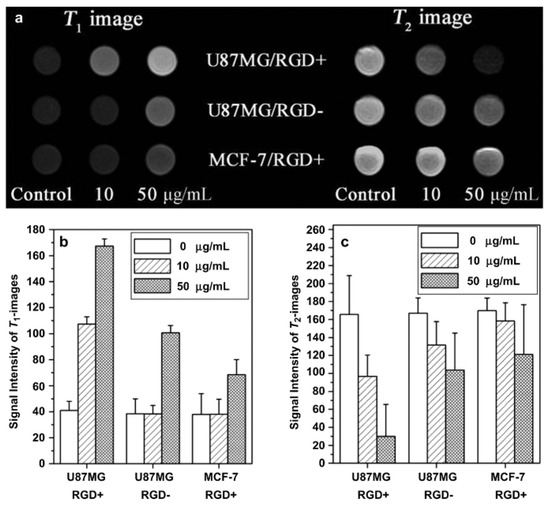
Figure 7.
(a) T1- and T2-weighted MR images of Fe3O4@SiO2 (Gd-DTPA)-RGD NPs in U87MG and MCF-7 cells at different concentrations of NPs after incubation for 6 h on a 3T MRI system (RGD+: Fe3O4@SiO2 (Gd-DTPA)-RGD). RGD-: Fe3O4@SiO2(Gd-DTPA); (b) signal intensity analysis for T1-weighted MR images; (c) signal intensity analysis for T2-weighted MR images. Reproduced with permission from [117]. Copyright from Elsevier.
In other studies, core–shell NPs with different metal ions in the shell and/or in the core were studied (Table 5). Superparamagnetic SPION core−porous silica shell nanoparticles, containing the paramagnetic complexes ([Ln(btfa)3(H2O)2]) (btfa = 4,4,4-trifluoro-L-phenyl-1,3-butanedione, Ln = Gd/Eu) (γ-Fe2O3@SiO2/[Gd/Eu(btfa)3(H2O)2]) imbedded in the shell, with a 50 nm diameter and a 10 nm core, performed as a promising trimodal T1−T2 MRI and optical imaging contrast agent in Hela cells suspensions in vitro [118]. Also, colloidal suspensions of Fe/Fe2O3 NPs capable of providing both T1w and T2w MR images were synthesized [119]. Fe@Fe3O4-Gd(DOTA) NPs are strongly paramagnetic at room temperature with a Ms = 55 emu.g−1, and r2 and r1 values higher than those of Fe@Fe3O4 and Gd(DOTA) alone. These increased relaxivities suggest that the NPs are potential T1–T2 DMCAs for MRI [120]. Avocado-like Fe3+/Fe2O3 NPs were developed for working as T1–T2 DMCAs, based on a PDA-Fe3+-TA (tannic acid) coordination network (CNMN) embedded with DOX (Fe2O3@PDA-Fe3+-TA-CNMN/DOX). These NPs had suitable r1 and r2 values and the strong heat generated by Fe3+-TA allowed the CNMN to act as a photothermal agent and to effectively deliver the chemotherapeutic drug DOX to achieve chemo-photothermal combination therapy [121]. Finally, an iron core (with its subsequent oxidation giving a ferrite shell) with added Ni2+ ions to form a superparamagnetic nickel ferrite shell NP has been studied; with its surface treated with dopamine-PEG to make it dispersible, and it acts as a T1–T2 DMCA [122].
Table 5 shows that many of the systems described using this strategy have r2/r1 values above the values recommended for efficient DMCAs [107,109,110,113,115,118,120,121]. Besides this, the systems based on the release of toxic free Mn2+ in the TME, although interesting for animal studies, are not suited for clinical applications [110,111].

Table 5.
Summary of basic properties of the examples of NPs with a T2 material inside a paramagnetic T1 material.
Table 5.
Summary of basic properties of the examples of NPs with a T2 material inside a paramagnetic T1 material.
| NPs Components | r1 (mM−1.s−1) | r2 (mM−1.s−1) | r2/r1 | Bo (T) | dH (nm); ζ (mV) | Therapeutic Modality | In Vitro/In Vivo Model for MRI | Ref. |
|---|---|---|---|---|---|---|---|---|
| MnFe2O4@SiO2@ Gd2O(CO3)2 | 2.0 → 32.5 | 332 → 213 | 166 → 6.5 | 4.7 | 26 → 58 | - | In vitro phantoms | [105] |
| MnFe2O4@SiO2@ Gd2O(CO3)2 | 3.7 → 32.3 | 312 → 208 | 84.3 → 6.4 | 3.0 | 31 → 55 | - | In vitro phantoms | [106] |
| Fe3O4@mSiO2/PDDA/BSA-Gd2O3 | 11.47 | 195.1 | 17.0 | 3.0 | 345.6; +26.9 | - | In vitro 786-0 cells In vivo BALB/c mice | [107] |
| Fe3O4@Gd2O3 nanocubes | 45.24 | 186.51 | 4.1 | 1.5 | 9.2; -* | - | In vitro phantoms In vivo SD rats (3 T) | [108] |
| Fe3O4@MnO-PEG | 1.3 | 35.8 | 28 | 3.0 | 5.0; -* | - | In vivo BALB/c mice (7.0 T) | [109] |
| Fe3O4@Mn3O4 → Fe3O4 +Mn2+ (GSH) | 2.4 → 16.1 | 92 → 258 | 38.4 → 16.1 | 1.5 | 22; -* | - | In vivo MKN-45 tumor-bearing mice | [110] |
| MnOx-SPION @MSN@CPT → SPION +Mn2+ (GSH, pH) | 2 → 13.6 | 102.2 → -* | * | 0.5 | 120; -* | ChT (CPT) | In vivo pancreatic-tumor- bearing mice | [111] |
| Fe3O4@ALA-GdDOTA (NP5) Fe3O4@ALA-Mn-DOTA (NP6) | 31.6 | 836.7 | 26.4 | 1.47 | 6; * | - | In vitro phantoms | [113] |
| 14.2 | 324.5 | 22.8 | ||||||
| Fe3O4@DOPA-GdDTPA-PEG | 11.17 | 30.32 | 2.7 | 3.0 | 73.8; −5.5 | - | In vivo BALB/c nude mice. | [114] |
| Fe3O4/CuInS2@SiO2- (GdDTPA)-RGD | 1.56 | 23.22 | 14.9 | 3.0 | 45; +8.16 | - | In vivo BSPC-3 pancreatic tumor mice | [115] |
| MnFe2O4@Gd: FA-DTPA-PEG-DIB- | 20.59 | 68.48 | 3.32 | 0.55 | 18; * | - | In vitro Hela and 3T3 cells | [116] |
| Fe3O4@SiO2-GdDTPA-RGD | 4.2 | 17.4 | 4.1 | 3.0 | 27; +7.25 | - | In vitro U87MG cells In vivo U87MG tumor mice | [117] |
| γ-Fe2O3@SiO2/[Gd/Eu (btfa)3(H2O)2] | 1.0 | 75.9 | 78 | 9.4 | 50; −40 | - | In vitro Hela cells | [118] |
| Fe@Fe3O4-GdDOTA | 7.2 | 109.4 | 15.2 | 0.5 | 358; +24.6 | - | In vitro 4T1 cells In vivo 4T1 tumor mice | [120] |
| Fe2O3@PDA-Fe3+-TA-CNMN/DOX | 5.01 | 125.45 | 25.0 | 7.0 | 95.6; −30.3 | ChT(DOX)/ PTT | In vivo tumor mice | [121] |
| Fe@NiFe2O4-PEG/dopamine | 7.19 | 9.96 | 1.4 | 2.4 | 10–15; * | - | In vitro phantoms | [122] |
* Not available.
- 2.
- Doping superparamagnetic T2 contrast NPs with paramagnetic T1 contrast materials inside
For systems where the paramagnetic material resides inside the superparamagnetic iron oxide, the magnetic fields of both materials reinforce each other simultaneously, strongly enhancing the r1 value and causing a synergistic T1–T2 enhancement effect (Figure 4, right) [103]. The basic properties of systems using this strategy are shown in Table 6, some of which are discussed in some detail. The theory of synergistic T1–T2 enhancement effect discussed above was confirmed by Gao et al. using Gd2O3-embedded Fe3O4-HDA-G2 NPs (GdIO-HDA-G2), which showed a synergistic enhancement of r1 and r2. The GdIO had higher r2 (146.5 mM−1.s−1) than Fe3O4 (125.4 mM−1.s−1) of similar size and also higher r1 (69.5 mM−1.s−1) than Gd2O3 (12.1 mM−1.s−1) of similar size. Furthermore, the Gd2O3 NPs showed no enhanced T2 contrast, while Fe3O4 nanoparticles showed limited enhanced T1 contrast. Simultaneous in vivo T1w and T2w MRI of BALB/c mice upon i.v. injection of GdIO showed simultaneous strong MRI contrast enhancement of liver in both types of images due to the high accumulation of NPs in the hepatic Kupffer cells of the liver mononuclear phagocyte system (MPS). The same MRI experiment in HepG2 tumor mice detected the liver lesions through pseudo-negative and pseudo-positive contrast effects because the contrast between lesions and surrounding normal liver tissue increased due to the very low uptake by hepatic tumors, which contain few active Kupffer cells and macrophages (Figure 8). This work validated the new strategy for the design of new T1–T2 DMCAs [103].

Table 6.
Summary of basic properties of NPs made of a paramagnetic T1 material inside a superparamagnetic T2 material or forming hybrid oligomers of different shapes (in bold). Their properties are compared with those of the NPs made of their components.
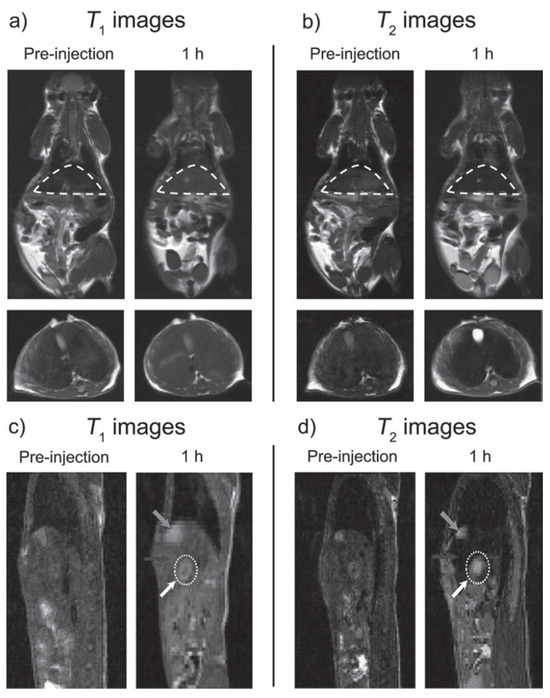
Figure 8.
Simultaneous T1 and T2 MRI imaging of liver and hepatic tumor (7 T), respectively. (a) T1w and (b) T2w in vivo MR images of BALB/c mice (top: coronal plane, bottom: transverse plane) before and after iv. injection of GdIO nanoparticles with a dose of 2.0 mg kg−1. The regions of liver in the coronal planes were circled by dash lines. (c) T1w and (d) T2w in vivo MR images of nude mice orthotopically inoculated with HepG2 liver cancer cells (sagittal plane) before and after iv. injection of GdIO nanoparticles with a dose of 2.0 mg Fe kg−1. Grey arrows: gallbladder, black arrows: liver, white dotted circles and white arrows: liver tumor. Reproduced from [103].
Another example of the use of the same design strategy consisted of water-dispersible GdIO NPs stearic acid modified low molecular weight polyethyleneimine (stPEI) (GdIO–stPEI). This nanoplatform was capable of binding and delivering siRNA for gene knockdown and work as T1–T2 DMCA with HCT-116 cells in vitro [123]. Zwitterion dopamine sulfonate-coated superparamagnetic GdIO NPs (GdIO-ZDS) with small core size (2.8–4.8 nm) showed partial paramagnetism at room temperature, with a decreased MS value relative to IOs of the same core size. This resulted from the combination of surface canting with the effect of the embedded Gd2O3 nanoclusters which disturbs the long-range order of magnetic spins in the small GdIO NPs. It also led to a significantly increased r1 value and decreased r2 value relative to IO-ZDS of the same core size. For example, GdIO NPs with a 4.8 nm diameter, had a high r1 = 7.85 mM−1.s−1 and a low r2/r1 = 5.24 relative to IO (r2/r1 = 9.59). These NPs caused a strong positive tumor contrast effect in T1w MRI images of SKOV3 human ovarian tumor mice through an enhanced permeation and retention (EPR) effect [124].
Superparamagnetic Fe3O4 NPs have also been doped with other paramagnetic ions, such as Mn2+ (MnIO) and Eu3+ (EuIO) [125,126]. The MRI contrast abilities of uniform Mn2+-doped iron oxide (MnIO) NPs nanoparticles with 5, 7, 9 and 12 nm size were studied. The NPs were superparamagnetic at 300 K, with Ms values which decreased with the decrease in the MnIO NP sizes, from 71.0 emu g−1 for the 12 nm NPs to 39.7 emu g−1 for the 5 nm NPs due to the spin canting effect at their surface. Their r1 and r2 values were highly size-dependent, with r2/r1 values decreasing from 7.4 for the 12 nm NPs to 2.6 for the 5 nm NPs. Thus, by controlling the size of the MnIO NPs, T1-dominated, T2-dominated, and T1–T2 DMCAs could be obtained with much higher contrast enhancement than the corresponding conventional iron oxide nanoparticles, as verified by in vivo MRI of BALB/c mice [125]. Finally, Eu3+-dopped iron oxide nanocubes (EuIO) were developed as T1–T2 DMCAs for in vivo MRI. The EuIO nanocubes were composed of mixed Fe3O4 (magnetite) and Eu2O3 nanoclusters of 10.0, 14.0 and 20.1 nm size, due to the large ionic radius of Eu3+ ions (94.7 pm) which prevented them from occupying either the tetrahedral or the octahedral interstitial sites in the spinel structure. The EuIO nanocubes are partially paramagnetic at 300 K, which is different from the superparamagnetism of magnetite NPs, due to increased spin canting on their surface layer after Eu2O3 embedding. The Eu2O3 clusters located inside the iron oxide NPs nanoparticles disturbed the local magnetic field intensity of the whole NPS and reduced their Ms values (~39.6 emu g−1) relative to magnetite NPs with a similar size (~53.4 emu g−1) at 300 K. The larger EuIO NPs had higher Ms values due to the loss of the spin canting effect on the particle surface. As a result, both r1 and r2 values of EuIO nanocubes could be tuned by varying their sizes and Eu doping ratios. Larger EuIO nanocubes had higher r1 and r2 values. The Eu/Fe molar ratio also had an important role in the r1 and r2 values of EuIO nanocubes: raising the Eu molar ratio increased r1 due to the spin order of Eu3+ ions which had the same orientation as the local magnetic field, while it decreased r2 values due to the reduction in the Ms values after Eu embedding. For instance, EuIO nanocubes of 14 nm diameter showed a high r1 = 36.8 mM−1.s−1, which is approximately 3 times higher than that of Fe3O4 NPs (12.47 mM−1.s−1) of similar size. After citrate coating, EuIO nanocubes produced enhanced T1 and T2 MRI contrast effects in Sprague Dawley rats as models for in vivo MRI studies, in particular in the cardiac and liver regions [126].
In summary, Table 6 shows that the r2/r1 values of the composite NPs (in bold) are more suitable as DMCAs when compared with those of the NPs made of their components.
- 3.
- T1 and T2 contrast materials connected side-by-side forming hybrid oligomers of different shapes
A third strategy to control the interference by magnetic coupling between T1 and T2 materials present in a single-composite nanostructure is to engineer the architecture of heterogeneous NPs forming hybrid trimers and oligomers of different geometries, like dumbbell-shaped NPs [109,127] or nanoflowers [109] (Table 6). Dumbbell-shaped NPs, or so-called ‘Janus’ NPs, were synthesized, with two different components within one single structure, as solid-state analogues of bifunctional organic molecules to construct hybrid nanotrimers (HNTs) (Figure 9a, right panel), in which iron oxide and Au nanocrystals were connected by a platinum nanocube (Au-IONP). The surface of its Au component was covalently immobilized with Gd- cystamine-DOTA2 (dithiol derivative of DOTA) as a T1 material (Gd(DOTA)-HNTs). To reduce its magnetic coupling with the IONP (T2 material), the size of the Au nanocrystals was increased by controlling the seed-mediated growth processes during the synthesis and the size of the Pt cubes was also increased to increase the distance (D, Figure 9a, right panel) between the IONP and the Au nanocrystals. The resulting heterotrimers with large Au crystals had a dumbbell structure, (Gd(DOTA)-DB-HNTs and Gd(DOTA)-XDB-HNTs, the latter with a larger Au component and thus a larger number of Gd per single NP). The IO component was covered with PEG chains to make the whole NPs water-soluble and biocompatible (Figure 9b). The Gd(DOTA)-DB-HNTs and Gd(DOTA)-XDB-HNTs had increased r1 values due to the reduced magnetic coupling between the T1 and T2 components as their D values increased. Their r2 values were similar due to the similar sizes and shapes of the iron oxide components. The calculation of the r1 relaxivity of each particle was based on different concentrations of Gd: r1 (mM [Fe + Gd]−1.s−1) or r1′ (mM [Gd]−1.s−1). Even though the r2/r1 ratio of Gd(DOTA)-DB-HNTs was 33, their r2/r1´was only 4.2, indicating that they could be T1–T2 DMCAs. Although Gd(DOTA)-XDB-HNTs have slightly higher r1′ (32.1 mM [Gd]−1.s−1), they did not have a better r2/r1´ ratio (4.2) than Gd(DOTA)-DB-HNTs because of their larger size of the IO component, which increased r2. The hybrid heterotrimers were highly stable in physiological conditions and induced simultaneous positive and negative contrast enhancements in in vivo MRI images of HT-29 tumor-bearing mice upon i.v. injection of Gd(DOTA)-DB-HNTs, showing their potential as T1–T2 DMCAs [127].
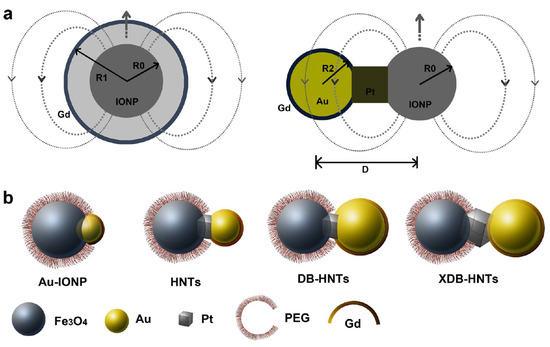
Figure 9.
(a) Engineering the heterogeneous nanostructures for magnetic coupling of T1 and T2 contrast agents with (left) core/shell structures or (right) dumbbell structures. R0 is the radius of T2 contrast agent (iron oxide nanoparticles), R1 is the radius with the Gd shell, R2 is the radius of gold nanoparticles, and D represents the center-to-center distance between iron oxide and gold nanoparticles. (b) Design and characterization of dumbbell heterostructures for dual T1 and T2-weighted MRI. Illustration of constructions of four different types of dumbbell-like or dumbbell heterostructures (Au_IONPs, HNTs, DB-HNTs, and extra-large DB-HNTs (XDB-HNTs)). Adapted from [127].
Fe3O4/MnO hybrid nanocrystals were prepared based on seed-mediated growth of MnO on the surface of a Fe3O4, where the resulting structures depended on the size of the seed NP, producing core/shell spherical Fe3O4 (5 nm)@MnO, dumbbell shaped Fe3O4 (11 nm)/MnO, and flower-shaped Fe3O4 (21 nm)/MnO hybrid NPs from 5 nm, 11 nm, and 21 nm Fe3O4 NPs, respectively (Figure 10). All the NPs had a high r2/r1 ratio and the dumbbell shaped Fe3O4/MnO NPs produced a negative contrast effect in T2*w MRI images and a positive contrast effect in T1w MRI images upon releasing Mn2+ ions in a low pH environment, in vitro (aqueous phantoms) and in vivo (normal brain of a nude mouse) obtained after i.v. injection. The same study using an orthotopic xenograft model of human hepatocellular carcinoma (HCC) showed high contrast between relatively hyperintense HCC and hypointense background liver parenchyma in T2w MRI HCC and hypointense background liver parenchyma due to the presence of Kupffer cells in the later, while in T1w MRI images a bright signal of the HCC tumor was observed after injection, due to HCC cell uptake of the Mn2+ ions liberated from the NPs in the low pH environment of the TME (Figure 11). This induced organ-specific contrast enhancement showed the potential of the hybrid NPs and T1–T2 DMCAs [109].
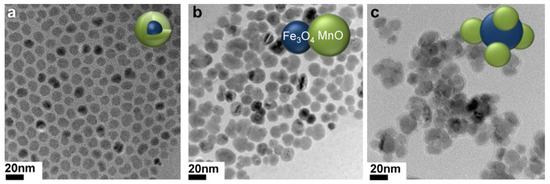
Figure 10.
TEM images of (a) Fe3O4 (5 nm)@MnO, (b) Fe3O4 (11 nm)/MnO dumbbell-like, and (c) Fe3O4 (21 nm)/MnO flower. Reproduced with permission from [109]. Copyright from Elsevier.
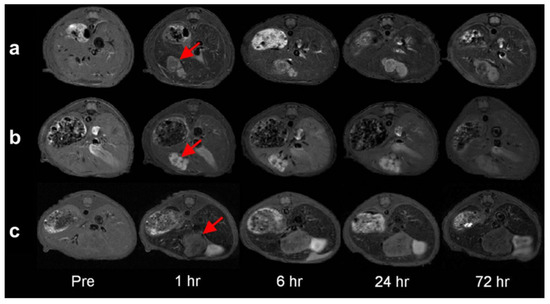
Figure 11.
Serial T2-weighted MR images of the orthotopic xenograft model of a nude mouse liver for human HCC (arrows) before as well as 1 h, 6 h, 24 h and 72 h after the contrast injection of (a) Fe3O4/MnO dumbbell nanocrystals, (b) MnO nanocrystals, and (c) and Fe3O4 nanocrystals. Reproduced with permission from [109]. Copyright from Elsevier.
In summary, the synthetically challenging strategy of designing DMCAs based on forming hybrid oligomers of different shapes using T1 and T2 contrast materials, although scientifically original, has so far produced nanosystems with too high r2/r1 values [109,127].
4. General Issues of In Vivo Use of NPs as MRI Contrast Agents
Besides the problems of design and contrast efficacy described in previous sections, the use of MNPs as MRI CAs in vivo involves many other issues, including their colloidal stability, biocompatibility, absorption, distribution, metabolism, and excretion (ADME) [128], toxicity, targeting and potential theranostic use.
Several intrinsic properties of MNPs, such as size, shape, coating, charge and presence of surface ligands, influence their biodistribution, elimination and target site accumulation. MNPs should be colloidally stable in aqueous media and in body fluids, as in vivo agglomeration and precipitation causes unacceptable safety issues and limits their high-performance MRI function. The colloidal stability is ensured by high zeta potentials and coating with hydrophilic and biocompatible ligands, such as PEG, which also maintains their non-toxicity. The biocompatibility of the materials used in DMCAs is also quite variable. Fe3+-based NPs are more biocompatible than those containing Gd3+, Dy3+, Ho3+, Tb3+ and Mn2+, because iron is an essential element in the human body, participating in a wide variety of metabolic processes, including oxygen transport, DNA synthesis, and electron transport. However, its concentration in body tissues must be tightly regulated because in excessive amounts, Fe2+ is toxic through the generation of reactive oxygen species (ROS) via the so-called Fenton reaction, which eventually results in cell damage, cell death, and organ failure, primarily affecting the liver, heart, pancreas, thyroid, and central nervous system [129]. Although Mn is also an essential trace element, excessive doses of Mn2+ can be neurotoxic, and its build-up in the brain may lead to manganism, a neurological disorder similar to Parkinsonism [130]. Also, free Gd3+ ions liberated from Gd chelates or NPs into the body could cause nephrogenic systemic fibrosis (NSF) [14,15]. Non-toxicity of metal-based NPs is critical for their in vivo use as MRI CAs [131].
Because MRI CAs are usually intravenously injected, the preferable excretion of MNPs is through the renal system rather than the hepatobiliary pathway because the later one is relatively slow and the MNPs could be taken up by the liver reticuloendothelial system (RES) and be partially decomposed during the excretion process, which would be toxic to the body. This metabolization process of small (<10 nm) NPs can occur through direct penetration of the cell membrane (phagocytosis) and delivery to the cytoplasm and nucleus, while for larger NPs (10–200 nm) the uptake is mediated by clathrin-dependent endocytosis- Both mechanisms facilitate the cell lysosomal internalization and degradation of the MNPs [128].
For renal excretion, MNPs should be ultrasmall with hydrodynamic diameters less than 5 nm because the glomerular filtration diameter in the kidneys is 4.5–5 nm [132]. The kinetic stability of MNPs should also be high to avoid their decomposition until they are excreted as urine through the renal system.
In summary, MNPs as MRI CAs for safe in vivo applications should be kinetically stable (i.e., no decomposition), coated with hydrophilic and biocompatible polymers for non-toxicity and colloidal stability, and ultrasmall with hydrodynamic diameters less than 5 nm for renal excretion. This excretion route, avoiding as far as possible their liver retention in the hepatobiliary pathway, is also very important for targeting and theranostic drug delivery by MNPs [133,134]. In this review, several examples of in vivo use of theranostic DMCAs were presented, e.g., using ferroptosis inhibition [78], immunotherapy [79] and photodynamic therapy (PTT) associated with chemotherapy (ChT) [82] and chemodynamic therapy (CDT) [84]. As far as nanocarriers for drug delivery and theranostics are concerned, the inappropriate release, internal instability and tissue non-targeting effects, sometimes observed due to biological barriers, are the main restrictions for their in vivo application [128,135].
5. Conclusions
This review describes recent developments and optimization of MNPs containing Gd, Mn, Fe and other lanthanide ions as potential dual-mode T1–T2 MRI contrast agents (DMCAs). Their high performance was highlighted by describing selected in vivo MRI studies. However, the development stage of most of the reported MNP-based MRI CAs is still quite limited, both in vitro and in preclinical in vivo small animal studies. To improve their chances to reach the clinical trials stage, several key issues must be solved. These include long-term colloidal stability in aqueous media, toxicity and pharmacokinetics, which depend on their coating with hydrophilic and biocompatible ligands. In comparison with MNPs containing Mn, or lanthanides (such as Gd, Dy, Ho, and Tb), Fe-based NPs are less toxic because iron is an essential element, and several IONPs have been approved and commercialized as MRI CAs, such as Feridex®, Sinerem®, and Resovist®, before being discontinued due to lack of commercial interest. The understanding of the correlation between the physicochemical properties of MNPs and their biological behavior in vivo must also be improved using appropriate preclinical small animal studies.
Another major problem faced by the clinical introduction of new MRI CAs in general, and MNP-based MRI CAs in particular, due to their molecular complexity and the consequent expensive synthesis, is to solve the main regulatory challenges of controlled NPs synthesis, uniformity, batch-to-batch reproducibility and upscaling of production. This leads to large development costs, especially for phase III studies, compared with the post-approval revenues [136,137]. However, the large amount of information obtainable with molecular imaging using MNP-based MRI CAs, such as those described in this review, may contribute to early diagnosis and personalized therapies, which ultimately will reduce health care costs.
Funding
This research was supported by FUNDAÇÃO PARA A CIÊNCIA E TECNOLOGIA (FCT-PORTUGAL) through funding the CQC-IMS project (grant numbers UIDB/00313/2020 and UIDP/00313/2020).
Conflicts of Interest
The author declares no conflicts of interest.
References
- Reimer, P.; Parizel, P.M.; Meaney, J.F.M.; Stichnoth, F.A. Clinical MR Imaging, a Practical Approach; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Weissleder, R.; Mahmood, U. Molecular imaging. Radiology 2001, 219, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium (III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] [PubMed]
- Merbach, A.E.; Helm, L.; Tóth, É. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2013. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef]
- Aime, S.; Botta, M.; Terreno, E. Gd(III)-Based Contrast Agents for MRI. Adv. Inorg. Chem. 2005, 57, 173–237. [Google Scholar] [CrossRef]
- Geraldes, C.F.G.C.; Laurent, S. Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media Mol. Imaging 2009, 4, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef]
- Amoroso, A.J.; Pope, S.J.A. Using lanthanide ions in molecular bioimaging. Chem. Soc. Rev. 2015, 44, 4723–4742. [Google Scholar] [CrossRef]
- Villaraza, A.J.; Bumb, A.; Brechbiel, M.W. Macromolecules, Dendrimers, and Nanomaterials in Magnetic Resonance Imaging: The Interplay between Size, Function, and Pharmacokinetics. Chem. Rev. 2010, 110, 2921–2959. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Lee, N.; Hyeon, T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2012, 41, 2575–2589. [Google Scholar] [CrossRef]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef]
- Sherry, A.D.; Caravan, P.; Lenkinski, R.E. Primer on gadolinium chemistry. J. Magn. Reson. Imaging 2009, 30, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, H.S.; Morcos, S.K.; Almén, T.; Bellin, M.-F.; Bertolotto, M.; Bongartz, G.; Clement, O.; Leander, P.; Heinz-Peer, G.; Reimer, P.; et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: Updated ESUR Contrast Medium Safety Committee guidelines. Eur. Radiol. 2013, 23, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, H.S. Nephrogenic Systemic Fibrosis: History and Epidemiology. Radiol. Clin. N. Am. 2009, 47, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Berstein, E.J.; Schmidt-Lauber, C.; Kay, J. Nephrogenic systemic fibrosis: A systemic fibrosing disease resulting from gadolinium exposure. Best Pract. Res. Clin. Rheumatol. 2012, 26, 489–503. [Google Scholar] [CrossRef]
- Gianolio, E.; Bardini, P.; Arena, F.; Stefania, R.; Di Gregorio, E.; Iani, R.; Aime, S. Gadolinium Retention in the Rat Brain: Assessment of the Amounts of Insoluble Gadolinium-containing Species and Intact Gadolinium Complexes after Repeated Administration of Gadolinium-based Contrast Agents. Radiology 2017, 285, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Khairinisa, M.A.; Ariyani, W.; Tsushima, Y.; Koibuchi, N. Effects of gadolinium deposits in the cerebellum: Reviewing the literature from in vitro laboratory studies to in vivo human investigations. Int. J. Environ. Res. Public Health 2021, 18, 7214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.J.; Idée, J.-M. A comprehensive literature update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant. Imaging Med. Surg. 2017, 7, 88–122. [Google Scholar] [CrossRef]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. [Google Scholar] [CrossRef]
- Helm, L. Relaxivity in paramagnetic systems: Theory and mechanisms. Prog. Nucl. Magn. Reson. Spectrosc. 2006, 49, 45–64. [Google Scholar] [CrossRef]
- Norek, M.; Peters, J.A. MRI contrast agents based on dysprosium or holmium. Prog. Nucl. Magn. Reason. Spectrosc. 2011, 59, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Luchinat, C.; Parigi, G.; Ravera, E. NMR of Paramagnetic Molecules, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Gueron, M. Nuclear relaxation in macromolecules by paramagnetic ions: A novel mechanism. J. Magn. Reson. 1975, 19, 58–66. [Google Scholar] [CrossRef]
- Vega, A.J.; Fiat, D. Nuclear relaxation processes of paramagnetic complexes-The slow-motion case. Mol. Phys. 1976, 31, 347–355. [Google Scholar] [CrossRef]
- Roch, A.; Muller, R.N.; Gillis, P. Theory of proton relaxation induced by superparamagnetic particles. J. Chem. Phys. 1999, 110, 5403–5411. [Google Scholar] [CrossRef]
- Vuong, Q.L.; Berret, J.-F.; Fresnais, J.; Gossuin, Y.; Sandre, O. A Universal Scaling Law to Predict the Efficiency of Magnetic Nanoparticles as MRI T2-Contrast, Agents. Adv. Healthc. Mater. 2012, 1, 502–512. [Google Scholar] [CrossRef]
- Vuong, Q.L.; Gossuin, Y.; Gillis, P.; Delangre, S. New simulation approach using classical formalism to water nuclear magnetic relaxation dispersions in presence of superparamagnetic particles used as MRI contrast agents. J. Chem. Phys. 2012, 137, 114505. [Google Scholar] [CrossRef]
- Brown, M.A.; Semalka, R.C. MRI: Basic Principles and Applications; Wiley-Liss: New York, NY, USA, 2003. [Google Scholar]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, B.H.; Na, H.B.; Hyeon, T. Paramagnetic inorganic nanoparticles as T1 MRI contrast agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 196–209. [Google Scholar] [CrossRef]
- Bridot, J.-L.; Faure, A.-C.; Laurent, S.; Rivière, C.; Billotey, C.; Hiba, B.; Janier, M.; Josserand, V.; Coll, J.-L.; Vander Elst, L.; et al. Hybrid gadolinium oxide nanoparticles: Multimodal contrast agents for in vivo imaging. J. Am. Chem. Soc. 2007, 129, 5076–5084. [Google Scholar] [CrossRef]
- Zhang, W.; Martinelli, J.; Mayer, F.; Bonnet, C.S.; Szeremeta, F.; Djanashvili, K. Molecular architecture control in synthesis of spherical Ln-containing nanoparticles. RSC Adv. 2015, 5, 69861–69869. [Google Scholar] [CrossRef][Green Version]
- Osseni, S.A.; Lechevallier, S.; Verelst, M.; Perriat, P.; Dexpert-Ghys, J.; Neumeyer, D.; Garcia, R.; Mayer, F.; Djanashvili, K.; Peters, J.A.; et al. Gadolinium oxysulfide nanoparticles as multimodal imaging agents for T2-weighted MR, X-ray tomography and photoluminescence. Nanoscale 2014, 6, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.; Peters, J.A.; Djanashvili, K. Microwave-assisted seeded growth of lanthanide-based nanoparticles for imaging and therapy. Chem. Eur. J. 2012, 18, 8004–8007. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.N.M.; Alvares, R.D.A.; Oakden, W.; Chaudhary, R.; Hill, M.L.; Pichaandi, J.; Mo, G.C.H.; Yip, C.; Macdonald, P.M.; Stanisz, G.P.; et al. Polymer-Stabilized Lanthanide Fluoride Nanoparticle Aggregates as Contrast Agents for Magnetic Resonance Imaging and Computed Tomography. Chem. Mater. 2010, 22, 4728–4739. [Google Scholar] [CrossRef]
- Evanics, F.; Diamente, P.R.; Van Veggel, F.C.J.M.; Stanisz, G.J.; Prosser, R.S. Water-soluble GdF3 and GdF3/LaF3 nanoparticles-physical characterization and NMR relaxation properties. Chem. Mater. 2006, 18, 2499–2505. [Google Scholar] [CrossRef]
- Hifumi, H.; Yamaoka, S.; Tanimoto, A.; Citterio, D.; Suzuki, K. Gadolinium-based hybrid nanoparticles as a positive MR contrast agent. J. Am. Chem. Soc. 2006, 128, 15090–15091. [Google Scholar] [CrossRef]
- Frangville, C.; Gallois, M.; Li, Y.; Nguyen, H.H.; Lauth-de Viguerie, N.; Talham, D.R.; Mingotaud, C.; Marty, J.-D. Hyperbranched polymer mediated size-controlled synthesis of gadolinium phosphate nanoparticles: Colloidal properties and particle size-dependence on MRI relaxivity. Nanoscale 2016, 8, 4252–4259. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Baek, M.J.; Choi, E.S.; Woo, S.; Kim, J.H.; Kim, T.J.; Jung, J.C.; Chae, K.S.; Chang, Y.; Lee, G.H. Paramagnetic ultrasmall gadolinium oxide nanoparticles as advanced T1 MRI contrast agent: Account for large longitudinal relaxivity, optimal particle diameter, and in vivo T1 MR images. ACS Nano 2009, 3, 3663–3669. [Google Scholar] [CrossRef]
- Yang, J.; Shan, P.; Zhao, Q.; Zhang, S.; Li, L.; Yang, X.; Yu, X.; Lu, Z.; Wang, Z.; Zhang, X. A design strategy of ultrasmall Gd2O3 nanoparticles for T1 MRI with high performance. New J. Chem. 2021, 45, 7270–7277. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, C.; Wang, S.; Li, Q.; Zhang, M.; Li, J.; Xu, K. Comparative study on in vivo behavior of PEGylated gadolinium oxide nanoparticles and Magnevist as MRI contrast agent. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 547–555. [Google Scholar] [CrossRef]
- Park, Y.; Kim, H.M.; Kim, J.H.; Moon, K.C.; Yoo, B.; Lee, K.T.; Lee, N.; Choi, Y.; Park, W.; Ling, D.; et al. Theranostic probe based on lanthanide-doped nanoparticles for simultaneous in vivo dual-modal imaging and photodynamic therapy. Adv. Mater. 2012, 24, 5755–5761. [Google Scholar] [CrossRef]
- Pan, D.; Schmieder, A.H.; Wickline, S.A.; Lanza, G.M. Manganese-based MRI contrast agents: Past, present and future. Tetrahedron 2011, 67, 8431–8444. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhu, Q.; Zeng, Y.; Zeng, Q.; Chen, X.; Zhan, Y. Manganese Oxide Nanoparticles as MRI Contrast Agents in tumour multimodal imaging and therapy. Int. J. Nanomed. 2019, 14, 8321–8344. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Zheng, P.; Ma, P.A.; Lin, J. Manganese Oxide Nanomaterials: Synthesis, Properties, and Theranostic Applications. Adv. Mater. 2020, 32, 51905823. [Google Scholar] [CrossRef]
- Shin, J.; Anisur, R.M.; Ko, M.K.; Im, G.H.; Lee, J.H.; Lee, I.S. Hollow Manganese Oxide Nanoparticles as Multifunctional Agents for Magnetic Resonance Imaging and Drug Delivery. Angew. Chem. Int. Ed. 2009, 48, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Liu, K.; Zhang, K.; Fan, Y.; Lin, H.; Gao, J. Zwitterion-Coated Ultrasmall MnO Nanoparticles Enable Highly Sensitive T1-Weighted Contrast-Enhanced Brain Imaging. ACS Appl. Mater. Interfaces 2022, 14, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, C.; Hou, P.; Zhang, M.; Xu, K. One-pot preparation of hydrophilic manganese oxide nanoparticles as T1 nano-contrast agent for molecular magnetic resonance imaging of renal carcinoma in vitro and in vivo. Biosens. Bioelectron. 2018, 102, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Tian, X.M.; Yang, C.; Liu, P.; Luo, N.Q.; Liang, Y.; Li, H.B.; Chen, D.H.; Wang, C.X.; Li, L.; et al. Ultrahigh relaxivity and safe probes of manganese oxide nanoparticles for in vivo imaging. Sci. Rep. 2013, 3, 3424. [Google Scholar] [CrossRef]
- Botta, M.; Geraldes, C.F.G.C.; Tei, L. High spin Fe(III)-doped nanostructures as T1 MR imaging probes. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 15, e1858. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, Z.; Bao, J.; Wang, Z.; Hu, J.; Chi, X.; Ni, K.; Wang, R.; Chen, X.; Chen, Z.; et al. Octapod iron oxide nanoparticles as high-performance T2 contrast agents for magnetic resonance imaging. Nat. Commun. 2013, 4, 2266. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Chang, Y.; Zhao, L.; Zhang, K.; Zhao, Y.; Gao, F.; Gao, X. Ultrasmall Superparamagnetic Iron Oxide Nanoparticle for T2-Weighted Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2017, 9, 28959–28966. [Google Scholar] [CrossRef]
- Leal, M.P.; Muñoz-Hernández, C.; Berry, C.C.; García-Martín, M.L. In vivo pharmacokinetics of T2 contrast agents based on iron oxide nanoparticles: Optimization of blood circulation times. RSC Adv. 2015, 5, 76883–76891. [Google Scholar] [CrossRef]
- Lee, N.; Choi, Y.; Lee, Y.; Park, M.; Moon, W.K.; Choi, S.H.; Hyeon, T. Water-dispersible ferrimagnetic iron oxide nanocubes with extremely high r2 relaxivity for highly sensitive in vivo MRI of tumors. Nano Lett. 2012, 12, 3127–3131. [Google Scholar] [CrossRef] [PubMed]
- Caro, C.; Paez-Muñoz, J.M.; Beltrán, A.M.; Leal, M.P.; García-Martín, M.L. PEGylated Terbium-Based Nanorods as Multimodal Bioimaging Contrast Agents. ACS Appl. Nano Mater. 2021, 4, 4199–4207. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Y.; Shukla, S.; Gu, Y.; Yu, X.; Steinmetz, N.F. Dysprosium-Modified Tobacco Mosaic Virus Nanoparticles for Ultra-High-Field Magnetic Resonance and Near-Infrared Fluorescence Imaging of Prostate Cancer. ACS Nano 2017, 11, 9249–9258. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-Y.; Pellico, J.; Khrapitchev, A.A.; Sibson, N.R.; Davis, J.J. Dy-DOTA integrated mesoporous silica nanoparticles as promising ultrahigh field magnetic resonance imaging contrast agents. Nanoscale 2018, 10, 21041–21045. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Zhang, J.; Bu, W.; Zhang, C.; Yao, Z.; Xing, H.; Wang, J.; Duan, F.; Liu, Y.; Fan, W.; et al. PEGylated NaHoF4 nanoparticles as contrast agents for both X-ray computed tomography and ultra-high field magnetic resonance imaging. Biomaterials 2016, 76, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Caravan, P.; Greenfield, M.T.; Bulte, J.W.M. Molecular factors that determine Curie spin relaxation in dysprosium complexes. Magn. Reson. Med. 2001, 46, 917–922. [Google Scholar] [CrossRef]
- Norek, M.; Kampert, E.; Zeitler, U.; Peters, J.A. Tuning of the size of Dy2O3 nanoparticles for optimal performance as an MRI contrast agent. J. Am. Chem. Soc. 2008, 130, 5335–5340. [Google Scholar] [CrossRef]
- Wang, G.; Peng, Q.; Li, Y. Lanthanide-doped nanocrystals: Synthesis, optical-magnetic properties, and applications. Acc. Chem. Res. 2011, 44, 322–332. [Google Scholar] [CrossRef]
- Das, G.K.; Johnson, N.J.J.; Cramen, J.; Blasiak, B.; Latta, P.; Tomanek, B.; van Veggel, F.C.J.M. NaDyF4 Nanoparticles as T2 Contrast Agents for Ultrahigh Field Magnetic Resonance Imaging. J. Phys. Chem. Lett. 2012, 3, 524–529. [Google Scholar] [CrossRef]
- Jun, Y.-W.; Seo, J.-W.; Cheon, J. Nanoscaling Laws of Magnetic Nanoparticles and Their Applicabilities in Biomedical Sciences. Acc. Chem. Res. 2008, 41, 179–189. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Weissleder, R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv. Drug Deliv. Rev. 2008, 60, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gu, H.; Xu, B. Multifunctional Magnetic Nanoparticles: Design, Synthesis, and Biomedical Applications. Acc. Chem. Res. 2009, 42, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, R.; Peng, H.; Li, P.; Xu, Z.; Whittaker, A.K. The evolution of gadolinium-based contrast agents: From single-modality to multi-modality. Nanoscale 2016, 8, 10491–10510. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Lee, J.-H.; Shin, T.-H.; Cheon, J. Theranostic Magnetic Nanoparticles. Acc. Chem. Res. 2011, 44, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kattel, K.; Park, Y.; Chang, Y.; Kim, T.; Gang, J.; Lee, H. Paramagnetic nanoparticle T1 and T2 MRI contrast agents. Phys. Chem. Chem. Phys. 2012, 14, 12687–12700. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Bai, R.; Munasinghe, J.; Shen, Z.; Nie, L.; Chen, X. T1–T2 Dual-Modal Magnetic Resonance Imaging: From Molecular Basis to Contrast Agents. ACS Nano 2017, 11, 5227–5232. [Google Scholar] [CrossRef]
- Tegafaw, S.L.; Ahmad, M.Y.; Al Saidi, A.K.A.; Zhao, D.; Liu, Y.; Nam, S.-W.; Chang, Y.; Lee, G.H. Magnetic Nanoparticle-Based High-Performance Positive and Negative Magnetic Resonance Imaging Contrast Agents. Pharmaceutics 2023, 15, 1745. [Google Scholar] [CrossRef]
- Courant, T.; Roullin, V.G.; Cadiou, C.; Callewaert, M.; Andry, M.C.; Portefaix, C.; Hoeffel, C.; de Goltstein, M.C.; Port, M.; Laurent, S.; et al. Hydrogels Incorporating GdDOTA: Towards Highly Efficient Dual T1/T2 MRI Contrast Agents. Angew. Chem. Int. Ed. 2012, 51, 9119–9122. [Google Scholar] [CrossRef]
- Marasini, S.; Yue, H.; Ghazanfari, A.; Ho, S.L.; Park, J.A.; Kim, S.; Cha, H.; Liu, S.; Tegafaw, T.; Ahmad, M.Y.; et al. Polyaspartic Acid-Coated Paramagnetic Gadolinium Oxide Nanoparticles as a Dual-Modal T1 and T2 Magnetic Resonance Imaging Contrast Agent. Appl. Sci. 2021, 11, 8222. [Google Scholar] [CrossRef]
- Mekuria, S.L.; Debele, T.A.; Tsai, H.-C. Encapsulation of Gadolinium Oxide Nanoparticle (Gd2O3) Contrasting Agents in PAMAM Dendrimer Templates for Enhanced Magnetic Resonance Imaging in vivo. ACS Appl. Mater. Interfaces 2017, 9, 6782–6795. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, H.; Ma, L.; Jin, J.; Shen, T.; Wei, R.; Wang, X.; Ai, H.; Chen, Z.; Gao, J. Albumin-Based Nanoparticles Loaded with Hydrophobic Gadolinium Chelates as T1−T2 Dual-Mode Contrast Agents for Accurate Liver Tumor Imaging. Nanoscale 2017, 9, 4516–4523. [Google Scholar] [CrossRef]
- Niu, D.; Luo, X.; Li, Y.; Liu, X.; Wang, X.; Shi, J. Manganese-loaded dual-mesoporous silica spheres for efficient T1- and T2-weighted dual mode magnetic resonance imaging. ACS Appl. Mater. Interfaces 2013, 5, 9942–9948. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Liang, P.; Guan, G.; Yin, B.; Wang, B.; Yue, R.; Zhang, X.; Song, G. Overcoming Hypoxia-Induced Ferroptosis Resistance via a 19F/1HMRI Traceable Core-Shell Nanostructure. Angew. Chem. Int. Ed. 2022, 61, e202206074. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Yang, X.; Xu, Y.; Lin, J.; Zhang, F.; Duan, X.; Liu, S.; Liu, J.; Shen, J.; Shuai, X.; et al. Manganese-doped mesoporous polydopamine nanoagent for T1–T2 magnetic resonance imaging and tumor therapy. Nano Res. 2023, 16, 2991–3003. [Google Scholar] [CrossRef]
- Tegafaw, T.; Xu, W.; Ahmad, M.W.; Baeck, J.S.; Chang, Y.; Bae, J.E.; Chae, K.S.; Kim, T.J.; Lee, G.H. Dual-mode T1 and T2 magnetic resonance imaging contrast agent based on ultrasmall mixed gadolinium-dysprosium oxide nanoparticles: Synthesis, characterization, and in vivo application. Nanotechnology 2015, 26, 365102. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Fang, F.; Liu, J.; Jiang, C.; Han, X.; Song, Z.; Chen, J.; Sun, G.; Lei, H.; Lu, L. An ultrasmall and metabolizable PEGylated NaGdF4:Dy nanoprobe for high-performance T1/T2-weighted MR and CT multimodal imaging. Nanoscale 2015, 7, 15680–15688. [Google Scholar] [CrossRef]
- Chen, Y.; Ai, K.; Liu, J.; Ren, X.; Jiang, C.; Lu, L. Polydopamine-Based Coordination Nanocomplex for T1/T2 Dual Mode Magnetic Resonance Imaging-Guided Chemo-Photothermal Synergistic Therapy. Biomaterials 2016, 77, 198–206. [Google Scholar] [CrossRef]
- Suárez-García, S.; Arias-Ramos, N.; Frias, C.; Candiota, A.P.; Arús, C.; Lorenzo, J.; Ruiz-Molina, D.; Novio, F. Dual T1/T2 Nanoscale Coordination Polymers as Novel Contrast Agents for MRI: A Preclinical Study for Brain Tumor. ACS Appl. Mater. Interfaces 2018, 10, 38819–38832. [Google Scholar] [CrossRef]
- Chen, C.; Huang, C.; Liu, J.; Tao, J.; Chen, Y.; Deng, K.; Xu, Y.; Lin, B.; Zhao, P. Hofmeister Effect-Based T1−T2 Dual-Mode MRI and Enhanced Synergistic Therapy of Tumor. ACS Appl. Mater. Interfaces 2022, 14, 49568–49581. [Google Scholar] [CrossRef]
- Li, Z.; Yi, P.W.; Sun, Q.; Lei, H.; Zhao, H.L.; Zhu, Z.H.; Smith, S.C.; Lan, M.B.; Lu, G.Q. Ultrasmall Water-Soluble and Biocompatible Magnetic Iron Oxide Nanoparticles as Positive and Negative Dual Contrast Agents. Adv. Funct. Mater. 2012, 22, 2387–2393. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, X.; Skallberg, A.; Liu, Y.; Hu, Z.; Mei, X.; Uvdal, K. One-step synthesis of water-dispersible ultra-small Fe3O4 nanoparticles as contrast agents for T1 and T2 magnetic resonance imaging. Nanoscale 2014, 6, 2953–2963. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Hu, F.; Rui, Y.; Duan, Y.; Gu, H. A T1/T2 dual functional iron oxide MRI contrast agent with super stability and low hypersensitivity benefited by ultrahigh carboxyl group density. J. Mater. Chem. B 2019, 7, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, Y.; Wang, Q.; Liang, Z.; Han, G.; Wang, Z.; Lee, J.; Zhao, M.; Li, F.; Bai, R.; et al. An Ultrahigh-Field-Tailored T1–T2 Dual-Mode MRI Contrast Agent for High-Performance Vascular Imaging. Adv Mater. 2021, 33, e2004917. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhao, Z.; Zhang, H.; Wang, Z.; Chen, X.; Wang, R.; Chen, Z.; Gao, J. Interplay between longitudinal and transverse contrasts in Fe3O4 nanoplates with (111) exposed surfaces. ACS Nano 2014, 8, 7976–7985. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Alipour, A.; Soran-Erdem, Z.; Aykut, Z.G.; Demir, H.V. Highly monodisperse low-magnetization magnetite nanocubes as simultaneous T1–T2 MRI contrast agents. Nanoscale 2015, 7, 10519–10526. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.; Diaz-Diestra, D.; Santiago-Medina, C.; Kumar, N.; Tu, K.; Beltran-Huarac, J.; Jadwisienczak, W.M.; Weiner, B.R.; Morell, G. T1- and T2-weighted Magnetic Resonance Dual Contrast by Single Core Truncated Cubic Iron Oxide Nanoparticles with Abrupt Cellular Internalization and Immune Evasion. ACS Appl. Bio. Mater. 2018, 1, 79–89. [Google Scholar] [CrossRef]
- Li, F.; Liang, Z.; Liu, J.; Sun, J.; Hu, X.; Zhao, M.; Liu, J.; Bai, R.; Kim, D.; Sun, X.; et al. Dynamically Reversible Iron Oxide Nanoparticle Assemblies for Targeted Amplification of T1-Weighted Magnetic Resonance Imaging of Tumors. Nano Lett. 2019, 19, 4213–4220. [Google Scholar] [CrossRef]
- Li, X.; Lu, S.; Xiong, Z.; Hu, Y.; Ma, D.; Lou, W.; Peng, C.; Shen, M.; Shi, X. Light-Addressable Nanoclusters of Ultrasmall Iron Oxide Nanoparticles for Enhanced and Dynamic Magnetic Resonance Imaging of Arthritis. Adv. Sci. 2019, 6, 1901800. [Google Scholar] [CrossRef]
- Cao, Y.; He, Y.; Mao, Z.; Kuang, Y.; Liu, M.; Zhang, Y.; Pei, R. Synergistic regulation of longitudinal and transverse relaxivity of extremely small iron oxide nanoparticles (ESIONPs) using pH-responsive nanoassemblies. Nanoscale 2020, 12, 17502–17516. [Google Scholar] [CrossRef]
- He, Y.; Cao, Y.; Mao, Z.; Zhou, Y.; Zhang, Y.; Pei, R. Redox-triggered aggregation of ESIONPs with switchable T1 to T2 contrast effect for T2-weighted magnetic resonance imaging. J. Mater. Chem. B 2021, 9, 1821–1832. [Google Scholar] [CrossRef]
- He, Y.; Mao, Z.; Lu, Z.; Yan, J.; Zhang, Y.; Bianco, A.; Cao, Y.; Pei, R. Extremely Small Iron Oxide Nanoparticles with pH-Dependent Solubility Transition as T1/T2 Switchable Contrast Agents for MRI. ACS Appl. Nano Mater. 2022, 5, 15826–15836. [Google Scholar] [CrossRef]
- He, Y.; Mao, Z.; Zhang, Y.; Lu, H.; Yan, J.; Cao, Y.; Pei, R. Tumor Acid Microenvironment-Triggered Self-Assembly of ESIONPs for T1/T2 Switchable Magnetic Resonance Imaging. ACS Appl. Bio Mater. 2020, 3, 7752–7761. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, J.; Chen, H.; Wu, H.; Xu, Y.; Li, Y.; Yi, H.; Wang, Y.A.; Yang, L.; Mao, H. Exerting Enhanced Permeability and Retention Effect Driven Delivery by Ultrafine Iron Oxide Nanoparticles with T1–T2 Switchable Magnetic Resonance Imaging Contrast. ACS Nano 2017, 11, 4582–4592. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yan, C.; An, L.; Zhao, H.; Song, S.; Yang, S. Fe3O4 assembly for tumor accurate diagnosis by endogenous GSH responsive T2/T1 magnetic relaxation conversion. J. Mater. Chem. B 2021, 9, 7734–7740. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tang, J.; Li, J.; Li, W.; Liu, Y.; Chen, C. In vivo aggregation-induced transition between T1 and T2 relaxations of magnetic ultra-small iron oxide nanoparticles in tumor microenvironment. Nanoscale 2017, 9, 3040–3050. [Google Scholar] [CrossRef] [PubMed]
- Si, G.; Hapuarachchige, S.; Artemov, D. Ultrasmall Superparamagnetic Iron Oxide Nanoparticles as Nanocarriers for Magnetic Resonance Imaging: Development and In Vivo Characterization. ACS Appl. Nano Mater. 2022, 5, 9625–9632. [Google Scholar] [CrossRef]
- Choi, J.-s.; Kim, S.; Yoo, D.; Shin, T.-H.; Kim, H.; Gomes, M.D.; Kim, S.H.; Pines, A.; Cheon, J. Distance-dependent magnetic resonance tuning as a versatile MRI sensing platform for biological targets. Nat. Mater. 2017, 16, 537. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, D.; Bao, J.; Chen, Q.; Liu, G.; Chen, Z.; Chen, X.; Gao, J. A Synergistically Enhanced T1–T2 Dual-Modal Contrast Agent. Adv. Mater. 2012, 24, 6223–6228. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Choi, J.-s.; Lee, J.-H.; Shin, T.-H.; Song, H.-T.; Kim, E.Y.; Cheon, J. Self-confirming “AND” logic nanoparticles for fault-free MRI. J. Am. Chem. Soc. 2010, 132, 11015–11017. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Gao, L.; Liu, K.; Luo, C.; Wang, Y.; Yu, L.; Peng, H.; Zhang, W. Characterization of Fe3O4/SiO2/Gd2O(CO3)2 core/shell/shell nanoparticles as T1 and T2 dual mode MRI contrast agent. Talanta 2015, 131, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; You, J.; Wu, C.; Dai, Y.; Shi, M.; Dong, L.; Xu, K. T1–T2 molecular magnetic resonance imaging of renal carcinoma cells based on nano-contrast agents. Int. J. Nanomed. 2018, 13, 4607–4625. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhi, D.; Luo, Y.; Zhang, J.; Nan, X.; Zhang, Y.; Zhou, W.; Qiu, B.; Wen, L.; Liang, G. Core/shell Fe3O4/Gd2O3 nanocubes as T1–T2 dual modal MRI contrast agents. Nanoscale 2016, 8, 12826–12833. [Google Scholar] [CrossRef] [PubMed]
- Im, G.H.; Kim, S.M.; Lee, D.-G.; Lee, W.J.; Lee, J.H.; Lee, I.S. Fe3O4/MnO hybrid nanocrystals as a dual contrast agent for both T1- and T2-weighted liver MRI. Biomaterials 2013, 34, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Son, H.-Y.; Kim, G.-Y.; Park, K.; Huh, Y.-M.; Haam, S. Redoxable heteronanocrystals functioning magnetic relaxation switch for activatable T1 and T2 dual-mode magnetic resonance imaging. Biomaterials 2016, 101, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Yang, J.; Ma, J.; Li, X.; Wu, W.; Liu, C.; He, J.; Miao, L. Ternary-Responsive Drug Delivery with Activatable Dual Mode Contrast Enhanced in vivo Imaging. ACS Appl. Mater. Interfaces 2018, 10, 31947–31958. [Google Scholar] [CrossRef]
- Shin, T.-H.; Choi, J.-s.; Yun, S.; Kim, I.-S.; Song, H.-T.; Kim, Y.; Park, K.I.; Cheon, J. T1 and T2 dual-mode MRI contrast agent for enhancing accuracy by engineered nanomaterials. ACS Nano 2014, 8, 3393–3401. [Google Scholar] [CrossRef]
- Keasberry, N.A.; Bañobre-López, M.; Wood, C.; Stasiuk, G.J.; Gallo, J.; Long, N.J. Tuning the relaxation rates of dual-mode T1/T2 nanoparticle contrast agents: A study into the ideal system. Nanoscale 2015, 7, 16119–16128. [Google Scholar] [CrossRef]
- Bae, K.H.; Kim, Y.B.; Lee, Y.; Hwang, J.; Park, H.; Park, T.G. Bioinspired Synthesis and Characterization of Gadolinium-Labeled Magnetite Nanoparticles for Dual Contrast T1- and T2-Weighted Magnetic Resonance Imaging. Bioconjug. Chem. 2010, 21, 505–512. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.; Zhu, Y.; Yang, X.; Yao, X.; Li, J.; Huang, G.; Li, C. Multifunctional gadolinium-labeled silica-coated Fe3O4 and CuInS2 nanoparticles as a platform for in vivo tri-modality magnetic resonance and fluorescence imaging. J. Mater. Chem. B 2015, 3, 2873–2882. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Li, T.; Liu, J.; Wang, B. Controlled synthesis of MnFe2O4 nanoparticles and Gd complex-based nanocomposites as tunable and enhanced T1/T2-weighted MRI contrast agents. J. Mater. Chem. B 2014, 2, 4748–4753. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhuang, Y.; Sun, Y.; Dai, A.; Shi, X.; Wu, D.; Li, F.; Hu, H.; Yang, S. Targeted dual contrast T1- and T2-weighted magnetic resonance imaging of tumors using multifunctional gadolinium-labeled superparamagnetic iron oxide nanoparticles. Biomaterials 2011, 32, 4584–4593. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.L.C.; Sereno, J.; Abrunhosa, A.J.; Delville, M.H.; Rocha, J.; Carlos, L.D.; Geraldes, C.F.G.C. Gd- and Eu-Loaded Iron Oxide@Silica Core−Shell Nanocomposites as Trimodal Contrast Agents for Magnetic Resonance Imaging and Optical Imaging. Inorg. Chem. 2019, 58, 16618–16628. [Google Scholar] [CrossRef] [PubMed]
- Bomatí, M.O.; Gossuin, Y.; Morales, M.P.; Gillis, P.; Muller, R.N.; Veintemillas-Verdaguer, S. Comparative analysis of the 1H NMR relaxation enhancement produced by iron oxide and core-shell iron–iron oxide nanoparticles. Magn. Reson. Imaging 2007, 25, 1437–1441. [Google Scholar] [CrossRef]
- Wang, K.; An, L.; Tian, Q.; Lin, J.; Yang, S. Gadolinium-labelled iron/iron oxide core/shell nanoparticles as T1–T2 contrast agent for magnetic resonance imaging. RSC Adv. 2018, 8, 26764–26770. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Gong, S.; Zhang, C.; Qian, C.; Qiao, H.; Sun, M. Dual-Mode Avocado-like All-Iron Nanoplatform for Enhanced T1/T2 MRI-Guided Cancer Theranostic Therapy. Nano Lett. 2020, 20, 4842–4849. [Google Scholar] [CrossRef] [PubMed]
- Shultz, M.D.; Calvin, S.; Fatouros, P.P.; Morrison, S.A.; Carpenter, E.E. Enhanced ferrite nanoparticles as MRI contrast agents. J. Magn. Magn. Mater. 2007, 311, 464–468. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Z.; Wang, Z.; Xue, Y.; Zeng, Y.; Gao, J.; Zhu, L.; Zhang, X.; Liu, G.; Chen, X. Gadolinium embedded iron oxide nanoclusters as T1–T2 dual-modal MRI-visible vectors for safe and efficient siRNA delivery. Nanoscale 2013, 5, 8098–8104. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Chi, X.; Bao, J.; Yang, L.; Zhao, W.; Chen, Z.; Wang, X.; Chen, X.; Gao, J. Engineered Iron-Oxide-Based Nanoparticles as Enhanced T1 Contrast Agents for Efficient Tumor Imaging. ACS Nano 2013, 7, 3287–3296. [Google Scholar] [CrossRef]
- Huang, G.; Li, H.; Chen, J.; Zhao, Z.; Yang, L.; Chi, X.; Chen, Z.; Wang, X.; Gao, J. Tunable T1 and T2 contrast abilities of manganese-engineered iron oxide nanoparticles through size control. Nanoscale 2014, 6, 10404–10412. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, Z.; Liu, H.; Wu, C.; Zhang, H.; Huang, G.; Ai, H.; Gao, J. Europium-engineered iron oxide nanocubes with high T1 and T2 contrast abilities for MRI in living subjects. Nanoscale 2015, 7, 6843–6850. [Google Scholar] [CrossRef]
- Cheng, K.; Yang, M.; Zhang, R.; Qin, C.; Su, X.; Cheng, Z. Hybrid nanotrimers for dual T1 and T2-weighted magnetic resonance imaging. ACS Nano 2014, 8, 9884–9896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Meng, K.; Liu, Y.; Pan, Y.; Qu, W.; Chen, D.; Xie, S. Absorption, distribution, metabolism, and excretion of nanocarriers in vivo and their influences. Adv. Colloid Interface Sci. 2020, 284, 102261. [Google Scholar] [CrossRef] [PubMed]
- Roemhild, K.; von Maltzahn, F.; Weiskirchen, R.; Knüchel, R.; von Stillfried, S.; Lammers, T. Iron metabolism: Pathophysiology and Pharmacology. Trends Pharmacol. Sci. 2021, 42, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, R.G.; Martin, C.J.; Doney, B.C. From Manganism to Manganese-Induced Parkinsonism: A Conceptual Model Based on the Evolution of Exposure. Neuromol. Med. 2009, 11, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Alromi, D.A.; Madani, S.Y.; Seifalian, A. Emerging application of magnetic nanoparticles for diagnosis and treatment of cancer. Polymers 2021, 13, 4146. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of nanoparticles. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Mou, X.; Ali, Z.; Li, S.; He, N. Applications of Magnetic Nanoparticles in Targeted Drug Delivery System. J. Nanosci. Nanotechnol. 2015, 15, 54–62. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Z.; Zhang, Y.S.; Ding, J.; Chen, X. Smart transformable nanoparticles for enhanced tumor theranostics. Appl. Phys. Rev. 2021, 8, 041321. [Google Scholar] [CrossRef]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef]
- Nunn, A.D.P. The cost of developing imaging agents for routine clinical use. Investig. Radiol. 2006, 41, 206–212. [Google Scholar] [CrossRef]
- Josephson, L.; Rudin, M. Barriers to clinical translation with diagnostic drugs. J. Nucl. Med. 2013, 54, 329–332. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).