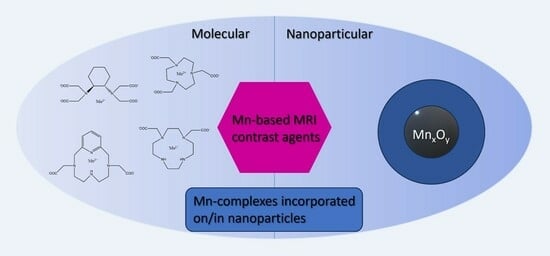
Mn-Based MRI Contrast Agents: An Overview
Abstract
1. Introduction
2. Molecular Mn-Based Contrast Agents
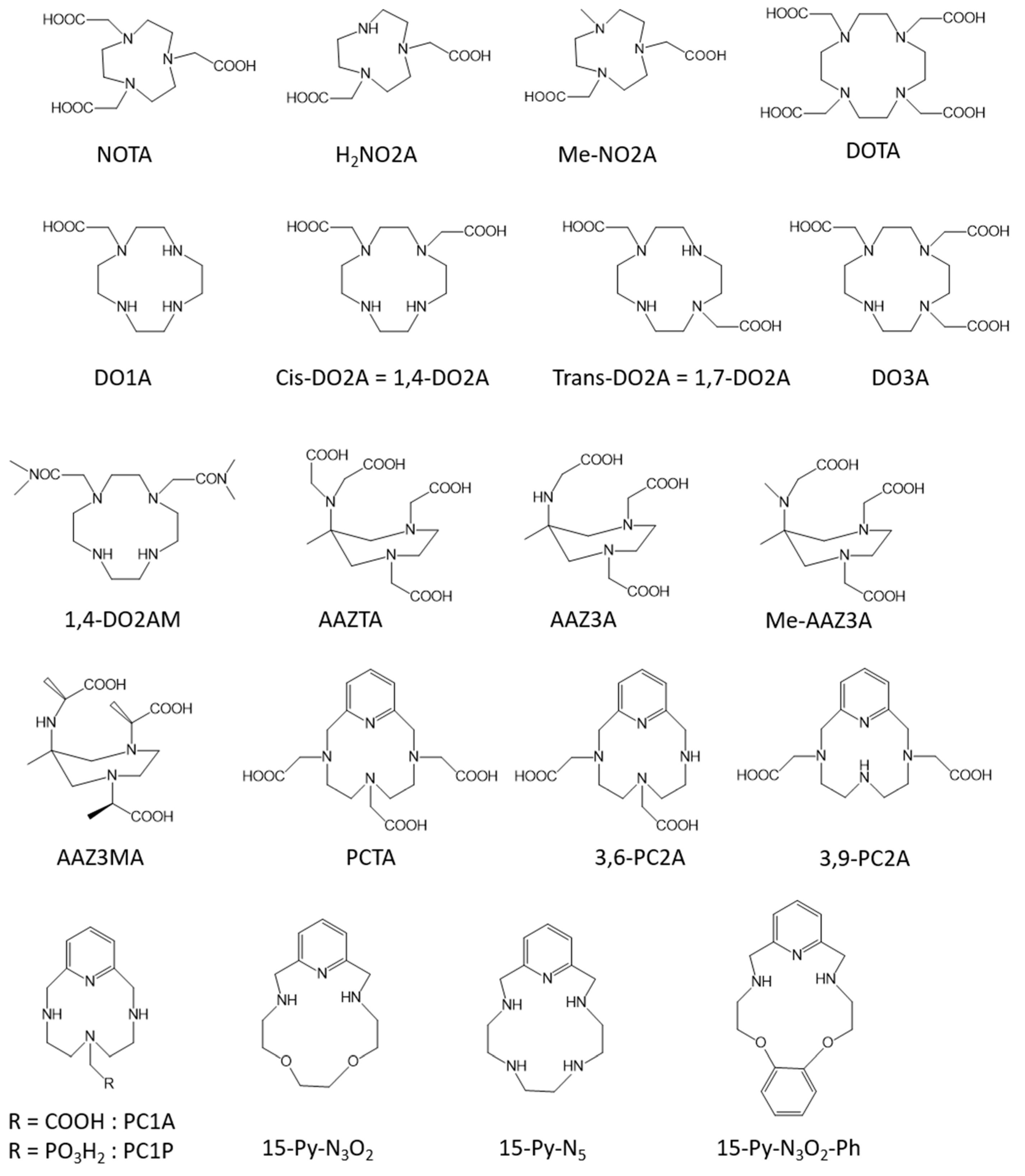
2.1. Non-Specific Contrast Agents
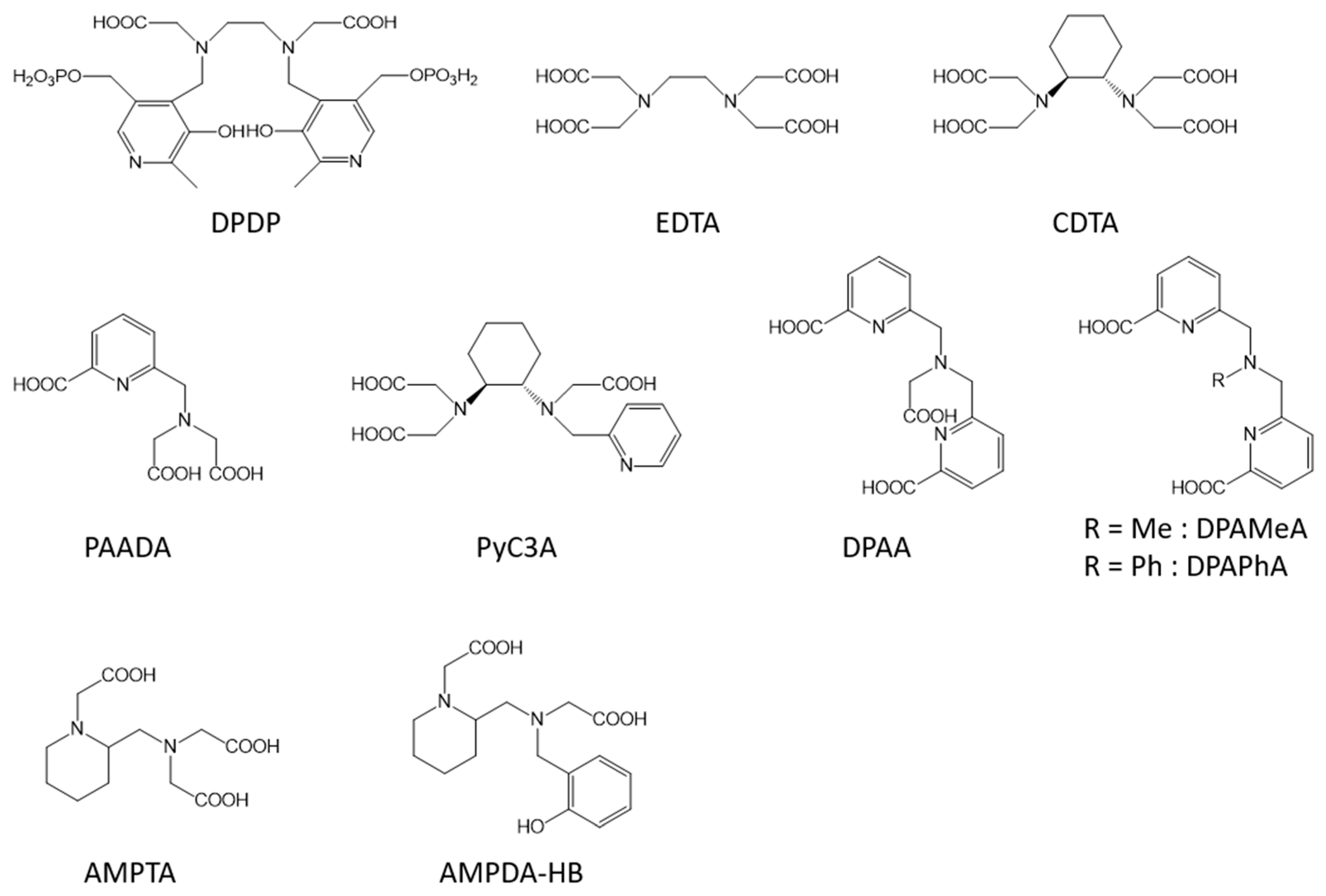
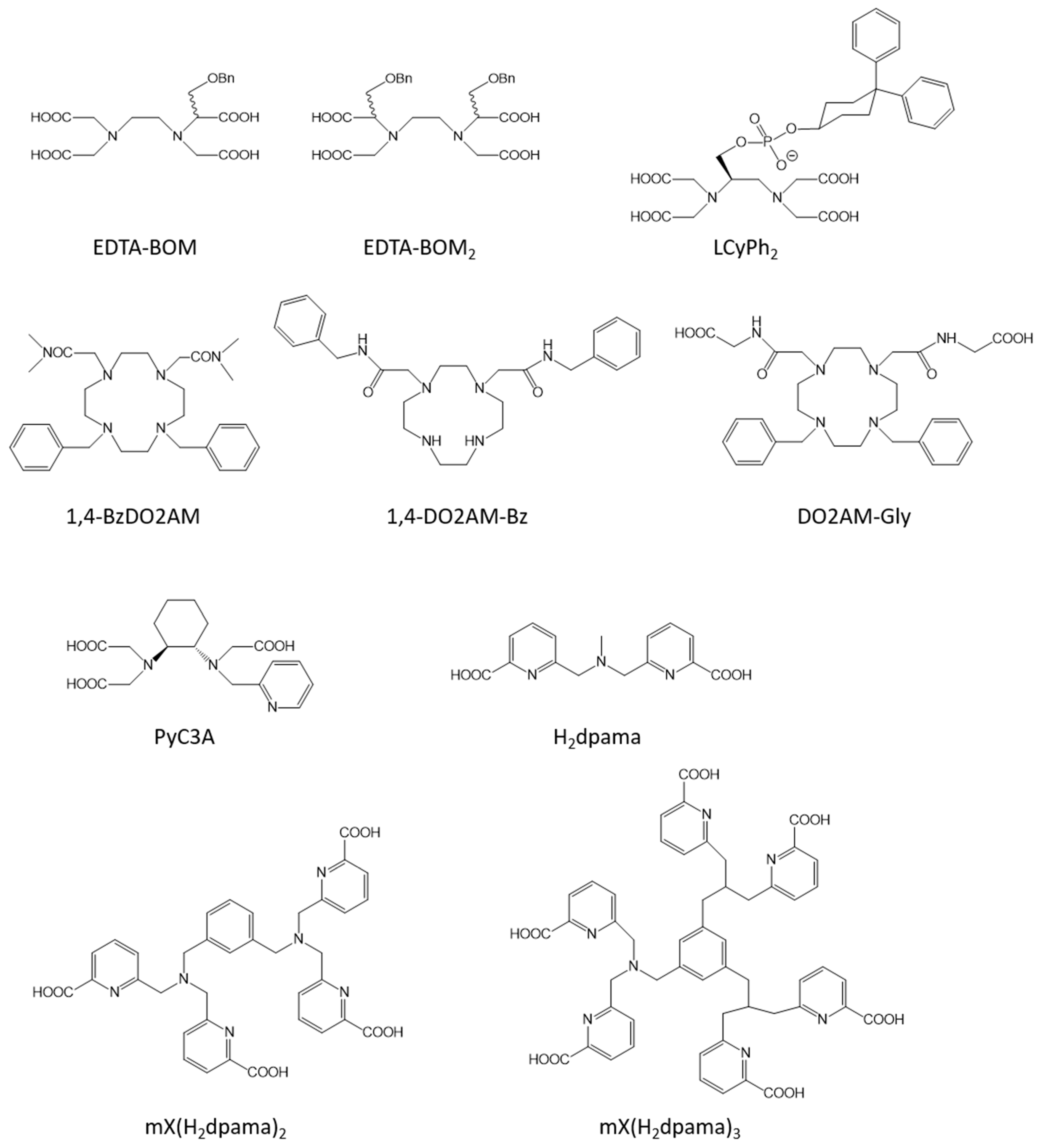
2.2. Liver Targeted Contrast Agents
2.3. Blood Pool Agents
2.4. Responsive Contrast Agents
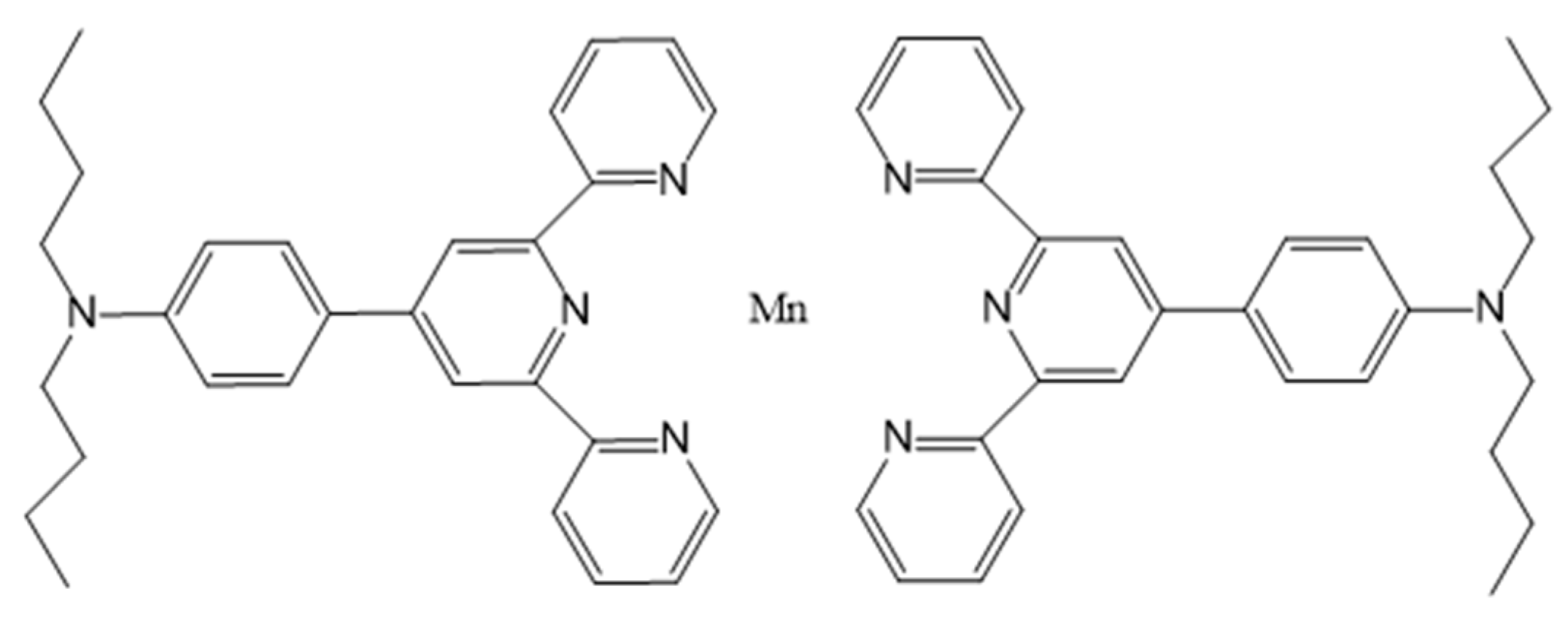
2.5. Multimodal Contrast Agents
| q | r1 in Water or Buffer (s−1 mM−1) | r1 in the Presence of HSA (s−1 mM−1) | Application Area | Tested In Vitro and/or In Vivo | |
|---|---|---|---|---|---|
| Mn-EDTA | 1 | 2.9 (0.47 T, 35 °C, [27]) | extracellular | no | |
| Mn-CDTA | 1 | 3.0 (0.47 T, 40 °C, [30]) | extracellular | no | |
| Mn-PyC3A | 1 | 2.1 (1.4 T, 37 °C, [31]) | 3.5 (1.4 T, 37 °C, [31]) | extracellular/blood pool | yes [33,34] |
| Mn-DPAA | 1 | 2.7 (0.47 T, 37 °C, [35]) | extracellular | no | |
| Mn-DPAMeA | 2 | 5.1 (0.47 T, 37 °C, [35]) | extracellular | no | |
| Mn-DPAPhA | 2 | 4.2 (0.47 T, 37 °C, [35]) | extracellular | no | |
| Mn-PAADA | 2 | 3.3 (0.47 T, 37 °C, [36]) | extracellular | no | |
| Mn-AMPTA | 1 | 2.6 (0.47 T, 37 °C, [37]) | extracellular | no | |
| Mn-AMPDA-HB | 1 | 2.7 (0.47 T, 37 °C, [37]) | extracellular | no | |
| Mn-MeNO2A | 1 | 2.2 (0.47 T, 37 °C, [46]) | extracellular | no | |
| Mn-DO3A | 0 | 1.3 (0.47 T, 37 °C, [49]) | extracellular | no | |
| Mn-1,7-DO2A | 0 | 1.3 (0.47 T, 37 °C, [49]) | extracellular | no | |
| Mn-1,4-DO2A | 1 | 1.7 (0.47 T, 37 °C, [49]) | extracellular | no | |
| Mn-1,4-DO2AM | 1 | 2.0 (0.47 T, 37 °C, [51]) | extracellular | no | |
| Mn-AAZTA | 0 | 1.6 (0.47 T, 25 °C, [53]) | extracellular | no | |
| Mn-AAZ3A | 1 | 2.5 (0.47 T, 25 °C, [53]) | extracellular | no | |
| Mn-MeAAZ3A | 1 | 2.0 (0.47 T, 25 °C, [53]) | extracellular | no | |
| Mn-AAZ3MA | 1 | 1.9 (0.47 T, 25 °C, [53]) | extracellular | no | |
| Mn-3,6-PC2A | 1 | 2.7 (0.47 T, 25 °C, [54]) | extracellular | no | |
| Mn-3,9-PC2A | 1 | 2.9 (0.47 T, 25 °C, [54]) | extracellular | no | |
| Mn-15-pyN5 | 2 | 3.1 (0.47 T, 37 °C, [57]) | extracellular | no | |
| Mn-15-pyN3O2 | 2 | 3.6 (0.47 T, 37 °C, [57]) | extracellular | no | |
| Mn-EDTA-BTA | 1 | 3.5 (1.5 T, 24 °C, [64]) | 15.1 (1.5 T, 24 °C, [64]) | liver | yes [63] |
| Mn-EDTA-EOB | 1 | 2.3 (1.5 T, 24 °C, [64]) | 6.3 (1.5 T, 24 °C, [64]) | liver | yes [64] |
| Mn-EOB-PC2A | 1 | 2.8 (1.5 T, 25 °C, [66]) | 5.9 (1.5 T, 25 °C, [66]) | liver | yes [66] |
| Mn-NOTA-NP | 1 | 3.6 (3 T, 25 °C, [67]) | 9.0 (3 T, 25 °C, [67]) | liver | yes [67] |
| Mn-PyC3A-3-Obn | 1 | 2.6 (1.4 T, 37 °C, [68]) | 9.0 (1.4 T, 37 °C, [68]) | liver | yes [68] |
| Mn-BnO-TyEDTA | 1 | 4.3 (0.47 T, 32 °C, [69]) | 15.8 (0.47 T, 32 °C, [69]) | liver | yes [69,70] |
| Mn-EDTA-BOM | 1 | 3.6 (0.47 T, 25 °C, [72]) | 55.3 (0.47 T, 25 °C, [72]) | blood pool | no |
| Mn-LCyPh2 | 1 | 5.8 (0.47 T, 37 °C, [73]) | 48.0 (0.47 T, 37 °C, [73]) | blood pool | yes [73] |
| Mn-1,4-BzDO2AM | 1 | 3.8 (0.47 T, 25 °C, [76]) | 18.5 (0.47 T, 25 °C, [76]) | blood pool | no |
| Mn-1,4-DO2AM-Bz | 1 | 3.5 (0.47 T, 25 °C, [76]) | 27.4 (0.47 T, 25 °C, [76]) | blood pool | no |
| Mn-DO2AM-Gly | 1 | 4.5 (1 T, 25 °C, [77]) | 14.0 (1 T, 25 °C, [77]) | blood pool | yes [77] |
| Mn-dpama | 2 | 4.2 (0.47 T, 37 °C, [79]) | 12.2 (0.47 T, 37 °C, [79]) | blood pool | no |
| mX(Mn-dpama)2 | 2 | 6.1 (0.47 T, 37 °C, [79]) | 39.0 (0.47 T, 37 °C, [79]) | blood pool | no |
| mX(Mn-dpama)3 | 2 | 8.3 (0.47 T, 37 °C, [79]) | 45.2 (0.47 T, 37 °C, [79]) | blood pool | no |
| Mn-PC2A-EA | 1 | 3.5/2.1 (0.47 T, 25 °C, [86]) | pH responsive | no | |
| MnII/III-HBET | 1 | 1.0/2.8 (1.4 T, 37 °C, [88]) | redox responsive | no | |
| MnII/III-JED | 1 | 0.5/3.3 (1.4 T, 37 °C, [90]) | redox responsive | no | |
| Mn-Hptp1 | 1/2 | 4.7/5.3 (3 T, 25 °C, [92]) | redox responsive | no | |
| Mn-Tyr-EDTA | 1 | 3.3/8.5 (0.47 T, 32 °C, [93]) | 8.0 (0.47 T, 32 °C, [93]) | redox responsive | yes [93] |
| Mn-3,9-PC2A-DPA | 1 | 3.2 (1.4 T, 37 °C, [94]) | 12.1 (1.4 T, 37 °C, [94]) | Zn responsive | yes [94] |
2.6. In Vitro/In Vivo Studies and Toxicity Issues
3. Nanoparticular Contrast Agents
3.1. Nanoparticles Incorporating Mn Complexes
3.2. Mn-Based Organic/Inorganic Nanoparticles
3.3. In Vitro/In Vivo Tests and Toxicity Issues
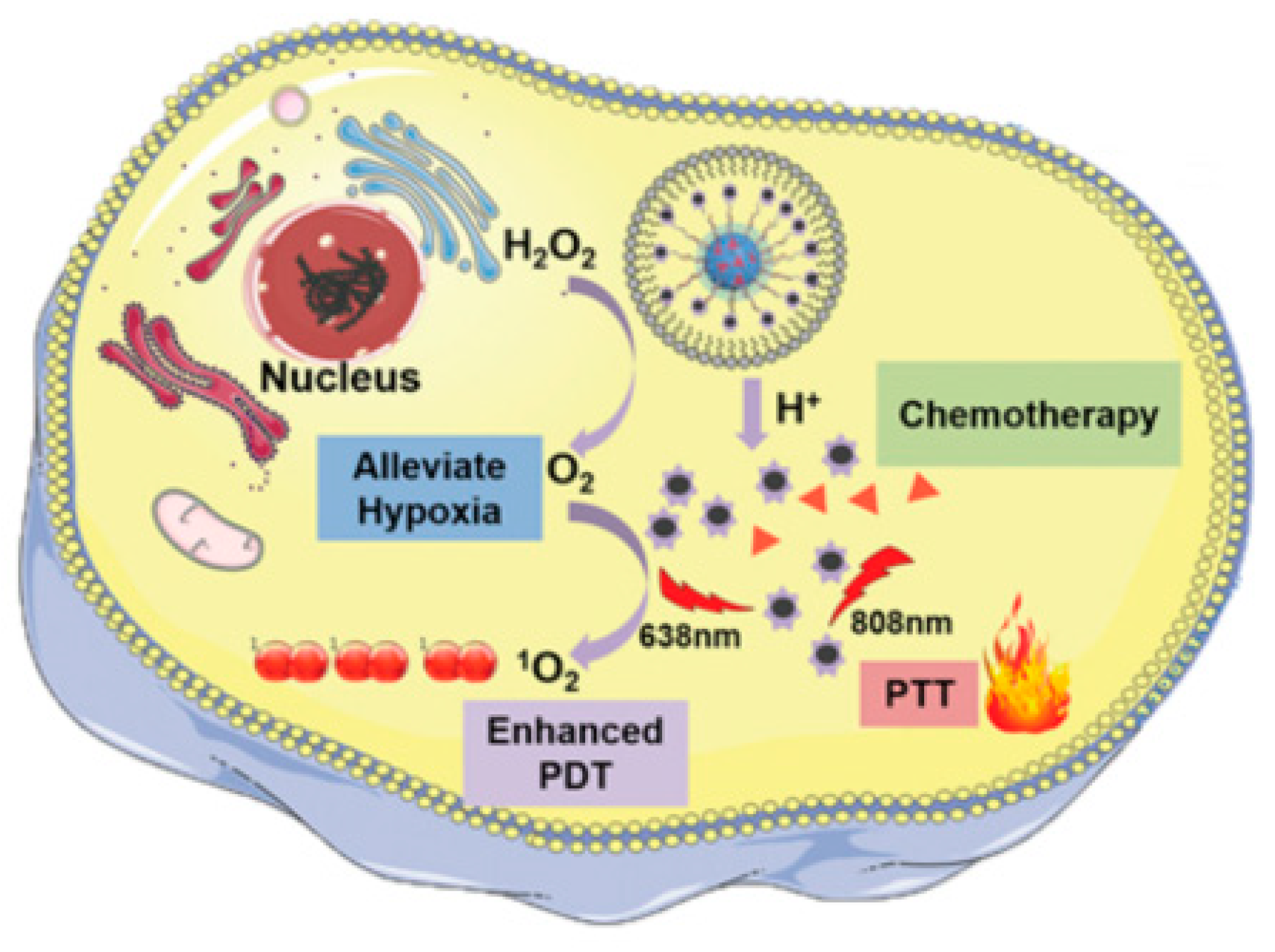
4. Theranostic Agents
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Washner, J.; Gale, E.M.; Rodrigez-Rodrigez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Iyad, N.; Ahmad, M.S.; Alkhatib, S.G.; Hjouj, M. Gadolinium contrast agents-challenges and opportunities of a multidisciplinary approach: Literature review. Eur. J. Radiol. Open 2023, 11, 100503. [Google Scholar] [CrossRef] [PubMed]
- Bauerle, T.; Saake, M.; Uder, M. Gadolinium-based contrast agents: What we learned from acute adverse events, nephrogenic systemic fibrosis and brain retention. RoFo Fortschr. Auf Dem Geb. Rontgenstrahlen Bildgeb. Verfahr. 2021, 193, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Amici, G.; Rosner, M.; Ronco, C.; Novara, G. Gadolinium-Based Contrast Media Nephrotoxicity in Kidney Impairment: The Physio-Pathological Conditions for the Perfect Murder. J. Clin. Med. 2021, 10, 271. [Google Scholar] [CrossRef]
- McDonald, J.S.; McDonald, R.J. MR imaging safety considerations of gadolinium-based contrast agents: Gadolinium retention and nephrogenic systemic fibrosis. Magn. Reason. Imaging Clin. N. Am. 2020, 28, 497–507. [Google Scholar] [CrossRef]
- Lancelot, E.; Raynaud, J.-S.; Desché, P. Current and future MR contrast agents: Seeking a better chemical stability and relaxivity for optimal safety and efficacy. Investig. Radiol. 2020, 55, 578–588. [Google Scholar] [CrossRef]
- Blomqvist, L.; Nordberg, G.F.; Nurchi, V.M.; Aaseth, J.O. Gadolinium in Medical Imaging—Usefulness, Toxic Reactions and Possible Countermeasures—A Review. Biomolecules 2022, 12, 742. [Google Scholar] [CrossRef]
- Gianolio, E.; Gregorio, E.D.; Aime, S. Chemical Insights into the Issues of Gd Retention in the Brain and Other Tissues Upon the Administration of Gd-Containing MRI Contrast Agents. Eur. J. Inorg. Chem. 2019, 2019, 137–151. [Google Scholar] [CrossRef]
- Chehabeddine, L.; Al Saleh, T.; Baalbaki, M.; Saleh, E.; Khoury, S.J.; Hannoun, S. Cumulative administrations of gadolinium-based contrast agent: Risks of accumulation and toxicity of linear vs macrocyclic agents. Crit. Rev. Toxicol. 2019, 49, 262–279. [Google Scholar] [CrossRef]
- Davies, J.; Siebenhandl-Wolff, P.; Tranquart, F.; Jones, P.; Evans, P. Gadolinium: Pharmacokinetics and toxicity in humans and laboratory animals following contrast agent administration. Arch. Toxicol. 2022, 96, 403–429. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Mo, Y.; Huang, C.; Liu, C.; Duan, Z.; Liu, J.; Wu, D. Recent Research Progress of 19F Magnetic Resonance Imaging Probes: Principle, Design, and Their Application. Macromol. Rapid Commun. 2023, 44, 2200744. [Google Scholar] [CrossRef]
- Akakuru, O.U.; Iqbal, M.Z.; Saeed, M.; Liu, C.; Paunesku, T.; Woloschak, G.; Hosmane, N.S.; Wu, A. The transition from metal-based to metal-free contrast agents for T1 magnetic resonance imaging enhancement. Bioconjug. Chem. 2019, 30, 2264–2286. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Caravan, P.; Price, W.S.; Platas-Iglesias, C.; Gale, E.M. Applications for transition-metal chemistry in contrast-enhanced magnetic resonance imaging. Inorg. Chem. 2020, 59, 6648–6678. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Wu, L.; Chen, X.; Zhang, Y.; Jiang, L. Research progress on manganese complexes as contrast agents for magnetic resonance imaging. Polyhedron 2023, 242, 116489. [Google Scholar] [CrossRef]
- Botta, M.; Carniato, F.; Esteban-Gómez, D.; Platas-Iglesias, C.; Tei, L. Mn(II) Compounds as an Alternative to Gd-Based MRI Probes. Future Med. Chem. 2019, 11, 1461–1483. [Google Scholar] [CrossRef]
- Crossgrove, J.; Zheng, W. Manganese Toxicity upon Overexposure. NMR Biomed. 2004, 17, 544–553. [Google Scholar] [CrossRef]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef]
- Castets, C.R.; Koonjoo, N.; Hertanu, A.; Voisin, P.; Franconi, J.M.; Miraux, S.; Ribot, E.J. In Vivo MEMRI Characterization of Brain Metastases Using a 3D Look-Locker T1-Mapping Sequence. Sci. Rep. 2016, 6, 39449. [Google Scholar] [CrossRef]
- Bianchi, A.; Gobbo, O.L.; Dufort, S.; Sancey, L.; Lux, F.; Tillement, O.; Coll, J.L.; Crémillieux, Y. Orotracheal Manganese-Enhanced MRI (MEMRI): An Effective Approach for Lung Tumor Detection. NMR Biomed. 2017, 30, e3790. [Google Scholar] [CrossRef]
- Rocklage, S.M.; Cacheris, W.P.; Quay, S.C.; Hahn, F.E.; Raymond, K.N. Manganese(II) N,N′-dipyridoxylethylenediamine-N,N′-diacetate 5,5′-bis(phosphate). Synthesis and characterization of a paramagnetic chelate for magnetic resonance imaging enhancement. Inorg. Chem. 1989, 28, 477–485. [Google Scholar] [CrossRef]
- Elizondo, G.; Fretz, C.J.; Stark, D.D.; Rocklage, S.M.; Quay, S.C.; Worah, D.; Tsang, D.D.; Chen, M.C.; Ferrucci, J.T. Preclinical Evaluation of MnDPDP: New Paramagnetic Hepatobiliary Contrast Agent for MR Imaging. Radiology 1991, 178, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Vander Elst, L.; Muller, R.N. Comparative Study of the Physicochemical Properties of Six Clinical Low Molecular Weight Gadolinium Contrast Agents. Contrast Media Mol. Imaging 2006, 1, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Barandov, A.; Bartelle, B.B.; Gonzales, B.A.; White, W.L.; Lippard, S.J.; Jasanoff, A. Membrane-permeable Mn(III) complexes for molecular magnetic resonance imaging of intracellular targets. J. Am. Chem. Soc. 2016, 138, 5483–5486. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, C.F.G.C.; Castro, M.M.C.A.; Peters, J. Mn(III) porphyrins as potential MRI contrast agents for diagnosis and MRI-guided therapy. Coord. Chem. Rev. 2021, 445, 214069. [Google Scholar] [CrossRef]
- Wolf, G.L.; Burnett, K.R.; Goldstein, E.J.; Joseph, P.M. Magnetic Resonance Annual 1985; Kressel, H., Ed.; Raven: New York, NY, USA, 1985; p. 231. [Google Scholar]
- Koenig, S.H.; Baglin, C.; Brown, R.D., III; Brewer, C.F. Magnetic field dependence of solvent proton relaxation induced by Gd3+ and Mn2+ complexes. Magn. Reson. Med. 1984, 1, 496–501. [Google Scholar] [CrossRef]
- Zhu, J.; Gale, E.M.; Atanasova, I.; Rietz, T.A.; Caravan, P. Hexameric MnII Dendrimer as MRI Contrast Agent. Chem. Eur. J. 2014, 20, 14507–14513. [Google Scholar] [CrossRef]
- Kálmán, F.K.; Tircsó, G. Kinetic inertness of the Mn2+ complexes formed with AAZTA and some open-chain EDTA derivatives. Inorg. Chem. 2012, 51, 10065–10067. [Google Scholar] [CrossRef]
- Borodin, O.Y.; Sannikov, M.Y.; Belyanin, M.L.; Filimonov, V.D.; Usov, V.Y.; Rybakov, Y.L.; Gukasov, V.M.; Shimanovskii, N.L. Relaxivity of Paramagnetic complexes of Manganese and Gadolinium. Pharm. Chem. J. 2019, 53, 635–637. [Google Scholar] [CrossRef]
- Gale, E.M.; Atanasova, I.P.; Blasi, F.; Ay, I.; Caravan, P. A Manganese Alternative to Gadolinium for MRI Contrast. J. Am. Chem. Soc. 2015, 137, 15548–15557. [Google Scholar] [CrossRef]
- Gale, E.M.; Wey, H.Y.; Ramsay, I.A.; Yen, Y.F.; Sosnovik, D.E.; Caravan, P. A manganese-based alternative to gadolinium: Contrast-enhanced MR angiography, excretion, pharmacokinetics, and metabolism. Radiology 2018, 286, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Erstad, D.J.; Ramsay, I.A.; Jordan, V.C.; Sojoodi, M.; Fuchs, B.C.; Tanabe, K.K.; Caravan, P.; Gale, E.M. Tumor Contrast Enhancement and Whole-Body Elimination of the Manganese-Based Magnetic Resonance Imaging Contrast Agent Mn-PyC3A. Investig. Radiol. 2019, 54, 697–703. [Google Scholar] [CrossRef]
- Zhou, I.Y.; Ramsay, I.A.; Ay, I.; Pantazopoulos, P.; Rotile, N.J.; Wong, A.; Caravan, P.; Gale, E.M. Positron Emission Tomography–Magnetic Resonance Imaging Pharmacokinetics, In Vivo Biodistribution, and Whole-Body Elimination of Mn-PyC3A. Investig. Radiol. 2021, 56, 261–270. [Google Scholar] [CrossRef]
- Forgács, A.; Pujales-Paradela, R.; Regueiro-Figueroa, M.; Valencia, L.; Esteban, D.; Botta, M.; Platas-Iglesias, C. Platas-Iglesias. Developing the family of picolinate ligands for Mn2+ complexation. Dalton Trans. 2017, 46, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Pujales-Paradela, R.; Carniato, F.; Uzal-Varela, R.; Brandariz, I.; Iglesias, E.; Platas-Iglesias, C.; Botta, M.; Esteban-Gómez, D. A pentadentate member of the picolinate family for Mn(ii) complexation and an amphiphilic derivative. Dalton Trans. 2019, 48, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, J.; Callegari, E.; Baranyai, Z.; Fraccarollo, A.; Cossi, M.; Tei, L. Semi-Rigid (Aminomethyl) Piperidine-Based Pentadentate Ligands for Mn(II) Complexation. Molecules 2021, 26, 5993. [Google Scholar] [CrossRef]
- Bonner, B.P.; Yurista, S.R.; Coll-Font, J.; Chen, S.; Eder, R.A.; Foster, A.N.; Nguyen, K.D.; Caravan, P.; Gale, E.M.; Nguyen, C. Contrast-Enhanced Cardiac Magnetic Resonance Imaging with a Manganese-Based Alternative to Gadolinium for Tissue Characterization of Acute Myocardial Infarction. J. Am. Heart Assoc. 2023, 12, e026923. [Google Scholar] [CrossRef]
- Cieslik, P.; Comba, P.; Dittmar, B.; Ndiaye, D.; Toth, E.; Velmurugan, G.; Wadepohl, H. Exceptional Manganese (II) Stability and Manganese(II)/Zinc(II) Selectivity with Rigid Polydentate Ligands. Angew. Chem. Int. Ed. 2022, 61, e202115580. [Google Scholar] [CrossRef]
- Anbu, S.; Hoffmann, S.H.L.; Carniato, F.; Kenning, L.; Price, T.W.; Prior, T.J.; Botta, M.; Martins, A.F.; Stasiuk, G.J. A single-pot template reaction towards a manganese-based T1 contrast agent. Angew. Chem. Int. Ed. 2021, 60, 10736–10744. [Google Scholar] [CrossRef]
- Drahos, B.; Kubicek, V.; Bonnet, C.S.; Hermann, P.; Lukes, I.; Toth, E. Dissociation Kinetics of Mn2+ complexes of NOTA and DOTA. Dalton Trans. 2011, 40, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Uzal-Varela, R.; Rodriguez-Rodriguez, A.; Martinez-Calvo, M.; Carniato, F.; Lalli, D.; Esteban-Gomez, D.; Brandariz, I.; Pérez-Lourido, P.; Botta, M.; Platas-Iglesias, C. Mn2+ complexes containing sulfonamide groups with pH-responsive relaxivity. Inorg. Chem. 2020, 59, 14306–14317. [Google Scholar] [CrossRef]
- Uzal-Varela, R.; Valencia, L.; Lalli, D.; Maneiro, M.; Esteban-Gomez, D.; Platas-Iglesias, C.; Botta, M.; Rodriguez-Rodriguez, A. Understanding the effect of the electron spin relaxation on the relaxivities of Mn(II) complexes with triazacyclononane derivatives. Inorg. Chem. 2021, 60, 15055–15068. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.; He, Z.; Hsieh, W.; Liu, S.; Tóth, E. Dinuclear Complexes Formed with the Triazacyclononane Derivative ENOTA4−: High-Pressure 17O NMR Evidence of an Associative Water Exchange on [MnII2(ENOTA)(H2O)2]. Inorg. Chem. 2007, 46, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Ducommun, Y.; Newman, K.E.; Merbach, A.E. High-pressure oxygen-17 NMR evidence for a gradual mechanistic changeover from Ia to Id for water exchange on divalent octahedral metal ions going from manganese(II) to nickel(II). Inorg. Chem. 1980, 19, 3696–3703. [Google Scholar] [CrossRef]
- Patinec, V.; Rolla, G.A.; Botta, M.; Tripier, R.; Esteban-Gomez, D.; Platas-Iglesias, C. Hyperfine Coupling Constants on Inner-Sphere Water Molecules of a Triazacyclononane-based Mn(II) Complex and Related Systems Relevant as MRI Contrast Agents. Inorg. Chem. 2013, 52, 11173–11184. [Google Scholar] [CrossRef] [PubMed]
- de Sá, A.; Bonnet, C.S.; Geraldes, C.F.G.C.; Tóth, E.; Ferreira, P.M.T.; André, J.P. Thermodynamic stability and relaxation studies of small, triaza-macrocyclic Mn(ii) chelates. Dalton Trans. 2013, 42, 4522–4532. [Google Scholar] [CrossRef]
- Pujales-Paradela, R.; Carniato, F.; Esteban-Gomez, D.; Botta, M.; Platas-Iglesias, C. Controlling water exchange rates in potential Mn2+-based MRI agents derived from NO2A2−. Dalton Trans. 2019, 48, 3962–3972. [Google Scholar] [CrossRef] [PubMed]
- Rolla, G.A.; Platas-Iglesias, C.; Botta, M.; Tei, L.; Helm, L. 1H and 17O NMR Relaxometric and Computational Study on Macrocyclic Mn(II) Complexes. Inorg. Chem. 2013, 52, 3268–3279. [Google Scholar] [CrossRef] [PubMed]
- Garda, Z.; Forgacs, A.; Do, Q.N.; Kalman, F.K.; Timari, S.; Baranyai, Z.; Tei, L.; Toth, I.; Kovacs, Z.; Tircso, G. Physico-chemical properties of MnII complexes formed with cis- and trans-DO2A: Thermodynamic, electrochemical and kinetic studies. J. Inorg. Biochem. 2016, 163, 206–213. [Google Scholar] [CrossRef]
- Forgacs, A.; Tei, L.; Baranyai, Z.; Toth, I.; Zekany, L.; Botta, M. A Bisamide Derivative of [Mn(1,4-DO2A)]–Solution Thermodynamic, Kinetic, and NMR Relaxometric Studies. Eur. J. Inorg. Chem. 2016, 2016, 1165–1174. [Google Scholar] [CrossRef]
- Garda, Z.; Molnár, E.; Kálmán, F.K.; Botár, R.; Nagy, V.; Baranyai, Z.; Brücher, E.; Kovács, Z.; Tóth, I.; Tircsó, G. Effect of the Nature of Donor Atoms on the Thermodynamic, Kinetic and Relaxation Properties of Mn(II) Complexes Formed with Some Trisubstituted 12-Membered Macrocyclic Ligands. Front. Chem. 2018, 6, 232. [Google Scholar] [CrossRef]
- Tei, L.; Gugliotta, G.; Fekete, M.; Kalman, F.K.; Botta, M. Mn(II) complexes of novel hexadentate AAZTA-like chelators: A solution thermodynamics and relaxometric study. Dalton Trans. 2011, 40, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Garda, Z.; Molnar, E.; Hamon, N.; Barriada, J.L.; Esteban-Gomez, D.; Varadi, B.; Nagy, V.; Pota, K.; Kalman, F.K.; Toth, I.; et al. Complexation of Mn(II) by Rigid Pyclen Diacetates: Equilibrium, Kinetic, Relaxometric, Density Functional Theory, and Superoxide Dismutase Activity Studies. Inorg. Chem. 2021, 60, 1133–1148. [Google Scholar] [CrossRef]
- Devreux, M.; Henoumont, C.; Dioury, F.; Boutry, S.; Vacher, O.; Vander Elst, L.; Port, M.; Muller, R.N.; Sandre, O.; Laurent, S. Mn2+ complexes with Pyclen-based derivatives as contrast agents for magnetic resonance imaging: Synthesis and relaxometry characterization. Inorg. Chem. 2021, 60, 3604–3619. [Google Scholar] [CrossRef] [PubMed]
- Drahoš, B.; Kotek, J.; Cíśarǒvá, I.; Hermann, P.; Helm, L.; Lukeš, I.; Tóth, É. Mn2+ Complexes with 12-Membered Pyridine Based Macrocycles Bearing Carboxylate or Phosphonate Pendant Arm: Crystallographic, Thermodynamic, Kinetic, Redox, and 1H/17O Relaxation Studies. Inorg. Chem. 2011, 50, 12785–12801. [Google Scholar] [CrossRef]
- Drahos, B.; Kotek, J.; Hermann, P.; Lukes, I.; Toth, E. Mn2+ Complexes with Pyridine-Containing 15-Membered Macrocycles: Thermodynamic, Kinetic, Crystallographic, and 1H/17O Relaxation Studies. Inorg. Chem. 2010, 49, 3224–3238. [Google Scholar] [CrossRef] [PubMed]
- Pota, K.; Molnar, E.; Kalman, F.K.; Freire, D.M.; Tircso, G.; Green, K.N. Manganese Complex of a Rigidified 15-Membered Macrocycle: A Comprehensive Study. Inorg. Chem. 2020, 59, 11366–11376. [Google Scholar] [CrossRef]
- Prazakova, M.; Ndiaye, D.; Toth, E.; Drahos, B. A seven-coordinate Mn(II) complex with a pyridine-based 15-membered macrocyclic ligand containing one acetate pendant arm: Structure, stability and relaxation properties. Dalton Trans. 2023, 52, 7936–7947. [Google Scholar] [CrossRef]
- Nagendraraj, T.; Kumaran, S.S.; Mayilmurugan, R. Mn(II) complexes of phenylenediamine based macrocyclic ligands as T1-MRI contrast agents. J. Inorg. Biochem. 2022, 228, 111684–111692. [Google Scholar] [CrossRef]
- Reale, G.; Calderoni, F.; Ghirardi, T.; Porto, F.; Illuminati, F.; Marvelli, L.; Martini, P.; Uccelli, L.; Tonini, E.; Del Bianco, L.; et al. Development and evaluation of the magnetic properties of a new manganese (II) complex: A potential MRI contrast agent. Int. J. Mol. Sci. 2023, 24, 3461. [Google Scholar] [CrossRef]
- Uzal-Varela, R.; Perez-Fernandez, F.; Valencia, L.; Rodriguez-Rodriguez, A.; Platas-Iglesias, C.; Caravan, P.; Esteban-Gomez, D. Thermodynamic stability of Mn(II) complexes with aminocarboxylate ligands analyzed using structural descriptors. Inorg. Chem. 2022, 61, 14173–14186. [Google Scholar] [CrossRef]
- Islam, M.K.; Kim, S.; Kim, H.K.; Park, S.; Lee, G.H.; Kang, H.J.; Jung, J.C.; Park, J.S.; Kim, T.J.; Chang, Y. Manganese Complex of Ethylenediaminetetra acetic acid (EDTA)-Benzothiazole Aniline (BTA) Conjugate as a Potential Liver-Targeting MRI Contrast Agent. J. Med. Chem. 2017, 60, 2993–3001. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Kim, S.; Kim, H.K.; Kim, Y.H.; Lee, Y.M.; Choi, G.; Baek, A.R.; Sung, B.K.; Kim, M.; Cho, A.E.; et al. Synthesis and Evaluation of Manganese (II)-based Ethylenediaminetetraacetic Acid-Ethoxybenzyl Conjugate as a Highly Stable Hepatobiliary Magnetic Resonance Imaging Contrast Agent. Bioconjug. Chem. 2018, 29, 3614. [Google Scholar] [CrossRef] [PubMed]
- McRae, S.W.; Cleary, M.; DeRoche, D.; Martinez, F.M.; Xia, Y.; Caravan, P.; Gale, E.M.; Ronald, J.A.; Scholl, T.J. Development of a Suite of Gadolinium-Free OATP1-Targeted Paramagnetic Probes for Liver MRI. J. Med. Chem. 2023, 66, 6567–6576. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.C.; Qin, J.; Laney, V.; Ayat, N.; Lu, Z.R. Manganese(II) EOB-Pyclen Diacetate for Liver-Specific MRI. ACS Appl. Bio Mater. 2022, 5, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Baek, M.K.; Yang, A.R.; Kim, B.W.; Hwang, S.; Nam, D.W.; Lee, S.W.; Chang, G.H. Manganese (II) Complex of 1,4,7-Triazacyclononane-1,4,7- Triacetic Acid (NOTA) as a Hepatobiliary MRI Contrast Agent. Pharmaceuticals 2023, 16, 602. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Ramsay, I.A.; Erstad, D.J.; Fuchs, B.C.; Tanabe, K.K.; Caravan, P.; Gale, E.M. Manganese-Based Contrast Agents for Magnetic Resonance Imaging of Liver Tumors: Structure Activity Relationships and Lead Candidate Evaluation. J. Med. Chem. 2018, 61, 8811–8824. [Google Scholar] [CrossRef]
- Chen, K.; Li, P.; Zhu, C.; Xia, Z.; Xia, Q.; Zhong, L.; Xiao, B.; Cheng, T.; Wu, C.; Shen, C.; et al. Mn(II) Complex of Lipophilic Group-Modified Ethylenediaminetetraacetic Acid (EDTA) as a New Hepatobiliary MRI Contrast Agent. J. Med. Chem. 2021, 64, 9182–9192. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xiao, B.; Xia, Z.; Dai, L.; Xia, Q.; Zhong, L.; Zhu, C.; Zhu, J. A New OATP-mediated hepatobliliary-specific Mn(II)-based MRI contrast agent for hepatocellular carcinoma in mice: A comparison with Gd-EOB-DTPA. J. Magn. Reson. Imaging 2023, 58, 926–933. [Google Scholar] [CrossRef]
- Ning, Y.; Zhou, I.Y.; Rotile, N.J.; Pantazopoulos, P.; Wang, H.; Barrett, S.C.; Sojoodi, M.; Tanabe, K.K.; Caravan, P. Dual Hydrazine-Equipped Turn-On Manganese-Based Probes for Magnetic Resonance Imaging of Liver Fibrogenesis. J. Am. Chem. Soc. 2022, 144, 16553–16558. [Google Scholar] [CrossRef] [PubMed]
- Aime, S.; Anelli, P.L.; Botta, M.; Brocchetta, M.; Canton, S.; Fedeli, F.; Gianolio, E.; Terreno, E. Relaxometric evaluation of novel manganese(III) complexes for application as contrast agents in magnetic resonance imaging. J. Biol. Inorg. Chem. 2002, 7, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Troughton, J.S.; Greenfield, M.T.; Greenwood, J.M.; Dumas, S.; Wiethoff, A.J.; Wang, J.; Spiller, M.; McMurry, T.J.; Caravan, P. Synthesis and Evaluation of a High Relaxivity Manganese(II)-Based MRI Contrast Agent. Inorg. Chem. 2004, 43, 6313–6323. [Google Scholar] [CrossRef]
- Baroni, S.; Serra, S.C.; Mingo, A.F.; Lux, G.; Giovenzana, G.B.; Lattuada, L. Synthesis and Relaxometric Characterization of a New Mn(II)-EDTA-Deoxycholic Acid Conjugate Complex as a Potential MRI Blood Pool Agent. Chem. Sel. 2016, 1, 1607–1612. [Google Scholar] [CrossRef]
- Kalman, F.K.; Nagy, V.; Varadi, B.; Garda, Z.; Molnar, E.; Trencsenyi, G.; Kiss, J.; Même, S.; Meme, W.; Toth, E.; et al. Mn(II)-based MRI contrats agent candidate for vascular imaging. J. Med. Chem. 2020, 63, 6057. [Google Scholar] [CrossRef]
- Forgacs, A.; Tei, L.; Baranyai, Z.; Esteban-Gomez, D.; Platas-Iglesias, C.; Botta, M. Optimising the relaxivities of Mn2+ complexes by targeting human serum albumin (HSA). Dalton Trans. 2017, 46, 8494–8504. [Google Scholar] [CrossRef]
- Leone, L.; Anemone, A.; Carella, A.; Botto, E.; Longo, D.L.; Tei, L. A neutral and stable macrocyclic Mn(II) complex for MRI tumor visualization. ChemMedChem 2022, 17, e202200508. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Bai, R.; Wang, Z.; Bryant, H.; Lang, L.; Merkle, H.; Munasinghe, J.; Tang, L.; Tang, W.; Tian, R.; et al. An albumin-binding T1-T2 dual-modal MRI contrast agents for improved sensitivity and accuracy in tumor imaging. Bioconjug. Chem. 2019, 30, 1821–1829. [Google Scholar] [CrossRef]
- Forgacs, A.; Regueiro-Figueroa, M.; Barriada, J.L.; Esteban-Gomez, D.; de Blas, A.; Rodriguez-Blas, T.; Botta, M.; Platas-Iglesis, C. Mono-, Bi-, and Trinuclear Bis-Hydrated Mn2+ Complexes as Potential MRI Contrast Agents. Inorg. Chem. 2015, 54, 9576. [Google Scholar] [CrossRef]
- Su, H.; Wu, C.; Zhu, J.; Miao, T.; Wang, D.; Xia, C.; Zhao, X.; Gong, Q.; Song, B.; Ai, H. Rigid Mn(II) chelate as efficient MRI contrast agent for vascular imaging. Dalton Trans. 2012, 41, 14480. [Google Scholar] [CrossRef]
- Rolla, G.; De Biasio, V.; Giovenzana, G.B.; Botta, M.; Tei, L. Supramolecular assemblies based on amphiphilic Mn2+-complexes as high relaxivity MRI probes. Dalton Trans. 2018, 47, 10660–10670. [Google Scholar] [CrossRef]
- Mulas, G.; Rolla, G.A.; Geraldes, C.F.G.C.; Starmans, L.W.E.; Botta, M.; Terreno, E.; Tei, L. Mn(II)-Based Lipidic Nanovesicles as High-Efficiency MRI Probes. ACS Appl. Bio Mater. 2020, 3, 2401–2409. [Google Scholar] [CrossRef]
- Liu, X.; Fu, S.; Xia, C.; Li, M.; Cai, Z.; Wu, C.; Lu, F.; Zhu, J.; Song, B.; Gong, Q.; et al. PEGylated amphiphilic polymeric manganese(II) complexes as magnetic resonance angiographic agents. J. Mater. Chem. B 2022, 10, 2204–2214. [Google Scholar] [CrossRef]
- Chen, K.; Cai, Z.; Cao, Y.; Jiang, L.; Jiang, Y.; Gu, H.; Fu, S.; Xia, C.; Lui, S.; Gong, Q.; et al. Kinetically inert manganese (II)-based hybrid micellar complexes for magnetic resonance imaging of lymph node metastasis. Regen. Biomater. 2023, 10, rbad053. [Google Scholar] [CrossRef]
- Swietach, P.; Vaughan-Jones, R.D.; Harris, A.L.; Hulikova, A. The chemistry, physiology, and pathology of pH in cancer. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130099. [Google Scholar] [CrossRef]
- Botar, R.; Molnar, E.; Trencsenyi, G.; Kiss, J.; Kalman, F.K.; Tircso, G. Stable and inert Mn(II)-based and pH responsive contrast agents. J. Am. Chem. Soc. 2020, 142, 1662. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Pan, Y.; Liang, G. Development of macrocyclic Mn(II)-Bispyridine complexes as pH responsive magnetic resonance imaging contrast agents. Eur. J. Inorg. Chem. 2023, 26, e202200786. [Google Scholar] [CrossRef]
- Loving, G.S.; Mukherjee, S.; Caravan, P. Redox-activated manganese-based MR contrast agent. J. Am. Chem. Soc. 2013, 135, 4620–4623. [Google Scholar] [CrossRef] [PubMed]
- Gale, M.E.; Mukherjee, S.; Liu, C.; Loving, G.S.; Caravan, P. Structure-redox-relaxivity relationships for redox responsive manganese-based magnetic resonance imaging probes. Inorg. Chem. 2014, 53, 10748–10761. [Google Scholar] [CrossRef] [PubMed]
- Gale, E.M.; Jones, C.M.; Ramsay, I.; Farrar, C.T.; Caravan, P. A Janus Chelator Enables Biochemically Responsive MRI Contrast with Exceptional Dynamic Range. J. Am. Chem. Soc. 2016, 138, 15861–15864. [Google Scholar] [CrossRef]
- Yu, M.; Beyers, R.J.; Gorden, J.D.; Cross, J.N.; Goldsmith, C.R. A Magnetic Resonance Imaging Contrast Agent Capable of DetectingHydrogen Peroxide. Inorg. Chem. 2012, 51, 9153–9155. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Ambrose, S.L.; Whaley, Z.L.; Fan, S.; Gorden, J.D.; Beyers, R.J.; Schwartz, D.D.; Goldsmith, C.R. A Mononuclear Manganese(II) Complex Demonstrates a Strategy ToSimultaneously Image and Treat Oxidative Stress. J. Am. Chem. Soc. 2014, 136, 12836–12839. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Q.; Zhu, C.; Cao, W.; Xia, Z.; Liu, X.; Xiao, B.; Chen, K.; Liu, Y.; Zhong, L.; et al. An activatable Mn(II) MRI probe for detecting peroxidase activity in vitro and in vivo. J. Inorg. Biochem. 2022, 236, 111979. [Google Scholar] [CrossRef]
- Botar, R.; Molnar, E.; Garda, Z.; Madarasi, E.; Trencsenyi, G.; Kiss, J.; Kalman, F.K.; Tircso, G. Synthesis and characterization of a stable and inert MnII-based ZnII responsive MRI probe for molecular imaging of glucose stimulated zinc secretion (GSZS). Inorg. Chem. Front. 2022, 9, 577–583. [Google Scholar] [CrossRef]
- Vitor, T.; Martins, K.M.; Ionescu, T.M.; Cunha, M.L.; Baroni, R.H.; Garcia, M.R.T.; Wagner, J.; Campos Neto, G.d.C.; Nogueira, S.A.; Guerra, E.G.; et al. PET/MRI: A novel hybrid imaging technique. Major clinical indications and preliminary experience in Brazil. Einstein 2017, 15, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Vanasschen, C.; Monar, E.; Tircso, G.; Kalman, F.K.; Toth, E.; Brandt, M.; Coenen, H.H.; Neumaier, B. Novel CDTA-based, Bifunctional Chelators for Stable and Inert MnII Complexation: Synthesis and Physicochemical Characterization. Inorg. Chem. 2017, 56, 7746–7760. [Google Scholar] [CrossRef] [PubMed]
- Csupasz, T.; Szücs, D.; Kalman, F.K.; Holloczki, O.; Fekete, A.; Szikra, D.; Toth, E.; Toth, I.; Tircso, G. A new oxygen containing pyclen-type ligand as a manganese(II) binder for MRI and 52Mn PET applications: Equilibrium, kinetic, relaxometric, structural and radiochemical studies. Molecules 2022, 27, 371. [Google Scholar] [CrossRef] [PubMed]
- Sathiyajith, C.; Hallett, A.J.; Edwards, P.G. Synthesis, photophysical characterization, relaxometric studies and molecular docking studies of gadolinium-free contrast agents for dual modal imaging. Results Chem. 2022, 4, 100307. [Google Scholar] [CrossRef]
- Feng, Z.; Zhu, T.; Wang, L.; Yuan, T.; Jiang, Y.; Tian, X.; Tian, Y.; Zhang, Q. Coordination-Regulated Terpyridine–Mn(II) Complexes for Photodynamic Therapy Guided by Multiphoton Fluorescence/Magnetic Resonance Imaging. Inorg. Chem. 2022, 61, 12652–12661. [Google Scholar] [CrossRef]
- Tan, M.; Wu, X.; Jeong, E.-K.; Chen, Q.; Parker, D.L.; Lu, Z.-R. An effective targeted nanoglobular manganese(II) chelate conjugate for magnetic resonance molecular imaging of tumor extracellular matrix. Mol. Pharm. 2010, 7, 936–943. [Google Scholar] [CrossRef][Green Version]
- Tan, M.; Ye, Z.; Jeong, E.-K.; Wu, X.; Parker, D.L.; Lu, Z.-R. Synthesis and evaluation of nanoglobular macrocyclic Mn(II) chelate conjugates as non-gadolinium(III) MRI contrast agents. Bioconjug. Chem. 2011, 22, 931–937. [Google Scholar] [CrossRef]
- Sun, C.; Lin, H.; Gong, X.; Yang, Z.; Mo, Y.; Chen, X.; Gao, J. DOTA-branched organic frameworks as giant and potent metal chelators. J. Am. Chem. Soc. 2020, 142, 198–206. [Google Scholar] [CrossRef]
- Sun, C.; Yang, Z.; Wu, P.; Luo, X.; Liu, K.; Wang, B.; Lin, H.; Gao, J. Multinuclear Mn(II) united-DOTA complexes with enhanced inertness and high MRI contrast ability. Cell Rep. Phys. Sci. 2022, 3, 100920. [Google Scholar] [CrossRef]
- Carniato, F.; Ricci, M.; Tei, L.; Garello, F.; Furlan, C.; Terreno, E.; Ravera, E.; Parigi, G.; Luchinat, C.; Botta, M. Novel nanogels loaded with Mn(II) chelates as effective and biologically stable MRI probes. Small 2023, 19, 2302868. [Google Scholar] [CrossRef] [PubMed]
- Mallik, R.; Saha, M.; Mukherjee, C. Porous Silica Nanospheres with a Confined Mono(aquated) Mn(II)-Complex: A Potential T1–T2 Dual Contrast Agent for Magnetic Resonance Imaging. ACS Appl. Bio Mater. 2021, 4, 8356–8367. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.-O.; Aaltonen, E.; Petoral, R., Jr.; Lauritzson, P.; Miyazaki, H.; Pietras, K.; Mansson, S.; Hansson, L.; Leander, P.; Axelsson, O. Novel Nano-Sized MR Contrast Agent Mediates Strong Tumor Contrast Enhancement in an Oncogene-Driven Breast Cancer Model. PLoS ONE 2014, 9, e107762. [Google Scholar] [CrossRef]
- Gianolio, E.; Bäckström, S.; Petoral, R.M., Jr.; Olsson, A.; Aime, S.; Axelsson, O. Characterization of a Manganese-Containing Nanoparticle as an MRI Contrast Agent. Eur. J. Inorg. Chem. 2019, 13, 1759–1766. [Google Scholar] [CrossRef]
- Palmai, M.; Petho, A.; Nagy, L.N.; Klébert, S.; May, Z.; Mihaly, J.; Wacha, A.; Jemnitz, K.; Veres, Z.; Horvath, I.; et al. Direct immobilization of manganese chelates on silica nanospheres for MRI applications. J. Colloid Interface Sci. 2017, 498, 298–305. [Google Scholar] [CrossRef]
- Lalli, D.; Ferrauto, G.; Terreno, E.; Carniato, F.; Botta, M. Mn(II)-conjugated silica nanoparticles as potential MRI probes. J. Mater. Chem. B 2021, 9, 8994. [Google Scholar] [CrossRef]
- Farinha, P.; Coelho, J.M.P.; Reis, C.P.; Gaspar, M.M. A Comprehensive Updated Review on Magnetic Nanoparticles in Diagnostics. Nanomaterials 2021, 11, 3432. [Google Scholar] [CrossRef]
- Caspani, S.; Magalhaes, R.; Araujo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef]
- Cai, X.; Zhu, Q.; Zeng, Y.; Zeng, Q.; Chen, X.; Zhan, Y. Manganese oxide nanoparticles as MRI contrast agents in tumor multimodal imaging and therapy. Int. J. Nanomed. 2019, 14, 8321–8344. [Google Scholar] [CrossRef]
- Hashemzadeh, S.; Akbari, M.E.; Astani, S.A.; Hashemzadeh, J.; Hafez, A.A. Engineering effects on efficacy and toxicity of manganese oxide nanostructures, as a contrast agent, in magnetic resonance imaging: A review. Nano 2022, 17, 2230003. [Google Scholar] [CrossRef]
- Zhen, Z.; Xie, J. Development of manganese-based nanoparticles as contrast probes for magnetic resonance imaging. Theranostics 2012, 2, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, C.; Hou, P.; Zhang, M.; Xu, K. One-pot preparation of hydrophilic manganese oxide nanoparticles as T1 nano-contrast agent for molecular magnetic resonance imaging of renal carcinoma in vitro and in vivo. Biosens. Bioelectron. 2018, 102, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.T.; Yue, T.; Xu, K.; Golzarian, J.; Yu, J.H.; Huang, J. Fabrication and evaluation of tumor-targeted positive MRI contrast agent based on ultrasmall MnO nanoparticles. Colloid Surf. B 2015, 131, 148–154. [Google Scholar] [CrossRef]
- Huang, H.T.; Yue, T.; Xu, Y.Y.; Xu, K.; Xu, H.; Liu, S.; Yu, J.; Huang, J. PEGylation of MnO nanoparticles via catechol-Mn chelation to improving T1 weighted magnetic resonance application. J. Appl. Polym. Sci. 2015, 132, 42360. [Google Scholar] [CrossRef]
- Hsu, B.Y.W.; Wang, M.; Zhang, Y.; Vijayaragavan, V.; Wong, S.Y.; Chang, A.Y.C.; Bhakoo, K.K.; Li, X.; Wang, J. Silica-F127 nanohybrid-encapsulated manganese oxide nanoparticles for optimized T1 magnetic resonance relaxivity. Nanoscale 2014, 6, 293–299. [Google Scholar] [CrossRef]
- Costanzo, M.; Scolaro, L.; Berlier, G.; Marengo, A.; Grecchi, S.; Zancanaro, C.; Malatesta, M.; Arpicco, S. Cell uptake and intracellular fate of phospholipidic manganese-based nanoparticles. Int. J. Pharmaceut. 2016, 508, 83–91. [Google Scholar] [CrossRef]
- Gallo, J.; Alam, I.S.; Lavdas, I.; Wylezinska-Arridge, M.; Aboagye, E.O.; Long, N.J. RGD-targeted MnO nanoparticles as T1 contrast agents for cancer imaging–the effect of PEG length in vivo. J. Mater. Chem. B 2014, 2, 868–876. [Google Scholar] [CrossRef]
- Peng, Y.K.; Lui, C.N.P.; Chen, Y.W.; Chou, S.W.; Raine, E.; Chou, P.T.; Yung, K.L.; Tsang, E. Engineering of single magnetic particle carrier for living brain cell imaging: A tunable T1/T2 dual-modal contrast agent for magnetic resonance imaging application. Chem. Mater. 2017, 29, 4411–4417. [Google Scholar] [CrossRef]
- Peng, E.; Wang, F.H.; Tan, S.H.; Zheng, B.W.; Li, S.F.Y.; Xue, J.M. Tailoring a two-dimensional graphene-oxide surface: Dual T1 and T2 MRI contrast agent materials. J. Mater. Chem. B 2015, 3, 5678–5682. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Zhang, H.; Hu, Y.P.; Bai, L.; Xue, J.Y. MnO nanoparticles with potential application in magnetic resonance imaging and drug delivery for myocardial infarction. Int. J. Nanomed. 2018, 13, 6177–6188. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Shao, C.; Li, S.; Wang, Z.; Qu, Y.; Gu, W.; Yu, C.; Ye, L. Cy5.5 conjugated MnO nanoparticles for magnetic resonance/near-infrared fluorescence dual-modal imaging of brain gliomas. J. Colloid Interface Sci. 2015, 457, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.; Prado, Y.; Dia, N.; Rivière, E.; Laurent, S.; Roch, M.; Vander Elst, L.; Muller, R.N.; Sancey, L.; Perriat, P.; et al. MnII-containing coordination nanoparticles as highly efficient T1 contrast agents for magnetic resonance imaging. Chem. Commun. 2014, 50, 6740–6743. [Google Scholar] [CrossRef]
- Panich, A.M.; Shames, A.I.; Aleksenskii, A.E.; Yudina, E.B.; Vul, A.Y. Manganese-grafted detonation nanodiamond, a novel potential MRI contrast agent. Diam. Relat. Mater. 2021, 119, 108590. [Google Scholar] [CrossRef]
- Panich, A.M.; Salti, M.; Aleksenskii, A.E.; Kulvelis, Y.V.; Chizhikova, A.; Vul, A.Y.; Shames, A.I. Suspensions of manganese-grafted nanodiamonds: Preparation, NMR, and MRI study. Diam. Relat. Mater. 2023, 131, 109591. [Google Scholar] [CrossRef]
- Qin, R.; Li, S.; Qiu, Y.; Feng, Y.; Liu, Y.; Ding, D.; Xu, L.; Ma, X.; Sun, W.; Chen, H. Carbonized paramagnetic complexes of Mn(II) as contrast agents for precise magnetic resonance imaging of sub-millimeter-sized orthotopic tumors. Nat. Commun. 2022, 13, 1938. [Google Scholar] [CrossRef]
- Stepanidenko, E.A.; Vedernikova, A.A.; Badrieva, Z.F.; Brui, E.A.; Ondar, S.O.; Miruschenko, M.D.; Volina, O.V.; Koroleva, A.V.; Zhizhin, E.V.; Ushakova, E.V. Manganese-doped carbon dots as a promising nanoprobe for luminescent and magnetic resonance imaging. Photonics 2023, 10, 757. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Z.; Li, S.; Lin, S.; Zhang, L.; Meng, Z.; Zhang, X.; Sun, S.-K. Non-invasive diagnosis of acute kidney injury using Mn-doped carbon dots-based magnetic resonance imaging. Biomater. Sci. 2023, 11, 4289–4297. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Li, W.; Zhang, C.; Song, T.; Wang, P.; Sun, D.; Huang, X.; Qin, X.; Ran, L.; et al. PDGFB-targeted functional MRI nanoswitch for activatable T1-T2 dual-modal ultra-sensitive diagnosis of cancer. J. Nanobiotechnol. 2023, 21, 9. [Google Scholar] [CrossRef]
- Iki, N.; Nakane, R.; Masuya-Suzuki, A.; Ozawa, Y.; Maruoka, T.; Iiyama, M.; Sumiyoshi, A.; Aoki, I. MRI contrasting agent based on Mn-MOF-74 nanoparticles with coordinatively unsaturated sites. Mol. Imaging Biol. 2023, 25, 968–976. [Google Scholar] [CrossRef]
- Sun, M.; Chen, G.; Ouyang, S.; Chen, C.; Zheng, Z.; Lin, P.; Song, X.; Chen, H.; Chen, Y.; You, Y.; et al. Magnetic Resonance Diagnosis of Early Triple-Negative Breast Cancer Based on the Ionic Covalent Organic Framework with High Relaxivity and Long Retention Time. Anal. Chem. 2023, 95, 8267–8276. [Google Scholar] [CrossRef]
- Tullio, C.; Salvioni, L.; Bellini, M.; Degrassi, A.; Fiandra, L.; D’Arienzo, M.; Garbujo, S.; Rotem, R.; Testa, F.; Prosperi, D.; et al. Development of an effective tumor-targeted contrast agent for magnetic resonance imaging based on Mn/H-ferritin. ACS Appl. Bio Mater. 2021, 4, 7800–7810. [Google Scholar] [CrossRef] [PubMed]
- Pashkunova-Martic, I.; Kukeva, R.; Stoyanova, R.; Pantcheva, I.; Dorkov, P.; Friske, J.; Hejl, M.; Jakupec, M.; Hohagen, M.; Legin, A.; et al. Novel salinomycin-based paramagnetic complexes–First evaluation of their potential theranostic properties. Pharmaceutics 2022, 14, 2319. [Google Scholar] [CrossRef]
- Antwi-Baah, R.; Wang, Y.; Chen, X.; Liu, H.; Yu, K. Hybrid morphologies of paramagnetic manganese-based nanoparticles as theranostics. Chem. Eng. J. 2023, 466, 142970. [Google Scholar] [CrossRef]
- Liu, X.; Rong, P. Recent advances of manganese-based hybrid nanomaterials for cancer precision medicine. Front. Oncol. 2021, 11, 707618. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Guo, J.; Cai, X.; Bin, L.; Lu, C.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Manganese complexes and manganese-based metal-organic frameworks as contrast agents in MRI and chemotherapeutics agents: Applications and prospects. Colloids Surf. B Biointerfaces 2022, 213, 112432. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Abnous, K.; Nekooei, S.; Taghdisi, S.M.; Farzad, S.A.; Ramezani, M.; Alibolandi, M. Synthesis of manganese-incorporated polycaprolactone-poly (glyceryl methacrylate) theranostic smart hybrid polymersomes for efficient colon adenocarcinoma treatment. Int. J. Pharm. 2022, 623, 121963. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Pang, L.; Yu, B.; Cong, H.; Shen, Y. Mn-dox metal-organic nanoparticles for cancer therapy and magnetic resonance imaging. Dye. Pigment. 2022, 199, 110080. [Google Scholar] [CrossRef]
- Arkaban, H.; Shervedani, R.K.; Torabi, M.; Norouzi-Barough, L. Fabrication of a biocompatible and biodegradable targeted theranostic nanocomposite with pH-controlled drug release ability. J. Drug Deliv. Sci. Technol. 2022, 72, 103403. [Google Scholar] [CrossRef]
- Jiang, G.; Fan, D.; Tian, J.; Xiang, Z.; Fang, Q. Self-confirming magnetosomes for tumor-targeted T1/T2 dual-mode MRI and MRI-guided photothermal therapy. Adv. Healthc. Mater. 2022, 11, 2200841. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Huang, H.; Lan, Y.; Gan, L.; Huang, J. Embedding Mn2+ in polymer coating on rod-like cellulose nanocrystal to integrate MRI and photothermal function. Carbohydr. Polym. 2022, 297, 120061. [Google Scholar] [CrossRef]
- Li, M.; Huo, L.; Zeng, J.; Zhu, G.; Liu, X.; Zhu, X.; Huang, G.; Wang, Y.; Ni, K.; Zhao, Z. Switchable ROS scavenger/generator for MRI-guided anti-inflammation and anti-tumor therapy with enhanced therapeutic efficacy and reduced side effects. Adv. Healthc. Mater. 2023, 12, 2202043. [Google Scholar] [CrossRef] [PubMed]
- Rehman, Z.U.; Iqbal, M.Z.; Hou, J.; Butt, F.K.; AlFaify, S.; Haq, B.U.; Tariq, Z.; Rehman, S.U.; Bilal, M.; Akram, M.; et al. Graphitic carbon nitride-manganese oxide nanoflowers as promising T1 magnetic resonance imaging contrast material. Appl. Phys. A 2022, 128, 926. [Google Scholar] [CrossRef]
- Wang, H.; Bremner, D.H.; Wu, K.; Gong, X.; Fan, Q.; Xie, X.; Zhang, H.; Wu, J.; Zhu, L.-M. Platelet membrane biomimetic bufalin-loaded hollow MnO2 nanoparticles for MRI-guided chemo-chemodynamic combined therapy of cancer. Chem. Eng. J. 2020, 382, 122848. [Google Scholar] [CrossRef]
- Jiang, S.; Li, X.; Zhang, F.; Mao, J.; Cao, M.; Zhang, X.; Huang, S.; Duan, X.; Shen, J. Manganese dioxide-based nanocarrier delivers Paclitaxel to enhance chemotherapy against orthotopic glioma through hypoxia relief. Small Methods 2022, 6, e2101531. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Jiang, G.; He, J.; Zhang, Y.; He, G.; Wu, J.; Lin, J.; Zhou, X.; Huang, P. Manganese-dioxide-coating-instructed plasmonic modulation of gold nanorods for activatable duplex-imaging-guided NIR-II photothermal-chemodynamic therapy. Adv. Mater. 2021, 33, 2008540. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.; Yao, Z.; Zhao, K.; Shao, F.; Su, J.; Liu, S. Black Phosphorus Quantum Dots Encapsulated Biodegradable Hollow Mesoporous MnO2: Dual-Modality Cancer Imaging and Synergistic Chemo-Phototherapy. Adv. Funct. Mater. 2021, 31, 2104643. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henoumont, C.; Devreux, M.; Laurent, S. Mn-Based MRI Contrast Agents: An Overview. Molecules 2023, 28, 7275. https://doi.org/10.3390/molecules28217275
Henoumont C, Devreux M, Laurent S. Mn-Based MRI Contrast Agents: An Overview. Molecules. 2023; 28(21):7275. https://doi.org/10.3390/molecules28217275
Chicago/Turabian StyleHenoumont, Céline, Marie Devreux, and Sophie Laurent. 2023. "Mn-Based MRI Contrast Agents: An Overview" Molecules 28, no. 21: 7275. https://doi.org/10.3390/molecules28217275
APA StyleHenoumont, C., Devreux, M., & Laurent, S. (2023). Mn-Based MRI Contrast Agents: An Overview. Molecules, 28(21), 7275. https://doi.org/10.3390/molecules28217275