Microwave-Assisted Grafting of Coal onto Nitrogen-Doped Carbon Dots with a High Quantum Yield and Enhanced Photoluminescence Properties
Abstract
1. Introduction
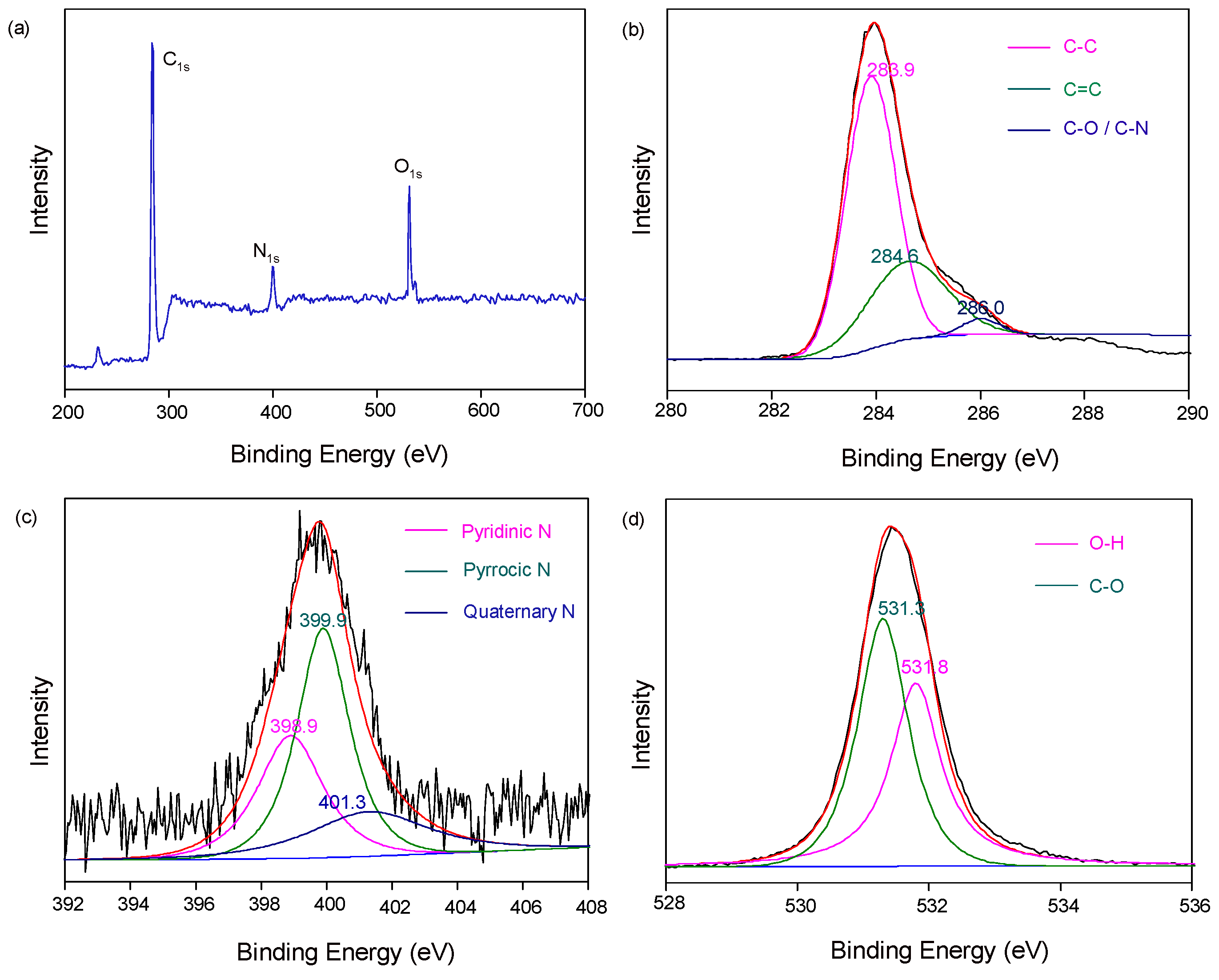
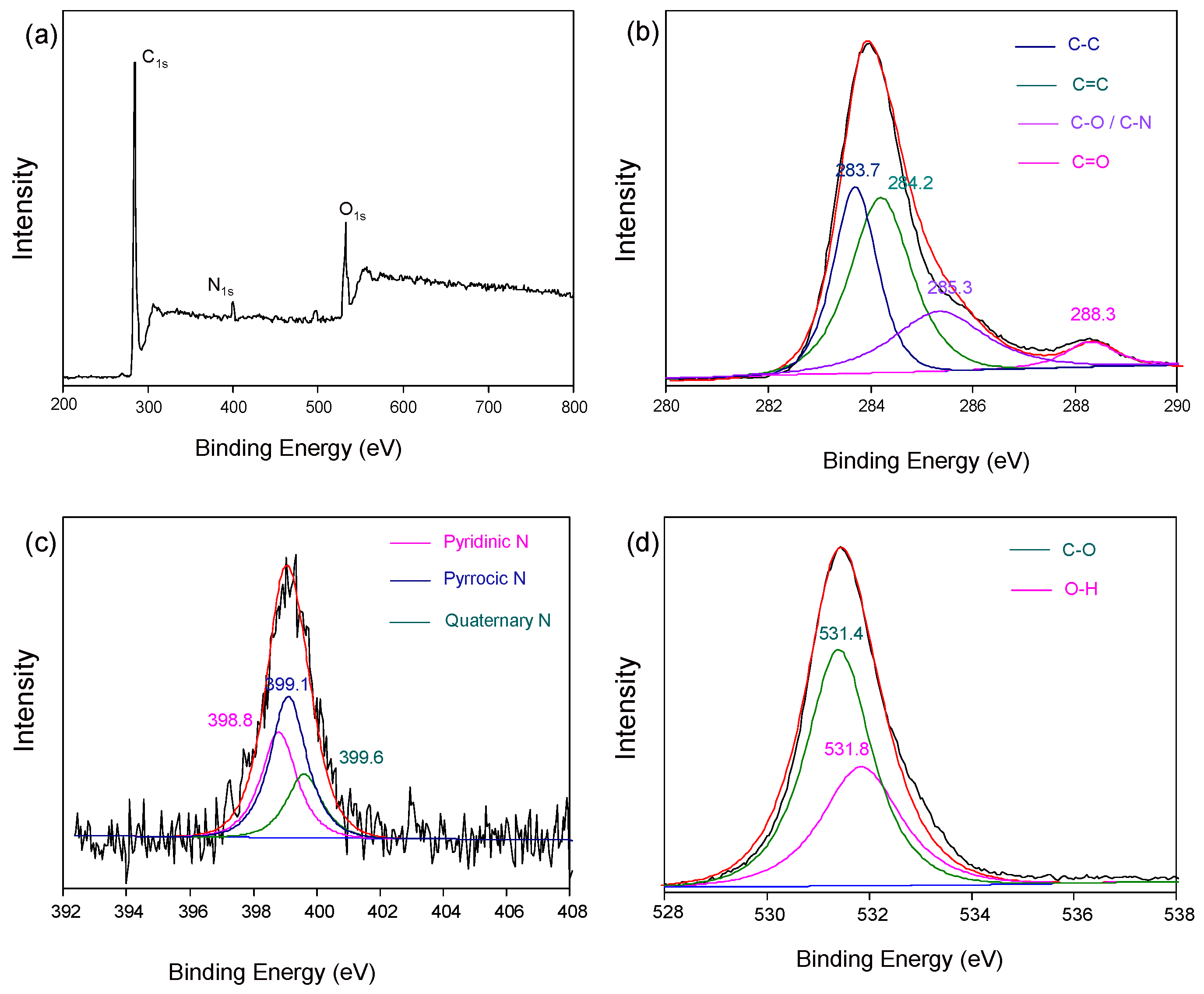
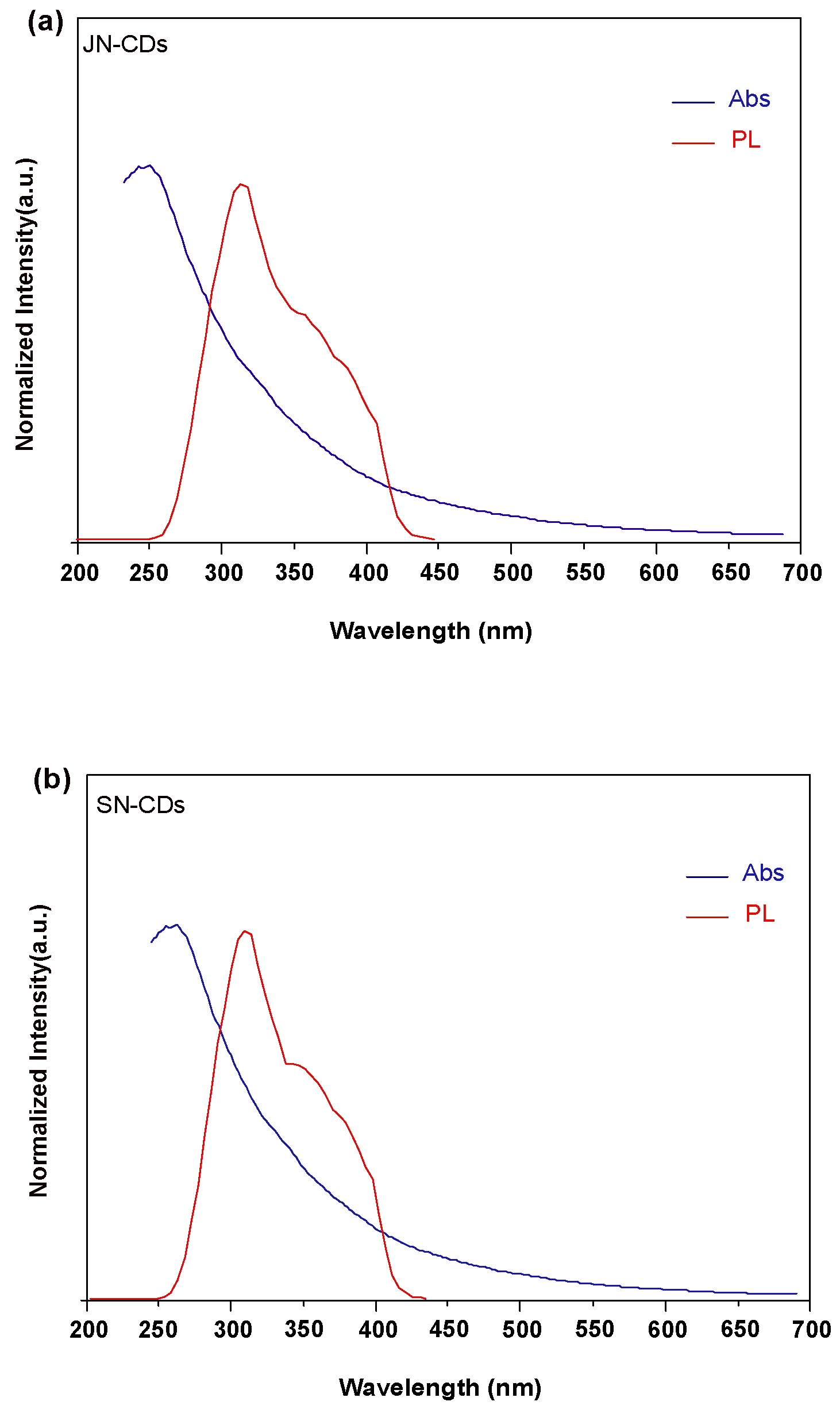
2. Results
3. Materials and Methods
3.1. Materials
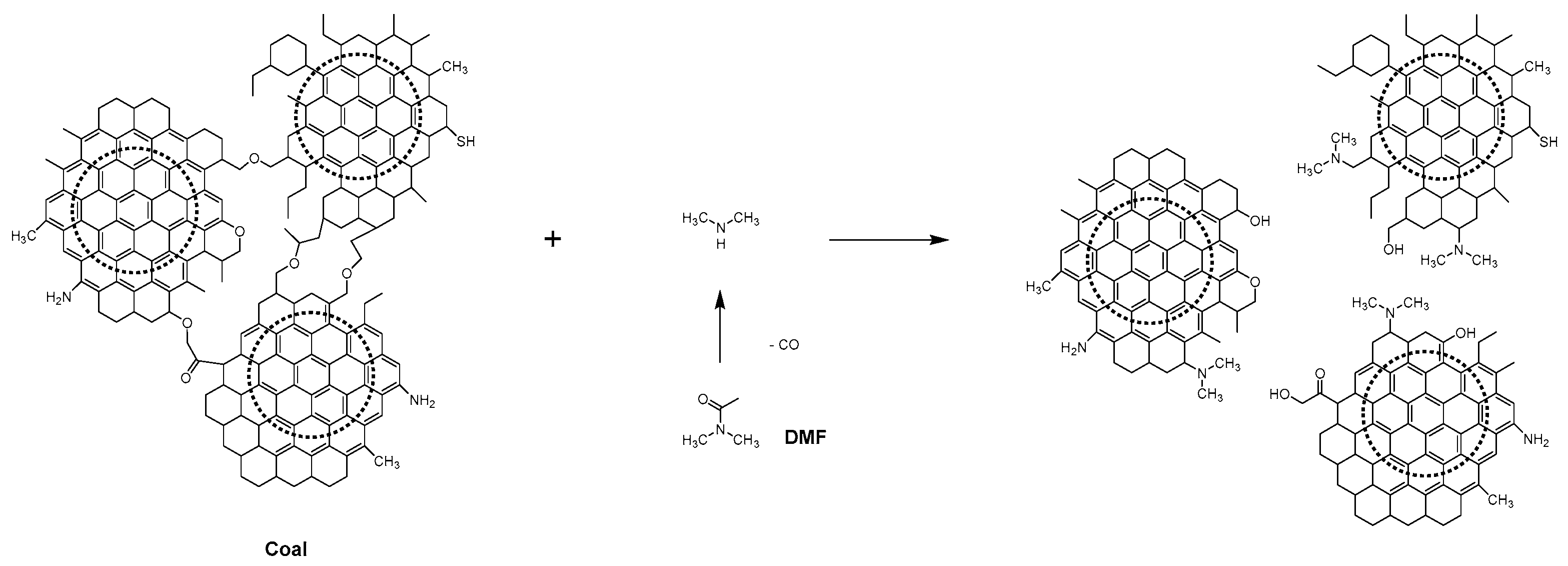
3.2. Synthesis of the of JN-CDs and SN-CDs
3.3. Synthesis of N-CDs–Polymethyl Methacrylate (N-CDs-PMMA) Composites
3.4. Characterization
3.5. Quantum Yield Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.Y.; Yu, C.; Hu, C. Solvothermal conversion of coal into nitrogen-doped carbon dots with singlet oxygen generation and high quantum yield. Chem. Eng. J. 2017, 320, 570–575. [Google Scholar] [CrossRef]
- Shi, C.; Wei, X.Y.; Liu, F.J. Carbon Dots Derived from Facile Tailoring of Shaerhu Lignite as a Novel Fluorescence Sensor with High-Selectivity and Sensitivity for Cu2+ Detection. ChemistrySelect 2020, 5, 12125–12130. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Dev, A.; Karmakar, S. Nanosensors and nanobiosensors in food and agriculture. Environ. Chem. Lett. 2017, 16, 161–182. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, J.; Liu, B.; Wu, S.; Liu, J. Nucleoside-based fluorescent carbon dots for discrimination of metal ions. J. Mater. Chem. B 2020, 8, 3640–3646. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Chen, N.; Qu, L. Graphene quantum dots: An emerging material for energy-related applications and beyond. Energy Environ. Sci. 2012, 5, 8869–8890. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q. Glowing grapheme quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620. [Google Scholar] [CrossRef]
- Nasser, A.; Parinaz, N.-M.; Esmaiel, J. Microwave-assisted and one-step synthesis of PEG passivated fluorescent carbon dots from gelatin as an efficient nanocarrier for methotrexate delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 540–547. [Google Scholar]
- Yu, D.; Li, G.; Liu, W.; Li, Y.; Song, Z. A fluorescent pickering-emulsion stabilizer prepared using carbon nitride quantum dots and laponite nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 310–317. [Google Scholar] [CrossRef]
- Tan, H.; Liu, W.; Gong, B.; Zhang, W.; Li, H. Unique dual functions for carbon dots in emulsion preparations: Costabilization and fluorescence probing. Langmuir 2015, 31, 9537–9545. [Google Scholar] [CrossRef]
- Stachurski, C.D.; Click, S.M.; Wolfe, K.D.; Dervishogullari, D.; Rosenthal, S.J. Optical and electrochemical tuning of hydrothermally synthesized nitrogen-doped carbon dots. Nanoscale Adv. 2020, 2, 3375. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, M.; Bhandari, B.; Yang, C.H. Food waste as a carbon source in carbon quantum dots technology and their applications in food safety detection. Trends Food Sci. Technol. 2019, 95, 86–96. [Google Scholar] [CrossRef]
- Man, Y.; Ban, M.; Jin, X.; Li, A.; Tao, J.; Pan, L. An integrated distance-based microfluidic aptasensor for visual quantitative detection of Salmonella with sample-in-answer-out capability. Sens. Actuators B Chem. 2023, 381, 133480. [Google Scholar] [CrossRef]
- Jiao, Z.; Zhang, H.; Jiao, S.; Guo, Z.; Zhu, D.; Zhao, X. A Turn-on Biosensor-Based Aptamer-Mediated Carbon Quantum Dots Nanoaggregate for Acetamiprid Detection in Complex Samples. Food Anal. Methods 2019, 12, 668–676. [Google Scholar] [CrossRef]
- Wang, B.; Lin, Y.; Tan, H.; Luo, M.; Dai, S.; Lu, H.; Huang, Z. One-pot synthesis of N-doped carbon dots by pyrolyzing the gel composed of ethanolamine and 1-carboxyethyl-3-methylimidazolium chloride and their selective fluorescence sensing for Cr(VI) ions. Analyst 2018, 143, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Doñate-Buendía, C.; Torres-Mendieta, R.; Pyatenko, A.; Falomir, E.; Fernández-Alonso, M.; Mínguez-Vega, G. Fabrication by Laser Irradiation in a Continuous Flow Jet of Carbon Quantum Dots for Fluorescence Imaging. ACS Omega 2018, 3, 2735–2742. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Sun, L.; Zhang, J.; Yang, X.; Ma, H. Defects coordination triggers red-shifted photoluminescence in carbon dots and their application in ratiometric Cr (VI) sensing. Microchem. J. 2021, 169, 106552. [Google Scholar] [CrossRef]
- Zeng, L.; Li, X.; Fan, S.; Li, J.; Mu, J.; Qin, M.; Wang, L.; Gan, G.; Tadé, M.O.; Liu, S. The bioelectrochemical synthesis of high-quality carbon dots with strengthened electricity output and excellent catalytic performance. Nanoscale 2019, 11, 4428–4437. [Google Scholar] [CrossRef]
- Gunjal, D.B.; Gurav, Y.M.; Gore, A.H.; Naik, V.M.; Kolekar, G.B. Nitrogen doped waste tea residue derived carbon dots for selective quantification of tetracycline in urine and pharmaceutical samples and yeast cell imaging application. Opt. Mater. 2019, 98, 109484. [Google Scholar] [CrossRef]
- Sabet, M.; Mahdavi, K. Green synthesis of high photoluminescence nitrogen-doped carbon quantum dots from grass via a simple hydrothermal method for removing organic and inorganic water pollutions. Appl. Surf. Sci. 2019, 463, 283–291. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Ding, Y.Y.; Gong, X.J.; Liu, Y.; Lu, W.J.; Gao, Y.F.; Xian, M.; Shuang, S.M.; Dong, C. Facile preparation of bright orange fluorescent carbon dots and the constructed biosensing platform for the detection of pH in living cells. Talanta 2018, 189, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.J. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Wu, W.; Li, C.; Ma, H. A Green, Economic “Switch-On” Sensor for Cefixime Analysis Based on Black Soya Bean Carbon Quantum Dots. J. AOAC Int. 2020, 103, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Tuerhong, M.; Yang, X.; Xue-Bo, Y. Review on carbon dots and their applications. Chin. J. Anal. Chem. 2017, 45, 139–150. [Google Scholar] [CrossRef]
- Xu, C.; Gan, N.; Zhiqiang, Z.; Liu, Y.; Xiao, Q.; Huang, S. Bright Mn-doped carbon dots for the determination of permanganate and L-ascorbic acid by a fluorescence on-off-on strategy. Microchim. Acta 2020, 187, 659. [Google Scholar]
- Zhang, Y.; Zhang, J.; Sun, L.; Tian, R.; Ma, H. Ratiometric detection of propafenone hydrochloride with one-pot synthesized dual emissive carbon dots. Chem. Pap. 2023, 77, 375–383. [Google Scholar] [CrossRef]
- Lin, F.; Jia, C.; Wu, F.-G. Carbon dots for intracellular sensing. Small Struct. 2022, 3, 2200033. [Google Scholar] [CrossRef]
- Li, L.-P.; Ren, X.-F.; Bai, P.-R.; Liu, Y.; Xu, W.-Y.; Xie, J.; Zhang, R.-P. Near-infrared emission carbon dots for bio-imagin applications. New Carbon Mater. 2021, 36, 632–638. [Google Scholar] [CrossRef]
- Atchudan, R.; Gangadaran, P.; Perumal, S.; Edison, T.N.J.I.; Sundramoorthy, A.K.; Rajendran, R.L.; Ahn, B.-C.; Lee, Y.R. Green Synthesis of Multicolor Emissive Nitrogen-Doped Carbon Dots for Bioimaging of Human Cancer Cells. J. Clust. Sci. 2023, 34, 1583–1594. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, X.; Liu, Y.; Liang, L.; Peng, Y.; Wu, S.; Zhao, Y. N-Doped Carbon Dots as Fluorescent “Turn-Off” Nanosensors for Ascorbic Acid and Fe3+ Detection. ACS Appl. Nano Mater. 2022, 5, 7268–7277. [Google Scholar] [CrossRef]
- Liao, X.; Chen, C.; Wang, P.; Zhou, R.; Zhao, X.; Fan, H.; Huang, Z. Carbon dots derived from cellobiose for temperature and phosalone detection. Mater. Res. Bull. 2022, 151, 111790. [Google Scholar] [CrossRef]
- Guo, Z.; Luo, J.; Zhu, Z.; Sun, Z.; Zhang, X.; Wu, Z.-C.; Mo, F.; Guan, A. A facile synthesis of high-efficient N, S co-doped carbon dots for temperature sensing application. Dye Pigment. 2020, 173, 107952. [Google Scholar] [CrossRef]
- Shi, J.; Tong, L.; Tong, W.; Chen, H.; Lan, M.; Sun, X.; Zhu, Y. Current progress in long-term and continuous cell metabolite detection using microfluidics. TrAC Trend Anal. Chem. 2019, 117, 263–279. [Google Scholar] [CrossRef]
- Koklu, A.; Ohayon, D.; Wustoni, S.; Druet, V.; Saleh, A.; Inal, S. Organic bioelectronic devices for metabolite sensing. Chem. Rev. 2022, 122, 4581–4635. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Hu, F.; Zhang, Y.; Dong, B.; Li, Z. Carbon quantum dots/UiO-66@ polysulfone porous microspheres fabricated via pickering emulsion template for pollutant removal. Sep. Purif. Technol. 2024, 337, 126367. [Google Scholar] [CrossRef]
- Gong, B.; Liu, W.; Chen, X.; Tan, H.; Zhang, X. Stabilizing alkenyl succinic anhydride (ASA) emulsions with starch nanocrystals and fluorescent carbon dots. Carbohydr. Polym. 2017, 165, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, Z.; Zhang, X.; Shi, Y.; Zhou, N. Nitrogen-Doped Carbon Quantum Dots as Fluorescent Probes for Sensitive and Selective Detection of Nitrite. Molecules 2017, 22, 2061. [Google Scholar] [CrossRef]
- Nguyen, K.G.; Baragau, I.A.; Gromicova, R. Investigating the effect of N-doping on carbon quantum dots structure, optical properties and metal ion screening. Sci. Rep. 2022, 12, 13806. [Google Scholar] [CrossRef]
- Munusamy, S.; Mandlimath, T.R.; Swetha, P. A Nitrogen-doped carbon dots: Recent developments in its fluorescent sensor applications. Environ. Res. 2023, 231, 116046. [Google Scholar] [CrossRef]




| λabs nm a | λex nm b | λem nm c | Φ (%) | Yield | |
|---|---|---|---|---|---|
| JN-CDs | 270 | 310 | 445 | 49.5 | 25.7 |
| SN-CDs | 270 | 310 | 465 | 40.0 | 35.1 |
| Carbogenic dots | 275 | 365 | 445 | 18.6 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, C.; Wei, X.-Y. Microwave-Assisted Grafting of Coal onto Nitrogen-Doped Carbon Dots with a High Quantum Yield and Enhanced Photoluminescence Properties. Molecules 2024, 29, 1349. https://doi.org/10.3390/molecules29061349
Shi C, Wei X-Y. Microwave-Assisted Grafting of Coal onto Nitrogen-Doped Carbon Dots with a High Quantum Yield and Enhanced Photoluminescence Properties. Molecules. 2024; 29(6):1349. https://doi.org/10.3390/molecules29061349
Chicago/Turabian StyleShi, Chong, and Xian-Yong Wei. 2024. "Microwave-Assisted Grafting of Coal onto Nitrogen-Doped Carbon Dots with a High Quantum Yield and Enhanced Photoluminescence Properties" Molecules 29, no. 6: 1349. https://doi.org/10.3390/molecules29061349
APA StyleShi, C., & Wei, X.-Y. (2024). Microwave-Assisted Grafting of Coal onto Nitrogen-Doped Carbon Dots with a High Quantum Yield and Enhanced Photoluminescence Properties. Molecules, 29(6), 1349. https://doi.org/10.3390/molecules29061349





