The Importance of Substituent Position for Antibacterial Activity in the Group of Thiosemicarbazide Derivatives
Abstract
1. Introduction
2. Results and Discussion
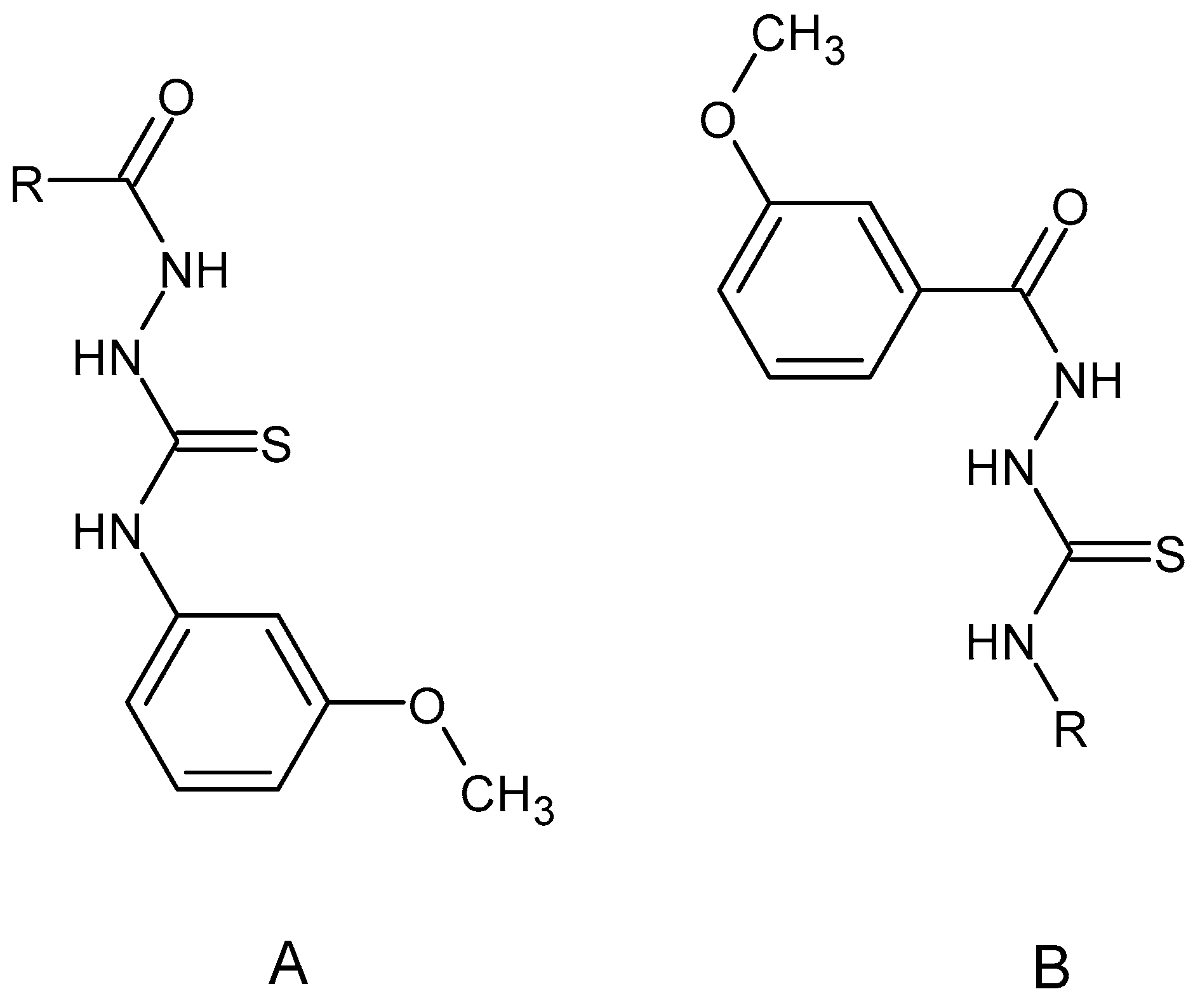
2.1. Chemistry
2.2. Antibacterial Evaluation
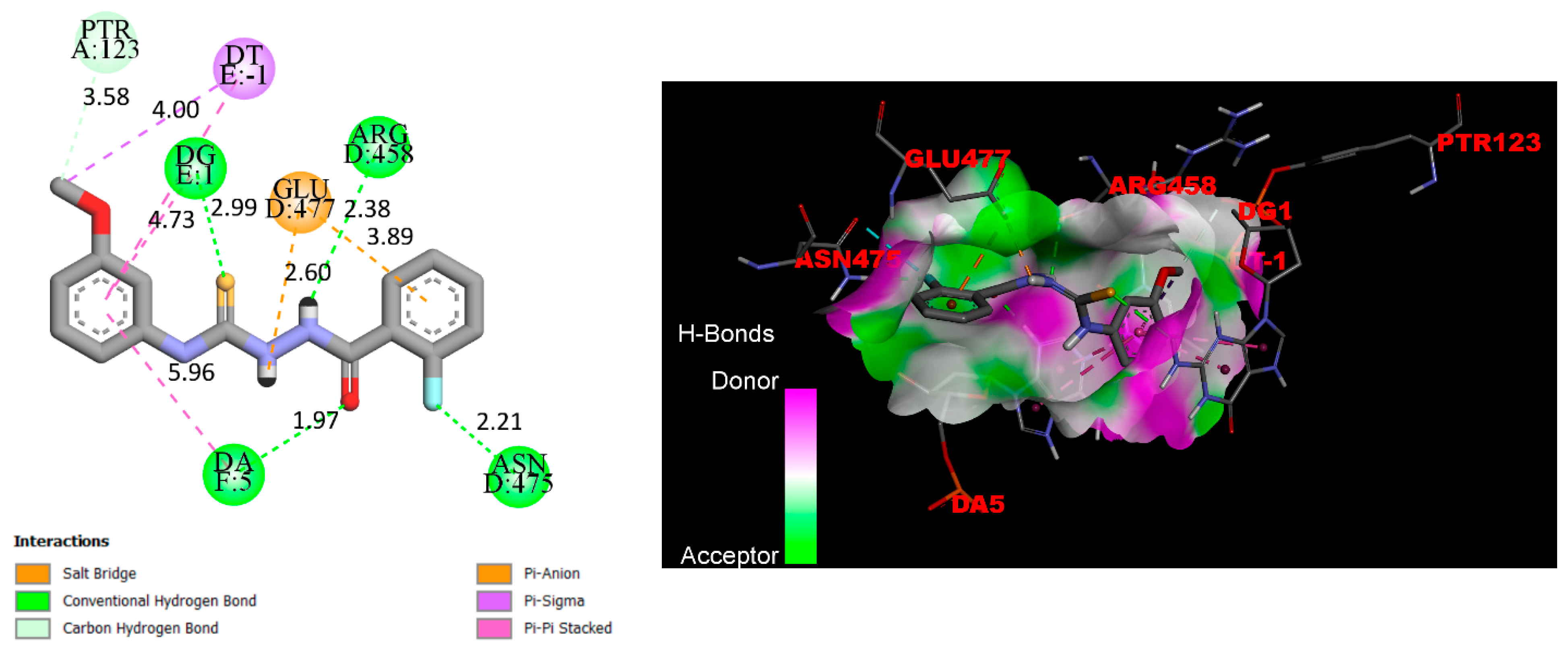
2.3. Docking
3. Experiment
3.1. Chemistry
3.1.1. General Comments
3.1.2. Synthesis of Thiosemicarbazide Derivatives
- Yield 64%, m.p. 171–172 °C. Spectral data were as follows: IR (cm−1) KBr: 3320 (NH); 1669 (C=O), 1573 (CHarom); 1362 (C=S); 1263 (C-O-C). 1H NMR (DMSO-d6) δ (ppm): 3.75 (s, 3H, CH3), 6.75 (d, 1H, CHarom, J = 8.7 Hz), 7.05 (d, 1H, CHarom, J = 7.8 Hz), 7.18 (bs, 1H, CHarom,), 7.25 (t, 1H, CHarom, J = 8.1 Hz), 7.33 (d, 1H, CHarom, J = 7.9 Hz), 7.35–7.36 (m, 1H, CHarom), 7.59–7.62 (m, 1H, CHarom), 7.85 (bs, 1H, CHarom,), 9.84 (bs, 2H, 2NH), 10.33 (s, 1H, NH). 13C NMR (DMSO-d6) δ (ppm): 55.5; 110.90; 111.8; 116.7 (d, J = 22.0 Hz); 118.3; 122.2; 124.4; 129.3; 131.1; 133.7; 140.7; 159.5; 160.1 (d, J = 250.5 Hz); 163.9; 181.4. Elemental analysis for C15H14FN3O2S. Calculated: C 56.41; H 4.42; N 13.16. Found: C 56.43; H 4.40; N 13.15.
- Yield 93%, m.p. 172–173 °C. Spectral data were as follows: IR (cm−1) KBr: 3319 (NH); 1638 (C=O), 1581 (CHarom); 1361 (C=S); 1259 (C-O-C). 1H NMR (DMSO-d6) δ (ppm): 3.75 (s, 3H, CH3), 6.75 (d, 1H, CHarom, J = 7.2 Hz), 7.04 (d, 1H, CHarom, J = 8.0 Hz), 7.12 (bs, 1H, CHarom,), 7.24 (t, 1H, CHarom, J = 8.1 Hz), 7.46 (t, 1H, CHarom, J = 8.5 Hz), 7.59–7.62 (m, 1H, CHarom J = 7.7 Hz), 7.77 (d, 1H, CHarom J = 9.7 Hz), 7.80 (d, 1H, CHarom, J = 6.3 Hz), 9.77 (bs, 2H, 2NH), 10.65 (s, 1H, NH). 13C NMR (DMSO-d6) δ (ppm): 55.5; 111.0; 112.1; 115.2 (d, J = 24.0 Hz); 118.5; 119.2 (d, J = 20.8 Hz); 124.5; 129.1; 130.9; 135.3; 140.7; 159.4; 162.2 (d, J = 243.0 Hz), 165.2; 181.3. Elemental analysis for C15H14FN3O2S. Calculated: C 56.41; H 4.42; N 13.16. Found: C 56.42; H 4.39; N 13.13.
- Yield 87% (0.24 g), m.p. 173–175 °C. Spectral data were as follows: IR (cm−1) KBr: 3323 (NH); 1670 (C=O), 1573 (CHarom); 1365 (C=S); 1264 (C-O-C).1H NMR (DMSO-d6) δ ppm: 3.75 (s, 3H, CH3), 6.76 (d, 1H, CHarom, J = 8.3 Hz), 7.03 (d, 1H, CHarom, J = 8.0 Hz), 7.11 (bs, 1H, CHarom,), 7.25 (t, 1H, CHarom, J = 8.1 Hz), 7.78 (t, 1H, CHarom, J = 7.8 Hz), 7.98 (d, 1H, CHarom J = 7.8 Hz), 8.24 (d, 1H, CHarom J = 7.8 Hz), 8.30 (s, 1H, CHarom), 9.80 (s, 2H, 2NH), 10.82 (s, 1H, NH). 13C NMR (DMSO-d6) δ (ppm): 55.5; 111.0; 112.2; 118.6; 121.7; 125.1 (d, J = 49.4 Hz); 127.1; 128.8; 129.54 (q, J = 31.5 Hz); 130.1; 132.4; 134.0; 140.7; 159.4; 165.1, 181.4. Elemental analysis for C16H14F3N3O2S. Calculated: C 52.03; H 3.82; N 11.38. Found: C 52.00; H 3.79; N 11.36.
3.2. Microbiology
3.3. Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Othman, A.A.; Kihel, M.; Amara, S. 1,3,4-Oxadiazole, 1,3,4-Thiadiazole and 1,2,4-Triazole Derivatives as Potential Antibacterial Agents. Arab. J. Chem. 2019, 12, 1660–1675. [Google Scholar] [CrossRef]
- Walsh, C. Molecular Mechanisms That Confer Antibacterial Drug Resistance. Nature 2000, 406, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Neu, H.C. The Crisis in Antibiotic Resistance. Science 1992, 257, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef] [PubMed]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic Resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Shiradkar, M.R.; Murahari, K.K.; Gangadasu, H.R.; Suresh, T.; Kalyan, C.A.; Panchal, D.; Kaur, R.; Burange, P.; Ghogare, J.; Mokale, V.; et al. Synthesis of New S-Derivatives of Clubbed Triazolyl Thiazole as Anti-Mycobacterium Tuberculosis Agents. Bioorgan. Med. Chem. 2007, 15, 3997–4008. [Google Scholar] [CrossRef] [PubMed]
- Altun, A.; Kumru, M.; Dimoglo, A. Study of Electronic and Structural Features of Thiosemicarbazone and Thiosemicarbazide Derivatives Demonstrating Anti-HSV-1 Activity. J. Mol. Struct.-Theochem 2001, 535, 235–246. [Google Scholar] [CrossRef]
- Han, M.İ.; Ince, U.; Gündüz, M.G.; Küçükgüzel, G. Synthesis, Antimicrobial Evaluation, and Molecular Modeling Studies of New Thiosemicarbazide-Triazole Hybrid Derivatives of (S)-Naproxen. Chem. Biodivers. 2022, 19, e202100900. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.; Tomić, M.; Pavić, V. Coumarinyl Thiosemicarbazides as Antimicrobial Agents. Pharm. Chem. J. 2018, 51, 1078–1081. [Google Scholar] [CrossRef]
- Plech, T.; Paneth, A.; Kaproń, B.; Kosikowska, U.; Malm, A.; Strzelczyk, A.; Stączek, P. Structure-Activity Relationship Studies of Microbiologically Active Thiosemicarbazides Derived from Hydroxybenzoic Acid Hydrazides. Chem. Biol. Drug Des. 2015, 85, 315–325. [Google Scholar] [CrossRef]
- Janowska, S.; Khylyuk, D.; Andrzejczuk, S.; Wujec, M. Design, Synthesis, Antibacterial Evaluations and In Silico Studies of Novel Thiosemicarbazides and 1,3,4-Thiadiazoles. Molecules 2022, 27, 3161. [Google Scholar] [CrossRef] [PubMed]
- Ameryckx, A.; Pochet, L.; Wang, G.; Yildiz, E.; Saadi, B.E.; Wouters, J.; Van Bambeke, F.; Frédérick, R. Pharmacomodulations of the Benzoyl-Thiosemicarbazide Scaffold Reveal Antimicrobial Agents Targeting D-Alanyl-d-Alanine Ligase in Bacterio. Eur. J. Med. Chem. 2020, 200, 112444. [Google Scholar] [CrossRef] [PubMed]
- Abhale, Y.K.; Shinde, A.; Deshmukh, K.K.; Nawale, L.; Sarkar, D.; Mhaske, P.C. Synthesis, Antitubercular and Antimicrobial Potential of Some New Thiazole Substituted Thiosemicarbazide Derivatives. Med. Chem. Res. 2017, 26, 2557–2567. [Google Scholar] [CrossRef]
- Bhat, M.A.; Khan, A.A.; Ghabbour, H.A.; Quah, C.K.; Fun, H.K. Synthesis, Characterization, X-ray Structure and Antimicrobial Activity of N-(4-Chlorophenyl)-2-(Pyridin-4-Ylcarbonyl) Hydrazinecarbothioamide. Trop. J. Pharm. Res. 2016, 15, 1751–1757. [Google Scholar] [CrossRef]
- Li, Y.; Geng, J.; Liu, Y.; Yu, S.; Zhao, G. Thiadiazole-a Promising Structure in Medicinal Chemistry. ChemMedChem 2013, 8, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Janowska, S.; Khylyuk, D.; Gornowicz, A.; Bielawska, A.; Janowski, M.; Czarnomysy, R.; Bielawski, K.; Wujec, M. Synthesis and Anticancer Activity of 1,3,4-Thiadiazoles with 3-Methoxyphenyl Substituent. Molecules 2022, 27, 6977. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.X.; Nguyen, B.N.; Drewe, J.; Reddy, P.S.; Kasibhatla, S.; Pervin, A. Preparation of 4-Substituted-1-(Arylmethylidene)Thiosemicarbazides and 4-Substituted-1-(Arylcarbonyl)Thiosemicarbazides as Activators of Caspases and Inducers of Apoptosis. Patent WO2002098420, 12 December 2002. [Google Scholar]
- Ameryckx, A.; Thabault, L.; Pochet, L.; Leimanis, S.; Poupaert, J.H.; Wouters, J.; Joris, B.; Van Bambeke, F.; Frédérick, R. 1-(2-Hydroxybenzoyl)-Thiosemicarbazides Are Promising Antimicrobial Agents Targeting d-Alanine-d-Alanine Ligase in Bacterio. Eur. J. Med. Chem. 2018, 159, 324–338. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI Supplement M100; Clinical Lab. Standards Institute: Malvern, PA, USA, 2022; Volume 42, pp. 34–78. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing: Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 13.1; EUCAST: Växjö, Sweden, 2023; Available online: https://www.eucast.org/ (accessed on 2 February 2023).
- Laponogov, I.; Pan, X.-S.; Veselkov, D.A.; McAuley, K.E.; Fisher, L.M.; Sanderson, M.R. Correction: Structural Basis of Gate-DNA Breakage and Resealing by Type II Topoisomerases. PLoS ONE 2010, 5, e11338. [Google Scholar] [CrossRef]
- Germe, T.; Vörös, J.; Jeannot, F.; Taillier, T.; Stavenger, R.A.; Bacqué, E.; Maxwell, A.; Bax, B.D. A New Class of Antibacterials, the Imidazopyrazinones, Reveal Structural Transitions Involved in DNA Gyrase Poisoning and Mechanisms of Resistance. Nucleic Acids Res. 2018, 46, 4114–4128. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Park, I.-S.; Walsh, C.T.; Knox, J.R. D-Alanine: D-Alanine Ligase: Phosphonate and Phosphinate Intermediates with Wild Type and the Y216F Mutant. Biochemistry 1997, 36, 2531–2538. [Google Scholar] [CrossRef] [PubMed]


| MIC—Minimal Inhibitory Concentration [µg/mL] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Microorganism | T1A | T2A | T3A | T4A | T5A | T6A | T7A | T8A | T9A | CIP | |
| Gram-positive bacteria | S. aureus NCTC 4163 | >256 | >256 | 64 | 32 | 128 | 256 | 64 | 64 | 64 | 0.125 |
| S. aureus ATCC 25923 | >256 | >256 | 64 | 32 | 128 | 256 | 64 | 64 | 64 | 0.25 | |
| S. aureus ATCC 6538 | >256 | >256 | 64 | 64 | 128 | 256 | 64 | 128 | 64 | 0.125 | |
| S. aureus ATCC 29213 | >256 | >256 | 128 | 64 | 128 | 256 | 128 | 128 | 64 | 0.5 | |
| S. epidermidis ATCC 12228 | >256 | >256 | >256 | 128 | 256 | 256 | >256 | 256 | 64 | 0.25 | |
| S. epidermidis ATCC 35984 | >256 | >256 | >256 | 256 | 256 | 256 | >256 | 256 | 128 | 0.125 | |
| Gram-negative bacteria | E. coli ATCC 25922 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | 0.008 |
| P. aeruginosa ATCC 15442 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | 0.125 | |
 | ||
| Compounds | S. aureus ATCC 25923 | S. epidermidis ATCC 12228 |
| MIC [µg/mL] | MIC [µg/mL] | |
| T1A | >256 | >256 |
| T1B | 62.5 | 62.5 |
| T2A | >256 | >256 |
| T2B | 1000 | 500 |
| T3A | 64 | >256 |
| T3B | 1000 | 125 |
| T4A | 32 | 256 |
| T4B | 1000 | 1000 |
| T5A | 128 | 256 |
| T5B | 125 | >1000 |
| T6A | 256 | 256 |
| T6B | 500 | 250 |
| T7A | 64 | >256 |
| T7B | 250 | 62.5 |
| T8A | 64 | 256 |
| T8B | 250 | 31.25 |
| T9A | 64 | 128 |
| T9B | 500 | 125 |
| Compounds | Topoisomerase IV (3LTN) | DNA Gyrase (6FQM) | Ddl (1IOV) | |||
|---|---|---|---|---|---|---|
| Binding Energy Kcal/mol | Inhibition Constant, Ki uM | Binding Energy Kcal/mol | Inhibition Constant, Ki uM | Binding Energy Kcal/mol | Inhibition Constant, Ki uM | |
| T1A | −8.32 | 0.79 | −7.84 | 1.79 | −8.73 | 0.39 |
| T2A | −7.86 | 1.85 | −8.73 | 0.39 | −7.83 | 1.83 |
| T3A | −8.03 | 1.3 | −9.92 | 0.053 | −8.64 | 0.46 |
| T4A | −8.55 | 0.15 | −9.78 | 0.068 | −8.99 | 0.26 |
| T5A | −6.59 | 14.87 | −6.64 | 13.50 | −8.88 | 0.31 |
| T6A | −7.36 | 4.03 | −8.48 | 0.20 | −8.19 | 0.99 |
| T7A | −6.64 | 13.50 | −8.67 | 0.45 | −9.03 | 0.24 |
| T8A | −7.83 | 1.82 | −9.85 | 0.60 | −8.78 | 0.35 |
| T9A | −8.48 | 0.19 | −9.50 | 0.11 | −9.55 | 0.10 |
| PD 0305970 | −8.38 | 0.72 | - | - | - | - |
| E32 | - | - | −9.29 | 0.155 | - | - |
| POV | - | - | - | - | −9.50 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janowska, S.; Stefańska, J.; Khylyuk, D.; Wujec, M. The Importance of Substituent Position for Antibacterial Activity in the Group of Thiosemicarbazide Derivatives. Molecules 2024, 29, 1333. https://doi.org/10.3390/molecules29061333
Janowska S, Stefańska J, Khylyuk D, Wujec M. The Importance of Substituent Position for Antibacterial Activity in the Group of Thiosemicarbazide Derivatives. Molecules. 2024; 29(6):1333. https://doi.org/10.3390/molecules29061333
Chicago/Turabian StyleJanowska, Sara, Joanna Stefańska, Dmytro Khylyuk, and Monika Wujec. 2024. "The Importance of Substituent Position for Antibacterial Activity in the Group of Thiosemicarbazide Derivatives" Molecules 29, no. 6: 1333. https://doi.org/10.3390/molecules29061333
APA StyleJanowska, S., Stefańska, J., Khylyuk, D., & Wujec, M. (2024). The Importance of Substituent Position for Antibacterial Activity in the Group of Thiosemicarbazide Derivatives. Molecules, 29(6), 1333. https://doi.org/10.3390/molecules29061333







