Enhanced Photovoltaic Performance of Asymmetrical Benzo Dithiophene Homopolymer Donor Materials in Nonfullerene Acceptor-Based Organic Photovoltaics
Abstract
1. Introduction
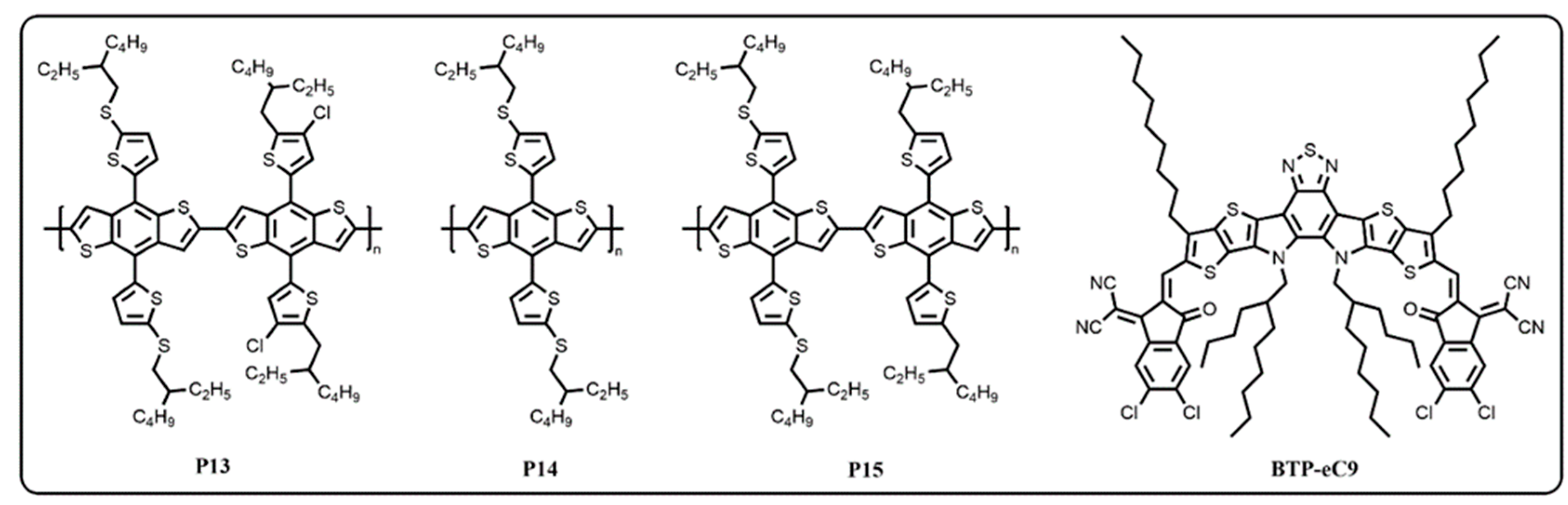
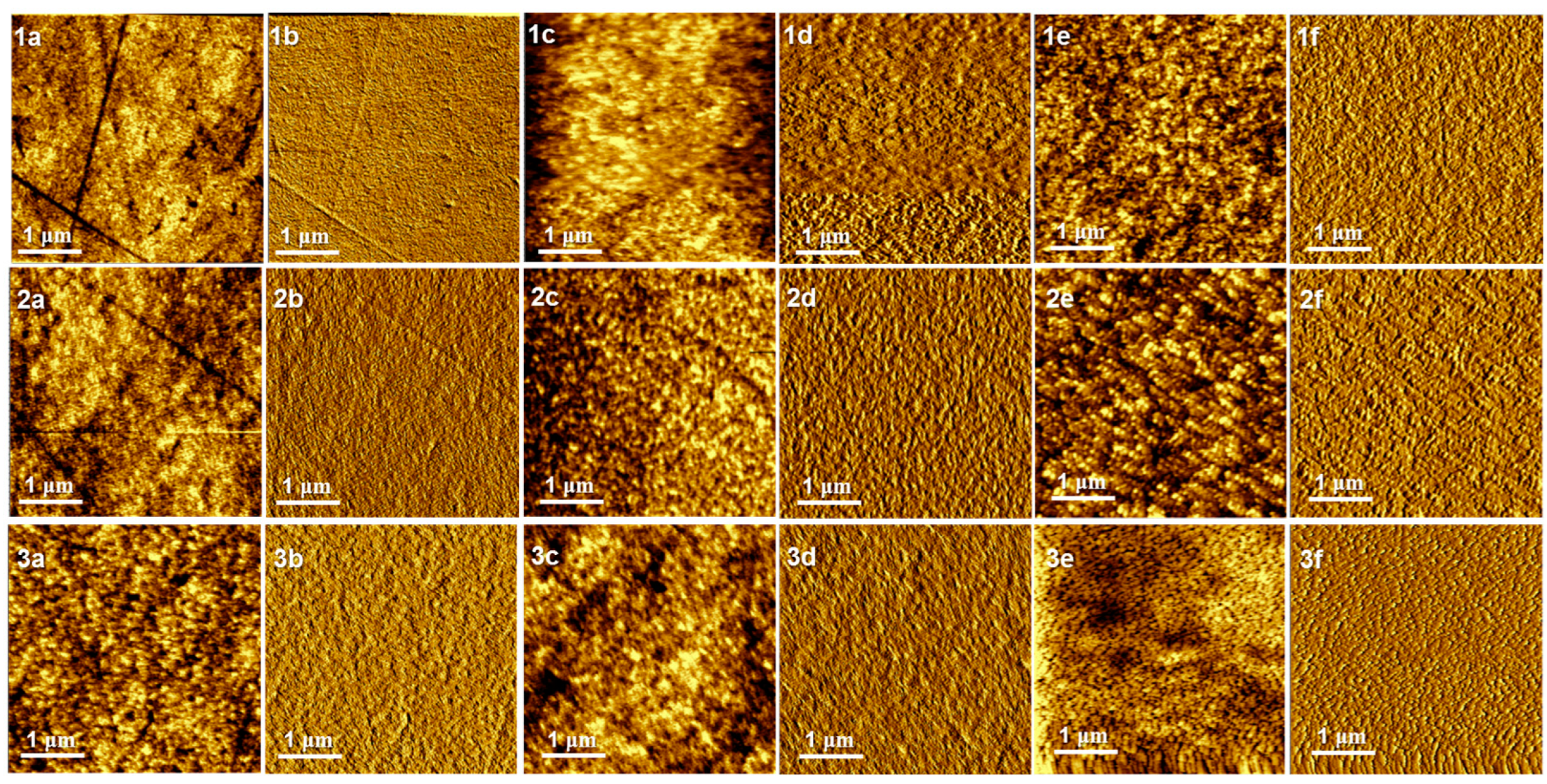
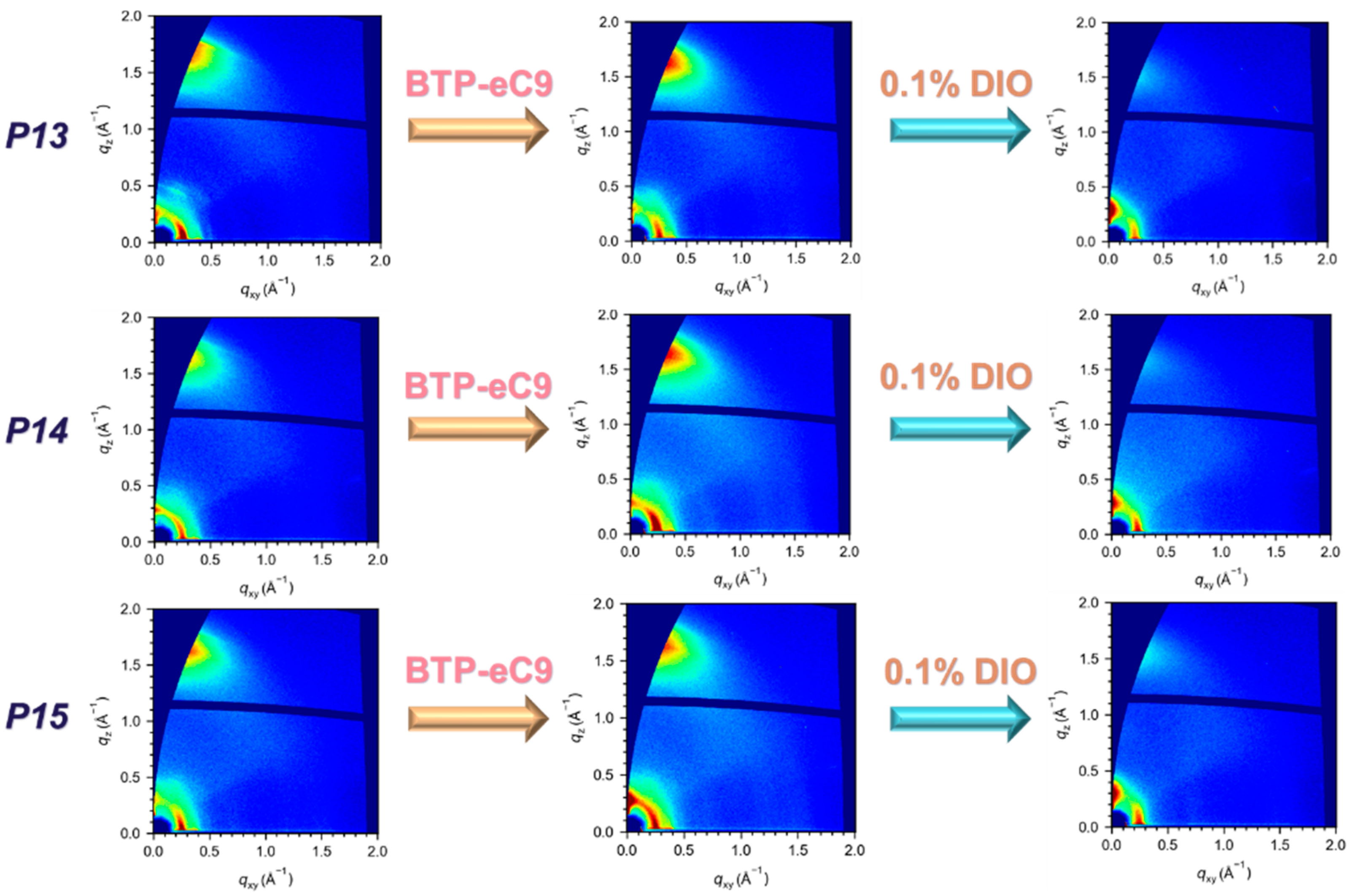
2. Results and Discussion
3. Materials and Methods
- Fabrication of OSC devices
- Charge carrier mobility, dissociation, and recombination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y. Molecular Design of Photovoltaic Materials for Polymer Solar Cells: Toward Suitable Electronic Energy Levels and Broad Absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Hu, Y.; Qin, M.; Li, J.; Wang, Y.; Qin, J.; Cheng, P. Toward High-Performance Organic Photovoltaics: The New Cooperation of Sequential Solution-Processing and Promising Non-Fullerene Acceptors. Mater. Horiz. 2022, 9, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, Y.; Yao, H.; Zhang, J.; Bi, P.; Chen, Z.; Wang, J.; Cui, Y.; Ma, L.; Xian, K.; et al. Suppressing the Energetic Disorder of All-Polymer Solar Cells Enables over 18% Efficiency. Energy Environ. Sci. 2023, 16, 1581–1589. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Y.; Zheng, Q. Benzodithiophene with Multiple Side-Chains for Efficient Wide-Bandgap D–A Copolymers. J. Mater. Chem. A 2023, 11, 5127–5134. [Google Scholar] [CrossRef]
- Shao, Y.; Gao, Y.; Sun, R.; Zhang, M.; Min, J. A Versatile and Low-Cost Polymer Donor Based on 4-Chlorothiazole for Highly Efficient Polymer Solar Cells. Adv. Mater. 2023, 35, 2208750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guo, X.; Ma, W.; Ade, H.; Hou, J. A Polythiophene Derivative with Superior Properties for Practical Application in Polymer Solar Cells. Adv. Mater. 2014, 26, 5880–5885. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, S.; Ren, J.; Gao, M.; Bi, P.; Ye, L.; Hou, J. Molecular Design of a Non-Fullerene Acceptor Enables a P3HT-Based Organic Solar Cell with 9.46% Efficiency. Energy Environ. Sci. 2020, 13, 2864–2869. [Google Scholar] [CrossRef]
- Yang, C.; Yu, R.; Liu, C.; Li, H.; Zhang, S.; Hou, J. Achieving over 10 % Efficiency in Poly(3-hexylthiophene)-Based Organic Solar Cells via Solid Additives. ChemSusChem 2021, 14, 3607–3613. [Google Scholar] [CrossRef]
- Kang, T.E.; Kim, T.; Wang, C.; Yoo, S.; Kim, B.J. Poly(Benzodithiophene) Homopolymer for High-Performance Polymer Solar Cells with Open-Circuit Voltage of Near 1 V: A Superior Candidate To Substitute for Poly(3-Hexylthiophene) as Wide Bandgap Polymer. Chem. Mater. 2015, 27, 2653–2658. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, J.B.; Yoon, S.C.; Jung, I.H.; Hwang, D.-H. Enhanced and Controllable Open-Circuit Voltage Using 2D-Conjugated Benzodithiophene (BDT) Homopolymers by Alkylthio Substitution. J. Mater. Chem. C 2016, 4, 2170–2177. [Google Scholar] [CrossRef]
- Tao, P.; Lü, X.; Zhou, G.; Wong, W.-Y. Asymmetric Tris-Heteroleptic Cyclometalated Phosphorescent Iridium(III) Complexes: An Emerging Class of Metallophosphors. Acc. Mater. Res. 2022, 3, 830–842. [Google Scholar] [CrossRef]
- Schmitt, J.; Heitz, V.; Sour, A.; Bolze, F.; Kessler, P.; Flamigni, L.; Ventura, B.; Bonnet, C.S.; Tóth, É. A Theranostic Agent Combining a Two-Photon-Absorbing Photosensitizer for Photodynamic Therapy and a Gadolinium(III) Complex for MRI Detection. Chem. Eur. J. 2016, 22, 2775–2786. [Google Scholar] [CrossRef]
- Pun, A.B.; Campos, L.M.; Congreve, D.N. Tunable Emission from Triplet Fusion Upconversion in Diketopyrrolopyrroles. J. Am. Chem. Soc. 2019, 141, 3777–3781. [Google Scholar] [CrossRef] [PubMed]
- Guo, E.Q.; Ren, P.H.; Zhang, Y.L.; Zhang, H.C.; Yang, W.J. Diphenylamine End-Capped 1,4-Diketo-3,6-Diphenylpyrrolo[3,4-c]Pyrrole (DPP) Derivatives with Large Two-Photon Absorption Cross-Sections and Strong Two-Photon Excitation Red Fluorescence. Chem. Commun. 2009, 45, 5859–5861. [Google Scholar] [CrossRef] [PubMed]
- Bürgi, L.; Turbiez, M.; Pfeiffer, R.; Bienewald, F.; Kirner, H.-J.; Winnewisser, C. High-Mobility Ambipolar Near-Infrared Light-Emitting Polymer Field-Effect Transistors. Adv. Mater. 2008, 20, 2217–2224. [Google Scholar] [CrossRef]
- Bao, W.W.; Li, R.; Dai, Z.C.; Tang, J.; Shi, X.; Geng, J.T.; Deng, Z.F.; Hua, J. Diketopyrrolopyrrole (DPP)-Based Materials and Its Applications: A Review. Front. Chem. 2020, 8, 679. [Google Scholar] [CrossRef] [PubMed]
- Stolte, M.; Suraru, S.-L.; Diemer, P.; He, T.; Burschka, C.; Zschieschang, U.; Klauk, H.; Würthner, F. Diketopyrrolopyrrole Organic Thin-Film Transistors: Impact of Alkyl Substituents and Tolerance of Ethylhexyl Stereoisomers. Adv. Funct. Mater. 2016, 26, 7415–7422. [Google Scholar] [CrossRef]
- Liu, Q.; Bottle, S.E.; Sonar, P. Developments of Diketopyrrolopyrrole-Dye-Based Organic Semiconductors for a Wide Range of Applications in Electronics. Adv. Mater. 2020, 32, 1903882. [Google Scholar] [CrossRef]
- He, T.; Leowanawat, P.; Burschka, C.; Stepanenko, V.; Stolte, M.; Würthner, F. Impact of 2-Ethylhexyl Stereoisomers on the Electrical Performance of Single-Crystal Field-Effect Transistors. Adv. Mater. 2018, 30, 1804032. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, H.; Blaikie, C.; Caporale, C.; Manzhos, S.; Feron, K.; MacLeod, J.M.; Massi, M.; Bottle, S.E.; Bell, J.; et al. Naphthalene Flanked Diketopyrrolopyrrole Based Organic Semiconductors for High Performance Organic Field Effect Transistors. New J. Chem. 2018, 42, 12374–12385. [Google Scholar] [CrossRef]
- Di Carlo Rasi, D.; Janssen, R.A.J. Advances in Solution-Processed Multijunction Organic Solar Cells. Adv. Mater. 2019, 31, 1806499. [Google Scholar] [CrossRef]
- Xie, B.; Chen, Z.; Ying, L.; Huang, F.; Cao, Y. Near-Infrared Organic Photoelectric Materials for Light-Harvesting Systems: Organic Photovoltaics and Organic Photodiodes. InfoMat 2020, 2, 57–91. [Google Scholar] [CrossRef]
- Shin, D.H.; Choi, S.-H. Recent Studies of Semitransparent Solar Cells. Coatings 2018, 8, 329. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, G.; Xu, Y.; Xu, X.; Yu, L. Power Generation, Evaporation Mitigation, and Thermal Insulation of Semitransparent Polymer Solar Cells: A Potential for Floating Photovoltaic Applications. ACS Appl. Energy Mater. 2019, 2, 6060–6070. [Google Scholar] [CrossRef]
- Chen, M.; Yan, L.; Zhao, Y.; Murtaza, I.; Meng, H.; Huang, W. Anthracene-Based Semiconductors for Organic Field-Effect Transistors. J. Mater. Chem. C. 2018, 6, 7416–7444. [Google Scholar] [CrossRef]
- Melzer, C.; Koop, E.J.; Mihailetchi, V.D.; Blom, P.W.M. Hole Transport in Poly(Phenylene Vinylene)/Methanofullerene Bulk-Heterojunction Solar Cells. Adv. Funct. Mater. 2004, 14, 865–870. [Google Scholar] [CrossRef]
- Blom, P.W.M.; Mihailetchi, V.D.; Koster, L.J.A.; Markov, D.E. Device Physics of Polymer:Fullerene Bulk Heterojunction Solar Cells. Adv. Mater. 2007, 19, 1551–1566. [Google Scholar] [CrossRef]
- Huo, L.; Liu, T.; Sun, X.; Cai, Y.; Heeger, A.J.; Sun, Y. Single-Junction Organic Solar Cells Based on a Novel Wide-Bandgap Polymer with Efficiency of 9.7%. Adv. Mater. 2015, 27, 2938–2944. [Google Scholar] [CrossRef]
- Koster, L.J.A.; Mihailetchi, V.D.; Ramaker, R.; Blom, P.W.M. Light Intensity Dependence of Open-Circuit Voltage of Polymer:Fullerene Solar Cells. Appl. Phys. Lett. 2005, 86, 123509. [Google Scholar] [CrossRef]
- Kyaw, A.K.K.; Wang, D.H.; Wynands, D.; Zhang, J.; Nguyen, T.-Q.; Bazan, G.C.; Heeger, A.J. Improved Light Harvesting and Improved Efficiency by Insertion of an Optical Spacer (ZnO) in Solution-Processed Small-Molecule Solar Cells. Nano Lett. 2013, 13, 3796–3801. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.T.; Li, Y.Y.; Colberts, F.J.M.; Lo, M.M.; Zhang, J.J.; Yang, F.; Jin, Y.Z.; Zhang, F.L.; Janssen, R.A.J.; Li, C.; et al. “Double-Cable” Conjugated Polymers with Linear Backbone toward High Quantum Efficiencies in Single-Component Polymer Solar Cells. J. Am. Chem. Soc. 2017, 139, 18647–18656. [Google Scholar] [CrossRef] [PubMed]
- Kyeong, M.K.; Lee, J.H.; Lee, K.H.; Hong, S.K. BODIPY-Based Conjugated Polymers for Use as Dopant-Free Hole Transporting Materials for Durable Perovskite Solar Cells: Selective Tuning of HOMO/LUMO Levels. ACS Appl. Mater. Interfaces 2018, 10, 23254–23262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, S.L.; Xu, Z.; Qiao, B.; Huang, D.; Zhao, L.; Li, Y.; Zhu, Y.Q.; Wang, P. Revealing the Effect of Additives with Different Solubility on the Morphology and the Donor Crystalline Structures of Organic Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 18231–18237. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, Y.; Maffei, L.P.; Cruciani, F.; Muller, M.A.; Liu, S.J.; Lopatin, S.; Wehbe, N.; Ndjawa, G.O.N.G.; Amassian, A.; Laquai, F.; et al. Polymer Main-chain Substitution Effects on the Efficiency of Nonfullerence BHJ Solar Cells. Adv. Energy Mater. 2017, 7, 1700834. [Google Scholar] [CrossRef]
- Xia, D.D.; Wu, Y.; Wang, Q.; Zhang, A.D.; Li, C.; Lin, Y.Z.; Colberts, F.J.M.; van Franeker, J.J.; Janssen, R.A.J.; Zhan, X.W.; et al. Effect of Alkyl Side Chains of Conjugated Polymer Donors on the Device Performance of Non-Fullerene Solar Cells. Macromolecules 2016, 49, 6445–6454. [Google Scholar] [CrossRef]
- Kong, R.; Xiao, Z.; Xie, F.Y.; Jiang, J.X.; Ding, L.M. A D–A copolymer donor containing an alkylthio-substituted thieno[3,2-b]thiophene unit. N. J. Chem. 2017, 41, 2895–2898. [Google Scholar] [CrossRef]
- An, Y.K.; Liao, X.F.; Cjem, L.; Yi, J.P.; Ai, Q.Y.; Xie, Q.; Huang, B.; Liu, F.; Jen, A.K.Y.; Chen, Y.W. Nonhalogen Solvent-Processed Asymmetric Wide-Bandgap Polymers for Nonfullerene Organic Solar Cells with Over 10% Efficiency. Adv. Func. Mater. 2018, 28, 1706517. [Google Scholar] [CrossRef]
- Liao, X.F.; Yao, Z.Y.; Gao, K.; Shi, X.L.; Zuo, L.J.; Zhu, Z.L.; Chen, L.; Liu, F.; Chen, Y.W.; Jen, A.K.Y. Mapping Nonfullerene Acceptors with a Novel Wide Bandgap Polymer for High Performance Polymer Solar Cells. Adv. Energy Mater. 2018, 8, 1801214. [Google Scholar] [CrossRef]
- Hao, D.; Li, M.; Liu, Y.H.; Li, C.H.; Bo, Z.S. Bis(carboxylate) substituted benzodithiophene based wide-bandgap polymers for high performance nonfullerene polymer solar cells. Dye. Pigment. 2019, 162, 120–125. [Google Scholar] [CrossRef]
- Li, G.D.; Xu, Q.Q.; Chang, C.M.; Fan, Q.P.; Zhu, X.Q.; Li, W.B.; Guo, X.; Zhang, M.J.; Wong, W.Y. High-Performance Nonfullerene Polymer Solar Cells Based on a Wide-Bandgap Polymer without Extra Treatment. Macromol. Rapid. Commun. 2018, 39, 1800660. [Google Scholar] [CrossRef]


| Sample | HOMO [eV] a | LUMO [eV] a | Eg [eV] b | λedge [nm] | Egopt [eV] c | HOMO [eV] d | LUMO [eV] d |
|---|---|---|---|---|---|---|---|
| P13 | −5.51 | −3.63 | 1.88 | 630 | 1.97 | −5.23 | −2.18 |
| P14 | −5.53 | −3.60 | 1.93 | 635 | 1.95 | −5.27 | −2.14 |
| P15 | −5.48 | −3.61 | 1.87 | 640 | 1.94 | −5.15 | −2.05 |
| Polymer/ BTP-eC9 | PCE (%) a | FF (%) | VOC (V) | JSC (mA·cm−2) b |
|---|---|---|---|---|
| P13 | 9.18 (8.63) | 57.10 | 0.88 | 18.33 (17.68) |
| P14 | 9.07 (8.49) | 53.15 | 0.86 | 19.94 (19.33) |
| P15 | 11.53 (11.08) | 65.87 | 0.79 | 22.04 (21.44) |
| Items | µh (cm2 V−1 s−1) | α | KT/q | Pdiss a |
|---|---|---|---|---|
| P13:BTP-eC9 | 5.03 × 10−5 | 0.99 | 1.45 | 0.97 |
| P13:BTP-eC9, 0.1% DIO | 3.65 × 10−5 | 0.98 | 1.25 | 0.97 |
| P14:BTP-eC9 | 1.26 × 10−5 | 0.98 | 1.97 | 0.99 |
| P14:BTP-eC9, 0.1% DIO | 7.86 × 10−6 | 0.97 | 1.83 | 0.98 |
| P15:BTP-eC9 | 4.88 × 10−5 | 0.99 | 1.45 | 0.99 |
| P15:BTP-eC9, 0.1% DIO | 2.30 × 10−4 | 0.98 | 1.25 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Du, L.; Du, Z.; He, W.; Li, H.; Li, G.; Yang, C.; Cheng, P.; Cao, Z.; Yu, D. Enhanced Photovoltaic Performance of Asymmetrical Benzo Dithiophene Homopolymer Donor Materials in Nonfullerene Acceptor-Based Organic Photovoltaics. Molecules 2024, 29, 1332. https://doi.org/10.3390/molecules29061332
Xu W, Du L, Du Z, He W, Li H, Li G, Yang C, Cheng P, Cao Z, Yu D. Enhanced Photovoltaic Performance of Asymmetrical Benzo Dithiophene Homopolymer Donor Materials in Nonfullerene Acceptor-Based Organic Photovoltaics. Molecules. 2024; 29(6):1332. https://doi.org/10.3390/molecules29061332
Chicago/Turabian StyleXu, Wei, Li Du, Zhengkun Du, Wei He, Hongxiang Li, Guojuan Li, Cheng Yang, Pei Cheng, Zhong Cao, and Donghong Yu. 2024. "Enhanced Photovoltaic Performance of Asymmetrical Benzo Dithiophene Homopolymer Donor Materials in Nonfullerene Acceptor-Based Organic Photovoltaics" Molecules 29, no. 6: 1332. https://doi.org/10.3390/molecules29061332
APA StyleXu, W., Du, L., Du, Z., He, W., Li, H., Li, G., Yang, C., Cheng, P., Cao, Z., & Yu, D. (2024). Enhanced Photovoltaic Performance of Asymmetrical Benzo Dithiophene Homopolymer Donor Materials in Nonfullerene Acceptor-Based Organic Photovoltaics. Molecules, 29(6), 1332. https://doi.org/10.3390/molecules29061332











