Selective Generation of Aldimine and Ketimine Tautomers of the Schiff Base Condensates of Amino Acids with Imidazole Aldehydes or of Imidazole Methanamines with Pyruvates—Isomeric Control with 2- vs. 4-Substituted Imidazoles
Abstract
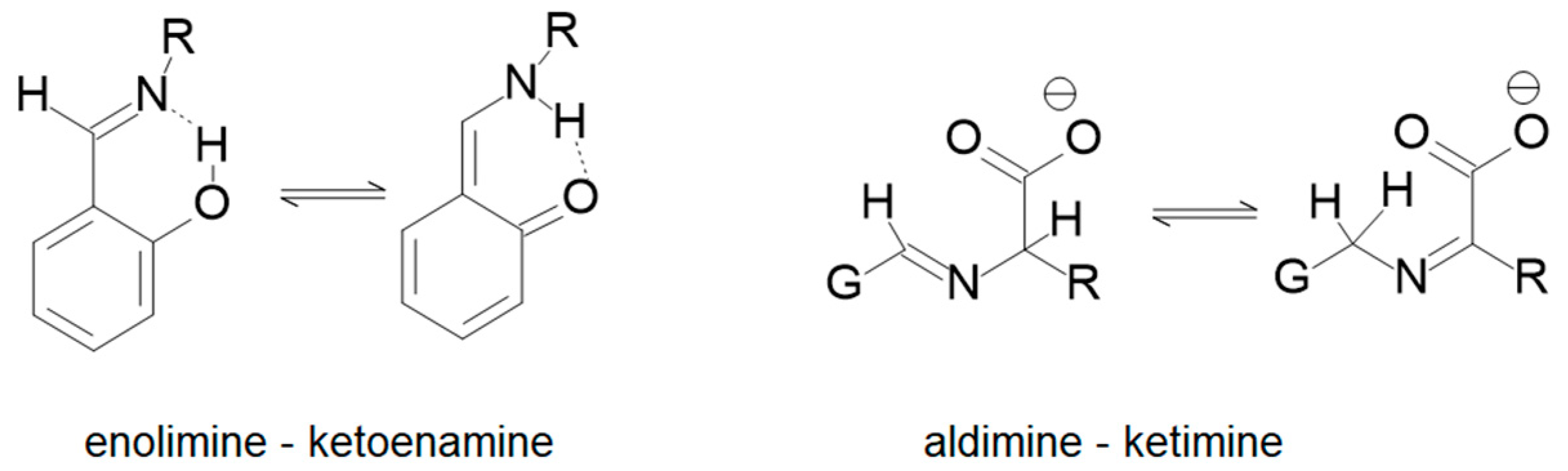
1. Introduction
2. Results and Discussion
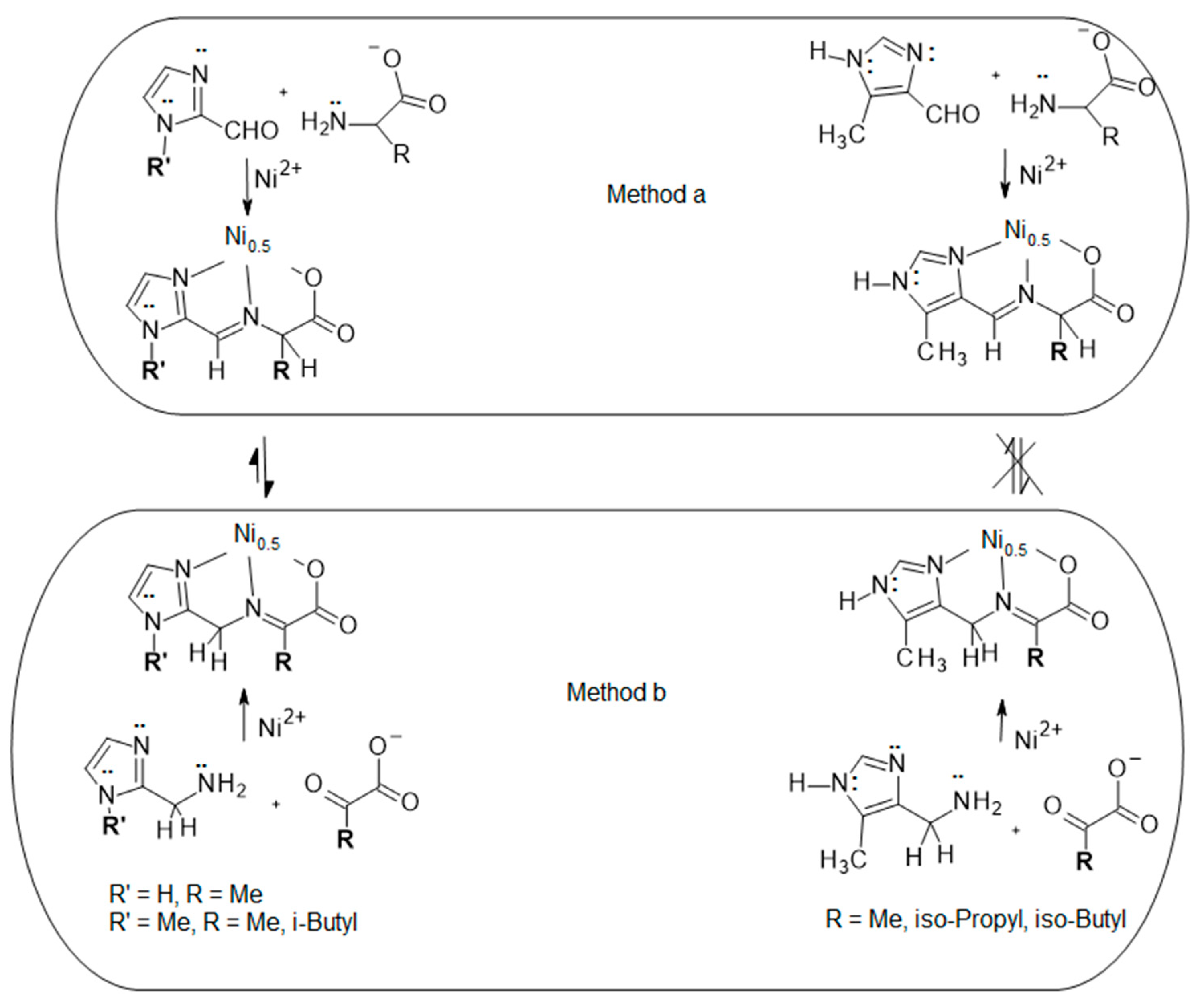
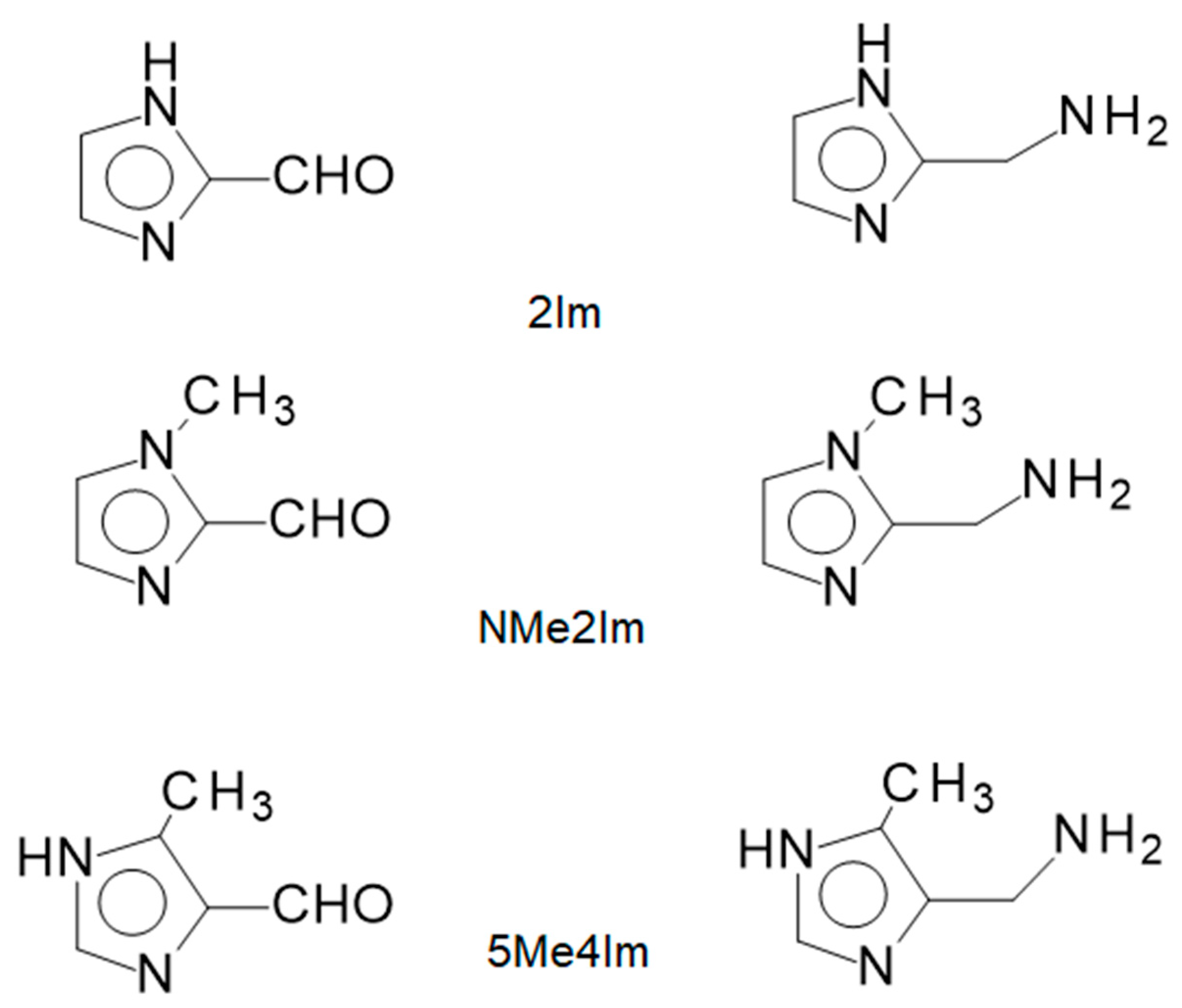
2.1. Ligands Employed and Reactions Examined
2.2. Methods (a) and (b) for 2Im and NMe2Im
2.3. Methods (a) and (b) for 5Me4Im
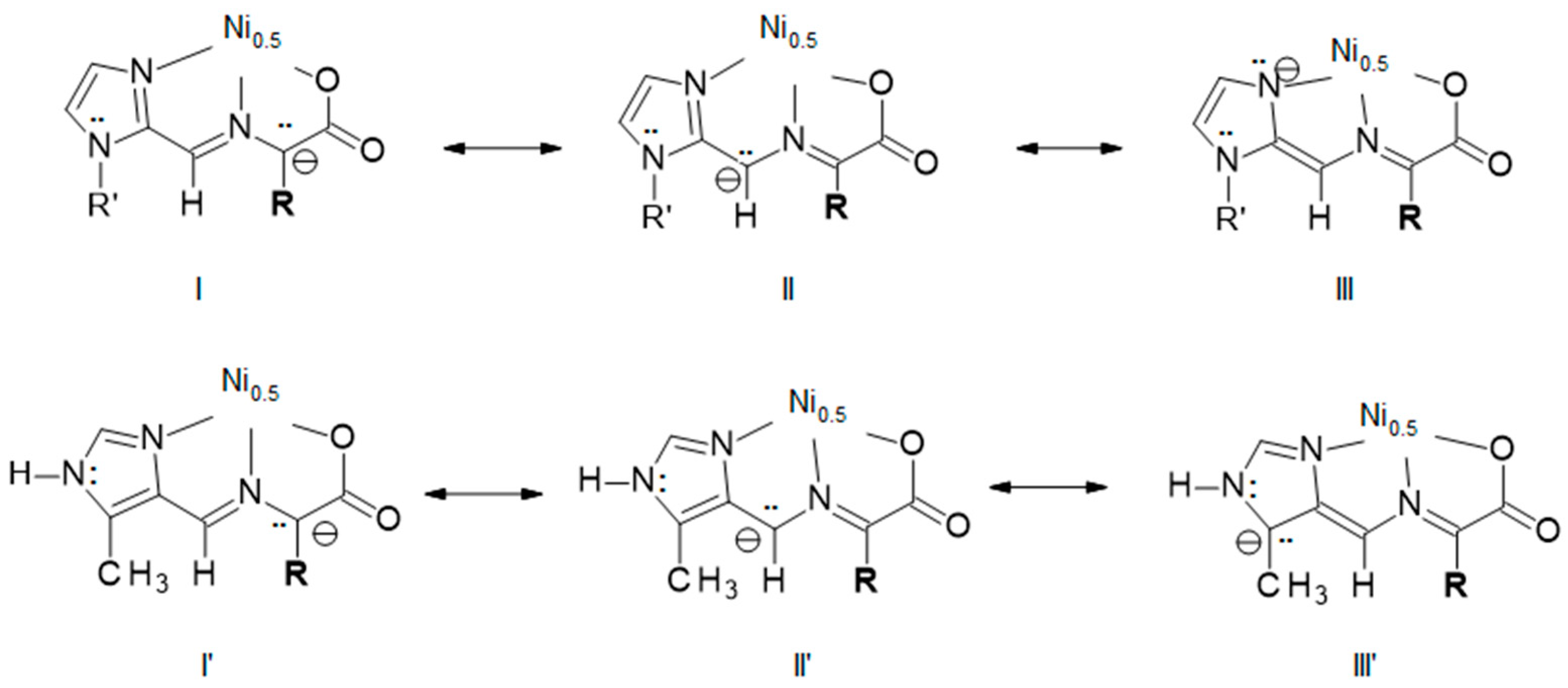
2.4. Explanation for Reactivity Difference between the 2- and 4-Substituted Imidazoles
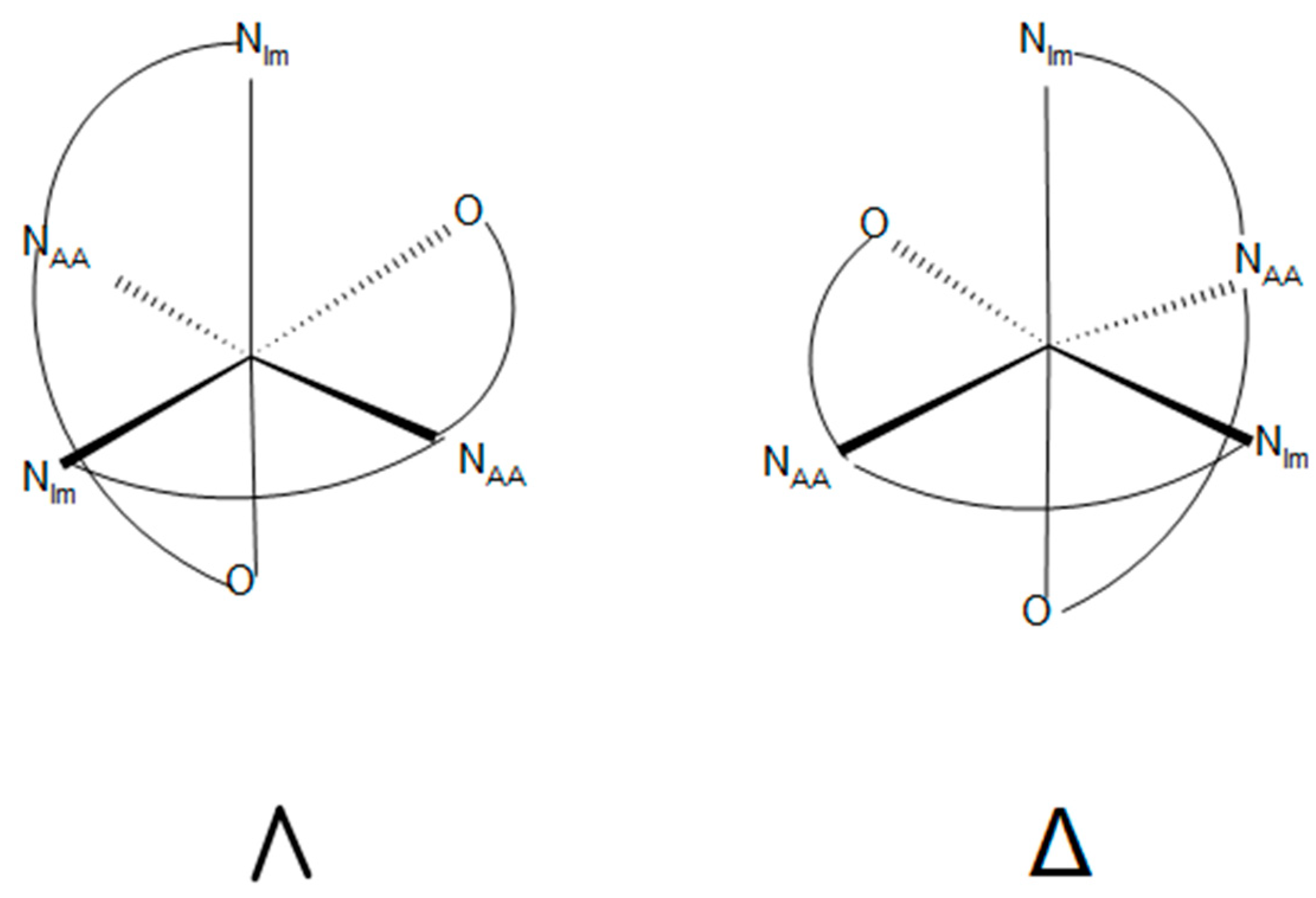
2.5. Chirality Correlation
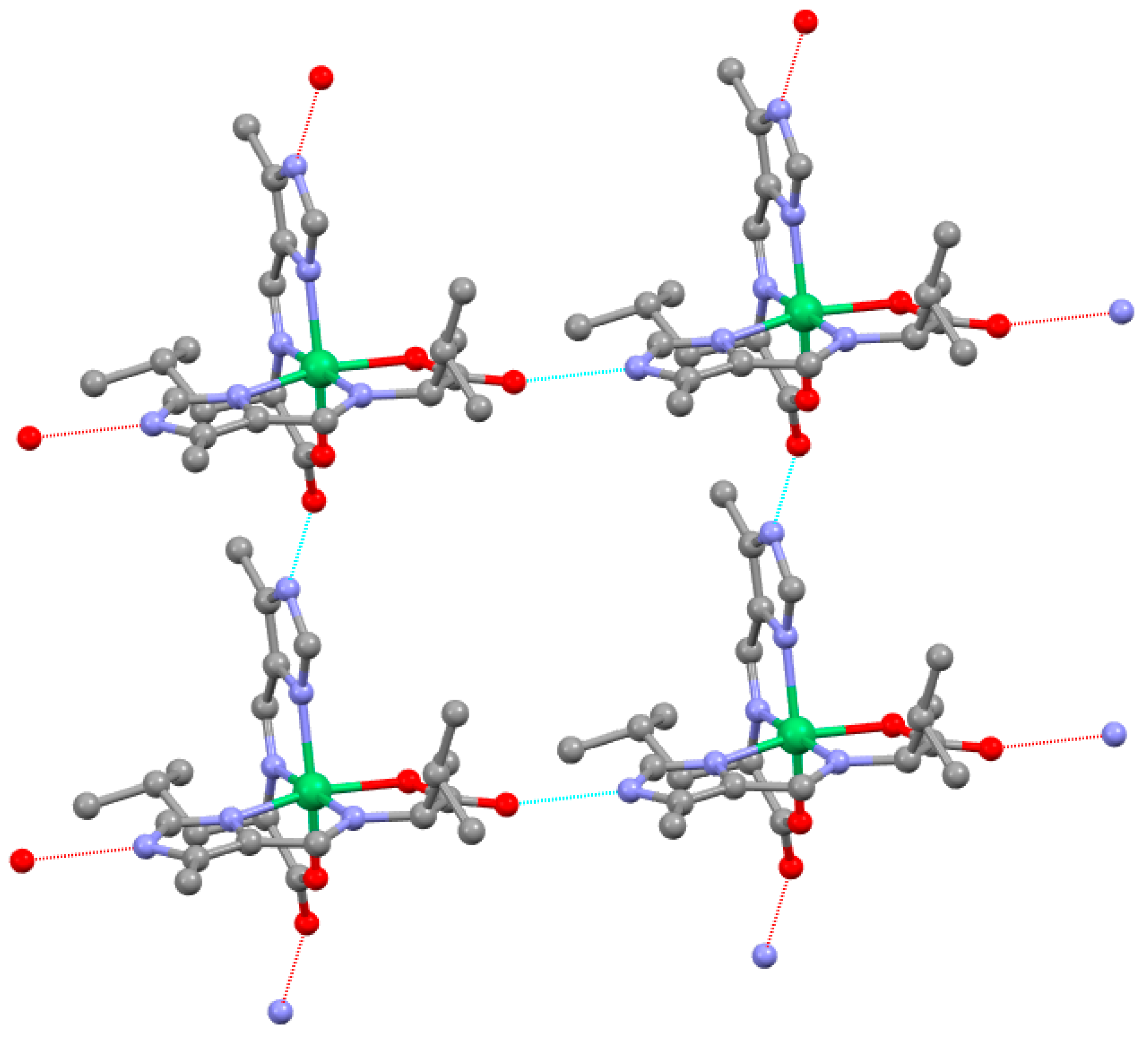
2.6. Supramolecular Structure
2.7. Visible Spectroscopy of NiN4O2 Complexes
3. Experimental
3.1. General
3.2. ESI-MS Were Obtained by Axis Pharm Laboratory, San Diego, CA
3.3. X-ray Crystallography
3.4. Syntheses
3.5. Available Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sunatsuki, Y.; Ikuta, Y.; Matsumoto, N.; Ohta, H.; Kojima, M.; Iijima, S.; Hayami, S.; Maeda, Y.; Kaizaki, S.; Dahan, F.; et al. An Unprecedented Homochiral Mixed-Valence Spin-Crossover Compound. Angew. Chem. Int. Ed. 2003, 42, 1614–1618. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, Y.; Ooidemizu, M.; Yamahata, Y.; Yamada, M.; Osa, S.; Matsumoto, N.; Iijima, S.; Sunatsuki, Y.; Kojima, M.; Dahan, F.; et al. A New Family of Spin Crossover Complexes with a Tripod Ligand Containing Three Imidazoles: Synthesis, Characterization, and Magnetic Properties of [FeIIH3LMe](NO3)2·1.5H2O, [FeIIILMe]·3.5H2O, [FeIIH3LMe][FeIILMe]NO3, and [FeIIH3LMe][FeIIILMe](NO3)2 (H3LMe = Tris [2-(((2-methylimidazol-4-yll)methylidene)amino)ethyl]amine). Inorg. Chem. 2003, 42, 7001–7017. [Google Scholar]
- Yamada, M.; Ooidemizu, M.; Ikuta, Y.; Osa, S.; Matsumoto, N.; Iijima, S.; Kojima, M.; Dahan, F.; Tuchagues, J.P. Interlayer Interaction of Two-Dimensional Layered Spin Crossover Complexes [FeIIH3LMe][FeIILMe]X (X− = ClO4−, BF4−, PF6−, AsF6−, and SbF6−; H3LMe = Tris [2-(((2-methylimidazol-4-yl)methylidene)amino)ethyl]amine). Inorg. Chem. 2003, 42, 8406–8416. [Google Scholar] [CrossRef]
- Sunatsuki, Y.; Ohta, H.; Kojima, M.; Ikuta, Y.; Goto, Y.; Matsumoto, N.; Iijima, S.; Akashi, H.; Kaizaki, S.; Dahan, F.; et al. Supramolecular SpinCrossover Iron Complexes Based on Imidazole−Imidazolate Hydrogen Bond. Inorg. Chem. 2004, 43, 4154–4171. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G. Structural Evidence of Spin State Selection and Spin Crossover Behavior of Tripodal Schiff Base Complexes of tris(2-aminoethyl)amine and Related Tripodal Amines. Magnetochemistry 2020, 6, 28. [Google Scholar] [CrossRef]
- Shahraki, S. Schiff Base Compounds as Artificial Metalloenzymes. Colloids Surf. B Biointerfaces 2022, 218, 112727–112743. [Google Scholar] [CrossRef]
- Bos, J.; Roelfes, G. Artificial Metalloenzymes for Enantioselective Catalysis. Curr. Opin. Chem. Biol. 2014, 19, 135–143. [Google Scholar] [CrossRef]
- Erxleben, A. Transition Metal Salen Complexes in Bioinorganic and Medicinal Chemistry. Inorganica Chim. Acta 2018, 472, 40–57. [Google Scholar] [CrossRef]
- Yoshida, N.; Oshio, H.; Ito, T. Supramolecular Motifs in Metal Complexes of Schiff Bases. Part 6.1 Topology of Two Types of Self-assembly of Bis-N,O-bidentate Schiff Base Ligands by Copper(II) Ions. J. Chem. Soc. Perkin Trans. 2001, 2, 1674–1678. [Google Scholar] [CrossRef]
- Alcazar, J.J.; Geue, N.; Valladares, V.; Canete, A.; Perez, E.G.; Garcia-Rio, L.; Santos, J.G.; Aliaga, M.E. Supramolecular Control of Reactivity toward Hydrolysis of 7-Diethylaminocoumarin Schiff Bases by Cucurbit [7]uril Encapsulation. ACS Omega 2021, 6, 10333–10342. [Google Scholar] [CrossRef]
- Nagy, P.L. Tautomerism; Antonov, L., Ed.; Wiley, VCH, Verlag GmbH & Co.: Weinheim, Germany, 2016; Chapter 6; p. 140. [Google Scholar]
- Yu, J.; Daru, A.; Deng, M.; Blackmond, D. Prebiotic Access to Enantioenriched Amino Acids via Peptide-Mediated Transamination Reactions. ChemRxiv 2023, preprint. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Gil, D.M.; Echeverría, G.A.; Piro, O.E.; Jios, J.L.; Ulic, S.E. Enol-imino-keto-enamine Tautomerism in a Diazepine Derivative: How Decisive Are the Intermolecular Interactions in the Equilibrium? J. Org. Chem. 2019, 84, 11042–11053. [Google Scholar] [CrossRef] [PubMed]
- Shepelenko, E.N.; Tsukanov, A.V.; Revinskii, Y.V.; Dubonosov, A.D.; Bren, V.A.; Minkin, V.I. Benzoid–Quinoid Tautomerism of Schiff Bases and Their Structural Analogs: LIII. Schiff Bases Derived from 5-Hydroxy- and 5-Hydroxy-6-nitro-2,3-diphenyl-1-benzofuran-4-carbaldehydes. Russ. J. Org. Chem. 2007, 43, 559–563. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, C.; Zhang, Z.; Usadi, A.K.; Calabro, D.C.; Baugh, L.S.; Yuan, Y.D.; Zhao, D. Evaluation of Schiff-Base Covalent Organic Frameworks for CO2 Capture: Structure–Performance Relationships, Stability, and Performance under Wet Conditions. ACS Sustain. Chem. Eng. 2022, 10, 332–341. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Wu, J.; Li, J.; Yu, X.; Zhang, Q. Highly Stable β-Ketoenamine-based Covalent Organic Frameworks (COFs): Synthesis and Optoelectrical Applications. Front. Optoelectron. 2022, 15, 38. [Google Scholar] [CrossRef]
- Gansow, O.A.; Holm, R.H. A Proton Resonance Investigation of Equilibria, Solute Structures, and Transamination in the Aqueous Systems Pyridoxamine-Pyruvate-Zinc(II) and –Aluminum(III). J. Am. Chem. Soc. 1969, 91, 5984–5993. [Google Scholar] [CrossRef]
- Viedma, C.; Ortiz, J.E.; de Torres, T.; Izumi, T.; Blackmond, D.G. Evolution of Solid Phase Homochirality for a Proteinogenic Amino Acid. J. Am. Chem. Soc. 2008, 130, 15274–15275. [Google Scholar] [CrossRef]
- Klussman, M.; Iwamura, H.; Mathew, S.P.; Wells, D.H., Jr.; Pandya, U.; Armstrong, A.; Blackmond, D.G. Thermodynamic Control of Asymmetric Amplification in Amino Acid Catalysis. Nature 2006, 441, 621–623. [Google Scholar] [CrossRef]
- Hein, J.E.; Blackmond, D.G. On the Origin of Single Chirality of Amino Acids and Sugars in Biogenesis. Acc. Chem. Res. 2012, 45, 2045–2054. [Google Scholar] [CrossRef]
- Brewer, G.; Brewer, C.; Butcher, R.J.; Zavalij, P. Formation of Ketimines from Aldimines in Schiff Base Condensation of Amino Acids and Imidazole-2-Carboxaldehydes: Tautomerization of Schiff Bases of Amino Acids Resulting in the Loss of Stereogenic Center. Inorganics 2023, 11, 381. [Google Scholar] [CrossRef]
- Jung, M.J. Enzyme Inhibition by Amino Acids and their Derivatives. In Chemistry and Biochemistry of the Amino Acids; Barrett, G.C., Ed.; Chapman and Hall Ltd.: London, UK, 1985; pp. 228–234. [Google Scholar]
- Voet, D.; Voet, J.G.; Pratt, C.W. Amino Acid Metabolism. In Fundamentals of Biochemistry; John Wiley and Sons: New York, NY, USA, 2002; pp. 616–619. [Google Scholar]
- Hopgood, D. Transamination in Pyridoxal Phosphate Systems. Kinetic Studies of the Influence of the Side-chains of Several Amino Acids on 1,3-Prototropic Shift in Zinc(II) Aldimine Complexes. J. Chem. Soc. Dalton Trans. 1972, 4, 482–487. [Google Scholar] [CrossRef]
- Dherbussy, Q.; Mayer, R.J.; Muchkowska, K.B.; Moran, J. Metal-pyridoxal Cooperativity in Nonenzymatic Transamination. J. Am. Chem. Soc. 2023, 145, 13357–13370. [Google Scholar] [CrossRef]
- Weinstein, G.N.; Holm, R.H. Synthesis of Tautomeric Schiff Base Complexes and Ketimine Aldimine Conversion Rates of Copper(II) Complexes. Inorg. Chem. 1972, 11, 2553–2556. [Google Scholar] [CrossRef]
- Weinstein, G.N.; O’Connor, M.J.; Holm, R.H. Preparation, Properties, and Racemization Kinetics of Copper(II)-Schiff Base-Amino Acid Complexes Related to Vitamin B6 Catalysis. Inorg. Chem. 1970, 9, 2104–2112. [Google Scholar] [CrossRef]
- Cotton, F.A.; Francis, J.N.; Frenz, B.A.; Tsutsul, M.F. The Structure of a Dihapto(vinyl alcohol) Complex of Pt(II). J. Am. Chem. Soc. 1973, 95, 2483–2486. [Google Scholar] [CrossRef]
- Giordano, F.; Ruffo, F.; Saporito, A.; Panunzi, A. Stabilization of Unstable Unsaturated Molecules in Five-coordinate TBP Complexes of Pt(II): Vinyl Alcohol Derivatives. Inorg. Chim. Acta 1997, 264, 231–237. [Google Scholar] [CrossRef]
- Wang, A.; Xie, X.; Zheng, C.; Liu, Y. Gold/Lewis Acid Catalyzed Oxidative Cyclization Involving Activation of Nitriles. Chem. Commun. 2020, 56, 15581–15584. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Weng, Z.; Yang, D. One-Pot Construction of 1-Phenylchromeno [3,4-b]pyrrol-4(3 H)-one: Application to Total Synthesis of Ningalin B and a Pyrrolocoumarin-Based Electrochromic Switch. Org. Lett. 2019, 21, 5225–5228. [Google Scholar] [CrossRef] [PubMed]
- Narode, A.S.; Ho, Y.; Cheng, M.; Liu, R. Gold-Catalyzed Addition of β-Oxo Enols at Tethered Alkynes via a Non-Conia-ene Pathway: Observation of a Formal 1,3-Hydroxymethylidene Migration. Org. Lett. 2023, 25, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Jamshaid, S.; Devkota, S.; Lee, Y.R. Catalyst- and Substituent-Controlled Regio- and Stereoselective Synthesis of Indolyl Acrylates by Lewis-Acid-Catalyzed Direct Functionalization of 3-Formylindoles with Diazo Esters. Org. Lett. 2021, 23, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki; Ozaki, N.; Saika, Y.; Ohta, K.; Goboh, K.; Nakamura, F.; Hashimoto, M.; Oteya, S. First Transition Metal Complex of 1,8-Bis(dimethylamino)naphalene (proton Sponge). Chem. Lett. 2004, 33, 928–929. [Google Scholar] [CrossRef]
- Fujino, M.; Hasegawa, S.; Akutsu, H.; Yamada, J.; Nakatsuji, S. Radical Compounds and Metal Complexes with Azobenzene Chromophore. Polyhedron 2007, 26, 1989–1992. [Google Scholar] [CrossRef]
- Basseti, M.; Cerichelli, G.; Floris, B. Substituent Effects in Keto-Enol Tautomerism. Part 3. Influence of Substitution on the Equilibrium Composition of β-Dicarbonyl Compounds. Tetrahedron 1988, 44, 2997–3004. [Google Scholar]
- Tollec, J. The Chemistry of the Enols; Rappoport, Z., Ed.; John Wiley and Sons: New York, NY, USA, 1990; p. 366. [Google Scholar]
- Dwyer, D.S. Electronic Properties of Amino Acid Side Chains: Quantum Mechanics Calculation of Substituent Effects. BMC Chem. Biol. 2005, 5, 2. [Google Scholar] [CrossRef]
- Weider, M.; Fass, J.; Chodera, J.D. Fitting quantum machine learning potentials to experimental free energy data: Predicting tautomer ratios in solution. R. Soc. Chem. Chem. Sci. 2021, 12, 11364–11381. [Google Scholar] [CrossRef]
- Iihoshi, T.; Sato, T.; Towatari, M.; Matsumoto, N.; Kojima, M. Enantioselective Assembly Structures of Copper(II) and Zinc(II) Complexes with the 1:1 Condensation Products of Imidazole-4-carbaldehyde Derivatives and DL-Phenylalanine. Bull. Chem. Soc. Jpn. 2009, 82, 458–466. [Google Scholar] [CrossRef]
- Cruz, C.; Gonzalez, C.; Rubio, F.; Erices, J.; Wrighton-Araneda, K.; Cortés-Arriagada, D.; Venegas-Yazigi, D.; Audebrand, N.; Paredes-García, V. Chiral 1D Metal–Organic Materials Based on Cu(II) and Amino Acid Schiff Bases. Cryst. Growth Des. 2022, 22, 237–250. [Google Scholar] [CrossRef]
- Shintoya, S.; Yamauchi, S.; Oishi, T.; Matsumoto, N.; Fujiami, T. Assembly structures of 1:2 nickel(II) complex of 5-methylimidazol-4-yl-methylidene-l-phenylalanine and 1:2 nickel(II) complex of its racemic ligand. Inorg. Chim. Acta 2013, 406, 59–64. [Google Scholar] [CrossRef]
- Brewer, G.; Butcher, R.J.; Zemba, M. Experimental Crystal Structure Determination CCDC 961032, HOVRAM01; CSD Communication: Cambridge, UK, 2018. [Google Scholar]
- Yamouchi, S.; Fujinami, T.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatski, Y.; Watanabe, M.; Tsuchimoto, M.; Coletti, C.; Re, N. Synthesis, Structure, Luminescence, and Magnetic Properties of a Single-Ion Magnet “mer”-[Tris(N-[(imidazol-4-yl)-methylidene]-DL-phenylalaninato)terbium(III) and Related“fac”-DL-Alaninato Derivative. Inorg. Chem. 2014, 53, 5961–5971. [Google Scholar] [CrossRef] [PubMed]
- Hamada, D.; Fujinami, T.; Yamauchi, S.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatski, Y.; Watanabe, M.; Tsuchimoto, M.; Coletti, C.; et al. Luminescent DyIII single ion magnets with same N6O3 donor atoms but different donor atom arrangements, ‘fac’-[DyIII(HLDL-ala)3].8H2O and ‘mer’-[DyIII(HLDL-phe)3].7H2O. Polyhedron 2016, 109, 120–128. [Google Scholar] [CrossRef]
- Crutcher, T.; Larionov, V.A. Assymmetric Catalysts with Octahedral stereogenic-at-Metal Complexes Featuring chiral Ligands. Coord. Chem. Rev. 2018, 376, 95–113. [Google Scholar]
- Brewer, G.; Brewer, C.; Butcher, R.J.; Robichaux, G.T.; Viragh, C. Correlation of nitrogen chirality, R or S, with metal chelate chirality, Δ or Λ, in a series of reduced tripodal Schiff base complexes. A route to total spontaneous resolution. Inorg. Chim. Acta 2014, 410, 171–177. [Google Scholar] [CrossRef]
- Brewer, G.; (Department of Chemistry, The Catholic University of America, Washington, DC, USA); Brewer, C.; (Department of Chemistry, The Catholic University of America, Washington, DC, USA); Zavalij, P.; (Department of Chemistry, University of Maryland, College Park, MD, USA). Unpublished work. 2022.
- Brewer, G.; (Department of Chemistry, The Catholic University of America, Washington, DC, USA); Brewer, C.; (Department of Chemistry, The Catholic University of America, Washington, DC, USA); Zavalij, P.; (Department of Chemistry, University of Maryland, College Park, MD, USA). Unpublished work. 2023.
- Brewer, G.; (Department of Chemistry, The Catholic University of America, Washington, DC, USA); Brewer, C.; (Department of Chemistry, The Catholic University of America, Washington, DC, USA); Butcher, R.J.; (Department of Chemistry, Howard University, Washington, DC 20059, USA). Unpublished work. 2023.
- Felber, T.; Schafer, T.; Herrmann, H. Five-Membered Heterocycles as Potential Photosensitizers in the Tropospheric Aqueous Phase: Photophysical Properties of Imidazole-2-carboxaldehyde, 2-Furaldehyde, and 2-acetylfuran. J. Phys. Chem. A 2020, 124, 10029–10039. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Huang, M.; Lu, T.; Zhao, W.; Hu, C.; Gu, X.; Zhang, W. Characterization of Imidazole Compounds in Aqueous Secondary Organic Aerosol Generated from Evaporation of Droplets Containing Pyruvaldehyde and Inorganic Ammonium. Atmosphere 2022, 12, 970. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Phase annealing in SHELX-90: Direct methods for larger structures. Acta Crystallogr. 1990, A46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97: Program for Crystal Structure Refinement; ScienceOpen, Inc.: Boston, MA, USA, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97: FORTRAN Program for Crystal Structure Analysis; Göttingen University, Institute Fr Anorganisheche Chemie der Universitat: Göttingen, Germany, 1998. [Google Scholar]


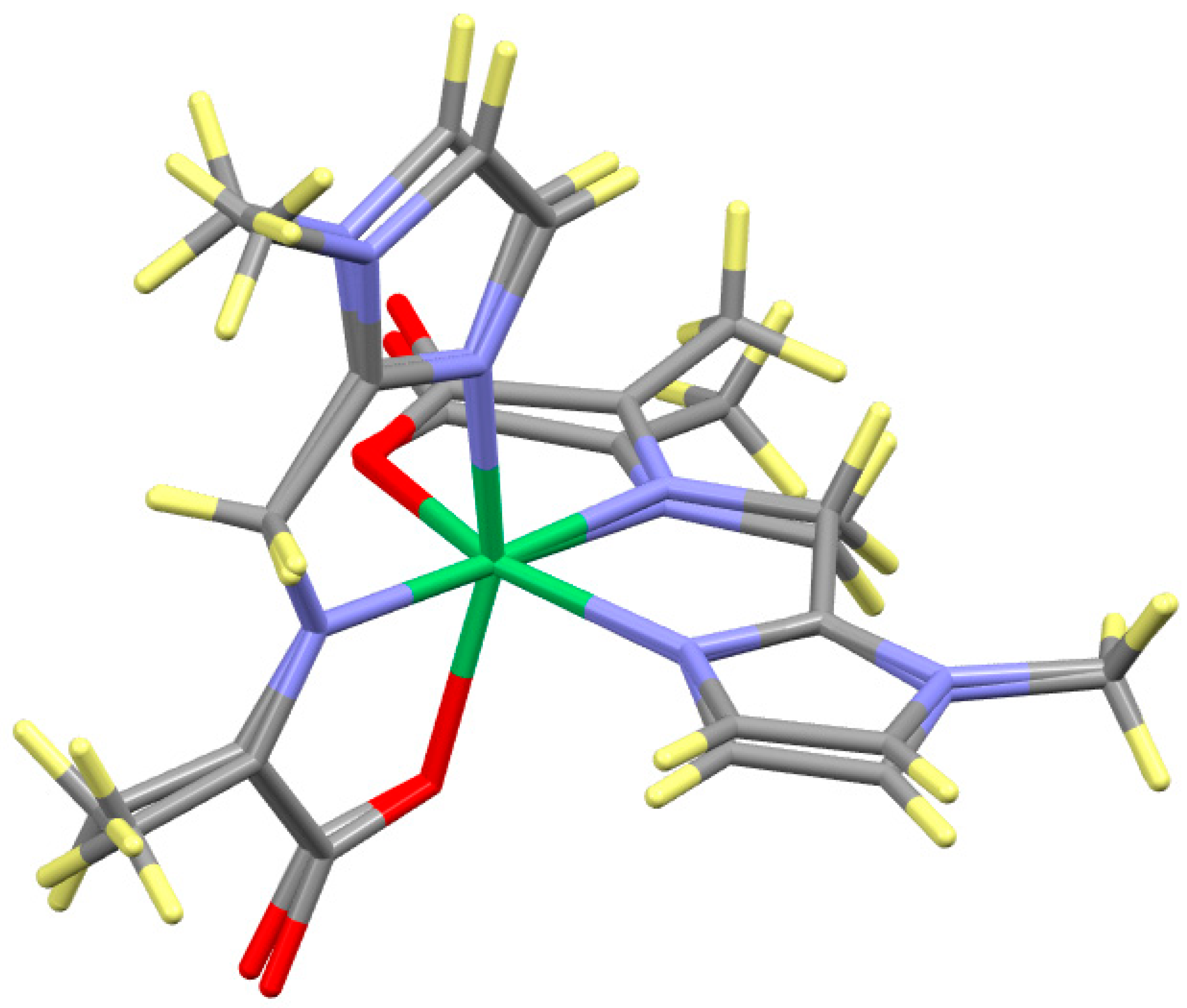


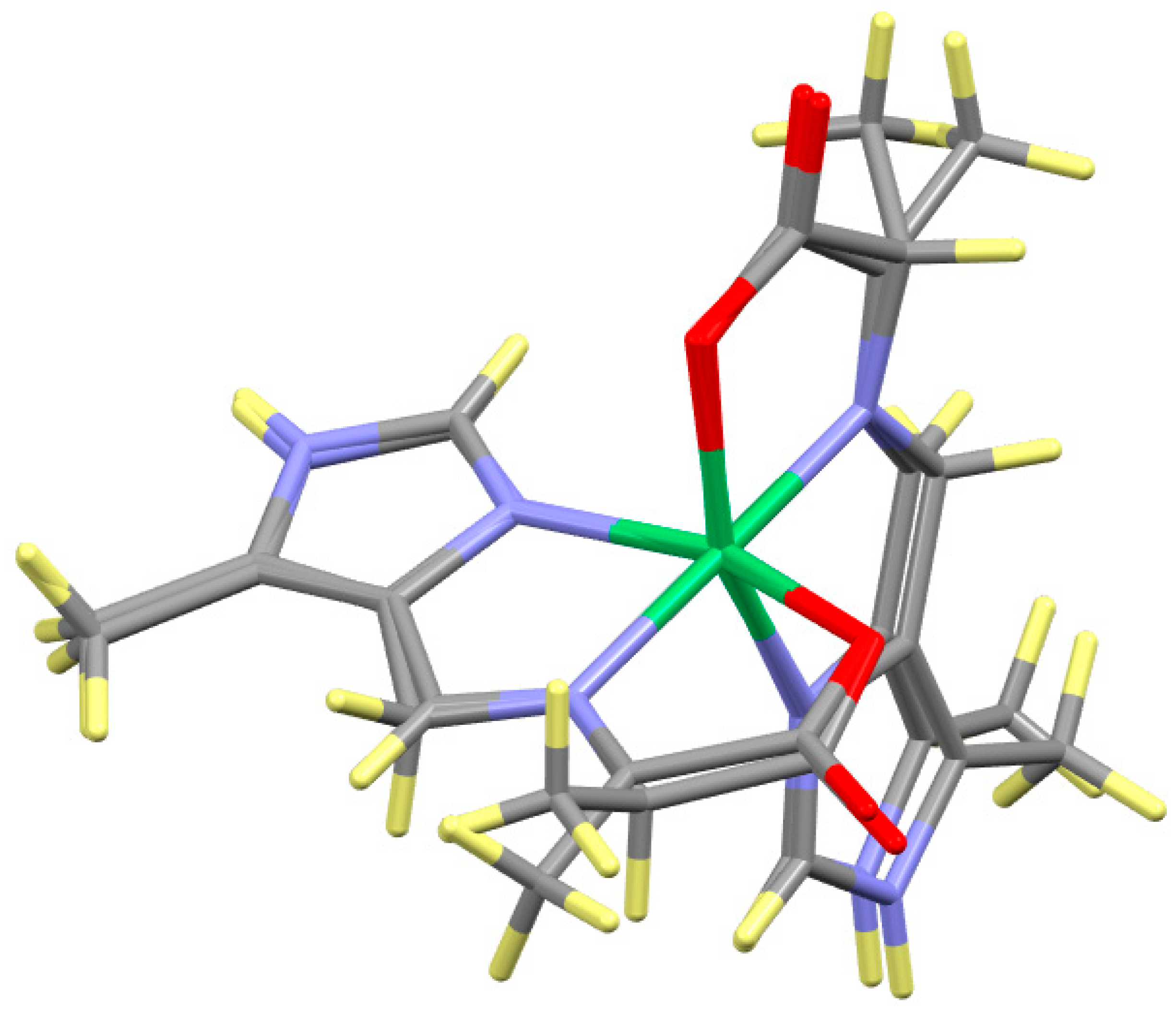

| Alanine, R = Me | |||||
|---|---|---|---|---|---|
| Distance | Aldimine | Ketimine | Angle | Aldimine | Ketimine |
| Cald-CIm | 1.451(6) 1.444(6) | 1.490(13) 1.497(3) | CIm–Cald–NAA | 116.1(4) 116.6(4) | 108.32(19) 107.87(17) |
| Cald-NAA | 1.263(6) 1.264(6) | 1.442(3) 1.451(3) | CIm–Cald–H | 121.9 121.7 | 110.0 110.1 |
| Cald-H | 0.9300 0.9300 | 0.9700 0.9700 | CIm–Cald–H’ | xxxxx | 110.0 110.1 |
| Cald-H’ | xxxxxx | 0.9700 0.9700 | NAA–Cald–H | 121.9 121.7 | 110.0 110.1 |
| NAA–Cald–H’ | xxxxx | 110.0 110.1 | |||
| H–Cald–H’ | xxxxx | 108.4 108.4 | |||
| NAA-Cald | 1.263(6) 1.264(6) | 1.442(3) 1.451(3) | Ni–NAA–Cald | 117.0(3) 117.1(3) | 118.26(15) 118.48(12) |
| NAA-Cα | 1.484(7) 1.456(5) | 1.278(3) 1.271(3) | Ni–NAA–Cα | 116.7(3) 116.5(3) | 117.63(15) 117.94(15) |
| NAA-Ni | 2.010(4) 2.012(4) | 2.0259(17) 2.0212(17) | Cα–NAA–Cald | 126.3(4) 125.7(4) | 124.05(19) 123.42(18) |
| Cα-NAA | 1.484(7) 1.456(5) | 1.278(3) 1.271(3) | NAA–Cα–Cβ | 110.5(6) 113.6(5) | 125.6(2) 126.0(2) |
| Cα-Cβ | 1.503(9) 1.476(8) | 1.463(4) 1.473(3) | NAA–Cα–CCA | 107.5(5) 108.7(4) | 113.88(19) 114.04(19) |
| Cα-CCA | 1.543(9) 1.536(7) | 1.538(3) 1.530(3) | NAA–Cα–H | 109.0 107.6 | xxxxx |
| Cα-H | 0.9800 0.9800 | xxxxxx | Cβ–Cα–CCA | 111.7(6) 111.5(5) | 120.6(2) 119.95(19) |
| Cβ–Cα–H | 109.0 107.6 | xxxxx | |||
| CCA–Cα–H | 109.0 107.6 | xxxxx | |||
| Complex | Amino Acid Used | CCDC Code or Deposition Number | SG | Chirality of Ni and Alpha C Atom(s) Found in Crystal | Chiral or Racemic |
|---|---|---|---|---|---|
| Ni(VAld-5Me4Im)2 | DL Valine | 2206541 | C2/c | ΔSS and ɅRR in equal quantity in crystal | Racemic |
| Ni(LAld-5Me4Im)2 | DL leucine | 2323329 | C2/c | ΔSS and ɅRR in equal quantity in crystal | Racemic |
| Ni(FAld-5Me4Im)2 | DL Phenylalanine | OFIJOE ZEXHUJ | P21/c | ΔSS and ɅRR in equal quantity in crystal | Racemic |
| Ni(FAld-5Me4Im)2 | L Phenylalanine | OFIJIY | P21 | ΔSS only | Chiral in bulk due to the source of AA |
| Ni(FAld-4Im)2 | DL Phenylalanine | 986005 | P41212 | ɅRR only in crystal | Homochiral in crystal, separation achieved at the level of crystal. Bulk racemic |
| Ni(VAld-2Im)2 | DL valine | 961029 | P41212 | ɅRR only in crystal | Homochiral in the crystal, separation achieved at the level of crystal. Bulk racemic |
| Ni(VAld-2Im)2 | DL Valine | 961030 | P43212 | ΔSS only in crystal | Homochiral in the crystal, separation achieved at the level of crystal. Bulk racemic |
| Ni(LKet–Nme2Im) (LAld-NMe2Im) | DL Leucine | NISXOG 967169 | P-1 | ΔS and ɅR in equal quantity in crystal | Racemic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brewer, G.; Brewer, C.; Butcher, R.J.; Zavalij, P. Selective Generation of Aldimine and Ketimine Tautomers of the Schiff Base Condensates of Amino Acids with Imidazole Aldehydes or of Imidazole Methanamines with Pyruvates—Isomeric Control with 2- vs. 4-Substituted Imidazoles. Molecules 2024, 29, 1324. https://doi.org/10.3390/molecules29061324
Brewer G, Brewer C, Butcher RJ, Zavalij P. Selective Generation of Aldimine and Ketimine Tautomers of the Schiff Base Condensates of Amino Acids with Imidazole Aldehydes or of Imidazole Methanamines with Pyruvates—Isomeric Control with 2- vs. 4-Substituted Imidazoles. Molecules. 2024; 29(6):1324. https://doi.org/10.3390/molecules29061324
Chicago/Turabian StyleBrewer, Greg, Cynthia Brewer, Raymond J. Butcher, and Peter Zavalij. 2024. "Selective Generation of Aldimine and Ketimine Tautomers of the Schiff Base Condensates of Amino Acids with Imidazole Aldehydes or of Imidazole Methanamines with Pyruvates—Isomeric Control with 2- vs. 4-Substituted Imidazoles" Molecules 29, no. 6: 1324. https://doi.org/10.3390/molecules29061324
APA StyleBrewer, G., Brewer, C., Butcher, R. J., & Zavalij, P. (2024). Selective Generation of Aldimine and Ketimine Tautomers of the Schiff Base Condensates of Amino Acids with Imidazole Aldehydes or of Imidazole Methanamines with Pyruvates—Isomeric Control with 2- vs. 4-Substituted Imidazoles. Molecules, 29(6), 1324. https://doi.org/10.3390/molecules29061324








