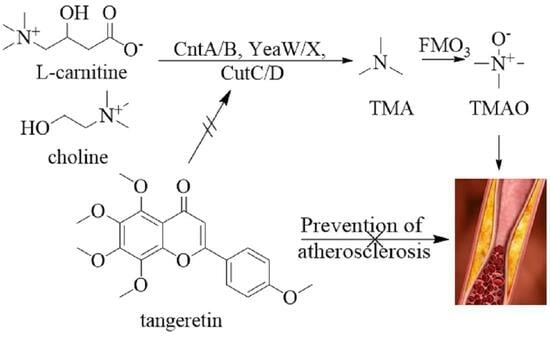
Tangeretin Mitigates Trimethylamine Oxide Induced Arterial Inflammation by Disrupting Choline–Trimethylamine Conversion through Specific Manipulation of Intestinal Microflora
Abstract
1. Introduction
2. Results and Discussion
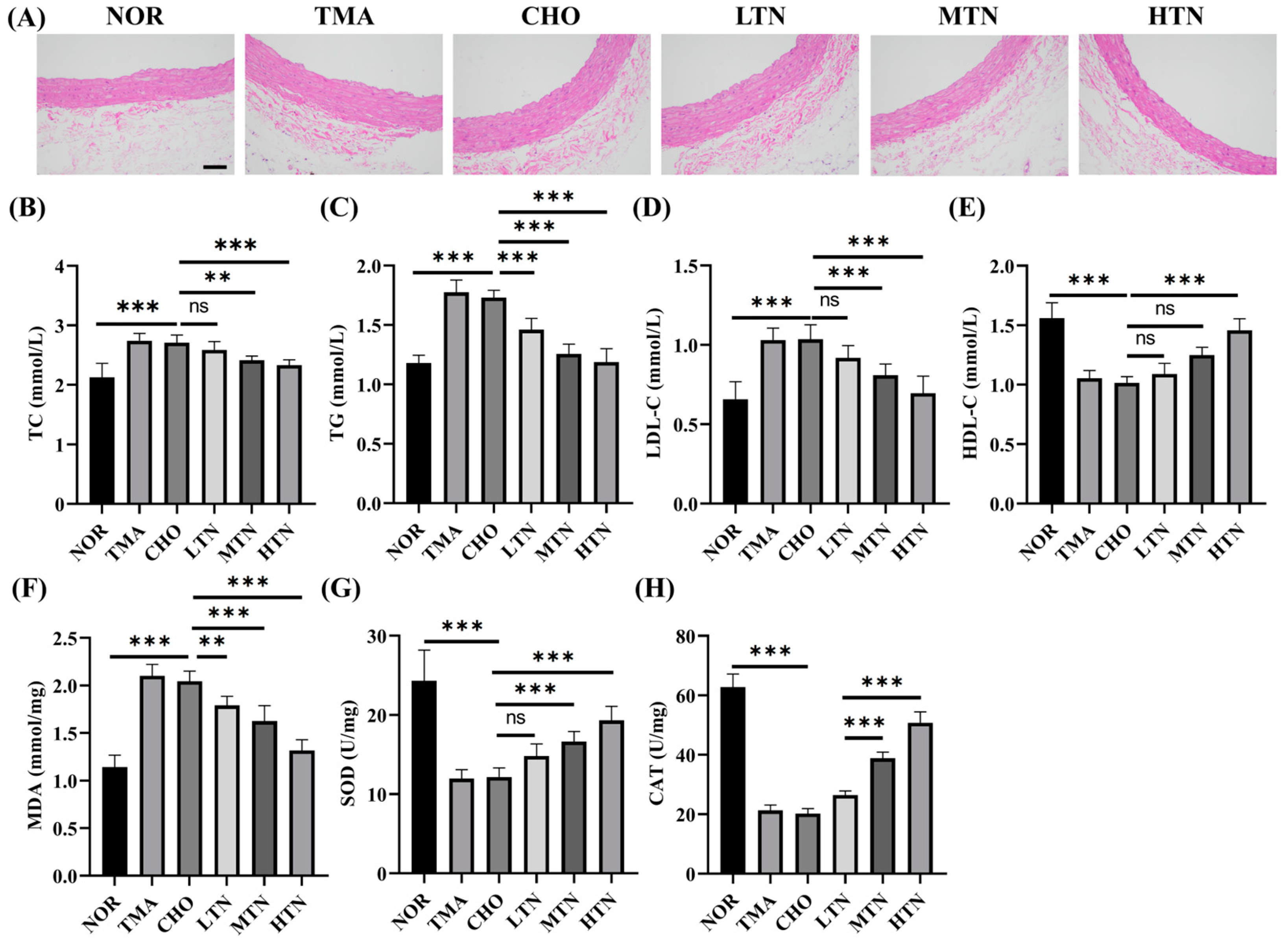
2.1. Tangeretin Effectively Counteracted the Negative Effects of Choline Chloride-Induced Inflammation
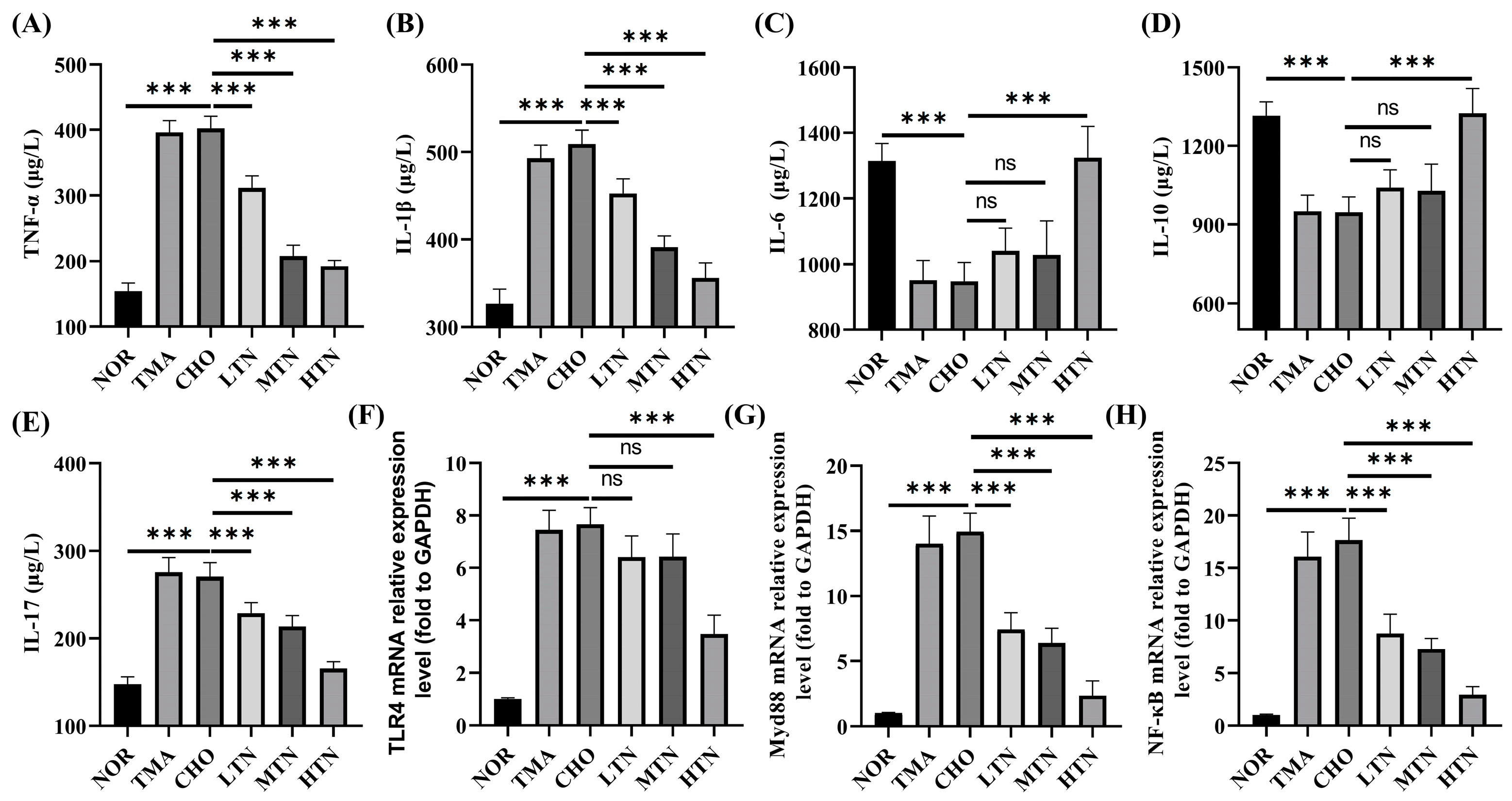
2.2. Tangeretin Suppressed Choline Chloride-Stimulated Inflammation
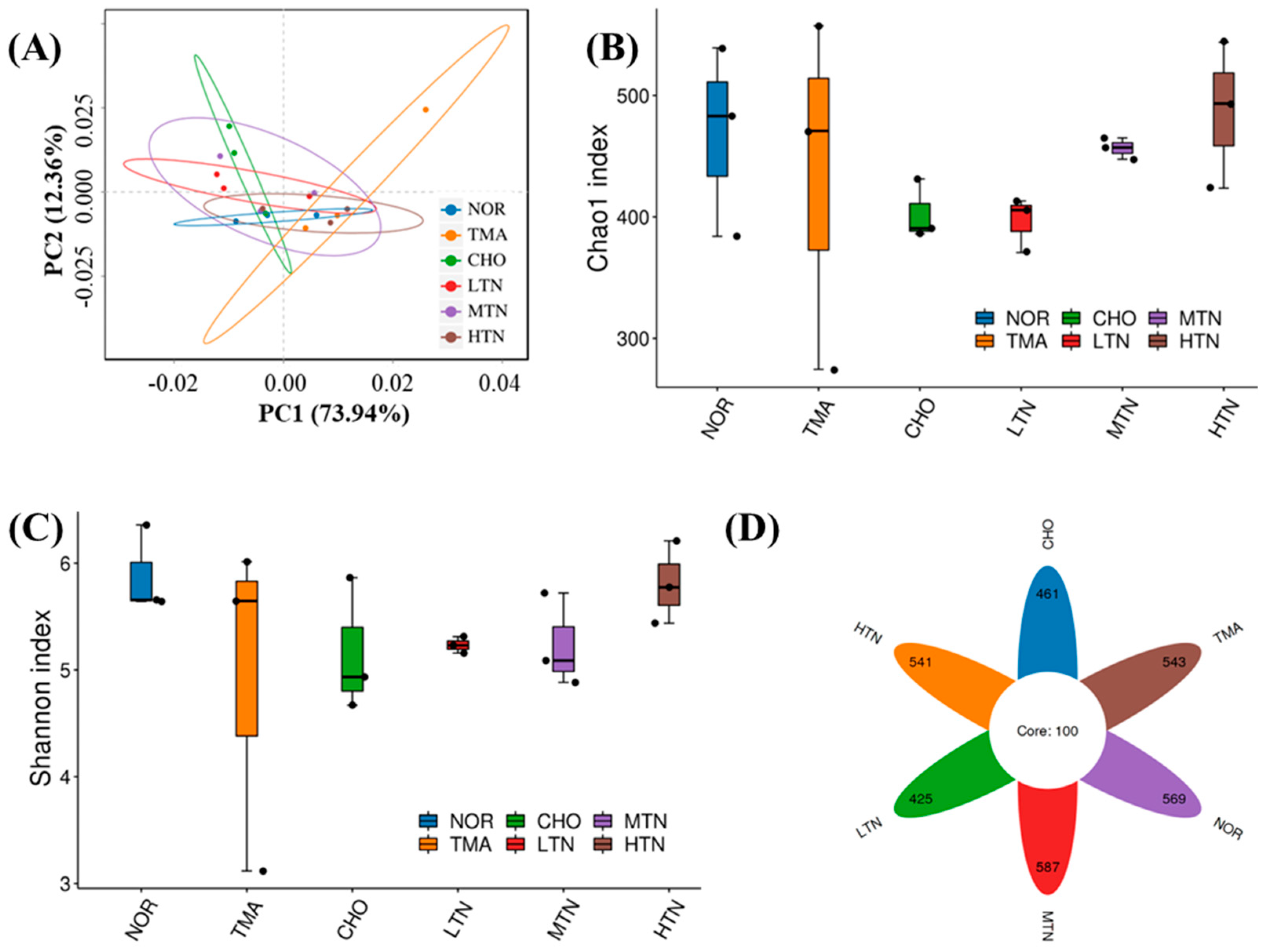
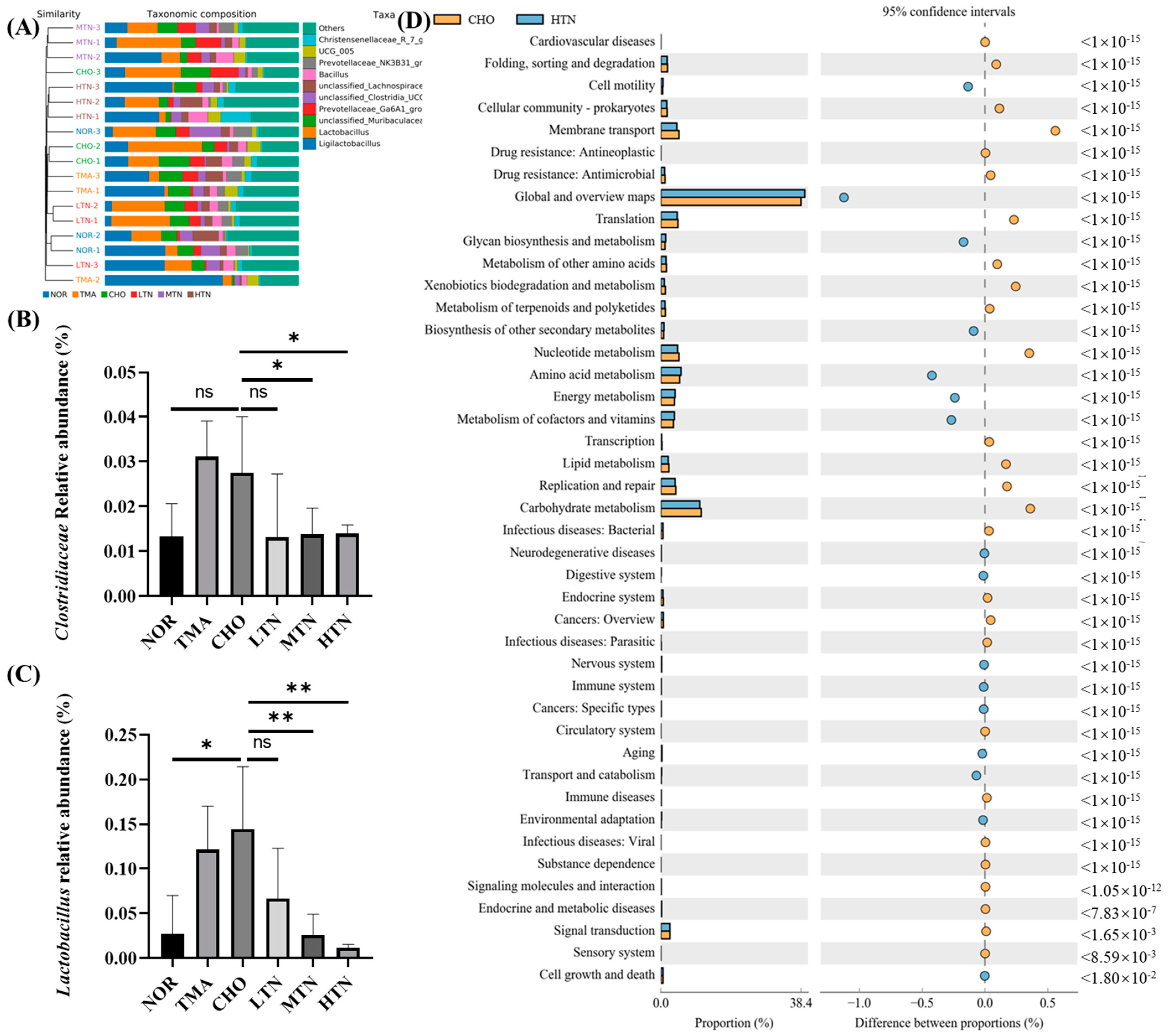
2.3. Tangeretin Regulated the Diversity of Intestinal Flora
2.4. Tangeretin Modulated the Overall Structure and Composition of Gut Microbiota
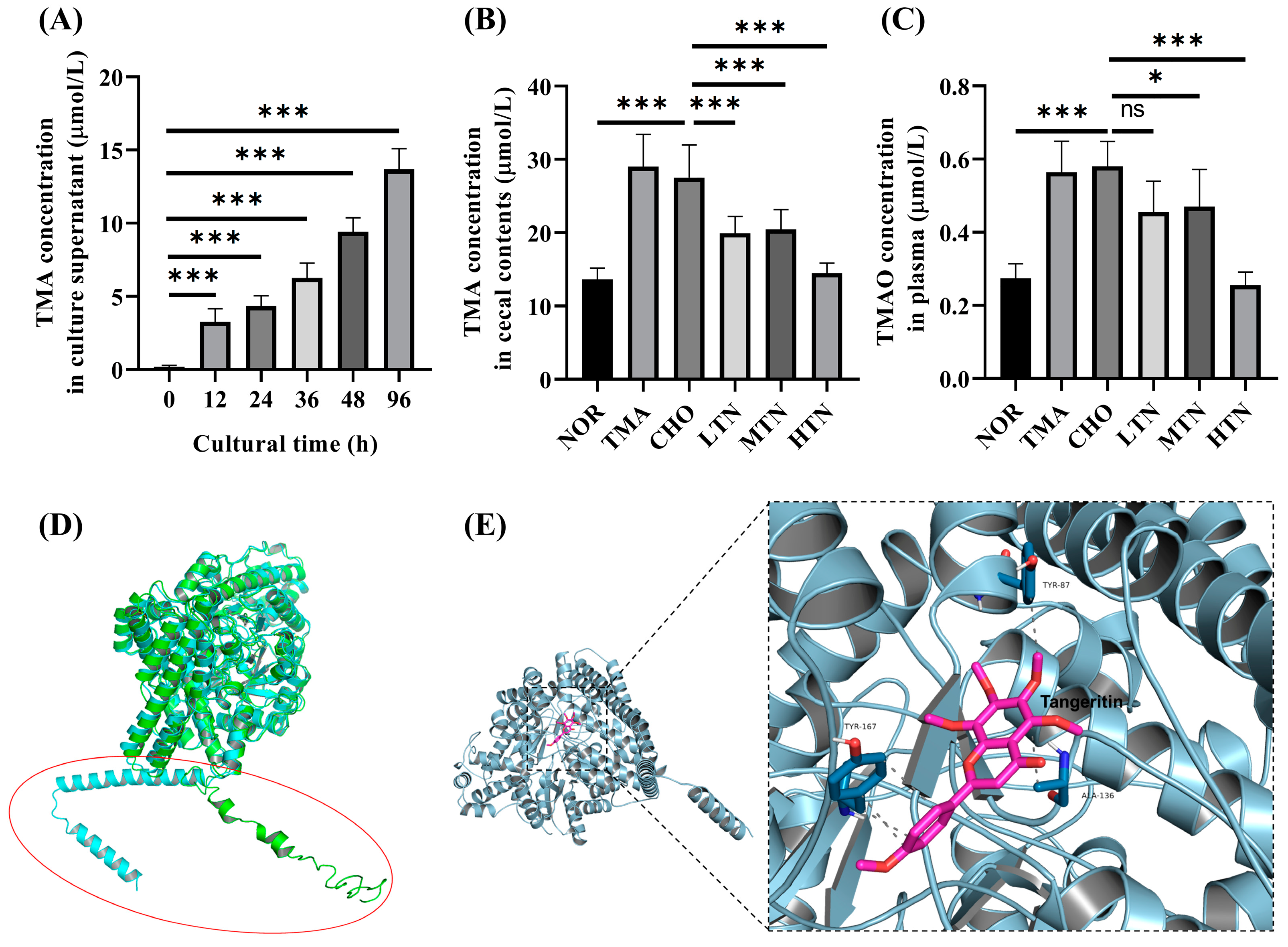
2.5. Tangeretin Inhibited the Conversion of Choline Chloride to TMA via CutC
3. Materials and Methods
3.1. Materials and Reagents
3.2. Experimental Animal Model
3.3. Histomorphological Staining
3.4. Immunohistochemical Detection
3.5. RNA Isolation and Quantitative RT-qPCR
3.6. Cytokine Analysis
3.7. Intestinal Microbiota Sequencing Procedure
3.8. Bacterial Species and Habitats
3.9. Molecular Docking
3.10. Measurement Quantification of TMA and TMAO
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Brennan, L.; Castro, S.; Brownson, R.C.; Claus, J.; Orleans, C.T. Accelerating evidence reviews and broadening evidence standards to identify effective, promising, and emerging policy and environmental strategies for prevention of childhood obesity. Annu. Rev. Public Health 2011, 32, 199–223. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lin, C.C.; Yang, Y.; Yuan, L.; Wang, P.; Wen, X.; Pan, M.H.; Zhao, H.; Ho, C.T.; Li, S. Nobiletin prevents trimethylamine oxide-induced vascular inflammation via inhibition of the NF-κB/MAPK pathways. J. Agric. Food Chem. 2019, 67, 6169–6176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Y.; Huang, F.Q.; Lao, X.; Lu, Y.; Gao, X.; Alolga, R.N.; Yin, K.; Zhou, X.; Wang, Y.; Liu, B.; et al. Integrated metagenomics identifies a crucial role for trimethylamine-producing Lachnoclostridium in promoting atherosclerosis. NPJ Biofilms Microbiomes 2022, 8, 11. [Google Scholar] [CrossRef]
- Jonsson, A.L.; Bäckhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 79–87. [Google Scholar] [CrossRef]
- Roberts, A.B.; Gu, X.; Buffa, J.A.; Hurd, A.G.; Wang, Z.; Zhu, W.; Gupta, N.; Skye, S.M.; Cody, D.B.; Levison, B.S.; et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat. Med. 2018, 24, 1407–1417. [Google Scholar] [CrossRef]
- Yang, G.; Xia, X.; Zhong, H.; Shen, J.; Li, S. Protective Effect of Tangeretin and 5-Hydroxy-6,7,8,3’,4’-Pentamethoxyflavone on Collagen-Induced Arthritis by Inhibiting Autophagy via Activation of the ROS-AKT/mTOR Signaling Pathway. J. Agric. Food Chem. 2021, 69, 259–266. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Zhu, J.; Zhao, D.G.; Ma, Y.Y.; Li, D.; Ho, C.T.; Huang, Q. Bidirectional interaction of nobiletin and gut microbiota in mice fed with a high-fat diet. Food Funct. 2021, 12, 3516–3526. [Google Scholar] [CrossRef]
- Alsaif, M.A.; Khan, L.A.; Alhamdan, A.A.; Alorf, S.; Al-Othman, A.M.; Alawami, S. Effects of dietary flavonoids intake in saudi patients with coronary heart disease. J. Family Community Med. 2007, 14, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Gómez, O.; García-Rodríguez, R.V.; Pérez-Gutierrez, S.; Rivero-Ramírez, N.L.; García-Martínez, Y.; Pablo-Pérez, S.S.; Pérez-Pastén-Borja, R.; Cristóbal-Luna, J.M.; Chamorro-Cevallos, G. Protective effect of the phycobiliproteins from arthrospira maxima on indomethacin-induced gastric ulcer in a rat model. Plants 2023, 12, 1586. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, N.; Poetzl, C.; von den Driesch, P.; Wladykowski, E.; Moll, I.; Behne, M.J.; Brandner, J.M. Alteration of tight junction proteins is an early event in psoriasis: Putative involvement of proinflammatory cytokines. Am. J. Pathol. 2009, 175, 1095–1106. [Google Scholar] [CrossRef]
- You, G.; Zheng, L.; Zhang, Y.; Zhang, Y.; Wang, Y.; Guo, W.; Liu, H.; Tatiana, P.; Vladimir, K.; Zan, J. Tangeretin Attenuates Cerebral Ischemia-Reperfusion-Induced Neuronal Pyroptosis by Inhibiting AIM2 Inflammasome Activation via Regulating NRF2. Inflammation 2024, 47, 145–158. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; You, G.; Zheng, D.; He, Z.; Guo, W.; Antonina, K.; Shukhrat, Z.; Ding, B.; Zan, J.; et al. Tangeretin attenuates acute lung injury in septic mice by inhibiting ROS-mediated NLRP3 inflammasome activation via regulating PLK1/AMPK/DRP1 signaling axis. Inflamm Res 2024, 73, 47–63. [Google Scholar] [CrossRef]
- Li, J.; Wei, Q.; Song, K.; Wang, Y.; Yang, Y.; Li, M.; Yu, J.; Su, G.; Peng, L.; Fu, B.; et al. Tangeretin attenuates bleomycin-induced pulmonary fibrosis by inhibiting epithelial-mesenchymal transition via the PI3K/Akt pathway. Front. Pharmacol. 2023, 14, 1247800. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yang, X.; Zhan, M.; Chen, Y.; Xu, J.; Xiao, J.; Xiao, H.; Song, M. Dietary tangeretin improved antibiotic-associated diarrhea in mice by enhancing the intestinal barrier function, regulating the gut microbiota, and metabolic homeostasis. Food Funct. 2023, 14, 10731–10746. [Google Scholar] [CrossRef]
- Ripp, S.L.; Itagaki, K.; Philpot, R.M.; Elfarra, A.A. Species and sex differences in expression of flavin-containing monooxygenase form 3 in liver and kidney microsomes. Drug Metab. Dispos. 1999, 27, 46–52. [Google Scholar]
- Janmohamed, A.; Hernandez, D.; Phillips, I.R.; Shephard, E.A. Cell-, tissue-, sex- and developmental stage-specific expression of mouse flavin-containing monooxygenases (Fmos). Biochem. Pharmacol. 2004, 68, 73–83. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Sniffen, S.; McGill Percy, K.C.; Pallaval, V.B.; Chidipi, B. Gut Dysbiosis and Immune System in Atherosclerotic Cardiovascular Disease (ACVD). Microorganisms 2022, 10, 108. [Google Scholar] [CrossRef]
- He, S.; He, X.; Pan, S.; Jiang, W. Exploring the mechanism of chuanxiong rhizoma against thrombosis based on network pharmacology, molecular docking and experimental verification. Molecules 2023, 28, 6702. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef]
- Huson, D.H.; Mitra, S.; Ruscheweyh, H.J.; Weber, N.; Schuster, S.C. Integrative analysis of environmental sequences using MEGAN4. Genome Res. 2011, 21, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Asnicar, F.; Weingart, G.; Tickle, T.L.; Huttenhower, C.; Segata, N. Compact graphical representation of phylogenetic data and metadata with GraPhlAn. PeerJ 2015, 3, e1029. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, B.; Wallace, K.; Vasu, C.; Alekseyenko, A.V. W(∗)(d)-test: Robust distance-based multivariate analysis of variance. Microbiome 2019, 7, 51. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Essenmacher, L.A.; Racine, K.C.; Neilson, A.P. Development of a high-throughput method to study the inhibitory effect of phytochemicals on trimethylamine formation. Nutrients 2021, 13, 1466. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Racine, K.C.; Neilson, A.P. Phenolic-rich beverages reduce bacterial TMA formation in an ex vivo-in vitro colonic fermentation model. Food Funct. 2022, 13, 8022–8037. [Google Scholar] [CrossRef]
- Ocque, A.J.; Stubbs, J.R.; Nolin, T.D. Development and validation of a simple UHPLC-MS/MS method for the simultaneous determination of trimethylamine N-oxide, choline, and betaine in human plasma and urine. J. Pharm. Biomed. Anal. 2015, 109, 128–135. [Google Scholar] [CrossRef]

| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| TLR4 | GAGGACTGGGTGAGAAACGA | GCAATGGCTACACCAGGAAT |
| MyD88 | TGTGTGTTTCCTTTGGGACA | TGCCACTACCTCATGCAAAG |
| NF-κB | GATGCAGTTAATGCCCCACT | TGCTGCTGGTGATTCTCTTG |
| GAPDH | ATGACTCTACCCACGGCAAG | GATCTCGCTCCTGGAAGATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Leng, C.; Lin, K.; Li, Y.; Zhou, M.; Zhou, M.; Shu, X.; Liu, W. Tangeretin Mitigates Trimethylamine Oxide Induced Arterial Inflammation by Disrupting Choline–Trimethylamine Conversion through Specific Manipulation of Intestinal Microflora. Molecules 2024, 29, 1323. https://doi.org/10.3390/molecules29061323
Cao Y, Leng C, Lin K, Li Y, Zhou M, Zhou M, Shu X, Liu W. Tangeretin Mitigates Trimethylamine Oxide Induced Arterial Inflammation by Disrupting Choline–Trimethylamine Conversion through Specific Manipulation of Intestinal Microflora. Molecules. 2024; 29(6):1323. https://doi.org/10.3390/molecules29061323
Chicago/Turabian StyleCao, Yu, Changlong Leng, Kuan Lin, Youwei Li, Meiling Zhou, Mei Zhou, Xiji Shu, and Wei Liu. 2024. "Tangeretin Mitigates Trimethylamine Oxide Induced Arterial Inflammation by Disrupting Choline–Trimethylamine Conversion through Specific Manipulation of Intestinal Microflora" Molecules 29, no. 6: 1323. https://doi.org/10.3390/molecules29061323
APA StyleCao, Y., Leng, C., Lin, K., Li, Y., Zhou, M., Zhou, M., Shu, X., & Liu, W. (2024). Tangeretin Mitigates Trimethylamine Oxide Induced Arterial Inflammation by Disrupting Choline–Trimethylamine Conversion through Specific Manipulation of Intestinal Microflora. Molecules, 29(6), 1323. https://doi.org/10.3390/molecules29061323