Biocatalytic Synthesis of Coumarin S-Glycosides: Towards Non-Cytotoxic Probes for Biomedical Imaging and Sensing
Abstract
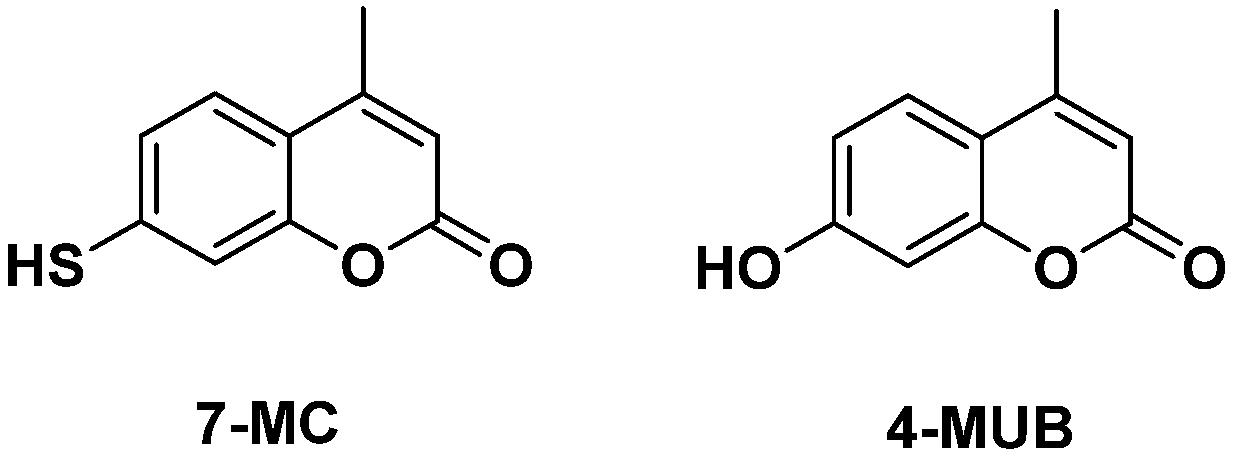
1. Introduction
2. Results and Discussion
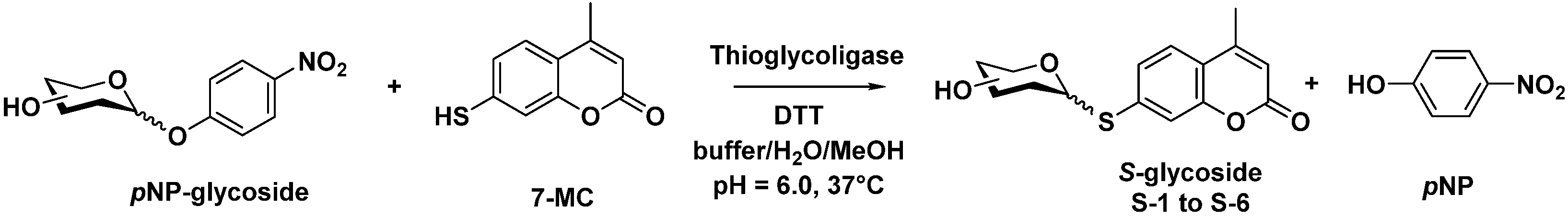
2.1. Biocatalyzed Synthesis of S-Glycosidic Coumarins
2.2. Cytotoxic Activity
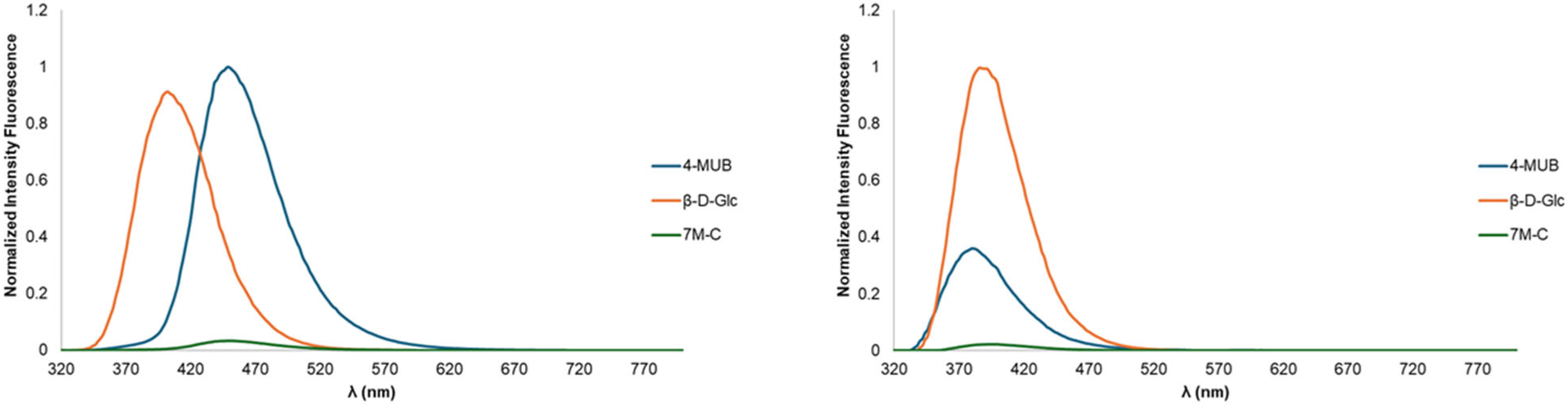
2.3. Optical Spectroscopy
| Compound | Absorbance | Fluorescence | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λmax abs (nm) | ε (103 M−1 cm−1) | λmax ex (nm) | λmax em (nm) | Φ | ||||||
| DMF | PBS | DMF | PBS | DMF | PBS | DMF | PBS | DMF | PBS | |
| 4-MUB | 322 | 324 | 17.0 | 20.2 | 320 | 333 | 380 | 448 | 0.081 a | 0.293 |
| 7-MC b | 328 | 368 | 10.4 | 28.9 | 334 | 321 | 392 | 448 | 0.009 | 0.020 |
| S-1 | 330 | 326 | 26.7 | 23.3 | 332 | 332 | 390 | 402 | 0.182 | 0.235 |
| S-2 | 330 | 324 | 19.5 | 26.0 | 332 | 326 | 386 | 402 | 0.186 | 0.181 |
| S-3 | 330 | 326 | 18.6 | 20.8 | 332 | 332 | 386 | 402 | 0.198 | 0.242 |
| S-4 | 330 | 326 | 22.3 | 18.4 | 334 | 332 | 388 | 402 | 0.223 | 0.271 |
| S-5 | 330 | 326 | 22.3 | 10.4 | 334 | 326 | 386 | 402 | 0.183 | 0.228 |
| S-6 | 330 | 326 | 22.3 | 19.8 | 334 | 332 | 388 | 416 | 0.223 | 0.185 |
3. Materials and Methods
3.1. Chemical Synthesis
3.2. Enzymatic Thioglycosylation
3.3. Protein Expression and Production
3.4. Cellular Assays
3.5. Optical Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Zhang, Y.; Lu, Q.; Xing, D.; Zhang, R. Exploring Carbohydrates for Therapeutics: A Review on Future Directions. Front. Pharmacol. 2021, 12, 756724. [Google Scholar] [CrossRef]
- Jiang, H.; Qin, X.; Wang, Q.; Xu, Q.; Wang, J.; Wu, Y.; Chen, W.; Wang, C.; Zhang, T.; Xing, D.; et al. Application of Carbohydrates in Approved Small Molecule Drugs: A Review. Eur. J. Med. Chem. 2021, 223, 113633. [Google Scholar] [CrossRef]
- Fernández-Tejada, A.; Cañada, F.J.; Jiménez-Barbero, J. Recent Developments in Synthetic Carbohydrate-Based Diagnostics, Vaccines, and Therapeutics. Chem. Eur. J. 2015, 21, 10616–10628. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, L.; Assaf, Z.; Pistorio, S.G.; Lafite, P.; Demchenko, A.V.; Daniellou, R. Hydrolysis of Glycosyl Thioimidates by Glycoside Hydrolase Requires Remote Activation for Efficient Activity. Catalysts 2019, 9, 826. [Google Scholar] [CrossRef]
- Driguez, H. Thiooligosaccharides as Tools for Structural Biology. ChemBioChem 2001, 2, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.R.; Bundle, D.R. S-Linked Ganglioside Analogues for Use in Conjugate Vaccines. Org. Lett. 2004, 6, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-F.; Yan, M.-C.; Chang, T.-C.; Lin, C.-C. Synthesis of S-Linked α(2→9) Octasialic Acid via Exclusive α S-Glycosidic Bond Formation. J. Am. Chem. Soc. 2009, 131, 3138–3139. [Google Scholar] [CrossRef] [PubMed]
- Wardrop, D.J.; Waidyarachchi, S.L. Synthesis and Biological Activity of Naturally Occurring α-Glucosidase Inhibitors. Nat. Prod. Rep. 2010, 27, 1431–1468. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.R.; Kartha, K.P.R. Solvent-Free Synthesis of Thioglycosides by Ball Milling. Green Chem. 2009, 11, 953–956. [Google Scholar] [CrossRef]
- Escopy, S.; Singh, Y.; Demchenko, A.V. Triflic Acid-Mediated Synthesis of Thioglycosides. Org. Biomol. Chem. 2019, 17, 8379–8383. [Google Scholar] [CrossRef]
- Brachet, E.; Brion, J.-D.; Alami, M.; Messaoudi, S. Nickel-Catalyzed Arylation, Alkenylation, and Alkynylation of Unprotected Thioglycosides at Room Temperature. Chem. Eur. J. 2013, 19, 15276–15280. [Google Scholar] [CrossRef]
- Bruneau, A.; Roche, M.; Hamze, A.; Brion, J.-D.; Alami, M.; Messaoudi, S. Stereoretentive Palladium-Catalyzed Arylation, Alkenylation, and Alkynylation of 1-Thiosugars and Thiols Using Aminobiphenyl Palladacycle Precatalyst at Room Temperature. Chem. Eur. J. 2015, 21, 8375–8379. [Google Scholar] [CrossRef]
- Ibrahim, N.; Alami, M.; Messaoudi, S. Recent Advances in Transition-Metal-Catalyzed Functionalization of 1-Thiosugars. Asian J. Org. Chem. 2018, 7, 2026–2038. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Jahn, M.; Marles, J.; Warren, R.A.J.; Withers, S.G. Thioglycoligases: Mutant Glycosidases for Thioglycoside Synthesis. Angew. Chem. Int. Ed. 2003, 42, 352–354. [Google Scholar] [CrossRef]
- Bretagne, D.; Pâris, A.; de Vaumas, R.; Lafite, P.; Daniellou, R. Crystal Structure of Dictyoglomus thermophilum β-d-Xylosidase DtXyl Unravels the Structural Determinants for Efficient Notoginsenoside R1 Hydrolysis. Biochimie 2021, 181, 34–41. [Google Scholar] [CrossRef]
- Kurdziel, M.; Kopeć, M.; Pâris, A.; Lewiński, K.; Lafite, P.; Daniellou, R. Thioglycoligation of Aromatic Thiols Using a Natural Glucuronide Donor. Org. Biomol. Chem. 2020, 18, 5582–5585. [Google Scholar] [CrossRef]
- Peyrot, C.; Didak, B.; Guillotin, L.; Landemarre, L.; Lafite, P.; Lemiègre, L.; Daniellou, R. Enzymatic Synthesis of a Series of Thioglycosides: Analogs of Arbutin with Efficient Antipigmentation Properties. Eur. J. Org. Chem. 2021, 2021, 3812–3818. [Google Scholar] [CrossRef]
- Chiu, H.-H.; Shen, M.-Y.; Liu, Y.-T.; Fu, Y.-L.; Chiu, Y.-A.; Chen, Y.-H.; Huang, C.; Li, Y.-K. Diversity of Sugar Acceptor of Glycosyltransferase 1 from Bacillus cereus and Its Application for Glucoside Synthesis. Appl. Microbiol. Biotechnol. 2016, 100, 4459–4471. [Google Scholar] [CrossRef] [PubMed]
- Rodrigue, J.; Ganne, G.; Blanchard, B.; Saucier, C.; Giguère, D.; Shiao, T.C.; Varrot, A.; Imberty, A.; Roy, R. Aromatic Thioglycoside Inhibitors against the Virulence Factor LecA from Pseudomonas Aeruginosa. Org. Biomol. Chem. 2013, 11, 6906–6918. [Google Scholar] [CrossRef] [PubMed]
- El-Sawy, E.R.; Abdelwahab, A.B.; Kirsch, G. Insight on Mercapto-Coumarins: Synthesis and Reactivity. Molecules 2022, 27, 2150. [Google Scholar] [CrossRef]
- Ibis, C.; Sahinler Ayla, S.; Bahar, H.; Stasevych, M.; Komarovska-Porokhnyavets, O.; Novikov, V. Synthesis, Characterization, and Biological Properties of Novel Piperidinolyl-, Piperidinyl-, and Piperazinyl-Substituted Naphthoquinone Compounds and Their Reactions With Some Thiols. Phosphorus Sulfur Silicon Relat. Elem. 2014, 190, 1422–1433. [Google Scholar] [CrossRef]
- Ibis, C.; Ayla, S.S.; Ozkok, F.; Bahar, H. Synthesis of New Piperazinyl and Piperidinolyl Substituted P-Chloranil Derivatives and Their Reactions with Thiols. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 2273–2282. [Google Scholar] [CrossRef]
- Feng, C.-H.; Lu, C.-Y. A New Matrix for Analyzing Low Molecular Mass Compounds and Its Application for Determination of Carcinogenic Areca Alkaloids by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry. Anal. Chim. Acta 2009, 649, 230–235. [Google Scholar] [CrossRef]
- Koktan, J.; Královec, K.; Havelek, R.; Kuličková, J.; Řezanka, P.; Kaman, O. Magnetic Oxide Particles with Gold Nanoshells: Synthesis, Properties and Cytotoxic Effects. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 922–932. [Google Scholar] [CrossRef]
- González-Béjar, M.; Frenette, M.; Jorge, L.; Scaiano, J.C. 7-Mercapto-4-Methylcoumarin as a Reporter of Thiol Binding to the CdSe Quantum Dot Surface. Chem. Commun. 2009, 22, 3202–3204. [Google Scholar] [CrossRef]
- Taglietti, A.; Diaz Fernandez, Y.A.; Galinetto, P.; Grisoli, P.; Milanese, C.; Pallavicini, P. Mixing Thiols on the Surface of Silver Nanoparticles: Preserving Antibacterial Properties While Introducing SERS Activity. J. Nanoparticle Res. 2013, 15, 2047. [Google Scholar] [CrossRef]
- Kamil Reza, K.; Wang, J.; Vaidyanathan, R.; Dey, S.; Wang, Y.; Trau, M. Electrohydrodynamic-Induced SERS Immunoassay for Extensive Multiplexed Biomarker Sensing. Small 2017, 13, 1602902. [Google Scholar] [CrossRef] [PubMed]
- Bassi, B.; Taglietti, A.; Galinetto, P.; Marchesi, N.; Pascale, A.; Cabrini, E.; Pallavicini, P.; Dacarro, G. Tunable Coating of Gold Nanostars: Tailoring Robust SERS Labels for Cell Imaging. Nanotechnology 2016, 27, 265302. [Google Scholar] [CrossRef]
- Bard, A.; Rondon, R.; Marquez, D.T.; Lanterna, A.E.; Scaiano, J.C. How Fast Can Thiols Bind to the Gold Nanoparticle Surface? Photochem. Photobiol. 2018, 94, 1109–1115. [Google Scholar] [CrossRef]
- Reddy, K.R.; El-Zein, A.; Airey, D.W.; Alonso-Marroquin, F.; Schubel, P.; Manalo, A. Self-Healing Polymers: Synthesis Methods and Applications. Nano-Struct. Nano-Objects 2020, 23, 100500. [Google Scholar] [CrossRef]
- Gulyuz, S.; Bayram, D.; Ozkose, U.U.; Bolat, Z.B.; Kocak, P.; Saka, O.M.; Devrim, B.; Parlak Khalily, M.; Telci, D.; Sahin, F.; et al. Synthesis, Biocompatibility and Gene Encapsulation of Poly(2-Ethyl 2-Oxazoline)-Dioleoyl Phosphatidylethanolamine (PEtOx-DOPE) and Post-Modifications with Peptides and Fluorescent Dye Coumarin. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 981–993. [Google Scholar] [CrossRef]
- Gospodova, T.; Rashkova, J.; Ivanova, D.; Viteva, L.; Duprat, C.; Mazières, M.-R.; Bakalova, S.; Kaneti, J. Synthetic Potentials of Heptamethine Merocyanine Dyes Containing an Active Chlorine Atom: Reactivity towards Nucleophiles. Eur. J. Org. Chem. 2009, 2009, 5063–5071. [Google Scholar] [CrossRef]
- Cara, E.; Mandrile, L.; Sacco, A.; Giovannozzi, A.M.; Rossi, A.M.; Celegato, F.; Leo, N.D.; Hönicke, P.; Kayser, Y.; Beckhoff, B.; et al. Towards a Traceable Enhancement Factor in Surface-Enhanced Raman Spectroscopy. J. Mater. Chem. C 2020, 8, 16513–16519. [Google Scholar] [CrossRef]
- Galardon, E.; Tomas, A.; Roussel, P.; Artaud, I. New Fluorescent Zinc Complexes: Towards Specific Sensors for Hydrogen Sulfide in Solution. Dalton Trans. 2009, 42, 9126–9130. [Google Scholar] [CrossRef] [PubMed]
- Varandas, P.A.M.M.; Cobb, A.J.A.; Segundo, M.A.; Silva, E.M.P. Emergent Glycerophospholipid Fluorescent Probes: Synthesis and Applications. Bioconjugate Chem. 2020, 31, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Wang, H.; Zhang, B.; Yao, J.; Li, X.; Feng, W.; Zhou, P.; Wang, Y.; Fang, J. A Thiol–Thiosulfonate Reaction Providing a Novel Strategy for Turn-on Thiol Sensing. Chem. Commun. 2015, 51, 14913–14916. [Google Scholar] [CrossRef]
- Barr, B.K.; Holewinski, R.J. 4-Methyl-7-Thioumbelliferyl-β-d-Cellobioside: A Fluorescent, Nonhydrolyzable Substrate Analogue for Cellulases. Biochemistry 2002, 41, 4447–4452. [Google Scholar] [CrossRef]
- Tanaka, T.; Matsumoto, T.; Noguchi, M.; Kobayashi, A.; Shoda, S. Direct Transformation of Unprotected Sugars to Aryl 1-Thio-β-Glycosides in Aqueous Media Using 2-Chloro-1,3-Dimethylimidazolinium Chloride. Chem. Lett. 2009, 38, 458–459. [Google Scholar] [CrossRef]
- Katayama, H.; Asahina, Y.; Hojo, H. Chemical Synthesis of the S-Linked Glycopeptide, Sublancin. J. Pept. Sci. 2011, 17, 818–821. [Google Scholar] [CrossRef]
- Venkateswarlu, C.; Gautam, V.; Chandrasekaran, S. Useful Approach to the Synthesis of Aryl Thio- and Selenoglycosides in the Presence of Rongalite. Carbohydr. Res. 2014, 396, 48–53. [Google Scholar] [CrossRef]
- Ardourel, M.; Felgerolle, C.; Pâris, A.; Acar, N.; Ramchani Ben Othman, K.; Ueda, N.; Rossignol, R.; Bazinet, A.; Hébert, B.; Briault, S.; et al. Dietary Supplement Enriched in Antioxidants and Omega-3 Promotes Glutamine Synthesis in Müller Cells: A Key Process against Oxidative Stress in Retina. Nutrients 2021, 13, 3216. [Google Scholar] [CrossRef]
- Holze, C.; Michaudel, C.; Mackowiak, C.; Haas, D.A.; Benda, C.; Hubel, P.; Pennemann, F.L.; Schnepf, D.; Wettmarshausen, J.; Braun, M.; et al. Oxeiptosis, a ROS-Induced Caspase-Independent Apoptosis-like Cell-Death Pathway. Nat. Immunol. 2018, 19, 130–140. [Google Scholar] [CrossRef]
- Ourhzif, E.-M.; Pâris, A.; Abrunhosa-Thomas, I.; Ketatni, E.M.; Chalard, P.; Khouili, M.; Daniellou, R.; Troin, Y.; Akssira, M. Design, Synthesis, and Evaluation of Cytotoxic Activities of Arylnaphthalene Lignans and Aza-Analogs. Arch. Der Pharm. 2021, 354, 2000479. [Google Scholar] [CrossRef]
- Cox, D.; O’Kennedy, R.; Thornes, R.D. The Rarity of Liver Toxicity in Patients Treated with Coumarin (1, 2-Benzopyrone). Hum. Toxicol. 1989, 8, 501–506. [Google Scholar] [CrossRef]
- Elinos-Báez, C.M.; León, F.; Santos, E. Effects of Coumarin and 7OH-Coumarin on Bcl-2 and Bax Expression in Two Human Lung Cancer Cell Lines in Vitro. Cell Biol. Int. 2005, 29, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Saidu, N.E.B.; Valente, S.; Bana, E.; Kirsch, G.; Bagrel, D.; Montenarh, M. Coumarin Polysulfides Inhibit Cell Growth and Induce Apoptosis in HCT116 Colon Cancer Cells. Bioorganic Med. Chem. 2012, 20, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Abu-Eittah, R.H.; El-Tawil, B.A.H. The Electronic Absorption Spectra of Some Coumarins. A Molecular Orbital Treatment. Can. J. Chem. 1985, 63, 1173–1179. [Google Scholar] [CrossRef]
- Lanterna, A.E.; González-Béjar, M.; Frenette, M.; Scaiano, J.C. Photophysics of 7-Mercapto-4-Methylcoumarin and Derivatives: Complementary Fluorescence Behaviour to 7-Hydroxycoumarins. Photochem. Photobiol. Sci. 2017, 16, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mottaleb, M.S.A.; El-Sayed, B.A.; Abo-Aly, M.M.; El-Kady, M.Y. Fluorescence-Properties and Excited State Interactions of 7-Hydroxy-4-Methylcoumarin Laser Dye. J. Photochem. Photobiol. A Chem. 1989, 46, 379–390. [Google Scholar] [CrossRef]
- Nasseri, S.A.; Betschart, L.; Opaleva, D.; Rahfeld, P.; Withers, S.G. A Mechanism-Based Approach to Screening Metagenomic Libraries for Discovery of Unconventional Glycosidases. Angew. Chem. 2018, 130, 11529–11534. [Google Scholar] [CrossRef]
- Buchheit, D.; Schmitt, E.I.; Bischoff, D.; Ebner, T.; Bureik, M. S-Glucuronidation of 7-Mercapto-4-Methylcoumarin by Human UDP Glycosyltransferases in Genetically Engineered Fission Yeast Cells. Biol. Chem. 2011, 392, 1089–1095. [Google Scholar] [CrossRef]
- Li, S.-S.; Xu, W.; Lin, F.-L.; Wang, Q.-Q.; Jiang, R.-W. Efficient Glycosylation of Coumarin by Plant Glycosyltransferase UGT74AN3. World J. Pharm. Pharm. Sci. 2021, 10, 1959–1967. [Google Scholar] [CrossRef]
- Yoshida, N.; Fujieda, T.; Kobayashi, A.; Ishihara, M.; Noguchi, M.; Shoda, S. Direct Introduction of Detachable Fluorescent Tag into Oligosaccharides. Chem. Lett. 2013, 42, 1038–1039. [Google Scholar] [CrossRef]
- Huang, W.; He, Y.; Jiang, R.; Deng, Z.; Long, F. Functional and Structural Dissection of a Plant Steroid 3-O-Glycosyltransferase Facilitated the Engineering Enhancement of Sugar Donor Promiscuity. ACS Catal. 2022, 12, 2927–2937. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Fery-Forgues, S.; Lavabre, D. Are Fluorescence Quantum Yields So Tricky to Measure? A Demonstration Using Familiar Stationery Products. J. Chem. Educ. 1999, 76, 1260–1264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statemebarrnts, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
| Substrate | Product | Conversion (%) | Yield (%) |
|---|---|---|---|
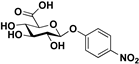 pNP-β-d-GlcA |  S-1 | 75 | 54 |
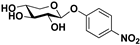 pNP-β-d-Xyl |  S-2 | 80 | 72 |
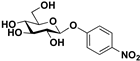 pNP-β-d-Glc | 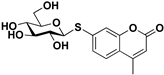 S-3 | 79 | 63 |
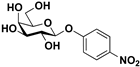 pNP-β-d-Gal | 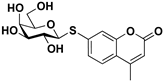 S-4 | 73 | 55 |
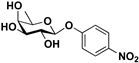 pNP-β-d-Fuc | 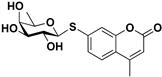 S-5 | 78 | 68 |
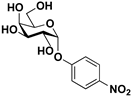 pNP-α-d-Gal |  S-6 | 81 | 63 |
| Compound | A-549 | HS683 | MCF-7 | SK-MEL-28 | HaCaT |
|---|---|---|---|---|---|
| S-1 | >1000 a | >1000 | >1000 | >1000 | >1000 |
| S-2 | 74.8 (20.5–273.1) | 275.0 (110.5–684.5) | 128.4 (28.7–575.6) | 227.6 (160.9–321.8) | 161.9 (120.6–732.7) |
| S-3 | >1000 | >1000 | >1000 | >1000 | >1000 |
| S-4 | >1000 | >1000 | >1000 | >1000 | >1000 |
| S-5 | >1000 | >1000 | >1000 | >1000 | >1000 |
| S-6 | >1000 | >1000 | >1000 | >1000 | >1000 |
| 7-MC | >1000 | n.d. b | >1000 | 306.1 (210.1–446.0) | n.d. |
| 5-FU | 8.9 (6.3–12.5) | 37.0 (29.8–45.9) | 4.8 (3.8–6.2) | 6.6 (5.2–8.3) | 0.4 (0.3–0.5) |
| Etoposide | 1.6 (1.3–2.0) | 1.6 (1.4–1.8) | 3.9 (2.8–5.4) | 3.2 (2.8–3.8) | 0.5 (0.4–0.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burrini, N.; Pâris, A.; Collet, G.; Lafite, P.; Daniellou, R. Biocatalytic Synthesis of Coumarin S-Glycosides: Towards Non-Cytotoxic Probes for Biomedical Imaging and Sensing. Molecules 2024, 29, 1322. https://doi.org/10.3390/molecules29061322
Burrini N, Pâris A, Collet G, Lafite P, Daniellou R. Biocatalytic Synthesis of Coumarin S-Glycosides: Towards Non-Cytotoxic Probes for Biomedical Imaging and Sensing. Molecules. 2024; 29(6):1322. https://doi.org/10.3390/molecules29061322
Chicago/Turabian StyleBurrini, Nastassja, Arnaud Pâris, Guillaume Collet, Pierre Lafite, and Richard Daniellou. 2024. "Biocatalytic Synthesis of Coumarin S-Glycosides: Towards Non-Cytotoxic Probes for Biomedical Imaging and Sensing" Molecules 29, no. 6: 1322. https://doi.org/10.3390/molecules29061322
APA StyleBurrini, N., Pâris, A., Collet, G., Lafite, P., & Daniellou, R. (2024). Biocatalytic Synthesis of Coumarin S-Glycosides: Towards Non-Cytotoxic Probes for Biomedical Imaging and Sensing. Molecules, 29(6), 1322. https://doi.org/10.3390/molecules29061322








