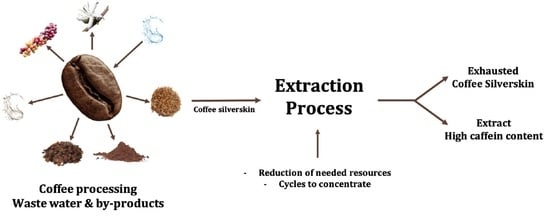
Valorization of Coffee Silverskin Using Extraction Cycles and Water as a Solvent: Design of Process
Abstract
1. Introduction
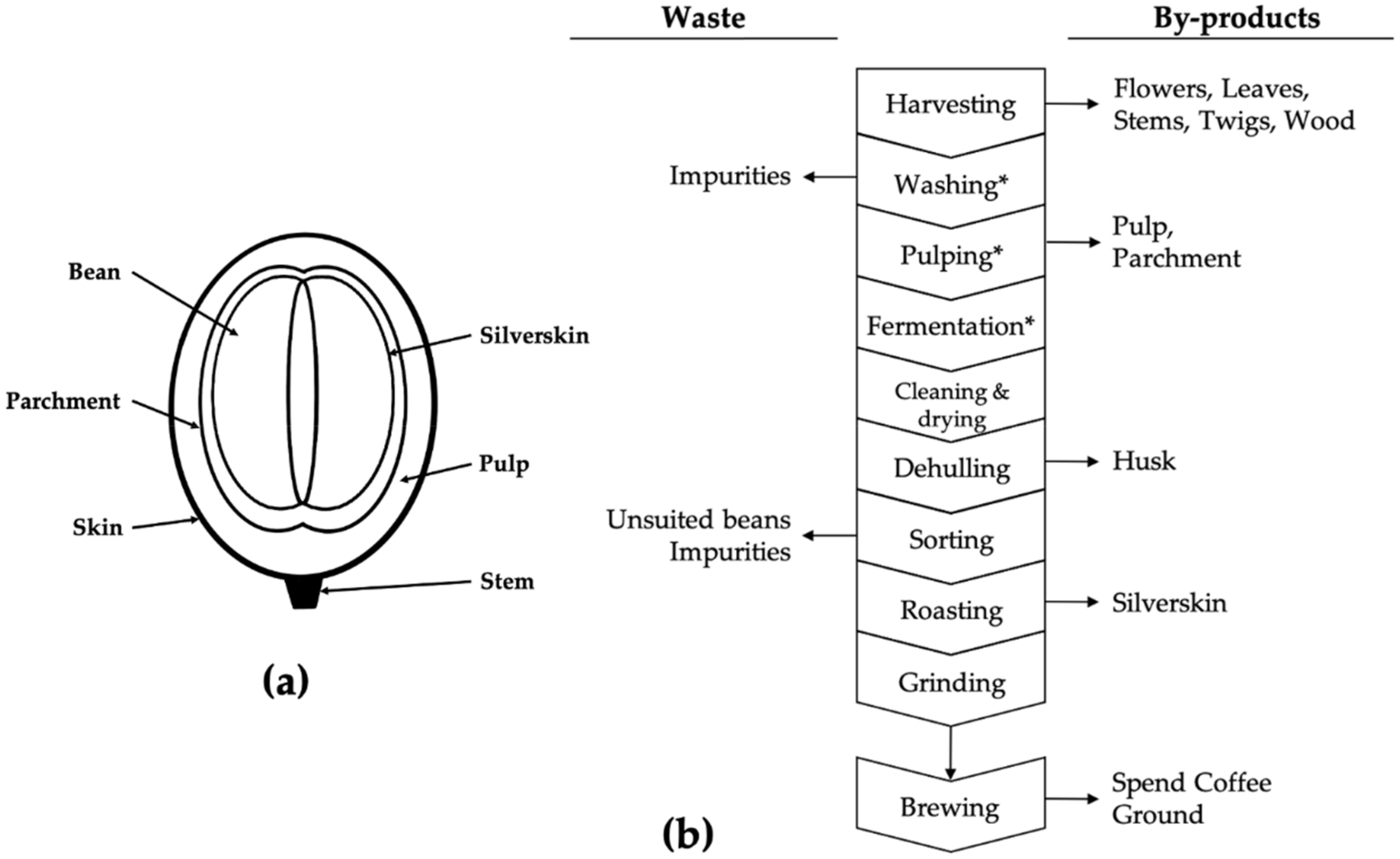
2. Results and Discussion
2.1. Extraction and Selection of Parameter Settings
2.2. Extraction Cycles and Limiting Parameters
2.3. Second Extraction of the Biomass
2.4. Thermogravimetric (TGA) Characterization of the Insoluble Fibers
2.5. Schematic Representation of the Process with Water Compensation and Potential Silverskin Valorizations
3. Materials and Methods
3.1. Materials
3.2. Standard Extraction from Silverskin and Water-Insoluble Fibers
3.3. Calculations for Hypothetical Number of Cycles
3.4. UV–Visible Spectrophotometric Determination of Caffeine Quantities Obtained by Water Extraction from Silverskin
3.5. UPLC UV–Visible Determination of Caffeine Quantities Obtained by Water Extraction from Silverskin
3.6. Thermogravimetric Analysis of the Water-Insoluble Fibers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arya, S.S.; Venkatram, R.; More, P.R.; Vijayan, P. The Wastes of Coffee Bean Processing for Utilization in Food: A Review. J. Food Sci. Technol. 2022, 59, 429–444. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Benítez, V.; Bartolomé, B.; Aguilera, Y.; Martín-Cabrejas, M.A. Revalorization of Coffee Husk: Modeling and Optimizing the Green Sustainable Extraction of Phenolic Compounds. Foods 2021, 10, 653. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Franca, A.S. An Overview of the Potential Uses for Coffee Husks. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 283–291. ISBN 978-0-12-409517-5. [Google Scholar]
- World Coffee Production by Country. Available online: https://www.atlasbig.com/en-us/countries-coffee-production#google_vignette (accessed on 25 February 2024).
- Coffee Consumption by Country. Available online: https://worldpopulationreview.com/country-rankings/coffee-consumption-by-country (accessed on 25 February 2024).
- Narita, Y.; Inouye, K. Review on Utilization and Composition of Coffee Silverskin. Food Res. Int. 2014, 61, 16–22. [Google Scholar] [CrossRef]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; Rajcic De Rezende, T.; Schwarz, S.; Lachenmeier, D.W. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Brand, D.; Mohan, R.; Roussos, S. Biotechnological Potential of Coffee Pulp and Coffee Husk for Bioprocesses. Biochem. Eng. J. 2000, 6, 153–162. [Google Scholar] [CrossRef]
- Campos, R.C.; Pinto, V.R.A.; Melo, L.F.; Rocha, S.J.S.S.D.; Coimbra, J.S. New Sustainable Perspectives for “Coffee Wastewater” and Other by-Products: A Critical Review. Future Foods 2021, 4, 100058. [Google Scholar] [CrossRef]
- Gathuo, B.; Rantala, P.; Määttä, R. Coffee Industry Wastes. Water Sci. Technol. 1991, 24, 53–60. [Google Scholar] [CrossRef]
- Ijanu, E.M.; Kamaruddin, M.A.; Norashiddin, F.A. Coffee Processing Wastewater Treatment: A Critical Review on Current Treatment Technologies with a Proposed Alternative. Appl. Water Sci. 2020, 10, 11. [Google Scholar] [CrossRef]
- Chemat, A.; Ravi, H.K.; Hostequin, A.C.; Burney, H.; Tomao, V.; Fabiano-Tixier, A.-S. Valorization of Spent Coffee Grounds by 2-Methyloxolane as Bio-Based Solvent Extraction. Viable Pathway towards Bioeconomy for Lipids and Biomaterials. OCL 2022, 29, 7. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Dried Coffee Husk (Cascara) from Coffea Arabica L. as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, e07085. [Google Scholar] [CrossRef]
- Sunanjaya, I.W.; Resiani, N.M.D.; Yasa, I.M.R. The Utilization of Coffee Waste in Supporting Family Food Security. E3S Web Conf. 2021, 306, 01025. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee Silverskin Extracts: Quantification of 30 Bioactive Compounds by a New HPLC-MS/MS Method and Evaluation of Their Antioxidant and Antibacterial Activities. Food Res. Int. 2020, 133, 109128. [Google Scholar] [CrossRef]
- Rodrigues, F.; Palmeira-de-Oliveira, A.; Das Neves, J.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B.P.P. Coffee Silverskin: A Possible Valuable Cosmetic Ingredient. Pharm. Biol. 2015, 53, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, C.; Annibaldi, A.; Girolametti, F.; Giovannini, L.; Riolo, P.; Ruschioni, S.; Olivotto, I.; Illuminati, S. A Chemically Safe Way to Produce Insect Biomass for Possible Application in Feed and Food Production. Int. J. Environ. Res. Public Health 2020, 17, 2121. [Google Scholar] [CrossRef] [PubMed]
- Gwon, S.; Choi, Y.C.; Shin, M. Effect of plant cellulose microfibers on hydration of cement composites. Constr. Build. Mater. 2021, 267, 121734. [Google Scholar] [CrossRef]
- Garcia, C.V.; Kim, Y.-T. Spent Coffee Grounds and Coffee Silverskin as Potential Materials for Packaging: A Review. J. Polym. Environ. 2021, 29, 2372–2384. [Google Scholar] [CrossRef]
- Ghazvini, A.K.A.; Ormondroyd, G.; Curling, S.; Saccani, A.; Sisti, L. An Investigation on the Possible Use of Coffee Silverskin in PLA/PBS Composites. J. Appl. Polym. Sci. 2022, 139, 52264. [Google Scholar] [CrossRef]
- Autorité Européenne de sécurité des aliments EFSA Autorité Européenne de Sécurité Des Aliments. Available online: https://www.efsa.europa.eu/fr (accessed on 9 June 2023).
- European Union Eur-Lex. Available online: https://eur-lex.europa.eu/homepage.html (accessed on 9 June 2023).
- European Comission Food Safety—EU Novel Food Catalogue. Available online: https://webgate.ec.europa.eu/fip/novel_food_catalogue/# (accessed on 9 June 2023).
- Iriondo-DeHond, A.; Haza, A.I.; Ávalos, A.; Del Castillo, M.D.; Morales, P. Validation of Coffee Silverskin Extract as a Food Ingredient by the Analysis of Cytotoxicity and Genotoxicity. Food Res. Int. 2017, 100, 791–797. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Esposito, F.; Napolitano, A.; Ritieni, A.; Fogliano, V. Characterization of a New Potential Functional Ingredient: Coffee Silverskin. J. Agric. Food Chem. 2004, 52, 1338–1343. [Google Scholar] [CrossRef]
- Bresciani, L.; Calani, L.; Bruni, R.; Brighenti, F.; Del Rio, D. Phenolic Composition, Caffeine Content and Antioxidant Capacity of Coffee Silverskin. Food Res. Int. 2014, 61, 196–201. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Ullate, M.; Martin-Cabrejas, M.A.; Martorell, P.; Genovés, S.; Ramon, D.; Del Castillo, M.D. A Novel Antioxidant Beverage for Body Weight Control Based on Coffee Silverskin. Food Chem. 2014, 150, 227–234. [Google Scholar] [CrossRef]
- Zhong, J.; Tang, N.; Asadzadeh, B.; Yan, W. Measurement and Correlation of Solubility of Theobromine, Theophylline, and Caffeine in Water and Organic Solvents at Various Temperatures. J. Chem. Eng. Data 2017, 62, 2570–2577. [Google Scholar] [CrossRef]
- Nonappa; Kolehmainen, E. Caffeine as a Gelator. Gels 2016, 2, 9. [Google Scholar] [CrossRef]
- Dabir, T.O.; Gaikar, V.G.; Jayaraman, S.; Mukherjee, S. Thermodynamic Modeling Studies of Aqueous Solubility of Caffeine, Gallic Acid and Their Cocrystal in the Temperature Range of 303 K–363 K. Fluid Phase Equilibria 2018, 456, 65–76. [Google Scholar] [CrossRef]
- Tello, J.; Viguera, M.; Calvo, L. Extraction of Caffeine from Robusta Coffee (Coffea Canephora Var. Robusta) Husks Using Supercritical Carbon Dioxide. J. Supercrit. Fluids 2011, 59, 53–60. [Google Scholar] [CrossRef]
- European Food Safety Authority. L’Évaluation des Risques Expliquée par l’EFSA: La Caféine; EFSA: Parma, Italy, 2015. [Google Scholar]
- Dauber, C.; Romero, M.; Chaparro, C.; Ureta, C.; Ferrari, C.; Lans, R.; Frugoni, L.; Echeverry, M.V.; Calvo, B.S.; Trostchansky, A.; et al. Cookies Enriched with Coffee Silverskin Powder and Coffee Silverskin Ultrasound Extract to Enhance Fiber Content and Antioxidant Properties. Appl. Food Res. 2024, 4, 100373. [Google Scholar] [CrossRef]
- Bianchi, S.; Vannini, M.; Sisti, L.; Marchese, P.; Mallegni, N.; Rodríguez, Ó.; Kohnen, S.; Tchoumtchoua, J.; Cinelli, P.; Celli, A. Valorization of Coffee Silverskin by Cascade Extraction of Valuable Biomolecules: Preparation of Eco-friendly Composites as the Ultimate Step. Biofuels Bioprod. Bioref. 2024, 18, 524–542. [Google Scholar] [CrossRef]
- Taweekayujan, S.; Somngam, S.; Pinnarat, T. Optimization and Kinetics Modeling of Phenolics Extraction from Coffee Silverskin in Deep Eutectic Solvent Using Ultrasound-Assisted Extraction. Heliyon 2023, 9, e17942. [Google Scholar] [CrossRef] [PubMed]
- Smyrnakis, G.; Stamoulis, G.; Palaiogiannis, D.; Chatzimitakos, T.; Athanasiadis, V.; Lalas, S.I.; Makris, D.P. Recovery of Polyphenolic Antioxidants from Coffee Silverskin Using Acid-Catalyzed Ethanol Organosolv Treatment. ChemEngineering 2023, 7, 72. [Google Scholar] [CrossRef]
- Castillo, M.; Ibáñez, E.; Amigo-Benavent, M.; Herrero, M.; Plaza, M.; Ullate, M. Application of Products of Coffee Silverskin in Anti-Ageing Cosmetics and Functional Food. Patent WO2013004873A1, 10 January 2013. [Google Scholar]
- Iriondo-DeHond, A.; Aparicio García, N.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.; San Andres, M.I.; Sanchez-Fortun, S.; Del Castillo, M.D. Validation of Coffee By-Products as Novel Food Ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- Garcia-Serna, E.; Martinez-Saez, N.; Mesias, M.; Morales, F.; Castillo, M. Use of Coffee Silverskin and Stevia to Improve the Formulation of Biscuits. Pol. J. Food Nutr. Sci. 2014, 64, 243–251. [Google Scholar] [CrossRef]
- Lajoie, L.; Fabiano-Tixier, A.-S.; Chemat, F. Water as Green Solvent: Methods of Solubilisation and Extraction of Natural Products—Past, Present and Future Solutions. Pharmaceuticals 2022, 15, 1507. [Google Scholar] [CrossRef]
- Al-Rawajfeh, A.E.; Al-Shamaileh, E.M. Assessment of Tap Water Resources Quality and Its Potential of Scale Formation and Corrosivity in Tafila Province, South Jordan. Desalination 2007, 206, 322–332. [Google Scholar] [CrossRef]
- Vandeponseele, A.; Draye, M.; Piot, C.; Chatel, G. Study of Influential Parameters of the Caffeine Extraction from Spent Coffee Grounds: From Brewing Coffee Method to the Waste Treatment Conditions. Clean Technol. 2021, 3, 335–350. [Google Scholar] [CrossRef]
- Rhoads, W.J.; Ji, P.; Pruden, A.; Edwards, M.A. Water Heater Temperature Set Point and Water Use Patterns Influence Legionella Pneumophila and Associated Microorganisms at the Tap. Microbiome 2015, 3, 67. [Google Scholar] [CrossRef]
- Belay, A.; Kim, H.K.; Hwang, Y. Binding of Caffeine with Caffeic Acid and Chlorogenic Acid Using Fluorescence Quenching, UV/Vis and FTIR Spectroscopic Techniques. Luminescence 2016, 31, 565–572. [Google Scholar] [CrossRef]
- Lau, O.-W.; Luk, S.-F.; Cheng, O.-M.; Chiu, T.P.Y. Background-Correction Methods for the Determination of Caffeine in Beverages, Coffee and Tea by Using Second-Derivative Ultraviolet Spectrophotometry. Analyst 1992, 117, 777. [Google Scholar] [CrossRef]
- Abbaspour, A.; Mirzajani, R. Simultaneous Determination of Phenytoin, Barbital and Caffeine in Pharmaceuticals by Absorption (Zero-Order) UV Spectra and First-Order Derivative Spectra—Multivariate Calibration Methods. J. Pharm. Biomed. Anal. 2005, 38, 420–427. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, N.; Fontanive, L.; Uggeri, F.; Suggi-Liverani, F.; Navarini, L. NMR Reinvestigation of the Caffeine–Chlorogenate Complex in Aqueous Solution and in Coffee Brews. Food Biophys. 2009, 4, 321–330. [Google Scholar] [CrossRef]
- Panusa, A.; Petrucci, R.; Lavecchia, R.; Zuorro, A. UHPLC-PDA-ESI-TOF/MS Metabolic Profiling and Antioxidant Capacity of Arabica and Robusta Coffee Silverskin: Antioxidants vs. Phytotoxins. Food Res. Int. 2017, 99, 155–165. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Alves, R.C.; Costa, A.S.G.; Nunes, M.A.; Oliveira, M.B.P.P. Coffea Canephora Silverskin from Different Geographical Origins: A Comparative Study. Sci. Total Environ. 2018, 645, 1021–1028. [Google Scholar] [CrossRef]
- Martuscelli, M.; Esposito, L.; Di Mattia, C.; Ricci, A.; Mastrocola, D. Characterization of Coffee Silver Skin as Potential Food-Safe Ingredient. Foods 2021, 10, 1367. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, A.; Squillante, J.; Velotto, S.; D’Auria, G.; Ferranti, P.; Mamone, G.; Errico, M.E.; Avolio, R.; Castaldo, R.; Cirillo, T.; et al. Valorization of Coffee Industry Wastes: Comprehensive Physicochemical Characterization of Coffee Silverskin and Multipurpose Recycling Applications. J. Clean. Prod. 2022, 370, 133520. [Google Scholar] [CrossRef]
- Górska, A.; Brzezińska, R.; Wirkowska-Wojdyła, M.; Bryś, J.; Domian, E.; Ostrowska-Ligęza, E. Application of Thermal Methods to Analyze the Properties of Coffee Silverskin and Oil Extracted from the Studied Roasting By-Product. Appl. Sci. 2020, 10, 8790. [Google Scholar] [CrossRef]
- Martuscelli, M.; Esposito, L.; Mastrocola, D. The Role of Coffee Silver Skin against Oxidative Phenomena in Newly Formulated Chicken Meat Burgers after Cooking. Foods 2021, 10, 1833. [Google Scholar] [CrossRef]
- Langenbahn, H.J. Volkswagen: Imitation Leather with Coffee Silverskin as Filler Material. Available online: https://www.thezerowastecoffeeproject.com/post/volkswagen-imitation-leather-with-coffee-silverskin-as-filler-material (accessed on 25 June 2023).
- Ramalakshmi, K.; Raghavan, B. Caffeine in Coffee: Its Removal. Why and How? Crit. Rev. Food Sci. Nutr. 1999, 39, 441–456. [Google Scholar] [CrossRef] [PubMed]
- European Commission, Directorate General for Health and Consumers. Opinion on Dichloromethane. Revision of 26 February 2013; Publications Office, LU European Commission: Brussel, Belgium, 2012. [Google Scholar]
- Derrien, L. L’utilisation de la Caféine dans les Compléments Alimentaires. Ph.D. Thesis, Université Bretagne Loire, Rennes, France, 2021. [Google Scholar]
- Food and Drug Administration. Highly Concentrated Caffeine in Dietary Supplements Guidance for Industry; Food and Drug Administration: Silver Spring, MD, USA, 2018. [Google Scholar]
- Wang, R.; Xue, J.; Meng, L.; Lee, J.-W.; Zhao, Z.; Sun, P.; Cai, L.; Huang, T.; Wang, Z.; Wang, Z.-K.; et al. Caffeine Improves the Performance and Thermal Stability of Perovskite Solar Cells. Joule 2019, 3, 1464–1477. [Google Scholar] [CrossRef]
- Huber, V.; Hioe, J.; Touraud, D.; Kunz, W. Uncovering the Curcumin Solubilization Ability of Selected Natural Deep Eutectic Solvents Based on Quaternary Ammonium Compounds. J. Mol. Liq. 2022, 361, 119661. [Google Scholar] [CrossRef]





| Cycles without Water Addition | Cycles with Water Compensation | ||
|---|---|---|---|
| Cycles | Water Left after Each Cycle (mL) | Cycles | Water Used during the Process (mL) |
| 1 | 200.6 | 1 | 200.0 |
| 2 | 167.2 | 2 | 233.7 |
| 3 | 134.8 | 3 | 264.1 |
| 4 | 101.3 | 4 | 293.5 |
| 5 | 72.4 | 5 | 320.7 |
| 6 | 346.2 | ||
| 7 | 371.7 | ||
| 8 | 397.0 | ||
| 9 | 425.5 | ||
| 10 | 449.9 | ||
| 11 | 476.1 | ||
| 12 | 500.3 | ||
| 13 | 529.7 | ||
| 14 | 551.9 | ||
| 15 | 576.5 | ||
| Cycles without Water Compensation | Cycles with Water Compensation | ||||||
|---|---|---|---|---|---|---|---|
| Cycles | Caffeine Content in Extract (g/200 mL) | Caffeine Content in 2nd Extraction (g/200 mL) | Ratio | Cycles | Caffeine Content in Extract (g/200 mL) | Caffeine Content in 2nd Extraction (g/200 mL) | Ratio |
| 1 | 0.156 | 0.066 | 2.4 | 1 | 0.146 | n.d. | |
| 2 | 0.324 | 0.090 | 3.6 | 5 | 0.575 | 0.122 | 4.7 |
| 3 | 0.534 | 0.134 | 4.0 | 10 | 0.869 | 0.163 | 5.3 |
| 4 | 0.810 | 0.148 | 5.5 | 15 | 0.922 | 0.170 | 5.4 |
| 5 | 1.138 | 0.175 | 6.5 | ||||
| Products | Caffeine Content (mg) | Caffeine Concentration (mg/mL) | Equivalence in Filter Coffee Cups |
|---|---|---|---|
| Filter coffee (200 mL) 1 | 90 | 0.45 | 1 |
| Espresso (60 mL) 1 | 80 | 1.33 | 3 |
| Energy beverage (250 mL) 1 | 80 | 0.32 | 1 |
| Extract after 1 cycle (170 mL) | 171 | 1.03 | 3 |
| Extract after 11 cycles (175 mL) | 748 | 4.25 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chemat, A.; Touraud, D.; Müller, R.; Kunz, W.; Fabiano-Tixier, A.-S. Valorization of Coffee Silverskin Using Extraction Cycles and Water as a Solvent: Design of Process. Molecules 2024, 29, 1318. https://doi.org/10.3390/molecules29061318
Chemat A, Touraud D, Müller R, Kunz W, Fabiano-Tixier A-S. Valorization of Coffee Silverskin Using Extraction Cycles and Water as a Solvent: Design of Process. Molecules. 2024; 29(6):1318. https://doi.org/10.3390/molecules29061318
Chicago/Turabian StyleChemat, Aziadé, Didier Touraud, Rainer Müller, Werner Kunz, and Anne-Sylvie Fabiano-Tixier. 2024. "Valorization of Coffee Silverskin Using Extraction Cycles and Water as a Solvent: Design of Process" Molecules 29, no. 6: 1318. https://doi.org/10.3390/molecules29061318
APA StyleChemat, A., Touraud, D., Müller, R., Kunz, W., & Fabiano-Tixier, A.-S. (2024). Valorization of Coffee Silverskin Using Extraction Cycles and Water as a Solvent: Design of Process. Molecules, 29(6), 1318. https://doi.org/10.3390/molecules29061318