Porous α-Fe2O3 Hollow Rods/Reduced Graphene Oxide Composites Templated by MoO3 Nanobelts for High-Performance Supercapacitor Applications
Abstract
1. Introduction
2. Results and Discussion
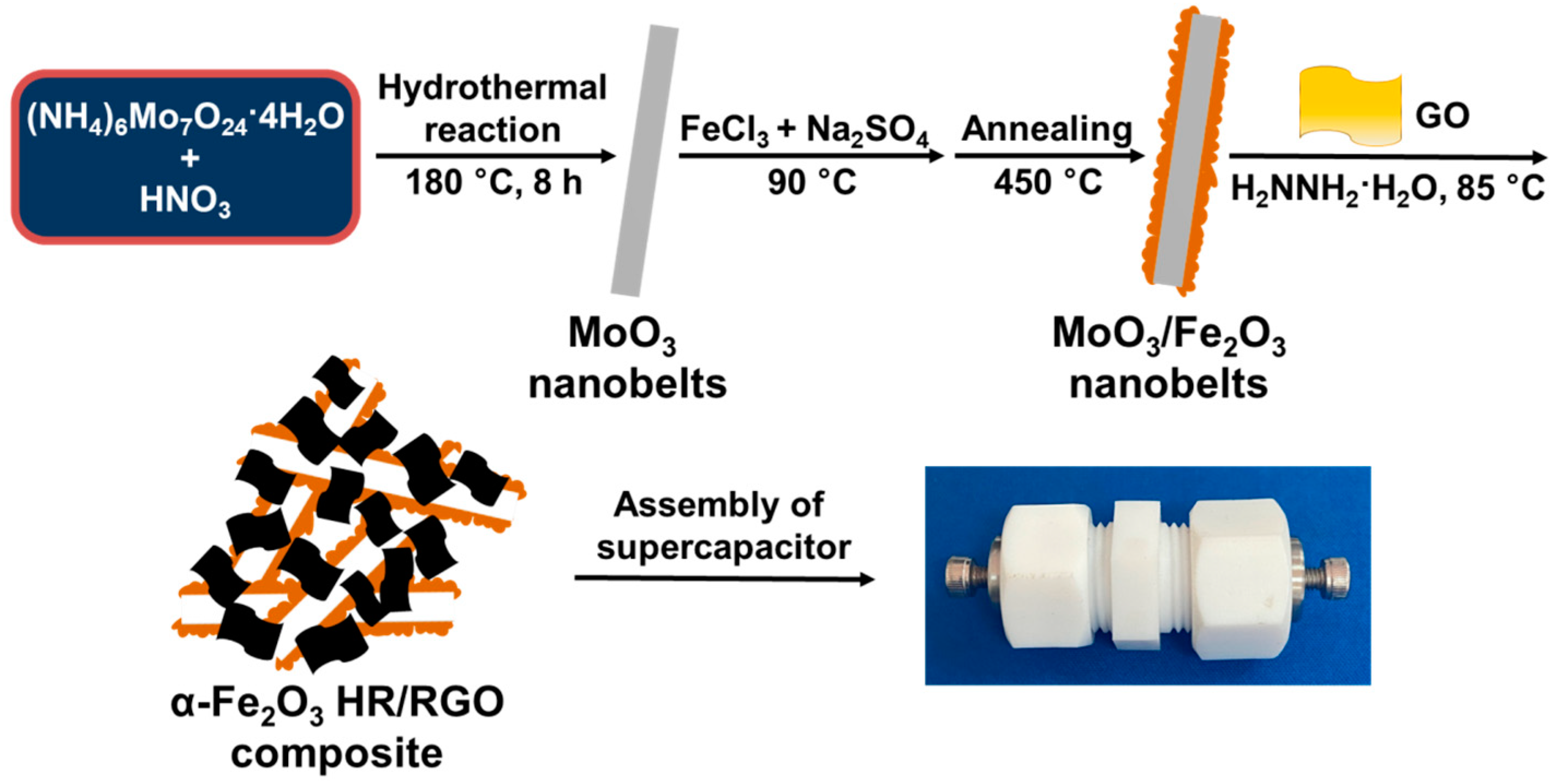
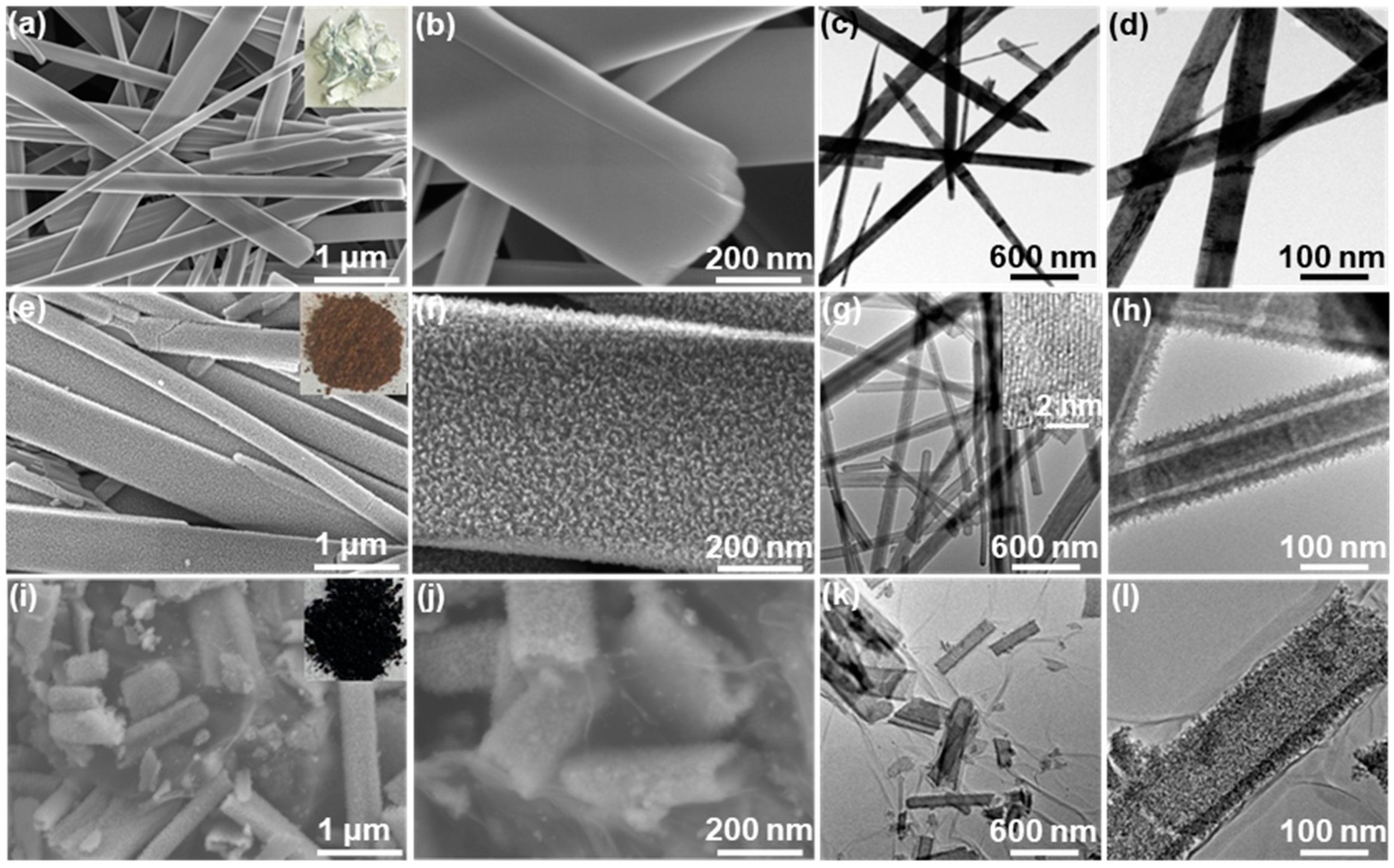
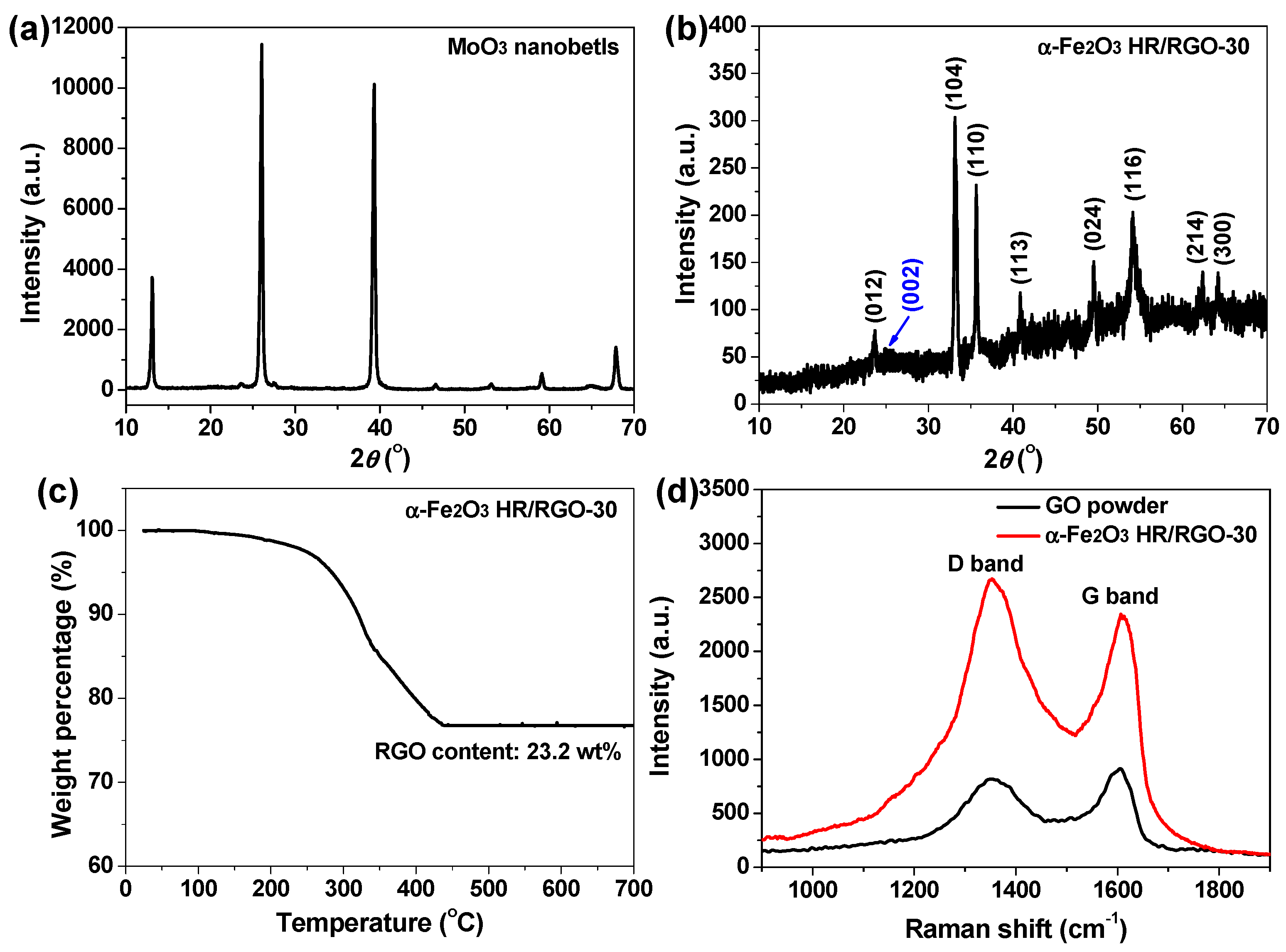
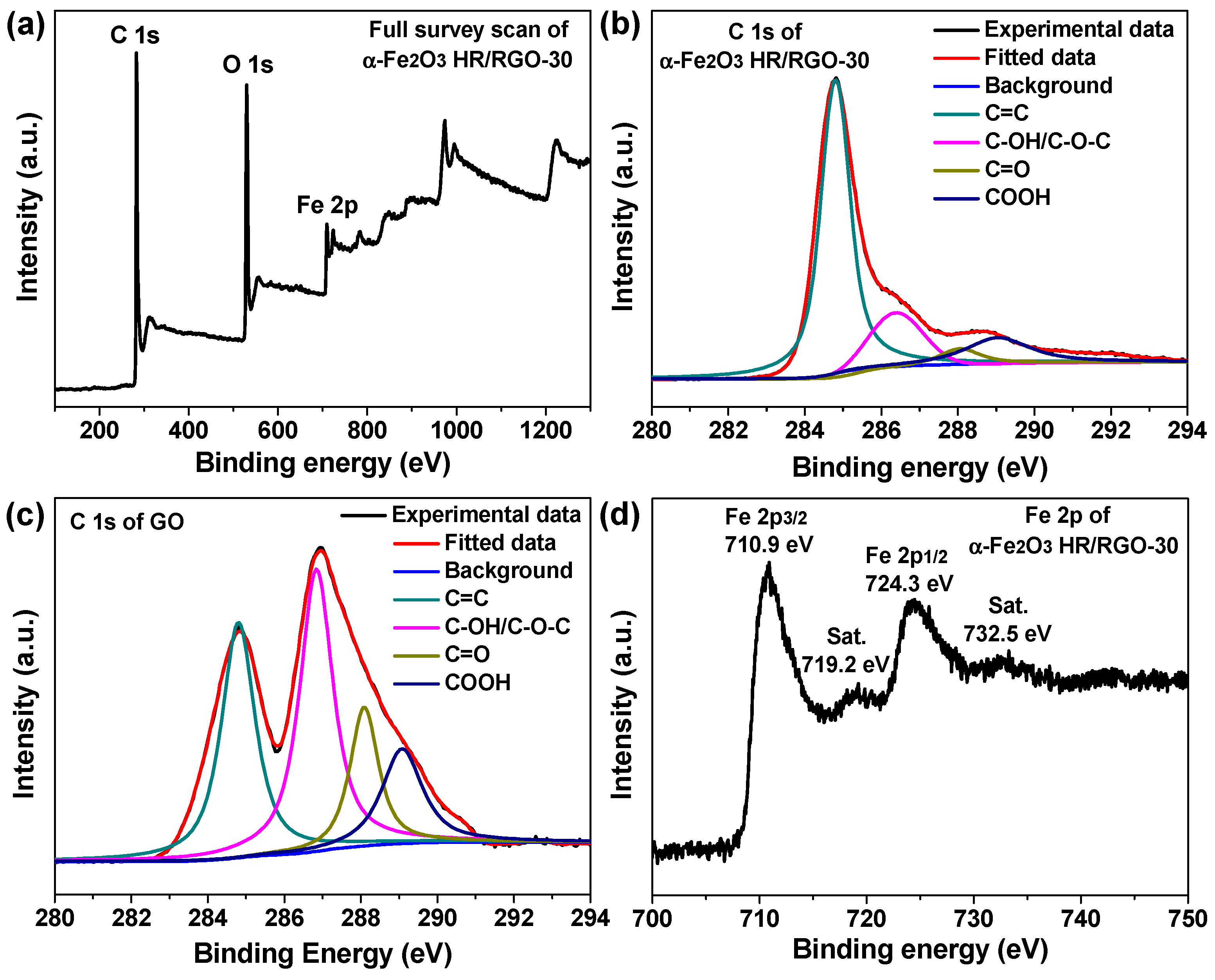
2.1. Materials Characterizations
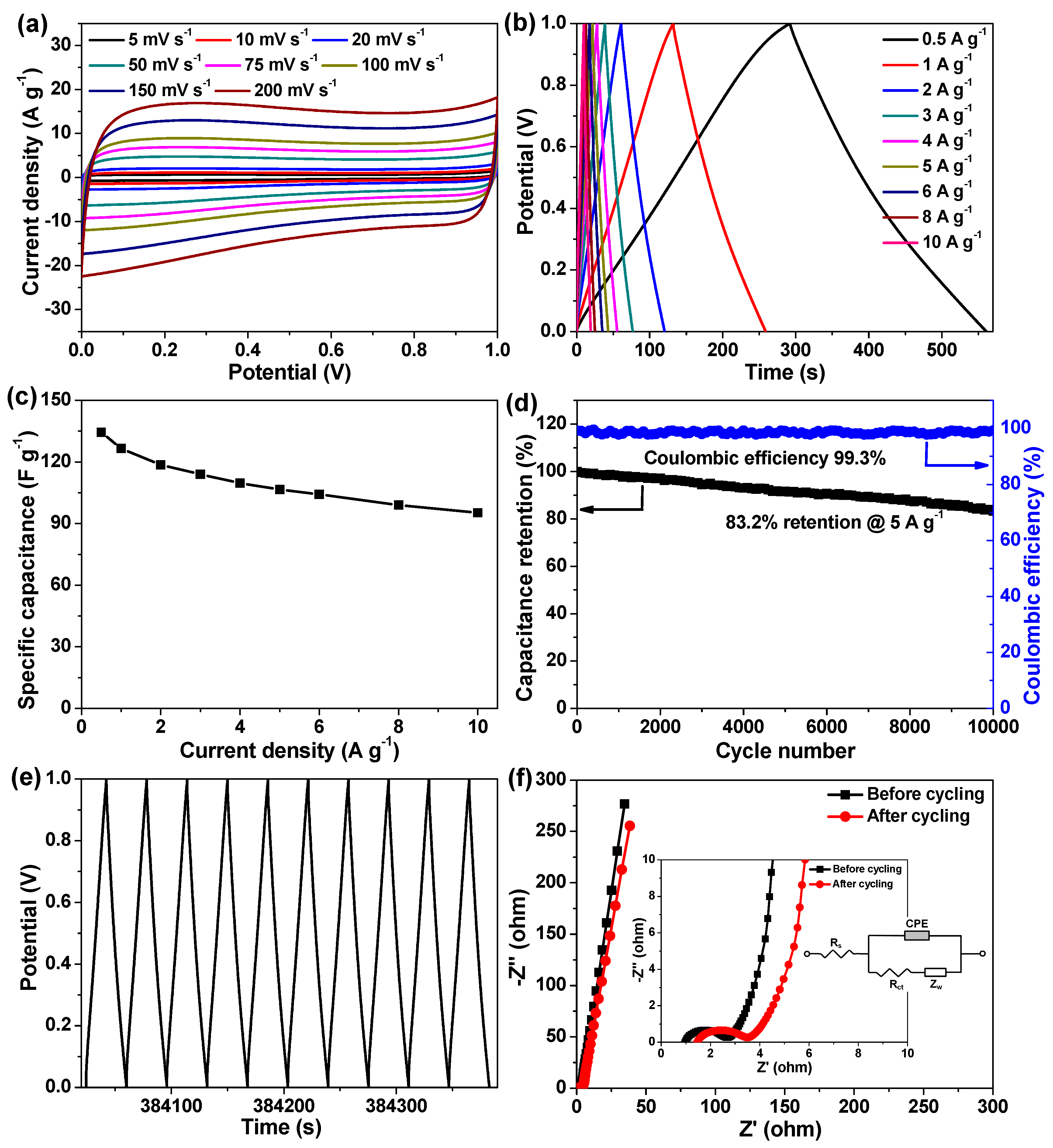
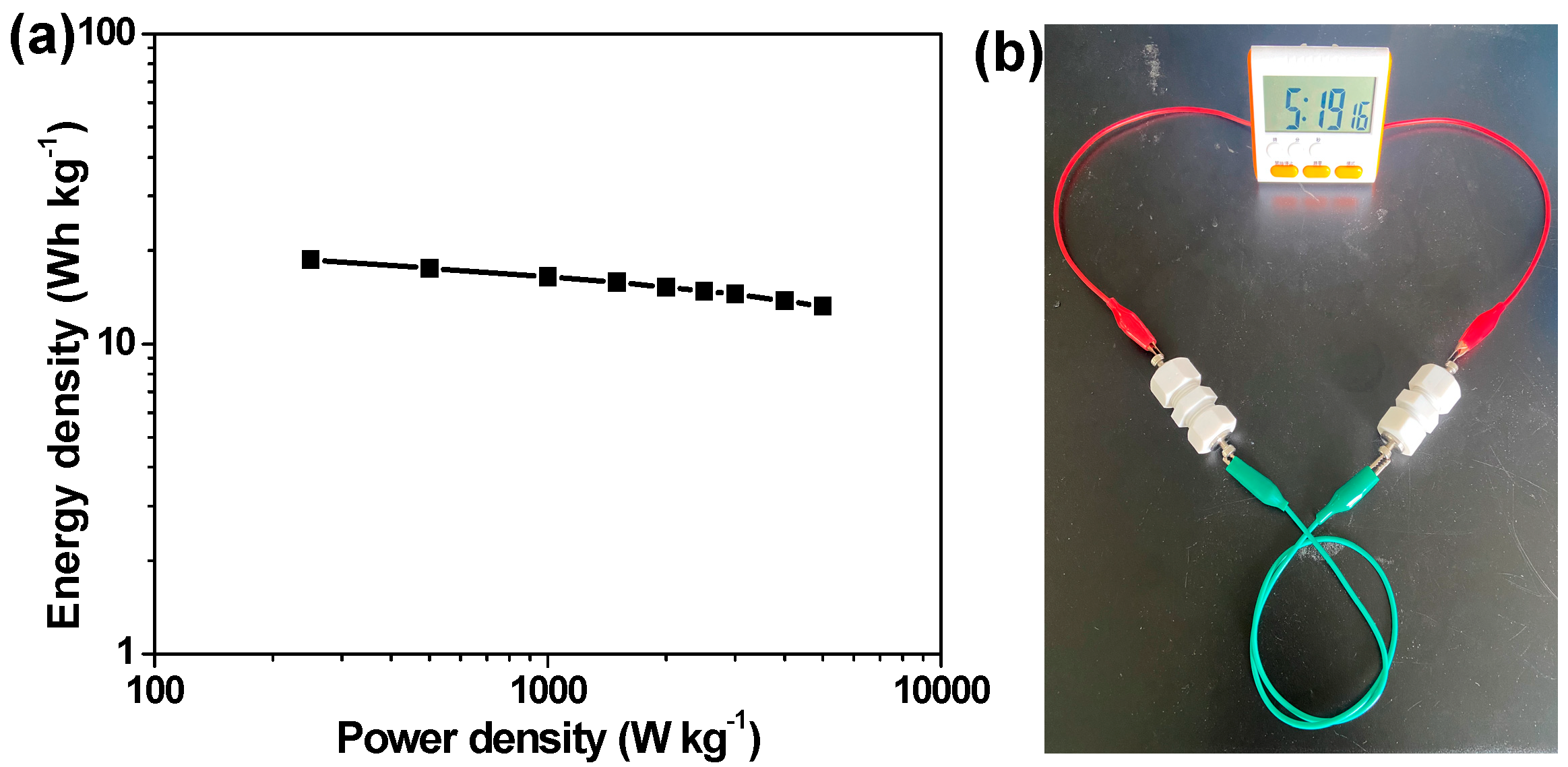
2.2. Electrochemical Evaluation
3. Materials and Methods
3.1. Chemicals
3.2. Fabrication of α-Fe2O3 HR/RGO
3.3. Characterizations
3.4. Electrochemical Performance Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Qi, H.; Yang, J.; Bo, Z.; Huang, F.; Islam, M.S.; Lu, X.; Dai, L.; Amal, R.; Wang, C.H.; et al. Two-birds-one-stone: Multifunctional supercapacitors beyond traditional energy storage. Energy Environ. Sci. 2021, 14, 1854–1896. [Google Scholar] [CrossRef]
- Tomy, M.; Rajappan, A.A.; VM, V.; Suryabai, X.T. Emergence of novel 2D materials for high-performance supercapacitor. Energy Fuels 2021, 35, 19881–19900. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Z.; Yang, G.; Zhang, Q.; Lan, T.; Zhang, C.; Li, P.; Liu, K.; He, S. Synthesis of chain-like nitrogen-doped carbon for high-performance supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133498. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, Q.; Yan, B.; Chen, J.; Chen, D.; Jiang, L.; Lan, T.; Zhang, C.; Yang, W.; He, S. N/O co-doped porous carbon synthesized by lewis acid salt activation for high rate performance supercapacitor. J. Energy Storage 2024, 80, 110322. [Google Scholar] [CrossRef]
- Yan, B.; Zhao, W.; Zhang, Q.; Kong, Q.; Chen, G.; Zhang, C.; Han, J.; Jiang, S.; He, S. One stone for four birds: A “chemical blowing” strategy to synthesis wood-derived carbon monoliths for high-mass loading capacitive energy storage in low temperature. J. Colloid Interface Sci. 2024, 653, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gong, Y.; Li, D.; Pan, C. Biomass-derived porous carbon materials: Synthesis, designing, and applications for supercapacitors. Green Chem. 2022, 24, 3864–3894. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest advances in supercapacitors: From new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Choudhary, N.; Jung, Y.; Thomas, J. Recent advances in two-dimensional nanomaterials for supercapacitor electrode applications. ACS Energy Lett. 2018, 3, 482–495. [Google Scholar] [CrossRef]
- Zou, Z.; Lei, Y.; Li, Y.; Zhang, Y.; Xiao, W. Nitrogen-doped hierarchical meso/microporous carbon from bamboo fungus for symmetric supercapacitor applications. Molecules 2019, 24, 3677. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Xian, X.; Xiao, W.; Zhang, Y.; Deng, Y.; Hu, R.; Tian, L.; Wang, Y.; Qu, J. Template-assisted synthesis of porous manganese dioxide hollow rods as supercapacitor electrode material. J. Electron. Mater. 2022, 51, 6792–6802. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Q.; Yang, K.; Tan, Y.; Tian, W.; Zhu, L.; Li, Z.; Yang, C. Solvothermally induced α-Fe2O3/graphene nanocomposites with ultrahigh capacitance and excellent rate capability for supercapacitors. J. Mater. Chem. A 2015, 3, 22005–22011. [Google Scholar] [CrossRef]
- Rehman, J.; Eid, K.; All, R.; Fan, X.; Murtaza, G.; Faizan, M.; Laref, A.; Zheng, W.; Varma, R.S. Engineering of transition metal sulfide nanostructures as efficient electrodes for high-performance supercapacitors. ACS Appl. Energy Mater. 2022, 5, 6481–6498. [Google Scholar] [CrossRef]
- Agarwal, A.; Sankapal, B.R. Metal phosphides: Topical advances in the design of supercapacitors. J. Mater. Chem. A 2021, 9, 20241–20276. [Google Scholar] [CrossRef]
- Zeng, G.; Wang, Y.; Lou, X.; Chen, H.; Jiang, S.; Zhou, W. Vanadium oxide/carbonized chestnut needle composites as cathode materials for advanced aqueous zinc-ion batteries. J. Energy Storage 2024, 77, 109859. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, K.; Zhou, W.; Deng, W.; Zhu, H.; Chen, H. High-voltage and long-life cathode material in aqueous ammonium-ion and hybrid-ion batteries. Small 2023, 19, 2308741. [Google Scholar] [CrossRef]
- Lu, X.; Zeng, Y.; Yu, M.; Zhai, T.; Liang, C.; Xie, S.; Balogun, M.-S.; Tong, Y. Oxygen-deficient hematite nanorods as high-performance and novel negative electrodes for flexible asymmetric supercapacitors. Adv. Mater. 2014, 26, 3148–3155. [Google Scholar] [CrossRef]
- Shivakumara, S.; Penki, T.R.; Munichandraiah, N. Preparation and electrochemical performance of porous hematite (α-Fe2O3) nanostructures as supercapacitor electrode material. J. Solid State Electrochem. 2014, 18, 1057–1066. [Google Scholar] [CrossRef]
- Sun, S.; Lang, J.; Wang, R.; Kong, L.; Li, X.; Yan, X. Identifying pseudocapacitance of Fe2O3 in an ionic liquid and its application in asymmetric supercapacitors. J. Mater. Chem. A 2014, 2, 14550–14556. [Google Scholar] [CrossRef]
- Chen, L.-F.; Yu, Z.-Y.; Ma, X.; Li, Z.-Y.; Yu, S.-H. In situ hydrothermal growth of ferric oxides on carbon cloth for low-cost and scalable high-energy-density supercapacitor. Nano Energy 2014, 9, 345–354. [Google Scholar] [CrossRef]
- Zheng, X.; Yan, X.; Sun, Y.; Yu, Y.; Zhang, G.; Shen, Y.; Liang, Q.; Liao, Q.; Zhang, Y. Temperature-dependent electrochemical capacitive performance of the α-Fe2O3 hollow nanoshuttles as supercapacitor electrodes. J. Colloid Interface Sci. 2016, 466, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, Y.; Xiao, W.; Tian, L.; Song, J. Preparation and electrochemical properties of mesoporous α-Fe2O3 nanowires for supercapacitor application. J. Mater. Sci. Mater. Electron. 2023, 34, 1098. [Google Scholar] [CrossRef]
- Li, T.; Yu, H.; Zhi, L.; Zhang, W.; Dang, L.; Liu, Z.; Lei, Z. Facile electrochemical fabrication of porous Fe2O3 nanosheets for flexible asymmetric supercapacitors. J. Phys. Chem. C 2017, 121, 18982–18991. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Chaudhari, S.; Yu, J.-S. Cube-like α-Fe2O3 supported on ordered multimodal porous carbon as high performance electrode material for supercapacitors. ChemSusChem 2014, 7, 3102–3111. [Google Scholar] [CrossRef]
- Shi, T.-Z.; Feng, Y.-L.; Peng, T.; Yuan, B.-G. Sea urchin-shaped Fe2O3 coupled with 2D MXene nanosheets as negative electrode for high-performance asymmetric supercapacitors. Electrochim. Acta 2021, 381, 138245. [Google Scholar] [CrossRef]
- Cheng, X.; Gui, X.; Lin, Z.; Zheng, Y.; Liu, M.; Zhan, R.; Zhu, Y.; Tang, Z. Three-dimensional α-Fe2O3/carbon nanotube sponges as flexible supercapacitor electrodes. J. Mater. Chem. A 2015, 3, 20927–20934. [Google Scholar] [CrossRef]
- Zou, Z.; Xiao, W.; Zhang, Y.; Yu, H.; Zhou, W. Facile synthesis of freestanding cellulose/RGO/silver/Fe2O3 hybrid film for ultrahigh-areal-energy-density flexible solid-state supercapacitor. Appl. Surf. Sci. 2020, 500, 144244. [Google Scholar] [CrossRef]
- Ma, Y.; Sheng, H.; Dou, W.; Su, Q.; Zhou, J.; Xie, E.; Lan, W. Fe2O3 nanoparticles anchored on the Ti3C2Tx MXene paper for flexible supercapacitors with ultrahigh volumetric capacitance. ACS Appl. Mater. Interfaces 2020, 12, 41410–41418. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hessel, C.M.; Ren, H.; Yu, R.; Jin, Q.; Yang, M.; Zhao, H.; Wang, D. α-Fe2O3 multi-shelled hollow microspheres for lithium ion battery anodes with superior capacity and charge retention. Energy Environ. Sci. 2014, 7, 632–637. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, X.; Wei, L.; Guo, X. MOF-derived porous hollow α-Fe2O3 microboxes modified by silver nanoclusters for enhanced pseudocapacitive storage. Appl. Surf. Sci. 2019, 463, 616–625. [Google Scholar] [CrossRef]
- Kong, D.; Cheng, C.; Wang, Y.; Liu, B.; Huang, Z.; Yang, H.Y. Seed-assisted growth of α-Fe2O3 nanorod arrays on reduced graphene oxide: A superior anode for high-performance Li-ion and Na-ion batteries. J. Mater. Chem. A 2016, 4, 11800–11811. [Google Scholar] [CrossRef]
- Li, C.-Q.; Shen, X.; Ding, R.-C.; Wang, G.-S. Excellent microwave absorption properties based on a composite of one dimensional Mo2C@C nanorods and a PVDF matrix. RSC Adv. 2019, 9, 21243–21248. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhao, P.; Li, Z.; Li, W.; Zhong, J.; Yu, J.; Yang, Z. A corn-inspired structure design for an iron oxide fiber/reduced graphene oxide composite as a high-performance anode material for Li-ion batteries. RSC Adv. 2017, 7, 44874–44883. [Google Scholar] [CrossRef]
- Kononenko, O.; Brzhezinskaya, M.; Zotov, A.; Korepanov, V.; Levashov, V.; Matveev, V.; Roshchupkin, D. Influence of numerous Moiré superlattices on transport properties of twisted multilayer graphene. Carbon 2022, 194, 52–61. [Google Scholar] [CrossRef]
- Zou, Z.; Zhou, W.; Zhang, Y.; Yu, H.; Hu, C.; Xiao, W. High-performance flexible all-solid-state supercapacitor constructed by free-standing cellulose/reduced graphene oxide/silver nanoparticles composite film. Chem. Eng. J. 2019, 357, 45–55. [Google Scholar] [CrossRef]
- Arora, K.; Karthikeyan, S.; Shiekh, B.A.; Kaur, M.; Singh, H.; Bhadu, G.R.; Kang, T.S. In situ preparation of a nanocomposite comprising graphene and α-Fe2O3 nanospindles for the photo-degradation of antibiotics under visible light. New J. Chem. 2020, 44, 15567–15573. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkow, S.A.; Besedina, N.A.; Brzhezinskaya, M.; Malkow, M.N.; Stolyarova, D.Y.; Arutyunyan, A.F.; Struchkov, N.S.; Saveliev, S.D.; Diankin, I.D.; et al. Guiding graphene derivation for covalent immobilization of aptamers. Carbon 2022, 196, 264–279. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. Graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Tian, S.; Sun, J.; Yang, S.; He, P.; Ding, S.; Ding, G.; Xie, X. Facile thermal annealing of graphite oxide in air for graphene with a higher C/O ratio. RSC Adv. 2015, 5, 69845–69860. [Google Scholar] [CrossRef]
- Purkait, T.; Singh, G.; Kumar, D.; Singh, M.; Dey, R.S. High-performance flexible supercapacitors based on electrochemically tailored three-dimensional reduced graphene oxide networks. Sci. Rep. 2018, 8, 640. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Yuan, X.; Yang, Z.; Zhang, M.; Meng, A.; Li, Q. A high-energy density asymmetric supercapacitor based on Fe2O3 nanoneedle arrays and NiCo2O4/Ni(OH)2 hybrid nanosheet arrays grown on SiC nanowire networks as free-standing advanced electrodes. Adv. Energy Mater. 2018, 8, 1702787. [Google Scholar] [CrossRef]
- Yang, P.; Ding, Y.; Lin, Z.; Chen, Z.; Li, Y.; Qiang, P.; Ebrahimi, M.; Mai, W.; Wong, C.P.; Wang, Z.L. Low-cost high-performance solid-state asymmetric supercapacitors based on MnO2 nanowires and Fe2O3 nanotubes. Nano Lett. 2014, 14, 731–736. [Google Scholar] [CrossRef]
- Gholipour-Ranjbar, H.; Ganjali, M.R.; Norouzi, P.; Naderi, H.R. Synthesis of cross-linked graphene aerogel/Fe2O3 nanocomposite with enhanced supercapacitive performance. Ceram. Int. 2016, 42, 12097–12104. [Google Scholar] [CrossRef]
- Nie, G.; Lu, X.; Lei, J.; Jiang, Z.; Wang, C. Electrospun V2O5-doped α-Fe2O3 composite nanotubes with tunable ferromagnetism for high-performance supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 15495–15501. [Google Scholar] [CrossRef]
- Quan, H.; Cheng, B.; Xiao, Y.; Lei, S. One-pot synthesis of α-Fe2O3 nanoplates-reduced graphene oxide composites for supercapacitor application. Chem. Eng. J. 2016, 286, 165–173. [Google Scholar] [CrossRef]
- Ali, A.; Hantanasirisakul, K.; Abdala, A.; Urbankowski, P.; Zhao, M.-Q.; Anasori, B.; Gogotsi, Y.; Aїssa, B.; Mahmoud, K.A. Effect of synthesis on performance of MXene/iron oxide material for lithium-ion batteries. Langmuir 2018, 34, 11325–11334. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, H.; Jung, H.M. Interfacial generation of plates assembled with α-Fe2O3 nano-flakes for electrochemical capacitors. J. Electroanal. Chem. 2016, 770, 44–49. [Google Scholar] [CrossRef]
- Fu, C.; Mahadevegowda, A.; Grant, P.S. Production of hollow and porous Fe2O3 from industrial mill scale and its potential for large-scale electrochemical energy storage applications. J. Mater. Chem. A 2016, 4, 2597–2604. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, C.; Niu, L.; Li, R.; Guo, H.; Shi, Y.; Li, C.; Liu, X.; Gong, Y. Hydrothermal synthesis of CuCo2O4/CuO nanowire arrays and rGO/Fe2O3 composites for high-performance aqueous asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 9977–9985. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, B.; Yin, Y.; Yin, T.; Cheng, J.; Zhan, K.; Yan, Y.; Yang, J.; Li, J. Fe2O3-decorated millimeter-long vertically aligned carbon nanotube arrays as advanced anode materials for asymmetric supercapacitors with high energy and power densities. J. Mater. Chem. A 2016, 4, 19026–19036. [Google Scholar] [CrossRef]
- Nie, G.; Lu, X.; Chi, M.; Zhu, Y.; Yang, Z.; Song, N.; Wang, C. Hierarchical α-Fe2O3@MnO2 core-shell nanotubes as electrode materials for high-performance supercapacitors. Electrochim. Acta 2017, 231, 36–43. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsu, J.H.; Lin, Y.G.; Hsu, Y.K. Synthesis of Fe2O3 nanorods/silver nanowires on coffee filter as low-cost and efficient electrodes for supercapacitors. J. Electroanal. Chem. 2017, 801, 65–71. [Google Scholar] [CrossRef]
- Yang, S.; Song, X.; Zhang, P.; Gao, L. Heating-rate-induced porous α-Fe2O3 with controllable pore size and crystallinity grown on graphene for supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.S.; Sankapal, B.R. Comparative studies on MWCNTs, Fe2O3 and Fe2O3/MWCNTs thin films towards supercapacitor application. New J. Chem. 2016, 40, 2619–2627. [Google Scholar] [CrossRef]
- Zeng, Y.; Han, Y.; Zhao, Y.; Zeng, Y.; Yu, M.; Liu, Y.; Tang, H.; Tong, Y.; Lu, X. Advanced Ti-doped Fe2O3@PEDOT core/shell anode for high-energy asymmetric supercapacitors. Adv. Energy Mater. 2015, 5, 1402176. [Google Scholar] [CrossRef]
- Xu, L.; Xia, J.; Xu, H.; Yin, S.; Wang, K.; Huang, L.; Wang, L.; Li, H. Reactable ionic liquid assisted solvothermal synthesis of graphite-like C3N4 hybridized α-Fe2O3 hollow microspheres with enhanced supercapacitive performance. J. Power Sources 2014, 245, 866–874. [Google Scholar] [CrossRef]



| Electrode Materials | Electrolyte | Maximum Specific Capacitance | Rate Performance | Cyclic Performance | References |
|---|---|---|---|---|---|
| Porous α-Fe2O3 nanostructure | 0.5 M Na2SO3 | 193 F g−1@1 A g−1 | 90 F g−1@5 A g−1 | 92%@2 A g−1 (1000 cycles) | [17] |
| α-Fe2O3 hollow nanoshuttles | 1 M KOH | 249 F g−1@0.5 A g−1 | ~90 F g−1@8 A g−1 | 93.6%@8 A g−1 (2000 cycles) | [20] |
| Mesoporous α-Fe2O3 nanowire | 1 M KOH | 330 F g−1@1 A g−1 | 99.6 F g−1@10 A g−1 | 87%@2 A g−1 (2000 cycles) | [21] |
| α-Fe2O3 nano-flakes | 1 M Na2SO3 | 171 F g−1@0.5 A g−1 | 36 F g−1@3 A g−1 | 85%@1 A g−1 (1000 cycles) | [46] |
| Hollow and porous Fe2O3 microrods | 0.5 M Na2SO3 | 213 F g−1@2 A g−1 | 120 F g−1@6 A g−1 | 88%@6 A g−1 (5000 cycles) | [47] |
| α-Fe2O3@Ag microboxes | 1 M Na2SO3 | 701 F g−1@0.1 A g−1 | 254 F g−1@5 A g−1 | 80%@10 A g−1 (2000 cycles) | [29] |
| RGO/α-Fe2O3 composite | 2 M KOH | 469.5 F g−1@4 A g−1 | 132.4 F g−1@16 A g−1 | 88%@8 A g−1 (5000 cycles) | [48] |
| V2O5-doped α-Fe2O3 nanotubes | 3 M KOH | 183 F g−1@4 A g−1 | ~110 F g−1@5 A g−1 | 81.5%@1 A g−1 (200 cycles) | [43] |
| Fe2O3/carbon nanotube arrays | 2 M KOH | 248 F g−1@8 A g−1 | 204 F g−1@24 A g−1 | 89%@8 A g−1 (5000 cycles) | [49] |
| α-Fe2O3 nanotube@MnO2 nanosheet | 3 M KOH | 289.9 F g−1@1 A g−1 | 118.3 F g−1@5 A g−1 | 85.3%@1 A g−1 (1200 cycles) | [50] |
| Fe2O3 nanorods/silver nanowires | 1 M Li2SO4 | 287.4 F g−1@0.67 A g−1 | 177.8 F g−1@2 A g−1 | 60%@2 A g−1 (5000 cycles) | [51] |
| Porous α-Fe2O3/graphene | 1 M Na2SO4 | 343.7 F g−1@3 A g−1 | 182.1 F g−1@10 A g−1 | 95.8%@10 A g−1 (50,000 cycles) | [52] |
| α-Fe2O3/porous carbon | 1 M H3PO4 | 372 F g−1@0.7 A g−1 | 294 F g−1@1.5 A g−1 | 82%@1.5 A g−1 (1000 cycles) | [23] |
| Fe2O3/multiwall carbon nanotube film | 1 M Na2SO3 | 431 F g−1@5 mV s−1 | ~160 F g−1@200 mV s−1 | 65%@100 mV s−1 (500 cycles) | [53] |
| α-Fe2O3/carbon nanotube sponge | 2 M KCl | 296.3 F g−1@5 mV s−1 | ~100 F g−1@300 mV s−1 | 60%@100 mV s−1 (1000 cycles) | [25] |
| Ti-doped Fe2O3@PEDOT nanorod arrays | 5 M LiCl | 311.6 F g−1@1 mA cm−2 | 208.1 F g−1@8 mA cm−2 | 96.1%@100 mV s−1 (30,000 cycles) | [54] |
| C3N4/Fe2O3 hollow microspheres | 2.5 M Li2SO4 | 260 F g−1@0.5 A g−1 | 87 F g−1@5 A g−1 | 92%@1 A g−1 (1000 cycles) | [55] |
| α-Fe2O3 HR/RGO-30 composite | 2 M KOH | 426.3 F g−1@1 A g−1 | 219 F g−1@20 A g−1 | 87.7%@10 A g−1 (10,000 cycles) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, G.; Liang, G.; Xiao, W.; Tian, L.; Zhang, Y.; Hu, R.; Wang, Y. Porous α-Fe2O3 Hollow Rods/Reduced Graphene Oxide Composites Templated by MoO3 Nanobelts for High-Performance Supercapacitor Applications. Molecules 2024, 29, 1262. https://doi.org/10.3390/molecules29061262
Zhou G, Liang G, Xiao W, Tian L, Zhang Y, Hu R, Wang Y. Porous α-Fe2O3 Hollow Rods/Reduced Graphene Oxide Composites Templated by MoO3 Nanobelts for High-Performance Supercapacitor Applications. Molecules. 2024; 29(6):1262. https://doi.org/10.3390/molecules29061262
Chicago/Turabian StyleZhou, Gangqiang, Guo Liang, Wei Xiao, Liangliang Tian, Yanhua Zhang, Rong Hu, and Yi Wang. 2024. "Porous α-Fe2O3 Hollow Rods/Reduced Graphene Oxide Composites Templated by MoO3 Nanobelts for High-Performance Supercapacitor Applications" Molecules 29, no. 6: 1262. https://doi.org/10.3390/molecules29061262
APA StyleZhou, G., Liang, G., Xiao, W., Tian, L., Zhang, Y., Hu, R., & Wang, Y. (2024). Porous α-Fe2O3 Hollow Rods/Reduced Graphene Oxide Composites Templated by MoO3 Nanobelts for High-Performance Supercapacitor Applications. Molecules, 29(6), 1262. https://doi.org/10.3390/molecules29061262






