Deep Eutectic Solvents as Agents for Improving the Solubility of Edaravone: Experimental and Theoretical Considerations
Abstract
1. Introduction
2. Results and Discussion
2.1. Solubility Determination
2.2. Instrumental Characteristics of Studied Systems
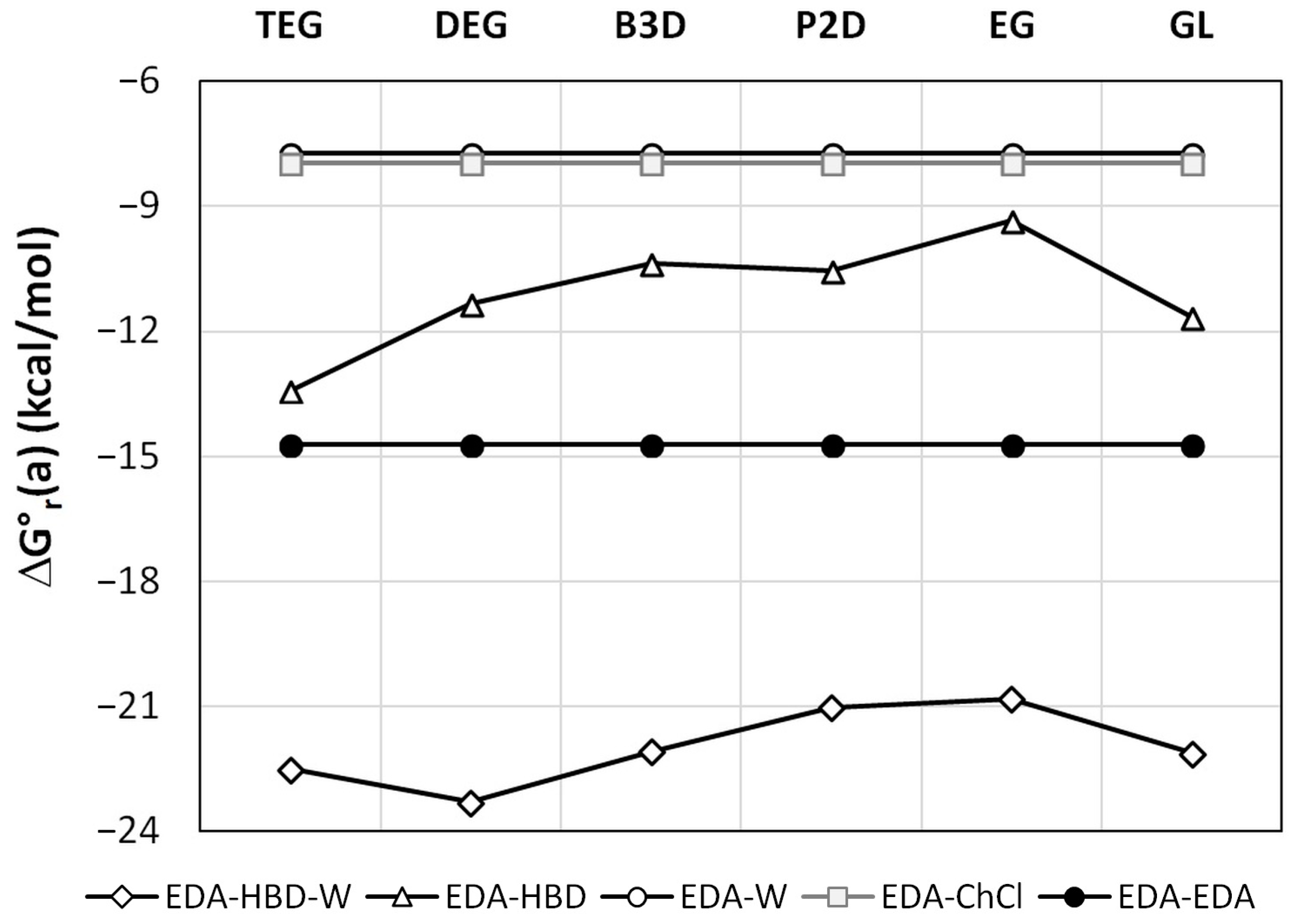
2.3. Intermolecular Interactions of Edaravone in Studied DESs
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Calibration Curve
3.3. Preparation of the Samples and Solubility Measurements
3.4. Instrumental Analysis of the Samples
3.5. Intermolecular Interactions Computations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishi, H.; Watanabe, T.; Sakurai, H.; Yuki, S.; Ishibashi, A. Effect of MCI-186 on brain edema in rats. Stroke 1989, 20, 1236–1240. [Google Scholar] [CrossRef]
- Abe, K.; Yuki, S.; Kogure, K. Strong attenuation of ischemic and postischemic brain edema in rats by a novel free radical scavenger. Stroke 1988, 19, 480–485. [Google Scholar] [CrossRef]
- Drugbank Edaravone. Available online: https://go.drugbank.com/drugs/DB12243 (accessed on 28 November 2022).
- Bhandari, R.; Kuhad, A.; Kuhad, A. Edaravone: A new hope for deadly amyotrophic lateral sclerosis. Drugs Today 2018, 54, 349–360. [Google Scholar] [CrossRef]
- Mao, Y.-F.; Yan, N.; Xu, H.; Sun, J.-H.; Xiong, Y.-C.; Deng, X.-M. Edaravone, a free radical scavenger, is effective on neuropathic pain in rats. Brain Res. 2009, 1248, 68–75. [Google Scholar] [CrossRef]
- Lin, M.; Katsumura, Y.; Hata, K.; Muroya, Y.; Nakagawa, K. Pulse radiolysis study on free radical scavenger edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one). J. Photochem. Photobiol. B Biol. 2007, 89, 36–43. [Google Scholar] [CrossRef]
- Kikuchi, K.; Tancharoen, S.; Takeshige, N.; Yoshitomi, M.; Morioka, M.; Murai, Y.; Tanaka, E. The Efficacy of Edaravone (Radicut), a Free Radical Scavenger, for Cardiovascular Disease. Int. J. Mol. Sci. 2013, 14, 13909–13930. [Google Scholar] [CrossRef]
- Demir, F.; Demir, M.; Aygun, H. Evaluation of the protective effect of edaravone on doxorubicin nephrotoxicity by [99mTc]DMSA renal scintigraphy and biochemical methods. Naunyn. Schmiedebergs. Arch. Pharmacol. 2020, 393, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Koike, N.; Sasaki, A.; Murakami, T.; Suzuki, K. Effect of edaravone against cisplatin-induced chronic renal injury. Drug Chem. Toxicol. 2019, 44, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, M.; Ricardo Pires, J.; Viseu, I.; Magalhães, P.; Gregório, H.; Afreixo, V.; Gregório, T. Edaravone for acute ischemic stroke–Systematic review with meta-analysis. Clin. Neurol. Neurosurg. 2022, 219, 107299. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Potential use of edaravone to reduce specific side effects of chemo-, radio- and immuno-therapy of cancers. Int. Immunopharmacol. 2019, 77, 105967. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. Equilibrium solubility of edaravone in some binary aqueous and non-aqueous solutions reconsidered: Extended Hildebrand solubility approach, transfer property and preferential solvation. J. Mol. Liq. 2021, 331, 115794. [Google Scholar] [CrossRef]
- Wu, X.; Yin, X.; Tang, T.; Zheng, H.; Xu, W.; Lin, Z.; Chen, X.; Li, R.; Zhao, J.; Han, D. Solubility of Edaravone in Four Mixed Solvents at 273.15-313.15 K and Correlation of Jouyban-Acree and CNIBS/R-K Models. J. Chem. Eng. Data 2020, 65, 1460–1467. [Google Scholar] [CrossRef]
- Qiu, J.; Huang, H.; He, H.; Liu, H.; Hu, S.; Han, J.; Yi, D.; An, M.; Guo, Y.; Wang, P. Solubility Determination and Thermodynamic Modeling of Edaravone in Different Solvent Systems and the Solvent Effect in Pure Solvents. J. Chem. Eng. Data 2020, 65, 3240–3251. [Google Scholar] [CrossRef]
- Li, R.; Yao, L.; Khan, A.; Zhao, B.; Wang, D.; Zhao, J.; Han, D. Co-solvence phenomenon and thermodynamic properties of edaravone in pure and mixed solvents. J. Chem. Thermodyn. 2019, 138, 304–312. [Google Scholar] [CrossRef]
- Abraham, R.J.; Cooper, M.A.; Aghamohammadi, A.; Afarinkia, K.; Liu, X. The Use of MM/QM Calculations of 13C Chemical Shifts in the Analysis of Edaravone Tautomers. J. Solution Chem. 2022, 51, 1162–1167. [Google Scholar] [CrossRef]
- Fakhraian, H.; Nafari, Y. Preparative, mechanistic and tautomeric investigation of 1-phenyl and 1-methyl derivative of 3-methyl-5-pyrazolone. J. Chem. Sci. 2021, 133, 40. [Google Scholar] [CrossRef]
- Freyer, W.; Köppel, H.; Radeglia, R.; Malewski, G. 1H-NMR-, 13C-NMR-, and IR-Investigations Concerning Tautomerism of 15N-Labeled 3-Methyl-1-phenyl-Δ2-pyrazolin-5-one. J. Prakt. Chem. 1983, 325, 238–250. [Google Scholar] [CrossRef]
- Martínez, F.; Jouyban, A.; Acree, W.E. Pharmaceuticals solubility is still nowadays widely studied everywhere. Pharm. Sci. 2017, 23, 1–2. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Tran, P.; Pyo, Y.-C.; Kim, D.-H.; Lee, S.-E.; Kim, J.-K.; Park, J.-S. Overview of the Manufacturing Methods of Solid Dispersion Technology for Improving the Solubility of Poorly Water-Soluble Drugs and Application to Anticancer Drugs. Pharmaceutics 2019, 11, 132. [Google Scholar] [CrossRef]
- Hancock, B.C.; York, P.; Rowe, R.C. The use of solubility parameters in pharmaceutical dosage form design. Int. J. Pharm. 1997, 148, 1–21. [Google Scholar] [CrossRef]
- Blagden, N.; de Matas, M.; Gavan, P.T.; York, P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv. Drug Deliv. Rev. 2007, 59, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Grossmann, L.; McClements, D.J. Current insights into protein solubility: A review of its importance for alternative proteins. Food Hydrocoll. 2023, 137, 108416. [Google Scholar] [CrossRef]
- Sou, T.; Bergström, C.A.S. Automated assays for thermodynamic (equilibrium) solubility determination. Drug Discov. Today Technol. 2018, 27, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Chen, H. Application of deep eutectic solvents (DESs) as trace level drug extractants and drug solubility enhancers: State-of-the-art, prospects and challenges. J. Mol. Liq. 2022, 349, 118105. [Google Scholar] [CrossRef]
- Suwanwong, Y.; Boonpangrak, S. Molecularly imprinted polymers for the extraction and determination of water-soluble vitamins: A review from 2001 to 2020. Eur. Polym. J. 2021, 161, 110835. [Google Scholar] [CrossRef]
- Huang, L.; Tong, W.-Q. Impact of solid state properties on developability assessment of drug candidates. Adv. Drug Deliv. Rev. 2004, 56, 321–334. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef]
- Scholz, A.; Abrahamsson, B.; Diebold, S.M.; Kostewicz, E.; Polentarutti, B.I.; Ungell, A.-L.; Dressman, J.B. Influence of hydrodynamics and particle size on the absorption of felodipine in labradors. Pharm. Res. 2002, 19, 42–46. [Google Scholar] [CrossRef]
- Janssens, S.; Van den Mooter, G. Review: Physical chemistry of solid dispersions. J. Pharm. Pharmacol. 2009, 61, 1571–1586. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Korn, C.; Balbach, S. Compound selection for development–Is salt formation the ultimate answer? Experiences with an extended concept of the “100mg approach”. Eur. J. Pharm. Sci. 2014, 57, 257–263. [Google Scholar] [CrossRef]
- Chadha, R.; Bhalla, Y.; Vashisht, M.K.; Chadha, K. Cocrystallization in Nutraceuticals. In Recrystallization in Materials Processing, 1st ed.; Glebovsky, V., Ed.; InTech: London, UK, 2015. [Google Scholar] [CrossRef]
- Seedher, N.; Kanojia, M. Co-solvent solubilization of some poorly-soluble antidiabetic drugs. Pharm. Dev. Technol. 2009, 14, 185–192. [Google Scholar] [CrossRef]
- Lovette, M.A. Solubility Model to Guide Solvent Selection in Synthetic Process Development. Cryst. Growth Des. 2022, 22, 4404–4420. [Google Scholar] [CrossRef]
- Modarresi, H.; Conte, E.; Abildskov, J.; Gani, R.; Crafts, P. Model-Based Calculation of Solid Solubility for Solvent Selection—A Review. Ind. Eng. Chem. Res. 2008, 47, 5234–5242. [Google Scholar] [CrossRef]
- Eckert, F.; Klamt, A. Fast solvent screening via quantum chemistry: COSMO-RS approach. AIChE J. 2002, 48, 369–385. [Google Scholar] [CrossRef]
- Przybyłek, M.; Miernicka, A.; Nowak, M.; Cysewski, P. New Screening Protocol for Effective Green Solvents Selection of Benzamide, Salicylamide and Ethenzamide. Molecules 2022, 27, 3323. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Ferreira, A.M.; Okura, T.; Pinheiro Rolemberg, M.; Mafra, M.R.; Coutinho, J.A.P. Using COSMO-RS to Predict Hansen Solubility Parameters. Ind. Eng. Chem. Res. 2022, 61, 15631–15638. [Google Scholar] [CrossRef]
- Buarque, F.S.; Lima, T.S.P.; Carniel, A.; Ribeiro, B.D.; Coelho, M.A.Z.; Souza, R.L.; Soares, C.M.F.; Pereira, M.M.; Lima, Á.S. Hormones Concentration in an Aqueous Two-Phase System: Experimental and Computational Analysis. Chem. Eng. Technol. 2024. [Google Scholar] [CrossRef]
- Buarque, F.S.; Lima, N.S.; Soares, C.M.F.; Marques, M.N.; Cavalcanti, E.B.; Pereira, M.M.; Souza, R.L.; Lima, Á.S. Preconcentration and chromatographic detection of atrazine in real water sample using aqueous two-phase system based on tetrahydrofuran and glycerol. Environ. Qual. Manag. 2021, 31, 39–48. [Google Scholar] [CrossRef]
- Guidetti, M.; Hilfiker, R.; Kuentz, M.; Bauer-Brandl, A.; Blatter, F. Exploring the Cocrystal Landscape of Posaconazole by Combining High-Throughput Screening Experimentation with Computational Chemistry. Cryst. Growth Des. 2023, 23, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, M.; Ziółkowska, D.; Mroczyńska, K.; Cysewski, P. Applicability of Phenolic Acids as Effective Enhancers of Cocrystal Solubility of Methylxanthines. Cryst. Growth Des. 2017, 17, 2186–2193. [Google Scholar] [CrossRef]
- DeSimone, J.M. Practical approaches to green solvents. Science 2002, 297, 799–803. [Google Scholar] [CrossRef]
- Jessop, P.G. Searching for green solvents. Green Chem. 2011, 13, 1391–1398. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Häckl, K.; Kunz, W. Some aspects of green solvents. Comptes Rendus Chim. 2018, 21, 572–580. [Google Scholar] [CrossRef]
- e Silva, A.P.S.; Pires, F.C.S.; Ferreira, M.C.R.; Silva, I.Q.; Aires, G.C.M.; Ribeiro, T.M.; Ortiz, E.G.; Martins, M.L.H.S.; de Carvalho, R.N. Case studies of green solvents in the pharmaceutical industry. In Green Sustainable Process for Chemical and Environmental Engineering and Science, Solvents for the Pharmaceutical Industry, 1st ed.; Innamiudin, Boddula, R., Ahamed, M.I., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 151–159. [Google Scholar] [CrossRef]
- Becker, J.; Manske, C.; Randl, S. Green chemistry and sustainability metrics in the pharmaceutical manufacturing sector. Curr. Opin. Green Sustain. Chem. 2022, 33, 100562. [Google Scholar] [CrossRef]
- Mishra, M.; Sharma, M.; Dubey, R.; Kumari, P.; Ranjan, V.; Pandey, J. Green synthesis interventions of pharmaceutical industries for sustainable development. Curr. Res. Green Sustain. Chem. 2021, 4, 100174. [Google Scholar] [CrossRef]
- Santana-Mayor, Á.; Rodríguez-Ramos, R.; Herrera-Herrera, A.V.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Deep eutectic solvents. The new generation of green solvents in analytical chemistry. TrAC Trends Anal. Chem. 2021, 134, 116108. [Google Scholar] [CrossRef]
- Perna, F.M.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sustain. Chem. 2020, 21, 27–33. [Google Scholar] [CrossRef]
- Vanda, H.; Dai, Y.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. Comptes Rendus Chim. 2018, 21, 628–638. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Jurić, T.; Uka, D.; Holló, B.B.; Jović, B.; Kordić, B.; Popović, B.M. Comprehensive physicochemical evaluation of choline chloride-based natural deep eutectic solvents. J. Mol. Liq. 2021, 343, 116968. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Zhao, Y.; Wang, J.; Yu, Z. Insights into the Hydrogen Bond Interactions in Deep Eutectic Solvents Composed of Choline Chloride and Polyols. ACS Sustain. Chem. Eng. 2019, 7, 7760–7767. [Google Scholar] [CrossRef]
- Biernacki, K.; Souza, H.K.S.; Almeida, C.M.R.; Magalhães, A.L.; Goncąlves, M.P. Physicochemical Properties of Choline Chloride-Based Deep Eutectic Solvents with Polyols: An Experimental and Theoretical Investigation. ACS Sustain. Chem. Eng. 2020, 8, 18712–18728. [Google Scholar] [CrossRef]
- Espino, M.; de los Ángeles Fernández, M.; Gomez, F.J.V.; Silva, M.F. Natural designer solvents for greening analytical chemistry. TrAC Trends Anal. Chem. 2016, 76, 126–136. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents–Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Lomba, L.; Ribate, M.P.; Zaragoza, E.; Concha, J.; Garralaga, M.P.; Errazquin, D.; García, C.B.; Giner, B. Deep Eutectic Solvents: Are They Safe? Appl. Sci. 2021, 11, 10061. [Google Scholar] [CrossRef]
- De Morais, P.; Gonçalves, F.; Coutinho, J.A.P.; Ventura, S.P.M. Ecotoxicity of Cholinium-Based Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2015, 3, 3398–3404. [Google Scholar] [CrossRef]
- Macário, I.P.E.; Jesus, F.; Pereira, J.L.; Ventura, S.P.M.; Gonçalves, A.M.M.; Coutinho, J.A.P.; Gonçalves, F.J.M. Unraveling the ecotoxicity of deep eutectic solvents using the mixture toxicity theory. Chemosphere 2018, 212, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Lapeña, D.; Errazquin, D.; Lomba, L.; Lafuente, C.; Giner, B. Ecotoxicity and biodegradability of pure and aqueous mixtures of deep eutectic solvents: Glyceline, ethaline, and reline. Environ. Sci. Pollut. Res. 2021, 28, 8812–8821. [Google Scholar] [CrossRef]
- Emami, S.; Shayanfar, A. Deep eutectic solvents for pharmaceutical formulation and drug delivery applications. Pharm. Dev. Technol. 2020, 25, 779–796. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Liu, J.; Wang, W.; Yang, Q.; Yang, G. Deep eutectic solvents: Recent advances in fabrication approaches and pharmaceutical applications. Int. J. Pharm. 2022, 622, 121811. [Google Scholar] [CrossRef] [PubMed]
- Cysewski, P.; Jeliński, T.; Przybyłek, M. Intermolecular Interactions of Edaravone in Aqueous Solutions of Ethaline and Glyceline Inferred from Experiments and Quantum Chemistry Computations. Molecules 2023, 28, 629. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, M.; Jeliński, T.; Mianowana, M.; Misiak, K.; Cysewski, P. Exploring the Solubility Limits of Edaravone in Neat Solvents and Binary Mixtures: Experimental and Machine Learning Study. Molecules 2023, 28, 6877. [Google Scholar] [CrossRef]
- Esfahani, H.S.; Khoshsima, A.; Pazuki, G. Choline chloride-based deep eutectic solvents as green extractant for the efficient extraction of 1-butanol or 2-butanol from azeotropic n-heptane + butanol mixtures. J. Mol. Liq. 2020, 313, 113524. [Google Scholar] [CrossRef]
- Chen, Q.; He, N.; Fan, J.; Song, F. Physical Properties of Betaine-1,2-Propanediol-Based Deep Eutectic Solvents. Polymers 2022, 14, 1783. [Google Scholar] [CrossRef]
- Bu, F.; Zhao, Y.; Li, B.; Zhang, X.; Li, J. The effect of choline chloride-butanediol based deep eutectic solvents on ultrasound-assisted extraction, antioxidant activity and stability of anthocyanins extracted from Perilla frutescens (L.) Britt. Sustain. Chem. Pharm. 2023, 32, 101000. [Google Scholar] [CrossRef]
- Basaiahgari, A.; Panda, S.; Gardas, R.L. Effect of Ethylene, Diethylene, and Triethylene Glycols and Glycerol on the Physicochemical Properties and Phase Behavior of Benzyltrimethyl and Benzyltributylammonium Chloride Based Deep Eutectic Solvents at 283.15–343.15 K. J. Chem. Eng. Data 2018, 63, 2613–2627. [Google Scholar] [CrossRef]
- Gabriele, F.; Chiarini, M.; Germani, R.; Tiecco, M.; Spreti, N. Effect of water addition on choline chloride/glycol deep eutectic solvents: Characterization of their structural and physicochemical properties. J. Mol. Liq. 2019, 291, 111301. [Google Scholar] [CrossRef]
- Aissaoui, T.; Benguerba, Y.; AlNashef, I.M. Theoretical investigation on the microstructure of triethylene glycol based deep eutectic solvents: COSMO-RS and TURBOMOLE prediction. J. Mol. Struct. 2017, 1141, 451–456. [Google Scholar] [CrossRef]
- Hayyan, M.; Aissaoui, T.; Hashim, M.A.; AlSaadi, M.A.H.; Hayyan, A. Triethylene glycol based deep eutectic solvents and their physical properties. J. Taiwan Inst. Chem. Eng. 2015, 50, 24–30. [Google Scholar] [CrossRef]
- Cysewski, P.; Jeliński, T.; Cymerman, P.; Przybyłek, M. Solvent Screening for Solubility Enhancement of Theophylline in Neat, Binary and Ternary NADES Solvents: New Measurements and Ensemble Machine Learning. Int. J. Mol. Sci. 2021, 22, 7347. [Google Scholar] [CrossRef]
- Nakamaru, Y.; Kinoshita, S.; Kawaguchi, A.; Takei, K.; Palumbo, J.; Suzuki, M. Pharmacokinetic profile of edaravone: A comparison between Japanese and Caucasian populations. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Shukla, S. Role of Edaravone as a Treatment Option for Patients with Amyotrophic Lateral Sclerosis. Pharmaceuticals 2020, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Isci, A.; Kaltschmitt, M. Recovery and recycling of deep eutectic solvents in biomass conversions: A review. Biomass Convers. Biorefinery 2022, 12, 197–226. [Google Scholar] [CrossRef]
- Nguyen, H.V.D.; De Vries, R.; Stoyanov, S.D. Natural Deep Eutectics as a “green” Cellulose Cosolvent. ACS Sustain. Chem. Eng. 2020, 8, 14166–14178. [Google Scholar] [CrossRef]
- Ratti, R. Industrial applications of green chemistry: Status, Challenges and Prospects. SN Appl. Sci. 2020, 2, 263. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, S.; Ma, P. Use of ionic liquids as ‘green’ solvents for extractions. J. Chem. Technol. Biotechnol. 2005, 80, 1089–1096. [Google Scholar] [CrossRef]
- Salehpour, S.; Dubé, M.A. Reaction Monitoring of Glycerol Step-Growth Polymerization Using ATR-FTIR Spectroscopy. Macromol. React. Eng. 2012, 6, 85–92. [Google Scholar] [CrossRef]
- Cysewski, P.; Jeliński, T.; Przybyłek, M. Application of COSMO-RS-DARE as a Tool for Testing Consistency of Solubility Data: Case of Coumarin in Neat Alcohols. Molecules 2022, 27, 5274. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, M.; Kowalska, A.; Tymorek, N.; Dziaman, T.; Cysewski, P. Thermodynamic Characteristics of Phenacetin in Solid State and Saturated Solutions in Several Neat and Binary Solvents. Molecules 2021, 26, 4078. [Google Scholar] [CrossRef] [PubMed]
- Cysewski, P.; Przybyłek, M.; Kowalska, A.; Tymorek, N. Thermodynamics and Intermolecular Interactions of Nicotinamide in Neat and Binary Solutions: Experimental Measurements and COSMO-RS Concentration Dependent Reactions Investigations. Int. J. Mol. Sci. 2021, 22, 7365. [Google Scholar] [CrossRef] [PubMed]
- Cysewski, P.; Przybyłek, M.; Rozalski, R. Experimental and theoretical screening for green solvents improving sulfamethizole solubility. Materials 2021, 14, 5915. [Google Scholar] [CrossRef] [PubMed]
- Cysewski, P.; Jeliński, T.; Przybyłek, M.; Nowak, W.; Olczak, M. Solubility Characteristics of Acetaminophen and Phenacetin in Binary Mixtures of Aqueous Organic Solvents: Experimental and Deep Machine Learning Screening of Green Dissolution Media. Pharmaceutics 2022, 14, 2828. [Google Scholar] [CrossRef]
- Cysewski, P.; Przybyłek, M.; Jeliński, T. Intermolecular Interactions as a Measure of Dapsone Solubility in Neat Solvents and Binary Solvent Mixtures. Materials 2023, 16, 6336. [Google Scholar] [CrossRef]
- Dassault Systèmes, Biovia. COSMOtherm, Version 22.0.0; Dassault Systèmes, Biovia: San Diego, CA, USA, 2022.
- TURBOMOLE GmbH. TURBOMOLE, Version 7.6.0; TURBOMOLE GmbH: Karlsruhe, Germany, 2021.
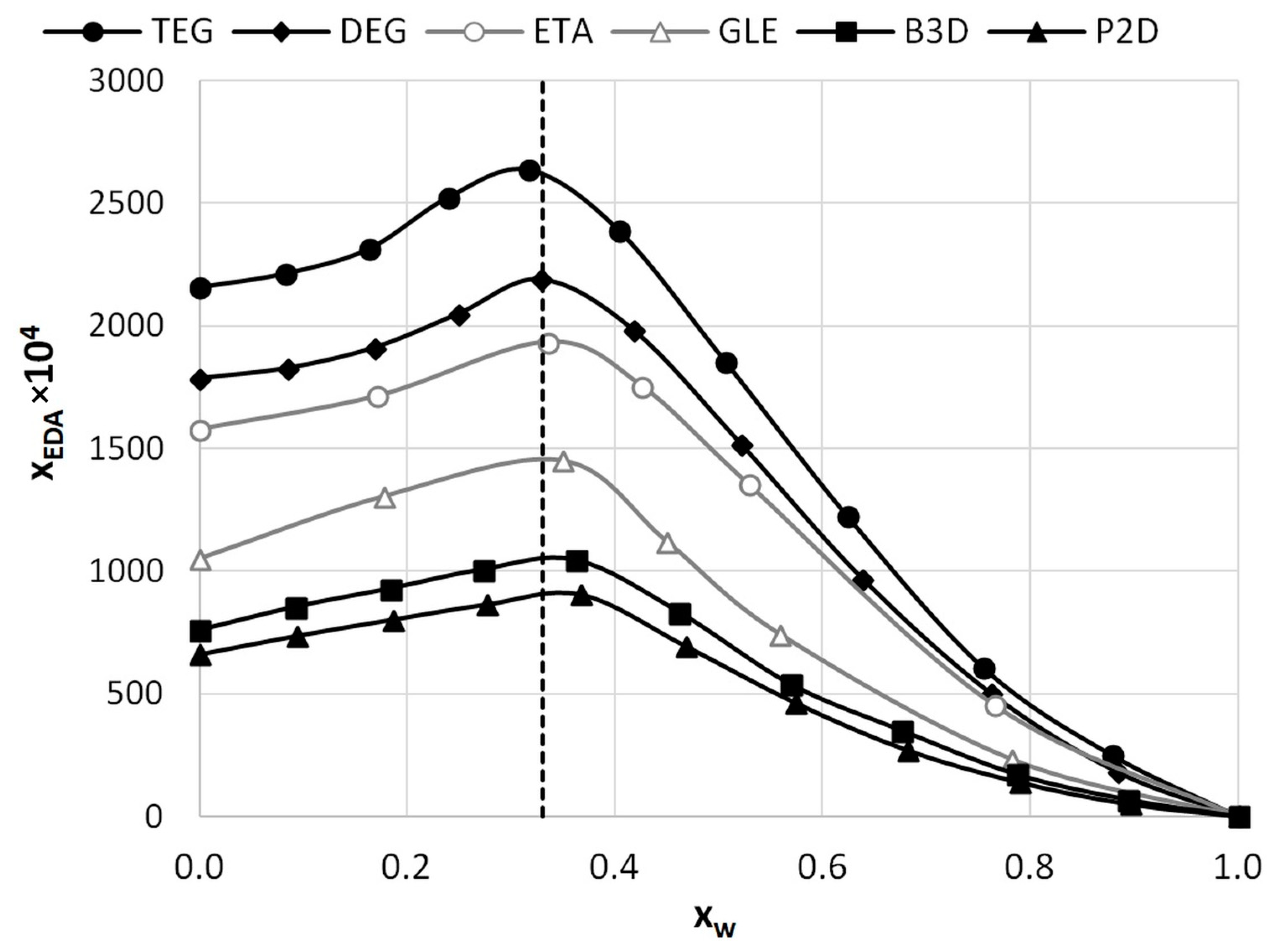



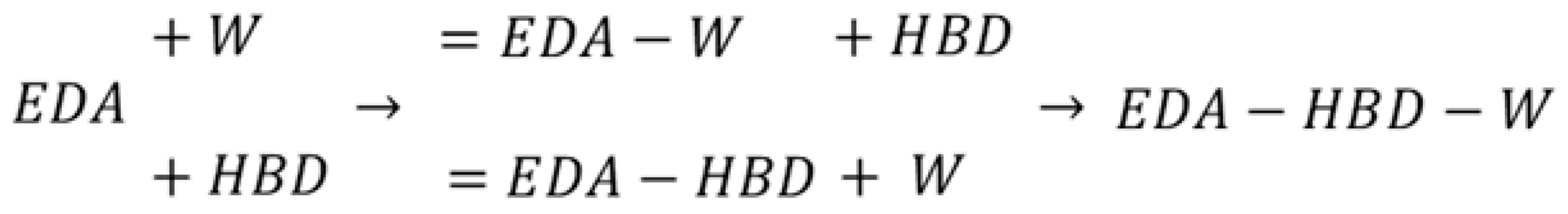
| HBD | 1:1 1 | 1:2 | 1:4 | |||
|---|---|---|---|---|---|---|
| xE (×104) | cE (mg/mL) | xE (×104) | cE (mg/mL) | xE (×104) | cE (mg/mL) | |
| P2D | 464.15 (±6.51) | 79.01 (±1.00) | 660.63 (±7.59) | 120.6 (±1.27) | 560.48 (±3.17) | 110.86 (±0.52) |
| B3D | 572.96 (±4.75) | 88.53 (±0.74) | 760.16 (±8.22) | 123.21 (±1.25) | 658.32 (±11.89) | 112.47 (±2.01) |
| DEG | 1142.86 (±12.68) | 175.47 (±2.24) | 1786.68 (±32.15) | 274.56 (±4.47) | 1430.41 (±33.48) | 227.6 (±5.31) |
| TEG | 1338.15 (±24.28) | 182.36 (±3.13) | 2158.65 (±28.83) | 283.35 (±3.78) | 1730.97 (±44.63) | 224.62 (±5.19) |
| x*DES 1 | P2D | B3D | DEG | TEG | ||||
|---|---|---|---|---|---|---|---|---|
| xE (×104) | cE (mg/mL) | xE (×104) | cE (mg/mL) | xE (×104) | cE (mg/mL) | xE (×104) | cE (mg/mL) | |
| 0.0 2 | 1.73 (±0.01) | 1.72 (±0.01) | 1.73 (±0.01) | 1.72 (±0.01) | 1.73 (±0.01) | 1.72 (±0.01) | 1.73 (±0.01) | 1.72 (±0.01) |
| 0.1 | 50.98 (±0.45) | 34.08 (±0.32) | 70.37 (±1.40) | 44.66 (±0.84) | 181.86 (±6.36) | 108.49 (±3.61) | 251.94 (±5.21) | 136.67 (±2.81) |
| 0.2 | 140.32 (±2.37) | 71.00 (±1.19) | 173.10 (±3.15) | 81.10 (±1.32) | 502.69 (±22.14) | 210.35 (±7.66) | 607.00 (±10.63) | 217.30 (±3.64) |
| 0.3 | 270.15 (±7.15) | 108.69 (±2.67) | 350.90 (±5.04) | 127.93 (±1.52) | 969.29 (±21.54) | 305.56 (±5.60) | 1223.75 (±19.47) | 325.47 (±3.52) |
| 0.4 | 463.56 (±8.70) | 153.13 (±2.61) | 538.01 (±9.72) | 161.55 (±2.67) | 1518.63 (±23.66) | 384.60 (±4.36) | 1852.86 (±13.62) | 398.73 (±2.73) |
| 0.5 | 694.74 (±26.13) | 195.31 (±6.52) | 831.86 (±8.04) | 209.27 (±1.69) | 1983.79 (±41.52) | 431.02 (±7.05) | 2388.18 (±23.29) | 444.40 (±4.09) |
| 0.6 | 904.23 (±9.14) | 222.13 (±1.86) | 1044.48 (±23.52) | 229.98 (±4.43) | 2189.62 (±40.44) | 429.00 (±6.24) | 2635.92 (±25.61) | 447.50 (±3.27) |
| 0.7 | 863.77 (±38.78) | 195.04 (±7.79) | 1007.49 (±15.00) | 203.22 (±2.83) | 2049.69 (±42.53) | 377.45 (±6.40) | 2524.97 (±37.72) | 397.24 (±4.90) |
| 0.8 | 801.94 (±10.87) | 168.23 (±1.97) | 928.82 (±34.85) | 173.25 (±6.06) | 1911.17 (±62.54) | 331.84 (±9.52) | 2313.80 (±40.79) | 343.41 (±5.34) |
| 0.9 | 736.17 (±10.62) | 143.75 (±1.93) | 854.66 (±13.27) | 148.11 (±2.21) | 1828.09 (±51.23) | 299.48 (±7.79) | 2213.70 (±45.66) | 308.19 (±6.00) |
| 1.0 | 660.63 (±7.59) | 120.60 (±1.27) | 760.16 (±8.22) | 123.21 (±1.25) | 1786.68 (±32.15) | 274.56 (±4.47) | 2158.65 (±28.83) | 283.35 (±3.78) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeliński, T.; Przybyłek, M.; Mianowana, M.; Misiak, K.; Cysewski, P. Deep Eutectic Solvents as Agents for Improving the Solubility of Edaravone: Experimental and Theoretical Considerations. Molecules 2024, 29, 1261. https://doi.org/10.3390/molecules29061261
Jeliński T, Przybyłek M, Mianowana M, Misiak K, Cysewski P. Deep Eutectic Solvents as Agents for Improving the Solubility of Edaravone: Experimental and Theoretical Considerations. Molecules. 2024; 29(6):1261. https://doi.org/10.3390/molecules29061261
Chicago/Turabian StyleJeliński, Tomasz, Maciej Przybyłek, Magdalena Mianowana, Kinga Misiak, and Piotr Cysewski. 2024. "Deep Eutectic Solvents as Agents for Improving the Solubility of Edaravone: Experimental and Theoretical Considerations" Molecules 29, no. 6: 1261. https://doi.org/10.3390/molecules29061261
APA StyleJeliński, T., Przybyłek, M., Mianowana, M., Misiak, K., & Cysewski, P. (2024). Deep Eutectic Solvents as Agents for Improving the Solubility of Edaravone: Experimental and Theoretical Considerations. Molecules, 29(6), 1261. https://doi.org/10.3390/molecules29061261








