2-Azidobenzaldehyde-Based [4+2] Annulation for the Synthesis of Quinoline Derivatives
Abstract
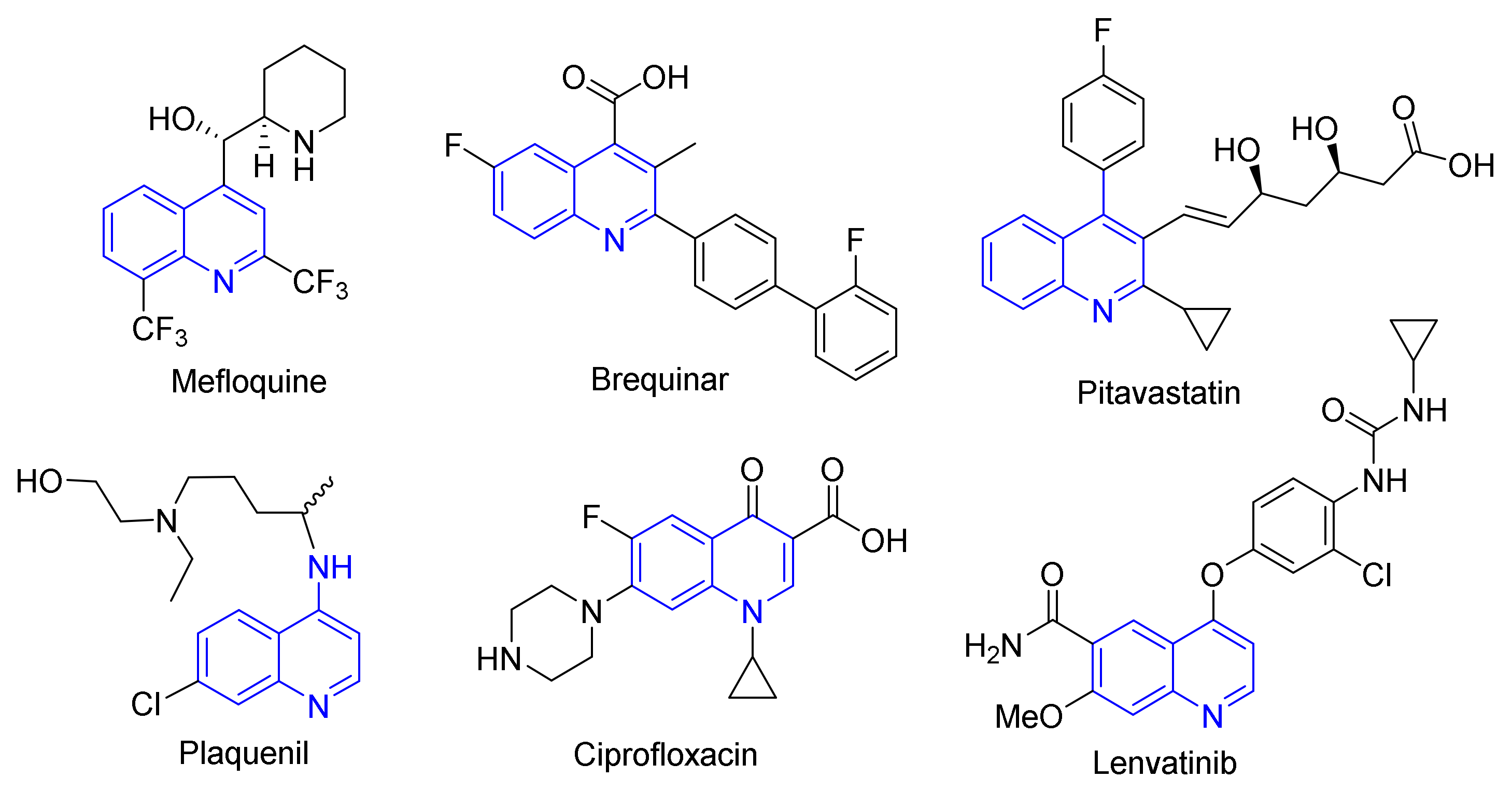
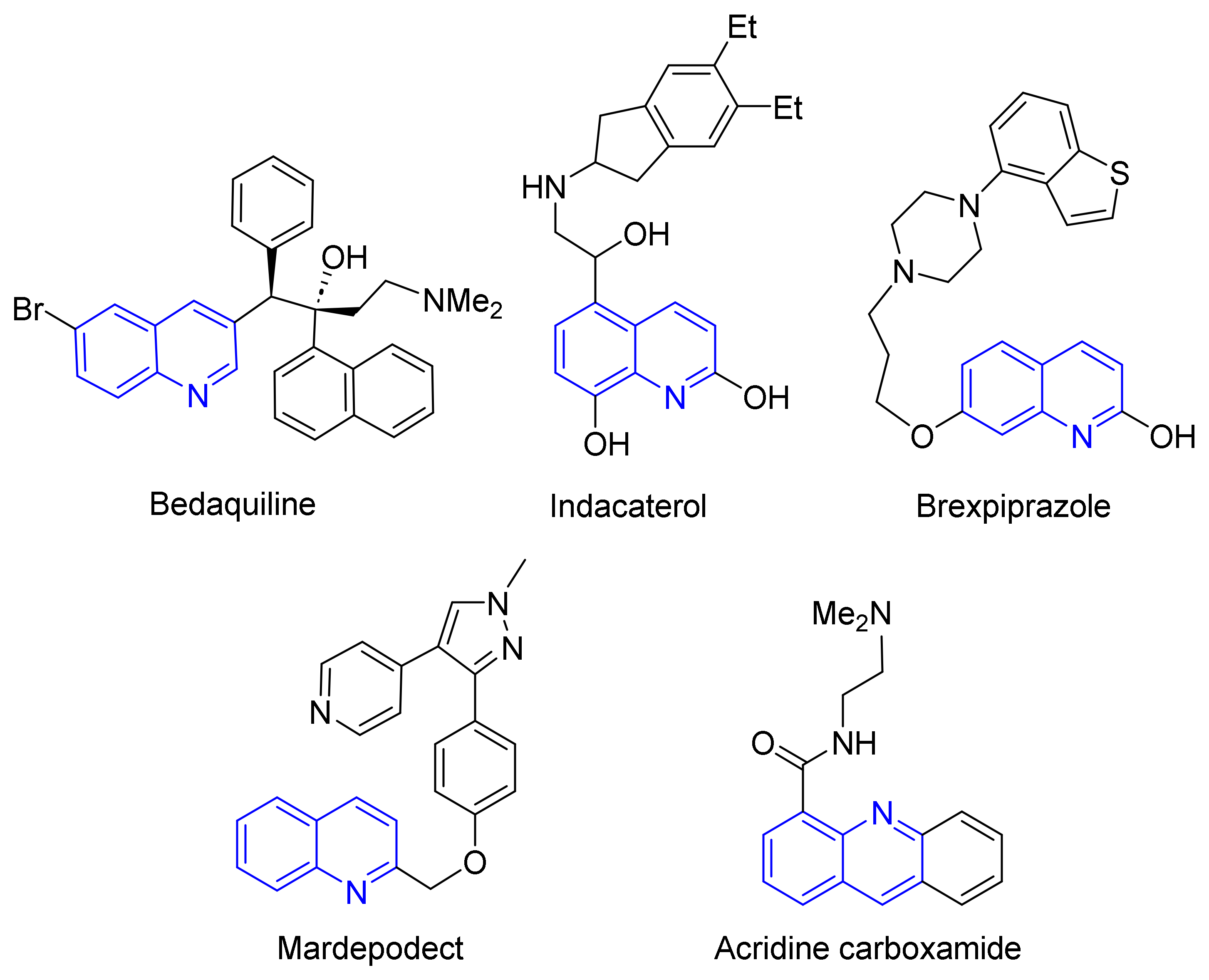
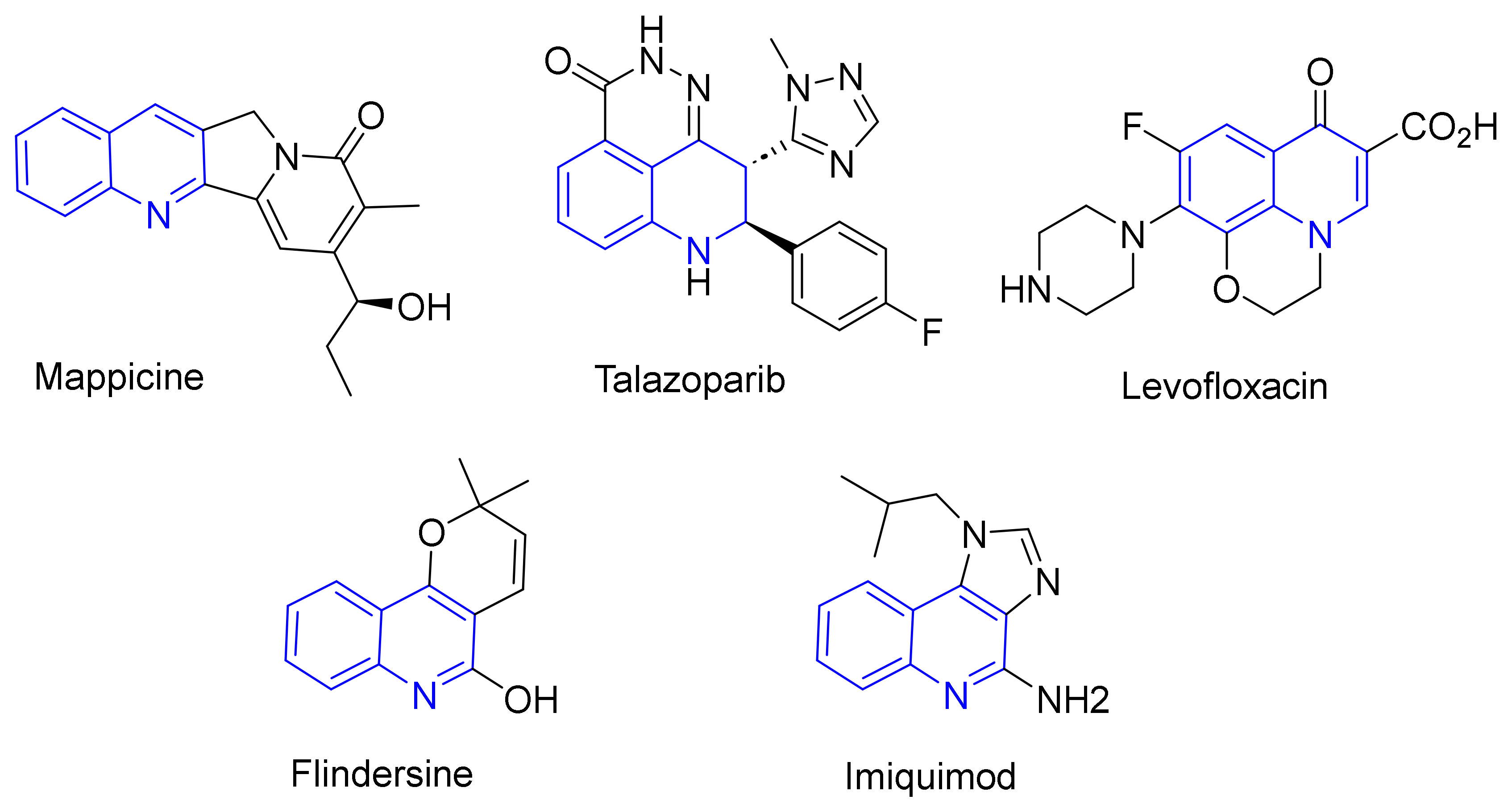
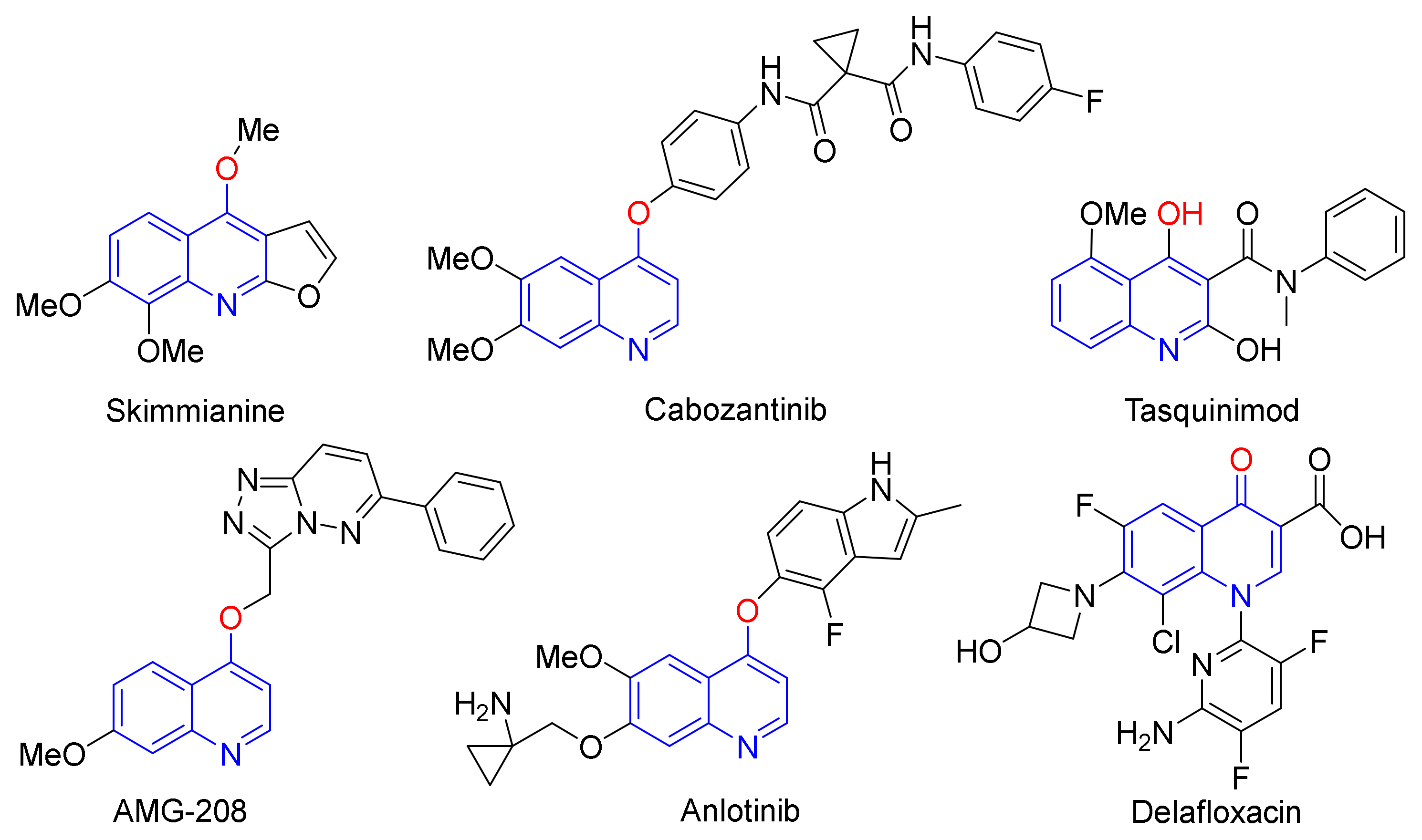
1. Introduction
2. Results
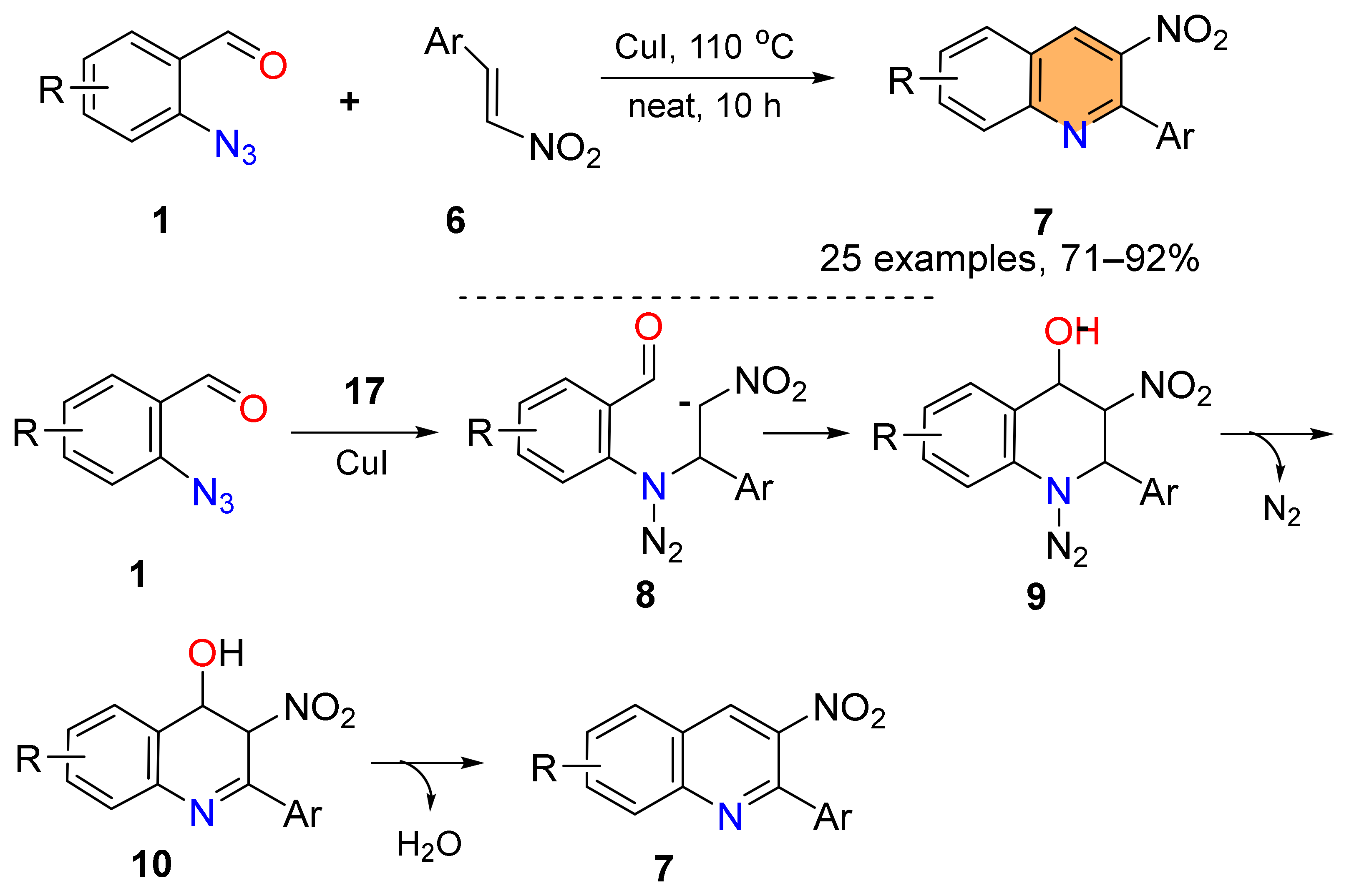
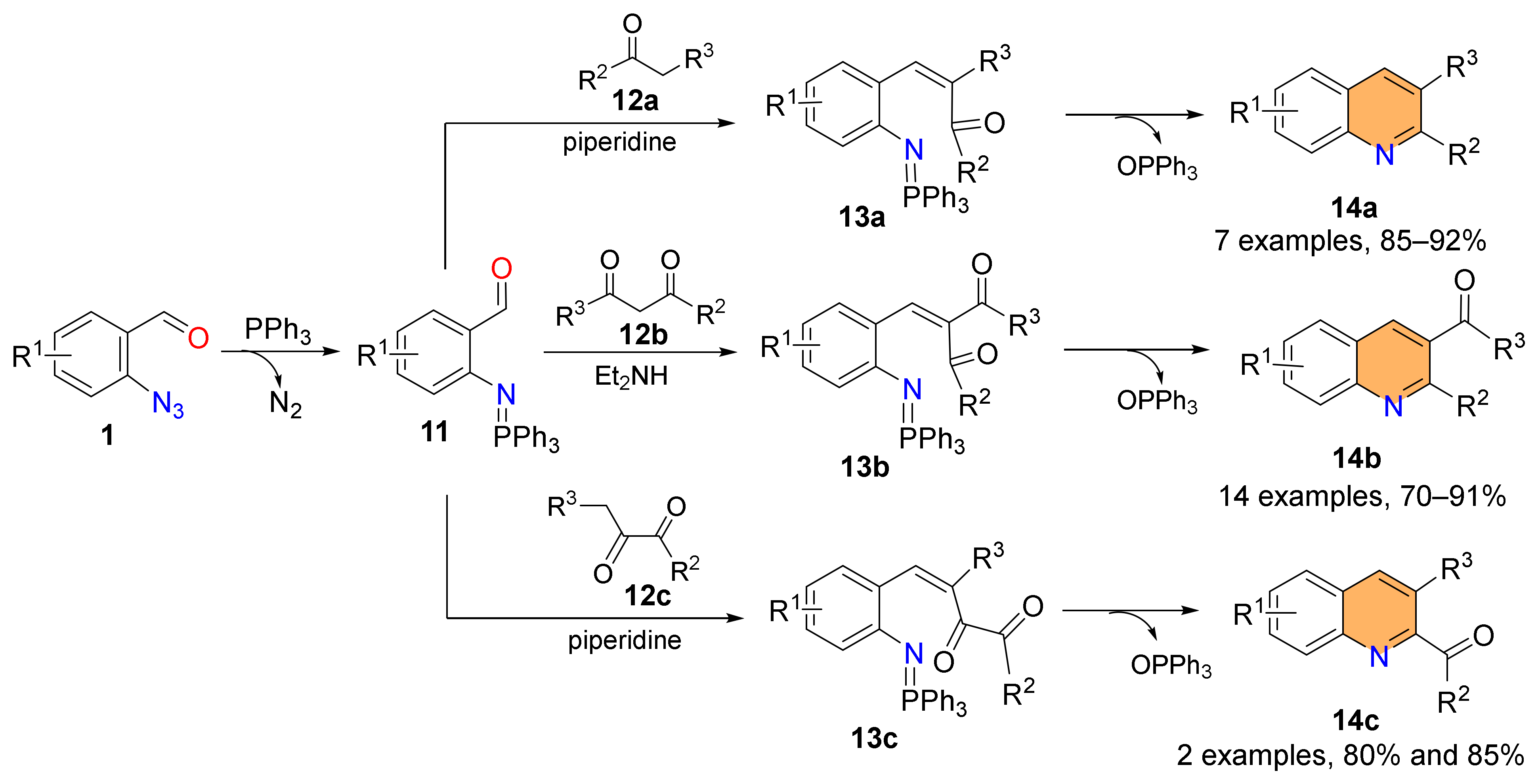
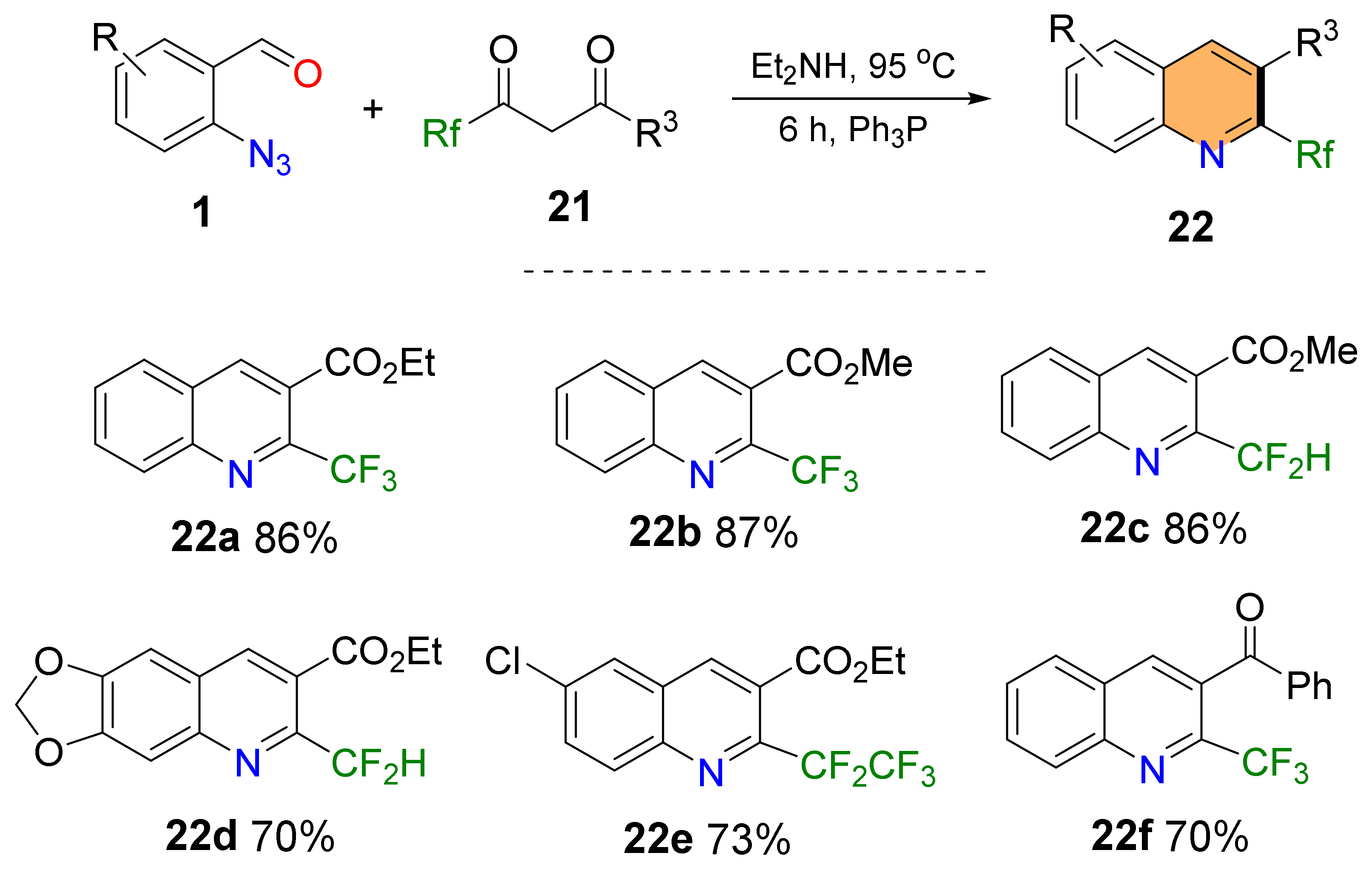
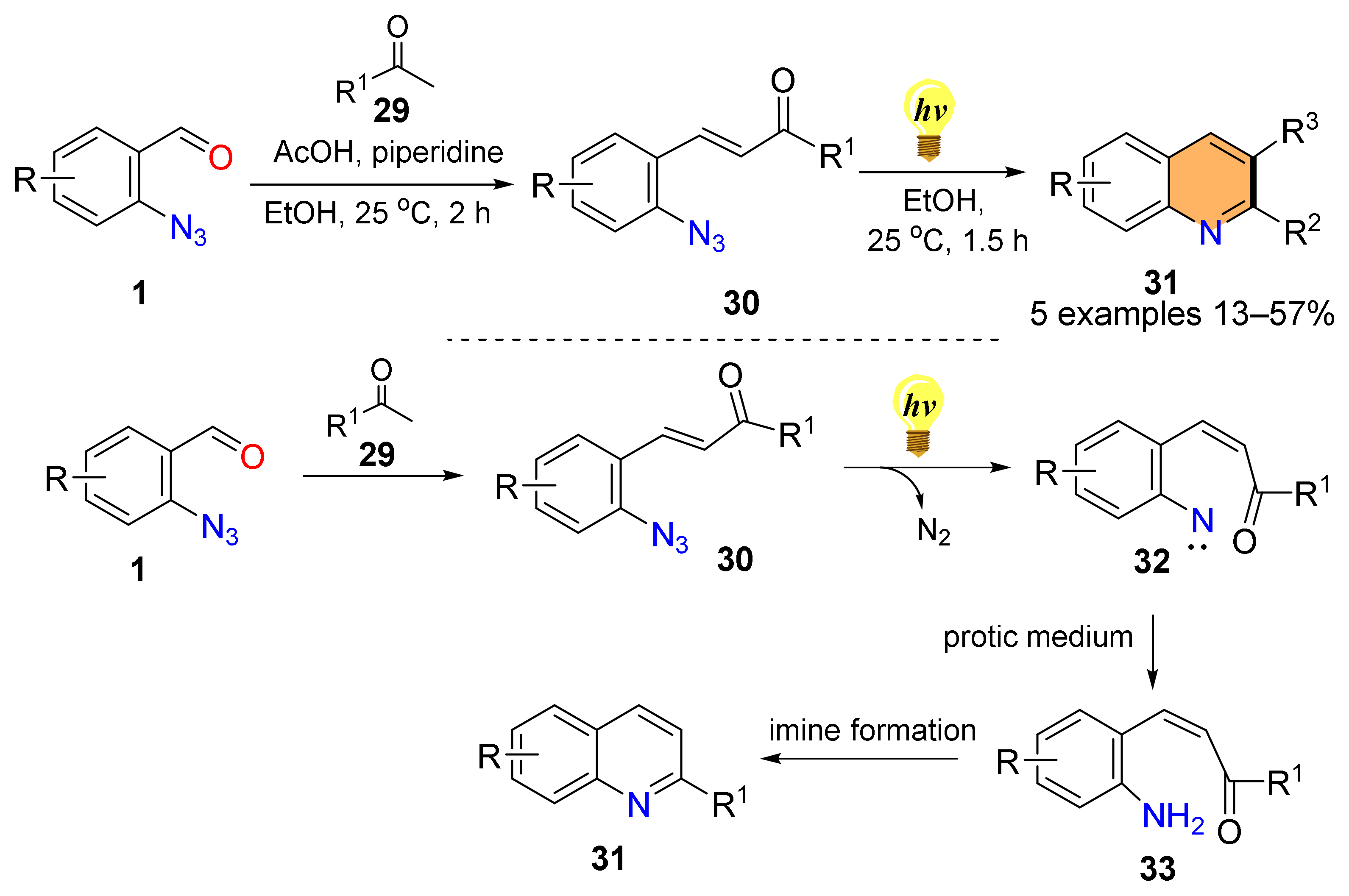
2.1. Synthesis of 4-Unsubstituted Quinolines
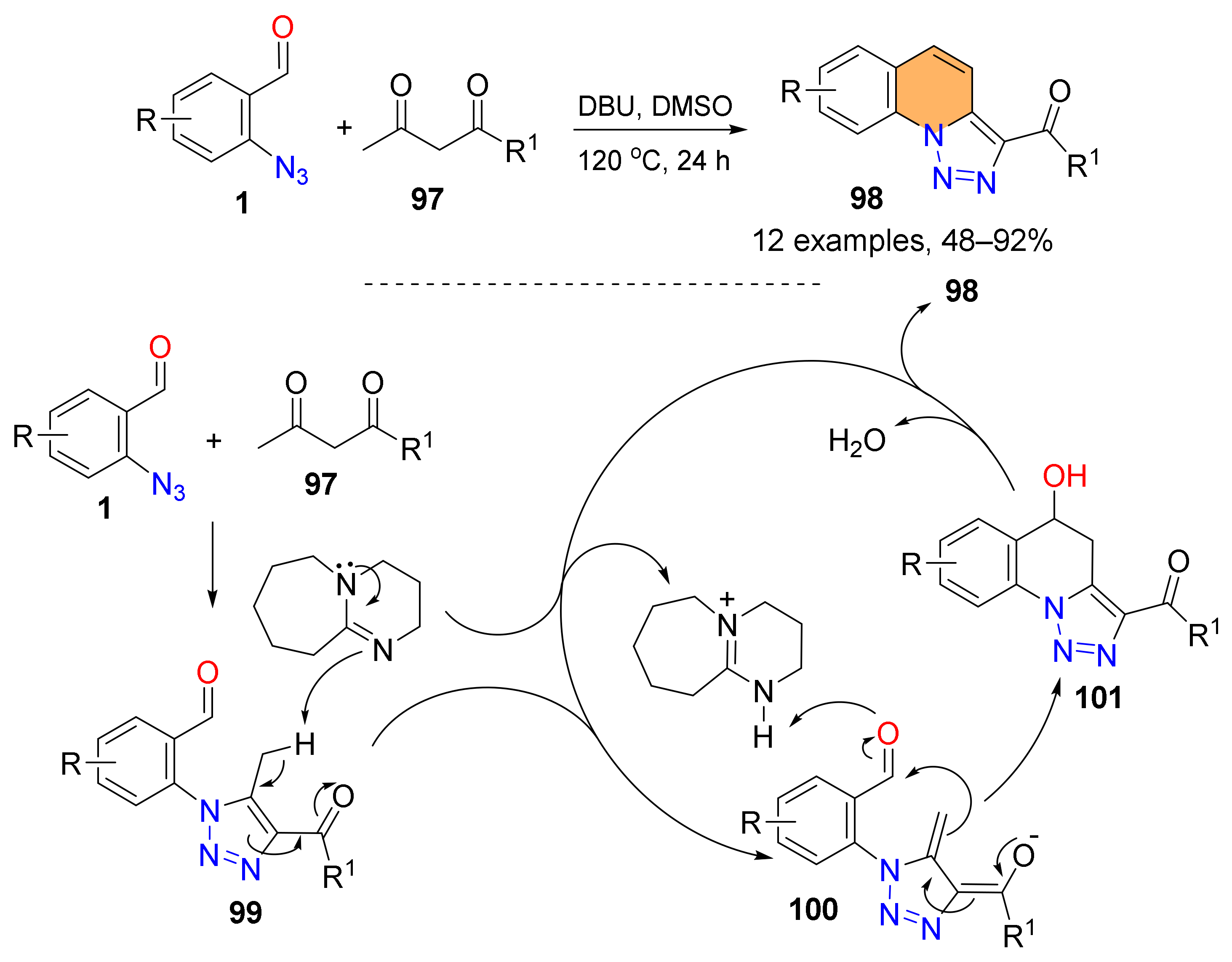
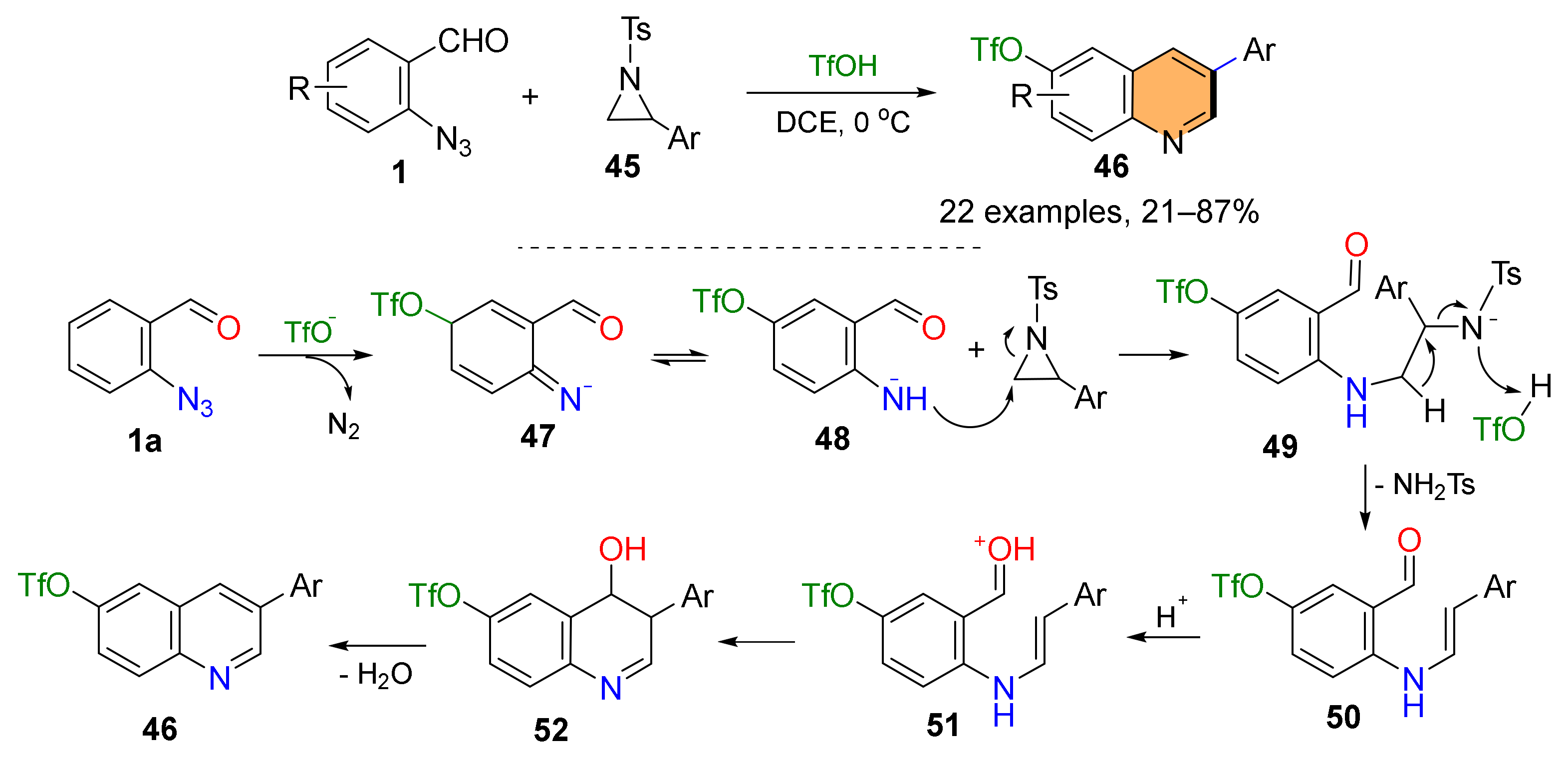
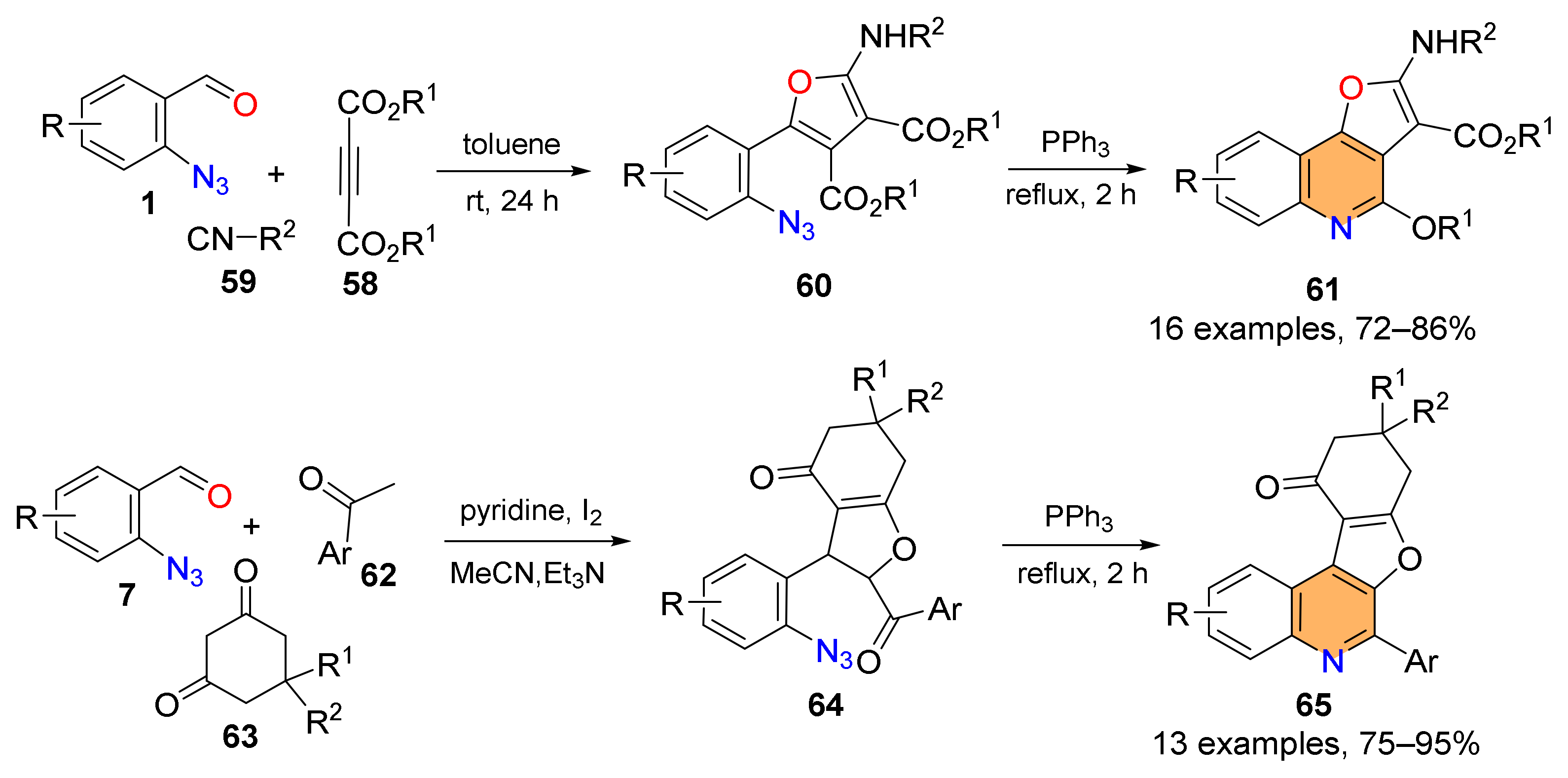
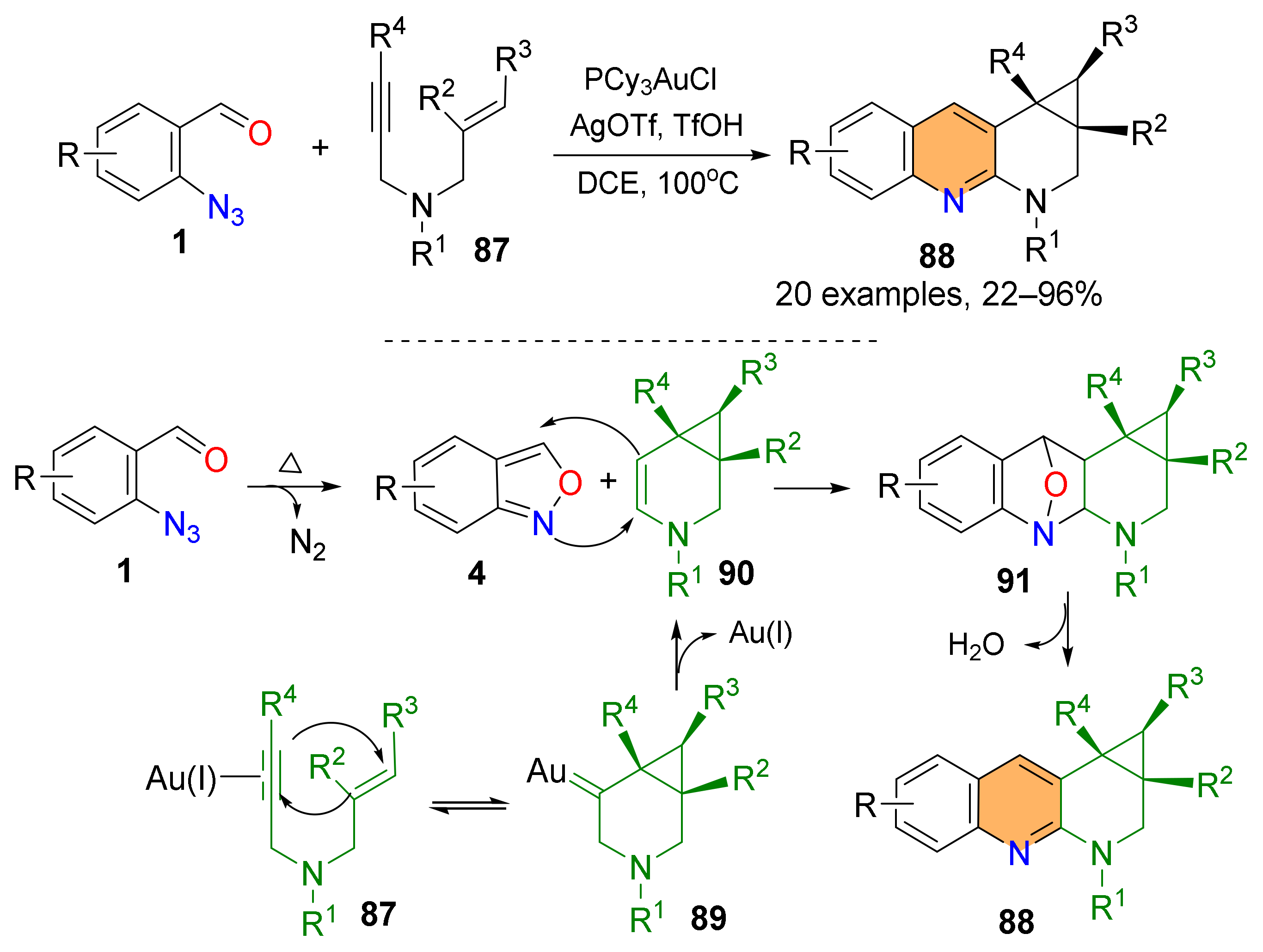
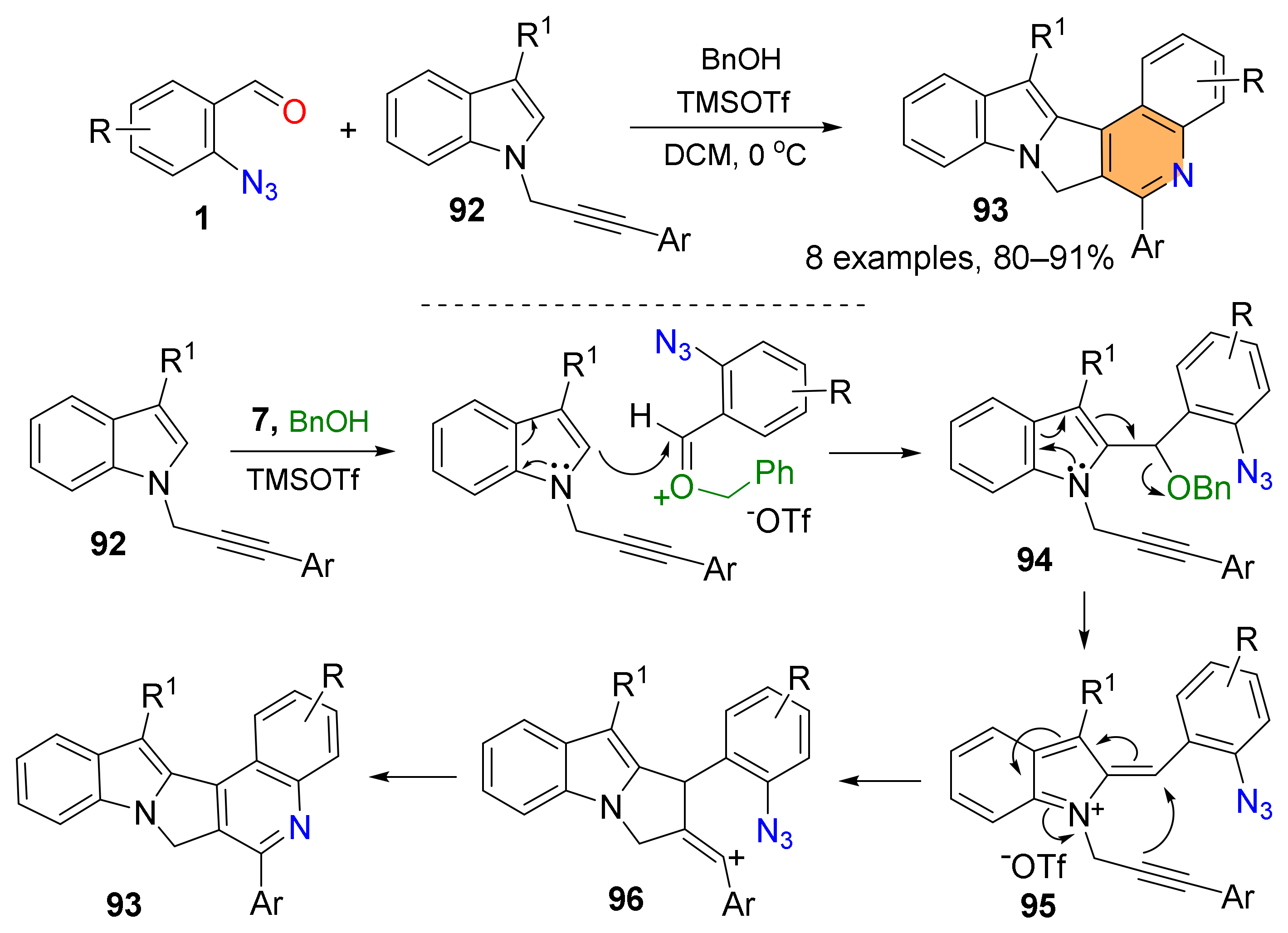
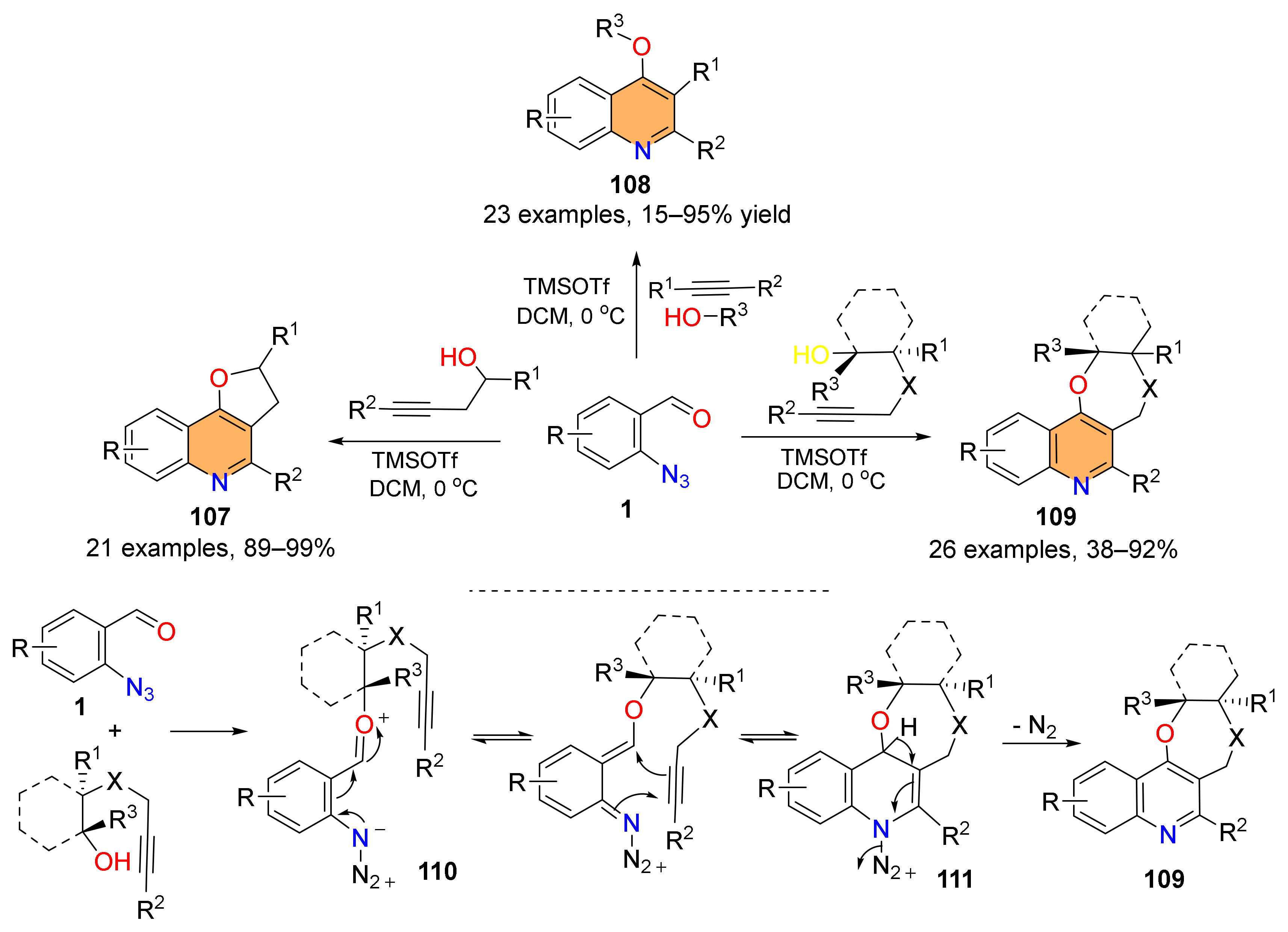
2.2. Synthesis of Polycyclic Quinolines
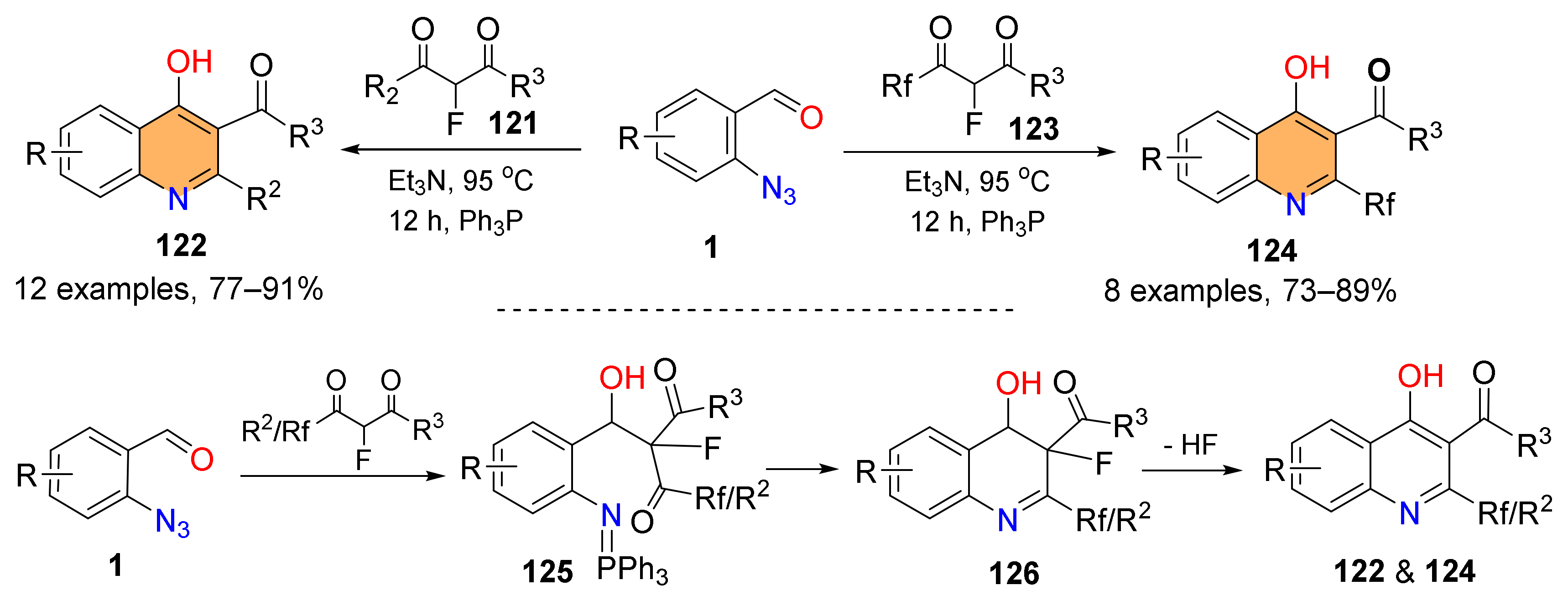
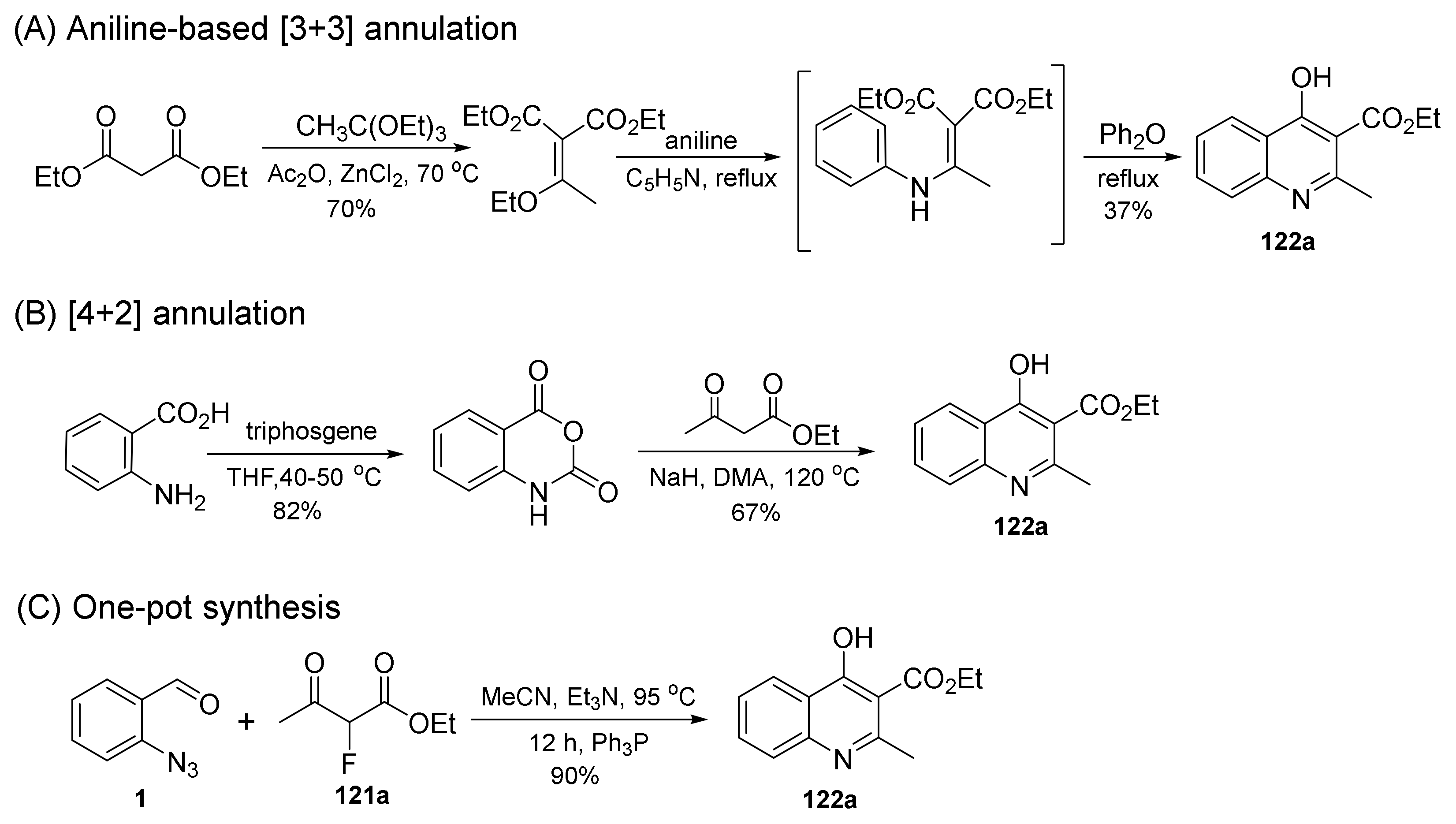
2.3. Synthesis of 4-Hydroxyquinoline Derivatives
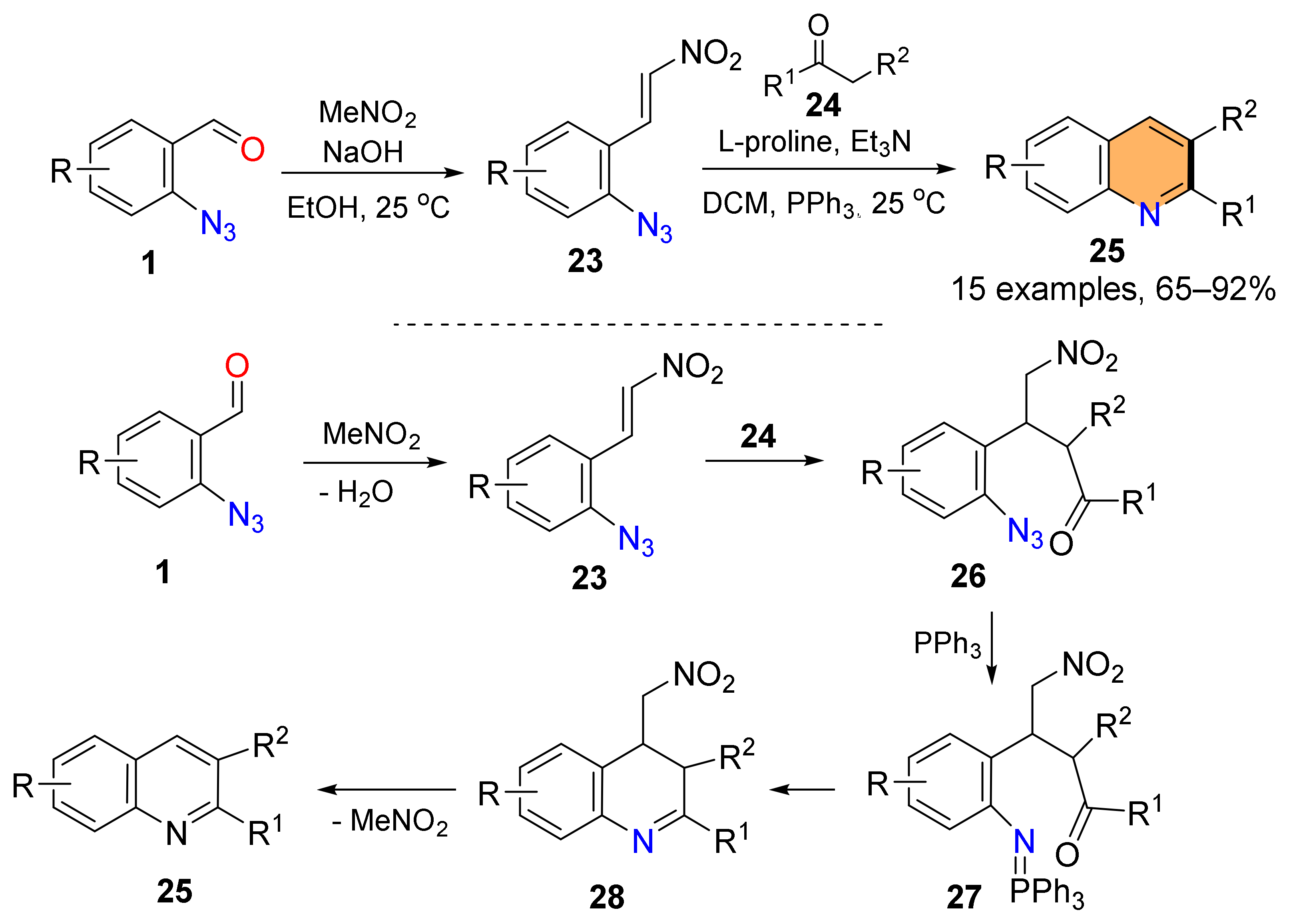
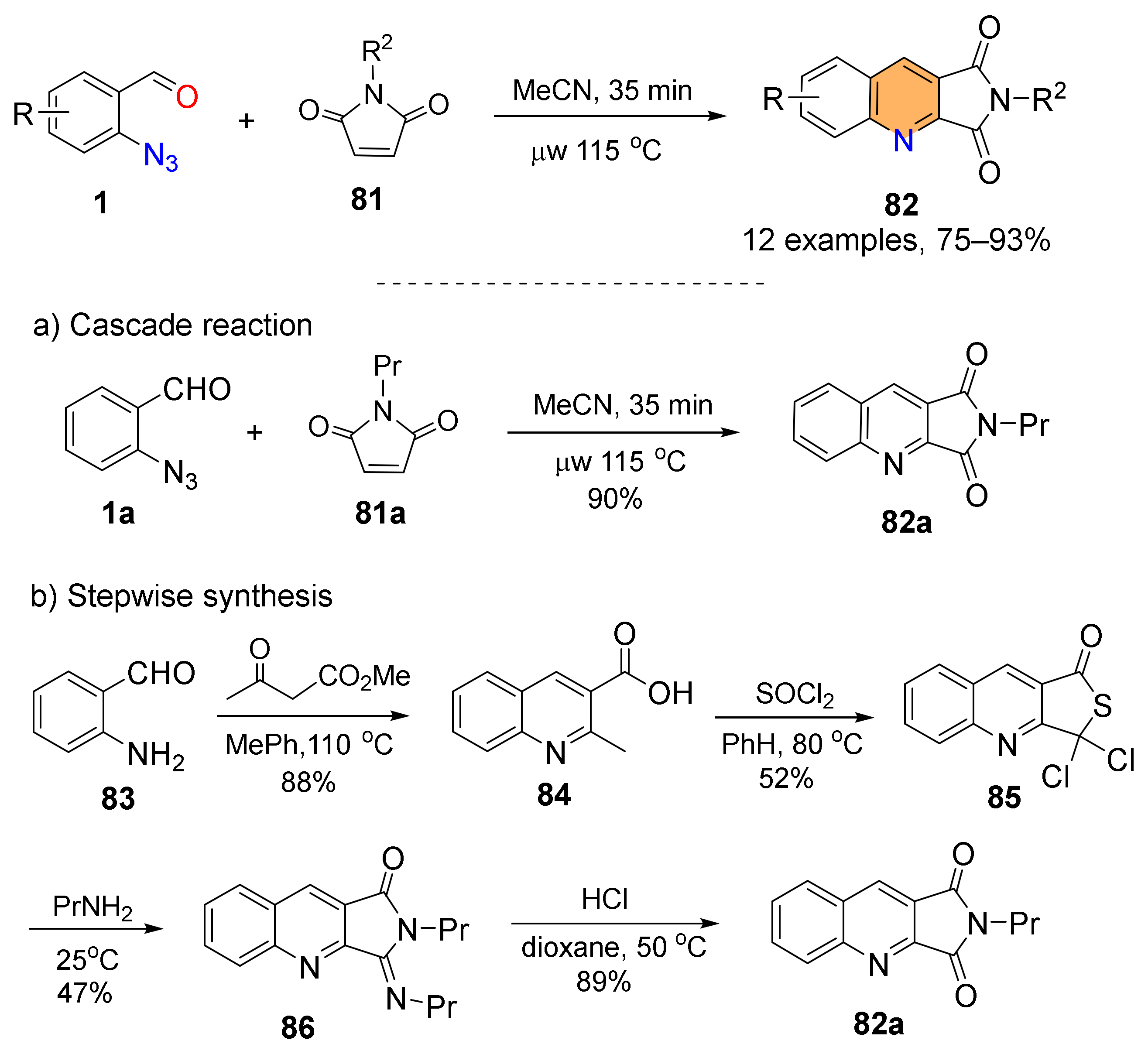
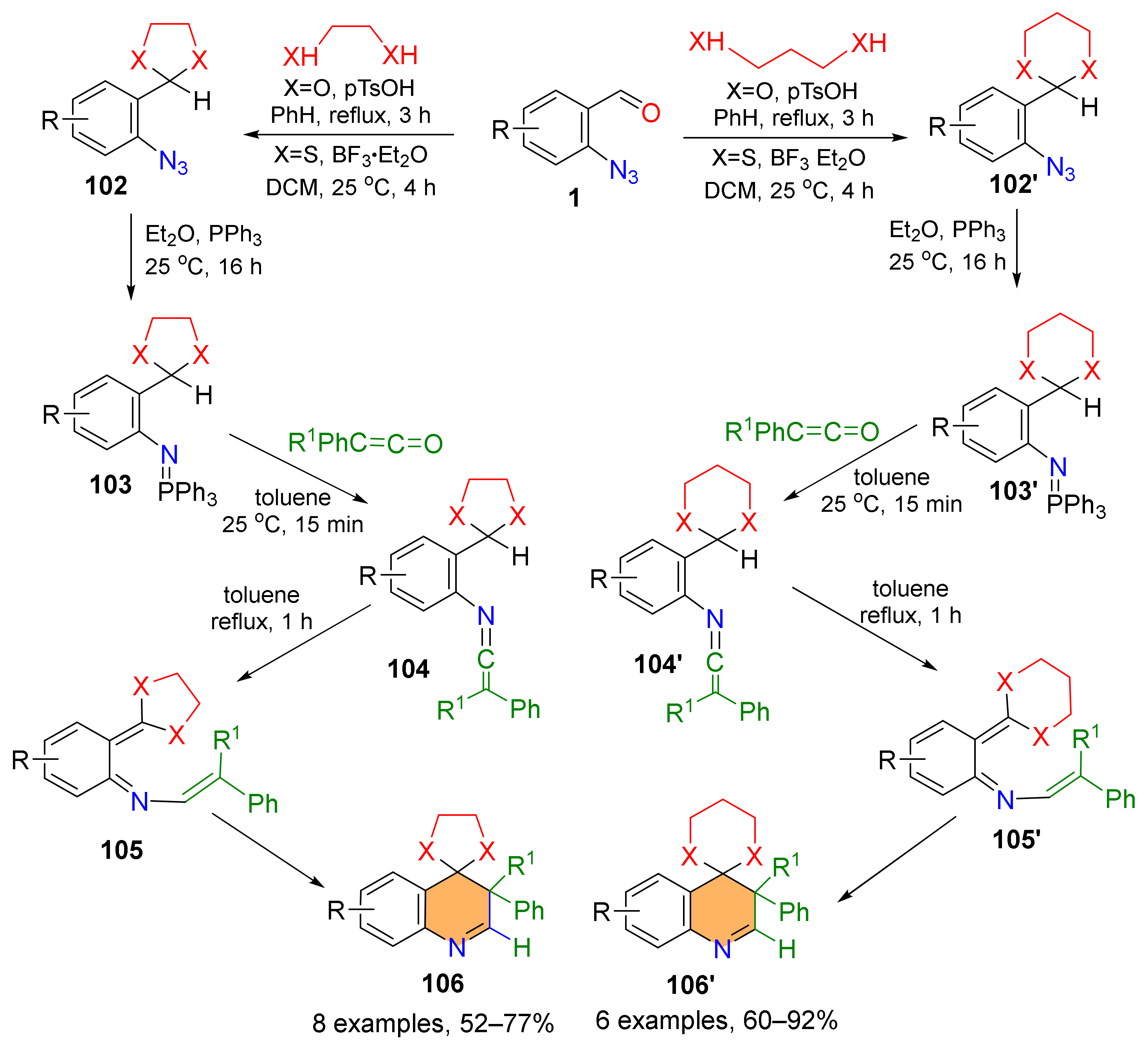
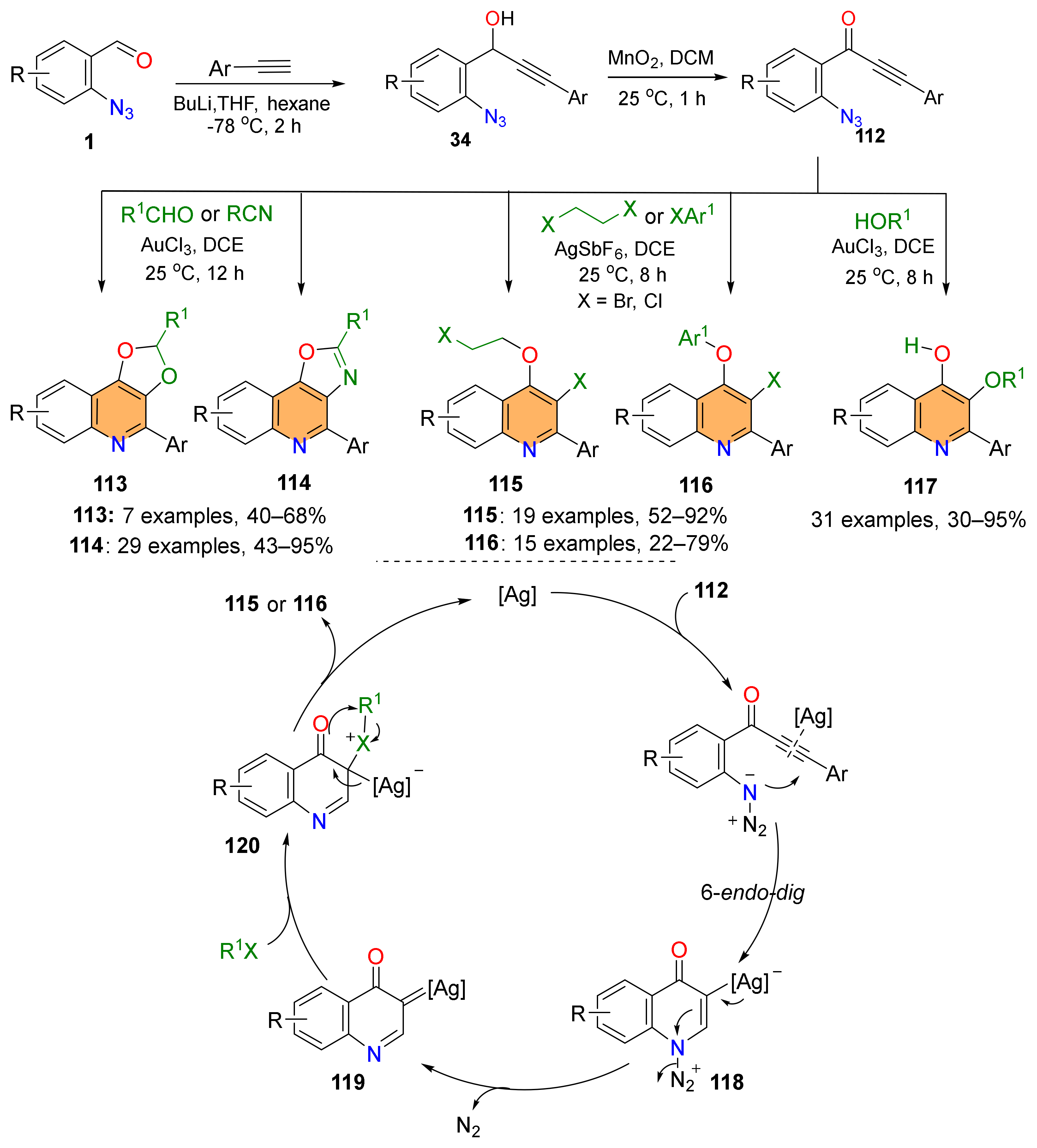
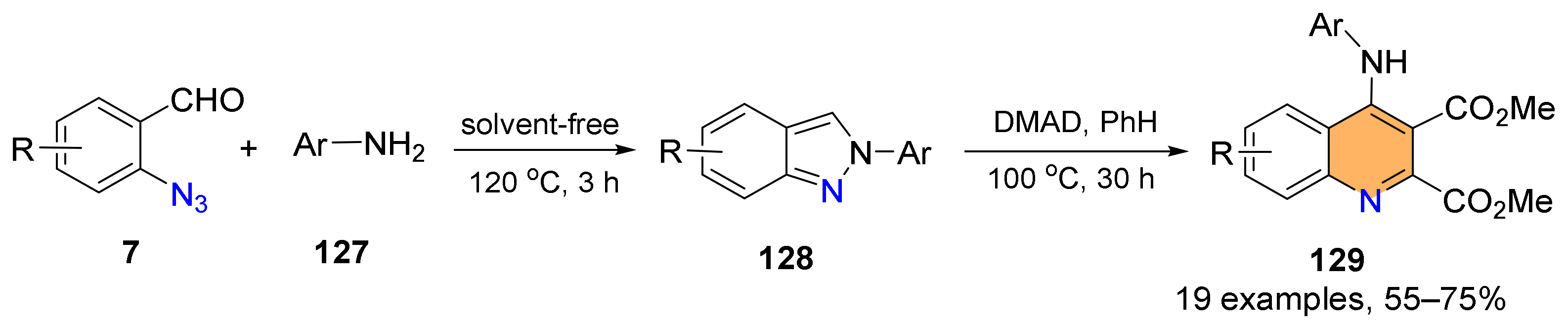
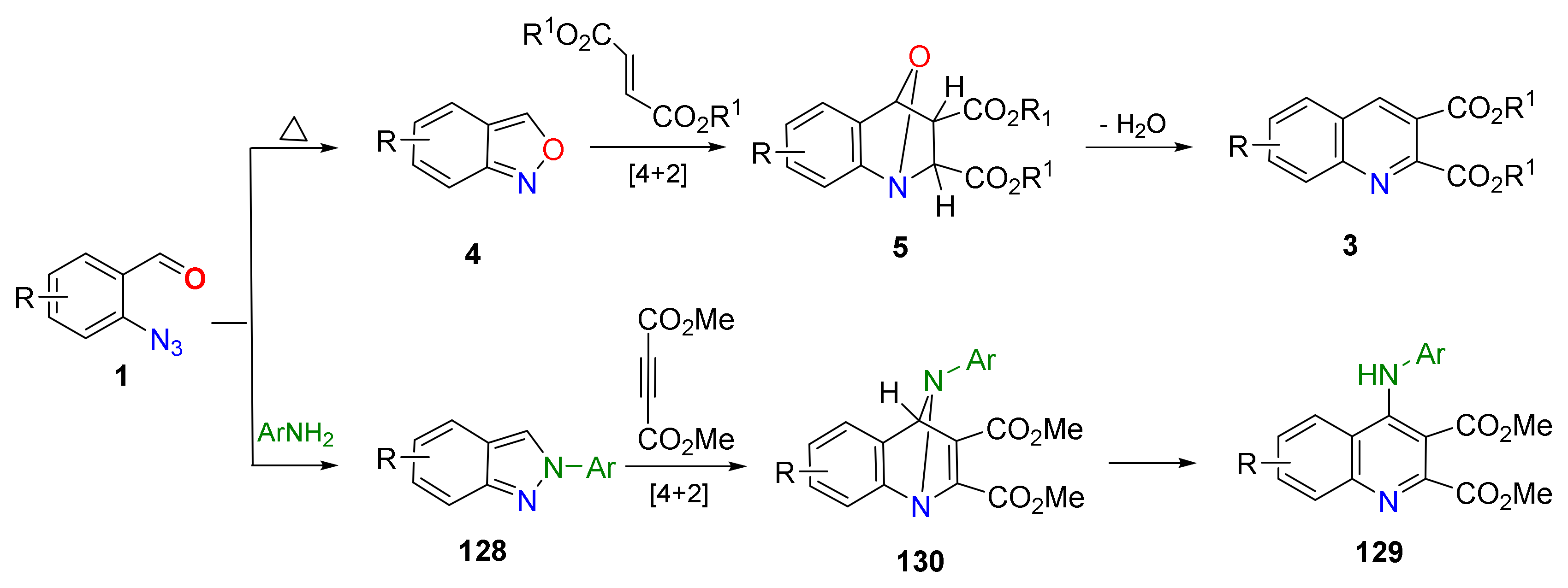
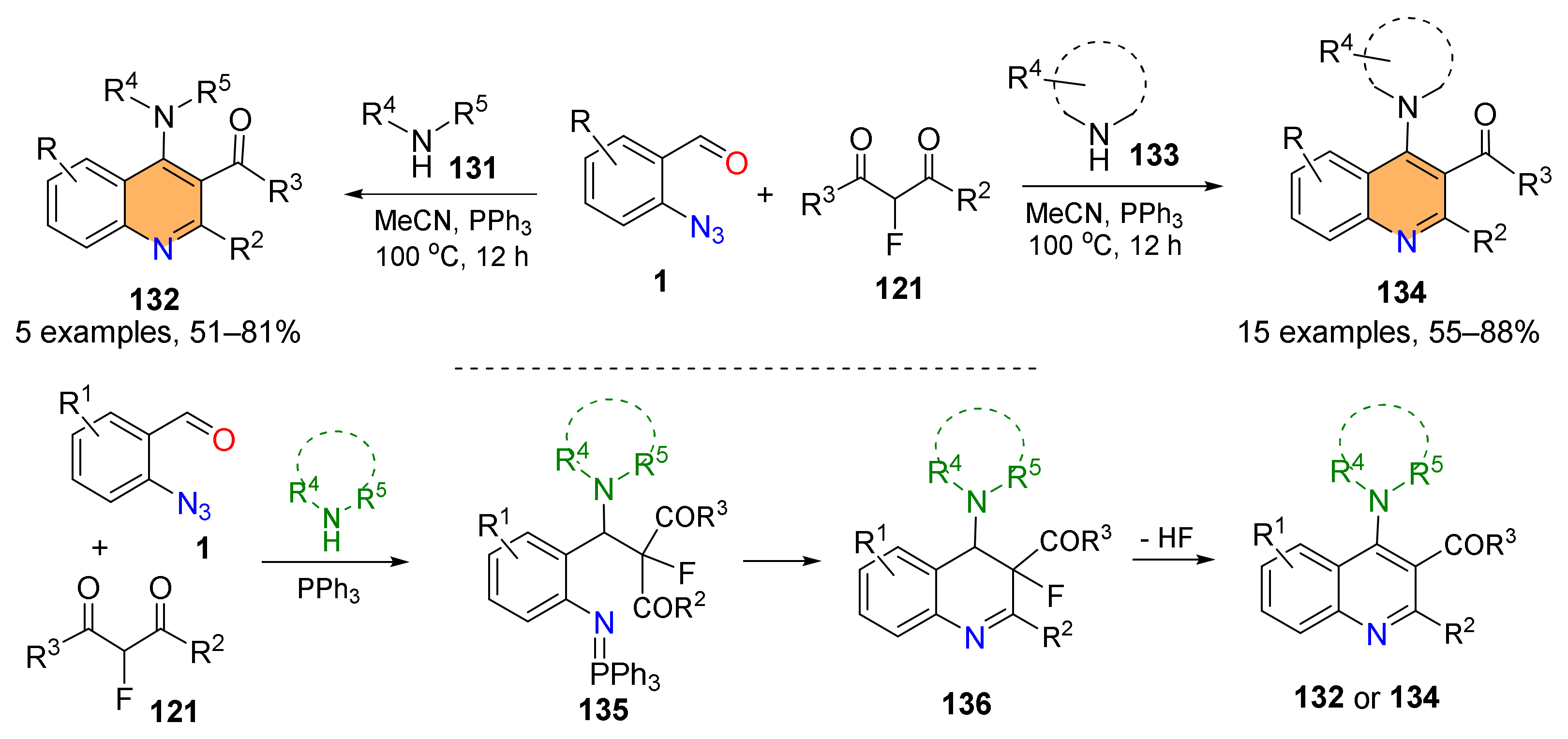
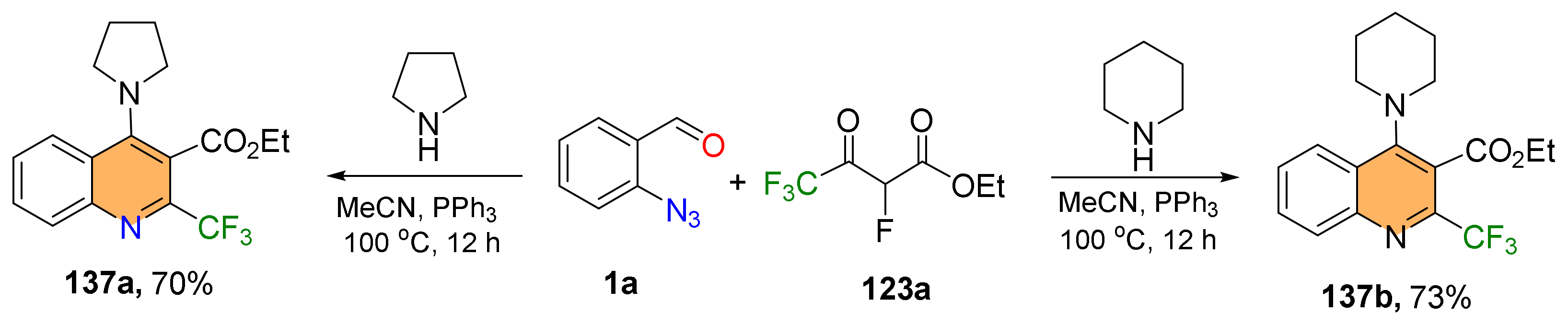
2.4. Synthesis of 4-Amino-Quinolines
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chung, P.Y.; Bian, Z.X.H.; Pun, Y.D.; Chan, A.S.; Chui, C.H.; Tang, J.C.; Lam, K.H. Recent advances in research of natural and synthetic bioactive quinolines. Future Med. Chem. 2015, 7, 947–967. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bawa, S.; Gupta, H. Biological activities of quinoline derivatives. Mini Rev. Med. Chem. 2009, 9, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Broch, S.; Henon, H.; Debaud, L.A.; Fogeron, M.L.; Bonnefoy-Berard, N.; Anizon, F.; Moreau, P. Synthesis and biological activities of new di- and trimeric quinoline derivatives. Bioorg. Med. Chem. 2010, 18, 7132–7143. [Google Scholar] [CrossRef] [PubMed]
- Dib, M.; Ouchetto, H.; Ouchetto, K.; Hafid, A.; Khouili, M. Recent Developments of Quinoline Derivatives and their Potential Biological Activities. Curr. Org. Synth. 2021, 18, 248–269. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.M.; Wang, C.; Liu, W.; Liang, L.L.; Gong, K.K.; Zhao, C.Y.; Sun, K.L. Quinoline and quinolone dimers and their biological activities: An overview. Eur. Med. Chem. 2019, 161, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Paoliello, M.M.B.; Docea, A.O.; Santamaria, A.; Tinkov, A.A.; Skalny, A.V.; Aschner, M. Review of the mechanism underlying mefloquine-induced neurotoxicity. Crit. Rev. Toxicol. 2021, 51, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, D.J.; Jaszczak, M.K.; Boratynski, P.J. A Review of Modifications of Quinoline Antimalarials: Mefloquine and (hydroxy)Chloroquine. Molecules 2022, 27, 1003. [Google Scholar] [CrossRef]
- Dos Santos, M.C.; Scaini, J.L.R.; Lopes, M.V.C.; Rodrigues, B.G.; Silva, N.O.; Borges, C.R.L.; Dos Santos, S.C.; Dos Santos Machado, K.; Werhli, A.V.; da Silva, P.E.A.; et al. Mefloquine synergism with anti-tuberculosis drugs and correlation to membrane effects: Biologic, spectroscopic and molecular dynamics simulations studies. Bioorg. Chem. 2021, 110, 104786. [Google Scholar] [CrossRef]
- Cifci, B.; Yildiz, Y.; Altin, E.; Habibi, H.; Kocer, B.; Dizbay, M. Successful treatment of HIV-associated progressive multifocal leukoencephalopathy (PML) with mirtazapine, mefloquine, and IVIG combination therapy: A case report. J. Neurovirol. 2023, 29, 111–115. [Google Scholar] [CrossRef]
- Dodion, P.F.; Wagener, T.; Stoter, G.; Drozd, A.; Lev, L.M.; Skovsgaard, T.; Renard, J.; Cavalli, F. Phase II trial with Brequinar (DUP-785, NSC 368390) in patients with metastatic colorectal cancer: A study of the Early Clinical Trials Group of the EORTC. Ann. Oncol. 1990, 1, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.I.; Krpina, K.; Ianevski, A.; Shtaida, N.; Yang, E.; Jo, J.; Koit, S.; Tenson, T.; Hukkanen, V.; Anthonsen, M.W.; et al. Novel Antiviral Activities of Obatoclax, Emetine, Niclosamide, Brequinar, and Homoharringtonine. Viruses 2019, 11, 964. [Google Scholar] [CrossRef] [PubMed]
- Horvat, N.K.; Lesinski, G.B. Bring on the brequinar: An approach to enforce the differentiation of myeloid-derived suppressor cells. J. Clin. Investig. 2022, 132, e158661. [Google Scholar] [CrossRef] [PubMed]
- Sadeq, A.; Elnour, A.A.; Farah, F.H.; Ramadan, A.; Baraka, M.A.; Don, J.; Amoodi, A.A.; Sam, K.G.; Mazrouei, N.A.; Alkaabi, M. A Systematic Review of Randomized Clinical Trials on the Efficacy and Safety of Pitavastatin. Curr. Rev. Clin. Exp. Pharmacol. 2023, 18, 120–147. [Google Scholar] [CrossRef] [PubMed]
- Katanasaka, Y.; Hirano, S.; Sunagawa, Y.; Miyazaki, Y.; Sato, H.; Funamoto, M.; Shimizu, K.; Shimizu, S.; Sari, N.; Hasegawa, K.; et al. Clinically Administered Doses of Pitavastatin and Rosuvastatin. Int. Heart J. 2021, 62, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Gowripattapu, S.; Sathis Kumar, D.; Selvamuthukumar, S. Formulation and Statistical Evaluation of Tablets Containing Pitavastatin- Self Nano Emulsifying Drug Delivery Systems. Curr. Drug Deliv. 2023, 20, 414–432. [Google Scholar] [CrossRef] [PubMed]
- Weisinger, H.S.; Pesudovs, K.; Collin, H.B. Management of patients undergoing hydroxychloroquine (Plaquenil) therapy. Clin. Exp. Optom. 2000, 83, 32–36. [Google Scholar] [CrossRef]
- Veld, A.E.I.; Grievink, H.W.; van der Plas, J.L.; Eveleens Maarse, B.C.; van Kraaij, S.J.W.; Woutman, T.D.; Schoonakker, M.; Klarenbeek, N.B.; de Kam, M.L.; Kamerling, I.M.C.; et al. Immunosuppression by hydroxychloroquine: Mechanistic proof in in vitro experiments but limited systemic activity in a randomized placebo-controlled clinical pharmacology study. Immunol. Res. 2023, 71, 617–627. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, J.; Wang, W.; Tang, X.; Zhou, L.; Lv, Y.; Zheng, Y. Investigation of the Pathogenic Mechanism of Ciprofloxacin in Aortic Aneurysm and Dissection by an Integrated Proteomics and Network Pharmacology Strategy. J. Clin. Med. 2023, 12, 1270. [Google Scholar] [CrossRef]
- Loupias, P.; Laumaille, P.; Morandat, S.; Mondange, L.; Guillier, S.; El Kirat, K.; Da Nascimento, S.; Biot, F.; Taudon, N.; Dassonville-Klimpt, A.; et al. Synthesis and study of new siderophore analog-ciprofloxacin conjugates with antibiotic activities against Pseudomonas aeruginosa and Burkholderia spp. Eur. J. Med. Chem. 2023, 245, 114921. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, H.; Wang, J.; Zhou, M.; Zhang, L.; Xiang, J.; Liao, Q.; Luo, L.; Qian, M.; Zhang, D. Pharmacokinetics, withdrawal time, and dietary risk assessment of enrofloxacin and its metabolite ciprofloxacin, and sulfachloropyridazine-trimethoprim in Taihe black-boned silky fowls. J. Food Sci. 2023, 88, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Chen, B.; Zhao, Z.; He, Q.; Mao, Y.; Yang, Y.; Yao, F.; Yang, Y.; Chen, Z.; Yang, J.; et al. Prediction of Response to Lenvatinib Monotherapy for Unresectable Hepatocellular Carcinoma by Machine Learning Radiomics: A Multicenter Cohort Study. Clin. Cancer Res. 2023, 29, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Fan, W.; Zhu, B.; Wang, G.; Sun, J.; Xiao, C.; Huang, F.; Tang, R.; Cheng, Y.; Huang, Z.; et al. Lenvatinib Combined with Transarterial Chemoembolization as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Phase III, Randomized Clinical Trial (LAUNCH). J. Clin. Oncol. 2023, 41, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Liu, C.J.; Wen, H.Q.; Duan, X.H.; Jiao, Y.Q.; Liu, Y.J.; Chen, M.S.; Zhu, K.S.; Mao, X.H.; Zhou, Q.F. Effectiveness of lenvatinib plus immune checkpoint inhibitors in primary advanced hepatocellular carcinoma beyond oligometastasis. Clin. Transl. Med. 2023, 13, e1214. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Kumar, S.; Haider, M.R.; Ali, M.R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar] [CrossRef] [PubMed]
- Arafa, R.K.; Hegazy, G.H.; Piazza, G.A.; Abadi, A.H. Synthesis and in vitro antiproliferative effect of novel quinoline-based potential anticancer agents. Eur. J. Med. Chem. 2013, 63, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.G.; Li, Z.W.; Hu, X.X.; Si, S.Y.; You, X.F.; Tang, S.; Wang, Y.X.; Song, D.Q. Synthesis and Biological Evaluation of Quinoline Derivatives as a Novel Class of Broad-Spectrum Antibacterial Agents. Molecules 2019, 24, 548. [Google Scholar] [CrossRef]
- Dorababu, A. Recent update on antibacterial and antifungal activity of quinoline scaffolds. Arch. Pharm. 2021, 354, e2000232. [Google Scholar] [CrossRef]
- Jin, G.; Xiao, F.; Li, Z.; Qi, X.; Zhao, L.; Sun, X. Design, Synthesis, and Dual Evaluation of Quinoline and Quinolinium Iodide Salt Derivatives as Potential Anticancer and Antibacterial Agents. ChemMedChem 2020, 15, 600–609. [Google Scholar] [CrossRef]
- Chen, Y.J.; Ma, K.Y.; Du, S.S.; Zhang, Z.J.; Wu, T.L.; Sun, Y.; Liu, Y.Q.; Yin, X.D.; Zhou, R.; Yan, Y.F.; et al. Antifungal Exploration of Quinoline Derivatives against Phytopathogenic Fungi Inspired by Quinine Alkaloids. J. Agric. Food Chem. 2021, 69, 12156–12170. [Google Scholar] [CrossRef]
- Cretton, S.; Dorsaz, S.; Azzollini, A.; Favre-Godal, Q.; Marcourt, L.; Ebrahimi, S.N.; Voinesco, F.; Michellod, E.; Sanglard, D.; Gindro, K.; et al. Antifungal Quinoline Alkaloids from Waltheria indica. J. Nat. Prod. 2016, 79, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Z.; Zhu, J.K.; Yin, X.D.; Yan, Y.F.; Wang, Y.L.; Shang, X.F.; Liu, Y.Q.; Zhao, Z.M.; Peng, J.W.; Liu, H. Design, Synthesis, and Antifungal Evaluation of Novel Quinoline Derivatives Inspired from Natural Quinine Alkaloids. J. Agric. Food Chem. 2019, 67, 11340–11353. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Deng, X.; Zhang, L.; Roth, M.G.; Fontoura, B.M.; Phillips, M.A.; De Brabander, J.K. SAR-Based Optimization of a 4-Quinoline Carboxylic Acid Analogue with Potent Antiviral Activity. ACS Med. Chem. Lett. 2013, 4, 517–521. [Google Scholar] [CrossRef] [PubMed]
- De la Guardia, C.; Stephens, D.E.; Dang, H.T.; Quijada, M.; Larionov, O.V.; Lleonart, R. Antiviral Activity of Novel Quinoline Derivatives against Dengue Virus Serotype 2. Molecules 2018, 23, 672. [Google Scholar] [CrossRef] [PubMed]
- Seliem, I.A.; Panda, S.S.; Girgis, A.S.; Moatasim, Y.; Kandeil, A.; Mostafa, A.; Ali, M.A.; Nossier, E.S.; Rasslan, F.; Srour, A.M.; et al. New quinoline-triazole conjugates: Synthesis, and antiviral properties against SARS-CoV-2. Bioorg. Chem. 2021, 114, 105117. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, G.; Zhao, J.; Cheng, N.; Wang, Y.; Fu, Y.; Zheng, Y.; Wang, J.; Zhu, M.; Cen, S.; et al. Synthesis and antiviral activity of a series of novel quinoline derivatives as anti-RSV or anti-IAV agents. Eur. J. Med. Chem. 2021, 214, 113208. [Google Scholar] [CrossRef] [PubMed]
- Vinindwa, B.; Dziwornu, G.A.; Masamba, W. Synthesis and Evaluation of Chalcone-Quinoline Based Molecular Hybrids as Potential Anti-Malarial Agents. Molecules 2021, 26, 4093. [Google Scholar] [CrossRef]
- Roy, D.; Anas, M.; Manhas, A.; Saha, S.; Kumar, N.; Panda, G. Synthesis, biological evaluation, Structure—Activity relationship studies of quinoline-imidazole derivatives as potent antimalarial agents. Bioorg. Chem. 2022, 121, 105671. [Google Scholar] [CrossRef]
- Jesu Jaya Sudan, R.; Lesitha Jeeva Kumari, J.; Iniyavan, P.; Sarveswari, S.; Vijayakumar, V. Evaluation of xanthene-appended quinoline hybrids as potential leads against antimalarial drug targets. Mol. Divers. 2023, 27, 709–727. [Google Scholar] [CrossRef]
- Orhan Puskullu, M.; Tekiner, B.; Suzen, S. Recent Studies of Antioxidant Quinoline Derivatives. Mini Rev. Med. Chem. 2013, 13, 365–372. [Google Scholar] [CrossRef]
- Parameswaran, K.; Sivaguru, P.; Lalitha, A. Synthesis of novel bis(pyrimido [5,4-c]quinoline-2,4(1H,3H)-dione) and its derivatives: Evaluation of their antioxidant properties. Bioorg. Med. Chem. Lett. 2013, 23, 3873–3878. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, L.; Nadaradjane, A.; Nicod, L.; Guyon, C.; Xicluna, A.; Robert, J.F.; Refouvelet, B. Synthesis and antioxidant activity evaluation of new hexahydropyrimido [5,4-c]quinoline-2,5-diones and 2-thioxohexahydropyrimido [5,4-c]quinoline-5-ones obtained by Biginelli reaction in two steps. Eur. J. Med. Chem. 2008, 43, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Tung, C.W.; Wu, C.H.; Tzeng, C.C.; Chen, Y.H.; Hwang, T.L.; Chen, Y.L. Discovery of Indeno [1,2-c]quinoline Derivatives as Potent Dual Antituberculosis and Anti-Inflammatory Agents. Molecules 2017, 22, 1001. [Google Scholar] [CrossRef] [PubMed]
- El-Feky, S.A.; Abd El-Samii, Z.K.; Osman, N.A.; Lashine, J.; Kamel, M.A.; Thabet, H. Synthesis, molecular docking and anti-inflammatory screening of novel quinoline incorporated pyrazole derivatives using the Pfitzinger reaction II. Bioorg. Chem. 2015, 58, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Chia, E.W.; Pearce, A.N.; Berridge, M.V.; Larsen, L.; Perry, N.B.; Sansom, C.E.; Godfrey, C.A.; Hanton, L.R.; Lu, G.L.; Walton, M.; et al. Synthesis and anti-inflammatory structure–activity relationships of thiazine–quinoline–quinones: Inhibitors of the neutrophil respiratory burst in a model of acute gouty arthritis. Bioorg. Med. Chem. 2008, 16, 9432–9442. [Google Scholar] [CrossRef] [PubMed]
- Bewley, B.R.; Spearing, P.K.; Weiner, R.L.; Luscombe, V.B.; Zhan, X.; Chang, S.; Cho, H.P.; Rodriguez, A.L.; Niswender, C.M.; Conn, P.J.; et al. Discovery of a novel, CNS penetrant M4 PAM chemotype based on a 6-fluoro-4-(piperidin-1-yl)quinoline-3-carbonitrile core. Bioorg. Med. Chem. Lett. 2017, 27, 4274–4279. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, P.; Marciniec, K.; Maslankiewicz, A.; Satala, G.; Duszynska, B.; Bojarski, A.J.; Partyka, A.; Jastrzebska-Wiesek, M.; Wrobel, D.; Wesolowska, A.; et al. Quinoline- and isoquinoline-sulfonamide derivatives of LCAP as potent CNS multi-receptor—5-HT1A/5-HT2A/5-HT7 and D2/D3/D4—Agents: The synthesis and pharmacological evaluation. Bioorg. Med. Chem. 2012, 20, 1545–1556. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, D.; Bawa, S.; Khan, S.A. Design, Synthesis and Screening of Quinoline-Incorporated Thiadiazole as a Potential Anticonvulsant. Chem. Biol. Drug Des. 2012, 79, 104–111. [Google Scholar] [CrossRef]
- Muruganantham, N.; Sivakumar, R.; Anbalagan, N.; Gunasekaran, V.; Leonard, J.T. Synthesis, Anticonvulsant and Antihypertensive Activities of 8-Substituted Quinoline Derivatives. Biol. Pharm. Bull. 2004, 27, 1683–1687. [Google Scholar] [CrossRef]
- Shanley, H.T.; Taki, A.C.; Byrne, J.J.; Jabbar, A.; Wells, T.N.C.; Samby, K.; Boag, P.R.; Nguyen, N.; Sleebs, B.E.; Gasser, R.B. A High-Throughput Phenotypic Screen of the ‘Pandemic Response Box’ Identifies a Quinoline Derivative with Significant Anthelmintic Activity. Pharmaceuticals 2022, 15, 257. [Google Scholar] [CrossRef]
- Williams, A.; Villamor, L.; Fussell, J.; Loveless, R.; Smeyne, D.; Philp, J.; Shaikh, A.; Sittaramane, V. Discovery of Quinoline-Derived Trifluoromethyl Alcohols as Antiepileptic and Analgesic Agents That Block Sodium Channels. ChemMedChem 2022, 17, e202100547. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsov, V.V.; Mendez, L.Y.V.; Gomez, C.M.M. Recent Progress in the Synthesis of Quinolines. Curr. Org. Chem. 2005, 9, 141–161. [Google Scholar] [CrossRef]
- Weyesa, A.; Mulugeta, E. Recent advances in the synthesis of biologically and pharmaceutically active quinoline and its analogues: A review. RSC Adv. 2020, 10, 20784–20793. [Google Scholar] [CrossRef] [PubMed]
- Nainwal, L.M.; Tasneem, S.; Akhtar, W.; Verma, G.; Khan, M.F.; Parvez, S.; Shaquiquzzaman, M.; Akhter, M.; Alam, M.M. Green recipes to quinoline: A review. Eur. J. Med. Chem. 2019, 164, 121–170. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.M.; Patel, K.D.; Vekariya, R.H.; Panchal, S.N.; Patel, H.D. Recent advances in the synthesis of quinolines: A review. RSC Adv. 2014, 4, 24463–24476. [Google Scholar] [CrossRef]
- Barluenga, J.; Rodriguez, F.; Fananas, F.J. Recent Advances in the Synthesis of Indole and Quinoline Derivatives through Cascade Reactions. Chem. Asian J. 2009, 4, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Ramann, G.A.; Cowen, B.J. Recent Advances in Metal-Free Quinoline Synthesis. Molecules 2016, 21, 986. [Google Scholar] [CrossRef]
- Bharate, J.B.; Vishwakarma, R.A.; Bharate, J.B. Metal-free domino one-pot protocols for quinoline synthesis. RSC Adv. 2015, 5, 42020–42053. [Google Scholar] [CrossRef]
- Teja, C.; Khan, F.R.N. Radical Transformations towards the Synthesis of Quinoline: A Review. Chem. Asian J. 2020, 15, 4153–4167. [Google Scholar] [CrossRef]
- Vessally, E.; Edjlali, L.; Hosseinian, A.; Bekhradnia, A.; Esrafili, M.D. Novel routes to quinoline derivatives from N-propargylamines. RSC Adv. 2016, 6, 49730–49746. [Google Scholar] [CrossRef]
- Doraghi, F.; Karimian, S.; Aledavoud, S.P.; Amini, A.; Larijani, B.; Mahdavi, M. 2-azidobenzaldehyde: A versatile scaffold for the generation of N-heterocyclic compounds. J. Mol. Struct. 2023, 1294, 136510. [Google Scholar] [CrossRef]
- Das, S.K.; Roy, S.; Khatua, H.; Chattopadhyay, B. Ir-Catalyzed Intramolecular Transannulation/C(sp2)–H Amination of 1,2,3,4-Tetrazoles by Electrocyclization. J. Am. Chem. Soc. 2018, 140, 8429–8433. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Das, S.K.; Khatua, H.; Das, S.; Singh, K.N.; Chattopadhyay, B. Iron-Catalyzed Radical Activation Mechanism for Denitrogenative Rearrangement Over C(sp3)–H Amination. Angew. Chem. Int. Ed. 2021, 60, 8772–8780. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.J.; Joshi, G.; Yadav, U.P.; Maurya, A.K.; Agnihotri, V.K.; Kalra, S.; Kumar, R.; Singh, S.; Sawant, D.M. Exploration of Pd-catalysed four-component tandem reaction for one-pot assembly of pyrazolo [1,5-c]quinazolines as potential EGFR inhibitors. Bioorg. Chem. 2019, 93, 103314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, M.L.; Liu, M.; Ding, M.W. New efficient synthesis of polysubstituted 3,4-dihydroquinazolines and 4H-3,1-benzothiazines through a Passerini/Staudinger/aza-Wittig/addition/nucleophilic substitution sequence. Beilstein J. Org. Chem. 2022, 18, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Vroemans, R.; Bamba, F.; Winters, J.; Thomas, J.; Jacobs, J.; Van Meervelt, L.; John, J.; Dehaen, W. Sequential Ugi reaction/base-induced ring closing/IAAC protocol toward triazolobenzodiazepine-fused diketopiperazines and hydantoins. Beilstein J. Org. Chem. 2018, 14, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Vidyacharan, S.; Murugan, A.; Sharada, D.S. C(sp2)–H Functionalization of 2H-Indazoles at C3-Position via Palladium(II)-Catalyzed Isocyanide Insertion Strategy Leading to Diverse Heterocycles. J. Org. Chem. 2016, 81, 2837–2848. [Google Scholar] [CrossRef]
- De Moliner, F.; Bigatti, M.; De Rosa, C.; Banfi, L.; Riva, R.; Basso, A. Synthesis of triazolo-fused benzoxazepines and benzoxazepinones via Passerini reactions followed by 1,3-dipolar cycloadditions. Mol. Divers. 2014, 18, 473–482. [Google Scholar] [CrossRef]
- Singh, B. Bedaquiline in Drug-Resistant Tuberculosis: A Mini-Review. Curr. Mol. Pharmacol. 2023, 16, 243–253. [Google Scholar] [CrossRef]
- Conradie, F.; Bagdasaryan, T.R.; Borisov, S.; Howell, P.; Mikiashvili, L.; Ngubane, N.; Samoilova, A.; Skornykova, S.; Tudor, E.; Variava, E.; et al. Bedaquiline–Pretomanid–Linezolid Regimens for Drug-Resistant Tuberculosis. N. Engl. J. Med. 2022, 387, 810–823. [Google Scholar] [CrossRef]
- Frampton, J.E. QVA149 (Indacaterol/Glycopyrronium Fixed-Dose Combination): A Review of Its Use in Patients with Chronic Obstructive Pulmonary Disease. Drugs 2014, 74, 465–488. [Google Scholar] [CrossRef]
- Cazzola, M.; Bardaro, F.; Stirpe, E. The role of indacaterol for chronic obstructive pulmonary disease (COPD). J. Thorac. Dis. 2013, 5, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Umezaki, Y.; Motomura, H.; Egashira, R.; Toyofuku, A.; Naito, T. A Case of Oral Cenesthopathy Treated with the Combination of Brexpiprazole and Mirtazapine. Clin. Neuropharmacol. 2023, 46, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Shapovalov, V.; Kopanitsa, L.; Pruteanu, L.L.; Ladds, G.; Bailey, D.S. Transcriptomics-Based Phenotypic Screening Supports Drug Discovery in Human Glioblastoma Cells. Cancers 2021, 13, 3780. [Google Scholar] [CrossRef] [PubMed]
- Murray, V.; Chen, J.K.; Galea, A.M. The Potential of Acridine Carboxamide Pt Complexes as Anti-Cancer Agents: A Review. Anti-Cancer Agent. Med. Chem. 2014, 14, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Menon, R.S.; Biju, A.T.; Abhilash, K.G. 1,2-Benzoquinones in Diels–Alder reactions, dipolar cycloadditions, nucleophilic additions, multicomponent reactions and more. Chem. Soc. Rev. 2012, 41, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.B.; Mudududdla, R.; Bharate, J.B.; Battini, N.; Battula, S.; Yadav, R.R.; Singh, B.; Vishwakarma, R.A. Tandem one-pot synthesis of flavans by recyclable silica–HClO4 catalyzed Knoevenagel condensation and [4+2]-Diels–Alder cycloaddition. Org. Biomol. Chem. 2012, 10, 5143–5150. [Google Scholar] [CrossRef] [PubMed]
- Houk, K.N.; Liu, F.; Yang, Z.; Seeman, J.I. Evolution of the Diels–Alder Reaction Mechanism since the 1930s: Woodward, Houk with Woodward, and the Influence of Computational Chemistry on Understanding Cycloadditions. Angew. Chem. 2021, 60, 12660–12681. [Google Scholar] [CrossRef]
- Zhang, X.F.; Dhawan, G.; Muthengi, A.; Liu, S.; Wang, W.; Legris, M.; Zhang, W. One-pot and catalyst-free synthesis of pyrroloquinolinediones and quinolinedicarboxylates. Green Chem. 2017, 19, 3851–3855. [Google Scholar] [CrossRef]
- Zheng, L.; Zeng, Z.G.; Yan, Q.; Jia, F.C.; Jia, L.; Chen, Y. Copper-Catalyzed Synthesis of 3-NO2 Quinolines from o-Azidobenzaldehyde and Nitro-olefins and its Application in the Concise Synthesis of Quindolines. Adv. Synth. Catal. 2018, 360, 4037–4042. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Awad, J.M.; Xie, G.; Qiu, W.; Muriph, R.E. Sequential decarboxylative [3+2] cycloaddition and Staudinger/aza-Wittig reactions for diastereoselective synthesis of tetrahydro-pyrroloquinazolines and tetrahedro-pyrrolobenzodiazepines. Tetrahedron Lett. 2020, 61, 151392. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.F.; Awad, J.M.; Xie, G.; Qiu, W.; Zhang, W. One-pot synthesis of tetrahydro-pyrrolobenzodiazepines and tetrahydro-pyrrolobenzodiazepinones through sequential 1,3-dipolar cycloaddition/N-alkylation (N-acylation)/Staudinger/aza-Wittig reactions. Green Chem. 2019, 21, 4489–4494. [Google Scholar] [CrossRef]
- Zhang, X.F.; Ma, X.; Qiu, W.; Evans, J.; Zhnag, W. Cascade Knoevenagel and aza-Wittig reactions for the synthesis of substituted quinolines and quinolin-4-ols. Green Chem. 2019, 21, 349–354. [Google Scholar] [CrossRef]
- Qu, F.; He, P.; Hu, R.; Cheng, X.; Wang, S.; Wu, J. Efficient Synthesis of Quinolines via a Knoevenagel/Staudinger/aza-Wittig Sequence. Synth. Commun. 2015, 45, 2802–2809. [Google Scholar] [CrossRef]
- Malkova, K.; Bubyrev, A.; Kalinin, S.; Dar’in, D. Facile access to 3-sulfonylquinolines via Knoevenagel condensation/aza-Wittig reaction cascade involving ortho-azidobenzaldehydes and β-ketosulfonamides and sulfones. Beilstein J. Org. Chem. 2023, 19, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Fakhfakh, M.A.; Fournet, A.; Prina, E.; Mouscadet, J.F.; Franck, X.; Hocquemiller, R.; Figadere, B. Synthesis and biological evaluation of substituted quinolines: Potential treatment of protozoal and retroviral co-infections. Bioorg. Med. Chem. 2003, 11, 5013–5023. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.S.; Pinjari, J.; Dere, R.T.; Verma, A.; Vishwakarma, P.; Shivahare, R.; Moger, M.; Kumar Goud, P.S.; Ramanathan, V.; Bose, P.; et al. Design, synthesis and biological evaluation of 2-substituted quinolines as potential antileishmanial agents. Eur. J. Med. Chem. 2013, 69, 527–536. [Google Scholar] [CrossRef]
- Gopaul, K.; Shintre, S.A.; Koorbanally, N.A. A Review on the Synthesis and Anti-cancer Activity of 2-substituted Quinolines. Anti-Cancer Agent. Med. Chem. 2015, 15, 631–646. [Google Scholar] [CrossRef]
- Swallow, S. Chapter Two—Fluorine in Medicinal Chemistry. Progress Med. Chem. 2015, 54, 65–133. [Google Scholar] [CrossRef]
- Ramachandran, P.V. Welcome to ‘Fluorine in Medicinal Chemistry’. Future Med. Chem. 2009, 1, 771–772. [Google Scholar] [CrossRef]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef] [PubMed]
- Bohm, H.J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Muller, K.; Obst-Sander, U.; Stahl, M. Fluorine in Medicinal Chemistry. ChemBioChem 2004, 5, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Acena, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II–III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef] [PubMed]
- Mai, A.; Rotili, D.; Tarantino, D.; Ornaghi, P.; Tosi, F.; Vicidomini, C.; Sbardella, G.; Nebbioso, A.; Miceli, M.; Altucci, L.; et al. Small-Molecule Inhibitors of Histone Acetyltransferase Activity: Identification and Biological Properties. J. Med. Chem. 2006, 49, 6897–6907. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, V.; Brannigan, J.A.; Whalley, D.; Ansell, K.H.; Saxty, B.; Holder, A.A.; Wilkinson, A.J.; Tate, E.W.; Leatherbarrow, R.J. Discovery of Plasmodium vivaxN-Myristoyltransferase Inhibitors: Screening, Synthesis, and Structural Characterization of their Binding Mode. J. Med. Chem. 2012, 55, 3578–3582. [Google Scholar] [CrossRef]
- Yu, Z.; Zheng, H.; Yuan, W.; Tang, Z.; Zhang, A.; Shi, D. An unexpected one-pot synthesis of multi-substituted quinolines via a cascade reaction of Michael/Staudinger/aza-Wittig/aromatization of ortho-azido-β-nitro-styrenes with various carbonyl compounds. Tetrahedron 2013, 69, 8137–8141. [Google Scholar] [CrossRef]
- Chaabouni, S.; Pinkerton, N.M.; Legrand, S.; Abid, S.; Galaup, C.; Chassaing, S.; Koivisto, J.; Hirvonen, J.; Kostiainen, M.A.; Bimbo, L.M. Photochemistry of ortho-azidocinnamoyl derivatives: Facile and modular synthesis of 2-acylated indoles and 2-substituted quinolines under solvent control. Synlett 2017, 28, 2614–2618. [Google Scholar] [CrossRef]
- Kumar, G.R.; Kumar, R.; Rajesh, M.; Reddy, M.S. A nickel-catalyzed anti-carbometallative cyclization of alkyne–azides with organoboronic acids: Synthesis of 2,3-diarylquinolines. Chem. Commun. 2018, 54, 759–762. [Google Scholar] [CrossRef]
- Huo, Z.; Gridnev, I.D.; Yamamoto, Y. A Method for the Synthesis of Substituted Quinolines via Electrophilic Cyclization of 1-Azido-2-(2-propynyl)benzene. J. Org. Chem. 2010, 75, 1266–1270. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.Z.; Liu, Y.; Yan, X.; Yan, Y.; Chao, S.; Shang, X.; Ni, T.; Zhou, P. Synthesis of Isoquinolylselenocyanates and Quinolylselenocyanates via Electrophilic Selenocyanogen Cyclization Induced by Pseudohalogen (SeCN)2 Generated in situ. Adv. Synth. Catal. 2022, 364, 187–192. [Google Scholar] [CrossRef]
- Qiu, Y.F.; Niu, Y.J.; Wei, X.; Cao, B.Q.; Wang, X.C.; Quan, Z.J. AgSCF3/Na2S2O8-Promoted Trifluoromethylthiolation/Cyclization of o-Propargyl Arylazides/o-Alkynyl Benzylazides: Synthesis of SCF3-Substituted Quinolines and Isoquinolines. J. Org. Chem. 2019, 84, 4165–4178. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Zhu, Y.M.; Yang, P.J.; Wang, S.; Wang, S.; Liu, Z.; Yang, G. Copper(I)-Catalyzed Kinetic Resolution of N-Sulfonylaziridines with Indoles: Efficient Construction of Pyrroloindolines. J. Am. Chem. Soc. 2015, 137, 10088–10091. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Miller, A.W.; Goyal, S.; Nguyen, S.T. Sc(OTf)3-catalyzed condensation of 2-alkyl-N-tosylaziridine with aldehydes or ketones: An efficient synthesis of 5-alkyl-1,3-oxazolidines. Chem. Commun. 2009, 26, 3928–3930. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, I.; Klotz, P.; Mann, A. Phenylaziridine as a Masked 1,3 Dipole in Reactions with Nonactivated Alkenes. Angew. Chem. 2000, 39, 4615–4617. [Google Scholar] [CrossRef]
- Wani, I.A.; Sayyad, M.; Ghorai, M.K. Domino ring-opening cyclization (DROC) of activated aziridines and epoxides with nitrones via dual-catalysis “on water”. Chem. Commun. 2017, 53, 4386–4389. [Google Scholar] [CrossRef]
- Yi, R.; Li, X.; Wan, B. Ring-opening and cyclization of aziridines with aryl azides: Metal-free synthesis of 6-(triflyloxy)quinolines. Org. Chem. Front. 2018, 5, 3488–3493. [Google Scholar] [CrossRef]
- Lin, C.H.; Tsai, M.R.; Wang, Y.S.; Chang, N.C. An Efficient Approach to 3,4-Disubstituted Pyridin-2-ones. Formal Synthesis of Mappicine Ketone. J. Org. Chem. 2003, 68, 5688–5691. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Singh, A.S.; Konecny, G.E.; McCann, K.E.; Hecht, J.R.; Goldman, J.; Chmielowski, B.; Finn, R.S.; O’Brien, N.; Von Euw, E.; et al. Preclinical and Clinical Trial Results Using Talazoparib and Low-Dose Chemotherapy. Clin. Cancer Res. 2023, 29, 40–49. [Google Scholar] [CrossRef]
- Wronska, N.; Majoral, J.P.; Appelhans, D.; Bryszewska, M.; Lisowska, K. Synergistic Effects of Anionic/Cationic Dendrimers and Levofloxacin on Antibacterial Activities. Molecules 2019, 24, 2894. [Google Scholar] [CrossRef]
- Gentili, P.L.; Ortica, F.; Romani, A.; Favaro, G. Effects of Proximity on the Relaxation Dynamics of Flindersine and 6(5H)-Phenanthridinone. J. Phys. Chem. A 2007, 111, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, D.; Stockfleth, E. Topical treatment of basal cell carcinoma with the immune response modifier imiquimod. Future Oncol. 2015, 11, 2985–2990. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; He, H.T.; Yang, H.Y.; Zeng, Z.G.; Zhong, C.R.; Shi, H.; Ouyang, M.L.; Tao, Y.Y.; Pang, Y.L.; Zhang, Y.H.; et al. Synthesis of 4-Tetrazolyl-Substituted 3,4-Dihydroquinazoline Derivatives with Anticancer Activity via a One-Pot Sequential Ugi-Azide/Palladium-Catalyzed Azide-Isocyanide Cross-Coupling/Cyclization Reaction. J. Org. Chem. 2022, 87, 9488–9496. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Arigela, R.K.; Samala, S.; Kundu, B. Diversity Oriented Synthesis of Indoloazepinobenzimidazole and Benzimidazotriazolobenzodiazepine from N1-Alkyne-1,2-diamines. Chem. A Eur. J. 2015, 21, 18828–18833. [Google Scholar] [CrossRef] [PubMed]
- Ramachary, D.B.; Shruthi, K.S. A Brønsted Acid-Amino Acid as a Synergistic Catalyst for Asymmetric List-Lerner-Barbas Aldol Reactions. J. Org. Chem. 2016, 81, 2405–2419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, W.; Zuo, G.; Li, X.; Zhang, X.F.; Zhang, W. One-pot Mannich/aza-Wittig/deaminative aromatization reactions for the synthesis of 1,2,3,4-tetrahydroacridines and cyclohepta[b]quinolines. New J. Chem. 2023, 47, 220–223. [Google Scholar] [CrossRef]
- Sun, M.; Lu, Y.; Zhao, L.; Ding, M. One-pot and divergent synthesis of furo [3,2-c]quinolines and quinazolin-4(3H)-ones via sequential isocyanide-based three-component/Staudinger/aza-Wittig reaction. Tetrahedron 2021, 80, 131868. [Google Scholar] [CrossRef]
- Wei, J.; Nie, B.; Peng, R.; Cheng, X.; Wang, S. A Facile Synthesis of Benzofuro [2,3-c]quinolines via a Multicomponent Reaction and Staudinger–Aza-Wittig–Dehydroaromatization Sequence. Synlett 2016, 27, 626–630. [Google Scholar] [CrossRef]
- Bharate, J.B.; Sharma, R.; Aravinda, S.; Gupta, V.K.; Bharate, S.B.; Vishwakarma, R.A. Montmorillonite clay catalyzed synthesis of functionalized pyrroles through domino four-component coupling of amines, aldehydes, 1,3-dicarbonyl compounds and nitroalkanes. RSC Adv. 2013, 3, 21736–21742. [Google Scholar] [CrossRef]
- Qu, F.; Hu, R.; Gao, L.; Wu, J.; Cheng, X.H.; Wang, S.; He, P. New and Efficient Synthesis of 2,3,4-Trisubstituted 2H-Pyrrolo [3,4-c]quinolines via a MCR/Staudinger/Aza-Wittig Sequence. Synthesis 2015, 47, 3701–3710. [Google Scholar] [CrossRef]
- Shi, Y.; Liao, L.; He, P.; Hu, Y.; Cheng, H.; Wang, S.; Wu, J. Synthesis of 2H-pyrrolo [3,4-C]quinoline via an Aldol/Van Leusen/Staudinger/aza-Wittig sequence. Synth. Commun. 2016, 46, 1357–1363. [Google Scholar] [CrossRef]
- Nie, B.; Wu, L.; Hu, R.; Sun, Y.; Wu, J.; He, P.; Huang, N. Synthesis of cyclopropa[c]indeno [1,2-b]quinolines through a MCR/Staudinger/aza-Wittig sequence. Synth. Commun. 2017, 47, 1368–1374. [Google Scholar] [CrossRef]
- Van Es, T.; Staskun, B. Chlorine- and Sulphur-substituted Pyrrolo [3, 4-b]quinolines and Related Derivatives arising from the Aminolysis of 3, 3, 9-Trichlorothieno [3, 4-b]quinolin-1(3H)-one. S. Afr. J. Chem. 2003, 56, 40–46. [Google Scholar]
- Yi, R.; Li, X.; Wan, B. Merging Gold Catalysis and Brønsted Acid Catalysis for the Synthesis of Tetrahydrobenzo[b][1,8]naphthyridines. Adv. Synth. Catal. 2018, 360, 875–880. [Google Scholar] [CrossRef]
- Gharpure, S.J.; Shelke, Y.G. Lewis Acid Mediated Cascade Friedel–Craft/Alkyne Indol-2-yl Cation Cyclization/Vinyl Cation Trapping for the Synthesis of N-Fused Indole Derivatives. Org. Lett. 2017, 19, 5406–5409. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.P.; Bach, M.F.; Moraes, M.C.; Barcellos, T.; Lenardao, E.J.; Silva, M.S.; Alve, D. Sequential Organocatalytic Synthesis of [1,2,3]Triazolo [1,5-a]quinolines. Adv. Synth. Catal. 2020, 362, 5044–5055. [Google Scholar] [CrossRef]
- Alajarin, M.; Bonillo, B.; Ortin, M.M.; Sanchez-Andrada, P.; Vidal, A. Hydricity-Promoted [1,5]-H Shifts in Acetalic Ketenimines and Carbodiimides. Org. Lett. 2006, 8, 5645–5648. [Google Scholar] [CrossRef]
- Alajarin, M.; Bonillo, B.; Ortin, M.M.; Sanchez-Andrada, P.; Vidal, A. Tandem 1,5-Hydride Shift/6π Electrocyclization of Ketenimines and Carbodiimides Substituted with Cyclic Acetal and Dithioacetal Functions: Experiments and Computations. Eur. J. Org. Chem. 2011, 2011, 1896–1913. [Google Scholar] [CrossRef]
- Ratheesh, M.; Sindhu, G.; Helen, A. Anti-inflammatory effect of quinoline alkaloid skimmianine isolated from Ruta graveolens L. Inflamm. Res. 2013, 62, 367–376. [Google Scholar] [CrossRef]
- Zuo, Y.; Pu, J.; Chen, G.; Shen, W.; Wang, B. Study on the activity and mechanism of skimmianine against human non-small cell lung cancer. Nat. Prod. Res. 2019, 33, 759–762. [Google Scholar] [CrossRef]
- Wang, L.H.; Wehland, M.; Wise, P.M.; Infanger, M.; Grimm, D.; Kreissl, M.C. Cabozantinib, Vandetanib, Pralsetinib and Selpercatinib as Treatment for Progressed Medullary Thyroid Cancer with a Main Focus on Hypertension as Adverse Effect. Int. J. Mol. Sci. 2023, 24, 2312. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Pili, R. Tasquinimod targets suppressive myeloid cells in the tumor microenvironment. Oncoimmunology 2019, 8, e1072672. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Rosen, P.; Lockhart, A.C.; Fu, S.; Janku, F.; Kurzrock, R.; Khan, R.; Amore, B.; Caudillo, I.; Deng, H.; et al. A first-in-human study of AMG 208, an oral MET inhibitor, in adult patients with advanced solid tumors. Oncotarget 2015, 6, 18693–18706. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Peng, L.; Liu, Y.; Till, B.G.; Yan, X.; Zhang, J.; Zhu, L.; Wang, H.; Zhang, S.; Li, H.; et al. Low-dose anlotinib confers improved survival in combination with immune checkpoint inhibitor in advanced non-small cell lung cancer patients. Cancer Immunol. Immunother. 2023, 72, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zou, W.; Zhao, X. Treatment of neurofibromatosis type II with anlotinib: A case report and literature review. Anti-Cancer Drugs 2023, 34, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Lin, F.; Yu, B.; Qiu, J.; Zheng, L. The efficacy and adverse events of delafloxacin in the treatment of acute bacterial infections: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2022, 13, 975578. [Google Scholar] [CrossRef] [PubMed]
- Gould, R.G.; Jacobs, W.A. The Synthesis of Certain Substituted Quinolines and 5,6-Benzoquinolines. J. Am. Chem. Soc. 1939, 10, 2890–2895. [Google Scholar] [CrossRef]
- Jentsch, N.G.; Hume, J.D.; Crull, E.B.; Beauti, S.M.; Pham, A.H.; Pigza, J.A.; Kessl, J.J.; Donahue, M.G. Quinolines from the cyclocondensation of isatoic anhydride with ethyl acetoacetate: Preparation of ethyl 4-hydroxy-2-methylquinoline-3-carboxylate and derivatives. Beilstein J. Org. Chem. 2018, 14, 2529–2536. [Google Scholar] [CrossRef]
- Gharpure, S.J.; Nanda, S.K.; Adate, P.A.; Shelke, Y.G. Lewis Acid Promoted Oxonium Ion Driven Carboamination of Alkynes for the Synthesis of 4-Alkoxy Quinolines. J. Org. Chem. 2017, 82, 2067–2080. [Google Scholar] [CrossRef]
- Hande, P.E.; Mishra, M.; Ali, F.; Kapoor, S.; Datta, A.; Gharpure, S.J. Design and Expeditious Synthesis of Quinoline-Pyrene-Based Ratiometric Fluorescent Probes for Targeting Lysosomal pH. ChemBioChem 2020, 21, 1492–1498. [Google Scholar] [CrossRef]
- Gharpure, S.J.; Nanda, S.K.; Fartade, D.J. Formal [4+2] Cycloaddition of o-Aza-Quinone Methide for the Synthesis of 1,4-Heterocycle-Fused Quinolines. Adv. Synth. Catal. 2021, 363, 2562–2567. [Google Scholar] [CrossRef]
- Su, H.; Bao, M.; Pei, C.; Hu, W.H.; Qiu, L.H.; Xu, X.F. Gold-catalyzed dual annulation of azide-tethered alkynes with nitriles: Expeditious synthesis of oxazolo [4,5-c]quinolines. Org. Chem. Front. 2019, 6, 2404–2409. [Google Scholar] [CrossRef]
- Su, H.; Bao, M.; Huang, J.J.; Qiu, L.H.; Xu, X.F. Silver-Catalyzed Carbocyclization of Azide-Tethered Alkynes: Expeditious Synthesis of Polysubstituted Quinolines. Adv. Synth. Catal. 2019, 361, 826–831. [Google Scholar] [CrossRef]
- Huang, J.; Su, H.; Bao, M.; Qiu, L.; Zhang, Y.; Xu, X. Gold(iii)-catalyzed azide-yne cyclization/O–H insertion cascade reaction for the expeditious construction of 3-alkoxy-4-quinolinone frameworks. Org. Biomol. Chem. 2020, 18, 3888–3892. [Google Scholar] [CrossRef]
- Lenoci, A.; Tomassi, S.; Conte, M.; Benedetti, R.; Rodriguez, V.; Carradori, S.; Secci, D.; Castellano, S.; Sbardella, G.; Filetici, P.; et al. Quinoline-Based p300 Histone Acetyltransferase Inhibitors with Pro-apoptotic Activity in Human Leukemia U937 Cells. ChemMedChem 2014, 9, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhou, W.; Liu, J. Synthesis and HMG-CoA reductase inhibition of 2-cyclopropyl-4-thiophenyl-quinoline mevalonolactones. Bioorg. Med. Chem. 2009, 17, 7915–7923. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qiu, W.; Liu, L.; Zhang, X.; Awad, J.M.; Evans, J.; Zhang, W. Synthesis of tetrahydropyrrolothiazoles through one-pot and four-component N,S-acetalation and decarboxylative [3+2] cycloaddition. Green Synth. Catal. 2021, 2, 74–77. [Google Scholar] [CrossRef]
- Ward, S.A. Mechanisms of chloroquine resistance in malarial chemotherapy. Trends Pharmacol. Sci. 1988, 9, 241–246. [Google Scholar] [CrossRef]
- Homewood, C.A.; Warhurst, D.C.; Peters, W.; Baggaley, V.C. Lysosomes, pH and the Anti-malarial Action of Chloroquine. Nature 1972, 235, 50–52. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, H.K.; Hwang, E.S. Novel anti-adipogenic activity of anti-malarial amodiaquine through suppression of PPARγ activity. Arch. Pharm. Res. 2017, 40, 1336–1343. [Google Scholar] [CrossRef]
- Brummendorf, T.H.; Cortes, J.E.; Milojkovic, D.; Gambacorti-Passerini, C.; Clark, R.E.; le Coutre, P.; Garcia-Gutierrez, V.; Chuah, C.; Kota, V.; Lipton, J.H.; et al. Bosutinib versus imatinib for newly diagnosed chronic phase chronic myeloid leukemia: Final results from the BFORE trial. Leukemia 2022, 36, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Mezger, K.; Ebert, S.; Muhle, H.E.; Stadt, U.Z.; Borkhardt, A.; Dilloo, D.; Faber, J.; Feuchtinger, T.; Imschweiler, T.; Jorch, N.; et al. Amsacrine combined with etoposide and methylprednisolone is a feasible and safe component in first-line intensified treatment of pediatric patients with high-risk acute lymphoblastic leukemia in CoALL08-09 trial. Pediatr. Blood Cancer 2022, 69, e29997. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Dalia Ricci, A.; Brandi, G. Neoadjuvant Dovitinib in Early- and Intermediate-Stage Hepatocellular Carcinoma. Oncologyst 2022, 27, e976. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.D.; Adhikari, A.V.; Shetty, N.S. Design, synthesis and antimicrobial activities of some new quinoline derivatives carrying 1,2,3-triazole moiety. Eur. J. Med. Chem. 2010, 45, 3803–3810. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.J.; Loo, T.L. Synthesis and antitumor activity of halogen-substituted 4-(3,3-dimethyl-1-triazeno)quinolines. J. Med. Chem. 1978, 21, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Lavrard, H.; Larini, P.; Popowycz, F. Superacidic Cyclization of Activated Anthranilonitriles into 2-Unsubstituted-4-aminoquinolines. Org. Lett. 2017, 19, 4203–4206. [Google Scholar] [CrossRef] [PubMed]
- Vidyacharan, S.; Sagar, A.; Sharada, D.S. A new route for the synthesis of highly substituted 4-aminoquinoline drug like molecules via aza hetero–Diels–Alder reaction. Org. Biomol. Chem. 2015, 13, 7614–7618. [Google Scholar] [CrossRef]
- Zhang, X.F.; Ma, X.M.; Qiu, W.; Award, J.M.; Evans, J.; Zhang, W. One-Pot Mannich, Aza-Wittig and Dehydrofluorinative Aromatization Reactions for Direct Synthesis of 2,3-Disubstituted 4-Aminoquinolines. Adv. Synth. Catal. 2020, 362, 5513–5517. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, M.; Qiu, W.; Zhang, W. 2-Azidobenzaldehyde-Based [4+2] Annulation for the Synthesis of Quinoline Derivatives. Molecules 2024, 29, 1241. https://doi.org/10.3390/molecules29061241
Zhang X, Liu M, Qiu W, Zhang W. 2-Azidobenzaldehyde-Based [4+2] Annulation for the Synthesis of Quinoline Derivatives. Molecules. 2024; 29(6):1241. https://doi.org/10.3390/molecules29061241
Chicago/Turabian StyleZhang, Xiaofeng, Miao Liu, Weiqi Qiu, and Wei Zhang. 2024. "2-Azidobenzaldehyde-Based [4+2] Annulation for the Synthesis of Quinoline Derivatives" Molecules 29, no. 6: 1241. https://doi.org/10.3390/molecules29061241
APA StyleZhang, X., Liu, M., Qiu, W., & Zhang, W. (2024). 2-Azidobenzaldehyde-Based [4+2] Annulation for the Synthesis of Quinoline Derivatives. Molecules, 29(6), 1241. https://doi.org/10.3390/molecules29061241