Abstract
The 1,4-benzodiazepine structural framework is a fascinating element commonly found in biologically active and pharmaceutically relevant compounds. A highly efficient method for synthesizing 1,4-benzodiazepin-3-ones is described, involving a [4+3]-cycloaddition reaction between 2-amino-β-nitrostyrenes and α-bromohydroxamate, with Cs2CO3 used as a base. This process yielded the desired 1,4-benzodiazepines in good yields. Furthermore, an organocatalytic asymmetric [4+3]-cycloaddition was successfully accomplished using a bifunctional squaramide-based catalyst. This approach enabled the enantioselective synthesis of chiral 1,4-benzodiazepines with commendable yields and moderate enantioselectivities, reaching up to 80% yield and 72% ee.
1. Introduction
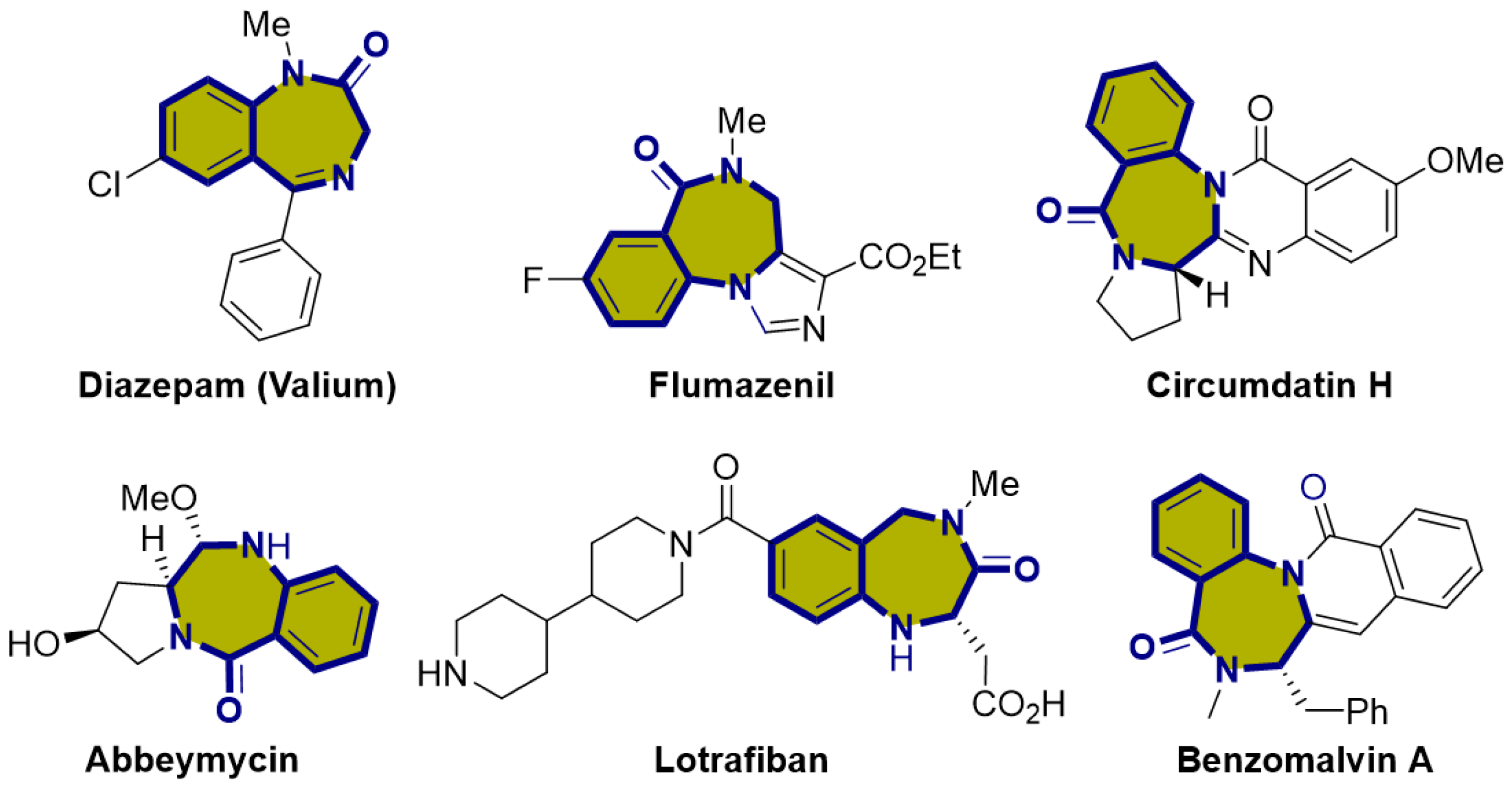
N,N-Heterocycles are prevalent in various natural products and pharmaceuticals, making them crucial structural elements in medicinal chemistry [1,2]. Among these, 1,4-benzodiazepinone, which is a seven-membered lactam, presents a structural framework frequently observed in numerous natural substances. Derivatives of this compound can exhibit a wide range of biological activities and valuable pharmaceutical characteristics, indicating their promise as potential candidates for drug discovery (Figure 1) [3,4,5,6,7,8,9]. Currently, this seven-membered structural framework is prevalent in numerous drug molecules with distinct bioactivities. For instance, Diazepam, initially introduced to the market as Valium, belongs to the benzodiazepine family and serves as an anxiolytic medication. It is frequently prescribed to address various conditions, such as anxiety, seizures, muscle spasms, and insomnia [10]. Additionally, Lotrafiban belongs to the latest generation of platelet GPIIb/IIIa blockers, representing a significant advancement in interventional cardiology and the treatment of acute ischemic syndrome, aimed at preventing vascular occlusion [11,12].
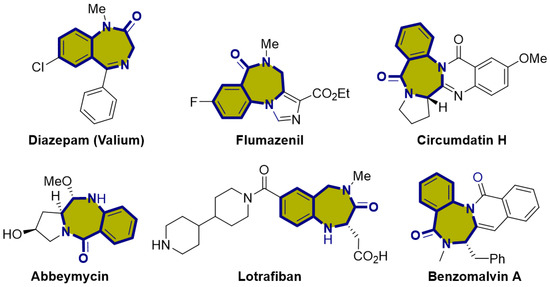
Figure 1.
Biologically active compounds containing the 1,4-benzodiazepione skeleton.
Indeed, the effective synthesis of a wide range of functionally diverse 1,4-benzodiazepinone derivatives has garnered considerable research attention [4,13,14,15,16,17]. However, the synthesis of a seven-membered ring 1,4-benzodiazepinone presents difficulties due to unfavorable enthalpic and entropic factors that hinder the ring closure process [18]. Consequently, achieving efficient access to 1,4-benzodiazepinone represents a significant and demanding task in the field of organic synthesis. While there have been numerous reported synthetic routes for 1,4-benzodiazepinones over the past decade, it is noteworthy that there are very limited instances of one-pot and asymmetric synthesis methods available [19].
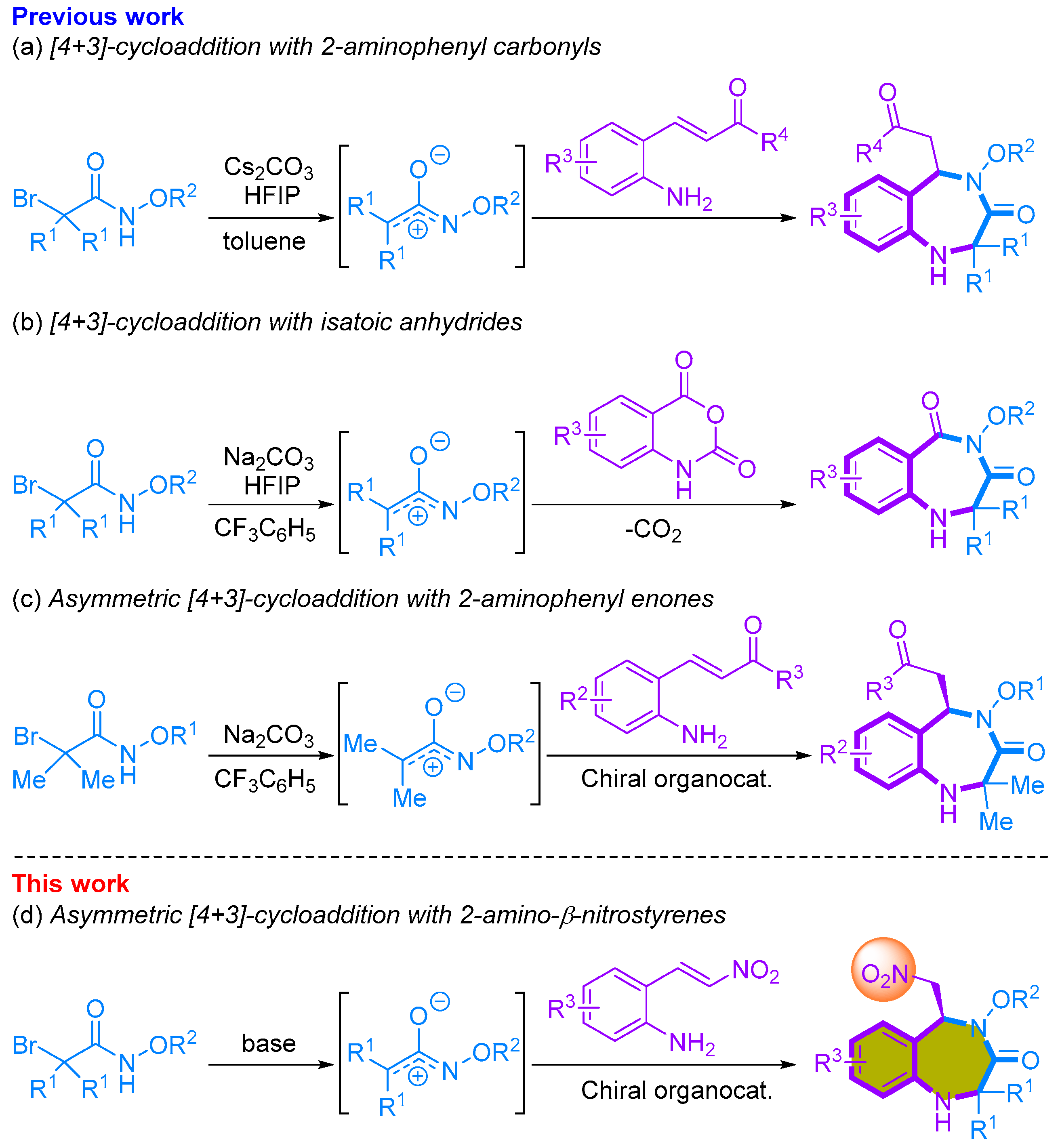
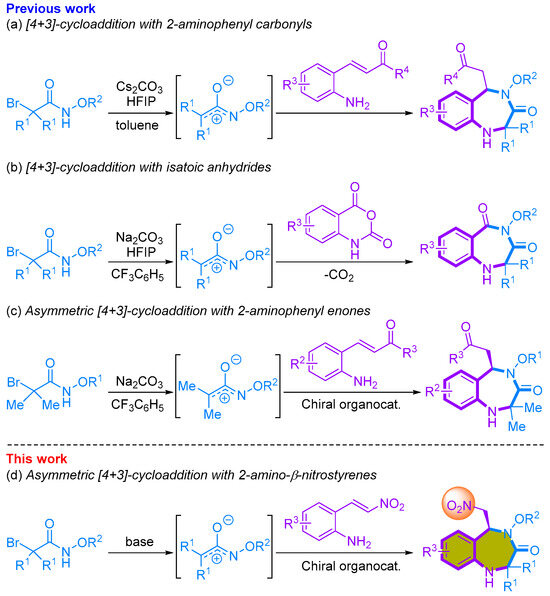
We recently reported research detailing synthetic methods for 1,4-benzodiazepinone through the utilization of a [4+3]-annulation approach involving azaoxyallyl cations [20,21,22,23]. We successfully achieved the one-pot synthesis of 1,4-benzodiazepine-3-ones by conducting aza/aza-[4+3]-cycloadditions of α-halohydroxamates with 2-aminophenyl α,β-unsaturated phenyl ketones. This reaction employed Cs2CO3 as the base and hexafluoroisopropanol (HFIP) as an additive, resulting in the formation of 1,4-benzodiazepine-3-ones in good yields (Scheme 1a) [20]. We also developed a [4+3]-annulation reaction involving in situ-generated azaoxyallyl cations and isatoic anhydride, leading to the synthesis of seven-membered 1,4-benzodiazepinediones in moderate to good yields. This reaction involves a sequential cascade, encompassing decarboxylative addition of HFIP to isatoic anhydride, addition to azaoxyallyl cation, and intramolecular substitution, ultimately yielding 1,4-benzodiazepinediones (Scheme 1b) [21]. In addition to these advancements, we reported an organocatalytic asymmetric [4+3]-cycloaddition of 2-aminophenyl α,β-unsaturated carbonyls with in situ generated azaoxyallyl cations. In this asymmetric reaction, we obtained enantioenriched functionalized seven-membered 1,4-benzodiazepine-3-ones in good yields and with excellent enantioselectivities (Scheme 1c). Furthermore, several of the resulting compounds displayed promising bioactivity in the prevention of peripheral nerve degeneration [23]. Continuing our exploration of organocatalytic asymmetric [4+3]-cycloaddition reactions for the synthesis of enantioenriched 1,4-benzodiazepine-3-ones, we present our achievement in the asymmetric [4+2]-cycloaddition of 2-amino-β-nitrostyrenes and α-bromohydroxamates (Scheme 1d). We anticipate that the resulting compounds will serve as a valuable foundation for the synthesis of diverse compounds, potentially enhancing their bioactivity by facilitating the conversion of the nitro group.
Scheme 1.
Synthetic of 1,4-benzodiazepiones using [4+3]-cycloaddition with azaoxyallyl cations.
2. Results and Discussion
2.1. Non-Enantioselective [4+3]-Cycloaddition of 2-Amino-β-nitrostyrenes with Azaoxyallyl Cations
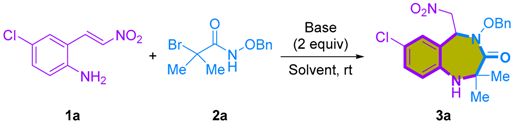
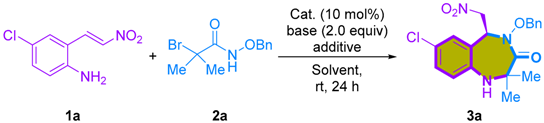
To begin our study, our primary objective was to determine the feasibility of the [4+3]-cycloaddition involving 2-amino-β-nitrostyrenes [24] and α-bromohydroxamates. In our initial experiment, we conducted a reaction involving 2-amino-β-nitrostyrene 1a and α-bromohydroxamate 2a, employing Cs2CO3 as a base and HFIP as an additive. This reaction was carried out in toluene at room temperature, resulting in the desired 1,4-benzodiazepine-3-ones 3a in an 18% yield (Table 1, entry 1). Despite the initial low yield, these promising results served as a source of motivation to drive further investigations. We embarked on a systematic approach to establish and optimize the reaction conditions for the [4+3]-cycloaddition involving 2-amino-β-nitrostyrene 1a and α-bromohydroxamate 2a. In our quest to identify the optimal reaction conditions for the [4+3]-cycloaddition reaction, we observed a notable improvement when we switched the solvent to CH2Cl2, resulting in an increased yield of 55% (Table 1, entry 2). Subsequently, we introduced HFIP as a co-solvent. It is worth noting that many cycloadditions involving azaoxyallyl cations have demonstrated enhanced performance in HFIP solutions. This can be attributed to HFIP’s distinctive properties, such as its strong hydrogen bond donation and high ionizing power, which effectively stabilize azaoxyallyl cations [25,26]. The incorporation of a CH2Cl2/HFIP co-solvent system significantly improved the yield of 1,4-benzodiazepine-3-one 3a, elevating it to 85%. Moreover, adjusting the solvent concentration to 0.2 M led to a further increase in yield, reaching an impressive 91%. (Table 1, entries 3 and 4). Further efforts to optimize the reaction efficiency involved exploring various solvents. However, the co-solvent system of HFIP with solvents such as CHCl3, CH3CN, toluene, and THF resulted in inferior yields of the product (Table 1, entries 5–8). Despite thorough exploration of various inorganic and organic bases (Table 1, entries 10–15), it was found that Cs2CO3 consistently delivered the most favorable results.

Table 1.
Optimization of the reaction conditions a.
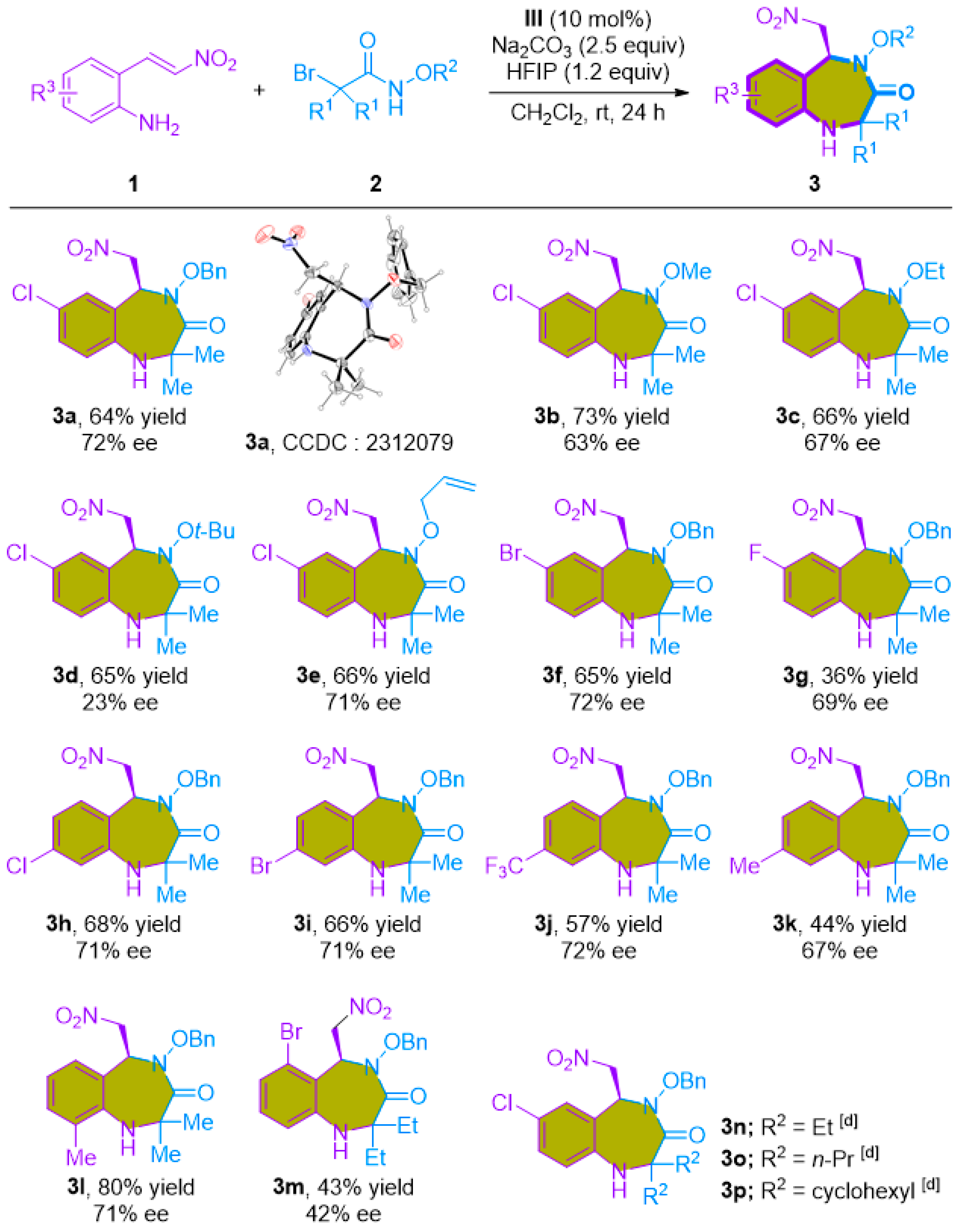
With the optimized reaction conditions in hand, we embarked on an exploration of the substrate scope and the generality of this reaction (Scheme 2). α-Bromohydroxamates bearing an array of N-protecting groups, including methoxy, ethoxy, tert-butyloxy, and allyloxy, exhibited excellent compatibility with the reaction, yielding the desired products (3a–3e) in yields ranging from good to high (75–91%). A range of 2-amino-β-nitrostyrenes (1b–1i) were employed in the cycloaddition, delivering 1,4-benzodiazepine-3-ones in moderate to good yields (41–83%). Our exploration of substituents positioned at the C4–C6 positions demonstrated a broad tolerance for diverse groups, including halides, -CF3, and methyl groups. Moreover, α-bromohydroxamates bearing diethyl, diisopropyl, and cyclohexyl substituents were also successfully accommodated under the reaction conditions, affording the desired products (3n–3p) in good yields (73–79%).

Scheme 2.
Substrate scope for the [4+3]-cycloaddition of 2-amino-β-nitrostyrenes with α-bromohydroxamates a,b. a Standard reaction conditions: 1a (0.20 mmol), 2 (0.30 mmol), Cs2CO3 (0.40 mmol), and CH2Cl2/HFIP (1:1, 1.0 mL), stirred at rt for 1 h. b Isolated yield after chromatographic purification.
2.2. Enantioselective [4+3]-Cycloaddition of 2-Amino-β-nitrostyrenes with Azaoxyallyl Cations
Having successfully pioneered the initial instance of [4+3]-cycloaddition between 2-amino-β-nitrostyrenes and azaoxyallyl cations, our research focus naturally transitioned towards developing an enantioselective reaction. This shift holds the promise of opening a new pathway to obtain enantioenriched chiral 1,4-benzodiazepine-3-ones. We envisioned achieving enantioselective [4+3]-cycloaddition through the use of a suitable bifunctional squaramide-based or thiourea-based organocatalyst, facilitating precise hydrogen bonding interactions [27,28,29,30].
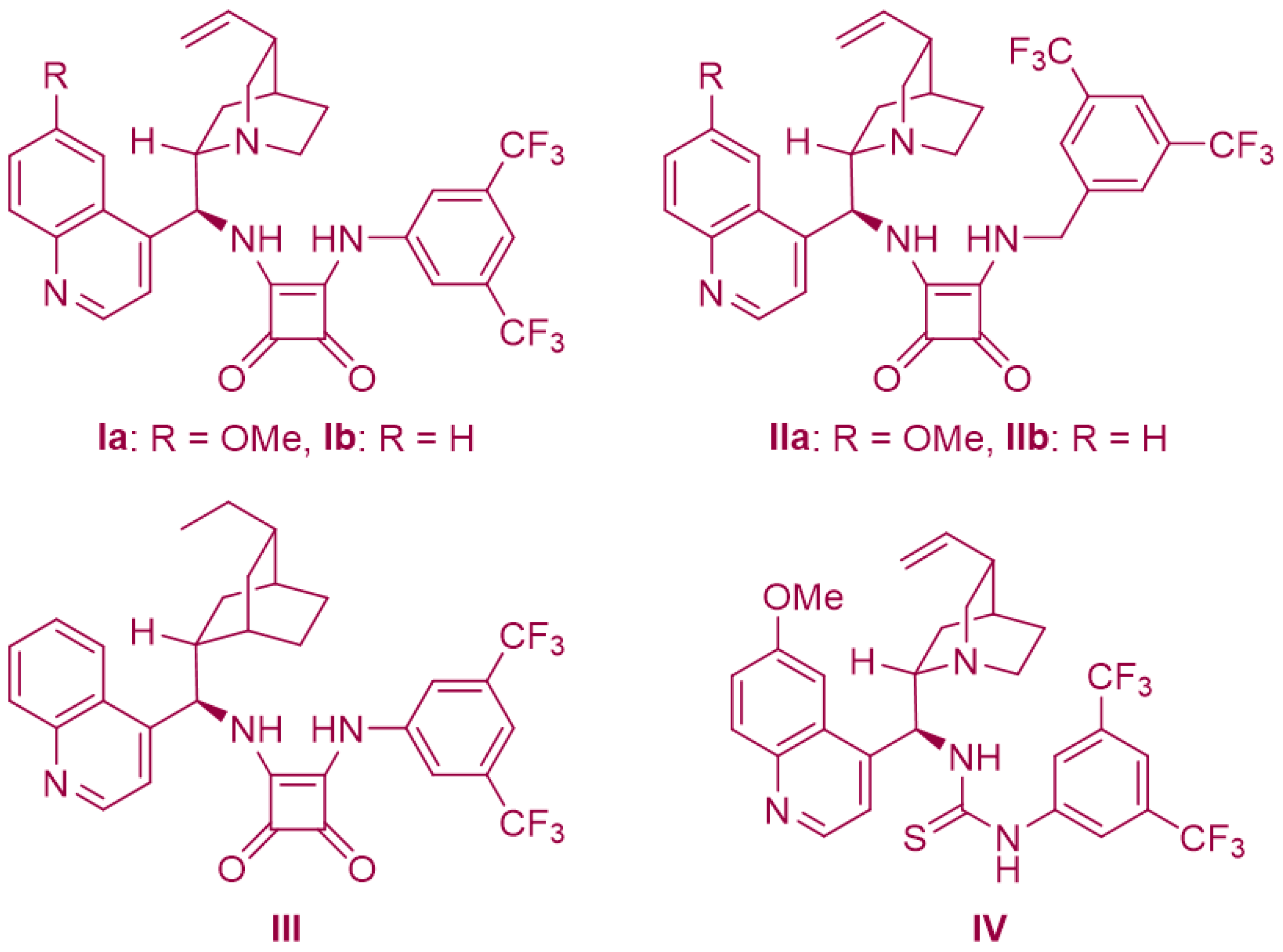
The reaction between 2-amino-β-nitrostyrene 1a and α-bromohydroxamate 2a, utilizing Na2CO3 as a base and a bifunctional squaramide-based organocatalyst Ia, was carried out in CF3C6H5 at room temperature, yielding the desired enantio-enriched 1,4-benzodiazepine-3-one 3a in a 14% yield with a 48% ee (Table 2, entry 1). Although the yield was modest, it confirmed the possibility of enantiomeric discrimination. Subsequently, to enhance both the reaction yield and enantioselectivity, we optimized the reaction conditions. A comprehensive exploration of various inorganic and organic bases was conducted (Table 2, entries 2–7). In terms of yield, Cs2CO3 displayed slightly superior results compared to Na2CO3, and with respect to enantioselectivity, K2CO3 outperformed Na2CO3. However, when considering the overall performance, it became evident that Na2CO3 yielded the most promising results. Moving forward, we further investigated various organic solvents, including toluene, CH2Cl2, CHCl3, ClCH2CH2Cl, THF, and CH3CN, employing catalyst Ia. Notably, when utilizing CH2Cl2 as the solvent, both the reaction yield and stereoselectivity were significantly higher compared to the other solvents tested, resulting in a 37% yield and a 78% ee (Table 2, entry 9). In our ongoing effort to optimize the reaction conditions, we conducted a screening of various chiral cinchona-derived squaramide-based catalysts (Figure 2). The cinchonidine-derived squaramide catalyst Ib, yielded results similar to those obtained with the quinine-derived squaramide catalyst Ia. However, the bis(trifluoromethyl)phenylmethylene-squaramide catalysts (Ic and Id) produced inferior results compared to the bis(trifluoromethyl)phenyl-squaramide catalysts (Ia and Ib). Among the bifunctional catalysts evaluated, cinchonidine-derived squaramide III emerged as the optimal choice due to its outstanding reactivity and enantioselectivity (Table 2, entry 17). On the other hand, the cinchona-derived thiourea catalyst IV exhibited good reactivity with a 72% yield, but unfortunately, it did not demonstrate enantioselectivity (Table 2, entry 18; 72% yield, 2% ee). Furthermore, our investigation revealed that the addition of HFIP as an additive in this reaction led to a slight decrease in enantioselectivity, but a notable increase in reactivity. After careful examination, we determined that the optimal reaction conditions entailed employing catalyst III, Na2CO3 as the base, HFIP as the additive, and CH2Cl2 as the solvent (Table 2, entry 22; 64% yield, and 72% ee).

Table 2.
Optimization of the asymmetric catalytic reaction conditions a.

Figure 2.
Chiral squaramide-based and thiourea-based catalysts.
Upon the optimized reaction conditions, the substrate scope and the generality of the 2-amino-β-nitrostyrene and α-bromohydroxamate were investigated, as shown in Scheme 3. α-Bromohydroxamates, featuring a range of N-protecting groups such as methoxy, ethoxy, and allyloxy demonstrated good reactivity and exhibited moderate enantioselectivity (63–71% ee), with only slightly reduced enantioselectivity observed for α-bromohydroxamate having tert-butyloxy group. A variety of 2-amino-β-nitrostyrenes (1b–1i) were utilized in the cycloaddition, yielding enantioenriched 1,4-benzodiazepine-3-ones with moderate to good yields (ranging from 36% to 80%) and enantioselectivities between 42% to 72% ee. Substituents positioned at C4-C6 exhibited a remarkable degree of adaptability, displaying a wide range of tolerance in terms of enantioselectivity for various groups. Regrettably, α-bromohydroxamates bearing diethyl, diisopropyl, and cyclohexyl substituents, which successfully underwent racemic reactions, did not exhibit reactivity in this asymmetric reaction. Finally, the relative and absolute configuration of the proposed 1,4-benzodiazepine-3-ones were determined by X-ray crystallographic analysis of 3a [31]. The configurations of the other products were assigned by analogy.

Scheme 3.
Substrate scope for asymmetric [4+3]-cycloaddition of 2-amino-β-nitrostyrenes with α-bromohydroxamates a,b,c. a Standard reaction conditions: 1 (0.10 mmol), 2 (0.30 mmol), Na2CO3 (0.25 mmol]), HFIP (0.12 mmol]), III (0.010 mmol) and CH2C12 (1.0 mL), stirred at rt. b Isolated yield after chromatographic purification. c ee was determined by chiral HPLC. d Trace amounts of the product were detected.
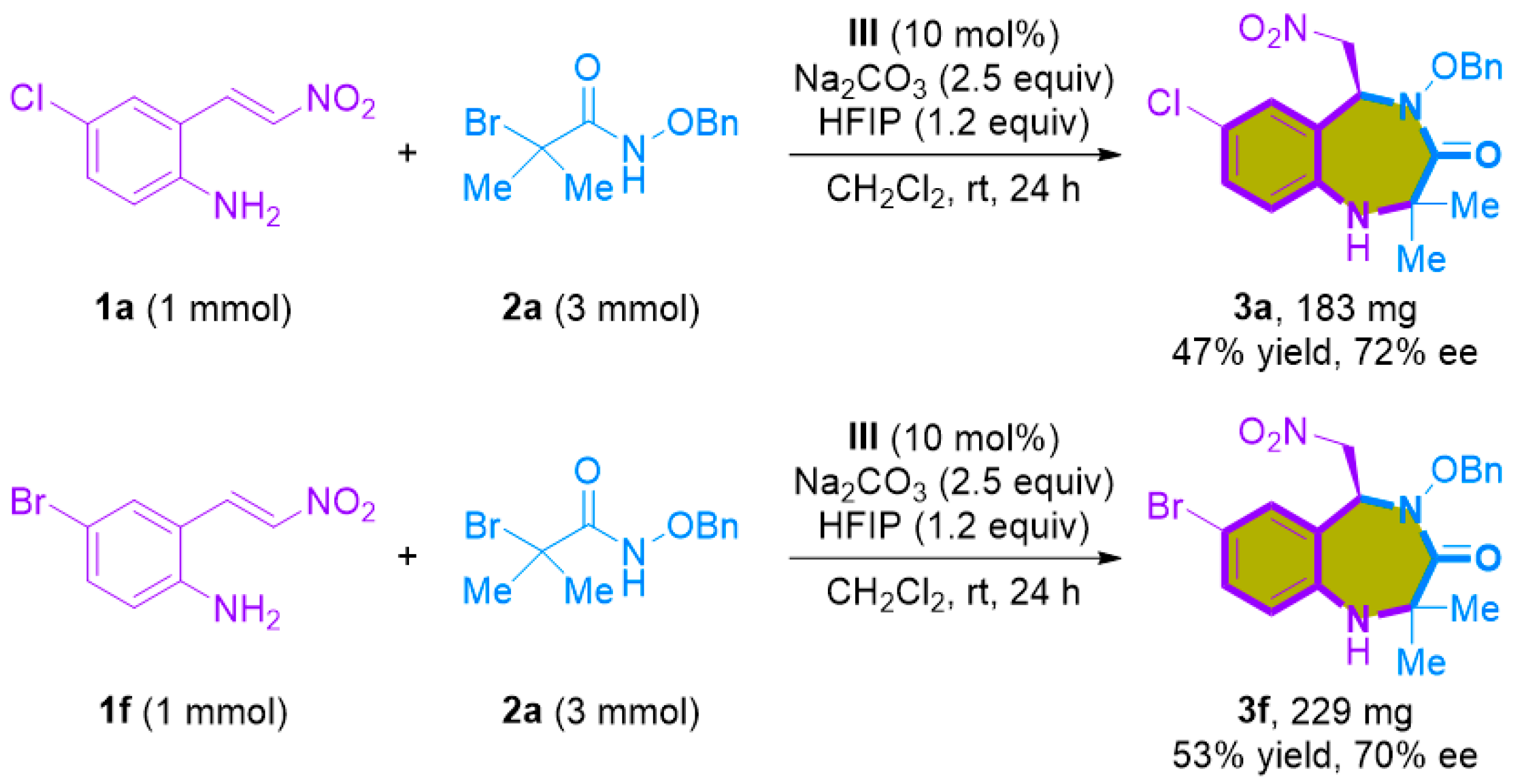
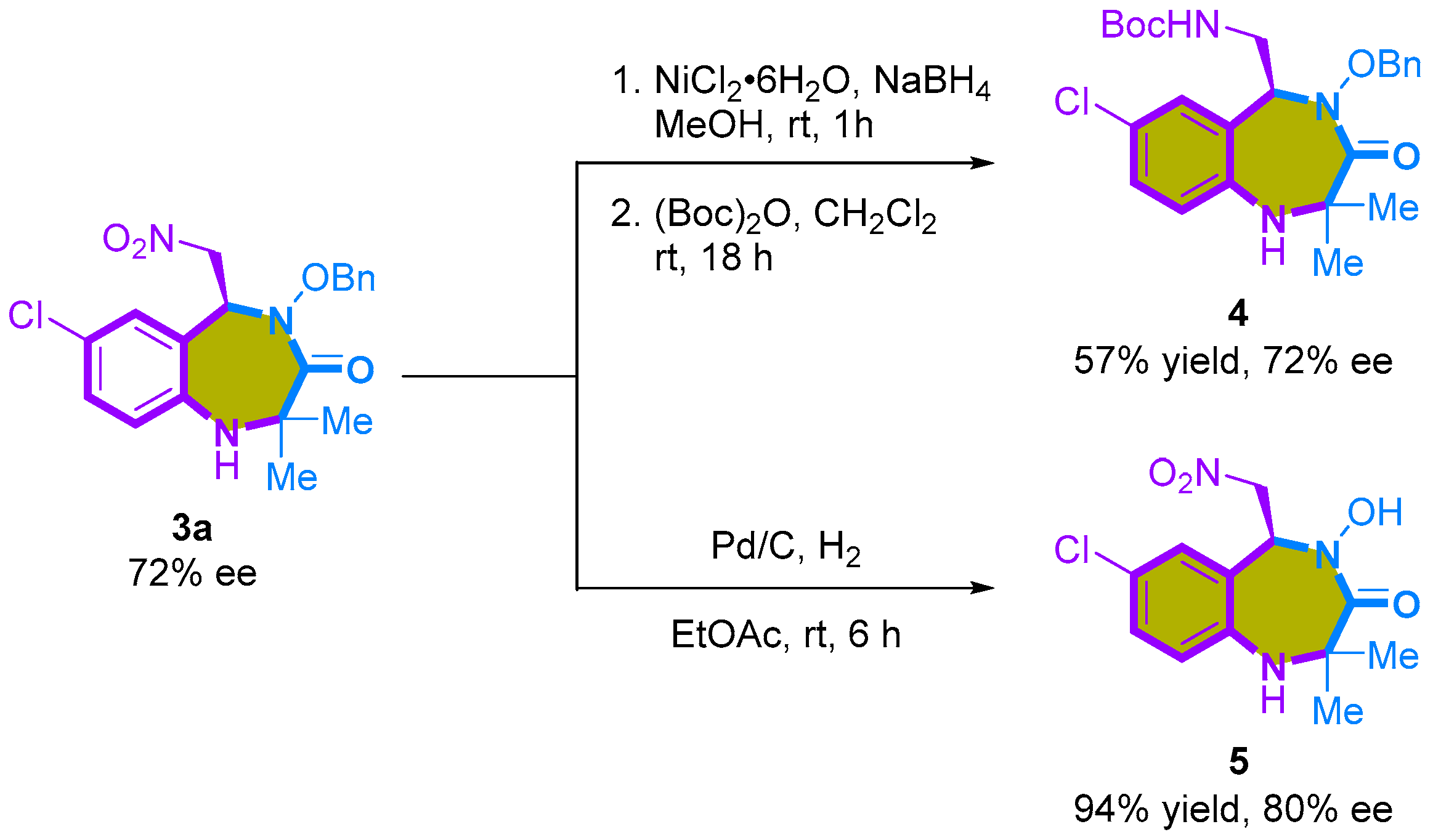
To illustrate the practicality of the [4+3]-cycloaddition, we conducted a one-mmol scale reaction and subsequent synthetic transformations. The scalability of the [4+3]-cycloaddition reaction was verified using 1 mmol of 1a and 1f under the optimized reaction conditions, resulting in a modest reduction in yield (47% and 53%, respectively), but with minimal loss of enantioselectivities (Scheme 4). Moreover, we demonstrated the versatility of the synthesized 1,4-benzodiazepine-3-ones 3 through subsequent transformations (Scheme 5). Successfully reducing the nitro group in the 1,4-benzodiazepine-3-one products, particularly 3a, was achieved using NiCl2 and NaBH4, in combination with (Boc)2O, yielding product 4 with a respectable 57% yield. Additionally, deprotecting the benzyl group of 3a via palladium-catalyzed hydrogenation produced N-hydroxylamide products 5 in high yield (94%) and a slightly enhanced enantioselectivity (80% ee).

Scheme 4.
One-mmol scale synthesis.

Scheme 5.
Synthetic transformations.
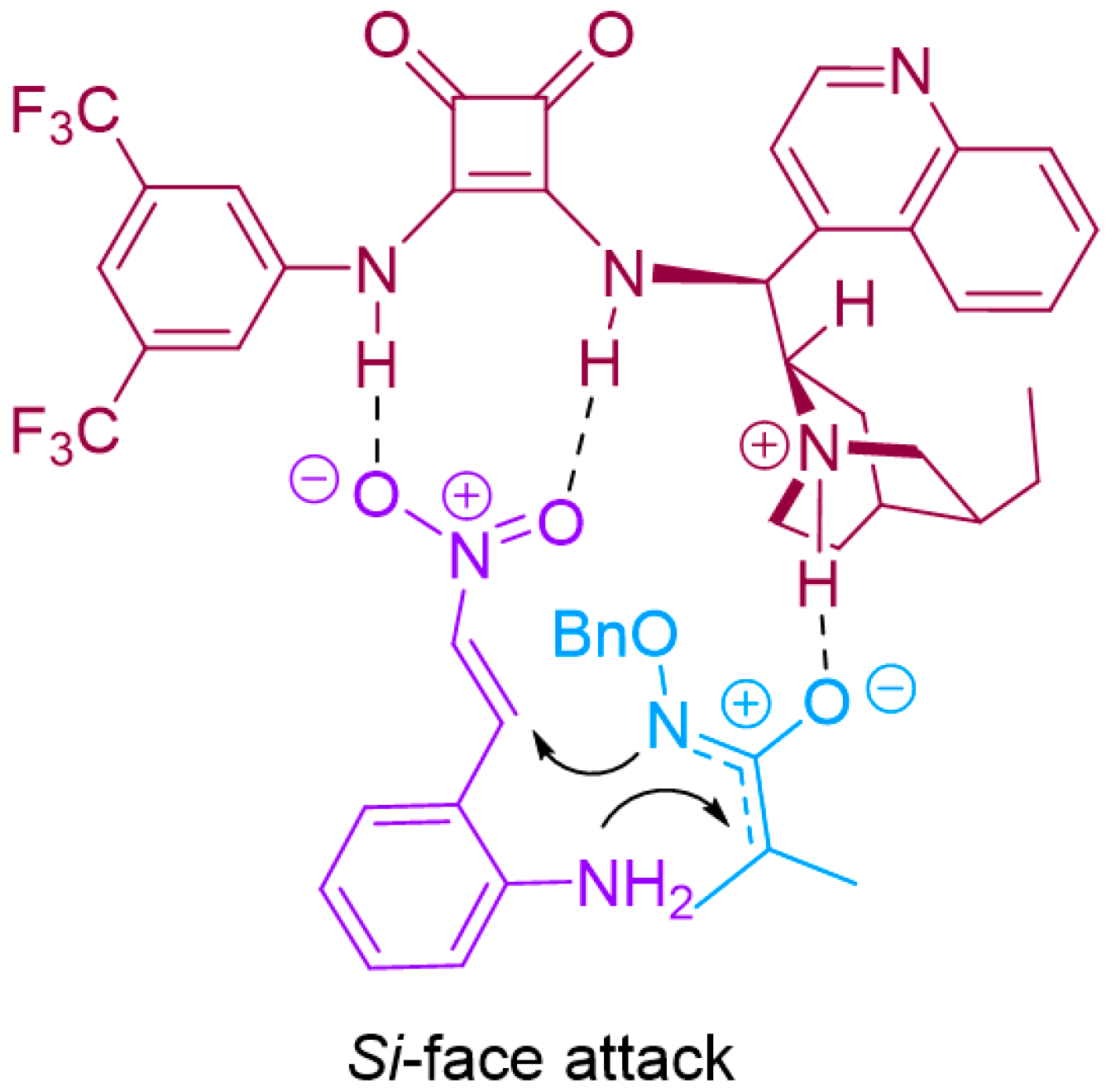
Based on our experimental findings and the absolute configuration of 1,4-benzodiazepine-3-ones, as illustrated in Figure 3, we propose a plausible transition state mechanism. In the reaction, the azaoxyallyl cation undergoes deprotonation, and the deprotonated oxygen atom forms a hydrogen bond with the protonated amine in squaramide catalyst III. Concurrently, 1,4-benzodiazepine-3-ones 1a engages with the catalyst through double hydrogen bonding, effectively stabilizing and activating the substrate in a bidentate interaction. This interaction positions the nitrogen atom of 1a to attack the α-carbon of the azaoxyallyl cation. Simultaneously, the nitrogen atom of the azaoxyallyl cation selectively attacks the Si face of the 2-amino-β-nitrostyrene, facilitating an intramolecular attack that leads to the formation of the desired product 3a with an S-configuration.

Figure 3.
The Plausible transition state of the reaction.
3. Experimental
3.1. General Information
Prior to usage, organic solvents (Daejung Chemicals & Metals Co, Siheung, Republic of Korea) underwent distillation. Under reduced pressure, organic solutions were concentrated using a rotary evaporator. Forced-flow chromatography on Merk silica gel 60 achieved chromatographic purification of products. EM Reagents 0.25 mm silica gel 60-F plates (Merk, Rahway, NJ, USA) were utilized for Thin-layer chromatography (TLC). Visualization of developed chromatograms occurred through fluorescence quenching and anisaldehyde stain. Recording at 400 MHz for 1H, 100 MHz for 13C, and 176 MHz for 19F, 1H, 13C, and 19F NMR spectra were internally referenced to residual protic solvent signals. 1H NMR data include chemical shift (δ ppm), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, br = broad singlet, dd = doublet of doublets, dt = doublet of triplets, qd = quartet of doublets, ddd = doublet of doublet of doublets, and m = multiplet), coupling constant (Hz), and integration. Chemical shift is used to report 13C NMR data. IR spectra were recorded on an FT IR spectrometer (Bruker, Karlsruhe, Germany). High-resolution mass spectra (HRMS) were obtained using a double-focusing mass spectrometer with EI-magnetic sector (Jeol Ltd., Tokyo, Japan). The crystal structure was determined by a single-crystal diffractometer (a Bruker D8 Venture PHOTON III M14 diffractometer) (Bruker, Karlsruhe, Germany) at the Western Seoul Center of Korea Basic Science Institute. Enantiomeric excesses were determined using an HPLC instrument with Chiralpak columns (Daicel Corporation, Tokyo, Japan), as indicated. α-Bromohydroxamates [32,33,34], 2-amino-β-nitrostyrens [24], and organocatalysts [35,36,37] were prepared according to the literature.
3.2. General Procedure I for the [4+3]-Cycloaddition of 2-Amino-β-nitrostyrenes with α-Bromohydroxamates
To a solution of 2-amino-β-nitrostyrene 1 (0.20 mmol, 1 equiv) and α-bromohydroxamate 2 (0.30 mmol, 1.5 equiv in CH2Cl2/HFIP (1:1, 1.0 mL, 0.2 M) was added Cs2CO3 (0.40 mmol, 2 equiv) at 0 °C. The reaction mixture was allowed to stir at room temperature. After stirring for 1 h, the resulting mixture was filtered through the plug of celite. The filtrate was concentrated in vacuo. The crude residue was purified by flash column chromatography with EtOAc/hexanes as eluent to afford desired product 3 (The spectra can be found in Supplementary Materials).
4-(Benzyloxy)-7-chloro-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3a). Following the general procedure I; 71 mg, yield 91%, White solid; m.p. 145–147 °C; Rf 0.3 (30% ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.47–7.37 (m, 5H), 7.18 (dd, J = 8.3, 2.4 Hz, 1H), 6.75 (d, J = 8.3 Hz, 1H), 6.57 (d, J = 2.4 Hz, 1H), 5.24–5.12 (m, 1H), 4.96 (s, 2H), 4.86–4.75 (m, 2H), 3.12 (s, 1H), 1.63 (s, 3H), 1.21 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 172.7, 142.8, 134.9, 130.5, 130.4, 130.2, 129.4, 129.0, 128.9, 128.6, 124.6, 76.6, 75.0, 65.8, 63.5, 29.7, 27.7; IR (neat) 3338, 2922, 2854, 1666, 1551, 1489, 1454, 1388, 1285, 1230, 1197, 1000 cm−1; HRMS (EI) m/z calcd for [M]+ C19H20ClN3O4: 389.1142 Found: 389.1123.
7-Chloro-4-methoxy-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3b). Following the general procedure I; 53 mg, yield 73%, White solid; m.p. 52–54 °C; Rf 0.3 (30% ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.29 (d, J = 2.3 Hz, 1H), 7.28–7.23 (m, 1H), 6.83 (d, J = 8.3 Hz, 1H), 5.34–5.15 (m, 2H), 5.04 (dd, J = 11.4, 4.5 Hz, 1H), 3.78 (s, 3H), 3.23 (s, 1H), 1.61 (s, 3H), 1.20 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 172.6, 143.1, 130.7, 130.0, 129.15, 129.11, 125.0, 75.5, 64.2, 63.5, 62.0, 29.8, 27.3; IR (neat) 3313, 2974, 2932, 1650, 1549, 1491, 1449, 1424, 1377, 1307, 1197, 1024 cm−1; HRMS (EI) m/z calcd for [M]+ C13H16ClN3O4: 313.0829 Found: 313.0806.
7-Chloro-4-ethoxy-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3c). Following the general procedure I; 56 mg, yield 85%, White solid; m.p. 118–120 °C; Rf 0.3 (30% ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.28 (d, J = 2.3 Hz, 1H), 7.27–7.23 (m, 1H), 6.83 (d, J = 8.2 Hz, 1H), 5.30 (dd, J = 12.1, 8.8 Hz, 1H), 5.18 (dd, J = 8.8, 4.9 Hz, 1H), 5.04 (dd, J = 12.1, 4.9 Hz, 1H), 4.01 (q, J = 7.1 Hz, 2H), 3.22 (s, 1H), 1.61 (s, 3H), 1.29 (t, J = 7.0 Hz, 3H), 1.20 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 172.7, 143.1, 130.7, 130.1, 129.1, 129.0, 124.9, 75.3, 70.1, 64.8, 63.4, 29.7, 27.6, 13.6; IR (neat) 3309, 2980, 2931, 1644, 1555, 1490, 1379, 1307, 1261, 1194, 1026 cm−1; HRMS (EI) m/z calcd for [M]+ C14H18ClN3O4: 327.0986 Found: 327.1006.
4-(tert-Butoxy)-7-chloro-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3d). Following the general procedure I; 54 mg, yield 75%, White solid; m.p. 129–131 °C; Rf 0.3 (30% ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.30–7.24 (m, 1H), 7.23 (d, J = 2.4 Hz, 1H), 6.83 (d, J = 8.2 Hz, 1H), 5.38 (dd, J = 12.3, 9.7 Hz, 1H), 5.13 (dd, J = 9.7, 4.2 Hz, 1H), 4.94 (dd, J = 12.4, 4.1 Hz, 1H), 3.35 (s, 1H), 1.60 (s, 3H), 1.32 (s, 9H), 1.22 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 176.1, 143.3, 130.7, 130.6, 128.5, 127.3, 124.2, 84.1, 74.0, 67.1, 63.0, 28.7, 28.6, 27.2; IR (neat) 3358, 2978, 2934, 1682, 1547, 1479, 1447, 1421, 1374, 1310, 1198, 1157, 1097, 977 cm−1; HRMS (EI) m/z calcd for [M]+ C16H22ClN3O4: 355.1299 Found: 355.1281.
4-(Allyloxy)-7-chloro-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3e). Following the general procedure I; 51 mg, yield 75%, Colorless gum; Rf 0.3 (30% ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.27 (d, J = 1.3 Hz, 1H), 7.26–7.24 (m, 1H), 6.87–6.77 (m, 1H), 6.05 (ddt, J = 17.4, 9.8, 6.7 Hz, 1H), 5.39–5.26 (m, 3H), 5.17 (dd, J = 8.9, 4.8 Hz, 1H), 5.03 (dd, J = 12.1, 4.8 Hz, 1H), 4.45 (dd, J = 6.7, 1.0 Hz, 2H), 3.18 (s, 1H), 1.62 (s, 3H), 1.19 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 172.8, 143.1, 131.8, 130.7, 130.3, 129.2, 128.9, 124.9, 121.8, 75.8, 75.2, 65.3, 63.5, 29.7, 27.6; IR (neat) 3311, 2973, 2928, 1650, 1549, 1491, 1451, 1422, 1377, 1306, 1285, 1198, 995 cm−1; HRMS (EI) m/z calcd for [M]+ C15H18ClN3O4: 339.0986 Found: 339.0963.
4-(Benzyloxy)-7-bromo-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3f). Following the general procedure I; 72 mg, yield 83%, White solid; m.p. 102–104 °C; Rf 0.3 (30% ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.48–7.42 (m, 1H), 7.42–7.36 (m, 4H), 7.32 (dd, J = 8.2, 2.2 Hz, 1H), 6.76–6.61 (m, 2H), 5.22–5.10 (m, 1H), 4.95 (s, 2H), 4.86–4.74 (m, 2H), 3.13 (s, 1H), 1.63 (s, 3H), 1.21 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 172.6, 143.3, 134.9, 133.4, 133.0, 130.4, 129.4, 129.0, 128.9, 125.0, 116.2, 76.5, 75.0, 65.7, 63.5, 29.6, 27.8; IR (neat) 3339, 2969, 2924, 1646, 1549, 1488, 1455, 1376, 1282, 1196, 999 cm−1; HRMS (EI) m/z calcd for [M]+ C19H20BrN3O4: 433.0637 Found: 433.0653.
4-(Benzyloxy)-7-fluoro-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3g). Following the general procedure I; 44 mg, yield 58%, White solid; m.p. 98–100 °C; Rf 0.3 (30% ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.45–7.36 (m, 5H), 6.93 (td, J = 8.3, 2.9 Hz, 1H), 6.77 (dd, J = 8.6, 4.7 Hz, 1H), 6.39 (dd, J = 8.3, 2.9 Hz, 1H), 5.21 (dd, J = 11.8, 8.5 Hz, 1H), 4.95 (d, J = 1.4 Hz, 2H), 4.80 (ddd, J = 16.6, 12.5, 4.9 Hz, 2H), 3.07 (s, 1H), 1.62 (s, 3H), 1.20 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 173.0, 158.7 (d, J1 = 244.1 Hz), 140.2 (d, J4 = 2.7 Hz), 134.9, 130.3, 129.4, 129.0, 128.8 (d, J3 = 8.0 Hz), 124.8 (d, J3 = 8.1 Hz), 117.2 (d, J2 = 22.3 Hz), 117.1 (d, J2 = 23.6 Hz), 76.5, 75.1, 65.8 (d, J4 = 1.7 Hz), 63.5, 29.7, 27.4; 19F NMR (376 MHz, CDCl3) δ -118.93; IR (neat) 3339, 2986, 2925, 1667, 1552, 1495, 1458, 1407, 1387, 1288, 1202, 1149, 999 cm−1; HRMS (EI) m/z calcd for [M]+ C19H20FN3O4: 373.1438 Found: 373.1448.
4-(Benzyloxy)-8-chloro-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3h). Following the general procedure I; 64 mg, yield 82%, White solid; m.p. 133–135 °C; Rf 0.4 (30% ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.45–7.35 (m, 5H), 6.89 (dd, J = 8.1, 2.0 Hz, 1H), 6.84 (d, J = 2.0 Hz, 1H), 6.58 (d, J = 8.1 Hz, 1H), 5.12 (dd, J = 12.0, 9.1 Hz, 1H), 4.95 (s, 2H), 4.85 (dd, J = 9.1, 4.8 Hz, 1H), 4.76 (dd, J = 12.1, 4.8 Hz, 1H), 3.21 (s, 1H), 1.63 (s, 3H), 1.23 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 172.8, 145.5, 136.1, 135.0, 131.4, 130.3, 129.3, 128.9, 125.4, 123.9, 123.3, 76.5, 75.0, 65.6, 63.5, 29.6, 27.7; IR (neat) 3336, 3001, 2977, 1657, 1595, 1548, 1454, 1416, 1383, 1297, 1201, 1088, 1002 cm−1; HRMS (EI) m/z calcd for [M]+ C19H20ClN3O4: 389.1142 Found: 389.1163.
4-(Benzyloxy)-8-bromo-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3i). 66 mg, yield 76%, White solid; m.p. 138–140 °C; Rf 0.5 (30% ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.43–7.35 (m, 5H), 7.04 (dd, J = 8.0, 1.9 Hz, 1H), 7.00 (d, J = 1.9 Hz, 1H), 6.51 (d, J = 8.0 Hz, 1H), 5.12 (dd, J = 12.1, 9.0 Hz, 1H), 4.95 (s, 2H), 4.84 (dd, J = 9.1, 4.8 Hz, 1H), 4.75 (dd, J = 12.1, 4.8 Hz, 1H), 3.21 (s, 1H), 1.63 (s, 3H), 1.23 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 172.7, 145.6, 134.9, 131.6, 130.3, 129.3, 128.9, 126.8, 126.2, 125.9, 124.1, 76.5, 74.9, 65.7, 63.5, 29.6, 27.8; IR (neat) 3337, 3000, 2976, 1651, 1590, 1548, 1453, 1416, 1380, 1309, 1228, 1200, 1079, 1002 cm−1; HRMS (EI) m/z calcd for [M]+ C19H20BrN3O4: 433.0637 Found: 433.0616.
4-(Benzyloxy)-2,2-dimethyl-5-(nitromethyl)-8-(trifluoromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3j). Following the general procedure I; 60 mg, yield 71%, White solid; m.p. 138–140 °C; Rf 0.4 (30% ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.45–7.35 (m, 5H), 7.17 (dd, J = 7.9, 1.7 Hz, 1H), 7.08 (d, J = 1.8 Hz, 1H), 6.76 (d, J = 7.8 Hz, 1H), 5.15 (dd, J = 12.4, 9.2 Hz, 1H), 4.96 (s, 2H), 4.93 (dd, J = 9.1, 4.8 Hz, 1H), 4.78 (dd, J = 12.3, 4.7 Hz, 1H), 3.35 (s, 1H), 1.66 (s, 3H), 1.23 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 172.7, 144.8, 134.9, 132.8 (q, J2 = 32.8 Hz), 131.1, 130.6, 130.3, 129.4, 129.0, 123.4 (q, J1 = 272.6 Hz). 120.5 (q, J3 = 3.8 Hz), 119.9 (q, J3 = 3.6 Hz), 76.6, 74.8, 65.8, 63.6, 29.6, 27.8; 19F NMR (376 MHz, CDCl3) δ -62.8; IR (neat) 3339, 2976, 1650, 1550, 1454, 1414, 1379, 1327, 1284, 1232, 1203, 1163, 1121, 1075 cm−1; HRMS (EI) m/z calcd for [M]+ C20H20F3N3O4: 423.1406 Found: 423.1396.
4-(Benzyloxy)-2,2,8-trimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3k). Following the general procedure I; 30 mg, yield 41%, White solid; m.p. 110–112 °C; Rf 0.4 (30% ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.47–7.41 (m, 2H), 7.41–7.35 (m, 3H), 6.73 (ddd, J = 7.7, 1.7, 0.8 Hz, 1H), 6.67–6.59 (m, 2H), 5.15 (dd, J = 11.8, 8.9 Hz, 1H), 4.95 (s, 2H), 4.91 (dd, J = 8.9, 5.0 Hz, 1H), 4.80 (dd, J = 11.8, 5.0 Hz, 1H), 3.05 (s, 1H), 2.27 (s, 3H), 1.63 (s, 3H), 1.20 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 173.3, 144.2, 140.9, 135.1, 130.22, 130.19, 129.2, 128.9, 124.5, 124.0 (two peaks overlapping), 76.4, 75.6, 66.0, 63.4, 29.9, 27.6, 21.3; IR (neat) 3335, 2919, 1659, 1620, 1548, 1454, 1414, 1380, 1287, 1204, 1184, 1000 cm−1; HRMS (EI) m/z calcd for [M]+ C20H23N3O4: 369.1689 Found: 369.1667.
4-(Benzyloxy)-2,2,9-trimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3l). Following the general procedure I; 63 mg, yield 75%, White solid; m.p. 152–154 °C; Rf 0.4 (30% ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.47–7.41 (m, 2H), 7.41–7.34 (m, 3H), 7.18–7.11 (m, 1H), 6.83 (t, J = 7.6 Hz, 1H), 6.62 (dd, J = 7.6, 1.5 Hz, 1H), 5.15 (dd, J = 11.9, 8.8 Hz, 1H), 5.00–4.92 (m, 3H), 4.82 (dd, J = 11.9, 5.0 Hz, 1H), 3.19 (s, 1H), 2.22 (s, 3H), 1.69 (s, 3H), 1.18 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 173.2, 142.8, 135.1, 132.2, 130.2, 130.0, 129.2, 128.9, 128.5, 127.4, 123.4, 76.4, 75.8, 66.5, 63.7, 29.9, 26.8, 17.5; IR (neat) 3344, 2987, 2972, 1658, 1543, 1469, 1453, 1373, 1303, 1241, 1214, 1175, 986 cm−1; HRMS (EI) m/z calcd for [M]+ C20H23N3O4: 369.1689 Found: 369.1700.
4-(Benzyloxy)-6-bromo-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3m). Following the general procedure I; 48 mg, yield 55%, White solid; m.p. 68–70 °C; Rf 0.4 (30% ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.54–7.44 (m, 2H), 7.42–7.30 (m, 4H), 7.12 (t, J = 7.9 Hz, 1H), 6.84 (dd, J = 7.8, 1.1 Hz, 1H), 6.13 (dd, J = 7.9, 5.7 Hz, 1H), 5.26 (dd, J = 12.1, 7.9 Hz, 1H), 5.07–4.92 (m, 3H), 3.27 (s, 1H), 1.63 (s, 3H), 1.20 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 172.4, 146.3, 134.6, 131.4, 129.9, 129.1, 128.8, 128.7, 128.1, 124.6, 123.4, 76.7, 75.5, 63.7, 63.7, 30.0, 27.4; IR (neat) 3298, 2970, 2919, 1650, 1548, 1453, 1415, 1374, 1305, 1198, 989, 969 cm−1; HRMS (EI) m/z calcd for [M]+ C19H20BrN3O4: 433.0637 Found: 433.0653.
4-(Benzyloxy)-7-chloro-2,2-diethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3n). Following the general procedure I; 61 mg, yield 73%, White solid; m.p. 188–190 °C; Rf 0.4 (30% ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.48–7.36 (m, 5H), 7.16 (dd, J = 8.3, 2.4 Hz, 1H), 6.76 (d, J = 8.3 Hz, 1H), 6.47 (d, J = 2.4 Hz, 1H), 5.13 (dd, J = 12.2, 8.8 Hz, 1H), 4.96 (d, J = 1.0 Hz, 2H), 4.85 (dd, J = 12.2, 5.0 Hz, 1H), 4.77 (dd, J = 8.9, 5.0 Hz, 1H), 3.27 (s, 1H), 2.25 (dq, J = 14.6, 7.4 Hz, 1H), 1.65 (dt, J = 14.9, 7.5 Hz, 1H), 1.54 (dq, J = 14.4, 7.2 Hz, 1H), 1.44 (dq, J = 14.7, 7.4 Hz, 1H), 1.08 (t, J = 7.3 Hz, 3H), 0.81 (t, J = 7.5 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 171.8, 143.0, 143.0, 134.9, 130.4, 130.15, 130.13, 129.4, 129.0, 128.2, 128.0, 124.2, 75.4, 70.2, 65.8, 32.01, 31.99, 9.1, 7.6; IR (neat) 3343, 2966, 2935, 2878, 1666, 1551, 1485, 1454, 1383, 1279, 1227, 1182, 1005 cm−1; HRMS (EI) m/z calcd for [M]+ C21H24ClN3O4: 417.1455 Found: 417.1452.
4-(Benzyloxy)-7-chloro-5-(nitromethyl)-2,2-dipropyl-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3o). Following the general procedure I; 71 mg, yield 79%, White solid; m.p. 98–100 °C; Rf 0.7 (30% ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.49–7.34 (m, 5H), 7.16 (dd, J = 8.3, 2.4 Hz, 1H), 6.74 (d, J = 8.3 Hz, 1H), 6.50 (d, J = 2.4 Hz, 1H), 5.14 (dd, J = 12.0, 8.7 Hz, 1H), 4.96 (s, 2H), 4.87–4.75 (m, 2H), 3.28 (s, 1H), 2.20 (td, J = 12.6, 3.1 Hz, 1H), 1.73–1.52 (m, 2H), 1.52–1.35 (m, 2H), 1.35–1.18 (m, 3H), 0.96 (t, J = 7.1 Hz, 3H), 0.79 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 171.9, 143.0, 134.8, 130.41, 130.38, 130.1, 129.4, 129.0, 128.2, 128.1, 124.1, 76.8, 75.3, 69.8, 65.7, 42.0, 41.7, 18.0, 16.3, 14.5, 14.3; IR (neat) 3341, 2967, 2872, 1655, 1549, 1483, 1453, 1431, 1381, 1284, 1178, 1014 cm−1; HRMS (EI) m/z calcd for [M]+ C23H28ClN3O4: 445.1768 Found: 445.1765.
4-(Benzyloxy)-7-chloro-5-(nitromethyl)-4,5-dihydrospiro[benzo[e][1,4]diazepine-2,1’-cyclohexan]-3(1H)-one (3p). Following the general procedure I; 66 mg, yield 77%, White solid; m.p. 125–127 °C; Rf 0.5 (30% ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.48–7.35 (m, 5H), 7.18 (dd, J = 8.3, 2.4 Hz, 1H), 6.85 (d, J = 8.4 Hz, 1H), 6.57 (d, J = 2.4 Hz, 1H), 5.19–5.06 (m, 1H), 4.94 (s, 2H), 4.83–4.71 (m, 2H), 3.71 (s, 1H), 2.41 (td, J = 13.9, 4.7 Hz, 1H), 1.92–1.79 (m, 2H), 1.79–1.60 (m, 2H), 1.59–1.47 (m, 1H), 1.47–1.24 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 173.6, 142.0, 135.0, 130.4, 130.3, 130.2, 129.3, 129.0 (two peaks overlapping), 128.5, 124.3, 76.5, 75.1, 65.8, 65.3, 34.2, 32.2, 24.6, 21.3, 20.6; IR (neat) 3385, 2932, 2859, 1656, 1541, 1492, 1455, 1436, 1379, 1318, 1267, 1178, 980 cm−1; HRMS (EI) m/z calcd for [M]+ C22H24ClN3O4: 429.1455 Found: 429.1484.
3.3. General Procedure II for the Asymmetric [4+3]-Cycloaddition of 2-Amino-β-nitrostyrenes with α-Bromohydroxamates
A solution of 2-amino-β-nitrostyrene 1 (0.10 mmol, 1 equiv), α-bromohydroxamate 2 (0.2 mmol, 2 equiv), HFIP (0.12 mmol, 1.2 equiv), and catalyst III (0.01 mmol, 0.1 equiv) in CH2Cl2 (1.0 mL, 0.1 M) were stirred for 5 min at 0 °C, then added Na2CO3 (0.15 mmol, 1.5 equiv) at 0 °C and allowed to stir at room temperature. After stirring for 1 h and then again for 1 more hours, α-bromohydroxamate 2 (0.05 mmol, 0.5 equiv) and Na2CO3 (0.05 mmol, 0.5 equiv) were added to the reaction mixture twice. After stirring for an additional 22 h, the resulting mixture was filtered through the plug of celite. The filtrate was concentrated in vacuo. The crude residue was purified by flash column chromatography with EtOAc/hexanes as eluent to afford the desired product 3. The enantiomeric ratio was determined using HPLC analysis.
(S)-4-(Benzyloxy)-7-chloro-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3a). Following the general procedure II; 25 mg, yield 64%, = +150.7 (c = 0.91, CHCl3); 72% ee; Chiralpak IA column and IA guard column (10% EtOH:hexanes, 1.0 mL/min flow, λ = 254 nm); major-isomer tr = 13.8 min and minor-isomer tr = 42.0 min.
(S)-7-Chloro-4-methoxy-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3b). Following the general procedure II; 23 mg, yield 73%, = +93.8 (c = 0.84, CHCl3); 63% ee; Chiralpak IA column and IA guard column (20% EtOH:hexanes, 1.0 mL/min flow, λ = 254 nm); major-isomer tr = 8.7 min and minor-isomer tr = 18.6 min.
(S)-7-Chloro-4-ethoxy-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3c). Following the general procedure II; 22 mg, yield 66%, = +103.2 (c = 0.62, CHCl3); 67% ee; Chiralpak IA column and IA guard column (20% EtOH:hexanes, 1.0 mL/min flow, λ = 254 nm); major-isomer tr = 7.6 min and minor-isomer tr = 17.9 min.
(S)-4-(tert-butoxy)-7-chloro-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3d). Following the general procedure II; 23 mg, yield 65%, = +16.6 (c = 0.84, CHCl3); 23% ee; Chiralpak IA column and IA guard column (2% EtOH:hexanes, 1.0 mL/min flow, λ = 254 nm); major-isomer tr = 19.9 min and minor-isomer tr = 36.5 min.
(S)-4-(Allyloxy)-7-chloro-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3e). Following the general procedure II; 22 mg, yield 66%, = +91.8 (c = 0.64, CHCl3); 71% ee; Chiralpak IA column and IA guard column (30% EtOH:hexanes, 1.0 mL/min flow, λ = 254 nm); major-isomer tr = 6.9 min and minor-isomer tr = 14.1 min.
(S)-4-(Benzyloxy)-7-bromo-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3f). Following the general procedure II; 28 mg, yield 65%, = +130.5 (c = 1.07, CHCl3); 72% ee; Chiralpak IA column and IA guard column (10% EtOH:hexanes, 1.0 mL/min flow, λ = 254 nm); major-isomer tr = 14.2 min and minor-isomer tr = 42.1 min.
(S)-4-(Benzyloxy)-7-fluoro-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3g). Following the general procedure II; 14 mg, yield 36%, = +102.5 (c = 0.48, CHCl3); 69% ee; Chiralpak IA column and IA guard column (10% EtOH:hexanes, 1.0 mL/min flow, λ = 254 nm); major-isomer tr = 13.6 min and minor-isomer tr = 42.3 min.
(S)-4-(Benzyloxy)-8-chloro-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3h). Following the general procedure II; 27 mg, yield 68%, = +158.8 (c = 0.86, CHCl3); 71% ee; Chiralpak IA column and IA guard column (10% EtOH:hexanes, 1.0 mL/min flow, λ = 254 nm); major-isomer tr = 13.2 min and minor-isomer tr = 24.7 min.
(S)-4-(Benzyloxy)-8-bromo-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3i). Following the general procedure II; 29 mg, yield 66%, = +146.3 (c = 0.86, CHCl3); 71% ee; Chiralpak IA column and IA guard column (10% EtOH:hexanes, 1.0 mL/min flow, λ = 254 nm); major-isomer tr = 13.6 min and minor-isomer tr = 24.0 min.
(S)-4-(Benzyloxy)-2,2-dimethyl-5-(nitromethyl)-8-(trifluoromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3j). Following the general procedure II; 24 mg, yield 57%, = +121.3 (c = 0.71, CHCl3); 72% ee; Chiralpak IA column and IA guard column (10% EtOH:hexanes, 1.0 mL/min flow, λ = 254 nm); major-isomer tr = 10.5 min and minor-isomer tr = 14.6 min.
(S)-4-(Benzyloxy)-2,2,8-trimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3k). Following the general procedure II; 16 mg, yield 44%, = +136.9 (c = 0.47, CHCl3); 67% ee; Chiralpak IA column and IA guard column (10% EtOH:hexanes, 1.0 mL/min flow, λ = 254 nm); major-isomer tr = 12.8 min and minor-isomer tr = 27.0 min.
(S)-4-(Benzyloxy)-2,2,9-trimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3l). Following the general procedure II; 30 mg, yield 80%, = +148.0 (c = 0.93, CHCl3); 71 ee; Chiralcel OJ-H column and OJ-H guard column (10% EtOH:hexanes, 1.0 mL/min flow, λ = 254 nm); minor-isomer tr = 28.9 min and major-isomer tr = 35.6 min.
(S)-4-(Benzyloxy)-6-bromo-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (3m). Following the general procedure II; 19 mg, yield 43%, = +44.6 (c = 0.68, CHCl3); 42% ee; Chiralpak IA column and IA guard column (10% EtOH:hexanes, 1.0 mL/min flow, λ = 254 nm); major-isomer tr = 13.7 min and minor-isomer tr = 28.3 min.
3.4. Procedure for a One-mmol Scale Synthesis of 3a
A solution of 2-amino-β-nitrostyrene 1a (199 mg, 1.0 mmol), α-bromohydroxamate 2a (544 mg, 2.0 mmol), HFIP (0.13 mL, 1.2 mmol), and catalyst III (60 mg, 0.10 mmol) in CH2Cl2 (10 mL, 0.1 M) were stirred for 5 min at 0 °C, then added Na2CO3 (159 mg, 1.5 mmol) at 0 °C and allowed to stir at room temperature. After stirring for 1 h and then again for 1 more hour, α-bromohydroxamate 2 (136 mg, 0.5 mmol), and Na2CO3 (53 mg, 0.5 mmol) were added to the reaction mixture twice. After stirring for an additional 22 h, the resulting mixture was filtered through the plug of celite. The filtrate was concentrated in vacuo. The crude residue was purified by flash column chromatography (EtOAc/hexanes = 1:4) as eluent to afford the desired product 3a (183 mg, 47% yield, 72% ee) as a white solid.
3.5. Procedure for a One-mmol Scale Synthesis of 3f
A solution of 2-amino-β-nitrostyrene 1f (243 mg, 0.10 mmol), α-bromohydroxamate 2a (544 mg, 2.0 mmol), HFIP (0.13 mL, 1.2 mmol), and catalyst III (60 mg, 0.10 mmol) in CH2Cl2 (10 mL, 0.1 M) were stirred for 5 min at 0 °C, then added Na2CO3 (159 mg, 1.5 mmol) at 0 °C and allowed to stir at room temperature. After stirring for 1 hour and then again for 1 more hour, α-bromohydroxamate 2 (136 mg, 0.5 mmol) and Na2CO3 (53 mg, 0.5 mmol) were added to the reaction mixture twice. After stirring for an additional 22 h, the resulting mixture was filtered through the plug of celite. The filtrate was concentrated in vacuo. The crude residue was purified by flash column chromatography (EtOAc/hexanes = 1:4) as eluent to afford the desired product 3f (229 mg, 53% yield, 70 % ee) as a white solid.
3.6. Procedure for Reduction of 3a
Step 1: To a solution of 3a (39 mg, 0.10 mmol, 1 equiv) in MeOH (1.0 mL, 0.1 M) at 0 °C was added NiCl2•6H2O (48 mg, 0.20 mmol, 2 equiv). After stirring for 5 min at 0 °C, NaBH4 (38 mg, 1.0 mmol, 10 equiv) was added in portions to the reaction mixture. The mixture was allowed to stir at room temperature for 1 h. After then, the resulting mixture was quenched with deionized water (1 mL) and added CH2Cl2 (2 mL). The mixture was filtered through the plug of celite and the filtrate was extracted with CH2Cl2. The combined organic layers were washed with brine, dried (anhydrous Na2SO4), and concentrated in vacuo. The crude residue was purified by flash column chromatography with 3% MeOH/EtOAc (with 2% Et3N) as eluent to afford nitro reduction products. Step 2: A solution of the crude primary amine in CH2Cl2 (1.0 mL, 0.1 M) was added (Boc)2O and stirred for 18 h at room temperature. Then, the resulting mixture was concentrated in vacuo and was purified by flash column chromatography (EtOAc/hexanes = 1:3) as eluent to afford desired product 4 (26 mg, yield 57%) as a white solid.
tert-Butyl (S)-((4-(benzyloxy)-7-chloro-2,2-dimethyl-3-oxo-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-5-yl)methyl)carbamate (4). = +103.5 (c = 0.85, CHCl3); 72% ee; White solid; m.p. 76–78 °C; Rf 0.5 (50% EtOAc in hexanes); 1H NMR (400 MHz, CDCl3) δ 7.35 (dtd, J = 13.4, 7.7, 5.8 Hz, 5H), 7.10 (dd, J = 8.3, 2.4 Hz, 1H), 6.69 (d, J = 8.2 Hz, 1H), 6.55 (d, J = 2.5 Hz, 1H), 4.92 (s, 2H), 4.84 (d, J = 6.5 Hz, 1H), 4.35 (t, J = 7.1 Hz, 1H), 3.80–3.64 (m, 2H), 3.10 (s, 1H), 1.61 (s, 3H), 1.36 (s, 9H), 1.22 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 172.3, 155.8, 143.1, 135.2, 131.1, 130.2, 129.7, 129.3, 128.9, 128.7, 128.1, 124.1, 79.4, 76.0, 67.8, 63.0, 42.7, 29.7, 28.4, 28.0; IR (neat) 3316, 2973, 2929, 2865, 1698, 1633, 1492, 1453, 1392, 1365, 1248, 1164, 995 cm−1; HRMS (EI) m/z calcd for [M]+ C24H30ClN3O4: 459.1925 Found: 459.1903; Chiralpak AD-H column and AD-H guard column (5% EtOH:hexanes, 1.0 mL/min flow, λ = 254 nm); minor-isomer tr = 21.3 min and major-isomer tr = 31.8 min.
3.7. Procedure for Debenzylation of 3a
A solution of 3a (39 mg, 0.10 mmol) and 5% Pd/C (21 mg, 10 mol%) in EtOAc (1.0 mL, 0.1 M) was stirred under H2 atmosphere for 6 h at room temperature. After that, the resulting mixture was filtered through the plug of celite and concentrated in vacuo to afford desired product 7 (28 mg, yield 94%) as a white solid.
(S)-7-Chloro-4-hydroxy-2,2-dimethyl-5-(nitromethyl)-1,2,4,5-tetrahydro-3H-benzo[e][1,4]diazepin-3-one (5). = +133.9 (c = 0.96, CHCl3); 80% ee; White solid; m.p. 135–137 °C; Rf 0.4 (50% Ethyl acetate in hexanes); 1H NMR (400 MHz, CDCl3) δ 8.83 (s, 1H), 7.34–7.26 (m, 2H), 6.85 (d, J = 8.2 Hz, 1H), 5.41 (dd, J = 8.4, 5.5 Hz, 1H), 5.16 (dd, J = 12.4, 8.4 Hz, 1H), 5.07 (dd, J = 12.4, 5.6 Hz, 1H), 3.20 (s, 1H), 1.64 (s, 3H), 1.21 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 170.0, 142.6, 130.9, 130.3, 129.5, 128.7, 125.3, 75.3, 62.4, 61.8, 29.5, 27.3; IR (neat) 3339, 2920, 2853, 1608, 1546, 1492, 1431, 1377, 1320, 1259, 1198, 1093,1011 cm−1; HRMS (EI) m/z calcd for [M]+ C12H14ClN3O4: 299.0673 Found: 299.0675; Chiralcel OD-H column and OD-H guard column (10% EtOH:hexanes, 1.0 mL/min flow, λ = 254 nm); major-isomer tr = 14.7 min and minor-isomer tr = 22.8 min.
4. Conclusions
In summary, we have established a highly effective [4+3]-cycloaddition reaction involving 2-amino-β-nitrostyrenes and α-bromohydroxamates using Cs2CO3 as a base. This methodology has proven to be a reliable route for the synthesis of 1,4-benzodiazepin-3-ones, consistently delivering good yields. Furthermore, we have achieved an organocatalytic asymmetric [4+3]-cycloaddition employing a bifunctional squaramide-based catalyst. This innovative approach has paved the way for the enantioselective synthesis of chiral 1,4-benzodiazepines, yielding impressive results in terms of both yields and enantioselectivities, (up to 80% yield and 72% ee). The resulting seven-membered benzodiazepin-3-one compounds are anticipated to provide a valuable foundation for the synthesis of a wide range of diverse compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29061221/s1, X-ray crystallography data of 3a, NMR spectra, and HPLC spectra of the products (3a–3m, 4, and 5).
Author Contributions
Conceptualization, resources, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, and funding acquisition, S.-G.K.; methodology, validation, formal analysis, and investigation, Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Kyonggi University Research Grant 2023 (2023-024).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Garg, M.; Chauhan, M.; Singh, P.K.; Alex, J.M.; Kumar, R. Pyrazoloquinazolines: Synthetic strategies and bioactivities. Eur. J. Med. Chem. 2015, 97, 444–461. [Google Scholar] [CrossRef] [PubMed]
- Vila, N.; Besada, P.; Costas, T.; Costas-Lago, M.C.; Terán, C. Phthalazin-1(2H)-one as a remarkable scaffold in drug discovery. Eur. J. Med. Chem. 2015, 97, 462–482. [Google Scholar] [CrossRef]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The Combinatorial Synthesis of Bicyclic Privileged Structures or Privileged Substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Dhiman, P.; Kumar, S.; Singh, G.; Monga, V. Recent advances in synthesis and medicinal chemistry of benzodiazepines. Bioorg. Chem. 2020, 97, 103668. [Google Scholar] [CrossRef]
- Magi, E.; Fattorusso, C.; Persico, M.; Corvino, A.; Esposito, G.; Fiorino, F.; Luciano, P.; Perissutti, E.; Santagada, V.; Severino, B.; et al. New Insights into the Structure−Activity Relationship and Neuroprotective Profile of Benzodiazepinone Derivatives of Neurounina-1 as Modulators of the Na+/Ca2+ Exchanger Isoforms. J. Med. Chem. 2021, 64, 17901–17919. [Google Scholar] [CrossRef] [PubMed]
- Vézina-Dawod, S.; Perreault, M.; Guay, L.-D.; Gerber, N.; Gobeil, S.; Biron, E. Synthesis and biological evaluation of novel 1,4-benzodiazepin-3-one derivatives as potential antitumor agents against prostate cancer. Bioorg. Med. Chem. 2021, 45, 116314. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.I.; Quasimi, H.; Kumar, A.; Alam, A.; Bhagat, S.; Alam, M.S.; Khan, G.A.; Dhulap, A.; Ansari, M.A. Protective effects of novel diazepinone derivatives in snake venom induced sterile inflammation in experimental animals. Eur. J. Pharmacol. 2022, 928, 175095. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Alam, M.S.; Hamid, H.; Chugh, V.; Tikla, T.; Kaul, R.; Dhulap, A.; Sharma, S.K. Design and synthesis of anti–inflammatory 1,2,3–triazolylpyrrolobenzodiazepinone derivatives and impact of molecular structure on COX–2 selective targeting. J. Mol. Struct. 2023, 1272, 134151. [Google Scholar] [CrossRef]
- Lee, S.; Love, M.S.; Modukuri, R.; Chatterjee, A.K.; Huerta, L.; Lawson, A.P.; McNamara, C.W.; Mead, J.R.; Hedstrom, L.; Cuny, G.D. Structure-activity relationship of BMS906024 derivatives for Cryptosporidium parvum growth inhibition. Bioorg. Med. Chem. Lett. 2023, 90, 129328. [Google Scholar] [CrossRef]
- Calcaterra, N.E.; Barrow, J.C. Classics in Chemical Neuroscience: Diazepam (Valium). ACS Chem. Neurosci. 2014, 5, 253–260. [Google Scholar] [CrossRef]
- Liu, F.; Craft, R.M.; Morris, S.A.; Carroll, R.C. Lotrafiban: An oral platelet glycoprotein IIb/IIIa blocker. Expert Opin. Investig. Drugs 2000, 9, 2673–2687. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, R.M.; Gretler, D.D. Platelet glycoprotein IIb-IIIa antagonists as prototypical integrin blockers: Novel parenteral and potential oral antithrombotic agents. J. Med. Chem. 2000, 43, 3453–3473. [Google Scholar] [CrossRef] [PubMed]
- Saranya, P.V.; Neetha, M.; Radhika, S.; Anilkumar, G. An overview of palladium-catalyzed synthesis of seven-membered heterocycles. J. Heterocycl. Chem. 2021, 58, 673–684. [Google Scholar] [CrossRef]
- Sasiambarrena, L.D.; Barri, I.A.; Fraga, G.G.; Bravo, R.D.; Ponzinibbio, A. Facile synthesis of 4-substituted 1,2,4,5-tetrahydro-1,4-benzodiazepin-3-ones by reductive cyclization of 2-chloro-N-(2-nitrobenzyl)acetamides. Tetrahedron Lett. 2019, 60, 264–267. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Awad, J.M.; Xie, G.; Qiu, W.; Zhang, W. One-pot synthesis of tetrahydro-pyrrolobenzodiazepines and tetrahydro-pyrrolobenzodiazepinones through sequential 1,3-dipolar cycloaddition/N-alkylation (N-acylation)/Staudinger/aza-Wittig reactions. Green Chem. 2019, 21, 4489–4494. [Google Scholar] [CrossRef]
- Velasco-Rubio, Á.; Varela, J.; Saá, C. Pd-Catalyzed allylic C–H activation to seven-membered N,O-heterocycles. Chem. Commun. 2021, 57, 10915–10918. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, P.S.K.P.; Muthuraja, P.; Gopinath, P. Rh(III) Catalyzed Redox-Neutral C-H Activation/[5 + 2] Annulation of Aroyl Hydrazides and Sulfoxonium Ylides: Synthesis of Benzodiazepinones. Org. Lett. 2023, 25, 8361–8366. [Google Scholar] [CrossRef] [PubMed]
- Illuminati, G.; Mandolini, L. Ring closure reactions of bifunctional chain molecules. Acc. Chem. Res. 1981, 14, 95–102. [Google Scholar] [CrossRef]
- Moyano, A.; Rios, R. Asymmetric Organocatalytic Cyclization and Cycloaddition Reactions. Chem. Rev. 2011, 111, 4703–4832. [Google Scholar] [CrossRef]
- Kwon, Y.I.; Choi, S.; Jang, H.S.; Kim, S.-G. Rapid Access to Hindered α-Amino Acid Derivatives and Benzodiazepin-3-ones from Aza-Oxyallyl Cations. Org. Lett. 2020, 22, 1420–1425. [Google Scholar] [CrossRef]
- Kim, E.; Lee, C.Y.; Kim, S.-G. HFIP-Mediated Decarboxylative [4+3]-Annulation of Azaoxyallyl Cations with Isatoic Anhydride. Adv. Synth. Catal. 2020, 362, 3594–3603. [Google Scholar] [CrossRef]
- Jang, H.S.; Kwon, Y.I.; Kim, S.-G. Facile Synthesis of Functionalized 1,4-Benzodiazepine-3-one-5-acetates via [4+3]-Annulation of Azaoxyallyl Cations with 2-Aminophenyl α,β-Unsaturated Esters. Bull. Korean Chem. Soc. 2020, 41, 727–734. [Google Scholar] [CrossRef]
- Lee, C.Y.; Kwon, Y.I.; Jang, H.S.; Lee, S.; Chun, Y.L.; Jung, J.; Kim, S.-G. Organocatalytic Enantioselective [4+3]-Cycloadditions of Azaoxyallyl Cations with 2-Aminophenyl Enones. Adv. Synth. Catal. 2021, 363, 4197–4203. [Google Scholar] [CrossRef]
- Kim, Y.; Han, J.W.; Kim, S.-G. Organocatalytic Enantioselective Synthesis of Polycyclic Benzosultams from 2-Amino-β-nitrostyrenes with Cyclic N-Sulfonyl Ketimines. Org. Lett. 2024, 24, 1472–1477. [Google Scholar] [CrossRef]
- Khaksar, S. Fluorinated alcohols: A magic medium for the synthesis of heterocyclic compounds. J. Fluor. Chem. 2015, 172, 51–61. [Google Scholar] [CrossRef]
- Shuklov, I.A.; Dubrovina, N.V.; Börner, A. Fluorinated Alcohols as Solvents, Cosolvents and Additives in Homogeneous Catalysis. Synthesis 2007, 2007, 2925–2943. [Google Scholar] [CrossRef]
- Chauhan, P.; Mahajan, S.; Kaya, U.; Hack, D.; Enders, D. Bifunctional Amine-Squaramides: Powerful Hydrogen-Bonding Organocatalysts for Asymmetric Domino/Cascade Reactions. Adv. Synth. Catal. 2015, 357, 253–281. [Google Scholar] [CrossRef]
- Fang, X.; Wang, C.-J. Recent advances in asymmetric organocatalysis mediated by bifunctional amine–thioureas bearing multiple hydrogen-bonding donors. Chem. Commun. 2015, 51, 1185–1197. [Google Scholar] [CrossRef]
- Zhao, B.-L.; Li, J.-H.; Du, D.-M. Squaramide-Catalyzed Asymmetric Reactions. Chem. Rec. 2017, 17, 994–1018. [Google Scholar] [CrossRef] [PubMed]
- Parvin, T.; Yadav, R.; Choudhury, L.H. Recent applications of thiourea-based organocatalysts in asymmetric multicomponent reactions (AMCRs). Org. Biomol. Chem. 2020, 18, 5513–5532. [Google Scholar] [CrossRef]
- CCDC 2312079 (3a) Contains the Supplementary Crystallographic Data for This Paper. These Data Can Be Obtained Free of Charge from the Cambridge Crystallographic Data Centre. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 5 December 2023).
- Jeffrey, C.S.; Barnes, K.; Eickhoff, J.; Carson, C. Generation and Reactivity of Aza-Oxyallyl Cationic Intermediates: Aza-[4 + 3] Cycloaddition Reactions for Heterocycle Synthesis. J. Am. Chem. Soc. 2011, 133, 7688–7691. [Google Scholar] [CrossRef] [PubMed]
- DiPoto, M.C.; Wu, J. Synthesis of 2-Aminoimidazolones and Imidazolones by (3 + 2) Annulation of Azaoxyallyl Cations. Org. Lett. 2018, 20, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Takeuchi, K.; Nishikata, T. The synthetic protocol for α-bromocarbonyl compounds via brominations. Tetrahedron 2019, 75, 2726–2736. [Google Scholar] [CrossRef]
- Malerich, J.P.; Hagihara, K.; Rawal, V.H. Chiral Squaramide Derivatives are Excellent Hydrogen Bond Donor Catalysts. J. Am. Chem. Soc. 2008, 130, 14416–14417. [Google Scholar] [CrossRef]
- Yang, W.; Du, D.M. Highly enantioselective Michael addition of nitroalkanes to chalcones using chiral squaramides as hydrogen bonding organocatalysts. Org. Lett. 2010, 12, 5450–5453. [Google Scholar] [CrossRef]
- Zhu, Y.; Malerich, J.P.; Rawal, V.H. Squaramide-catalyzed enantioselective Michael addition of diphenyl phosphite to nitroalkenes. Angew. Chem. Int. Ed. 2010, 49, 153–156. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).