Responsive Microgels through RAFT-HDA Dynamic Covalent Bonding Chemistry
Abstract
1. Introduction
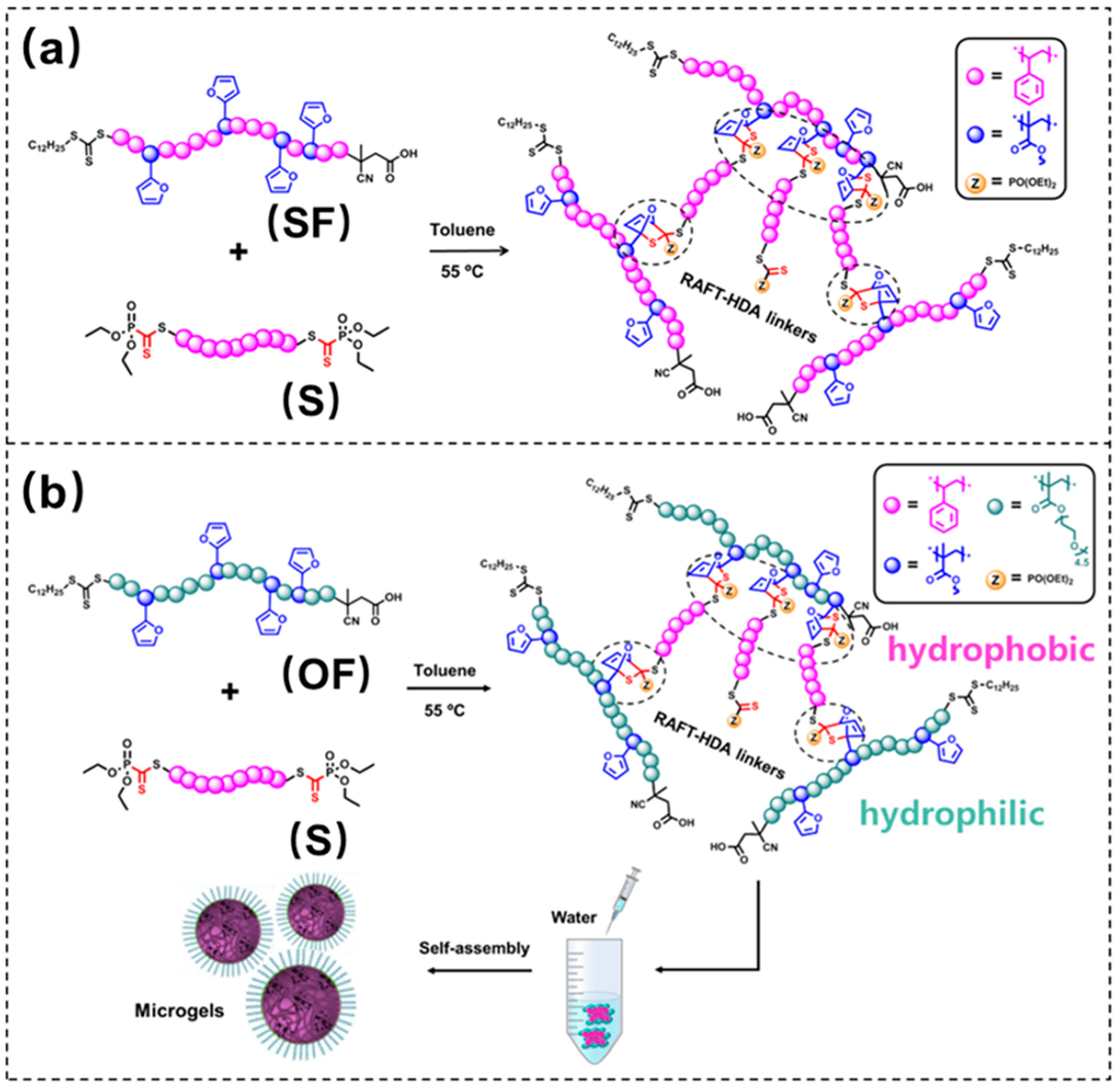
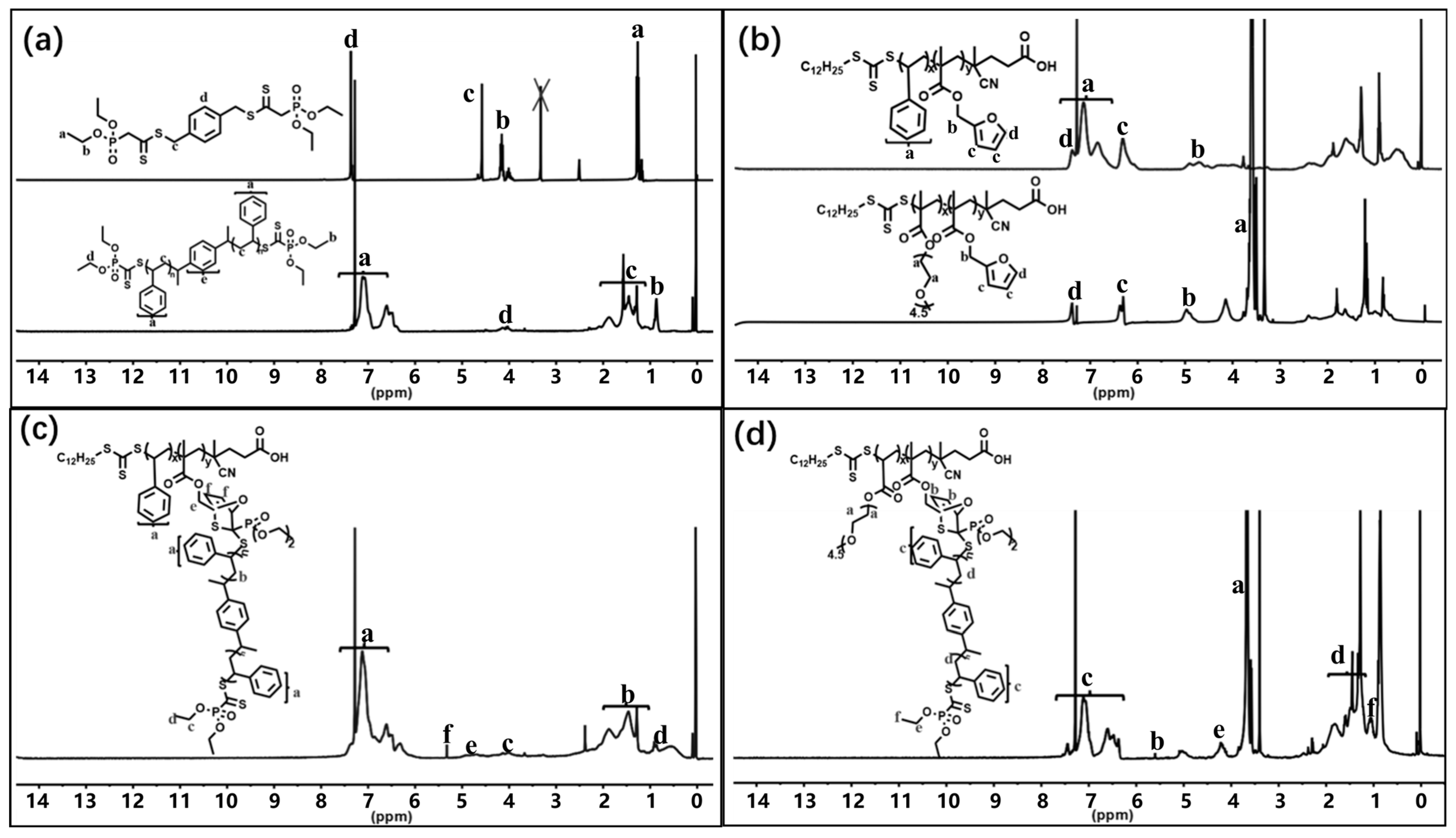
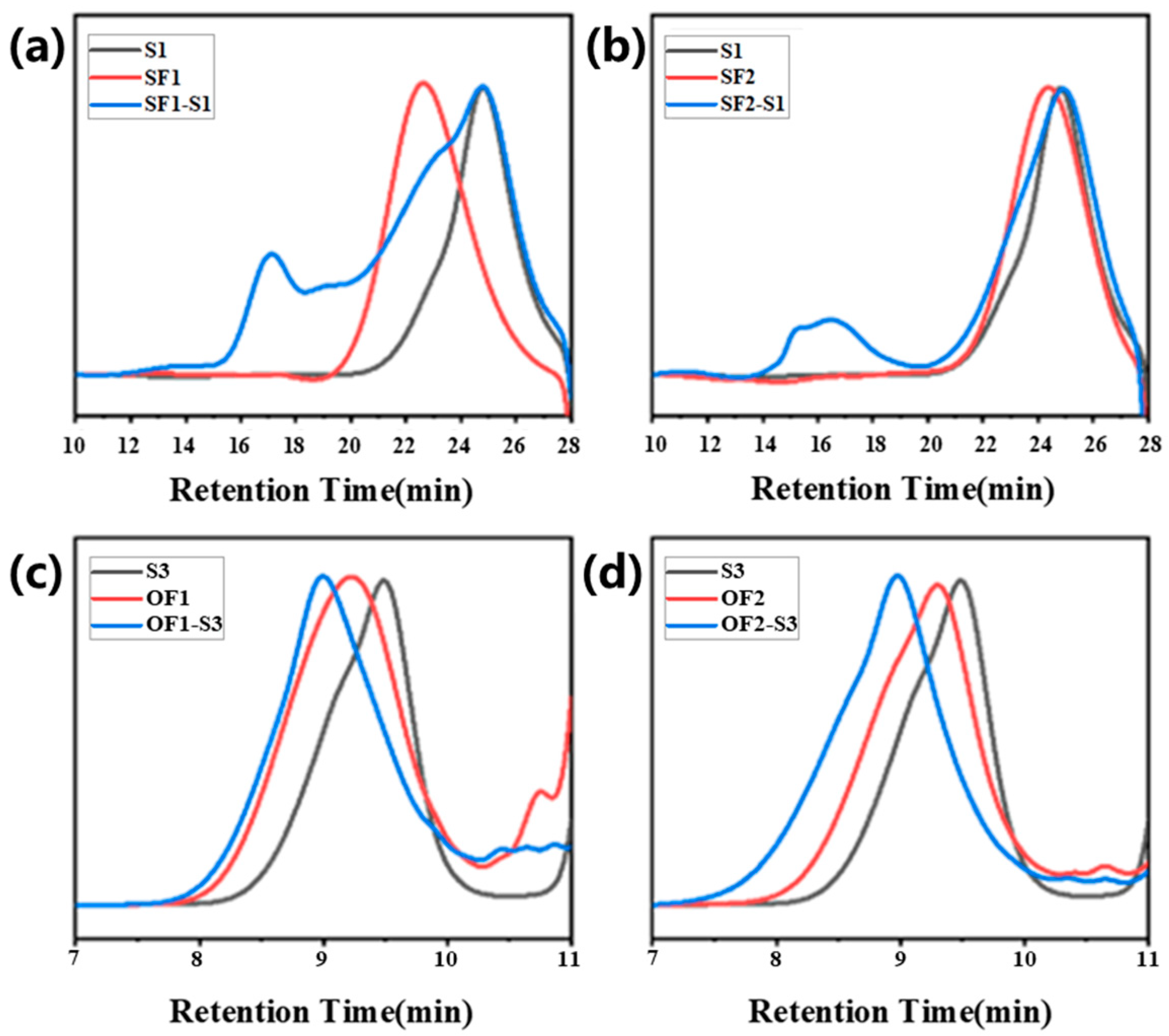
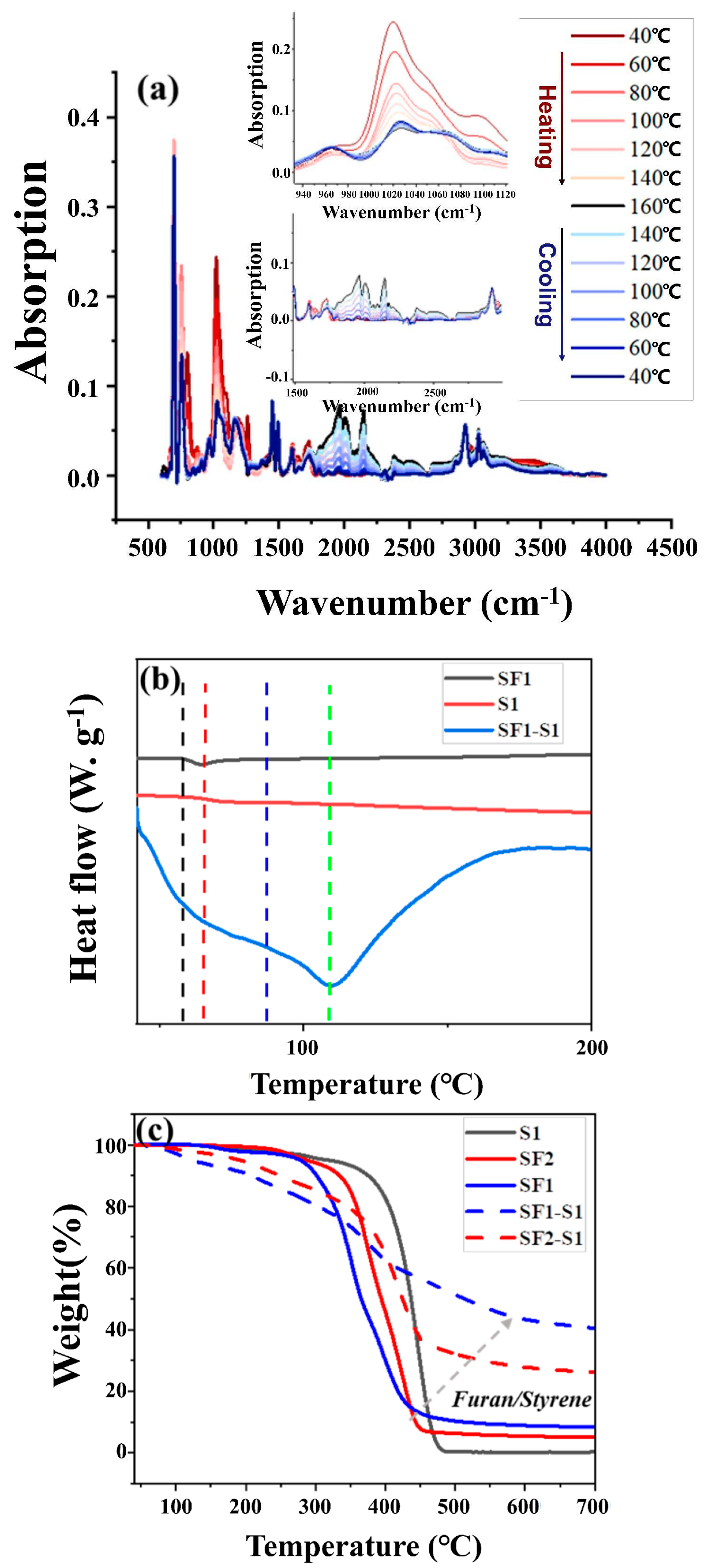
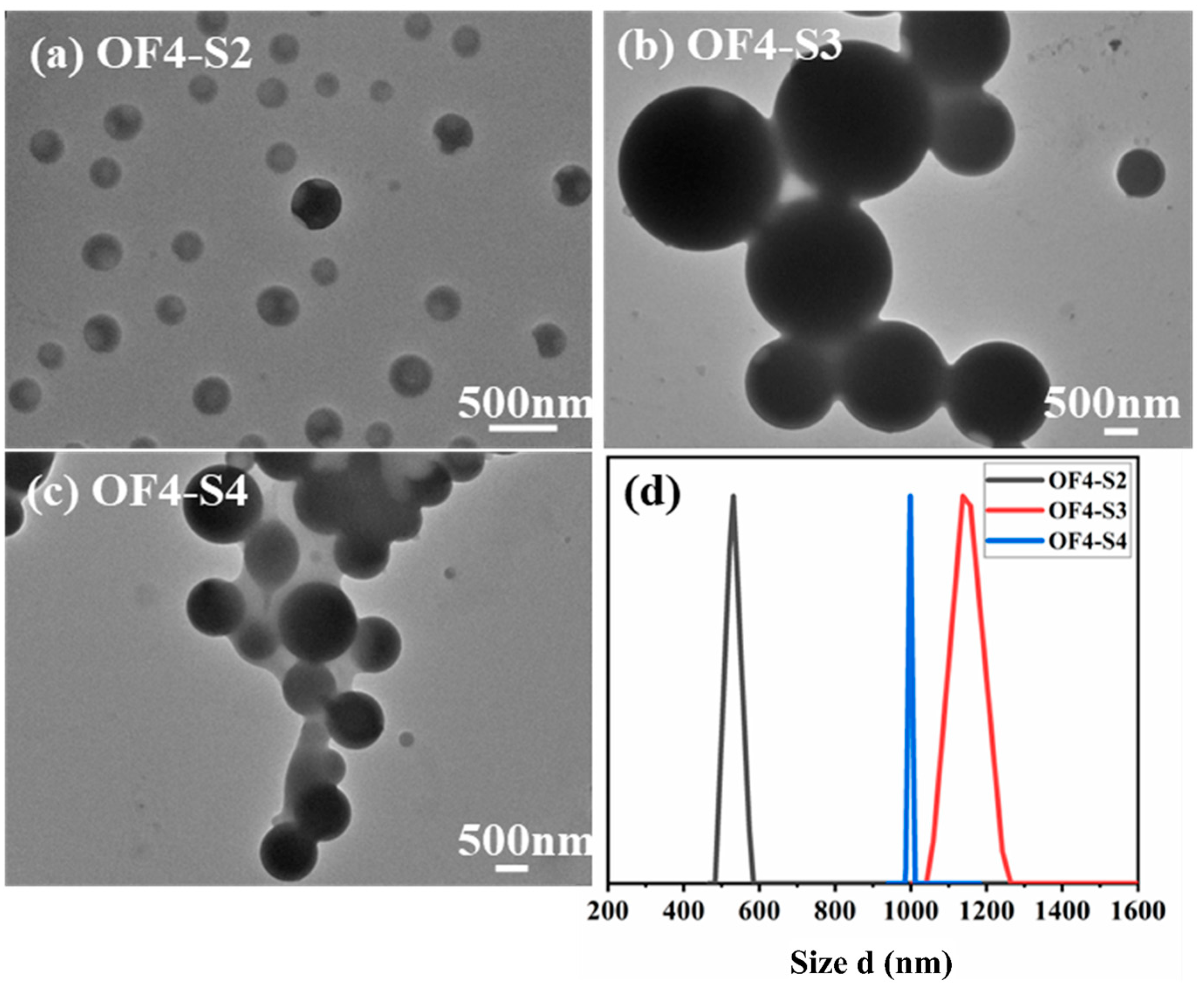
2. Results and Discussion
3. Materials and Methods
3.1. Characterizations
3.2. Synthesis of Phosphoryl Disulfide Terminated Polystyrene (PS1-PS4)
3.3. Synthesis of a Long Chain with Furan as the Group Vertical Side Group
- (SF1-[S]0/[F]0/[RAFT]0/[AIBN]0 = 150:44:1:0.2, conversiona(conversion rate of the previous component) of 40%, conversionb(conversion rate of the previous component) = 79.5%,
- [S]/[F] = 60:35; SF2-[S]0/[F]0/[RAFT]0/[AIBN]0 = 115:20:1:0.2, conversiona = 37.4%, conversionb = 84.6%, [S]/[F] = 43:17; OF1-[O]0/[F]0/[RAFT]0/[AIBN]0 = 55:5:1:0.2, conversiona = 81.8%,
- conversionb = 40%, [O]/[F] = 45:2OF2-[O]0/[F]0/[RAFT]0/[AIBN]0 = 55:10:1:0.2, conversiona = 81.8%, conversionb = 40%,
- [O]/[F] = 45:4; OF3-[O]0/[F]0/[RAFT]0/[AIBN]0 = 55:15:1:0.2, conversiona = 81.8%, conversionb = 46.7%, [O]/[F] = 45:7; OF4-[O]0/[F]0/[RAFT]0/[AIBN]0 = 55:10:1:0.2, conversiona = 72.7%, conversionb = 50%, [O]/[F] = 40:5).
3.4. Reversible Addition Fragmentation Chain Transfer Hydro Diels–Alder Cyclization between Phosphoryl Disulfides and Furan Rings
3.5. Preparation of Polymeric Microgels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition−fragmentation chain transfer: the raft process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the raft process—A third update. Aust. J. Chem. 2012, 65, 985–1076. [Google Scholar] [CrossRef]
- Hu, W.; Ren, Z.; Li, J.; Askounis, E.; Xie, Z.; Pei, Q. New dielectric elastomers with variable moduli. Adv. Funct. Mater. 2015, 25, 4827–4836. [Google Scholar] [CrossRef]
- Molev, G.; Lu, Y.; Kim, K.S.; Majdalani, I.C.; Guerin, G.; Petrov, S.; Walker, G.; Manners, I.; Winnik, M.A. Organometallic–polypeptide diblock copolymers: Synthesis by Diels–Alder coupling and crystallization-driven self-assembly to uniform truncated elliptical lamellae. Macromolecules 2014, 47, 2604–2615. [Google Scholar] [CrossRef]
- Chang, H.; Kim, M.S.; Huber, G.W.; Dumesic, J.A. Design of closed-loop recycling production of a Diels-Alder Polymer from a biomass-derived difuran as a functional additive for polyurethanes. Green Chem. 2021, 23, 9479–9488. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.; Du Prez, F.; Kalow, J. Introduction to chemistry for covalent adaptable networks. Polym. Chem. 2020, 11, 5295–5296. [Google Scholar] [CrossRef]
- Xu, X.; Ma, S.; Wang, S.; Wang, B.; Feng, H.; Li, P.; Liu, Y.; Yu, Z.; Zhu, J. Fast-reprocessing, postadjustable, self-healing covalent adaptable networks with Schiff base and Diels-Alder adduct. Macromol. Rapid Commun. 2022, 43, e2100777. [Google Scholar] [CrossRef]
- Rinehart, J.M.; Reynolds, J.R.; Yee, S.K. Limitations of Diels–Alder dynamic covalent networks as thermal conductivity switches. ACS Appl. Polym. Mater. 2022, 4, 1218–1224. [Google Scholar] [CrossRef]
- Li, X.; Yu, R.; He, Y.; Zhang, Y.; Yang, X.; Zhao, X.; Huang, W. Four-dimensional printing of shape memory polyurethanes with high strength and recyclability based on Diels-Alder chemistry. Polymer 2020, 200, 122532. [Google Scholar] [CrossRef]
- Hirschbiel, A.F.; Konrad, W.; Schulze-Suenninghausen, D.; Wiedmann, S.; Luy, B.; Schmidt, B.V.K.J.; Barner-Kowollik, C. Access to multiblock copolymers via supramolecular host-guest chemistry and photochemical ligation. ACS Macro Lett. 2015, 4, 1062–1066. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Miao, Y.; Wen, X.; Wang, J.; Sun, Y.; Chen, Y.; Lin, J.; Qiu, L.; Guo, K.; et al. Enzyme-mediated in situ self-assembly promotes in vivo bioorthogonal reaction for pretargeted multimodality imaging. Angew Chem. Int. Ed. Engl. 2021, 60, 18082–18093. [Google Scholar] [CrossRef]
- Masson, G.; Lalli, C.; Benohoud, M.; Dagousset, G. Catalytic enantioselective [4+2]-cycloaddition: A strategy to access aza-hexacycles. Chem. Soc. Rev. 2013, 42, 902–923. [Google Scholar] [CrossRef]
- Knall, A.C.; Slugovc, C. Inverse electron demand Diels-Alder (Iedda)-initiated conjugation: A (high) potential click chemistry scheme. Chem. Soc. Rev. 2013, 42, 5131–5142. [Google Scholar] [CrossRef]
- Geng, Z.; Shin, J.J.; Xi, Y.; Hawker, C.J. Click chemistry strategies for the accelerated synthesis of functional macromolecules. J. Polym. Sci. 2021, 59, 963–1042. [Google Scholar] [CrossRef]
- Sinnwell, S.; Inglis, A.J.; Davis, T.P.; Stenzel, M.H.; Barner-Kowollik, C. An atom-efficient conjugation approach to well-defined block copolymers using RAFT chemistry and hetero Diels-Alder cycloaddition. Chem. Commun. 2008, 17, 2052–2054. [Google Scholar] [CrossRef]
- Espinosa, E.; Glassner, M.; Boisson, C.; Barner-Kowollik, C.; D’Agosto, F. Synthesis of cyclopentadienyl capped polyethylene and subsequent block copolymer formation via hetero Diels-Alder (Hda) chemistry. Macromol. Rapid Commun. 2011, 32, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Nebhani, L.; Schmiedl, D.; Barner, L.; Bamer-Kowollik, C. Quantification of grafting densities achieved via modular ‘grafting-to’ approaches onto divinylbenzene microspheres. Adv. Funct. Mater. 2010, 20, 2010–2020. [Google Scholar] [CrossRef]
- Tischer, T.; Goldmann, A.S.; Linkert, K.; Trouillet, V.; Brner, H.G.; Barner-Kowollik, C. Modular ligation of thioamide functional peptides onto solid cellulose substrates. Adv. Funct. Mater. 2012, 22, 3853–3864. [Google Scholar] [CrossRef]
- Kaupp, M.; Vogt, A.P.; Natterodt, J.C.; Trouillet, V.; Gruendling, T.; Hofe, T.; Barner, L.; Barner-Kowollik, C. Modular design of glyco-microspheres via mild pericyclic reactions and their quantitative analysis. Polym. Chem. 2012, 3, 2605–2614. [Google Scholar] [CrossRef]
- Inglis, A.J.; Sinnwell, S.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. Reversible addition fragmentation chain transfer (RAFT) and Hetero-Diels Alder chemistry as a convenient conjugation tool for access to complex macromolecular designs. Macromolecules 2008, 41, 4120–4126. [Google Scholar] [CrossRef]
- Sinnwell, S.; Inglis, A.J.; Stenzel, M.H.; Barner-Kowollik, C. Access to three-arm star block copolymers by a consecutive combination of the Copper(I)-Catalyzed Azide–Alkyne cycloaddition and the RAFT Hetero Diels–Alder concept. Macromol. Rapid Commun. 2008, 29, 1090–1096. [Google Scholar] [CrossRef]
- Bousquet, A.; Barner-Kowollik, C.; Stenzel, M.H. Synthesis of comb polymers via Grafting-onto macromolecules bearing pendant diene groups via the Hetero-Diels-Alder-Raft click concept. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1773–1781. [Google Scholar] [CrossRef]
- Sinnwell, S.; Lammens, M.; Stenzel, M.H.; Prez, F.E.D.; Barner-Kowollik, C. Efficient access to multi-arm star block copolymers by a combination of ATRP and RAFT-HDA click chemistry. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 2207–2213. [Google Scholar] [CrossRef]
- Beloqui, A.; Mane, S.R.; Langer, M.; Glassner, M.; Bauer, D.M.; Fruk, L.; Barner-Kowollik, C.; Delaittre, G. Hetero-Diels–Alder cycloaddition with RAFT polymers as bioconjugation platform. Angew Chem. Int. Ed. 2020, 59, 19951–19955. [Google Scholar] [CrossRef]
- Inglis, A.J.; Paulöhrl, T.; Barner-Kowollik, C. Ambient temperature synthesis of a versatile macromolecular building block: Cyclopentadienyl-Capped polymers. Macromolecules 2009, 43, 33–36. [Google Scholar] [CrossRef]
- Inglis, A.J.; Stenzel, M.H.; Barner-Kowollik, C. Ultra-Fast Raft-Hda Click Conjugation: An efficient route to high molecular weight block copolymers. Macromol. Rapid. Commun. 2009, 30, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Langer, M.; Brandt, J.; Lederer, A. Amphiphilic Block Copolymers Featuring a Reversible Hetero Diels-Alder Linkage. Polym. Chem. 2014, 5, 5330–5338. [Google Scholar] [CrossRef]
- Dürr, C.J.; Hlalele, L.; Kaiser, A.; Brandau, S.; Barner-Kowollik, C. Mild and efficient modular synthesis of poly(acrylonitrile-co-butadiene) block and miktoarm star copolymer architectures. Macromolecules 2012, 46, 49–62. [Google Scholar] [CrossRef]
- Zydziak, N.; Hübner, C.; Bruns, M.; Barner-Kowollik, C. One-Step functionalization of single-walled carbon nanotubes (SWCNTs) with cyclopentadienyl-capped macromolecules via Diels−Alder chemistry. Macromolecules 2011, 44, 3374–3380. [Google Scholar] [CrossRef]
- Zydziak, N.; Preuss, C.M.; Winkler, V.; Bruns, M.; Hübner, C.; Barner-Kowollik, C. Hetero Diels-Alder chemistry for the functionalization of Single-Walled carbon nanotubes with cyclopentadienyl End-Capped polymer strands. Macromol. Rapid Commun. 2013, 34, 672–680. [Google Scholar] [CrossRef]
- Guimard, N.K.; Ho, J.; Brandt, J.; Lin, C.Y.; Namazian, M.; Mueller, J.O.; Oehlenschlaeger, K.K.; Hilf, S.; Lederer, A.; Schmidt, F.G.; et al. Harnessing entropy to direct the bonding/debonding of polymer systems based on reversible chemistry. Chem. Sci. 2013, 4, 2752–2759. [Google Scholar] [CrossRef]
- Schenzel, A.M.; Klein, C.; Rist, K.; Moszner, N.; Barner-Kowollik, C. Reversing adhesion: A triggered release self-reporting adhesive. Adv. Sci. 2016, 3, 1500361. [Google Scholar] [CrossRef]
- Glassner, M.; Kempe, K.; Schubert, U.S.; Hoogenboom, R.; Barner-Kowollik, C. One-Pot synthesis of cyclopentadienyl endcapped poly(2-ethyl-2-oxazoline) and subsequent ambient temperature Diels-Alder conjugations. Chem. Commun. 2011, 47, 10620–10622. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.; Lenz, J.; Pahnke, K.; Schmidt, F.G.; Barner-Kowollik, C.; Lederer, A. Investigation of thermoreversible polymer networks by temperature dependent size exclusion chromatography. Polym. Chem. 2017, 8, 6598–6605. [Google Scholar] [CrossRef]
- Oehlenschlaeger, K.K.; Mueller, J.O.; Brandt, J.; Hilf, S.; Barner-Kowollik, C. Adaptable hetero Diels-Alder networks for fast Self-Healing under mild conditions. Adv. Mater. 2014, 26, 3561–3566. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, H.; Schmitt, C.W.; Wedler-Jasinski, N.; Woehlk, H.; Fairfull-Smith, K.E.; Blinco, J.P.; Barner-Kowollik, C. Spin fluorescence silencing enables an efficient thermally driven self-reporting polymer release system. Polym. Chem. 2017, 8, 6199–6203. [Google Scholar] [CrossRef]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Oh, J.K.; Lee, D.I.; Park, J.M. Biopolymer-based microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2009, 34, 1261–1282. [Google Scholar] [CrossRef]
- Wiemer, K.; Dörmbach, K.; Slabu, I.; Agrawal, G.; Schrader, F.; Caumanns, T.; Bourone, S.D.M.; Mayer, J.; Steitz, J.; Simona, U.; et al. Hydrophobic superparamagnetic fept nanoparticles in hydrophilic poly(N-vinylcaprolactam) microgels: A new multifunctional hybrid system. J. Mater. Chem. B 2017, 5, 1284–1292. [Google Scholar] [CrossRef]
- Karg, M. Multifunctional inorganic/organic hybrid microgels: An overview of recent developments in synthesis, characterization, and application. Colloid Polym. Sci. 2012, 290, 673–688. [Google Scholar] [CrossRef]
- Peng, L.; Feng, A.; Liu, S.; Huo, M.; Yuan, J. Electrochemical stimulated pickering emulsion for recycling of enzyme in biocatalysis. ACS Appl. Mater. Interfaces 2016, 8, 29203–29207. [Google Scholar] [CrossRef]
- Zhang, H.; Niu, C.; Zhang, Y.; Wang, X.; Yang, B. A mechanically strong polyvinyl alcohol/poly(2-(N, N′-Dimethyl Amino) ethyl methacrylate)-poly (acrylic acid) hydrogel with ph-responsiveness. Colloid Polym. Sci. 2020, 298, 619–628. [Google Scholar] [CrossRef]
- Sasaki, Y.; Akiyoshi, K. Nanogel Engineering for new nanobiomaterials: From chaperoning engineering to biomedical applications. Chem. Rec. 2010, 10, 366–376. [Google Scholar] [CrossRef]
- Rossow, T.; Heyman, J.A.; Ehrlicher, A.J.; Langhoff, A.; Weitz, D.A.; Haag, R.; Seiffert, S. Controlled synthesis of Cell-Laden microgels by radical-free gelation in droplet microfluidics. J. Am. Chem. Soc. 2012, 134, 4983–4989. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jiang, X.; Yin, J. Multi-Responsive Microgel of hyperbranched poly(ether amine) (Hpea-Mgel) for the selective adsorption and separation of hydrophilic fluorescein dyes. J. Mater. Chem. 2012, 22, 17976–17983. [Google Scholar] [CrossRef]
- Steinhilber, D.; Rossow, T.; Wedepohl, S.; Paulus, F.; Seiffert, S.; Haag, R. A microgel construction kit for bioorthogonal encapsulation and ph-controlled release of living cells. Angew. Chem. Int. Ed. Engl. 2013, 52, 13538–13543. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, K.C.R.; Xu, P. Multicompartment intracellular Self-Expanding nanogel for targeted delivery of drug cocktail. Adv. Mater. 2012, 24, 6479–6483. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, F.; Shen, H.; Wu, D. Controlled formation of microgels/nanogels from a disulfide-linked core/shell hyperbranched polymer. ACS Macro Lett. 2012, 1, 1295–1299. [Google Scholar] [CrossRef]
- Yan, L.; Tao, W. One-Step Synthesis of pegylated cationic nanogels of poly(N, N′-dimethylaminoethyl methacrylate) in aqueous solution via self-stabilizing micelles using an amphiphilic macroraft agent. Polymer 2010, 51, 2161–2167. [Google Scholar] [CrossRef]
- Heller, D.A.; Levi, Y.; Pelet, J.M.; Doloff, J.C.; Wallas, J.; Pratt, G.W.; Jiang, S.; Sahay, G.; Schroeder, A.; Schroeder, J.E.; et al. Modular ‘click-in-emulsion’ bone-targeted nanogels. Adv. Mater. 2013, 25, 1449–1454. [Google Scholar] [CrossRef]
- Tan, H.; Jin, H.; Mei, H.; Zhu, L.; Wei, W.; Wang, Q.; Liang, F.; Zhang, C.; Li, J.; Qu, X.; et al. Peg-Urokinase nanogels with enhanced stability and controllable bioactivity. Soft Matter 2012, 8, 2644–2650. [Google Scholar] [CrossRef]
- Kim, K.; Bae, B.; Kang, Y.J.; Nam, J.M.; Kang, S.; Ryu, J.H. Natural polypeptide-based supramolecular nanogels for stable noncovalent encapsulation. Biomacromolecules 2013, 14, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Nakai, T.; Hirakura, T.; Sakurai, Y.; Shimoboji, T.; Ishigai, M.; Akiyoshi, K. Injectable hydrogel for sustained protein release by salt-induced association of hyaluronic acid nanogel. Macromol. Biosci. 2012, 12, 475–483. [Google Scholar] [CrossRef]
- Hasegawa, U.; Sawada, S.; Shimizu, T.; Kishida, T.; Otsuji, E.; Mazda, O.; Akiyoshi, K. Raspberry-Like assembly of Cross-Linked nanogels for protein delivery. J. Control. Release 2009, 140, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Hirakura, T.; Yasugi, K.; Nemoto, T.; Sato, M.; Shimoboji, T.; Aso, Y.; Morimoto, N.; Akiyoshi, K. Hybrid hyaluronan hydrogel encapsulating nanogel as a protein nanocarrier: New system for sustained delivery of protein with a chaperone-like function. J. Control. Release 2010, 142, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Asayama, W.; Niwa, T.; Sawada, S.-i.; Ueda, T.; Taguchi, H.; Akiyoshi, K. Amphiphilic Polysaccharide Nanogels as Artificial Chaperones in Cell-Free Protein Synthesis. Macromol. Biosci. 2011, 11, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Moritani, Y.; Ikeda-Fukazawa, T.; Sasaki, Y.; Akiyoshi, K. A hybrid hydrogel biomaterial by nanogel engineering: Bottom-up design with nanogel and liposome building blocks to develop a multidrug delivery system. Adv. Healthc. Mater. 2012, 1, 722–728. [Google Scholar] [CrossRef]
- Siegwart, D.J.; Srinivasan, A.; Bencherif, S.I.; Karunanidhi, A.; Matyjaszewski, K. Cellular uptake of functional nanogels prepared by inverse miniemulsion atrp with encapsulated proteins, carbohydrates, and gold nanoparticles. Biomacromolecules 2009, 10, 2300–2309. [Google Scholar] [CrossRef]
- Wang, Z.J.; Ma, Z.Y.; Zhang, Z.N.; Wu, F.; Jiang, H.; Jia, X.R. Mechanical activation of a dithioester derivative-based retro RAFT-HDA reaction. Polym. Chem. 2014, 5, 6893–6897. [Google Scholar] [CrossRef]




| Component | ƒ | M/ƒ (g∙mol−1) | DP a | Mn a | Mn b | Đ b | Blocks | r ([F]0/[S]0) | [F]0 (mol∙Ml−1) | [S]0 (mol∙Ml−1) | T (°C) | Mn b | Đ b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSt1(S1) | 2 | 3344 | 66 | 6689 | 6838 | 1.15 | |||||||
| PSt2(S2) | 2 | 1416 | 28 | 2832 | 2765 | 1.15 | OF4-S2 | 1 | 0.06 | 0.06 | 55 | 8696 | 1.11 |
| PSt3(S3) | 2 | 1993 | 39 | 3987 | 3767 | 1.4 | OF4-S3 | 1 | 0.06 | 0.06 | 55 | 12,586 | 1.33 |
| PSt4(S4) | 2 | 3781 | 75 | 7563 | 7209 | 1.13 | OF4-S4 | 1 | 0.06 | 0.06 | 55 | 15,428 | 1.33 |
| P(St60-co-FMA35)(SF1) | 35 | 351 | 95 | 10,473 | 12,289 | 1.19 | SF1-S1 | 1 | 0.06 | 0.06 | 55 | 154,080 | 1.18 |
| P(St43-co-FMA17)(SF2) | 17 | 443 | 60 | 7531 | 7483 | 1.15 | SF2-S1 | 1 | 0.06 | 0.06 | 55 | 213,879 | 1.52 |
| P(OEGMA45-co-FMA2)(OF1) | 2 | 2566 | 47 | 5133 | 4743 | 1.54 | OF1-S3 | 1 | 0.06 | 0.06 | 55 | 10,434 | 1.4 |
| P(OEGMA45-co-FMA4)(OF2) | 4 | 1375 | 49 | 5501 | 5498 | 1.26 | OF2-S3 | 1 | 0.06 | 0.06 | 55 | 19,380 | 1.14 |
| P(OEGMA45-co-FMA7)(OF3) | 7 | 860 | 52 | 6021 | 7902 | 1.03 | OF3-S3 | 1 | 0.06 | 0.06 | 55 | 3162 | 1.03 |
| P(OEGMA40-co-FMA5)(OF4) | 5 | 952 | 42 | 4763 | 4477 | 1.38 | OF4-S2 | 2 | 0.12 | 0.06 | 55 | gel | |
| OF4-S3 | |||||||||||||
| OF4-S4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, J.; Yin, H.; Cao, R.; Huang, C.; Luo, X.; Ji, J. Responsive Microgels through RAFT-HDA Dynamic Covalent Bonding Chemistry. Molecules 2024, 29, 1217. https://doi.org/10.3390/molecules29061217
Nie J, Yin H, Cao R, Huang C, Luo X, Ji J. Responsive Microgels through RAFT-HDA Dynamic Covalent Bonding Chemistry. Molecules. 2024; 29(6):1217. https://doi.org/10.3390/molecules29061217
Chicago/Turabian StyleNie, Jingkai, Hang Yin, Ruyue Cao, Changyuan Huang, Xiang Luo, and Jun Ji. 2024. "Responsive Microgels through RAFT-HDA Dynamic Covalent Bonding Chemistry" Molecules 29, no. 6: 1217. https://doi.org/10.3390/molecules29061217
APA StyleNie, J., Yin, H., Cao, R., Huang, C., Luo, X., & Ji, J. (2024). Responsive Microgels through RAFT-HDA Dynamic Covalent Bonding Chemistry. Molecules, 29(6), 1217. https://doi.org/10.3390/molecules29061217





