Research Progress on Benzimidazole Fungicides: A Review
Abstract
1. Introduction
2. Physical and Chemical Properties
3. Disease Prevention and Control
4. Toxicological Properties
5. Pesticide Residue and Detection Technology
5.1. Spectroscopic Analysis Method
5.2. Gas Chromatography Technology
5.3. High-Performance Liquid Chromatography Technology
5.4. Enzyme-Linked Immunosorbent Assay
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, X.; Liu, C.J.; Yang, B.B.; Geng, R.H.; Wang, D.L. Residue analysis and risk assessment of dietary intake of thiophanate-methyl and its metabolite in Hemerocallis citrina Baroni. J. Food Saf. Qual. 2022, 13, 3907–3915. [Google Scholar]
- Xiao, L.L.; Wang, Y.X.; Wang, Y.; Zhang, X.; Lv, Y.T.; Liu, Z.Q.; Liu, C.L.; Liu, Y.P.; Li, X.G. Residues and dissipation of thiophanate-methyl and its metabolites carbendazim in citrus. Agrochemicals 2018, 57, 276–278. [Google Scholar]
- Ahmed, M.U.; Hasan, Q.; Hossain, M.M.; Saito, M.; Tamiya, E. Meat species identification based on the loop mediated isothermal amplification and electrochemical DNA sensor. Food Control 2010, 21, 599–605. [Google Scholar] [CrossRef]
- Fan, S.; Zhao, P.; Zhang, F.; Yu, C.; Pan, C. Spinach or amaranth may represent highest residue of thiophanate-methyl with open field application on six leaf vegetables. Bull. Environ. Contam. Toxicol. 2013, 90, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, H.Q.; Wang, Y.L. Progress in the synthesis and application of benzimidazoles and their derivatives. Chinese J. Org. Chem. 2008, 28, 210. [Google Scholar]
- Danaher, M.; De Ruyck, H.; Crooks, S.R.; Dowling, G.; O’Keeffe, M. Review of methodology for the determination of benzimidazole residues in biological matrices. J. Chromatogr. B 2007, 845, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Li, F.J.; Komura, R.; Nakashima, C.; Shimizu, M.; Kageyama, K.; Suga, H. Molecular diagnosis of thiophanate-methyl-resistant strains of Fusarium fujikuroi in Japan. Plant Dis. 2002, 106, 634–640. [Google Scholar] [CrossRef]
- Prousalis, K.P.; Polygenis, D.A.; Syrokou, A.; Lamari, F.N.; Tsegenidis, T. Determination of carbendazim, thiabendazole and o-phenylphenol residues in lemons by HPLC following sample clean-up by ion-pairing. Anal. Bioanal. Chem. 2004, 379, 458–463. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Y.; Han, J.; Chen, D.; Yang, G.; Lan, T.; Li, J.; Zhang, K. Determination, dissipation dynamics, terminal residues and dietary risk assessment of thiophanate-methyl and its metabolite carbendazim in cowpeas collected from different locations in China under field conditions. J. Sci. Food Agr. 2021, 101, 5498–5507. [Google Scholar] [CrossRef]
- Zhang, Y.X. Determination of benzimidazoles residues in beef by HPLC. Livest. Pouliry Ind. 2006, 6–7. [Google Scholar]
- Zhang, S.X.; Li, J.S.; Qian, C.F. Matrix solid-phase dispersion-high performance liquid chromatographic multi-residue analysis of benzimidazoles in bovine muscle tissue. Chin. J. Vet. Sci. 2000, 20, 569–571. [Google Scholar]
- Diao, C.L.; Liu, F.; Song, B.A. Advances in the mechanism of action of agricultural fungicides. Agrochemicals 2006, 45, 374–377. [Google Scholar]
- Liu, C.L. World Book of Pesticides (Fungicides Volume); Chemical Industry Press: Beijing, China, 2008. [Google Scholar]
- Davidse, L.C. Benzimidazole fungcides: Mechanism of action and biological impact. Annu. Rev. Phytopathol. 1986, 24, 43–65. [Google Scholar] [CrossRef]
- Chen, Y. Study on analysis method of benzimidazole fungicide residues and residue status in fruits. CAAS Diss. 2008. [Google Scholar]
- Fujimura, M.; Oeda, K.; Inoue, H.; Kato, T. A single amino-acid substitution in the beta-tubulin gene of Neurospora confers both carbendazim resistance and diethofencarb sensitivity. Curr. Genet. 1992, 21, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Albertini, C.; Gredt, M.; Leroux, P. Mutations of the β-tubulin gene associated with different phenotypes of benzimidazole resistance in the cereal eyespot fungi Tapesia yallundae and Tapesia acuformis. Pestic. Biochem. Phys. 1999, 64, 17–31. [Google Scholar] [CrossRef]
- Li, H.X.; Lu, Y.J.; Zhou, M.G. Research progress on benzimidazole fungicides. In Proceedings of the Third Academic Seminar on Chemical Control of Plant Diseases; Phytopathology Society: Zhangjiajie, China, 2002. [Google Scholar]
- Mao, Y.S.; Duan, Y.B.; Zhou, M.G. Research progress of the resistance to succinate dehydrogenase inhibitors. Chin. J. Pestic. Sci. 2022, 24, 937–948. [Google Scholar]
- Huang, W.W. Study on the LC-MS/MS Methods for Detecting Benzimidazole Fungicides and Metabolites in Concentrated Fruit Juice; Guangxi University: Nanning, China, 2013. [Google Scholar]
- Sun, J.L.; Qi, J.S. Modern Pesticide Application Technology Series: Fungicides Volume; Chinese Chemical Industry Press: Beijing, China, 2014. [Google Scholar]
- Wang, B. Determination of Benzimidazoles Residues in Foodstuffs by HPLC-MS/MS; Jimei University: Guangdong, China, 2010. [Google Scholar]
- Duan, L.F. FAO standards for the identification of highly hazardous pesticides. Pestic. Sci. Adm. 2019, 40, 8. [Google Scholar]
- Xu, D.G.; Feng, C.G. Pesticide toxicity classification and recommendations. Plant Dr. 2015, 28, 35–37. [Google Scholar]
- Liu, W.P.; Zhang, Y. Progress in potential toxicity of chiral pesticides. J. Zhejiang Univ. 2012, 38, 63–70. [Google Scholar]
- Xu, P.Y.; Wu, D.S. New progress in the study of organophosphorus pesticide toxicity. J. Prev. Med. Health Inf. 2004, 20, 389–392. [Google Scholar]
- Yu, G.C.; Wang, X.F. Research progress on the mechanisms of male reproductive toxicity induced by benzimidazole. Occup. Health 2014, 30, 2661–2663. [Google Scholar]
- Yoon, C.S.; Jin, J.H.; Park, J.H.; Yeo, C.Y.; Kim, S.J.; Hwang, Y.G.; Hong, S.J.; Cheong, S.W. Toxic effects of carbendazim and n-butyl isocyanate, metabolites of the fungicide benomyl, on early development in the African clawed frog, Xenopus laevis. Environ. Toxicol. 2008, 23, 131–144. [Google Scholar] [CrossRef]
- Wei, Z.H.; Xu, J.; Guo, M.X.; Shi, A.M.; Sun, J.Z. Research progress on domestic carbendazim. J. Anhui Agri. Sci. 2015, 43, 125–127+141. [Google Scholar]
- Roepcke, C.B.; Muench, S.B.; Schulze, H.; Bachmann, T.; Schmid, R.D.; Hauer, B. Analysis of phosphorothionate pesticides using a chloroperoxidase pretreatment and acetylcholinesterase biosensor detection. J. Agric. Food Chem. 2010, 58, 8748–8756. [Google Scholar] [CrossRef] [PubMed]
- Knabel, A.; Meyer, K.; Rapp, J.; Schulz, R. Fungicide field concentrations exceed focus surface water predictions: Urgent need of model improvement. Environ. Sci. Technol. 2014, 48, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.C.; Wang, X.F. Research progress of toxicology of carbendazim. Occup. Health 2008, 24, 1834–1835. [Google Scholar]
- Song, Y.C.; Yu, C.G.; Wang, X.F. Research progress on reproductive toxicity of carbendazim. Chin. Occup. Med. 2010, 37, 505–507. [Google Scholar]
- Song, Y.C. Study on the Mechanism of the Carbendazim-Induced Reproductive Toxicity in Male Rats; University of Jinan: Jinan, China, 2011. [Google Scholar]
- Sun, Y.X. Pesticide residue and hazard analysis in vegetables. Mod. Agric. 2020, 9, 64–66. [Google Scholar]
- Gao, Z.X.; Wu, Y.H.; Zheng, H.F.; Ao, K.H.; Liu, Y.; Zeng, Q.H.; Huang, C. Residue analysis of triabendazole in peruvian ground cherry by RP-HPLC-PDA. Food Sci. 2012, 10, 204–207. [Google Scholar]
- Ekman, E.; Faniband, M.H.; Littorin, M.; Maxe, M.; Jönsson, B.A.; Lindh, C.H. Determination of 5-hydroxythiabendazole in human urine as a biomarker of exposure to thiabendazole using LC/MS/MS. J. Chromatogr. B 2014, 973, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.D. Fluorescence Approaches for Rapid Determination of Benzimidazole Fungicides in Food; Xiamen University: Xiamen, China, 2014. [Google Scholar]
- GB 2763-2021; National Food Safety Standard—Maximum Residue Limits for Pesticides in Food. Haichuang Technology Center: Hangzhou, China, 2021.
- Ministry of Food and Drug Safety of the South Korea. Pesticide MRLs in Food. Available online: http://www.mfds.go.kr/index.do#info (accessed on 14 December 2023).
- European Commission (EU). European Commission. EU-Pesticides Database. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/mrls/?event=search.pr (accessed on 14 December 2023).
- Del Pozo, M.; Hernández, L.; Quintana, C. A selective spectrofluorimetric method for carbendazim determination in oranges involving inclusion-complex formation with cucurbit[7]uril. Talanta 2010, 81, 1542–1546. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zou, Q.L.; Zhong, G.J.; Yang, Y.S. Determination of carbendazim in water samples by derivative gas chromatography method. Environ. Monit. Chin. 2012, 28, 41–43. [Google Scholar]
- Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Rexing, D.J.; Snyder, S.A. Broad range analysis of endocrine disruptors and pharmaceuticals using gas chromatography and liquid chromatography tandem mass spectrometry. Chemosphere 2006, 65, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Zamora, O.; Paniagua, E.E.; Cacho, C.; Vera-Avila, L.E.; Perez-Conde, C. Determination of benzimidazole fungicides in water samples by on-line MISPE–HPLC. Anal. Bioanal. Chem. 2009, 393, 1745–1753. [Google Scholar] [CrossRef]
- Mezcua, M.; Agüera, A.; Lliberia, J.L.; Cortés, M.A.; Bagó, B.; Fernández-Alba, A.R. Application of ultra performance liquid chromatography–tandem mass spectrometry to the analysis of priority pesticides in groundwater. J. Chromatogr. A 2006, 1109, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Soler, C.; Mañes, J.; Picó, Y. Comparison of liquid chromatography using triple quadrupole and quadrupole ion trap mass analyzers to determine pesticide residues in oranges. J. Chromatogr. A 2005, 1067, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Picó, Y.; la Farré, M.; Soler, C.; Barceló, D. Identification of unknown pesticides in fruits using ultra-performance liquid chromatography–quadrupole time-of-flight mass spectrometry: Imazalil as a case study of quantification. J. Chromatogr. A 2007, 1176, 123–134. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Liu, X.G.; Xu, J.; Dong, F.S.; Liang, X.Y.; Li, M.M.; Duan, L.F.; Zheng, Y.Q. Simultaneous determination of spirotetramat and its four metabolites in fruits and vegetables using a modified quick, easy, cheap, effective, rugged, and safe method and liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2013, 1299, 71–77. [Google Scholar] [CrossRef]
- Schenck, F.J.; Hobbs, J.E. Evaluation of the quick, easy, cheap, effective, rugged, and safe (QuEChERS) approach to pesticide residue analysis. B. Environ. Contam. Tox. 2004, 73, 24–30. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Son, K.A.; Kwon, H.; Koesukwiwat, U.; Fu, W.; Mastovska, K.; Hoh, E.; Leepipatpiboon, N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J. Chromatogr. A 2010, 1217, 2548–2560. [Google Scholar] [CrossRef] [PubMed]
- Wilkowska, A.; Biziuk, M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011, 125, 803–812. [Google Scholar] [CrossRef]
- Subhani, Q.; Huang, Z.P.; Zhu, Z.Y.; Zhu, Y. Simultaneous determination of imidacloprid and carbendazim in water samples by ion chromatography with fluorescence detector and post-column photochemical reactor. Talanta 2013, 116, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, C.H.; Wu, N.C.; Yin, G.H.; Wu, X.F. Determination of thiabendazole, paclobutrazol and hexaconazole pesticide residues in longan using GC-MS/MS. Agrochemicals 2014, 53, 423–425. [Google Scholar]
- Gao, R.; Zhao, R.Z.; Yang, J.; Chen, J.H.; Xiao, H. Determination of carbendazim residues in rapeseed by high performance liquid chromatography. J. Nanjing Med. Univ.: Nat. Sci. Ed. 2004, 24, 425–426. [Google Scholar]
- Liu, C.L.; Liu, F.M.; Li, L.; Lan, R.H.; Jiang, S.R. The determination of carbendazim and imidacloprid residues in asparagus by high performance liquid chromatography. Chin. J. Pestic. Sci. 2004, 6, 93–96. [Google Scholar]
- Peng, F.; Tian, J.G.; Jin, H.Y.; Du, Q.P. Determ ination of residual carbendazim in chinese traditional medicine of ginseng by HPLC. Chin. Pharm. J. 2007, 42, 475–477. [Google Scholar]
- Caprioli, G.; Cristalli, G.; Galarini, R.; Giacobbe, D.; Ricciutelli, M.; Vittori, S.; Zou, Y.T.; Sagratini, G. Comparison of two different isolation methods of benzimidazoles and their metabolites in the bovine liver by solid-phase extraction and liquid chromatography–diode array detection. J. Chromatogr. A 2010, 1217, 1779–1785. [Google Scholar] [CrossRef]
- Al-Ebaisat, H. Determination of some benzimidazole fungicides in tomato puree by high performance liquid chromatography with SampliQ polymer SCX solid phase extraction. Arab. J. Chem. 2011, 4, 115–117. [Google Scholar] [CrossRef]
- Liang, P.; Zhao, Y.X.; Li, P.; Yu, Q.L. Matrix solid-phase dispersion based on cucurbit[7]uril-assisted dispersive liquid–liquid microextraction coupled with high performance liquid chromatography for the determination of benzimidazole fungicides from vegetables. J. Chromatogr. A 2021, 1658, 462592. [Google Scholar] [CrossRef]
- Wu, M.; Hu, J. Residue analysis of albendazole in watermelon and soil by solid phase extraction and HPLC. Anal. Lett. 2014, 47, 356–366. [Google Scholar] [CrossRef]
- Kim, K.G.; Park, D.W.; Kang, G.R.; Kim, T.S.; Yang, Y.; Moon, S.J.; Choi, E.A.; Ha, D.R.; Kim, E.S.; Cho, B.S. Simultaneous determination of plant growth regulator and pesticides in bean sprouts by liquid chromatography–tandem mass spectrometry. Food Chem. 2016, 208, 239–244. [Google Scholar] [CrossRef]
- Blasco, C.; Fernández, M.; Picó, Y.; Font, G.; Mañes, J. Simultaneous determination of imidacloprid, carbendazim, methiocarb and hexythiazox in peaches and nectarines by liquid chromatography–mass spectrometry. Anal. Chim. Acta 2002, 461, 109–116. [Google Scholar] [CrossRef]
- Bean, K.A.; Henion, J.D. Determination of carbendazim in soil and lake water by immunoaffinity extraction and coupled-column liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 1997, 791, 119–126. [Google Scholar] [CrossRef]
- Wang, X. Determination of carbendazim in orange juice using QuEChERS and LC/MS/MS. Lc Gc Europe 2012, 11. [Google Scholar]
- Dong, B.; Hu, J. Residue dissipation and dietary intake risk assessment of thiophanate-methyl and its metabolite carbendazim in watercress under Chinese field conditions. Int. J. Environ. Anal. Chem. 2023, 103, 561–574. [Google Scholar] [CrossRef]
- Shi, C.; Lv, C.X.; Feng, X.Q.; Li, M.M.; Liu, S.S. Research progress of enzyme-linked immunosorbent assay in food testing and analysis. J. Food Saf. Qual. 2014, 7, 3269–3275. [Google Scholar]
- You, J.; Guo, H.B.; Zeng, S.D.; Liu, Y.J.; Wang, M.Y. Properties and detection technologies of benzimidazole fungicide. Agrochemicals 2016, 55, 859–863. [Google Scholar]
- Šmídová, Z.; Blažková, M.; Fukal, L.; Rauch, P. Pesticides in food–immunochromatographic detection of thiabendazole and methiocarb. Czech J. Food Sci. 2009, 27, S414–S416. [Google Scholar] [CrossRef]
- Blažková, M.; Rauch, P.; Fukal, L. Strip-based immunoassay for rapid detection of thiabendazole. Biosens. Bioelectron. 2010, 25, 2122–2128. [Google Scholar] [CrossRef]
- Estevez, M.C.; Belenguer, J.; Gomez-Montes, S.; Miralles, J.; Escuela, A.M.; Montoya, A.; Lechuga, L.M. Indirect competitive immunoassay for the detection of fungicide thiabendazole in whole orange samples by Surface Plasmon Resonance. Analyst 2012, 137, 5659–5665. [Google Scholar] [CrossRef]
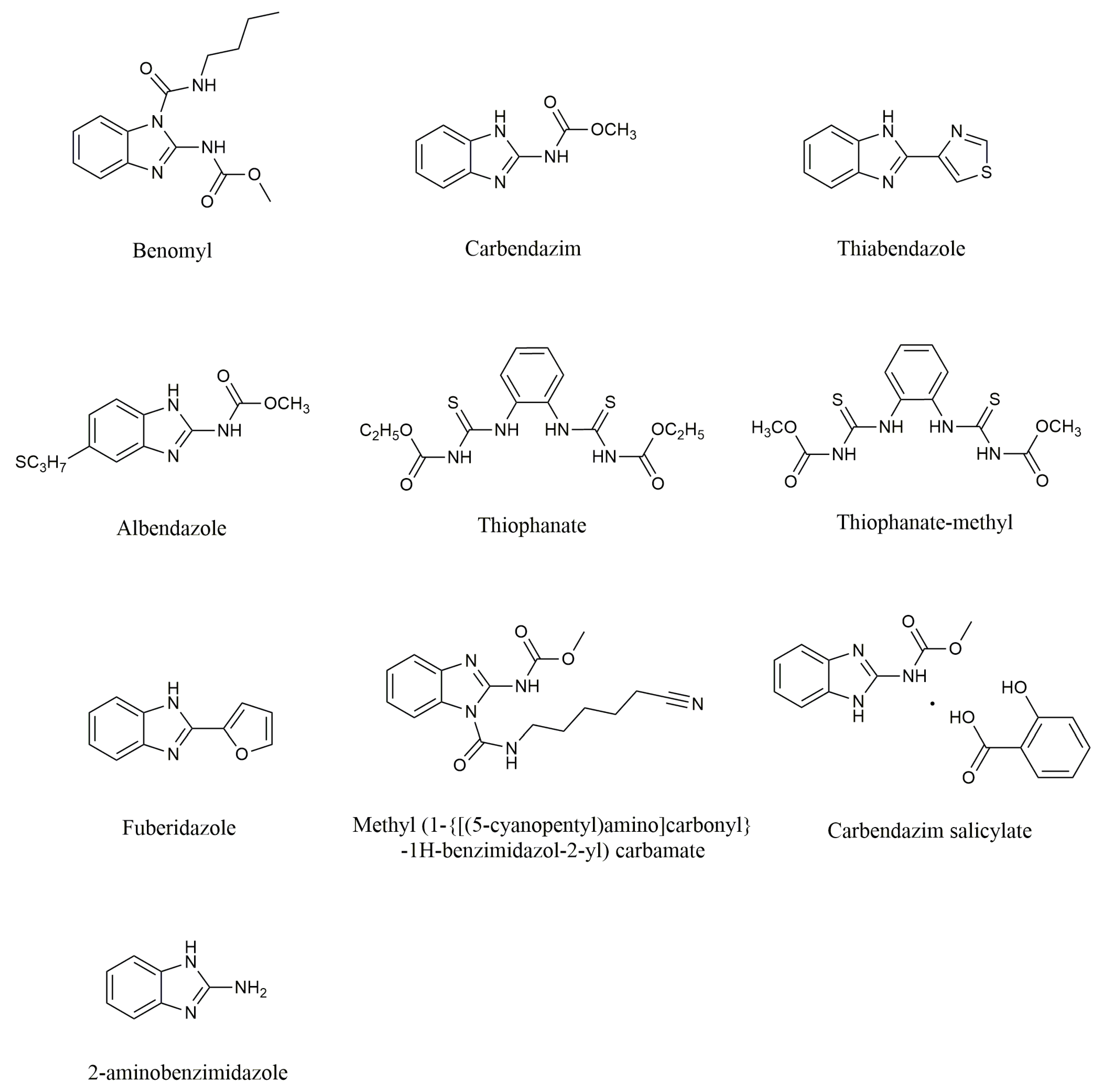
| Compounds | Chemical Formula | Molecular Mass | Melting Points | Solubility | Properties | CAS Number |
|---|---|---|---|---|---|---|
| Benomyl | C14H18N4O3 | 290.318 | 140 °C | Insoluble in water and oil; soluble in chloroform, acetone, dimethylformamide | White crystalline powder | 17804-35-2 |
| Carbendazim | C9H9N3O2 | 191.187 | 302–307 °C | At 24 °C, slightly soluble in water; soluble in dimethylformamide, acetone, ethanol, chloroform, ethyl acetate | Light-gray or beige powder | 10605-21-7 |
| Thiabendazole | C10H7N3S | 201.25 | 298–301 °C | At 20 °C, slightly soluble in water; soluble in acetone, methanol, xylene, ethyl acetate, octanol | White crystalline powder | 148-79-8 |
| Albendazole | C12H15N3O2S | 265.33 | 206–212 °C | Insoluble in water; slightly soluble in ethanol, chloroform, hot dilute hydrochloric acid, dilute sulfuric acid; soluble in glacial acetic acid | White powder | 54965-21-8 |
| Thiophanate | C14H18N4O4S2 | 370.447 | 195 °C | Insoluble in water; soluble in dimethylformamide, acetonitrile, cyclohexane, ethanol, acetone, and other organic solvents | Colorless flaky crystals | 23564-06-9 |
| Thiophanate-methyl | C12H14N4O4S2 | 342.39 | 172 °C | Slightly soluble in water; soluble in dimethylformamide, dioxane, chloroform, acetone, methanol, ethanol, ethyl acetate, and so on | Colorless crystalline solid | 23564-05-8 |
| Fuberidazole | C11H8N2O | 184.19 | 286 °C | At room temperature, insoluble in water; soluble in methanol, ethanol, acetone, dichloromethane, isopropanol, and other organic solvents | Colorless crystalline solid | 3878-19-1 |
| Methyl (1-{[(5-cyanopentyl)amino]carbonyl}-1H-benzimidazol-2-yl) carbamate | C16H19N5O3 | 329.354 | / | Insoluble in water; soluble in toluene, dichloromethane, dimethylformamide, cyclohexane, and other organic solvents | Colorless crystals | 28559-00-4 |
| Carbendazim salicylate | C16H15N3O5 | 329.28 | / | Soluble in water | Deep-brown liquid (soluble solvent), grayish-white powder (wettable powder) | / |
| 2-Aminobenzimidazole | C7H7N3 | 133.15 | 226–230 °C | Soluble in water, ethanol, acetone; insoluble in ether, benzene | White or pale-yellow crystalline solid | 934-32-7 |
| Compounds | Disease Control |
|---|---|
| Benomyl | Apples (powdery mildew, scab), pears (powdery mildew, scab), wheat (wheat scab), rice (rice blast), cucurbits (cucurbit scab, anthracnose), eggplant (eggplant gray mold), tomatoes (tomato leaf mold), allium (allium gray-mold rot), celery (celery gray-spot disease), asparagus (asparagus stem blight), citrus (scab, gray mold), soybean (soybean sclerotinia), peanut (peanut brown-spot disease), sweet potato (black-spot disease, dry rot) [21] |
| Carbendazim | Wheat (head blight, loose smut, glume blotch), cereal (stem rot), apples (powdery mildew, scab), pears (powdery mildew, black-spot, ring rot), grape (powdery mildew, gray mold, white rot), peach (powdery mildew), cotton (cotton seedling blight, boll rot), peanut (peanut leaf spot and root rot), tobacco (tobacco anthracnose), tomatoes (early blight, gray mold), sugarcane (pineapple disease), beet (beet leaf spot), rice (rice blast, sheath blight), sesame (sesame leaf spot) [21] |
| Thiabendazole | Citrus (citrus green mold, blue mold, stem-end rot) [21] |
| Albendazole | Rice (rice blast), tobacco (anthracnose) [21] |
| Thiophanate | Cereal crops (Fusarium head blight, powdery mildew, smut), rice (rice blast, sheath blight, kernel smut) [21,22] |
| Thiophanate-methyl | Rice (rice blast, sheath blight), wheat (rust, powdery mildew), cereal crops (Fusarium head blight, smut), rapeseed (sclerotinia), tomatoes (downy mildew), vegetables (anthracnose, leaf spot), peanut (scab), fruit trees (powdery mildew, anthracnose) [21,22] |
| Fuberidazole | Wheat (black head mold, snow mold) [21,22] |
| Methyl (1-{[(5-cyanopentyl)amino]carbonyl}-1H-benzimidazol-2-yl) carbamate | Rice (rice bakanae disease), apples (powdery mildew), pears (powdery mildew) [21] |
| Carbendazim salicylate | Wheat (wheat scab), cotton (cotton wilt) [21] |
| Toxicity Level | Oral LD50 (mg/kg) | Dermal LD50 (mg/kg) | LD50 Inhalation (mg/m3) |
|---|---|---|---|
| Extremely Toxic | ≤5 | ≤20 | ≤20 |
| Highly Toxic | >5~50 | >20~200 | >20~200 |
| Moderately Low Toxicity | >50~500 | >200~2000 | >200~2000 |
| Low Toxicity | >500~5000 | >2000~5000 | >2000~5000 |
| Slightly Toxic | >5000 | >5000 | >5000 |
| Compounds | Toxicological Properties |
|---|---|
| Benomyl | Rat acute oral LD50 > 5000 mg/kg; rabbit acute dermal LD50 > 5000 mg/kg; temporary irritant to rabbit eyes; no abnormalities found in dogs fed with 500 mg/kg for two years; non-toxic to earthworms. |
| Carbendazim | Rat acute oral LD50 > 15,000 mg/kg; rat acute percutaneous LD50 > 2000 mg/kg; rabbit acute oral LD50 > 10,000 mg/kg; no irritation to rabbit’s eyes and skin; no abnormality was found when feeding 300 mg/kg to dogs for two years; and it is non-toxic to earthworms. |
| Thiabendazole | Rat acute oral LD50 > 3100 mg/kg; mouse acute oral LD50 > 3600 mg/kg; rabbit acute percutaneous LD50 > 2000 mg/kg; no irritation to rabbit eyes and skin; 40 mg/kg fed to a dog for two years without abnormalities; no teratogenicity, mutagenicity, carcinogenicity to animals. |
| Albendazole | Rat acute oral LD50 > 4287 mg/kg; rat acute percutaneous LD50 > 608 mg/kg; mouse acute oral LD50 > 17,531 mg/kg. |
| Thiophanate | Mouse acute oral LD50 > 15,000 mg/kg. |
| Thiophanate-methyl | Rat acute oral LD50 > 7500 mg/kg; rat acute percutaneous LD50 > 10,000 mg/kg; mouse acute oral LD50 > 17,531 mg/kg. |
| Fuberidazole | Rat acute oral LD50 > 1100 mg/kg; rat acute percutaneous LD50 > 1000 mg/kg. |
| Methyl (1-{[(5-cyanopentyl)amino]carbonyl}-1H-benzimidazol-2-yl) carbamate | Rat acute oral LD50 >2500 mg/kg; rabbit acute oral LD50 >1000 mg/kg; dog acute oral LD50 > 500 mg/kg. |
| Carbendazim salicylate | Rat acute oral LD50 > 500 mg/kg. |
| 2-aminobenzimidazole | Rat acute oral LD50 > 500 mg/kg. |
| Compounds | China’s ADI (mg/kg bw) | China’s MRL (mg/kg) | Korea’s ADI (mg/kg bw) | Korea’s MRL (mg/kg) | EU’s MRL (mg/kg) |
|---|---|---|---|---|---|
| Benomyl | 0.1 | 0.5–5 | 0.1 | 0.01–20 * | 0.05–2 |
| Carbendazim | 0.03 | 0.02–20 | 0.03 | 0.01–20 * | 0.05–2 |
| Thiabendazole | 0.1 | 0.05–15 | 0.1 | 0.2–40 | 0.01–20 |
| Albendazole | 0.05 | 0.1–5 | / | / | / |
| Thiophanate | / | / | / | / | / |
| Thiophanate-methyl | 0.09 | 0.1–20 | 0.08 | 0.01–20 * | 0.05–6 |
| Fuberidazole | / | / | / | / | 0.01–0.05 |
| Methyl (1-{[(5-cyanopentyl)amino]carbonyl}-1H-benzimidazol-2-yl) carbamate | / | / | / | / | / |
| Carbendazim salicylate | / | / | / | / | / |
| 2-Aminobenzimidazole | 0.1 | 0.5–5 | 0.1 | 0.01–20 | 0.05–2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, S.; Zhang, M.; Tang, S.; Li, M.; Wu, R.; Wan, S.; Chen, L.; Wei, X.; Li, F. Research Progress on Benzimidazole Fungicides: A Review. Molecules 2024, 29, 1218. https://doi.org/10.3390/molecules29061218
Bai S, Zhang M, Tang S, Li M, Wu R, Wan S, Chen L, Wei X, Li F. Research Progress on Benzimidazole Fungicides: A Review. Molecules. 2024; 29(6):1218. https://doi.org/10.3390/molecules29061218
Chicago/Turabian StyleBai, Song, Miaohe Zhang, Shouying Tang, Miao Li, Rong Wu, Suran Wan, Lijun Chen, Xian Wei, and Feifei Li. 2024. "Research Progress on Benzimidazole Fungicides: A Review" Molecules 29, no. 6: 1218. https://doi.org/10.3390/molecules29061218
APA StyleBai, S., Zhang, M., Tang, S., Li, M., Wu, R., Wan, S., Chen, L., Wei, X., & Li, F. (2024). Research Progress on Benzimidazole Fungicides: A Review. Molecules, 29(6), 1218. https://doi.org/10.3390/molecules29061218







