Characterization of the Volatile Constituents of Plai (Zingiber purpureum) by Gas Chromatography–Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Chemicals
3.4. Extraction and Isolation
3.4.1. 3,4-Dimethoxybenzaldehyde (12)
3.4.2. (E)-1-(3′,4′-Dimethoxyphenyl)but-1-ene (14)
3.4.3. (E)-1-(3′,4′-Dimethoxyphenyl)buta-1,3-diene (15)
3.4.4. (E)-1-(2′,4′,5′-Trimethoxyphenyl)but-1-ene (17)
3.4.5. (E)-3-(3′,4′-Dimethoxyphenyl)propenal (18)
3.4.6. (E)-1-(2′,4′,5′-Trimethoxyphenyl)buta-1,3-diene (19)
3.4.7. (E)-4-(3′,4′-Dimethoxyphenyl)but-3-en-1-ol (20)
3.4.8. Cassumunol H (21)
3.4.9. (E)-4-(3′,4′-Dimethoxyphenyl)but-3-en-1-yl acetate (22)
3.4.10. cis-Banglene (23)
3.4.11. trans-Banglene (24)
3.4.12. 2′-Methoxy cis-banglene (25)
3.4.13. 2′-Methoxy trans-banglene (26)
3.4.14. 2‴-Methoxy cis-banglene (27)
3.4.15. 2‴-Methoxy trans-banglene (28)
3.4.16. 2′, 2‴-Dimethoxy cis-banglene (29)
3.4.17. 2′, 2‴-Dimethoxy trans-banglene (30)
3.5. GC–MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waris, M.; Koçak, E.; Gonulalan, E.M.; Demirezer, L.O.; Kır, S.; Nemutlu, E. Metabolomics Analysis Insight into Medicinal Plant Science. Trends Analyt. Chem. 2022, 157, 116795. [Google Scholar] [CrossRef]
- Okada, T.; Afendi, F.M.; Altaf-Ul-Amin, M.; Takahashi, H.; Nakamura, K.; Kanaya, S. Metabolomics of Medicinal Plants: The Importance of Multivariate Analysis of Analytical Chemistry Data. Curr. Comput. Aided Drug Des. 2010, 6, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Creek, D.J.; Dunn, W.B.; Fiehn, O.; Griffin, J.L.; Hall, R.D.; Lei, Z.; Mistrik, R.; Neumann, S.; Schymanski, E.L.; Sumner, L.W.; et al. Metabolite Identification: Are You Sure? And How Do Your Peers Gauge Your Confidence? Metabolomics 2014, 10, 350–353. [Google Scholar] [CrossRef]
- Dunn, W.B.; Erban, A.; Weber, R.J.M.; Creek, D.J.; Brown, M.; Breitling, R.; Hankemeier, T.; Goodacre, R.; Neumann, S.; Kopka, J.; et al. Mass Appeal: Metabolite Identification in Mass Spectrometry-Focused Untargeted Metabolomics. Metabolomics 2013, 9, 44–66. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.; Wei, X.; Li, X.; Wei, X.; Wu, S.; Huang, W.; Koidis, A.; Xu, Z.; Lei, H. Untargeted Metabolomics by Liquid Chromatography-Mass Spectrometry for Food Authentication: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2455–2488. [Google Scholar] [CrossRef] [PubMed]
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software Tools and Approaches for Compound Identification of LC-MS/MS Data in Metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Salek, R.M.; Steinbeck, C.; Viant, M.R.; Goodacre, R.; Dunn, W.B. The Role of Reporting Standards for Metabolite Annotation and Identification in Metabolomic Studies. Gigascience 2013, 2, 13. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Cham, Switzerland, 2016; pp. 443–468. ISBN 978-3-319-26065-5. [Google Scholar] [CrossRef]
- Chongmelaxme, B.; Sruamsiri, R.; Dilokthornsakul, P.; Dhippayom, T.; Kongkaew, C.; Saokaew, S.; Chuthaputti, A.; Chaiyakunapruk, N. Clinical Effects of Zingiber cassumunar (Plai): A Systematic Review. Complement. Ther. Med. 2017, 35, 70–77. [Google Scholar] [CrossRef]
- Ardiyani, M.; Senjaya, S.K.; Maruzy, A.; Widiyastuti, Y.; Sulistyaningsih, L.D.; Susila. Genetic Diversity of ‘Bangle’ (Zingiber montanum (J. Koenig) Link Ex A. Dietr.) Inferred from Sequence-Related Amplified Polymorphism Markers. Agric. Nat. Resour. 2021, 55, 105–112. [Google Scholar] [CrossRef]
- Bai, L.; Maslin, B.R.; Triboun, P.; Xia, N.; Leong-Škorničková, J. Unravelling the Identity and Nomenclatural History of Zingiber montanum, and Establishing Z. purpureum as the Correct Name for Cassumunar Ginger. Taxon 2019, 68, 1334–1349. [Google Scholar] [CrossRef]
- Singh, C.; Manglembi, N.; Swapana, N.; Chanu, S. Ethnobotany, Phytochemistry and Pharmacology of Zingiber cassumunar Roxb. (Zingiberaceae). J. Pharmacogn. Phytochem. 2015, 4, 1–6. [Google Scholar]
- Bureau of Drug and Narcotic, Department of Medicine Sciences, Ministry of Public Health. Thai Herbal Pharmacopoeia; Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public Health: Bangkok, Thailand, 2021; pp. 560–567. Available online: https://bdn.go.th/thp/home (accessed on 6 March 2024).
- Leelarungrayub, J.; Manorsoi, J.; Manorsoi, A. Anti-Inflammatory Activity of Niosomes Entrapped with Plai Oil (Zingiber cassumunar Roxb.) by Therapeutic Ultrasound in a Rat Model. Int. J. Nanomed. 2017, 12, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Purwanto, U.R.E.; Sholikhah, M.; Munisih, S. Formulation and Physical Characterization of Essential Oil Bangle (Zingiber cassumunar Roxb.) Nanoemulsion Gel. J. Sci. Technol. Res. Pharm. 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Ozaki, Y.; Kawahara, N.; Harada, M. Anti-Inflammatory Effect of Zingiber cassumunar ROXB. and Its Active Principles. Chem. Pharm. Bull. 1991, 39, 2353–2356. [Google Scholar] [CrossRef]
- Han, A.; Kim, H.; Piao, D.; Jung, C.; Seo, E.K. Phytochemicals and Bioactivities of Zingiber cassumunar Roxb. Molecules 2021, 26, 2377. [Google Scholar] [CrossRef]
- Seaho, B.; Lekwongphaiboon, C.; Inthakusol, W.; Prateeptongkum, S.; Harnying, W.; Berkessel, A.; Duangdee, N. NMR-Based Stability Evaluation of (E)-1-(3′,4′-Dimethoxyphenyl)Butadiene (DMPBD) from Zingiber cassumunar Roxb. Rhizome. Phytochem. Anal. 2023. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.S.; Joshi, N.; Padalia, R.C.; Singh, V.R.; Goswami, P.; Verma, S.K.; Iqbal, H.; Chanda, D.; Verma, R.K.; Darokar, M.P.; et al. Chemical Composition and Antibacterial, Antifungal, Allelopathic and Acetylcholinesterase Inhibitory Activities of Cassumunar-Ginger. J. Sci. Food Agric. 2018, 98, 321–327. [Google Scholar] [CrossRef]
- Khruengsai, S.; Sripahco, T.; Pripdeevech, P. Antibacterial Activity and Synergic Effects of the Essential Oils of Amomum verum Blackw and Zanthoxylum limonella (Dennst.) Alston. Arch. Microbiol. 2023, 205, 102. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Anuchapreeda, S.; Leelapornpisid, P.; Phongpradist, R.; Viernstein, H.; Mueller, M. Development of Microemulsion Delivery System of Essential Oil from Zingiber cassumunar Roxb. Rhizome for Improvement of Stability and Anti-Inflammatory Activity. AAPS Pharm. Sci. Tech. 2017, 18, 1332–1342. [Google Scholar] [CrossRef]
- Mahayothee, B.; Thamsala, T.; Khuwijitjaru, P.; Janjai, S. Effect of Drying Temperature and Drying Method on Drying Rate and Bioactive Compounds in Cassumunar Ginger (Zingiber montanum). J. Appl. Res. Med. Aromat. Plants 2020, 18, 100262. [Google Scholar] [CrossRef]
- Sukatta, U.; Rugthaworn, P.; Punjee, P.; Chidchenchey, S.; Keeratinijakal, V. Chemical Composition and Physical Properties of Oil from Plai (Zingiber cassumunar Roxb.) Obtained by Hydro Distillation and Hexane Extraction. Agric. Nat. Resour. 2009, 43, 212–217. [Google Scholar]
- Wang, C.; Zhang, Y.; Ding, H.; Song, M.; Yin, J.; Yu, H.; Li, Z.; Han, L.; Zhang, Z. Authentication of Zingiber Species Based on Analysis of Metabolite Profiles. Front. Plant Sci. 2021, 12, 705446. [Google Scholar] [CrossRef]
- Bora, P.K.; Saikia, J.; Kemprai, P.; Saikia, S.P.; Banik, D.; Haldar, S. Evaluation of Postharvest Drying, Key Odorants, and Phytotoxins in Plai (Zingiber montanum) Essential Oil. J. Agric. Food Chem. 2021, 69, 5500–5509. [Google Scholar] [CrossRef]
- Truong, V.; Manochai, B.; Pham, T.; Jeong, W. Antioxidant and Anti-Inflammatory Activities of Zingiber montanum Oil in HepG2 Cells and Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. J. Med. Food 2021, 24, 595–605. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.I.; Chowdhury, J.U.; Jaripa, B. Volatile Constituents of Essential Oils Isolated from Leaf and Rhizome of Zingiber cassumunar Roxb. Bangladesh J. Pharmacol. 2008, 3, 69–73. [Google Scholar] [CrossRef]
- Bordoloi, A.K.; Sperkova, J.; Leclercq, P.A. Essential Oils of Zingiber cassumunar Roxb. from Northeast India. J. Essent. Oil Res. 1999, 11, 441–445. [Google Scholar] [CrossRef]
- Thepthong, P.; Rattakarn, K.; Ritchaiyaphum, N.; Intachai, S.; Chanasit, W. Effect of Extraction Solvents on Antioxidant and Antibacterial Activity of Zingiber montanum Rhizomes. ASEAN J. Sci. Technol. Rep. 2023, 26, 1–9. [Google Scholar] [CrossRef]
- Mektrirat, R.; Yano, T.; Okonogi, S.; Katip, W.; Pikulkaew, S. Phytochemical and Safety Evaluations of Volatile Terpenoids from Zingiber cassumunar Roxb. on Mature Carp Peripheral Blood Mononuclear Cells and Embryonic Zebrafish. Molecules 2020, 25, 613. [Google Scholar] [CrossRef] [PubMed]
- Risnawati, E.; Ainurofiq, A.; Wartono, W.M. Study of Antibacterial Activity and Identification of the Most Active Fraction from Ethanol Extraction of Zingiber cassumunar Roxb. Rhizomes by Vacuum Liquid Chromatography. J. Chem. Pharm. Res. 2014, 6, 101–107. [Google Scholar]
- Aji, N.; Kumala, S.; Mumpuni, E.; Rahmat, D. Antibacterial Activity and Active Fraction of Zingiber officinale Roscoe, Zingiber montanum (J. Koenig) Link Ex A., and Zingiber zzerumbet (L.) Roscoe Ex Sm. Against Propionibacterium Acnes. Pharmacogn. J. 2022, 14, 103–111. [Google Scholar] [CrossRef]
- Nishidono, Y.; Fujita, T.; Kawanami, A.; Nishizawa, M.; Tanaka, K. Identification of PGC-1α Activating Constituents in Zingiberaceous Crude Drugs. Fitoterapia 2017, 122, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Manaprasersak, A.; Karpkird, T. Selectivity Encapsulation of Zingiber cassumunar Roxb. Oil by Beta-Cyclodextrin Derivatives. Mater. Today Proc. 2020, 23, 659–665. [Google Scholar] [CrossRef]
- Septama, W.A.; Chiara, M.A.; Turnip, G.; Tasfiyati, A.N.; Dewi, R.T.; Sianipar, E.A.; Jaisi, A. Essential Oil of Zingiber cassumunar Roxb. and Zingiber officinale Rosc.: A Comparative Study on Chemical Constituents, Antibacterial Activity, Biofilm Formation, and Inhibition of Pseudomonas Aeruginosa Quorum Sensing System. Chem. Biodivers. 2023, 20, e202201205. [Google Scholar] [CrossRef]
- Taroeno; Brophy, J.J.; Zwaving, J.H. Analysis of the Essential Oil of Zingiber cassumunar Roxb. from Indonesia. Flavour Fragr. J. 1991, 6, 161–163. [Google Scholar] [CrossRef]
- Thongphasuk, P.; Thongphasuk, J.; Bavovada, R.; Chamulitrat, W. Effects of Gamma Irradiation on Active Components, Free Radicals and Toxicity of Cassumunar Ginger Rhizomes. Int. J. Pharm. Pharm. Sci. 2014, 6, 432–436. [Google Scholar]
- Bua-in, S.; Paisooksantivatana, Y. Essential Oil and Antioxidant Activity of Cassumunar Ginger (Zingiberaceae: Zingiber montanum (Koenig) Link Ex Dietr.) Collected from Various Parts of Thailand. Agric. Nat. Resour. 2009, 43, 467–475. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 978-1932633214. [Google Scholar]
- Gavara, L.; Boisse, T.; Hénichart, J.; Daïch, A.; Rigo, B.; Gautret, P. Toward New Camptothecins. Part 6: Synthesis of Crucial Ketones and Their Use in Friedländer Reaction. Tetrahedron 2010, 66, 7544–7561. [Google Scholar] [CrossRef]
- Galopin, C.C. A Short and Efficient Synthesis of (±)-trans-Sabinene Hydrate. Tetrahedron Lett. 2001, 42, 5589–5591. [Google Scholar] [CrossRef]
- Cheng, D.; Knox, K.R.; Cohen, T. Tandem Lithium-Ene Cyclization and Thiophenoxide Expulsion to Produce Fused Vinylcyclopropanes: First Observation of Allylic Lithium Oxyanion- Induced Reactivity and Stereoselectivity in Intramolecular Carbolithiation. J. Am. Chem. Soc. 2000, 122, 412–413. [Google Scholar] [CrossRef]
- Adams, R.P.; Weyerstahl, P. cis- and trans-Sabinene Hydrate: Comparisons of Quadrupole and Ion Trap Mass Spectra. J. Essent. Oil Res. 1992, 4, 197–200. [Google Scholar] [CrossRef]
- Mladenović, M.Z.; Radulović, N.S. The Essential Oil of Achillea ageratifolia (Sm.) Boiss. subsp. serbica (Nyman) Heimerl (Asteraceae) Revisited: The Stereochemical Nomenclature Issues, Structural Elucidation and Synthesis of (New) Sabinyl Esters. Flavour Fragr. J. 2017, 32, 5–23. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Fukushima, S.; Yoshihira, K.; Natori, S.; Dechatiwongse, T.; Mihashi, K.; Nishi, M.; Hara, S. Further Characterization of the Constituents of a Thai Medicinal Plant, Zingiber cassumunar ROXB. Chem. Pharm. Bull. 1980, 28, 2948–2959. [Google Scholar] [CrossRef]
- Tangyuenyongwatana, P.; Gritsanapan, W. A Study on Artifacts Formation in the Thai Traditional Medicine Prasaplai. Planta Med. 2008, 74, 1403–1405. [Google Scholar] [CrossRef]
- Joshi, B.P.; Singh, N.P.; Sharma, A.; Shinha, A.K. Microwave-Assisted Minutes Synthesis of Bioactive Phenylbutanoids Occurring in Zingiber cassumunar. Chem. Nat. Compd. 2005, 41, 370–373. [Google Scholar] [CrossRef]
- Paolini, J.; Tomi, P.; Bernardini, A.; Bradesi, P.; Casanova, J.; Kaloustian, J. Detailed Analysis of the Essential Oil from Cistus albidus L. by Combination of GC/RI, GC/MS and 13C-NMR Spectroscopy. Nat. Prod. Res. 2008, 22, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Cheah, Y.H.; Nordin, F.J.; Sarip, R.; Tee, T.T.; Azimahtol, H.L.P.; Sirat, H.M.; Rashid, B.A.A.; Abdullah, N.R.; Ismail, Z. Combined Xanthorrhizol-Curcumin Exhibits Synergistic Growth Inhibitory Activity via Apoptosis Induction in Human Breast Cancer Cells MDA-MB-231. Cancer Cell Int. 2009, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Proença, C.; Albuquerque, H.M.T.; Ribeiro, D.; Freitas, M.; Santos, C.M.M.; Silva, A.M.S.; Fernandes, E. Novel Chromone and Xanthone Derivatives: Synthesis and ROS/RNS Scavenging Activities. Eur. J. Med. Chem. 2016, 115, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Jitoe, A. Phenylbutenoid Monomers from the Rhizomes of Zingiber cassumunar. Phytochemistry 1995, 39, 459–461. [Google Scholar] [CrossRef]
- Matsuda, H.; Nakamura, S.; Iwami, J.; Li, X.; Pongpiriyadacha, Y.; Nakai, M.; Kubo, M.; Fukuyama, Y.; Yoshikawa, M. Invasion Inhibitors of Human Fibrosarcoma HT 1080 Cells from the Rhizomes of Zingiber cassumunar: Structures of Phenylbutanoids, Cassumunols. Chem. Pharm. Bull. 2011, 59, 365–370. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Masuda, T.; Andoh, T.; Yonemori, S.; Takeda, Y. Phenylbutenoids from the Rhizomes of Alpinia flabellata. Phytochemistry 1999, 50, 163–166. [Google Scholar] [CrossRef]
- Jitoe, A.; Masuda, T.; Nakatani, N. Phenylbutenoid Dimers from the Rhizomes of Zingiber cassumunar. Phytochemistry 1993, 32, 357–363. [Google Scholar] [CrossRef]
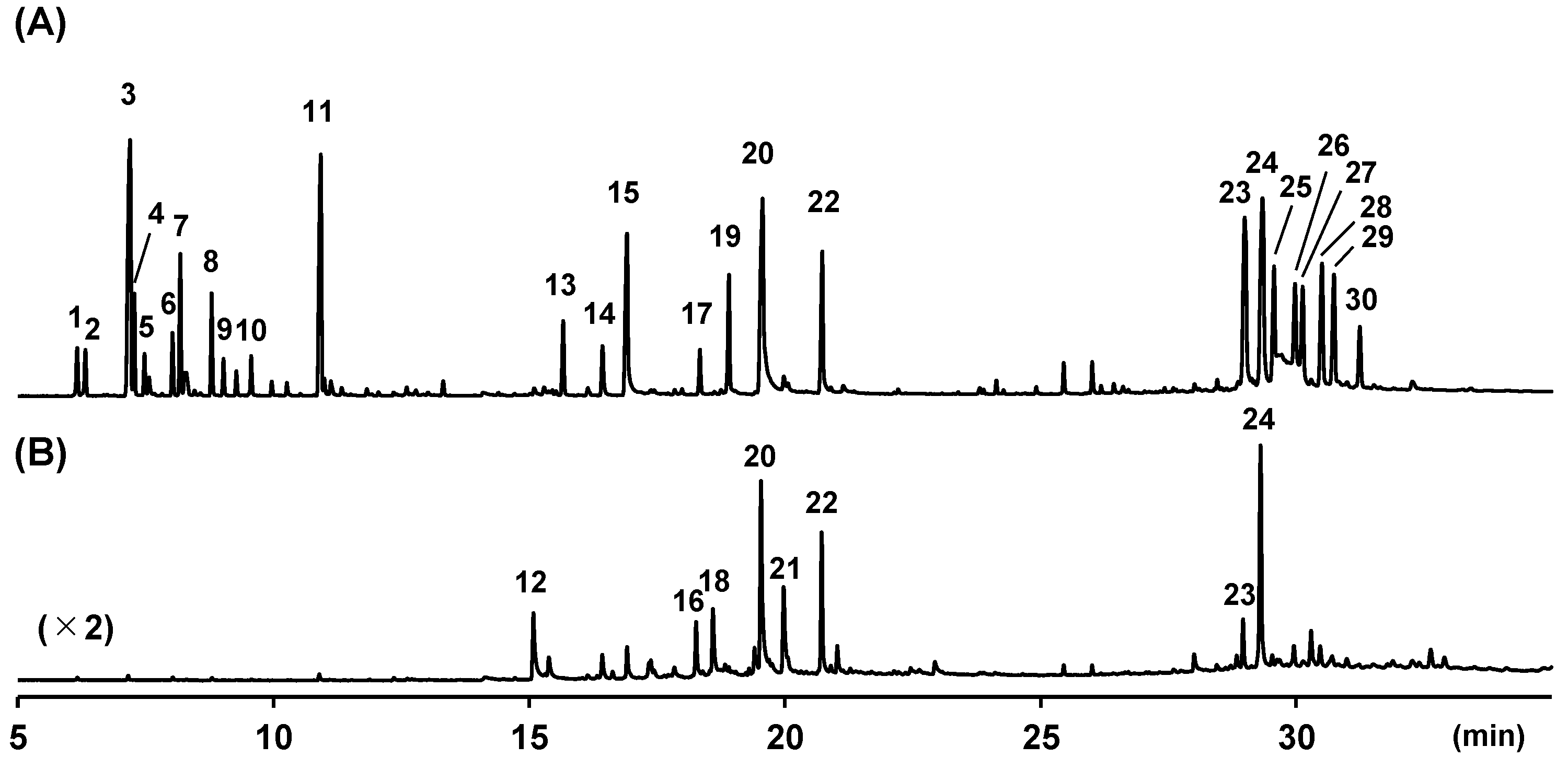
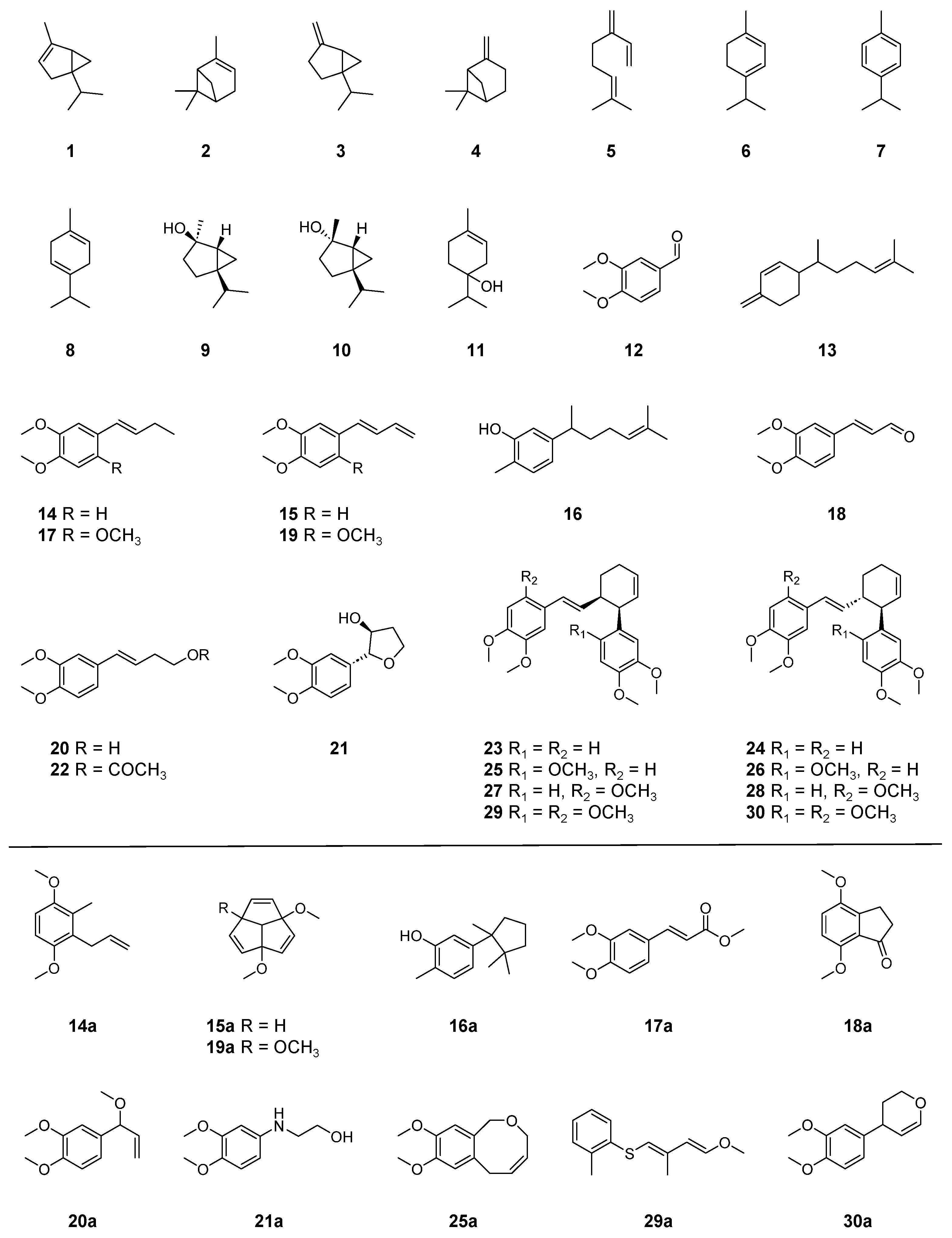
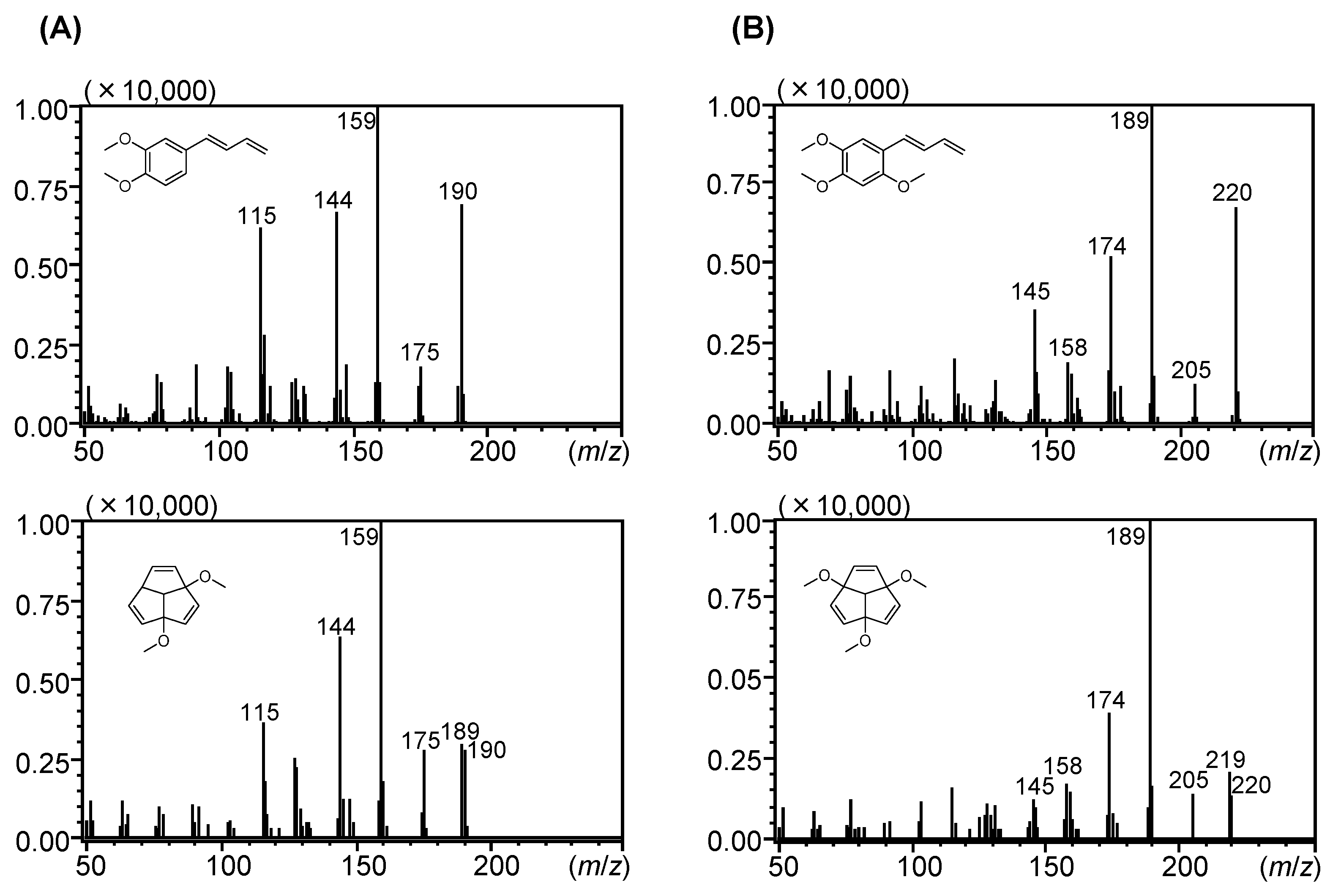
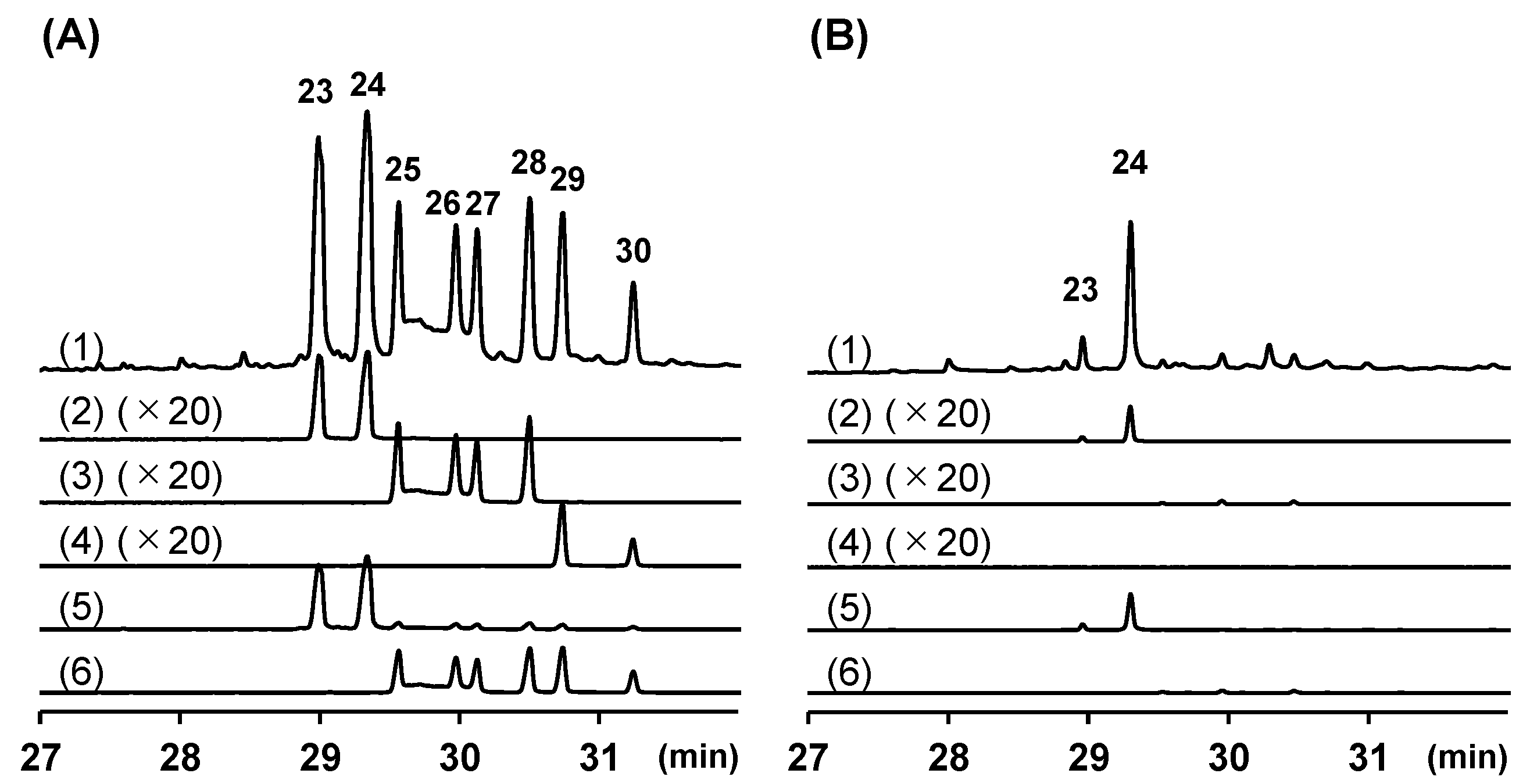
| Peak | RI | Compounds Annotated via Library Search | Similarity | Compounds Annotated or Identified via This Study | MSI 1 |
|---|---|---|---|---|---|
| 1 | 931 | α-Thujene (1) | 95 | α-Thujene (1) | 2 |
| 2 | 939 | α-Pinene (2) | 98 | α-Pinene (2) | 2 |
| 3 | 979 | Sabinene (3) | 96 | Sabinene (3) | 1 |
| 4 | 982 | β-Pinene (4) | 98 | β-Pinene (4) | 2 |
| 5 | 991 | Myrcene (5) | 97 | Myrcene (5) | 2 |
| 6 | 1019 | α-Terpinene (6) | 96 | α-Terpinene (6) | 2 |
| 7 | 1028 | p-Cymene (7) | 97 | p-Cymene (7) | 2 |
| 8 | 1063 | γ-Terpinene (8) | 98 | γ-Terpinene (8) | 2 |
| 9 | 1075 | 4-Thujanol (9 or 10) | 97 | trans-4-Thujanol (9) | 1 |
| 10 | 1103 | 4-Thujanol (9 or 10) | 96 | cis-4-Thujanol (10) | 2 |
| 11 | 1188 | Terpinen-4-ol (11) | 96 | Terpinen-4-ol (11) | 1 |
| 12 | 1483 | 3,4-Dimethoxybenzaldehyde (12) | 93 | 3,4-Dimethoxybenzaldehyde (12) | 0 |
| 13 | 1530 | β-Sesquiphellandrene (13) | 93 | β-Sesquiphellandrene (13) | 2 |
| 14 | 1592 | 1,4-Dimethoxy-2-methyl-3-(2-propen-1-yl)benzene (14a) | 86 | (E)-1-(3′,4′-Dimethoxyphenyl)but-1-ene (14) | 0 |
| 15 | 1633 | 1,4-Dimethoxytriquinacene (15a) | 86 | (E)-1-(3′,4′-Dimethoxyphenyl)buta-1,3-diene (15) | 0 |
| 16 | 1752 | δ-Cuparenol (16a) | 80 | Xanthorrhizol (16) | 1 |
| 17 | 1759 | Methyl 3,4-dimethoxycinnamate (17a) | 72 | (E)-1-(2′,4′,5′-Trimethoxyphenyl)but-1-ene (17) | 0 |
| 18 | 1782 | 4,7-Dimethoxy-1-indanone (18a) | 78 | (E)-3-(3′,4′-Dimethoxyphenyl)propenal (18) | 0 |
| 19 | 1811 | 1,4,7-Trimethoxytriquinacene (19a) | 80 | (E)-1-(2′,4′,5′-Trimethoxyphenyl)buta-1,3-diene (19) | 0 |
| 20 | 1872 | 1,2-Dimethoxy-4-(1-methoxy-2-propen-1-yl)benzene (20a) | 76 | (E)-4-(3′,4′-Dimethoxyphenyl)but-3-en-1-ol (20) | 0 |
| 21 | 1914 | 2-[(3,4-Dimethoxyphenyl)amino]ethanol (21a) | 73 | Cassumunol H (21) | 0 |
| 22 | 1988 | 1,4-Dimethoxytriquinacene (15a) | 76 | (E)-4-(3′,4′-Dimethoxyphenyl)but-3-en-1-yl acetate (22) | 0 |
| 23 | 3007 | (E)-1-(3′,4′-Dimethoxyphenyl)buta-1,3-diene (15) | 76 | cis-Banglene (23) | 0 |
| 24 | 3048 | (E)-1-(3′,4′-Dimethoxyphenyl)buta-1,3-diene (15) | 76 | trans-Banglene (24) | 0 |
| 25 | 3074 | 3,6-Dihydro-8,9-dimethoxy-1H-2-benzoxocin (25a) | 73 | 2′-Methoxy cis-banglene (25) | 0 |
| 26 | 3120 | 3,6-Dihydro-8,9-dimethoxy-1H-2-benzoxocin (25a) | 72 | 2′-Methoxy trans-banglene (26) | 0 |
| 27 | 3134 | 3,6-Dihydro-8,9-dimethoxy-1H-2-benzoxocin (25a) | 72 | 2‴-Methoxy cis-banglene (27) | 0 |
| 28 | 3172 | 3,6-Dihydro-8,9-dimethoxy-1H-2-benzoxocin (25a) | 74 | 2‴-Methoxy trans-banglene (28) | 0 |
| 29 | 3195 | 1-[[(1E,3E)-4-Methoxy-2-methyl-1,3-butadien-1-yl]thio]-2-methylbenzene (29a) | 75 | 2′, 2‴-Dimethoxy cis-banglene (29) | 0 |
| 30 | 3239 | 4-(3,4-Dimethoxyphenyl)-3,4-dihydro-2H-pyran (30a) | 73 | 2′, 2‴-Dimethoxy trans-banglene (30) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishidono, Y.; Saifudin, A.; Tanaka, K. Characterization of the Volatile Constituents of Plai (Zingiber purpureum) by Gas Chromatography–Mass Spectrometry. Molecules 2024, 29, 1216. https://doi.org/10.3390/molecules29061216
Nishidono Y, Saifudin A, Tanaka K. Characterization of the Volatile Constituents of Plai (Zingiber purpureum) by Gas Chromatography–Mass Spectrometry. Molecules. 2024; 29(6):1216. https://doi.org/10.3390/molecules29061216
Chicago/Turabian StyleNishidono, Yuto, Azis Saifudin, and Ken Tanaka. 2024. "Characterization of the Volatile Constituents of Plai (Zingiber purpureum) by Gas Chromatography–Mass Spectrometry" Molecules 29, no. 6: 1216. https://doi.org/10.3390/molecules29061216
APA StyleNishidono, Y., Saifudin, A., & Tanaka, K. (2024). Characterization of the Volatile Constituents of Plai (Zingiber purpureum) by Gas Chromatography–Mass Spectrometry. Molecules, 29(6), 1216. https://doi.org/10.3390/molecules29061216






