tert-Butyl Nitrite-Induced Radical Nitrile Oxidation Cycloaddition: Synthesis of Isoxazole/Isoxazoline-Fused Benzo 6/7/8-membered Oxacyclic Ketones
Abstract
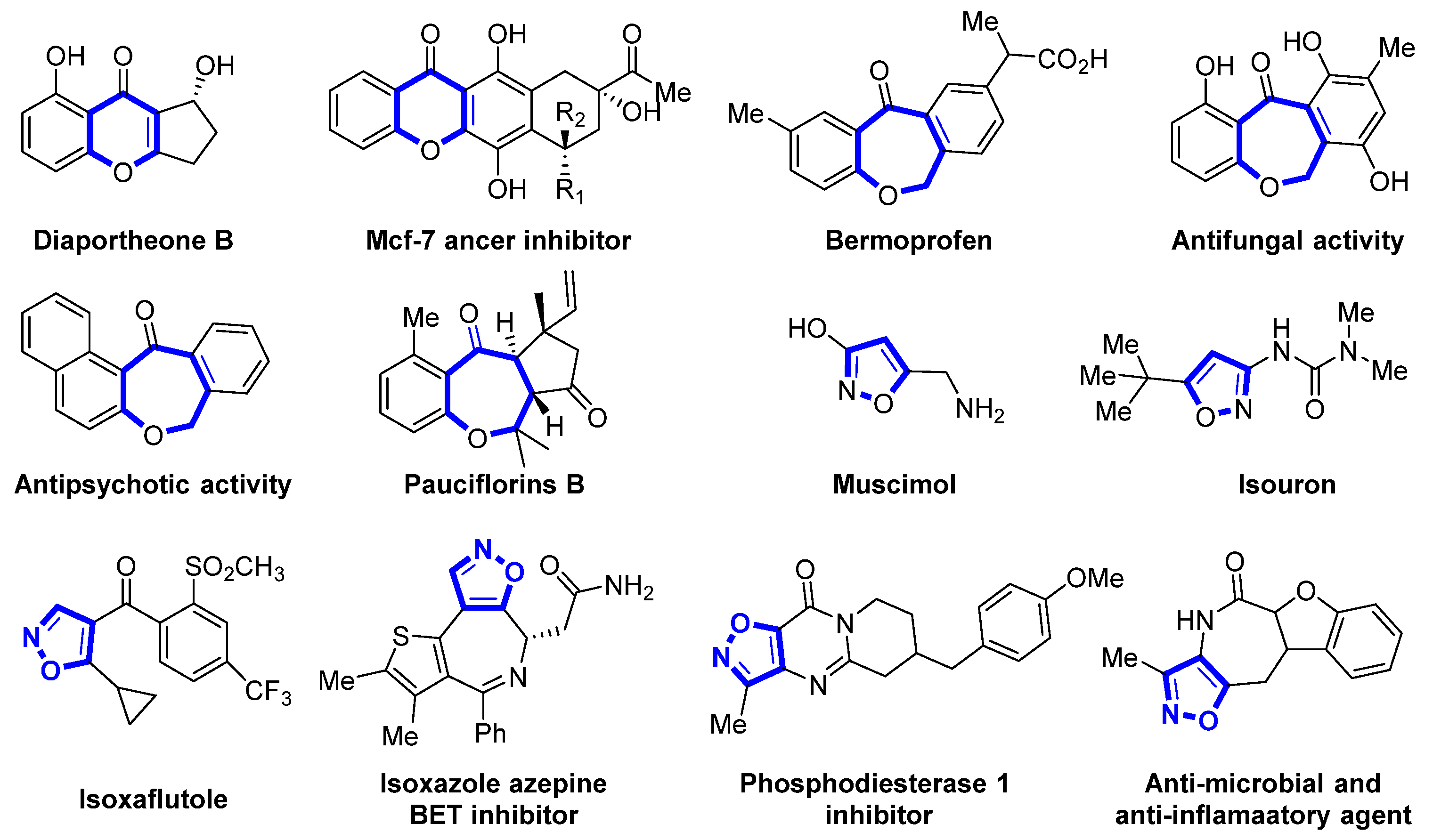
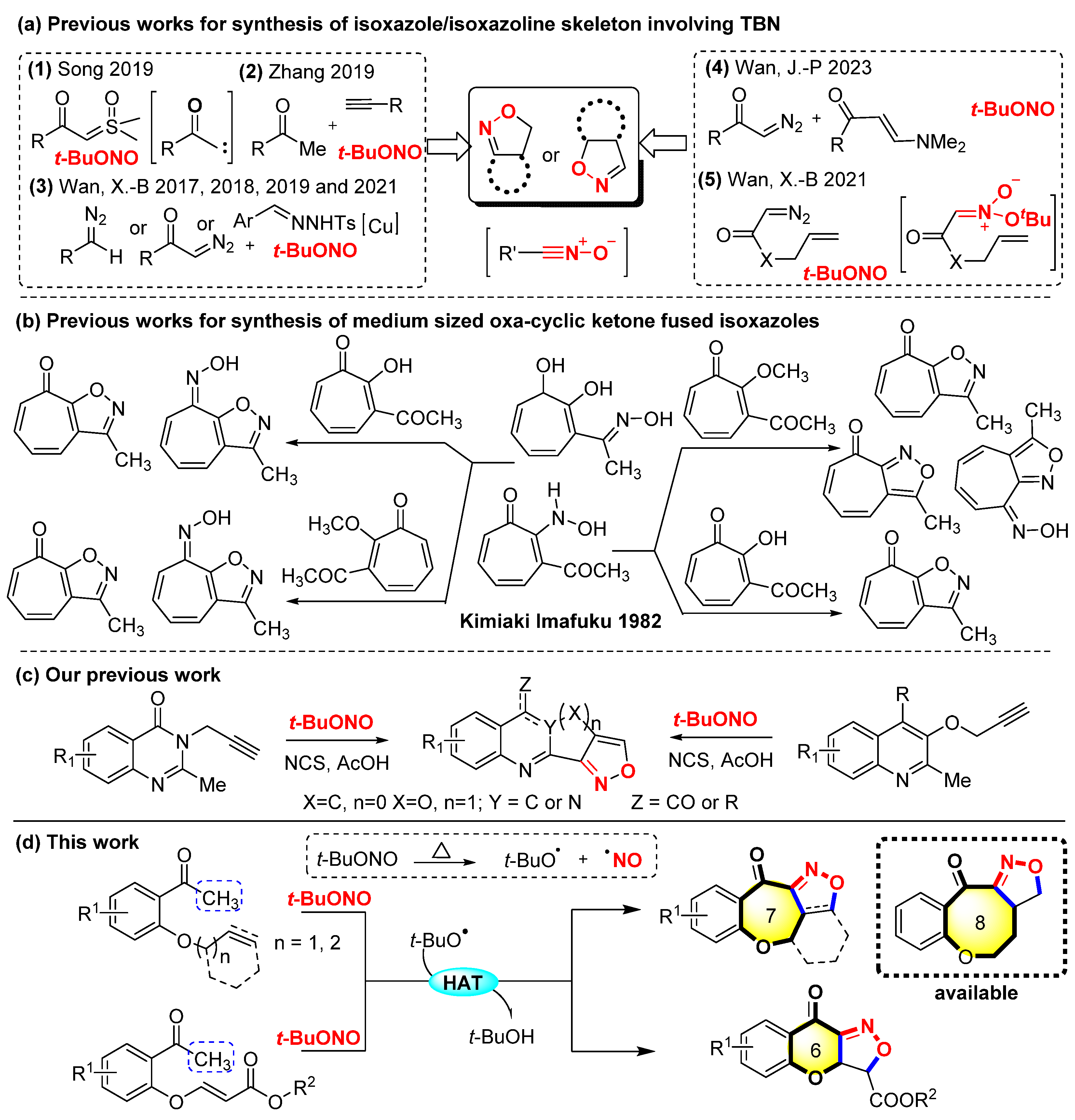
1. Introduction
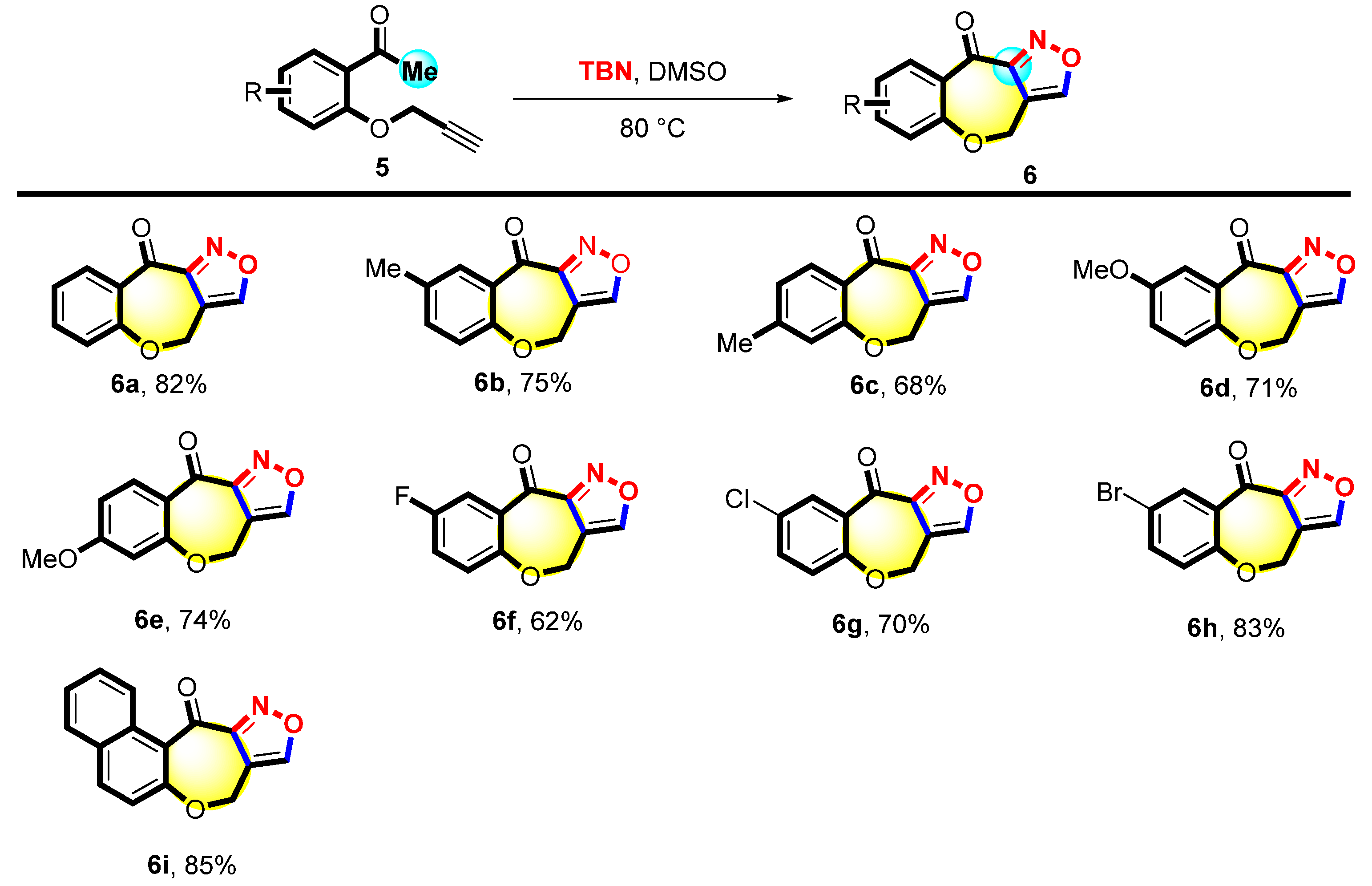
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthetic Procedures
3.3. Characterization of Products
- 2a,2a1,3,4,5,5a-Hexahydro-11H-2,6-dioxa-1-azadibenzo[cd,g]azulen-11-one (2a), 38 mg, 95%, white solid, m.p.: 118–119 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.99 (dd, J = 7.9, 1.8 Hz, 1H), 7.57 (td, J = 7.7, 1.8 Hz, 1H), 7.31–7.21 (m, 1H), 7.12 (dd, J = 8.1, 1.1 Hz, 1H), 4.87 (dt, J = 10.4, 4.4 Hz, 1H), 4.31 (td, J = 6.7, 4.1 Hz, 1H), 3.70 (dd, J = 10.4, 7.2 Hz, 1H), 2.26–2.00 (m, 2H), 1.96–1.80 (m, 2H), 1.79–1.54 (m, 2H). 13C NMR (126 MHz, CDCl3) δ (ppm) 184.9, 160.7, 158.4, 136.1, 129.9, 129.8, 124.9, 123.3, 81.3, 78.0, 49.2, 27.8, 23.7, 14.8. HRMS (ESI): m/z [M + H]+ calcd for C14H14NO3: 244.0968; found: 244.0966.
- 8-Methyl-2a,2a1,3,4,5,5a-hexahydro-11H-2,6-dioxa-1-azadibenzo[cd,g]azulen-11-one (2b), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1–5:1), 31 mg, 87%, light yellow solid, m.p.: 135–136 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.90 (d, J = 8.0 Hz, 1H), 7.05 (dd, J = 8.0, 1.6 Hz, 1H), 6.92 (d, J = 1.6 Hz, 1H), 4.83 (dt, J = 10.3, 4.4 Hz, 1H), 4.27 (ddd, J = 7.1, 5.8, 4.0 Hz, 1H), 3.69 (dd, J = 10.4, 7.1 Hz, 1H), 2.38 (s, 3H), 2.14 (dddd, J = 37.4, 13.8, 10.9, 5.7 Hz, 2H), 1.95–1.75 (m, 3H), 1.74–1.53 (m, 2H). 13C NMR (126 MHz, CDCl3) δ (ppm) 184.5, 161.1, 158.5, 147.9, 129.8, 126.9, 125.9, 123.6, 80.9, 77.8, 49.6, 27.9, 23.9, 21.7, 14.7. HRMS (ESI): m/z [M + H]+ calcd for C15H16NO3: 258.1125; found: 258.1123.
- 9-Chloro-2a,2a1,3,4,5,5a-hexahydro-11H-2,6-dioxa-1-azadibenzo[cd,g]azulen-11-one (2c), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1–5:1), 24 mg, 85%, light brown solid, m.p.: 135–136 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.89 (d, J = 2.7 Hz, 1H), 7.48 (dd, J = 8.6, 2.6 Hz, 1H), 7.06 (d, J = 8.6 Hz, 1H), 4.86 (dt, J = 10.3, 4.4 Hz, 1H), 4.29 (td, J = 6.7, 4.1 Hz, 1H), 3.70 (dd, J = 10.4, 7.2 Hz, 1H), 2.23–1.97 (m, 2H), 1.84 (ddq, J = 23.1, 9.2, 4.5, 3.9 Hz, 2H), 1.77–1.54 (m, 2H). 13C NMR (126 MHz, CDCl3) δ (ppm) 183.6, 158.9, 157.9, 135.7, 130.7, 130.5, 129.2, 125.0, 81.6, 78.4, 48.9, 27.6, 23.5, 14.7. HRMS (ESI): m/z [M + Na]+ calcd for C14H12ClNO3Na: 300.0398; found: 300.0398.
- 8-Chloro-2a,2a1,3,4,5,5a-hexahydro-11H-2,6-dioxa-1-azadibenzo[cd,g]azulen-11-one (2d), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1–5:1), 24 mg, 79%, white solid, m.p.: 179–181 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.94 (d, J = 8.5 Hz, 1H), 7.24 (dd, J = 8.5, 2.0 Hz, 1H), 7.15 (d, J = 2.0 Hz, 1H), 4.87 (dt, J = 10.5, 4.4 Hz, 1H), 4.33 (ddd, J = 7.1, 6.1, 4.0 Hz, 1H), 3.71 (dd, J = 10.4, 7.1 Hz, 1H), 2.2 –2.04 (m, 2H), 1.93–1.79 (m, 2H), 1.78–1.60 (m, 2H). 13C NMR (101 MHz, CDCl3) δ (ppm) 183.6, 161.2, 158.0, 141.9, 131.0, 128.1, 125.5, 123.7, 81.3, 78.5, 49.3, 27.7, 23.7, 14.7. HRMS (ESI): m/z [M + H]+ calcd for C14H13ClNO3: 278.0578; found: 278.0580.
- 9-Fluoro-2a,2a1,3,4,5,5a-hexahydro-11H-2,6-dioxa-1-azadibenzo[cd,g]azulen-11-one (2e), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1–5:1), 33 mg, 88%, white solid, m.p.: 132–133 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.62 (dd, J = 8.3, 3.2 Hz, 1H), 7.30–7.22 (m, 1H), 7.11 (dd, J = 8.9, 4.4 Hz, 1H), 4.88 (dt, J = 10.4, 4.3 Hz, 1H), 4.29 (td, J = 6.7, 4.0 Hz, 1H), 3.70 (dd, J = 10.4, 7.3 Hz, 1H), 2.23–2.00 (m, 2H), 1.93–1.78 (m, 3H), 1.81–1.54 (m, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 183.9, 159.2 (d, Jc–f = 246.8 Hz), 158.0, 156.7 (d, Jc–f = 2.7 Hz), 130.8 (d, Jc–f = 7.2 Hz), 125.1 (d, Jc–f = 8.1 Hz), 123.0 (d, Jc–f = 23.3 Hz), 115.4 (d, Jc–f = 24.1 Hz), 81.5, 78.3, 48.8, 27.7, 23.6, 14.7. HRMS (ESI): m/z [M + H]+ calcd for C14H13FNO3: 262.0874; found: 262.0873.
- 9-Bromo-2a,2a1,3,4,5,5a-hexahydro-11H-2,6-dioxa-1-azadibenzo[cd,g]azulen-11-one (2f), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1–5:1), 25 mg, 79%, white solid, m.p.: 134–135 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.07 (d, J = 2.6 Hz, 1H), 7.64 (dd, J = 8.6, 2.6 Hz, 1H), 7.01 (d, J = 8.6 Hz, 1H), 4.88 (dt, J = 10.4, 4.4 Hz, 1H), 4.30 (td, J = 6.8, 4.0 Hz, 1H), 3.70 (dd, J = 10.4, 7.2 Hz, 1H), 2.21–2.00 (m, 2H), 1.93–1.80 (m, 2H), 1.79–1.57 (m, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 183.5, 159.4, 157.8, 138.7, 132.4, 131.1, 125.3, 118.0, 81.6, 78.3, 49.0, 27.6, 23.6, 14.8. HRMS (ESI): m/z [M + H]+ calcd for C14H13BrNO3: 322.0073; found: 322.0073.
- 10-Methoxy-2a,2a1,3,4,5,5a-hexahydro-11H-2,6-dioxa-1-azadibenzo[cd,g]azulen-11-one (2g), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1–5:1), 38 mg, 91%, white solid, m.p.: 194–195 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.43 (t, J = 8.3 Hz, 1H), 6.80 (dd, J = 8.5, 0.9 Hz, 1H), 6.73 (dd, J = 8.1, 0.8 Hz, 1H), 4.92 (dt, J = 10.5, 4.4 Hz, 1H), 4.25 (ddd, J = 10.7, 7.6, 4.9 Hz, 1H), 3.86 (s, 3H), 3.64 (dd, J = 10.5, 7.6 Hz, 1H), 2.10–1.96 (m, 2H), 1.95–1.78 (m, 2H), 1.77–1.52 (m, 2H). 13C NMR (126 MHz, CDCl3) δ (ppm) 183.0, 159.2, 158.2, 157.4, 134.6, 122.0, 115.0, 108.7, 83.4, 78.0, 56.3, 47.4, 26.4, 22.5, 16.2. HRMS (ESI): m/z [M + H]+ calcd for C15H16NO4: 274.1074; found: 274.1074.
- 9-Methoxy-2a,2a1,3,4,5,5a-hexahydro-11H-2,6-dioxa-1-azadibenzo[cd,g]azulen-11-one (2h), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1–5:1), 29 mg, 81%, white solid, m.p.: 137–139 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.41 (d, J = 3.2 Hz, 1H), 7.11 (dd, J = 8.8, 3.2 Hz, 1H), 7.04 (d, J = 8.8 Hz, 1H), 4.85 (dt, J = 10.3, 4.3 Hz, 1H), 4.23 (ddd, J = 7.3, 6.1, 4.1 Hz, 1H), 3.83 (s, 3H), 3.66 (dd, J = 10.4, 7.3 Hz, 1H), 2.23–2.01 (m, 2H), 1.93–1.78 (m, 2H), 1.75–1.55 (m, 2H). 13C NMR (101 MHz, CDCl3) δ (ppm) 184.9, 158.4, 156.5, 154.9, 130.0, 124.4, 123.8, 111.2, 81.3, 78.1, 55.8, 49.1, 27.9, 23.7, 14.8. HRMS (ESI): m/z [M + H]+ calcd for C15H16NO4: 274.1074; found: 274.1072.
- 8-Methoxy-2a,2a1,3,4,5,5a-hexahydro-11H-2,6-dioxa-1-azadibenzo[cd,g]azulen-11-one (2i), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1–5:1), 26 mg, 81%, light yellow solid, m.p.: 98–100 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.99 (d, J = 8.8 Hz, 1H), 6.77 (dd, J = 8.9, 2.5 Hz, 1H), 6.56 (d, J = 2.4 Hz, 1H), 4.80 (dt, J = 10.4, 4.5 Hz, 1H), 4.28 (ddd, J = 7.0, 5.0, 3.9 Hz, 1H), 3.85 (s, 3H), 3.70 (dd, J = 10.4, 7.0 Hz, 1H), 2.16 (ddq, J = 18.8, 14.5, 4.9 Hz, 2H), 1.92–1.73 (m, 2H), 1.72–1.56 (m, 2H). 13C NMR (126 MHz, CDCl3) δ (ppm) 183.3, 166.2, 163.7, 158.6, 131.6, 122.3, 111.9, 107.1, 80.3, 77.9, 55.8, 50.0, 28.1, 24.1, 14.4. HRMS (ESI): m/z [M + H]+ calcd for C15H16NO4: 274.1074; found: 274.1074.
- 2a,2a1,3,4,5,5a-Hexahydro-13H-2,6-dioxa-1-azabenzo[cd]naphtho[2,1-g]azulen-13-one (2j), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1–5:1), 29 mg, 86%, brown solid, m.p.: 147–150 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.65 (d, J = 8.7 Hz, 1H), 7.99 (d, J = 8.8 Hz, 1H), 7.82 (dd, J = 8.2, 1.3 Hz, 1H), 7.60 (ddd, J = 8.5, 6.7, 1.4 Hz, 1H), 7.49 (ddd, J = 8.0, 6.8, 1.2 Hz, 1H), 7.23 (d, J = 8.8 Hz, 1H), 4.91 (dt, J = 10.5, 4.2 Hz, 1H), 4.34 (ddd, J = 9.0, 7.6, 4.8 Hz, 1H), 3.63 (dd, J = 10.5, 7.6 Hz, 1H), 2.07 (dddd, J = 26.6, 13.3, 10.1, 5.4 Hz, 2H), 1.91 (dddd, J = 15.2, 13.3, 7.4, 3.8 Hz, 2H), 1.85–1.54 (m, 2H). 13C NMR (126 MHz, CDCl3) δ (ppm) 186.1, 158.3, 158.0, 136.0, 131.3, 131.0, 129.0, 128.3, 126.0, 126.0, 124.9, 122.0, 83.1, 78.0, 47.5, 26.9, 22.7, 15.9. HRMS (ESI): m/z [M + H]+ calcd for C18H16NO3: 294.1124; found: 294.1123.
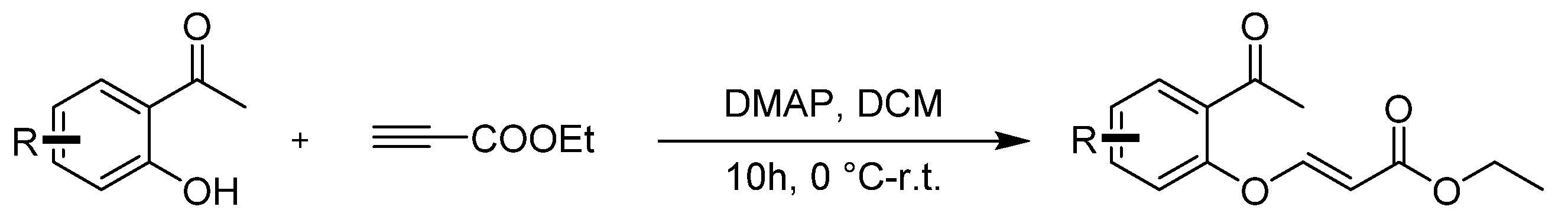
- Ethyl 9-oxo-3,3a-dihydro-9H-chromeno[3,2-c]isoxazole-3-carboxylate (4a), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1–2:1), 35 mg, 95%, yellow oil. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.04 (dd, J = 7.9, 1.8 Hz, 1H), 7.61 (ddd, J = 8.7, 7.2, 1.8 Hz, 1H), 7.19 (ddd, J = 8.1, 7.2, 1.0 Hz, 1H), 7.09 (dd, J = 8.4, 1.0 Hz, 1H), 6.03 (d, J = 6.9 Hz, 1H), 5.35 (d, J = 6.9 Hz, 1H), 4.36 (q, J = 7.1 Hz, 2H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 174.4, 166.2, 159.2, 151.4, 137.6, 128.0, 123.6, 123.4, 118.7, 86.7, 85.3, 63.1, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C13H12NO5: 274.0710; found: 274.0712.
- Ethyl 7-methyl-9-oxo-3,3a-dihydro-9H-chromeno[3,2-c]isoxazole-3-carboxylate (4b), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1–2:1), 31 mg, 91%, yellow solid, m.p.: 129–130 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.83–7.77 (m, 1H), 7.41 (dd, J = 8.5, 2.4 Hz, 1H), 6.98 (d, J = 8.4 Hz, 1H), 5.98 (d, J = 7.0 Hz, 1H), 5.32 (d, J = 7.0 Hz, 1H), 4.35 (q, J = 7.1 Hz, 2H), 2.35 (s, 3H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 174.5, 166.3, 157.3, 151.6, 138.7, 133.4, 127.4, 123.0, 118.4, 86.7, 85.2, 63.0, 20.4, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C14H14NO5: 276.0867; found: 276.0867.
- Ethyl 6-methyl-9-oxo-3,3a-dihydro-9H-chromeno[3,2-c]isoxazole-3-carboxylate (4c), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1–2:1), 26 mg, 82%, white solid, m.p.: 95–97 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.88 (d, J = 8.1 Hz, 1H), 6.98 (dd, J = 8.2, 1.5 Hz, 1H), 6.86 (s, 1H), 5.97 (d, J = 7.0 Hz, 1H), 5.30 (d, J = 7.0 Hz, 1H), 4.34 (q, J = 7.1 Hz, 2H), 2.39 (s, 3H), 1.36 (t, J = 7.2 Hz, 3H). 13C NMR (126 MHz, CDCl3)) δ (ppm) 174.0, 166.3, 159.2, 151.5, 149.8, 127.7, 124.9, 121.1, 118.6, 86.7, 85.1, 63.0, 22.0, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C14H14NO5: 276.0867; found: 274.0867.
- Ethyl 7-methoxy-9-oxo-3,3a-dihydro-9H-chromeno[3,2-c]isoxazole-3-carboxylate (4d), 30 mg, 86%, yellow solid, m.p.: 167–168 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.40 (d, J = 3.1 Hz, 1H), 7.19 (dd, J = 9.1, 3.2 Hz, 1H), 7.01 (d, J = 9.1 Hz, 1H), 5.97 (d, J = 7.0 Hz, 1H), 5.31 (d, J = 7.1 Hz, 1H), 4.35 (q, J = 7.1 Hz, 2H), 3.83 (s, 3H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 174.3, 166.3, 155.6, 153.9, 151.7, 126.9, 123.5, 120.0, 107.8, 86.8, 85.2, 63.1, 55.9, 14.1. HRMS (ESI): m/z [M + H]+ calcd for C14H14NO6: 292.0816; found: 292.0816.
- Ethyl 6-methoxy-9-oxo-3,3a-dihydro-9H-chromeno[3,2-c]isoxazole-3-carboxylate (4e), 29 mg, 87%, white solid, m.p.: 120–121 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.93 (d, J = 8.9 Hz, 1H), 6.71 (dd, J = 8.9, 2.4 Hz, 1H), 6.49 (d, J = 2.3 Hz, 1H), 5.98 (d, J = 7.1 Hz, 1H), 5.28 (d, J = 7.2 Hz, 1H), 4.34 (q, J = 7.1 Hz, 2H), 3.86 (s, 3H), 1.36 (t, J = 7.2 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 172.9, 167.3, 166.3, 161.4, 151.4, 129.6, 117.1, 111.9, 101.7, 86.9, 84.9, 63.0, 55.9, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C14H14NO6: 292.0816; found: 292.0817.
- Ethyl 7-bromo-9-oxo-3,3a-dihydro-9H-chromeno[3,2-c]isoxazole-3-carboxylate (4f), 24 mg, 80%, yellow solid, m.p.: 139–142 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 11.76 (s, 1H), 8.72 (d, J = 2.5 Hz, 1H), 7.66 (d, J = 11.4 Hz, 1H), 7.41 (s, 1H), 6.98 (d, J = 8.9 Hz, 1H), 4.49 (q, J = 7.1 Hz, 2H), 1.45 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 187.3, 163.0, 161.8, 161.5, 156.0, 140.7, 135.3, 120.5, 119.5, 111.4, 110.0, 62.9, 14.1. HRMS (ESI): m/z [M + H]+ calcd for C13H11BrNO5: 339.9815; found: 339.9817.
- Ethyl 7-chloro-9-oxo-3,3a-dihydro-9H-chromeno[3,2-c]isoxazole-3-carboxylate (4g), 27 mg, 83%, yellow solid, m.p.: 142–144 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.97 (d, J = 2.7 Hz, 1H), 7.54 (dd, J = 8.9, 2.7 Hz, 1H), 7.05 (d, J = 8.9 Hz, 1H), 6.03 (d, J = 6.9 Hz, 1H), 5.35 (d, J = 6.9 Hz, 1H), 4.36 (q, J = 7.2 Hz, 2H), 1.37 (t, J = 7.2 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 173.3, 166.0, 157.6, 150.8, 137.4, 129.3, 127.1, 124.1, 120.4, 86.7, 85.4, 63.2, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C13H11ClNO5: 296.0320; found: 296.0320.
- Ethyl 7-fluoro-9-oxo-3,3a-dihydro-9H-chromeno[3,2-c]isoxazole-3-carboxylate (4h), 20 mg, 78%, white solid, m.p.: 149–151 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.66 (dd, J = 8.0, 3.1 Hz, 1H), 7.32 (ddd, J = 9.0, 7.4, 3.2 Hz, 1H), 7.08 (dd, J = 9.1, 4.1 Hz, 1H), 6.02 (d, J = 6.9 Hz, 1H), 5.34 (d, J = 6.9 Hz, 1H), 4.35 (q, J = 7.1 Hz, 2H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 173.6, 166.0, 159.2 (d, Jc–f = 246.1 Hz), 155.4, 151.0, 125.1 (d, Jc–f = 24.8 Hz), 124.0 (d, Jc–f = 7.3 Hz), 120.5 (d, Jc–f = 7.4 Hz), 113.1 (d, Jc–f = 24.5 Hz), 86.8, 85.3, 63.1, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C13H11FNO5: 280.0616; found: 280.0616.
- Ethyl 11-oxo-7a,8-dihydro-11H-benzo[5,6]chromeno[3,2-c]isoxazole-8-carboxylate (4i), 14 mg, 70%, yellow solid, m.p.: 194–195 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 9.47 (d, J = 8.7 Hz, 1H), 8.04 (d, J = 9.0 Hz, 1H), 7.81 (d, J = 8.1 Hz, 1H), 7.76–7.70 (m, 1H), 7.53 (t, J = 7.5 Hz, 1H), 7.16 (d, J = 9.0 Hz, 1H), 6.09 (d, J = 6.9 Hz, 1H), 5.40 (d, J = 6.9 Hz, 1H), 4.38 (q, J = 7.1 Hz, 2H), 1.39 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 175.0, 166.3, 161.8, 152.1, 139.5, 131.4, 130.7, 129.9, 128.7, 126.3, 126.2, 118.5, 115.7, 86.4, 85.3, 63.1, 14.1. HRMS (ESI): m/z [M + H]+ calcd for C17H14NO5: 312.0866; found: 312.0866.
- Methyl 9-oxo-3,3a-dihydro-9H-chromeno[3,2-c]isoxazole-3-carboxylate (4j), 29 mg, 90%, white solid, m.p.: 138–139 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 8.04 (dd, J = 7.9, 1.8 Hz, 1H), 7.61 (ddd, J = 8.4, 7.2, 1.8 Hz, 1H), 7.19 (ddd, J = 8.1, 7.2, 1.1 Hz, 1H), 7.08 (dd, J = 8.4, 1.1 Hz, 1H), 6.03 (d, J = 6.9 Hz, 1H), 5.37 (d, J = 7.0 Hz, 1H), 3.91 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 174.3, 166.6, 159.1, 151.4, 137.6, 1287.0, 123.6, 123.4, 118.7, 86.7, 85.1, 53.6. HRMS (ESI): m/z [M + H]+ calcd for C12H10NO5: 248.0554; found: 248.0553.
- Methyl 7-methyl-9-oxo-3,3a-dihydro-9H-chromeno[3,2-c]isoxazole-3-carboxylate (4k), 32 mg, 92%, yellow solid, m.p.: 160–162 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 7.80 (d, J = 2.4 Hz, 1H), 7.40 (dd, J = 8.5, 2.4 Hz, 1H), 6.97 (d, J = 8.5 Hz, 1H), 5.98 (d, J = 7.2 Hz, 1H), 5.34 (d, J = 7.1 Hz, 1H), 3.90 (s, 3H), 2.35 (s, 3H). 13C NMR (101 MHz, CDCl3) δ (ppm) 174.4, 166.8, 157.3, 151.6, 138.7, 133.4, 127.4, 123.0, 118.4, 86.7, 85.0, 53.5, 20.4. HRMS (ESI): m/z [M + H]+ calcd for C13H12NO5: 262.0710; found: 262.0710.
- Methyl 7-methoxy-9-oxo-3,3a-dihydro-9H-chromeno[3,2-c]isoxazole-3-carboxylate (4l), 28 mg, 89%, yellow solid, m.p.: 193–194 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.41 (d, J = 3.2 Hz, 1H), 7.20 (dd, J = 9.1, 3.2 Hz, 1H), 7.01 (d, J = 9.0 Hz, 1H), 5.98 (d, J = 7.2 Hz, 1H), 5.34 (d, J = 7.2 Hz, 1H), 3.91 (s, 3H), 3.84 (s, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 174.2, 166.8, 155.6, 153.9, 151.7, 126.9, 1235, 120.0, 107.8, 86.8, 85.0, 55.9, 53.6. HRMS (ESI): m/z [M + H]+ calcd for C13H12NO6: 278.0659; found: 278.0657.
- Methyl 6-chloro-9-oxo-3,3a-dihydro-9H-chromeno[3,2-c]isoxazole-3-carboxylate (4m), 29 mg, 89%, yellow solid, m.p.: 121–123 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 11.97 (d, J = 1.5 Hz, 1H), 8.55 (dd, J = 8.8, 1.5 Hz, 1H), 7.43 (d, J = 1.4 Hz, 1H), 7.08 (t, J = 1.8 Hz, 1H), 6.98 (dd, J = 8.8, 2.0 Hz, 1H), 4.03 (d, J = 1.5 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 187.2, 164.6, 162.1, 161.0, 156.5, 144.4, 134.4, 120.5, 118.5, 117.0, 110.3, 53.3. HRMS (ESI): m/z [M + H]+ calcd for C12H9ClNO5: 282.0164; found: 282.0164.
- Methyl 7-chloro-9-oxo-3,3a-dihydro-9H-chromeno[3,2-c]isoxazole-3-carboxylate (4n), 29 mg, 89%, yellow solid, m.p.: 176–178 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.98 (d, J = 2.7 Hz, 1H), 7.55 (dd, J = 8.9, 2.6 Hz, 1H), 7.05 (d, J = 8.8 Hz, 1H), 6.03 (d, J = 7.0 Hz, 1H), 5.38 (d, J = 6.9 Hz, 1H), 3.91 (s, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 173.3, 166.5, 157.6, 150.9, 137.4, 129.4, 127.2, 124.1, 120.4, 86.8, 85.2, 53.7. HRMS (ESI): m/z [M + H]+ calcd for C12H9ClNO5: 282.0164; found: 282.0164.
- Methyl 11-oxo-7a,8-dihydro-11H-benzo[5,6]chromeno[3,2-c]isoxazole-8-carboxylate (4o), 30 mg, 86%, light yellow solid, m.p.: 145–146 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.68 (dd, J = 7.9, 3.1 Hz, 1H), 7.33 (ddd, J = 9.1, 7.4, 3.2 Hz, 1H), 7.09 (dd, J = 9.1, 4.1 Hz, 1H), 6.03 (d, J = 6.9 Hz, 1H), 5.38 (d, J = 6.9 Hz, 1H), 3.92 (s, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 173.5, 166.5, 159.3, 155.4, 151.0, 125.3, 124.1, 120.56, 113.2, 86.8, 85.2, 53.7. HRMS (ESI): m/z [M + H]+ calcd for C12H9FNO5: 266.0459; found: 266.0459.
- Methyl 7-bromo-9-oxo-3,3a-dihydro-9H-chromeno[3,2-c]isoxazole-3-carboxylate (4p), 18 mg, 77%, yellow solid, m.p.: 173–174 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.13 (d, J = 2.6 Hz, 1H), 7.68 (dd, J = 8.8, 2.6 Hz, 1H), 6.99 (d, J = 8.8 Hz, 1H), 6.03 (d, J = 6.9 Hz, 1H), 5.38 (d, J = 6.9 Hz, 1H), 3.91 (s, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 173.2, 166.4, 158.0, 150.8, 140.2, 130.3, 124.5, 120.6, 116.5, 86.7, 85.2, 53.7. HRMS (ESI): m/z [M + H]+ calcd for C12H9BrNO5: 325.9658; found: 325.9658.
- Methyl 11-oxo-7a,8-dihydro-11H-benzo[5,6]chromeno[3,2-c]isoxazole-8-carboxylate (4q), 20 mg, 67%, yellow solid, m.p.: 183–184 °C. 1H NMR (500 MHz, CDCl3) δ 9.48 (d, J = 8.7 Hz, 1H), 8.05 (d, J = 8.9 Hz, 1H), 7.82 (d, J = 8.1 Hz, 1H), 7.73 (ddd, J = 8.6, 6.9, 1.5 Hz, 1H), 7.54 (t, J = 7.5 Hz, 1H), 7.17 (d, J = 9.2 Hz, 1H), 6.10 (d, J = 6.9 Hz, 1H), 5.43 (d, J = 6.8 Hz, 1H), 3.93 (s, 3H). 13C NMR (126 MHz, Chloroform-d) δ 174.9, 166.8, 161.8, 152.1, 139.5, 131.4, 130.8, 129.9, 128.7, 126.3, 126.2, 118.4, 115.8, 86.42, 9.12, 53.6. HRMS (ESI): m/z [M + H]+ calcd for C16H12NO5: 298.07100; found:298.07100.
- 3,3a,4,5-Tetrahydro-11H-benzo[7,8]oxocino[5,4-c]isoxazol-11-one (4r), 22 mg, 78%, light brown solid, m.p.: 178–179 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 7.78 (dd, J = 7.8, 1.7 Hz, 1H), 7.55 (ddd, J = 8.1, 7.4, 1.8 Hz, 1H), 7.21 (td, J = 7.5, 1.0 Hz, 1H), 7.11 (dd, J = 8.2, 1.0 Hz, 1H), 5.08 (dd, J = 8.5, 4.7 Hz, 1H), 4.57 (dt, J = 9.4, 2.7 Hz, 1H), 3.83 (ddd, J = 12.0, 9.7, 2.3 Hz, 1H), 3.45 (ddd, J = 14.7, 8.5, 1.2 Hz, 1H), 3.32 (dd, J = 14.5, 0.9 Hz, 1H), 2.03–1.78 (m, 2H). 13C NMR (101 MHz, CDCl3) δ (ppm) 190.4, 161.2, 159.0, 135.5, 132.4, 128.7, 124.2, 121.4, 79.4, 70.3, 41.8, 34.5. HRMS (ESI): m/z [M + H]+ calcd for C12H12NO3: 218.0811; found: 218.0813.
- 4H,10H-Benzo[6,7]oxepino[4,3-c]isoxazol-10-one (6a), 22 mg, 82%, white solid, m.p.: 166–167 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.57 (s, 1H), 8.26 (dd, J = 8.1, 1.8 Hz, 1H), 7.58 (ddd, J = 8.5, 7.2, 1.8 Hz, 1H), 7.31–7.23 (m, 1H), 7.16 (dd, J = 8.1, 1.2 Hz, 1H), 5.15 (s, 2H). 13C NMR (126 MHz, CDCl3) δ (ppm) 180.2, 160.1, 155.6, 136.3, 132.1, 127.7, 124.5, 122.8, 117.5, 63.6. HRMS (ESI): m/z [M + H]+ calcd for C11H8NO3: 202.0498; found: 202.0498.
- Methyl-4H,10H-benzo[6,7]oxepino[4,3-c]isoxazol-10-one (6b), 15 mg, 75%, light yellow solid, m.p.: 193–196 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.56 (s, 1H), 8.02 (d, J = 2.5 Hz, 1H), 7.37 (dd, J = 8.3, 2.4 Hz, 1H), 7.05 (d, J = 8.2 Hz, 1H), 5.10 (s, 2H), 2.37 (s, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 180.4, 160.0, 158.0, 155.6, 137.2, 134.2, 131.8, 127.4, 122.6, 117.7, 63.6, 20.5. HRMS (ESI): m/z [M + H]+ calcd for C12H10NO3: 216.0655; found: 216.0655.
- 7-Methyl-4H,10H-benzo[6,7]oxepino[4,3-c]isoxazol-10-one (6c), 10 mg, 68%, white solid, m.p.: 196–197 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.56 (s, 1H), 8.02 (d, J = 2.5 Hz, 1H), 7.37 (dd, J = 8.3, 2.4 Hz, 1H), 7.05 (d, J = 8.2 Hz, 1H), 5.10 (s, 2H), 2.37 (s, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 179.7, 160.2, 160.1, 155.5, 148.2, 132.2, 125.6, 125.1, 122.9, 117.4, 63.4, 21.5. HRMS (ESI): m/z [M + H]+ calcd for C12H10NO3: 216.0655; found: 216.0654.
- 8-Methoxy-4H,10H-benzo[6,7]oxepino[4,3-c]isoxazol-10-one (6d), 32 mg, 71%, yellow solid, m.p.: 157–159 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.56 (s, 1H), 7.66 (d, J = 3.2 Hz, 1H), 7.14 (dd, J = 8.9, 3.1 Hz, 1H), 7.09 (d, J = 8.9 Hz, 1H), 5.15–5.04 (m, 2H), 3.86 (s, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 180.1, 159.7, 156.1, 155.6 154.3, 128.3, 124.4, 124.1, 117.8, 113.1, 63.8, 55.9. HRMS (ESI): m/z [M + H]+ calcd for C12H10NO4: 232.0604; found: 232.0605.
- 7-Methoxy-4H,10H-benzo[6,7]oxepino[4,3-c]isoxazol-10-one (6e), 20 mg, 74%, white solid, m.p.: 206–207 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.56 (s, 1H), 8.27 (d, J = 9.0 Hz, 1H), 6.80 (dd, J = 9.1, 2.5 Hz, 1H), 6.59 (d, J = 2.5 Hz, 1H), 5.12 (d, J = 0.7 Hz, 2H), 3.88 (s, 3H). 13C NMR (126 MHz, CDCl3) δ (ppm) 178.5, 166.2, 162.3, 160.4, 155.5, 134.3, 120.7, 117.0, 111.8, 106.1, 63.3, 55.8. HRMS (ESI): m/z [M + H]+ calcd for C12H10NO4: 232.0604; found: 232.0604.
- Fluoro-4H,10H-benzo[6,7]oxepino[4,3-c]isoxazol-10-one (6f), 15 mg, 62%, white solid, m.p.: 187–189 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.59 (s, 1H), 7.91 (dd, J = 9.2, 3.3 Hz, 1H), 7.31–7.26 (m, 1H), 7.16 (dd, J = 8.9, 4.6 Hz, 1H), 5.13 (s, 2H). 13C NMR (126 MHz, CDCl3) δ (ppm) 179.1, 159.4, 159.4 (d, Jc–f = 245.3 Hz), 156.2 (d, Jc–f = 2.6 Hz), 155.8, 128.9 (d, Jc–f = 7.3 Hz), 124.7 (d, Jc–f = 7.4 Hz), 123.5 (d, Jc–f = 23.3 Hz), 117.5 (d, Jc–f = 24.9 Hz), 63.8. HRMS (ESI): m/z [M + H]+ calcd for C11H7FNO3: 220.0405; found: 220.0404.
- 8-Chloro-4H,10H-benzo[6,7]oxepino[4,3-c]isoxazol-10-one (6g), 20 mg, 70%, white solid, m.p.: 202–203 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.59 (s, 1H), 8.22 (d, J = 2.8 Hz, 1H), 7.51 (dd, J = 8.7, 2.8 Hz, 1H), 7.13 (d, J = 8.7 Hz, 1H), 5.14 (s, 2H). 13C NMR (126 MHz, CDCl3) δ (ppm) 178.9, 159.6, 158.6, 155.9, 136.1, 131.4, 130.2, 128.5, 124.6, 117.2, 63.7. HRMS (ESI): m/z [M + H]+ calcd for C11H7ClNO3: 236.0109; found: 236.0109.
- 8-Bromo-4H,10H-benzo[6,7]oxepino[4,3-c]isoxazol-10-one (6h), 31 mg, 83%, white solid, m.p.: 193–194 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.59 (s, 1H), 8.36 (d, J = 2.6 Hz, 1H), 7.65 (dd, J = 8.7, 2.6 Hz, 1H), 7.06 (d, J = 8.6 Hz, 1H), 5.14 (s, 2H). 13C NMR (126 MHz, CDCl3) δ (ppm) 178.8, 159.6, 159.1, 155.9, 139.9, 134.4, 128.8, 124.8, 117.5, 117.2, 63.6. HRMS (ESI): m/z [M + H]+ calcd for C11H7BrNO3: 279.9603; found: 279.9602.
- 8H,12H-Naphtho[1′,2′:6,7]oxepino[4,3-c]isoxazol-12-one (6i), 27 mg, 85%, light brown solid, m.p.: 156–158 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.53–8.42 (m, 2H), 7.98 (d, J = 8.8 Hz, 1H), 7.82 (dd, J = 8.1, 1.4 Hz, 1H), 7.59 (ddd, J = 8.7, 6.9, 1.5 Hz, 1H), 7.49 (ddd, J = 8.1, 6.9, 1.2 Hz, 1H), 7.28 (d, J = 8.8 Hz, 1H), 5.18 (d, J = 1.2 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ (ppm) 184.2, 159.9, 158.1, 154.8, 135.9, 131.6, 131.5, 128.8, 128.3, 126.5, 126.1, 125.5, 121.6, 117.8, 65.2. HRMS (ESI): m/z [M + H]+ calcd for C15H10NO3: 252.0655; found: 252.0653.
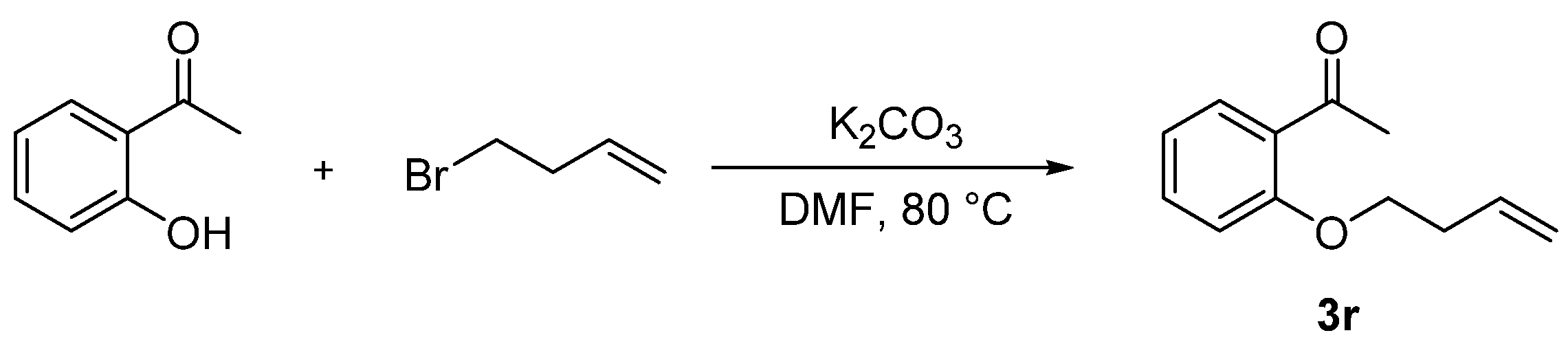
- 1-(2-(But-3-en-1-yloxy)phenyl)ethan-1-one (3r), 24 mg, 67%, light yellow oil. 1H NMR (400 MHz, CDCl3) δ (ppm) 7.73 (dd, J = 7.7, 1.9 Hz, 1H), 7.43 (ddd, J = 8.3, 7.3, 1.9 Hz, 1H), 7.05–6.84 (m, 2H), 5.90 (ddt, J = 16.9, 10.2, 6.6 Hz, 1H), 5.30–5.02 (m, 2H), 4.12 (t, J = 6.4 Hz, 2H), 2.63–2.57 (m, 5H). 13C NMR (101 MHz, CDCl3) δ (ppm) 199.8, 158.2, 134.3, 133.6, 130.4, 128.2, 120.5, 117.4, 112.1, 67.6, 33.6, 32.1. HRMS (ESI): m/z [M + H]+ calcd for C12H15NO2: 191.1066; found: 191.1066.
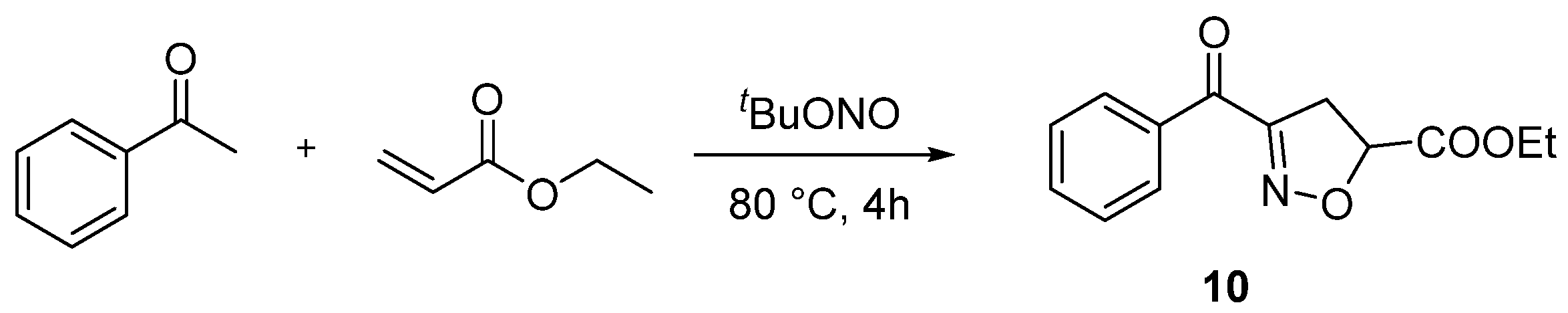
- Ethyl 3-benzoyl-4,5-dihydroisoxazole-5-carboxylate (10), 27 mg, 58%, light yellow oil, m.p.: 156–158 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 8.25–8.16 (m, 2H), 7.60 (td, J = 7.3, 1.5 Hz, 1H), 7.47 (td, J = 7.9, 1.7 Hz, 2H), 5.22–5.14 (m, 1H), 4.32–4.22 (m, 2H), 3.73–3.56 (m, 2H), 1.32 (td, J = 7.1, 1.6 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ (ppm) 185.4, 169.1, 156.8, 135.4, 133.8, 130.3, 128.4, 78.9, 62.2, 38.4, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C13H14NO4: 248.0917; found: 248.0918.
- (E)-2-Oxo-2-phenylacetaldehyde oxime (11), 24 mg, 67%, light yellow solid, m.p.: 150–151 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 8.68 (s, 1H), 8.07–8.04 (m, 2H), 8.03 (d, J = 1.4 Hz, 1H), 7.64–7.58 (m, 1H), 7.48 (dd, J = 8.4, 7.2 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ (ppm) 188.6, 148.7, 135.8, 133.6, 129.9, 128.5. HRMS (ESI): m/z [M + H]+ calcd for C8H8NO2: 150.0550; found: 150.0550.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morita, T.; Fukuhara, S.; Fuse, S.; Nakamura, H. Gold(I)-Catalyzed Intramolecular SEAr Reaction: Efficienct Synthesis of Isoxazole-Containing Fused Heterocycles. Org. Lett. 2018, 20, 433–436. [Google Scholar] [CrossRef]
- Ibrahim, K.T.; Neetha, M.; Anilkumar, G. Advancements in the synthesis of oxazolines. Monatsh. Chem. 2022, 153, 837–871. [Google Scholar] [CrossRef]
- Jiang, B.; Dai, M. Concise Total Syntheses of the 6–7–5 Hamigeran Natural Products. J. Am. Chem. Soc. 2023, 145, 18731–18736. [Google Scholar] [CrossRef]
- Jiang, B.; Dai, M. Synthetic Studies toward the Hamigerans with a 6–7–5 Tricyclic Core. Org. Lett. 2020, 22, 4176–4179. [Google Scholar] [CrossRef]
- Guo, Y.; Xiang, Y.; Wei, L.; Wan, J.-P. Thermoinduced Free-Radical C–H Acyloxylation of Tertiary Enaminones: Catalyst-Free Synthesis of Acyloxyl Chromones and Enaminones. Org. Lett. 2018, 20, 3971–3974. [Google Scholar] [CrossRef]
- Lin, Y.; Wan, J.-P.; Liu, Y. Cascade in Situ Iodination, Chromone Annulation, and Cyanation for Site-Selective Synthesis of 2-Cyanochromones. J. Org. Chem. 2023, 88, 4017–4023. [Google Scholar] [CrossRef]
- Luo, T.; Wan, J.-P.; Liu, Y. Toward C2-nitrogenated chromones by copper-catalyzed β-C(sp2)–H N-heteroarylation of enaminones. Org. Chem. Front. 2020, 7, 1107–1112. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, Y.; Wan, J.-P. Transition metal-free synthesis of 3-trifluoromethyl chromones via tandem C–H trifluoromethylation and chromone annulation of enaminones. Org. Chem. Front. 2020, 7, 2770–2775. [Google Scholar] [CrossRef]
- Haji, M.; Hosseinzadeh, M. Cyclohepta[b]pyran: An important scaffold in biologically active natural products. Med. Chem. Res. 2022, 31, 2059–2073. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Wang, M.-M.; Zhang, Y.-Q.; Xu, H.; Dai, H.-X. Construction of Indole-Fused Seven- and Eight-Membered Azaheterocycles via a Tandem Pd/NBE-Catalyzed Decarbonylation and Dual C–H Activation Sequence. Org. Lett. 2023, 25, 5406–5410. [Google Scholar] [CrossRef] [PubMed]
- Osipyan, A.; Sapegin, A.; Novikov, A.S.; Krasavin, M. Rare Medium-Sized Rings Prepared via Hydrolytic Imidazoline Ring Expansion (HIRE). J. Org. Chem. 2018, 83, 9707–9717. [Google Scholar] [CrossRef]
- Gao, M.; Gan, Y.; Xu, B. From Alkenes to Isoxazolines via Copper-Mediated Alkene Cleavage and Dipolar Cycloaddition. Org. Lett. 2019, 21, 7435–7439. [Google Scholar] [CrossRef]
- Meng, L.; Liu, H.; Lin, Z.; Wang, J. Synthetic and Computational Study of the Enantioselective [3 + 2]-Cycloaddition of Chromones with MBH Carbonates. Org. Lett. 2022, 24, 5890–5895. [Google Scholar] [CrossRef]
- Krstić, G.; Saidu, M.B.; Bombicz, P.; De, S.; Ali, H.; Zupkó, I.; Berkecz, R.; Gallah, U.S.; Rédei, D.; Hohmann, J. Pauciflorins A–E, Unexpected Chromone–Monoterpene-Derived Meroterpenoids from Centrapalus pauciflorus. J. Nat. Prod. 2023, 86, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.; Tang, J.; Wei, Y.; Zeng, X. Ring Expansion via Cleavage of Benzylic C−C Bonds Enabling Direct Synthesis of Medium Ring-Fused Benzocarbocycles. Chem. Asian J. 2016, 11, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qi, T.; He, S.; Huang, W.; Peng, C.; Zhan, G.; Han, B. Catalyst-Controlled Switchable (5 + 4)/(3 + 4) Cycloadditions for the Divergent Synthesis of Pyrazole-Fused Seven- and Nine-Membered Heterocycles. ACS Catal. 2023, 13, 10694–10704. [Google Scholar] [CrossRef]
- Reyes, R.L.; Iwai, T.; Sawamura, M. Construction of Medium-Sized Rings by Gold Catalysis. Chem. Rev. 2021, 121, 8926–8947. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Lu, L.; Zhuang, W.; Huang, Q. Synthesis of Indole-Fused Six-, Seven-, or Eight-Membered N,O-Heterocycles via Rhodium-Catalyzed NH-Indole-Directed C–H Acetoxylation/Hydrolysis/Annulation. J. Org. Chem. 2021, 86, 16753–16763. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-Z.; Guan, Y.-L.; Huang, Q.-W.; Qi, T.; Xiang, P.; Zhang, X.; Leng, H.-J.; Li, J.-L. Temperature-Controlled Divergent Asymmetric Synthesis of Indole-Based Medium-Sized Heterocycles through Palladium Catalysis. ACS Catal. 2023, 13, 1164–1172. [Google Scholar] [CrossRef]
- Li, P.; Jia, X. tert-Butyl Nitrite (TBN) as a Versatile Reagent in Organic Synthesis. Synthesis 2018, 50, 711–722. [Google Scholar] [CrossRef]
- Khaligh, G.N. Recent Advances and Applications of tert-Butyl Nitrite (TBN) in Organic Synthesis. Mini-Rev. Org. Chem. 2020, 17, 3–25. [Google Scholar] [CrossRef]
- Dahiya, A.; Sahoo, A.K.; Alam, T.; Patel, B.K. tert-Butyl Nitrite (TBN), a Multitasking Reagent in Organic Synthesis. Chem. Asian J. 2019, 14, 4454–4492. [Google Scholar] [CrossRef]
- Guo, X.; Xu, G.; Zhou, L.; Yan, H.; Hao, X.-Q.; Wang, Q. Synthesis and application of α-carbonyl nitrile oxides. Org. Chem. Front. 2020, 7, 2467–2473. [Google Scholar] [CrossRef]
- Pan, J.; Li, X.; Lin, F.; Liu, J.; Jiao, N. Chemoselective Nitrosylation of Anilines and Alkynes via Fragmentary or Complete NO Incorporation. Chem 2018, 4, 1427–1442. [Google Scholar] [CrossRef]
- Wang, X.-D.; Zhu, L.-H.; Liu, P.; Wang, X.-Y.; Yuan, H.-Y.; Zhao, Y.-L. Copper-Catalyzed Cascade Cyclization Reactions of Diazo Compounds with tert-Butyl Nitrite and Alkynes: One-Pot Synthesis of Isoxazoles. J. Org. Chem. 2019, 84, 16214–16221. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Liang, X.; Gao, Z.; Lei, A.; Pan, Y. Synthesis of Isoxazolines and Oxazines by Electrochemical Intermolecular [2 + 1 + n] Annulation: Diazo Compounds Act as Radical Acceptors. Org. Lett. 2019, 21, 9300–9305. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhou, Y.; Song, Q. Synthesis of Furoxans and Isoxazoles via Divergent [2 + 1 + 1 + 1] Annulations of Sulfoxonium Ylides and tBuONO. Org. Lett. 2019, 21, 5273–5276. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Tan, X.; Luo, Q.; Yu, X.; Zhang, S.; Liu, F.; Zhang, W.-H. Synthesis of 3-Acyl-isoxazoles and Δ2-Isoxazolines from Methyl Ketones, Alkynes or Alkenes, and tert-Butyl Nitrite via a Csp3–H Radical Functionalization/Cycloaddition Cascade. Org. Lett. 2019, 21, 5096–5100. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Jin, F.; Cheng, X.; Tao, S.; Jiang, G.; Li, X.; Yang, J.; Bao, X.; Wan, X. [2 + 2 + 1] Cycloaddition of N-tosylhydrazones, tert-butyl nitrite and alkenes: A general and practical access to isoxazolines. Chem. Sci. 2021, 12, 9823–9830. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhao, Y.; Fang, S.; Long, W.; Sun, H.; Wan, X. Coupling Reaction of Cu-Based Carbene and Nitroso Radical: A Tandem Reaction to Construct Isoxazolines. Org. Lett. 2017, 19, 5896–5899. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ogunlana, A.A.; Fang, S.; Long, W.; Sun, H.; Bao, X.; Wan, X. In situ generation of nitrile oxides from copper carbene and tert-butyl nitrite: Synthesis of fully substituted isoxazoles. Org. Biomol. Chem. 2018, 16, 4683–4687. [Google Scholar] [CrossRef]
- Song, W.; Liu, Y.; Yan, N.; Wan, J.-P. Tunable Key [3 + 2] and [2 + 1] Cycloaddition of Enaminones and α-Diazo Compounds for the Synthesis of Isomeric Isoxazoles: Metal-Controlled Selectivity. Org. Lett. 2023, 25, 2139–2144. [Google Scholar] [CrossRef]
- Ma, L.; Kou, L.; Jin, F.; Cheng, X.; Tao, S.; Jiang, G.; Bao, X.; Wan, X. Acyclic nitronate olefin cycloaddition (ANOC): Regio- and stereospecific synthesis of isoxazolines. Chem. Sci. 2021, 12, 774–779. [Google Scholar] [CrossRef]
- Sudoh, Y.; Jin, Z.-T.; Imafuku, K.; Matsumura, H. Reactions of 3-acetyltropolone and its methyl ethers with hydroxylamine. Formation of 8H-cyclohept[d]isoxazol-8-one and 8H-cyclohept[c]isoxazol-8-one. J. Heterocycl. Chem. 1982, 19, 525–528. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Chen, J.; Chen, M.; Li, X.; Jiang, T.; Liu, F.; Yang, X.; Sun, Y.; Zhu, Y. One-Pot Synthesis of Isoxazole-Fused Tricyclic Quinazoline Alkaloid Derivatives via Intramolecular Cycloaddition of Propargyl-Substituted Methyl Azaarenes under Metal-Free Conditions. Molecules 2023, 28, 2787. [Google Scholar] [CrossRef]
- Zhao, P.; Wu, X.; Zhou, Y.; Geng, X.; Wang, C.; Wu, Y.-d.; Wu, A.-X. Direct Synthesis of 2,3-Diaroyl Quinolines and Pyridazino[4,5-b]quinolines via an I2-Promoted One-Pot Multicomponent Reaction. Org. Lett. 2019, 21, 2708–2711. [Google Scholar] [CrossRef]
- Huang, H.-M.; Bellotti, P.; Daniliuc, C.G.; Glorius, F. Radical Carbonyl Propargylation by Dual Catalysis. Angew. Chem. Int. Ed. 2021, 60, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.R.; Manna, M.S.; Mukherjee, S. Nitro-enabled catalytic enantioselective formal umpolung alkenylation of β-ketoesters. Chem. Sci. 2017, 8, 6686–6690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Wang, Z.; Zhang, H.; Gao, J.-J.; Yang, K.-R.; Fan, W.-Y.; Wu, R.-X.; Feng, M.-L.; Zhu, W.; Zhu, Y.-P. Iodine-Mediated Domino Cyclization for One-Pot Synthesis of Indolizine-Fused Chromones via Metal-Free sp3 C–H Functionalization. J. Org. Chem. 2022, 87, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.-H.; Zhang, X.-J.; Li, Y.-M.; Wu, R.-X.; Zhang, H.-R.; Qin, L.-Y.; Ni, X.; Yan, Y.; Wu, A.-X.; Zhu, Y.-P. One-Pot Synthesis of Chromone-Fused Pyrrolo[2,1-a]isoquinolines and Indolizino[8,7-b]indoles: Iodine-Promoted Oxidative [2 + 2 + 1] Annulation of O-Acetylphenoxyacrylates with Tetrahydroisoquinolines and Noreleagnines. J. Org. Chem. 2021, 86, 15733–15742. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dang, H.; Rose, J.A.; Rablen, P.; Herzon, S.B. Hydroheteroarylation of Unactivated Alkenes Using N-Methoxyheteroarenium Salts. J. Am. Chem. Soc. 2017, 139, 5998–6007. [Google Scholar] [CrossRef]
- Sutariya, T.R.; Labana, B.M.; Parmar, B.D.; Parmar, N.J.; Kant, R.; Gupta, V.K. A domino synthetic approach for new, angular pyrazol- and isoxazol-heterocycles using [DBU][Ac] as an effective reaction medium. RSC Adv. 2015, 5, 23519–23529. [Google Scholar] [CrossRef]
- Shaikh, M.H.; Subhedar, D.D.; Khedkar, V.M.; Jha, P.C.; Khan, F.A.K.; Sangshetti, J.N.; Shingate, B.B. 1,2,3-Triazole tethered acetophenones: Synthesis, bioevaluation and molecular docking study. Chin. Chem. Lett. 2016, 27, 1058–1063. [Google Scholar] [CrossRef]
- Purushothaman, S.; Prasanna, R.; Raghunathan, R. Regioselective synthesis of spiropyrrolidine/spiropyrrolizidine/spirothiazolidine-grafted macrocycles through 1,3-dipolar cycloaddition methodology. Tetrahedron 2013, 69, 9742–9750. [Google Scholar] [CrossRef]
- Dimirjian, C.A.; Castiñeira Reis, M.; Balmond, E.I.; Turman, N.C.; Rodriguez, E.P.; Di Maso, M.J.; Fettinger, J.C.; Tantillo, D.J.; Shaw, J.T. Synthesis of Spirobicyclic Pyrazoles by Intramolecular Dipolar Cycloadditions/[1s, 5s] Sigmatropic Rearrangements. Org. Lett. 2019, 21, 7209–7212. [Google Scholar] [CrossRef] [PubMed]
- James, M.J.; Schwarz, J.L.; Strieth-Kalthoff, F.; Wibbeling, B.; Glorius, F. Dearomative Cascade Photocatalysis: Divergent Synthesis through Catalyst Selective Energy Transfer. J. Am. Chem. Soc. 2018, 140, 8624–8628. [Google Scholar] [CrossRef] [PubMed]




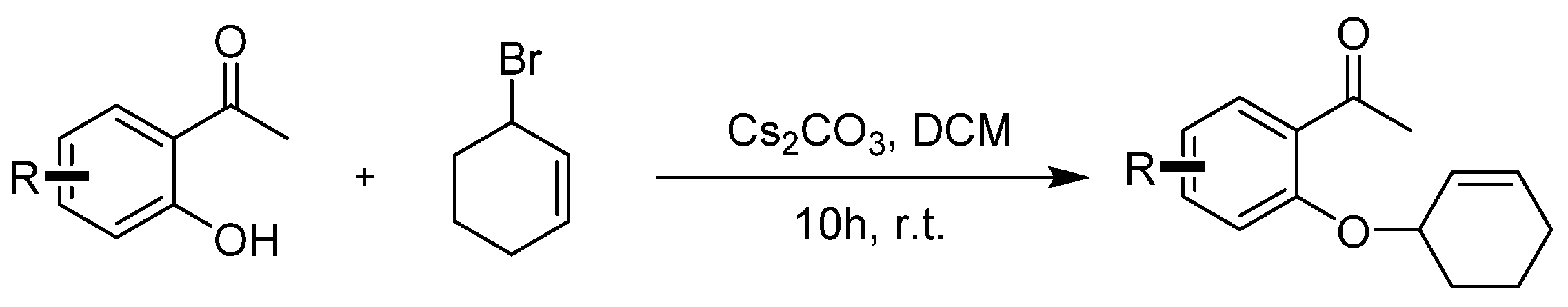
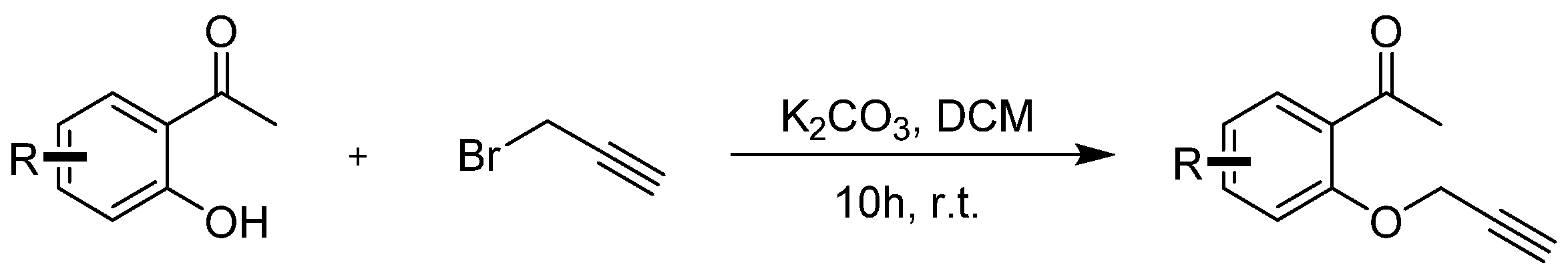
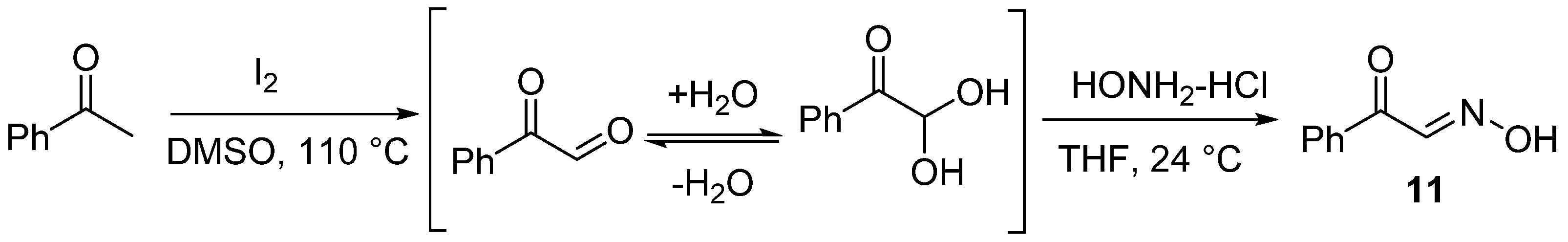
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.-K.; Cao, T.-Z.; Yue, Q.-W.; Ma, Y.; Yang, C.-M.; Zhang, H.-X.; Li, Y.-C.; Dong, Q.-K.; Zhu, Y.-P.; Sun, Y.-Y. tert-Butyl Nitrite-Induced Radical Nitrile Oxidation Cycloaddition: Synthesis of Isoxazole/Isoxazoline-Fused Benzo 6/7/8-membered Oxacyclic Ketones. Molecules 2024, 29, 1202. https://doi.org/10.3390/molecules29061202
Cao J-K, Cao T-Z, Yue Q-W, Ma Y, Yang C-M, Zhang H-X, Li Y-C, Dong Q-K, Zhu Y-P, Sun Y-Y. tert-Butyl Nitrite-Induced Radical Nitrile Oxidation Cycloaddition: Synthesis of Isoxazole/Isoxazoline-Fused Benzo 6/7/8-membered Oxacyclic Ketones. Molecules. 2024; 29(6):1202. https://doi.org/10.3390/molecules29061202
Chicago/Turabian StyleCao, Jian-Kang, Tian-Zheng Cao, Qian-Wen Yue, Ying Ma, Chuan-Ming Yang, Hong-Xi Zhang, Ya-Chen Li, Qiao-Ke Dong, Yan-Ping Zhu, and Yuan-Yuan Sun. 2024. "tert-Butyl Nitrite-Induced Radical Nitrile Oxidation Cycloaddition: Synthesis of Isoxazole/Isoxazoline-Fused Benzo 6/7/8-membered Oxacyclic Ketones" Molecules 29, no. 6: 1202. https://doi.org/10.3390/molecules29061202
APA StyleCao, J.-K., Cao, T.-Z., Yue, Q.-W., Ma, Y., Yang, C.-M., Zhang, H.-X., Li, Y.-C., Dong, Q.-K., Zhu, Y.-P., & Sun, Y.-Y. (2024). tert-Butyl Nitrite-Induced Radical Nitrile Oxidation Cycloaddition: Synthesis of Isoxazole/Isoxazoline-Fused Benzo 6/7/8-membered Oxacyclic Ketones. Molecules, 29(6), 1202. https://doi.org/10.3390/molecules29061202






