Redox Reactivity of Nonsymbiotic Phytoglobins towards Nitrite
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Particularities and Ferric Phytoglobins Binding Nitrite
2.2. Ferrous Phytoglobins Nitrite Reductase Activity in Anaerobic Conditions
2.3. Oxy Phytoglobins Nitrite Oxidase Activity in Aerobic Conditions
2.4. EPR Measurements in Phytoglobins Reactivity towards Nitrite
2.5. Mechanistic Considerations in Phytoglobins Reactivity towards Nitrite
3. Materials and Methods
3.1. Cloning and Expression of the Recombinant nsHs Phytoglobins
3.2. Purification of the Recombinant nsHs Plant Hemoglobins
3.3. Preparation of Oxy- and Deoxyglobins
3.4. UV-Vis Spectrophotometric Measurements
3.4.1. The Reaction of Deoxyglobins (GlbFe2+) with Nitrite
3.4.2. The Reaction of Oxyhemoglobins (GlbFe2+-O2) with Nitrite
3.5. EPR Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delledonne, M.; Xia, Y.; Dixon, R.A.; Lamb, C. Nitric Oxide Functions as a Signal in Plant Disease Resistance. Nature 1998, 394, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Beligni, M.V.; Lamattina, L. Is Nitric Oxide Toxic or Protective? Trends Plant Sci. 1999, 4, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Basalah, M.O. Role of Nitric Oxide in Tolerance of Plants to Abiotic Stress. Protoplasma 2011, 248, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Hartman, S.; Liu, Z.; van Veen, H.; Vicente, J.; Reinen, E.; Martopawiro, S.; Zhang, H.; van Dongen, N.; Bosman, F.; Bassel, G.W.; et al. Ethylene-Mediated Nitric Oxide Depletion Pre-Adapts Plants to Hypoxia Stress. Nat. Commun. 2019, 10, 4020. [Google Scholar] [CrossRef]
- Khan, M.; Imran, Q.M.; Shahid, M.; Mun, B.G.; Lee, S.U.; Khan, M.A.; Hussain, A.; Lee, I.J.; Yun, B.W. Nitric Oxide- Induced AtAO3 Differentially Regulates Plant Defense and Drought Tolerance in Arabidopsis thaliana. BMC Plant Biol. 2019, 19, 602. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.H.M.B.; Hasanuzzaman, M.; Parvin, K.; Mohsin, S.M.; Al Mahmud, J.; Nahar, K.; Fujita, M. Nitric Oxide and Hydrogen Sulfide: Two Intimate Collaborators Regulating Plant Defense against Abiotic Stress. Plant Growth Regul. 2020, 90, 409–424. [Google Scholar] [CrossRef]
- Gupta, K.J.; Mur, L.A.J.; Wany, A.; Kumari, A.; Fernie, A.R.; Ratcliffe, R.G. The Role of Nitrite and Nitric Oxide under Low Oxygen Conditions in Plants. New Phytol. 2020, 225, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Inafuku, M.; Nahar, K.; Fujita, M.; Oku, H. Nitric Oxide Regulates Plant Growth, Physiology, Antioxidant Defense, and Ion Homeostasis to Confer Salt Tolerance in the Mangrove Species, Kandelia obovata. Antioxidants 2021, 10, 611. [Google Scholar] [CrossRef]
- Manrique-Gil, I.; Sánchez-Vicente, I.; Torres-Quezada, I.; Lorenzo, O. Nitric Oxide Function during Oxygen Deprivation in Physiological and Stress Processes. J. Exp. Bot. 2021, 72, 904–916. [Google Scholar] [CrossRef]
- Sanchez-Corrionero, A.; Sánchez-Vicente, I.; Arteaga, N.; Manrique-Gil, I.; Gómez-Jiménez, S.; Torres-Quezada, I.; Albertos, P.; Lorenzo, O. Fine-Tuned Nitric Oxide and Hormone Interface in Plant Root Development and Regeneration. J. Exp. Bot. 2022, 74, 6104–6118. [Google Scholar] [CrossRef]
- Zeng, J.; Zhao, X.; Liang, Z.; Hidalgo, I.; Gebert, M.; Fan, P.; Wenzl, C.; Gornik, S.G.; Lohmann, J.U. Nitric Oxide Controls Shoot Meristem Activity via Regulation of DNA Methylation. Nat. Commun. 2023, 14, 8001. [Google Scholar] [CrossRef]
- Palmer, R.M.J.; Ferrige, A.G.; Moncada, S. Nitric Oxide Release Accounts for the Biological Activity of Endothelium-Derived Relaxing Factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef]
- Santolini, J.; André, F.; Jeandroz, S.; Wendehenne, D. Nitric Oxide Synthase in Plants: Where Do We Stand? Nitric Oxide 2017, 63, 30–38. [Google Scholar] [CrossRef]
- Stöhr, C.; Strube, F.; Marx, G.; Ullrich, W.R.; Rockel, P. A Plasma Membrane-Bound Enzyme of Tobacco Roots Catalyses the Formation of Nitric Oxide from Nitrite. Planta 2001, 212, 835–841. [Google Scholar] [CrossRef]
- Planchet, E.; Gupta, K.J.; Sonoda, M.; Kaiser, W.M. Nitric Oxide Emission from Tobacco Leaves and Cell Suspensions: Rate Limiting Factors and Evidence for the Involvement of Mitochondrial Electron Transport. Plant J. 2005, 41, 732–743. [Google Scholar] [CrossRef]
- Rümer, S.; Gupta Kapuganti, J.; Kaiser, W.M. Oxidation of Hydroxylamines to NO by Plant Cells. Plant Signal. Behav. 2009, 4, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; van Dongen, J.T. On the Origins of Nitric Oxide. Trends Plant Sci. 2011, 16, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Sturms, R.; Dispirito, A.A.; Hargrove, M.S. Plant and Cyanobacterial Hemoglobins Reduce Nitrite to Nitric Oxide under Anoxic Conditions. Biochemistry 2011, 50, 3873–3878. [Google Scholar] [CrossRef] [PubMed]
- Astier, J.; Gross, I.; Durner, J. Nitric Oxide Production in Plants: An Update. J. Exp. Bot. 2018, 69, 3401–3411. [Google Scholar] [CrossRef]
- Allagulova, C.R.; Lubyanova, A.R.; Avalbaev, A.M. Multiple Ways of Nitric Oxide Production in Plants and Its Functional Activity under Abiotic Stress Conditions. Int. J. Mol. Sci. 2023, 24, 11637. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.B.; Moura, J.J.G. How Biology Handles Nitrite. Chem. Rev. 2014, 114, 5273–5357. [Google Scholar] [CrossRef]
- Vinogradov, S.N.; Hoogewijs, D.; Bailly, X.; Arredondo-Peter, R.; Guertin, M.; Gough, J.; Dewilde, S.; Moens, L.; Vanfleteren, J.R. Three Globin Lineages Belonging to Two Structural Classes in Genomes from the Three Kingdoms of Life. Proc. Natl. Acad. Sci. USA 2005, 102, 11385–11389. [Google Scholar] [CrossRef] [PubMed]
- Hoy, J.A.; Hargrove, M.S. The Structure and Function of Plant Hemoglobins. Plant Physiol. Biochem. 2008, 46, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Arredondo-Peter, R.; Hill, R.; Hargrove, M. Phytoglobin: A Novel Nomenclature for Plant Globins Accepted by the Globin Community at the 2014 XVIII Conference on Oxygen-Binding and Sensing Proteins. F1000Research 2016, 5, 212. [Google Scholar] [CrossRef]
- Becana, M.; Yruela, I.; Sarath, G.; Catalán, P.; Hargrove, M.S. Plant Hemoglobins: A Journey from Unicellular Green Algae to Vascular Plants. New Phytol. 2020, 227, 1618–1635. [Google Scholar] [CrossRef]
- Garrocho-Villegas, V.; Gopalasubramaniam, S.K.; Arredondo-Peter, R. Plant Hemoglobins: What We Know Six Decades after Their Discovery. Gene 2007, 398, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Dordas, C. Nonsymbiotic Hemoglobins and Stress Tolerance in Plants. Plant Sci. 2009, 176, 433–440. [Google Scholar] [CrossRef]
- Seregélyes, C.; Igamberdiev, A.U.; Maassen, A.; Hennig, J.; Dudits, D.; Hill, R.D. NO-Degradation by Alfalfa Class 1 Hemoglobin (Mhb1): A Possible Link to PR-1a Gene Expression in Mhb1-Overproducing Tobacco Plants. FEBS Lett. 2004, 571, 61–66. [Google Scholar] [CrossRef]
- Qu, Z.L.; Zhong, N.Q.; Wang, H.Y.; Chen, A.P.; Jian, G.L.; Xia, G.X. Ectopic Expression of the Cotton Non-Symbiotic Hemoglobin Gene GhHbd1 Triggers Defense Responses and Increases Disease Tolerance in Arabidopsis. Plant Cell Physiol. 2006, 47, 1058–1068. [Google Scholar] [CrossRef]
- Zhao, L.; Gu, R.; Gao, P.; Wang, G. A Nonsymbiotic Hemoglobin Gene from Maize, ZmHb, Is Involved in Response to Submergence, High-Salt and Osmotic Stresses. Plant Cell Tissue Organ Cult. 2008, 95, 227–237. [Google Scholar] [CrossRef]
- Hebelstrup, K.H.; Shah, J.K.; Simpson, C.; Schjoerring, J.K.; Mandon, J.; Cristescu, S.M.; Harren, F.J.M.; Christiansen, M.W.; Mur, L.A.J.; Igamberdiev, A.U. An Assessment of the Biotechnological Use of Hemoglobin Modulation in Cereals. Physiol. Plant. 2014, 150, 593–603. [Google Scholar] [CrossRef]
- Lee, B.R.; Hwang, S. Over-Expression of NtHb1 Encoding a Non-Symbiotic Class 1 Hemoglobin of Tobacco Enhances a Tolerance to Cadmium by Decreasing NO (Nitric Oxide) and Cd Levels in Nicotiana tabacum. Environ. Exp. Bot. 2015, 113, 18–27. [Google Scholar] [CrossRef]
- Spyrakis, F.; Bruno, S.; Bidon-Chanal, A.; Luque, F.J.; Abbruzzetti, S.; Viappiani, C.; Dominici, P.; Mozzarelli, A. Oxygen Binding to Arabidopsis thaliana AHb2 Nonsymbiotic Hemoglobin: Evidence for a Role in Oxygen Transport. IUBMB Life 2011, 63, 355–362. [Google Scholar] [CrossRef]
- Vigeolas, H.; Hühn, D.H.; Geigenberger, P. Nonsymbiotic Hemoglobin-2 Leads to an Elevated Energy State and to a Combined Increase in Polyunsaturated Fatty Acids and Total Oil Content When Overexpressed in Developing Seeds of Transgenic Arabidopsis Plants. Plant Physiol. 2011, 155, 1435–1444. [Google Scholar] [CrossRef]
- Gisbert, C.; Timoneda, A.; Porcel, R.; Ros, R.; Mulet, J.M. Overexpression of BvHb2, a Class 2 Non-Symbiotic Hemoglobin from Sugar Beet, Confers Drought-Induced Withering Resistance and Alters Iron Content in Tomato. Agronomy 2020, 10, 1754. [Google Scholar] [CrossRef]
- Watts, R.A.; Hunt, P.W.; Hvitved, A.N.; Hargrove, M.S.; Peacock, W.J.; Dennis, E.S. A Hemoglobin from Plants Homologous to Truncated Hemoglobins of Microorganisms. Proc. Natl. Acad. Sci. USA 2001, 98, 10119–10124. [Google Scholar] [CrossRef]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite Reduction to Nitric Oxide by Deoxyhemoglobin Vasodilates the Human Circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef]
- Kim-Shapiro, D.B.; Gladwin, M.T.; Patel, R.P.; Hogg, N. The Reaction between Nitrite and Hemoglobin: The Role of Nitrite in Hemoglobin-Mediated Hypoxic Vasodilation. J. Inorg. Biochem. 2005, 99, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Kim-Shapiro, D.B. The Functional Nitrite Reductase Activity of the Heme-Globins. Blood 2008, 112, 2636–2647. [Google Scholar] [CrossRef] [PubMed]
- Tiso, M.; Tejero, J.; Kenney, C.; Frizzell, S.; Gladwin, M.T. Nitrite Reductase Activity of Nonsymbiotic Hemoglobins from Arabidopsis thaliana. Biochemistry 2012, 51, 5285–5292. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.W.; Klok, E.J.; Trevaskis, B.; Watts, R.A.; Ellis, M.H.; Peacock, W.J.; Dennis, E.S. Increased Level of Hemoglobin 1 Enhances Survival of Hypoxic Stress and Promotes Early Growth in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2002, 99, 17197–17202. [Google Scholar] [CrossRef]
- Dordas, C.; Hasinoff, B.B.; Igamberdiev, A.U.; Manac’h, N.; Rivoal, J.; Hill, R.D. Expression of a Stress-Induced Hemoglobin Affects NO Levels Produced by Alfalfa Root Cultures under Hypoxic Stress. Plant J. 2003, 35, 763–770. [Google Scholar] [CrossRef]
- Van Dongen, J.T.; Fröhlich, A.; Ramírez-Aguilar, S.J.; Schauer, N.; Fernie, A.R.; Erban, A.; Kopka, J.; Clark, J.; Langer, A.; Geigenberger, P. Transcript and Metabolite Profiling of the Adaptive Response to Mild Decreases in Oxygen Concentration in the Roots of Arabidopsis Plants. Ann. Bot. 2009, 103, 269–280. [Google Scholar] [CrossRef]
- Cochrane, D.W.; Shah, J.K.; Hebelstrup, K.H.; Igamberdiev, A.U. Expression of Phytoglobin Affects Nitric Oxide Metabolism and Energy State of Barley Plants Exposed to Anoxia. Plant Sci. 2017, 265, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Loreti, E.; Poggi, A.; Novi, G.; Alpi, A.; Perata, P. A Genome-Wide Analysis of the Effects of Sucrose on Gene Expression in Arabidopsis Seedlings under Anoxia. Plant Physiol. 2005, 137, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Kreuzwieser, J.; Hauberg, J.; Howell, K.A.; Carroll, A.; Rennenberg, H.; Millar, A.H.; Whelan, J. Differential Response of Gray Poplar Leaves and Roots Underpins Stress Adaptation during Hypoxia. Plant Physiol. 2009, 149, 461. [Google Scholar] [CrossRef]
- Tejero, J.; Gladwin, M.T. The Globin Superfamily: Functions in Nitric Oxide Formation and Decay. Biol. Chem. 2014, 395, 631. [Google Scholar] [CrossRef] [PubMed]
- Moţ, A.C. Nonsymbiotic Plant Hemoglobins: Synopsis and Perspectives of Their Structure and Enzymatic Activities. Acta Met. 2014, Tome XI, 163–169. [Google Scholar]
- Mot, A.C.; Puscas, C.; Miclea, P.; Naumova-Letia, G.; Dorneanu, S.; Podar, D.; Dissmeyer, N.; Silaghi-Dumitrescu, R. Redox Control and Autoxidation of Class 1, 2 and 3 Phytoglobins from Arabidopsis thaliana. Sci. Rep. 2018, 8, 13714. [Google Scholar] [CrossRef]
- Olson, J.S.; Mathews, A.J.; Rohlfs, R.J.; Springer, B.A.; Egeberg, K.D.; Sligar, S.G.; Tame, J.; Renaud, J.P.; Nagai, K. The Role of the Distal Histidine in Myoglobin and Haemoglobin. Nature 1988, 336, 265–266. [Google Scholar] [CrossRef]
- Sturms, R.; DiSpirito, A.A.; Fulton, D.B.; Hargrove, M.S. Hydroxylamine Reduction to Ammonium by Plant and Cyanobacterial Hemoglobins. Biochemistry 2011, 50, 10829–10835. [Google Scholar] [CrossRef] [PubMed]
- Copeland, D.M.; Soares, A.S.; West, A.H.; Richter-Addo, G.B. Crystal Structures of the Nitrite and Nitric Oxide Complexes of Horse Heart Myoglobin. J. Inorg. Biochem. 2006, 100, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shi, Y.; Tejero, J.; Powell, S.M.; Thomas, L.M.; Gladwin, M.T.; Shiva, S.; Zhang, Y.; Richter-Addo, G.B. Nitrosyl Myoglobins and Their Nitrite Precursors: Crystal Structural and Quantum Mechanics and Molecular Mechanics Theoretical Investigations of Preferred Fe-NO Ligand Orientations in Myoglobin Distal Pockets. Biochemistry 2018, 57, 4788–4802. [Google Scholar] [CrossRef] [PubMed]
- Valianti, V.K.; Tselios, C.; Pinakoulaki, E. Reversible Thermally Induced Spin Crossover in the Myoglobin–Nitrito Adduct Directly Monitored by Resonance Raman Spectroscopy. RSC Adv. 2023, 13, 9020–9025. [Google Scholar] [CrossRef]
- Hendgen-Cotta, U.B.; Merx, M.W.; Shiva, S.; Schmitz, J.; Becher, S.; Klare, J.P.; Steinhoff, H.J.; Goedecke, A.; Schrader, J.; Gladwin, M.T.; et al. Nitrite Reductase Activity of Myoglobin Regulates Respiration and Cellular Viability in Myocardial Ischemia-Reperfusion Injury. Proc. Natl. Acad. Sci. USA 2008, 105, 10256–10261. [Google Scholar] [CrossRef]
- Yi, J.; Orville, A.M.; Skinner, J.M.; Skinner, M.J.; Richter-Addo, G.B. Synchrotron X-Ray-Induced Photoreduction of Ferric Myoglobin Nitrite Crystals Gives the Ferrous Derivative with Retention of the O-Bonded Nitrite Ligand. Biochemistry 2010, 49, 5969. [Google Scholar] [CrossRef]
- Salhany, J.M. Kinetics of Reaction of Nitrite with Deoxy Hemoglobin after Rapid Deoxygenation or Predeoxygenation by Dithionite Measured in Solution and Bound to the Cytoplasmic Domain of Band 3 (SLC4A1). Biochemistry 2008, 47, 6059–6072. [Google Scholar] [CrossRef]
- Feng, X.U.; Quandt, K.S.; Hultquist, D.E. Characterization of NADPH-Dependent Methemoglobin Reductase as a Heme-Binding Protein Present in Erythrocytes and Liver. Proc. Natl. Acad. Sci. USA 1992, 89, 2130–2134. [Google Scholar] [CrossRef]
- Naumova-Leția, G.; Moț, A.C. Probing Reducing Power for Ferryl Phytoglobins of Several Phenolic Compounds Using Their Kinetic Profiles Assisted by Chemometric Methods. Stud. Univ. Babes-Bolyai Chem. 2017, 62, 49–66. [Google Scholar] [CrossRef]
- Mot, A.C.; Bischin, C.; Damian, G.; Attia, A.A.A.; Gal, E.; Dina, N.; Leopold, N.; Silaghi-Dumitrescu, R. Fe(III)—Sulfide Interaction in Globins: Characterization and Quest for a Putative Fe(IV)-Sulfide Species. J. Inorg. Biochem. 2018, 179, 32–39. [Google Scholar] [CrossRef]
- Puscas, C.; Radu, L.; Carrascoza, F.; Mot, A.C.; Amariei, D.; Lungu, O.; Scurtu, F.; Podea, P.; Septelean, R.; Matei, A.; et al. The High Affinity of Small-Molecule Antioxidants for Hemoglobin. Free Radic. Biol. Med. 2018, 124, 260–274. [Google Scholar] [CrossRef]
- Mot, A.C.; Puscas, C.; Dorneanu, S.A.; Silaghi-Dumitrescu, R. EPR Detection of Sulfanyl Radical during Sulfhemoglobin Formation—Influence of Catalase. Free Radic. Biol. Med. 2019, 137, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, H.; Tyuma, I. Mechanism of Autocatalytic Oxidation of Oxyhemoglobin by Nitrite. Environ. Health Perspect. 1987, 73, 147. [Google Scholar] [CrossRef] [PubMed]
- Keszler, A.; Piknova, B.; Schechter, A.N.; Hogg, N. The Reaction between Nitrite and Oxyhemoglobin: A Mechanistic Study. J. Biol. Chem. 2008, 283, 9615–9622. [Google Scholar] [CrossRef]
- Hathazi, D.; Scurtu, F.; Bischin, C.; Mot, A.; Attia, A.A.A.; Kongsted, J.; Silaghi-Dumitrescu, R. The Reaction of Oxy Hemoglobin with Nitrite: Mechanism, Antioxidant-Modulated Effect, and Implications for Blood Substitute Evaluation. Molecules 2018, 23, 350. [Google Scholar] [CrossRef]
- Silaghi-Dumitrescu, R.; Reeder, B.J.J.; Nicholls, P.; Cooper, C.E.E.; Wilson, M.T.T. Ferryl Haem Protonation Gates Peroxidatic Reactivity in Globins. Biochem. J. 2007, 403, 391–395. [Google Scholar] [CrossRef]
- Bawn, M.; MacMillan, F. Electron Paramagnetic Resonance Investigation of Nitrite Binding in Myoglobin. bioRxiv 2018, 252775. [Google Scholar] [CrossRef]
- Kakar, S.; Hoffman, F.G.; Storz, J.F.; Fabian, M.; Hargrove, M.S. Structure and Reactivity of Hexacoordinate Hemoglobins. Biophys. Chem. 2010, 152, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Deng, W.; Liu, D.; Li, Y.; Peng, K.; Lorimer, G.H.; Wang, J. Redox and Spectroscopic Properties of Mammalian Nitrite Reductase-like Hemoproteins. J. Inorg. Biochem. 2022, 237, 111982. [Google Scholar] [CrossRef]
- Huang, K.T.; Keszler, A.; Patel, N.; Patell, R.P.; Gladwin, M.T.; Kim-Shapiro, D.B.; Hogg, N. The Reaction between Nitrite and Deoxyhemoglobin: Reassessment of Reaction Kinetics and Stoichiometry. J. Biol. Chem. 2005, 280, 31126–31131. [Google Scholar] [CrossRef]
- Hathazi, D.; Mahuţ, S.D.; Scurtu, F.V.; Bischin, C.; Stanciu, C.; Attia, A.A.; Damian, G.; Silaghi-Dumitrescu, R. Involvement of Ferryl in the Reaction between Nitrite and the Oxy Forms of Globins. J. Biol. Inorg. Chem. 2014, 19, 1233–1239. [Google Scholar] [CrossRef]
- Morard, P.; Silvestre, J.; Lacoste, L.; Caumes, E.; Lamaze, T. Nitrate Uptake and Nitrite Release by Tomato Roots in Response to Anoxia. J. Plant Physiol. 2004, 161, 855–865. [Google Scholar] [CrossRef]
- Horchani, F.; Aschi-Smiti, S.; Brouquisse, R. Involvement of Nitrate Reduction in the Tolerance of Tomato (Solanum lycopersicum L.) Plants to Prolonged Root Hypoxia. Acta Physiol. Plant. 2010, 32, 1113–1123. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Freschi, L.; Sodek, L. Nitrogen Metabolism and Translocation in Soybean Plants Subjected to Root Oxygen Deficiency. Plant Physiol. Biochem. PPB 2013, 66, 141–149. [Google Scholar] [CrossRef]
- Limami, A.M.; Diab, H.; Lothier, J. Nitrogen Metabolism in Plants under Low Oxygen Stress. Planta 2014, 239, 531–541. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Hill, R.D. Nitrate, NO and Haemoglobin in Plant Adaptation to Hypoxia: An Alternative to Classic Fermentation Pathways. J. Exp. Bot. 2004, 55, 2473–2482. [Google Scholar] [CrossRef]
- Wang, Y.; Loake, G.J.; Chu, C. Cross-Talk of Nitric Oxide and Reactive Oxygen Species in Plant Programed Cell Death. Front. Plant Sci. 2013, 4, 57327. [Google Scholar] [CrossRef] [PubMed]
- Hebelstrup, K.H.; Hunt, P.; Dennis, E.; Jensen, S.B.; Jensen, E.Ø. Hemoglobin Is Essential for Normal Growth of Arabidopsis Organs. Physiol. Plant. 2006, 127, 157–166. [Google Scholar] [CrossRef]
- Raran-Kurussi, S.; Waugh, D.S. The Ability to Enhance the Solubility of Its Fusion Partners Is an Intrinsic Property of Maltose-Binding Protein but Their Folding Is Either Spontaneous or Chaperone-Mediated. PLoS ONE 2012, 7, e49589. [Google Scholar] [CrossRef] [PubMed]
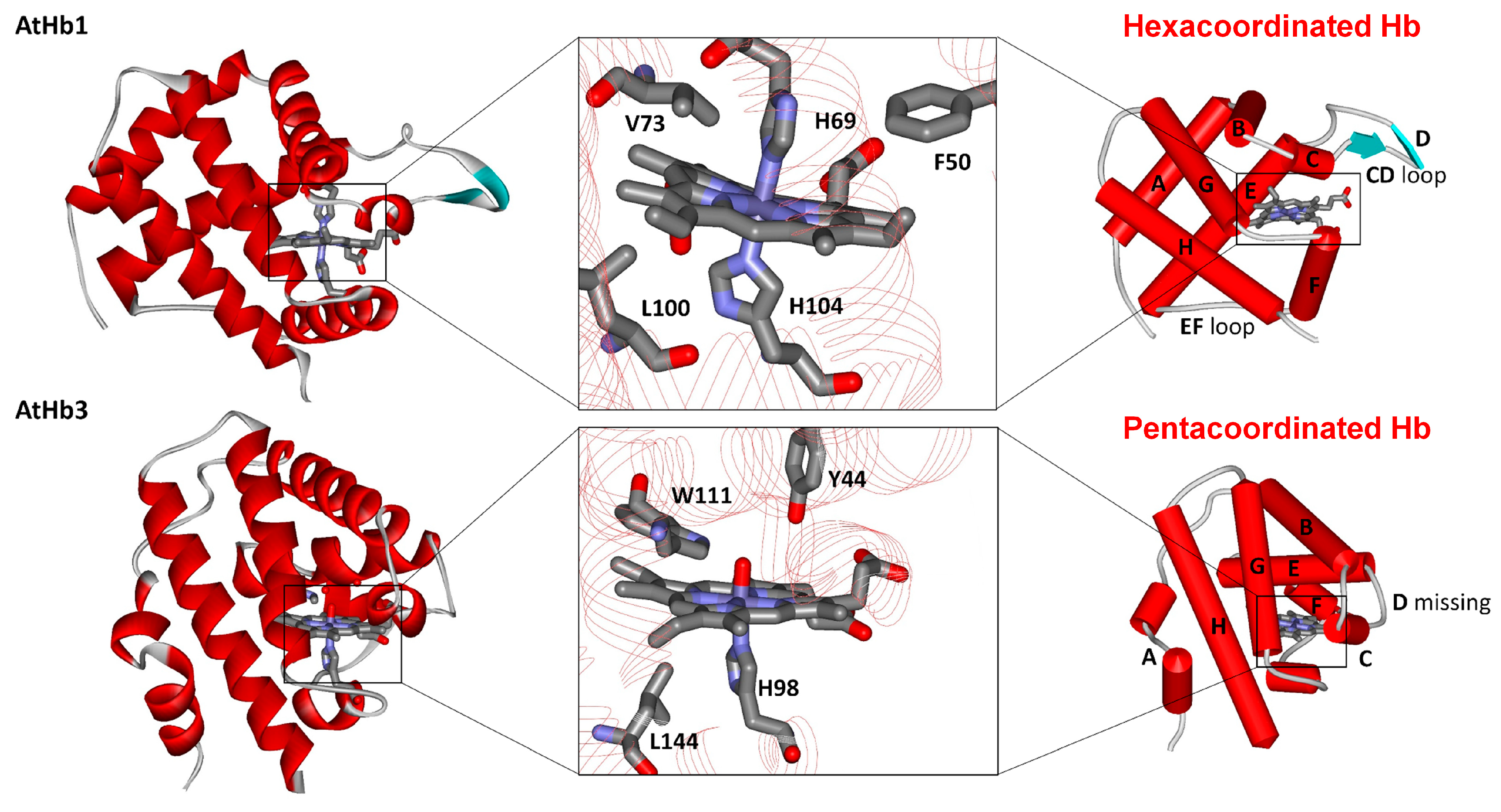


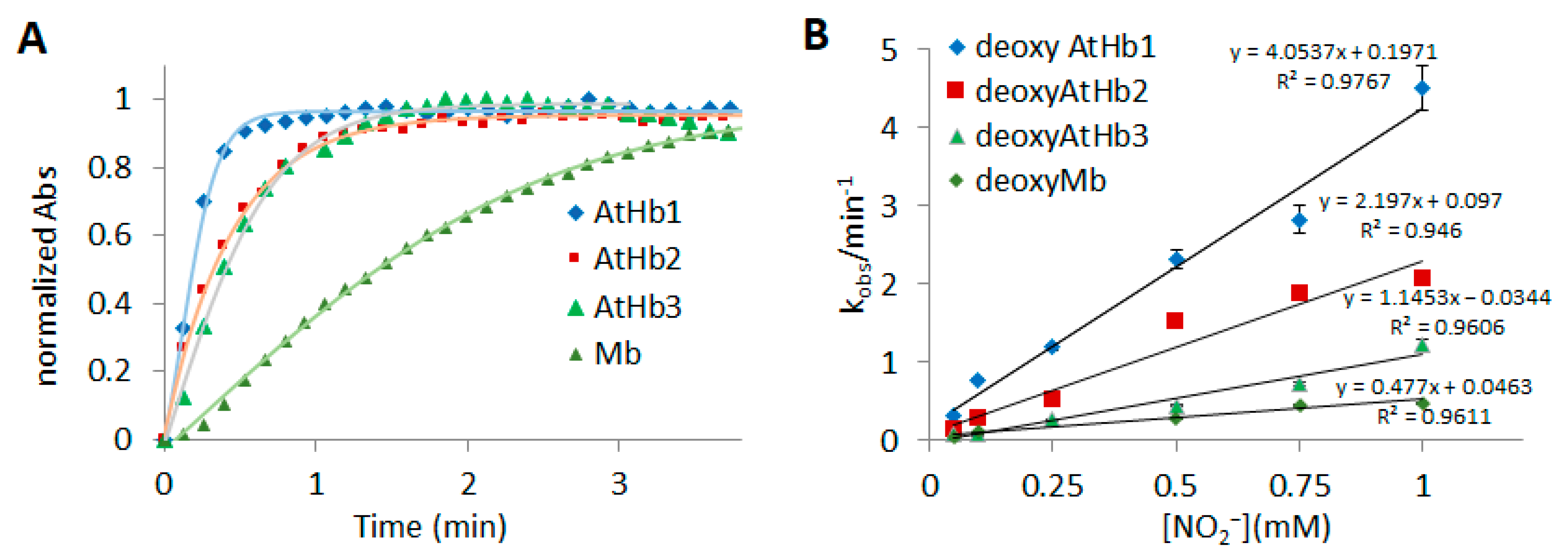



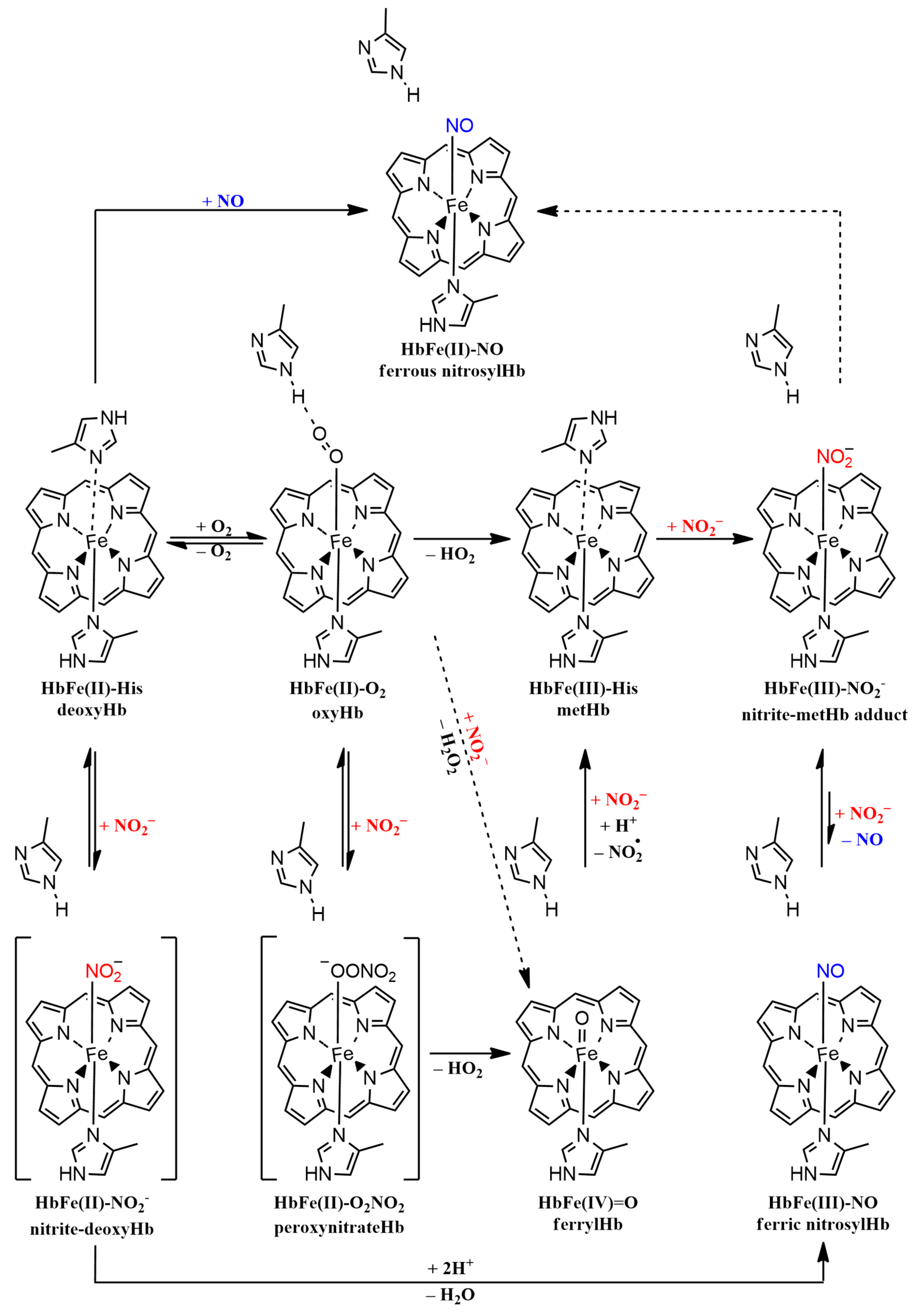
| AtHb1 | AtHb2 | AtHb3 | Mb |
|---|---|---|---|
| 67.56 | 36.62 | 19.09 | 7.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagrean-Tuza, C.; Pato, G.; Damian, G.; Silaghi-Dumitrescu, R.; Mot, A.C. Redox Reactivity of Nonsymbiotic Phytoglobins towards Nitrite. Molecules 2024, 29, 1200. https://doi.org/10.3390/molecules29061200
Zagrean-Tuza C, Pato G, Damian G, Silaghi-Dumitrescu R, Mot AC. Redox Reactivity of Nonsymbiotic Phytoglobins towards Nitrite. Molecules. 2024; 29(6):1200. https://doi.org/10.3390/molecules29061200
Chicago/Turabian StyleZagrean-Tuza, Cezara, Galaba Pato, Grigore Damian, Radu Silaghi-Dumitrescu, and Augustin C. Mot. 2024. "Redox Reactivity of Nonsymbiotic Phytoglobins towards Nitrite" Molecules 29, no. 6: 1200. https://doi.org/10.3390/molecules29061200
APA StyleZagrean-Tuza, C., Pato, G., Damian, G., Silaghi-Dumitrescu, R., & Mot, A. C. (2024). Redox Reactivity of Nonsymbiotic Phytoglobins towards Nitrite. Molecules, 29(6), 1200. https://doi.org/10.3390/molecules29061200







