The Roles of Exosome-Derived microRNAs in Cardiac Fibrosis
Abstract
1. Introduction
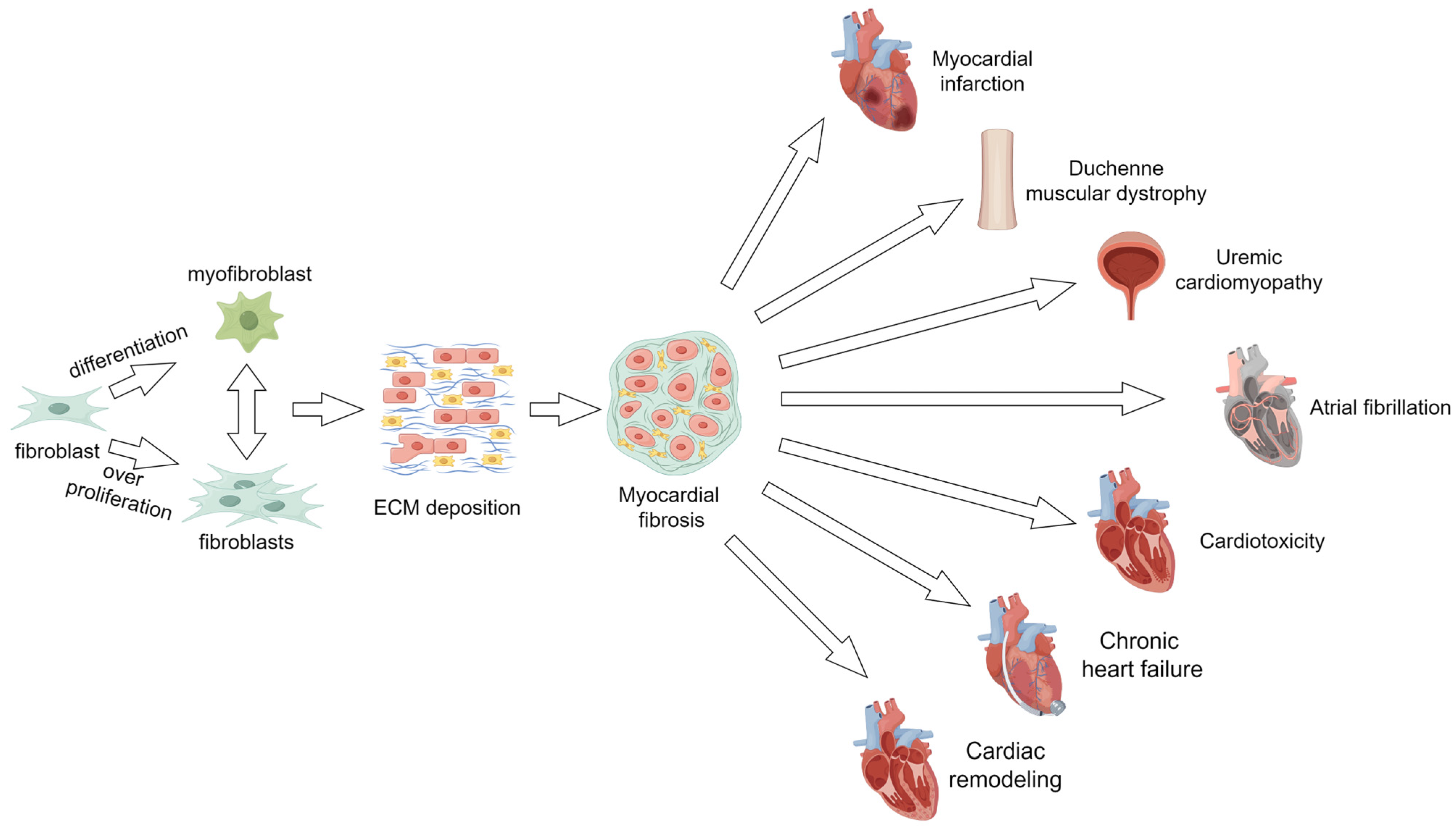
2. Myocardial Fibrosis
2.1. Myocardial Fibrosis and Myocardial Infarction
2.2. Myocardial Fibrosis and Duchenne Muscular Dystrophy
2.3. Myocardial Fibrosis and Uremic Cardiomyopathy
2.4. Myocardial Fibrosis and Atrial Fibrillation
2.5. Myocardial Fibrosis and Cardiotoxicity of Antitumor Drugs
2.6. Myocardial Fibrosis and Chronic Heart Failure
2.7. Myocardial Fibrosis and Cardiac Remodeling
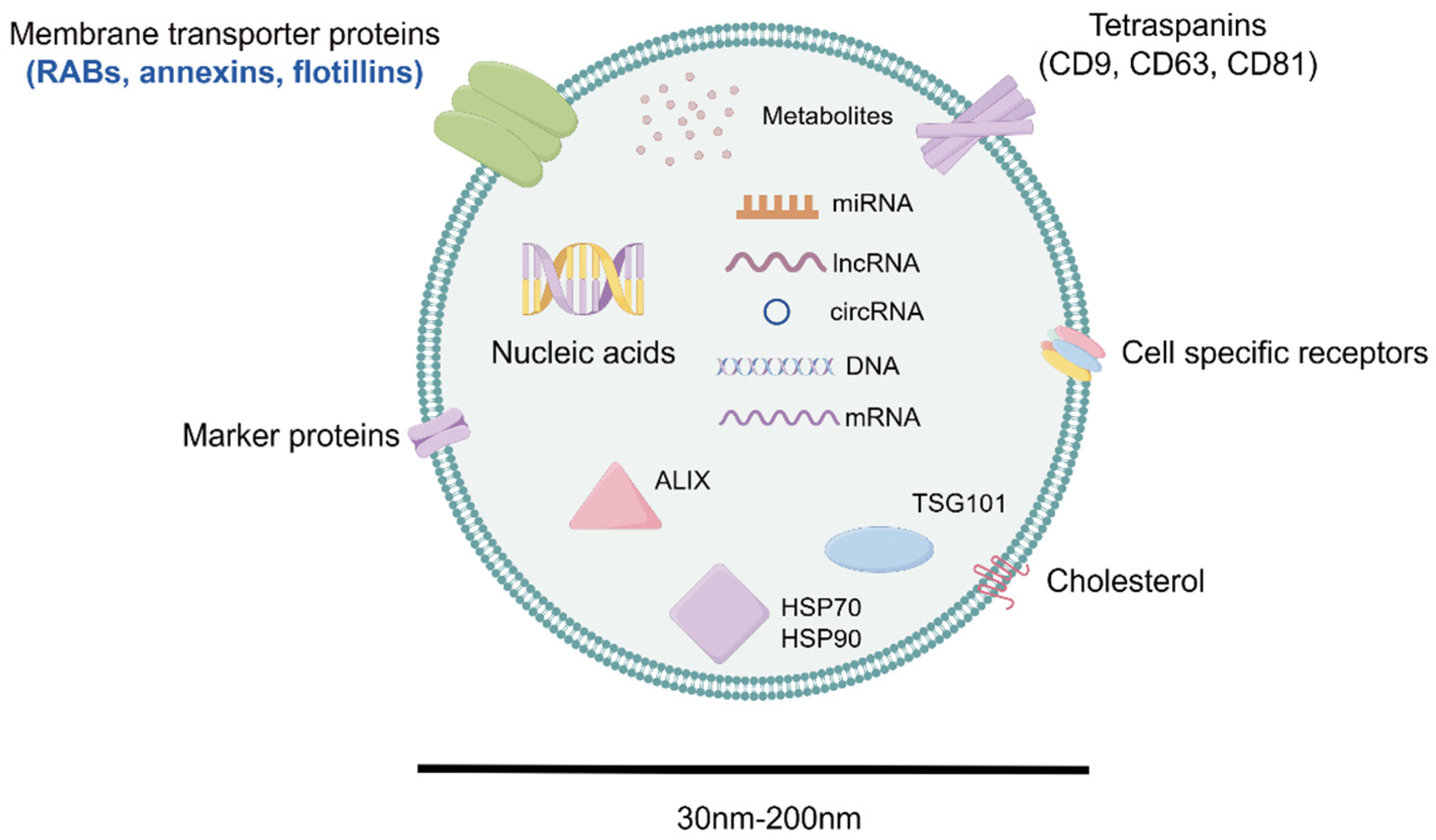
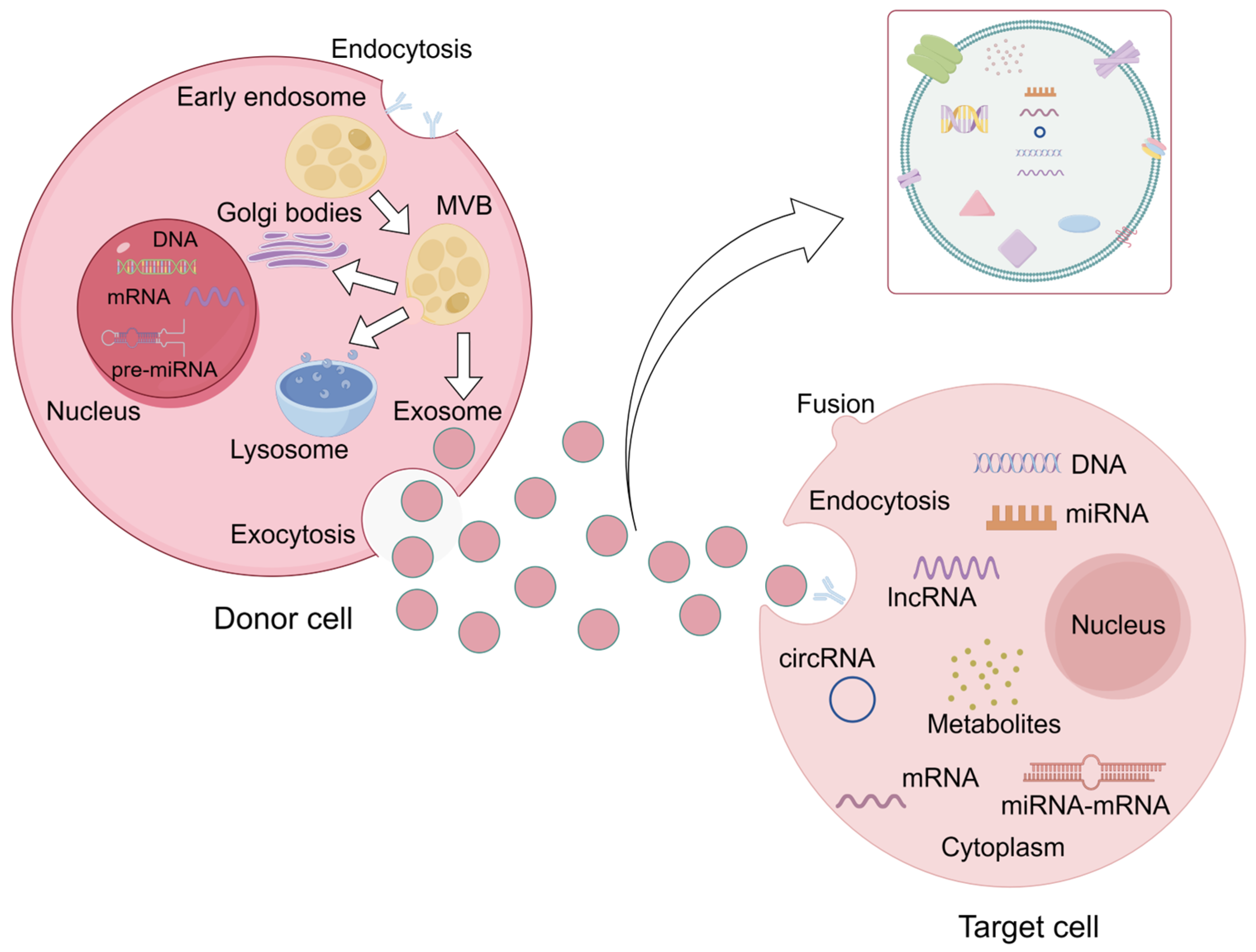
3. The Biogenesis and Composition of Exosomes
3.1. Exosomal Biogenesis
3.2. Exosomal Composition
4. Exosomal miRNA
4.1. Exosomal miRNA Transport
4.2. Exosomal miRNA Function
5. Exosomal miRNAs from Different Sources and Myocardial Fibrosis
5.1. Endothelial Progenitor Cells-Derived Exosomal miRNA
5.1.1. miR-1246, miR-1290
5.1.2. miR-133
5.1.3. miR-218-5p, miR-363-3p
5.2. Mesenchymal Stem Cell-Derived Exosomal miRNA
5.2.1. miR-29c
5.2.2. miR-22
5.2.3. miR-126
5.2.4. miR-146a
5.2.5. miR-210
5.2.6. miR-24
5.2.7. miR-671
5.3. Macrophages-Derived Exosomal miRNA
miR-155
5.4. Endothelial Cells-Derived Exosomal miRNA
5.4.1. miR-19a-3p
5.4.2. miR-10b-5p
5.5. Cardiomyocytes-Derived Exosomal miRNA
5.5.1. miR-208a
5.5.2. miR-494-3p
5.5.3. miR-378
5.5.4. miR-29b, miR-455
5.5.5. miR-210-3p
5.5.6. miR-23a
5.6. Cardiosphere-Derived Cells-Derived Exosomal miRNA
miR-92a
5.7. Cardiac Progenitor Cells-Derived Exosomal miRNA
5.7.1. miR-133a
5.7.2. miR-146a-5p
5.8. Pluripotent Stem Cell-Derived Exosomal miRNA
5.8.1. miR-290-295 Cluster
5.8.2. miR-373
5.9. Immune Cells-Derived Exosomal miRNA
miR-142-3p
5.10. Serum-Derived Exosomal miRNA
5.10.1. miR-21
5.10.2. miR-320a
5.10.3. miR-425, miR-744
5.10.4. miR-124-3p
5.11. Adipocyte-Derived Exosomal miRNA
miR-23a-3p
5.12. Others
5.12.1. miR-29b
5.12.2. miR-450a-2-3p
6. Conclusions and Prospect
Author Contributions
Funding
Conflicts of Interest
References
- Yellon, D.M.; Davidson, S.M. Exosomes: Nanoparticles involved in cardioprotection? Circ. Res. 2014, 114, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef]
- Qin, X.J.; Zhang, J.X.; Wang, R.L. Exosomes as mediators and biomarkers in fibrosis. Biomark. Med. 2020, 14, 697–712. [Google Scholar] [CrossRef]
- Jiang, W.; Xiong, Y.; Li, X.; Yang, Y. Cardiac Fibrosis: Cellular Effectors, Molecular Pathways, and Exosomal Roles. Front. Cardiovasc. Med. 2021, 8, 715258. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Loyer, X.; Vion, A.C.; Tedgui, A.; Boulanger, C.M. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ. Res. 2014, 114, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef]
- Raĭskina, M.E. Dinamika patologicheskikh izmeneniĭ v serdtse v ostroĭ éksperimental’nogo infarkta miokarda [Dynamics of the pathological changes in the heart during the acute stage of experimental myocardial infarct]. Kardiologiia 1967, 7, 3–13. [Google Scholar]
- Uusimaa, P.; Risteli, J.; Niemelä, M.; Lumme, J.; Ikäheimo, M.; Jounela, A.; Peuhkurinen, K. Collagen scar formation after acute myocardial infarction: Relationships to infarct size, left ventricular function, and coronary artery patency. Circulation 1997, 96, 2565–2572. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Warren, S.E.; Royal, H.D.; Markis, J.E.; Grossman, W.; McKay, R.G. Time course of left ventricular dilation after myocardial infarction: Influence of infarct-related artery and success of coronary thrombolysis. J. Am. Coll. Cardiol. 1988, 11, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Dambrink, J.H.; Beukema, W.P.; Van Gilst, W.H.; Peels, K.H.; Lie, K.I.; Kingma, J.H. Left ventricular dilatation and high-grade ventricular arrhythmias in the first year after myocardial infarction. J. Card. Fail. 1994, 1, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.R.; Daniel, L.L.; Daniels, C.R.; Dalal, S.; Singh, M.; Singh, K. Deficiency of ataxia telangiectasia mutated kinase modulates cardiac remodeling following myocardial infarction: Involvement in fibrosis and apoptosis. PLoS ONE 2013, 8, e83513. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yates, C.C.; Lockyer, P.; Xie, L.; Bevilacqua, A.; He, J.; Lander, C.; Patterson, C.; Willis, M. MMI-0100 inhibits cardiac fibrosis in myocardial infarction by direct actions on cardiomyocytes and fibroblasts via MK2 inhibition. J. Mol. Cell. Cardiol. 2014, 77, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, H.; Takai, S.; Tsuneyoshi, H.; Nishina, T.; Yoshikawa, K.; Miyazaki, M.; Ikeda, T.; Komeda, M. Chymase inhibition prevents cardiac fibrosis and dysfunction after myocardial infarction in rats. Hypertens. Res. 2006, 29, 57–64. [Google Scholar] [CrossRef]
- Fan, G.P.; Wang, W.; Zhao, H.; Cai, L.; Zhang, P.D.; Yang, Z.H.; Zhang, J.; Wang, X. Pharmacological Inhibition of Focal Adhesion Kinase Attenuates Cardiac Fibrosis in Mice Cardiac Fibroblast and Post-Myocardial-Infarction Models. Cell. Physiol. Biochem. 2015, 37, 515–526. [Google Scholar] [CrossRef]
- Emery, A.E. The muscular dystrophies. Lancet 2002, 359, 687–695. [Google Scholar] [CrossRef]
- Silva, M.C.; Magalhães, T.A.; Meira, Z.M.; Rassi, C.H.; Andrade, A.C.; Gutierrez, P.S.; Azevedo, C.F.; Gurgel-Giannetti, J.; Vainzof, M.; Zatz, M.; et al. Myocardial Fibrosis Progression in Duchenne and Becker Muscular Dystrophy: A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 190–199. [Google Scholar] [CrossRef]
- Semple, D.; Smith, K.; Bhandari, S.; Seymour, A.M. Uremic cardiomyopathy and insulin resistance: A critical role for akt? J. Am. Soc. Nephrol. 2011, 22, 207–215. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Gillings, N.; Flores, B.; Takahashi, M.; Kuro-O, M.; Moe, O.W. Recombinant α-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int. 2017, 91, 1104–1114. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.M.; Ji, J.L.; Gan, W.; Zhang, A.; Shi, H.J.; Wang, H.; Lv, L.; Li, Z.; Tang, T.; et al. Macrophage-Derived Exosomal Mir-155 Regulating Cardiomyocyte Pyroptosis and Hypertrophy in Uremic Cardiomyopathy. JACC Basic Transl. Sci. 2020, 5, 148–166, Erratum in JACC Basic Transl. Sci. 2020, 5, 547. [Google Scholar] [CrossRef]
- Lip, G.Y.; Tse, H.F.; Lane, D.A. Atrial fibrillation. Lancet 2012, 379, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Sohns, C.; Marrouche, N.F. Atrial fibrillation and cardiac fibrosis. Eur. Heart J. 2020, 41, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Owens, M.A.; Horten, B.C.; Da Silva, M.M. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin. Breast Cancer 2004, 5, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E., Jr.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- De Keulenaer, G.W.; Doggen, K.; Lemmens, K. The vulnerability of the heart as a pluricellular paracrine organ: Lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ. Res. 2010, 106, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Ewer, S.M. Cardiotoxicity of anticancer treatments. Nat. Rev. Cardiol. 2015, 12, 547–558, Erratum in Nat. Rev. Cardiol. 2015, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Rajesh, M.; Bátkai, S.; Kashiwaya, Y.; Haskó, G.; Liaudet, L.; Szabó, C.; Pacher, P. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1466–H1483. [Google Scholar] [CrossRef]
- Belmonte, F.; Das, S.; Sysa-Shah, P.; Sivakumaran, V.; Stanley, B.; Guo, X.; Paolocci, N.; Aon, M.A.; Nagane, M.; Kuppusamy, P.; et al. ErbB2 overexpression upregulates antioxidant enzymes, reduces basal levels of reactive oxygen species, and protects against doxorubicin cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1271–H1280. [Google Scholar] [CrossRef]
- Vejpongsa, P.; Yeh, E.T. Prevention of anthracycline-induced cardiotoxicity: Challenges and opportunities. J. Am. Coll. Cardiol. 2014, 64, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Sochalski, J.; Jaarsma, T.; Krumholz, H.M.; Laramee, A.; McMurray, J.J.; Naylor, M.D.; Rich, M.W.; Riegel, B.; Stewart, S. What works in chronic care management: The case of heart failure. Health Aff. 2009, 28, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, P.S.; Polegato, B.F.; Minicucci, M.F.; Paiva, S.A.; Zornoff, L.A. Cardiac Remodeling: Concepts, Clinical Impact, Pathophysiological Mechanisms and Pharmacologic Treatment. Arq. Bras. Cardiol. 2016, 106, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Kramer, D.G.; Patel, A.R.; Maron, M.S.; Udelson, J.E. Left ventricular remodeling in heart failure: Current concepts in clinical significance and assessment. JACC Cardiovasc. Imaging 2011, 4, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Schmitter, D.; Cotter, G.; Voors, A.A. Clinical use of novel biomarkers in heart failure: Towards personalized medicine. Heart Fail. Rev. 2014, 19, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Vegter, E.L.; van der Meer, P.; de Windt, L.J.; Pinto, Y.M.; Voors, A.A. MicroRNAs in heart failure: From biomarker to target for therapy. Eur. J. Heart Fail. 2016, 18, 457–468. [Google Scholar] [CrossRef]
- Huber, H.J.; Holvoet, P. Exosomes: Emerging roles in communication between blood cells and vascular tissues during atherosclerosis. Curr. Opin. Lipidol. 2015, 26, 412–419. [Google Scholar] [CrossRef]
- Trams, E.G.; Lauter, C.J.; Salem, N., Jr.; Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Gruenberg, J.; van der Goot, F.G. Mechanisms of pathogen entry through the endosomal compartments. Nat. Rev. Mol. Cell Biol. 2006, 7, 495–504. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Gusachenko, O.N.; Zenkova, M.A.; Vlassov, V.V. Nucleic acids in exosomes: Disease markers and intercellular communication molecules. Biochemistry 2013, 78, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sadik, N.; Cruz, L.; Gurtner, A.; Rodosthenous, R.S.; Dusoswa, S.A.; Ziegler, O.; Van Solinge, T.S.; Wei, Z.; Salvador-Garicano, A.M.; Gyorgy, B.; et al. Extracellular RNAs: A New Awareness of Old Perspectives. Methods Mol. Biol. 2018, 1740, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.P., Jr.; Zigon, E.; Rogers, G.; Davodian, D.; Lu, S.; Jovanovic-Talisman, T.; Jones, J.; Tigges, J.; Tyagi, S.; Ghiran, I.C. Detection of Extracellular Vesicle RNA Using Molecular Beacons. iScience 2020, 23, 100782. [Google Scholar] [CrossRef] [PubMed]
- Churov, A.; Summerhill, V.; Grechko, A.; Orekhova, V.; Orekhov, A. MicroRNAs as Potential Biomarkers in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 5547. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- Hill, M.; Tran, N. miRNA interplay: Mechanisms and consequences in cancer. Dis. Models Mech. 2021, 14, dmm047662. [Google Scholar] [CrossRef] [PubMed]
- Stoorvogel, W. Functional transfer of microRNA by exosomes. Blood 2012, 119, 646–648. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Fabbri, M.; Paone, A.; Calore, F.; Galli, R.; Gaudio, E.; Santhanam, R.; Lovat, F.; Fadda, P.; Mao, C.; Nuovo, G.J.; et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA 2012, 109, E2110–E2116. [Google Scholar] [CrossRef]
- Sahoo, S.; Adamiak, M.; Mathiyalagan, P.; Kenneweg, F.; Kafert-Kasting, S.; Thum, T. Therapeutic and Diagnostic Translation of Extracellular Vesicles in Cardiovascular Diseases: Roadmap to the Clinic. Circulation 2021, 143, 1426–1449. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Klychko, E.; Thorne, T.; Misener, S.; Schultz, K.M.; Millay, M.; Ito, A.; Liu, T.; Kamide, C.; Agrawal, H.; et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ. Res. 2011, 109, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.R.; Klyachko, E.; Thorne, T.; Schultz, K.M.; Millay, M.; Ito, A.; Kamide, C.E.; Liu, T.; Gupta, R.; Sahoo, S.; et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ. Res. 2012, 111, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Nickoloff, E.; Abramova, T.; Johnson, J.; Verma, S.K.; Krishnamurthy, P.; Mackie, A.R.; Vaughan, E.; Garikipati, V.N.S.; Benedict, C.; et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 2015, 117, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Tseliou, E.; Fouad, J.; Reich, H.; Slipczuk, L.; de Couto, G.; Aminzadeh, M.; Middleton, R.; Valle, J.; Weixin, L.; Marbán, E. Fibroblasts Rendered Antifibrotic, Antiapoptotic, and Angiogenic by Priming With Cardiosphere-Derived Extracellular Membrane Vesicles. J. Am. Coll. Cardiol. 2015, 66, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Garikipati, V.N.S.; Shoja-Taheri, F.; Davis, M.E.; Kishore, R. Extracellular Vesicles and the Application of System Biology and Computational Modeling in Cardiac Repair. Circ. Res. 2018, 123, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Chen, G.H.; Yang, Y.J. Exosomes: A Rising Star in Falling Hearts. Front. Physiol. 2017, 8, 494, Erratum in Front. Physiol. 2017, 8, 620. [Google Scholar] [CrossRef] [PubMed]
- Cosme, J.; Guo, H.; Hadipour-Lakmehsari, S.; Emili, A.; Gramolini, A.O. Hypoxia-Induced Changes in the Fibroblast Secretome, Exosome, and Whole-Cell Proteome Using Cultured, Cardiac-Derived Cells Isolated from Neonatal Mice. J. Proteome Res. 2017, 16, 2836–2847. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, L.; Feng, Z.; Chen, W.; Yan, S.; Yang, R.; Xiao, J.; Gao, J.; Zhang, D.; Ke, X. EPC-Derived Exosomal miR-1246 and miR-1290 Regulate Phenotypic Changes of Fibroblasts to Endothelial Cells to Exert Protective Effects on Myocardial Infarction by Targeting ELF5 and SP1. Front. Cell Dev. Biol. 2021, 9, 647763. [Google Scholar] [CrossRef]
- Lin, F.; Zeng, Z.; Song, Y.; Li, L.; Wu, Z.; Zhang, X.; Li, Z.; Ke, X.; Hu, X. YBX-1 mediated sorting of miR-133 into hypoxia/reoxygenation-induced EPC-derived exosomes to increase fibroblast angiogenesis and MEndoT. Stem Cell Res. Ther. 2019, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Yang, R.; Wu, F.; Wang, X.; Liang, J.; Hu, X.; Hu, C. Exosomal miR-218-5p/miR-363-3p from Endothelial Progenitor Cells Ameliorate Myocardial Infarction by Targeting the p53/JMY Signaling Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 5529430. [Google Scholar] [CrossRef] [PubMed]
- Bier, A.; Berenstein, P.; Kronfeld, N.; Morgoulis, D.; Ziv-Av, A.; Goldstein, H.; Kazimirsky, G.; Cazacu, S.; Meir, R.; Popovtzer, R.; et al. Placenta-derived mesenchymal stromal cells and their exosomes exert therapeutic effects in Duchenne muscular dystrophy. Biomaterials 2018, 174, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, W.; Wani, M.; Yu, X.; Ashraf, M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE 2014, 9, e88685. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Guo, D.; Liu, G.; Chen, G.; Hang, M.; Jin, M. Exosomes from MiR-126-Overexpressing Adscs Are Therapeutic in Relieving Acute Myocardial Ischaemic Injury. Cell. Physiol. Biochem. 2017, 44, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Alimujiang, M.; Chen, Q.; Shi, H.; Luo, X. Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction-induced myocardial damage via downregulation of early growth response factor 1. J. Cell. Biochem. 2019, 120, 4433–4443. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, K.; Zhang, N.; Zhao, Y.; Ma, Q.; Shen, J.; Lin, Y.; Xiang, P.; Tang, Y.; Hu, X.; et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1659–1670. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, Y.; Pan, X.; Liu, B.; Liang, C.; Zhang, Y.; Wang, Y.; Yan, B.; Xie, W.; Sun, Y.; et al. Knockout of beta-2 microglobulin enhances cardiac repair by modulating exosome imprinting and inhibiting stem cell-induced immune rejection. Cell. Mol. Life Sci. 2020, 77, 937–952. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Wu, C.; Liu, W.; He, Y.; Yang, Q. Adipose-Derived Mesenchymal Stem Cells-Derived Exosomes Carry MicroRNA-671 to Alleviate Myocardial Infarction Through Inactivating the TGFBR2/Smad2 Axis. Inflammation 2021, 44, 1815–1830. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Liu, L.; Xi, A.; Chen, B.; Li, Y.; Du, J. Macrophage-Derived mir-155-Containing Exosomes Suppress Fibroblast Proliferation and Promote Fibroblast Inflammation during Cardiac Injury. Mol. Ther. 2017, 25, 192–204. [Google Scholar] [CrossRef]
- Gollmann-Tepeköylü, C.; Pölzl, L.; Graber, M.; Hirsch, J.; Nägele, F.; Lobenwein, D.; Hess, M.W.; Blumer, M.J.; Kirchmair, E.; Zipperle, J.; et al. miR-19a-3p containing exosomes improve function of ischaemic myocardium upon shock wave therapy. Cardiovasc. Res. 2020, 116, 1226–1236. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H.; Mai, J.; Chen, Z.; Huang, T.; Wang, S.; Chen, Y.; Wang, J. Distinct Anti-Fibrotic Effects of Exosomes Derived from Endothelial Colony-Forming Cells Cultured Under Normoxia and Hypoxia. Med. Sci. Monit. 2018, 24, 6187–6199. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.; Xue, F.; Li, Y.; Liu, W.; Zhang, S. Exosomes derived from cardiomyocytes promote cardiac fibrosis via myocyte-fibroblast cross-talk. Am. J. Transl. Res. 2018, 10, 4350–4366. [Google Scholar]
- Tang, C.; Hou, Y.X.; Shi, P.X.; Zhu, C.H.; Lu, X.; Wang, X.L.; Que, L.L.; Zhu, G.Q.; Liu, L.; Chen, Q.; et al. Cardiomyocyte-specific Peli1 contributes to the pressure overload-induced cardiac fibrosis through miR-494-3p-dependent exosomal communication. FASEB J. 2023, 37, e22699, Erratum in FASEB J. 2023, 37, e22860. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, H.; Gao, W.; Zhang, L.; Ye, Y.; Yuan, L.; Ding, Z.; Wu, J.; Kang, L.; Zhang, X.; et al. MicroRNA-378 suppresses myocardial fibrosis through a paracrine mechanism at the early stage of cardiac hypertrophy following mechanical stress. Theranostics 2018, 8, 2565–2582. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Kalani, A.; Medina, I.; Familtseva, A.; Tyagi, S.C. Cardiosome mediated regulation of MMP9 in diabetic heart: Role of mir29b and mir455 in exercise. J. Cell. Mol. Med. 2015, 19, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Yan, S.; Zhao, X.; Han, X.; Fang, N.; Zhang, Y.; Dai, C.; Li, W.; Yu, H.; Gao, Y.; et al. Atrial myocyte-derived exosomal microRNA contributes to atrial fibrosis in atrial fibrillation. J. Transl. Med. 2022, 20, 407. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Q.; Jiao, H. LncRNA NRON promotes M2 macrophage polarization and alleviates atrial fibrosis through suppressing exosomal miR-23a derived from atrial myocytes. J. Formos. Med. Assoc. 2021, 120, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.G.E.; Li, C.; Rogers, R.; Fournier, M.; Li, L.; Vaturi, S.D.; Antes, T.; Sanchez, L.; Akhmerov, A.; Moseley, J.J.; et al. Augmenting canonical Wnt signalling in therapeutically inert cells converts them into therapeutically potent exosome factories. Nat. Biomed. Eng. 2019, 3, 695–705. [Google Scholar] [CrossRef]
- Izarra, A.; Moscoso, I.; Levent, E.; Cañón, S.; Cerrada, I.; Díez-Juan, A.; Blanca, V.; Núñez-Gil, I.J.; Valiente, I.; Ruíz-Sauri, A.; et al. miR-133a enhances the protective capacity of cardiac progenitors cells after myocardial infarction. Stem Cell Rep. 2014, 3, 1029–1042. [Google Scholar] [CrossRef]
- Milano, G.; Biemmi, V.; Lazzarini, E.; Balbi, C.; Ciullo, A.; Bolis, S.; Ameri, P.; Di Silvestre, D.; Mauri, P.; Barile, L.; et al. Intravenous administration of cardiac progenitor cell-derived exosomes protects against doxorubicin/trastuzumab-induced cardiac toxicity. Cardiovasc. Res. 2020, 116, 383–392. [Google Scholar] [CrossRef]
- Xuan, W.; Wang, L.; Xu, M.; Weintraub, N.L.; Ashraf, M. miRNAs in Extracellular Vesicles from iPS-Derived Cardiac Progenitor Cells Effectively Reduce Fibrosis and Promote Angiogenesis in Infarcted Heart. Stem Cells Int. 2019, 2019, 3726392. [Google Scholar] [CrossRef]
- Cai, L.; Chao, G.; Li, W.; Zhu, J.; Li, F.; Qi, B.; Wei, Y.; Chen, S.; Zhou, G.; Lu, X.; et al. Activated CD4+ T cells-derived exosomal miR-142-3p boosts post-ischemic ventricular remodeling by activating myofibroblast. Aging 2020, 12, 7380–7396. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Park, H.; Kim, H.; Mun, D.; Park, H.; Yun, N.; Joung, B. Human peripheral blood-derived exosomes for microRNA delivery. Int. J. Mol. Med. 2019, 43, 2319–2328, Erratum in Int. J. Mol. Med. 2019, 44, 358. [Google Scholar] [CrossRef]
- Wang, Q.G.; Cheng, B.C.; He, Y.Z.; Li, L.J.; Ling, Y.; Luo, G.; Wang, L.; Liang, S.; Zhang, Y. miR-320a in serum exosomes promotes myocardial fibroblast proliferation via regulating the PIK3CA/Akt/mTOR signaling pathway in HEH2 cells. Exp. Ther. Med. 2021, 22, 873. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Xu, B.; Liu, Y.L.; Liu, Z. Reduced exosome miR-425 and miR-744 in the plasma represents the progression of fibrosis and heart failure. Kaohsiung J. Med. Sci. 2018, 34, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Li, H.; Zhang, A.; Li, Z.; Zhang, Y.; Ren, M.; Zhang, Y.; Hou, Y. MicroRNAs sequencing of plasma exosomes derived from patients with atrial fibrillation: miR-124-3p promotes cardiac fibroblast activation and proliferation by regulating AXIN1. J. Physiol. Biochem. 2022, 78, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Li, W.; Yuan, Y.; Liu, S.; Liang, C.; Liu, H.E.; Zhang, R.; Liu, Y.; Sun, L.I.; Wei, Y.; et al. Epididymal white adipose tissue promotes angiotensin II-induced cardiac fibrosis in an exosome-dependent manner. Transl. Res. 2022, 248, 51–67. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, H.; Liu, C.; Shao, L.; Zhang, H.; Lu, K.; Wang, J.; Wang, Y.; Yu, Q.; Zhang, Y.; et al. Microneedle Patch Loaded with Exosomes Containing MicroRNA-29b Prevents Cardiac Fibrosis after Myocardial Infarction. Adv. Healthc. Mater. 2023, 12, e2202959. [Google Scholar] [CrossRef]
- Liu, L.; Luo, F.; Lei, K. Exosomes Containing LINC00636 Inhibit MAPK1 through the miR-450a-2-3p Overexpression in Human Pericardial Fluid and Improve Cardiac Fibrosis in Patients with Atrial Fibrillation. Mediat. Inflamm. 2021, 2021, 9960241. [Google Scholar] [CrossRef]
- Zucconi, E.; Vieira, N.M.; Bueno, C.R., Jr.; Secco, M.; Jazedje, T.; Costa Valadares, M.; Fussae Suzuki, M.; Bartolini, P.; Vainzof, M.; Zatz, M. Preclinical studies with umbilical cord mesenchymal stromal cells in different animal models for muscular dystrophy. J. Biomed. Biotechnol. 2011, 2011, 715251. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Di Giuseppe, M.; Njah, J.; Sala, E.; Shiva, S.; St Croix, C.M.; Stolz, D.B.; Watkins, S.C.; Di, Y.P.; Leikauf, G.D.; et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015, 6, 8472. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, S.H.; Levy, O.; Inamdar, M.S.; Karp, J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 2012, 10, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Chen, L.; Chen, W.; Yang, J.; Yang, Z.; Shen, Z. Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cell. Physiol. Biochem. 2015, 37, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Joo, H.J.; Kim, M.; Choi, S.C.; Lee, J.I.; Hong, S.J.; Lim, D.S. Transplantation of Adipose-Derived Stem Cell Sheet Attenuates Adverse Cardiac Remodeling in Acute Myocardial Infarction. Tissue Eng. Part A 2017, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Lee, H.C.; Park, J.H.; Kim, B.W.; Ahn, J.; Kim, J.H.; Park, J.S.; Oh, J.H.; Choi, J.H.; Cha, K.S.; et al. Effects of Intracoronary Administration of Autologous Adipose Tissue-Derived Stem Cells on Acute Myocardial Infarction in a Porcine Model. Yonsei Med. J. 2015, 56, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, Y.; Zhong, Z.; Wu, Y.; Zhao, J.; Wang, Y.; Cheng, H.; Kong, M.; Zhang, F.; Chen, Q.; et al. A Large-Scale Investigation of Hypoxia-Preconditioned Allogeneic Mesenchymal Stem Cells for Myocardial Repair in NonA Large-Scale Investigation of Hypoxia-Preconditioned Allogeneic Mesenchymal Stem Cells for Myocardial Repair in Nonhuman Primates: Paracrine Activity without Remuscularization. Circ. Res. 2016, 118, 970–983. [Google Scholar] [CrossRef]
- Hu, X.; Wu, R.; Shehadeh, L.A.; Zhou, Q.; Jiang, C.; Huang, X.; Zhang, L.; Gao, F.; Liu, X.; Yu, H.; et al. Severe hypoxia exerts parallel and cell-specific regulation of gene expression and alternative splicing in human mesenchymal stem cells. BMC Genom. 2014, 15, 303. [Google Scholar] [CrossRef]
- Hu, X.; Wu, R.; Jiang, Z.; Wang, L.; Chen, P.; Zhang, L.; Yang, L.; Wu, Y.; Chen, H.; Chen, H.; et al. Leptin signaling is required for augmented therapeutic properties of mesenchymal stem cells conferred by hypoxia preconditioning. Stem Cells 2014, 32, 2702–2713, Erratum in Stem Cells 2014, 32, 328. [Google Scholar] [CrossRef]
- Sahoo, S.; Losordo, D.W. Exosomes and cardiac repair after myocardial infarction. Circ. Res. 2014, 114, 333–344. [Google Scholar] [CrossRef]
- Lv, Z.; Yang, L. MiR-124 inhibits the growth of glioblastoma through the downregulation of SOS1. Mol. Med. Rep. 2013, 8, 345–349. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Z.; Qu, C.; Cui, W.; Wang, X.; Du, J. CD8 T cells are involved in skeletal muscle regeneration through facilitating MCP-1 secretion and Gr1(high) macrophage infiltration. J. Immunol. 2014, 193, 5149–5160. [Google Scholar] [CrossRef]
- Frairia, R.; Berta, L. Biological effects of extracorporeal shock waves on fibroblasts. A review. Muscles Ligaments Tendons J. 2012, 1, 138–147. [Google Scholar] [PubMed]
- Tepeköylü, C.; Primessnig, U.; Pölzl, L.; Graber, M.; Lobenwein, D.; Nägele, F.; Kirchmair, E.; Pechriggl, E.; Grimm, M.; Holfeld, J. Shockwaves prevent from heart failure after acute myocardial ischaemia via RNA/protein complexes. J. Cell. Mol. Med. 2017, 21, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.; Sarkar, S.; Vellaichamy, E.; Sen, S. Role of myocytes in myocardial collagen production. Hypertension 2001, 37, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhu, Y.; Li, J.; Liu, J.; Gao, Y.; Ha, T.; Que, L.; Liu, L.; Zhu, G.; Chen, Q.; et al. Pellino1-mediated TGF-β1 synthesis contributes to mechanical stress induced cardiac fibroblast activation. J. Mol. Cell. Cardiol. 2015, 79, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Fiuza-Luces, C.; Garatachea, N.; Berger, N.A.; Lucia, A. Exercise is the real polypill. Physiology 2013, 28, 330–358. [Google Scholar] [CrossRef] [PubMed]
- Moshal, K.S.; Rodriguez, W.E.; Sen, U.; Tyagi, S.C. Targeted deletion of MMP-9 attenuates myocardial contractile dysfunction in heart failure. Physiol. Res. 2008, 57, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Chavali, V.; Metreveli, N.; Tyagi, S.C. Ablation of MMP9 induces survival and differentiation of cardiac stem cells into cardiomyocytes in the heart of diabetics: A role of extracellular matrix. Can. J. Physiol. Pharmacol. 2012, 90, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Shon, S.M.; Park, J.H.; Nahrendorf, M.; Schellingerhout, D.; Kim, J.Y.; Kang, B.T.; Jeong, S.W.; Kim, E.J.; Ryu, J.H.; Kim, K.; et al. Exercise attenuates matrix metalloproteinase activity in preexisting atherosclerotic plaque. Atherosclerosis 2011, 216, 67–73. [Google Scholar] [CrossRef]
- Kreke, M.; Smith, R.R.; Marbán, L.; Marbán, E. Cardiospheres and cardiosphere-derived cells as therapeutic agents following myocardial infarction. Expert Rev. Cardiovasc. Ther. 2012, 10, 1185–1194, Erratum in Expert Rev. Cardiovasc. Ther. 2013, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.R.; Barile, L.; Cho, H.C.; Leppo, M.K.; Hare, J.M.; Messina, E.; Giacomello, A.; Abraham, M.R.; Marbán, E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 2007, 115, 896–908. [Google Scholar] [CrossRef]
- Chimenti, I.; Smith, R.R.; Li, T.S.; Gerstenblith, G.; Messina, E.; Giacomello, A.; Marbán, E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ. Res. 2010, 106, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Sanganalmath, S.K.; Bolli, R. Cell therapy for heart failure: A comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ. Res. 2013, 113, 810–834. [Google Scholar] [CrossRef] [PubMed]
- Chugh, A.R.; Beache, G.M.; Loughran, J.H.; Mewton, N.; Elmore, J.B.; Kajstura, J.; Pappas, P.; Tatooles, A.; Stoddard, M.F.; Lima, J.A.; et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: The SCIPIO trial: Surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 2012, 126 (Suppl. S1), S54–S64. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.G.; Cheng, K.; Marbán, E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2014, 2, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Mayhall, E.A.; Paffett-Lugassy, N.; Zon, L.I. The clinical potential of stem cells. Curr. Opin. Cell Biol. 2004, 16, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Lalit, P.A.; Salick, M.R.; Nelson, D.O.; Squirrell, J.M.; Shafer, C.M.; Patel, N.G.; Saeed, I.; Schmuck, E.G.; Markandeya, Y.S.; Wong, R.; et al. Lineage Reprogramming of Fibroblasts into Proliferative Induced Cardiac Progenitor Cells by Defined Factors. Cell Stem Cell 2016, 18, 354–367. [Google Scholar] [CrossRef]
- Menasché, P.; Vanneaux, V.; Fabreguettes, J.R.; Bel, A.; Tosca, L.; Garcia, S.; Bellamy, V.; Farouz, Y.; Pouly, J.; Damour, O.; et al. Towards a clinical use of human embryonic stem cell-derived cardiac progenitors: A translational experience. Eur. Heart J. 2015, 36, 743–750. [Google Scholar] [CrossRef]
- Savvatis, K.; Pappritz, K.; Becher, P.M.; Lindner, D.; Zietsch, C.; Volk, H.D.; Westermann, D.; Schultheiss, H.P.; Tschöpe, C. Interleukin-23 deficiency leads to impaired wound healing and adverse prognosis after myocardial infarction. Circ. Heart Fail. 2014, 7, 161–171. [Google Scholar] [CrossRef]
- Laroumanie, F.; Douin-Echinard, V.; Pozzo, J.; Lairez, O.; Tortosa, F.; Vinel, C.; Delage, C.; Calise, D.; Dutaur, M.; Parini, A.; et al. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation 2014, 129, 2111–2124. [Google Scholar] [CrossRef]
- Roy, S.; Khanna, S.; Hussain, S.R.; Biswas, S.; Azad, A.; Rink, C.; Gnyawali, S.; Shilo, S.; Nuovo, G.J.; Sen, C.K. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc. Res. 2009, 82, 21–29. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, H.; Ge, D.; Xu, Y.; Xu, H.; Yang, Y.; Gu, M.; Zhou, Y.; Zhu, J.; Ge, T.; et al. Mir-21 Promotes Cardiac Fibrosis After Myocardial Infarction Via Targeting Smad7. Cell. Physiol. Biochem. 2017, 42, 2207–2219. [Google Scholar] [CrossRef]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Schellings, M.W.; Vanhoutte, D.; van Almen, G.C.; Swinnen, M.; Leenders, J.J.; Kubben, N.; van Leeuwen, R.E.; Hofstra, L.; Heymans, S.; Pinto, Y.M. Syndecan-1 amplifies angiotensin II-induced cardiac fibrosis. Hypertension 2010, 55, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, P.J.; van Pelt, J.F.; Fagard, R.H. Stimulation of reactive oxygen species and collagen synthesis by angiotensin II in cardiac fibroblasts. Cardiovasc. Ther. 2012, 30, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Poyan Mehr, A.; Kreutz, R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.E. The many secret lives of adipocytes: Implications for diabetes. Diabetologia 2019, 62, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Sawaki, D.; Czibik, G.; Pini, M.; Ternacle, J.; Suffee, N.; Mercedes, R.; Marcelin, G.; Surenaud, M.; Marcos, E.; Gual, P.; et al. Visceral Adipose Tissue Drives Cardiac Aging Through Modulation of Fibroblast Senescence by Osteopontin Production. Circulation 2018, 138, 809–822. [Google Scholar] [CrossRef]
- Chevillet, J.R.; Kang, Q.; Ruf, I.K.; Briggs, H.A.; Vojtech, L.N.; Hughes, S.M.; Cheng, H.H.; Arroyo, J.D.; Meredith, E.K.; Gallichotte, E.N.; et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. USA 2014, 111, 14888–14893. [Google Scholar] [CrossRef]
- Wei, Z.; Batagov, A.O.; Schinelli, S.; Wang, J.; Wang, Y.; El Fatimy, R.; Rabinovsky, R.; Balaj, L.; Chen, C.C.; Hochberg, F.; et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat. Commun. 2017, 8, 1145. [Google Scholar] [CrossRef] [PubMed]
- Kogure, T.; Yan, I.K.; Lin, W.L.; Patel, T. Extracellular Vesicle-Mediated Transfer of a Novel Long Noncoding RNA TUC339: A Mechanism of Intercellular Signaling in Human Hepatocellular Cancer. Genes Cancer 2013, 4, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
| The Origin of Exosomes | miRNA | Target Gene/Pathway | Role | Disease Models | References |
|---|---|---|---|---|---|
| Endothelial progenitor cells | miR-1246, miR-1290 | ELF5 and SP1 | Anti-fibrosis | Myocardial infarction | [60] |
| miR-133 | YBX-1 | Anti-fibrosis | Hypoxia/reoxygenation | [61] | |
| miR-218-5p, miR-363-3p | p53 and JMY | Anti-fibrosis | Myocardial infarction | [62] | |
| Mesenchymal stromal cells | miR-29c | TGF-β | Anti-fibrosis | Duchenne muscular dystrophy | [63] |
| miR-22 | Mecp2 | Anti-fibrosis | Ischemic heart disease | [64] | |
| miR-126 | Unknown | Anti-fibrosis | Acute myocardial infarction | [65] | |
| miR-146a | EGR1/TLR4/NFκB | Anti-fibrosis | Acute myocardial infarction | [66] | |
| miR-210 | HIF-1α | Anti-fibrosis | Myocardial infarction | [67] | |
| miR-24 | Bim | Anti-fibrosis | Myocardial infarction | [68] | |
| miR-671 | TGFBR2 | Anti-fibrosis | Myocardial infarction | [69] | |
| Macrophages | miR-155 | FoxO3a | Pro-fibrosis | Uremic cardiomyopathy | [70] |
| Endothelial cells | miR-19a-3p | Akt/ERK | Anti-fibrosis | Myocardial infarction | [71] |
| miR-10b-5p | Smurf1 and HDAC4 | Anti-fibrosis | Ischemic heart disease | [72] | |
| Cardiomyocytes | miR-208a | Dyrk2 | Pro-fibrosis | Fibrosis | [73] |
| miR-494-3p | PTEN | Pro-fibrosis | Fibrosis | [74] | |
| miR-378 | MKK6/P38 MAPK pathway | Anti-fibrosis | Fibrosis | [75] | |
| miR-29b, miR-455 | MMP9 | Anti-fibrosis | Type 2 diabete | [76] | |
| miR-210-3p | GPD1L/PI3K/AKT signaling pathway | Pro-fibrosis | Atrial fibrillation | [77] | |
| miR-23a | Unknown | Pro-fibrosis | Atrial fibrillation | [78] | |
| Cardiosphere-derived cells | miR-92a | BMP2 | Anti-fibrosis | Duchenne muscular dystrophy | [79] |
| Cardiac progenitor cells | miR-133a | Bim, Bmf, bFgf and Vegf | Anti-fibrosis | Myocardial infarction | [80] |
| miR-146a-5p | Traf6, Smad4, Irak1, Nox4, and Mpo | Anti-fibrosis | Doxorubicin/trastuzumab-induced cardiac toxicity | [81] | |
| Pluripotent stem cells | miR-290-295 cluster | Unknown | Anti-fibrosis | acute myocardial infarction | [55] |
| miR-373 | GDF-11 and ROCK-2 | Anti-fibrosis | Myocardial infarction | [82] | |
| Immune cells | miR-142-3p | APC/WNT | Pro-fibrosis | Myocardial infarction | [83] |
| Serum | miR-21 | Smad7, PTEN and MMP2 | Pro-fibrosis | Myocardial infarction | [84] |
| miR-320a | PIK3CA/Akt/mTOR signaling pathway | Pro-fibrosis | Chronic heart failure | [85] | |
| miR-425, miR-744 | TGF-β1 | Anti-fibrosis | Heart failure | [86] | |
| miR-124-3p | AXIN1/WNT/β-catenin signaling pathway | Pro-fibrosis | Atrial fibrillation | [87] | |
| Adipocytes | miR-23a-3p | RAP1 | Pro-fibrosis | Fibrosis | [88] |
| Others | miR-29b | Unknown | Anti-fibrosis | Myocardial infarction | [89] |
| miR-450a-2-3p | MAPK1 | Anti-fibrosis | Atrial Fibrillation | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Leng, M.; Tang, W.; Cai, Z.; Yang, L.; Wang, L.; Zhang, Y.; Guo, J. The Roles of Exosome-Derived microRNAs in Cardiac Fibrosis. Molecules 2024, 29, 1199. https://doi.org/10.3390/molecules29061199
Tang X, Leng M, Tang W, Cai Z, Yang L, Wang L, Zhang Y, Guo J. The Roles of Exosome-Derived microRNAs in Cardiac Fibrosis. Molecules. 2024; 29(6):1199. https://doi.org/10.3390/molecules29061199
Chicago/Turabian StyleTang, Xinyuan, Mingyang Leng, Wenyue Tang, Zhenlu Cai, Lin Yang, Liang Wang, Yue Zhang, and Jiao Guo. 2024. "The Roles of Exosome-Derived microRNAs in Cardiac Fibrosis" Molecules 29, no. 6: 1199. https://doi.org/10.3390/molecules29061199
APA StyleTang, X., Leng, M., Tang, W., Cai, Z., Yang, L., Wang, L., Zhang, Y., & Guo, J. (2024). The Roles of Exosome-Derived microRNAs in Cardiac Fibrosis. Molecules, 29(6), 1199. https://doi.org/10.3390/molecules29061199





