Antimicrobial Activity of Syzygium aromaticum Essential Oil in Human Health Treatment
Abstract
1. Introduction
1.1. Syzygium Aromaticum: Botanical Aspects
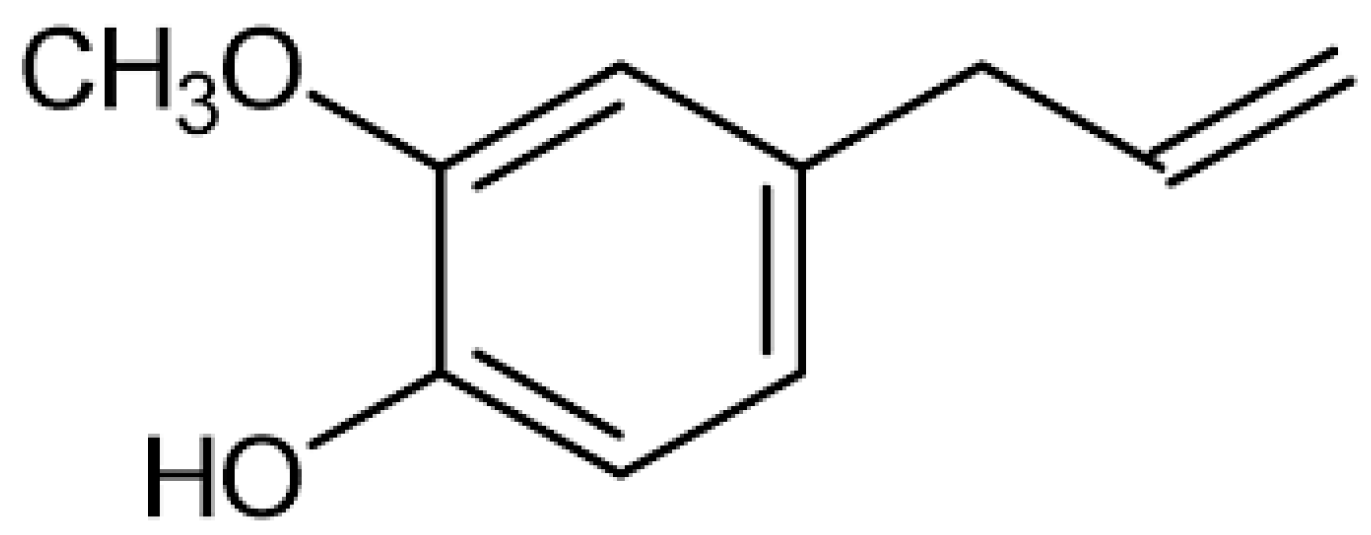
1.2. Chemical Composition of S. aromaticum EO
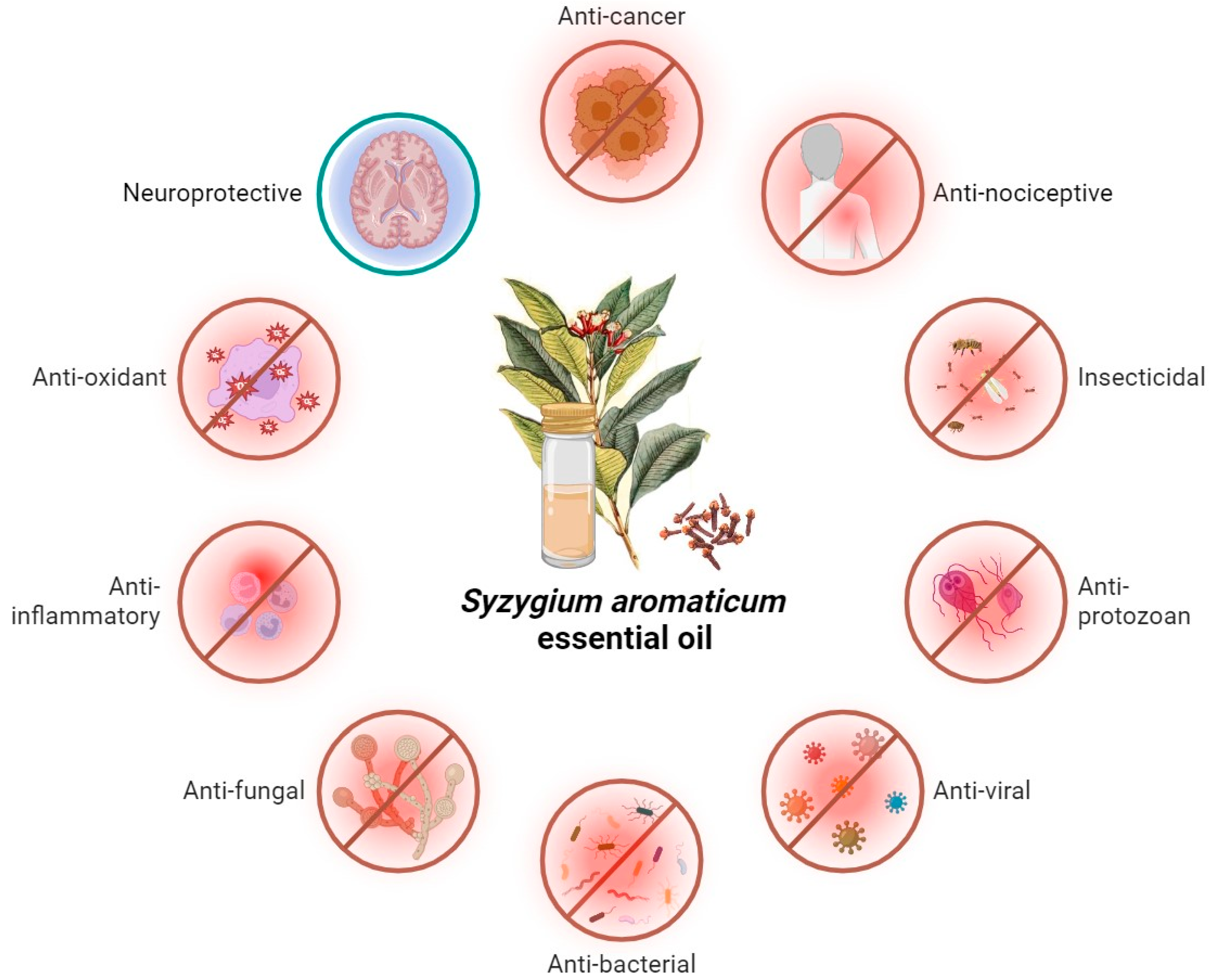
2. Biological Activities of S. aromaticum
2.1. Antimicrobial Activity of S. aromaticum EO
2.1.1. Antibacterial Activity
| Year | Product from S. aromaticum | Biological Activities | Mechanisms of Action | Reference |
|---|---|---|---|---|
| 2021 | Essential oil hydrodistilled from buds (São Luís, Maranhão, Brazil) | antioxidant, antibacterial, antitrypanosomal | membrane protein inactivation by eugenol hydroxyl group, cell membrane ruptures, modulation of NO production, downregulation of NF-kB and AP-1 | [14] |
| 2018 | Solid lipid nanoparticles containing essential oil, water-distilled from buds (Karaj city, Alborz province, Iran) | antibacterial, antifungal | interaction with microbial cell membrane | [17] |
| 2021 | Essential oil-encapsulated nanofiber formulation (Sigma Aldrich, Darmstadt, Germany) | antibacterial, wound healing | interaction with microbial cell membrane, antioxidant effect | [18] |
| 2020 | Essential oil (Fagron, Wichita, KS, USA, L150102014) | antibacterial | interaction with microbial cell membrane, synergy with antibiotics | [19] |
| 2017 | Essential oil (Apple Flavor & Fragrance Group Co. Ltd., Shanghai, China) | antibacterial | modulation of cell membrane permeability, biofilm inhibition | [20] |
| 2010 | Essential oil, hydro-distilled from buds | antibacterial, antifungal, anticarcinogenic | interaction with microbial cell membrane, eugenol cytotoxicity | [21] |
| 2007 | Essential oil, hydro-distilled from buds (Monastir, Tunisia) | antioxidant, antifungal, antibiofilm | free radical quenching, interaction with microbial cell membrane, | [22] |
| 2005 | Essential oil | antifungal | interaction with microbial cell membrane | [23] |
| 2018 | Vaginal gel containing plant extracts | antibacterial, antifungal, antibiofilm | interaction with microbial cell membrane | [24] |
| 2011 | Essential oil, obtained from buds (Segredo da Planta’, Paio Pires, Portugal) | anti-Giardia | Loss of cell polarity, adherence inhibition | [25] |
2.1.2. Nanofibers and Nanoparticle Formulations
2.1.3. Nosocomial Pathogens
2.1.4. S. aromaticum EO in the Treatment of Oral Cavity Diseases
2.1.5. Antifungal Activity against Different Species of Candida
2.1.6. Bacterial Vaginosis and Vulvovaginal Candidiasis
2.1.7. Anti-Giardia Activity
3. Conclusions
4. Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Firenzuoli, F. Fitoterapia Guida All’uso Clinico Delle Piante Medicinali, 4th ed.; Edra S.p.A: Milan, Italy, 2008. [Google Scholar]
- Nuñez, L.; D’Aquino, M. Microbicide Activity of Clove Essential Oil (Eugenia caryophyllata). Braz. J. Microbiol. 2012, 43, 1255–1260. [Google Scholar] [CrossRef]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Ben Kahla-Nakbi, A.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The Chemical Composition and Biological Activity of Clove Essential Oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A Short Review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef] [PubMed]
- MacLean, R.C.; Millan, A.S. The Evolution of Antibiotic Resistance. Science 2019, 365, 1082–1083. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Mariani, M.; Condò, C.; Sabia, C.; Messi, P. Essential Oils: A Natural Weapon against Antibiotic-Resistant Bacteria Responsible for Nosocomial Infections. Antibiotics 2021, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Rojas, D.F.; de Souza, C.R.F.; Oliveira, W.P. Clove (Syzygium aromaticum): A Precious Spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Maugini, E.; Maleci Bini, L.; Mariotti Lippi, M. Botanica Farmaceutica, 9th ed.; Piccin: Padova, Italy, 2014. [Google Scholar]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Hastuti, L.T.; Saepudin, E.; Cahyana, A.H.; Rahayu, D.U.C.; Murni, V.W.; Haib, J. The Influence of Sun Drying Process and Prolonged Storage on Composition of Essential Oil from Clove Buds (Syzygium aromaticum). In AIP Conference Proceedings, Proceedings of the 2nd International Symposium on Current Progress in Mathematics and Sciences (ISCPMS 2016), Depok, Indonesia, 1–2 November 2016; Sugeng, K.A., Triyono, D., Mart, T., Eds.; American Institute of Physics Inc.: College Park, MD, USA, 2017; p. 030092. [Google Scholar]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological Properties and Health Benefits of Eugenol: A Comprehensive Review. Oxid. Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef]
- Khalil, A.A.; ur Rahman, U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential Oil Eugenol: Sources, Extraction Techniques and Nutraceutical Perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef]
- Pramod, K.; Ansari, S.H.; Ali, J. Eugenol: A Natural Compound with Versatile Pharmacological Actions. Nat. Prod. Commun. 2010, 5, 1999–2006. [Google Scholar] [CrossRef]
- Teles, A.M.; Silva-Silva, J.V.; Fernandes, J.M.P.; Abreu-Silva, A.L.; da Silva Calabrese, K.; Mendes Filho, N.E.; Mouchrek, A.N.; Almeida-Souza, F. GC-MS Characterization of Antibacterial, Antioxidant, and Antitrypanosomal Activity of Syzygium aromaticum Essential Oil and Eugenol. Evid.-Based Complement. Altern. Med. 2021, 2021, 6663255. [Google Scholar] [CrossRef] [PubMed]
- Briozzo, J.; Núncez, L.; Chirife, J.; Herszage, L.; D’aquino, M. Antimicrobial Activity of Clove Oil Dispersed in a Concentrated Sugar Solution. J. Appl. Bacteriol. 1989, 66, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.O.; Suisted, J.R. The Effects of Temperature and Growth Rate on the Proportion of Unsaturated Fatty Acids in Bacterial Lipids. J. Gen. Microbiol. 1978, 104, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Fazly Bazzaz, B.S.; Khameneh, B.; Namazi, N.; Iranshahi, M.; Davoodi, D.; Golmohammadzadeh, S. Solid Lipid Nanoparticles Carrying Eugenia caryophyllata Essential Oil: The Novel Nanoparticulate Systems with Broad-Spectrum Antimicrobial Activity. Lett. Appl. Microbiol. 2018, 66, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Rasul, A.; Waqas, M.; Saadullah, M.; Aslam, N.; Abbas, G.; Latif, S.; Afzal, H.; Inam, S.; Akhtar Shah, P. Formulation and Evaluation of a Clove Oil-Encapsulated Nanofiber Formulation for Effective Wound-Healing. Molecules 2021, 26, 2491. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Ucha, J.C.; Martínez-Guitián, M.; Lasarte-Monterrubio, C.; Conde-Pérez, K.; Arca-Suárez, J.; Álvarez-Fraga, L.; Pérez, A.; Crecente-Campo, J.; Alonso, M.J.; Bou, G.; et al. Syzygium aromaticum (Clove) and Thymus zygis (Thyme) Essential Oils Increase Susceptibility to Colistin in the Nosocomial Pathogens Acinetobacter baumannii and Klebsiella pneumoniae. Biomed. Pharmacother. 2020, 130, 110606. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhu, X.; Cao, P.; Wei, S.; Lu, Y. Antibacterial and Antibiofilm Activities of Eugenol from Essential Oil of Syzygium aromaticum (L.) Merr. & L. M. Perry (Clove) Leaf against Periodontal Pathogen Porphyromonas gingivalis. Microb. Pathog. 2017, 113, 396–402. [Google Scholar]
- Kouidhi, B.; Zmantar, T.; Bakhrouf, A. Anticariogenic and Cytotoxic Activity of Clove Essential Oil (Eugenia caryophyllata) against a Large Number of Oral Pathogens. Ann. Microbiol. 2010, 60, 599–604. [Google Scholar] [CrossRef]
- Chaieb, K.; Zmantar, T.; Ksouri, R.; Hajlaoui, H.; Mahdouani, K.; Abdelly, C.; Bakhrouf, A. Antioxidant Properties of the Essential Oil of Eugenia caryophyllata and Its Antifungal Activity against a Large Number of Clinical Candida Species. Mycoses 2007, 50, 403–406. [Google Scholar] [CrossRef]
- Gayoso, C.W.; Lima, E.O.; Oliveira, V.T.; Pereira, F.O.; Souza, E.L.; Lima, I.O.; Navarro, D.F. Sensitivity of Fungi Isolated from Onychomycosis to Eugenia cariophyllata Essential Oil and Eugenol. Fitoterapia 2005, 76, 247–249. [Google Scholar] [CrossRef]
- Murina, F.; Vicariotto, F.; Di Francesco, S. Thymol, Eugenol and Lactobacilli in a Medical Device for the Treatment of Bacterial Vaginosis and Vulvovaginal Candidiasis. New Microbiol. 2018, 41, 220–224. [Google Scholar]
- Machado, M.; Dinis, A.M.; Salgueiro, L.; Custódio, J.B.A.; Cavaleiro, C.; Sousa, M.C. Anti-Giardia Activity of Syzygium aromaticum Essential Oil and Eugenol: Effects on Growth, Viability, Adherence and Ultrastructure. Exp. Parasitol. 2011, 127, 732–739. [Google Scholar] [CrossRef]
- Lu, H.; Hong, T.; Jiang, Y.; Whiteway, M.; Zhang, S. Candidiasis: From Cutaneous to Systemic, New Perspectives of Potential Targets and Therapeutic Strategies. Adv. Drug Deliv. Rev. 2023, 199, 114960. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Boyle, C. What Are Endophytes? In Microbial Root Endophytes; Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 9, pp. 1–13. [Google Scholar]
- Kandel, S.L.; Joubert, P.M.; Doty, S.L. Bacterial Endophyte Colonization and Distribution within Plants. Microorganisms 2017, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.A. Endophytes as Sources of Bioactive Products. Microbes Infect. 2003, 5, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Klimova, E.; Rodríguez-Peña, K.; Sánchez, S. Endophytes as Sources of Antibiotics. Biochem. Pharmacol. 2017, 134, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Triandriani, W.; Sogandi; Saputri, D.D.; Suhendar, U. Antioxidant Activity of Endophytic Bacterial Extract Isolated from Clove Leaf (Syzygium aromaticum L.). J. Agric. Appl. Biol. 2020, 1, 9–17. [Google Scholar] [CrossRef]
- Utami, L.A.; Wahyuni, W.T.; Mubarik, N.R.; Astuti, R.I. Endophytic Bacteria of Clove (Syzygium aromaticum L.) Leaves Produce Metabolites with Antioxidant and Antiaging Properties. J. Appl. Pharm. Sci. 2023, 13, 241–250. [Google Scholar] [CrossRef]
- Castronovo, L.M.; Calonico, C.; Ascrizzi, R.; Del Duca, S.; Delfino, V.; Chioccioli, S.; Vassallo, A.; Strozza, I.; De Leo, M.; Biffi, S.; et al. The Cultivable Bacterial Microbiota Associated to the Medicinal Plant Origanum vulgare L.: From Antibiotic Resistance to Growth-Inhibitory Properties. Front. Microbiol. 2020, 11, 862. [Google Scholar] [CrossRef]
- Polito, G.; Semenzato, G.; Del Duca, S.; Castronovo, L.M.; Vassallo, A.; Chioccioli, S.; Borsetti, D.; Calabretta, V.; Puglia, A.M.; Fani, R.; et al. Endophytic Bacteria and Essential Oil from Origanum vulgare Ssp. Vulgare Share Some VOCs with an Antibacterial Activity. Microorganisms 2022, 10, 1424. [Google Scholar] [CrossRef] [PubMed]
- Alvin, A.; Miller, K.I.; Neilan, B.A. Exploring the Potential of Endophytes from Medicinal Plants as Sources of Antimycobacterial Compounds. Microbiol. Res. 2014, 169, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Song, C.; Li, Z.; Kuipers, O.P. Antimicrobial activity screening of rhizosphere soil bacteria from tomato and genome-based analysis of their antimicrobial biosynthetic potential. BMC Genom. 2021, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Remali, J.; Sarmin, N.I.M.; Ng, C.L.; Tiong, J.J.L.; Aizat, W.M.; Keong, L.K.; Zin, N.M. Genomic characterization of a new endophytic Streptomyces kebangsaanensis identifies biosynthetic pathway gene clusters for novel phenazine antibiotic production. PeerJ 2017, 5, e3738. [Google Scholar] [CrossRef]
- Maggini, V.; De Leo, M.; Granchi, C.; Tuccinardi, T.; Mengoni, A.; Gallo, E.R.; Biffi, S.; Fani, R.; Pistelli, L.; Firenzuoli, F.; et al. The Influence of Echinacea purpurea Leaf Microbiota on Chicoric Acid Level. Sci. Rep. 2019, 9, 10897. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggini, V.; Semenzato, G.; Gallo, E.; Nunziata, A.; Fani, R.; Firenzuoli, F. Antimicrobial Activity of Syzygium aromaticum Essential Oil in Human Health Treatment. Molecules 2024, 29, 999. https://doi.org/10.3390/molecules29050999
Maggini V, Semenzato G, Gallo E, Nunziata A, Fani R, Firenzuoli F. Antimicrobial Activity of Syzygium aromaticum Essential Oil in Human Health Treatment. Molecules. 2024; 29(5):999. https://doi.org/10.3390/molecules29050999
Chicago/Turabian StyleMaggini, Valentina, Giulia Semenzato, Eugenia Gallo, Alessia Nunziata, Renato Fani, and Fabio Firenzuoli. 2024. "Antimicrobial Activity of Syzygium aromaticum Essential Oil in Human Health Treatment" Molecules 29, no. 5: 999. https://doi.org/10.3390/molecules29050999
APA StyleMaggini, V., Semenzato, G., Gallo, E., Nunziata, A., Fani, R., & Firenzuoli, F. (2024). Antimicrobial Activity of Syzygium aromaticum Essential Oil in Human Health Treatment. Molecules, 29(5), 999. https://doi.org/10.3390/molecules29050999








