Composition and Anti-Acetylcholinesterase Properties of the Essential Oil of the Ecuadorian Endemic Species Eugenia valvata McVaugh
Abstract
:1. Introduction
2. Results
2.1. Yield and Chemical Composition
2.2. Enantiomeric Distribution
2.3. Cholinesterase Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oil Isolation
4.3. Identification and Quantification of Essential Oil
4.4. Enantiomeric Analysis
4.5. Cholinesterase Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fokunang, C.N.; Ndikum, V.; Tabi, O.Y.; Jiofack, R.B.; Ngameni, B.; Guedje, N.M.; Tembe-Fokunang, E.A.; Tomkins, P.; Barkwan, S.; Kechia, F.; et al. Traditional medicine: Past, present and future research and development prospects and integration in the National Health System of Cameroon. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Che, C.T.; George, V.; Ijinu, T.P.; Pushpangadan, P.; Andrae-Marobela, K. Traditional Medicine. In Pharmacognosy, Fundamentals, Applications and Strategies; Badal McCreath, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 15–30. [Google Scholar]
- Elizagaray, B.; Castro, R. Cuban scientific production about medicinal plants and natural products from PlantMedCUBA database. Rev. Cub. Plant. Med. 2013, 18, 348–360. [Google Scholar]
- Martínez, Y.; Gómez, L.L. Social impact of an intervention strategy for the rational prescription of natural medicines implemented in Céspedes during 2011. Rev. Cub. Plant. Med. 2013, 18, 609–618. [Google Scholar]
- Ministerio del Ambiente del Ecuador.2015.Quinto Informe Nacional Para el Convenio Sobre la Diversidad Biológica. Quito. Ecuador. Available online: https://www.ambiente.gob.ec/wp-content/uploads/downloads/2015/06/QUINTO-INFORME-BAJA-FINAL-19.06.2015.pdf (accessed on 10 August 2023).
- León, S.; Valencia, R.; Pitman, N.; Endara, L.; Ulloa, C.U.; Navarrete, H. Libro Rojo de Plantas Endémicas del Ecuador, 2a edición. In Publicaciones del Herbario QCA, Pontificia Universidad Católica del Ecuador 2011. Available online: https://ddrn.dk/wp-content/uploads/2018/01/LIBRO_ROJO_de_las_plantas_endemicas_del-1.pdf (accessed on 9 August 2023).
- Fischer, D.C.H.; Limberger, R.P.; Henriques, A.T.; Moreno, P.R.H. Essential oils from leaves of two Eugenia brasiliensis specimens from southeastern Brazil. J. Essent. Oil Res. 2005, 17, 499–500. [Google Scholar] [CrossRef]
- Fernanda Mazine, F.; Quintino Faria, J.E.; Giaretta, A.; Vasconcelos, T.; Forest, F.; Lucas, E. Phylogeny and biogeography of the hyper–diverse genus Eugenia (Myrtaceae: Myrteae), with emphasis on E. sect. Umbellatae, the most unmanageable clade. Taxon 2018, 67, 752–769. [Google Scholar] [CrossRef]
- Araujo, N.M.P.; Arruda, H.S.; de Paulo Farias, D.; Molina, G.; Pereira, G.A.; Pastore, G.M. Plants from the genus Eugenia as promising therapeutic agents for the management of diabetes mellitus: A review. Food Res. Int. 2021, 142, 110182. [Google Scholar] [CrossRef]
- Wilson, P.G. Myrtaceae. In The Families and Genera of Vascular Plants; Kubitzki, K., Ed.; Springer Press: Berlin/Heidelberg, Germany, 2011; pp. 212–271. [Google Scholar]
- Da Silva, A.P.G.; Sganzerla, W.G.; Jacomino, A.P.; da Silva, E.P.; Xiao, J.; Simal-Gandara, J. Chemical composition, bioactive compounds, and perspectives for the industrial formulation of health products from uvaia (Eugenia pyriformis Cambess–Myrtaceae): A comprehensive review. J. Food Compos. Anal. 2022, 109, 104500. [Google Scholar] [CrossRef]
- Öztürk, A.; Özbek, H. The Anti-Inflammatory Activity of Eugenia Caryophllata Essential Oil: An animal model of anti-inflammatory activity. Eur. J. Gen. Med. 2005, 2, 159–163. [Google Scholar]
- Slowing, K.; Carretero, E.; Villar, A. Anti-inflammatory compounds of Eugenia jambos. Phytother. Res. 1996, 10 (Suppl. S1), S126–S127. [Google Scholar]
- Sumono, A.; Wulan, A. The use of bay leaf (Eugenia polyantha Wight) in dentistry. Dent. J. (Majalah Kedokteran Gigi). 2008, 41, 147–150. [Google Scholar] [CrossRef]
- Gasca, C.A.; Castillo, W.O.; Takahashi, C.S.; Fagg, C.W.; Magallanes, P.O.; Fonseca-Bazzo, Y.M.; Silveira, D. Assessment of anti-cholinesterase activity and cytotoxicity of cagaita (Eugenia dysenterica) leaves. Food Chem. Toxicol. 2017, 109, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Park, J.H.; Shin, Y.G.; Shin, U.K.; Kim, K.H.; Yakhak, H. Chemical constituents of Eugenia caryophyllata. Pharmaceut. Soc. Korea 1997, 41, 149–152. [Google Scholar]
- Son, K.; Kwon, S.Y.; Kim, H.P.; Chang, H.W.; Kang, S.S. Constituents from Syzygium aromaticum Merr. et Perry. Nat. Prod. Sci. 1998, 4, 263–267. [Google Scholar]
- Tanaka, T.; Nonaka, G.I.; Nishioka, I.; Kouno, I. Syzyginins A and B, two ellagitannins from Syzygium aromaticum. Phytochemistry 1996, 43, 1345–1348. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G. Flavonoids from Calycorectes, Campomanesia, Eugenia and Hexachlamys species. Fitoterapia 1995, 66, 373–374. [Google Scholar]
- Bravi, V.S.; Valle, E. Revisión de constituyentes químicos y propiedades biológicas en especies del género Eugenia (Myrtaceae) Review of chemical constituents and biological properties in species of the genus Eugenia (Myrtaceae). Dominguezia 2021, 37, 5–19. [Google Scholar]
- Jorgensen, P.; Leon-Yanez, S. Catalogue of the Vascular Plants of Ecuador; Missouri Botanical Garden Press: St. Louis, MO, USA, 1999; Volume 75, pp. 1–1182. [Google Scholar]
- Dobetsberger, C.; Buchbauer, G. Actions of essential oils on the central nervous system: An updated review. Flavour Fragr. J. 2011, 26, 300–316. [Google Scholar] [CrossRef]
- Lahlou, M. Essential oils and fragrance compounds: Bioactivity and mechanisms of action. Flavour Fragr. J. 2004, 19, 159–165. [Google Scholar] [CrossRef]
- Ayaz, M.; Junaid, M.; Ullah, F.; Sadiq, A.; Khan, M.A.; Ahmad, W.; Shah, M.R.; Imran, M.; Ahmad, S. Comparative chemical profiling, cholinesterase inhibitions and anti-radicals properties of essential oils from Polygonum hydropiper L.: A preliminary anti-alzheimer’s study. Lipids Health Dis. 2015, 14, 141. [Google Scholar] [CrossRef]
- Fung, J.K.K.; Tsang, H.W.; Chung, R.C. A Systematic review of the use of aromatherapy in treatment of behavioral problems in dementia. Geriatr. Gerontol. Int. 2012, 12, 372–382. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 10-193263321. [Google Scholar]
- Silva, J.; Andrade, E.; Barreto, L.; Silva, N.; Ribeiro, A.; Montenegro, R.; Maia, J. Chemical Composition of Four Essential Oils of Eugenia from the Brazilian Amazon and Their Cytotoxic and Antioxidant Activity. Medicines 2017, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- De Rojas, Y.E.C.; Lucena, M.E.; Bustamante, M.Y.G.; Guerrero, K.Y.R.; Zambrano, R.L.A.; De González, C.D.; Ustaríz, F.J.; Araujo, R.M.; Chacón, M.R.I. Composición química y actividad antifúngica del aceite esencial de hojas de Eugenia uniflora L. (Myrtaceae). Rev. Cub. Farm. 2022, 55, e796. [Google Scholar]
- Calva, J.; Ludeña, C.; Bec, N.; Larroque, C.; Salinas, M.; Vidari, G.; Armijos, C. Constituents and Selective BuChE Inhibitory Activity of the Essential Oil from Hypericum aciculare Kunth. Plants 2023, 12, 2621. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, M.; Guilhon, G.; Sarges, F.; Pereira, R.; Oliveira, J. Variabilidad química de los volátiles de las hojas de Eugenia protenta McVaugh (Myrtaceae) que crecen de forma silvestre en el norte de Brasil. Bioquím. Sist. Ecol. 2011, 39, 660–665. [Google Scholar] [CrossRef]
- Magina, M.D.A.; Dalmarco, E.M.; Dalmarco, J.B.; Colla, G.; Pizzolatti, M.G.; Brighente, I. Bioactive triterpenes and phenolics of leaves of Eugenia brasiliensis. Quım. Nova 2012, 35, 1184–1188. [Google Scholar] [CrossRef]
- Apel, M.A.; Limberger, R.P.; Sobral, M.; Henriques, A.T.; Ntalani, H.; Verin, P.; Menut, C.; Bessiere, J.M. Chemical composition of the essential oils from southern Brazilian Eugenia species. Part III. J. Essent. Oil Res. 2002, 14, 259–262. [Google Scholar] [CrossRef]
- Stefanello, M.É.A.; Cervi, A.C.; Ito, I.Y.; Salvador, M.J.; Wisniewski, A.; Simionatto, E.L. Chemical composition and antimicrobial activity of essential oils of Eugenia chlorophylla (myrtaceae). J. Essent. Oil Res. 2008, 20, 75–78. [Google Scholar] [CrossRef]
- Da Silva Rivas, A.C.; Lopes, P.M.; De Azevedo Barros, M.M.; Costa Machado, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar]
- Allenspach, M.; Valder, C.; Flamm, D.; Grisoni, F.; Steuer, C. Verification of chromatographic profile of primary essential oil of Pinus sylvestris L. combined with chemometric analysis. Molecules 2020, 25, 2973. [Google Scholar] [CrossRef] [PubMed]
- Vespermann, K.A.; Paulino, B.N.; Barcelos, M.C.; Pessôa, M.G.; Pastore, G.M.; Molina, G. Biotransformation of α-and β-pinene into flavor compounds. Appl. Microbiol. Biotechnol. 2017, 101, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Eduardo, L.; Farias, T.C.; Ferreira, S.B.; Ferreira, P.B.; Lima, Z.N.; Ferreira, S.B. Antibacterial activity and time-kill kinetics of positive enantiomer of α-pinene against strains of Staphylococcus aureus and Escherichia coli. Curr. Top. Med. Chem. 2018, 18, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Ložienė, K.; Švedienė, J.; Paškevičius, A.; Raudonienė, V.; Sytar, O.; Kosyan, A. Influence of plant origin natural α-pinene with different enantiomeric composition on bacteria, yeasts and fungi. Fitoterapia 2018, 127, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, L.E.; Startseva, V.A.; Vakulenko, I.A.; Khismatulina, I.M.; Lisovskaya, S.A.; Glushko, N.P.; Fassakhov, R.S. Synthesis and antifungal activity of compounds of the pinane series. Pharm. Chem. J. 2009, 43, 251–254. [Google Scholar] [CrossRef]
- Van Zyl, R.L.; Seatlholo, S.T.; Van Vuuren, S.F.; Viljoen, A.M. The biological activities of 20 nature identical essential oil constituents. J. Essent. Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and chondroprotective activity of (+)-α-pinene: Structural and enantiomeric selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Yamafuji, C. Inhibition of acetylcholinesterase activity by bicyclic monoterpenoids. J. Agric. Food Chem. 2005, 53, 1765–1768. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, N.; Zu, Y.; Fu, Y. Comparative anti-infectious bronchitis virus (IBV) activity of (-)-pinene: Effect on nucleocapsid (N) protein. Molecules 2011, 16, 1044–1054. [Google Scholar] [CrossRef]
- Tenfen, A.; Vechi, G.; Cechinel-Zanchett, C.C.; Lorenzett, T.S.; Reginato-Couto, C.E.; Siebert, D.A.; Vitali, L.; Micke, G.; Klein-Júnior, L.C.; Cechinel Filho, V. Phenolic profile by HPLC-ESI-MS/MS of six Brazilian Eugenia species and their potential as cholinesterase inhibitors. Nat. Prod. Res. 2021, 35, 2608–2611. [Google Scholar] [CrossRef]
- Knez, D.; Coquelle, N.; Pišlar, A.; Žakelj, S.; Jukič, M.; Sova, M.; Mravljak, J.; Nachon, F.; Brazzolotto, X.; Kos, J.; et al. Multi-target-directed ligands for treating Alzheimer’s disease: Butyrylcholinesterase inhibitors displaying antioxidant and neuroprotective activities. Eur. J. Med. Chem. 2018, 156, 598–617. [Google Scholar] [CrossRef] [PubMed]
- Moneim, A.E. Oxidant/Antioxidant imbalance and the risk of Alzheimer’s disease. Curr. Alzheimer Res. 2015, 12, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.G.; Tietbohl, L.A.C.; Fernandes, C.P.; Cruz, R.A.S.; Da Botas, G.S.; Santos, M.G.; Silva-Filho, M.V.; Rocha, L. Chemical composition of essential oils and anticholinesterasic activity of Eugenia sulcata spring ex mart. Lat. Am. J. Pharm. 2012, 31, 152–155. [Google Scholar]
- Souza, A.D.; Lopes, E.M.C.; Silva, M.C.D.; Cordeiro, I.; Young, M.C.M.; Sobral, M.E.G.; Moreno, P.R.H. Chemical composition and acetylcholinesterase inhibitory activity of essential oils of Myrceugenia myrcioides (Cambess.) O. Berg and Eugenia riedeliana O. Berg, Myrtaceae. Rev. Bras. Farmacogn. 2010, 20, 175–179. [Google Scholar] [CrossRef]
- Siebert, D.A.; Tenfen, A.; Yamanaka, C.N.; De Cordova, C.M.M.; Scharf, D.R.; Simionatto, E.; Alberton, M.D. Evaluation of seasonal chemical composition, antibacterial, antioxidant and anticholinesterase activity of essential oil from Eugenia brasiliensis Lam. Nat. Prod. Res. 2014, 29, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Ahn, Y.J. Laboratory and simulated field bioassays to evaluate larvicidal activity of Pinus densiflora hydrodistillate, its constituents and structurally related compounds against Aedes albopictus, Aedes aegypti and Culex pipiens pallens in relation to their inhibitory effects on acetylcholinesterase activity. Insects 2013, 30, 217–229. [Google Scholar]
- Miyazawa, M.; Watanabe, H.; Kameoka, H. Inhibition of acetylcholinesterase activity by monoterpenoids with a p-menthane skeleton. J. Agric. Food Chem. 1997, 45, 677–679. [Google Scholar] [CrossRef]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant and Antiacetylcholinesterase Activities of Some Commercial Essential Oils and Their Major Compounds. Molecules 2011, 16, 7672–7690. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Tougo, H.; Ishihara, M. Inhibition of acetylcholinesterase activity by essential oil from Citrus paradisi. Nat. Prod. Lett. 2001, 15, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Calva, J.; Cartuche, L.; Castillo, L.N.; Morocho, V. Biological Activities and Chemical Composition of Essential Oil from Hedyosmum purpurascens (Todzia)—An Endemic Plant in Ecuador. Molecules 2023, 28, 2366. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
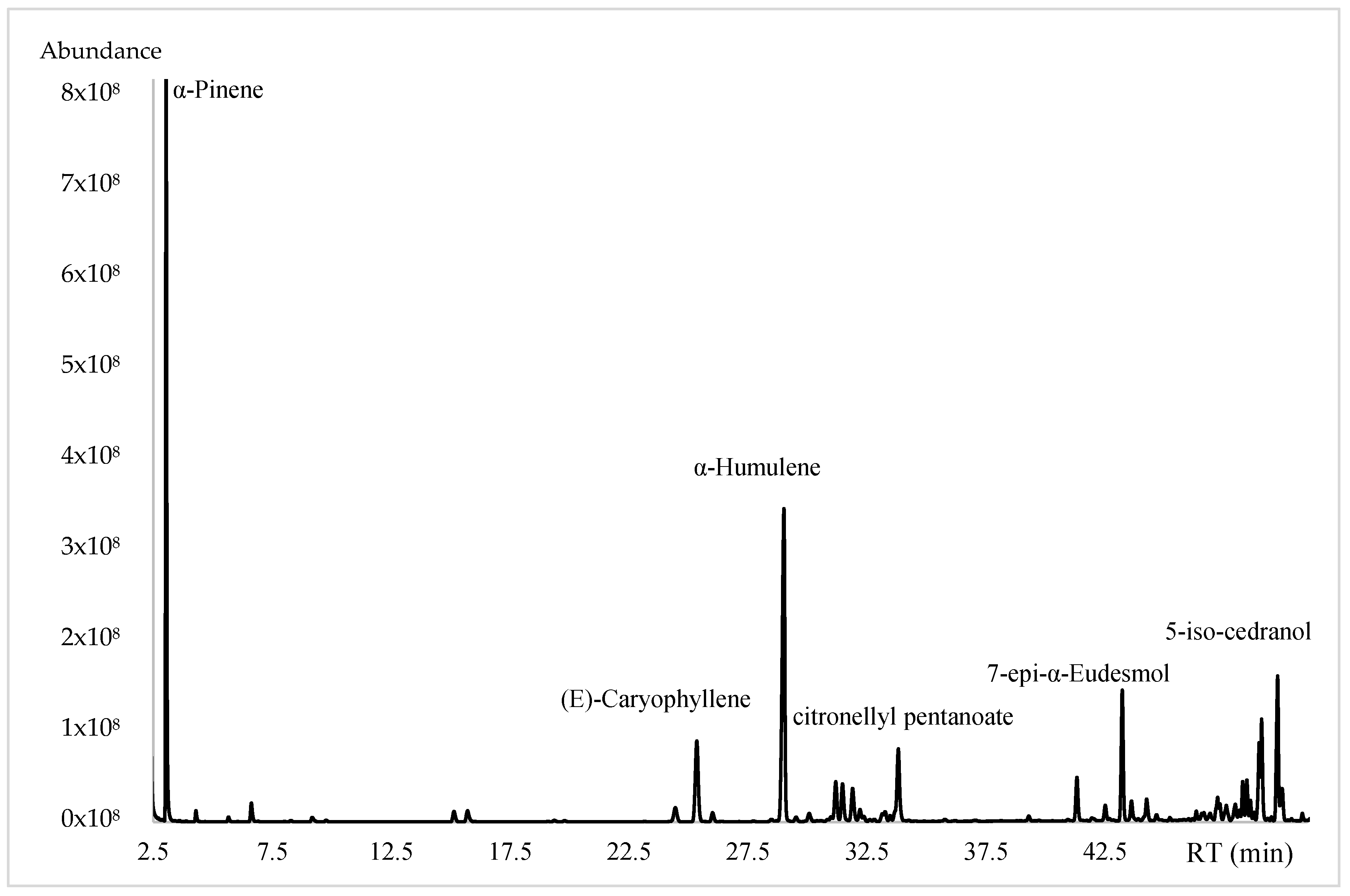
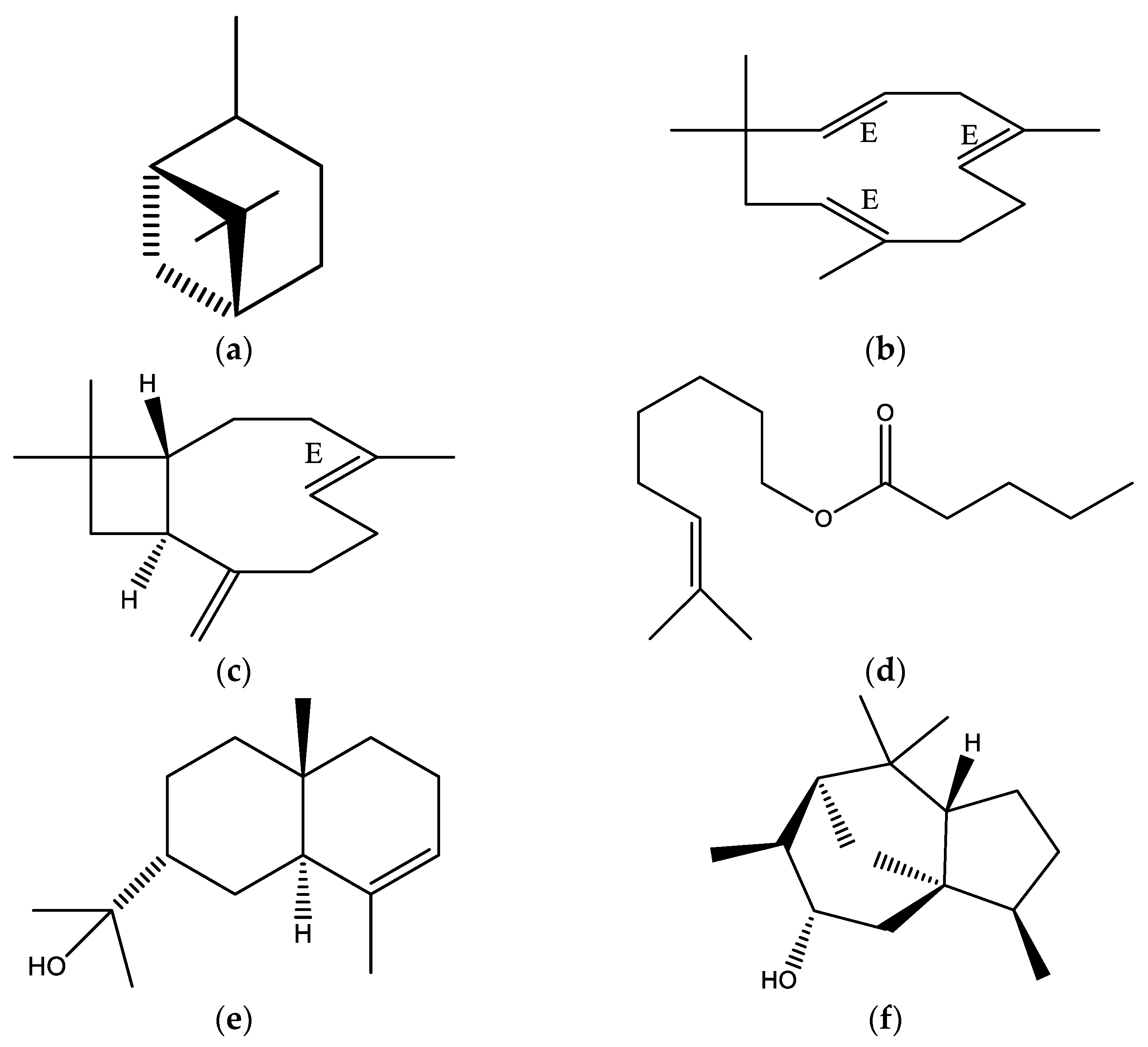


| Compounds | LRIcal 1 | LRIlit 2 | % ± SD 3 | MF |
|---|---|---|---|---|
| Terpenes | ||||
| α-Pinene | 932 | 939 | 22.70 ± 1.878 | C10H16 |
| β-Pinene | 978 | 974 | 0.34 ± 0.022 | C10H16 |
| Myrcene | 990 | 988 | 0.21 ± 0.010 | C10H16 |
| Limonene | 1030 | 1024 | 0.85 ± 0.054 | C10H16 |
| α-terpineol | 1203 | 1186 | 0.20 ± 0.003 | C10H18O |
| Cyclosativene | 1365 | 1371 | 0.27 ± 0.274 | C15H24 |
| α-cis-Bergamotene | 1413 | 1411 | 0.25 ± 0.003 | C15H24 |
| (E)-Caryophyllene | 1419 | 1417 | 6.02 ± 0.084 | C15H24 |
| α-trans-Bergamotene | 1433 | 1432 | 0.44 ± 0.005 | C15H24 |
| α-Humulene | 1458 | 1452 | 17.20 ± 0.25 | C15H24 |
| β-Santalene | 1461 | 1457 | 0.20 ± 0.030 | C15H24 |
| β-Chamigrene | 1475 | 1476 | 0.15 ± 0.006 | C15H24 |
| Widdra-2,4(14)-diene | 1478 | 1481 | 0.29 ± 0.171 | C15H24 |
| γ-Himachalene | 1485 | 1481 | 0.20 ± 0.074 | C15H24 |
| β-Selinene | 1491 | 1489 | 2.20 ± 0.029 | C15H24 |
| δ-Selinene | 1498 | 1492 | 1.80 ± 0.049 | C15H24 |
| β-Macrocarpene | 1503 | 1499 | 0.10 ± 0.001 | C15H24 |
| Epizonarene | 1505 | 1501 | 0.46 ± 0.325 | C15H24 |
| β-Bisabolene | 1510 | 1506 | 0.58 ± 0.059 | C15H24 |
| α-Alaskene | 1512 | 1512 | 0.38 ± 0.296 | C15H24 |
| 7-epi-α-Selinene | 1516 | 1520 | 0.37 ± 0.321 | C15H24 |
| δ-Cadinene | 1521 | 1522 | 1.84 ± 0.057 | C15H24 |
| β-Sesquiphellandene | 1527 | 1521 | 0.35 ± 0.003 | C15H24 |
| (E)-γ-Bisabolene | 1529 | 1529 | 0.28 ± 0.008 | C15H24 |
| Zonarene | 1536 | 1528 | 0.40 ± 0.008 | C15H24 |
| α-Cadinene | 1541 | 1537 | 1.71 ± 0.026 | C15H24 |
| Selina-3,7(11)-diene | 1545 | 1545 | 1.71 ± 0.027 | C15H24 |
| α-Calacorene | 1548 | 1544 | 0.08 ± 0.016 | C15H20 |
| Terpenoids and Oxygenated Terpenes | ||||
| (E)-Nerolidol | 1567 | 1561 | 0.75 ± 0.020 | C15H26O |
| Maaliol | 1571 | 1566 | 0.08 ± 0.004 | C15H26O |
| Caryophyllene oxide | 1589 | 1582 | 2.32 ± 0.110 | C15H24O |
| Gleenol | 1594 | 1589 | 0.08 ± 0.006 | C15H26O |
| Guaiol | 1602 | 1600 | 0.09 ± 0.020 | C15H26O |
| Geranyl 2-methyl butanoate | 1607 | 1601 | 0.87 ± 0.029 | C15H26O2 |
| trans-β-Elemenone | 1614 | 1602 | 0.08 ± 0.114 | C15H22O |
| Citronellyl pentanoate | 1620 | 1625 | 5.76 ± 0.361 | C15H28O2 |
| Eremoligenol | 1627 | 1629 | 0.59 ± 0.041 | C15H26O |
| γ-Eudesmol | 1635 | 1630 | 0.16 ± 0.067 | C15H26O |
| Cubenol | 1637 | 1645 | 0.82 ± 0.028 | C15H26O |
| Agarospirol | 1642 | 1646 | 1.56 ± 0.091 | C15H26O |
| Himachalol | 1645 | 1652 | 2.71 ± 0.154 | C15H26O |
| Cedr-8(15)-en-10-ol | 1649 | 1650 | 0.72 ± 0.042 | C15H24O |
| Selin-11-en-4-α-ol | 1650 | 1658 | 0.01 ± 0.017 | C15H26O |
| Valerianol | 1654 | 1656 | 1.12 ± 0.297 | C15H26O |
| α-Cadinol | 1656 | 1652 | 0.08 ± 0.129 | C15H26O |
| cis-Guaia-3,9-dien-11-ol | 1660 | 1648 | 1.38 ± 0.072 | C15H24O |
| 7-epi-α-Eudesmol | 1667 | 1662 | 4.34 ± 0.487 | C15H26O |
| Intermedeol | 1671 | 1665 | 2.66 ± 0.152 | C15H26O |
| 5-iso-Cedranol | 1684 | 1672 | 3.64 ± 0.256 | C15H26O |
| 5-neo-Cedranol | 1694 | 1684 | 0.45 ± 0.088 | C15H26O |
| cis-Thujopsenal | 1710 | 1708 | 0.62 ± 0.079 | C15H22O |
| (E)-Apritone | 1716 | 1708 | 0.07 ± 0.24 | C15H24O |
| Other compounds | ||||
| 2-Heptyl acetate | 1041 | 1038 | 0.23 ± 0.006 | C9H18O2 |
| Methyl octanoate | 1129 | 1123 | 0.60 ± 0.020 | C9H18O2 |
| Heptyl isobutanoate | 1216 | 1246 | 0.66 ± 0.005 | C11H22O2 |
| n-Tetradecane | 1403 | 1400 | 0.56 ± 0.008 | C14H30 |
| Occidentalol acetate | 1678 | 1681 | 0.58 ± 0.045 | C17H26O2 |
| Longiborneol acetate (=Juniperol acetate) | 1680 | 1684 | 0.40 ± 0.072 | C17H28O2 |
| Hydrocarbon monoterpenes | 24.14% | |||
| Oxygenated monoterpene | 0.20% | |||
| Hydrocarbon sesquiterpenes | 37.43% | |||
| Oxygenated sesquiterpenes | 31.08% | |||
| Other | 3.06% | |||
| Total identified | 95.91% |
| Compound | RT | LRI | ED (%) | e.e (%) |
|---|---|---|---|---|
| α-(+)-Pinene | 4.238 | 939 | 58.77 | 17.53 |
| α-(−)-Pinene | 4.282 | 941 | 41.23 | |
| (+)-Limonene | 8.503 | 1057 | 34.23 | 31.53 |
| (−)-Limonene | 8.701 | 1062 | 65.77 | |
| α-(+)-Cadinene | 37.569 | 1558 | 21.63 | 56.73 |
| α-(−)-Cadinene | 37.599 | 1559 | 78.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calva, J.; Silva, M.; Morocho, V. Composition and Anti-Acetylcholinesterase Properties of the Essential Oil of the Ecuadorian Endemic Species Eugenia valvata McVaugh. Molecules 2023, 28, 8112. https://doi.org/10.3390/molecules28248112
Calva J, Silva M, Morocho V. Composition and Anti-Acetylcholinesterase Properties of the Essential Oil of the Ecuadorian Endemic Species Eugenia valvata McVaugh. Molecules. 2023; 28(24):8112. https://doi.org/10.3390/molecules28248112
Chicago/Turabian StyleCalva, James, Maricarmen Silva, and Vladimir Morocho. 2023. "Composition and Anti-Acetylcholinesterase Properties of the Essential Oil of the Ecuadorian Endemic Species Eugenia valvata McVaugh" Molecules 28, no. 24: 8112. https://doi.org/10.3390/molecules28248112
APA StyleCalva, J., Silva, M., & Morocho, V. (2023). Composition and Anti-Acetylcholinesterase Properties of the Essential Oil of the Ecuadorian Endemic Species Eugenia valvata McVaugh. Molecules, 28(24), 8112. https://doi.org/10.3390/molecules28248112









