Chemometric Analysis Evidencing the Variability in the Composition of Essential Oils in 10 Salvia Species from Different Taxonomic Sections or Phylogenetic Clades
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Analysis of EOs
2.1.1. Salvia Members of Section Aethiopis/I-C Clade
2.1.2. Salvia Members of Section Eusphace/I-D Clade
2.1.3. Salvia Members of Section Hemisphace/I-B Clade
2.1.4. Salvia Members of Section Plethiosphace/I-C Clade
2.2. Chemometric Analysis
3. Materials and Methods
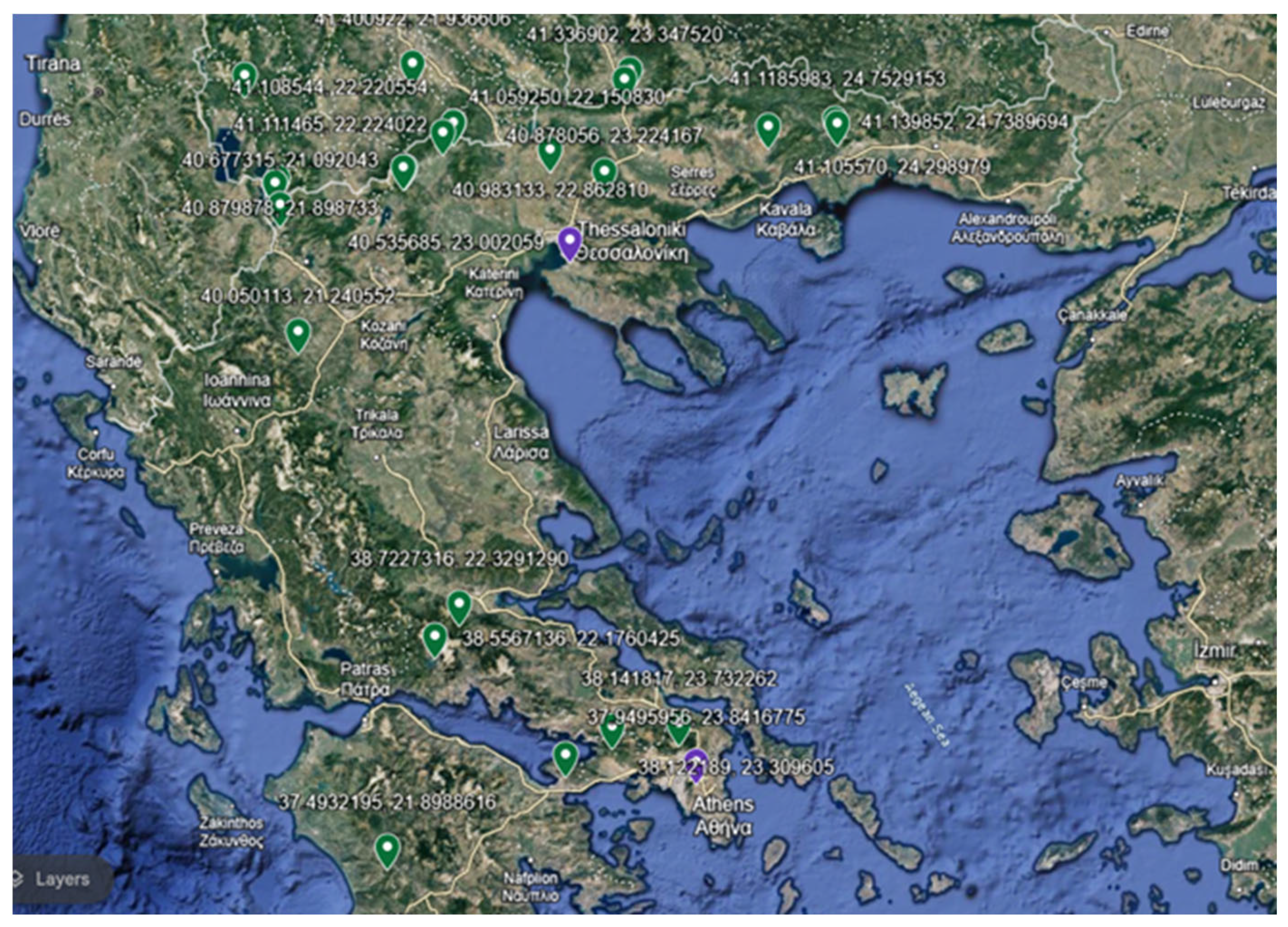
3.1. Plant Materials
| Taxon | Section 1 | Clade 2 |
|---|---|---|
| Salvia aethiopis L. | Aethiopis | I-C |
| Salvia argentea L. | Aethiopis | I-C |
| Salvia candidissima Vahl | Aethiopis | I-C |
| Salvia sclarea L. | Aethiopis | I-C |
| Salvia teddii Turill | Aethiopis | I-C |
| Salvia ringens Sm. | Eusphace | I-D |
| Salvia verticillata L. | Hemisphace | I-B |
| Salvia amplexicaulis Lam. | Plethiosphace | I-C |
| Salvia pratensis L. | Plethiosphace | I-C |
| Salvia virgata Jacq. | Plethiosphace | I-C |
3.2. EO Isolation
3.3. GC–MS Analysis
3.4. Chemometric Analysis
3.5. Literature Review
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Will, M.; Claßen-Bockhoff, R. Time to split Salvia s.l. (Lamiaceae)—New insights from Old World Salvia phylogeny. Mol. Phylogen. Evol. 2017, 109, 33–58. [Google Scholar] [CrossRef]
- Plants of the World Online (POWO). Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30000096-2 (accessed on 31 January 2024).
- Walker, J.B.; Sytsma, K.J.; Treutlein, J.; Wink, M. Salvia (Lamiaceae) is not monophyletic: Implications for the systematics, radiation, and ecological specializations of Salvia and Tribe Mentheae. Amer. J. Bot. 2004, 91, 1115–1125. [Google Scholar] [CrossRef]
- Sutton, J. The Gardener’s Guide to Growing Salvias; Workman Publishing Company: Newton Abbot, UK, Portland, 2000; ISBN 978-0-88192-474-9. [Google Scholar]
- Drew, B.T.; González-Gallegos, J.G.; Xiang, C.-L.; Kriebel, R.; Drummond, C.P.; Walked, J.B.; Sytsma, K.J. Salvia united: The greatest good for the greatest number. Taxon 2017, 66, 133–145. [Google Scholar] [CrossRef]
- Vascular Plants of Greece Checklist|Flora of Greece—An Annotated Checklist. Available online: https://portal.cybertaxonomy.org/flora-greece/intro (accessed on 31 January 2024).
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crops Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Alves Borges Leal, A.L.; et al. Salvia spp. Plants—From farm to food applications and phytopharmacotherapy. Trends Food Sci. Techn. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Wu, Y.-B.; Ni, Z.-Y.; Shi, Q.-W.; Dong, M.; Kiyota, H.; Gu, Y.-C.; Cong, B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M.; Gono-Bwalya, A.B.; van Zyl, R.L.; van Vuuren, S.F.; Lourens, A.C.U.; Başer, K.H.C.; Demirci, B.; Lindsey, K.L.; van Staden, J.; et al. The in vitro pharmacological activities and a chemical investigation of three South African Salvia species. J. Ethnopharm. 2005, 102, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Askari, S.F.; Avan, R.; Tayarani-Najaran, Z.; Sahebkar, A.; Eghbali, S. Iranian Salvia species: A phytochemical and pharmacological update. Phytochemistry 2021, 183, 112619. [Google Scholar] [CrossRef]
- Assessment report on Salvia officinalis L., Folium and Salvia officinalis L., Aetheroleum. EMA/HMPC/150801/2015 Committee on Herbal Medicinal Products (HMPC). Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-salvia-officinalis-l-folium-and-salvia-officinalis-l-aetheroleum-revision-1_en.pdf (accessed on 31 January 2024).
- Lu, Y.; Yeap Foo, L. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, H.; Hu, X.; Sun, Z.; Han, C. The pharmacological properties of Salvia essential oils. J. Appl. Pharm. Sci. 2013, 3, 122–127. [Google Scholar] [CrossRef]
- Lorenzo, D.; Paz, D.; Davies, P.; Villamil, J.; Vila, R.; Cañigueral, S.; Dellacassa, E. Characterization and enantiomeric distribution of some terpenes in the essential oil of a Uruguayan biotype of Salvia sclarea L. Flavour Fragr. J. 2004, 19, 303–307. [Google Scholar] [CrossRef]
- Couladis, M.; Tzakou, O.; Mimica-Dukić, N.; Jančić, R.; Stojanović, D. Essential oil of Salvia officinalis L. from Serbia and Montenegro. Flavour Fragr. J. 2002, 17, 119–126. [Google Scholar] [CrossRef]
- Leontaritou, P.; Lamari, F.N.; Papasotiropoulos, V.; Iatrou, G. Morphological, genetic and essential oil variation of Greek sage (Salvia fruticosa Mill.) populations from Greece. Ind. Crops Prod. 2020, 150, 112346. [Google Scholar] [CrossRef]
- Rajabi, Z.; Ebrahimi, M.; Farajpour, M.; Mirza, M.; Ramshini, H. Compositions and yield variation of essential oils among and within nine Salvia species from various areas of Iran. Ind. Crops Prod. 2014, 61, 233–239. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Masoudi, S.; Monfared, A.; Komeilizadeh, H. Volatile constituents of three Salvia species grown wild in Iran. Flavour Fragr. J. 1999, 14, 276–278. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Goodarzi, A.; Azadbakht, M. The essential oil of Salvia aethiopis L. J. Essent. Oil Res. 2005, 17, 274–275. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Rineh, A.; Khalilzadeh, M.A.; Eslami, B. Chemical constituents of the essential oils from leaves, flowers, stem and aerial parts of Salvia aethiopis L. from Iran. J. Essent. Oil Res. 2007, 19, 569–571. [Google Scholar] [CrossRef]
- Velickovic, A.; Ristic, M.; Velickovic, D.; Ilic, S.; Mitic, N. The possibilities of the application of some species of sage (Salvia L.) as auxiliaries in the treatment of some diseases. J. Serb. Chem. Soc. 2003, 68, 435–445. [Google Scholar] [CrossRef]
- Velickovic, D.; Ristic, M.; Velickovic, A. Chemical composition of the essential oils obtained from the flower, leaf and stem of Salvia aethiopis L. and Salvia glutinosa L. originating from the southeast region of Serbia. J. Essent. Oil Res. 2003, 15, 346–349. [Google Scholar] [CrossRef]
- Kostic, E.; Kitic, D.; Vujovic, M.; Markovic, M.; Pavlovic, A.; Stojanovic, G. A Chemometric approach to the headspace sampled volatiles of selected Salvia species from southeastern Serbia. Bot. Serb. 2022, 46, 285–294. [Google Scholar] [CrossRef]
- Torres, M.E.; Velasco-Negueruela, A.; Pérez-Alonso, M.J.; Pinilla, M.G. Volatile constituents of two Salvia species grown wild in Spain. J. Essent. Oil Res. 1997, 9, 27–33. [Google Scholar] [CrossRef]
- Güllüce, M.; Özer, H.; Barifi, Ö.; Daferera, D. Chemical composition of the essential oil of Salvia aethiopis L. Turk. J. Biol. 2006, 30, 231–233. [Google Scholar]
- Göze, İ.; Vural, N.; Ercan, N. Characterization of essential oil and antioxidant activities of some species of Salvia in Turkey. Nat. Volatiles Essent. Oils 2016, 3, 1–7. [Google Scholar]
- Hatipoglu, S.D.; Zorlu, N.; Dirmenci, T.; Goren, A.C.; Ozturk, T.; Topcu, G. Determination of volatile organic compounds in fourty five Salvia species by thermal desorption-GC-MS technique. Rec. Nat. Prod. 2016, 10, 659–700. [Google Scholar]
- Ivanič, R.; Savin, K. A comparative analysis of essential oils from several wild species of Salvia. Planta Med. 1976, 30, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Chalchat, J.-C.; Gorunovic, M.S.; Petrovic, S.D.; Maksimovic, Z.A. Chemical compositions of two wild pecies of the genus Salvia L. from Yugoslavia: Salvia aethiopis and Salvia verticillata. J. Essent. Oil Res. 2001, 13, 416–418. [Google Scholar] [CrossRef]
- Lioliou, A.E. Comparative Study of the Chemical Composition of Essential Oils as well as Hydroalcoholic Extracts of the Genus Salvia and Their Bioactivity. Master’s Thesis, Agricultural University of Athens, Athens, Greece, 2021. (In Greek). [Google Scholar]
- Riccobono, L.; Maggio, A.; Rosselli, S.; Ilardi, V.; Senatore, F.; Bruno, M. Chemical composition of volatile and fixed oils from of Salvia argentea L. (Lamiaceae) growing wild in Sicily. Nat. Prod. Res. 2016, 30, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Holeman, M.A.; Berrada, M.; Bellakhdar, J.; Ilidrissi, A.; Pinel, R. Comparative chemical study on essential oils from Salvia officinalis, S. aucheri, S. verbenaca, S. phlomoides and S. argentea. Fitoterapia 1984, 55, 143–148. [Google Scholar]
- Couladis, M.; Tzakou, O.; Stojanovic, D.; Mimica-Dukic, N.; Jancic, R. The essential oil composition of Salvia argentea L. Flavour Fragr. J. 2001, 16, 227–229. [Google Scholar] [CrossRef]
- Velickovic, D.; Ristic, M.; Milosavljevic, N.; Davidovic, D.; Bogdanovic, S. Chemical composition of the essential oil of Salvia argentea L. Agro. Food Ind. Hi-Tech. 2014, 26, 70–72. [Google Scholar]
- Farhat, M.B.; Landoulsi, A.; Chaouch-Hamada, R.; Sotomayor, J.A.; Jordán, M.J. Profiling of essential oils and polyphenolics of Salvia argentea and evaluation of its by-products antioxidant activity. Ind. Crops Prod. 2013, 47, 106–112. [Google Scholar] [CrossRef]
- Rayouf, M.B.T.; Msaada, K.; Hosni, K.; Marzouk, B. Essential oil constituents of Salvia argentea L. from Tunisia: Phenological variations. Med. Arom. Plant Sci. Biotechnol. 2012, 7, 40–44. [Google Scholar]
- Tiğli Kaytanlioğlu, E.H.; Özderīn, S.; Fakīr, H.; Erbaş, S. Determination of the essential oil components of some sage (Salvia sp.) species naturally distributed in the Isparta province. Kastamonu Üniv. Orman Fak. Dergisi 2023, 23, 1–10. [Google Scholar] [CrossRef]
- Pitarokili, D.; Tzakou, O.; Loukis, A. Essential oil composition of Salvia verticillata, S. verbenaca, S. glutinosa and S. candidissima growing wild in Greece. Flavour Fragr. J. 2006, 21, 670–673. [Google Scholar] [CrossRef]
- Bayrak, A.; Akgul, A. Composition of essential oils from Turkish Salvia species. Phytochemistry 1987, 26, 846–847. [Google Scholar] [CrossRef]
- Özler, M.A.; Duru, M.E.; Diri, H.A.; Harmandar, M. Antioxidant activity and chemical composition of the essential oil of Salvia candidissima Vahl. growing wild in Turkey. Acta Hort. 2006, 826, 363–370. [Google Scholar] [CrossRef]
- Bayar, Y.; Genc, N. Determination of the chemical components, antioxidant and antifungal activities of essential oil and plant extract of Salvia candidissima Vahl. Medit. Agric. Sci. 2018, 31, 93–99. [Google Scholar] [CrossRef][Green Version]
- El-Gohary, A.E.; Amer, H.M.; Salama, A.B.; Wahba, H.E.; Khalid, K.A. Characterization of the essential oil components of adapted Salvia sclarea L. (clary sage) plant under Egyptian environmental conditions. J. Essent. Oil Bear. Plants 2020, 23, 788–794. [Google Scholar] [CrossRef]
- Foray, L.; Bertrand, C.; Pinguet, F.; Soulier, M.; Astre, C.; Marion, C.; Pélissier, Y.; Bessière, J.-M. In Vitro cytotoxic activity of three essential oils from Salvia species. J. Essent. Oil Res. 1999, 11, 522–526. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Satyal, P.; Setzer, W.N.; Wink, M. Chemical compositions of the essential oils of three Salvia species cultivated in Germany. AJEONP 2015, 3, 26–29. [Google Scholar]
- Souleles, C.; Argyriadou, N. Constituents of the essential oil of Salvia sclarea growing wild in Greece. Int. J. Pharmacogn. 1997, 35, 218–220. [Google Scholar] [CrossRef]
- Pitarokili, D.; Couladis, M.; Petsikos-Panayotarou, N.; Tzakou, O. Composition and antifungal activity on soil-borne pathogens of the essential oil of Salvia sclarea from Greece. J. Agric. Food Chem. 2002, 50, 6688–6691. [Google Scholar] [CrossRef]
- Koutsaviti, A.; Tzini, D.I.; Tzakou, O. Greek Salvia sclarea L. essential oils: Effect of hydrodistillation time, comparison of the aroma chemicals using hydrodistillation and HS-SPME techniques. Rec. Nat. Prod. 2016, 10, 800–805. [Google Scholar]
- Grigoriadou, K.; Trikka, F.A.; Tsoktouridis, G.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Maloupa, E.; Makris, A.M. Μicropropagation and cultivation of Salvia sclarea for essential oil and sclareol production in Northern Greece. Vitr. Cell. Dev.Biol.-Plant 2020, 56, 51–59. [Google Scholar] [CrossRef]
- Yousefzadi, M.; Sonboli, A.; Karimi, F.; Ebrahimi, S.N.; Asghari, B.; Zeinali, A. Antimicrobial activity of some Salvia species essential oils from Iran. Zeit. Naturforschung C 2007, 62, 514–518. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Masoomi, R.; Jookar Kashi, F.; Batooli, H. Bioactivity of the essential oil and methanol extracts of flowers and leaves of Salvia sclarea L. from Central Iran. J. Essent. Oil Bear. Plants 2016, 19, 885–896. [Google Scholar] [CrossRef]
- Peana, A.T.; Moretti, M.D.L.; Juliano, C. Chemical composition and antimicrobial action of the essential oils of Salvia desoleana and S. sclarea. Planta Med. 1999, 65, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Carrubba, A.; La Torre, R.; Piccaglia, R.; Marotti, M. Characterization of an Italian biotype of clary sage (Salvia sclarea L.) grown in a semi-arid Mediterranean environment. Flavour Fragr. J. 2002, 17, 191–194. [Google Scholar] [CrossRef]
- Fraternale, D.; Giamperi, L.; Bucchini, A.; Ricci, D.; Epifano, F.; Genovese, S.; Curini, M. Composition and antifungal activity of essential oil of Salvia sclarea from Italy. Chem. Nat. Compd. 2005, 41, 604–606. [Google Scholar] [CrossRef]
- Ben Akacha, B.; Ben Hsouna, A.; Generalić Mekinić, I.; Ben Belgacem, A.; Ben Saad, R.; Mnif, W.; Kačániová, M.; Garzoli, S. Salvia officinalis L. and Salvia sclarea essential oils: Chemical composition, biological activities and preservative effects against Listeria monocytogenes inoculated into minced beef meat. Plants 2023, 12, 3385. [Google Scholar] [CrossRef]
- Raafat, K.; Habib, J. Phytochemical compositions and antidiabetic potentials of Salvia sclarea L. essential oils. J. Oleo Sci. 2018, 67, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Rzepa, J.; Wojtal, L.; Staszek, D.; Grygierczyk, G.; Labe, K.; Hajnos, M.; Kowalska, T.; Waksmundzka-Hajnos, M. Fingerprint of selected Salvia species by HS-GC-MS analysis of their volatile fraction. J. Chromatogr. Sci. 2009, 47, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Acimovic, M.G.; Loncar, B.L.; Jeliazkov, V.D.; Pezo, L.L.; Ljujic, J.P.; Miljkovic, A.R.; Vujisic, L.V. Comparison of volatile compounds from clary sage (Salvia sclarea L.) verticillasters essential oil and hydrolate. J. Essent. Oil Bear. Plants 2022, 25, 555–570. [Google Scholar] [CrossRef]
- Farkaš, P.; Hollá, M.; Tekel, J.; Mellen, S.; Vaverková, Š. Composition of the essential oils from the flowers and leaves of Salvia sclarea L. (Lamiaceae) cultivated in Slovak Republic. J. Essent. Oil Res. 2005, 17, 141–144. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Setzer, W.N. The essential oil of Salvia sclarea L. from Tajikistan. Rec. Nat. Prod. 2012, 6, 75–79. [Google Scholar]
- Yuce, E.; Yildirim, N.; Yildirim, N.C.; Paksoy, M.Y.; Bagci, E. Essential oil composition, antioxidant and antifungal activities of Salvia sclarea L. from Munzur valley in Tunceli, Turkey. Cell. Mol. Biol. 2014, 60, 1–5. [Google Scholar] [PubMed]
- Doga, G.; Hayta, S.; Yuce, E.; Bagci, E. Composition of the essential oil of two Salvia taxa (Salvia sclarea and Salvia verticillata subsp. verticillata) from Turkey. Nat. Sci. Discovery 2015, 1, 62–67. [Google Scholar] [CrossRef][Green Version]
- Dzumayev, K.K.; Tsibulskaya, I.A.; Zenkevich, I.G.; Tkachenko, K.G.; Satzyperova, I.F. Essential oils of Salvia sclarea L. produced from plants grown in Southern Uzbekistan. J. Essent. Oil Res. 1995, 7, 597–604. [Google Scholar] [CrossRef]
- Gad, H.A.; Mamadalieva, R.Z.; Khalil, N.; Zengin, G.; Najar, B.; Khojimatov, O.K.; Al Musayeib, N.M.; Ashour, M.L.; Mamadalieva, N.Z. GC-MS Chemical profiling, biological investigation of three Salvia species growing in Uzbekistan. Molecules 2022, 27, 5365. [Google Scholar] [CrossRef]
- Tzakou, O.; Pitarokili, D.; Chinou, I.B.; Harvala, C. Composition and antimicrobial activity of the essential oil of Salvia ringens. Planta Med. 2001, 67, 81–83. [Google Scholar] [CrossRef]
- Stojanovic, D.; Marcetic, M.; Usjak, D.; Milenkovic, M. Composition and antimicrobial activity of essential oils of Salvia fruticosa and Salvia ringens (Lamiaceae). VSP 2022, 79, 62–68. [Google Scholar] [CrossRef]
- Georgiev, V.; Marchev, A.; Nikolova, M.; Ivanov, I.; Gochev, V.; Stoyanova, A.; Pavlov, A. Chemical compositions of essential oils from leaves and flowers of Salvia ringens Sibth. et Sm. growing wild in Bulgaria. J. Essent. Oil Bear. Plants 2013, 16, 624–629. [Google Scholar] [CrossRef]
- Alimpić, A.; Pljevljakušić, D.; Šavikin, K.; Knežević, A.; Ćurčić, M.; Veličković, D.; Stević, T.; Petrović, G.; Matevski, V.; Vukojević, J.; et al. Composition and biological effects of Salvia ringens (Lamiaceae) essential oil and extracts. Ind. Crops Prod. 2015, 76, 702–709. [Google Scholar] [CrossRef]
- Sefidkon, F.; Khajavi, M.S. Chemical composition of the essential oils of two Salvia species from Iran: Salvia verticillata L. and Salvia santolinifolia Boiss. Flavour Fragr. J. 1999, 14, 77–78. [Google Scholar] [CrossRef]
- Nasermoadeli, S.; Rowshan, V.; Abotalebi, A.; Nasermoadeli, L.; Charkhchian, M.M. Comparison of Salvia verticillata essential oil components in wild and cultivated population. Ann. Biol. Res. 2013, 4, 252–255. [Google Scholar]
- Dehaghi, N.K.; Ostad, S.N.; Maafi, N.; Pedram, S.; Ajani, Y.; Hadjiakhoondi, A. Cytotoxic activity of the essential oil of Salvia verticillata L. Res. J. Pharmacogn. 2014, 1, 27–33. [Google Scholar]
- Forouzin, F.; Jamei, R.; Heidari, R. Compositional analysis and antioxidant activity of volatile components of two Salvia spp. Trop. J. Pharm. Res. 2015, 14, 2009. [Google Scholar] [CrossRef][Green Version]
- Kameli, M.; Hejazi, S.M.H.; Majd, A.; Mirza, M.; Nejadsattari, T. Study of chemical composition of different populations of Salvia verticillata L. in Iran. IJALS 2017, 10, 299–306. [Google Scholar] [CrossRef]
- Asgarpanah, J. A review on the essential oil chemical profile of Salvia genus from Iran. Nat. Volatiles Essent. Oils 2021, 8, 1–28. [Google Scholar] [CrossRef]
- Tabanca, N.; Demirci, B.; Aytaç, Z.; Can, K.H. The chemical composition of Salvia verticillata L. subsp. verticillata from Turkey. Nat. Volatiles Essent. Oils 2017, 4, 18–28. [Google Scholar]
- Krstic, L.; Malencic, D.; Anackov, G. Structural investigations of trichomes and essential oil composition of Salvia verticillata. Bot. Helv. 2006, 116, 159–168. [Google Scholar] [CrossRef]
- Giuliani, C.; Ascrizzi, R.; Lupi, D.; Tassera, G.; Santagostini, L.; Giovanetti, M.; Flamini, G.; Fico, G. Salvia Verticillata: Linking glandular trichomes, volatiles and pollinators. Phytochemistry 2018, 155, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Smékalová, K.; Dušek, K.; Dušková, E. Salvia verticillata L. and Salvia pratensis L.—the variability of essential oil content in the Czech Republic. Acta Hortic. 2010, 860, 51–60. [Google Scholar] [CrossRef]
- Petrović, S.; Pavlović, M.; Tzakou, O.; Couladis, M.; Milenković, M.; Vučićević, D.; Niketić, M. Composition and antimicrobial activity of Salvia amplexicaulis Lam. essential oil. J. Essent. Oil Res. 2009, 21, 563–566. [Google Scholar] [CrossRef]
- Veličković, D.T.; Ristić, M.S.; Milosavljević, N.P.; Karabegović, I.T.; Stojičević, S.S.; Lazić, M.L. Chemical composition of the essential oils of Salvia austriaca Jacq. and Salvia amplexicaulis Lam. from Serbia. Agro Food Ind. Hi-Tech 2012, 23, 8–10. [Google Scholar]
- Plants of the World Online (POWO). Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:455763-1 (accessed on 31 January 2024).
- Anačkov, G.; Božin, B.; Zorić, L.; Vukov, D.; Mimica-Dukić, N.; Merkulov, L.; Igić, R.; Jovanović, M.; Boža, P. Chemical composition of essential oil and leaf anatomy of Salvia bertolonii Vis. and Salvia pratensis L. (Sect. Plethiosphace, Lamiaceae). Molecules 2008, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sefidkon, F.; Mirza, M. Chemical composition of the essential oils of two Salvia species from Iran, Salvia virgata Jacq. and Salvia syriaca L. Flavour Fragr. J. 1999, 14, 45–46. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Saeedi, M.; Changizi, S.; Vosoughi, M. Essential oil composition of Salvia virgata Jacq. from Iran. J. Essent. Oil Bear. Plants 2005, 8, 330–333. [Google Scholar] [CrossRef]
- Javidnia, K.; Miri, R.; Soltani, M.; Gholami, M.; Khosravi, A.R. Antimicrobial activity and chemical composition of the essential oils of six Iranian Salvia species. Chem. Nat. Compd. 2008, 44, 654–658. [Google Scholar] [CrossRef]
- Baharfar, R.; Tajbakhsh, M.; Azimi, R.; Khalilzadeh, M.A.; Eslami, B. Chemical constituents of essential oils from the leaves, stems and aerial parts of Salvia virgata Jacq. from Iran. J. Essent. Oil Res. 2009, 21, 448–450. [Google Scholar] [CrossRef]
- Alizadeh, A. Essential oil constituents, antioxidant and antimicrobial ativities of Salvia virgata Jacq. from Iran. J. Essent. Oil Bear. Plants 2013, 16, 172–182. [Google Scholar] [CrossRef]
- Golparvar, A.R.; Hadipanah, A.; Gheisari, M.M.; Naderi, D.; Rahmaniyan, S.; Khorrami, M. Chemical composition and antimicrobial activity of essential oil of Salvia officinalis L. and Salvia virgata Jacq. J. Herb. Drugs 2017, 08, 71–78. [Google Scholar] [CrossRef]
- Saadatjoo, B.; Saeidi, K.; Mohammadkhani, A.; Shirmardi, H.A. Assessment of variation in essential oil content and composition within and among Salvia sp. from southwest Iran. J. Essent. Oil Bear. Plants 2018, 21, 237–245. [Google Scholar] [CrossRef]
- Coşge Şenkal, B.; Uskutoğlu, T.; Cesur, C.; Özavci, V.; Doğan, H. Determination of essential oil components, mineral matter, and heavy metal content of Salvia virgata Jacq. grown in culture conditions. Turk. J. Agric. For. 2019, 43, 395–404. [Google Scholar] [CrossRef]
- Altun, M.; Ünal, M.; Kocagöz, T.; Gören, A.C. Essential oil compositions and antimicrobial activity of Salvia species. J. Essent. Oil Bear. Plants 2007, 10, 251–258. [Google Scholar] [CrossRef]
- Kilic, O. Chemical composition of four Salvia L. species from Turkey: A chemotaxonomic approach. J. Essent. Oil Bear, Plants 2016, 19, 229–235. [Google Scholar] [CrossRef]
- Bentham, G. Labiatarum Genera et Species, 1st ed.; Ridgway and Sons: London, UK, 1832; pp. 190–312. [Google Scholar]
- Hellenic Pharmacopoeia, 5th ed.; National Organization for Medicines of Greece: Athens, Greece, 2002; Chapter 28.12.
- van Den Dool, H.; Kratz, P.D. A Generalization of the retention index system including linear temperature programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P.M. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4.1 ed.; Allured Publishing: Carol Stream, IL, USA, 2017. [Google Scholar]

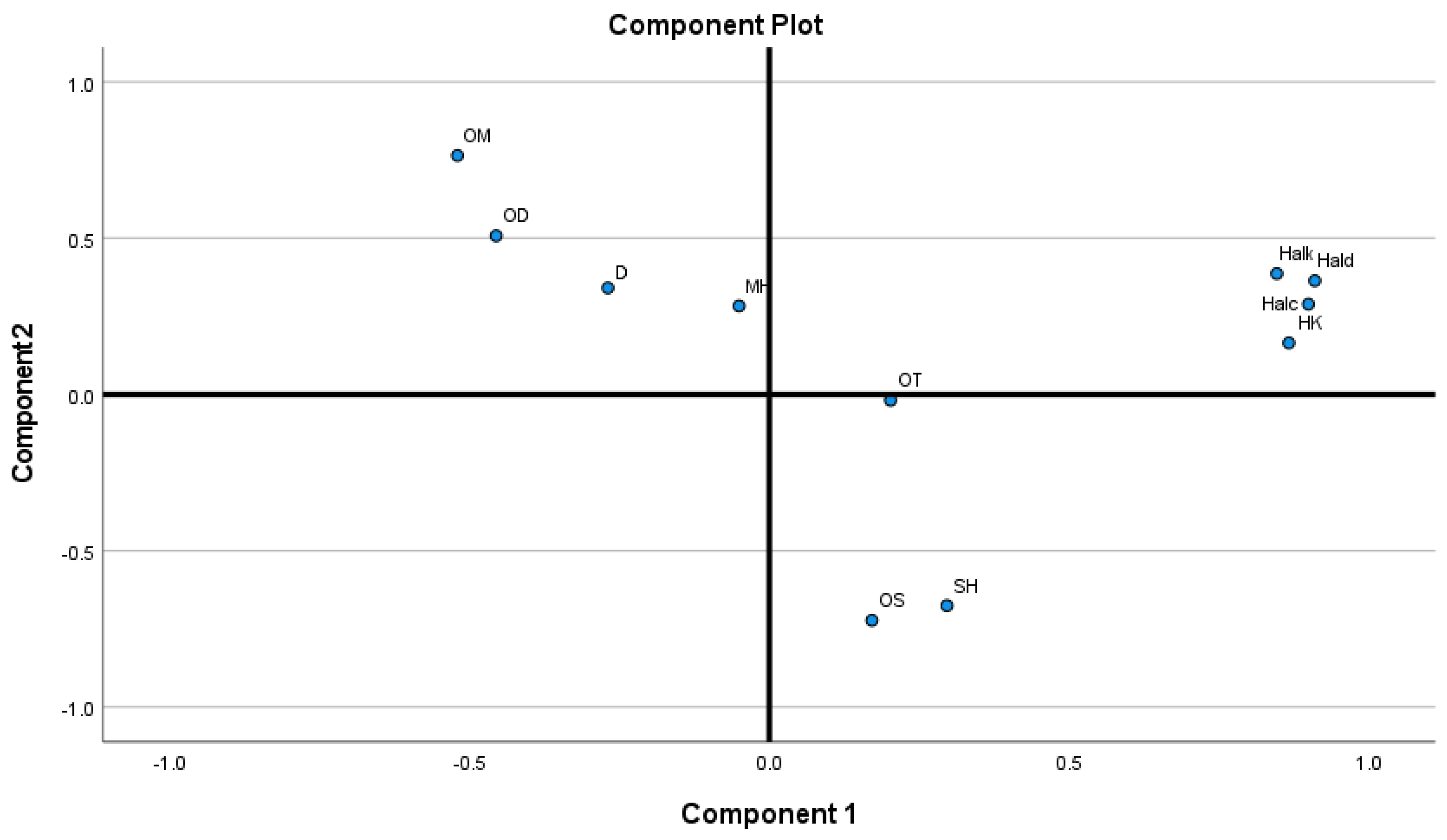

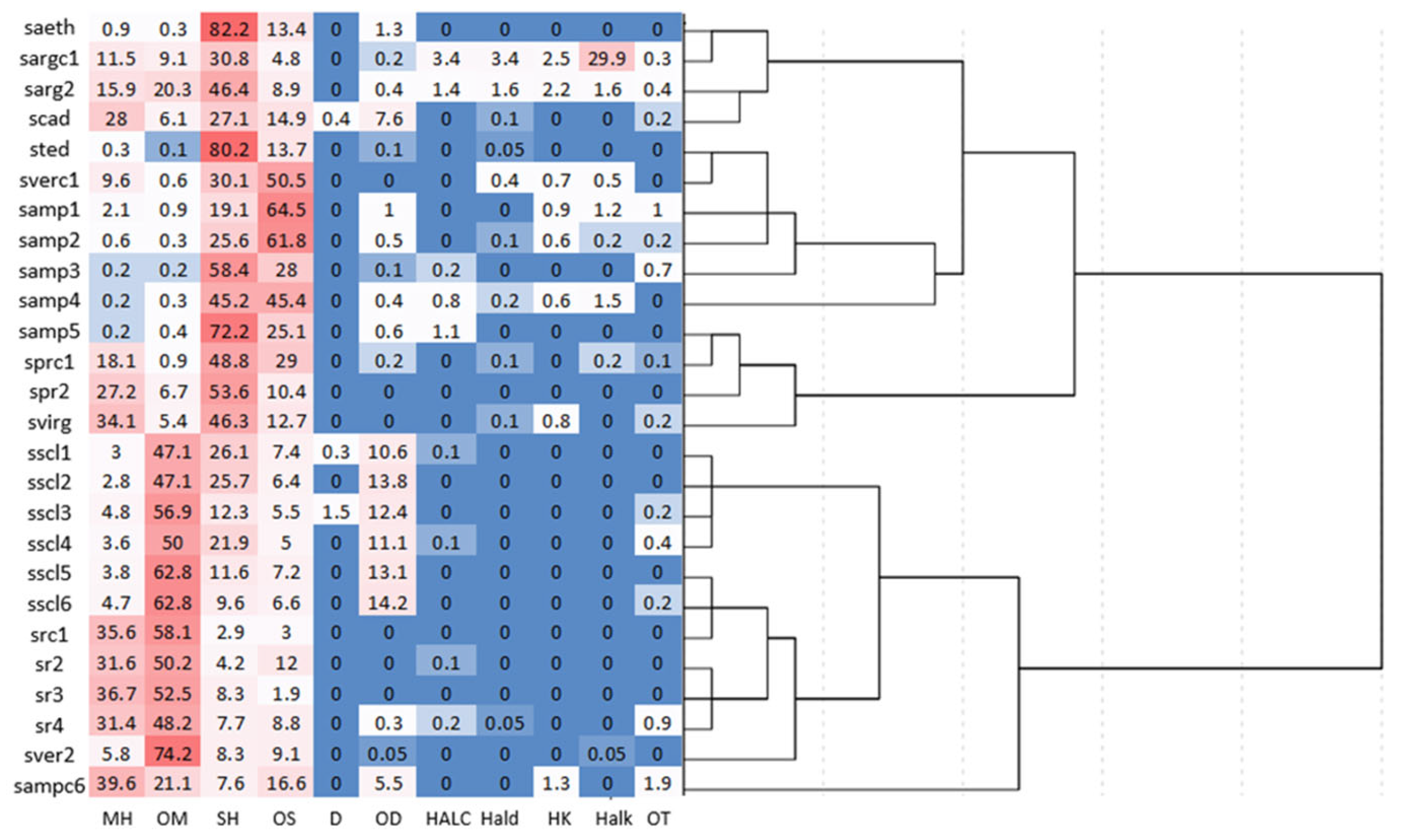
| No | RIC | RIL | Compounds | saeth | sargc1 | sarg2 | scad | sted | sscl1 | sscl2 | sscl3 | sscl4 | sscl5 | sscl6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentage (%) | ||||||||||||||
| 1 | 920 | 921 | Tricyclene | 0.1 | 0.2 | tr | ||||||||
| 2 | 924 | 924 | α-Thujene | tr | tr | 0.2 | tr | |||||||
| 3 | 930 | 932 | α-Pinene | 0.3 | 4.3 | 5.7 | 7.5 | tr | 0.1 | tr | tr | |||
| 4 | 944 | 946 | Camphene | 0.1 | 2.0 | 3.0 | 0.8 | tr | tr | |||||
| 5 | 968 | 969 | Sabinene | 0.3 | 0.7 | 0.9 | 6.2 | 0.2 | tr | tr | ||||
| 6 | 972 | 974 | β-Pinene | 0.2 | 3.2 | 4.3 | 9.7 | 0.1 | 0.1 | 0.1 | ||||
| 7 | 975 | 977 | 1-Octen-3-ol | 0.1 | 0.1 | |||||||||
| 8 | 988 | 988 | Myrcene | 0.2 | 0.3 | 0.2 | 0.9 | 0.8 | 1.4 | 1.2 | 1.2 | 1.4 | ||
| 9 | 1001 | 1002 | α-Phellandrene | 0.1 | ||||||||||
| 10 | 1013 | 1014 | α-Terpinene | 0.1 | 0.2 | 0.2 | tr | tr | tr | |||||
| 11 | 1023 | 1024 | Limonene | 0.5 | 0.6 | 2.0 | 0.3 | 0.3 | 0.4 | 0.4 | 0.4 | 0.4 | ||
| 12 | 1025 | 1026 | 1,8-Cineole | tr | 0.1 | |||||||||
| 13 | 1032 | 1032 | (Z)-β-Ocimene | 0.5 | 0.5 | 0.8 | 0.7 | 0.6 | 0.8 | |||||
| 14 | 1043 | 1044 | (E)-β-Ocimene | tr | 1.0 | 0.9 | 1.5 | 1.3 | 1.2 | 1.5 | ||||
| 15 | 1052 | 1054 | γ-Terpinene | 0.2 | 0.3 | 0.6 | tr | tr | tr | |||||
| 16 | 1063 | 1065 | cis-Sabinene hydrate | 0.2 | ||||||||||
| 17 | 1083 | 1086 | Terpinolene | 0.1 | 0.2 | 0.2 | 0.3 | 0.3 | 0.5 | tr | 0.4 | 0.5 | ||
| 18 | 1087 | 1089 | p-Cymenene | 0.1 | 0.2 | 0.3 | tr | tr | ||||||
| 19 | 1095 | 1095 | Linalool | 0.5 | 3.5 | 0.7 | tr | 14.6 | 13.6 | 18.2 | 16.3 | 24.5 | 21.7 | |
| 20 | 1096 | 1098 | trans-Sabinene hydrate | 0.2 | ||||||||||
| 21 | 1098 | 1100 | n-Nonanal | 3.2 | 1.5 | 0.1 | tr | |||||||
| 22 | 1115 | 1118 | cis-p-Menth-2-en-1-ol | 0.1 | tr | |||||||||
| 23 | 1119 | 1122 | α-Campholenal | 0.1 | 0.4 | 0.3 | ||||||||
| 24 | 1126 | 1128 | allo-Ocimene | tr | tr | tr | ||||||||
| 25 | 1131 | 1135 | trans-Pinocarveol | 0.1 | 0.5 | 0.4 | ||||||||
| 26 | 1135 | 1137 | trans-Sabinol | 0.4 | ||||||||||
| 27 | 1138 | 1140 | trans-Verbenol | 0.1 | 0.4 | 0.3 | ||||||||
| 28 | 1139 | 1141 | Camphor | tr | 0.3 | tr | ||||||||
| 29 | 1149 | 1154 | Nerol oxide | tr | tr | |||||||||
| 30 | 1156 | 1160 | Pinocarvone | tr | 0.4 | 0.4 | ||||||||
| 31 | 1161 | 1165 | Borneol | 0.6 | 2.5 | 0.8 | 0.1 | tr | ||||||
| 32 | 1167 | 1170 | cis-Linalool oxide | 0.1 | 0.1 | |||||||||
| 33 | 1171 | 1174 | Terpinen-4-ol | 0.2 | 0.4 | 0.7 | 0.1 | 0.1 | tr | 0.1 | ||||
| 34 | 1184 | 1186 | α-Terpineol | 0.1 | 0.1 | 0.3 | 5.1 | 4.8 | 6.2 | 6.1 | 7.4 | 6.8 | ||
| 35 | 1189 | 1194 | Myrtenol | 0.2 | 0.2 | 0.4 | ||||||||
| 36 | 1192 | 1195 | Myrtenal | 0.4 | 0.4 | |||||||||
| 37 | 1198 | 1200 | trans-Dihydrocarvone | tr | ||||||||||
| 38 | 1200 | 1201 | n-Decanal | 0.2 | 0.1 | |||||||||
| 39 | 1202 | 1204 | Verbenone | 0.1 | ||||||||||
| 40 | 1205 | 1207 | trans-Piperitol | tr | ||||||||||
| 41 | 1210 | 1214 | Linalool formate | 0.1 | 0.1 | 0.1 | ||||||||
| 42 | 1211 | 1215 | trans-Carveol | 0.1 | ||||||||||
| 43 | 1223 | 1227 | Nerol | 0.8 | 0.6 | 1.1 | 1.1 | 0.9 | 1.2 | |||||
| 44 | 1230 | 1235 | Isobornyl formate | 0.1 | ||||||||||
| 45 | 1234 | 1239 | Carvone | tr | ||||||||||
| 46 | 1250 | 1254 | Linalool acetate | 0.2 | 0.2 | 21.2 | 23.9 | 24.4 | 22.3 | 24.1 | 25.7 | |||
| 47 | 1277 | 1280 | Neryl formate | tr | ||||||||||
| 48 | 1279 | 1284 | Bornyl acetate | 0.3 | 6.8 | 10.4 | 0.2 | tr | ||||||
| 49 | 1289 | 1289 | Thymol | 0.2 | 0.1 | |||||||||
| 50 | 1295 | 1298 | Carvacrol | tr | 0.2 | |||||||||
| 51 | 1298 | 1298 | Geranyl formate | 0.1 | 0.1 | 0.1 | ||||||||
| 52 | 1330 | 1335 | δ-Elemene | 0.9 | 0.2 | 0.2 | ||||||||
| 53 | 1342 | 1348 | α-Cubebene | 0.4 | tr | 0.2 | ||||||||
| 54 | 1350 | 1356 | Eugenol | tr | ||||||||||
| 55 | 1355 | 1357 | 4aα,7α,7aα-Nepetalactone | 0.1 | tr | |||||||||
| 56 | 1359 | 1359 | Neryl acetate | 1.6 | 1.5 | 2.2 | tr | 2.1 | 2.4 | |||||
| 57 | 1372 | 1374 | α-Copaene | 13.7 | 3.1 | 5.4 | 0.7 | 0.2 | 1.5 | 1.7 | 0.8 | 1.4 | 0.9 | 0.8 |
| 58 | 1377 | 1379 | Geranyl acetate | 3.5 | 2.7 | 4.2 | 4.0 | 3.8 | 4.6 | |||||
| 59 | 1384 | 1387 | β-Bourbonene | 0.7 | 0.5 | 0.5 | 0.5 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 | ||
| 60 | 1386 | 1387 | β-Cubebene | 4.4 | 0.2 | 0.4 | 0.2 | 0.1 | 0.4 | 0.6 | 0.2 | 0.2 | ||
| 61 | 1388 | 1389 | β-Elemene | 1.1 | 2.2 | 3.1 | 0.5 | 0.2 | 0.4 | 0.4 | 0.2 | 0.3 | 0.1 | |
| 62 | 1402 | 1409 | α-Gurjunene | 1.6 | 2.4 | 0.4 | ||||||||
| 63 | 1412 | 1417 | (E)-Caryophyllene | 30.6 | 2.9 | 3.0 | 4.7 | 59.6 | 4.5 | 6.3 | 1.8 | 3.2 | 2.7 | 1.8 |
| 64 | 1424 | 1430 | β-Copaene | 0.2 | 0.1 | 0.2 | 0.1 | |||||||
| 65 | 1426 | 1431 | β-Gurjunene (Calarene) | 0.1 | 0.1 | 0.1 | ||||||||
| 66 | 1429 | 1432 | α-trans-Bergamotene | 0.2 | ||||||||||
| 67 | 1435 | 1439 | Aromadendrene | 0.3 | 0.5 | 0.3 | 0.1 | 0.1 | ||||||
| 68 | 1438 | 1440 | (Z)-β-Farnesene | 0.2 | ||||||||||
| 69 | 1440 | 1442 | Guaia-6,9-diene | 0.4 | 0.4 | 0.3 | ||||||||
| 70 | 1447 | 1452 | α-Humulene | 6.4 | 0.6 | 0.5 | 0.4 | 4.8 | 0.2 | 0.3 | 0.1 | 0.2 | 0.1 | |
| 71 | 1449 | 1453 | Geranyl acetone | 0.2 | 0.2 | 0.1 | ||||||||
| 72 | 1452 | 1454 | (E)-β-Farnesene | 0.5 | 0.1 | 5.4 | tr | tr | ||||||
| 73 | 1455 | 1458 | Alloaromadendrene | tr | 1.1 | 0.1 | tr | tr | ||||||
| 74 | 1476 | 1480 | Germacrene D | 20.0 | 13.6 | 20.0 | 15.9 | 6.2 | 14.7 | 14.7 | 7.6 | 14.2 | 5.8 | 5.1 |
| 75 | 1479 | 1482 | epi-Bicyclosesquiphellandrene | 0.1 | 0.1 | |||||||||
| 76 | 1484 | 1487 | (E)-β-Ionone | 0.3 | 0.4 | |||||||||
| 77 | 1488 | 1490 | Phenyl ethyl 3-methyl butanoate | 0.2 | ||||||||||
| 78 | 1492 | 1496 | Valencene | 0.4 | 0.7 | 0.1 | ||||||||
| 79 | 1494 | 1496 | Viridiflorene (Ledene) | 0.1 | ||||||||||
| 80 | 1498 | 1500 | Bicyclogermacrene | 0.6 | 0.5 | 1.0 | 1.6 | 1.4 | 2.7 | 0.9 | 0.5 | 0.6 | 1.0 | 0.2 |
| 81 | 1499 | 1500 | α-Muurolene | 0.1 | tr | |||||||||
| 82 | 1502 | 1505 | β-Bisabolene | 0.1 | ||||||||||
| 83 | 1503 | 1505 | (E,E)-α-Farnesene | 0.3 | 0.4 | |||||||||
| 84 | 1504 | 1508 | Germacrene A | 0.5 | 0.7 | 1.1 | 0.2 | 0.1 | 0.2 | 0.2 | 0.1 | 0.2 | 0.1 | |
| 85 | 1510 | 1513 | γ-Cadinene | 0.3 | 0.1 | 0.3 | 0.3 | |||||||
| 86 | 1512 | 1514 | Cubebol | 0.6 | ||||||||||
| 87 | 1521 | 1522 | δ-Cadinene | 3.6 | 3.7 | 5.9 | 0.9 | 0.2 | 0.5 | 0.6 | 0.3 | 0.5 | 0.3 | 0.3 |
| 88 | 1523 | 1528 | cis-Calamenene | 0.1 | ||||||||||
| 89 | 1541 | 1544 | α-Calacorene | tr | tr | 0.5 | 0.1 | |||||||
| 90 | 1546 | 1548 | Elemol | 0.1 | 0.1 | 0.1 | ||||||||
| 91 | 1550 | 1557 | 1,5-Epoxysalvial-4(14)-ene | 0.4 | 0.2 | 0.2 | 0.1 | 0.1 | 0.3 | 0.3 | ||||
| 92 | 1554 | 1559 | Germacrene B | 0.1 | ||||||||||
| 93 | 1557 | 1561 | (E)-Nerolidol | tr | tr | |||||||||
| 94 | 1569 | 1573 | epi-Globulol | 0.8 | 1.2 | |||||||||
| 95 | 1571 | 1574 | Germacrene D-4-ol | 0.5 | ||||||||||
| 96 | 1574 | 1577 | Spathulenol | 0.4 | 1.0 | 5.3 | 1.5 | 0.6 | 0.4 | 0.4 | 1.6 | 0.4 | ||
| 97 | 1579 | 1582 | Caryophyllene oxide | 8.4 | 3.3 | 4.4 | 3.3 | 11.2 | 1.6 | 2.3 | 0.5 | 0.7 | 3.6 | 2.1 |
| 98 | 1585 | 1590 | Globulol | 0.4 | ||||||||||
| 99 | 1588 | 1590 | β-Copaen-4-α-ol | 0.4 | 0.4 | 0.1 | ||||||||
| 100 | 1590 | 1594 | Salvial-4(14)-en-1-one | 0.6 | 1.1 | 0.1 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | ||
| 101 | 1598 | 1600 | n-Hexadecane | 0.1 | ||||||||||
| 102 | 1601 | 1602 | Ledol | 0.4 | ||||||||||
| 103 | 1603 | 1604 | (2R,5E)-Caryophyll-5-en-12-al | 0.4 | 0.5 | 0.1 | 0.2 | |||||||
| 104 | 1604 | 1607 | β-Oplopenone | 0.2 | 0.2 | 0.3 | 0.2 | 0.1 | ||||||
| 105 | 1605 | 1608 | Humulene epoxide II | 1.3 | 0.3 | 0.3 | 0.5 | 0.7 | 0.2 | 0.2 | 0.1 | |||
| 106 | 1617 | 1622 | 10-epi-γ-Eudesmol | 0.1 | 0.1 | 0.1 | 0.1 | |||||||
| 107 | 1625 | 1626 | (2S,5E)-Caryophyll-5-en-12-al | 0.2 | 0.5 | |||||||||
| 108 | 1626 | 1627 | 1-epi-Cubenol | 0.3 | ||||||||||
| 109 | 1627 | 1628 | Isospathulenol | 0.4 | 0.3 | 0.2 | ||||||||
| 110 | 1629 | 1631 | Allo-aromadendrene epoxide | 0.5 | ||||||||||
| 111 | 1635 | 1638 | epi-α-Cadinol | 0.2 | 0.3 | |||||||||
| 112 | 1637 | 1639 | Caryophylla-4(12),8(13)-dien-5α-ol or Caryophylla-4(12),8(13)-dien-5β-ol | 0.2 | 0.1 | 0.7 | ||||||||
| 113 | 1639 | 1640 | epi-α-Muurolol (tau muurolol) | 0.4 | 0.7 | 0.3 | 0.2 | |||||||
| 114 | 1641 | 1644 | α-Muurolol | 0.1 | ||||||||||
| 115 | 1644 | 1649 | β-Selinene | 0.2 | 0.1 | 0.1 | 0.1 | |||||||
| 116 | 1645 | 1649 | β-Eudesmol | 1.1 | 1.0 | 1.0 | 0.6 | 0.5 | 0.9 | |||||
| 117 | 1648 | 1650 | Caryophyllenol-II | 0.2 | ||||||||||
| 118 | 1649 | 1652 | α-Cadinol | 0.4 | tr | 0.2 | 1.2 | 0.3 | 0.3 | 0.7 | ||||
| 119 | 1651 | 1652 | α-Eudesmol | 0.8 | 0.7 | 0.7 | 1.2 | 0.7 | ||||||
| 120 | 1654 | 1656 | Valerianol | 0.5 | 0.7 | |||||||||
| 121 | 1657 | 1661 | 14-Hydroxy-9-epi-(E)-caryophyllene | 0.6 | ||||||||||
| 122 | 1664 | 1666 | 14-Hydroxy-(Z)-caryophyllene | 0.2 | 0.6 | |||||||||
| 123 | 1680 | 1685 | Germacra-4(15),5,10(14)-trien-1α-ol | 0.2 | ||||||||||
| 124 | 1683 | 1685 | α-Bisabolol | 0.1 | ||||||||||
| 125 | 1684 | 1688 | 2,3-Dihydro-farnesol | tr | ||||||||||
| 126 | 1688 | 1690 | Endo-8-hydroxy-cycloisolongifolene | 0.3 | 0.2 | |||||||||
| 127 | 1690 | 1691 | Vulgarol B | 0.3 | 0.2 | 0.3 | ||||||||
| 128 | 1695 | 1697 | 2-Pentadecanone | 1.4 | 1.1 | |||||||||
| 129 | 1697 | 1698 | (2Z,6Z)-Farnesol | 0.1 | ||||||||||
| 130 | 1699 | 1704 | δ-Dodecalactone | 0.2 | 0.4 | 0.2 | ||||||||
| 131 | 1710 | 1711 | Valerenol | 0.4 | 0.2 | 0.2 | 0.4 | 0.3 | ||||||
| 132 | 1740 | 1743 | Aromadendrene epoxide | tr | 0.1 | |||||||||
| 133 | 1764 | 1765 | β-Costol | 0.1 | ||||||||||
| 134 | 1766 | 1768 | β-Bisabolenal | 0.2 | ||||||||||
| 135 | 1778 | 1779 | 14-Hydroxy-α-muurolene | 0.1 | ||||||||||
| 136 | 1799 | 1800 | n-Octadecane | 0.2 | ||||||||||
| 137 | 1800 | 1803 | 14-Hydroxy-δ-cadinene | 0.1 | ||||||||||
| 138 | 1820 | 1826 | 8,13-Epoxy-15,16-dinorlab-12-ene (sclareol oxide) | 0.2 | 1.3 | 0.1 | 1.6 | 1.5 | 1.0 | 0.9 | 1.9 | 1.4 | ||
| 139 | 1842 | 1845 | 6,10,14-Trimethyl-2-pentadecanone (Hexahydrofarnesyl acetone) | 1.1 | 1.1 | |||||||||
| 140 | 1914 | β-Springene | 0.4 | 0.3 | 1.5 | |||||||||
| 141 | 1935 | 1942 | Phytol | 0.6 | 0.2 | 0.2 | 0.1 | 0.3 | 0.1 | 0.1 | ||||
| 142 | 1974 | Ledene oxide-(I) | 0.1 | 0.2 | ||||||||||
| 143 | 1985 | 1987 | Manool oxide | 0.4 | 0.2 | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 | ||||
| 144 | 1999 | 2000 | n-Eicosane | 0.1 | ||||||||||
| 145 | 2002 | 2009 | 13-epi-Manool oxide | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 | |||||
| 146 | 2023 | 2026 | (E,E)-Geranyl linalool | 0.2 | 0.2 | tr | ||||||||
| 147 | 2050 | 2056 | Manool | 0.6 | 0.8 | 0.9 | 0.6 | 0.9 | ||||||
| 148 | 2055 | 2059 | 13-epi-Manool | 1.0 | 0.7 | |||||||||
| 149 | 2075 | 2077 | n-Octadecanol | 3.4 | 1.4 | |||||||||
| 150 | 2099 | 2100 | n-Heneicosane | 0.2 | 0.1 | |||||||||
| 151 | 2143 | 2149 | Abienol | 0.1 | 0.1 | 0.2 | 0.1 | |||||||
| 152 | 2200 | 2200 | n-Docosane | 0.1 | ||||||||||
| 153 | 2215 | 2218 | (E)-Phytol acetate | 0.2 | ||||||||||
| 154 | 2220 | 2222 | Sclareol | 5.1 | 7.6 | 10.8 | 9.8 | 8.6 | 10.3 | 11.2 | ||||
| 155 | 2299 | 2300 | n-Tricosane | 0.2 | ||||||||||
| 156 | 2399 | 2400 | n-Tetracosane | 20.0 | ||||||||||
| 157 | 2500 | 2500 | n-Pentacosane | 8.8 | 0.4 | |||||||||
| 158 | 2699 | 2700 | Heptacosane | 0.7 | 0.6 | |||||||||
| Total | 98.1 | 95.9 | 99.1 | 84.4 | 94.4 | 94.6 | 95.8 | 93.6 | 92.1 | 98.7 | 98.1 | |||
| Grouped components | saeth | sargc1 | sarg2 | scad | sted | sscl1 | sscl2 | sscl3 | sscl4 | sscl5 | sscl6 | |||
| Monoterpene Hydrocarbons | 0.9 | 11.5 | 15.9 | 28.0 | 0.3 | 3.0 | 2.8 | 4.8 | 3.6 | 3.8 | 4.7 | |||
| Oxygenated Monoterpenes | 0.3 | 9.1 | 20.3 | 6.1 | 0.1 | 47.1 | 47.1 | 56.9 | 50.0 | 62.8 | 62.8 | |||
| Sesquiterpene Hydrocarbons | 82.2 | 30.8 | 46.4 | 27.1 | 80.2 | 26.1 | 25.7 | 12.3 | 21.9 | 11.6 | 9.6 | |||
| Oxygenated Sesquiterpenes | 13.4 | 4.8 | 8.9 | 14.9 | 13.7 | 7.4 | 6.4 | 5.5 | 5.0 | 7.2 | 6.6 | |||
| Diterpenes | 0.4 | 0.3 | 1.5 | |||||||||||
| Oxygenated diterpenes | 1.3 | 0.2 | 0.4 | 7.6 | 0.1 | 10.6 | 13.8 | 12.4 | 11.1 | 13.1 | 14.2 | |||
| Hydrocarbons–Alcohols | 3.4 | 1.4 | 0.1 | 0.1 | ||||||||||
| Hydrocarbons–Aldehydes | 3.4 | 1.6 | 0.1 | tr | ||||||||||
| Hydrocarbons–Ketones | 2.5 | 2.2 | ||||||||||||
| Hydrocarbons–Alkanes | 29.9 | 1.6 | ||||||||||||
| Esters | 0.2 | |||||||||||||
| Phenylpropanoids | tr | |||||||||||||
| Others | 0.3 | 0.4 | 0.2 | 0.4 | 0.2 | |||||||||
| Total | 98.1 | 95.9 | 99.1 | 84.4 | 94.4 | 94.6 | 95.8 | 93.6 | 92.1 | 98.7 | 98.1 | |||
| No | RIC | RIL | Compounds | src1 | sr2 | sr3 | sr4 |
|---|---|---|---|---|---|---|---|
| Percentage (%) | |||||||
| 1 | 920 | 921 | Tricyclene | 0.8 | 0.6 | 0.6 | 1.1 |
| 2 | 924 | 924 | α-Thujene | 0.2 | 0.6 | ||
| 3 | 930 | 932 | α-Pinene | 9.1 | 12.5 | 11.2 | 11.6 |
| 4 | 944 | 946 | Camphene | 15.6 | 5.5 | 11.1 | 10.3 |
| 5 | 968 | 969 | Sabinene | 0.1 | 1.4 | ||
| 6 | 972 | 974 | β-Pinene | 4.8 | 6.6 | 7.7 | 5.5 |
| 7 | 975 | 977 | 1-Octen-3-ol | 0.1 | 0.2 | ||
| 8 | 988 | 988 | Myrcene | 2.2 | 5.7 | 1.2 | 2.5 |
| 9 | 1001 | 1002 | α-Phellandrene | 0.2 | 0.3 | 0.1 | |
| 10 | 1013 | 1014 | α-Terpinene | 0.2 | 0.2 | 0.4 | 0.1 |
| 11 | 1025 | 1026 | 1,8-Cineole | 17.9 | 34.4 | 40.2 | 20.5 |
| 12 | 1029 | 1031 | Benzene acetaldehyde | tr | |||
| 13 | 1032 | 1032 | (Z)-β-Ocimene | 0.3 | |||
| 14 | 1043 | 1044 | (E)-β-Ocimene | tr | |||
| 15 | 1052 | 1054 | γ-Terpinene | tr | 0.1 | 0.4 | 0.1 |
| 16 | 1063 | 1065 | cis-Sabinene hydrate | 0.1 | tr | tr | |
| 17 | 1083 | 1086 | Terpinolene | 0.1 | 0.1 | ||
| 18 | 1087 | 1089 | p-Cymenene | 2.4 | 1.8 | ||
| 19 | 1095 | 1095 | Linalool | tr | 0.4 | 1.0 | 0.5 |
| 20 | 1096 | 1098 | trans-Sabinene hydrate | tr | tr | ||
| 21 | 1099 | 1101 | cis-Thujone | 1.4 | |||
| 22 | 1110 | 1112 | trans-Thujone | 0.3 | |||
| 23 | 1113 | 1114 | endo-Fenchol | 0.1 | |||
| 24 | 1115 | 1118 | cis-p-Menth-2-en-1-ol | 0.9 | 0.2 | 0.5 | |
| 25 | 1119 | 1122 | α-Campholenal | 0.1 | |||
| 26 | 1135 | 1137 | trans-Sabinol | 1.0 | 0.2 | ||
| 27 | 1139 | 1141 | Camphor | 1.8 | 1.8 | 4.5 | 2.7 |
| 28 | 1143 | 1145 | Camphene hydrate | 0.1 | 0.1 | ||
| 29 | 1156 | 1160 | Pinocarvone | 0.2 | tr | ||
| 30 | 1161 | 1165 | Borneol | 12.9 | 5.5 | 1.6 | 12.7 |
| 31 | 1167 | 1170 | cis-Linalool oxide | tr | tr | ||
| 32 | 1171 | 1174 | Terpinen-4-ol | 1.2 | 1.5 | 0.8 | 1.2 |
| 33 | 1178 | 1179 | p-Cymen-8-ol | tr | |||
| 34 | 1184 | 1186 | α-Terpineol | 1.5 | 0.5 | 0.8 | 0.5 |
| 35 | 1189 | 1194 | Myrtenol | 0.2 | |||
| 36 | 1192 | 1195 | Myrtenal | 0.1 | 0.2 | ||
| 37 | 1193 | 1195 | cis-Piperitol | 0.4 | 0.4 | ||
| 38 | 1205 | 1207 | trans-Piperitol | 0.6 | 0.4 | 0.2 | |
| 39 | 1211 | 1215 | trans-Carveol | 0.1 | 0.1 | ||
| 40 | 1222 | 1226 | cis-Carveol | tr | tr | ||
| 41 | 1223 | 1227 | Nerol | tr | |||
| 42 | 1230 | 1235 | Isobornyl formate | 0.1 | |||
| 43 | 1234 | 1239 | Carvone | tr | |||
| 44 | 1243 | 1249 | Piperitone | 0.1 | 0.1 | ||
| 45 | 1250 | 1254 | Linalool acetate | 0.1 | 0.1 | ||
| 46 | 1279 | 1284 | Bornyl acetate | 20.3 | 3.0 | 2.0 | 7.9 |
| 47 | 1289 | 1289 | Thymol | 0.1 | 0.1 | ||
| 48 | 1295 | 1298 | Carvacrol | 0.4 | 0.1 | 0.1 | |
| 49 | 1320 | 1324 | Myrtenyl acetate | tr | |||
| 50 | 1332 | 1339 | trans-Carvyl acetate | tr | |||
| 51 | 1350 | 1356 | Eugenol | 0.9 | |||
| 52 | 1355 | 1357 | 4aα,7α,7aα-Nepetalactone | tr | |||
| 53 | 1359 | 1359 | Neryl acetate | tr | |||
| 54 | 1367 | 1373 | α-Ylangene | 0.1 | 0.1 | ||
| 55 | 1372 | 1374 | α-Copaene | 0.1 | 0.5 | 0.3 | 0.2 |
| 56 | 1377 | 1379 | Geranyl acetate | 0.1 | 0.1 | ||
| 57 | 1384 | 1387 | β-Bourbonene | 0.3 | 0.1 | 0.5 | 0.1 |
| 58 | 1399 | 1402 | 1,7-di-epi-α-Cedrene | tr | |||
| 59 | 1402 | 1409 | α-Gurjunene | 0.1 | 0.2 | ||
| 60 | 1408 | 1410 | α-Cedrene | 0.2 | |||
| 61 | 1412 | 1417 | (E)-Caryophyllene | 0.8 | 1.1 | 2.7 | 0.7 |
| 62 | 1424 | 1430 | β-Copaene | 0.1 | 0.1 | ||
| 63 | 1426 | 1431 | β-Gurjunene (Calarene) | 0.1 | |||
| 64 | 1429 | 1432 | α-trans-Bergamotene | 0.6 | |||
| 65 | 1435 | 1439 | Aromadendrene | 0.2 | |||
| 66 | 1447 | 1452 | α-Humulene | 0.4 | 0.3 | 1.4 | 0.9 |
| 67 | 1449 | 1453 | Geranyl acetone | tr | |||
| 68 | 1452 | 1454 | (E)-β-Farnesene | tr | 0.2 | ||
| 69 | 1455 | 1458 | Alloaromadendrene | 0.1 | 0.1 | ||
| 70 | 1464 | 1469 | β-Acoradiene | 0.2 | |||
| 71 | 1472 | 1478 | γ-Muurolene | 0.4 | 0.7 | 0.4 | |
| 72 | 1475 | 1479 | ar-Curcumene | 1.4 | |||
| 73 | 1476 | 1480 | Germacrene D | 0.9 | |||
| 74 | 1478 | 1481 | γ-Curcumene | 0.1 | |||
| 75 | 1480 | 1483 | α-Amorphene | 0.4 | 0.1 | 0.2 | |
| 76 | 1492 | 1496 | Valencene | 0.2 | |||
| 77 | 1499 | 1500 | α-Muurolene | 0.1 | 0.1 | 0.2 | 0.2 |
| 78 | 1502 | 1505 | β-Bisabolene | 0.3 | |||
| 79 | 1510 | 1513 | γ-Cadinene | 0.3 | 0.2 | 0.3 | 0.4 |
| 80 | 1520 | 1521 | trans-Calamenene | 0.3 | 0.7 | ||
| 81 | 1521 | 1522 | δ-Cadinene | 0.3 | 0.1 | 0.6 | 0.5 |
| 82 | 1523 | 1528 | cis-Calamenene | 0.2 | 0.3 | ||
| 83 | 1531 | 1537 | α-Cadinene | tr | 0.1 | ||
| 84 | 1539 | 1542 | cis-Sesquisabinene hydrate | 0.3 | |||
| 85 | 1541 | 1544 | α-Calacorene | 0.1 | 0.1 | ||
| 86 | 1569 | 1573 | epi-Globulol | 0.1 | |||
| 87 | 1571 | 1574 | Germacrene D-4-ol | 0.2 | |||
| 88 | 1574 | 1577 | Spathulenol | 0.7 | 1.1 | ||
| 89 | 1579 | 1582 | Caryophyllene oxide | 1.4 | 2.0 | 1.2 | 0.7 |
| 90 | 1580 | 1586 | Gleenol | 0.1 | 0.1 | ||
| 91 | 1589 | 1592 | Viridiflorol | 0.9 | 0.2 | ||
| 92 | 1597 | 1600 | Rosifoliol | 0.2 | |||
| 93 | 1601 | 1602 | Ledol | 0.3 | |||
| 94 | 1605 | 1608 | Humulene epoxide II | 0.8 | 0.5 | 0.5 | 1.4 |
| 95 | 1615 | 1618 | 1,10-di-epi-Cubenol | 0.2 | |||
| 96 | 1626 | 1627 | 1-epi-Cubenol | 0.2 | |||
| 97 | 1630 | 1632 | α-Acorenol | 0.2 | |||
| 98 | 1635 | 1638 | epi-α-Cadinol | 0.4 | |||
| 99 | 1637 | 1639 | Caryophylla-4(12),8(13)-dien-5α-ol or Caryophylla-4(12),8(13)-dien-5β-ol | 0.2 | 0.4 | ||
| 100 | 1639 | 1640 | epi-α-Muurolol (tau muurolol) | 0.1 | 0.3 | ||
| 101 | 1641 | 1644 | α-Muurolol | 0.5 | |||
| 102 | 1644 | 1649 | β-Selinene | 0.1 | |||
| 103 | 1649 | 1652 | α-Cadinol | 0.6 | 0.3 | 0.2 | 0.6 |
| 104 | 1665 | 1670 | epi-β-Bisabolol | 1.6 | |||
| 105 | 1671 | 1674 | Valeranone | 6.4 | |||
| 106 | 1683 | 1685 | α-Bisabolol | 0.3 | 0.5 | ||
| 107 | 1985 | 1987 | Manool oxide | tr | 0.1 | ||
| 108 | 2002 | 2009 | 13-epi-Manool oxide | tr | 0.1 | ||
| 109 | 2025 | 2030 | (6Z,10E)-Pseudo phytol | tr | |||
| 110 | 2050 | 2056 | Manool | tr | |||
| 111 | 2053 | 2058 | (6Ε,10E)-Pseudo phytol | tr | |||
| 112 | 2220 | 2222 | Sclareol | tr | 0.1 | ||
| Total | 99.6 | 98.1 | 99.4 | 97.5 | |||
| Grouped components | src1 | sr2 | sr3 | sr4 | |||
| Monoterpene Hydrocarbons | 35.6 | 31.6 | 36.7 | 31.4 | |||
| Oxygenated Monoterpenes | 58.1 | 50.2 | 52.5 | 48.2 | |||
| Sesquiterpene Hydrocarbons | 2.9 | 4.2 | 8.3 | 7.7 | |||
| Oxygenated Sesquiterpenes | 3.0 | 12.0 | 1.9 | 8.8 | |||
| Oxygenated diterpenes | 0.3 | ||||||
| Hydrocarbons–Alcohols | 0.1 | 0.2 | |||||
| Hydrocarbons–Aldehydes | tr | ||||||
| Phenylpropanoids | 0.9 | ||||||
| Total | 99.6 | 98.1 | 99.4 | 97.5 | |||
| No | RIC | RIL | Compounds | sverc1 | sver2 |
|---|---|---|---|---|---|
| Percentage (%) | |||||
| 1 | 930 | 932 | α-Pinene | 1.0 | 0.6 |
| 2 | 944 | 946 | Camphene | tr | |
| 3 | 960 | 961 | Verbenene | 0.2 | |
| 4 | 968 | 969 | Sabinene | 0.2 | 0.6 |
| 5 | 972 | 974 | β-Pinene | 6.1 | 1.6 |
| 6 | 988 | 988 | Myrcene | 0.3 | 0.2 |
| 7 | 1013 | 1014 | α-Terpinene | 0.1 | |
| 8 | 1023 | 1024 | Limonene | 1.8 | |
| 9 | 1025 | 1026 | 1,8-Cineole | 18.4 | |
| 10 | 1032 | 1032 | (Z)-β-Ocimene | 0.7 | |
| 11 | 1043 | 1044 | (E)-β-Ocimene | 1.3 | |
| 12 | 1052 | 1054 | γ-Terpinene | 0.7 | |
| 13 | 1063 | 1065 | cis-Sabinene hydrate | 0.1 | |
| 14 | 1083 | 1086 | Terpinolene | tr | |
| 15 | 1095 | 1095 | Linalool | 0.2 | 0.1 |
| 16 | 1098 | 1100 | n-Nonanal | 0.4 | |
| 17 | 1099 | 1101 | cis-Thujone | 0.1 | |
| 18 | 1119 | 1122 | α-Campholenal | 0.1 | |
| 19 | 1131 | 1135 | trans-Pinocarveol | 0.1 | |
| 20 | 1134 | 1137 | cis-Verbenol | 0.4 | 0.1 |
| 21 | 1139 | 1141 | Camphor | 0.1 | |
| 22 | 1156 | 1160 | Pinocarvone | 0.1 | |
| 23 | 1171 | 1174 | Terpinen-4-ol | 0.2 | |
| 24 | 1184 | 1186 | α-Terpineol | 1.2 | |
| 25 | 1189 | 1194 | Myrtenol | 0.1 | |
| 26 | 1192 | 1195 | Myrtenal | 0.2 | |
| 27 | 1198 | 1200 | trans-Dihydrocarvone | tr | |
| 28 | 1295 | 1298 | Carvacrol | 0.1 | |
| 29 | 1300 | 1300 | n-Tridecane | 0.3 | |
| 30 | 1342 | 1348 | α-Cubebene | 0.4 | |
| 31 | 1355 | 1357 | 4aα,7α,7aα-Nepetalactone | 51.4 | |
| 32 | 1372 | 1374 | α-Copaene | 2.3 | 0.2 |
| 33 | 1380 | 1386 | 4aα,7α,7aβ-Nepetalactone | 1.2 | |
| 34 | 1384 | 1387 | β-Bourbonene | 3.0 | 1.3 |
| 35 | 1386 | 1387 | β-Cubebene | 0.8 | |
| 36 | 1388 | 1389 | β-Elemene | 0.7 | 0.2 |
| 37 | 1389 | 1391 | 4a-α,7β,7aα-Nepetalactone | 0.6 | |
| 38 | 1402 | 1409 | α-Gurjunene | 0.4 | |
| 39 | 1412 | 1417 | (E)-Caryophyllene | 3.8 | 1.3 |
| 40 | 1424 | 1430 | β-Copaene | 0.1 | |
| 41 | 1426 | 1431 | β-Gurjunene (Calarene) | 2.0 | |
| 42 | 1447 | 1452 | α-Humulene | 0.7 | 0.4 |
| 43 | 1452 | 1454 | (E)-β-Farnesene | 1.5 | |
| 44 | 1455 | 1458 | Alloaromadendrene | 0.4 | |
| 45 | 1472 | 1478 | γ-Muurolene | 1.2 | |
| 46 | 1476 | 1480 | Germacrene D | 11.5 | 2.8 |
| 47 | 1479 | 1482 | epi-Bicyclosesquiphellandrene | 0.4 | |
| 48 | 1480 | 1483 | α-Amorphene | 0.4 | |
| 49 | 1498 | 1500 | Bicyclogermacrene | 0.1 | |
| 50 | 1499 | 1500 | α-Muurolene | 0.1 | |
| 51 | 1502 | 1505 | β-Bisabolene | 0.1 | |
| 52 | 1504 | 1508 | Germacrene A | 0.1 | |
| 53 | 1510 | 1513 | γ-Cadinene | 0.8 | |
| 54 | 1521 | 1522 | δ-Cadinene | 1.2 | 0.2 |
| 55 | 1541 | 1544 | α-Calacorene | tr | |
| 56 | 1550 | 1557 | 1,5-Epoxy-salvial(4)14-ene | 0.1 | |
| 57 | 1557 | 1561 | (E)-Nerolidol | 35.0 | |
| 58 | 1574 | 1577 | Spathulenol | 1.1 | 0.1 |
| 59 | 1579 | 1582 | Caryophyllene oxide | 2.6 | 6.0 |
| 60 | 1585 | 1590 | Globulol | 1.6 | |
| 61 | 1588 | 1590 | β-Copaen-4α-ol | 1.0 | 0.1 |
| 62 | 1589 | 1592 | Viridiflorol | 0.2 | |
| 63 | 1590 | 1594 | Salvial-4(14)-en-1-one | 1.8 | 0.2 |
| 64 | 1605 | 1608 | Humulene epoxide II | 0.4 | 1.0 |
| 65 | 1629 | 1631 | Allo-aromadendrene epoxide | 0.5 | |
| 66 | 1635 | 1638 | epi-α-Cadinol | 0.6 | 0.3 |
| 67 | 1637 | 1639 | Caryophylla-4(12),8(13)-dien-5α-ol or Caryophylla-4(12),8(13)-dien-5β-ol | 0.2 | 0.2 |
| 68 | 1639 | 1640 | epi-α-Muurolol (tau muurolol) | 2.2 | 0.4 |
| 69 | 1649 | 1652 | α-Cadinol | 0.3 | 0.1 |
| 70 | 1654 | 1656 | Valerianol | 0.5 | |
| 71 | 1656 | 1660 | cis-Calamenen-10-ol | 0.1 | |
| 72 | 1657 | 1661 | 14-Hydroxy-9-epi-(E)-caryophyllene | 0.4 | 0.1 |
| 73 | 1664 | 1666 | 14-Hydroxy-(Z)-caryophyllene | 0.7 | |
| 74 | 1710 | 1711 | Valerenol | 1.6 | 0.1 |
| 75 | 1800 | 1803 | 14-Hydroxy-δ-cadinene | 0.1 | |
| 76 | 1842 | 1845 | 6,10,14-Trimethyl-2-pentadecanone (hexahydrofarnesyl acetone) | 0.7 | |
| 77 | 1935 | 1942 | Phytol | tr | |
| 78 | 2399 | 2400 | n-Tetracosane | tr | |
| 79 | 2500 | 2500 | n-Pentacosane | 0.2 | tr |
| Total | 92.4 | 97.4 | |||
| Grouped components | sverc1 | sver2 | |||
| Monoterpene Hydrocarbons | 9.6 | 5.8 | |||
| Oxygenated Monoterpenes | 0.6 | 74.2 | |||
| Sesquiterpene Hydrocarbons | 30.1 | 8.3 | |||
| Oxygenated Sesquiterpenes | 50.5 | 9.1 | |||
| Oxygenated diterpenes | tr | ||||
| Hydrocarbons–Aldehydes | 0.4 | ||||
| Hydrocarbons–Ketones | 0.7 | ||||
| Alkanes | 0.5 | tr | |||
| Total | 92.4 | 97.4 | |||
| No | RIC | RIL | Compounds | samp1 | samp2 | samp3 | samp4 | samp5 | sampc6 | sprc1 | spr2 | svirg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentage (%) | ||||||||||||
| 1 | 920 | 921 | Tricyclene | 0.6 | ||||||||
| 2 | 924 | 924 | α-Thujene | 0.9 | 2.3 | 2.6 | ||||||
| 3 | 930 | 932 | α-Pinene | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 14.4 | 0.1 | 1.6 | 1.9 |
| 4 | 944 | 946 | Camphene | 12.9 | 1.8 | 2.2 | ||||||
| 5 | 968 | 969 | Sabinene | 0.5 | 0.1 | 0.1 | tr | tr | 14.8 | 15.0 | 21.2 | |
| 6 | 972 | 974 | β-Pinene | 8.6 | 1.8 | 1.3 | ||||||
| 7 | 975 | 977 | 1-Octen-3-ol | 0.2 | 0.6 | 1.1 | ||||||
| 8 | 988 | 988 | Myrcene | 0.8 | 0.3 | 1.8 | 0.4 | 0.8 | ||||
| 9 | 1013 | 1014 | α-Terpinene | 0.4 | 1.0 | 0.9 | ||||||
| 10 | 1023 | 1024 | Limonene | 0.2 | 0.8 | |||||||
| 11 | 1025 | 1026 | 1,8-Cineole | 0.4 | 0.1 | 0.1 | 0.4 | 19.6 | 1.7 | |||
| 12 | 1052 | 1054 | γ-Terpinene | 0.3 | tr | 0.8 | 3.3 | 1.9 | ||||
| 13 | 1063 | 1065 | cis-Sabinene hydrate | 0.1 | 0.5 | 0.2 | ||||||
| 14 | 1083 | 1086 | Terpinolene | 0.1 | 0.3 | |||||||
| 15 | 1087 | 1089 | p-Cymenene | 0.3 | tr | 1.3 | 0.4 | 0.4 | 0.2 | |||
| 16 | 1095 | 1095 | Linalool | 0.1 | 0.1 | tr | ||||||
| 17 | 1096 | 1098 | trans-Sabinene hydrate | tr | 0.1 | 0.3 | ||||||
| 18 | 1098 | 1100 | n-Nonanal | 0.1 | 0.2 | 0.1 | 0.1 | |||||
| 19 | 1139 | 1141 | Camphor | 0.5 | ||||||||
| 20 | 1161 | 1165 | Borneol | 0.4 | 2.7 | 0.3 | ||||||
| 21 | 1171 | 1174 | Terpinen-4-ol | 0.3 | tr | 0.4 | 1.5 | 0.6 | ||||
| 22 | 1184 | 1186 | α-Terpineol | tr | ||||||||
| 23 | 1198 | 1200 | trans-Dihydrocarvone | tr | ||||||||
| 24 | 1200 | 1201 | n-Decanal | 0.1 | ||||||||
| 25 | 1250 | 1254 | Linalool acetate | tr | tr | |||||||
| 26 | 1279 | 1284 | Bornyl acetate | 0.6 | 3.7 | |||||||
| 27 | 1295 | 1298 | Carvacrol | 0.1 | tr | 0.3 | ||||||
| 28 | 1342 | 1348 | α-Cubebene | 0.3 | 0.2 | 0.1 | 0.4 | tr | 0.2 | |||
| 29 | 1355 | 1357 | 4aα,7α,7aα-Nepetalactone | 0.6 | ||||||||
| 30 | 1367 | 1373 | α-Ylangene | tr | 0.1 | 0.2 | tr | |||||
| 31 | 1372 | 1374 | α-Copaene | 0.9 | 0.6 | tr | 0.9 | 0.9 | 0.4 | 0.4 | ||
| 32 | 1384 | 1387 | β-Bourbonene | 1.0 | 0.7 | 0.3 | 0.9 | 1.3 | 0.4 | |||
| 33 | 1386 | 1387 | β-Cubebene | tr | 0.2 | 0.3 | 0.3 | tr | 0.5 | |||
| 34 | 1388 | 1389 | β-Elemene | 0.3 | 0.5 | 1.3 | 0.7 | 1.1 | 0.3 | |||
| 35 | 1412 | 1417 | (E)-Caryophyllene | 5.7 | 6.5 | 7.2 | 6.7 | 14.8 | 1.2 | 45.5 | 46.9 | 20.8 |
| 36 | 1424 | 1430 | β-Copaene | 0.4 | 0.4 | 0.3 | 0.6 | 0.7 | ||||
| 37 | 1426 | 1431 | β-Gurjunene (Calarene) | 0.4 | 0.3 | |||||||
| 38 | 1435 | 1439 | Aromadendrene | 0.1 | 1.1 | |||||||
| 39 | 1447 | 1452 | α-Humulene | 0.8 | 0.4 | 0.9 | 0.9 | 1.7 | tr | 2.2 | 1.4 | 1.1 |
| 40 | 1449 | 1453 | Geranyl acetone | 0.2 | 0.2 | |||||||
| 41 | 1452 | 1454 | (E)-β-Farnesene | 0.5 | 1.0 | 0.8 | 1.3 | 2.0 | tr | 0.5 | ||
| 42 | 1455 | 1458 | Alloaromadendrene | tr | 0.4 | 2.7 | 5.1 | 4.7 | 3.8 | 15.2 | ||
| 43 | 1472 | 1478 | γ-Muurolene | 1.7 | tr | |||||||
| 44 | 1476 | 1480 | Germacrene D | 4.0 | 10.0 | 39.0 | 20.1 | 40.2 | tr | 2.9 | 6.1 | |
| 45 | 1480 | 1483 | α-Amorphene | 0.8 | 1.5 | 1.2 | 0.8 | |||||
| 46 | 1484 | 1487 | (E)-β-Ionone | 1.0 | 0.2 | 0.7 | 1.9 | 0.1 | 0.2 | |||
| 47 | 1498 | 1500 | Bicyclogermacrene | 1.3 | 1.9 | 0.4 | 0.6 | |||||
| 48 | 1499 | 1500 | α-Muurolene | 0.3 | 0.2 | 0.4 | 0.6 | |||||
| 49 | 1503 | 1505 | (E,E)-α-Farnesene | 0.7 | ||||||||
| 50 | 1504 | 1508 | Germacrene A | 0.1 | 0.8 | 0.2 | ||||||
| 51 | 1510 | 1513 | γ-Cadinene | 1.1 | 0.8 | 0.9 | 1.6 | 1.0 | 0.5 | |||
| 52 | 1521 | 1522 | δ-Cadinene | 1.3 | 1.4 | 1.5 | 2.4 | 2.0 | 0.5 | 0.3 | ||
| 53 | 1529 | 1531 | (Z)-Nerolidol | 0.2 | ||||||||
| 54 | 1531 | 1537 | α-Cadinene | 0.2 | ||||||||
| 55 | 1541 | 1544 | α-Calacorene | tr | 0.2 | 0.2 | ||||||
| 56 | 1550 | 1557 | 1,5-Epoxy-salvial(4)14-ene | 0.7 | 0.6 | 0.5 | 0.5 | 0.6 | ||||
| 57 | 1553 | 1559 | Germacrene B | 1.3 | ||||||||
| 58 | 1557 | 1561 | (E)-Nerolidol | 0.3 | 0.7 | 0.4 | 3.7 | |||||
| 59 | 1574 | 1577 | Spathulenol | 0.7 | 18.7 | 2.4 | 1.8 | 0.7 | 0.4 | 0.6 | ||
| 60 | 1579 | 1582 | Caryophyllene oxide | 35.1 | 10.0 | 8.0 | 7.5 | 6.8 | 5.2 | 25.8 | 10.4 | 6.6 |
| 61 | 1589 | 1592 | Viridiflorol | 0.6 | 0.4 | 12.1 | 0.6 | |||||
| 62 | 1590 | 1594 | Salvial-4(14)-en-1-one | 7.0 | 5.8 | 3.4 | 3.7 | 3.7 | 0.4 | |||
| 63 | 1604 | 1607 | β-Oplopenone | 4.4 | 3.4 | 1.8 | ||||||
| 64 | 1605 | 1608 | Humulene epoxide II | 3.6 | 1.3 | 1.4 | 1.0 | 0.9 | 0.3 | |||
| 65 | 1627 | 1628 | Isospathulenol | 2.0 | 0.2 | |||||||
| 66 | 1629 | 1631 | Allo-aromadendrene epoxide | 2.3 | ||||||||
| 67 | 1635 | 1638 | epi-α-Cadinol | 1.1 | 1.1 | 1.0 | 0.3 | |||||
| 68 | 1637 | 1639 | Caryophylla-4(12),8(13)-dien-5α-ol or Caryophylla-4(12),8(13)-dien-5β-ol | 0.4 | 1.0 | 1.2 | 0.3 | |||||
| 69 | 1639 | 1640 | epi-α-Muurolol (tau muurolol) | 1.0 | 0.8 | |||||||
| 70 | 1640 | 1643 | Caryophylla-3,8(15)-dien-5α-ol | 5.2 | 0.5 | |||||||
| 71 | 1641 | 1644 | α-Muurolol | 2.1 | ||||||||
| 72 | 1644 | 1649 | β-Selinene | 0.3 | 0.6 | |||||||
| 73 | 1648 | 1650 | Caryophyllenol-II | 3.4 | 0.9 | |||||||
| 74 | 1649 | 1652 | α-Cadinol | 0.7 | 0.9 | 2.4 | 1.3 | 4.4 | ||||
| 75 | 1656 | 1660 | cis-Calamenen-10-ol | 0.4 | ||||||||
| 76 | 1666 | 1668 | trans-Calamenen-10-ol | 0.5 | ||||||||
| 77 | 1685 | 1687 | Eudesma-4(15),7-dien-1β-ol | 1.3 | ||||||||
| 78 | 1688 | 1690 | Endo-8-hydroxy-cycloisolongifolene | 4.0 | ||||||||
| 79 | 1690 | 1691 | Vulgarol B | 6.4 | 0.6 | 1.1 | 4.4 | 2.9 | ||||
| 80 | 1691 | 1691 | Eudesma-4,11-dien-2-ol | 6.3 | 1.1 | |||||||
| 81 | 1710 | 1711 | Valerenol | 1.3 | 3.7 | 1.3 | 1.1 | 2.0 | ||||
| 82 | 1740 | 1743 | Aromadendrene epoxide | 0.6 | 0.5 | |||||||
| 83 | 1764 | 1765 | β-Costol | 0.8 | 0.5 | 0.3 | ||||||
| 84 | 1770 | 1773 | α-Costol | 0.3 | ||||||||
| 85 | 1842 | 1845 | 6,10,14-Trimethyl-2-pentadecanone (hexahydrofarnesyl acetone) | 0.9 | 0.6 | 0.6 | 1.3 | 0.8 | ||||
| 86 | 1900 | 1900 | n-Nonadecane | 0.2 | ||||||||
| 87 | 1935 | 1942 | Phytol | 1.0 | 0.2 | 0.1 | 0.4 | 0.6 | 5.5 | 0.2 | ||
| 88 | 1944 | 1946 | Isophytol | 0.3 | ||||||||
| 89 | 1974 | Ledene oxide-(I) | 6.3 | 3.7 | 1.5 | 1.6 | ||||||
| 90 | 2299 | 2300 | n-Tricosane | 0.1 | ||||||||
| 91 | 2399 | 2400 | n-Tetracosane | 0.3 | 0.1 | |||||||
| 92 | 2500 | 2500 | n-Pentacosane | 0.9 | 0.2 | 0.2 | ||||||
| 93 | 2600 | 2600 | Hexacosane | 0.2 | 0.2 | |||||||
| 94 | 2699 | 2700 | Heptacosane | 0.5 | ||||||||
| 95 | 2800 | 2800 | Octacosane | 0.1 | ||||||||
| 96 | 2900 | 2900 | Nonacosane | 0.3 | ||||||||
| Total | 90.7 | 89.9 | 87.8 | 94.6 | 99.6 | 93.6 | 97.4 | 97.9 | 99.6 | |||
| Grouped components | samp1 | samp2 | samp3 | samp4 | samp5 | sampc6 | sprc1 | spr2 | svirg | |||
| Monoterpene Hydrocarbons | 2.1 | 0.6 | 0.2 | 0.2 | 0.2 | 39.6 | 18.1 | 27.2 | 34.1 | |||
| Oxygenated Monoterpenes | 0.9 | 0.3 | 0.2 | 0.3 | 0.4 | 21.1 | 0.9 | 6.7 | 5.4 | |||
| Sesquiterpene Hydrocarbons | 19.1 | 25.6 | 58.4 | 45.2 | 72.2 | 7.6 | 48.8 | 53.6 | 46.3 | |||
| Oxygenated Sesquiterpenes | 64.5 | 61.8 | 28.0 | 45.4 | 25.1 | 16.6 | 29.0 | 10.4 | 12.7 | |||
| Oxygenated diterpenes | 1.0 | 0.5 | 0.1 | 0.4 | 0.6 | 5.5 | 0.2 | |||||
| Hydrocarbons–Alcohols | 0.2 | 0.8 | 1.1 | |||||||||
| Hydrocarbons–Aldehydes | 0.1 | 0.2 | 0.1 | 0.1 | ||||||||
| Hydrocarbons–Ketones | 0.9 | 0.6 | 0.6 | 1.3 | 0.8 | |||||||
| Alkanes | 1.2 | 0.2 | 1.5 | 0.2 | ||||||||
| Others | 1.0 | 0.2 | 0.7 | 1.9 | 0.1 | 0.2 | ||||||
| Total | 90.7 | 89.9 | 87.8 | 94.6 | 99.6 | 93.6 | 97.4 | 97.9 | 99.6 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomou, E.-M.; Fraskou, P.; Dimakopoulou, K.; Dariotis, E.; Krigas, N.; Skaltsa, H. Chemometric Analysis Evidencing the Variability in the Composition of Essential Oils in 10 Salvia Species from Different Taxonomic Sections or Phylogenetic Clades. Molecules 2024, 29, 1547. https://doi.org/10.3390/molecules29071547
Tomou E-M, Fraskou P, Dimakopoulou K, Dariotis E, Krigas N, Skaltsa H. Chemometric Analysis Evidencing the Variability in the Composition of Essential Oils in 10 Salvia Species from Different Taxonomic Sections or Phylogenetic Clades. Molecules. 2024; 29(7):1547. https://doi.org/10.3390/molecules29071547
Chicago/Turabian StyleTomou, Ekaterina-Michaela, Panagiota Fraskou, Konstantina Dimakopoulou, Eleftherios Dariotis, Nikos Krigas, and Helen Skaltsa. 2024. "Chemometric Analysis Evidencing the Variability in the Composition of Essential Oils in 10 Salvia Species from Different Taxonomic Sections or Phylogenetic Clades" Molecules 29, no. 7: 1547. https://doi.org/10.3390/molecules29071547
APA StyleTomou, E.-M., Fraskou, P., Dimakopoulou, K., Dariotis, E., Krigas, N., & Skaltsa, H. (2024). Chemometric Analysis Evidencing the Variability in the Composition of Essential Oils in 10 Salvia Species from Different Taxonomic Sections or Phylogenetic Clades. Molecules, 29(7), 1547. https://doi.org/10.3390/molecules29071547









