Exploring Novel Antidepressants Targeting G Protein-Coupled Receptors and Key Membrane Receptors Based on Molecular Structures
Abstract
1. Introduction
2. Challenges in MDD: Cross-Scale Abnormalities
3. Controversial ADs: Psychedelics and Ketamine
3.1. Psychedelics: 5-HT2A Receptor Agonists
| Class | Representation | Compounds | Binding Structure (PDB ID) | Clinical Trials |
|---|---|---|---|---|
 |  | LSD, ergotamine (ERG), dihydroergotamine (DHE) | 5-HT1BR-ERG (7C61) 5-HT1BR-DHE (4IAQ) 5-HT2AR-LSD (7WC6) 5-HT2BR-LSD (7SRS) 5-HT2BR-methysergide (6DRZ) 5-HT2BR-methylergonovine (6DRY) 5-HT2BR-ERG (5TUD) 5-HT2BR-LSD (5TVN) 5-HT2BR-ERG (4NC3) 5-HT2CR-ERG (6BQG) 5-HT5AR-methylergonovine (7UM7) | a. LSD-assisted therapy with anxiety and ratings of depression symptoms [109]. b. Single microdoses of orderly produced LSD, dose-related subjective effects [110]. c. The link between psychosis model and therapeutic model seems to lie in LSD mystical experiences [111]. |
 |  | DMT, 5-MeO-DMT, psilocin, psilocybin | 5-HT2AR-psilocin (7WC5) 5-HT2CR-psilocin (8DPG) | a. Compared trial: psilocybin versus escitalopram for depression [112]. b. Assisted therapy: psilocybin was given in the context of supportive psychotherapy [113]. c. Psilocybin for TRD [114]. d. After psilocybin therapy for depression, global integration in the brain is increased [115]. |
 |  | Mescaline, DOM, DOI, DOB, NBOMes | 5-HT2AR-25CN-NBOH (6WHA) | (none) |
3.2. Ketamine: An Antagonist of the NMDAR
4. Advancements in Cryo-EM and VDS
4.1. Cryo-EM: Resolving Active Receptors
4.2. Molecular Docking and Virtual Drug Libraries
4.3. Predicting Structures via Artificial Intelligence
5. Non-Hallucinogenic Psychedelics
5.1. Functionally Directed Approach and Fluorescence Sensors
5.2. Structures of the 5-HT2A Receptor
5.3. Removal of Hallucinogenic Effects
6. Designing ADs for the 5-HT1A Receptor
6.1. Structure of the 5-HT1A Receptor
6.2. Structure of the Aripiprazole-5-HT2A Receptor
6.3. Brain Region Specificity of the 5-HT1A Receptor
7. Ketamine: Ca2+ Influx and Synaptic Plasticity
7.1. NMDAR-Centered Glutamate Hypothesis
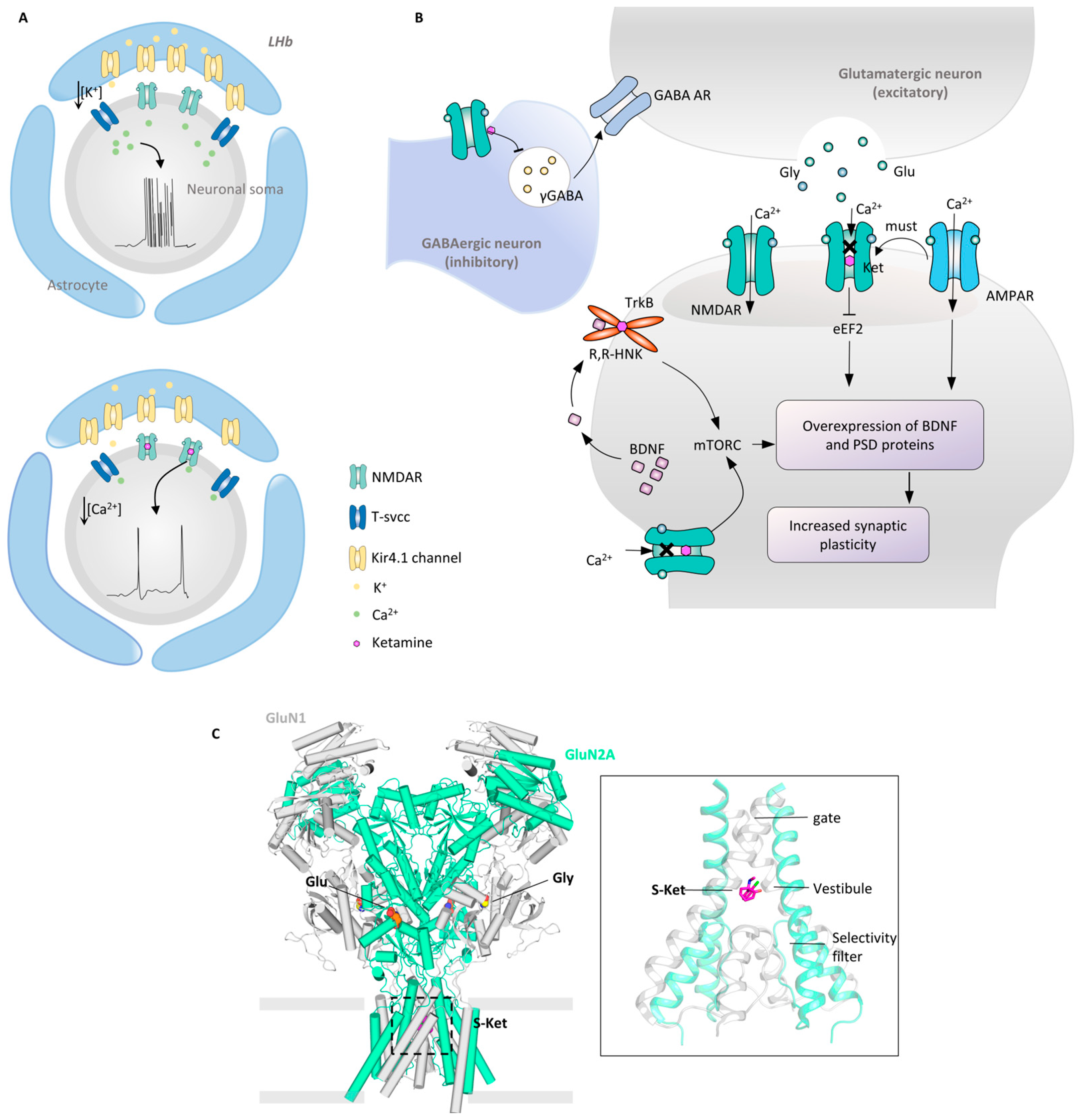
7.2. Synaptic Plasticity: The AMPAR and TrkB
7.3. Structural Mechanism of the S-Ketamine NMDAR
7.4. Ketamine Targets Multiple Types of Receptors
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.T.; Perry, K.W.; Bymaster, F.P. Case history: The discovery of fluoxetine hydrochloride (Prozac). Nat. Rev. Drug Discov. 2005, 4, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 2023, 28, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Fornaro, M.; Anastasia, A.; Novello, S.; Fusco, A.; Pariano, R.; De Berardis, D.; Solmi, M.; Veronese, N.; Stubbs, B.; Vieta, E.J.P.r. The emergence of loss of efficacy during antidepressant drug treatment for major depressive disorder: An integrative review of evidence, mechanisms, and clinical implications. Pharmacol. Res. 2019, 139, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.; Lau, W.K.; Sim, J.; Sum, M.Y.; Baldessarini, R.J. Prevention of Relapse and Recurrence in Adults with Major Depressive Disorder: Systematic Review and Meta-Analyses of Controlled Trials. Int. J. Neuropsychopharmacol. 2016, 19, pyv076. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.H.; Fava, M.; Wisniewski, S.R.; Thase, M.E.; Quitkin, F.; Warden, D.; Ritz, L.; Nierenberg, A.A.; Lebowitz, B.D.; Biggs, M.M.; et al. Medication augmentation after the failure of SSRIs for depression. N. Engl. J. Med. 2006, 354, 1243–1252. [Google Scholar] [CrossRef]
- Gaynes, B.N.; Lux, L.; Gartlehner, G.; Asher, G.; Forman-Hoffman, V.; Green, J.; Boland, E.; Weber, R.P.; Randolph, C.; Bann, C.; et al. Defining treatment-resistant depression. Depress. Anxiety 2020, 37, 134–145. [Google Scholar] [CrossRef]
- Roth, B.L. Molecular pharmacology of metabotropic receptors targeted by neuropsychiatric drugs. Nat. Struct. Mol. Biol. 2019, 26, 535–544. [Google Scholar] [CrossRef]
- Ballante, F.; Kooistra, A.J.; Kampen, S.; de Graaf, C.; Carlsson, J. Structure-Based Virtual Screening for Ligands of G Protein-Coupled Receptors: What Can Molecular Docking Do for You? Pharmacol. Rev. 2021, 73, 527–565. [Google Scholar] [CrossRef]
- Blay, V.; Tolani, B.; Ho, S.P.; Arkin, M.R. High-Throughput Screening: Today’s biochemical and cell-based approaches. Drug Discov. Today 2020, 25, 1807–1821. [Google Scholar] [CrossRef]
- Wootten, D.; Christopoulos, A.; Marti-Solano, M.; Babu, M.M.; Sexton, P.M. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2018, 19, 638–653. [Google Scholar] [CrossRef]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schioth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef]
- Rosenbaum, D.M.; Cherezov, V.; Hanson, M.A.; Rasmussen, S.G.; Thian, F.S.; Kobilka, T.S.; Choi, H.J.; Yao, X.J.; Weis, W.I.; Stevens, R.C.; et al. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science 2007, 318, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, B.; Feng, D.; Hu, H.; Chu, M.; Qu, Q.; Tarrasch, J.T.; Li, S.; Sun Kobilka, T.; Kobilka, B.K.; et al. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 2017, 546, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.P.; Chari, A.; Ciferri, C.; Liu, W.T.; Remigy, H.W.; Stark, H.; Wiesmann, C. Cryo-EM in drug discovery: Achievements, limitations and prospects. Nat. Rev. Drug Discov. 2018, 17, 471–492. [Google Scholar] [CrossRef]
- Lyu, J.; Wang, S.; Balius, T.E.; Singh, I.; Levit, A.; Moroz, Y.S.; O’Meara, M.J.; Che, T.; Algaa, E.; Tolmachova, K.; et al. Ultra-large library docking for discovering new chemotypes. Nature 2019, 566, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Irwin, J.J.; Shoichet, B.K. Modeling the expansion of virtual screening libraries. Nat. Chem. Biol. 2023, 19, 712–718. [Google Scholar] [CrossRef]
- Turner, E.H. Esketamine for treatment-resistant depression: Seven concerns about efficacy and FDA approval. Lancet Psychiatry 2019, 6, 977–979. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A.; et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet. Psychiatry 2016, 3, 619–627. [Google Scholar] [CrossRef]
- McClure-Begley, T.D.; Roth, B.L. The promises and perils of psychedelic pharmacology for psychiatry. Nat. Rev. Drug Discov. 2022, 21, 463–473. [Google Scholar] [CrossRef]
- Osmond, H. A review of the clinical effects of psychotomimetic agents. Ann. N. Y. Acad. Sci. 1957, 66, 418–434. [Google Scholar] [CrossRef]
- Cole, J.O.; Katz, M.M. The psychotomimetic drugs. An overview. Jama 1964, 187, 758–761. [Google Scholar] [CrossRef]
- Popova, V.; Daly, E.J.; Trivedi, M.; Cooper, K.; Lane, R.; Lim, P.; Mazzucco, C.; Hough, D.; Thase, M.E.; Shelton, R.C.; et al. Efficacy and Safety of Flexibly Dosed Esketamine Nasal Spray Combined With a Newly Initiated Oral Antidepressant in Treatment-Resistant Depression: A Randomized Double-Blind Active-Controlled Study. Am. J. Psychiatry 2019, 176, 428–438. [Google Scholar] [CrossRef]
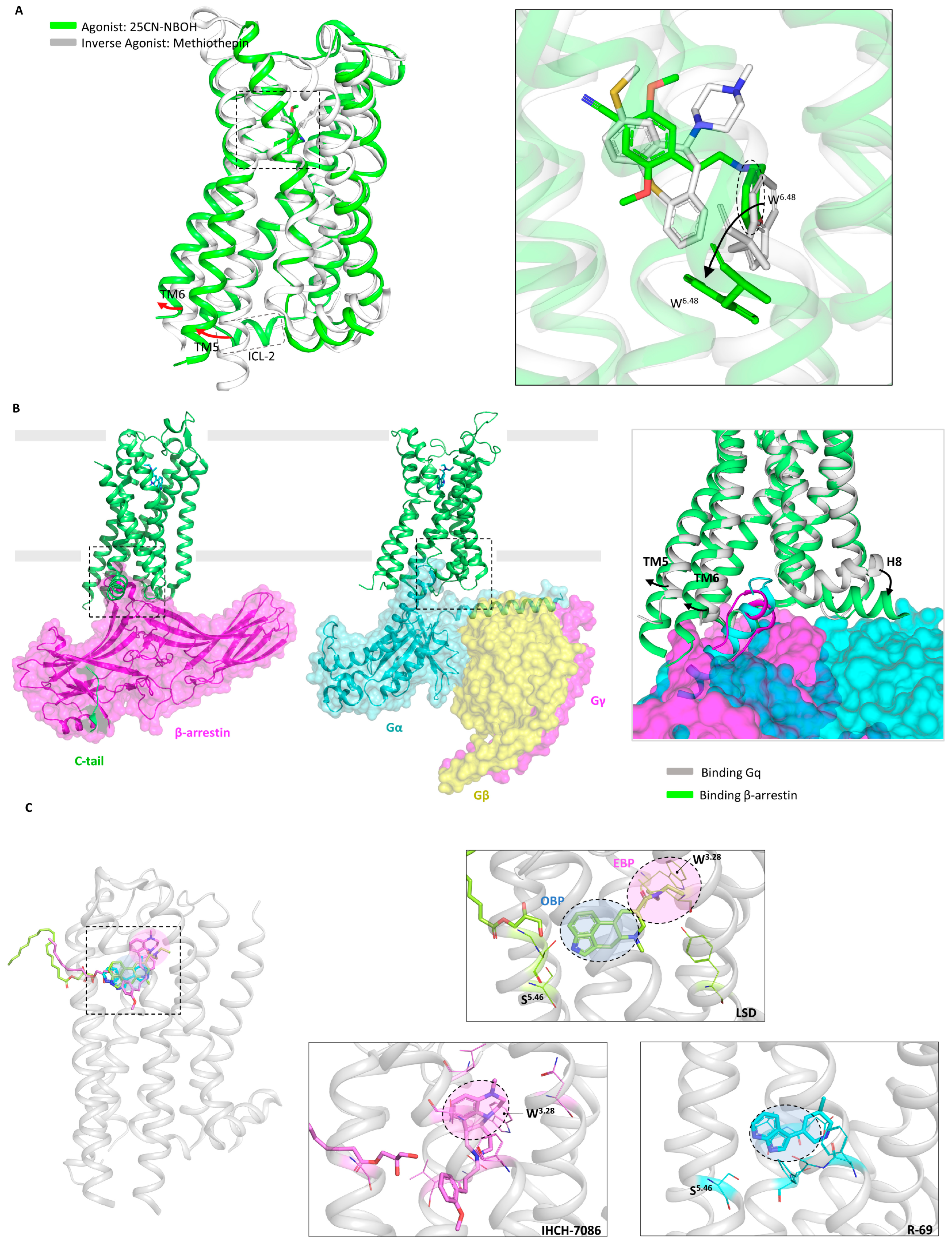
- Kim, K.; Che, T.; Panova, O.; DiBerto, J.F.; Lyu, J.; Krumm, B.E.; Wacker, D.; Robertson, M.J.; Seven, A.B.; Nichols, D.E.; et al. Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor. Cell 2020, 182, 1574–1588.e19. [Google Scholar] [CrossRef]
- Cao, D.; Yu, J.; Wang, H.; Luo, Z.; Liu, X.; He, L.; Qi, J.; Fan, L.; Tang, L.; Chen, Z.; et al. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science 2022, 375, 403–411. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, F.; Zhang, T.; Lv, S.; Zhou, L.; Du, D.; Lin, H.; Guo, F.; Luo, C.; Zhu, S. Structural basis of ketamine action on human NMDA receptors. Nature 2021, 596, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Sala, D.; Batebi, H.; Ledwitch, K.; Hildebrand, P.W.; Meiler, J. Targeting in silico GPCR conformations with ultra-large library screening for hit discovery. Trends Pharmacol. Sci. 2023, 44, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, J.C. Diagnostic Issues and Controversies in DSM-5: Return of the False Positives Problem. Annu. Rev. Clin. Psychol. 2016, 12, 105–132. [Google Scholar] [CrossRef] [PubMed]
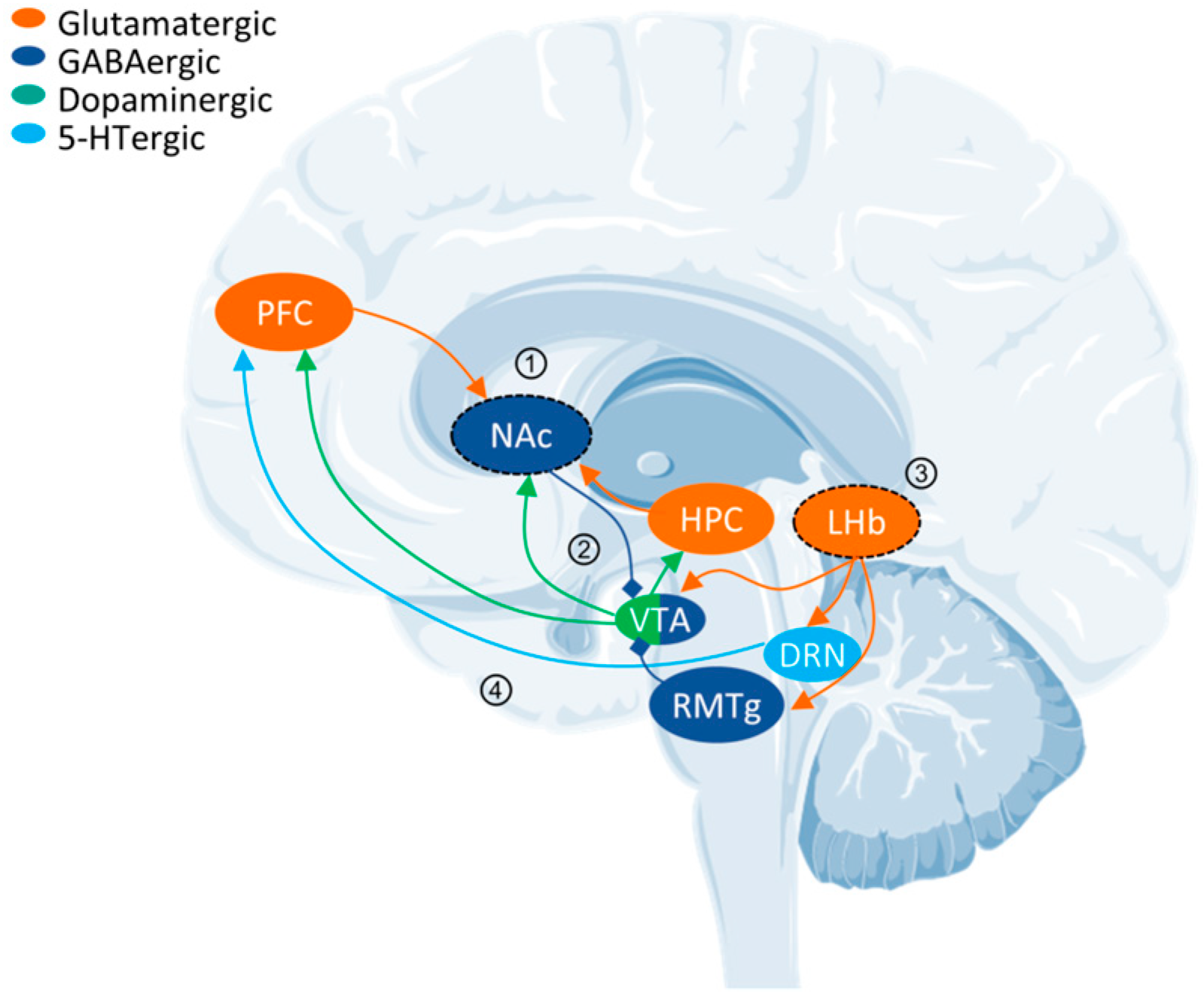
- Hu, H.; Cui, Y.; Yang, Y. Circuits and functions of the lateral habenula in health and in disease. Nat. Rev. Neurosci. 2020, 21, 277–295. [Google Scholar] [CrossRef]
- Russo, S.J.; Nestler, E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013, 14, 609–625. [Google Scholar] [CrossRef]
- Marwaha, S.; Palmer, E.; Suppes, T.; Cons, E.; Young, A.H.; Upthegrove, R. Novel and emerging treatments for major depression. Lancet 2023, 401, 141–153. [Google Scholar] [CrossRef]
- Kaiser, R.H.; Andrews-Hanna, J.R.; Wager, T.D.; Pizzagalli, D.A. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry 2015, 72, 603–611. [Google Scholar] [CrossRef]
- Wang, Y.; LeDue, J.M.; Murphy, T.H. Multiscale imaging informs translational mouse modeling of neurological disease. Neuron 2022, 110, 3688–3710. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.J.; Feinberg, M.; Greden, J.F.; Tarika, J.; Albala, A.A.; Haskett, R.F.; James, N.M.; Kronfol, Z.; Lohr, N.; Steiner, M.; et al. A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch. Gen. Psychiatry 1981, 38, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.W.; Chrousos, G.P. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs low CRH/NE states. Mol. Psychiatry 2002, 7, 254–275. [Google Scholar] [CrossRef] [PubMed]
- Mongan, D.; Raj Susai, S.; Föcking, M.; Byrne, J.F.; Zammit, S.; Cannon, M.; Cotter, D.R. Associations between plasma inflammatory markers and psychotic disorder, depressive disorder and generalised anxiety disorder in early adulthood: A nested case-control study. Brain Behav. Immun. 2023, 111, 90–100. [Google Scholar] [CrossRef]
- Mousten, I.V.; Sørensen, N.V.; Christensen, R.H.B.; Benros, M.E. Cerebrospinal Fluid Biomarkers in Patients With Unipolar Depression Compared With Healthy Control Individuals: A Systematic Review and Meta-analysis. JAMA Psychiatry 2022, 79, 571–581. [Google Scholar] [CrossRef]
- Xie, X.H.; Lai, W.T.; Xu, S.X.; Di Forti, M.; Zhang, J.Y.; Chen, M.M.; Yao, L.H.; Wang, P.; Hao, K.K.; Rong, H. Hyper-inflammation of astrocytes in patients of major depressive disorder: Evidence from serum astrocyte-derived extracellular vesicles. Brain Behav. Immun. 2023, 109, 51–62. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Schmaal, L.; Veltman, D.J.; van Erp, T.G.; Sämann, P.G.; Frodl, T.; Jahanshad, N.; Loehrer, E.; Tiemeier, H.; Hofman, A.; Niessen, W.J.; et al. Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA Major Depressive Disorder working group. Mol. Psychiatry 2016, 21, 806–812. [Google Scholar] [CrossRef]
- Belleau, E.L.; Treadway, M.T.; Pizzagalli, D.A. The Impact of Stress and Major Depressive Disorder on Hippocampal and Medial Prefrontal Cortex Morphology. Biol. Psychiatry 2019, 85, 443–453. [Google Scholar] [CrossRef]
- Mayberg, H.S.; Lozano, A.M.; Voon, V.; McNeely, H.E.; Seminowicz, D.; Hamani, C.; Schwalb, J.M.; Kennedy, S.H. Deep brain stimulation for treatment-resistant depression. Neuron 2005, 45, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Disner, S.G.; Beevers, C.G.; Haigh, E.A.; Beck, A.T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 2011, 12, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Francis, T.C.; Lobo, M.K. Emerging Role for Nucleus Accumbens Medium Spiny Neuron Subtypes in Depression. Biol. Psychiatry 2017, 81, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C.; Videen, T.O.; Price, J.L.; Preskorn, S.H.; Carmichael, S.T.; Raichle, M.E. A functional anatomical study of unipolar depression. J. Neurosci. Off. J. Soc. Neurosci. 1992, 12, 3628–3641. [Google Scholar] [CrossRef]
- Chaudhury, D.; Walsh, J.J.; Friedman, A.K.; Juarez, B.; Ku, S.M.; Koo, J.W.; Ferguson, D.; Tsai, H.C.; Pomeranz, L.; Christoffel, D.J.; et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 2013, 493, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Jhou, T.C.; Fields, H.L.; Baxter, M.G.; Saper, C.B.; Holland, P.C. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 2009, 61, 786–800. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Aghajanian, G.K. Physiological evidence for habenula as major link between forebrain and midbrain raphe. Science 1977, 197, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Hikosaka, O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 2007, 447, 1111–1115. [Google Scholar] [CrossRef]
- Hong, S.; Jhou, T.C.; Smith, M.; Saleem, K.S.; Hikosaka, O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 11457–11471. [Google Scholar] [CrossRef]
- Li, H.; Pullmann, D.; Cho, J.Y.; Eid, M.; Jhou, T.C. Generality and opponency of rostromedial tegmental (RMTg) roles in valence processing. Elife 2019, 8, e41542. [Google Scholar] [CrossRef]
- Stamatakis, A.M.; Stuber, G.D. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat. Neurosci. 2012, 15, 1105–1107. [Google Scholar] [CrossRef]
- Li, B.; Piriz, J.; Mirrione, M.; Chung, C.; Proulx, C.D.; Schulz, D.; Henn, F.; Malinow, R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature 2011, 470, 535–539. [Google Scholar] [CrossRef]
- Hu, H. Reward and Aversion. Annu. Rev. Neurosci. 2016, 39, 297–324. [Google Scholar] [CrossRef]
- Tye, K.M.; Mirzabekov, J.J.; Warden, M.R.; Ferenczi, E.A.; Tsai, H.C.; Finkelstein, J.; Kim, S.Y.; Adhikari, A.; Thompson, K.R.; Andalman, A.S.; et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 2013, 493, 537–541. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, Y.; Ni, Z.; Dong, Y.; Cai, G.; Foncelle, A.; Ma, S.; Sang, K.; Tang, S.; Li, Y.; et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 2018, 554, 323–327. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, Y.; Sang, K.; Dong, Y.; Ni, Z.; Ma, S.; Hu, H. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 2018, 554, 317–322. [Google Scholar] [CrossRef]
- Fan, Z.; Chang, J.; Liang, Y.; Zhu, H.; Zhang, C.; Zheng, D.; Wang, J.; Xu, Y.; Li, Q.J.; Hu, H. Neural mechanism underlying depressive-like state associated with social status loss. Cell 2023, 186, 560–576.e17. [Google Scholar] [CrossRef]
- Wang, C.S.; Kavalali, E.T.; Monteggia, L.M. BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell 2022, 185, 62–76. [Google Scholar] [CrossRef]
- Hebb, D.O. The Organization of Behavior: A Neuropsychological Theory; Psychology Press: London, UK, 2005. [Google Scholar]
- Morris, R.G. Long-term potentiation and memory. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2003, 358, 643–647. [Google Scholar] [CrossRef]
- Malenka, R.C.; Bear, M.F. LTP and LTD: An embarrassment of riches. Neuron 2004, 44, 5–21. [Google Scholar] [CrossRef]
- El-Husseini Ael, D.; Schnell, E.; Dakoji, S.; Sweeney, N.; Zhou, Q.; Prange, O.; Gauthier-Campbell, C.; Aguilera-Moreno, A.; Nicoll, R.A.; Bredt, D.S. Synaptic strength regulated by palmitate cycling on PSD-95. Cell 2002, 108, 849–863. [Google Scholar] [CrossRef]
- Yoshii, A.; Constantine-Paton, M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat. Neurosci. 2007, 10, 702–711. [Google Scholar] [CrossRef]
- Harward, S.C.; Hedrick, N.G.; Hall, C.E.; Parra-Bueno, P.; Milner, T.A.; Pan, E.; Laviv, T.; Hempstead, B.L.; Yasuda, R.; McNamara, J.O. Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature 2016, 538, 99–103. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K. Synaptic dysfunction in depression: Potential therapeutic targets. Science 2012, 338, 68–72. [Google Scholar] [CrossRef]
- Kim, J.W.; Autry, A.E.; Na, E.S.; Adachi, M.; Björkholm, C.; Kavalali, E.T.; Monteggia, L.M. Sustained effects of rapidly acting antidepressants require BDNF-dependent MeCP2 phosphorylation. Nat. Neurosci. 2021, 24, 1100–1109. [Google Scholar] [CrossRef]
- Rantamäki, T.; Hendolin, P.; Kankaanpää, A.; Mijatovic, J.; Piepponen, P.; Domenici, E.; Chao, M.V.; Männistö, P.T.; Castrén, E. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2007, 32, 2152–2162. [Google Scholar] [CrossRef]
- Vargas, M.V.; Dunlap, L.E.; Dong, C.; Carter, S.J.; Tombari, R.J.; Jami, S.A.; Cameron, L.P.; Patel, S.D.; Hennessey, J.J.; Saeger, H.N.; et al. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science 2023, 379, 700–706. [Google Scholar] [CrossRef]
- Lapin, I.P.; Oxenkrug, G.F. Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet 1969, 1, 132–136. [Google Scholar] [CrossRef]
- Okaty, B.W.; Commons, K.G.; Dymecki, S.M. Embracing diversity in the 5-HT neuronal system. Nat. Rev. Neurosci. 2019, 20, 397–424. [Google Scholar] [CrossRef]
- Roth, B.L.; Nakaki, T.; Chuang, D.M.; Costa, E. Aortic recognition sites for serotonin (5HT) are coupled to phospholipase C and modulate phosphatidylinositol turnover. Neuropharmacology 1984, 23, 1223–1225. [Google Scholar] [CrossRef]
- Roth, B.L.; Nakaki, T.; Chuang, D.M.; Costa, E. 5-Hydroxytryptamine2 receptors coupled to phospholipase C in rat aorta: Modulation of phosphoinositide turnover by phorbol ester. J. Pharmacol. Exp. Ther. 1986, 238, 480–485. [Google Scholar]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- Murrough, J.W.; Abdallah, C.G.; Mathew, S.J. Targeting glutamate signalling in depression: Progress and prospects. Nat. Rev. Drug Discov. 2017, 16, 472–486. [Google Scholar] [CrossRef]
- Papouin, T.; Ladépêche, L.; Ruel, J.; Sacchi, S.; Labasque, M.; Hanini, M.; Groc, L.; Pollegioni, L.; Mothet, J.P.; Oliet, S.H. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 2012, 150, 633–646. [Google Scholar] [CrossRef]
- Chou, T.-H.; Epstein, M.; Michalski, K.; Fine, E.; Biggin, P.C.; Furukawa, H. Structural insights into binding of therapeutic channel blockers in NMDA receptors. Nat. Struct. Mol. Biol. 2022, 29, 507–518. [Google Scholar] [CrossRef]
- Woolley, D.W.; Shaw, E.J.P.o.t.N.A.o.S. A biochemical and pharmacological suggestion about certain mental disorders. Proc. Natl. Acad. Sci. 1954, 40, 228–231. [Google Scholar] [CrossRef]
- Ballentine, G.; Friedman, S.F.; Bzdok, D. Trips and neurotransmitters: Discovering principled patterns across 6850 hallucinogenic experiences. Sci. Adv. 2022, 8, eabl6989. [Google Scholar] [CrossRef]
- Stevens, J. Storming Heaven: LSD and the American Dream; Grove Press: New York, NY, USA, 1987. [Google Scholar]
- Glennon, R.A.; Titeler, M.; McKenney, J.D. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984, 35, 2505–2511. [Google Scholar] [CrossRef]
- Titeler, M.; Lyon, R.A.; Glennon, R.A. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology 1988, 94, 213–216. [Google Scholar] [CrossRef]
- Yaden, D.B.; Yaden, M.E.; Griffiths, R.R. Psychedelics in Psychiatry-Keeping the Renaissance From Going Off the Rails. JAMA Psychiatry 2021, 78, 469–470. [Google Scholar] [CrossRef]
- Heffter, A. Ueber Pellote: Ein Beitrag zur pharmakologischen Kenntniss der Cacteen. Arch. Für Exp. Pathol. Und Pharmakol. 1894, 34, 65–86. [Google Scholar] [CrossRef]
- Hofmann, A. How LSD originated. J. Psychedelic Drugs 1979, 11, 53–60. [Google Scholar] [CrossRef]
- Wasson, R.G. Notes on the present status of ololiuhqui and the other hallucinogens of Mexico. Bot. Mus. Leafl. Harv. Univ. 1963, 20, 161–193. [Google Scholar] [CrossRef]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef]
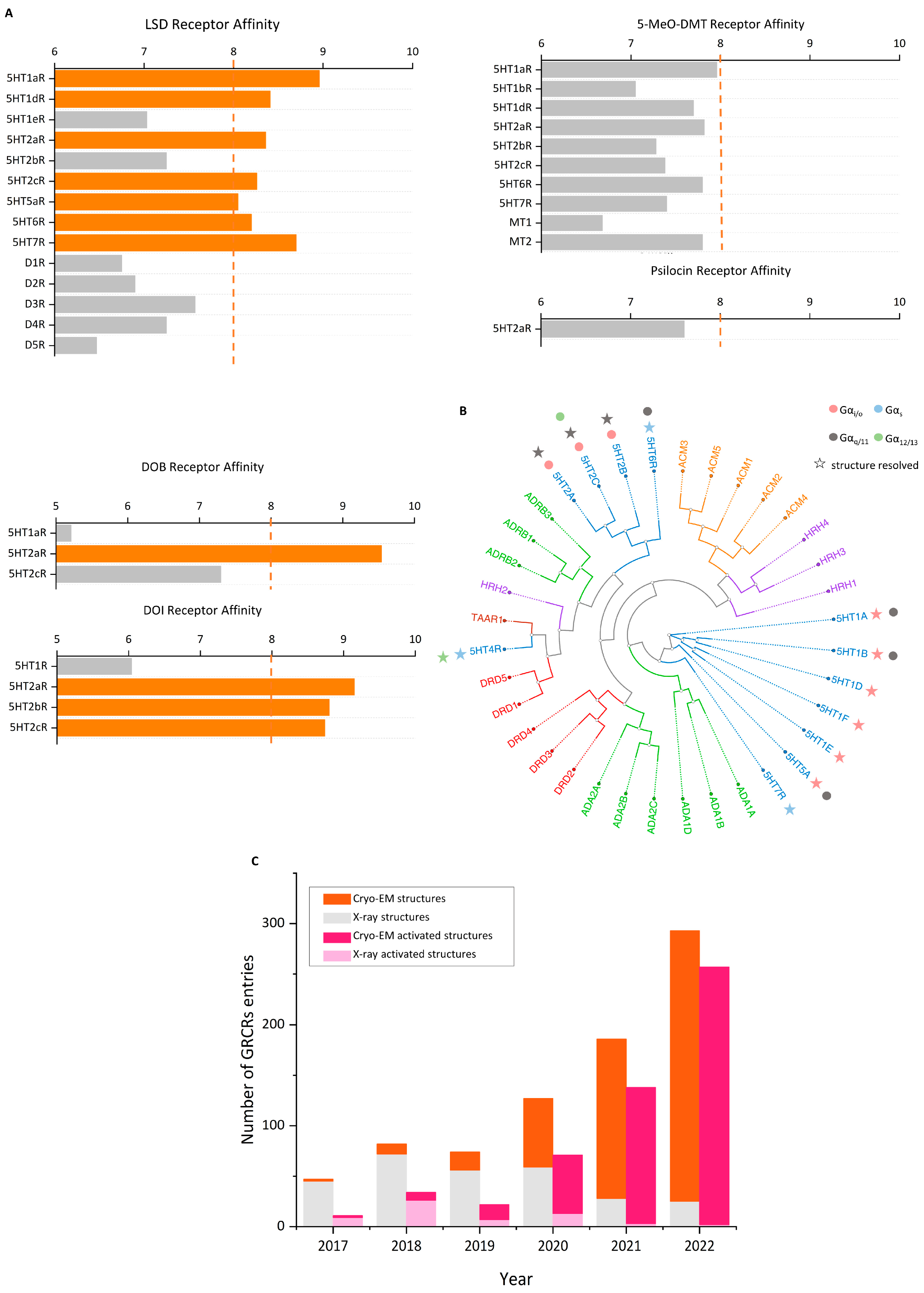
- Rickli, A.; Luethi, D.; Reinisch, J.; Buchy, D.; Hoener, M.C.; Liechti, M.E. Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs). Neuropharmacology 2015, 99, 546–553. [Google Scholar] [CrossRef]
- Hansen, M.; Phonekeo, K.; Paine, J.S.; Leth-Petersen, S.; Begtrup, M.; Bräuner-Osborne, H.; Kristensen, J.L. Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists. ACS Chem. Neurosci. 2014, 5, 243–249. [Google Scholar] [CrossRef]
- Porter, R.H.; Benwell, K.R.; Lamb, H.; Malcolm, C.S.; Allen, N.H.; Revell, D.F.; Adams, D.R.; Sheardown, M.J. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br. J. Pharmacol. 1999, 128, 13–20. [Google Scholar] [CrossRef]
- Rickli, A.; Moning, O.D.; Hoener, M.C.; Liechti, M.E. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2016, 26, 1327–1337. [Google Scholar] [CrossRef]
- Keiser, M.J.; Setola, V.; Irwin, J.J.; Laggner, C.; Abbas, A.I.; Hufeisen, S.J.; Jensen, N.H.; Kuijer, M.B.; Matos, R.C.; Tran, T.B.; et al. Predicting new molecular targets for known drugs. Nature 2009, 462, 175–181. [Google Scholar] [CrossRef]
- Simmler, L.D.; Buchy, D.; Chaboz, S.; Hoener, M.C.; Liechti, M.E. In Vitro Characterization of Psychoactive Substances at Rat, Mouse, and Human Trace Amine-Associated Receptor 1. J. Pharmacol. Exp. Ther. 2016, 357, 134–144. [Google Scholar] [CrossRef]
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 2001, 60, 1181–1188. [Google Scholar] [CrossRef]
- Cao, C.; Barros-Alvarez, X.; Zhang, S.; Kim, K.; Damgen, M.A.; Panova, O.; Suomivuori, C.M.; Fay, J.F.; Zhong, X.; Krumm, B.E.; et al. Signaling snapshots of a serotonin receptor activated by the prototypical psychedelic LSD. Neuron 2022, 110, 3154–3167.e7. [Google Scholar] [CrossRef]
- Gumpper, R.H.; Fay, J.F.; Roth, B.L. Molecular insights into the regulation of constitutive activity by RNA editing of 5HT(2C) serotonin receptors. Cell Rep. 2022, 40, 111211. [Google Scholar] [CrossRef]
- Nichols, D.E. Hallucinogens. Pharmacol. Ther. 2004, 101, 131–181. [Google Scholar] [CrossRef]
- González-Maeso, J.; Weisstaub, N.V.; Zhou, M.; Chan, P.; Ivic, L.; Ang, R.; Lira, A.; Bradley-Moore, M.; Ge, Y.; Zhou, Q.; et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 2007, 53, 439–452. [Google Scholar] [CrossRef]
- Rodriguiz, R.M.; Nadkarni, V.; Means, C.R.; Pogorelov, V.M.; Chiu, Y.-T.; Roth, B.L.; Wetsel, W.C.J.S.R. LSD-stimulated behaviors in mice require β-arrestin 2 but not β-arrestin 1. Sci. Rep. 2021, 11, 17690. [Google Scholar] [CrossRef]
- De Gregorio, D.; Posa, L.; Ochoa-Sanchez, R.; McLaughlin, R.; Maione, S.; Comai, S.; Gobbi, G. The hallucinogen d-lysergic diethylamide (LSD) decreases dopamine firing activity through 5-HT(1A), D(2) and TAAR(1) receptors. Pharmacol. Res. 2016, 113, 81–91. [Google Scholar] [CrossRef]
- Pierce, P.A.; Peroutka, S.J. Antagonist properties of d-LSD at 5-hydroxytryptamine2 receptors. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 1990, 3, 503–508. [Google Scholar]
- Erkizia-Santamaría, I.; Alles-Pascual, R.; Horrillo, I.; Meana, J.J.; Ortega, J.E. Serotonin 5-HT(2A), 5-HT(2c) and 5-HT(1A) receptor involvement in the acute effects of psilocybin in mice. In vitro pharmacological profile and modulation of thermoregulation and head-twich response. Biomed. Pharmacother. = Biomed. Pharmacother. 2022, 154, 113612. [Google Scholar]
- Fortier, J.H.; Pizzarotti, B.; Shaw, R.E.; Levy, R.J.; Ferrari, G.; Grau, J. Drug-associated valvular heart diseases and serotonin-related pathways: A meta-analysis. Heart (Br. Card. Soc.) 2019, 105, 1140–1148. [Google Scholar] [CrossRef]
- Smith, S.R.; Weissman, N.J.; Anderson, C.M.; Sanchez, M.; Chuang, E.; Stubbe, S.; Bays, H.; Shanahan, W.R. Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 2010, 363, 245–256. [Google Scholar] [CrossRef]
- Thomsen, W.J.; Grottick, A.J.; Menzaghi, F.; Reyes-Saldana, H.; Espitia, S.; Yuskin, D.; Whelan, K.; Martin, M.; Morgan, M.; Chen, W.; et al. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: In vitro and in vivo pharmacological characterization. J. Pharmacol. Exp. Ther. 2008, 325, 577–587. [Google Scholar] [CrossRef]
- Boyer, E.W.; Shannon, M. The serotonin syndrome. N. Engl. J. Med. 2005, 352, 1112–1120. [Google Scholar] [CrossRef]
- Holze, F.; Gasser, P.; Müller, F.; Dolder, P.C.; Liechti, M.E. Lysergic Acid Diethylamide-Assisted Therapy in Patients With Anxiety With and Without a Life-Threatening Illness: A Randomized, Double-Blind, Placebo-Controlled Phase II Study. Biol. Psychiatry 2023, 93, 215–223. [Google Scholar] [CrossRef]
- Bershad, A.K.; Schepers, S.T.; Bremmer, M.P.; Lee, R.; de Wit, H. Acute Subjective and Behavioral Effects of Microdoses of Lysergic Acid Diethylamide in Healthy Human Volunteers. Biol. Psychiatry 2019, 86, 792–800. [Google Scholar] [CrossRef]
- Wießner, I.; Falchi, M.; Palhano-Fontes, F.; Feilding, A.; Ribeiro, S.; Tófoli, L.F. LSD, madness and healing: Mystical experiences as possible link between psychosis model and therapy model. Psychol. Med. 2023, 53, 1151–1165. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.M.; Aaronson, S.T.; Alvarez, O.; Arden, P.C.; Baker, A.; Bennett, J.C.; Bird, C.; Blom, R.E.; Brennan, C.; Brusch, D.; et al. Single-Dose Psilocybin for a Treatment-Resistant Episode of Major Depression. N. Engl. J. Med. 2022, 387, 1637–1648. [Google Scholar] [CrossRef]
- Daws, R.E.; Timmermann, C.; Giribaldi, B.; Sexton, J.D.; Wall, M.B.; Erritzoe, D.; Roseman, L.; Nutt, D.; Carhart-Harris, R. Increased global integration in the brain after psilocybin therapy for depression. Nat. Med. 2022, 28, 844–851. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 2013, 504, 107–112. [Google Scholar] [CrossRef]
- Domino, E.F.; Chodoff, P.; Corssen, G. Pharmacologic effects of CI-581, A new dissociative anesthetic, in man. Clin. Pharmacol. Ther. 1965, 6, 279–291. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Riggs, L.M.; Highland, J.N.; Georgiou, P.; Pereira, E.F.R.; Albuquerque, E.X.; Thomas, C.J.; Zarate, C.A., Jr.; et al. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol. Rev. 2018, 70, 621–660. [Google Scholar] [CrossRef]
- Krystal, J.H.; Karper, L.P.; Seibyl, J.P.; Freeman, G.K.; Delaney, R.; Bremner, J.D.; Heninger, G.R.; Bowers, M.B., Jr.; Charney, D.S. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry 1994, 51, 199–214. [Google Scholar] [CrossRef]
- Reier, C.E. Ketamine-“dissociative agent” or hallucinogen? N. Engl. J. Med. 1971, 284, 791–792. [Google Scholar]
- Yoon, G.; Petrakis, I.L.; Krystal, J.H. Association of Combined Naltrexone and Ketamine With Depressive Symptoms in a Case series of Patients With Depression and Alcohol Use Disorder. JAMA Psychiatry 2019, 76, 337–338. [Google Scholar] [CrossRef]
- Sofia, R.D.; Harakal, J.J. Evaluation of ketamine HCl for anti-depressant activity. Arch. Int. Pharmacodyn. Ther. 1975, 214, 68–74. [Google Scholar]
- Khorramzadeh, E.; Lotfy, A.O. The use of ketamine in psychiatry. Psychosomatics 1973, 14, 344–346. [Google Scholar] [CrossRef]
- Domino, E.F. Taming the ketamine tiger. 1965. Anesthesiology 2010, 113, 678–684. [Google Scholar] [CrossRef]
- Zarate, C.A., Jr.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 2006, 63, 856–864. [Google Scholar] [CrossRef]
- Murrough, J.W.; Iosifescu, D.V.; Chang, L.C.; Al Jurdi, R.K.; Green, C.E.; Perez, A.M.; Iqbal, S.; Pillemer, S.; Foulkes, A.; Shah, A.; et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am. J. Psychiatry 2013, 170, 1134–1142. [Google Scholar] [CrossRef]
- Anis, N.A.; Berry, S.C.; Burton, N.R.; Lodge, D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br. J. Pharmacol. 1983, 79, 565–575. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wang, D.; Wu, Z.; Huang, C.; Xu, X.; Xu, X.; Liu, C.; Hashimoto, K.; Yang, C. A bibliometric analysis of research on (R)-ketamine from 2002 to 2021. Neuropharmacology 2022, 218, 109207. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Cao, Q.; Luo, S.; He, L.; Yang, C.; Chen, J.; Qi, Q.; Hashimoto, K.; Zhang, J.C. Microglial ERK-NRBP1-CREB-BDNF signaling in sustained antidepressant actions of (R)-ketamine. Mol. Psychiatry 2022, 27, 1618–1629. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Georgiou, P.; Fischell, J.; Elmer, G.I.; Alkondon, M.; Yuan, P.; Pribut, H.J.; Singh, N.S.; et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016, 533, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Skiteva, O.; Zhang, X.; Svenningsson, P.; Chergui, K. Ketamine and its metabolite (2R,6R)-hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Mol. Psychiatry 2018, 23, 2066–2077. [Google Scholar] [CrossRef] [PubMed]
- Casarotto, P.C.; Girych, M.; Fred, S.M.; Kovaleva, V.; Moliner, R.; Enkavi, G.; Biojone, C.; Cannarozzo, C.; Sahu, M.P.; Kaurinkoski, K.; et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 2021, 184, 1299–1313.e19. [Google Scholar] [CrossRef] [PubMed]
- Cao, E.; Liao, M.; Cheng, Y.; Julius, D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 2013, 504, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Neumann, P.; Konevega, A.L.; Bock, L.V.; Ficner, R.; Rodnina, M.V.; Stark, H. Structure of the E. coli ribosome-EF-Tu complex at <3 Å resolution by Cs-corrected cryo-EM. Nature 2015, 520, 567–570. [Google Scholar] [PubMed]
- Grant, T.; Grigorieff, N. Automatic estimation and correction of anisotropic magnification distortion in electron microscopes. J. Struct. Biol. 2015, 192, 204–208. [Google Scholar] [CrossRef]
- Zheng, S.Q.; Palovcak, E.; Armache, J.P.; Verba, K.A.; Cheng, Y.; Agard, D.A. MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 2017, 14, 331–332. [Google Scholar] [CrossRef]
- Kurzbaum, E.; Raizner, Y.; Cohen, O.; Suckeveriene, R.Y.; Kulikov, A.; Hakimi, B.; Iasur Kruh, L.; Armon, R.; Farber, Y.; Menashe, O. Encapsulated Pseudomonas putida for phenol biodegradation: Use of a structural membrane for construction of a well-organized confined particle. Water Res. 2017, 121, 37–45. [Google Scholar] [CrossRef]
- Liang, Y.L.; Khoshouei, M.; Radjainia, M.; Zhang, Y.; Glukhova, A.; Tarrasch, J.; Thal, D.M.; Furness, S.G.B.; Christopoulos, G.; Coudrat, T.; et al. Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature 2017, 546, 118–123. [Google Scholar] [CrossRef]
- Cai, K.; Zhang, X.; Bai, X.C. Cryo-electron Microscopic Analysis of Single-Pass Transmembrane Receptors. Chem. Rev. 2022, 122, 13952–13988. [Google Scholar] [CrossRef]
- Cherezov, V.; Rosenbaum, D.M.; Hanson, M.A.; Rasmussen, S.G.; Thian, F.S.; Kobilka, T.S.; Choi, H.J.; Kuhn, P.; Weis, W.I.; Kobilka, B.K.; et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 2007, 318, 1258–1265. [Google Scholar] [CrossRef]
- Wacker, D.; Wang, C.; Katritch, V.; Han, G.W.; Huang, X.P.; Vardy, E.; McCorvy, J.D.; Jiang, Y.; Chu, M.; Siu, F.Y.; et al. Structural features for functional selectivity at serotonin receptors. Science 2013, 340, 615–619. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, Y.; Ma, J.; Wu, H.; Wacker, D.; Katritch, V.; Han, G.W.; Liu, W.; Huang, X.P.; Vardy, E.; et al. Structural basis for molecular recognition at serotonin receptors. Science 2013, 340, 610–614. [Google Scholar] [CrossRef]
- Pándy-Szekeres, G.; Caroli, J.; Mamyrbekov, A.; Kermani, A.A.; Keserű, G.M.; Kooistra, A.J.; Gloriam, D.E. GPCRdb in 2023: State-specific structure models using AlphaFold2 and new ligand resources. Nucleic Acids Res. 2022, 51, D395–D402. [Google Scholar] [CrossRef]
- Rasmussen, S.G.; Choi, H.J.; Rosenbaum, D.M.; Kobilka, T.S.; Thian, F.S.; Edwards, P.C.; Burghammer, M.; Ratnala, V.R.; Sanishvili, R.; Fischetti, R.F.; et al. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature 2007, 450, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Palczewski, K.; Kumasaka, T.; Hori, T.; Behnke, C.A.; Motoshima, H.; Fox, B.A.; Le Trong, I.; Teller, D.C.; Okada, T.; Stenkamp, R.E.; et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science 2000, 289, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Irwin, J.J.; Shoichet, B.K. ZINC—A free database of commercially available compounds for virtual screening. J. Chem. Inf. Model. 2005, 45, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Irwin, J.J.; Tang, K.G.; Young, J.; Dandarchuluun, C.; Wong, B.R.; Khurelbaatar, M.; Moroz, Y.S.; Mayfield, J.; Sayle, R.A. ZINC20-A Free Ultralarge-Scale Chemical Database for Ligand Discovery. J. Chem. Inf. Model. 2020, 60, 6065–6073. [Google Scholar] [CrossRef] [PubMed]
- Bender, B.J.; Gahbauer, S.; Luttens, A.; Lyu, J.; Webb, C.M.; Stein, R.M.; Fink, E.A.; Balius, T.E.; Carlsson, J.; Irwin, J.J.; et al. A practical guide to large-scale docking. Nat. Protoc. 2021, 16, 4799–4832. [Google Scholar] [CrossRef]
- Macarron, R.; Banks, M.N.; Bojanic, D.; Burns, D.J.; Cirovic, D.A.; Garyantes, T.; Green, D.V.; Hertzberg, R.P.; Janzen, W.P.; Paslay, J.W.; et al. Impact of high-throughput screening in biomedical research. Nat. Rev. Drug Discov. 2011, 10, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef]
- Coleman, R.G.; Carchia, M.; Sterling, T.; Irwin, J.J.; Shoichet, B.K. Ligand pose and orientational sampling in molecular docking. PLoS ONE 2013, 8, e75992. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, H.; Yao, X.; Li, D.; Xu, L.; Li, Y.; Tian, S.; Hou, T. Comprehensive evaluation of ten docking programs on a diverse set of protein-ligand complexes: The prediction accuracy of sampling power and scoring power. Phys. Chem. Chem. Phys. 2016, 18, 12964–12975. [Google Scholar] [CrossRef]
- Vass, M.; Schmidt, É.; Horti, F.; Keserű, G.M. Virtual fragment screening on GPCRs: A case study on dopamine D3 and histamine H4 receptors. Eur. J. Med. Chem. 2014, 77, 38–46. [Google Scholar] [CrossRef]
- Latorraca, N.R.; Venkatakrishnan, A.J.; Dror, R.O. GPCR Dynamics: Structures in Motion. Chem. Rev. 2017, 117, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Sterling, T.; Irwin, J.J. ZINC 15--Ligand Discovery for Everyone. J. Chem. Inf. Model. 2015, 55, 2324–2337. [Google Scholar] [CrossRef]
- Gorgulla, C.; Boeszoermenyi, A.; Wang, Z.F.; Fischer, P.D.; Coote, P.W.; Padmanabha Das, K.M.; Malets, Y.S.; Radchenko, D.S.; Moroz, Y.S.; Scott, D.A.; et al. An open-source drug discovery platform enables ultra-large virtual screens. Nature 2020, 580, 663–668. [Google Scholar] [CrossRef]
- Sadybekov, A.A.; Sadybekov, A.V.; Liu, Y.; Iliopoulos-Tsoutsouvas, C.; Huang, X.P.; Pickett, J.; Houser, B.; Patel, N.; Tran, N.K.; Tong, F.; et al. Synthon-based ligand discovery in virtual libraries of over 11 billion compounds. Nature 2022, 601, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.M.; Kang, H.J.; McCorvy, J.D.; Glatfelter, G.C.; Jones, A.J.; Che, T.; Slocum, S.; Huang, X.P.; Savych, O.; Moroz, Y.S.; et al. Virtual discovery of melatonin receptor ligands to modulate circadian rhythms. Nature 2020, 579, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Alon, A.; Lyu, J.; Braz, J.M.; Tummino, T.A.; Craik, V.; O'Meara, M.J.; Webb, C.M.; Radchenko, D.S.; Moroz, Y.S.; Huang, X.P.; et al. Structures of the sigma(2) receptor enable docking for bioactive ligand discovery. Nature 2021, 600, 759–764. [Google Scholar] [CrossRef]
- Che, T.; Roth, B.L. Structural Insights Accelerate the Discovery of Opioid Alternatives. Annu. Rev. Biochem. 2021, 90, 739–761. [Google Scholar] [CrossRef]
- Fink, E.A.; Xu, J.; Hubner, H.; Braz, J.M.; Seemann, P.; Avet, C.; Craik, V.; Weikert, D.; Schmidt, M.F.; Webb, C.M.; et al. Structure-based discovery of nonopioid analgesics acting through the alpha(2A)-adrenergic receptor. Science 2022, 377, eabn7065. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.L.; Confair, D.N.; Kim, K.; Barros-Alvarez, X.; Rodriguiz, R.M.; Yang, Y.; Kweon, O.S.; Che, T.; McCorvy, J.D.; Kamber, D.N.; et al. Bespoke library docking for 5-HT2A receptor agonists with antidepressant activity. Nature 2022, 610, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Manivet, P.; Nishio, H.; Pratuangdejkul, J.; Rajab, M.; Ishiguro, M.; Launay, J.M.; Nagatomo, T. Identification of the binding sites and selectivity of sarpogrelate, a novel 5-HT2 antagonist, to human 5-HT2A, 5-HT2B and 5-HT2C receptor subtypes by molecular modeling. Life Sci. 2003, 73, 193–207. [Google Scholar] [CrossRef]
- Vacher, B.; Bonnaud, B.; Funes, P.; Jubault, N.; Koek, W.; Assié, M.B.; Cosi, C. Design and synthesis of a series of 6-substituted-2-pyridinylmethylamine derivatives as novel, high-affinity, selective agonists at 5-HT1A receptors. J. Med. Chem. 1998, 41, 5070–5083. [Google Scholar] [CrossRef]
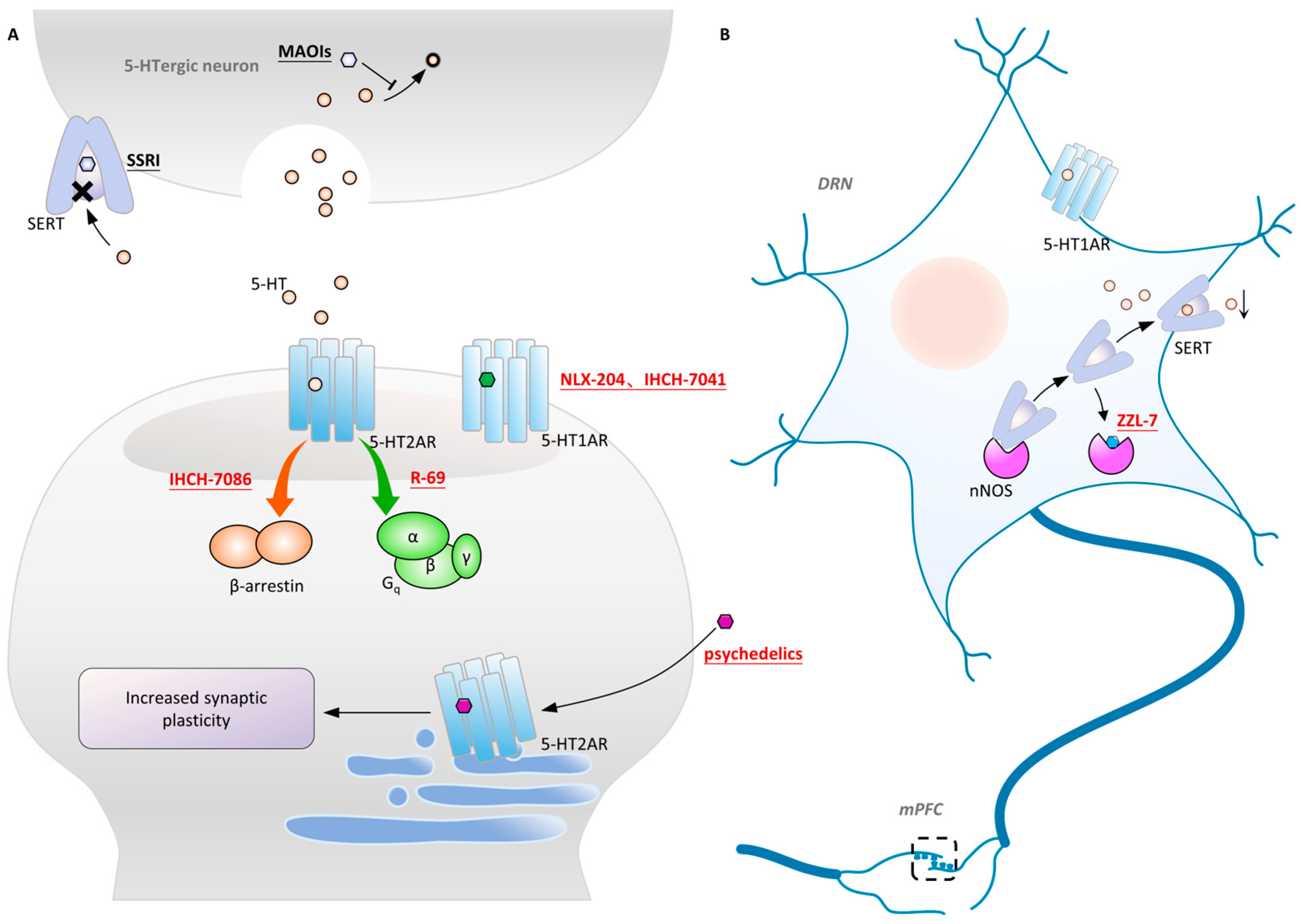
- Sniecikowska, J.; Gluch-Lutwin, M.; Bucki, A.; Więckowska, A.; Siwek, A.; Jastrzebska-Wiesek, M.; Partyka, A.; Wilczyńska, D.; Pytka, K.; Latacz, G.; et al. Discovery of Novel pERK1/2- or β-Arrestin-Preferring 5-HT(1A) Receptor-Biased Agonists: Diversified Therapeutic-like versus Side Effect Profile. J. Med. Chem. 2020, 63, 10946–10971. [Google Scholar] [CrossRef]
- Persechino, M.; Hedderich, J.B.; Kolb, P.; Hilger, D. Allosteric modulation of GPCRs: From structural insights to in silico drug discovery. Pharmacol. Ther. 2022, 237, 108242. [Google Scholar] [CrossRef] [PubMed]
- Elofsson, A. Progress at protein structure prediction, as seen in CASP15. Curr. Opin. Struct. Biol. 2023, 80, 102594. [Google Scholar] [CrossRef]
- Senior, A.W.; Evans, R.; Jumper, J.; Kirkpatrick, J.; Sifre, L.; Green, T.; Qin, C.; Žídek, A.; Nelson, A.W.R.; Bridgland, A.; et al. Improved protein structure prediction using potentials from deep learning. Nature 2020, 577, 706–710. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Lee, C.; Su, B.H.; Tseng, Y.J. Comparative studies of AlphaFold, RoseTTAFold and Modeller: A case study involving the use of G-protein-coupled receptors. Brief. Bioinform. 2022, 23, bbac308. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.; Coleman, R.G.; Setola, V.; Irwin, J.J.; Fan, H.; Schlessinger, A.; Sali, A.; Roth, B.L.; Shoichet, B.K. Ligand discovery from a dopamine D3 receptor homology model and crystal structure. Nat. Chem. Biol. 2011, 7, 769–778. [Google Scholar] [CrossRef] [PubMed]
- He, X.H.; You, C.Z.; Jiang, H.L.; Jiang, Y.; Xu, H.E.; Cheng, X. AlphaFold2 versus experimental structures: Evaluation on G protein-coupled receptors. Acta Pharmacol. Sin. 2022, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Del Alamo, D.; Sala, D.; McHaourab, H.S.; Meiler, J. Sampling alternative conformational states of transporters and receptors with AlphaFold2. Elife 2022, 11, e75751. [Google Scholar] [CrossRef]
- Stein, R.A.; McHaourab, H.S. SPEACH_AF: Sampling protein ensembles and conformational heterogeneity with Alphafold2. PLoS Comput. Biol. 2022, 18, e1010483. [Google Scholar] [CrossRef] [PubMed]
- Heo, L.; Feig, M. Multi-state modeling of G-protein coupled receptors at experimental accuracy. Proteins 2022, 90, 1873–1885. [Google Scholar] [CrossRef]
- Rodríguez-Espigares, I.; Torrens-Fontanals, M.; Tiemann, J.K.S.; Aranda-García, D.; Ramírez-Anguita, J.M.; Stepniewski, T.M.; Worp, N.; Varela-Rial, A.; Morales-Pastor, A.; Medel-Lacruz, B.; et al. GPCRmd uncovers the dynamics of the 3D-GPCRome. Nat. Methods 2020, 17, 777–787. [Google Scholar] [CrossRef]
- Zhang, Y.; Vass, M.; Shi, D.; Abualrous, E.; Chambers, J.M.; Chopra, N.; Higgs, C.; Kasavajhala, K.; Li, H.; Nandekar, P.; et al. Benchmarking Refined and Unrefined AlphaFold2 Structures for Hit Discovery. J. Chem. Inf. Model. 2023, 63, 1656–1667. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, G.; Zeng, C.; Zhan, X.; Liang, K.; Xu, Q.; Zhao, Y.; Wang, P.; Wang, Q.; Zhou, Q.; et al. Structure of the cytoplasmic ring of the Xenopus laevis nuclear pore complex. Science 2022, 376, eabl8280. [Google Scholar] [CrossRef]
- Humphreys, I.R.; Pei, J.; Baek, M.; Krishnakumar, A.; Anishchenko, I.; Ovchinnikov, S.; Zhang, J.; Ness, T.J.; Banjade, S.; Bagde, S.R.; et al. Computed structures of core eukaryotic protein complexes. Science 2021, 374, eabm4805. [Google Scholar] [CrossRef]
- Richard, E.; Michael, O.N.; Alexander, P.; Natasha, A.; Andrew, S.; Tim, G.; Augustin, Ž.; Russ, B.; Sam, B.; Jason, Y.; et al. Protein complex prediction with AlphaFold-Multimer. bioRxiv 2022. [Google Scholar] [CrossRef]
- Gao, M.; Nakajima An, D.; Parks, J.M.; Skolnick, J. AF2Complex predicts direct physical interactions in multimeric proteins with deep learning. Nat. Commun. 2022, 13, 1744. [Google Scholar] [CrossRef]
- Asher, W.B.; Terry, D.S.; Gregorio, G.G.A.; Kahsai, A.W.; Borgia, A.; Xie, B.; Modak, A.; Zhu, Y.; Jang, W.; Govindaraju, A.; et al. GPCR-mediated beta-arrestin activation deconvoluted with single-molecule precision. Cell 2022, 185, 1661–1675.e16. [Google Scholar] [CrossRef] [PubMed]
- Lutomski, C.A.; El-Baba, T.J.; Robinson, C.V.; Riek, R.; Scheres, S.H.W.; Yan, N.; AlQuraishi, M.; Gan, L. The next decade of protein structure. Cell 2022, 185, 2617–2620. [Google Scholar] [CrossRef] [PubMed]
- Congreve, M.; de Graaf, C.; Swain, N.A.; Tate, C.G. Impact of GPCR Structures on Drug Discovery. Cell 2020, 181, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Tingle, B.I.; Tang, K.G.; Castanon, M.; Gutierrez, J.J.; Khurelbaatar, M.; Dandarchuluun, C.; Moroz, Y.S.; Irwin, J.J. ZINC-22─A Free Multi-Billion-Scale Database of Tangible Compounds for Ligand Discovery. J. Chem. Inf. Model. 2023, 63, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Mendez, D.; Gaulton, A.; Bento, A.P.; Chambers, J.; De Veij, M.; Félix, E.; Magariños, M.P.; Mosquera, J.F.; Mutowo, P.; Nowotka, M.; et al. ChEMBL: Towards direct deposition of bioassay data. Nucleic Acids Res. 2019, 47, D930–D940. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Tagari, M.; Newman, R.; Chagoyen, M.; Carazo, J.M.; Henrick, K. New electron microscopy database and deposition system. Trends Biochem. Sci. 2002, 27, 589. [Google Scholar] [CrossRef] [PubMed]
- Tunyasuvunakool, K.; Adler, J.; Wu, Z.; Green, T.; Zielinski, M.; Zidek, A.; Bridgland, A.; Cowie, A.; Meyer, C.; Laydon, A.; et al. Highly accurate protein structure prediction for the human proteome. Nature 2021, 596, 590–596. [Google Scholar] [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Mysinger, M.M.; Shoichet, B.K. Rapid context-dependent ligand desolvation in molecular docking. J. Chem. Inf. Model. 2010, 50, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
- Cameron, L.P.; Tombari, R.J.; Lu, J.; Pell, A.J.; Hurley, Z.Q.; Ehinger, Y.; Vargas, M.V.; McCarroll, M.N.; Taylor, J.C.; Myers-Turnbull, D.; et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 2021, 589, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Ly, C.; Dunlap, L.E.; Vargas, M.V.; Sun, J.; Hwang, I.W.; Azinfar, A.; Oh, W.C.; Wetsel, W.C.; Olson, D.E.; et al. Psychedelic-inspired drug discovery using an engineered biosensor. Cell 2021, 184, 2779–2792. [Google Scholar] [CrossRef] [PubMed]
- Wasko, M.J.; Witt-Enderby, P.A.; Surratt, C.K. DARK Classics in Chemical Neuroscience: Ibogaine. ACS Chem. Neurosci. 2018, 9, 2475–2483. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.N.; Favela, D.; Zhang, G.; Olson, D.E. The iboga enigma: The chemistry and neuropharmacology of iboga alkaloids and related analogs. Nat. Prod. Rep. 2021, 38, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Koenig, X.; Kovar, M.; Boehm, S.; Sandtner, W.; Hilber, K. Anti-addiction drug ibogaine inhibits hERG channels: A cardiac arrhythmia risk. Addict. Biol. 2014, 19, 237–239. [Google Scholar] [CrossRef]
- Thurner, P.; Stary-Weinzinger, A.; Gafar, H.; Gawali, V.S.; Kudlacek, O.; Zezula, J.; Hilber, K.; Boehm, S.; Sandtner, W.; Koenig, X. Mechanism of hERG channel block by the psychoactive indole alkaloid ibogaine. J. Pharmacol. Exp. Ther. 2014, 348, 346–358. [Google Scholar] [CrossRef]
- Patriarchi, T.; Cho, J.R.; Merten, K.; Howe, M.W.; Marley, A.; Xiong, W.H.; Folk, R.W.; Broussard, G.J.; Liang, R.; Jang, M.J.; et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 2018, 360, eaat4422. [Google Scholar] [CrossRef]
- Cameron, L.P.; Benson, C.J.; DeFelice, B.C.; Fiehn, O.; Olson, D.E. Chronic, Intermittent Microdoses of the Psychedelic N,N-Dimethyltryptamine (DMT) Produce Positive Effects on Mood and Anxiety in Rodents. ACS Chem. Neurosci. 2019, 10, 3261–3270. [Google Scholar] [CrossRef]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Soltanzadeh Zarandi, S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef]
- Kwan, A.C.; Olson, D.E.; Preller, K.H.; Roth, B.L. The neural basis of psychedelic action. Nat. Neurosci. 2022, 25, 1407–1419. [Google Scholar] [CrossRef]
- Ly, C.; Greb, A.C.; Vargas, M.V.; Duim, W.C.; Grodzki, A.C.G.; Lein, P.J.; Olson, D.E. Transient Stimulation with Psychoplastogens Is Sufficient to Initiate Neuronal Growth. ACS Pharmacol. Transl. Sci. 2021, 4, 452–460. [Google Scholar] [CrossRef]
- Hesselgrave, N.; Troppoli, T.A.; Wulff, A.B.; Cole, A.B.; Thompson, S.M. Harnessing psilocybin: Antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2022489118. [Google Scholar] [CrossRef] [PubMed]
- Cavus, I.; Duman, R.S. Influence of estradiol, stress, and 5-HT2A agonist treatment on brain-derived neurotrophic factor expression in female rats. Biol. Psychiatry 2003, 54, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Barre, A.; Berthoux, C.; De Bundel, D.; Valjent, E.; Bockaert, J.; Marin, P.; Bécamel, C. Presynaptic serotonin 2A receptors modulate thalamocortical plasticity and associative learning. Proc. Natl. Acad. Sci. USA 2016, 113, E1382–E1391. [Google Scholar] [CrossRef] [PubMed]
- Stoliker, D.; Egan, G.F.; Friston, K.J.; Razi, A. Neural Mechanisms and Psychology of Psychedelic Ego Dissolution. Pharmacol. Rev. 2022, 74, 876–917. [Google Scholar] [CrossRef] [PubMed]
- Wacker, D.; Wang, S.; McCorvy, J.D.; Betz, R.M.; Venkatakrishnan, A.J.; Levit, A.; Lansu, K.; Schools, Z.L.; Che, T.; Nichols, D.E.; et al. Crystal Structure of an LSD-Bound Human Serotonin Receptor. Cell 2017, 168, 377–389.e12. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, J.; Weinstein, H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 1995, 25, 366–428. [Google Scholar]
- Smith, J.S.; Lefkowitz, R.J.; Rajagopal, S. Biased signalling: From simple switches to allosteric microprocessors. Nat. Rev. Drug Discov. 2018, 17, 243–260. [Google Scholar] [CrossRef]
- Sandhu, M.; Cho, A.; Ma, N.; Mukhaleva, E.; Namkung, Y.; Lee, S.; Ghosh, S.; Lee, J.H.; Gloriam, D.E.; Laporte, S.A.; et al. Dynamic spatiotemporal determinants modulate GPCR:G protein coupling selectivity and promiscuity. Nat. Commun. 2022, 13, 7428. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.P.; Scully, C.C.G.; de Graaf, C.; Brown, A.J.H.; Maguire, J.J. Advances in therapeutic peptides targeting G protein-coupled receptors. Nat. Rev. Drug Discov. 2020, 19, 389–413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, Z.G.; Zhang, K.; Kiselev, E.; Crane, S.; Wang, J.; Paoletta, S.; Yi, C.; Ma, L.; Zhang, W.; et al. Two disparate ligand-binding sites in the human P2Y1 receptor. Nature 2015, 520, 317–321. [Google Scholar] [CrossRef]
- Ho, J.D.; Chau, B.; Rodgers, L.; Lu, F.; Wilbur, K.L.; Otto, K.A.; Chen, Y.; Song, M.; Riley, J.P.; Yang, H.C.; et al. Structural basis for GPR40 allosteric agonism and incretin stimulation. Nat. Commun. 2018, 9, 1645. [Google Scholar] [CrossRef]
- Periole, X. Interplay of G Protein-Coupled Receptors with the Membrane: Insights from Supra-Atomic Coarse Grain Molecular Dynamics Simulations. Chem. Rev. 2017, 117, 156–185. [Google Scholar] [CrossRef]
- Gentile, F.; Yaacoub, J.C.; Gleave, J.; Fernandez, M.; Ton, A.T.; Ban, F.; Stern, A.; Cherkasov, A. Artificial intelligence-enabled virtual screening of ultra-large chemical libraries with deep docking. Nat. Protoc. 2022, 17, 672–697. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Huang, X.P.; Chen, G.; Whaley, R.; Peng, S.; Wang, Y.; Zhang, G.; Wang, S.X.; Wang, S.; Roth, B.L.; et al. Life beyond kinases: Structure-based discovery of sorafenib as nanomolar antagonist of 5-HT receptors. J. Med. Chem. 2012, 55, 5749–5759. [Google Scholar] [CrossRef]
- Gandhimathi, A.; Sowdhamini, R. Molecular modelling of human 5-hydroxytryptamine receptor (5-HT2A) and virtual screening studies towards the identification of agonist and antagonist molecules. J. Biomol. Struct. Dyn. 2016, 34, 952–970. [Google Scholar] [CrossRef]
- Xu, P.; Huang, S.; Zhang, H.; Mao, C.; Zhou, X.E.; Cheng, X.; Simon, I.A.; Shen, D.D.; Yen, H.Y.; Robinson, C.V.; et al. Structural insights into the lipid and ligand regulation of serotonin receptors. Nature 2021, 592, 469–473. [Google Scholar] [CrossRef]
- de Rubio, R.G.; Ransom, R.F.; Malik, S.; Yule, D.I.; Anantharam, A.; Smrcka, A.V. Phosphatidylinositol 4-phosphate is a major source of GPCR-stimulated phosphoinositide production. Sci. Signal 2018, 11, eaan1210. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, G.; De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 2006, 443, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Khelashvili, G.; Menon, A.K. Phospholipid Scrambling by G Protein-Coupled Receptors. Annu. Rev. Biophys. 2022, 51, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.L.; Cropper, J.D.; Berg, K.A.; Clarke, W.P. Mechanisms of regulation of agonist efficacy at the 5-HT(1A) receptor by phospholipid-derived signaling components. J. Pharmacol. Exp. Ther. 2001, 297, 1025–1035. [Google Scholar] [PubMed]
- Pucadyil, T.J.; Chattopadhyay, A. Cholesterol modulates the antagonist-binding function of hippocampal serotonin1A receptors. Biochim. Biophys. Acta 2005, 1714, 35–42. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, D.; Wu, M.; Guo, Y.; Guo, W.; Zhong, L.; Cai, X.; Dai, A.; Jang, W.; Shakhnovich, E.I.; et al. Common activation mechanism of class A GPCRs. Elife 2019, 8, e50279. [Google Scholar] [CrossRef]
- Huang, S.; Xu, P.; Tan, Y.; You, C.; Zhang, Y.; Jiang, Y.; Xu, H.E. Structural basis for recognition of anti-migraine drug lasmiditan by the serotonin receptor 5-HT1F–G protein complex. Cell Res. 2021, 31, 1036–1038. [Google Scholar] [CrossRef]
- Duncan, A.L.; Song, W.; Sansom, M.S.P. Lipid-Dependent Regulation of Ion Channels and G Protein-Coupled Receptors: Insights from Structures and Simulations. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 31–50. [Google Scholar] [CrossRef]
- Ebdrup, B.H.; Rasmussen, H.; Arnt, J.; Glenthøj, B. Serotonin 2A receptor antagonists for treatment of schizophrenia. Expert. Opin. Investig. Drugs 2011, 20, 1211–1223. [Google Scholar] [CrossRef]
- Leucht, S.; Cipriani, A.; Spineli, L.; Mavridis, D.; Orey, D.; Richter, F.; Samara, M.; Barbui, C.; Engel, R.R.; Geddes, J.R.; et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet 2013, 382, 951–962. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, L.; Wang, H.; Yu, J.; Lu, D.; Qi, J.; Nie, F.; Luo, Z.; Liu, Z.; Cheng, J.; et al. Structure-based design of a novel third-generation antipsychotic drug lead with potential antidepressant properties. Nat. Neurosci. 2022, 25, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Celada, P.; Bortolozzi, A.; Artigas, F. Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: Rationale and current status of research. CNS Drugs 2013, 27, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Samuels, B.A.; Anacker, C.; Hu, A.; Levinstein, M.R.; Pickenhagen, A.; Tsetsenis, T.; Madroñal, N.; Donaldson, Z.R.; Drew, L.J.; Dranovsky, A.; et al. 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat. Neurosci. 2015, 18, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.; Boothman, L.; Raley, J.; Quérée, P. Important messages in the ‘post’: Recent discoveries in 5-HT neurone feedback control. Trends Pharmacol. Sci. 2007, 28, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Richardson-Jones, J.W.; Craige, C.P.; Guiard, B.P.; Stephen, A.; Metzger, K.L.; Kung, H.F.; Gardier, A.M.; Dranovsky, A.; David, D.J.; Beck, S.G.; et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 2010, 65, 40–52. [Google Scholar] [CrossRef]
- Descarries, L.; Riad, M. Effects of the antidepressant fluoxetine on the subcellular localization of 5-HT1A receptors and SERT. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2012, 367, 2416–2425. [Google Scholar] [CrossRef]
- Gray, N.A.; Milak, M.S.; DeLorenzo, C.; Ogden, R.T.; Huang, Y.Y.; Mann, J.J.; Parsey, R.V. Antidepressant treatment reduces serotonin-1A autoreceptor binding in major depressive disorder. Biol. Psychiatry 2013, 74, 26–31. [Google Scholar] [CrossRef]
- Gettys, T.W.; Fields, T.A.; Raymond, J.R. Selective activation of inhibitory G-protein alpha-subunits by partial agonists of the human 5-HT1A receptor. Biochemistry 1994, 33, 4283–4290. [Google Scholar] [CrossRef]
- Mannoury la Cour, C.; El Mestikawy, S.; Hanoun, N.; Hamon, M.; Lanfumey, L. Regional differences in the coupling of 5-hydroxytryptamine-1A receptors to G proteins in the rat brain. Mol. Pharmacol. 2006, 70, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Valdizán, E.M.; Castro, E.; Pazos, A. Agonist-dependent modulation of G-protein coupling and transduction of 5-HT1A receptors in rat dorsal raphe nucleus. Int. J. Neuropsychopharmacol. 2010, 13, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Gammans, R.E.; Mayol, R.F.; LaBudde, J.A. Metabolism and disposition of buspirone. Am. J. Med. 1986, 80, 41–51. [Google Scholar] [CrossRef]
- Perrone, R.; Berardi, F.; Leopoldo, M.; Tortorella, V.; Fornaretto, M.G.; Caccia, C.; McArthur, R.A. 1-aryl-4-[(1-tetralinyl)alkyl]piperazines: Alkylamido and alkylamino derivatives. Synthesis, 5-HT1A receptor affinity, and selectivity. 3. J. Med. Chem. 1996, 39, 3195–3202. [Google Scholar] [CrossRef]
- Osman, R.; Topiol, S.; Rubenstein, L.; Weinstein, H. A molecular model for activation of a 5-hydroxytryptamine receptor. Mol. Pharmacol. 1987, 32, 699–705. [Google Scholar] [PubMed]
- Lemoine, L.; Verdurand, M.; Vacher, B.; Blanc, E.; Le Bars, D.; Newman-Tancredi, A.; Zimmer, L. [18F]F15599, a novel 5-HT1A receptor agonist, as a radioligand for PET neuroimaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 594–605. [Google Scholar] [CrossRef]
- Maurel, J.L.; Autin, J.M.; Funes, P.; Newman-Tancredi, A.; Colpaert, F.; Vacher, B. High-efficacy 5-HT1A agonists for antidepressant treatment: A renewed opportunity. J. Med. Chem. 2007, 50, 5024–5033. [Google Scholar] [CrossRef]
- Lladó-Pelfort, L.; Assié, M.B.; Newman-Tancredi, A.; Artigas, F.; Celada, P. Preferential in vivo action of F15599, a novel 5-HT(1A) receptor agonist, at postsynaptic 5-HT(1A) receptors. Br. J. Pharmacol. 2010, 160, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Sniecikowska, J.; Gluch-Lutwin, M.; Bucki, A.; Więckowska, A.; Siwek, A.; Jastrzebska-Wiesek, M.; Partyka, A.; Wilczyńska, D.; Pytka, K.; Pociecha, K.; et al. Novel Aryloxyethyl Derivatives of 1-(1-Benzoylpiperidin-4-yl)methanamine as the Extracellular Regulated Kinases 1/2 (ERK1/2) Phosphorylation-Preferring Serotonin 5-HT(1A) Receptor-Biased Agonists with Robust Antidepressant-like Activity. J. Med. Chem. 2019, 62, 2750–2771. [Google Scholar] [CrossRef]
- Karasawa, J.; Shimazaki, T.; Kawashima, N.; Chaki, S. AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res. 2005, 1042, 92–98. [Google Scholar] [CrossRef]
- Li, N.; Lee, B.; Liu, R.J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.Y.; Aghajanian, G.; Duman, R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Castren, E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor Signaling in Depression and Antidepressant Action. Biol. Psychiatry 2021, 90, 128–136. [Google Scholar] [CrossRef]
- Sun, N.; Qin, Y.J.; Xu, C.; Xia, T.; Du, Z.W.; Zheng, L.P.; Li, A.A.; Meng, F.; Zhang, Y.; Zhang, J.; et al. Design of fast-onset antidepressant by dissociating SERT from nNOS in the DRN. Science 2022, 378, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.J.; Wu, D.L.; Chen, R.; Li, N.; Zhu, L.J. Requirement of hippocampal DG nNOS-CAPON dissociation for the anxiolytic and antidepressant effects of fluoxetine. Theranostics 2022, 12, 3656–3675. [Google Scholar] [CrossRef] [PubMed]
- Poulie, C.B.M.; Pottie, E.; Simon, I.A.; Harpsoe, K.; D’Andrea, L.; Komarov, I.V.; Gloriam, D.E.; Jensen, A.A.; Stove, C.P.; Kristensen, J.L. Discovery of beta-Arrestin-Biased 25CN-NBOH-Derived 5-HT(2A) Receptor Agonists. J. Med. Chem. 2022, 65, 12031–12043. [Google Scholar] [CrossRef] [PubMed]
- Parekh, P.K.; Johnson, S.B.; Liston, C. Synaptic Mechanisms Regulating Mood State Transitions in Depression. Annu. Rev. Neurosci. 2022, 45, 581–601. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, D.M.; Pothula, S.; Liu, R.J.; Wu, M.; Li, X.Y.; Girgenti, M.J.; Taylor, S.R.; Duman, C.H.; Delpire, E.; Picciotto, M.; et al. GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J. Clin. Investig. 2020, 130, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.E.; Guo, T.Z.; Lu, J.; Saper, C.B.; Franks, N.P.; Maze, M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat. Neurosci. 2002, 5, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Homayoun, H.; Moghaddam, B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 11496–11500. [Google Scholar] [CrossRef]
- Autry, A.E.; Adachi, M.; Nosyreva, E.; Na, E.S.; Los, M.F.; Cheng, P.F.; Kavalali, E.T.; Monteggia, L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011, 475, 91–95. [Google Scholar] [CrossRef]
- Sanacora, G.; Zarate, C.A.; Krystal, J.H.; Manji, H.K. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 2008, 7, 426–437. [Google Scholar] [CrossRef]
- Karolewicz, B.; Stockmeier, C.A.; Ordway, G.A. Elevated levels of the NR2C subunit of the NMDA receptor in the locus coeruleus in depression. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2005, 30, 1557–1567. [Google Scholar] [CrossRef][Green Version]
- Karolewicz, B.; Szebeni, K.; Gilmore, T.; Maciag, D.; Stockmeier, C.A.; Ordway, G.A. Elevated levels of NR2A and PSD-95 in the lateral amygdala in depression. Int. J. Neuropsychopharmacol. 2009, 12, 143–153. [Google Scholar] [CrossRef]
- Gray, A.L.; Hyde, T.M.; Deep-Soboslay, A.; Kleinman, J.E.; Sodhi, M.S. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol. Psychiatry 2015, 20, 1057–1068. [Google Scholar] [CrossRef]
- Aguilar-Valles, A.; De Gregorio, D.; Matta-Camacho, E.; Eslamizade, M.J.; Khlaifia, A.; Skaleka, A.; Lopez-Canul, M.; Torres-Berrio, A.; Bermudez, S.; Rurak, G.M.; et al. Antidepressant actions of ketamine engage cell-specific translation via eIF4E. Nature 2021, 590, 315–319. [Google Scholar] [CrossRef]
- Tartt, A.N.; Mariani, M.B.; Hen, R.; Mann, J.J.; Boldrini, M. Dysregulation of adult hippocampal neuroplasticity in major depression: Pathogenesis and therapeutic implications. Mol. Psychiatry 2022, 27, 2689–2699. [Google Scholar] [CrossRef]
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2011, 13, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Turrigiano, G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu. Rev. Neurosci. 2011, 34, 89–103. [Google Scholar] [CrossRef]
- Manji, H.K.; Quiroz, J.A.; Sporn, J.; Payne, J.L.; Denicoff, K.; Gray, N.A.; Zarate, C.A., Jr.; Charney, D.S. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol. Psychiatry 2003, 53, 707–742. [Google Scholar] [CrossRef] [PubMed]
- Price, R.B.; Nock, M.K.; Charney, D.S.; Mathew, S.J. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry 2009, 66, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, G.M.; Zhang, J.; Thomas, M.; Banasr, M.; Ma, X.; Pittman, B.; Bristow, L.; Schaeffer, E.; Duman, R.S.; Rothman, D.L.; et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol. Psychiatry 2017, 22, 120–126. [Google Scholar] [CrossRef]
- Voleti, B.; Navarria, A.; Liu, R.J.; Banasr, M.; Li, N.; Terwilliger, R.; Sanacora, G.; Eid, T.; Aghajanian, G.; Duman, R.S. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol. Psychiatry 2013, 74, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-J.; Duman, C.; Kato, T.; Hare, B.; Lopresto, D.; Bang, E.; Burgdorf, J.; Moskal, J.; Taylor, J.; Aghajanian, G.; et al. GLYX-13 Produces Rapid Antidepressant Responses with Key Synaptic and Behavioral Effects Distinct from Ketamine. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017, 42, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C.A., Jr.; Singh, J.B.; Quiroz, J.A.; De Jesus, G.; Denicoff, K.K.; Luckenbaugh, D.A.; Manji, H.K.; Charney, D.S. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am. J. Psychiatry 2006, 163, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Gideons, E.S.; Kavalali, E.T.; Monteggia, L.M. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc. Natl. Acad. Sci. USA 2014, 111, 8649–8654. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.B.; Chao, M.V. The tyrosine kinase Fyn determines the localization of TrkB receptors in lipid rafts. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 4859–4869. [Google Scholar] [CrossRef]
- Pralle, A.; Keller, P.; Florin, E.L.; Simons, K.; Hörber, J.K. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 2000, 148, 997–1008. [Google Scholar] [CrossRef]
- Abbar, M.; Demattei, C.; El-Hage, W.; Llorca, P.M.; Samalin, L.; Demaricourt, P.; Gaillard, R.; Courtet, P.; Vaiva, G.; Gorwood, P.; et al. Ketamine for the acute treatment of severe suicidal ideation: Double blind, randomised placebo controlled trial. BMJ (Clin. Res. Ed.) 2022, 376, e067194. [Google Scholar] [CrossRef]
- Lee, C.H.; Lu, W.; Michel, J.C.; Goehring, A.; Du, J.; Song, X.; Gouaux, E. NMDA receptor structures reveal subunit arrangement and pore architecture. Nature 2014, 511, 191–197. [Google Scholar] [CrossRef]
- Lü, W.; Du, J.; Goehring, A.; Gouaux, E. Cryo-EM structures of the triheteromeric NMDA receptor and its allosteric modulation. Science 2017, 355, eaal3729. [Google Scholar] [CrossRef]
- Wilkinson, D. A review of the effects of memantine on clinical progression in Alzheimer's disease. Int. J. Geriatr. Psychiatry 2012, 27, 769–776. [Google Scholar] [CrossRef]
- Lodge, D.; Mercier, M.S. Ketamine and phencyclidine: The good, the bad and the unexpected. Br. J. Pharmacol. 2015, 172, 4254–4276. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Wang, Q.; Wen, H.; Liu, Z.; Wang, F.; Wang, Y.; Yao, F.; Song, N.; Kou, Z.; et al. Distinct structure and gating mechanism in diverse NMDA receptors with GluN2C and GluN2D subunits. Nat. Struct. Mol. Biol. 2023, 30, 629–639. [Google Scholar] [CrossRef]
- Jilin, Z.; Jinjin, D.; Luyu, Y.; Wei, L.; Haitao, Z.; Fang, L.; Tian, X.; Yang, X.; Yiming, H.; Yidi, S.; et al. A novel antidepressant acting via allosteric inhibition of GluN2D-incorporated NMDA receptors at GABAergic interneurons. bioRxiv 2022. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, F.; Fu, Z.W.; Zhang, B.; Huang, C.G.; Li, Y. Timosaponin derivative YY-23 acts as a non-competitive NMDA receptor antagonist and exerts a rapid antidepressant-like effect in mice. Acta Pharmacol. Sin. 2016, 37, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Yao, K.; Zhang, Y.; Zhu, S. NMDA receptors as therapeutic targets for depression treatment: Evidence from clinical to basic research. Neuropharmacology 2023, 225, 109378. [Google Scholar] [CrossRef] [PubMed]
- Wahl-Schott, C.; Biel, M. HCN channels: Structure, cellular regulation and physiological function. Cell. Mol. Life Sci. CMLS 2009, 66, 470–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shu, S.; Bayliss, D.A. HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, F.F.; Chen, X.D.; Zhou, C. Inhibition of HCN1 channels by ketamine accounts for its antidepressant actions. Sichuan Da Xue Xue Bao. Yi Xue Ban. = J. Sichuan University. Med. Sci. Ed. 2014, 45, 888–892, 932. [Google Scholar]
- Langmead, C.J.; Watson, J.; Reavill, C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol. Ther. 2008, 117, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef]
- Flood, P.; Krasowski, M.D. Intravenous anesthetics differentially modulate ligand-gated ion channels. Anesthesiology 2000, 92, 1418–1425. [Google Scholar] [CrossRef]
- Yamakura, T.; Chavez-Noriega, L.E.; Harris, R.A. Subunit-dependent inhibition of human neuronal nicotinic acetylcholine receptors and other ligand-gated ion channels by dissociative anesthetics ketamine and dizocilpine. Anesthesiology 2000, 92, 1144–1153. [Google Scholar] [CrossRef]
- Coates, K.M.; Flood, P. Ketamine and its preservative, benzethonium chloride, both inhibit human recombinant alpha7 and alpha4beta2 neuronal nicotinic acetylcholine receptors in Xenopus oocytes. Br. J. Pharmacol. 2001, 134, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak, K.; Haginaka, J.; Moaddel, R.; Wainer, I.W. Displacement and nonlinear chromatographic techniques in the investigation of interaction of noncompetitive inhibitors with an immobilized alpha3beta4 nicotinic acetylcholine receptor liquid chromatographic stationary phase. Anal. Chem. 2002, 74, 4618–4624. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.F.; Hilmas, C.; Santos, M.D.; Alkondon, M.; Maelicke, A.; Albuquerque, E.X. Unconventional ligands and modulators of nicotinic receptors. J. Neurobiol. 2002, 53, 479–500. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Picciotto, M.R. Nicotine receptors and depression: Revisiting and revising the cholinergic hypothesis. Trends Pharmacol. Sci. 2010, 31, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Philip, N.S.; Carpenter, L.L.; Tyrka, A.R.; Price, L.H. Nicotinic acetylcholine receptors and depression: A review of the preclinical and clinical literature. Psychopharmacology 2010, 212, 1–12. [Google Scholar] [CrossRef]
- Hirota, K.; Okawa, H.; Appadu, B.L.; Grandy, D.K.; Devi, L.A.; Lambert, D.G. Stereoselective interaction of ketamine with recombinant mu, kappa, and delta opioid receptors expressed in Chinese hamster ovary cells. Anesthesiology 1999, 90, 174–182. [Google Scholar] [CrossRef]
- Klein, M.E.; Chandra, J.; Sheriff, S.; Malinow, R. Opioid system is necessary but not sufficient for antidepressive actions of ketamine in rodents. Proc. Natl. Acad. Sci. USA 2020, 117, 2656–2662. [Google Scholar] [CrossRef]
- Yuan, Y.; Zaidi, S.A.; Elbegdorj, O.; Aschenbach, L.C.; Li, G.; Stevens, D.L.; Scoggins, K.L.; Dewey, W.L.; Selley, D.E.; Zhang, Y. Design, synthesis, and biological evaluation of 14-heteroaromatic-substituted naltrexone derivatives: Pharmacological profile switch from mu opioid receptor selectivity to mu/kappa opioid receptor dual selectivity. J. Med. Chem. 2013, 56, 9156–9169. [Google Scholar] [CrossRef]
- Williams, N.R.; Heifets, B.D.; Bentzley, B.S.; Blasey, C.; Sudheimer, K.D.; Hawkins, J.; Lyons, D.M.; Schatzberg, A.F. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol. Psychiatry 2019, 24, 1779–1786. [Google Scholar] [CrossRef]
- Williams, N.R.; Heifets, B.D.; Blasey, C.; Sudheimer, K.; Pannu, J.; Pankow, H.; Hawkins, J.; Birnbaum, J.; Lyons, D.M.; Rodriguez, C.I.; et al. Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism. Am. J. Psychiatry 2018, 175, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
| Name | Website * | Introduction | Reference |
|---|---|---|---|
| Virtual drug libraries | |||
| ZICN 15/20/22 | https://zinc15.docking.org, https://zinc20.docking.org https://cartblanche22.docking.org/ | Zinc 15/20 contains over 980 million compounds, of which 230 million are available for purchase. ZINC-22 focuses on make-on-demand compounds and has about 37 billion molecules in 2D and 4.5 billion in 3D. | [149,158,188] |
| ChEMBL | https://www.ebi.ac.uk/chembl/ | ChEMBL is a manually curated database of bioactive molecules with drug-like properties. It brings together chemical properties and bioactivity and includes 2.4 million compounds and 1.5 million assays. | [189] |
| Drugbank | https://go.drugbank.com/ | DrugBank is a web resource containing detailed drug, drug target, drug action, and drug interaction information about FDA-approved drugs. | [190] |
| Protein structure databases | |||
| EMDB | https://www.ebi.ac.uk/emdb/ | EMDB is a public repository for electron cryo-microscopy volume maps and tomograms of macromolecular complexes and subcellular structures, which contains more than 26,000 entries. | [191] |
| RCSB PDB | https://www.rcsb.org/ | RCSB PDB is an archive of 3D structure data for large biological molecules (proteins, DNA, and RNA). It contains more than 203,863 experimental structures and 1,068,577 computed structure models. | [116] |
| GPCRdb | https://gpcrdb.org/ | GPCRdb contains all human non-olfactory GPCRs (and >27,000 orthologs), G-proteins, and arrestins. It includes drugs, in-trial agents, and ligands, with activity and availability data. GPCRdb annotates all published GPCR structures and provides structure models. | [145] |
| Protein structure prediction programs | |||
| Alphafold2 (v2.3.0) | https://alphafold.com/ (database) https://github.com/deepmind/alphafold (program) | AlphaFold utilizes a machine learning method, enabling prediction of a protein’s 3D structure from its sequence. The database has released 200 million protein structure predictions, covering virtually all proteins. | [192] |
| Rosettafold | https://github.com/RosettaCommons/RoseTTAFold | Rosettafold accurately predicts protein structures and interactions using a 3-track neural network. The simultaneous processing of sequence, distance, and coordinate information by the three-track architecture assists with incorporating constraints and experimental information. | [193] |
| GPCRdb | https://gpcrdb.org/ | GPCRdb contains all human non-olfactory GPCRs (and >27,000 orthologs), G-proteins, and arrestins. It includes drugs, in-trial agents, and ligands, with activity and availability data. GPCRdb annotates all published GPCR structures and provides structure models. | [145] |
| Molecular docking tools | |||
| Dock (3.6) | https://dock.compbio.ucsf.edu/DOCK3.6/ | The DOCK algorithm addresses rigid body docking using a geometric matching algorithm to superimpose the ligand onto a negative image of the binding pocket. It is suitable for tackling large library screens. | [194] |
| Schrödinger Glide (2023-4) | https://www.schrodinger.com/products/glide | Glide is a commercial docking software from Schrödinger. It can perform flexible ligand docking. Glide offers multiple speed vs. accuracy options for scoring modes. | [195] |
| VirtualFlow | https://virtual-flow.org/ | VirtualFlow, a highly automated and versatile open-source platform, scales linearly with the number of CPUs that can prepare and efficiently screen ultra-large libraries of compounds. | [159] |
| V-SYNTHES | https://github.com/katritchlab/V-SYNTHES | A modular synthon-based approach—V-SYNTHES—for performing hierarchical screening. V-SYNTHES identifies the best scaffold–synthon combinations as seeds and iteratively elaborates these seeds to select complete molecules. | [160] |
| Name and Structure | Prototype | Target and Biased Selectivity | Discovery Method | Pharmacology | References |
|---|---|---|---|---|---|
TBG | Ibogaine | High selectivity for 5-HT2 receptors and weak or no opioid agonist activity. | Applying the principles of function-oriented synthesis. | Zebrafish toxicity assay: low cardiotoxicity, low lethality. HTR assays: not hallucinogenic. Transcranial 2-photon imaging: increased spine formation. Forced swim test behavior: significantly reduced immobility. Alcohol- and Heroin-seeking behavior: reduced both intakes. | [196] |
AAZ-A-154 | DMT | Binds 5-HT2AR but not the hallucinogenic conformation. | Using psychLight2-expressing cell line imaging screening platform. | HTR assays: not hallucinogenic. Forced swim test: rapid and long-lasting antidepressant-like effects after a single administration. Sucrose preference: reduced anhedonia in depressive mice for at least 12 days. | [197] |
IHCH-7086 | Lumateperone | 5-HT2AR β-arrestin-biased. | Based on the position of Psilocin, identifying crucial residues for β-arrestin. | HTR assays: failed to produce any HTR, even at high doses. Forced swim test and tail suspension test: significantly attenuated acute restraint stress-induced/corticosterone-induced depression-like behavior. | [25] |
R-69 | THP | Highly selective activation of 5-HT2AR and stimulated Gq signaling. | Docking a designed 75 million THP scaffold library against a 5-HT2AR model. | HTR assays: induced very low levels of HTRs, blocked the HTRs induced by LSD. Open field locomotion: did not possess locomotor-stimulating or reinforcing activity. Forced swim test and tail suspension test: antidepressant-like actions at least for 24 h. Sucrose preference: substantially increased. | [165] |
NLX-204 | NLX-101 | A 5-HT1AR ERK-biased agonist in PFC. | Characterization of NLX-101 specific activation of ERK signaling in the PFC/HPC. | ERK1/2 phosphorylation: dose-dependent activation in the rat PFC. Forced swim test: antidepressant-like effects. Sucrose preference: rapid effect, reversing sucrose consumption deficit. | [255] |
ZZL-7 | Sakura-6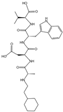 | nNOS PDZ domain in DRN. | Dissociating the SERT from nNOS reduced intercellular 5-HT concentration in DRN. | In vivo electrophysiology: increased firing frequency of serotonergic neurons. Forced swim test and tail suspension test: reduced immobility time. General activity: no effect and no other side effects. | [253] |
IHCH-7041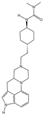 | Aripiprazole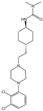 | A partial agonist at DRD2/3 and 5-HT1AR with negligible 5-HT2AR binding. | Based on TGAs adopting an unexpected “upside-down” posture in the 5-HT2AR binding site. | Locomotor responses: displayed antipsychotic-like effects. Forced swim test and tail suspension test: Significantly attenuated immobility. Novel object recognition: significantly attenuated deficits. Morris water maze: restored their spatial navigation ability. In vivo electrophysiology: inhibited glutamatergic transmission through 5-HT1AR. | [233] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, H.; Wang, X.; Chi, J.; Chen, H.; Liu, Y.; Yang, J.; Yu, J.; Ruan, Y.; Xiang, X.; Pi, J.; et al. Exploring Novel Antidepressants Targeting G Protein-Coupled Receptors and Key Membrane Receptors Based on Molecular Structures. Molecules 2024, 29, 964. https://doi.org/10.3390/molecules29050964
Yao H, Wang X, Chi J, Chen H, Liu Y, Yang J, Yu J, Ruan Y, Xiang X, Pi J, et al. Exploring Novel Antidepressants Targeting G Protein-Coupled Receptors and Key Membrane Receptors Based on Molecular Structures. Molecules. 2024; 29(5):964. https://doi.org/10.3390/molecules29050964
Chicago/Turabian StyleYao, Hanbo, Xiaodong Wang, Jiaxin Chi, Haorong Chen, Yilin Liu, Jiayi Yang, Jiaqi Yu, Yongdui Ruan, Xufu Xiang, Jiang Pi, and et al. 2024. "Exploring Novel Antidepressants Targeting G Protein-Coupled Receptors and Key Membrane Receptors Based on Molecular Structures" Molecules 29, no. 5: 964. https://doi.org/10.3390/molecules29050964
APA StyleYao, H., Wang, X., Chi, J., Chen, H., Liu, Y., Yang, J., Yu, J., Ruan, Y., Xiang, X., Pi, J., & Xu, J.-F. (2024). Exploring Novel Antidepressants Targeting G Protein-Coupled Receptors and Key Membrane Receptors Based on Molecular Structures. Molecules, 29(5), 964. https://doi.org/10.3390/molecules29050964








