Synthesis and Anti-Inflammatory Activity of Ferulic Acid-Sesquiterpene Lactone Hybrids
Abstract
1. Introduction
2. Results
2.1. Chemistry
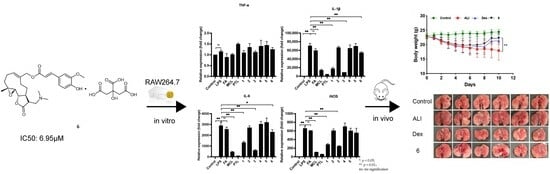
2.2. Assessment of Cytotoxicity
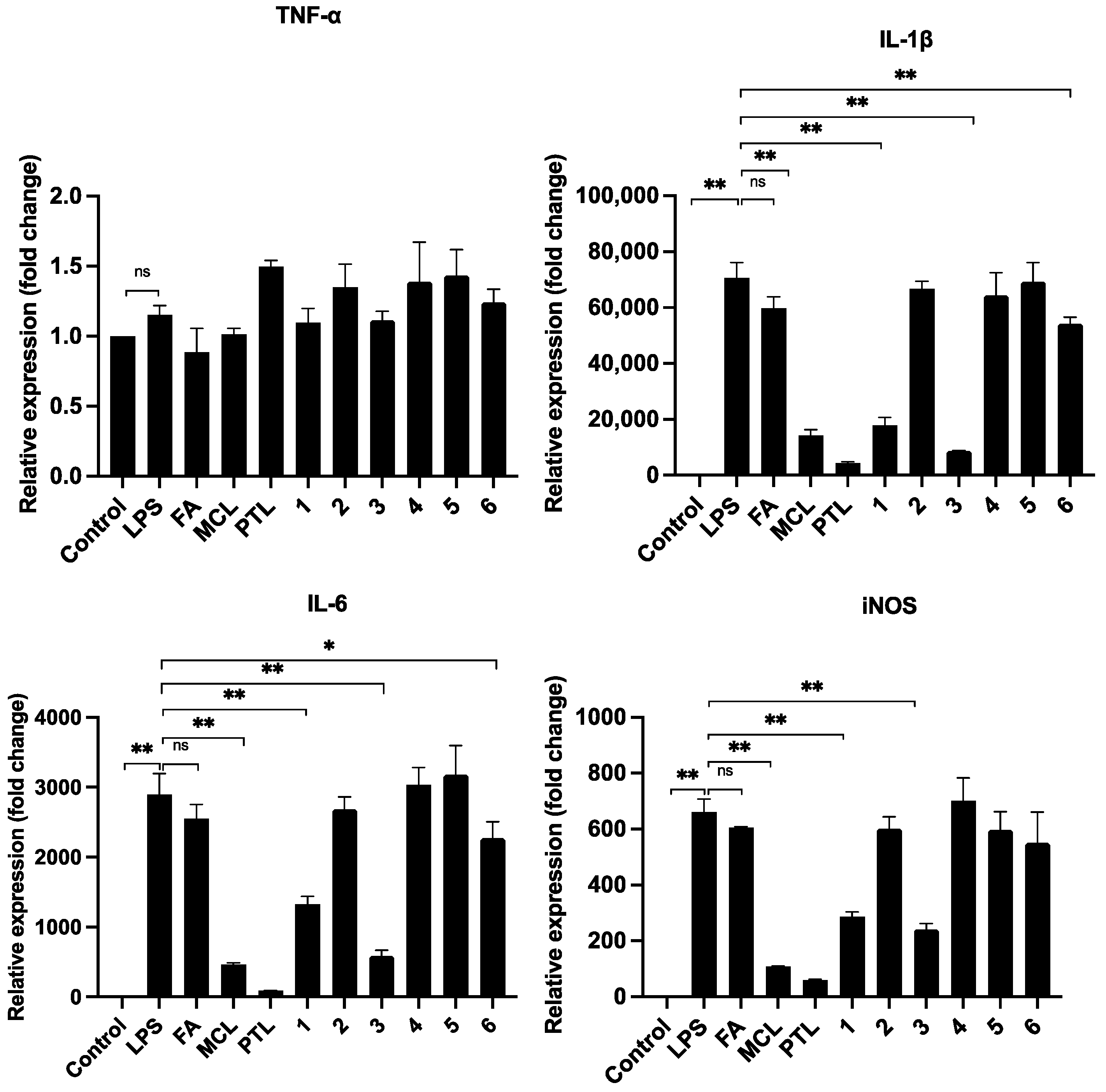
2.3. Anti-Inflammation of Compounds in RAW264.7 Cells
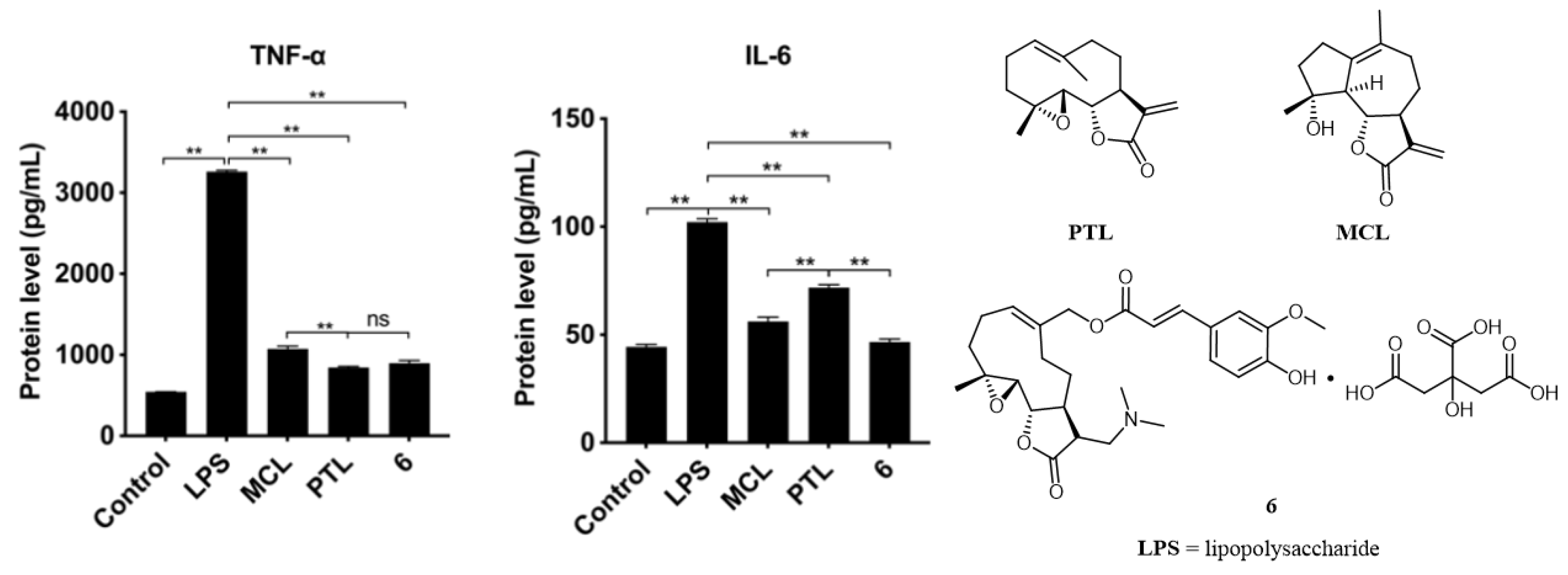
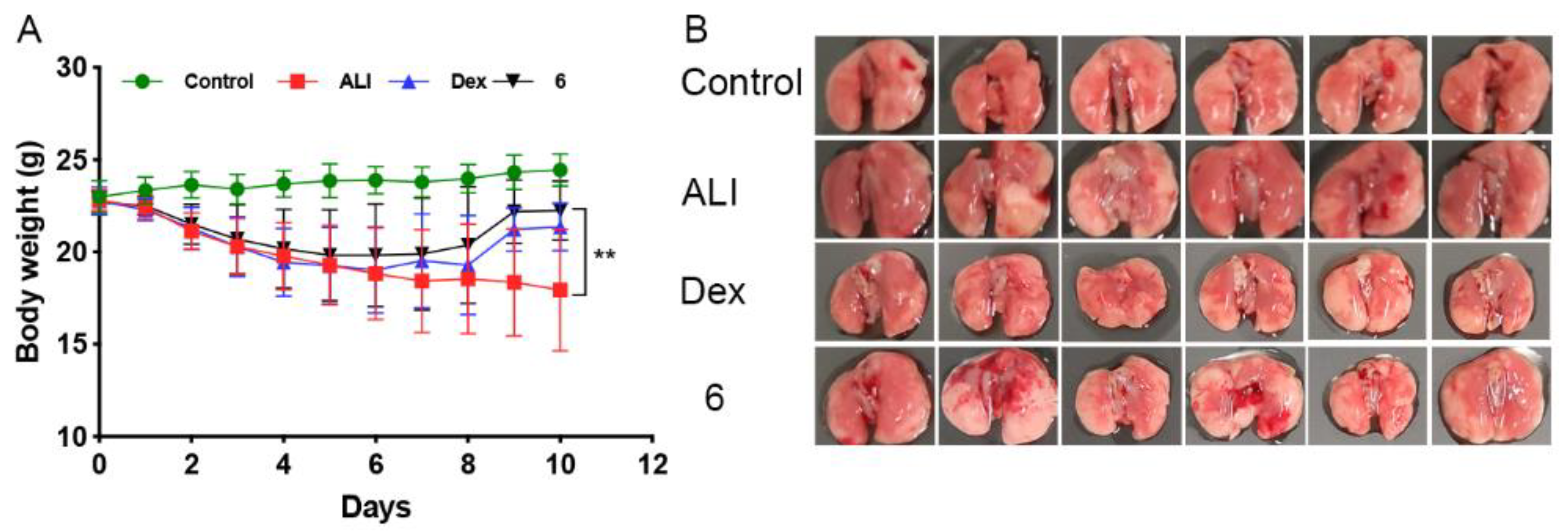
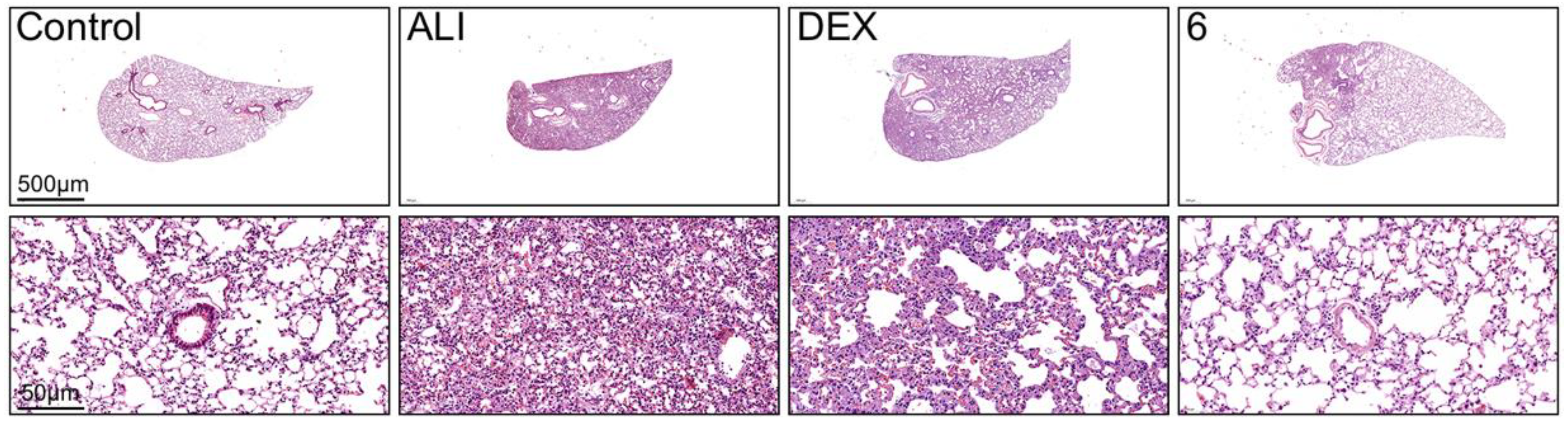
2.4. Compound 6 Mitigated the Inflammation of BLM-Induced Mice
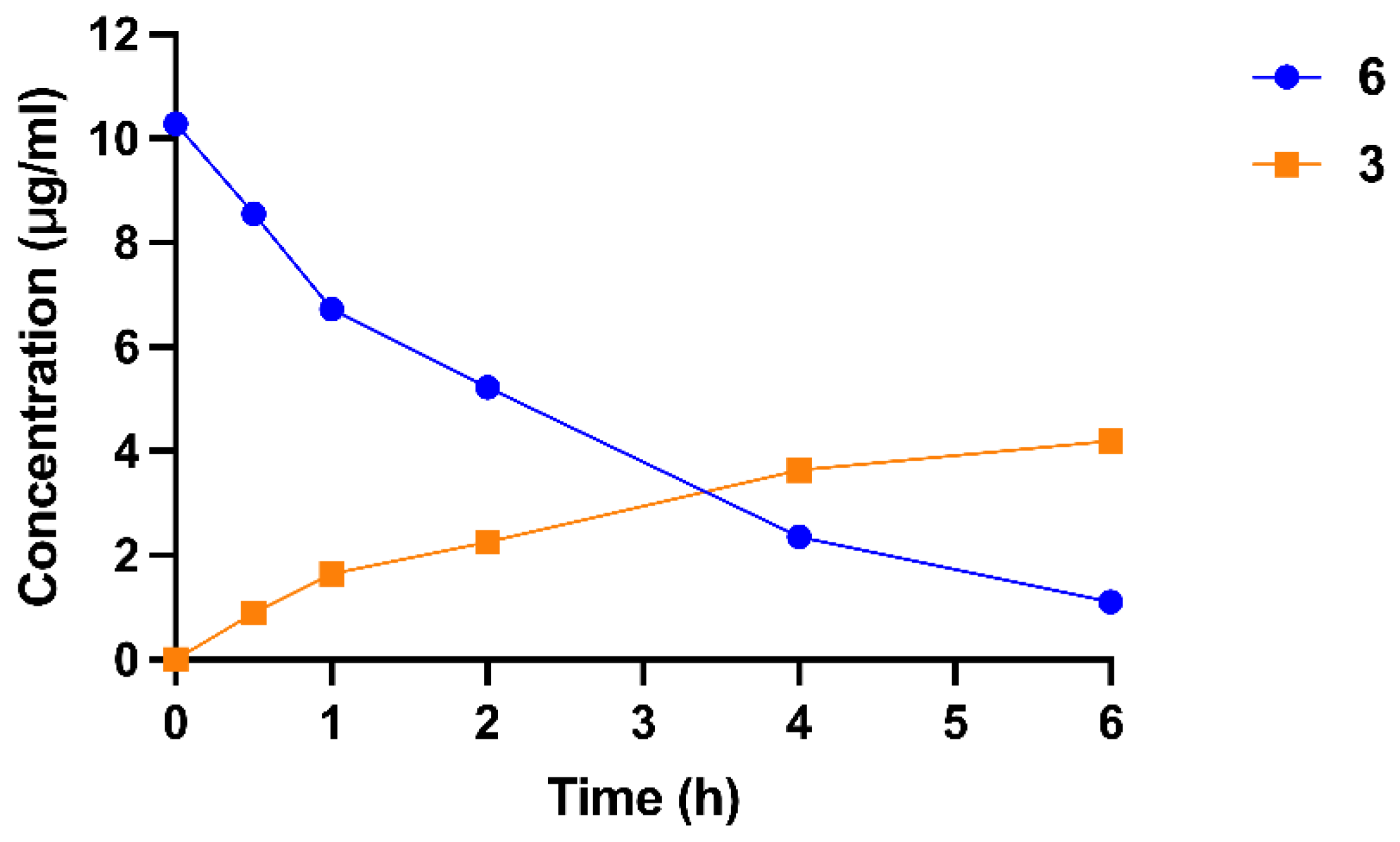
2.5. Transformation from Prodrug to Active Drug
3. Experimental Procedure and Methods
3.1. Chemistry
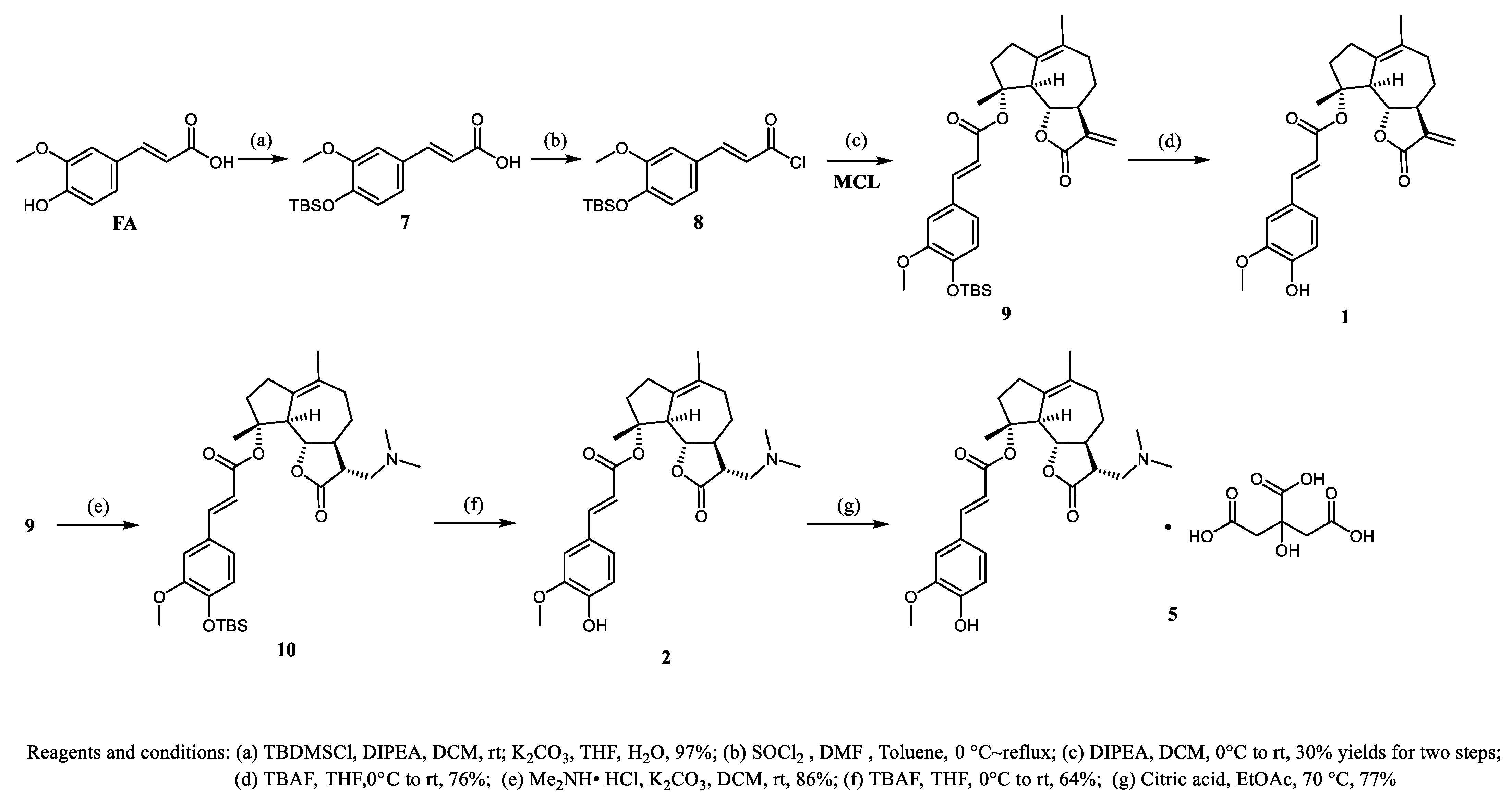
3.1.1. Compound 9
- (3aS,9R,9bS)-6,9-dimethyl-3-methylene-2-oxo-2,3,3a,4,5,7,8,9,9a,9b-decahydroazuleno[4,5-b]furan-9-yl (E)-3-(4-((tert-butyldimethylsilyl)oxy)-3-methoxyphenyl)acrylate (9). To a solution of compound 7 (5 mmol) in Toluene (2 mL) was added SOCl2 (2 mL) and DMF (a drop) at 0 °C under N2 atmosphere. Then the mixture was stirred at 80 °C for 1 h. Removal of the organic layer under vacuum gave crude acyl chloride.
3.1.2. Compound 1
- (3aS,9R,9bS)-6,9-dimethyl-3-methylene-2-oxo-2,3,3a,4,5,7,8,9,9a,9b-decahydroazuleno[4,5-b]furan-9-yl (E)-3-(4-hydroxy-3-methoxyphenyl)acrylate (1). To a solution of compound 9 (0.9 mmol) in THF (3 mL) was added TBAF (1.8 mmol) at 0 °C. Then the reaction mixture was stirred at room temperature for 40 min. The reaction was quenched with saturated ammonium chloride solution and extracted with EtOAc (3 × 15 mL). The combined organic layers were washed with saturated brine, dried over Na2SO4, and concentrated to give an oily crude product, which was purified on a silica gel column (hexanes: EtOAc = 3:1) to yield compound 1 as a yellow oil. Yield: 76%; 1H NMR (400 MHz, CDCl3): δ = 7.66 (d, J = 15.9 Hz, 1H), 7.09–7.07 (m, 2H), 6.89 (d, J = 8.7 Hz, 1H), 6.27–6.21 (m, 2H), 5.49 (d, J = 3.1 Hz, 1H), 3.92 (s, 3H), 3.86 (t, J = 10.1 Hz, 1H), 3.15 (d, J = 10.2 Hz, 1H), 2.75–2.70 (m, 1H), 2.63–2.58 (m, 1H), 2.51–2.45 (m, 1H), 2.28 (s, 3H), 2.12–2.09 (m, 1H), 2.04–1.95 (m, 1H), 1.72 (d, J = 1.8 Hz, 3H), 1.59 (s, 3H), 1.43–1.33 (m, 1H), 1.28–1.21 (m, 1H).13C NMR (100 MHz, CDCl3): δ = 170.4, 166.6, 147.7, 146.7, 144.7, 139.5, 131.6, 129.9, 127.3, 123.1, 118.8, 117.0, 114.6, 109.6, 88.5, 83.1, 57.3, 55.9, 49.9, 36.7, 35.0, 30.6, 25.9, 24.2, 18.5. HRMS (ESI): m/z [M + H]+ calcd for C25H29O6: 425.1959; found: 425.1957.
3.1.3. Compound 10
- (3R,3aS,9R,9bS)-3-((dimethylamino)methyl)-6,9-dimethyl-2-oxo-2,3,3a,4,5,7,8,9,9a,9b-decahydroazuleno[4,5-b]furan-9-yl (E)-3-(4-((tert-butyldiphenylsilyl)oxy)-3-methoxyphenyl)acrylate (10). Compound 9 (0.6 mmol), K2CO3 (9 mmol) and Me2NH·HCl (4.8 mmol) were added to dry DCM (5 mL) at room temperature and the resulting solution was stirred at this temperature for 5 h. The solid in the mixture was filtered off, and the resulting solution was concentrated under reduced pressure. The residue was dissolved in CH2Cl2, and then washed with water. The organic layer was dried with Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using ethyl acetate−petroleum ether as the eluent to give the desired product 10 as a yellow oil. Yield: 86%; 1H NMR (400 MHz, CDCl3):δ = 7.66 (d, J = 15.9 Hz, 1H), 7.09–7.01 (m, 2H), 6.81 (d, J = 8.1 Hz, 1H), 6.24 (d, J = 15.9 Hz, 1H), 3.87–3.80 (m, 4H), 3.07 (d, J = 9.0 Hz, 1H), 2.74 (dd, J = 12.9, 4.8 Hz, 1H), 2.64–2.54 (m, 2H), 2.50–2.35 (m, 2H), 2.26 (s, 6H), 2.19–1.89 (m, 5H), 1.70 (s, 3H), 1.58 (s, 3H), 1.37–1.13 (m, 2H), 0.98 (s, 9H), 0.16 (s, 6H).13C NMR (100 MHz, CDCl3): δ = 177.5, 166.6, 151.1, 147.1, 144.7, 131.9, 129.9, 128.7, 122.1, 120.9, 117.5, 111.1, 88.6, 82.5, 58.5, 56.9, 55.4, 51.5, 46.0, 45.9, 44.94, 36.8, 35.4, 30.4, 27.4, 25.7, 23.9, 18.6, 18.5, −4.6. HRMS (ESI): m/z [M + H]+ calcd for chemical formula: C33H50NO6Si: 584.3402; found: 584.3399.
3.1.4. Compound 2
- (3R,3aS,9R,9aS,9bS)-3-((dimethylamino)methyl)-6,9-dimethyl-2-oxo-2,3,3a,4,5,7,8,9,9a,9b-decahydroazuleno[4,5-b]furan-9-yl (E)-3-(4-hydroxy-3-methoxyphenyl)acrylate (2). To a solution of compound 10 (0.6 mmol) in THF (3 mL) was added TBAF at 0 °C. Then the reaction mixture was stirred at room temperature for 40 min. The reaction was quenched with saturated ammonium chloride solution and extracted with EtOAc (3 × 15 mL). The combined organic layers were washed with saturated brine, dried over Na2SO4, and concentrated to give an oily crude product, which was purified on a silica gel column (CH2Cl2: MeOH = 20:1) to yield compound 2 as a yellow solid. Yield: 64%. mp: 139−141 °C. 1H NMR (400 MHz, CDCl3):δ = 7.64 (d, J = 15.9 Hz, 1H), 7.06 (d, J = 8.6 Hz, 2H), 6.86 (d, J = 7.7 Hz, 1H), 6.22 (d, J = 15.9 Hz, 1H), 3.90 (s, 3H), 3.84 (t, J = 10.1 Hz, 1H), 3.05 (d, J = 10.0 Hz, 1H), 2.74 (dd, J = 13.0, 4.9 Hz, 1H), 2.67–2.53 (m, 2H), 2.50–2.35 (m, 2H), 2.27–1.96 (m, 12H), 1.69 (s, 3H), 1.57 (s, 3H), 1.38–1.19 (m, 2H). 13C NMR (100 MHz, CDCl3): δ = 177.5, 166.7, 147.8, 146.8, 144.7, 131.9, 129.8, 127.1, 122.9, 116.9, 114.7, 109.8, 88.6, 82.5, 58.2, 56.9, 55.9, 51.4, 45.9, 44.9, 36.7, 35.3, 30.4, 27.3, 23.9, 18.5. HRMS (ESI): m/z [M + H]+ calcd for chemical formula: C27H36NO6: 470.2537; found: 470.2538.
3.1.5. Compound 5
- (3R,3aS,9R,9aS,9bS)-3-((dimethylamino)methyl)-6,9-dimethyl-2-oxo-2,3,3a,4,5,7,8,9,9a,9b-decahydroazuleno[4,5-b]furan-9-yl (E)-3-(4-hydroxy-3-methoxyphenyl)acrylate 2-hydroxypropane-1,2,3-tricarboxylate (5). To a solution of compound 2 (1.0 mmol) in EtOAc (10 mL) was added citric acid (1.0 mmol) at room temperature. Then the reaction mixture was stirred at room temperature for 30 min. The white solid in the mixture was filtered off, and washed with EtOAc to obtain compound 5. Yield: 77%. mp: 96−98 °C. 1H NMR (400 MHz, DMSO-d6): 9.58 (s, 1H), 7.57 (d, J = 15.8 Hz, 1H), 7.28 (d, J = 1.8 Hz, 1H), 7.08 (dd, J = 8.2, 1.8 Hz, 1H), 6.78 (d, J = 8.1 Hz, 1H), 6.31 (d, J = 15.8 Hz, 1H), 4.0–3.95 (m, 1H), 3.81 (s, 3H), 3.07 (d, J = 9.8 Hz, 1H), 2.83–2.76 (m, 3H), 2.65–2.49 (m, 4H), 2.48–2.28 (m, 8H), 2.27–2.00 (m, 5H), 1.95–1.87 (m, 1H), 1.68 (s, 3H), 1.49 (s, 3H), 1.36–1.29 (m, 1H). 13C NMR (100 MHz, DMSO-d6): δ = 177.5, 176.9, 170.8, 166.4, 149.7, 148.4, 145.4, 132.1, 130.1, 126.2, 123.5, 116.4, 115.9, 111.6, 88.7, 82.2, 71.9, 57.4, 56.3, 56.1, 50.9, 45.4, 44.4, 43.6, 36.8, 35.2, 30.3, 26.8, 24.3, 21.2, 14.6. HRMS (ESI): m/z [M + H]+ calcd for chemical formula: C27H36NO13: 470.2537; found: 470.2539.
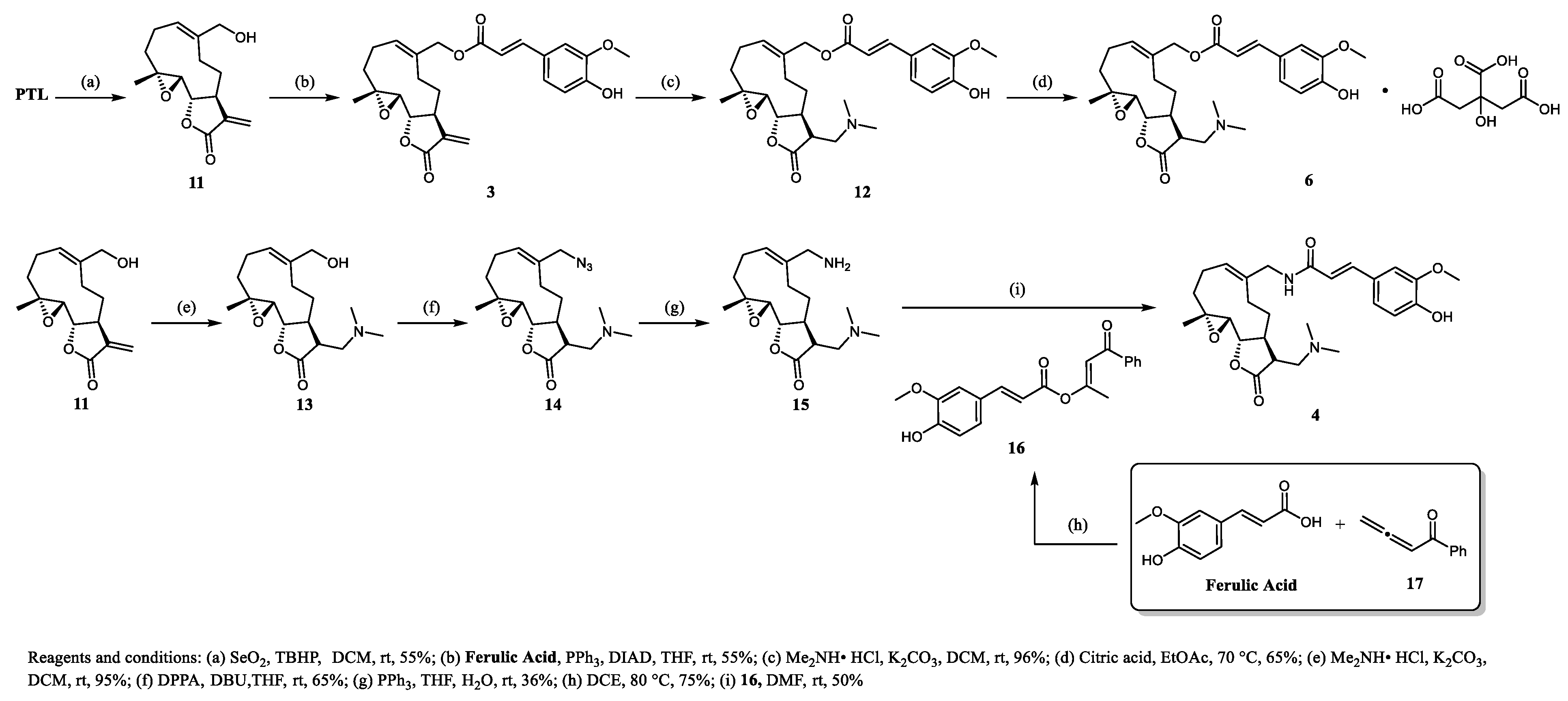
3.1.6. Compound 3
- ((1aR,7aS,10aS,10bS,E)-1a-methyl-8-methylene-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2′,3′:9,10]cyclodeca[1,2-b]furan-5-yl)methyl (E)-3-(4-hydroxy-3-methoxyphenyl)acrylate (3). To a solution of MMB (5 mmol), Ferulic acid (7.5 mmol) and PPh3 (7.5 mmol) in anhydrous THF (50 mL) was added DIAD (5 mmol) at room temperature under N2 atmosphere. The reaction was stirred for 4h at room temperature. The reaction was quenched with saturated ammonium chloride solution and extracted with EtOAc (3 × 30 mL). The combined organic layers were washed with saturated brine, dried over Na2SO4, and concentrated. The crude product was purified on a silica gel column (PE: EA = 2:1) to yield compound 3 as a yellow solid. Yield: 55%; mp: 96−98 °C. 1H NMR (400 MHz, CDCl3) δ = 7.60 (d, J = 15.9 Hz, 1H), 7.05 (dd, J = 8.2, 1.8 Hz, 1H), 6.99 (d, J = 1.8 Hz, 1H), 6.91 (d, J = 8.2 Hz, 1H), 6.24 (dd, J = 9.7, 6.2 Hz, 2H), 6.03 (s, 1H), 5.73 (t, J = 8.1 Hz, 1H), 5.55 (d, J = 3.2 Hz, 1H), 4.76 (d, J = 12.5 Hz, 1H), 4.59 (d, J = 12.5 Hz, 1H), 3.92 (s, 3H), 3.87 (t, J = 9.3 Hz, 1H), 3.01 (m, J = 9.1, 4.7 Hz, 1H), 2.90 (d, J = 9.4 Hz, 1H), 2.47–2.16 (m, 6H), 1.72–1.65 (m, 1H), 1.55 (s, 3H), 1.12 (t, J = 12.6 Hz, 1H).
3.1.7. Compound 12
- ((1aR,7aS,8R,10aS,10bS,E)-8-((dimethylamino)methyl)-1a-methyl-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2′,3′:9,10]cyclodeca[1,2-b]furan-5-yl)methyl (E)-3-(4-hydroxy-3-methoxyphenyl)acrylate (12). Compound 3 (1 mmol), K2CO3 (15 mmol) and Me2NH·HCl (8 mmol) were added to dry DCM (10 mL) at room temperature and the resulting solution was stirred at this temperature for 5 h. The solid in the mixture was filtered off, and the resulting solution was concentrated under reduced pressure. The residue was dissolved in CH2Cl2, and then washed with water. The organic layer was dried with Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using ethyl acetate−petroleum ether as the eluent to give the desired product 12 as a yellow oil. Yield: 96%; 1H NMR (400 MHz, CDCl3): δ = 7.62 (d, J = 15.9 Hz, 1H), 7.08 (dd, J = 8.2, 1.8 Hz, 1H), 7.03–7.02 (m, 1H), 6.92 (d, J = 8.2 Hz, 1H), 6.30 (d, J = 15.9 Hz, 1H), 5.66 (t, J = 8.0 Hz, 1H), 4.87 (d, J = 12.8 Hz, 1H), 4.66 (d, J = 12.9 Hz, 1H), 3.92 (s, 3H), 3.90–3.84 (m, 2H), 2.81 (d, J = 9.4 Hz, 1H), 2.78–2.74 (m, 1H), 2.64 (dd, J = 12.9, 5.7 Hz, 1H), 2.51–2.28 (m, 6H), 2.24 (s, 6H), 2.18–2.12 (m, 2H), 1.59–1.54 (m, 4H), 1.10 (t, J = 12.7 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ = 176.9, 166.8, 148.1, 146.8, 145.2, 135.9, 128.5, 126.8, 123.1, 114.9, 114.7, 109.3, 81.2, 66.2, 63.8, 59.8, 58.2, 55.9, 45.7, 44.4, 42.9, 36.9, 26.9, 24.5, 23.7, 17.9. HRMS (ESI): m/z [M + H]+ calcd for chemical formula: C27H36NO7: 486.2486; found: 486.2485.
3.1.8. Compound 6
- ((1aR,7aS,8R,10aS,10bS,E)-8-((dimethylamino)methyl)-1a-methyl-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2′,3′:9,10]cyclodeca[1,2-b]furan-5-yl)methyl (E)-3-(4-hydroxy-3-methoxyphenyl)acrylate 2-hydroxypropane-1,2,3-tricarboxylate (6). To a solution of compound 12 (1 mmol) in EtOAc (10 mL) was added citric acid (1 mmol) at room temperature. Then the reaction mixture was stirred at 70 °C for 8 h. The white solid in the mixture was filtered off, and washed with EtOAc to obtain compound 6. Yield: 65%. 1H NMR (400 MHz, D2O): δ = 7.49 (d, J = 15.9 Hz, 1H), 7.08 (d, J = 1.7 Hz, 1H), 7.01 (dd, J = 8.2, 1.6 Hz, 1H), 6.82 (d, J = 8.2 Hz, 1H), 6.27 (d, J = 16.0 Hz, 1H), 5.56 (t, J = 8.0 Hz, 1H), 4.59 (d, J = 13.1 Hz, 1H), 4.43 (d, J = 13.0 Hz, 1H), 4.21 (t, J = 9.6 Hz, 1H), 3.78 (s, 3H), 3.45–3.39 (m, 1H), 3.28 (dd, J = 13.2, 3.5 Hz, 1H), 3.16–3.06 (m, 1H), 2.92–2.84 (m, 7H), 2.79–2.63 (m, 4H), 2.38–2.12 (m, 4H), 2.09–1.95 (m, 4H), 1.76–1.70 (m, 1H), 1.49 (s, 3H), 1.16 (t, J = 7.2 Hz, 1H), 0.93 (t, J = 12.2 Hz, 1H). 13C NMR (100 MHz, D2O): δ = 177.8, 177.4, 174.0, 168.7, 148.2, 147.7, 146.2, 134.4, 129.5, 126.5, 123.2, 115.6, 114.2, 111.2, 82.2, 73.5, 66.6, 63.4, 62.7, 61.7, 55.8, 55.5, 43.4, 42.4, 41.3, 35.7, 24.9, 24.0, 22.9, 20.5, 16.6, 13.2. HRMS (ESI): m/z [M + H]+ calcd for Chemical Formula: C27H36NO7: 486.2486; found: 486.2481.
3.1.9. Compound 13
- (1aR,7aS,8R,10aS,10bS,E)-8-((dimethylamino)methyl)-5-(hydroxymethyl)-1a-methyl-2,3,6,7,7a,8,10a,10b-octahydrooxireno[2′,3′:9,10]cyclodeca[1,2-b]furan-9(1aH)-one (13). MMB (3 mmol), K2CO3 (45 mmol) and Me2NH·HCl (24 mmol) were added to dry DCM (25 mL) at room temperature and the resulting solution was stirred at this temperature for 5 h. The solid in the mixture was filtered off, and the resulting solution was concentrated under reduced pressure. The residue was dissolved in CH2Cl2, and then washed with water. The organic layer was dried with Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using ethyl acetate−petroleum ether as the eluent to give the desired product 13 as a yellow oil. Yield: 95%; 1H NMR (400 MHz, CDCl3): δ = 5.57 (t, J = 8.0 Hz, 1H), 4.09 (dd, J = 31.7, 13.1 Hz, 2H), 3.84 (t, J = 9.0 Hz, 1H), 2.81–2.78 (m, 1H), 2.74–2.68 (m, 1H), 2.65–2.55 (m, 1H), 2.49–2.23 (m, 6H), 2.23–2.20 (m, 7H), 2.14–2.07 (m, 2H), 1.62–1.54 (m, 1H), 1.51 (s, 3H), 1.06 (t, J = 12.8 Hz, 1H). 13C NMR (100 MHz, CDCl3): δ = 176.9, 140.8, 127.0, 81.4, 65.8, 63.9, 59.9, 57.5, 45.6, 44.0, 42.0, 36.9, 27.3, 25.6, 23.6, 17.8.
3.1.10. Compound 14
- (1aR,7aS,8R,10aS,10bS,E)-5-(azidomethyl)-8-((dimethylamino)methyl)-1a-methyl-2,3,6,7,7a,8,10a,10b-octahydrooxireno[2′,3′:9,10]cyclodeca[1,2-b]furan-9(1aH)-one (14). To a solution of compound 13 (1 mmol) and DBU (2 mmol) in THF (10 mL) was added DPPA at room temperature. The reaction mixture was stirred at room temperature for 8h. Removal of the organic layer under vacuum gave a yellow oil. Then the crude product was added to EtOAc (10 mL)/H2O (10 mL) at room temperature. The reaction was extracted with EtOAc (3 × 30 mL). The combined organic layers were washed with saturated brine, dried over Na2SO4, and concentrated. The crude product was purified on a silica gel column (PE: EA = 1:1) to yield compound 14 as a colorless oil. Yield: 65%. 1H NMR (400 MHz, CDCl3): δ = 5.60 (t, J = 8.1 Hz, 1H), 3.95 (d, J = 13.2 Hz, 1H), 3.87–3.83 (m, 2H), 2.76–2.56 (m, 2H), 2.61–2.56 (m, 1H), 2.47–2.35 (m, 3H), 2.34–2.08 (m, 11H), 1.64–1.56 (m, 1H), 1.54 (s, 3H), 1.07 (t, J = 12.6 Hz, 1H).13C NMR (100 MHz, CDCl3): δ = 176.8, 135.7, 129.6, 81.0, 63.9, 59.8, 58.1, 55.4, 45.6, 44.1, 42.6, 36.9, 26.6, 24.4, 23.7, 17.8. HRMS (ESI): m/z [M + H]+ calcd for chemical formula: C17H27N4O3: 335.2083; found: 335.2085.
3.1.11. Compound 15
- (1aR,7aS,8R,10aS,10bS,E)-5-(aminomethyl)-8-((dimethylamino)methyl)-1a-methyl-2,3,6,7,7a,8,10a,10b-octahydrooxireno[2′,3′:9,10]cyclodeca[1,2-b]furan-9(1aH)-one (15). To a solution of compound 14 (0.4 mmol) in THF (3 mL) was added PPh3 (0.44 mmol) and H2O (45 µL). The reaction mixture was stirred at room temperature overnight. Removal of the organic layer under vacuum gave a yellow oil. The crude product was purified on a silica gel column (DCM: CH2Cl2 = 6:1) to yield compound 15 as a colorless oil. Yield: 36%. 1H NMR (400 MHz, CDCl3): δ = 5.50 (t, J = 7.9 Hz, 1H), 3.82 (d, J = 9.5 Hz, 1H), 3.45 (s, 1H), 3.33 (d, J = 6.3 Hz, 1H), 2.80–2.71 (m, 2H), 2.57 (dd, J = 12.9, 6.5 Hz, 1H), 2.49 (s, 2H), 2.44–2.34 (m, 3H), 2.28–2.25 (m, 3H), 2.22 (s, 6H), 2.18–2.08 (m, 3H), 1.53 (s, 3H), 1.06 (t, J = 12.3 Hz, 1H).13C NMR (100 MHz, CDCl3): δ = 176.9, 141.2, 124.8, 81.2, 63.7, 60.0, 58.4, 45.7, 45.4, 44.3, 43.2, 37.1, 26.8, 24.6, 23.7, 17.9. HRMS (ESI): m/z [M + H]+ calcd for chemical formula: C17H29N2O3: 309.2173; found: 309.2174.
3.1.12. Compound 16
- (E)-4-oxo-4-phenylbut-2-en-2-yl (E)-3-(4-hydroxy-3-methoxyphenyl)acrylate (16). To a solution of ferulic acid (1.1 mmol) in DCE (5 mL) was added allenone 17 (1 mmol) at room temperature. The reaction mixture was allowed to stir at 80 °C until the allenone 17 was fully consumed. The reaction mixture was purified by flash silica gel chromatography (PE: EA = 5:1) to afford the compound 16 as a brown oil. Yield: 75%. 1H NMR (400 MHz, CDCl3): δ = 7.95–7.92 (m, 2H), 7.74 (d, J = 15.9 Hz, 1H), 7.57–7.50 (m, 1H), 7.47–7.43 (m, 2H), 7.13–7.10 (m, 1H), 7.06 (d, J = 1.6 Hz, 1H), 6.95–6.90 (m, 2H), 6.36 (d, J = 15.9 Hz, 1H), 6.17–6.09 (m, 1H), 3.92 (s, 3H), 2.48 (d, J = 0.8 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ = 190.4, 164.5, 164.2, 148.6, 147.3, 146.9, 138.7, 132.7, 128.5, 128.1, 126.4, 123.5, 114.9, 113.9, 113.3, 109.6, 55.9, 19.1.; HRMS (ESI): m/z [M + H]+ calcd for chemical formula: C20H18NaO5: 361.1046; found: 361.1045.
3.1.13. Compound 4
- (E)-N-(((1aR,7aS,8R,10aS,10bS,E)-8-((dimethylamino)methyl)-1a-methyl-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2′,3′:9,10]cyclodeca[1,2-b]furan-5-yl)methyl)-3-(4-hydroxy-3-methoxyphenyl)acrylamide (4). To a solution of compound 15 (2 mmol) in dry DMF (10 mL) was added compound 16 at room temperature. The reaction mixture was allowed to stir at room temperature until the compound 16 was fully consumed. The reaction was quenched with brine and extracted with EtOAc (3 × 15 mL). The combined organic layers were washed with saturated brine, dried over Na2SO4, and concentrated. The crude product was purified on a silica gel column (DCM: CH2Cl2 = 15:1) to yield compound 4 as a yellow solid. mp: 132−134 °C. Yield: 50%. 1H NMR (400 MHz, DMSO-d6): δ = 9.40 (s, 1H), 8.00 (t, J = 5.8 Hz, 1H), 7.34 (d, J = 15.7 Hz, 1H), 7.13 (d, J = 1.8 Hz, 1H), 6.99 (dd, J = 8.2, 1.8 Hz, 1H), 6.78 (d, J = 8.1 Hz, 1H), 6.49 (d, J = 15.7 Hz, 1H), 5.41–5.29 (m, 1H), 4.01 (t, J = 9.5 Hz, 1H), 3.90–3.84 (m, 1H), 3.80 (s, 3H), 2.72 (d, J = 9.5 Hz, 1H), 2.64–2.55 (m, 3H), 2.47–2.19 (m, 5H), 2.17 (s, 6H), 2.08–2.00 (m, 3H), 1.64–1.54 (m, 1H), 1.47 (s, 3H), 0.94–0.84 (m, 1H). 13C NMR (100 MHz, DMSO-d6): δ = 177.8, 165.7, 148.7, 148.3, 139.6, 138.3, 126.9, 124.1, 121.9, 119.3, 116.1, 111.2, 81.1, 63.7, 60.2, 58.7, 55.9, 45.9, 43.9, 42.8, 42.3, 37.4, 26.2, 24.9, 23.6, 17.9. HRMS (ESI): m/z [M + H]+ calcd for chemical formula: C27H37N2O6: 485.2646; found: 485.2641.
3.1.14. Compound 18
- (E)-N-(((1aR,7aS,8R,10aS,10bS,E)-8-((dimethylamino)methyl)-1a-methyl-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2′,3′:9,10]cyclodeca[1,2-b]furan-5-yl)methyl)-3-(4-hydroxy-3-methoxyphenyl)acrylamide 2-hydroxypropane-1,2,3-tricarboxylate (18). To a solution of compound 4 (2.0 mmol) in EtOAc (20 mL) was added citric acid (2.0 mmol) at room temperature. Then the reaction mixture was stirred at room temperature for 30 min. The white solid in the mixture was filtered off, and washed with EtOAc to obtain compound 18. Yield: 70%. mp: 113−115 °C. 1H NMR (400 MHz, DMSO-d6): 9.42 (s, 1H), 8.02 (t, J = 5.7 Hz, 1H), 7.35 (d, J = 15.7 Hz, 1H), 7.13 (d, J = 1.8 Hz, 1H), 6.99 (dd, J = 8.2, 1.8 Hz, 1H), 6.79 (d, J = 8.1 Hz, 1H), 6.49 (d, J = 15.7 Hz, 1H), 5.36 (t, J = 7.4 Hz, 1H), 4.06–3.98 (m, 2H), 3.93–3.88 (m, 1H), 3.80 (s, 3H), 3.78–3.73 (m, 1H), 2.79–2.62 (m, 4H), 2.62–2.54 (m, 1H), 2.51–2.49 (m, 2H), 2.47–2.32 (m, 3H), 2.28 (s, 6H), 2.25–2.21 (m, 1H), 2.05–2.02 (m, 3H), 1.66–1.55 (m, 1H), 1.47 (s, 3H), 0.95–0.82 (m, 1H). 13C NMR (100 MHz, DMSO-d6): δ = 177.63, 177.08, 170.82, 165.79, 148.74, 148.28, 139.68, 138.27, 126.88, 124.42, 122.02, 119.28, 116.13, 111.22, 81.21, 71.83, 63.56, 60.23, 58.22, 55.98, 45.59, 44.58, 43.74, 42.78, 42.41, 37.37, 26.13, 24.75, 23.65, 21.24, 17.98, 14.57. HRMS (ESI): m/z [M + H]+ calcd for chemical formula: C27H37N2O6: 485.2646; found: 485.2643.
3.2. Cell Culture and Treatment
3.3. Cell Viability Assay
3.4. Isolation of Total RNA and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
3.5. Enzyme-Linked Immunosorbent Assay (ELISA)
3.6. Animals
3.7. Mice Survival Rates and Body Weight
3.8. Studies on the Release of Prodrugs into Active Drugs
3.9. Aqueous Solubility Measurement
4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiss, U. Inflammation. Nature 2008, 454, 427. [Google Scholar] [CrossRef]
- Van der Poll, T.; van de Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Kolb, R.; Sutterwala, F.S.; Zhang, W. Obesity and cancer: Inflammation bridges the two. Curr. Opin. Pharmacol. 2016, 29, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.P.; Bernard, G.R. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet 2007, 369, 1553–1564. [Google Scholar] [CrossRef]
- Qian, J.; Chen, X.; Shu, S.; Zhang, W.; Fang, B.; Chen, X.; Zhao, Y.; Liu, Z.; Liang, G. Design and synthesis novel di-carbonyl analogs of curcumin (DACs) act as potent anti-inflammatory agents against LPS-induced acute lung injury (ALI). Eur. J. Med. Chem. 2019, 167, 414–425. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, Y.; Zhang, W.; Zhan, L.; Yu, Y.; Chen, Y.; Jia, W.; Liu, Z.; Qian, J.; Zhang, Y.; et al. Base promoted synthesis of novel indole-dithiocarbamate compounds as potential anti-inflammatory therapeutic agents for treatment of acute lung injury. Eur. J. Med. Chem. 2019, 171, 54–65. [Google Scholar] [CrossRef]
- Yu, P.; Dong, L.; Zhang, Y.; Chen, W.; Xu, S.; Wang, Z.; Shan, X.; Zhou, J.; Liu, Z.; Liang, G. Design, synthesis and biological activity of novel asymmetric C66 analogs as anti-inflammatory agents for the treatment of acute lung injury. Eur. J. Med. Chem. 2015, 94, 436–446. [Google Scholar] [CrossRef]
- Ge, W.; Hao, X.; Han, F.; Liu, Z.; Wang, T.; Wang, M.; Chen, N.; Ding, Y.; Chen, Y.; Zhang, Q. Synthesis and structure-activity relationship studies of parthenolide derivatives as potential anti-triple negative breast cancer agents. Eur. J. Med. Chem. 2019, 166, 445–469. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, X.; Liu, C.; Ge, W.; Wang, Q.; Hao, X.; Wang, M.; Chen, Y.; Zhang, Q. Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells. J. Hematol. Oncol. 2021, 14, 19. [Google Scholar] [CrossRef]
- Cui, M.; Wang, Z.; Huang, L.T.; Wang, J.H. Parthenolide leads to proteomic differences in thyroid cancer cells and promotes apoptosis. BMC Complement. Med. Ther. 2022, 22, 99. [Google Scholar] [CrossRef]
- Wang, M.; Li, Q. Parthenolide could become a promising and stable drug with anti-inflammatory effects. Nat. Prod. Res. 2015, 29, 1092–1101. [Google Scholar] [CrossRef]
- Liu, Y.J.; Tang, B.; Wang, F.C.; Tang, L.; Lei, Y.Y.; Luo, Y.; Huang, S.J.; Yang, M.; Wu, L.Y.; Wang, W.; et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics 2020, 10, 5225–5241. [Google Scholar] [CrossRef]
- Fan, M.; Wang, C.; Zhao, X.; Jiang, Y.; Wang, C. Parthenolide alleviates microglia-mediated neuroinflammation via MAPK/TRIM31/NLRP3 signaling to ameliorate cognitive disorder. Int. Immunopharmacol. 2023, 120, 110287. [Google Scholar] [CrossRef]
- Ding, W.; Cai, C.; Zhu, X.; Wang, J.; Jiang, Q. Parthenolide ameliorates neurological deficits and neuroinflammation in mice with traumatic brain injury by suppressing STAT3/NF-κB and inflammasome activation. Int. Immunopharmacol. 2022, 108, 108913. [Google Scholar] [CrossRef]
- Li, X.H.; Xiao, T.; Yang, J.H.; Qin, Y.; Gao, J.J.; Liu, H.J.; Zhou, H.G. Parthenolide attenuated bleomycin-induced pulmonary fibrosis via the NF-κB/Snail signaling pathway. Respir. Res. 2018, 19, 111. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Shi, Q.Q.; Ding, Y.H.; Long, J.; Zhang, Q.; Chen, Y. Synthesis of micheliolide derivatives and their activities against AML progenitor cells. Molecules 2013, 18, 5980–5992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, Y.; Ding, Y.; Zhai, J.; Ji, Q.; Ma, W.; Yang, M.; Fan, H.; Long, J.; Tong, Z.; et al. Guaianolide sesquiterpene lactones, a source to discover agents that selectively inhibit acute myelogenous leukemia stem and progenitor cells. J. Med. Chem. 2012, 55, 8757–8769. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, G.; Tong, T.; Chen, J. Micheliolide suppresses LPS-induced neuroinflammatory responses. PLoS ONE 2017, 12, e0186592. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ding, Q.; Chen, C.; Chen, F.; Zhou, X.; Hong, C.J.; Pan, W. Micheliolide inhibits gastric cancer growth in vitro and in vivo via blockade of the IL-6/STAT3 pathway. Pharmazie 2019, 74, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Jaffar, J.; Glaspole, I.; Symons, K.; Westall, G. Inhibition of NF-κB by ACT001 reduces fibroblast activity in idiopathic pulmonary fibrosis. Biomed. Pharmacother. 2021, 138, 111471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Z.; Zhu, J.; Peng, Z.; Tang, C. Ferulic acid regulates miR-17/PTEN axis to inhibit LPS-induced pulmonary microvascular endothelial cells apoptosis through activation of PI3K/Akt pathway. J. Toxicol. Sci. 2022, 47, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Matowane, G.R.; Ramorobi, L.M.; Mashele, S.S.; Bonnet, S.L.; Noreljaleel, A.E.M.; Swain, S.S.; Makhafola, T.J.; Chukwuma, C.I. Complexation potentiated promising anti-diabetic and anti-oxidative synergism between ZN(ii) and ferulic acid: A multimode study. Diabet. Med. 2022, 39, e14905. [Google Scholar] [CrossRef] [PubMed]
- El-Gogary, R.I.; Nasr, M.; Rahsed, L.A.; Hamzawy, M.A. Ferulic acid nanocapsules as a promising treatment modality for colorectal cancer: Preparation and in vitro/in vivo appraisal. Life Sci. 2022, 298, 120500. [Google Scholar] [CrossRef]
- Liu, X.; Qi, K.; Gong, Y.; Long, X.; Zhu, S.; Lu, F.; Lin, K.; Xu, J. Ferulic Acid Alleviates Myocardial Ischemia Reperfusion Injury Via Upregulating AMPKα2 Expression-Mediated Ferroptosis Depression. J. Cardiovasc. Pharmacol. 2021, 79, 489–500. [Google Scholar] [CrossRef]
- Lampiasi, N.; Montana, G. The molecular events behind ferulic acid mediated modulation of IL-6 expression in LPS-activated Raw 264.7 cells. Immunobiology 2016, 221, 486–493. [Google Scholar] [CrossRef]
- Cao, L.; Li, Z.; Yang, Z.; Wang, M.; Zhang, W.; Ren, Y.; Li, L.; Hu, J.; Sun, Z.; Nie, S. Ferulic acid positively modulates the inflammatory response to septic liver injury through the GSK-3β/NF-κB/CREB pathway. Life Sci. 2021, 277, 119584. [Google Scholar] [CrossRef]
- Wu, X.; Lin, L.; Wu, H. Ferulic acid alleviates lipopolysaccharide-induced acute lung injury through inhibiting TLR4/NF-κB signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Wang, P.; Zhao, J. Allenone-Mediated Racemization/Epimerization-Free Peptide Bond Formation and Its Application in Peptide Synthesis. J. Am. Chem. Soc. 2021, 143, 10374–10381. [Google Scholar] [CrossRef] [PubMed]
- Golden, T.N.; Venosa, A.; Gow, A.J. Cell Origin and iNOS Function Are Critical to Macrophage Activation Following Acute Lung Injury. Front. Pharmacol. 2021, 12, 761496. [Google Scholar] [CrossRef]
- Guo, C.; Atochina-Vasserman, E.; Abramova, H.; George, B.; Manoj, V.; Scott, P.; Gow, A. Role of NOS2 in pulmonary injury and repair in response to bleomycin. Free Radic. Biol. Med. 2016, 91, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Li, J.; Li, Q.; Wang, X.; Medikonda, R.; Zhao, T.; Li, T.; Ma, H.; Yi, L.; Liu, P.; et al. ACT001 reduces the expression of PD-L1 by inhibiting the phosphorylation of STAT3 in glioblastoma. Theranostics 2020, 10, 5943–5956. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Payne, D.T.; Ampolu, B.; Bland, N.; Brown, J.T.; Dutton, M.J.; Fitton, C.A.; Gulliver, A.; Hale, L.; Hamza, D.; et al. Derivatisation of parthenolide to address chemoresistant chronic lymphocytic leukaemia. Medchemcomm 2019, 10, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yang, Z.; Ge, W.; Kuang, B.; Xu, J.; Yang, J.; Chen, Y.; Zhang, Q. Synthesis and biological evaluation of dithiocarbamate esters of parthenolide as potential anti-acute myelogenous leukaemia agents. J. Enzyme Inhib. Med. Chem. 2018, 33, 1376–1391. [Google Scholar] [CrossRef]
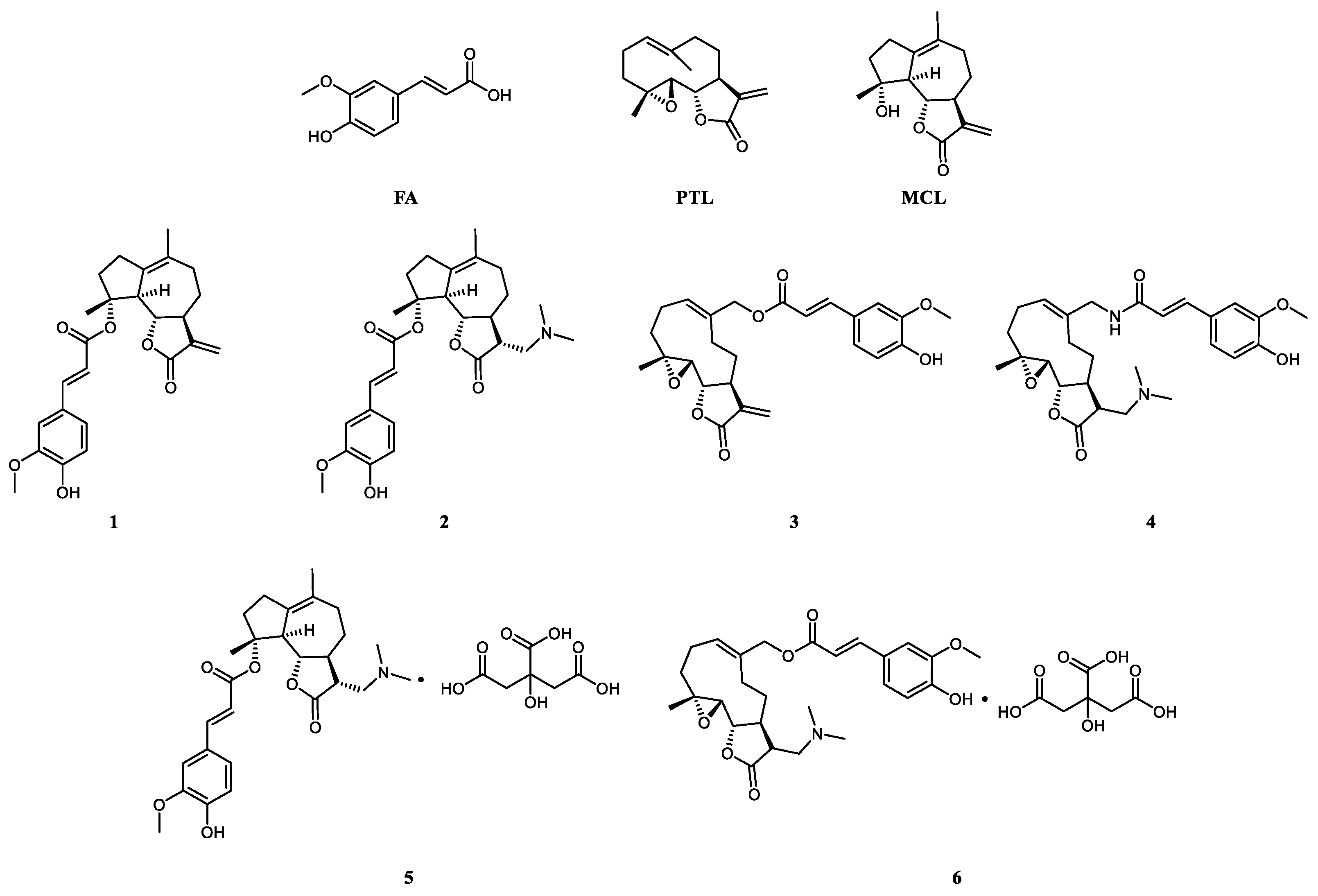
| Entry | Compounds | MW | IC50 (μM) |
|---|---|---|---|
| 1 | FA | 194.19 | >100 |
| 2 | MCL | 248.14 | 9.03 ± 0.16 |
| 3 | PTL | 248.32 | 5.35 ± 0.06 |
| 4 | 1 | 424.18 | 6.14 ± 0.09 |
| 5 | 2 | 469.25 | 19.58 ± 0.21 |
| 6 | 3 | 440.18 | 3.04 ± 0.08 |
| 7 | 4 | 484.26 | 38.39 ± 1.14 |
| 8 | 5 | 661.27 | 28.80 ± 0.21 |
| 9 | 6 | 677.25 | 6.95 ± 1.09 |
| Compounds | Aqueous Solubility IC50 (μg/mL) |
|---|---|
| pH = 7.0 | |
| PTL | 50 |
| MCL | 50 |
| 1 | <10 |
| 2 | <10 |
| 3 | <10 |
| 4 | <10 |
| 5 | 2500 |
| 6 | 2500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, X.; Liu, N.; Lv, K.; Wang, J.; Li, M.; Zhang, Y.; Huo, X.; Bao, S.; Shen, Z.; Zhang, X. Synthesis and Anti-Inflammatory Activity of Ferulic Acid-Sesquiterpene Lactone Hybrids. Molecules 2024, 29, 936. https://doi.org/10.3390/molecules29050936
Duan X, Liu N, Lv K, Wang J, Li M, Zhang Y, Huo X, Bao S, Shen Z, Zhang X. Synthesis and Anti-Inflammatory Activity of Ferulic Acid-Sesquiterpene Lactone Hybrids. Molecules. 2024; 29(5):936. https://doi.org/10.3390/molecules29050936
Chicago/Turabian StyleDuan, Xiyan, Ning Liu, Ke Lv, Junqi Wang, Mingyue Li, Yanwei Zhang, Xiaoguang Huo, Shiqi Bao, Zhuo Shen, and Xuemei Zhang. 2024. "Synthesis and Anti-Inflammatory Activity of Ferulic Acid-Sesquiterpene Lactone Hybrids" Molecules 29, no. 5: 936. https://doi.org/10.3390/molecules29050936
APA StyleDuan, X., Liu, N., Lv, K., Wang, J., Li, M., Zhang, Y., Huo, X., Bao, S., Shen, Z., & Zhang, X. (2024). Synthesis and Anti-Inflammatory Activity of Ferulic Acid-Sesquiterpene Lactone Hybrids. Molecules, 29(5), 936. https://doi.org/10.3390/molecules29050936