Adsorption Mechanisms of TM3 (TM = Mo, Ru, Au)-Decorated Tin Sulfide Monolayers for the Decomposition of Gas Components under Fault Conditions in Oil-Immersed Transformers
Abstract
1. Introduction
2. Results and Discussion
2.1. Geometric Structures and Electronic Properties of Pure SnS and TM3-SnS (TM = Mo, Ru, Au)
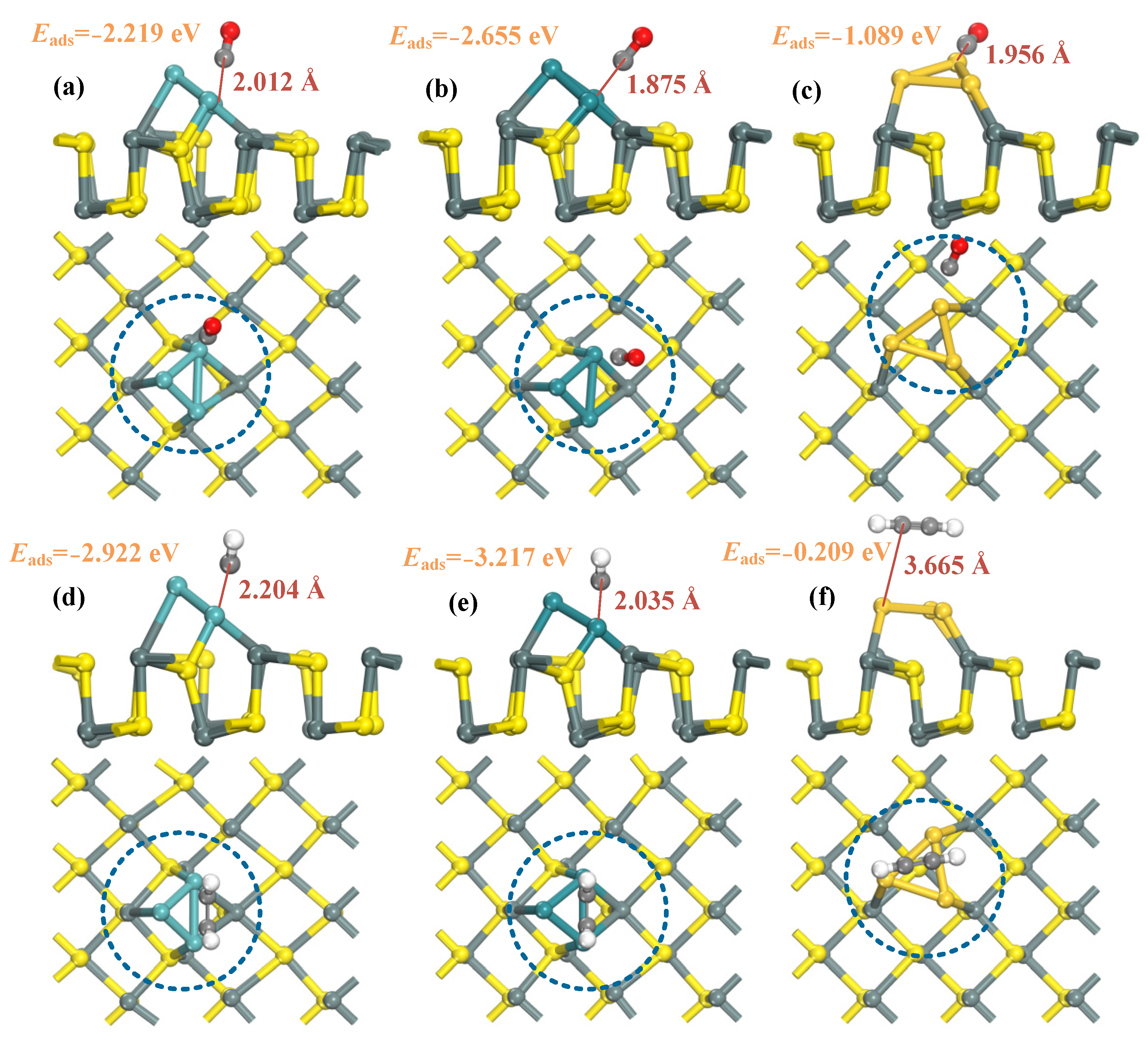
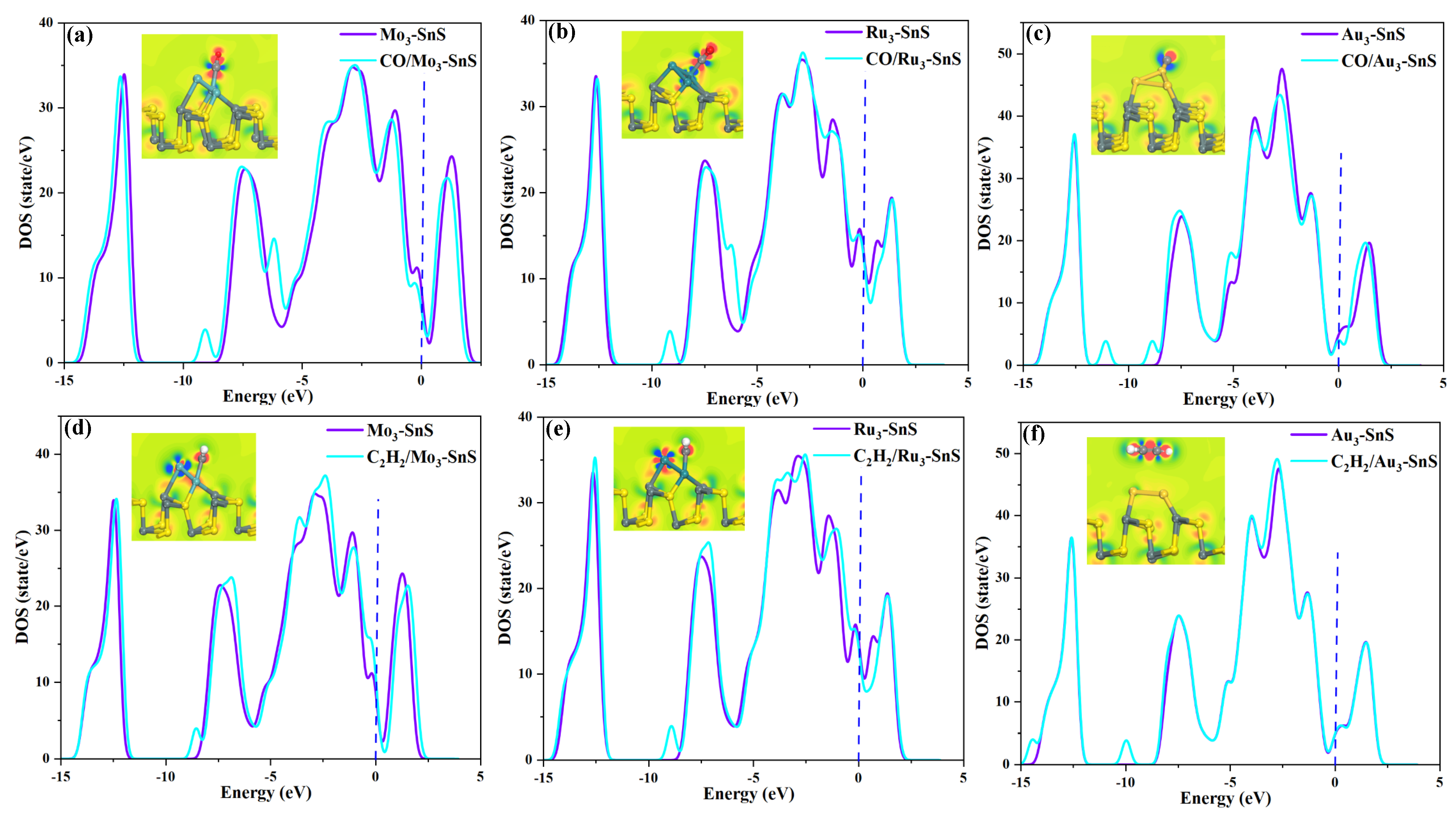
2.2. CO and C2H2 Adsorption on TM3-SnS (TM = Mo, Ru, Au) and Electronic Properties Analysis
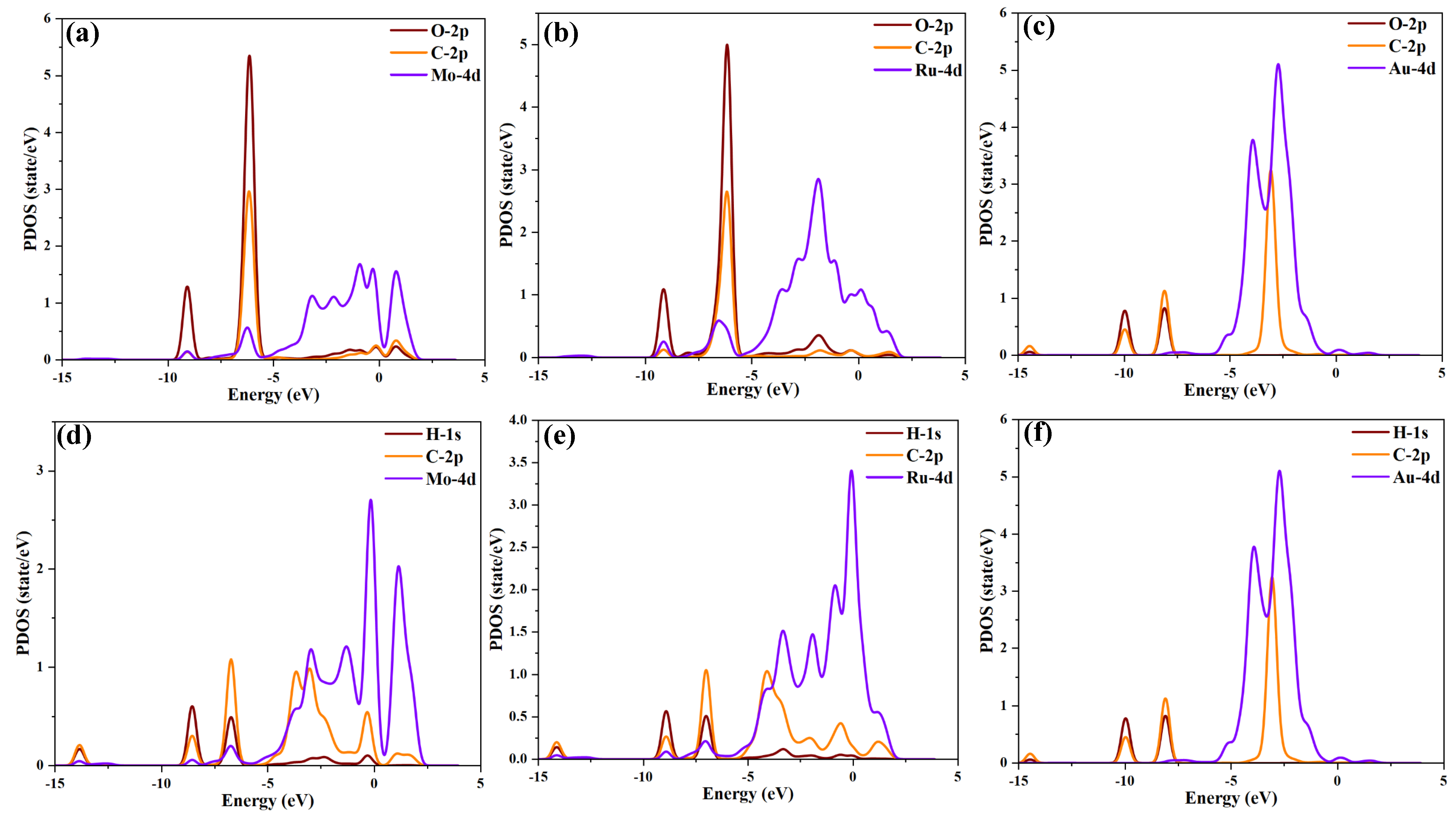
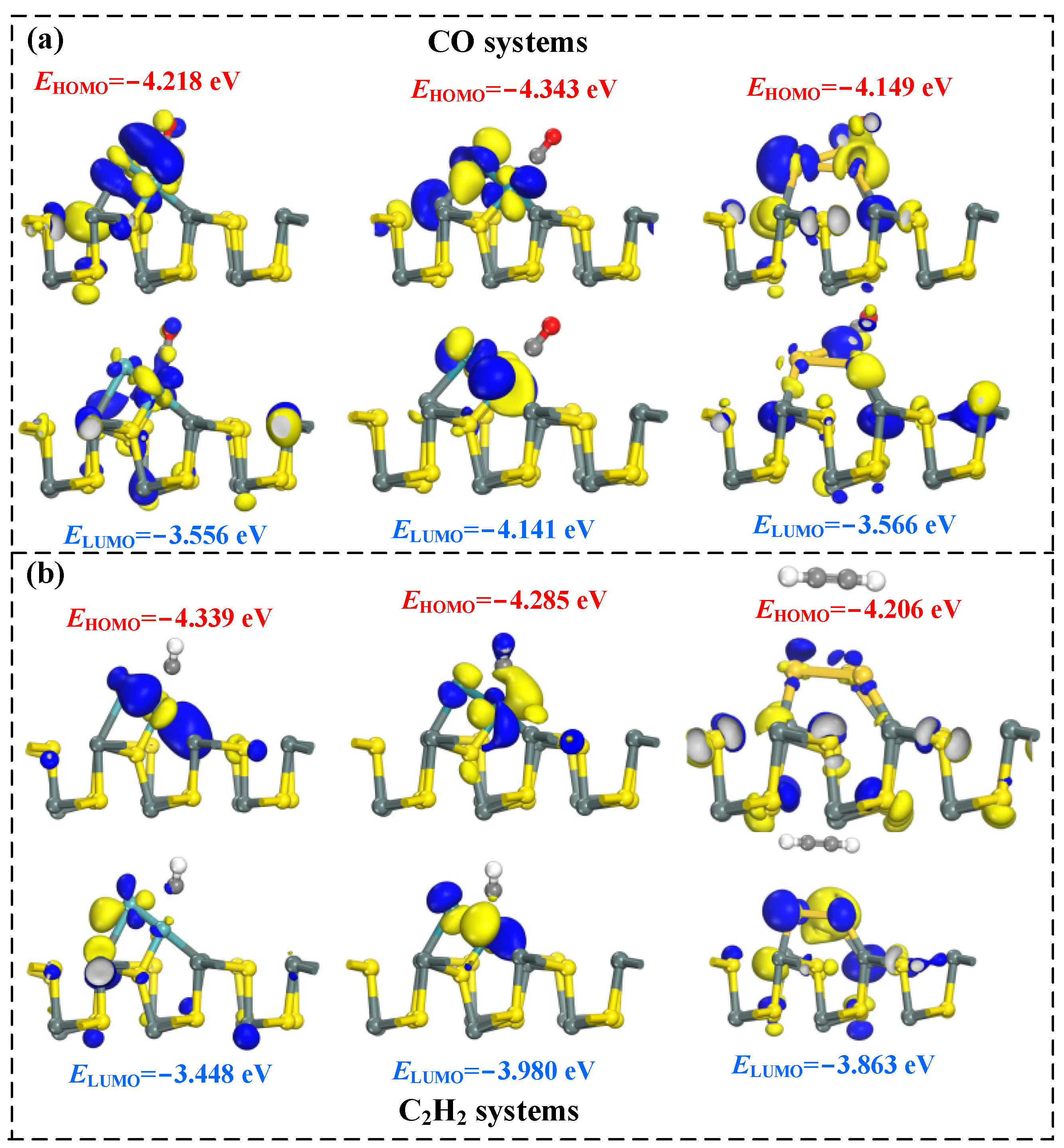
2.3. Molecular Orbital Theory Analysis of TM3-SnS (TM = Mo, Ru, Au)
3. Computational Details
4. Conclusions
- (1)
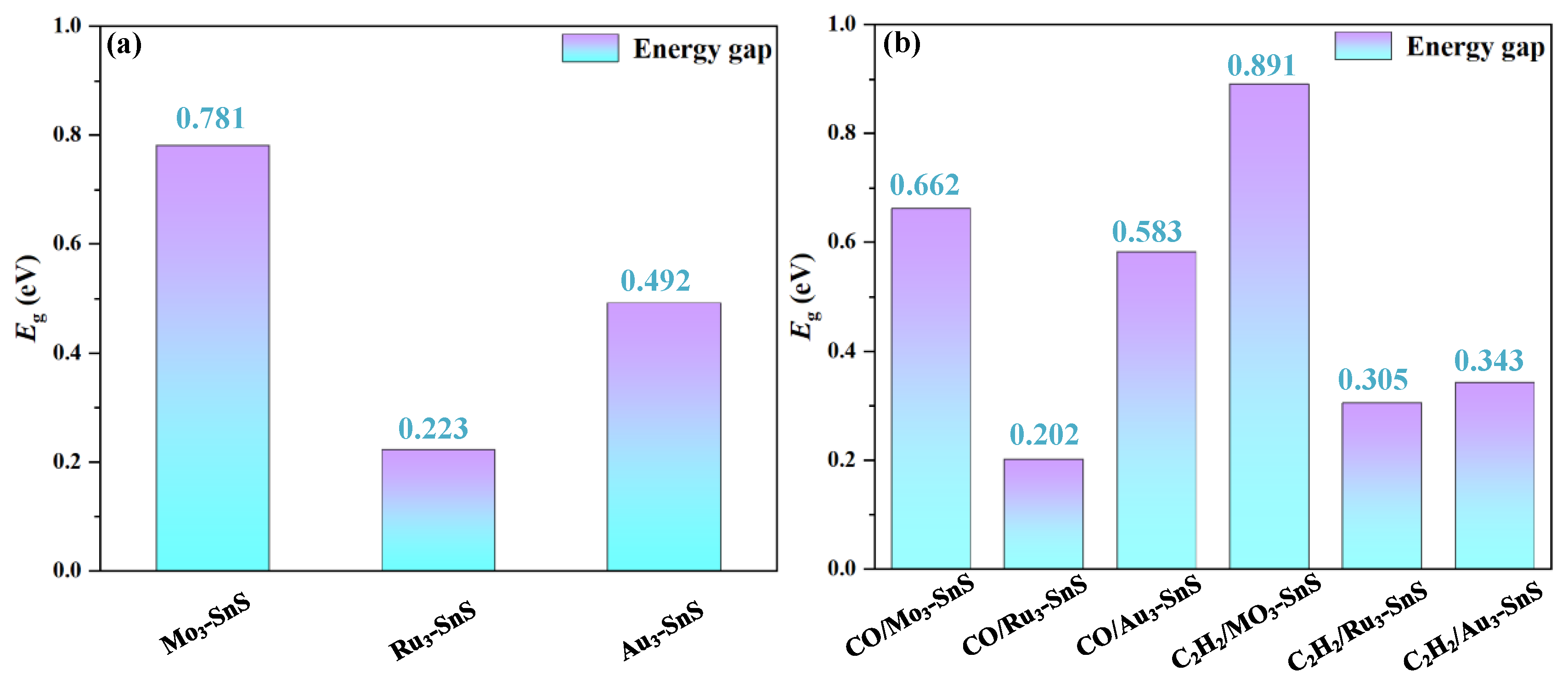
- The presence of Mo3 metal particles has a minimal effect on the band structure, while Ru3 and Au3 doping lead to a reduction in the bandgap by 71.4% and 44.4%, respectively. Nanoparticle doping enhances the surface electronic performance of the SnS monolayer and augments its potential for gas adsorption.
- (2)
- The modified SnS exhibits adsorption capacity in the order of Ru3-SnS > Mo3-SnS > Au3-SnS, the adsorption competitiveness of the mixture for C2H2 is better than that of CO, and C2H2 adsorption is physico-chemical while CO is physically adsorbed.
- (3)
- The electronic activity of the metal particle’s 4d orbitals is the fundamental reason for enhancing the adsorption capacity of SnS, and different metal elements have a differentiated impact on the overall electronic distribution of the system.
- (4)
- Molecular orbital theory analysis reveals significant differences in molecular orbitals after the adsorption of different target molecules, indicating the strong selectivity and adsorption capability of TM3-SnS (TM = Mo, Ru, Au) for CO and C2H2.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huynh, T.L.D.; Hille, E.; Nasir, M.A. Diversification in the age of the 4th industrial revolution: The role of artificial intelligence, green bonds and cryptocurrencies. Technol. Forecast. Soc. Chang. 2020, 159, 120188. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhang, Y.R.; Pan, A.; Han, M.C.; Veglianti, E. Carbon emission reduction effects of industrial robot applications: Heterogeneity characteristics and influencing mechanisms. Technol. Soc. 2022, 70, 102034. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Wang, S.S.; Chen, J.; Gong, C.Y. Impedance-Phased Dynamic Control Method for Grid-Connected Inverters in a Weak Grid. IEEE Trans. Power Electron. 2017, 32, 274–283. [Google Scholar] [CrossRef]
- Tan, K.M.; Ramachandaramurthy, V.K.; Yong, J.Y. Integration of electric vehicles in smart grid: A review on vehicle to grid technologies and optimization techniques. Renew. Sustain. Energy Rev. 2016, 53, 720–732. [Google Scholar] [CrossRef]
- Xie, Y.M.; Ruan, J.J.; Shi, Y.; Jin, S.; Tian, Y.; Zhu, L. Inversion Detection Method for Resistivity of Oil-Immersed Paper in Transformer. IEEE Trans. Power Deliv. 2019, 34, 1757–1765. [Google Scholar] [CrossRef]
- Liu, J.F.; Fan, X.H.; Zhang, Y.Y.; Zheng, H.B.; Zhang, C.H. Condition prediction for oil-immersed cellulose insulation in field transformer using fitting fingerprint database. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 279–287. [Google Scholar] [CrossRef]
- Gui, Y.G.; Xu, L.N.; Ding, Z.Y.; Ran, L.; Chen, X.P.; Tang, C. Co, Rh decorated GaNNTs for online monitoring of characteristic decomposition products in oil-immersed transformer. Appl. Surf. Sci. 2021, 561, 150072. [Google Scholar] [CrossRef]
- Gui, Y.G.; Shi, J.Z.; Xu, L.N.; Ran, L.; Chen, X.P. Aun (n = 1–4) cluster doped MoSe2 nanosheet as a promising gas-sensing material for C2H4 gas in oil-immersed transformer. Appl. Surf. Sci. 2021, 541, 14835. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.X.; Zhang, G.Z.; Tang, J. Pd-doped MoS2 monolayer: A promising candidate for DGA in transformer oil based on DFT method. Appl. Surf. Sci. 2019, 470, 1035–1042. [Google Scholar] [CrossRef]
- Chen, Y.; Gui, Y.G.; Chen, X.P. Adsorption and gas-sensing properties of C2H4, CH4, H2, H2O on metal oxides (CuO, NiO) modified SnS2 monolayer: A DFT study. Results Phys. 2021, 28, 104680. [Google Scholar] [CrossRef]
- Jiang, T.Y.; Zhang, W.T.; Zhang, T.; Yuan, H.X.; Bi, M.Q.; Zhou, X. Adsorption and gas-sensing performances of C2H2, C2H4, CO, H2 in transformer oil on Pt-doped MoTe2 monolayer: A DFT study. Phys. E-Low-Dimens. Syst. Nanostruct. 2023, 146, 115568. [Google Scholar] [CrossRef]
- Chen, Y.; Gui, Y.G.; Ding, Z.Y.; Xu, L.N.; Chen, X.P. Adsorption and gas-sensing properties of Pdn-GaNNTs to C2H2 and H2 gases. Phys. E-Low-Dimens. Syst. Nanostruct. 2022, 136, 115004. [Google Scholar] [CrossRef]
- Wang, J.F.; Jin, X.X.; Li, C.H.; Wang, W.J.; Wu, H.; Guo, S.Y. Graphene and graphene derivatives toughening polymers: Toward high toughness and strength. Chem. Eng. J. 2019, 370, 831–854. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, X.H.; Liu, B.; Deng, S.J.; Xie, D.; Liu, Q.; Wang, Y.D.; Wu, J.B.; Wang, X.L.; Tu, J.P. Multiscale Graphene-Based Materials for Applications in Sodium Ion Batteries. Adv. Energy Mater. 2019, 9, 1803342. [Google Scholar] [CrossRef]
- Tang, L.; Meng, X.G.; Deng, D.H.; Bao, X.H. Confinement Catalysis with 2D Materials for Energy Conversion. Adv. Mater. 2019, 31, 1901996. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.J.; Fan, Y.Y.; Zhang, Y.F.; Mei, L.; Zhu, R.S.; Qin, J.Q.; Hu, J.G.; Chen, Z.X.; Ng, Y.H.; Voiry, D.; et al. 2D Transition Metal Dichalcogenides for Photocatalysis. Angew. Chem.-Int. Ed. 2023, 62, e202218016. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.S.; Stratakis, E. Recent Advances in 2D Metal Monochalcogenides. Adv. Sci. 2020, 7, 2001655. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.D.; Liu, Y.; Huang, B.L.; Wu, R.X.; Zhang, Z.W.; Zhao, B.; Ma, H.F.; Dang, W.Q.; Wei, Z.; et al. General synthesis of two-dimensional van der Waals heterostructure arrays. Nature 2020, 579, 368–374. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, X.F.; Shi, W.; Wu, J.B.; Jiang, D.S.; Tan, P.H. Phonon and Raman scattering of two-dimensional transition metal dichalcogenides from monolayer, multilayer to bulk material. Chem. Soc. Rev. 2015, 44, 2757–2785. [Google Scholar] [CrossRef]
- Zhou, T.F.; Pang, W.K.; Zhang, C.F.; Yang, J.P.; Chen, Z.X.; Liu, H.K.; Guo, Z.P. Enhanced Sodium-Ion Battery Performance by Structural Phase Transition from Two-Dimensional Hexagonal-SnS2 to Orthorhombic-SnS. Acs Nano 2014, 8, 8323–8333. [Google Scholar] [CrossRef] [PubMed]
- Shafique, A.; Shin, Y.H. Thermoelectric and phonon transport properties of two-dimensional IV–VI compounds. Sci. Rep. 2017, 7, 506. [Google Scholar] [CrossRef]
- Zeng, R.J.; Lian, K.K.; Su, B.; Lu, L.L.; Lin, J.W.; Tang, D.P.; Lin, S.; Wang, X.C. Versatile Synthesis of Hollow Metal Sulfides via Reverse Cation Exchange Reactions for Photocatalytic CO2 Reduction. Angew. Chem.-Int. Ed. 2021, 60, 25055–25062. [Google Scholar] [CrossRef]
- Sarkar, A.S.; Konidakis, I.; Gagaoudakis, E.; Maragkakis, G.M.; Psilodimitrakopoulos, S.; Katerinopoulou, D.; Sygellou, L.; Deligeorgis, G.; Binas, V.; Oikonomou, I.M.; et al. Liquid Phase Isolation of SnS Monolayers with Enhanced Optoelectronic Properties. Adv. Sci. 2023, 10, 2201842. [Google Scholar] [CrossRef]
- Barraza-Lopez, S.; Fregoso, B.M.; Villanova, J.W.; Parkin, S.S.P.; Chang, K. Colloquium: Physical properties of group-IV monochalcogenide monolayers. Rev. Mod. Phys. 2021, 93, 011001. [Google Scholar] [CrossRef]
- Wang, P.T.; Ge, W.Y.; Lin, L.; Jia, X.H.; Zhang, X.M.; Lu, J. Ultra-sensitive NO2 detection based on SnS nanosheets: Experimental and DFT investigation. Vacuum 2023, 209, 111777. [Google Scholar] [CrossRef]
- Luyen, Q.V.; Bui, P.T.; Chu, V.T.; Hung, N.M.; Arepalli, V.K.; Bui, V.D.; Nguyen, T.D. Fabrication of Ag embedded-SnS films via the RF approach: First study on NO2 gas-sensing performance. Sens. Actuators A-Phys. 2022, 334, 113319. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Shi, Y.Y.; Shi, Z.D.; Xia, H.; Ma, M.Y.; Wang, Y.L.; Huang, K.; Wu, Y.; Gong, Y.J.; Fei, H.L.; et al. High-Pressure Synthesis of Single-Crystalline SnS Nanoribbons. Nano Lett. 2023, 23, 7449–7455. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.S.; Mushtaq, A.; Kushavah, D.; Pal, S.K. Liquid exfoliation of electronic grade ultrathin tin(II) sulfide (SnS) with intriguing optical response. Npj 2d Mater. Appl. 2020, 4, 1. [Google Scholar] [CrossRef]
- Li, T.; Hu, S.L.; Ma, R.; Sang, T.Y.; Chen, Q.L.; Ma, L.; Chen, Y.; Liao, Y.; Yang, G.L.; Huang, Y.F.; et al. The electronic properties and adsorption mechanism of Agn, Aun (n = 1–4) modified GeSe monolayer towards hazardous gases (H2S, NH3, NO2 and SOF2): A first-principles study. Surf. Interfaces 2022, 32, 102150. [Google Scholar] [CrossRef]
- Gui, Y.G.; Li, T.; He, X.; Ding, Z.Y.; Yang, P.G. Pt Cluster Modified h-BN for Gas Sensing and Adsorption of Dissolved Gases in Transformer Oil: A Density Functional Theory Study. Nanomaterials 2019, 9, 1746. [Google Scholar] [CrossRef]
- Wang, Y.; Gui, Y.G.; Ji, C.; Tang, C.; Zhou, Q.; Li, J.; Zhang, X.X. Adsorption of SF6 decomposition components on Pt3-TiO2 (101) surface: A DFT study. Appl. Surf. Sci. 2018, 459, 242–248. [Google Scholar] [CrossRef]
- Li, J.; Pang, L.; Cai, F.W.; Yuan, X.Y.; Kong, F.Y. Adsorption Properties of Pd3-Modified Double-Vacancy Defect Graphene toward SF6 Decomposition Products. Sensors 2020, 20, 4188. [Google Scholar] [CrossRef]
- Mi, H.W.; Zhou, Q.; Zeng, W. A density functional theory study of the adsorption of Cl2, NH3, and NO2 on Ag3-doped WSe2 monolayers. Appl. Surf. Sci. 2021, 563, 150329. [Google Scholar] [CrossRef]
- Liu, Z.C.; Gui, Y.G.; Xu, L.N.; Chen, X.P. Adsorption and gas-sensing properties of Aun (n = 1–3) cluster doped MoTe2 for NH3, NO2, and SO2 gas molecules. Surf. Interfaces 2022, 30, 101883. [Google Scholar] [CrossRef]
- Liu, H.C.; Wang, F.P.; Hu, K.L.; Li, T.; Yan, Y. Pd4 cluster decorated SnO2 nanowire for detecting characteristic gases in oil-immersed transformers: A theoretical and experimental study. Appl. Surf. Sci. 2022, 590, 153122. [Google Scholar] [CrossRef]
- Patel, K.; Roondhe, B.; Dabhi, S.D.; Jha, P.K. A new flatland buddy as toxic gas scavenger: A first principles study. J. Hazard. Mater. 2018, 351, 337–345. [Google Scholar] [CrossRef]
- Li, T.; Gui, Y.G.; Zhao, W.H.; Tang, C.; Dong, X.C. Palladium modified MoS2 monolayer for adsorption and scavenging of SF6 decomposition products: A DFT study. Phys. E-Low-Dimens. Syst. Nanostruct. 2020, 123, 114178. [Google Scholar] [CrossRef]
- Kim, M.C.; Sim, E.; Burke, K. Understanding and Reducing Errors in Density Functional Calculations. Phys. Rev. Lett. 2013, 111, 073003. [Google Scholar] [CrossRef] [PubMed]
- Lejaeghere, K.; Bihlmayer, G.; Björkman, T.; Blaha, P.; Blügel, S.; Blum, V.; Caliste, D.; Castelli, I.E.; Clark, S.J.; Dal Corso, A.; et al. Reproducibility in density functional theory calculations of solids. Science 2016, 351, aad3000. [Google Scholar] [CrossRef] [PubMed]
- Lehtola, S.; Steigemann, C.; Oliveira, M.J.T.; Marques, M.A.L. Recent developments in LIBXC—A comprehensive library of functionals for density functional theory. Softwarex 2018, 7, 1–5. [Google Scholar] [CrossRef]
- Lin, L.; Hu, C.C.; Deng, C.; Xu, Y.H.; Tao, H.L.; Zhang, Z.Y. Adsorption behavior of transition metal (Pd, Pt, Ag and Au) doped SnS monolayers on SF6 decomposed species and the effects of applied electric field and biaxial strain. Flatchem 2022, 36, 100438. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zhu, X.D.; Zhu, K.X.; Shen, J.B.; Xu, Y.; Chen, D.; Wang, P. Adsorption of NO2 and NH3 on single-atom (Co, Pd, Pt)-decorated 2H-MoS2 monolayer: A DFT study. Results Phys. 2023, 51, 106694. [Google Scholar] [CrossRef]
- Li, T.; Pan, S.X.; Ma, R.; Sang, T.Y.; Ma, L.; Wang, M.Z.; Zeng, W.; Huang, W.H.; Jiang, X.L.; Yang, G.L. The gas sensing and adsorption properties of XO2 (X = Ti, Zr, Hf) doped C3N towards H2S, SO2, SOF2: A first-principles study. Diam. Relat. Mater. 2022, 130, 109553. [Google Scholar] [CrossRef]
- Qian, H.; Lu, W.H.; Wei, X.X.; Chen, W.; Deng, J. H2S and SO2 adsorption on Pt-MoS2 adsorbent for partial discharge elimination: A DFT study. Results Phys. 2019, 12, 107–112. [Google Scholar] [CrossRef]
- Yang, G.L.; Jiang, X.L.; Liu, L.; Han, X.B.; Li, T.; Liao, Y.; Bi, C.L.; Li, Y.T.; Ren, X.D. Adsorption Properties of Metal Oxides (ZnO, TiO2, Ag2O) Modified SnS2 Toward Dissolved Gas Analysis in Transformer Oil. IEEE Sens. J. 2023, 23, 20992–21000. [Google Scholar] [CrossRef]
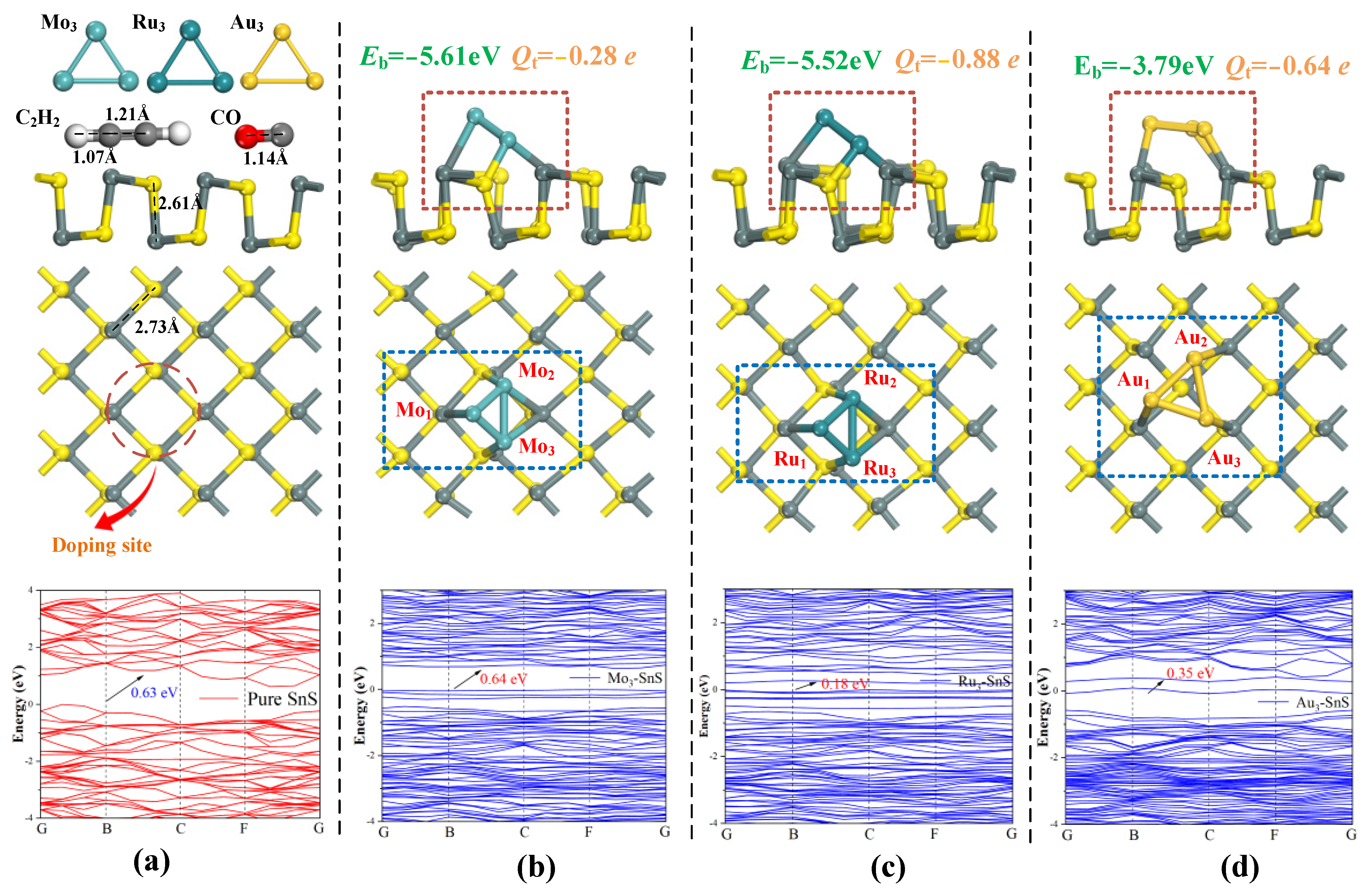

| Structure | Eb (eV) | Qt (e) |
|---|---|---|
| Mo3-SnS | −5.61 | −0.28 |
| Ru3-SnS | −5.52 | −0.88 |
| Au3-SnS | −3.79 | −0.64 |
| Structure | Eads (eV) | d (Å) |
|---|---|---|
| CO/Mo3-SnS | −2.219 | 2.012 |
| CO/Ru3-SnS | −2.655 | 1.875 |
| CO/Au3-SnS | −1.089 | 1.956 |
| C2H2/Mo3-SnS | −2.922 | 2.204 |
| C2H2/Ru3-SnS | −3.217 | 2.035 |
| C2H2/Au3-SnS | −0.209 | 3.665 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wang, B.; Ma, H.; Ma, F.; Wang, H.; Wang, X. Adsorption Mechanisms of TM3 (TM = Mo, Ru, Au)-Decorated Tin Sulfide Monolayers for the Decomposition of Gas Components under Fault Conditions in Oil-Immersed Transformers. Molecules 2024, 29, 934. https://doi.org/10.3390/molecules29050934
Li M, Wang B, Ma H, Ma F, Wang H, Wang X. Adsorption Mechanisms of TM3 (TM = Mo, Ru, Au)-Decorated Tin Sulfide Monolayers for the Decomposition of Gas Components under Fault Conditions in Oil-Immersed Transformers. Molecules. 2024; 29(5):934. https://doi.org/10.3390/molecules29050934
Chicago/Turabian StyleLi, Min, Bo Wang, Hengrui Ma, Fuqi Ma, Hongxia Wang, and Xiao Wang. 2024. "Adsorption Mechanisms of TM3 (TM = Mo, Ru, Au)-Decorated Tin Sulfide Monolayers for the Decomposition of Gas Components under Fault Conditions in Oil-Immersed Transformers" Molecules 29, no. 5: 934. https://doi.org/10.3390/molecules29050934
APA StyleLi, M., Wang, B., Ma, H., Ma, F., Wang, H., & Wang, X. (2024). Adsorption Mechanisms of TM3 (TM = Mo, Ru, Au)-Decorated Tin Sulfide Monolayers for the Decomposition of Gas Components under Fault Conditions in Oil-Immersed Transformers. Molecules, 29(5), 934. https://doi.org/10.3390/molecules29050934






