Dyeing of Tussah Silk with Reactive Dyes: Dye Selection, Dyeing Conditions, Dye Fixation Characteristics, and Comparison with Mulberry Silk
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of Reactive Dyes for the Dyeing of Tussah Silk
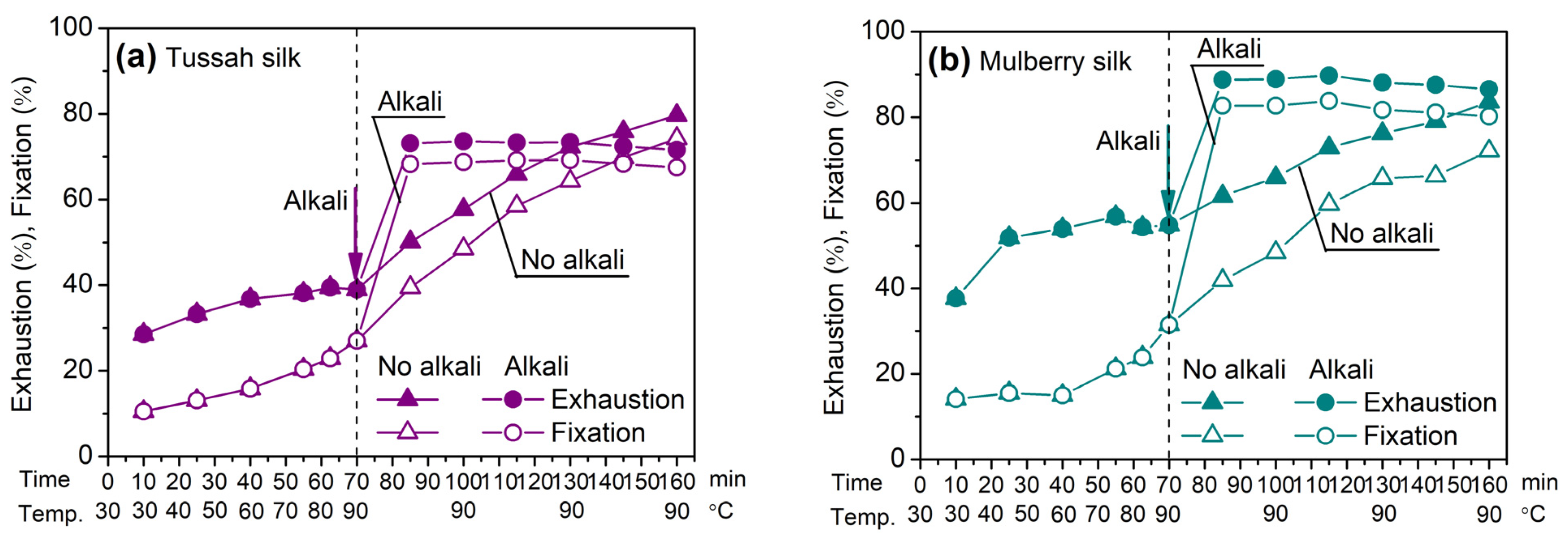
2.1.1. SES/MCT Dyes: Effect of Temperature and Alkali
2.1.2. SES, MCT, and Bis(MCT) Dyes: Effect of Temperature and Alkali
2.1.3. Dyeing Results of Various Reactive Dyes
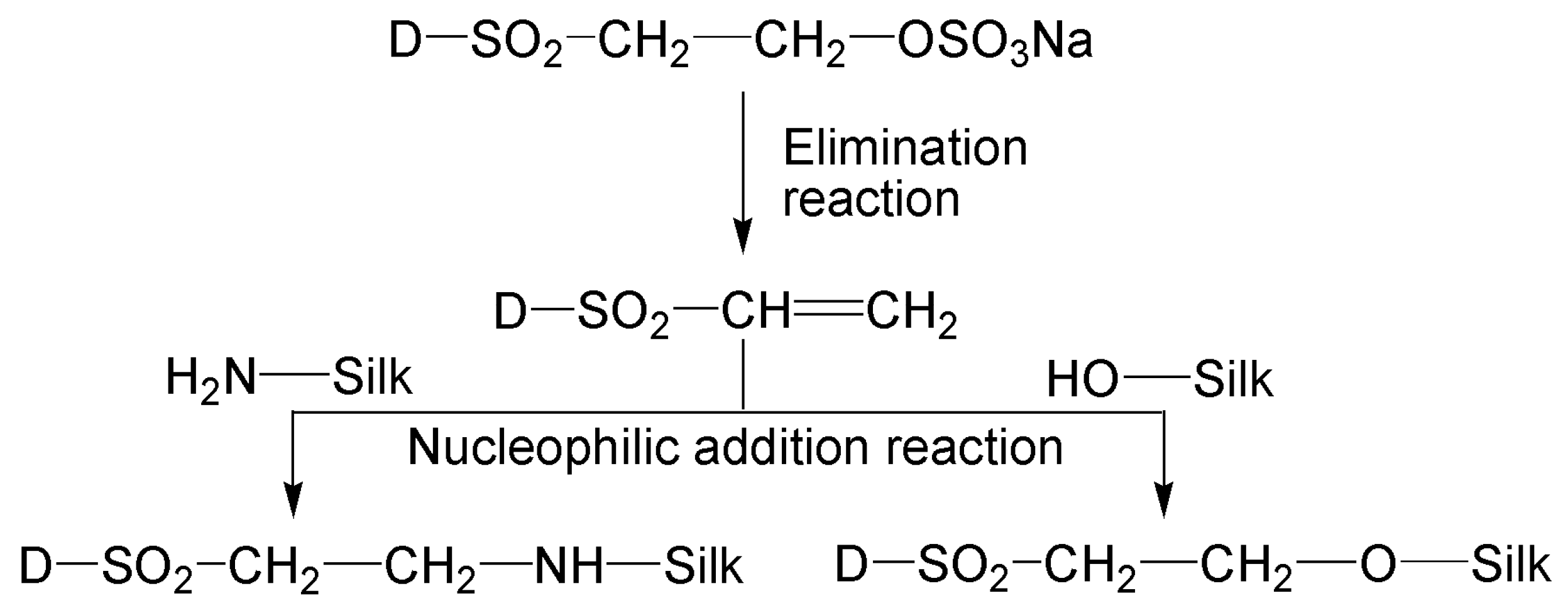
2.2. Dyeing of Tussah Silk with SES Dyes under Alkaline Conditions
2.2.1. Effect of Neutral Electrolyte Dosage
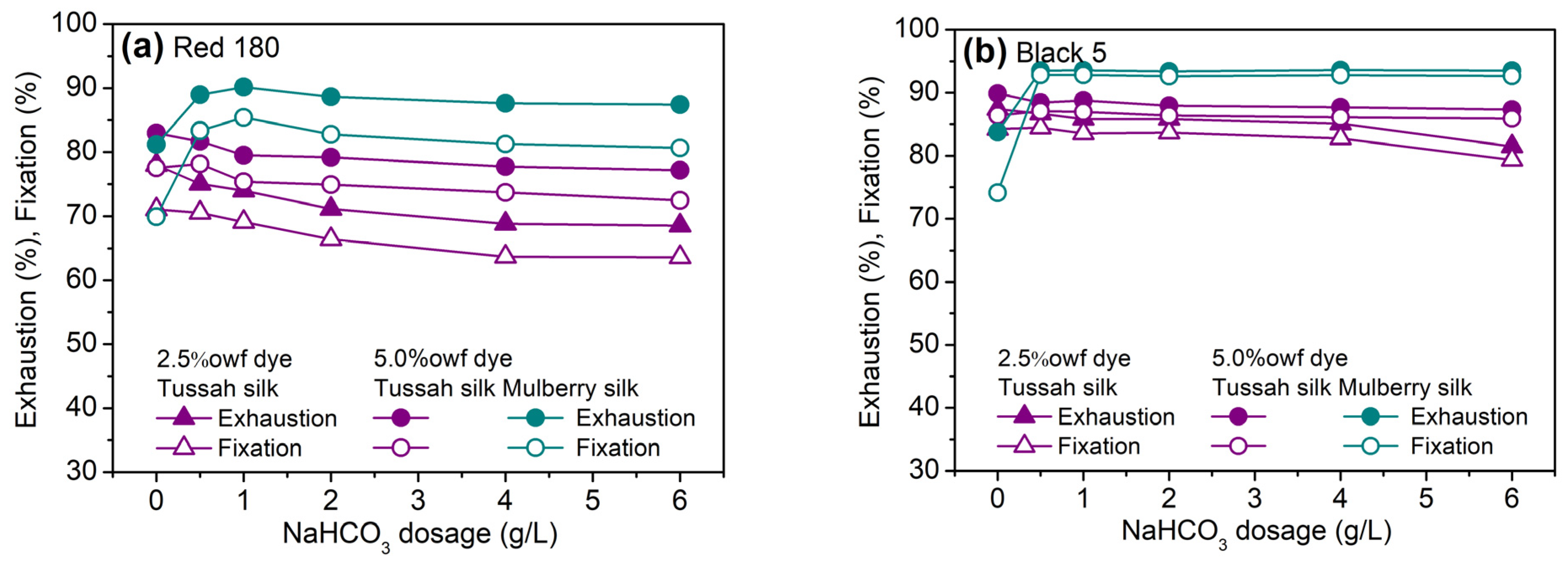
2.2.2. Effect of Sodium Bicarbonate Dosage
2.2.3. Exhaustion and Fixation Rate
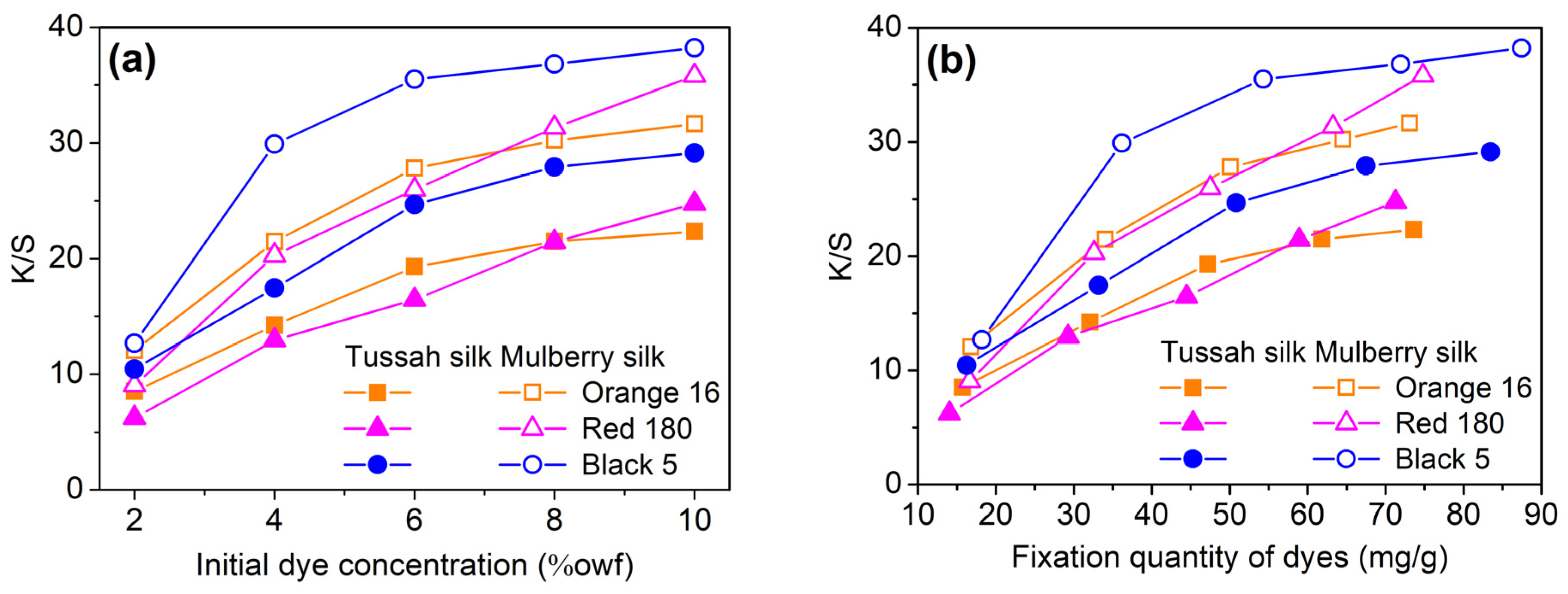
2.2.4. Build-Up Property
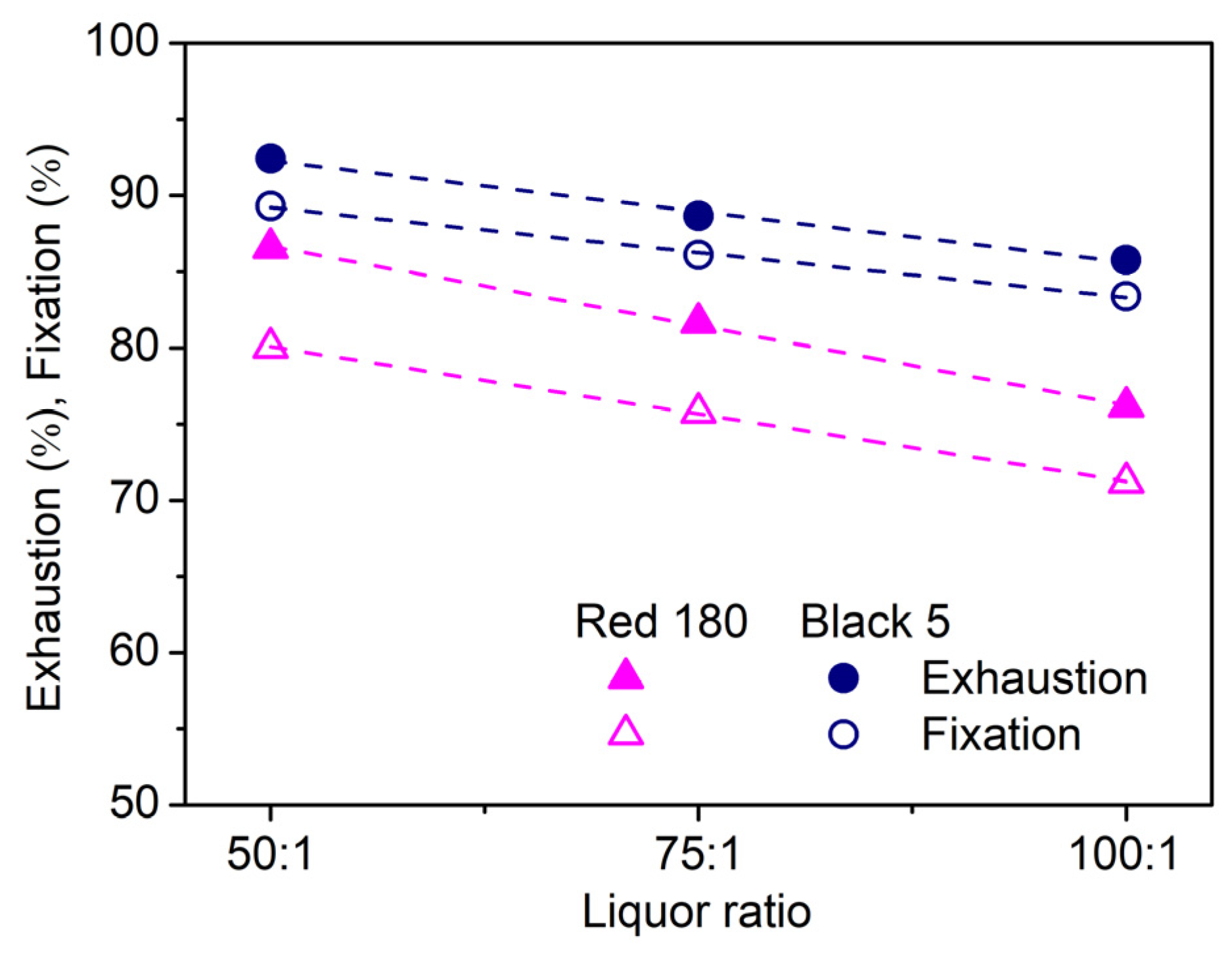
2.2.5. Effect of Liquor Ratio
2.2.6. Color Fastness to Soaping
2.3. Dyeing of Tussah Silk with SES Dyes under Neutral Conditions
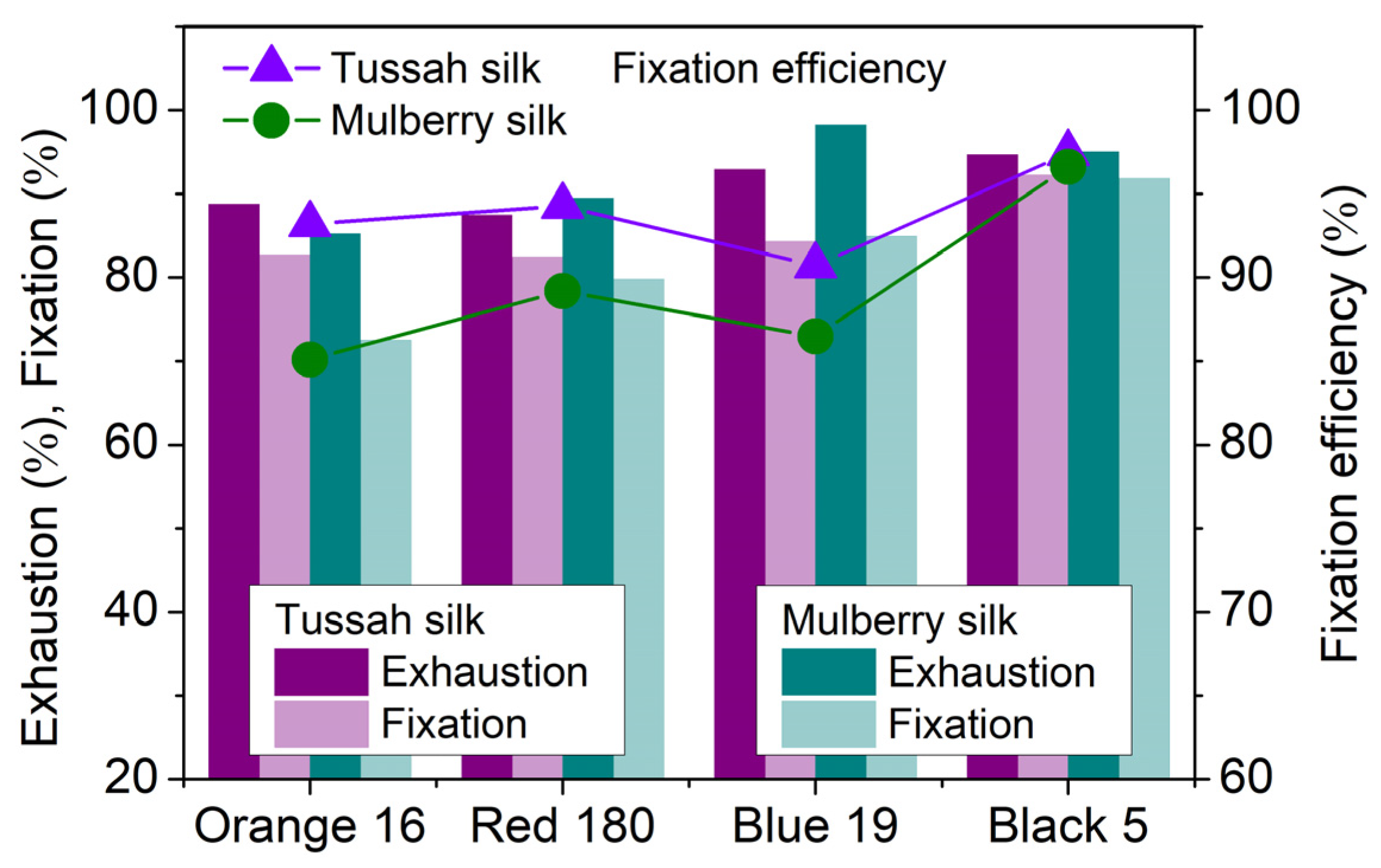
2.3.1. Comparison of the Boiling Dyeing of Tussah and Mulberry Silks
2.3.2. Effect of Holding Time for Boiling Dyeing
2.3.3. Color Fastness to Soaping
3. Materials and Methods
3.1. Materials
3.2. Dyeing Methods
3.2.1. Reactive Dyeing under Alkaline Conditions
3.2.2. Reactive Dyeing under Neutral Conditions
3.3. Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| a* | Redness/greenness index |
| b* | Yellowness/blueness index |
| h | Hue angle |
| K/S | Apparent color depth |
| MCT | Monochlorotriazine |
| Bis(MCT) | Bis(monochlorotriazine) |
| Bis(SES) | Bis(sulfatoethylsulfone) |
| owf | On the weight of fiber |
| SES | Sulfatoethylsulfone |
| SES/MCT | Sulfatoethylsulfone/monochlorotriazine |
| UV | Ultraviolet |
| VS | Vinylsulfone |
References
- Malay, A.D.; Sato, R.; Yazawa, K.; Watanabe, H.; Ifuku, N.; Masunaga, H.; Hikima, T.; Guan, J.; Mandal, B.B.; Damrongsakkul, S.; et al. Relationships between physical properties and sequence in silkworm silks. Sci. Rep. 2016, 6, 27573. [Google Scholar] [CrossRef]
- Liddiard, A.G. Preparation, dyeing and finishing or wild silk. Color. Technol. 1982, 98, 394–397. [Google Scholar] [CrossRef]
- Kako, T.; Katayama, A. Color fastnesses of mulberry and tussah yarns. J. Seric. Sci. Jpn. 1986, 55, 118–125. (In Japanese) [Google Scholar] [CrossRef]
- Kako, T. Dyeing property of chitosan-treated tussah silk fabrics by direct dyes. J. Seric. Sci. Jpn. 2001, 70, 117–122. (In Japanese) [Google Scholar] [CrossRef]
- Anis, P.; Toprak, T.; Yener, E.; Capar, G. Investigation of the effects of environmentally friendly degumming methods on silk dyeing performance. Text. Res. J. 2019, 89, 1286–1296. [Google Scholar] [CrossRef]
- Zhou, F.; Huang, Y. Analysis and improvement of color fastness of silk fabrics. Text. Dye. Finish. J. 2019, 41, 32–34. (In Chinese) [Google Scholar] [CrossRef]
- Lewis, D.M. Developments in the chemistry of reactive dyes and their application processes. Color. Technol. 2014, 130, 382–412. [Google Scholar] [CrossRef]
- Wu, Z. Recent developments of reactive dyes and reactive dyeing of silk. Rev. Prog. Color. 1998, 28, 32–38. [Google Scholar] [CrossRef]
- Lou, W.-D. Study on dyeing behavior of Reactive Dye Red M-3BE for protein fiber. Silk 2006, 9, 28–30. (In Chinese) [Google Scholar] [CrossRef]
- Lin, J.; Liu, Z.-M.; Lu, X.-J.; Jia, Y.-T. Effect of chitosan on the dyeing of tussah silk with reactive dyes. Silk 2007, 8, 45–47. (In Chinese) [Google Scholar] [CrossRef]
- Lin, J.; Fu, Y.-N.; Cui, Y.-F. Tussah silk dyed with B-type reactive dyes. Dyest. Color. 2015, 52, 34–36+54. (In Chinese) [Google Scholar]
- You, K.-F. Study on dyeing process of silk fabrics finish with chitosan with reactive dyes. Silk 2004, 4, 29–31. (In Chinese) [Google Scholar] [CrossRef]
- Kato, H.; Hata, T. Dyeing of silk with mononicotinic acid triazine reactive dyes. J. Insect Biotechnol. Sericology 2002, 71, 161–166. [Google Scholar] [CrossRef]
- Lu, S. Dyeing modified tussah silk with reactive dye. J. East. Liaoning Univ. (Nat. Sci.) 2013, 20, 1–4+18. (In Chinese) [Google Scholar] [CrossRef]
- Agarwal, D.; Sen, K.; Gulrajani, M.L. Application of heterobifunctional reactive dyes on silk. Color. Technol. 1996, 112, 10–16. [Google Scholar] [CrossRef]
- Dohmyo, M.; Shimizu, Y.; Kimura, M. Reaction mechanism of reactive dye with silk. J. Seric. Sci. Jpn. 1985, 54, 181–185. [Google Scholar] [CrossRef]
- Burkinshaw, S.M.; Paraskevas, M. The dyeing of silk: Part 3 the application and wash-off of modified vinyl sulfone dyes. Dye. Pigment. 2011, 88, 212–219. [Google Scholar] [CrossRef]
- Burkinshaw, S.M.; Paraskevas, M. The dyeing of silk: Part 4 heterobifunctional dyes. Dye. Pigment. 2011, 88, 396–402. [Google Scholar] [CrossRef]
- Shen, Y.-F.; Lin, H.-M.; Yang, A.-Q.; Tang, L.-Y. A study on the dyeing of silk with bifunctional reactive dyes. Dyest. Color. 2004, 41, 105–108. (In Chinese) [Google Scholar] [CrossRef]
- Wang, H.-F.; Shao, J.-Z. Dyeing behaviors of silk fabric with wool reactive dyestuffs. China Dye. Print. 2005, 31, 4–6. (In Chinese) [Google Scholar] [CrossRef]
- Yanagi, Y.; Kondo, Y.; Hirabayashi, K. Deterioration of silk fabrics and their crystallinity. Text. Res. J. 2000, 70, 871–875. [Google Scholar] [CrossRef]
- Lewis, D.M. The dyeing of wool with reactive dyes. Color. Technol. 1982, 98, 165–175. [Google Scholar] [CrossRef]
- Derbyshire, A.N.; Tristram, G.R. Chemical reactions between dyes and wool. Color. Technol. 1965, 81, 584–591. [Google Scholar] [CrossRef]
- Schroeder, W.A.; Kay, L.M.; Lewis, B.; Munger, N. The amino acid composition of Bombyx mori silk fibroin and of tussah silk fibroin. J. Am. Chem. Soc. 1955, 77, 3908–3913. [Google Scholar] [CrossRef]
- Simmonds, D.H. The amino acid composition of keratins I. The amino acid analysis of merino 64’s quality virgin wool. Aust. J. Biol. Sci. 1954, 7, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-F.; Shao, J.-Z.; Wang, G.-M. Study on dyeing of silk fabrics with α-bromoacrylamide reactive dyes. Silk 2005, 1, 38–40. (In Chinese) [Google Scholar]
- Gulrajani, M.L. Dyeing of silk with reactive dyes. Rev. Prog. Color. 1993, 23, 51–56. [Google Scholar] [CrossRef]
- Jiang, H.-C.; Tang, R.-C. Low temperature reactive dyeing of silk: An investigation into dyeing conditions, and strength loss and friction damage of fabric. J. Ind. Eng. Chem 2023, in press. [Google Scholar] [CrossRef]
- Burkinshaw, S.M.; Son, Y.-A.; Bide, M.J. The application of heterobifunctional reactive dyes to nylon 6,6: Process modifications to achieve high efficiencies. Dye. Pigment. 2001, 48, 245–251. [Google Scholar] [CrossRef]
- Kawahara, Y.; Nakajima, S.; Furuta, H. Dyeing behavior of tannic acid-treated wild silk fibers with acid dyes. Sen’i Gakkaishi 1993, 49, 444–447. (In Japanese) [Google Scholar] [CrossRef] [PubMed]
- Uzumcu, M.B.; Celik, P.; Gulumser, T.; Kadoglu, H. A comparison of color fastness properties of mulberry silk and tussah silk fabrics in blends with cellulosic fibers. J. Nat. Fibers 2021, 18, 1834–1843. [Google Scholar] [CrossRef]
- Kawahara, Y.; Shioya, M.; Kikutani, T.; Takaku, A. Analysis of swelling behavior of silk fibers by small-angle X-ray scattering. Sen’i Gakkaishi 1994, 50, 199–207. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Kawahara, Y.; Shioya, M.; Takaku, A. Influence of swelling of noncrystalline regions in silk fibers on modification with methacrylamide. J. Appl. Polym. Sci. 1996, 59, 51–56. [Google Scholar] [CrossRef]
- Das, S. The preparation and processing of tussah silk. Color. Technol. 1992, 108, 481–486. [Google Scholar] [CrossRef]






| Reactive Dye | Depth (%owf) | Temperature (°C) | Accelerant Alkali | Fixation (%) | Reference |
|---|---|---|---|---|---|
| Sulfatoethylsulfone/monochlorotriazine dye (SES/MCT) | |||||
| Reactive Red M-3BE | 2, 8 | 80 | Na2SO4 60 g/L NaHCO3 2.5 g/L | 37% | [9] |
| Reactive Yellow B-3BD, Red B-2BF and Blue B-KV | 1 | 60 | Na2SO4 20 g/L Na2CO3 15 g/L | 60–65% | [10] |
| Yellow B-4RFN and Red B-2BF | – | 60 | Na2SO4 40 g/L Na2CO3 + NaHCO3 15 g/L | 55–65% | [11] |
| Bis(monochlorotriazine) dye (Bis(MCT)) | |||||
| Reactive Yellow KE-3G, Red KE-3B, and Blue KE-R | 1 | 85 | CH3COOH (pH 4.6) CH3COOH (pH 4.1) | 56–66% 57–77% | [12] |
| Mononicotinic acid triazine dye (MNT) | |||||
| Kayacelon React Red CN-3B and Blue CN-BL | 1–2 | 99 | Na2HPO4 + NaH2PO4 2 g/L (pH 6.5–7.5) Na2SO4 20–80 g/L | 60–75% | [13] |
| Dichlorotriazine dye (DCT) | |||||
| Reactive Brilliant Red X-3B | – | Room temperature | NaCl 40 g/L Na2CO3 10 g/L | 41% | [14] |
| Amino Acid | Content (%wt) | |||
|---|---|---|---|---|
| Tussah Silk | Mulberry Silk | Wool | ||
| Hydroxy amino acids | ||||
| Serine | Ser | 14.80 | 15.98 | 9.04 |
| Tyrosine | Tyr | 10.60 | 11.29 | 6.38 |
| Threonine | Thr | 0.20 | 1.49 | 6.55 |
| Total | 25.60 | 28.76 | 21.97 | |
| Alkaline amino acids | ||||
| Lysine | Lys | 0.17 | 0.56 | 2.82 |
| Argnine | Arg | 5.41 | 0.98 | 10.49 |
| Histidine | His | 1.55 | 0.30 | 0.90 |
| Total | 7.13 | 1.84 | 14.21 | |
| C.I. Reactive | Trade Name | Chromophore | Molecular Mass (g/mol) | Number of –SO3Na |
|---|---|---|---|---|
| Sulfatoethylsulfone dye (SES) | ||||
| Orange 16 | Reactive Orange 16 | Monoazo | 617.5 | 1 |
| Red 180 | Reactive Red 180 | Monoazo | 933.8 | 3 |
| Black 5 | Reactive Black 5 (Bis(SES)) | Bisazo | 991.8 | 2 |
| Blue 19 | Starzol Brill. Blue KN-RHG | Anthraquinone | 626.6 | 1 |
| Monochlorotriazine dye (MCT) | ||||
| Yellow 3 | Reactive Yellow 3 | Monoazo | 637.0 | 2 |
| Red 24 | Reactive Red 24 | Monoazo | 788.1 | 3 |
| Blue 13 | Procion Blue H-5R | Monoazo (1:1 Cu complex) | 942.2 | 4 |
| Sulfatoethylsulfone/monochlorotriazine dye (SES/MCT) | ||||
| Yellow 160 | Starzol Brill. Yellow LB-4GLN | – | – | – |
| – | Starzol Yellow LB-3RD | Monoazo | – | – |
| Yellow 145 | Starzol Yellow LB-4RFN | Monoazo | 1026.3 | 3 |
| – | Starzol Scarlet LB-3G | Monoazo | – | – |
| Red 195 | Starzol Red LB-3BF | Monoazo | 1136.3 | 4 |
| Blue 194 | Starzol Dark Blue LB-2GLN | Bisazo | 1205.4 | 4 |
| Blue 231 | Starzol Turquoise Blue LB-BGFN | Phthalocyanine | – | – |
| – | Starzol Black LB-ED | Azo | – | – |
| Bis(monochlorotriazine) dye (Bis(MCT)) | ||||
| Orange 84 | Evercion Orange H-ER | Bisazo | 1850.3 | 8 |
| Red 141 | Evercion Red H-E7B | Bisazo | 1774.2 | 8 |
| Blue 171 | Evercion Navy Blue H-ER | Bisazo | 1418.9 | 6 |
| Dyeing Method | Dye | Exhaustion (%) | Fixation (%) | Fixation Efficiency (%) | Fastness | ||
|---|---|---|---|---|---|---|---|
| Color Change | Staining | ||||||
| Silk | Cotton | ||||||
| Alkali (NaHCO3) | Oange 16 | 84.37 | 79.61 | 94.36 | 5 | 4–5 | 4–5 |
| Red 180 | 77.39 | 72.59 | 93.80 | 4–5 | 5 | 4–5 | |
| Blue 19 | 91.54 | 85.19 | 93.06 | 4–5 | 5 | 5 | |
| Black 5 | 84.65 | 82.80 | 97.81 | 4–5 | 4–5 | 5 | |
| No alkali | Oange 16 | 66.93 | 58.70 | 87.70 | 5 | 5 | 4–5 |
| Red 180 | 78.87 | 73.22 | 92.84 | 4–5 | 4–5 | 4–5 | |
| Blue 19 | 77.97 | 65.21 | 83.63 | 4–5 | 4–5 | 5 | |
| Black 5 | 82.99 | 78.27 | 94.31 | 4–5 | 4–5 | 5 | |
| Dye | Color Change | Staining | |
|---|---|---|---|
| Silk | Cotton | ||
| Oange 16 | 5 | 5 | 4–5 |
| Red 180 | 4–5 | 4–5 | 4–5 |
| Blue 19 | 5 | 4–5 | 5 |
| Black 5 | 4–5 | 4–5 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Tang, R. Dyeing of Tussah Silk with Reactive Dyes: Dye Selection, Dyeing Conditions, Dye Fixation Characteristics, and Comparison with Mulberry Silk. Molecules 2024, 29, 1151. https://doi.org/10.3390/molecules29051151
Yu Y, Tang R. Dyeing of Tussah Silk with Reactive Dyes: Dye Selection, Dyeing Conditions, Dye Fixation Characteristics, and Comparison with Mulberry Silk. Molecules. 2024; 29(5):1151. https://doi.org/10.3390/molecules29051151
Chicago/Turabian StyleYu, Yingjie, and Rencheng Tang. 2024. "Dyeing of Tussah Silk with Reactive Dyes: Dye Selection, Dyeing Conditions, Dye Fixation Characteristics, and Comparison with Mulberry Silk" Molecules 29, no. 5: 1151. https://doi.org/10.3390/molecules29051151
APA StyleYu, Y., & Tang, R. (2024). Dyeing of Tussah Silk with Reactive Dyes: Dye Selection, Dyeing Conditions, Dye Fixation Characteristics, and Comparison with Mulberry Silk. Molecules, 29(5), 1151. https://doi.org/10.3390/molecules29051151







