Investigating the Effects of Donors and Alkyne Spacer on the Properties of Donor-Acceptor-Donor Xanthene-Based Dyes
Abstract
1. Introduction
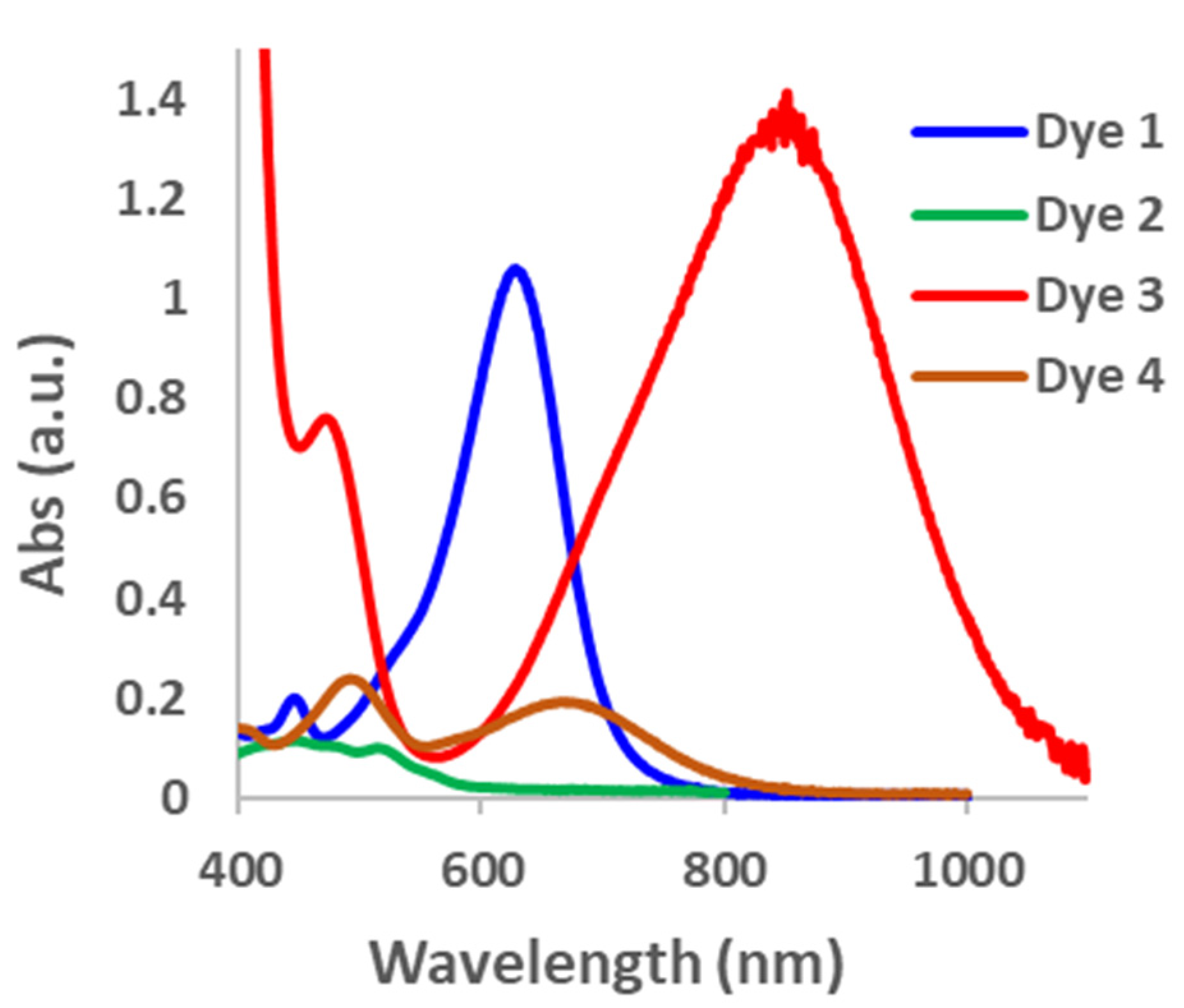
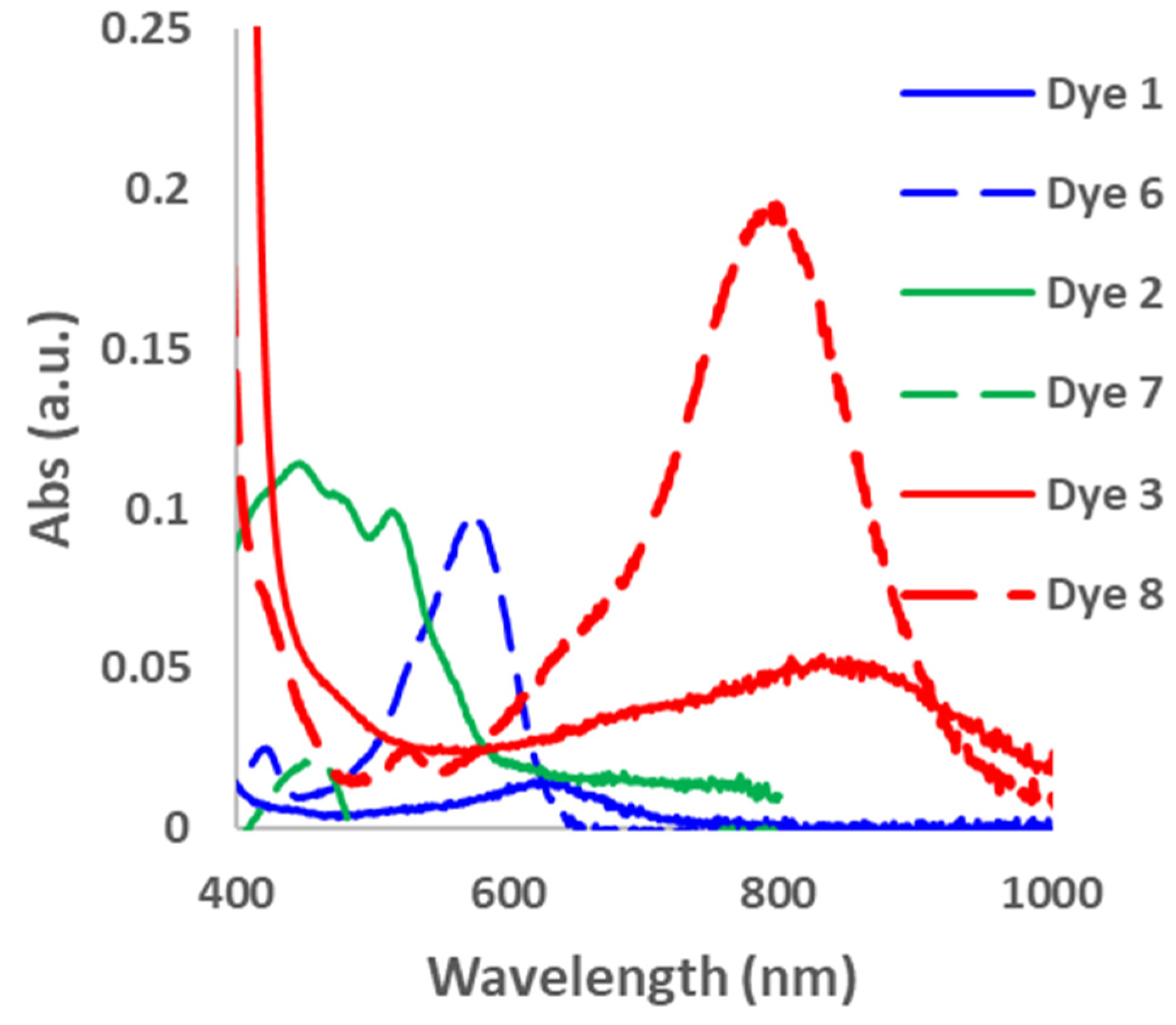
2. Results and Discussions
3. Materials and Methods
- Synthesis
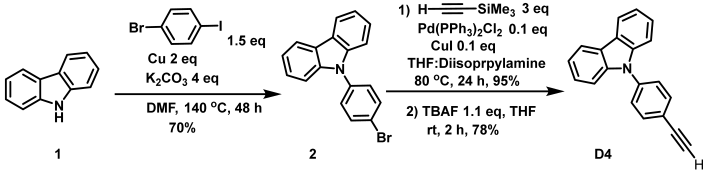
- Synthesis of 9-(4-ethynylphenyl)-9H-carbazole (D4)
- General procedure for synthesis of Dyes 1–4
- 3′,6′-bis((4-methoxyphenyl)ethynyl)-3H-spiro[isobenzofuran-1,9’-xanthen]-3-one (Dye 1)
- Synthesis of 3’,6’-bis((4-(dimethylamino)phenyl)ethynyl)-3H-spiro[isobenzofuran-1,9’-xanthen]-3-one (Dye 2)
- Synthesis of 3’,6’-bis((4-(diphenylamino)phenyl)ethynyl)-3H-spiro[isobenzofuran-1,9’-xanthen]-3-one (Dye 3)
- Synthesis of 3’,6’-bis((4-(9H-carbazol-9-yl)phenyl)ethynyl)-3H-spiro[isobenzofuran-1,9’-xanthen]-3-one (Dye 4)
- General procedure for synthesis of Dyes 6–8
- 3′,6′-bis(4-methoxyphenyl)-3H-spiro[isobenzofuran-1,9’-xanthen]-3-one (Dye 6)
- Synthesis of 3’,6’-bis(4-(dimethylamino)phenyl)-3H-spiro[isobenzofuran-1,9’-xanthen]-3-one (Dye 7)
- Synthesis of 3’,6’-bis(4-(diphenylamino)phenyl)-3H-spiro[isobenzofuran-1,9’-xanthen]-3-one (Dye 8)
- Analysis of the ring opening of each probe using different equivalents of TFA
- Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kim, J.H.; Liess, A.; Stolte, M.; Krause, A.-M.; Stepanenko, V.; Zhong, C.; Bialas, D.; Spano, F.; Würthner, F. An Efficient Narrowband Near-Infrared at 1040 nm Organic Photodetector Realized by Intermolecular Charge Transfer Mediated Coupling Based on a Squaraine Dye. Adv. Mater. 2021, 33, 2100582. [Google Scholar] [CrossRef]
- Vashishtha, P.; Bishnoi, S.; Li, C.H.A.; Jagadeeswararao, M.; Hooper, T.J.N.; Lohia, N.; Shivarudraiah, S.B.; Ansari, M.S.; Sharma, S.N.; Halpert, J.E. Recent Advancements in Near-Infrared Perovskite Light-Emitting Diodes. ACS Appl. Electron. Mater. 2020, 2, 3470–3490. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Fakharuddin, A.; García de Arquer, F.P.; Georgiadou, D.G.; Kim, H.; Mohd Yusoff ARb Gao, F.; Nazeeruddin, M.K.; Bolink, H.J.; Sargent, E.H. Advances in solution-processed near-infrared light-emitting diodes. Nat. Photonics 2021, 15, 656–669. [Google Scholar] [CrossRef]
- Koumura, N.; Wang, Z.-S.; Mori, S.; Miyashita, M.; Suzuki, E.; Hara, K. Alkyl-Functionalized Organic Dyes for Efficient Molecular Photovoltaics. J. Am. Chem. Soc. 2006, 128, 14256–14257. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Zheng, R.; Zhao, Y.; Zhang, E.; Dou, L.; Yang, Y. Near-Infrared Materials: The Turning Point of Organic Photovoltaics. Adv. Mater. 2022, 34, 2107330. [Google Scholar] [CrossRef]
- Khalid, M.; Khan, M.U.; Razia E-t Shafiq, Z.; Alam, M.M.; Imran, M.; Akram, M.S. Exploration of efficient electron acceptors for organic solar cells: Rational design of indacenodithiophene based non-fullerene compounds. Sci. Rep. 2021, 11, 19931. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-P.; Li, C.-T.; Ho, K.-C. Use of organic materials in dye-sensitized solar cells. Mater. Today 2017, 20, 267–283. [Google Scholar] [CrossRef]
- Li, W.; Liu, Z.; Xu, X.; Cheng, Y.-B.; Zhao, Z.; He, H. Near-infrared absorbing porphyrin dyes with perpendicularly extended π-conjugation for dye-sensitized solar cells. RSC Adv. 2014, 4, 50897–50905. [Google Scholar] [CrossRef]
- Kolemen, S.; Cakmak, Y.; Erten-Ela, S.; Altay, Y.; Brendel, J.; Thelakkat, M.; Akkaya, E.U. Solid-State Dye-Sensitized Solar Cells Using Red and Near-IR Absorbing Bodipy Sensitizers. Org. Lett. 2010, 12, 3812–3815. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Chen, W.; Tan, Y.; Chen, H.; Yin, J. Photodynamic therapy based on organic small molecular fluorescent dyes. Chin. Chem. Lett. 2019, 30, 1689–1703. [Google Scholar] [CrossRef]
- Lange, N.; Szlasa, W.; Saczko, J.; Chwiłkowska, A. Potential of Cyanine Derived Dyes in Photodynamic Therapy2021. Pharmaceutics 2021, 13, 818. [Google Scholar] [CrossRef] [PubMed]
- Bassan, E.; Gualandi, A.; Cozzi, P.G.; Ceroni, P. Design of BODIPY dyes as triplet photosensitizers: Electronic properties tailored for solar energy conversion, photoredox catalysis and photodynamic therapy. Chem. Sci. 2021, 12, 6607–6628. [Google Scholar] [CrossRef] [PubMed]
- Schnermann, M.J. Organic dyes for deep bioimaging. Nature 2017, 551, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, J.O.; Rusin, O.; Lim, S.; Strongin, R.M. NIR dyes for bioimaging applications. Curr. Opin. Chem. Biol. 2010, 14, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Tian, R.; Antaris, A.L.; Chen, X.; Dai, H. Near-Infrared-II Molecular Dyes for Cancer Imaging and Surgery. Adv. Mater. 2019, 31, 1900321. [Google Scholar] [CrossRef] [PubMed]
- Ilina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine Dyes: Molecular Design for Different Applications and Remaining Challenges. Bioconjugate Chem. 2020, 31, 194–213. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Moradi, R.; Lashgari, N.; Kruger, H.G. Chapter 15—Squaraine Dyes. In Metal-Free Synthetic Organic Dyes; Ziarani, G.M., Moradi, R., Lashgari, N., Kruger, H.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 193–196. [Google Scholar]
- Mujumdar, R.B.; Ernst, L.A.; Mujumdar, S.R.; Waggoner, A.S. Cyanine dye labeling reagents containing isothiocyanate groups. Cytometry 1989, 10, 11–19. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Yue, X.; Dai, Z. Cyanine Conjugate-Based Biomedical Imaging Probes. Adv. Healthc. Mater. 2020, 9, 2001327. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Moradi, R.; Lashgari, N.; Kruger, H.G. Chapter 8—Cyanine Dyes. In Metal-Free Synthetic Organic Dyes; Ziarani, G.M., Moradi, R., Lashgari, N., Kruger, H.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 127–152. [Google Scholar]
- Gopika, G.S.; Prasad, P.M.H.; Lekshmi, A.G.; Lekshmypriya, S.; Sreesaila, S.; Arunima, C.; Kumar, M.S.; Anil, A.; Sreekumar, A.; Pillai, Z.S. Chemistry of cyanine dyes—A review. Mater. Today Proc. 2021, 46, 3102–3108. [Google Scholar] [CrossRef]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Sun, X.; Wang, W.; Yang, D.; Yang, C.; Shen, Q.; Shao, J. Diketopyrrolopyrrole-derived organic small molecular dyes for tumor phototheranostics. Chin. Chem. Lett. 2022, 33, 1681–1692. [Google Scholar] [CrossRef]
- Grzybowski, M.; Gryko, D.T. Diketopyrrolopyrroles: Synthesis, Reactivity, and Optical Properties. Adv. Opt. Mater. 2015, 3, 280–320. [Google Scholar] [CrossRef]
- Auwalu, M.A.; Cheng, S. Diketopyrrolopyrrole Fluorescent Probes, Photophysical and Biological Applications. Chemosensors 2021, 9, 44. [Google Scholar] [CrossRef]
- Shabir, G.; Saeed, A.; Ali Channar, P. A Review on the Recent Trends in Synthetic Strategies and Applications of Xanthene Dyes. Mini Rev. Org. Chem. 2018, 15, 166–197. [Google Scholar] [CrossRef]
- Karaman, O.; Alkan, G.A.; Kizilenis, C.; Akgul, C.C.; Gunbas, G. Xanthene dyes for cancer imaging and treatment: A material odyssey. Coord. Chem. Rev. 2023, 475, 214841. [Google Scholar] [CrossRef]
- Kamino, S.; Uchiyama, M. Xanthene-based functional dyes: Towards new molecules operating in the near-infrared region. Org. Biomol. Chem. 2023, 21, 2458–2471. [Google Scholar] [CrossRef]
- Kim, H.N.; Lee, M.H.; Kim, H.J.; Kim, J.S.; Yoon, J. A new trend in rhodamine-based chemosensors: Application of spirolactam ring-opening to sensing ions. Chem. Soc. Rev. 2008, 37, 1465–1472. [Google Scholar] [CrossRef]
- Fu, M.; Xiao, Y.; Qian, X.; Zhao, D.; Xu, Y. A design concept of long-wavelength fluorescent analogs of rhodamine dyes: Replacement of oxygen with silicon atom. Chem. Commun. 2008, 15, 1780–1782. [Google Scholar] [CrossRef]
- Kushida, Y.; Nagano, T.; Hanaoka, K. Silicon-substituted xanthene dyes and their applications in bioimaging. Analyst 2015, 140, 685–695. [Google Scholar] [CrossRef]
- Ikeno, T.; Nagano, T.; Hanaoka, K. Silicon-substituted Xanthene Dyes and Their Unique Photophysical Properties for Fluorescent Probes. Chem.–Asian J. 2017, 12, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, A.; Suda, S.; Taki, M.; Yamaguchi, E.; Grzybowski, M.; Sato, Y.; Higashiyama, T.; Yamaguchi, S. Phospha-fluorescein: A red-emissive fluorescein analogue with high photobleaching resistance. Chem. Commun. 2016, 52, 1120–1123. [Google Scholar] [CrossRef]
- Grzybowski, M.; Taki, M.; Senda, K.; Sato, Y.; Ariyoshi, T.; Okada, Y.; Kawakami, R.; Imamura, T.; Yamaguchi, S. A Highly Photostable Near-Infrared Labeling Agent Based on a Phospha-rhodamine for Long-Term and Deep Imaging. Angew. Chem. Int. Ed. 2018, 57, 10137–10141. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, H.; Tanaka, Y.; Taki, M.; Yamaguchi, S. Late-stage functionalisation of alkyne-modified phospha-xanthene dyes: Lysosomal imaging using an off–on–off type of pH probe. Chem. Sci. 2021, 12, 7902–7907. [Google Scholar] [CrossRef] [PubMed]
- Martineau, M.; Somasundaram, A.; Grimm, J.B.; Gruber, T.D.; Choquet, D.; Taraska, J.W.; Lavis, L.D.; Perrais, D. Semisynthetic fluorescent pH sensors for imaging exocytosis and endocytosis. Nat. Commun. 2017, 8, 1412. [Google Scholar] [CrossRef]
- Grimm, J.B.; Sung, A.J.; Legant, W.R.; Hulamm, P.; Matlosz, S.M.; Betzig, E.; Lavis, L.D. Carbofluoresceins and Carborhodamines as Scaffolds for High-Contrast Fluorogenic Probes. ACS Chem. Biol. 2013, 8, 1303–1310. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Yang, Y.; Chen, H. A Unique Class of Near-Infrared Functional Fluorescent Dyes with Carboxylic-Acid-Modulated Fluorescence ON/OFF Switching: Rational Design, Synthesis, Optical Properties, Theoretical Calculations, and Applications for Fluorescence Imaging in Living Animals. J. Am. Chem. Soc. 2012, 134, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, I.; Chang, H.; Xiong, Y.; Marder, S.; Gwaltney, S.R.; Scott, C.N. New Design Strategy Toward NIR I Xanthene-Based Dyes. J. Org. Chem. 2020, 85, 12108–12116. [Google Scholar] [CrossRef]
- Huang, T.; Wang, Q.; Meng, G.; Duan, L.; Zhang, D. Accelerating Radiative Decay in Blue through-Space Charge Transfer Emitters by Minimizing the Face-to-Face Donor–Acceptor Distances. Angew. Chem. Int. Ed. 2022, 61, e202200059. [Google Scholar]
- Rathnamalala, C.S.L.; Gayton, J.N.; Dorris, A.L.; Autry, S.A.; Meador, W.; Hammer, N.I.; Delcamp, J.H.; Scott, C.N. Donor–Acceptor–Donor NIR II Emissive Rhodindolizine Dye Synthesized by C–H Bond Functionalization. J. Org. Chem. 2019, 84, 13186–13193. [Google Scholar] [CrossRef]
- Koide, Y.; Urano, Y.; Hanaoka, K.; Terai, T.; Nagano, T. Evolution of Group 14 Rhodamines as Platforms for Near-Infrared Fluorescence Probes Utilizing Photoinduced Electron Transfer. ACS Chem. Biol. 2011, 6, 600–608. [Google Scholar] [CrossRef]
- Best, Q.A.; Sattenapally, N.; Dyer, D.J.; Scott, C.N.; McCarroll, M.E. pH-Dependent Si-Fluorescein Hypochlorous Acid Fluorescent Probe: Spirocycle Ring-Opening and Excess Hypochlorous Acid-Induced Chlorination. J. Am. Chem. Soc. 2013, 135, 13365–13370. [Google Scholar] [CrossRef] [PubMed]
- Rathnamalala, C.S.L.; Pino, N.W.; Herring, B.S.; Hooper, M.; Gwaltney, S.R.; Chan, J.; Scott, C.N. Thienylpiperidine Donor NIR Xanthene-Based Dye for Photoacoustic Imaging. Org. Lett. 2021, 23, 7640–7644. [Google Scholar] [CrossRef] [PubMed]
- Rathnamalala, C.S.L.; Hernandez, S.; Lucero, M.Y.; Swartchick, C.B.; Kalam Shaik, A.; Hammer, N.I.; East, A.K.; Gwaltney, S.R.; Chan, J.; Scott, C.N. Xanthene-Based Nitric Oxide-Responsive Nanosensor for Photoacoustic Imaging in the SWIR Window. Angew. Chem. Int. Ed. 2023, 62, e202214855. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; He, Z.; Zhao, Y.; Yang, Y.; Shi, W.; Li, X.; Ma, H. Xanthene-Based NIR-II Dyes for In Vivo Dynamic Imaging of Blood Circulation. J. Am. Chem. Soc. 2021, 143, 17136–17143. [Google Scholar] [CrossRef]
- Krishna, J.G.; Ojha, P.K.; Kar, S.; Roy, K.; Leszczynski, J. Chemometric modeling of power conversion efficiency of organic dyes in dye sensitized solar cells for the future renewable energy. Nano Energy 2020, 70, 104537. [Google Scholar] [CrossRef]
- Estrada, L.A.; Neckers, D.C. Synthesis and Photophysics of Dibenz[a,c]phenazine Derivatives. Org. Lett. 2011, 13, 3304–3307. [Google Scholar] [CrossRef]
- Woodroofe, C.C.; Lim, M.H.; Bu, W.; Lippard, S.J. Synthesis of isomerically pure carboxylate- and sulfonate-substituted xanthene fluorophores. Tetrahedron 2005, 61, 3097–3105. [Google Scholar] [CrossRef]
- Ramsingh Girase, T.; Bhilare, S.; Sankar Murthy Bandaru, S.; Chrysochos, N.; Schulzke, C.; Sanghvi, Y.S.; Kapdi, A.R. Carbazole-Based N-Heterocyclic Carbenes for the Promotion of Copper-Catalyzed Palladium-Free Homo-/Hetero-Coupling of Alkynes and Sonogashira Reactions. Asian J. Org. Chem. 2020, 9, 274–291. [Google Scholar] [CrossRef]
- Hebbar, N.; Fiol-Petit, C.; Ramondenc, Y.; Plé, G.; Plé, N. A new series of rod-like conjugated molecules with a pyrazine or a bipyrazine core. Synthesis and light emitting properties. Tetrahedron 2011, 67, 2287–2298. [Google Scholar] [CrossRef]
- Creamer, A.; Casey, A.; Marsh, A.V.; Shahid, M.; Gao, M.; Heeney, M. Systematic Tuning of 2,1,3-Benzothiadiazole Acceptor Strength by Monofunctionalization with Alkylamine, Thioalkyl, or Alkoxy Groups in Carbazole Donor–Acceptor Polymers. Macromolecules 2017, 50, 2736–2746. [Google Scholar] [CrossRef]
- Kwon, O.; Barlow, S.; Odom, S.A.; Beverina, L.; Thompson, N.J.; Zojer, E.; Brédas, J.-L.; Marder, S.R. Aromatic Amines: A Comparison of Electron-Donor Strengths. J. Phys. Chem. A 2005, 109, 9346–9352. [Google Scholar] [CrossRef] [PubMed]
- Hinckley, D.A.; Seybold, P.G. A spectroscopic/thermodynamic study of the rhodamine B lactone ⇌ zwitterion equilibrium. Spectrochim. Acta Part A Mol. Spectrosc. 1988, 44, 1053–1059. [Google Scholar] [CrossRef]
- Grimm, J.B.; Lavis, L.D. Synthesis of Rhodamines from Fluoresceins Using Pd-Catalyzed C–N Cross-Coupling. Org. Lett. 2011, 13, 6354–6357. [Google Scholar] [CrossRef]
- Breiten, B.; Wu, Y.-L.; Jarowski, P.D.; Gisselbrecht, J.-P.; Boudon, C.; Griesser, M.; Onitsch, C.; Gescheidt, G.; Schweizer, W.B.; Langer, N.; et al. Donor-substituted octacyano[4]dendralenes: A new class of cyano-rich non-planar organic acceptors. Chem. Sci. 2011, 2, 88–93. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Applications and validations of the Minnesota density functionals. Chem. Phys. Lett. 2011, 502, 1–13. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. ‘Contracted Gaussian basis sets for molecular calculations, I. Second row atoms, Z=11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods, X.X. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Hehre, W.J. Ab initio molecular orbital theory. Acc. Chem. Res. 1976, 9, 399–406. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J.; Mennucci, B.; Tomasi, J.; Cammi, R.; Barone, V. Geometries and properties of excited states in the gas phase and in solution: Theory and application of a time-dependent density functional theory polarizable continuum model. J. Chem. Phys. 2006, 124, 094107. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. GaussView, version 5.0; Gaussian, Inc.: Wallingford, CT, USA, 2016.



| Dyes | ΔH | ΔG | Keq |
|---|---|---|---|
| 1—OCH3 | 20.7 | 18.5 | 2.65677 × 10−14 |
| 2—N(CH3)2 | 23.6 | 21.2 | 2.77359 × 10−16 |
| 3—NPh2 | 22.1 | 20.1 | 1.77928 × 10−15 |
| 4—Carb | 23.2 | 21.4 | 1.97824 × 10−16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajapaksha, I.N.; Wang, J.; Leszczynski, J.; Scott, C.N. Investigating the Effects of Donors and Alkyne Spacer on the Properties of Donor-Acceptor-Donor Xanthene-Based Dyes. Molecules 2023, 28, 4929. https://doi.org/10.3390/molecules28134929
Rajapaksha IN, Wang J, Leszczynski J, Scott CN. Investigating the Effects of Donors and Alkyne Spacer on the Properties of Donor-Acceptor-Donor Xanthene-Based Dyes. Molecules. 2023; 28(13):4929. https://doi.org/10.3390/molecules28134929
Chicago/Turabian StyleRajapaksha, Ishanka N., Jing Wang, Jerzy Leszczynski, and Colleen N. Scott. 2023. "Investigating the Effects of Donors and Alkyne Spacer on the Properties of Donor-Acceptor-Donor Xanthene-Based Dyes" Molecules 28, no. 13: 4929. https://doi.org/10.3390/molecules28134929
APA StyleRajapaksha, I. N., Wang, J., Leszczynski, J., & Scott, C. N. (2023). Investigating the Effects of Donors and Alkyne Spacer on the Properties of Donor-Acceptor-Donor Xanthene-Based Dyes. Molecules, 28(13), 4929. https://doi.org/10.3390/molecules28134929