Abstract
Microbial cell factories, renowned for their economic and environmental benefits, have emerged as a key trend in academic and industrial areas, particularly in the fermentation of natural compounds. Among these, plant-derived terpenes stand out as a significant class of bioactive natural products. The large-scale production of such terpenes, exemplified by artemisinic acid—a crucial precursor to artemisinin—is now feasible through microbial cell factories. In the fermentation of terpenes, two-phase fermentation technology has been widely applied due to its unique advantages. It facilitates in situ product extraction or adsorption, effectively mitigating the detrimental impact of product accumulation on microbial cells, thereby significantly bolstering the efficiency of microbial production of plant-derived terpenes. This paper reviews the latest developments in two-phase fermentation system applications, focusing on microbial fermentation of plant-derived terpenes. It also discusses the mechanisms influencing microbial biosynthesis of terpenes. Moreover, we introduce some new two-phase fermentation techniques, currently unexplored in terpene fermentation, with the aim of providing more thoughts and explorations on the future applications of two-phase fermentation technology. Lastly, we discuss several challenges in the industrial application of two-phase fermentation systems, especially in downstream processing.
1. Introduction
Terpenes (hydrocarbons) or terpenoids (oxygen-containing derivatives), a highly chemically diverse family of natural products predominantly found in plants, possess a wide range of potent biological activities, including antimicrobial, antitumor, antiviral, antioxidant, anti-inflammatory, analgesic, digestive, and immunomodulatory effects [1,2,3]. Consequently, they have extensive applications in the medical field, food, and cosmetics. Notably, two terpene-based drugs, Taxol® (anticancer) and artemisinin (antimalarial), have achieved clinical acclaim [4,5]. Traditionally, terpenes have been sourced primarily through plant extraction. Nevertheless, this method is hampered by several factors, such as slow plant growth, geographical limitations, and environmental conditions, which ultimately lead to low purity and yield, inefficient processes, and elevated costs. For example, producing just 2 g of pure Taxol® requires about four trees of Pacific yew, highlighting the difficulty in meeting the escalating market and medicinal needs [4]. Chemical synthesis, as an alternative, often involves harsh conditions and lacks regioselectivity and stereoselectivity, complicating the synthesis of certain terpenes [6,7].
Recently, employing microorganisms for biosynthesizing plant-derived terpenes (PDTs) has proven effective [8,9,10,11]. Microorganisms offer advantages like rapid growth, genetic manipulability, environmental friendliness, and cost-effectiveness, making them suitable for terpene production [11,12,13]. Significant progress in this field includes constructing and regulating synthetic pathways, modifying key enzymes, and optimizing fermentation processes [11,13,14,15]. For example, Sudha Shukal and colleagues [16] achieved a significant milestone by biosynthesizing amorphadiene, a precursor of artemisinin, in Escherichia coli, yielding 30 g/L.
Between 1992 and 2001, several reviews addressed two-phase fermentation (TPF) systems, also known as “partitioning bioreactors”, “two-phase partitioning bioreactors (TPPB)”, extractive fermentation, or “in situ product removal (ISPR)” [17,18,19]. These systems, incorporating solvents or solids into cultures, effectively isolate specific metabolites, finding applications in various fields, including environmental biotechnology [20], and the microbial production of plant secondary metabolites [21,22]. A notable example is the use of TPF in amorphadiene production, where Keasling’s team [23] demonstrated that the two-phase culture significantly enhances volatile terpene yield by 8.5 times, markedly advancing terpene biosynthesis, particularly for volatile compounds.
Recently, several improved methods utilizing TPF systems have been developed for the biosynthesis of PDTs. For example, the resin Amberlite-XAD4, replacing organic solvents, has been employed for the TPF of α-humulene [24]. Additionally, alternative organic solvents like isopropyl myristate or methyl oleate have been used instead of n-dodecane for the fermentation of amorphadiene [25]. However, only a few reviews focusing on TPF systems for the production of PDTs have been reported. This review aims to discuss the recent advancements in TPF concerning the production of PDTs through microbial fermentation, with an emphasis on different types of TPF systems, their advantages, applications, influencing factors, limitations, and the economic considerations in downstream processing. For clarity and simplicity in subsequent discussions, the term ‘terpenes’ was chosen to collectively refer to all terpenoids produced by plants.
2. Types of TPF Systems
The culture medium, known as the aqueous phase, supports cell growth, while the alternative phase, which can be liquid, solid, or a combination of both, is referred to as the SP. TPF systems are typically classified into liquid–liquid and liquid–solid systems based on the distinctive properties of the SPs [17,19,20,21] (Figure 1). This discussion aims to provide a comprehensive overview of TPF applications beyond PDT fermentation, highlighting diverse TPF systems’ potential across various fields. For instance, the integration of immobilized cells as a solid phase in terpene fermentation, despite its promise, remains underexplored.
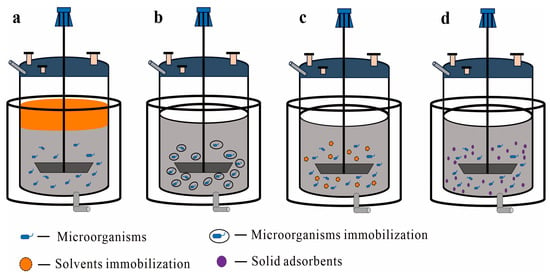
Figure 1.
A concept of different types of TPF in a bioreactor: (a) liquid–liquid TPF systems, where the orange color on the upper layer represents organic solvents or another aqueous solvent and the dark gray color on the lower layer represents the culture medium; (b–d) different types of liquid–solid TPF systems: (b) immobilized cells as the SP; (c) immobilized solvent as the SP; (d) solid adsorbents as the SP.
2.1. Liquid–Liquid TPF Systems
Liquid–liquid TPF systems consist of two immiscible phases: an aqueous phase containing the microorganisms and nutrients, and an SP comprising a mixture of compounds, that may be water-insoluble, such as organic solvents or liquid lipophilic compounds, or water-soluble, such as polymers or salts [21,26,27] (Figure 1a). Systems with water-insoluble organic compounds are termed aqueous–organic two-phase systems (AOTPS), while those containing water-soluble compounds are referred to as aqueous two-phase systems (ATPS) [26,28].
2.1.1. Aqueous-Organic TPF Systems
Aqueous-organic systems involve an aqueous and an immiscible organic phase, facilitating efficient product separation through in situ extraction [18,19,29]. The interaction between these phases allows for the dispersion of droplets, enhancing the extraction and separation of fermentation products [19,21] (Figure 2). Since Inoue and Horikoshi’s discovery in 1989 [30], which revealed varying tolerances of microorganisms to organic solvents and led to the isolation of the solvent-tolerant bacterium Pseudomonas putida IH 2000, organic solvents have been increasingly used in fermentation systems for in situ product extraction. For instance, Suzanne Verhoef and colleagues [31] utilized two solvent-tolerant P. putida S12 strains, employing glucose as the primary substrate to efficiently produce hydroxystyrene. This led to a final concentration of 21 mM, which was a fourfold increase compared to single-phase fed-batch cultivation. Similarly, Nicola Tan and colleagues [32] focused on trans-nerolidol, a valuable fragrance with antimalarial and anticancer properties, extensively used in cosmetics and agriculture. Under single-phase fed-batch fermentation, the strains produced over 6.8 g/L of nerolidol in 3 days. In contrast, two-phase extractive fed-batch fermentation yielded about 16 g/L of nerolidol in 4 days, with a carbon yield of approximately 9% (g/g), marking the highest yield achieved to date.
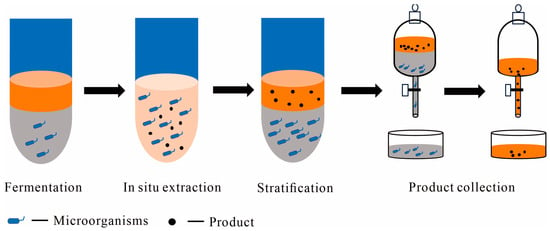
Figure 2.
Scheme of aqueous–organic TPF and post-treatment, with main steps including fermentation, in situ extraction, post-fermentation stratification, and product collection; the orange color on the upper layer represents organic solvent or another aqueous solvent, the dark gray color on the lower layer represents the culture medium, and the light orange color of in situ extraction represents two phases mixed during the in situ extractive fermentation.
2.1.2. Aqueous Two-Phase System
The aqueous two-phase system (ATPS), also referred to as an aqueous biphasic system (ABS), represents a biotechnological approach used in both fermentation and extraction processes [33,34]. It consists of two immiscible aqueous phases, usually formed by the combination of two water-soluble polymers, a polymer and a salt or two different salts. This system is environmentally preferable over traditional organic solvent-based TPF systems due to its aqueous nature and nonvolatile components [28,34]. The ATPS is primarily used in ex situ extraction processes, offering an alternative to conventional methods [28,35]. Ionic liquids (ILs), which are salts in the liquid state at low temperatures, have been effectively used for the extraction and purification of fermentation-derived components, showcasing sustainability, efficiency, and eco-friendliness [33,36]. However, the application of ATPSs for in situ extraction remains limited, mainly due to ATPSs’ cytotoxicity to cells and the challenge of finding an appropriate formulation for the fermentation process.
H. González-Peñas and coworkers [37] performed a solvent screening for in situ liquid extraction from acetone–butanol–ethanol (ABE) fermentation by Clostridium acetobutylicum. They selected methyltrioctylammonium chloride and trihexyl (tetradecyl) phosphonium chloride for their extraction capacity, demonstrating high distribution coefficients. Despite lower selectivity due to significant water extraction, this research highlighted ILs’ potential for in situ extraction processes. Deep eutectic solvents (DESs) represent an emerging class of eutectic mixtures of Lewis or Brønsted acids and bases, capable of forming a variety of anionic and cationic species [38,39]. Due to the similar characteristics and properties with ILs, DESs are widely known as IL analogs. For example, Liu Jingyang and his team [40] chose a DES composed of choline chloride and urea for the in situ extraction of L-valine produced by Brevibacterium flavum XV0505. Optimizing the timing and volumetric fraction of the IL addition, it was shown that adding 0.1% of this IL at the 16th hour of the fermentation process led to the XV0505 strain producing the highest yield of L-valine in both shake-flask and fed-batch fermentation experiments. Similarly, Parul Badhwar and colleagues [41] selected the Aureobasidium pullulans strain for cost-effective pullulan production and developed a new ATPS for fermentation. They conducted a comprehensive study of the effects of different molecular weights of polyethylene glycol (PEG) (400, 600, 4000, and 6000) and dextran or mono/di-sodium phosphate salts on the fermentation process. The PEG-dextran ATPS system was found to be suitable for the extractive fermentation of pullulan from A. pullulans, achieving a yield of 36.47 g/L. Although ILs showed lower selectivity, their high distribution coefficients indicate a strong potential for targeted extractions, highlighting the need for further optimization in selecting ILs for in situ fermentation processes [42].
2.2. Liquid–Solid TPF Systems
As defined by Sonia Malik et al. [21], liquid–solid TPF systems involve in situ adsorption with an aqueous medium and a solid phase comprising adsorbents or lipophilic materials. This review expands on the use of solids as an SP, including the integration of immobilized cells or solutions as the solid phase [43,44] (Figure 1b–d). Immobilization refers to the containment or fixation of cells or solutions on or within a matrix [44,45,46]. This process prevents their release during the fermentation while ensuring adequate permeability for the diffusion of substrates and products [45]. In this context, the immobilized cells or solutions effectively serve as a solid phase (Figure 1b,c). Liquid–solid systems show distinct advantages, such as simplified post-fermentation processing and the reusability of immobilized cells.
2.2.1. Immobilized Cells as the Solid Phase
Immobilized cells involve anchoring active cells (as shown in Figure 3), serving as biological catalysts, onto a carrier to create a stable structure [43]. Key carriers include solid particles, gels, and membranes [46]. This approach improves cell stability and reusability, thereby streamlining operational and control processes and enhancing their suitability for industrial applications.
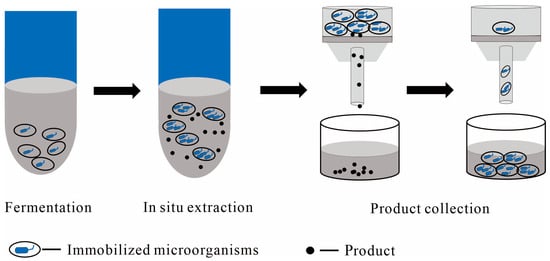
Figure 3.
Illustration of a flowchart using immobilized cells as the solid phase in the liquid–solid TPF and downstream processing, with main steps including fermentation, in situ extraction, post-fermentation filtration, and product collection; the dark gray color represents the culture medium.
Cell immobilization techniques encompass a variety of methods [43,47]. Adhesion and adsorptive immobilization entail the physical adsorption of cells onto a carrier’s surface. This process depends on the physicochemical properties of both the cells and the carrier. For instance, brewing yeast immobilization onto spent grains involves cell–carrier adhesion, cell–cell attachment, and cell adsorption inside carrier crevices, affected by factors like dilution rate and the hydrophobicity of the carrier [48]. Covalent bonding immobilization involves attaching cells to a carrier via covalent bonds. The efficiency of immobilization is significantly influenced by the quantity and accessibility of reactive groups on the cell surface, which are in turn affected by physiological factors. Thus, covalent coupling is not a major technique used for cell immobilization [47]. Encapsulation immobilization, also known as microencapsulation, involves encapsulating cells within a carrier to create anchored colloidal particles. This technique encompasses coating or entrapping microbial cells with a polymeric material, resulting in the formation of microspheres [49]. Microencapsulation provides several benefits, including increased cell loading capacity, improved cell survival, and a higher production rate of desired microbial products [50]. This technology has been widely applied in various fields involving microbial cells, including the microencapsulation of probiotics [51]. Gel immobilization is characterized by immobilizing cells within a gel-like substance, while entrapment immobilization traps cells within a matrix or polymer. The design of robust matrices, such as macroporous gels with immobilized microbial cells, has shown high efficiency and structural stability [46]. These gels allow for the high retained activity of yeast and E. coli cells even after drying and storage, demonstrating their practicality in stirred bioreactors [43,46].
Immobilized cells or enzymes are widely used in biotransformation, with recent papers offering comprehensive overviews of cell or enzyme immobilization techniques in this field [43,46,47]. However, the utilization of immobilized cell technology for producing PDTs remains limited. For example, El-Sayed R. et al. [52] immobilized two mutant strains of Aspergillus fumigatus and Alternaria tenuissima using five different entrapment carriers of calcium alginate, agar-agar, Na-CMC, gelatin, and arabic gum. Among these, calcium alginate gel beads proved to be the most effective and suitable entrapment carrier for maximum production of paclitaxel. Considering the limited cell immobilization reports, the present part aims to introduce several representative examples involving fermentative production via immobilized cell technology, with the goal of providing useful references and insights for the production of PDTs.
Product inhibition by butanol and acetone is a significant limitation in ABE fermentation. Rizki Fitria Darmayanti and colleagues [53] developed an innovative biobutanol extractive fermentation method using a large volume ratio of extractant with immobilized Clostridium saccharoperbutylacetonicum N1-4. The preculture cells of the N1-4 strain were fixed in calcium alginate beads, effectively maintaining a low butanol concentration in the aqueous phase and achieving a total butanol concentration of 64.6 g/L. In a study conducted by Sion Ham and colleagues [54], they utilized engineering techniques and immobilized whole cells of E. coli to establish a small-scale reactor system, successfully achieving continuous and efficient production of γ-aminobutyric acid (GABA). Remarkably, these anchored cells maintained high activity after 15 consecutive uses, whereas free cells lost activity after the ninth reaction. Moreover, after optimizing conditions such as buffer concentration, substrate concentration, and flow rate, the researchers successfully achieved continuous operation for 96 h in a 14 mL scale reactor, producing a total of 165 g of GABA. This research not only presents a viable method for producing high concentrations of GABA but also highlights the superior performance of immobilized microbial cells in the process. Weysser Felipe Cândido de Souza et al. [55] utilized an immobilization system comprising 2.0% w/v alginate, 2.0% w/v CaCl2, 2.0% w/v gelatin, and 0.2% w/v transglutaminase to immobilize Erwinia sp. D12 cells. Their experiments revealed that isomaltulose production reached its peak at 327.83 g/L within the first 24 h and that the cells remained stable over 72 h of continuous reaction, maintaining consistent isomaltulose output. This demonstrates that using ionic gelation to immobilize Erwinia sp. D12 cells are an effective method for enhancing sucrose-to-isomaltulose conversion. S. cerevisiae, a widely favored chassis cell, has shown tremendous potential in producing PDTs [56,57]. Although the technology for immobilizing S. cerevisiae cells has not yet been applied in the aforementioned fermentation field, the techniques for immobilizing or encapsulating S. cerevisiae cells are already quite mature in other biotransformation areas. These studies provide valuable experience and reference for future use of immobilized yeast in the production of terpenoid compounds.
2.2.2. Immobilized Solvent as the Solid Phase
The solution immobilization system involves integrating solution chemical substances with solid carriers to create solid particles or agglomerates, as illustrated in Figure 4. This technology is commonly used in separation and purification processes, enhancing product purity and minimizing waste. The previous discussion of liquid–liquid TPF introduced several biphasic systems, with a focus on the immobilization of ILs [44,58]. ILs are commonly immobilized onto materials like silica or polymers through physical confinement or covalent grafting, mainly enhancing organic catalysis and separation.
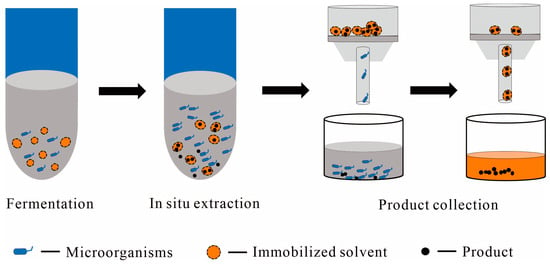
Figure 4.
A conceptual scheme of using immobilized solvent as the solid phase in the liquid–solid TPF and downstream processing, with main steps including fermentation, in situ extraction, post-fermentation filtration, and product collection; the dark gray color represents the culture medium and the orange color represents solvents, like ILs.
For example, Changhee Lee and colleagues [59] immobilized the lipase B (CALB) from Candida Antarctica and 1-octyl-3-methylimidazolium tetrafluoroborate in a polymeric hybrid monolith, obtaining an enzyme-SILP (e-SILP) catalyst. This catalyst was effective in continuous gas-phase transesterification of vinyl propionate and 2-propanol. Additionally, ILs were anchored on silica as a stationary phase for compound separation and purification. Another application of solution immobilization involved the same CALB in ester enzyme reaction systems. To address the solubility mismatch between enzymes and substrates, a Pickering gel emulsion stabilized by enzyme-modified polymer nanomaterials was developed, facilitating biphasic biocatalysis. These nanomaterials, produced surfactant-free via emulsion polymerization and covalently attached to CALB, were mixed with heptane to create an aqueous dispersion, enhancing nanoparticle decoration. Impressively, CALB immobilized in this emulsion achieved a 96.5% conversion rate and retained 92.5% of its activity after 10 reaction cycles [60]. Similarly, Susanne Wiese et al. [61] employed microgels in emulsions to improve the interaction between oil and water phases, forming droplets encapsulating both enzyme- and substrate-containing oil. The microgels positioned at the droplet interface facilitated substrate conversion. Post-reaction, heating beyond the microgels’ volume phase transition temperature induced emulsion breakdown, which allowed for product recovery via macroscopic phase separation.
2.2.3. Solid Adsorbents as the Solid Phase
Solid adsorbents like polymer beads and resins are preferred for in situ extractive fermentation due to their ability to efficiently adsorb and remove products from the aqueous phase, simplifying the process by eliminating extra separation steps (Figure 5). These adsorbents, particularly effective for volatile compounds, offer a nontoxic alternative to organic solvents.
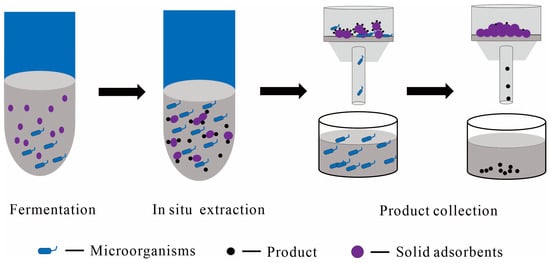
Figure 5.
A conceptual flowchart using solid adsorbents as the solid phase in the liquid–solid TPF and downstream processing, with main steps including fermentation, in situ extraction, post-fermentation filtration, and product collection; the dark gray color represents the culture medium.
As early as 2009, Guillermo Quijano et al. [20] detailed the use of solid-phase adsorbents in TPF for environmental biotechnology, notably in wastewater treatment. Sonia Malik et al. [21] provided insights into the application of adsorbent resins in plant cell fermentation, with a dedicated chapter focusing on the selection of appropriate adsorbents and operating conditions. Furthermore, Thomas Phillips and colleagues [22] explored the use of adsorbent resins in the microbial fermentation of natural products, employing in situ solid-phase adsorption techniques. Their paper not only delved into the underlying mechanisms but also examined the influence of in situ adsorption on the biosynthesis of microbial natural products. Given this extensive precedence, our objective here is to provide a concise overview of this technology, highlighting notable examples. The application of adsorbent resins in microbial fermentation systems for the production of PDTs will be discussed in detail in a subsequent section.
Jianxu Li and coworkers [62] evaluated an integrated in situ fermentation and in situ product recovery process aimed at enhancing the output of the antibiotic compound beauvericin (BEA) in Fusarium redolens Dzf2 mycelial cultivation. For this purpose, they employed macroporous polystyrene resin (X-5) as the adsorbent (encased in nylon bags), introducing it into flasks containing fungal mycelia. The findings indicated a significant increase in BEA volumetric production, from 194 mg/L to 265 mg/L by Day 7, with 65% of BEA adsorbed onto the resin. Renewing the resin and adding glucose on Day 7 further elevated BEA output to 400 mg/L by Day 9, effectively doubling the yield compared to the batch control culture. Haishan Qi et al. [63] introduced adsorbent resin HP20 during the fermentation of Streptomyces hygroscopicus var. ascomyceticus FS35. Following a metabolic profiling analysis and subsequent rational fermentation optimization, the production of ascomycin by S. hygroscopicus var. ascomyceticus FS35 significantly increased to 460 mg/L in a 168 h fermentation period. This represents a 53.3% enhancement compared to the yield under initial fermentation conditions. These case studies highlight the potential of solid adsorbent strategies, particularly adsorbent resins, in amplifying the production of significant natural products and refining processes.
3. The Advantages of TPF Systems
In microbial fermentation, increased yield is often hindered by the accumulation of fermentation products. Integrating fermentation with in situ extraction presents an effective strategy to mitigate this issue. This integrated approach accelerates product formation, boosts yield, and simplifies downstream processing. Among various two-phase systems, aqueous–organic and liquid–solid (resin) TPF technologies are particularly prominent and mature in microbial fermentation. Thus, subsequent chapters will extensively discuss the benefits of these TPF systems.
3.1. Enhance Productivity
Numerous studies have demonstrated that in situ product extraction, employing either a liquid (organic solvent) or solid (resin) phase, significantly enhances production. Microbial cells in the culture medium synthesize products, which are then extracted or adsorbed by the SP, disrupting the equilibrium and promoting product release. For instance, in β-elemene biosynthesis by E. coli, strategies like efflux protein enhancement and the use of n-dodecane as an organic phase in fermentation increased the β-elemene yield to 3.52 g/L [64].
3.1.1. Reducing Toxicity to Microbial Cells
Targeted products and harmful metabolites released during fermentation can inhibit microbial growth and production. Some monoterpenes and phenolic compounds can impair cell walls, membranes, and organelle membranes, diminish the activity of specific enzymes within the cells, obstruct normal cellular functions, and ultimately result in microbial death. TPF technology, by enabling simultaneous production and separation, efficiently extracts or adsorbs both products and nontarget metabolites, enhancing microbial tolerance and productivity. For example, Wei Liu and colleagues [65] discovered that during batch-fed fermentation of an engineered E. coli strain producing geraniol, introducing isopropyl myristate to establish an aqueous–organic two-phase system significantly prevented the volatilization of the target product and diminished its cellular toxicity. This method resulted in a notable increase in product yield.
3.1.2. Alleviating Feedback Inhibition
Product accumulation may lead to feedback inhibition, impeding the activity of enzymes in the biosynthetic pathway. While most secondary metabolites produced by microbial cells are hydrophobic with low solubility in the culture medium, even minimal concentrations can inhibit enzymes involved in their biosynthesis. TPF is instrumental in facilitating in situ extraction or adsorption of products, effectively alleviating feedback inhibition in the biosynthetic pathway or affecting cell membrane transport. For example, in the study conducted by Jorge H. Santoyo-Garcia et al. [66], it was found that the resin could remove the products/reactive oxygen species’ (ROS) effects in the production of paclitaxel by Taxus baccata vascular stem cells. This removal is crucial as it prevents the activation of secondary undesired pathways, inhibits cell growth, or diverts the metabolic flux towards side products.
3.1.3. Preventing Product Degradation and Loss
In the fermentation process, some enzymes in microbial cells or acidic substances in the fermentation system can degrade certain metabolites, particularly at high concentrations. The TPF systems can ensure that secondary metabolites are maximally protected from degradation by the microorganisms’ own enzymes, effectively limiting the loss of products in cell culture. Taking salinosporamide A as an example, this natural molecule is produced by marine actinomycete Salinispora tropica and has a half-life of 140 min. Adding 2% (w/v) XAD-7 resin at 24 h of fermentation increased the yield from 5.7 mg/L to 278 mg/L, suggesting that the resin may protect the product from degradation [67]. The hydrophobic and volatile characteristics of some terpenes primarily contribute to product loss in microbial production processes. To mitigate the volatile losses of terpenes, a prevalent strategy is employing TPF with organic solvents. These solvents not only decrease the volatility of terpenes but also reduce their toxicity to cells, thereby enhancing productivity.
3.2. Industrial Application: Cost-Effective and Downstream Processing
The primary goal of microbial fermentation research is scaling to industrial production, often hindered by complex and costly downstream processes. TPF technology can address this challenge, reducing post-fermentation costs and facilitating scale-up.
3.2.1. Increase in Cell Biomass and Recycling of the Second Phase
In TPF systems, the SP extracts or adsorbs cellular products, fostering cell growth and increasing microbial cell biomass and yield compared to traditional approaches. On the other hand, the recycling of the SP is another key feature of the TPF system. In industrial production, effective separation and recycling techniques allow multiple uses of the SP, minimizing downstream processing costs.
3.2.2. Reduction of Post-Processing Steps
Utilizing TPF systems obviates the need for intricate product harvesting procedures, preserving cell integrity and not interfering with the culture process, thereby minimizing the costs and time. Traditional fermentation typically necessitates numerous steps such as organic solvent extraction, concentration, and distillation to isolate the product. TPF technology streamlines these processes. For instance, in liquid–liquid TPF, the product, extracted by the SP organic solvent during fermentation, eliminates the need for extraction. The fermentation broth is centrifuged, and the organic solvent is directly concentrated, followed by distillation to retrieve the product. In solid–liquid TPF, a concentration step is unnecessary; organic solvents are directly employed to elute and extract from adsorbents like macroporous resins, followed by distillation, reducing industrial post-processing steps.
4. Applications of TPF in Microbial Production of Terpenes
Terpenes represent a very important class of secondary metabolites in plants, with over 80,000 structural types identified to date [68]. These compounds, composed of isoprene units (C5 units), vary in the number of isoprene units they contain [69], leading to classifications such as monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), sesterterpenes (C25), triterpenes (C30), sesquarterpenes (C35), tetraterpenes (C40), and polyterpenes (C > 40). Currently, there are several marketed plant-derived terpenoid drugs, such as paclitaxel, β-elemene, and artemisinin. Additionally, terpenes are highly favored in the fragrance and cosmetic industry, featuring components like menthol and ambergris [70].
The biosynthesis of terpenes in plants is complex but well understood [8,10,11,70]. As shown in Figure 6, isoprene isopentenyl diphosphate (IPP) and dimethyl allyl phosphate (DMAPP) are common precursors for all terpenes. One molecule of DMAPP and varying numbers of IPP condense under the influence of prenyltransferases to produce different terpene precursors. These precursors are then converted into various terpene skeletons under the action of various terpene synthases (TPs). The mevalonate (MVA) and 2-C-methyl-d-erythritol 4-phosphate (MEP) pathways are two distinct metabolic routes for the biosynthesis of terpenes in plants. The MVA pathway starts with acetyl-CoA and proceeds through six enzymatic steps to produce IPP and DMAPP, the basic building blocks for isoprenoid synthesis [71]. The MEP pathway uses pyruvate and glyceraldehyde 3-phosphate (G3P) as substrates and involves seven enzymatic reactions to produce IPP and DMAPP [72]. Given the well-characterized biosynthetic pathways of PDTs, the utilization of microbial engineering holds significant potential as an effective alternative for the production of desired terpenes.
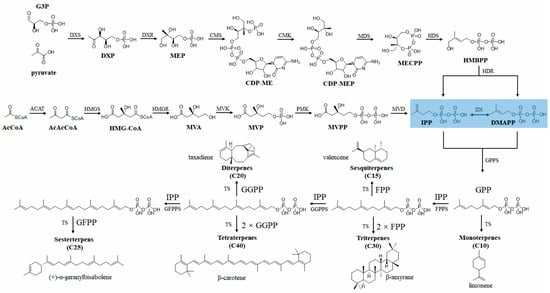
Figure 6.
Metabolic pathway of terpene biosynthesis. The terpenes’ skeletons are formed by the condensation of multiple units of IPP and its isomer, DMAPP. MEP biosynthetic pathway starts with pyruvate and G3P. Through a series of enzyme-catalyzed reactions, it ultimately produces IPP and DMAPP (shown on a light blue background). This process involves a variety of enzymes, DXS (1-deoxy-d-xylulose-5-phosphate synthase) and DXR (1-deoxy-d-xylulose-5-phosphate reductoisomerase), CMS (2-C-methyl-d-erythritol 4-phosphate cytidyltransferase), CMK (4-diphosphocytidyl-2-C-methyl-d-erythritol kinase), MDS (2-C-methyl-d-erythritol 2,4-cyclodiphosphate synthase), HDS (4-hydroxy-3-methylbut-2-enyl diphosphate synthase), and HDR (4-hydroxy-3-methylbut-2-enyl diphosphate reductase). The MVA biosynthetic pathway, which is another pathway for terpene biosynthesis, distinct from the MEP pathway, starts with acetyl-CoA. The primary enzymes involved in the MVA pathway are acetyl-CoA acetyltransferase (ACAT), hydroxymethylglutaryl-CoA synthase (HMGS), hydroxymethylglutaryl-CoA reductase (HMGR), mevalonate kinase (MVK), phosphomevalonate kinase (PMK), mevalonate-5-pyrophosphate decarboxylase (MVD), and isopentenyl-diphosphate delta-isomerase (IDI). Geranyl diphosphate synthase (GPPS), farnesyl diphosphate synthase (FPPS), geranylgeranyl diphosphate synthase (GGPPS), and farnesylgeranyl diphosphate synthase (GFPPS) TPs convert the basic terpene precursors, IPP and DMAPP, into various terpene compounds. Abbreviation of metabolites: DXP, 1-deoxy-d-xylulose-5-phosphate; MEP, 2-C-methyl-d-erythritol-4-phosphate; CDP-ME, 4-diphosphocytidyl-2-C-methyl-d-erythritol; CDP-MEP, 4-diphosphocytidyl-2-C-methyl-d-erythritol 2-phosphate; MEcPP, 2-C-methyl-d-erythritol-2,4-cyclodiphosphate; HMB-PP, 4-hydroxy-3-methylbut-2-enyl-diphosphate; AcAc-CoA, acetoacetyl-CoA; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; MVA, mevalonate; MVP, mevalonate-5-phosphate; MVPP, mevalonate-5-pyrophosphate; IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl pyrophosphate; GPP, geranyl pyrophosphate; FPP, farnesyl pyrophosphate; GGPP, geranylgeranyl diphosphate; GFPP, farnesylgeranyl diphosphate.
4.1. Microbial Production of Plant-Derived Terpenes
Microorganisms with a short growth cycle and minimal environmental impact are an ideal choice for PDT biosynthesis [73]. The microbial synthesis of terpenes, particularly with E. coli and S. cerevisiae, aligns with the goals of green and sustainable development due to their well-characterized metabolic pathways, genetic tractability, and suitability for large-scale fermentation [14]. These organisms offer the benefits of operational simplicity and cost-effectiveness, leveraging inexpensive substrates for efficient growth [74]. Further, the advancement in molecular and synthetic biology has led to the successful utilization of other microbial chassis such as Yarrowia lipolytica and Rhodosporidium toruloides in terpenoid biosynthesis. Y. lipolytica, recognized for its lipid production and “Generally Recognized as Safe” (GRAS) status [75], excels at utilizing renewable carbon sources and exhibits a high acetyl-CoA flux, making it a potent producer of acetyl-CoA-derived products [76]. R. toruloides, known for its broad substrate range and inhibitor tolerance, emerges as another viable host for high-value compound production [77]. TPF technology, encompassing both aqueous–organic solvent and aqueous–solid TPF systems, is pivotal in the microbial production of terpenes, enhancing the efficiency and quality of products such as β-elemene [78] and artemisinic acid [79]. The modifications of terpenes’ biosynthetic pathways in the microbial cell factory and the key enzymes engineering this process have been discussed by other comprehensive reviews. In this part, we focus on terpenes with validated fermentation experiments, emphasizing the preferred mode of microbial fermentation production, and exclude those only studied for their biosynthetic pathways, such as sesquiterpenes and sesquarterpenes.
Given the scope of this review, we have comprehensively collated most of the results concerning plant-derived terpenes in the process of microbial fermentation up to November 2023, which can be found in Supplementary Materials Tables S1–S5. For illustrative purposes, we have selectively highlighted the top-yielding examples of each terpene achieved using various chassis cells, employing different fermentation techniques (such as two-phase and non-two-phase fermentation), and across diverse fermentation systems and scales.
4.1.1. Monoterpenes
Monoterpenes, the simplest terpenes comprising two isoprene units, are key to the aromatic profiles of many plants’ essential oils [70]. Recent TPF applications have shown significant promise in enhancing monoterpene production, and Table 1 provides a comprehensive overview of these recent advancements. Taking geraniol as an example, it is not only an acyclic monoterpene isolated from plant essential oils that has been extensively utilized in the flavor industry for the past few decades but has also garnered considerable interest in recent years as a potential biofuel [65]. By overexpressing the synthase and heterologous MVA pathway in E. coli and subsequently employing fed-batch fermentation with isopropyl myristate as the organic phase, the production of geraniol was significantly enhanced, resulting in a yield of 2 g/L compared to the initial 78.8 mg/L obtained after basic fermentation in a bioreactor [65]. In another study, by adding the same SP in fed-batch fermentation, the production of geraniol was further increased to 13.19 g/L [80]. Different organic solvents, such as n-decane [81] and n-dodecane [82], had been employed as the SP in the TPF, which also increased the production of geraniol.

Table 1.
Summary of the fermentation results for monoterpenes, categorized by various chassis cells, fermentation scales, the second phase, and production outputs. This summary specifically highlights the results with the highest yield under each condition.
The demand for linalool, particularly as a flavoring agent, has been escalating, especially in the realm of processed foods and beverages. Achieving stable and cost-effective production of linalool is essential, as current extraction methods yield limited quantities and are not economically viable. To overcome the volatility of linalool in aqueous solutions and its high toxicity to microorganisms during fermentation production, an in situ extraction fermentation using isopropyl myristate as the SP was developed, resulting in 5.60 g/L (S)-linalool and 3.71 g/L (R)-linalool [113].
Limonene, the principal monoterpene in citrus fruit essential oils, is also found in oak, pine, and spearmint. Recently, it has garnered attention as a potential alternative or additive for jet fuel [125,126]. Although limonene is currently produced mainly as a by-product of orange juice manufacturing, the low concentration in natural sources makes its isolation economically unfeasible. Willrodt et al. [127] constructed an E. coli strain carrying a dual-plasmid system and performed a two-phase fed-batch operation in a bioreactor. The addition of an inert organic solvent prevented product inhibition, toxic effects, and limonene evaporation losses [90]. Diisononyl phthalate (DINP) has a good partition coefficient and has no detectable effect on E. coli growth [128]. When using DINP as the organic phase, the final limonene concentration reached 3630 mg/L [90]. The above examples showed that the appropriate use of biphasic fermentation technology and selection of suitable organic solvents as the SP can effectively enhance the monoterpenes’ productivity.
In the TPF systems, beyond utilizing organic solvents like n-dodecane as the SP, the resin can also be employed as an adsorbent in this phase to enhance monoterpene production. For example, by inducing cells with IPTG and arabinose to increase P450 expression levels and using Amberlite resin to extract the product, the production of perillyl alcohol can be boosted to 105 mg/L [102].
4.1.2. Sesquiterpenes
Sesquiterpenes, with their 15-carbon backbone derived from three isoprene units, exhibit a remarkable diversity in chemical structures and shapes, making them a significant class of terpene known for various structures and functions [129]. These compounds, found in numerous plants, contribute to the unique scents and flavors of many essential oils and have notable biological activities, including the antimalaria drug artemisinin. Unfortunately, the yield of artemisinin extracted from plant extracts is low, increasing the cost of treatments, and is affected by weather and environmental factors. Keasling’s team [130] focused on engineering microbes for the biosynthesis of artemisinin. By reconstructing the MVA pathway from S. cerevisiae into E. coli and regulating the relevant genes, they successfully produced amorphadiene in E. coli, which is the precursor of artemisinin. Using TPF technology, they innovatively used n-dodecane [79], isopropyl myristate [25], and methyl oleate [25] as the organic phase, providing new ideas and methods for the microbial metabolic synthesis of terpenoid compounds. The following Table 2 is a summary of the fermentation results for sesquiterpenes, categorized by various chassis cells, fermentation types and scales, the second phase, and production outputs. This summary specifically highlights the results with the highest yield under each condition; the fermentation data of most sesquiterpenes are attached in Supplementary Materials Table S2.
The isomers α-farnesene and β-farnesene play a crucial role in plant–insect interactions and possess significant economic value in pharmaceuticals, cosmetics, seasonings, and bioenergy [131]. Recent studies have successfully leveraged microbial metabolic engineering for the heterologous production of farnesene. E. coli, S. cerevisiae, and Y. Lipolytica have been successfully engineered for farnesene production [132,133,134,135,136]. Produced through batch fermentation with n-dodecane as the SP in a bioreactor, the production of α-farnesene reached 10.4 g/L [137]. An optimized S. cerevisiae strain in a 20,000 L bioreactor, with polymers and olefins as extractants, significantly increased α-farnesene yield to 130 g/L [138]. You et al. [136] engineered an E. coli strain overexpressing β-farnesene with IDI and FPPs, minimizing IPP accumulation. Using n-decane as the organic phase, this strain achieved a final titer of 8.74 g/L. In Y. lipolytica, the fusion expression of farnesene synthase and FPPs enhanced α-farnesene synthesis, reduced intracellular accumulation of mevalonate, and yielded 25.55 g/L of α-farnesene in TPF with n-dodecane [139]. Although the yield of α-farnesene through flask fermentation, employing resin as an adsorbent and Anabaena sp. as the cell factory, is presently limited to 305.4 μg/L [140], this observation suggests the viability of resin as an SP within the TPF system for sesquiterpene production. This research introduces a new concept and methodology into the realm of fermentation processes for sesquiterpenoid synthesis.

Table 2.
Summary of the selected highest yield of sesquiterpenes, categorized based on various chassis cells, fermentation scales, the second phase, and production outputs.
Table 2.
Summary of the selected highest yield of sesquiterpenes, categorized based on various chassis cells, fermentation scales, the second phase, and production outputs.
| Sesquiterpenes | Chassis Cells | Fermentation Types and Scales | Second Phases | Titers (mg/L) | References |
|---|---|---|---|---|---|
| amorphadiene | E. coli | flask | none | 112.2 | [130] |
| 14 mL tube | n-dodecane | 300 | [141] | ||
| 250 mL flask | n-dodecane | 1400 | [142] | ||
| 250 mL bioreactor | n-dodecane | 30,000 | [16] | ||
| S. cerevisiae | 250 mL flask | n-dodecane | 497 | [143] | |
| 2 L bioreactor | n-dodecane | 41,000 | [79] | ||
| flask | isopropyl myristate | 4000 | [25] | ||
| 2 L bioreactor | methyl oleate | 40,000 | [25] | ||
| Y. lipolytica | 250 mL flask | n-dodecane | 171.5 | [144] | |
| R. toruloides | 2 L bioreactor | n-dodecane | 36 | [145] | |
| B. subtilis | flask | n-dodecane | 20 | [146] | |
| S. elongatus | 100 mL flask | n-hexadecane | 19.8 | [147] | |
| α-farnesene | E. coli | 500 mL flask | n-decane | 1100 | [133] |
| S. cerevisiae | 250 mL flask | n-dodecane | 1477.2 | [137] | |
| 5 L bioreactor | n-dodecane | 10,400 | [137] | ||
| Y. lipolytica | 300 mL flask | n-dodecane | 1700 | [139] | |
| 1 L bioreactor | n-dodecane | 25,550 | [139] | ||
| S. elongatus | flask | n-dodecane | 4.6 | [148] | |
| Anabaena sp. | 250 mL flask | supelpak 2sv resin columns | 0.3054 | [140] | |
| P. pastoris | flask | n-dodecane | 2560 | [149] | |
| β-farnesene | E. coli | 5 L bioreactor | n-decane | 10,310 | [150] |
| 0.5 L flask | n-decane | 5290 | [151] | ||
| Y. lipolytica | 2.5 mL tubes | n-dodecane | 955 | [152] | |
| 2 L bioreactor | n-decane | 22,800 | [153] | ||
| bisabolene | E. coli | flask | n-dodecane | 1150 | [87] |
| 5 L bioreactor | n-dodecane | 9100 | [154] | ||
| S. cerevisiae | 125 mL flask | n-dodecane | 994 | [155] | |
| 2 L bioreactor | n-dodecane | 5200 | [79] | ||
| Synechococcus sp. | 250 mL flask | n-dodecane | 0.6 | [156] | |
| 3 L bioreactor | n-dodecane | 22.5 | [157] | ||
| R. toruloides | 2 L bioreactor | n-dodecane | 680 | [145] | |
| nerolidol | E. coli | 5 L bioreactor | n-dodecane | 16,000 | [32] |
| S. cerevisiae | 250 mL flask | n-dodecane | 497 | [158] | |
| 5 L bioreactor | n-dodecane | 7010 | [32] | ||
| α-humulene | E. coli | 2 L bioreactor | Amberlite XAD4 resin | 60.2 | [24] |
| bioreactor | n-dodecane | 0.958 | [159] | ||
| S. cerevisiae | 5 L bioreactor | n-dodecane | 1726.78 | [160] | |
| patchoulol | E. coli | 5 L bioreactor | n-dodecane | 970 | [161] |
| S. cerevisiae | 1.1 L flask | n-dodecane | 42.1 | [162] | |
| 5 L bioreactor | n-dodecane | 1632 | [163] | ||
| valencene | S. cerevisiae | 300 mL flask | n-dodecane | 31 | [164] |
| 3 L bioreactor | n-dodecane | 264.6 | [165] | ||
| Y. lipolytica | flask | n-dodecane | 22.8 | [166] | |
| C. glutamicum | 100 mL flask | n-dodecane | 2.41 | [167] | |
| R. sphaeroides | 250 mL flask | n-dodecane | 352 | [168] | |
| Synechocystis sp. | flask | isopropyl myristate | 9.6 | [169] | |
| germacrene A | E. coli | flask | none | 6.325 | [170] |
| 250 mL flask | n-dodecane | 364.26 | [171] | ||
| 4 L bioreactor | n-dodecane | 3520 | [64] | ||
| S. cerevisiae | flask | n-dodecane | 375 | [172] | |
| Y. lipolytica | 5 L bioreactor | isopropyl myristate | 39,000 | [78] | |
| P. pastoris | 1 L bioreactor | n-dodecane | 1900 | [173] | |
| O. polymorpha | 250 mL bioreactor | n-dodecane | 4700 | [174] | |
| α-santalene | E. coli | 1.3 L bioreactor | isopropyl myristate | 2916 | [175] |
| S. cerevisiae | 2.5 L flask | n-dodecane | 92 | [176] | |
| 5 L bioreactor | n-dodecane | 163 | [177] | ||
| Y. lipolytica | 5 L bioreactor | n-dodecane | 27.92 | [178] | |
| β-caryophyllene | E. coli | 25 mL flask | none | 100 | [179] |
| 5 L bioreactor | none | 1520 | [180] | ||
| 5 L bioreactor | n-dodecane | 5142 | [181] | ||
| S. cerevisiae | 1.3 L bioreactor | n-dodecane | 2949.1 | [182] | |
| α-cuprenene | X. dendrorhous | 100 mL flask | n-dodecane | 80 | [183] |
| viridiflorol | E. coli | 250 mL bioreactor | n-dodecane | 25,700 | [16] |
| longifolene | E. coli | 5 L bioreactor | n-decane | 382 | [184] |
| (+)-zizaene | E. coli | 2 L bioreactor | diaion HP20 resin | 211 | [185] |
| valerenadiene | E. coli | flask | n-decane | 62 | [186] |
| protoilludene | E. coli | flask | n-decane | 1199 | [187] |
| farnesol | S. cerevisiae | flask | none | 70 | [188] |
| E. coli | flask | methyl oleate | 1419 | [189] | |
| epi-isozizaene | E. coli | 4 L bioreactor | n-decane | 727.9 | [190] |
| α-isocomene | E. coli | bioreactor | n-decane | 77.5 | [190] |
| pentalenene | E. coli | 2.5 L bioreactor | n-decane | 780.3 | [190] |
| α-neoclovene | S. cerevisiae | 1.3 L bioreactor | n-dodecane | 487.1 | [182] |
| valerenic acid | S. cerevisiae | flask | n-dodecane | 4 | [191] |
| zerumbone | S. cerevisiae | 5 L bioreactor | n-dodecane | 40 | [192] |
| prespatane | R. toruloides | 2 L bioreactor | n-dodecane | 1173.6 | [77] |
| santalols | S. cerevisiae | 5 L bioreactor | n-dodecane | 1300 | [193] |
| z-α-Santalol | S. cerevisiae | 5 L bioreactor | n-dodecane | 1200 | [193] |
| zerumbone | S. cerevisiae | 5 L bioreactor | n-dodecane | 40 | [192] |
β-elemene is a sesquiterpene extracted from Curcuma aromatica Salisb. ‘Wenyujin’ and is one of the most widely used antitumor drugs for the treatment of various cancer tumors in China [194]. The heterologous MVA pathway and cyanobacterial enzyme genes were concurrently introduced into E. coli, resulting in a β-elemene yield of 6325.5 µg/L in shaking bottles [170]. However, this yield is insufficient for industrial production. Recent studies have identified efficient synthases from algae and integrated key pathway enzymes, export genes, and translational engineering to implement TPF technology in bioreactors with n-dodecane as the SP, achieving a β-elemene yield of 3.52 g/L [64]. Y. lipolytica, serving as an exceptional cell factory, has been engineered to reconstruct the endogenous mevalonate pathway and regulate lipid metabolism, resulting in a β-elemene titer of 39 g/L in a bioreactor containing an isopropyl myristate organic phase [78].
4.1.3. Diterpenes
Diterpenes, with 20 carbon atoms from four isoprene units, exhibit vast structural diversity and a wide range of biological activities, making them crucial in pharmaceuticals, food additives, fragrance synthesis, and agriculture [195]. Paclitaxel (Taxol®), a compound found in the bark of the Pacific yew tree, stands out for its effectiveness against breast and ovarian cancers. The overexpression of enzymes in the MEP pathway and paclitaxel synthase in E. coli led to the successful creation of a strain capable of producing taxadiene, a key precursor of paclitaxel, with a yield of 1 g/L achieved through two-phase fed-batch fermentation using n-dodecane as the SP [196]. In the case of S. cerevisiae, both liquid–liquid TPF with n-dodecane and solid–liquid fermentation using silica gel as an adsorbent were effective in enhancing taxadiene yield, which gave the yield of 129 mg/L [197] and 8 mg/L [66], respectively. These findings underscore the efficacy of TPF technology in enhancing productivity (Table 3).
Another example of fermentation paclitaxel has been discussed above, where the mutant strains of A. fumigatus and A. tenuissima were immobilized by five different entrapment materials and successfully applied for production enhancement of paclitaxel. The paclitaxel titers obtained by the immobilized mycelia of the respective mutants, 694.67 and 388.65 μg/L, were promising for fungal production of paclitaxel [52]. Thus, the immobilized cell technology has shown considerable potential for application in the industrial-scale production of paclitaxel through biotechnological processes.
In the production of miltiradiene using S. cerevisiae as the cell factory, the use of n-dodecane as the SP increased the yield by up to ten times, archiving at 3.5 g/L [198]. By strengthening upstream pathways, regulating central carbon metabolism and cofactor supply, fusing and truncating terpenoid synthase genes, knocking out related regulatory factors, and using TPF technology with n-hexane, the yield of sclareol in the bioreactor reached 11.4 g/L [199].

Table 3.
Summary of the selected highest yield of diterpenes, categorized based on various chassis cells, fermentation scales, the second phase, and production outputs.
Table 3.
Summary of the selected highest yield of diterpenes, categorized based on various chassis cells, fermentation scales, the second phase, and production outputs.
| Diterpenes | Chassis Cells | Fermentation Types and Scales | Second Phases | Titers (mg/L) | References |
|---|---|---|---|---|---|
| miltiradiene | S. cerevisiae | 5 L bioreactor | none | 488 | [195] |
| 10 mL flask | n-dodecane | 550 | [198] | ||
| 5 L bioreactor | n-dodecane | 3500 | [198] | ||
| taxadiene | E. coli | flask | none | 1.3 | [200] |
| 2 L flask | n-dodecane | 570 | [201] | ||
| 1 L bioreactor | n-dodecane | 1020 | [196] | ||
| S. cerevisiae | 500 mL bioreactor | none | 33 | [202] | |
| 500 mL flask | RP18 silica gel | 8 | [66] | ||
| 500 mL bioreactor | n-dodecane | 129 | [197] | ||
| A. fumigatus | 250 mL flask | immobilization | 0.694 | [52] | |
| A.tenuissima | 250 mL flask | immobilization | 0.388 | [52] | |
| oxygenated taxane | S. cerevisiae | 1 L bioreactor | n-dodecane | 78 | [203] |
| ent-Kaurene | E. coli | 1 L bioreactor | none | 578 | [204] |
| 3 L bioreactor | n-dodecane | 624 | [205] | ||
| R. toruloides | 2 L bioreactor | n-dodecane | 1400 | [206] | |
| geranylgeraniol | S. cerevisiae | bioreactor | none | 3300 | [207] |
| flask | n-dodecane | 772.98 | [208] | ||
| 5 L bioreactor | n-dodecane | 5070 | [208] | ||
| steviol | E. coli | 2q L bioreactor | none | 1100 | [209] |
| 3 L bioreactor | n-dodecane | 38.4 | [205] | ||
| sclareol | E. coli | bioreactor | n-dodecane | 1500 | [210] |
| S. cerevisiae | 100 mL flask | n-dodecane | 750 | [211] | |
| 0.4 L bioreactor | n-hexane | 11,400 | [199] | ||
| levopimaradiene | E. coli | 3 L bioreactor | n-dodecane | 700 | [212] |
| levopimaric acid | S. cerevisiae | 5 L bioreactor | n-dodecane | 400.3 | [213] |
| retinoids | E. coli | 14 mL tube | n-dodecane | 33 | [214] |
| retinol | S. cerevisiae | 5 L bioreactor | n-dodecane | 2349 | [215] |
| Y. lipolytica | 5 L bioreactor | n-dodecane | 4860 | [216] | |
| cis-abienol | E. coli | bioreactor | isopropyl myristate | 634 | [217] |
| 13R-manoyl oxide | S. cerevisiae | 5 L bioreactor | n-dodecane | 3000 | [218] |
| forskolin | S. cerevisiae | 5 L flask | n-hexane | 40 | [219] |
| gibberellic acid 3 | Y. lipolytica | 24-roundwell plates | none | 12.8 | [220] |
| gibberellic acid 4 | Y. lipolytica | 24-roundwell plates | none | 17.3 | [220] |
| carnosic acid | S. cerevisiae | 30 mL flask | none | 25 | [221] |
| 5 L bioreactor | none | 75.2 | [221] | ||
| rubusoside | S. cerevisiae | 250 mL bioreactor | none | 1400 | [222] |
| rebaudiosides | S. cerevisiae | 250 mL bioreactor | none | 132.7 | [222] |
4.1.4. Triterpenes and Tetraterpenes
Triterpenes and tetraterpenes, composed of six and eight isoprene units, respectively, play diverse roles in nature and human applications [223,224,225]. Triterpenes are recognized for their biological activities, often utilized in traditional medicine for their anti-inflammatory, antiviral, and anticancer properties. Tetraterpenes are best known for their presence in colorful plant pigments, such as carotenoids [225]. Most economically valuable triterpenoids are water-soluble, featuring hydrophilic groups like carboxyl or sugar moieties, enabling their dissolution in the aqueous phase during microbial fermentation such as ginsenosides, thus bypassing the need for an extraction solvent phase. This eliminates the need for an extract SP (Table 4). Conversely, lipophilic triterpenoids, such as squalene—used in cosmetics, dietary supplements, and as a vaccine adjuvant—require TPF for biosynthesis. Employing yeast with n-dodecane as the solvent phase has yielded significant squalene production (207.02 mg/L) [137]. Similarly, protopanaxadiol, the precursor to ginsenosides, has been biosynthesized using yeast as a cell factory, with methyl oleate or n-dodecane serving as in situ extraction solvents, resulting in a yield of 1189 mg/L [226]. In contrast, tetraterpenoids, particularly plant pigments, are fat-soluble substances due to their hydrophobic structures. β-Carotene, a naturally occurring red-orange pigment and one of the important tetraterpenoids, plays a crucial role in maintaining vision, skin health, and a properly functioning immune system owing to its conversion into vitamin A. In general, β-carotenoids produced by microorganisms are intracellularly stored, not released outside the cell. For instance, Y. lipolytica has a large intracellular organelle for lipid storage, referred to as the lipid body. A literature investigation reveals that only lycopene has been subject to TPF in the cells of Y. lipolytica, using n-dodecane as the organic solvent, with a yield of 4.2 g/L [227] (Table 4). However, the purpose of adding n-dodecane was solely to minimize the evaporation of isoprenol, which is an additional substrate, and not for in situ extraction.

Table 4.
Summary of the fermentation results for triterpenes and tetraterpenes, categorized by various chassis cells, fermentation scales, the second phase, and production outputs. This summary specifically highlights the results with the highest yield under each condition.
5. Factors Influencing TPF Systems
Factors influencing the efficiency and effectiveness of TPF systems are varied and complex, impacting the overall success of the fermentation process. When selecting materials for the SP, factors such as chemical stability, potential toxicity, interactions with aqueous phase components, solubility, and the ability to stabilize released products must be considered. The ideal SP choice varies depending on the specific product being accumulated.
5.1. Solvent or Adsorbent as the Second Phase
In TPF systems, organic solvents or macroporous resins are typically employed as the SP, especially in the fermentation of terpenes. ILs, while available, are less suitable due to their significant toxicity towards microbial cells [271]. The impact of physical parameters during fermentation, such as high-speed stirring, and post-fermentation processing issues also needs to be evaluated to ensure the SP does not disrupt cell integrity or impede industrial scalability. The selection of solvents or adsorbents as the SP greatly influences the efficiency of the system. Ensuring compatibility with the microbial culture and the aqueous phase is crucial. Similarly, the choice of microbial strain for fermentation is vital, as different strains possess distinct tolerances and metabolic capabilities, which significantly impact the yield and efficiency of the fermentation process.
5.1.1. Solvent Selection Considerations
Solvent toxicity is a primary concern when selecting an SP. The chosen solvent should be minimally toxic to microbial cells, and its biocompatibility and biodegradability are important considerations. Biodegradability means that the microbial cells do not degrade the organic solvent during the fermentation. For example, the solvents may potentially be degraded and used as a carbon source by some microorganisms [272,273]. Regarding biocompatibility, it has been widely accepted that the solvent tolerance of microorganisms correlates with the log p parameter, where p is the 1-octanol/water partition coefficient in the two-phase system [29,274,275,276]. In general, solvents with log p values below 2 are in general toxic, and organic solvents with a log p > 4 have been found to be compatible with microbial cells [275]. However, the tolerance of a particular strain to an organic solvent is not always evaluated in a straightforward way and can be influenced by medium composition, cultivation conditions, and inoculum history. For instance, Philipp Demiling et al. [277] screened 18 different kinds of organic solvents for biocompatibility and biodegradability and found that ethyl decanoate showed high biocompatibility and negligible biodegradability for the biosynthesis of rhamnolipids by Pseudomonas putida KT2440. Selecting an appropriate solvent is pivotal for TPF efficiency and yield, necessitating a comprehensive evaluation of potential solvents based on microbial strain characteristics and fermentation conditions. In Jacek Kujawski et al.’s [278] article, they present an online tool—the ALOGPS 2.1 program—for the calculation of the log p values of compounds.
The target product’s solubility in the solvent, ensuring efficient extraction from the fermentation broth, is another critical factor. For example, the log p values of oleic acid and DBP are 7.7 and 5.4, respectively, and the partition coefficients of paclitaxel in these solvents are 154 and 236, respectively [21]. In addition, the solvent should have low volatility; solvents with low volatility are preferred to minimize losses due to evaporation and to reduce the risk of flammability and other safety hazards.
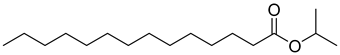
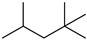
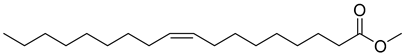
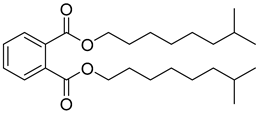
As indicated in Table 1, Table 2, Table 3 and Table 4 and Supplementary Materials, the majority of terpene fermentations utilize organic solvents as the SP, with n-dodecane being the most commonly used. Consequently, Table 5 was compiled, summarizing the nine different types of organic solvents selected for terpene compounds, including crucial log p values, as well as boiling points, which are essential for further discussion. However, we found that the log p results in different values, whether experimental or predicted, for the same chemical in different literatures. Despite these disparities, the specific numerical values will not differ significantly.

Table 5.
Summary of organic solvents in terpene water–organic TPF, detailing the names, CAS numbers, chemical structures, molecular formulas, and critical physical properties, including log p values and boiling points.
5.1.2. Adsorbent Selection Considerations
Compared to organic solvents, adsorbents offer lower toxicity and fewer biocompatibility issues. However, several additional factors must be considered, including the adsorption of components from the culture medium, the diversity of resin types, and their physical properties.
Adsorption of components in culture medium: The potential for adsorbents to absorb nutrients or other components from the culture media is a critical concern. For instance, aromatic acids may bind specifically to polystyrenic adsorbents. A notable example is the XAD-16 resin, known to bind methyl oleate, a primary carbon source in Myxococcus xanthus for epothilone production [285].
Diversity of resins: Adsorbents considered for in situ adsorption in fermentation may vary in chemical and physical properties, such as polymer chemistry, surface area, particle size, and pore size, as reviewed by Thomas Phillips et al. [22]. Thus, the product’s polarity and the resin properties are essential considerations. Resins can be categorized based on their adsorption mechanism—physical adsorption, which occurs without altering the chemical properties of the adsorbate, and chemical adsorption, involving chemical bond formation between the adsorbate and adsorbent [286]. Additionally, polymeric adsorbents are classified by their composition and functionality, including nonionic, anionic, cationic, and affinity resins.
In recent years, ion exchange resins have also been used in in situ fermentation [287]. Comprising tiny, porous beads made from an organic polymer matrix, these resins are functionalized with active groups to selectively bind and exchange specific ions in the fermentation system. The ion exchange process facilitates separation based on ion concentration and resin characteristics, with applications ranging from organic acid to amino acid separation. Examples of the latter are the anions of organic acids, produced by fermentation [288], e.g., lactic acid, citric acid, some amino acids, etc. A few reviews of the ion exchange resins have been published [287,289]. Thus, in this part, we only illustrated one typical example of lactic acid production by the application of ion exchange resins. Lactic acid is a monomer in the production of biodegradable polylactic acid (PLA), which is a well-known sustainable bioplastic material. Ahasa Yousuf and colleagues [290] employed Amberlite IRA-67, a weak ion exchange resin, for in situ extraction of lactic acid from 7-day dark fermentation broths of food waste. IRA-67 showed a maximum acid removal of 74%. However, the application in terpenoid fermentation is limited by the pH tolerance of terpenoids and the microbial resistance to acid or alkali.
Physical properties of resins: The high-speed agitation involved in fermentation processes poses a risk to the integrity of resin beads. While resin particle integrity may not directly impact adsorption capability, the initial recovery step often involves sieving, where physical degradation can lead to separation losses [22]. Frykman and his colleagues [285] compared two possible modes of resin bead breakage: collisions between resin particles and collisions between resin particles and the agitator. Theoretically, at an agitation speed of 800 rpm, these models suggest that most resin particle breakage is more likely caused by impeller blades hitting the beads rather than collisions between the beads themselves. The relationship between agitation rate and bead breakage was then experimentally evaluated using laser diffraction particle sizing to measure the size distribution of XAD-16 resin particles. Bead breakage was found to be negligible in the first 3 days when the agitation rate was 600 rpm (impeller tip speed = 2.0 m/s). However, after 10 days of agitation at 800 rpm (impeller tip speed = 2.7 m/s), the particle size was bimodally distributed, with 29% of particles having a diameter less than 250 μm and the remaining particles relatively unchanged (mean diameter of 700 μm) [22,285]. Thus, resin particle breakage (like cell breakage) may be influenced by other factors such as rheology and the length of fermentation. Particularly in industrial scale-up processes, this phenomenon should be given more attention. Although many considerations when selecting an appropriate adsorption resin have been discussed above, the statistical data in Table 1, Table 2, Table 3 and Table 4 showed that resin usage in terpene fermentation is exceptionally limited, pointing to a gap in the targeted research. Thus, selecting an appropriate resin for terpene fermentation in the TPF process relies on the existing literature and optimization through screening experiments.
5.2. Concentration and Timing of Second Phase Addition
Due to the different mechanisms of the effects of different concentrations of adsorbents and organic solvents on microbial TPF, a summary and overview of the addition concentrations of adsorbents and organic solvents are discussed separately below.
Concentration of adsorbent: Determining the optimal concentration of the adsorbent is crucial in TPF and requires experimental optimization. Typically quantified by weight per volume, resin concentrations in the literature range from 0.5% to 20% [22]. Insufficient adsorbent may lead to inadequate product sequestration, while excessive amounts can negatively impact cell growth and product concentration. For example, in the production of 10′-deoxymethynolide, the highest yield, reaching 280 mg/L, was obtained by adding HP2MGL resin at a concentration of 10% (ranging from 4% to 20%) [291]. Excessive resin not only adsorbs fermentation products from the fermentation liquid but also interrupts the adsorption of nutrients by producing microbes in the fermentation system, thereby inhibiting microbial growth and reducing the yield of the target compound.
In the TPF of PDTs, only a few volatile monoterpenes or sesquiterpenes selected resins as the adsorbents, which is summarized in Table 1, Table 2, Table 3 and Table 4. The concentration range of the resins is from 4% to 10%. Semra Alemdar et al. [24] employed hydrophobic resin Amberlite R XAD4 (10%) as the SP, increasing α-humulene yield by 2310% to 60.2 mg/L, using E. coli as the cell factory in a 2 L bioreactor. In the study of biosynthesis taxadiene by S. cerevisiae, Benedikt Engels et al. [66] chose 0.5% w/v RPC18 silica gel for product adsorption, resulting in a 40-fold increase in taxadiene to 8.7 mg/L. However, few studies investigate the effect of the concentration of resin addition on the TPF of PDTs.
Concentration of organic solvents: Organic solvents primarily act on the plasmalemma, affecting solute transport, energy maintenance, and intracellular homeostasis. The critical concentration of organic solvents is approximately 200 mM, with toxicity related to membrane distribution rather than specific chemical structure [292]. Concentrations that cause similar toxic effects are alike for compounds displaying different log p values, as can be seen from the evaluation of the biocompatible properties of different solvents discussed above. Hence, the membrane concentration of the solvent depends on key factors including the solvent’s concentration in the water phase, its partitioning from water into the membrane, and the volume ratio of the two liquid phases [293]. Therefore, the solvent’s membrane concentration can be calculated when the water phase concentration is known. Conventionally, organic solvents range from 4% to 20% in TPF based on prior studies.
Timing of second phase addition: Adding adsorbents or organic solvents as the SP to the fermentation system for in situ extraction of the product requires careful consideration of the timing. The efficiency of the process is influenced by when the SP is introduced. The considerations of the addition time for these two second terms will be discussed together.
The time for adding the SP needs to consider the growth cycle of microbial cells [294]. The SP should ideally be added at a point where the cells are most active and productive. This is often during the exponential growth phase [294]. When utilizing organic solvents as SPs in the experiments, they are typically introduced during the stage of inducing specific proteins’ expression in cells. Taking E. coli as an example, when the cell growth reaches a certain optical density (OD) range, such as between OD values of 1 and 2, the organic solvents are added simultaneously with inducers like isopropyl β-d-thiogalactoside (IPTG) [295]. This is because the introduction of the inducer activates specific proteins within the cells, initiating the biosynthesis of the product [296]. Similarly, in the S. cerevisiae system, organic solvents are introduced along with inducers, such as galactose, required for the GAL promoters [297], past a certain level of cell proliferation. In some cases, if the constitutive promoters are employed in S. cerevisiae, the addition of organic solvents is timed to coincide with a particular OD value when product biosynthesis commences.
The above discussion about addition time of the SP serves as a basic guideline. The essential principle is to delay the addition until the microorganisms attain a certain biomass. The timing for the SP addition is not static but is established through meticulous optimization, considering the microbial factory’s metabolic state and other pertinent factors. Taking the current highest yield production of β-elemene as an example, Qi Liu et al. [78] chose to add 10% isopropyl myristate (200 mL in a 2 L medium) after culturing Y. lipolytica for 24 h and then supplemented another 10% of IPM when the fermentation progressed to 96 h. This flexible adjustment of the addition strategy helps to improve the production efficiency of β-elemene. As for the use of adsorbents, they are typically added to the bioreactor along with the culture medium during the sterilization process due to their significantly lower cellular toxicity compared to organic solvents, facilitating earlier addition without impeding cell growth.
This timing is crucial to minimize interference with cell growth while ensuring their effective role in product synthesis onset. As discussed above, the added phase can alleviate feedback inhibition. If the product inhibits microbial activity, adding the SP early to remove the product from the system can be beneficial. Therefore, the optimal timing for the addition of the SP in a TPF system depends on a complex interplay of biological, chemical, and operational factors.
5.3. Economic Considerations and Downstream Processing
The cost of the solvent or adsorbent used in the SP is a significant factor for further industrial application. As discussed above, n-dodecane is a commonly used organic solvent but is not an economically viable extracting solvent. In the research by Gui Hwan Han et al. [154], natural vegetable oils, like canola, olive, corn, and soybean oil, showed a similar in situ extraction effect as n-dodecane, with extraction yields of (−)-α-bisabolol ranging from 96.6 to 98.8%, comparable to that of n-dodecane (99.5%). These findings suggest that vegetable oils can serve as natural, cost-effective, and biodegradable extractors during fermentation, reducing production costs. Considering the discussion on organic solvents above, it is feasible to explore more cost-effective alternatives. However, some microorganisms may use natural vegetable oil as a carbon source [298,299], potentially impacting in situ extraction. Therefore, it is essential to consider carbon source supplementation, especially in fed-batch fermentation, where constant monitoring of carbon source consumption is necessary to supplement new sources before depletion.
Downstream processing economic considerations include the costs associated with product recovery and purification, as well as the recovery, regeneration, and recycling associated with the SP. In fermentation processes involving organic solvents, the SP entails multiple critical procedures such as centrifugation for organic solvent collection, solvent concentration, and product purification like distillation. Although organic solvents are commonly used in microbial fermentation of PDTs (Table 1, Table 2, Table 3 and Table 4), they often form emulsion-like mixtures with water [300], especially under vigorous stirring in fermenters. While emulsification enhances substance transfer and biological reaction efficiency, it can pose operational challenges and increase processing costs by reducing recovery efficiency.
Many different downstream technologies have been applied to the separation and purification of terpenes, such as chromatography, distillation, and ion exchange. For instance, preparative HPLC and high-speed countercurrent chromatography are highly adept at separating terpenoids exhibiting high polarity, such as the valuable ginsenosides [301]. These methods are preferred as they minimize sample loss resulting from irreversible adsorption.
Distillation equipment, such as a distillation column, is suitable for volatile components like monoterpenes and sesquiterpenes. By consulting Table 5 for the boiling points of common organic solvents and details on high-value volatile terpenes, an optimal solvent can be chosen. For example, β-elemene is extracted from the plant C. aromatica and purified by the distillation column in the industry. The boiling point of β-elemene is predicted as 252.1 ± 35.0 °C at 760 mmHg using ACD/Labs Percepta Platform-PhysChem Module in ChemSpider. If the actual boiling point value of β-elemene is the lowest value of this predicted data, which is 217.1 °C, then using n-dodecane or methyl oleate as the SP for the TPF is not appropriate. The boiling point of the β-elemene is too close to the organic solvent, which will make more steps in the purification of distillation. Although the boiling point data of most volatile terpenes are based on model predictions, in Dustin Barton and James Chick’s paper [302], they have measured the enthalpy of evaporation of a series of sesquiterpenes, which can help to calculate and predict the boiling points of sesquiterpenes based on these data using the Clapeyron equation, and provide assistance for the selection of subsequent organic solvents. Employing resins as the SP simplifies and enhances the efficiency of subsequent processes. This advantage is primarily due to easy filtration collection and the convenience of dissolution using suitable organic solvents. In the research conducted by Francisco Aguilar et al. [185], they tested seven different solvents for elution of (+)-zizaene from the adsorber Diaion HP2; among them, isooctane showed the best elution effect. Economically, selecting resins as the SP offers advantages, with higher recovery and reuse rates compared to organic solvents, which typically incur losses post-processing.
6. Conclusions and Future Perspectives
In summary, TPF systems present a promising approach for microbial production of PDTs, providing advantages such as reducing microbial cell toxicity, facilitating product recovery, and simplifying post-fermentation processes. Despite these benefits, challenges like enhancing yields, improving recovery rates, and managing costs persist, highlighting areas for further development.
Current research extensively covers the use of organic solvents in TPF, but there is a lack of depth in selecting and optimizing solvents for specific terpenes. Similarly, the potential economic and processing benefits of resins remain underexplored, indicating a significant opportunity for future studies to focus on resin-based systems.
In the industrial production of terpenes, downstream-processing methods are still insufficient. For instance, the treatment of emulsification phenomena and the question of whether only distillation methods are suitable for the post-processing of volatile terpenes have not been fully explored in the published related fermentation research. This is particularly evident in Linhao Chen’s review [301], where only about 34 types of terpenes are discussed. Compared to the many terpenes listed in Table 1, Table 2, Table 3 and Table 4, it is clear that more work is needed to deepen the understanding of this field.
Emerging triphasic and multiphasic fermentation methods, such as the notable study achieving a yield of 64.6 g/L in butanol fermentation by a triphasic fermentation system consisting of immobilized cells, medium, and extraction agent, underscore the potential for innovative approaches in this field [53]. By addressing these highlighted gaps and leveraging new technologies, we can advance terpene fermentation toward higher efficiency and broader application. This overview aims to spur further innovation and research in TPF systems, emphasizing the need for a concerted effort to overcome existing hurdles and explore new fermentation strategies, ultimately driving the field toward more efficient and sustainable practices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29051127/s1, Table S1: Summary of the fermentation results for monoterpenes, categorized by various chassis cells, fermentation types, the second phase, and production outputs; Table S2: Summary of the fermentation results for sesquiterpenes, categorized by various chassis cells, fermentation types, the second phase, and production outputs; Table S3: Summary of the fermentation results for diterpenes, categorized by various chassis cells, fermentation types, the second phase, and production outputs; Table S4: Summary of the fermentation results for triterpenes, categorized by various chassis cells, fermentation types, the second phase, and production outputs; Table S5: Summary of the fermentation results for tetraterpenes, categorized by various chassis cells, fermentation types, the second phase, and production outputs.
Author Contributions
Conceptualization, T.L. and B.F.; methodology, T.L. and X.L. (Ximeng Liu); software, T.L.; validation, T.L., X.L. (Ximeng Liu) and H.X.; formal analysis, T.L., X.L. (Ximeng Liu) and X.L. (Xuan Lu); investigation, T.L., X.L. (Ximeng Liu), H.X., X.L. (Xuan Lu) and H.Z.; resources, T.L., X.L. (Ximeng Liu) and H.X.; data curation, T.L. and H.Z.; writing—original draft preparation, T.L., X.L. (Ximeng Liu) and H.X.; writing—review and editing, T.L. and B.F.; visualization, T.L.; supervision, T.L., X.L. (Xuan Lu) and B.F.; project administration, T.L. and B.F.; funding acquisition, T.L., X.L. (Xuan Lu) and B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Dalian Key Science and Technology Research and Development Plan (2021YF18SN024), the Dalian Science and Technology Innovation Fund Project (2022JJ12WZ059), and the Dalian University Research Platform Project (202101YB08 and 202301YB04).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Acknowledgments
This work was supported by Dalian University. We thank Chuan Tang from Dalian University for his assistance and suggestions in the emulsification and boiling point calculation parts of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants: From Farm to Pharmacy; Joshee, N., Dhekney, S.A., Parajuli, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 333–359. [Google Scholar]
- Brahmkshatriya, P.P.; Brahmkshatriya, P.S. Terpenes: Chemistry, Biological Role, and Therapeutic Applications. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2665–2691. [Google Scholar]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martinez, R.A.; Canovas-Diaz, M.; de Diego Puente, T. A Compressive Review about Taxol((R)): History and Future Challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef]
- Faurant, C. From bark to weed: The history of artemisinin. Parasite 2011, 18, 215–218. [Google Scholar] [CrossRef]
- Urabe, D.; Asaba, T.; Inoue, M. Convergent Strategies in Total Syntheses of Complex Terpenoids. Chem. Rev. 2015, 115, 9207–9231. [Google Scholar] [CrossRef] [PubMed]
- Hugelshofer, C.L.; Magauer, T. Bioinspired total syntheses of terpenoids. Org. Biomol. Chem. 2016, 15, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Ajikumar, P.K.; Tyo, K.; Carlsen, S.; Mucha, O.; Phon, T.H.; Stephanopoulos, G. Terpenoids: Opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol. Pharm. 2008, 5, 167–190. [Google Scholar] [CrossRef] [PubMed]
- Mewalal, R.; Rai, D.K.; Kainer, D.; Chen, F.; Kulheim, C.; Peter, G.F.; Tuskan, G.A. Plant-Derived Terpenes: A Feedstock for Specialty Biofuels. Trends Biotechnol. 2017, 35, 227–240. [Google Scholar] [CrossRef]
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef]
- Belcher, M.S.; Mahinthakumar, J.; Keasling, J.D. New frontiers: Harnessing pivotal advances in microbial engineering for the biosynthesis of plant-derived terpenoids. Curr. Opin. Biotechnol. 2020, 65, 88–93. [Google Scholar] [CrossRef]
- Kirby, J.; Keasling, J.D. Biosynthesis of plant isoprenoids: Perspectives for microbial engineering. Annu. Rev. Plant Biol. 2009, 60, 335–355. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, K.; Zhang, X.; Liu, G.; Zhu, T.; Che, Q.; Li, D.; Zhang, G. Heterologous expression and metabolic engineering tools for improving terpenoids production. Curr. Opin. Biotechnol. 2021, 69, 281–289. [Google Scholar] [CrossRef]
- Moser, S.; Pichler, H. Identifying and engineering the ideal microbial terpenoid production host. Appl. Microbiol. Biotechnol. 2019, 103, 5501–5516. [Google Scholar] [CrossRef]
- Daletos, G.; Stephanopoulos, G. Protein engineering strategies for microbial production of isoprenoids. Metab. Eng. Commun. 2020, 11, e00129. [Google Scholar] [CrossRef]
- Shukal, S.; Chen, X.; Zhang, C. Systematic engineering for high-yield production of viridiflorol and amorphadiene in auxotrophic Escherichia coli. Metab. Eng. 2019, 55, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Daugulis, A.J. Partitioning bioreactors. Curr. Opin. Biotechnol. 1997, 8, 169–174. [Google Scholar] [CrossRef]
- Freeman, A.; Woodley, J.M.; Lilly, M.D. In situ product removal as a tool for bioprocessing. Bio/Technology 1993, 11, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, J.J. Two-phase partitioning bioreactors in fermentation technology. Biotechnol. Adv. 2001, 19, 525–538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quijano, G.; Hernandez, M.; Thalasso, F.; Muñoz, R.; Villaverde, S. Two-phase partitioning bioreactors in environmental biotechnology. Appl. Microbiol. Biotechnol. 2009, 84, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Hossein Mirjalili, M.; Fett-Neto, A.G.; Mazzafera, P.; Bonfill, M. Living between two worlds: Two-phase culture systems for producing plant secondary metabolites. Crit. Rev. Biotechnol. 2012, 33, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.; Chase, M.; Wagner, S.; Renzi, C.; Powell, M.; DeAngelo, J.; Michels, P. Use of in situ solid-phase adsorption in microbial natural product fermentation development. J. Ind. Microbiol. Biotechnol. 2013, 40, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.D.; Marshall, J.; Chang, M.; Nowroozi, F.; Paradise, E.; Pitera, D.; Newman, K.L.; Keasling, J.D. High-level production of amorpha-4,11-diene in a two-phase partitioning bioreactor of metabolically engineered Escherichia coli. Biotechnol. Bioeng. 2006, 95, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, S.; König, J.C.; Hartwig, S.; Frister, T.; Scheper, T.; Beutel, S. Bioproduction of α-humulene in metabolically engineered Escherichia coli and application in zerumbone synthesis. Eng. Life Sci. 2017, 17, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Westfall, P.J.; Pitera, D.J.; Lenihan, J.R.; Eng, D.; Woolard, F.X.; Regentin, R.; Horning, T.; Tsuruta, H.; Melis, D.J.; Owens, A.; et al. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc. Natl. Acad. Sci. USA 2012, 109, E111–E118. [Google Scholar] [CrossRef] [PubMed]
- Drouin, C.M.; Cooper, D.G. Biosurfactants and aqueous two-phase fermentation. Biotechnol. Bioeng. 2004, 40, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, O.; Rito-Palomares, M. Aqueous two-phase systems strategies for the recovery and characterization of biological products from plants. J. Sci. Food Agric. 2010, 90, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B.; et al. Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Proced. Online 2016, 18, 18. [Google Scholar] [CrossRef]
- Rosinha Grundtvig, I.P.; Heintz, S.; Krühne, U.; Gernaey, K.V.; Adlercreutz, P.; Hayler, J.D.; Wells, A.S.; Woodley, J.M. Screening of organic solvents for bioprocesses using aqueous-organic two-phase systems. Biotechnol. Adv. 2018, 36, 1801–1814. [Google Scholar] [CrossRef]
- Inoue, A.; Horikoshi, K. A Pseudomonas thrives in high concentrations of toluene. Nature 1989, 338, 264–266. [Google Scholar] [CrossRef]
- Verhoef, S.; Wierckx, N.; Westerhof, R.G.M.; de Winde, J.H.; Ruijssenaars, H.J. Bioproduction of p-Hydroxystyrene from Glucose by the Solvent-Tolerant Bacterium Pseudomonas putida S12 in a Two-Phase Water-Decanol Fermentation. Appl. Environ. Microbiol. 2009, 75, 931–936. [Google Scholar] [CrossRef]
- Tan, N.; Ong, L.; Shukal, S.; Chen, X.; Zhang, C. High-Yield Biosynthesis of trans-Nerolidol from Sugar and Glycerol. J. Agric. Food Chem. 2023, 71, 8479–8487. [Google Scholar] [CrossRef]
- Freire, M.G.; Cláudio, A.F.M.; Araújo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Lopes, J.N.C.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. [Google Scholar] [CrossRef]
- Phong, W.N.; Show, P.L.; Chow, Y.H.; Ling, T.C. Recovery of biotechnological products using aqueous two phase systems. J. Biosci. Bioeng. 2018, 126, 273–281. [Google Scholar] [CrossRef]
- He, A.; Dong, B.; Feng, X.; Yao, S. Extraction of bioactive ginseng saponins using aqueous two-phase systems of ionic liquids and salts. Sep. Purif. Technol. 2018, 196, 270–280. [Google Scholar] [CrossRef]
- González-Valdez, J.; Mayolo-Deloisa, K.; Rito-Palomares, M. Novel aspects and future trends in the use of aqueous two-phase systems as a bioengineering tool. J. Chem. Technol. Biotechnol. 2017, 93, 1836–1844. [Google Scholar] [CrossRef]
- González-Peñas, H.; Lu-Chau, T.A.; Moreira, M.T.; Lema, J.M. Solvent screening methodology for in situ ABE extractive fermentation. Appl. Microbiol. Biotechnol. 2014, 98, 5915–5924. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Xu, Q.; Chen, N. Effect of a new ionic liguid on L-valine fermentation. Bull. Ferment. Sci. Technol. 2020, 49, 207–229. [Google Scholar] [CrossRef]
- Badhwar, P.; Kumar, P.; Dubey, K.K. Extractive Fermentation for Process integration and amplified pullulan production by Apullulans in Aqueous Two Phase Systems. Sci. Rep. 2019, 9, 32. [Google Scholar] [CrossRef]
- Haykir, N.I.; Nizan Shikh Zahari, S.M.S.; Harirchi, S.; Sar, T.; Awasthi, M.K.; Taherzadeh, M.J. Applications of ionic liquids for the biochemical transformation of lignocellulosic biomass into biofuels and biochemicals: A critical review. Biochem. Eng. J. 2023, 193, 108850. [Google Scholar] [CrossRef]
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell immobilization strategies for biotransformations. Curr. Opin. Green Sustain. Chem. 2022, 33, 100565. [Google Scholar] [CrossRef]
- Pedro, A.Q.; Coutinho, J.A.P.; Freire, M.G. Immobilization of Ionic Liquids, Types of Materials, and Applications. In Encyclopedia of Ionic Liquids; Springer Nature: Singapore, 2019; pp. 1–12. [Google Scholar]
- Hou, D.; Yu, W.; Zhang, D.; Zhao, L.; Liu, X.; Ma, X. Culture of yeast cells immobilized by alginate-chitosan microcapsules in aqueous-organic solvent biphasic system. J. Oceanol. Limnol. 2019, 37, 863–870. [Google Scholar] [CrossRef]
- Plieva, F.M.; Oknianska, A.; Degerman, E.; Mattiasson, B. Macroporous gel particles as robust macroporous matrices for cell immobilization. Biotechnol. J. 2008, 3, 410–417. [Google Scholar] [CrossRef]
- Lu, J.; Peng, W.; Lv, Y.; Jiang, Y.; Xu, B.; Zhang, W.; Zhou, J.; Dong, W.; Xin, F.; Jiang, M. Application of Cell Immobilization Technology in Microbial Cocultivation Systems for Biochemicals Production. Ind. Eng. Chem. Res. 2020, 59, 17026–17034. [Google Scholar] [CrossRef]
- Brányik, T.; Vicente, A.; Oliveira, R.; Teixeira, J. Physicochemical surface properties of brewing yeast influencing their immobilization onto spent grains in a continuous reactor. Biotechnol. Bioeng. 2004, 88, 84–93. [Google Scholar] [CrossRef]
- Dulieu, C.; Poncelet, D.; Neufeld, R.J. Encapsulation and Immobilization Techniques. In Cell Encapsulation Technology and Therapeutics; Kühtreiber, W.M., Lanza, R.P., Chick, W.L., Eds.; Birkhäuser: Boston, MA, USA, 1999; pp. 3–17. [Google Scholar]
- Coradello, G.; Tirelli, N. Yeast Cells in Microencapsulation. General Features and Controlling Factors of the Encapsulation Process. Molecules 2021, 26, 3123. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Desai, P.M.; Liew, C.V.; Chan, L.W.; Heng, P.W.S. Microencapsulation of microbial cells. J. Food Eng. 2013, 116, 369–381. [Google Scholar] [CrossRef]
- El-Sayed, E.-S.R.; Ahmed, A.S.; Hassan, I.A.; Ismaiel, A.A.; Karam El-Din, A.-Z.A. Strain improvement and immobilization technique for enhanced production of the anticancer drug paclitaxel by Aspergillus fumigatus and Alternaria tenuissima. Appl. Microbiol. Biotechnol. 2019, 103, 8923–8935. [Google Scholar] [CrossRef]
- Darmayanti, R.F.; Tashiro, Y.; Noguchi, T.; Gao, M.; Sakai, K.; Sonomoto, K. Novel biobutanol fermentation at a large extractant volume ratio using immobilized Clostridium saccharoperbutylacetonicum N1-4. J. Biosci. Bioeng. 2018, 126, 750–757. [Google Scholar] [CrossRef]
- Ham, S.; Kim, H.J.; Shin, N.; Hwang, J.H.; Oh, S.J.; Park, J.Y.; Joo, J.C.; Kim, H.T.; Bhatia, S.K.; Yang, Y.-H. Continuous production of gamma aminobutyric acid by engineered and immobilized Escherichia coli whole-cells in a small-scale reactor system. Enzym. Microb. Technol. 2023, 168, 110258. [Google Scholar] [CrossRef]
- de Souza, W.F.C.; Pereira, I.; de Lucena, F.A.; Martins, L.P.; Furtado, R.F.; da Castro, R.J.S.; Sato, H.H. A new system of Erwinia sp. D12 cells immobilized in a matrix of alginate and algaroba gum (Prosopis juliflora): An efficient way to improve isomaltulose production. Process Biochem. 2022, 114, 52–58. [Google Scholar] [CrossRef]
- Carsanba, E.; Pintado, M.; Oliveira, C. Fermentation Strategies for Production of Pharmaceutical Terpenoids in Engineered Yeast. Pharmaceuticals 2021, 14, 295. [Google Scholar] [CrossRef] [PubMed]
- Dusséaux, S.; Wajn, W.T.; Liu, Y.; Ignea, C.; Kampranis, S.C. Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids. Proc. Natl. Acad. Sci. USA 2020, 117, 31789–31799. [Google Scholar] [CrossRef]
- Li, H.; Bhadury, P.S.; Song, B.; Yang, S. Immobilized functional ionic liquids: Efficient, green, and reusable catalysts. RSC Adv. 2012, 2, 12525–12551. [Google Scholar] [CrossRef]
- Lee, C.; Sandig, B.; Buchmeiser, M.R.; Haumann, M. Supported ionic liquid phase (SILP) facilitated gas-phase enzyme catalysis—CALB catalyzed transesterification of vinyl propionate. Catal. Sci. Technol. 2018, 8, 2460–2466. [Google Scholar] [CrossRef]
- Wang, M.; Wang, M.; Zhang, S.; Chen, J. Pickering gel emulsion stabilized by enzyme immobilized polymeric nanoparticles: A robust and recyclable biocatalyst system for biphasic catalysis. React. Chem. Eng. 2019, 4, 1459–1465. [Google Scholar] [CrossRef]
- Wiese, S.; Spiess, A.C.; Richtering, W. Microgel-Stabilized Smart Emulsions for Biocatalysis. Angew. Chem. Int. Ed. 2012, 52, 576–579. [Google Scholar] [CrossRef]
- Xu, L.-J.; Liu, Y.-S.; Zhou, L.-G.; Wu, J.-Y. Enhanced beauvericin production with in situ adsorption in mycelial liquid culture of Fusarium redolens Dzf2. Process Biochem. 2009, 44, 1063–1067. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, S.; Fu, H.; Wen, J.; Jia, X. Enhancement of ascomycin production in Streptomyces hygroscopicus var. ascomyceticus by combining resin HP20 addition and metabolic profiling analysis. J. Ind. Microbiol. Biotechnol. 2014, 41, 1365–1374. [Google Scholar] [CrossRef]
- Fordjour, E.; Liu, C.L.; Hao, Y.P.; Sackey, I.; Yang, Y.K.; Liu, X.X.; Li, Y.; Tan, T.W.; Bai, Z.H. Engineering Escherichia coli BL21 (DE3) for high-yield production of germacrene A, a precursor of β-elemene via combinatorial metabolic engineering strategies. Biotechnol. Bioeng. 2023, 120, 3039–3056. [Google Scholar] [CrossRef]
- Liu, W.; Xu, X.; Zhang, R.; Cheng, T.; Cao, Y.; Li, X.; Guo, J.; Liu, H.; Xian, M. Engineering Escherichia coli for high-yield geraniol production with biotransformation of geranyl acetate to geraniol under fed-batch culture. Biotechnol. Biofuels 2016, 9, 58. [Google Scholar] [CrossRef]
- Engels, B.; Dahm, P.; Jennewein, S. Metabolic engineering of taxadiene biosynthesis in yeast as a first step towards Taxol (Paclitaxel) production. Metab. Eng. 2008, 10, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Tsueng, G.; Lam, K.S. Stabilization effect of resin on the production of potent proteasome inhibitor NPI-0052 during submerged fermentation of Salinispora tropica. J. Antibiot. 2007, 60, 469–472. [Google Scholar] [CrossRef]
- Pemberton, T.A.; Chen, M.B.; Harris, G.G.; Chou, W.K.W.; Duan, L.; Köksal, M.; Genshaft, A.S.; Cane, D.E.; Christianson, D.W. Exploring the Influence of Domain Architecture on the Catalytic Function of Diterpene Synthases. Biochemistry 2017, 56, 2010–2023. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Yuan, S.Q.; Li, L.S.; Zheng, J.; Zhao, D.; Wang, C.J.; Wang, H.; Liu, X.; Liu, J. Application of Terpenoid Compounds in Food and Pharmaceutical Products. Fermentation 2023, 9, 119. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X. Biosynthesis of monoterpenoid and sesquiterpenoid as natural flavors and fragrances. Biotechnol. Adv. 2023, 65, 22. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Liu, S.S.; Jin, G.J.; Yang, X.B.; Zhou, Y.J.J. Microbial production of limonene and its derivatives: Achievements and perspectives. Biotechnol. Adv. 2020, 44, 16. [Google Scholar] [CrossRef]
- Daletos, G.; Katsimpouras, C.; Stephanopoulos, G. Novel Strategies and Platforms for Industrial Isoprenoid Engineering. Trends Biotechnol. 2020, 38, 811–822. [Google Scholar] [CrossRef]
- Marienhagen, J.; Bott, M. Metabolic engineering of microorganisms for the synthesis of plant natural products. J. Biotechnol. 2013, 163, 166–178. [Google Scholar] [CrossRef]
- Chen, X.X.; Zhang, C.Q.; Lindley, N.D. Metabolic Engineering Strategies for Sustainable Terpenoid Flavor and Fragrance Synthesis. J. Agric. Food Chem. 2020, 68, 10252–10264. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Markham, K.A.; Palmer, C.M.; Liu, N.; Stephanopoulos, G.; Alper, H.S. Metabolic engineering in the host Yarrowia lipolytica. Metab. Eng. 2018, 50, 192–208. [Google Scholar] [CrossRef]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.W.M.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef]
- Geiselman, G.M.; Kirby, J.; Landera, A.; Otoupal, P.; Papa, G.; Barcelos, C.; Sundstrom, E.R.; Das, L.; Magurudeniya, H.D.; Wehrs, M.; et al. Conversion of poplar biomass into high-energy density tricyclic sesquiterpene jet fuel blendstocks. Microb. Cell Factories 2020, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, G.; Su, L.Q.; Liu, P.; Jia, S.R.; Wang, Q.H.; Dai, Z.J. Reprogramming the metabolism of oleaginous yeast for sustainably biosynthesizing the anticarcinogen precursor germacrene A. Green Chem. 2023, 25, 7988–7997. [Google Scholar] [CrossRef]
- Özaydin, B.; Burd, H.; Lee, T.S.; Keasling, J.D. Carotenoid-based phenotypic screen of the yeast deletion collection reveals new genes with roles in isoprenoid production. Metab. Eng. 2013, 15, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, L.J.; Zhang, X.Y.; Zhang, J.; Zhang, Y.; Wang, F.; Li, X. Combined bioderivatization and engineering approach to improve the efficiency of geraniol production. Green Chem. 2022, 24, 864–876. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, C.L.; Yang, L.Y.; Choi, E.S.; Kim, S.W. Geranyl diphosphate synthase: An important regulation point in balancing a recombinant monoterpene pathway in Escherichia coli. Enzym. Microb. Technol. 2015, 68, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Z.; Li, C.; Zhang, Y.; Shen, Y.; Hou, J.; Bao, X.M. Dynamic control of ERG20 expression combined with minimized endogenous downstream metabolism contributes to the improvement of geraniol production in Saccharomyces cerevisiae. Microb. Cell Factories 2017, 16, 11. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, X.; Zhang, J.; Zhou, Y.; Wang, F.; Zhang, Y.; Li, X. Co-localizing key pathway enzymes by protein scaffold to enhance geraniol production in Escherichia coli. Ind. Crops Prod. 2023, 203, 117144. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Du, G.; Chen, J.; Zhou, J. Overproduction of geraniol by enhanced precursor supply in Saccharomyces cerevisiae. J. Biotechnol. 2013, 168, 446–451. [Google Scholar] [CrossRef]
- Jiang, G.-Z.; Yao, M.-D.; Wang, Y.; Zhou, L.; Song, T.-Q.; Liu, H.; Xiao, W.-H.; Yuan, Y.-J. Manipulation of GES and ERG20 for geraniol overproduction in Saccharomyces cerevisiae. Metab. Eng. 2017, 41, 57–66. [Google Scholar] [CrossRef]
- Li, M.; Xu, S.; Lu, W. Engineering Corynebacterium glutamicum for Geraniol Production. Trans. Tianjin Univ. 2020, 27, 377–384. [Google Scholar] [CrossRef]
- Alonso-Gutierrez, J.; Kim, E.-M.; Batth, T.S.; Cho, N.; Hu, Q.; Chan, L.J.G.; Petzold, C.J.; Hillson, N.J.; Adams, P.D.; Keasling, J.D.; et al. Principal component analysis of proteomics (PCAP) as a tool to direct metabolic engineering. Metab. Eng. 2015, 28, 123–133. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, S.; Cao, J.; Qiao, J.; Zhao, G.-R. Systematic Optimization of Limonene Production in Engineered Escherichia coli. J. Agric. Food Chem. 2019, 67, 7087–7097. [Google Scholar] [CrossRef]
- Willrodt, C.; Hoschek, A.; Bühler, B.; Schmid, A.; Julsing, M.K. Decoupling production from growth by magnesium sulfate limitation boosts de novo limonene production. Biotechnol. Bioeng. 2015, 113, 1305–1314. [Google Scholar] [CrossRef]
- Rolf, J.; Julsing, M.K.; Rosenthal, K.; Lütz, S. A Gram-Scale Limonene Production Process with Engineered Escherichia coli. Molecules 2020, 25, 1881. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, H.; Weng, Y.; Li, P.; Zhang, C.; Xiao, D. Improve the production of d-limonene by regulating the mevalonate pathway of Saccharomyces cerevisiae during alcoholic beverage fermentation. J. Ind. Microbiol. Biotechnol. 2020, 47, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Meng, Y.; Zhang, L.; Qiao, J.; Zhao, G.-R. Combinatorial engineering of Saccharomyces cerevisiae for improving limonene production. Biochem. Eng. J. 2021, 176, 108155. [Google Scholar] [CrossRef]
- Kong, X.; Wu, Y.; Yu, W.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Efficient Synthesis of Limonene in Saccharomyces cerevisiae Using Combinatorial Metabolic Engineering Strategies. J. Agric. Food Chem. 2023, 71, 7752–7764. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Ren, Y.; Jin, G.; Tao, Y.; Lyu, L.; Zhao, Z.K.; Yang, X. Engineering Rhodosporidium toruloides for limonene production. Biotechnol. Biofuels 2021, 14, 243. [Google Scholar] [CrossRef]
- Zhao, D.; Gao, Q.; Zheng, X.; Liu, S.; Qi, Q.; Wang, X.; Yang, X. Optimization of Fermentation Conditions for Elevating Limonene Production with Engineered Rhodosporidium toruloides. Fermentation 2023, 9, 431. [Google Scholar] [CrossRef]
- Muñoz-Fernández, G.; Martínez-Buey, R.; Revuelta, J.L.; Jiménez, A. Metabolic engineering of Ashbya gossypii for limonene production from xylose. Biotechnol. Biofuels Bioprod. 2022, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.H.; Lv, Y.-B.; Chen, J.; Imanaka, T.; Wei, L.; Hua, Q. Metabolic engineering of oleaginous yeast Yarrowia lipolytica for limonene overproduction. Biotechnol. Biofuels 2016, 9, 214. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Wei, L.; Lv, Y.-B.; Chen, J.; Hua, Q. Elevating Limonene Production in Oleaginous Yeast Yarrowia lipolytica via Genetic Engineering of Limonene Biosynthesis Pathway and Optimization of Medium Composition. Biotechnol. Bioprocess Eng. 2019, 24, 500–506. [Google Scholar] [CrossRef]
- Lin, P.-C.; Saha, R.; Zhang, F.; Pakrasi, H.B. Metabolic engineering of the pentose phosphate pathway for enhanced limonene production in the cyanobacterium Synechocystis sp. PCC 6803. Sci. Rep. 2017, 7, 17503. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-C.; Zhang, F.; Pakrasi, H.B. Enhanced limonene production in a fast-growing cyanobacterium through combinatorial metabolic engineering. Metab. Eng. Commun. 2021, 12, e00164. [Google Scholar] [CrossRef]
- Sun, C.; Dong, X.; Zhang, R.; Xie, C. Effectiveness of recombinant Escherichia coli on the production of (R)-(+)-perillyl alcohol. BMC Biotechnol. 2020, 21, 3. [Google Scholar] [CrossRef]
- Alonso-Gutiérrez, J.; Chan, R.; Batth, T.S.; Adams, P.D.; Keasling, J.D.; Petzold, C.J.; Lee, T.S. Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metab. Eng. 2013, 19, 33–41. [Google Scholar] [CrossRef]
- Kong, S.; Fu, X.-Z.; Li, X.; Pan, H.; Guo, D. De novo biosynthesis of linalool from glucose in engineered Escherichia coli. Enzym. Microb. Technol. 2020, 140, 109614. [Google Scholar] [CrossRef]
- Ferraz, C.A.; Leferink, N.G.H.; Kosov, I.; Scrutton, N.S. Isopentenol Utilization Pathway for the Production of Linalool in Escherichia coli Using an Improved Bacterial Linalool/Nerolidol Synthase. Chembiochem 2021, 22, 2325–2334. [Google Scholar] [CrossRef]
- Mendez-Perez, D.; Alonso-Gutiérrez, J.; Hu, Q.; Molinas, M.; Baidoo, E.E.K.; Wang, G.; Chan, L.J.G.; Adams, P.D.; Petzold, C.J.; Keasling, J.D.; et al. Production of jet fuel precursor monoterpenoids from engineered Escherichia coli. Biotechnol. Bioeng. 2017, 114, 1703–1712. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Zhang, X.; Zhang, J.; Zhou, Y.; Wang, F.; Li, X. Efficient myrcene production using linalool dehydratase isomerase and rational biochemical process in Escherichia coli. J. Biotechnol. 2023, 371–372, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Xiao, L.; Wang, F.; Zhang, Y.; Li, X. Synthetic Protein Scaffolds for Improving R-(−)-Linalool Production in Escherichia coli. J. Agric. Food Chem. 2021, 69, 5663–5670. [Google Scholar] [CrossRef] [PubMed]
- Amiri, P.; Shahpiri, A.; Asadollahi, M.A.; Momenbeik, F.; Partow, S. Metabolic engineering of Saccharomyces cerevisiae for linalool production. Biotechnol. Lett. 2015, 38, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Wang, J.; Cao, X.; Liu, W.; Yu, H.; Ye, L. High-level production of linalool by engineered Saccharomyces cerevisiae harboring dual mevalonate pathways in mitochondria and cytoplasm. Enzym. Microb. Technol. 2020, 134, 109462. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Du, Y.; Fang, X.; Xu, N.; Yue, C.; Ye, L. Combinatorial Modulation of Linalool Synthase and Farnesyl Diphosphate Synthase for Linalool Overproduction in Saccharomyces cerevisiae. J. Agric. Food Chem. 2021, 69, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.H.; Wei, L.; Lin, J.; Hua, Q. Enhancing linalool production by engineering oleaginous yeast Yarrowia lipolytica. Bioresour. Technol. 2017, 245 Pt B, 1641–1644. [Google Scholar] [CrossRef]
- Taratynova, M.O.; Tikhonova, E.E.; Fedyaeva, I.M.; Dementev, D.A.; Yuzbashev, T.V.; Solovyev, A.I.; Sineoky, S.P.; Yuzbasheva, E.Y. Boosting Geranyl Diphosphate Synthesis for Linalool Production in Engineered Yarrowia lipolytica. Appl. Biochem. Biotechnol. 2023, 196, 1304–1315. [Google Scholar] [CrossRef]
- Hoshino, Y.; Moriya, M.; Matsudaira, A.; Katashkina, J.I.; Nitta, N.; Nishio, Y.; Usuda, Y. Stereospecific linalool production utilizing two-phase cultivation system in Pantoea ananatis. J. Biotechnol. 2020, 324, 21–27. [Google Scholar] [CrossRef]
- Nitta, N.; Tajima, Y.; Yamamoto, Y.; Moriya, M.; Matsudaira, A.; Hoshino, Y.; Nishio, Y.; Usuda, Y. Fermentative production of enantiopure (S)-linalool using a metabolically engineered Pantoea ananatis. Microb. Cell Factories 2021, 20, 54. [Google Scholar] [CrossRef]
- Karuppiah, V.; Ranaghan, K.E.; Leferink, N.G.H.; Johannissen, L.O.; Shanmugam, M.; Ní Cheallaigh, A.; Bennett, N.J.; Kearsey, L.J.; Takano, E.; Gardiner, J.M.; et al. Structural Basis of Catalysis in the Bacterial Monoterpene Synthases Linalool Synthase and 1,8-Cineole Synthase. ACS Catal. 2017, 7, 6268–6282. [Google Scholar] [CrossRef] [PubMed]
- Ignea, C.; Cvetković, I.; Loupassaki, S.; Kefalas, P.; Johnson, C.B.; Kampranis, S.C.; Makris, A.M. Improving yeast strains using recyclable integration cassettes, for the production of plant terpenoids. Microb. Cell Factories 2011, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Q.; Cao, Y.; Feng, X.; Zheng, Y.; Zou, H.; Liu, H.; Yang, J.; Xian, M. Microbial production of sabinene—A new terpene-based precursor of advanced biofuel. Microb. Cell Factories 2014, 13, 20. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, T.; Zou, H.; Zhang, H.; Xu, X.; Sun, C.; Aboulnaga, E.A.; Cheng, Z.; Zhao, G.; Xian, M. High titer mevalonate fermentation and its feeding as a building block for isoprenoids (isoprene and sabinene) production in engineered Escherichia coli. Process Biochem. 2017, 62, 1–9. [Google Scholar]
- Ignea, C.; Pontini, M.; Maffei, M.E.; Makris, A.M.; Kampranis, S.C. Engineering monoterpene production in yeast using a synthetic dominant negative geranyl diphosphate synthase. ACS Synth. Biol. 2014, 3, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, Q.; Ren, M.-F.; Feng, H.; Jiang, X.; Zheng, Y.; Liu, M.; Zhang, H.; Xian, M. Metabolic engineering of Escherichia coli for the biosynthesis of alpha-pinene. Biotechnol. Biofuels 2013, 6, 60. [Google Scholar] [CrossRef]
- Niu, F.-X.; He, X.; Wu, Y.; Liu, J.-Z. Enhancing Production of Pinene in Escherichia coli by Using a Combination of Tolerance, Evolution, and Modular Co-culture Engineering. Front. Microbiol. 2018, 9, 1623. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, R.S. Metabolic engineering of Saccharomyces cerevisiae for pinene production. CIESC 2019, 70, 179–188. [Google Scholar] [CrossRef]
- Ma, T.; Zong, H.; Lu, X.; Zhuge, B. Synthesis of pinene in the industrial strain Candida glycerinogenes by modification of its mevalonate pathway. J. Microbiol. 2022, 60, 1191–1200. [Google Scholar] [CrossRef]
- Kim, E.-M.; Eom, J.-H.; Um, Y.; Kim, Y.; Woo, H.M. Microbial Synthesis of Myrcene by Metabolically Engineered Escherichia coli. J. Agric. Food Chem. 2015, 63, 4606–4612. [Google Scholar] [CrossRef]
- Chuck, C.J.; Donnelly, J. The compatibility of potential bioderived fuels with Jet A-1 aviation kerosene. Appl. Energy 2014, 118, 83–91. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, C. A new approach for bio-jet fuel generation from palm oil and limonene in the absence of hydrogen. Chem. Commun. 2015, 51, 17249–17252. [Google Scholar] [CrossRef] [PubMed]
- Willrodt, C.; David, C.C.; Cornelissen, S.; Bühler, B.; Julsing, M.K.; Schmid, A. Engineering the productivity of recombinant Escherichia coli for limonene formation from glycerol in minimal media. Biotechnol. J. 2014, 9, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Schewe, H.; Holtmann, D.; Schrader, J. P450 BM-3-catalyzed whole-cell biotransformation of α-pinene with recombinant Escherichia coli in an aqueous-organic two-phase system. Appl. Microbiol. Biotechnol. 2009, 83, 849–857. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Tang, J.J.; Gao, J.M. Picrotoxane sesquiterpenoids: Chemistry, chemo- and bio-syntheses and biological activities. Nat. Product Rep. 2022, 39, 2096–2131. [Google Scholar] [CrossRef]
- Martin, V.J.J.; Pitera, D.J.; Withers, S.T.; Newman, J.D.; Keasling, J.D. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotechnol. 2003, 21, 796–802. [Google Scholar] [CrossRef]
- Crock, J.; Wildung, M.; Croteau, R. Isolation and bacterial expression of a sesquiterpene synthase cDNA clone from peppermint (Mentha x piperita, L.) that produces the aphid alarm pheromone (E)-beta-farnesene. Proc. Natl. Acad. Sci. USA 1997, 94, 12833–12838. [Google Scholar] [CrossRef]
- Wang, C.; Yoon, S.H.; Jang, H.J.; Chung, Y.R.; Kim, J.Y.; Choi, E.S.; Kim, S.W. Metabolic engineering of Escherichia coli for α-farnesene production. Metab. Eng. 2011, 13, 648–655. [Google Scholar] [CrossRef]
- Zhu, F.; Zhong, X.; Hu, M.; Lu, L.; Deng, Z.; Liu, T. In vitro reconstitution of mevalonate pathway and targeted engineering of farnesene overproduction in Escherichia coli. Biotechnol. Bioeng. 2014, 111, 1396–1405. [Google Scholar] [CrossRef]
- Tippmann, S.; Anfelt, J.; David, F.; Rand, J.M.; Siewers, V.; Uhlen, M.; Nielsen, J.; Hudson, E.P. Affibody Scaffolds Improve Sesquiterpene Production in Saccharomyces cerevisiae. ACS Synth. Biol. 2017, 6, 19–28. [Google Scholar] [CrossRef]
- Tippmann, S.; Ferreira, R.; Siewers, V.; Nielsen, J.; Chen, Y. Effects of acetoacetyl-CoA synthase expression on production of farnesene in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2017, 44, 911–922. [Google Scholar] [CrossRef]
- You, S.P.; Yin, Q.D.A.; Zhang, J.Y.; Zhang, C.Y.; Qi, W.; Gao, L.; Tao, Z.P.; Su, R.X.; He, Z.M. Utilization of biodiesel by-product as substrate for high-production of β-farnesene via relatively balanced mevalonate pathway in Escherichia coli. Bioresour. Technol. 2017, 243, 228–236. [Google Scholar] [CrossRef]
- Wang, J.H.; Jiang, W.; Liang, C.J.; Zhu, L.H.; Li, Y.R.; Mo, Q.; Xu, S.; Chu, A.; Zhang, L.; Ding, Z.Y.; et al. Overproduction of α-Farnesene in Saccharomyces cerevisiae by Farnesene Synthase Screening and Metabolic Engineering. J. Agric. Food Chem. 2021, 69, 3103–3113. [Google Scholar] [CrossRef]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, X.; Cui, Z.; Wang, Z.; Qi, Q.; Hou, J. Engineering the oleaginous yeast Yarrowia lipolytica for production of α-farnesene. Biotechnol. Biofuels 2019, 12, 296. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, C.; Gu, L.P.; Gibbons, W.; Zhou, R.B. Genetically engineering cyanobacteria to convert CO2, water, and light into the long-chain hydrocarbon farnesene. Appl. Microbiol. Biotechnol. 2014, 98, 9869–9877. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ching, C.B. Combinatorial engineering of mevalonate pathway for improved amorpha-4,11-diene production in budding yeast. Biotechnol. Bioeng. 2013, 111, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.H.; Zhang, F.; Alonso-Gutierrez, J.; Baidoo, E.; Batth, T.S.; Redding-Johanson, A.M.; Petzold, C.J.; Mukhopadhyay, A.; Lee, T.S.; Adams, P.D.; et al. Engineering dynamic pathway regulation using stress-response promoters. Nat. Biotechnol. 2013, 31, 1039–1046. [Google Scholar] [CrossRef]
- Kwak, S.; Yun, E.J.; Lane, S.; Oh, E.J.; Kim, K.H.; Jin, Y.S. Redirection of the Glycolytic Flux Enhances Isoprenoid Production in Saccharomyces cerevisiae. Biotechnol. J. 2019, 15, 1900173. [Google Scholar] [CrossRef] [PubMed]
- Marsafari, M.; Xu, P. Debottlenecking mevalonate pathway for antimalarial drug precursor amorphadiene biosynthesis in Yarrowia lipolytica. Metab. Eng. Commun. 2020, 10, e00121. [Google Scholar] [CrossRef]
- Yaegashi, J.; Kirby, J.; Ito, M.; Sun, J.; Dutta, T.; Mirsiaghi, M.; Sundstrom, E.R.; Rodriguez, A.; Baidoo, E.; Tanjore, D.; et al. Rhodosporidium toruloides: A new platform organism for conversion of lignocellulose into terpene biofuels and bioproducts. Biotechnol. Biofuels 2017, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zou, R.; Zhang, C.; Stephanopoulos, G.; Too, H.P. Optimization of amorphadiene synthesis in bacillus subtilis via transcriptional, translational, and media modulation. Biotechnol. Bioeng. 2013, 110, 2556–2561. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, H.J.; Choi, J.; Kim, J.; Sim, S.J.; Um, Y.; Kim, Y.; Lee, T.S.; Keasling, J.D.; Woo, H.M. Photosynthetic conversion of CO2 to farnesyl diphosphate-derived phytochemicals (amorpha-4,11-diene and squalene) by engineered cyanobacteria. Biotechnol. Biofuels 2016, 9, 202. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.; Lee, S.m.; Um, Y.; Kim, Y.; Sim, S.J.; Choi, J.-i.; Woo, H.M. Direct Conversion of CO2 to α-Farnesene Using Metabolically Engineered Synechococcus elongatus PCC 7942. J. Agric. Food Chem. 2017, 65, 10424–10428. [Google Scholar] [PubMed]
- Liu, H.; Chen, S.-L.; Xu, J.-Z.; Zhang, W.-G. Dual Regulation of Cytoplasm and Peroxisomes for Improved A-Farnesene Production in Recombinant Pichia pastoris. ACS Synth. Biol. 2021, 10, 1563–1573. [Google Scholar] [CrossRef]
- Yao, P.; You, S.; Qi, W.; Su, R.; He, Z. Investigation of fermentation conditions of biodiesel by-products for high production of β-farnesene by an engineered Escherichia coli. Environ. Sci. Pollut. Res. 2020, 27, 22758–22769. [Google Scholar] [CrossRef]
- Lv, J.; Wang, Y.; Zhang, C.; You, S.; Qi, W.; Su, R.; He, Z. Highly efficient production of FAMEs and β-farnesene from a two-stage biotransformation of waste cooking oils. Energy Convers. Manag. 2019, 199, 112001. [Google Scholar] [CrossRef]
- Arnesen, J.A.; Kildegaard, K.R.; Cernuda Pastor, M.; Jayachandran, S.; Kristensen, M.; Borodina, I. Yarrowia lipolytica Strains Engineered for the Production of Terpenoids. Front. Bioeng. Biotechnol. 2020, 8, 945. [Google Scholar] [CrossRef]
- Shi, T.; Li, Y.; Zhu, L.; Tong, Y.; Yang, J.; Fang, Y.; Wang, M.; Zhang, J.; Jiang, Y.; Yang, S. Engineering the oleaginous yeast Yarrowia lipolytica for β-farnesene overproduction. Biotechnol. J. 2021, 16, 2100097. [Google Scholar] [CrossRef]
- Han, G.H.; Kim, S.K.; Yoon, P.K.-S.; Kang, Y.; Kim, B.S.; Fu, Y.; Sung, B.H.; Jung, H.C.; Lee, D.-H.; Kim, S.-W.; et al. Fermentative production and direct extraction of (−)-α-bisabolol in metabolically engineered Escherichia coli. Microb. Cell Factories 2016, 15, 185. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Ouellet, M.; Chan, R.; Mukhopadhyay, A.; Keasling, J.D.; Lee, T.S. Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun. 2011, 2, 483. [Google Scholar] [CrossRef]
- Davies, F.K.; Work, V.H.; Beliaev, A.S.; Posewitz, M.C. Engineering Limonene and Bisabolene Production in Wild Type and a Glycogen-Deficient Mutant of Synechococcus sp. PCC 7002. Front. Bioeng. Biotechnol. 2014, 2, 21. [Google Scholar] [CrossRef]
- Sebesta, J.; Peebles, C.A.M. Improving heterologous protein expression in Synechocystis sp. PCC 6803 for alpha-bisabolene production. Metab. Eng. Commun. 2020, 10, e00117. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Zhang, L.; Zhu, S.; Yuan, W.; Hang, J.; Yin, D.; Tang, X.; Zheng, J.; Wang, Z.; Sun, J. Overexpression of the transcription factor HAC1 improves nerolidol production in engineered yeast. Enzym. Microb. Technol. 2020, 134, 109485. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Yu, F.; Okamoto, S.; Kuzuyama, T.; Utsumi, R.; Misawa, N. Efficient synthesis of functional isoprenoids from acetoacetate through metabolic pathway-engineered Escherichia coli. Appl. Microbiol. Biotechnol. 2009, 81, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, M.; Zhao, G.-R.; Lu, W. Harnessing Yeast Peroxisomes and Cytosol Acetyl-CoA for Sesquiterpene α-Humulene Production. J. Agric. Food Chem. 2020, 68, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, Y.; Han, L.; Wang, Q.; Liu, H.; Cheng, P.; Li, R.; Guo, X.; Zhou, Z. Enhancement of Patchoulol Production in Escherichia coli via Multiple Engineering Strategies. J. Agric. Food Chem. 2021, 69, 7572–7580. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, L.; Chen, Y.; Bach, L.S.; Rattleff, S.; Maury, J.; Brix, S.; Nielsen, J.; Mortensen, U.H. Diversion of Flux toward Sesquiterpene Production in Saccharomyces cerevisiae by Fusion of Host and Heterologous Enzymes. Appl. Environ. Microbiol. 2011, 77, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lin, Y.-C.; Guo, J.-J.; Du, M.-M.; Tao, X.; Gao, B.; Zhao, M.; Ma, Y.; Wang, F.-Q.; Wei, D.-Z. High-Level Production of Sesquiterpene Patchoulol in Saccharomyces cerevisiae. ACS Synth. Biol. 2021, 10, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Emmerstorfer, A.; Wimmer-Teubenbacher, M.; Wriessnegger, T.; Leitner, E.; Müller, M.; Kaluzna, I.A.; Schürmann, M.; Mink, D.G.; Zellnig, G.n.; Schwab, H.; et al. Over-expression of ICE2 stabilizes cytochrome P450 reductase in Saccharomyces cerevisiae and Pichia pastoris. Biotechnol. J. 2015, 10, 623–635. [Google Scholar] [CrossRef]
- Ouyang, X.; Li, W.; Cha, Y.; Zhu, C.; Li, S. Production of valencene and its derivatives by recombinant brewer’s yeast fermentation. Food Ferment. Ind. Editor. Staff 2019, 45, 9. [Google Scholar]
- Guo, X.; Sun, J.; Li, D.; Lu, W. Heterologous biosynthesis of (+)-nootkatone in unconventional yeast Yarrowia lipolytica. Biochem. Eng. J. 2018, 137, 125–131. [Google Scholar] [CrossRef]
- Frohwitter, J.; Heider, S.A.E.; Peters-Wendisch, P.; Beekwilder, J.; Wendisch, V.F. Production of the sesquiterpene (+)-valencene by metabolically engineered Corynebacterium glutamicum. J. Biotechnol. 2014, 191, 205–213. [Google Scholar] [CrossRef]
- Beekwilder, J.; van Houwelingen, A.; Cankar, K.; van Dijk, A.D.J.; de Jong, R.M.; Stoopen, G.; Bouwmeester, H.; Achkar, J.; Sonke, T.; Bosch, D. Valencene synthase from the heartwood of Nootka cypress (Callitropsis nootkatensis) for biotechnological production of valencene. Plant Biotechnol. J. 2013, 12, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Matsudaira, A.; Hoshino, Y.; Uesaka, K.; Takatani, N.; Omata, T.; Usuda, Y. Production of glutamate and stereospecific flavors, (S)-linalool and (+)-valencene, by Synechocystis sp. PCC6803. J. Biosci. Bioeng. 2020, 130, 464–470. [Google Scholar] [CrossRef]
- Gao, Y. The Study of Microbial Synthesis of Germacrene A the Precursor of β-Elemene. Master’s Thesis, Hangzhou Normal University, Hangzhou, China, 2012. [Google Scholar]
- Li, M.; Wen, Q.; Lv, S.; Yang, R.; Cheng, T.; Wang, Z.; Yang, J. Co-biosynthesis of germacrene A, a precursor of β-elemene, and lycopene in engineered Escherichia coli. Appl. Microbiol. Biotechnol. 2022, 106, 8053–8066. [Google Scholar] [CrossRef]
- Bröker, J.N.; Müller, B.; Prüfer, D.; Schulze Gronover, C. Combinatorial Metabolic Engineering in Saccharomyces cerevisiae for the Enhanced Production of the FPP-Derived Sesquiterpene Germacrene. Bioengineering 2020, 7, 135. [Google Scholar] [CrossRef]
- Cheng, J.; Zuo, Y.; Liu, G.; Li, D.; Gao, J.; Xiao, F.; Huang, L.; Xu, Z.; Lian, J. Development of a Pichia pastoris cell factory for efficient production of germacrene A: A precursor of β-elemene. Bioresour. Bioprocess. 2023, 10, 38. [Google Scholar] [CrossRef]
- Ye, M.; Gao, J.; Zhou, Y.J. Global metabolic rewiring of the nonconventional yeast Ogataea polymorpha for biosynthesis of the sesquiterpenoid β-elemene. Metab. Eng. 2023, 76, 225–231. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Zhang, X.; Zhang, Y.; Wang, F.; Li, X. Sesquiterpene Synthase Engineering and Targeted Engineering of α-Santalene Overproduction in Escherichia coli. J. Agric. Food Chem. 2022, 70, 5377–5385. [Google Scholar] [CrossRef]
- Scalcinati, G.; Knuf, C.; Partow, S.; Chen, Y.; Maury, J.; Schalk, M.; Daviet, L.; Nielsen, J.; Siewers, V. Dynamic control of gene expression in Saccharomyces cerevisiae engineered for the production of plant sesquitepene α-santalene in a fed-batch mode. Metab. Eng. 2012, 14, 91–103. [Google Scholar] [CrossRef]
- Asadollahi, M.A.; Maury, J.; Møller, K.; Nielsen, K.F.; Schalk, M.; Clark, A.; Nielsen, J. Production of plant sesquiterpenes in Saccharomyces cerevisiae: Effect of ERG9 repression on sesquiterpene biosynthesis. Biotechnol. Bioeng. 2007, 99, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Xu, S.; Sun, J.; Zhang, C.; Li, D.; Lu, W. Yarrowia lipolytica construction for heterologous synthesis of α-santalene and fermentation optimization. Appl. Microbiol. Biotechnol. 2019, 103, 3511–3520. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, F.; Davis, R.W. Engineering Escherichia coli for the production of terpene mixture enriched in caryophyllene and caryophyllene alcohol as potential aviation fuel compounds. Metab. Eng. Commun. 2018, 6, 13–21. [Google Scholar] [CrossRef]
- Yang, J.; Li, Z.; Guo, L.; Du, J.; Bae, H.-J. Biosynthesis of β-caryophyllene, a novel terpene-based high-density biofuel precursor, using engineered Escherichia coli. Renew. Energy 2016, 99, 216–223. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, K.; Guo, J.; Yang, Q.; Li, Y.; Xian, M.; Zhang, R. Highly efficient biosynthesis of β-caryophyllene with a new sesquiterpene synthase from tobacco. Biotechnol. Biofuels Bioprod. 2022, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, C.; Wang, P.; Yan, X.; Zhou, Z. Production of sesquiterpenoids α-neoclovene and β-caryophyllene by engineered Saccharomyces cerevisiae. Synth. Biol. J. 2021, 2, 792. [Google Scholar]
- Melillo, E.; Setroikromo, R.; Quax, W.J.; Kayser, O. Production of α-cuprenene in Xanthophyllomyces dendrorhous: A step closer to a potent terpene biofactory. Microb. Cell Factories 2013, 12, 13. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, R.; Liu, W.; Zhao, G.; Niu, W.; Guo, J.; Xian, M.; Liu, H. Manipulation of the precursor supply for high-level production of longifolene by metabolically engineered Escherichia coli. Sci. Rep. 2019, 9, 95. [Google Scholar] [CrossRef]
- Aguilar, F.; Scheper, T.; Beutel, S. Improved Production and In Situ Recovery of Sesquiterpene (+)-Zizaene from Metabolically-Engineered E. coli. Molecules 2019, 24, 3356. [Google Scholar] [CrossRef]
- Nybo, S.E.; Saunders, J.; McCormick, S.P. Metabolic engineering of Escherichia coli for production of valerenadiene. J. Biotechnol. 2017, 262, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, C.; Zhou, J.; Kim, S.-W. Combinatorial engineering of hybrid mevalonate pathways in Escherichia coli for protoilludene production. Microb. Cell Factories 2016, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Chappell, J. Building terpene production platforms in yeast. Biotechnol. Bioeng. 2015, 112, 1854–1864. [Google Scholar] [CrossRef]
- Zada, B.; Wang, C.; Park, J.-B.; Jeong, S.-H.; Park, J.-E.; Singh, H.B.; Kim, S.-W. Metabolic engineering of Escherichia coli for production of mixed isoprenoid alcohols and their derivatives. Biotechnol. Biofuels 2018, 11, 210. [Google Scholar] [CrossRef]
- Liu, C.-L.; Tian, T.; Alonso-Gutierrez, J.; Garabedian, B.; Wang, S.; Baidoo, E.E.K.; Benites, V.; Chen, Y.; Petzold, C.J.; Adams, P.D.; et al. Renewable production of high density jet fuel precursor sesquiterpenes from Escherichia coli. Biotechnol. Biofuels 2018, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; d’Espaux, L.; Dev, I.; van der Horst, C.; Keasling, J. De novo synthesis of the sedative valerenic acid in Saccharomyces cerevisiae. Metab. Eng. 2018, 47, 94–101. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Zhao, F.; Lu, C.; Zhao, G.-R.; Lu, W. Production of sesquiterpenoid zerumbone from metabolic engineered Saccharomyces cerevisiae. Metab. Eng. 2018, 49, 28–35. [Google Scholar] [CrossRef]
- Zha, W.; An, T.; Li, T.; Zhu, J.; Gao, K.; Sun, Z.; Xu, W.; Lin, P.; Zi, J. Reconstruction of the Biosynthetic Pathway of Santalols under Control of the GAL Regulatory System in Yeast. ACS Synth. Biol. 2020, 9, 449–456. [Google Scholar] [CrossRef]
- Adio, A.M. (-)trans-β-Elemene and related compounds: Occurrence, synthesis, and anticancer activity. Tetrahedron 2009, 65, 5145–5159. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, Y.; Huang, L.; Zhang, X. Production of miltiradiene by metabolically engineered Saccharomyces cerevisiae. Biotechnol. Bioeng. 2012, 109, 2845–2853. [Google Scholar] [CrossRef]
- Ajikumar, P.K.; Xiao, W.H.; Tyo, K.E.J.; Wang, Y.; Simeon, F.; Leonard, E.; Mucha, O.; Phon, T.H.; Pfeifer, B.; Stephanopoulos, G. Isoprenoid Pathway Optimization for Taxol Precursor Overproduction in Escherichia coli. Science 2010, 330, 70–74. [Google Scholar] [CrossRef]
- Nowrouzi, B.; Li, R.A.; Walls, L.E.; d’Espaux, L.; Malci, K.; Liang, L.G.; Jonguitud-Borrego, N.; Lerma-Escalera, A.I.; Morones-Ramirez, J.R.; Keasling, J.D.; et al. Enhanced production of taxadiene in Saccharomyces cerevisiae. Microb. Cell Factories 2020, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.Y.; Zhou, J.W.; Tong, Y.R.; Su, P.; Li, X.L.; Liu, Y.; Liu, N.; Wu, X.Y.; Zhang, Y.F.; Wang, J.D.; et al. Engineering chimeric diterpene synthases and isoprenoid biosynthetic pathways enables high-level production of miltiradiene in yeast. Metab. Eng. 2020, 60, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yu, W.; Chen, Y.; Yang, S.; Zhao, Z.K.; Nielsen, J.; Luan, H.W.; Zhou, Y.J. Engineering yeast for high-level production of diterpenoid sclareol. Metab. Eng. 2023, 75, 19–28. [Google Scholar] [CrossRef]
- Huang, Q.; Roessner, C.A.; Croteau, R.; Scott, A.I. Engineering Escherichia coli for the synthesis of taxadiene, a key intermediate in the biosynthesis of taxol. Bioorg. Med. Chem. 2001, 9, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Biggs, B.W.; Lim, C.G.; Sagliani, K.; Shankar, S.; Stephanopoulos, G.; De Mey, M.; Ajikumar, P.K. Overcoming heterologous protein interdependency to optimize P450-mediated Taxol precursor synthesis in Escherichia coli. Proc. Natl. Acad. Sci. USA 2016, 113, 3209–3214. [Google Scholar] [CrossRef]
- Zhou, K.; Qiao, K.; Edgar, S.; Stephanopoulos, G. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat. Biotechnol. 2015, 33, 377–383. [Google Scholar] [CrossRef]
- Walls, L.E.; Malcı, K.; Nowrouzi, B.; Li, R.A.; d’Espaux, L.; Wong, J.; Dennis, J.A.; Semião, A.J.C.; Wallace, S.; Martinez, J.L.; et al. Optimizing the biosynthesis of oxygenated and acetylated Taxol precursors in Saccharomyces cerevisiae using advanced bioprocessing strategies. Biotechnol. Bioeng. 2020, 118, 279–293. [Google Scholar] [CrossRef]
- Kong, M.K.; Kang, H.-J.; Kim, J.H.; Oh, S.H.; Lee, P.C. Metabolic engineering of the Stevia rebaudiana ent-kaurene biosynthetic pathway in recombinant Escherichia coli. J. Biotechnol. 2015, 214, 95–102. [Google Scholar] [CrossRef]
- Moon, J.H.; Lee, K.; Lee, J.H.; Lee, P.C. Redesign and reconstruction of a steviol-biosynthetic pathway for enhanced production of steviol in Escherichia coli. Microb. Cell Factories 2020, 19, 20. [Google Scholar] [CrossRef]
- Geiselman, G.M.; Zhuang, X.; Kirby, J.; Tran-Gyamfi, M.B.; Prahl, J.-P.; Sundstrom, E.R.; Gao, Y.; Munoz Munoz, N.; Nicora, C.D.; Clay, D.M.; et al. Production of ent-kaurene from lignocellulosic hydrolysate in Rhodosporidium toruloides. Microb. Cell Factories 2020, 19, 24. [Google Scholar] [CrossRef]
- Tokuhiro, K.; Muramatsu, M.; Ohto, C.; Kawaguchi, T.; Obata, S.; Muramoto, N.; Hirai, M.; Takahashi, H.; Kondo, A.; Sakuradani, E.; et al. Overproduction of Geranylgeraniol by Metabolically Engineered Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2009, 75, 5536–5543. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, L.; Li, Y.; Xu, S.; Jiang, W.; Liang, C.; Fang, Y.; Chu, A.; Zhang, L.; Ding, Z.; et al. Enhancing Geranylgeraniol Production by Metabolic Engineering and Utilization of Isoprenol as a Substrate in Saccharomyces cerevisiae. J. Agric. Food Chem. 2021, 69, 4480–4489. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Z.; Wang, G.; Lv, H.; Mao, Y.; Ma, K.; Wang, Y. De novo production of versatile oxidized kaurene diterpenes in Escherichia coli. Metab. Eng. 2022, 73, 201–213. [Google Scholar] [CrossRef]
- Schalk, M.; Pastore, L.; Mirata, M.A.; Khim, S.; Schouwey, M.; Deguerry, F.; Pineda, V.; Rocci, L.; Daviet, L. Toward a Biosynthetic Route to Sclareol and Amber Odorants. J. Am. Chem. Soc. 2012, 134, 18900–18903. [Google Scholar] [CrossRef]
- Ignea, C.; Trikka, F.A.; Nikolaidis, A.K.; Georgantea, P.; Ioannou, E.; Loupassaki, S.; Kefalas, P.; Kanellis, A.K.; Roussis, V.; Makris, A.M.; et al. Efficient diterpene production in yeast by engineering Erg20p into a geranylgeranyl diphosphate synthase. Metab. Eng. 2015, 27, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Leonard, E.; Ajikumar, P.K.; Thayer, K.; Xiao, W.-H.; Mo, J.D.; Tidor, B.; Stephanopoulos, G.; Prather, K.L.J. Combining metabolic and protein engineering of a terpenoid biosynthetic pathway for overproduction and selectivity control. Proc. Natl. Acad. Sci. USA 2010, 107, 13654–13659. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, C.; Lu, W. Heterologous production of levopimaric acid in Saccharomyces cerevisiae. Microb. Cell Factories 2018, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Lindley, N.; Too, H.-P. A “plug-n-play” modular metabolic system for the production of apocarotenoids. Biotechnol. Bioeng. 2018, 115, 174–183. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, T.; Yu, H.; Ye, L. Selective biosynthesis of retinol in S. cerevisiae. Bioresour. Bioprocess. 2022, 9, 22. [Google Scholar] [CrossRef]
- Park, H.; Lee, D.; Kim, J.-E.; Park, S.; Park, J.H.; Ha, C.W.; Baek, M.; Yoon, S.-H.; Park, K.H.; Lee, P.; et al. Efficient production of retinol in Yarrowia lipolytica by increasing stability using antioxidant and detergent extraction. Metab. Eng. 2022, 73, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, X.; Li, X.; Shi, H.; Wang, F.; Zhang, Y.; Li, X. Combinatorial Engineering of Mevalonate Pathway and Diterpenoid Synthases in Escherichia coli for cis-Abienol Production. J. Agric. Food Chem. 2019, 67, 6523–6531. [Google Scholar] [CrossRef]
- Zhang, C.; Ju, H.; Lu, C.-Z.; Zhao, F.; Liu, J.; Guo, X.; Wu, Y.; Zhao, G.-R.; Lu, W. High-titer production of 13R-manoyl oxide in metabolically engineered Saccharomyces cerevisiae. Microb. Cell Factories 2019, 18, 73. [Google Scholar] [CrossRef]
- Pateraki, I.; Andersen-Ranberg, J.; Jensen, N.B.; Wubshet, S.G.; Heskes, A.M.; Forman, V.; Hallström, B.; Hamberger, B.; Motawia, M.S.; Olsen, C.E.; et al. Total biosynthesis of the cyclic AMP booster forskolin from Coleus forskohlii. eLife 2017, 6, 23001. [Google Scholar] [CrossRef] [PubMed]
- Kildegaard, K.R.; Arnesen, J.A.; Adiego-Pérez, B.; Rago, D.; Kristensen, M.; Klitgaard, A.K.; Hansen, E.H.; Hansen, J.; Borodina, I. Tailored biosynthesis of gibberellin plant hormones in yeast. Metab. Eng. 2021, 66, 1–11. [Google Scholar] [CrossRef]
- Wei, P.; Zhang, C.; Bian, X.; Lu, W. Metabolic Engineering of Saccharomyces cerevisiae for Heterologous Carnosic Acid Production. Front. Bioeng. Biotechnol. 2022, 10, 916605. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X.; Zhang, C.; Zhou, X.; Xu, X.; Han, L.; Lv, X.; Liu, Y.; Liu, S.; Li, J.; et al. De novo biosynthesis of rubusoside and rebaudiosides in engineered yeasts. Nat. Commun. 2022, 13, 3040. [Google Scholar] [CrossRef]
- Yendo, A.C.A.; de Costa, F.; Gosmann, G.; Fett-Neto, A.G. Production of Plant Bioactive Triterpenoid Saponins: Elicitation Strategies and Target Genes to Improve Yields. Mol. Biotechnol. 2010, 46, 94–104. [Google Scholar] [CrossRef]
- Zhao, Y.-j.; Li, C. Biosynthesis of Plant Triterpenoid Saponins in Microbial Cell Factories. J. Agric. Food Chem. 2018, 66, 12155–12165. [Google Scholar] [CrossRef]
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C.-W. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12. [Google Scholar] [CrossRef]
- Dai, Z.B.; Liu, Y.; Zhang, X.A.; Shi, M.Y.; Wang, B.B.; Wang, D.; Huang, L.Q.; Zhang, X.L. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metab. Eng. 2013, 20, 146–156. [Google Scholar] [CrossRef]
- Luo, Z.S.; Liu, N.; Lazar, Z.; Chatzivasileiou, A.; Ward, V.; Chen, J.; Zhou, J.W.; Stephanopoulos, G. Enhancing isoprenoid synthesis in Yarrowia lipolytica by expressing the isopentenol utilization pathway and modulating intracellular hydrophobicity. Metab. Eng. 2020, 61, 344–351. [Google Scholar] [CrossRef]
- Li, T.; Liu, G.-S.; Zhou, W.; Jiang, M.; Ren, Y.-H.; Tao, X.-Y.; Liu, M.; Zhao, M.; Wang, F.-Q.; Gao, B.; et al. Metabolic Engineering of Saccharomyces cerevisiae To Overproduce Squalene. J. Agric. Food Chem. 2020, 68, 2132–2138. [Google Scholar] [CrossRef]
- Ke, D.; Caiyin, Q.; Zhao, F.; Liu, T.; Lu, W. Heterologous biosynthesis of triterpenoid ambrein in engineered Escherichia coli. Biotechnol. Lett. 2017, 40, 399–404. [Google Scholar] [CrossRef]
- Moser, S.; Strohmeier, G.A.; Leitner, E.; Plocek, T.J.; Vanhessche, K.; Pichler, H. Whole-cell (+)-ambrein production in the yeast Pichia pastoris. Metab. Eng. Commun. 2018, 7, e00077. [Google Scholar] [CrossRef]
- Huang, J.; Zha, W.; An, T.; Dong, H.; Huang, Y.; Wang, D.; Yu, R.; Duan, L.; Zhang, X.; Peters, R.J.; et al. Identification of RoCYP01 (CYP716A155) enables construction of engineered yeast for high-yield production of betulinic acid. Appl. Microbiol. Biotechnol. 2019, 103, 7029–7039. [Google Scholar] [CrossRef]
- Li, W.; Ma, X.; Li, G.; Zhang, A.; Wang, D.; Fan, F.; Ma, X.; Zhang, X.; Dai, Z.; Qian, Z. De Novo Biosynthesis of the Oleanane-Type Triterpenoids of Tunicosaponins in Yeast. ACS Synth. Biol. 2021, 10, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Zhou, Z.; Liang, Q.; Mosongo, I.; Li, C.; Zhang, Y. Improving lupeol production in yeast by recruiting pathway genes from different organisms. Sci. Rep. 2019, 9, 2992. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Rasool, A.; Liu, H.; Lv, B.; Chang, P.; Song, H.; Wang, Y.; Li, C. Engineering Saccharomyces cerevisiae for high yield production of α-amyrin via synergistic remodeling of α-amyrin synthase and expanding the storage pool. Metab. Eng. 2020, 62, 72–83. [Google Scholar] [CrossRef]
- Zhang, G.; Cao, Q.; Liu, J.; Liu, B.-x.; Li, J.; Li, C. Refactoring β-amyrin synthesis in Saccharomyces cerevisiae. Aiche J. 2015, 61, 3172–3179. [Google Scholar] [CrossRef]
- Kirby, J.; Romanini, D.W.; Paradise, E.M.; Keasling, J.D. Engineering triterpene production in Saccharomyces cerevisiae–β-amyrin synthase from Artemisia annua. FEBS J. 2008, 275, 1852–1859. [Google Scholar] [CrossRef]
- Gao, H.; Shao, M.; Zhang, X.; Yang, T.; Xu, M.; Gao, X.; Rao, Z. Construction of Saccharomyces cerevisiae cell factories for the production of pentacyclic triterpenoids. Food Ferment. Ind. 2021, 47, 8–14. [Google Scholar]
- Lu, C.; Zhang, C.; Zhao, F.; Li, D.; Lu, W. Biosynthesis of ursolic acid and oleanolic acid in Saccharomyces cerevisiae. AIChE J. 2018, 64, 3794–3802. [Google Scholar] [CrossRef]
- Jin, C.C.; Zhang, J.L.; Song, H.; Cao, Y.X. Boosting the biosynthesis of betulinic acid and related triterpenoids in Yarrowia lipolytica via multimodular metabolic engineering. Microb. Cell Factories 2019, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, J.; Wang, C.; Feng, X.; Li, C. Enhancing oleanolic acid production in engineered Saccharomyces cerevisiae. Bioresour. Technol. 2018, 257, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.F.; Xiao, H.; Zhong, J.J. Biosynthesis of a ganoderic acid in Saccharomyces cerevisiae by expressing a cytochrome P450 gene from Ganoderma lucidum. Biotechnol. Bioeng. 2018, 115, 1842–1854. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Liu, Y.; Sun, Z.; Wang, D.; Qu, G.; Ma, X.; Fan, F.; Zhang, L.; Li, S.; Zhang, X. Identification of a novel cytochrome P450 enzyme that catalyzes the C-2α hydroxylation of pentacyclic triterpenoids and its application in yeast cell factories. Metab. Eng. 2019, 51, 70–78. [Google Scholar] [CrossRef]
- Hansen, N.L.; Miettinen, K.; Zhao, Y.; Ignea, C.; Andreadelli, A.; Raadam, M.H.; Makris, A.M.; Møller, B.L.; Stærk, D.; Bak, S.; et al. Integrating pathway elucidation with yeast engineering to produce polpunonic acid the precursor of the anti-obesity agent celastrol. Microb. Cell Factories 2020, 19, 15. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, C.; Sun, W.; Zhou, A.; Wang, Y.; Zhang, G.; Zhou, X.; Huo, Y.; Li, C. Boosting 11-oxo-β-amyrin and glycyrrhetinic acid synthesis in Saccharomyces cerevisiae via pairing novel oxidation and reduction system from legume plants. Metab. Eng. 2018, 45, 43–50. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Xu, J.; Wang, J.; Dai, Z.; Zhang, X.; Huang, L. Construction of efficient yeast cell factories for production of ginsenosides precursor dammarenediol-II. Acta Pharm. Sin. B 2018, 53, 1233–1241. [Google Scholar]
- Zhang, X. Performance Optimization of Saccharomyces cerevisiae for Dammarendiol-II Production. Master’s Thesis, Tianjin University, Tianjin, China, 2015. [Google Scholar]
- Li, D.; Zhang, Q.; Zhou, Z.; Zhao, F.; Lu, W. Heterologous biosynthesis of triterpenoid dammarenediol-II in engineered Escherichia coli. Biotechnol. Lett. 2016, 38, 603–609. [Google Scholar] [CrossRef]
- Dai, Z.; Wang, B.; Liu, Y.; Shi, M.; Wang, D.; Zhang, X.; Liu, T.; Huang, L.; Zhang, X. Producing aglycons of ginsenosides in bakers’ yeast. Sci. Rep. 2014, 4, 3698. [Google Scholar] [CrossRef]
- Wang, P.; Wei, W.; Ye, W.; Li, X.; Zhao, W.; Yang, C.; Li, C.; Yan, X.; Zhou, Z. Synthesizing ginsenoside Rh2 in Saccharomyces cerevisiae cell factory at high-efficiency. Cell Discov. 2019, 5, 5. [Google Scholar] [CrossRef]
- Wang, P.; Wei, Y.; Fan, Y.; Liu, Q.; Wei, W.; Yang, C.; Zhang, L.; Zhao, G.; Yue, J.; Yan, X.; et al. Production of bioactive ginsenosides Rh2 and Rg3 by metabolically engineered yeasts. Metab. Eng. 2015, 29, 97–105. [Google Scholar] [CrossRef]
- Jung, S.C.; Kim, W.; Park, S.C.; Jeong, J.; Park, M.K.; Lim, S.; Lee, Y.; Im, W.T.; Lee, J.H.; Choi, G.; et al. Two Ginseng UDP-Glycosyltransferases Synthesize Ginsenoside Rg3 and Rd. Plant Cell Physiol. 2014, 55, 2177–2188. [Google Scholar] [CrossRef]
- Wei, W.; Wang, P.; Wei, Y.; Liu, Q.; Yang, C.; Zhao, G.; Yue, J.; Yan, X.; Zhou, Z. Characterization of Panax ginseng UDP-Glycosyltransferases Catalyzing Protopanaxatriol and Biosyntheses of Bioactive Ginsenosides F1 and Rh1 in Metabolically Engineered Yeasts. Mol. Plant 2015, 8, 1412–1424. [Google Scholar] [CrossRef]
- Yoon, S.H.; Park, H.M.; Kim, J.E.; Lee, S.H.; Choi, M.S.; Kim, J.Y.; Oh, D.K.; Keasling, J.D.; Kim, S.W. Increased β-carotene production in recombinant Escherichia coli harboring an engineered isoprenoid precursor pathway with mevalonate addition. Biotechnol. Progress 2007, 23, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, L. Biosynthesis of β-carotene in engineered E. coli using the MEP and MVA pathways. Microb. Cell Factories 2014, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Fathi, Z.; Tramontin, L.R.R.; Ebrahimipour, G.; Borodina, I.; Darvishi, F. Metabolic engineering of Saccharomyces cerevisiae for production of β-carotene from hydrophobic substrates. FEMS Yeast Res. 2020, 21, foaa068. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, H.; Cui, Y.-Y.; Chen, S.-G.; Weng, Z.; Zhao, M.; Liu, J.-Z. Chromosomal evolution of Escherichia coli for the efficient production of lycopene. BMC Biotechnol. 2013, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Miao, L.; Li, Q.; Dai, G.; Lu, F.; Liu, T.; Zhang, X.; Ma, Y. Production of lycopene by metabolically-engineered Escherichia coli. Biotechnol. Lett. 2014, 36, 1515–1522. [Google Scholar] [CrossRef]
- Ma, T.; Shi, B.; Ye, Z.; Li, X.; Liu, M.; Chen, Y.; Xia, J.; Nielsen, J.; Deng, Z.; Liu, T. Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metab. Eng. 2019, 52, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Nicolás-Molina, F.E.; Navarro, E.; Ruiz-Vázquez, R.M. Lycopene over-accumulation by disruption of the negative regulator gene crgA in Mucor circinelloides. Appl. Microbiol. Biotechnol. 2008, 78, 131–137. [Google Scholar] [CrossRef]
- Wang, G.-S.; Grammel, H.; Abou-Aisha, K.; Sägesser, R.; Ghosh, R. High-Level Production of the Industrial Product Lycopene by the Photosynthetic Bacterium Rhodospirillum rubrum. Appl. Environ. Microbiol. 2012, 78, 7205–7215. [Google Scholar] [CrossRef]
- Su, A.; Chi, S.; Li, Y.; Tan, S.; Qiang, S.; Chen, Z.; Meng, Y. Metabolic Redesign of Rhodobacter sphaeroides for Lycopene Production. J. Agric. Food Chem. 2018, 66, 5879–5885. [Google Scholar] [CrossRef]
- Zuo, Z.-Q.; Xue, Q.; Zhou, J.; Zhao, D.-H.; Han, J.; Xiang, H. Engineering Haloferax mediterranei as an Efficient Platform for High Level Production of Lycopene. Front. Microbiol. 2018, 9, 2893. [Google Scholar] [CrossRef]
- Bhataya, A.; Schmidt-Dannert, C.; Lee, P.C. Metabolic engineering of Pichia pastoris X-33 for lycopene production. Process Biochem. 2009, 44, 1095–1102. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Duan, Y.; Zheng, X.; Lin, Y.; Liang, S. Production of lycopene by metabolically engineered Pichia pastoris. Biosci. Biotechnol. Biochem. 2020, 84, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gong, Z.; Tang, J.; Lu, F.; Li, Q.; Zhang, X. Improving astaxanthin production in Escherichia coli by co-utilizing CrtZ enzymes with different substrate preference. Microb. Cell Factories 2022, 21, 71. [Google Scholar] [CrossRef]
- Chai, F.; Wang, Y.; Mei, X.; Yao, M.; Chen, Y.; Liu, H.; Xiao, W.; Yuan, Y. Heterologous biosynthesis and manipulation of crocetin in Saccharomyces cerevisiae. Microb. Cell Factories 2017, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-R.; Tian, G.-Q.; Shen, H.-J.; Liu, J.-Z. Metabolic engineering of Escherichia coli to produce zeaxanthin. J. Ind. Microbiol. Biotechnol. 2015, 42, 627–636. [Google Scholar] [CrossRef]
- Shen, H.-J.; Cheng, B.-Y.; Zhang, Y.-M.; Tang, L.; Li, Z.; Bu, Y.-F.; Li, X.-R.; Tian, G.-Q.; Liu, J.-Z. Dynamic control of the mevalonate pathway expression for improved zeaxanthin production in Escherichia coli and comparative proteome analysis. Metab. Eng. 2016, 38, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Ning, J.C.; Zhao, H. Coordinated induction of multi-gene pathways in Saccharomyces cerevisiae. Nucleic Acids Res. 2013, 41, e54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beuttler, H.; Hoffmann, J.; Jeske, M.; Hauer, B.; Schmid, R.D.; Altenbuchner, J.; Urlacher, V.B. Biosynthesis of zeaxanthin in recombinant Pseudomonas putida. Appl. Microbiol. Biotechnol. 2010, 89, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.P.M.; e Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Bardi, L.; Mattei, A.; Steffan, S.; Marzona, M. Hydrocarbon degradation by a soil microbial population with beta-cyclodextrin as surfactant to enhance bioavailability. Enzym. Microb. Technol. 2000, 27, 709–713. [Google Scholar] [CrossRef]
- Wu, M.; Li, W.; Dick, W.A.; Ye, X.; Chen, K.; Kost, D.; Chen, L. Bioremediation of hydrocarbon degradation in a petroleum-contaminated soil and microbial population and activity determination. Chemosphere 2017, 169, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Benfenati, E.; Gini, G.; Piclin, N.; Roncaglioni, A.; Vari, M.R. Predicting logP of pesticides using different software. Chemosphere 2003, 53, 1155–1164. [Google Scholar] [CrossRef]
- Akira Inoue, K.H. Estimation of Solvent-Tolerance of Bacteria by the Solvent Parameter Log P. J. Ferment. Bioeng. 1991, 71, 193–196. [Google Scholar]
- Laane, C.; Boeren, S.; Vos, K.; Veeger, C. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 1987, 30, 81–87. [Google Scholar] [CrossRef]
- Demling, P.; von Campenhausen, M.; Grütering, C.; Tiso, T.; Jupke, A.; Blank, L.M. Selection of a recyclablein situliquid–liquid extraction solvent for foam-free synthesis of rhamnolipids in a two-phase fermentation. Green Chem. 2020, 22, 8495–8510. [Google Scholar] [CrossRef]
- Kujawski, J.; Bernard, M.K.; Janusz, A.; Kuźma, W. Prediction of log P: ALOGPS Application in Medicinal Chemistry Education. J. Chem. Educ. 2011, 89, 64–67. [Google Scholar] [CrossRef]
- Yadav, D.K.; Yadav, M.; Rani, P.; Yadav, A.; Bhardwaj, N.; Bishnoi, N.R.; Singh, A. Demonstration of n-dodecane suitability for milking lipids from Chlorella vulgaris for the production of biodiesel. Bioresour. Technol. Rep. 2023, 23, 101550. [Google Scholar] [CrossRef]
- Zhang, C.; Seow, V.Y.; Chen, X.; Too, H.-P. Multidimensional heuristic process for high-yield production of astaxanthin and fragrance molecules in Escherichia coli. Nat. Commun. 2018, 9, 1858. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Braune, M.; Gröngröft, A. Liquid-Liquid Extraction of Caproic and Caprylic Acid: Solvent Properties and pH. Chem. Ing. Tech. 2023, 95, 1573–1579. [Google Scholar] [CrossRef]
- Wang, A.; Sun, Y.; Sun, Z.; Liu, X.; Yu, X.; Li, K.; Zhang, X.; Xu, Y.; Mu, W.; Li, B. Modification of sedimentation and bioaccumulation behavior as an efficient strategy to modulate the toxicity of pyraclostrobin to zebrafish (Danio rerio). Environ. Pollut. 2023, 322, 121164. [Google Scholar] [CrossRef] [PubMed]
- Ruelle, P. The n-octanol and n-hexane/water partition coefcient ofenvironmentally relevant chemicals predicted from the mobileorder and disorder(MOD)thermodynamics. Chemosphere 2000, 40, 457–512. [Google Scholar] [CrossRef]
- Gill, U.S.; Craan, A.G.; Subramanian, K.S.; Chu, I. Diisononyl Phthalate: Chemistry, Environmental Path, and Toxicology. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 172, pp. 87–127. [Google Scholar]
- Frykman, S.; Tsuruta, H.; Galazzo, J.; Licari, P. Characterization of product capture resin during microbial cultivations. J. Ind. Microbiol. Biotechnol. 2006, 33, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Pellenz, L.; de Oliveira, C.R.S.; da Silva Júnior, A.H.; da Silva, L.J.S.; da Silva, L.; Ulson de Souza, A.A.; de Souza, S.M.d.A.G.U.; Borba, F.H.; da Silva, A. A comprehensive guide for characterization of adsorbent materials. Sep. Purif. Technol. 2023, 305, 122435. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Baloch, H.A.; Mazari, S.A.; Mubarak, N.M.; Sabzoi, N.; Aziz, S.; Soomro, S.A.; Abro, R.; Shah, S.F. A review on extractive fermentation via ion exchange adsorption resins opportunities, challenges, and future prospects. Biomass Convers. Biorefinery 2021, 13, 3543–3554. [Google Scholar] [CrossRef]
- Luongo, V.; Palma, A.; Rene, E.R.; Fontana, A.; Pirozzi, F.; Esposito, G.; Lens, P.N.L. Lactic acid recovery from a model of Thermotoga neapolitana fermentation broth using ion exchange resins in batch and fixed-bed reactors. Sep. Sci. Technol. 2018, 54, 1008–1025. [Google Scholar] [CrossRef]
- Beschkov, V.N. Ion exchange in downstream processing in biotechnology. Phys. Sci. Rev. 2020, 5, 20180066. [Google Scholar] [CrossRef]
- Yousuf, A.; Bonk, F.; Bastidas-Oyanedel, J.-R.; Schmidt, J.E. Recovery of carboxylic acids produced during dark fermentation of food waste by adsorption on Amberlite IRA-67 and activated carbon. Bioresour. Technol. 2016, 217, 137–140. [Google Scholar] [CrossRef]
- Wo, J. Biosynthetic Mechanism of Streptonigrin and High Production of 10′-Desmethoxystreptonigrin. Ph.D. Thesis, Shanghai Jiaotong University, Shanghai, China, 2016. [Google Scholar]
- Isken, S.; Heipieper, H.J. Toxicity of Organic Solvents to Microorganisms; Wiley: New York, NY, USA, 2003. [Google Scholar]
- Osborne, S.J.; Leaver, J.; Turner, M.K.; Dunnill, P. Correlation of biocatalytic activity in an organic-aqueous two-liquid phase system with solvent concentration in the cell membrane. Enzym. Microb. Technol. 1990, 12, 281–291. [Google Scholar] [CrossRef]
- Huang, W.-C.; Tang, I.C. Chapter 8—Bacterial and Yeast Cultures—Process Characteristics, Products, and Applications. In Bioprocessing for Value-Added Products from Renewable Resources; Yang, S.-T., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 185–223. [Google Scholar]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Marbach, A.; Bettenbrock, K. lac operon induction in Escherichia coli: Systematic comparison of IPTG and TMG induction and influence of the transacetylase LacA. J. Biotechnol. 2012, 157, 82–88. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, J.; Zheng, Z.; Chen, N.; Luo, X.; Tang, H. Engineering an Efficient Expression Using Heterologous GAL Promoters and Transcriptional Activators in Saccharomyces cerevisiae. ACS Synth. Biol. 2023, 12, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Compagno, C.; Dashko, S.; Piškur, J. Introduction to Carbon Metabolism in Yeast. In Molecular Mechanisms in Yeast Carbon Metabolism; Piškur, J., Compagno, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–19. [Google Scholar]
- Lopez-Cuellar, M.R.; Alba-Flores, J.; Rodriguez, J.N.; Perez-Guevara, F. Production of polyhydroxyalkanoates (PHAs) with canola oil as carbon source. Int. J. Biol. Macromol. 2011, 48, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Ravera, F.; Dziza, K.; Santini, E.; Cristofolini, L.; Liggieri, L. Emulsification and emulsion stability: The role of the interfacial properties. Adv. Colloid Interface Sci. 2021, 288, 102344. [Google Scholar] [CrossRef]
- Chen, L.; Pang, Y.; Luo, Y.; Cheng, X.; Lv, B.; Li, C. Separation and purification of plant terpenoids from biotransformation. Eng. Life Sci. 2021, 21, 724–738. [Google Scholar] [CrossRef]
- Barton, D.; Chickos, J. The vapor pressure and vaporization enthalpy of (−) β-Elemene and (−) β-Bisabolene by correlation gas chromatography. J. Chem. Thermodyn. 2020, 148, 106139. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).