Preparation and Characterization of Bilayer Polymer-Dispersed Liquid Crystals Doped with Gd2O3 Nanoparticles and Rhodamine B Base Fluorescent Dye
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
- Preparing monolayer PDLC: LC cells were prepared using an 8 μm thick polyimide film as a spacer to hold two single-sided conductive glass substrates apart. Mixtures a1~a6 in Table 1 were injected into LC cells by capillary action, and monolayer PDLC samples were obtained after polymerization. The polymerization time, light intensity, and temperature for this set of samples were 6 min, 4.5 mW/cm2, and 35 °C, respectively;
- Preparing PVA films: A layer of aqueous PVA solution (0.05 wt%) was placed on the glass substrate with surface treatment using surface tension. Glass substrates with PVA films adhered to the surface were obtained by storing them at a temperature of 75 °C for 3 h;
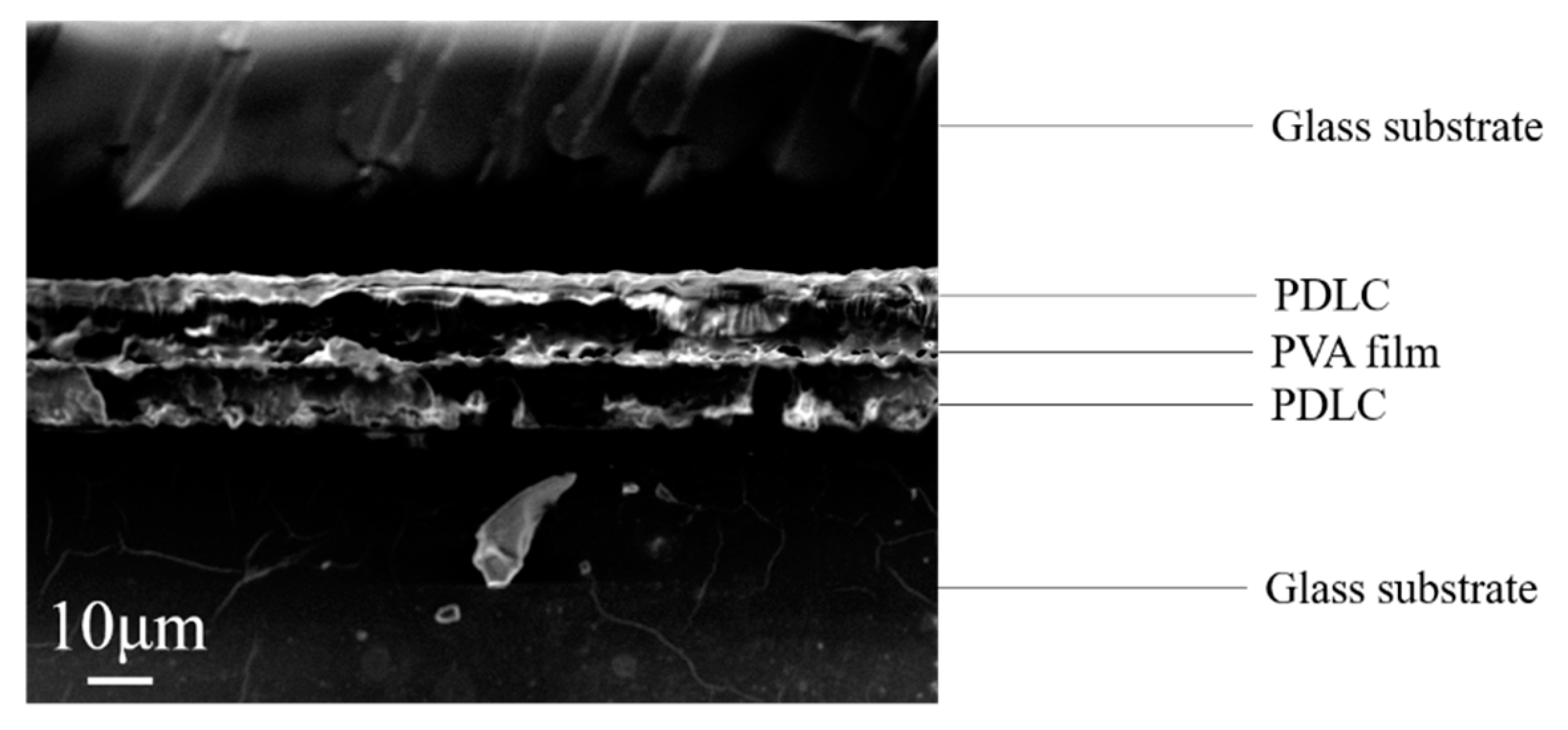
- Preparing bilayer PDLC: The ratios of various substances in the upper and lower layers of the bilayer PDLC are shown in Table 2 and Table 3, respectively. An 8 μm thick polyimide film was used to separate the glass substrate with PVA film on the surface from the unilateral conductive glass substrate and fixed to obtain the LC cell. The upper PDLC precursor was injected into the LC cell by capillary action, and PDLC-PVA composite films were obtained after curing. The glass substrates with PDLC-PVA composite film on the surface were obtained by peeling off the surface-treated glass substrates. The glass substrates attached with PDLC-PVA composite film on the surface were separated from the one-sided conductive glass substrates using 8 μm thick polyimide films to obtain new LC cells. The lower PDLC precursors were injected into new LC cells by capillary action, and bilayer PDLC films with a PDLC–PVA–PDLC structure were acquired after polymerization.
2.3. Characterization
3. Results and Discussions
3.1. Effect of the Crosslinker Content on the Property of Monolayer PDLC
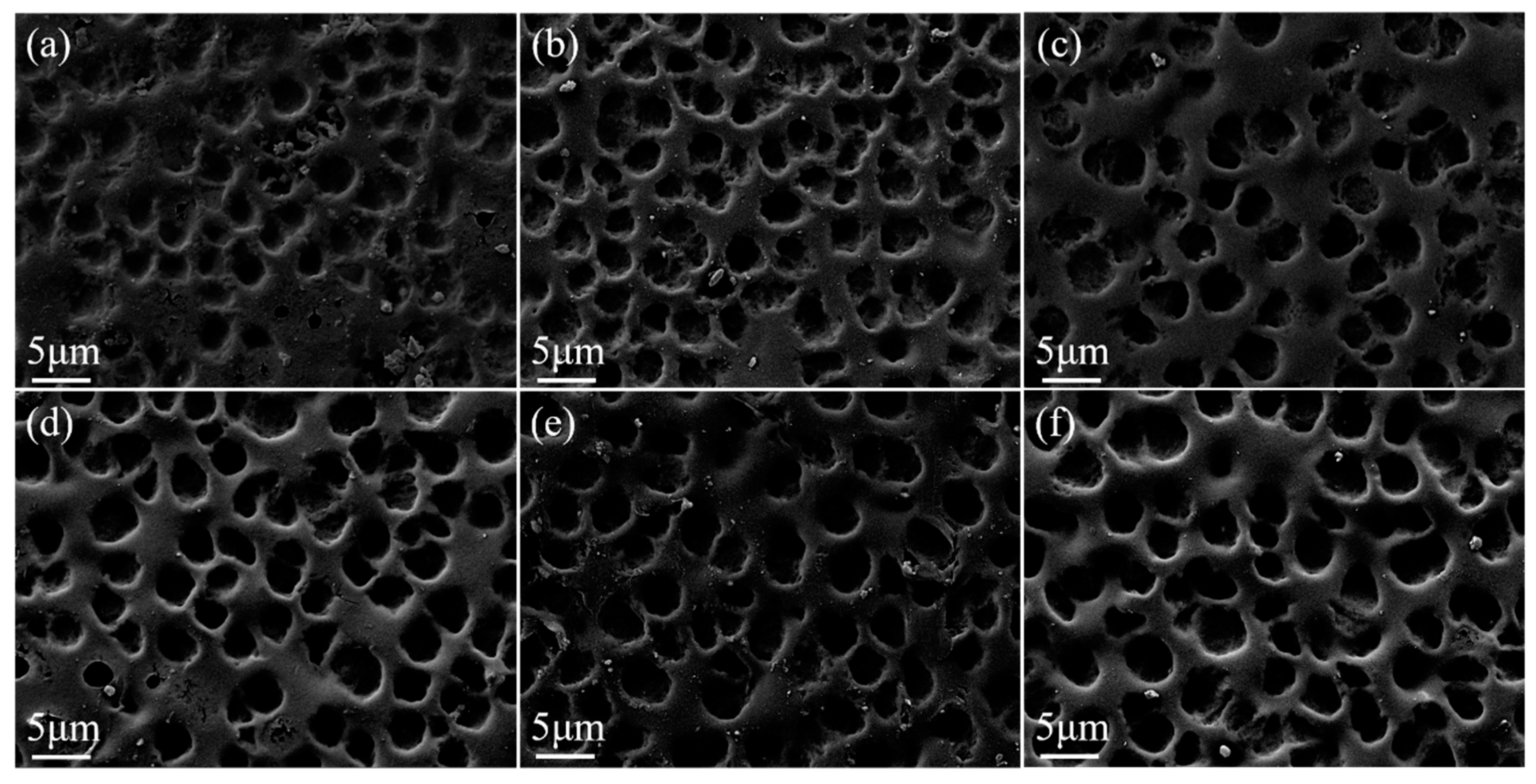
3.2. Gd2O3 Nanoparticles Doped Bilayer PDLC
3.2.1. Modification of Gd2O3 Nanoparticles
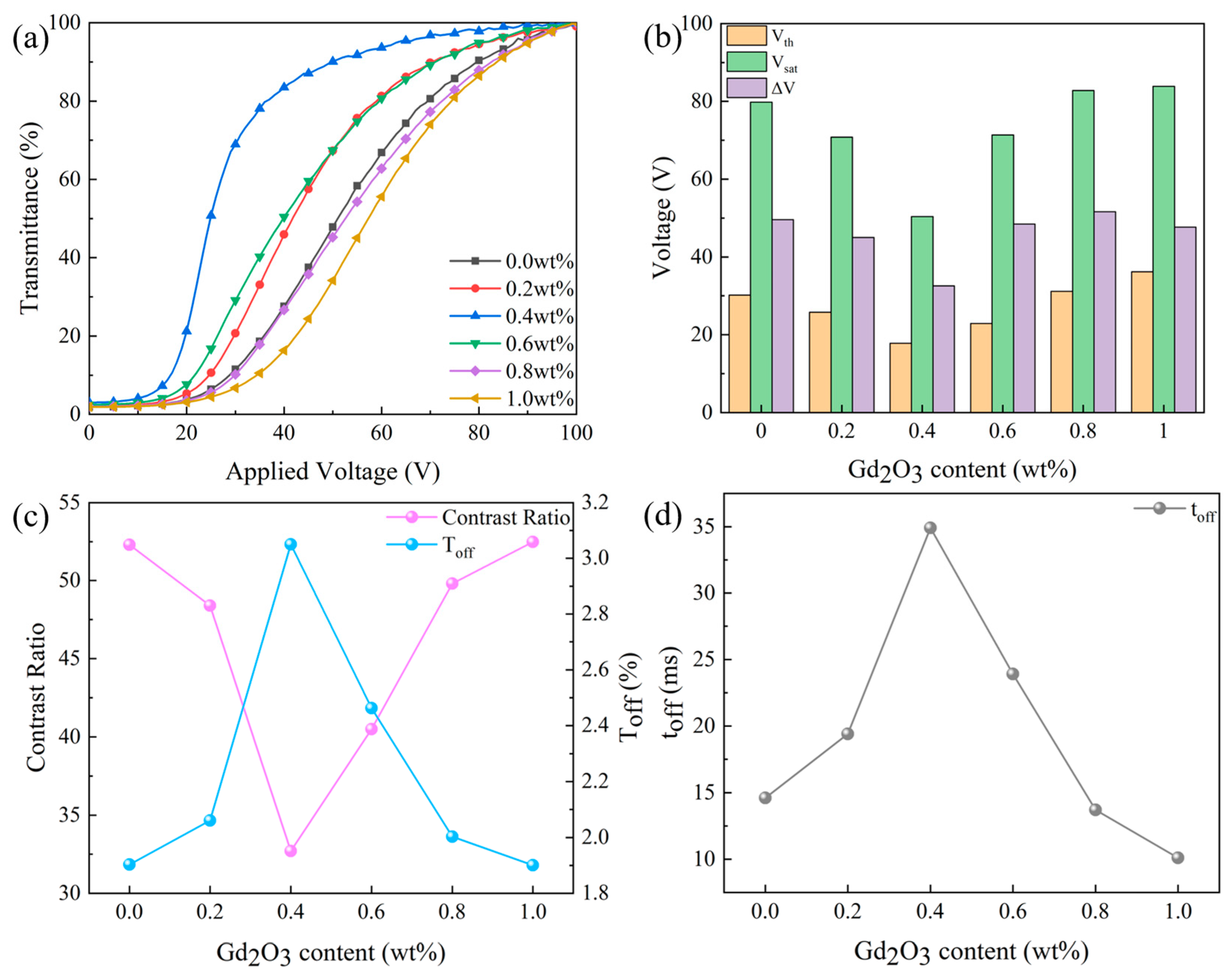
3.2.2. Effect of Gd2O3 Nanoparticle Content on the Properties of Bilayer PDLC
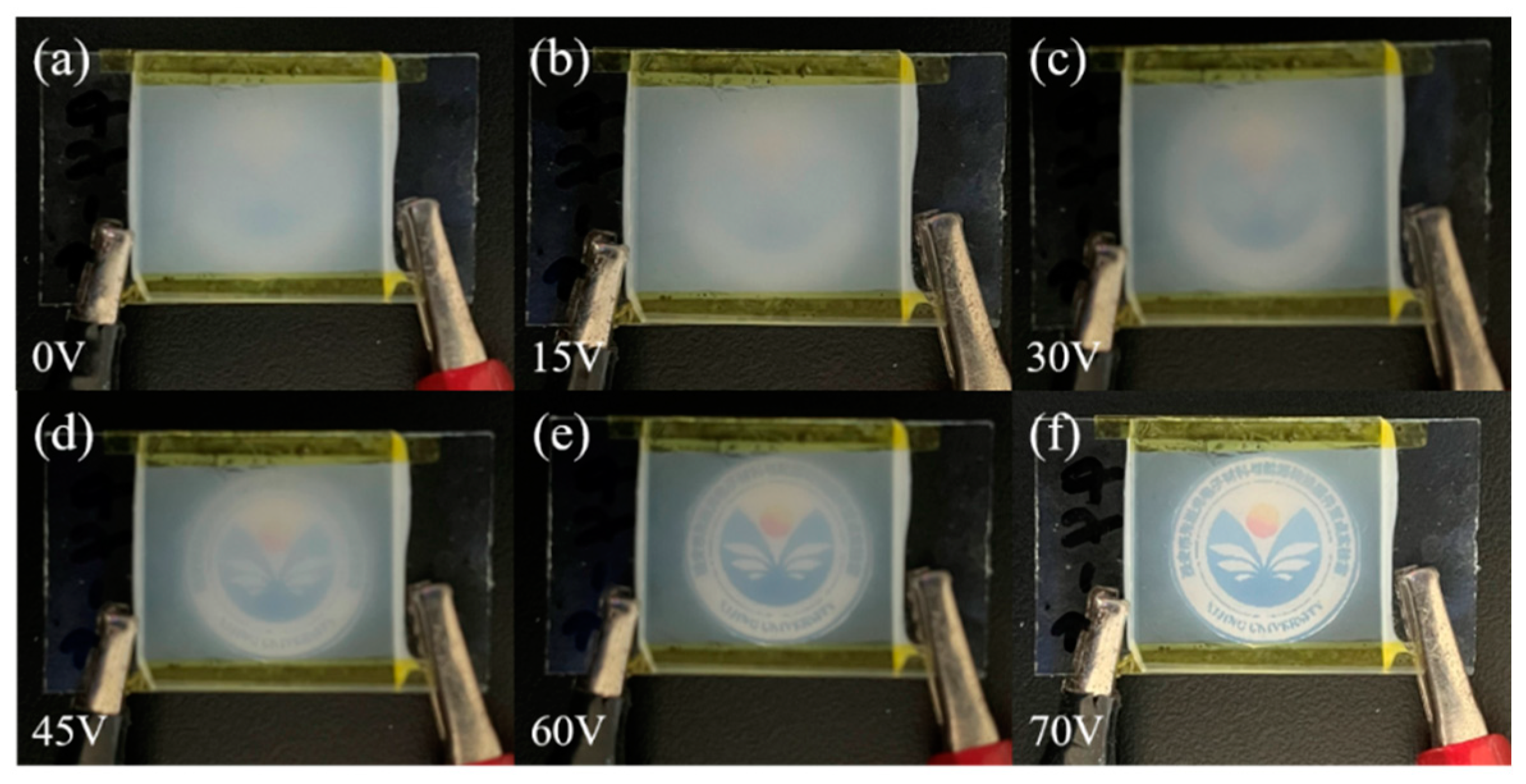
3.2.3. Progressive Driving Test of Bilayer PDLC Doped with Gd2O3 Nanoparticles
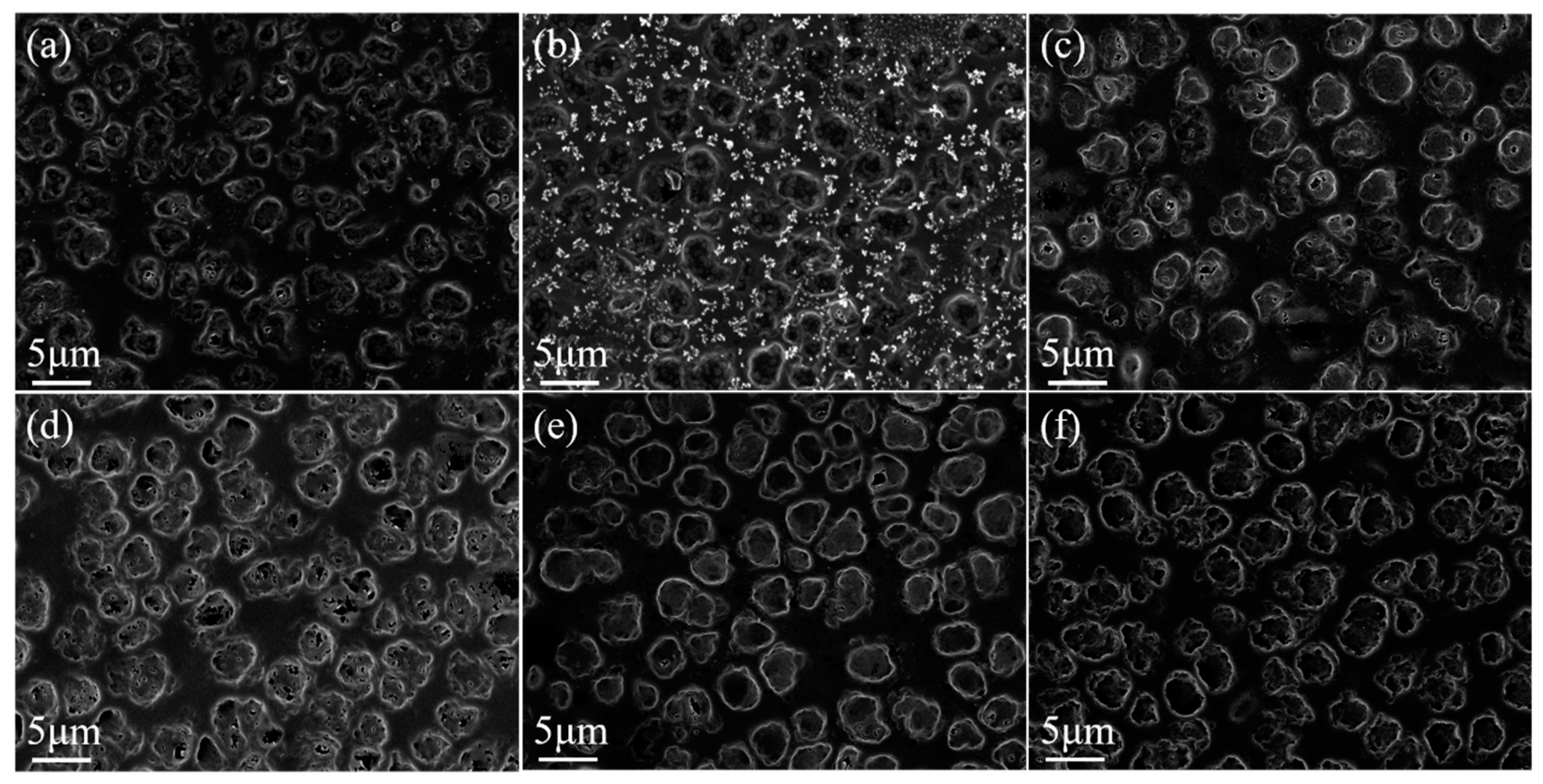
3.3. Fluorescent Dye Rhodamine B Base Doped Bilayer PDLC
3.3.1. Effect of the Fluorescent Dye Rhodamine B Base on Bilayer PDLC Properties
3.3.2. Progressive Driving Test of Bilayer PDLC Doped with Rhodamine B Base
3.4. High Temperature and Strong UV Light Radiation Tests
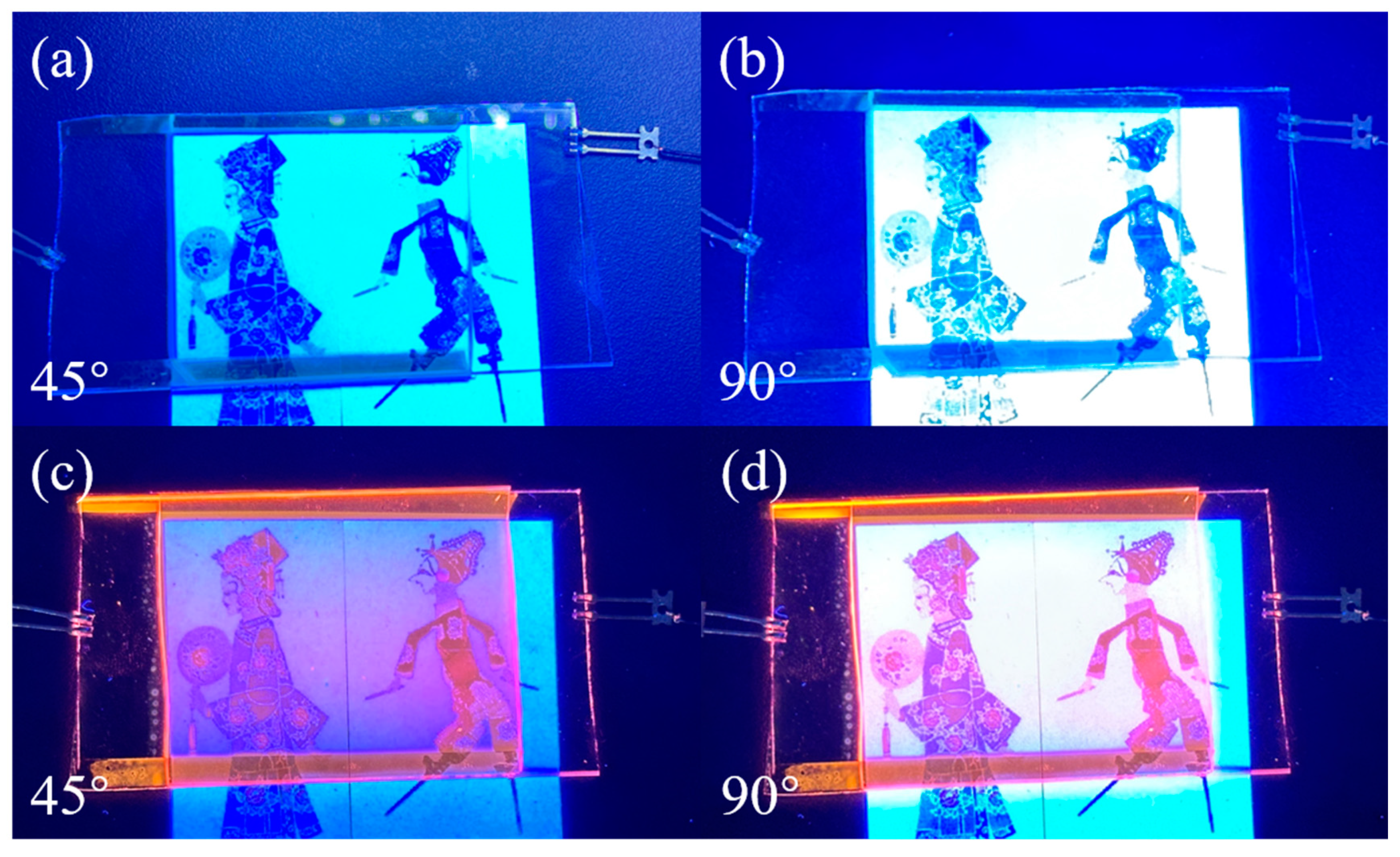
3.5. Application of Bilayer PDLC Doped with Rhodamine B Base in Multi-Angle Anti-Counterfeiting
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.M.; Miao, Z.C.; Shen, W.B. Development of polymer-dispersed liquid crystals: From mode innovation to applications. Compos. Part A Appl. Sci. Manuf. 2022, 163, 107234. [Google Scholar] [CrossRef]
- Hemaida, A.; Ghosh, A.; Sundaram, S.; Mallick, T.K. Simulation study for a switchable adaptive polymer dispersed liquid crystal smart window for two climate zones (Riyadh and London). Energ. Build. 2021, 251, 111381. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.W.; Cai, W.F.; He, H.L.; Cen, M.J.; Liu, J.X.; Luo, D.; Mu, Q.Q.; Gérard, D.; Liu, Y.J. Electrically switchable, polarization-sensitive encryption based on aluminum nanoaperture arrays integrated with polymer-dispersed liquid crystals. Nano Lett. 2021, 21, 7183–7190. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kang, D.; Nguyen, V.H.; Nasir, N.; Hong, H.; Kim, M.; Nguyen, D.C.; Lee, Y.J.; Lee, N.; Seo, Y. Application of titanium-carbide MXene-based transparent conducting electrodes in flexible smart windows. ACS Appl. Mater. Interfaces 2021, 13, 40976–40985. [Google Scholar] [CrossRef] [PubMed]
- Kamal, W.; Li, M.M.; Lin, J.D.; Parry, E.; Jin, Y.H.; Elston, S.J.; Castrejón-Pita, A.A.; Morris, S.M. Spatially patterned polymer dispersed liquid crystals for image-integrated smart windows. Adv. Opt. Mater. 2022, 10, 2101748. [Google Scholar] [CrossRef]
- Iluyemi, D.C.; Nundy, S.; Shaik, S.; Tahir, A.; Ghosh, A. Building energy analysis using EC and PDLC based smart switchable window in Oman. Sol. Energy 2022, 237, 301–312. [Google Scholar] [CrossRef]
- Gong, S.; Cao, Y.; Fang, X.; Wang, Y.F.; Liu, Q.Z.; Gui, H.; Shen, C.F.; Cao, X.; Kim, E.S.; Zhou, C.W. Carbon nanotube macroelectronics for active matrix polymer-dispersed liquid crystal displays. ACS Nano 2016, 10, 10068–10074. [Google Scholar]
- Manabe, H.; Date, M.; Takada, H.; Inamura, H. Techniques to reduce driving energy of 1-pixel displays. IEEE Trans. Ind. Appl. 2016, 52, 2638–2647. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.L.; Guan, S.; Guo, Z.H.; Zheng, X.X.; Fan, Y.J.; Wang, Y.; Qu, T.; Zhao, Y.B.; Chen, A.H.; et al. Self-powered optical switch based on triboelectrification-triggered liquid crystal alignment for wireless sensing. Adv. Funct. Mater. 2019, 29, 1808633. [Google Scholar] [CrossRef]
- Van, M.C.W.; Janssen, R.H.C.; Broer, D.J.; Wilderbeek, H.T.A.; Bastiaansen, C.W.M. Polymer-filled nematics: A new class of light-scattering materials for electro-optical switches. Adv. Mater. 2000, 12, 753–757. [Google Scholar]
- Baars, M.W.P.L.; Van, M.C.W.; Bastiaansen, C.W.M.; Broer, D.J.; Söntjens, S.H.M.; Meijer, E.W. A scattering electro-optical switch based on dendrimers dispersed in liquid crystals. Adv. Mater. 2000, 12, 715–719. [Google Scholar] [CrossRef]
- Ghosh, A. Diffuse transmission dominant smart and advanced windows for less energy-hungry building: A review. J. Build. Eng. 2023, 64, 105604. [Google Scholar] [CrossRef]
- Mesloub, A.; Ghosh, A.; Kolsi, L.; Alshenaifi, M. Polymer-dispersed liquid crystal (PDLC) smart switchable windows for less-energy hungry buildings and visual comfort in hot desert climate. J. Build. Eng. 2022, 59, 105101. [Google Scholar] [CrossRef]
- Ghosh, A. Investigation of vacuum-integrated switchable polymer dispersed liquid crystal glazing for smart window application for less energy-hungry building. Energy 2023, 265, 126396. [Google Scholar] [CrossRef]
- Ma, M.F.; Zhao, Y.Z.; Gao, H.; Wang, D.; Miao, Z.C.; Cao, H.; Yang, Z.; He, W.L. Role of semiconductor nanoparticles on electro-optical performance of PDLC devices. Liq. Cryst. 2022, 49, 2058–2068. [Google Scholar] [CrossRef]
- Seo, J.; Song, M.; Jeong, J.; Nam, S.; Heo, I.; Park, S.Y.; Kang, I.K.; Lee, J.H.; Kim, H.; Kim, Y. Broadband pH-sensing organic transistors with polymeric sensing layers featuring liquid crystal microdomains encapsulated by di-block copolymer chains. ACS Appl. Mater. Interfaces 2016, 8, 23862–23867. [Google Scholar] [CrossRef] [PubMed]
- Khlifi, S.; Bigeon, J.; Amela-Cortes, M.; Dumait, N.; Loas, G.; Cordier, S.; Molard, Y. Switchable two-dimensional waveguiding abilities of luminescent hybrid nanocomposites for active solar concentrators. ACS Appl. Mater. Interfaces 2020, 12, 14400–14407. [Google Scholar] [CrossRef]
- Ailincai, D.; Marin, L. Eco-friendly PDLC composites based on chitosan and cholesteryl acetate. J. Mol. Liq. 2021, 321, 114466. [Google Scholar] [CrossRef]
- Li, Y.; Gong, C.D.; Li, C.G.; Ruan, K.P.; Liu, C.; Liu, H.; Gu, J.W. Liquid crystalline texture and hydrogen bond on the thermal conductivities of intrinsic thermal conductive polymer films. J. Mater. Sci. Technol. 2021, 82, 250–256. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, Y.H.; Xiao, Y.H.; Xie, H.L.; Lan, R.C.; Zhang, L.Y.; Yang, H. Ultrafast switchable passive radiative cooling smart windows with synergistic optical modulation. Adv. Funct. Mater. 2023, 33, 2301319. [Google Scholar] [CrossRef]
- Cheng, Z.X.; Wang, T.J.; Li, X.; Zhang, Y.H.; Yu, H.F. NIR-Vis-UV light-responsive actuator films of polymer-dispersed liquid crystal/graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 27494–27501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tong, X.R.; Gao, Y.; Qian, Z.Y.; Ren, R.R.; Bian, C.C.; Wang, J.H.; Cai, G.F. A sensing and stretchable polymer-dispersed liquid crystal device based on spiderweb-inspired silver nanowires-micromesh transparent electrode. Adv. Funct. Mater. 2023, 33, 2303270. [Google Scholar] [CrossRef]
- Shivaraja, S.J.; Gupta, R.K.; Manjuladevi, V. Faster switching polymer dispersed liquid crystal devices incorporated with functionalized SWCNTs. J. Mol. Liq. 2022, 354, 118905. [Google Scholar] [CrossRef]
- Nasir, N.; Kumar, S.; Kim, M.; Nguyen, V.H.; Suleman, M.; Park, H.M.; Lee, S.; Kang, D.; Seo, Y. Effect of the photoinitiator concentration on the electro-optical properties of thiol-acrylate-based PDLC smart windows. ACS Appl. Energy Mater. 2022, 5, 6986–6995. [Google Scholar] [CrossRef]
- Shi, Z.H.; He, Z.M.; Li, C.S.; Miao, Z.C.; Wang, D.; Luan, Y.; Li, Y.Z.; Zhao, Y.Z. The role of nanomesh fibres loaded with fluorescent materials on the electro-optical performance of PDLC devices. Liq. Cryst. 2022, 49, 2037–2050. [Google Scholar] [CrossRef]
- Kim, E.; Liu, Y.; Hong, S.J.; Han, J.I. Effect of SiO2 nanoparticle doping on electro-optical properties of polymer dispersed liquid crystal lens for smart electronic glasses. Nano Converg. 2015, 2, 5. [Google Scholar] [CrossRef]
- Katariya-Jain, A.; Mhatre, M.M.; Dierking, I.; Deshmukh, R.R. Enhanced thermo-electro-optical and dielectric properties of carbon nanoparticle-doped polymer dispersed liquid crystal based switchable windows. J. Mol. Liq. 2024, 393, 123575. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Song, Y.Z.; Zhang, B.; Wang, L.; He, X.M. Metallized plastic foils: A Promising solution for high-energy lithium-ion battery current collectors. Adv. Energy Mater. 2023, 13, 2370146. [Google Scholar] [CrossRef]
- Peng, S.J.; Du, X.F.; Liang, Z.S.; Ma, M.B.; Guo, Y.; Xiong, L.L. Multilayer polymer nanocomposites based on interface engineering for high-performance capacitors. J. Energy Storage 2023, 60, 106636. [Google Scholar] [CrossRef]
- Khoi, T.M.; Tran, N.A.T.; Jung, H.B.; Huynh, V.P.; Kim, Y.; Hong, J.; Yoo, C.Y.; Kang, H.S.; Cho, Y. Selective and continuous ion recovery using flow electrode capacitive deionization with polymer multilayers functionalized ion exchange membrane. Desalination 2023, 558, 116626. [Google Scholar] [CrossRef]
- Culebras, M.; Byun, Y.Y.; Jang, J.; Serafin, A.; Collins, M.N.; Park, Y.T.; Cho, C. Nanostructured PEDOT-based multilayer thin films with high thermoelectric performances. Appl. Surf. Sci. 2023, 615, 156432. [Google Scholar] [CrossRef]
- Park, H.; Mong, A.L.; Kim, D. Single and multilayer composite electrolytes for enhanced Li-ion conductivity with restricted polysulfide diffusion for lithium-sulfur battery. Mater. Today Energy 2023, 33, 101274. [Google Scholar] [CrossRef]
- Eslami, H.; Mekonnen, T.H. Flexible and green multilayer paper coating for barrier enhancement of paper packaging. Sustain. Mater. Technol. 2023, 37, e00694. [Google Scholar] [CrossRef]
- Ogura, H.; Kawasaki, S.; Liu, Z.; Endo, T.; Maruyama, M.; Gao, Y.L.; Nakanishi, Y.; Lim, H.E.; Yanagi, K.; Irisawa, T.; et al. Multilayer in-plane heterostructures based on transition metal dichalcogenides for advanced electronics. ACS Nano 2023, 17, 6545–6554. [Google Scholar] [CrossRef]










| Sample | Weight Percentage (wt%) | ||
|---|---|---|---|
| SLC1717 | UV6301 | BDDMA | |
| Group A | |||
| a1 | 50 | 50 | 0 |
| a2 | 50 | 49 | 1 |
| a3 | 50 | 48 | 2 |
| a4 | 50 | 47 | 3 |
| a5 | 50 | 46 | 4 |
| a6 | 50 | 45 | 5 |
| Sample | Weight Percentage (wt%) | ||||
|---|---|---|---|---|---|
| SLC1717 | UV6301 | BDDMA | Gd2O3 Nanoparticles | Rhodamine B Base | |
| Group B | |||||
| b1 | 50 | 49 | 1 | 0.0 | 0.0 |
| b2 | 50 | 49 | 1 | 0.2 | 0.0 |
| b3 | 50 | 49 | 1 | 0.4 | 0.0 |
| b4 | 50 | 49 | 1 | 0.6 | 0.0 |
| b4 | 50 | 49 | 1 | 0.8 | 0.0 |
| b6 | 50 | 49 | 1 | 1.0 | 0.0 |
| Group C | |||||
| c1 | 50 | 49 | 1 | 0.2 | 0.0 |
| c2 | 50 | 49 | 1 | 0.2 | 0.2 |
| c3 | 50 | 49 | 1 | 0.2 | 0.4 |
| c4 | 50 | 49 | 1 | 0.2 | 0.6 |
| c5 | 50 | 49 | 1 | 0.2 | 0.8 |
| c6 | 50 | 49 | 1 | 0.2 | 1.0 |
| Sample | Weight Percentage (wt%) | ||||
|---|---|---|---|---|---|
| SLC1717 | UV6301 | BDDMA | Gd2O3 Nanoparticles | Rhodamine B Base | |
| Group B | |||||
| b1 | 50 | 47 | 3 | 0.0 | 0.0 |
| b2 | 50 | 47 | 3 | 0.2 | 0.0 |
| b3 | 50 | 47 | 3 | 0.4 | 0.0 |
| b4 | 50 | 47 | 3 | 0.6 | 0.0 |
| b4 | 50 | 47 | 3 | 0.8 | 0.0 |
| b6 | 50 | 47 | 3 | 1.0 | 0.0 |
| Group C | |||||
| c1 | 50 | 47 | 3 | 0.2 | 0.0 |
| c2 | 50 | 47 | 3 | 0.2 | 0.2 |
| c3 | 50 | 47 | 3 | 0.2 | 0.4 |
| c4 | 50 | 47 | 3 | 0.2 | 0.6 |
| c5 | 50 | 47 | 3 | 0.2 | 0.8 |
| c6 | 50 | 47 | 3 | 0.2 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhao, Y.; Li, X.; Gao, H.; Guo, Z.; Wang, D.; Luan, Y.; Wang, L. Preparation and Characterization of Bilayer Polymer-Dispersed Liquid Crystals Doped with Gd2O3 Nanoparticles and Rhodamine B Base Fluorescent Dye. Molecules 2024, 29, 1126. https://doi.org/10.3390/molecules29051126
Wu Y, Zhao Y, Li X, Gao H, Guo Z, Wang D, Luan Y, Wang L. Preparation and Characterization of Bilayer Polymer-Dispersed Liquid Crystals Doped with Gd2O3 Nanoparticles and Rhodamine B Base Fluorescent Dye. Molecules. 2024; 29(5):1126. https://doi.org/10.3390/molecules29051126
Chicago/Turabian StyleWu, Yongle, Yuzhen Zhao, Xun Li, Hong Gao, Zhun Guo, Dong Wang, Yi Luan, and Lei Wang. 2024. "Preparation and Characterization of Bilayer Polymer-Dispersed Liquid Crystals Doped with Gd2O3 Nanoparticles and Rhodamine B Base Fluorescent Dye" Molecules 29, no. 5: 1126. https://doi.org/10.3390/molecules29051126
APA StyleWu, Y., Zhao, Y., Li, X., Gao, H., Guo, Z., Wang, D., Luan, Y., & Wang, L. (2024). Preparation and Characterization of Bilayer Polymer-Dispersed Liquid Crystals Doped with Gd2O3 Nanoparticles and Rhodamine B Base Fluorescent Dye. Molecules, 29(5), 1126. https://doi.org/10.3390/molecules29051126








