Comparing Nutritional Values and Bioactivity of Kefir from Different Types of Animal Milk
Abstract
1. Introduction
2. Results
2.1. Microbial Counts
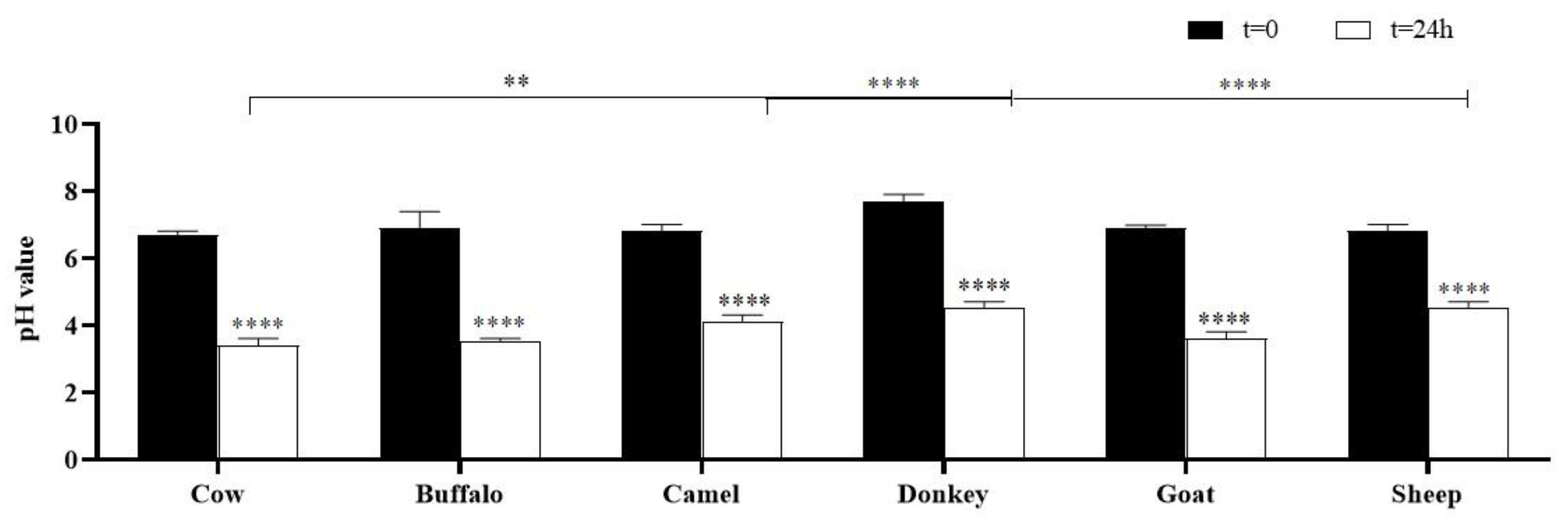
2.2. pH
2.3. Protein and Sugar Content
2.4. Fatty Acid Profile
2.5. Total Phenolic Content (TPC)
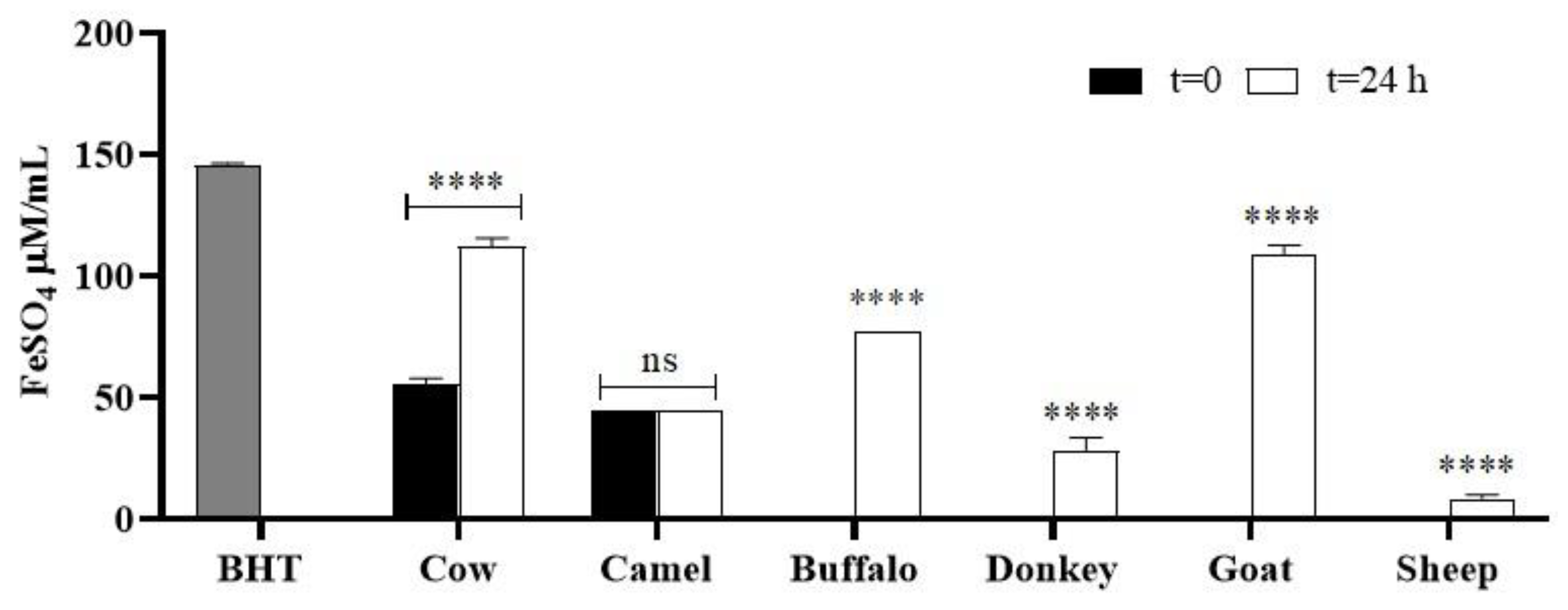
2.6. Antioxidants Activity: ABTS and Frap Assays
2.6.1. ABTS Assay
2.6.2. FRAP Assay
2.7. Rheology
2.8. Pearson Correlation
3. Discussion
4. Materials and Methods
4.1. Milks and Reagents
4.2. Kefir Grain Activation
4.3. Fermentation Process
4.4. Microbiological Analysis
- i.
- Lactobacilli were plated on MRS (Man Rogosa Sharpe) agar and incubated at 37 °C under anaerobic conditions for 5 days;
- ii.
- Mesophilic cocci were plated on M17 agar and incubated aerobically at 37 °C for 5 days;
- iii.
- Acetic acid bacteria (AAB) were plated on GYP medium containing 1% D-glucose, 0.8% yeast extract, 1.5% pepton, and 1.5% agar, and were incubated aerobically at 30 °C for 2 days;
- iv.
- Yeasts and molds were inoculated on PDA medium (potato dextrose agar) and incubated aerobically at 30 °C for 3 days;
- v.
- Total bacteria were plated on PCA medium (plate count agar) and incubated aerobically at 30 °C for 3 days;
4.5. pH
4.6. Protein Content Determination According to Kjeldahl Method
4.7. Sugar Content Determination by Phenol–Sulfuric Acid Assay
4.8. Fatty Acid Profile
4.9. Total Phenolic Content (TPC)
4.10. Antioxidant Activity
4.10.1. ABTS Assay
4.10.2. FRAP Assay
4.11. Rheology
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lajnaf, R.; Feki, S.; Ben Ameur, S.; Attia, H.; Kammoun, T.; Ayadi, M.; Masmoudi, H. Cow’s milk alternatives for children with cow’s milk protein allergy—Review of health benefits and risks of allergic reaction. Int. Dairy J. 2023, 141, 105624. [Google Scholar] [CrossRef]
- Aroua, M.; Koubaier, H.; Bouacida, S.; Ben Saïd, S.; Mahouachi, M.; Salimei, E. Chemical, Physicochemical, Microbiological, Bioactive, and Sensory Characteristics of Cow and Donkey Milk Kefir during Storage. Beverages 2023, 9, 2. [Google Scholar] [CrossRef]
- Medhammar, E.; Wijesinha-Bettoni, R.; Stadlmayr, B.; Nilsson, E.; Charrondiere, U.R.; Burlingame, B. Composition of milk from minor dairy animals and buffalo breeds: A biodiversity perspective. J. Sci. Food Agric. 2012, 92, 445–474. [Google Scholar] [CrossRef] [PubMed]
- Abid, J.; Ahmad, S.; Wasila, H.; Asif, T.; Farooq, M.; Jan, A. Minerals Composition, Phenolic Compounds and Antioxidant Activity of Fresh and Processed Milk Consumed in Khyber Pakhtunkhwa, Pakistan. J. Innov. Sci. 2022, 8, 167–174. [Google Scholar] [CrossRef]
- Murgia, A.; Scano, P.; Contu, M.; Ibba, I.; Altea, M.; Bussu, M.; Demuru, M.; Porcu, A.; Caboni, P. Characterization of donkey milk and metabolite profile comparison with human milk and formula milk. LWT Food Sci. Technol. 2016, 74, 427–433. [Google Scholar] [CrossRef]
- Živkov Baloš, M.; Ljubojević Pelić, D.; Jakšić, S.; Lazić, S. Donkey Milk: An Overview of its Chemical Composition and Main Nutritional Properties or Human Health Benefit Properties. J. Equine Vet. Sci. 2023, 121, 104225. [Google Scholar] [CrossRef] [PubMed]
- Gün, I. Comparison of composition, sensory properties and aroma compounds of kefir produced from donkey milk and cow milk. Agric. Food Sci. 2022, 72, 213–225. [Google Scholar] [CrossRef]
- Han, R.; Shi, R.; Yu, Z.; Ho, H.; Du, Q.; Sun, X.; Wang, J.; Jiang, H.; Fan, R.; Yang, Y. Distribution and variation in proteins of casein micellar fractions response to heat-treatment from five dairy species. Food Chem. 2021, 365, 130640. [Google Scholar] [CrossRef] [PubMed]
- Gallier, S.; Tolenaars, L.; Prosser, C. Whole Goat Milk as a Source of Fat and Milk Fat Globule Membrane in Infant Formula. Nutrients 2020, 12, 3486. [Google Scholar] [CrossRef]
- Mohapatra, A.; Shinde, A.K.; Singh, R. Sheep milk: A pertinent functional food. Small Rumin. Res. 2019, 181, 6–11. [Google Scholar] [CrossRef]
- Davati, N.; Tabatabaee Yazdi, F.; Zibaee, S.; Shahidi, F.; Edalatian, M.R. Study of Lactic Acid Bacteria Community from Raw Milk of Iranian One Humped Camel and Evaluation of Their Probiotic Properties. Jundishapur J. Microbiol. 2015, 8, e16750. [Google Scholar] [CrossRef]
- Meena, S.; Rajput, Y.S.; Sharma, R. Comparative fat digestibility of goat, camel, cow and buffalo milk. Int. Dairy J. 2014, 35, 153–156. [Google Scholar] [CrossRef]
- Larosa, C.; Fasura Balthazar, C.; Guimarães, J.; Pereira Margalho, L.; Lemos, F.; de Oliveira, F.; Abud, Y.; Sant’Anna, C.; Kasnowski Holanda Duarte, M.; Granato, D.; et al. Can sucrose-substitutes increase the antagonistic activity against foodborne pathogens, and improve the technological and functional properties of sheep milk kefir? Food Chem. 2021, 351, 129290. [Google Scholar] [CrossRef]
- de Lima, M.A.-O.; da Silva, R.A.; da Silva, M.F.; da Silva, P.A.B.; Costa, R.; Teixeira, J.A.C.; Porto, A.L.F.; Cavalcanti, M.T.H. Brazilian Kefir-Fermented Sheep’s Milk, a Source of Antimicrobial and Antioxidant Peptides. Probiot. Antimicrob. Prot. 2018, 10, 446–455. [Google Scholar] [CrossRef]
- Tomar, O.; Akarca, G.; Çağlar, A.; Beykaya, M.; Gok, V. The effects of kefir grain and starter culture on kefir produced from cow and buffalo milk during storage periods. Food Sci. Technol. 2019, 40, 238–244. [Google Scholar] [CrossRef]
- Baniasadi, M.; Azizkhani, M.; Saris, P.; Tooryan, F. Comparative antioxidant potential of kefir and yogurt of bovine and non-bovine origins. J. Food Sci. Technol. 2021, 59, 1307–1316. [Google Scholar] [CrossRef]
- Zhang, T.; Chang, M.; Zhou, Y.; Wang, M.; Yan, M.; Hou, X.; Liu, R.; Yuan, Y.; Yue, T. Dynamic alterations of flavor, functional nutrients, and microbial community during fermentation of different animal milk kefirs. Food Res. Int. 2024, 186, 114305. [Google Scholar] [CrossRef]
- Kesenkaş, H.; Dinkci, N.; SeÇKİN, A.K.; Kinik, Ö.; Gönç, S. Antioxidant Properties of Kefir Produced from Different Cow and Soy Milk Mixtures. Tarim. Bilimleri. Dergisi. 2011, 17, 253–259. [Google Scholar]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Alraddadi, F.A.J.; Ross, T.; Powell, S.M. Evaluation of the microbial communities in kefir grains and kefir over time. Int. Dairy J. 2023, 136, 105490. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Y.; Jia, H.; Wang, Z.; Gao, Z.; Luo, Y.; Sheng, Q.; Yuan, Y.; Yue, T. Metagenomic analysis of microflora structure and functional capacity in probiotic Tibetan kefir grains. Food Res. Int. 2021, 151, 110849. [Google Scholar] [CrossRef]
- Silva, K.R.; Rodrigues, S.A.; Filho, L.X.; Lima, Á.S. Antimicrobial activity of broth fermented with kefir grains. Appl. Biochem. Biotechnol. 2009, 152, 316–325. [Google Scholar] [CrossRef]
- Azizi, N.; Rajah Kumar, M.; Yeap, S.K.; Ong Abdullah, J.; Khalid, M.; Omar, A.; Osman, M.; Syed, S.; Alitheen, N. Kefir and Its Biological Activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Apalowo, O.E.; Adegoye, G.A.; Mbogori, T.; Kandiah, J.; Obuotor, T.M. Nutritional Characteristics, Health Impact, and Applications of Kefir. Foods 2024, 13, 1026. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- García-Burgos, M.; Moreno-Fernandez, J.; Alférez, M.; Díaz-Castro, J.; López-Aliaga, I. New perspectives in fermented dairy products and their health relevance. J. Funct. Foods 2020, 72, 104059. [Google Scholar] [CrossRef]
- Maftei, N.A.-O.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.A.-O.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef]
- Dong, J.; Liu, B.; Jiang, T.; Liu, Y.; Chen, L. The biofilm hypothesis: The formation mechanism of Tibetan kefir grains. Int. J. Dairy Technol. 2018, 71, 44–50. [Google Scholar] [CrossRef]
- D’Aimmo, M.R.; Satti, M.; Scarafile, D.; Modesto, M.; Pascarelli, S.; Biagini, S.A.; Luiselli, D.; Mattarelli, P.; Andlid, T. Folate-producing bifidobacteria: Metabolism, genetics, and relevance. Microbiome Res. Rep. 2023, 3, 11. [Google Scholar] [CrossRef]
- Ahmed, Z.; Wang, Y.; Ahmad, A.; Khan, S.; Nisa, M.; Ahmad, H.; Afreen, A. Kefir and Health: A Contemporary Perspective. Crit. Rev. Food Sci. Nutr. 2013, 53, 422–434. [Google Scholar] [CrossRef]
- M’Hir, S.; Ayed, L.A.-O.; De Pasquale, I.A.-O.; Fanizza, E.A.-O.; Tlais, A.Z.A.; Comparelli, R.A.-O.; Verni, M.A.-O.; Latronico, R.; Gobbetti, M.; Di Cagno, R.; et al. Comparison of Milk Kefirs Obtained from Cow’s, Ewe’s and Goat’s Milk: Antioxidant Role of Microbial-Derived Exopolysaccharides. Antioxidants 2024, 13, 335. [Google Scholar] [CrossRef]
- Gul, O.; Atalar, I.; Mortas, M.; Dervisoglu, M. Rheological, textural, colour and sensorial properties of kefir produced with buffalo milk using kefir grains and starter culture: A comparison with cows’ milk kefir. Int. J. Dairy Technol. 2018, 71, 73–80. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Baubekova, A.; Akhmetsadykova, S.; Faye, B. Traditional dairy fermented products in Central Asia. Int. Dairy J. 2022, 137, 105514. [Google Scholar] [CrossRef]
- Azizkhani, M.; Saris, P.; Baniasadi, M. An in-vitro assessment of antifungal and antibacterial activity of cow, camel, ewe, and goat milk kefir and probiotic yogurt. J. Food Meas. Charact. 2021, 15, 406–415. [Google Scholar] [CrossRef]
- Verruck, S.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Esmerino, E.A.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; da Cruz, A.G.; Prudencio, E.S. Dairy foods and positive impact on the consumer’s health. Adv. Food Nutr. Res. 2019, 89, 95–164. [Google Scholar]
- Baliyan, N.; Maurya, A.; Kumar, A.; Agnihotri, V.; Kumar, R. Probiotics from the bovine raw milk of Lahaul valley showed cis-9, trans-11 conjugated linoleic acid isomer and antioxidant activity with food formulation ability. LWT 2023, 176, 114553. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; Stanton, C.; Yang, B.; Ross, R.P.; Silva, C.C.G. Conjugated linoleic acid production and probiotic assessment of Lactobacillus plantarum isolated from Pico cheese. LWT 2018, 90, 403–411. [Google Scholar] [CrossRef]
- Wu, C.; Chen, H.; Mei, Y.; Yang, B.; Zhao, J.; Stanton, C.; Chen, W. Advances in research on microbial conjugated linoleic acid bioconversion. Prog. Lipid Res. 2024, 93, 101257. [Google Scholar] [CrossRef]
- Delgado-Fernández, P.; Corzo, N.; Lizasoain, S.; Olano, A.; Moreno, F.J. Fermentative properties of starter culture during manufacture of kefir with new prebiotics derived from lactulose. Int. Dairy J. 2019, 93, 22–29. [Google Scholar] [CrossRef]
- Cais-Sokolińska, D.; Pikul, J.; Wójtowski, J.; Danków, R.; Teichert, J.; Czyżak-Runowska, G.; Bagnicka, E. Evaluation of quality of kefir from milk obtained from goats supplemented with a diet rich in bioactive compounds. J. Sci. Food Agric. 2015, 95, 1343–1349. [Google Scholar] [CrossRef]
- Lopitz-Otsoa, F.; Rementeria, A.; Elguezabal, N.; Garaizar, J. Kefir: A symbiotic yeasts-bacteria community with alleged healthy capabilities. Rev. Iberoam. Micol. 2006, 23, 67–74. [Google Scholar] [CrossRef]
- Fan, D.; Stoyanova, L.; Netrusov, A. Microbiome and Metabiotic Properties of Kefir Grains and Kefirs Based on Them. Microbiology 2022, 91, 339–355. [Google Scholar]
- Tao, Z.; Yuan, H.; Liu, M.; Liu, Q.; Zhang, S.; Liu, H.; Jiang, Y.; Huang, D.; Wang, T. Yeast Extract: Characteristics, Production, Applications and Future Perspectives. J. Microbiol. Biotechnol. 2023, 33, 151–166. [Google Scholar] [CrossRef]
- Ryu, S.; Shin, M.; Yun, B.; Lee, W.; Choi, H.; Kang, M.; Oh, S.; Kim, Y. Bacterial Quality, Prevalence of Pathogens, and Molecular Characterization of Biofilm-Producing Staphylococcus aureus from Korean Dairy Farm Environments. Animals 2021, 11, 1306. [Google Scholar] [CrossRef]
- Magalhães-Guedes, K.; Pereira, G.; Campos, C.; Dragone, G.; Schwan, R. Brazilian kefir: Structure, microbial communities and chemical composition. Braz. J. Microbiol. 2011, 42, 693–702. [Google Scholar] [CrossRef]
- Kotova, I.; Cherdyntseva, T.; Netrusov, A. Russian Kefir Grains Microbial Composition and Its Changes during Production Process. Adv. Exp. Med. Biol. 2016, 932, 93–121. [Google Scholar]
- Wszolek, M.; Teahan, B.; Guldager, H.; Tamime, A.Y. Production of Kefir, Koumiss and other Related Products. In Fermented Milks; Tamime, A., Ed.; Wiley: Hoboken, NJ, USA, 2007; pp. 174–216. [Google Scholar]
- Wróblewska, B.; Kuliga, A.; Wnorowska, K. Bioactive Dairy-Fermented Products and Phenolic Compounds: Together or Apart. Molecules 2023, 28, 8081. [Google Scholar] [CrossRef] [PubMed]
- Iminov, A.; Tursinalievich, K.; Kuchkorovich, M.; Atabaev, M. Organic contents of residues and nutrients in the short-rowcrop rotation systems in typical agricultural lands of Uzbekistan. In Earth and Environmental Science; IOP Publishing: Atlanta, GA, USA, 2022; p. 1068. [Google Scholar]
- Sulistyaningtyas, A.; Lunggani, A.; Kusdiyantini, E. Kefir Produced from Red Rice Milk by Lactobacillus bulgaricus and Candida kefir Starter. IOP Conf. Ser. Earth Environ. Sci. 2019, 292, 012038. [Google Scholar] [CrossRef]
- Tyutkov, N.; Zhernyakova, A.; Birchenko, A.; Eminova, E.; Nadtochii, L.; Baranenko, D. Probiotics viability in frozen food products. Food Biosci. 2022, 50, 101996. [Google Scholar] [CrossRef]
- Grønnevik, H.; Falstad, M.; Narvhus, J. Microbiological and chemical properties of Norwegian kefir during storage. Int. Dairy J. 2011, 21, 601–606. [Google Scholar] [CrossRef]
- Das, M.; Santra, S.; Rajlakshmi; Saravanabhupathy, S.; Dey, S.; Banerjee, S.; Banerjee, R. Lactic Acid Production from Fungal Machineries and Mechanism of PLA Synthesis: Application of AI-Based Technology for Improved Productivity. In Fungi and Fungal Products in Human Welfare and Biotechnology; Satyanarayana, T., Deshmukh, S.K., Eds.; Springer Nature: Singapore, 2023; pp. 211–256. [Google Scholar]
- Ismaiel, A.; Ghaly, M.; El-Naggar, A. Some physicochemical analyses of kefir produced under different fermentation conditions. J. Sci. Ind. Res. 2013, 70, 365–372. [Google Scholar]
- Koohsari, H.; Ajam, F. The Effect of Some Fermentation Conditions on the Production of Kefiran by Kefir Grains in Fermented Milk. J. Res. Inn. 2021, 9, 399–410. [Google Scholar]
- Lee, S.J.; Jeon, H.S.; Yoo, J.Y.; Kim, J.H. Some Important Metabolites Produced by Lactic Acid Bacteria Originated from Kimchi. Foods 2021, 10, 2148. [Google Scholar] [CrossRef] [PubMed]
- Abdul Hakim, B.N.; Xuan, N.J.; Oslan, S.A.-O. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef] [PubMed]
- González-Orozco, B.D.; García-Cano, I.; Jiménez-Flores, R.; Alvárez, V.B. Invited review: Milk kefir microbiota-Direct and indirect antimicrobial effects. J. Dairy Sci. 2022, 105, 3703–3715. [Google Scholar] [CrossRef] [PubMed]
- Ayivi, R.D.; Ibrahim, S.A. Lactic acid bacteria: An essential probiotic and starter culture for the production of yoghurt. Int. J. Food Sci. Technol. 2022, 57, 7008–7025. [Google Scholar] [CrossRef]
- Li, A.; Zheng, J.; Han, X.; Yang, S.; Cheng, S.; Zhao, J.; Zhou, W.; Lu, Y. Advances in Low-Lactose/Lactose-Free Dairy Products and Their Production. Foods 2023, 12, 2553. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, M.; Hu, S.; Zhao, H.; Zhang, B. The Enzyme Gene Expression of Protein Utilization and Metabolism by Lactobacillus helveticus CICC 22171. Microorganisms 2022, 10, 1724. [Google Scholar] [CrossRef]
- Dallas, D.C.; Citerne, F.; Tian, T.; Silva, V.L.; Kalanetra, K.M.; Frese, S.A.; Robinson, R.C.; Mills, D.A.; Barile, D. Peptidomic analysis reveals proteolytic activity of kefir microorganisms on bovine milk proteins. Food Chem. 2016, 197, 273–284. [Google Scholar] [CrossRef]
- Bonczar, G.; Walczycka, M.; Duda, I. The changes of proteins fractions shares in milk and fermented milk drinks. Acta Sci. Polon. Technol. 2016, 15, 379–389. [Google Scholar] [CrossRef]
- Oskaybas Emlek, B.; ÖZbey, A. Effect of linoleic acid addition on the cis9-trans11-conjugated linoleic acid content of kefir. Gida J. Food 2021, 46, 895–902. [Google Scholar] [CrossRef]
- Yadav, H.; Jain, S.; Sinha, P. Production of free fatty acids and conjugated linoleic acid in probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei during fermentation and storage. Int. Dairy J. 2007, 17, 1006–1010. [Google Scholar] [CrossRef]
- Sumarmono, J.; Setyawardani, T.; Tianling, M.; Aini, N.; Wibowo, C.; Mohamed, T.; Sangsopha, J.; Jelan, Z. Comparative analysis of physical properties and fatty acid composition of set-yogurt manufactured from different milk types. Canrea J. Food Technol. Nutr. Culin. J. 2023, 6, 167–181. [Google Scholar] [CrossRef]
- Gedik, O.; Karahan, A. Physicochemical properties and survival assessment of potential probiotics in a novel dairy drink during storage. Food Sci. Nutr. 2023, 11, 7803–7815. [Google Scholar] [CrossRef]
- Vieira, C.P.; Álvares, T.S.; Gomes, L.S.; Torres, A.G.; Paschoalin, V.M.; Conte-Junior, C.A. Kefir Grains Change Fatty Acid Profile of Milk during Fermentation and Storage. PLoS ONE 2015, 10, e0139910. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, Y.; Li, K.; Zhang, W.; Liu, L. The relationship between dietary intake of ω-3 and ω-6 fatty acids and frailty risk in middle-aged and elderly individuals: A cross-sectional study from NHANES. Front. Nutr. 2024, 11, 1377910. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.P.; Cabral, C.C.; da Costa Lima, B.R.C.; Paschoalin, V.M.F.; Leandro, K.C.; Conte-Junior, C.A. Lactococcus lactis ssp. cremoris MRS47, a potential probiotic strain isolated from kefir grains, increases cis-9, trans-11-CLA and PUFA contents in fermented milk. J. Funct. Foods 2017, 31, 172–178. [Google Scholar] [CrossRef]
- Nasrollahzadeh, A.; Mollaei Tavani, S.; Arjeh, E.; Jafari, S.M. Production of conjugated linoleic acid by lactic acid bacteria; important factors and optimum conditions. Food Chem. X 2023, 20, 100942. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, T.; Şahin, S.; Akpinar Bayizit, A.; Yilmaz-Ersan, L. Assessment of antioxidant capacity by method comparison and amino acid characterisation in buffalo milk kefir. Int. J. Dairy Technol. 2018, 72, 65–73. [Google Scholar] [CrossRef]
- Torres-Guardado, R.; Esteve-Zarzoso, B.; Reguant, C.; Bordons, A. Microbial interactions in alcoholic beverages. Int. Microbiol. 2022, 25, 3. [Google Scholar] [CrossRef]
- Curiel, J.A.; Rodríguez, H.; Iranzo, J.M.; Rivas, B.; Muñoz, R. Ability of Lactobacillus brevis to degrade food phenolic acids. Food Chem. 2010, 120, 225–229. [Google Scholar] [CrossRef]
- Mousavi, Z.; Mousavi, M.; Razavi, S.; Emam-Djomeh, Z.; Kiani, H. Fermentation of pomegranate juice by probiotic lactic acid bacteria. World J. Microbiol. Biotechnol. 2011, 27, 123–128. [Google Scholar] [CrossRef]
- Ilyes, D.; Carlos, A.C.-J. Cheese’s Bioactive Peptide Content and Fatty Acids Profile. In Recent Trends on Cheese as Functional Food with Great Nutritive and Health Benefits; Adham, M.A., Ed.; IntechOpen: Rijeka, Croatia, 2023; pp. 1–21. [Google Scholar]
- Yirmibesoglu, S.; Tefon Öztürk, B. Comparing microbiological profiles, bioactivities, and physicochemical and sensory properties of donkey milk kefir and cow milk kefir. Turk. J. Vet. Anim. Sci. 2020, 44, 774–781. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Martini, S.; Solieri, L. Bioprospecting for Bioactive Peptide Production by Lactic Acid Bacteria Isolated from Fermented Dairy Food. Fermentation 2019, 5, 96. [Google Scholar] [CrossRef]
- Wang, Y.C.; Yu, R.C.; Chou, C.C. Antioxidative activities of soymilk fermented with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006, 23, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.A.-O.; Kokkiligadda, A.; Dasriya, V.; Naithani, H.A.-O. Functional relevance and health benefits of soymilk fermented by lactic acid bacteria. J. Appl. Microbiol. 2022, 133, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Irkin, R.; BerkkaÇAn, E. Improving Some Properties of Cow and Goat Mixed Milk Based Kefir with Inulin Addition as a Functional Food. Kahramanmaraş Sütçü İmam Üniversitesi Tarım Ve Doğa Derg. 2021, 25, 556–564. [Google Scholar] [CrossRef]
- Fazio, A.; La Torre, C.; Caroleo, M.C.; Caputo, P.; Cannataro, R.; Plastina, P.; Cione, E. Effect of Addition of Pectins from Jujubes (Ziziphus jujube Mill.) on Vitamin C Production during Heterolactic Fermentation. Molecules 2020, 25, 2706. [Google Scholar] [CrossRef] [PubMed]
- Kolakowski, P.; Ozimkiewicz, M. Restoration of kefir grains subjected to different treatments. Int. J. Dairy Technol. 2012, 65, 140–145. [Google Scholar] [CrossRef]
- Kazou, M.; Grafakou, A.; Tsakalidou, E.; Georgalaki, M. Zooming into the Microbiota of Home-Made and Industrial Kefir Produced in Greece Using Classical Microbiological and Amplicon-Based Metagenomics Analyses. Front. Microbiol. 2021, 12, 621069. [Google Scholar] [CrossRef]
- La Torre, C.; Fazio, A.; Caputo, P.; Tursi, A.; Formoso, P.; Cione, E. Influence of Three Extraction Methods on the Physicochemical Properties of Kefirans Isolated from Three Types of Animal Milk. Foods 2022, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Zirpoli, H.; Caputo, M.; Carraturo, A.; Torino, G.; Fazio, A.; Attya, M.; Rastrelli, L.; Tecce, M.F. Selective action of human sera differing in fatty acids and cholesterol content on in vitro gene expression. J. Cell Biochem. 2012, 113, 815–823. [Google Scholar] [CrossRef] [PubMed]
- La Torre, C.; Fazio, A.; Caputo, P.; Plastina, P.; Caroleo, M.C.; Cannataro, R.; Cione, E. Effects of Long-Term Storage on Radical Scavenging Properties and Phenolic Content of Kombucha from Black Tea. Molecules 2021, 26, 5474. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Barbarossa, A.; Carocci, A.; Fazio, A.; La Torre, C.; Caruso, A.; Ponassi, M.; Rosano, C.; et al. Synthesis, anticancer and antioxidant properties of new indole and pyranoindole derivatives. Bioorg. Chem. 2020, 105, 104440. [Google Scholar] [CrossRef] [PubMed]
- Malacaria, L.; La Torre, C.; Furia, E.; Fazio, A.; Caroleo, M.C.; Cione, E.; Gallelli, L.; Marino, T.; Plastina, P. Aluminum(III), iron(III) and copper(II) complexes of luteolin: Stability, antioxidant, and anti-inflammatory properties. J. Mol. Liq. 2022, 345, 117895. [Google Scholar] [CrossRef]
- Gutiérrez-Álzate, K.; Rosario, I.L.S.; de Jesus, R.L.C.; Maciel, L.F.; Santos, S.A.; de Souza, C.O.; Vieira, C.P.; Cavalheiro, C.P.; Costa, M.P.d. Physicochemical, Rheological, and Nutritional Quality of Artisanal Fermented Milk Beverages with Cupuassu (Theobroma grandiflorum) Pulp and Flour. Foods 2023, 12, 2217. [Google Scholar] [CrossRef]

| Samples | Total Bacteria | LAB | Lactococcus | AAB | Total Yeast |
|---|---|---|---|---|---|
| Cow | 8.60 ± 0.03 a,A | 4.48 ± 0.05 b,E | 4.61 ± 0.03 b,D | 5.00 ± 0.04 b,C | 5.49 ± 0.02 a,B |
| Camel | 4.54 ± 0.01 b,B | 2.90 ± 0.04 d,D | 4.0 ± 0.08 c,C | 5.77 ± 0.01 a,A | 0 c,E |
| Buffalo | 5.33 ± 0.01 b,A | 3.90 ± 0.01 c,C | 4.0 ± 0.05 c,B | 2.95 ± 0.05 c,D | 0 c,E |
| Donkey | 4.86 ± 0.05 b,C | 0 e,E | 6.28 ± 0.01 a,A | 5.62 ± 0.02 a,B | 2.84 ± 0.02 b,D |
| Goat | 4.82 ± 0.06 b,B | 4.90 ± 0.13 a,B | 6.50 ± 0.02 a,A | 3.84 ± 0.02 c,C | 2.70 ± 0.02 b,D |
| Sheep | 4.72 ± 0.02 b,B | 3.39 ± 0.03 c,C | 3.47 ± 0.02 c,C | 5.71 ± 0.02 a,A | 0 c,D |
| Samples | Total Bacteria | LAB | Lactococcus | AAB | Total Yeast |
|---|---|---|---|---|---|
| Cow | 6.09 ± 0.01 a,A | 4.78 ± 0.05 b,C | 3.30 ± 0.03 D | 5.82 ± 0.04 b,A,B | 5.76 ± 0.02 b,B |
| Camel | 5.88 ± 0.01 b,A | 2.38 ± 0.04 d,B | 5.02 ± 0.08 b,A | 5.50 ± 0.01 b,A | 5.93 ± 0.02 b,A |
| Buffalo | 6.11 ± 0.01 a,A | 2.55 ± 0.01 d,C | 0 d,D | 5.74 ± 0.05 b,A | 4.00 ± 0.03 d,B |
| Donkey | 4.74 ± 0.02 c,B | 3.74 ± 0.01 c,C | 4.28 ± 0.01 c,B | 6.35 ± 0.02 a,A | 6.41 ± 0.07 a,A |
| Goat | 4.94 ± 0.03 c,B | 3.90 ± 0.13 c,C | 4.50 ± 0.02 c,B | 6.14 ± 0.02 a,A | 4.30 ± 0.02 c,B |
| Sheep | 5.63 ± 0.01 b,B | 5.41 ± 0.03 a,B | 6.14 ± 0.02 a,A | 6.70 ± 0.02 a | 4.50 ± 0.03 c,C |
| Samples | Protein (g/100 mL) | Sugar (g/100 mL) | ||
|---|---|---|---|---|
| t = 0 | t = 24 h | t = 0 | t = 24 h | |
| Cow | 3.2 ± 0.3 b,B | 5.2 ± 0.5 a,A | 4.8 ± 0.1 a | 0.055 ± 0.007 b |
| Buffalo | 4.5 ± 0.1 a,A | 4.8 ± 0.1 a,A | 5.1 ± 0.1 a | 0.021 ± 0.002 b |
| Camel | 2.8 ± 0.8 a,B | 2.4 ± 0.3 a,C | 4.3 ± 0.1 a | 0.030 ± 0.001 b |
| Donkey | 1.7 ± 0.1 a,C | 2.4 ± 0.2 a,C | 6.0 ± 0.3 a | 0.051 ± 0.003 b |
| Goat | 3.4 ± 0.3 a,B | 3.4 ± 0.1 a,B | 4.4 ± 0.1 a | 0.041 ± 0.005 b |
| Sheep | 3.5 ± 0.4 a,B | 4.9 ± 0.1 b,A | 2.9 ± 0.1 a | 0.023 ± 0.004 b |
| Unfermented Milk | ||||||
| Fatty Acid | Cow | Camel | Buffalo | Donkey | Goat | Sheep |
| C4:0 | 0.8 ± 0.1 A | 0 A | 86.4 ± 0.5 A | 0 A | 0 A | 12.1 ± 0.1 B |
| C12:0 | 0.4 ± 0.03 B | 5.2 ± 0.1 A | 38.1 ± 1.2 A | 4.05 ± 0.1 A | 10.3 ± 0.1 B | 9.3 ± 2.2 B |
| C14:0 | 13.4 ± 2.2 B | 111.9 ± 1.0 A | 155.7 ± 3.3 A | 4.2 ± 0.7 A | 37.2 ± 3.4 B | 38.1 ± 4.6 B |
| C16:0 | 23.3 ± 3.8 B | 1.15 ± 1.1 B | 208.3 ± 1.0 A | 6.7 ± 2.2 A | 66.2 ± 4.8 B | 65.3 ± 0.6 B |
| C17:0 | 18.9 ± 0.6 A | 26.5 ± 0.3 A | 18.7 ± 1.2 B | 16.7 ± 0.5 A | 17.2 ± 0.5 A | 16.9 ± 0.1 A |
| C18:0 | 17.6 ± 1.9 A | 77.6 ± 0.1 A | 172.1 ± 5.3 A | 6.3 ± 0.9 B | 42.1 ± 0.7 B | 43.9 ± 0.2 B |
| C18:1n-9 | 25.3 ± 0.1 B | 149.0 ± 0.1 A | 231.7 ± 3.3 A | 8.1 ± 3.1 A | 77.2 ± 3.3 B | 130.7 ± 7.1 B |
| C16:1n-7 | 52.5 ± 0.1 A | 54.7 ± 0.1 A | 52.8 ± 0.2 A | 52.6 ± 0.1 A | 52.3 ± 0.1 A | 52.6 ± 0.1 A |
| C18:2n-6 | 2.8 ± 0.3 B | 15.3 ± 0.2 A | 28.4 ± 1.5 A | 2.6 ± 0.5 A | 7.2 ± 0.5 A | 21.4 ± 0.9 B |
| C20:4n-6 | 15.5 ± 0.2 A | 17.3 ± 0.1 A | 19.1 ± 2.1 A | 0 A | 15.9 ± 0.1 A | 15.8 ± 0.1 A |
| C18:3n-3 | 11.7 ± 0.1 A | 15.3 ± 0.2 A | 11.7 ± 0.1 A | 12.1 ± 0.2 A | 11.8 ± 0.1 A | 12.5 ± 1.1 A |
| C18:2c9t11 | 4.4 ± 0.1 A | 3.8 ± 0. 1 A | 2.8 ± 0.1 A | 0 A | 4.2 ± 0.1 A | 3.5 ± 0.1 A |
| C18:2c10t12 | 2.8 ± 0.1 A | 24.5 ± 0.1 A | 0 B | 0 A | 0 B | 36.7 ± 0.8 B |
| SFA | 74.6 ± 8.8 B | 398.5 ± 2.4 A | 679.2 ± 12.5 A | 37.8 ± 4.3 B | 173.2 ± 9.7 B | 185.7 ± 8.1 B |
| MUFA | 77.9 ± 4.1 B | 203.7 ± 0.1 A | 284.6 ± 3.6 A | 60.6 ± 3.05 A | 129.8 ± 3.3 B | 183.3 ± 7.1 B |
| PUFA | 30.1 ± 0.2 B | 48.0 ± 0.4 A | 59.4 ± 3.7 A | 15.0 ± 0.8 A | 34.9 ± 0.6 A | 49.8 ± 2.1 B |
| CLA | 7.1 ± 0.2 A | 28.3 ± 0.2 A | 2.8 ± 0.1 B | 0 A | 4.2 ± 0.1 B | 40.3 ± 1.0 B |
| Fermented Milk | ||||||
| Fatty Acid | Cow | Camel | Buffalo | Donkey | Goat | Sheep |
| C4:0 | 9.0 ± 0.1 A | 0.6 ± 0.2 A | 81.1 ± 0.5 A | 0 A | 0 A | 50.2 ± 0.4 A |
| C12:0 | 63.8 ± 1.2 A | 7. ± 0.5 A | 33.1 ± 1.2 A | 0 A | 41.1 ± 4.2 A | 87.1 ± 5.5 A |
| C14:0 | 198.5 ± 4.0 A | 108.4 ± 6.1 B | 141.5 ± 3.3 B | 5.2 ± 0.3 A | 86.1 ± 7.4 A | 202.2 ± 0.7 A |
| C16:0 | 350.5 ± 21.8 A | 44.1 ± 1.8 A | 210.1 ± 1.0 A | 8.7 ± 0.3 A | 134.5 ± 11.1 A | 300.1 ± 7.7 A |
| C17:0 | 26.1 ± 0.8 A | 17.5 ± 0.1 A | 59.5 ± 1.2 A | 16.6 ± 0. 1 A | 16.5 ± 0.1 A | 23.7 ± 0.2 A |
| C18:0 | 7.4 ± 1.3 A | 23.6 ± 1.0 B | 140.3 ± 5.3 B | 454.4 ± 3.4 A | 64.3 ± 4.5 A | 191.3 ± 4.1 B |
| C18:1n-9 | 453.7 ± 3.8 A | 38.5 ± 2.0 B | 218.7 ± 3.3 B | 13.1 ± 2.7 A | 155.1 ± 12.1 A | 382.8 ± 9.1 A |
| C16:1n-7 | 52.5 ± 0.1 A | 54.6 ± 0.8 A | 53.2 ± 0.3 A | 52.3 ± 1.4 A | 52.7 ± 0.1 A | 52.7 ± 0.1 A |
| C18:2n-6 | 32.7 ± 3.7 A | 15.6 ± 1.6 A | 15.5 ± 1.4 B | 3.2 ± 0.1 A | 12.1 ± 0.9 A | 32.8 ± 0.9 A |
| C20:4n-6 | 17.3 ± 0.1 A | 15.6 ± 0.2 A | 17.2 ± 2.1 A | 0 A | 16.3 ± 0.1 A | 18.5 ± 0.1 A |
| C18:3n-3 | 16.7 ± 0.4 A | 15.6 ± 1.6 A | 11.7 ± 0.1 A | 12.5 ± 0.1 A | 11.5 ± 0.1 A | 23.0 ± 0.2 A |
| C18:2c9t11 | 3.5 ± 0.1 A | 4.1 ± 0.1 A | 3.3 ± 0.1 A | 0 A | 4.1 ± 0.1 A | 2.2 ± 0.1 A |
| C18:2c10t12 | 5.6 ± 0.1 A | 18.4 ± 2.3 A | 78.2 ± 0.2 A | 0 A | 38.0 ± 0.7 A | 185.1 ± 5.9 A |
| SFA | 655.2 ± 34.3 A | 201.3 ± 12.7 B | 665.6 ± 12.5 A | 485.2 ± 4.0 A | 342.7 ± 27.1 A | 854.4 ± 18.7 A |
| MUFA | 506.4 ± 34.8 A | 93.1 ± 2.7 B | 272.0 ± 3.6 A | 65.6 ± 2.8 A | 207.6 ± 12.1 A | 435.4 ± 9.1 A |
| PUFA | 66.6 ± 4.1 A | 47.1 ± 3.5 A | 44.4 ± 3.6 B | 15.6 ± 0.1 A | 40.1 ± 1.0 A | 74.5 ± 1.3 A |
| CLA | 9.1 ± 0.2 A | 22.5 ± 2.4 B | 81.6 ± 0.3 A | 0 A | 42.1 ± 0.8 A | 187.4 ± 6.0 A |
| Samples | Concentrations (μg/mL) | |||||
|---|---|---|---|---|---|---|
| 333.33 | 166.67 | 33.33 | ||||
| t = 0 | t = 24 h | t = 0 | t = 24 h | t = 0 | t = 24 h | |
| Trolox | 100.0 ± 0.1 | 50.2 ± 0.1 | 30.1 ± 0.1 | |||
| Cow | 50.0 ± 1.4 b,A | 74.1 ± 0.6 a,A | 25.4 ± 1.4 b,B | 59.3 ± 0.5 a,A | 17.5 ± 1.4 b,A | 51.9 ± 0.5 a,A |
| Camel | 21.7 ± 0.8 a,B | 38.6 ± 4.4 a,B | 6.6 ± 0.8 a,B | 11.4 ± 2.3 a,B | 0 a,A | 0 a,C |
| Buffalo | 42.8 ± 0.1 b,A | 54.8 ± 1.4 a,A | 33.3 ± 0.1 a,B | 41.0 ± 0.5 a,A | 19.3 ± 2.2 a,A | 33.1 ± 0.5 a,A |
| Donkey | 63.5 ± 4.1 a,A | 62.7 ± 1.1 a,A | 41.0 ± 1.4 a,B | 46.7 ± 1.4 a,A | 26.5 ± 0.5 a,A | 30.7 ± 0.5 a,A |
| Goat | 64.0 ± 0.1 b,A | 76.2 ± 0.5 a,A | 60.1 ± 0.5 a,A | 61.2 ± 0.1 a,A | 14.7 ± 1.7 a,A | 21.2 ± 0.8 a,B |
| Sheep | 30.6 ± 5.1 b,A | 71.8 ± 0.8 a,A | 0 a,B | 0 a,B | 0 a,A | 0 a,C |
| Samples | EC50 (µg/mL) | |
|---|---|---|
| t = 0 h | t = 24 h | |
| Trolox | 1.8 ± 0.2 | |
| Cow | 365.6 ± 0.2 b,C | 63.6 ± 1.8 a,A |
| Camel | 1422.0 ± 3.0 b,E | 707.1 ± 2.3 a,F |
| Buffalo | 373.3 ± 2.6 b,C | 211.4 ± 2.3 a,D |
| Donkey | 186.7 ± 2.3 b,B | 162.8 ± 2.2 a,C |
| Goat | 146.7 ± 2.2 b,A | 109.8 ± 2.1 a,B |
| Sheep | 1167.0 ± 3.1 b,D | 402.5 ± 2.6 a,E |
| Sample | Fermentation Time (h) | Shear Rate (s−1) | |||
|---|---|---|---|---|---|
| 0.1 | 1.0 | 10 | 100 | ||
| Cow | 0 | 0.171 | 0.013 | 0.004 | 0.003 |
| 24 | 0.359 | 0.039 | 0.008 | 0.005 | |
| Camel | 0 | 0.148 | 0.007 | 0.002 | 0.002 |
| 24 | 0.195 | 0.016 | 0.004 | 0.003 | |
| Buffalo | 0 | 0.246 | 0.041 | 0.007 | 0.003 |
| 24 | 0.132 | 0.014 | 0.005 | 0.003 | |
| Donkey | 0 | 0.135 | 0.009 | 0.003 | 0.002 |
| 24 | 0.163 | 0.021 | 0.006 | 0.003 | |
| Goat | 0 | 0.312 | 0.028 | 0.005 | 0.002 |
| 24 | 0.382 | 0.026 | 0.006 | 0.003 | |
| Sheep | 0 | 0.185 | 0.008 | 0.005 | 0.004 |
| 24 | 0.509 | 0.072 | 0.016 | 0.008 | |
| Cow | Protein | TPC | FRAP | EC50 | SFA | MUFA | PUFA | CLA |
|---|---|---|---|---|---|---|---|---|
| Protein | ||||||||
| TPC | a - | |||||||
| FRAP | - | b + | ||||||
| EC50 | - | - | + | |||||
| SFA | - | - | - | - | ||||
| MUFA | - | + | + | + | - | |||
| PUFA | - | + | + | + | - | + | ||
| CLA | - | - | - | - | - | - | - | |
| Camel | Protein | TPC | FRAP | EC50 | SFA | MUFA | PUFA | CLA |
| Protein | ||||||||
| TPC | - | |||||||
| FRAP | - | - | ||||||
| EC50 | - | - | - | |||||
| SFA | - | - | - | - | ||||
| MUFA | - | + | - | - | - | |||
| PUFA | - | - | - | - | - | - | ||
| CLA | - | - | - | - | - | - | - | |
| Buffalo | Protein | TPC | FRAP | EC50 | SFA | MUFA | PUFA | CLA |
| Protein | ||||||||
| TPC | - | |||||||
| FRAP | - | - | ||||||
| EC50 | - | - | - | |||||
| SFA | - | - | - | - | ||||
| MUFA | - | - | - | - | - | |||
| PUFA | - | - | - | - | - | - | ||
| CLA | - | - | - | - | - | - | - | |
| Donkey | Protein | TPC | FRAP | EC50 | SFA | MUFA | PUFA | CLA |
| Protein | ||||||||
| TPC | - | |||||||
| FRAP | - | - | ||||||
| EC50 | - | - | + | |||||
| SFA | - | - | - | - | ||||
| MUFA | - | - | - | - | - | |||
| PUFA | - | - | - | - | - | - | ||
| CLA | - | - | - | - | - | - | ||
| Goat | Protein | TPC | FRAP | EC50 | SFA | MUFA | PUFA | CLA |
| Protein | ||||||||
| TPC | - | |||||||
| FRAP | - | - | ||||||
| EC50 | - | - | - | |||||
| SFA | - | - | - | - | ||||
| MUFA | - | - | - | - | - | |||
| PUFA | - | - | - | - | - | - | ||
| CLA | - | - | - | - | - | - | - | |
| Sheep | Protein | TPC | FRAP | EC50 | SFA | MUFA | PUFA | CLA |
| Protein | ||||||||
| TPC | + | |||||||
| FRAP | - | - | ||||||
| EC50 | - | - | - | |||||
| SFA | - | - | - | - | ||||
| MUFA | - | - | - | - | - | |||
| PUFA | - | - | - | - | - | - | ||
| CLA | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Torre, C.; Caputo, P.; Cione, E.; Fazio, A. Comparing Nutritional Values and Bioactivity of Kefir from Different Types of Animal Milk. Molecules 2024, 29, 2710. https://doi.org/10.3390/molecules29112710
La Torre C, Caputo P, Cione E, Fazio A. Comparing Nutritional Values and Bioactivity of Kefir from Different Types of Animal Milk. Molecules. 2024; 29(11):2710. https://doi.org/10.3390/molecules29112710
Chicago/Turabian StyleLa Torre, Chiara, Paolino Caputo, Erika Cione, and Alessia Fazio. 2024. "Comparing Nutritional Values and Bioactivity of Kefir from Different Types of Animal Milk" Molecules 29, no. 11: 2710. https://doi.org/10.3390/molecules29112710
APA StyleLa Torre, C., Caputo, P., Cione, E., & Fazio, A. (2024). Comparing Nutritional Values and Bioactivity of Kefir from Different Types of Animal Milk. Molecules, 29(11), 2710. https://doi.org/10.3390/molecules29112710









