Preparation and Application of Polymer-Dispersed Liquid Crystal Film with Step-Driven Display Capability
Abstract
1. Introduction
2. Results and Discussion
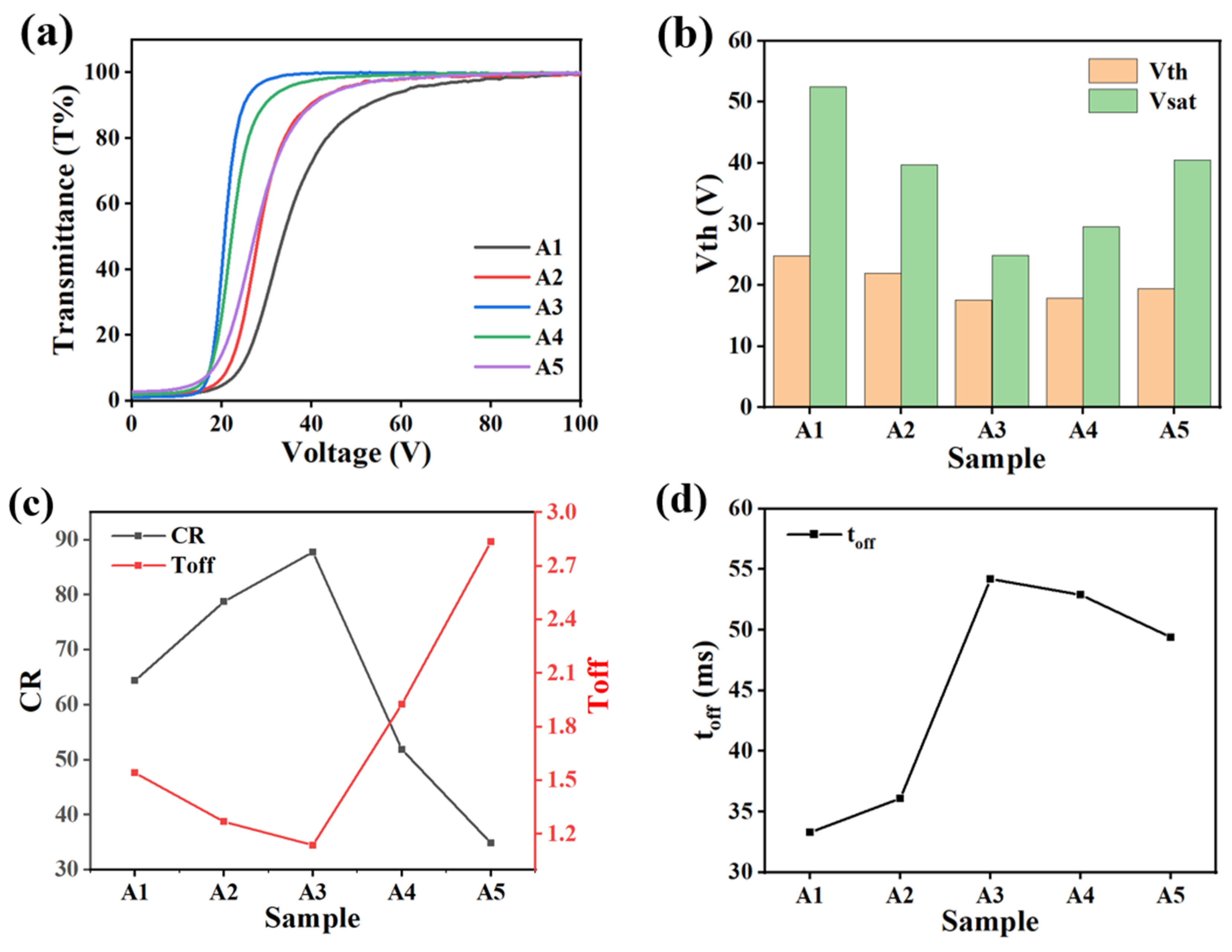
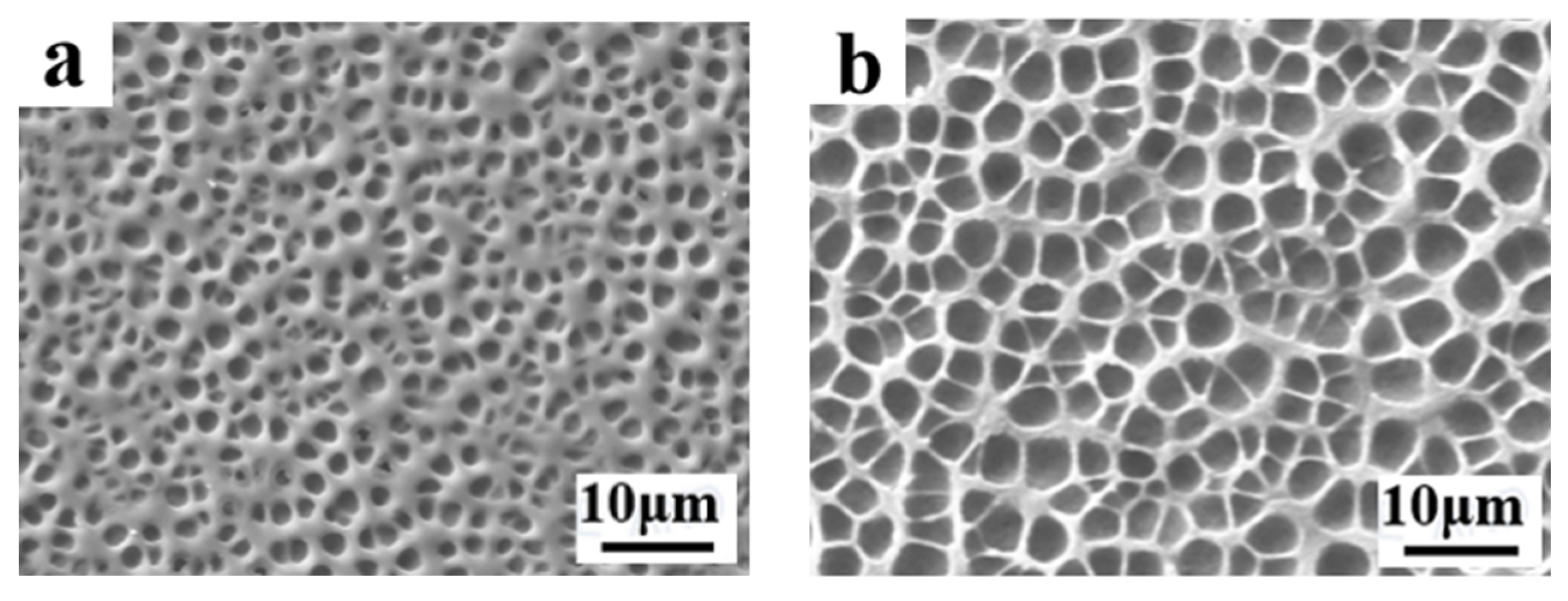
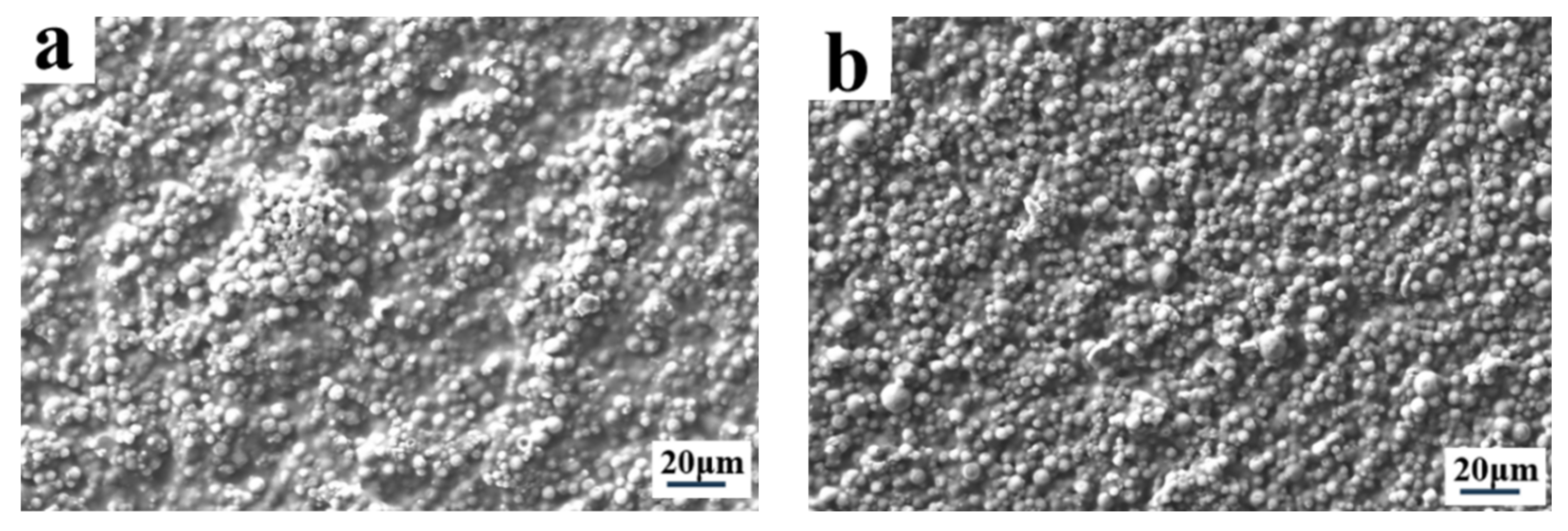
2.1. The Effect of Cross-Linker on Electro-Optical Properties of PDLC
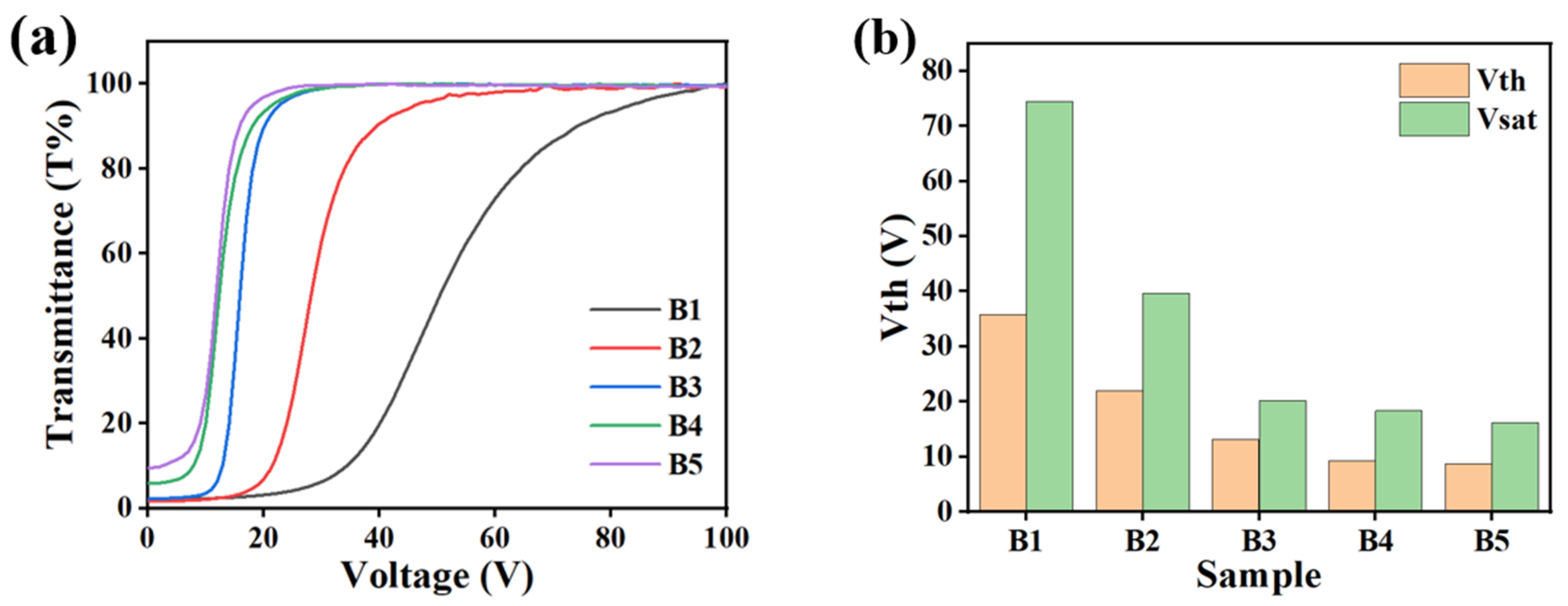
2.2. The Effect of LC Content on Electro-Optical Properties of PDLC
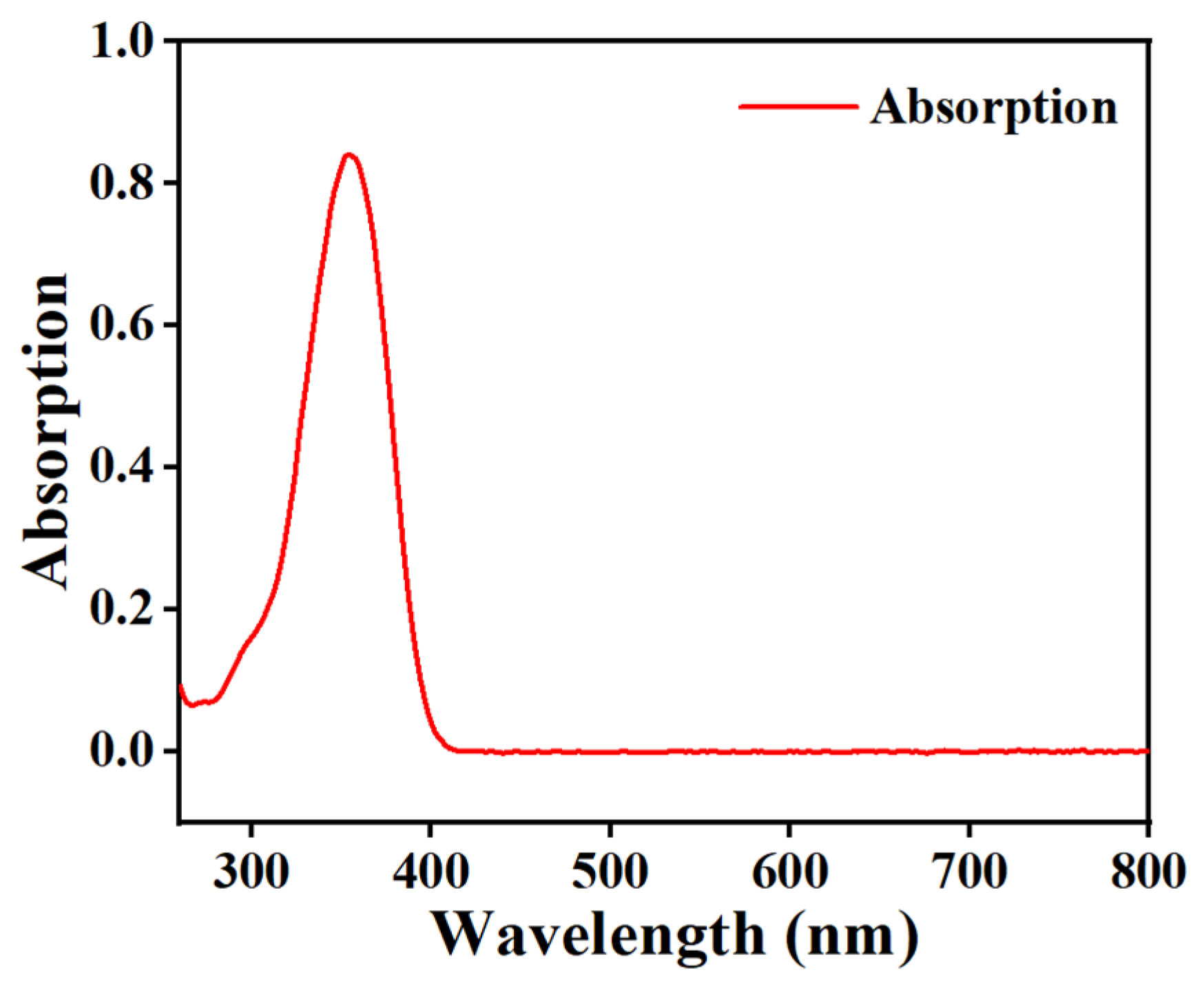
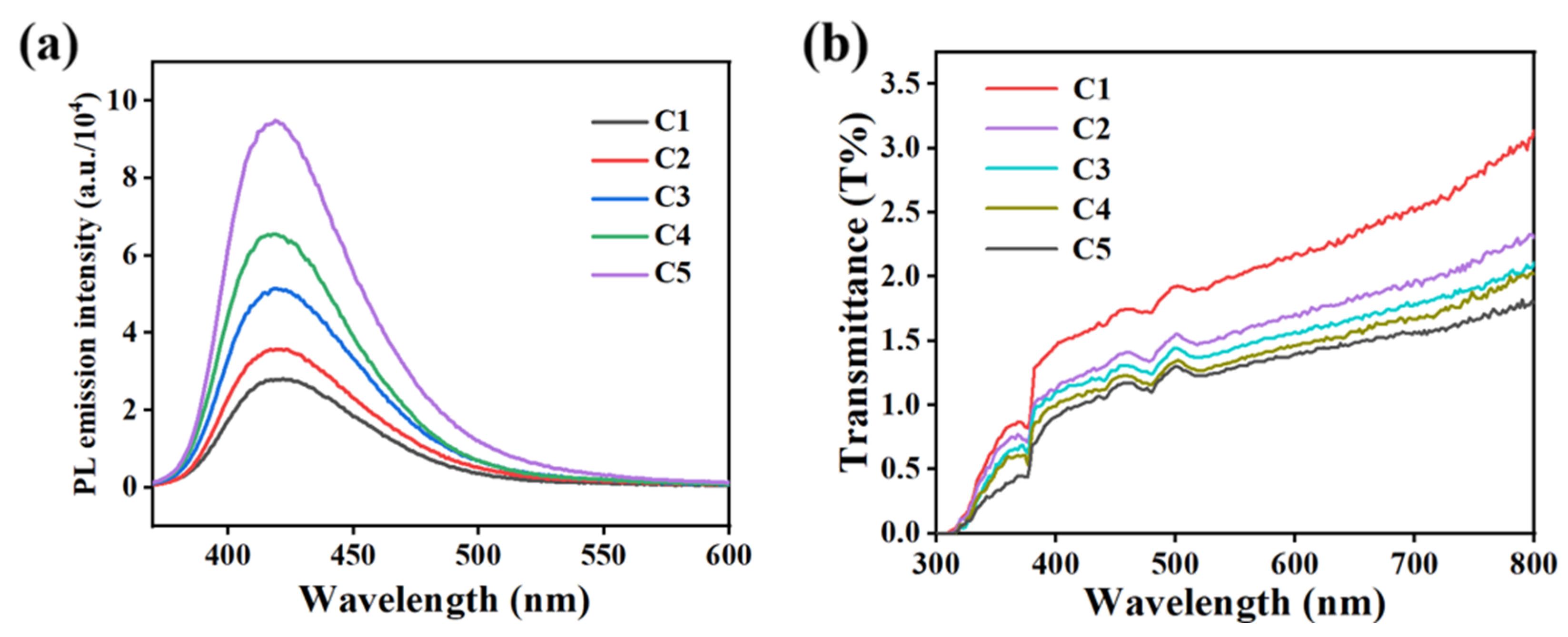
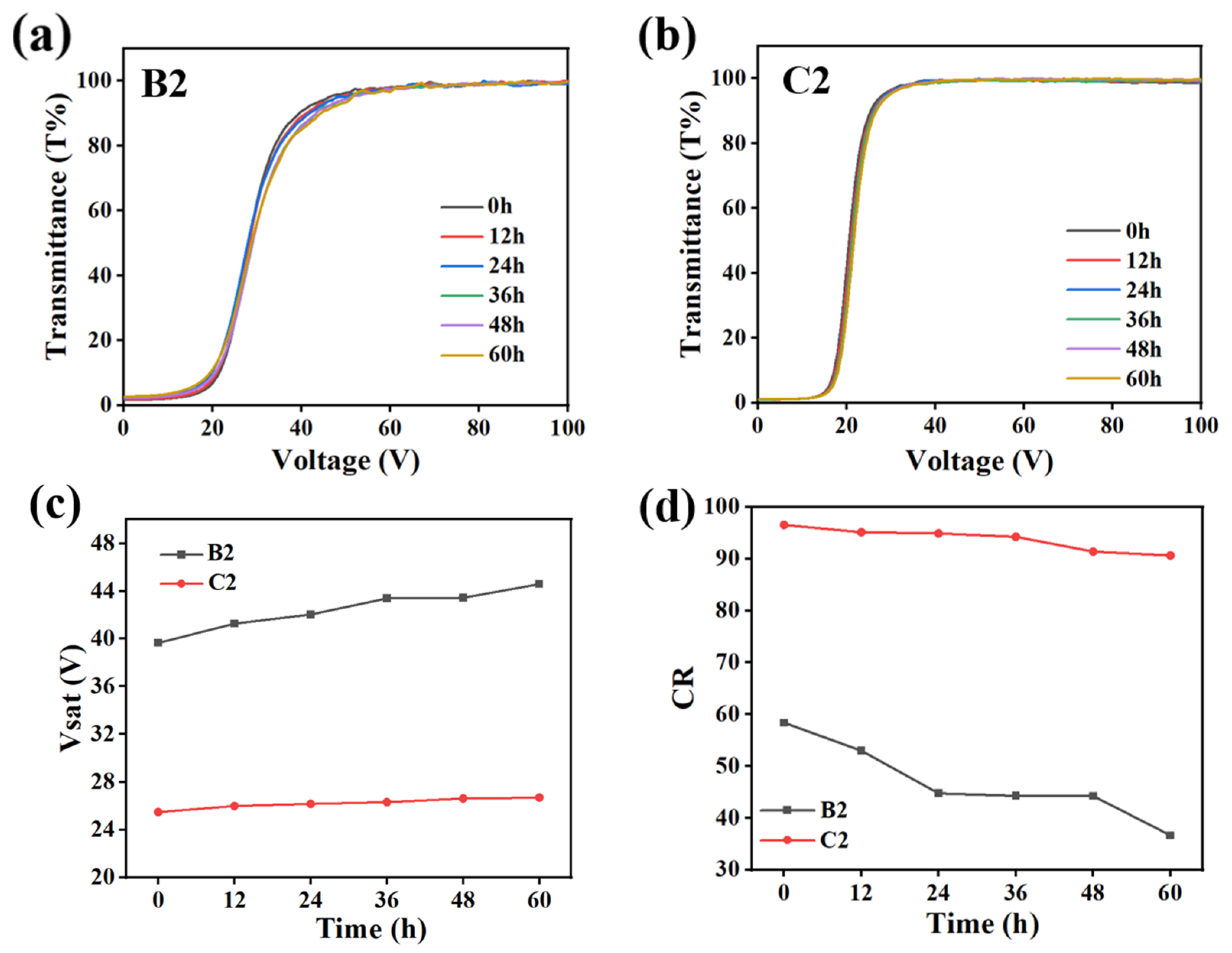
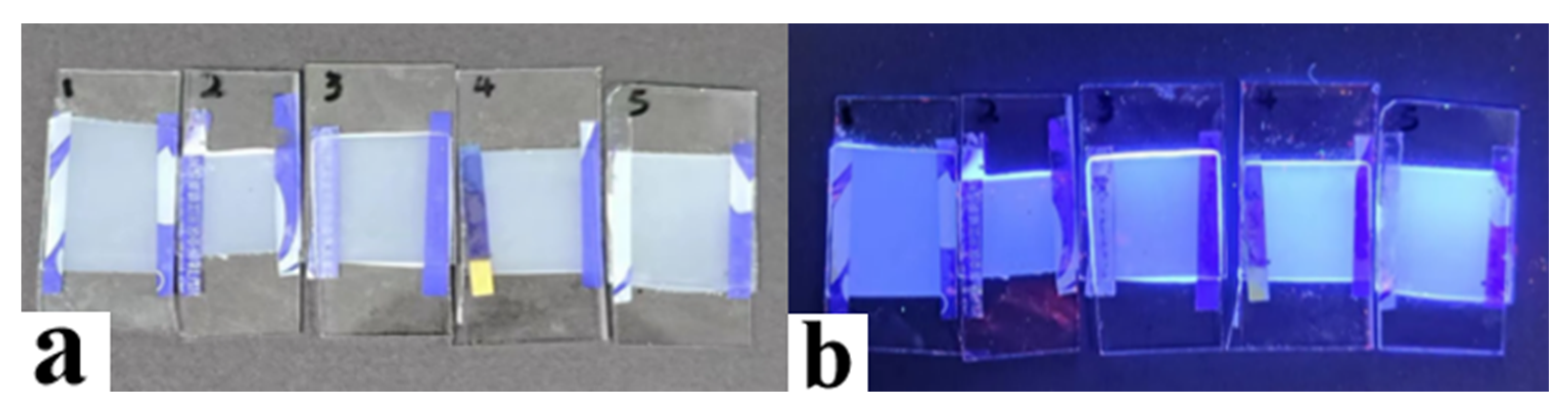
2.3. The Effect of Fluorescent Dye Content on Electro-Optical Properties of PDLC
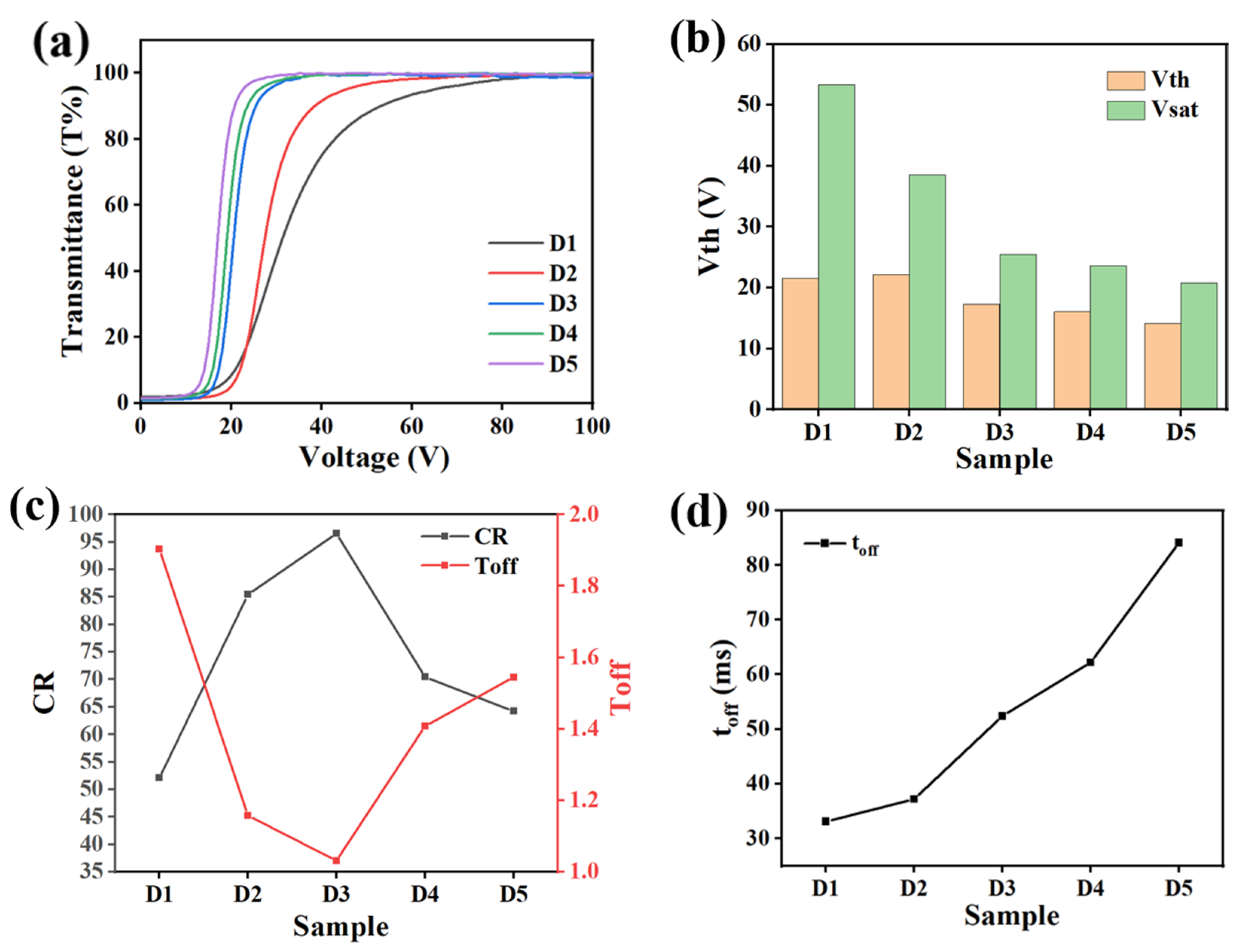
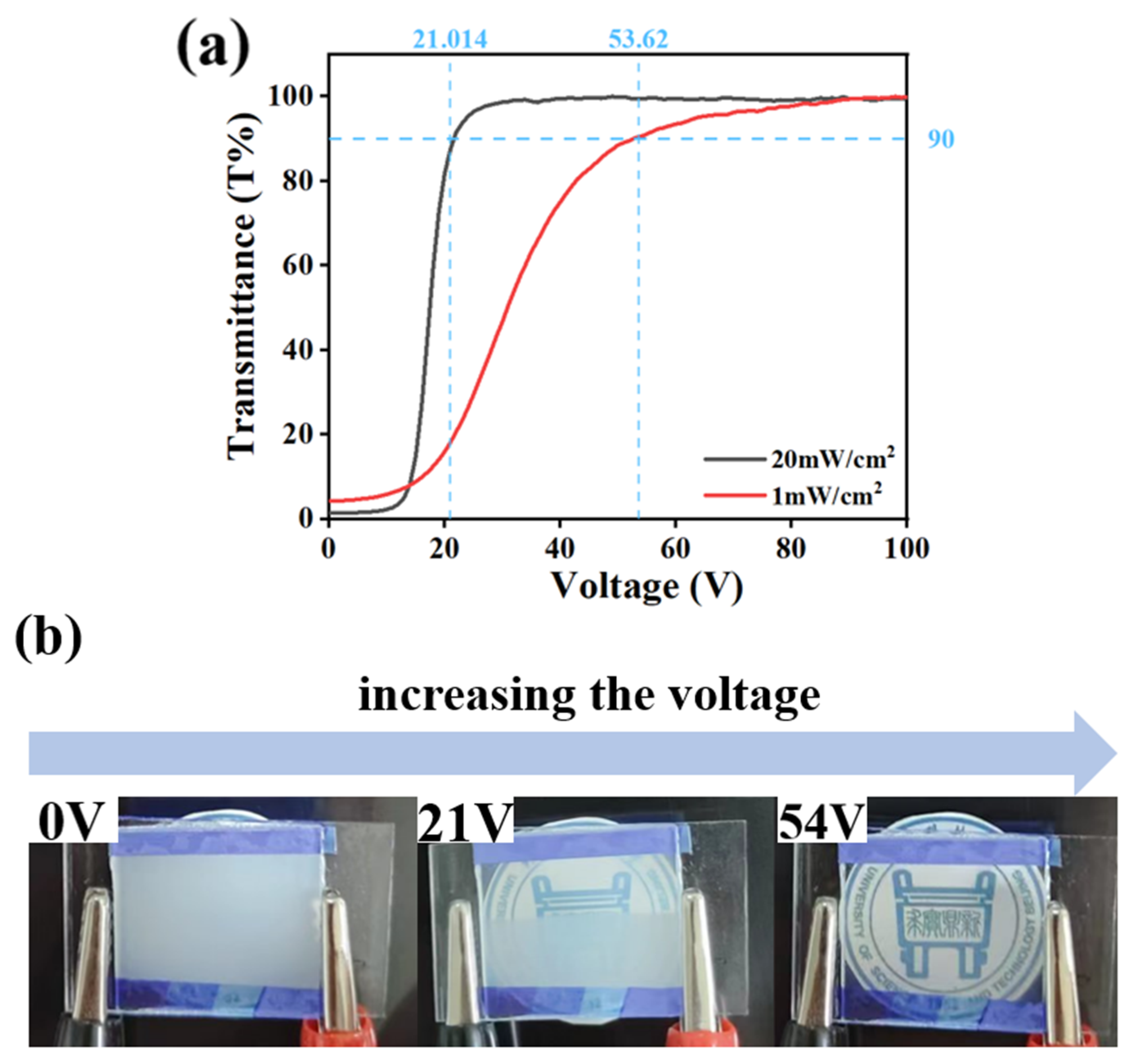
2.4. The Effect of UV-Light Intensity on Electro-Optical Properties of PDLC
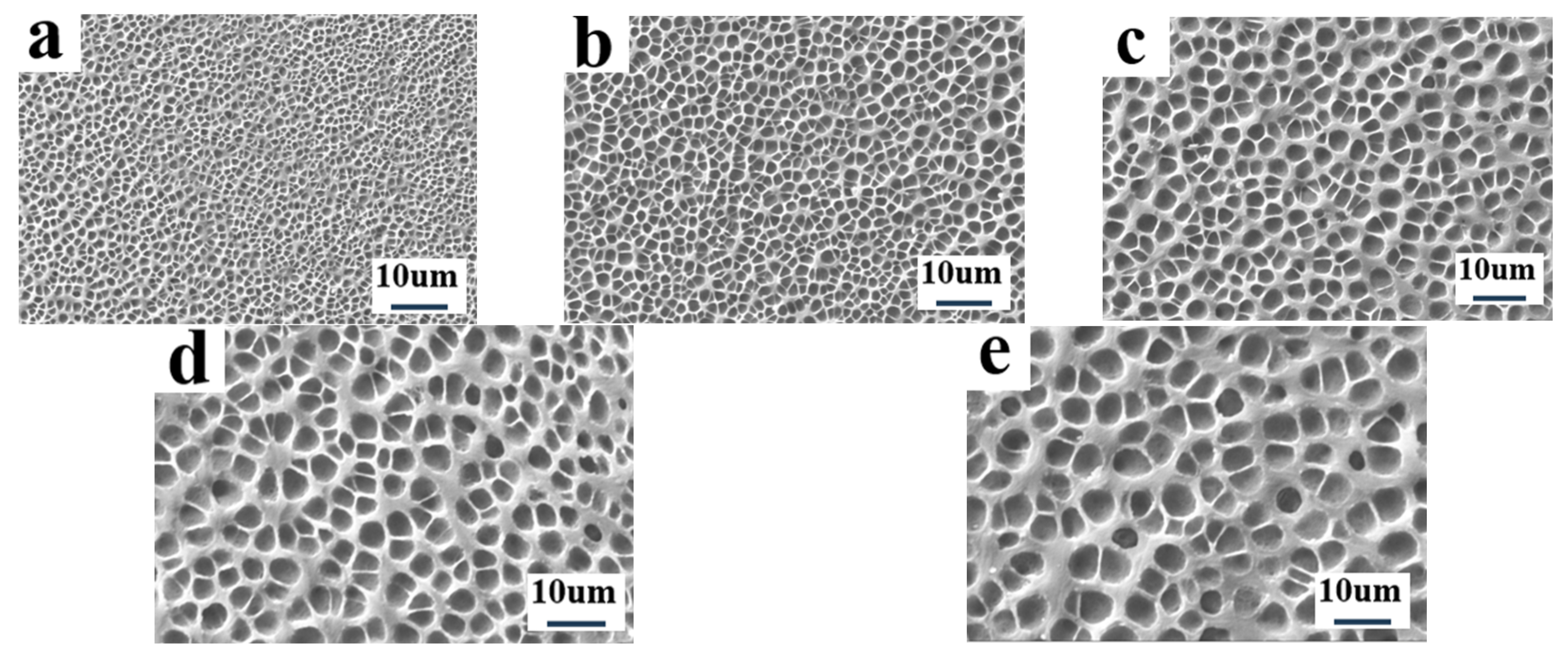
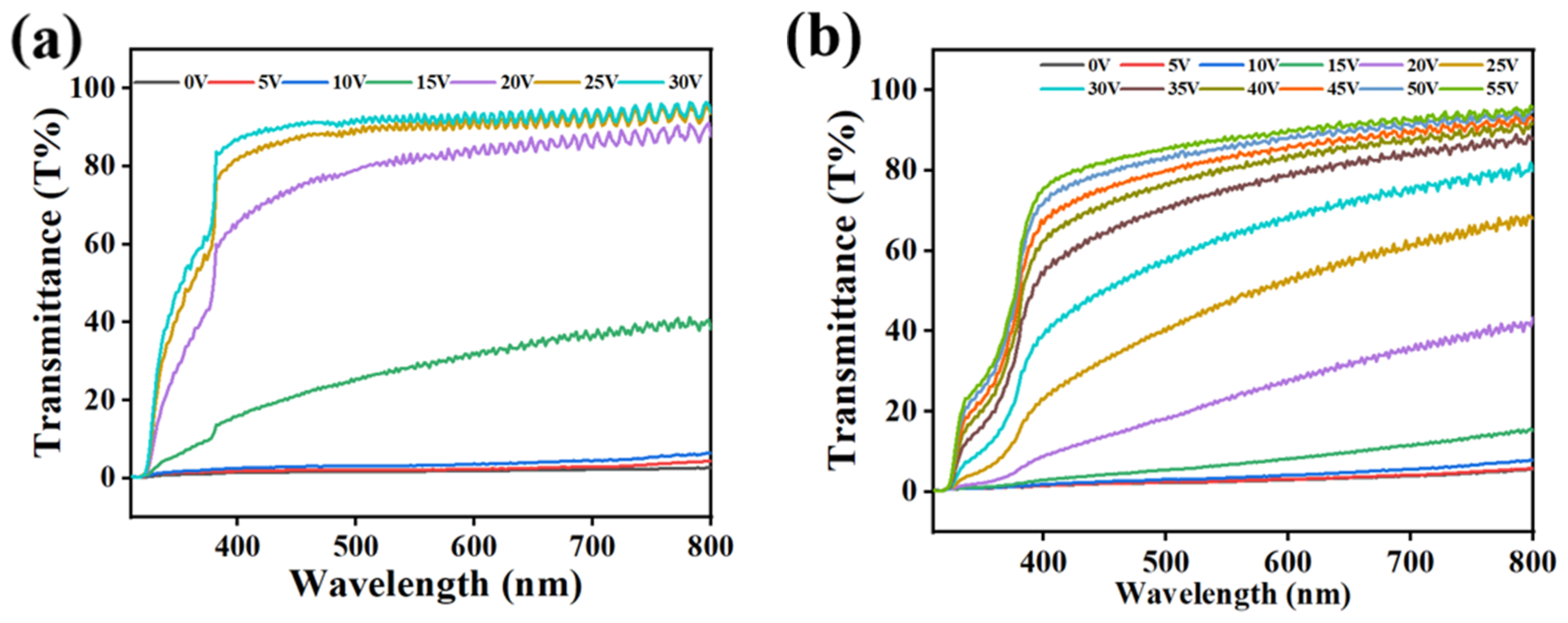
2.5. The Effect of Partitioned Polymerization on Electro-Optical Properties of PDLC
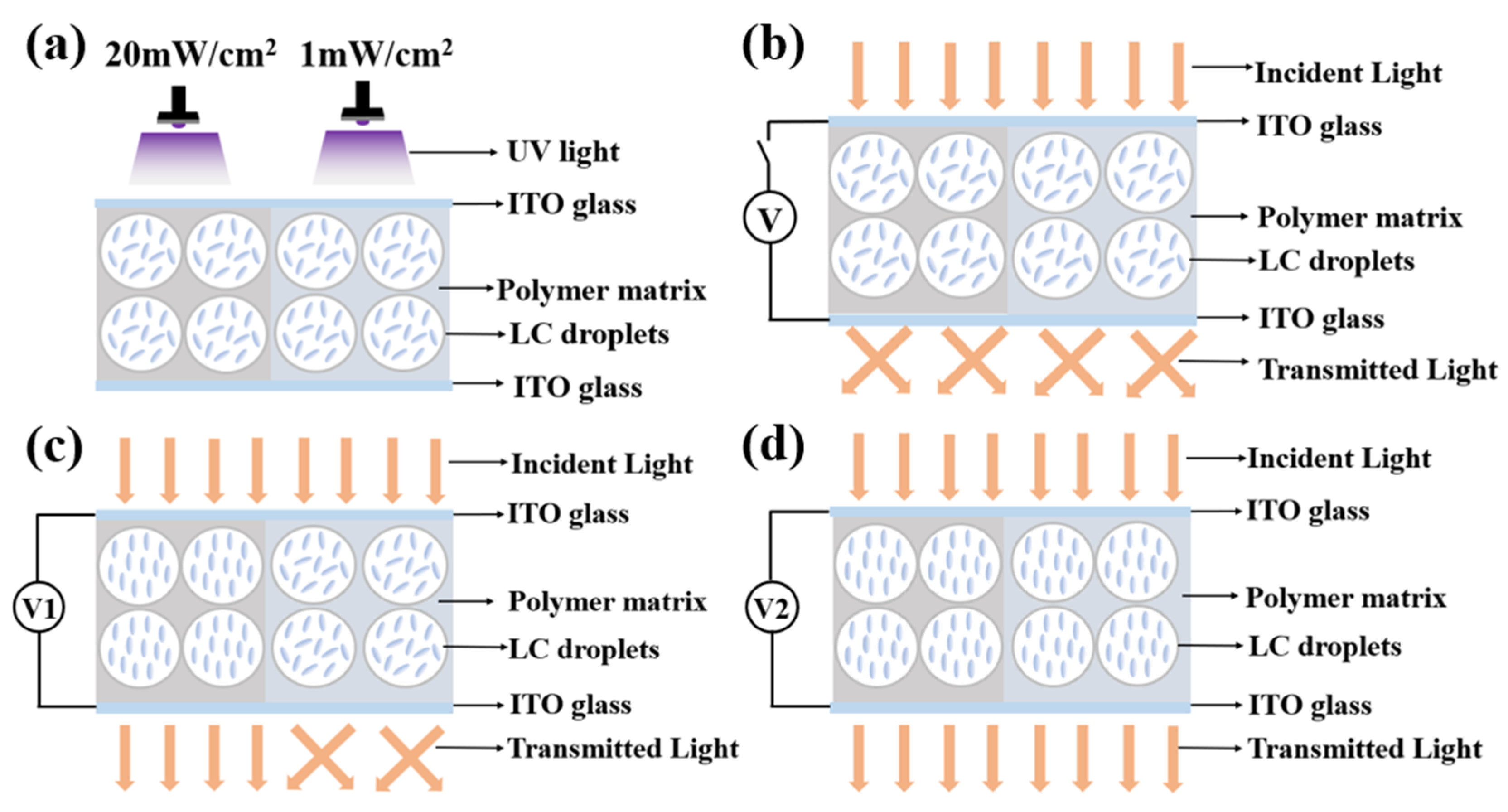
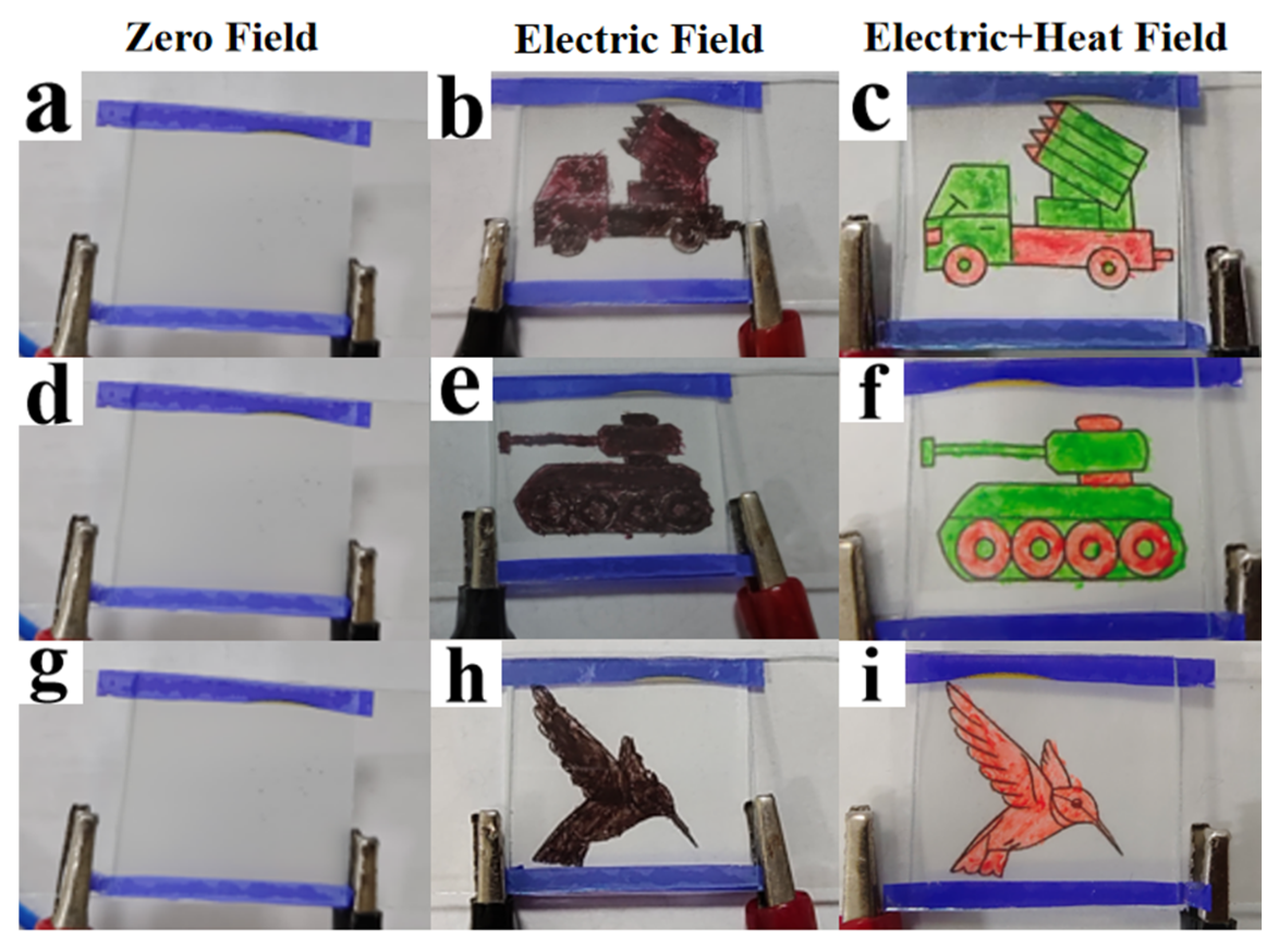
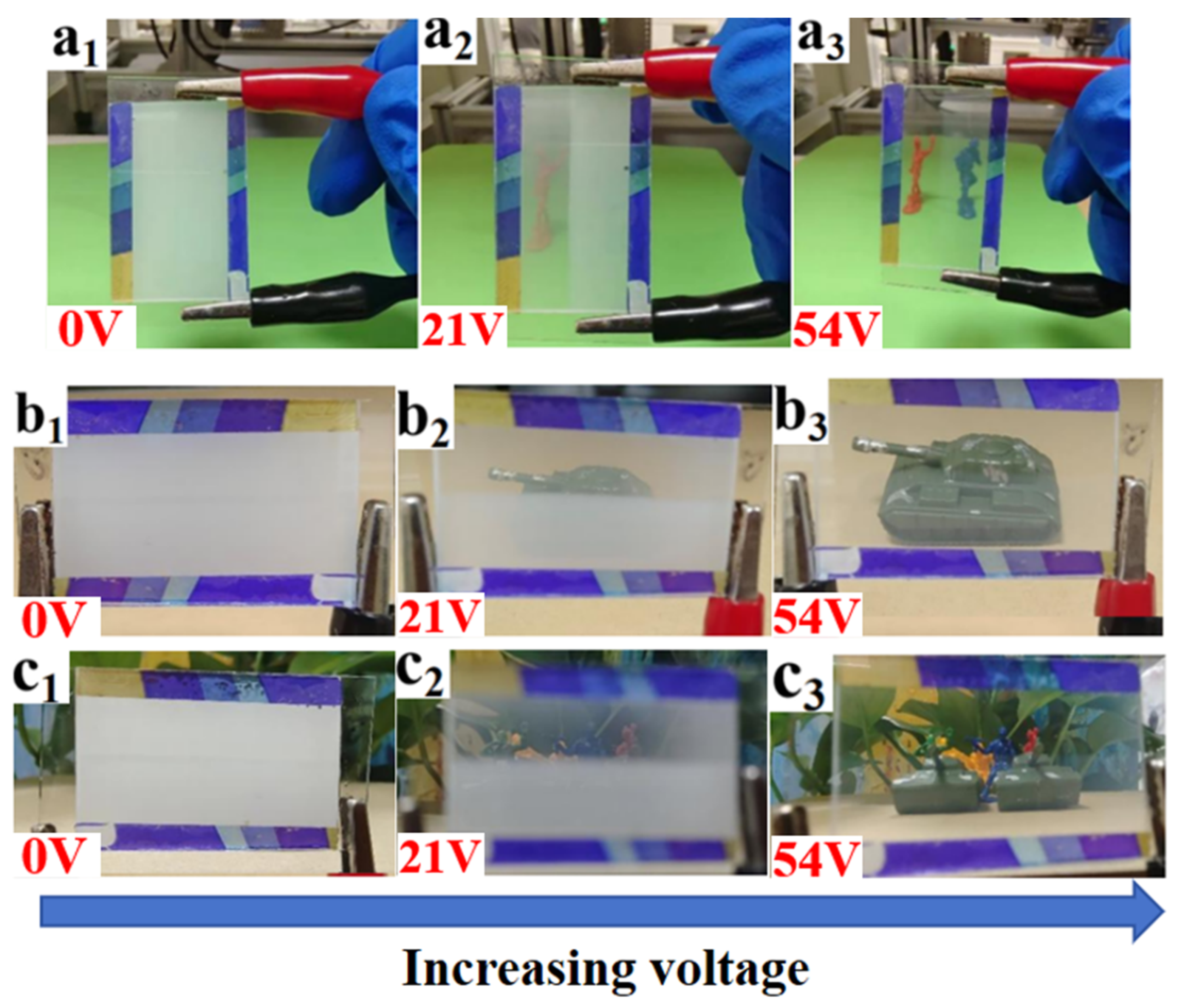
3. Mechanism and Applications
4. Experiment
4.1. Materials
4.2. Sample Preparation
4.3. Measurement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, C.W.; Chen, M.Y. Polymer-dispersed liquid crystal applied in active-matrix transparent display. J. Disp. Technol. 2014, 10, 683–687. [Google Scholar] [CrossRef]
- Kuo, C.W.; Lin, C.H.; Liao, Y.Y.; Lai, Y.H.; Chuang, C.T.; Yeh, C.N.; Lu, J.K.; Sugiura, N. Blur-free transparent LCD with hybrid transparency. Proc. Soc. Inf. Display 2013, 44, 70–73. [Google Scholar] [CrossRef]
- Song, S.H.; Hao, T.T.; Wang, B.; Liu, D.Q.; Ren, Z.C.; Zhang, Y.K.; Liu, W.C.; Zhang, L.P.; Li, Y. Multicolored displays with selective visible-infrared spectral modulation for optical information transmission. Adv. Opt. Mater. 2023, 11, 2301065. [Google Scholar] [CrossRef]
- Zhao, S.Q.; Chen, L.S.; Huang, W.B.; Liu, Y.H. Transparent multicolor electrochromic displays with ingenious hues adjustment by integrating cholesteric liquid crystal with viologen gel. Adv. Opt. Mater. 2023, 11, 2300503. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Guo, H.; He, D.G.; Sun, J.Q.; Chen, W.Q.; Song, Y.J.; Zhou, G.F. Development of cyclic tetrasiloxane polymer as a high-performance dielectric and hydrophobic layer for electrowetting displays. ACS Appl. Mater. Interfaces 2023, 15, 46470–46482. [Google Scholar] [CrossRef]
- Liu, A.Q.; Mukhin, I.S.; Islamova, R.M.; Tian, J.J. Flexible perovskite light-emitting diodes: Characteristics and performance. Adv. Funct. Mater. 2023, 2312209. [Google Scholar] [CrossRef]
- Zhan, X.Q.; Xu, F.F.; Zhou, Z.H.; Yan, Y.L.; Yao, J.N.; Zhao, Y.S. 3D laser displays based on circularly polarized lasing from cholesteric liquid crystal arrays. Adv. Mater. 2021, 33, 2104418. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Cao, Y.; Fang, X.; Wang, Y.F.; Liu, Q.Z.; Gui, H.; Shen, C.F.; Gao, X.; Kim, E.S.; Zhou, C.W. Carbon nanotube macroelectronics for active matrix polymer-dispersed liquid crystal displays. ACS Nano 2016, 10, 10068–10074. [Google Scholar] [CrossRef]
- Satoh, E.; Asaoka, Y.; Deguchi, K.; Ihara, I.; Minoura, K.; Fujiwara, S.; Miyata, A.; Itoh, Y.; Iyama, Y.C.; Shibasaki, M.; et al. 60-inch Highly transparent see-through active matrix display without polarizers. Proc. Soc. Inf. Display 2010, 41, 1192–1195. [Google Scholar] [CrossRef]
- He, Z.Q.; Yin, K.; Wu, S.T. Passive polymer-dispersed liquid crystal enabled multi-focal plane displays. Opt. Express 2020, 28, 15294–15299. [Google Scholar] [CrossRef]
- Xu, M.M.; Hua, H. Geometrical-lightguide-based head-mounted lightfield displays using polymer-dispersed liquid-crystal films. Opt. Express 2020, 28, 21165–21181. [Google Scholar] [CrossRef]
- Abdulhalim, I.; Madhuri, P.L.; Diab, M.; Mokari, T. Novel easy to fabricate liquid crystal composite with potential for electrically or thermally controlled transparency windows. Opt. Express 2019, 27, 17387–17401. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, P.; Sharma, A.; Chinky; Raina, K.K. Droplet configuration control with orange azo dichroic dye in polymer dispersed liquid crystal for advanced electro-optic characteristics. J. Mol. Liq. 2017, 233, 122–130. [Google Scholar] [CrossRef]
- Islam, M.S.; Chan, K.Y.; Thien, G.S.H.; Low, P.L.; Lee, C.L.; Wong, S.K.; Noor, E.E.M.; Au, B.W.C.; Ng, Z.N. Performances of polymer-dispersed liquid crystal films for smart glass applications. Polymers 2023, 15, 3420. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.M.; Kim, S.J.; Choi, G.B.; Lee, J.; Koo, M.M.; Kim, J.; Kim, Y.W.; Lee, J.; Kim, J.H.; Seo, T.H. A Graphene-Based Polymer Dispersed Liquid Crystal Device Enabled through a Water-Induced Interface Cleaning Process. Nanomaterials 2023, 13, 2309. [Google Scholar] [CrossRef]
- Zhang, D.X.; Zeng, J.T.; Zhu, S.T.; Ma, H.; Kang, X.X.; Lou, L.; He, Z.M. Effects of polyhedral oligomeric silsesquioxane and silicon microstructure on the electric-optical performance of polymer dispersed liquid crystals. Opt. Mater. 2023, 140, 113877. [Google Scholar] [CrossRef]
- Yu, M.A.; Xu, J.J.; Zhang, L.N.; Wang, Q.; Zou, C.; Gao, Y.Z.; Yang, H. Balanced electro-optical properties and off-axis haze performance of a polymer-dispersed liquid crystal film via refractive index matching. Phys. Chem. Chem. Phys. 2023, 25, 23770–23782. [Google Scholar] [CrossRef]
- Diao, Z.H.; Kong, L.S.; Yan, J.L.; Guo, J.D.; Liu, X.F.; Xuan, L.; Yu, L. Electrically tunable holographic waveguide display based on holographic polymer dispersed liquid crystal grating. Chin. Opt. Lett. 2019, 17, 012301. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.W.; Cai, W.F.; He, H.L.; Cen, M.J.; Liu, J.X.; Luo, D.; Mu, Q.Q.; Gérard, D.; Liu, Y.J. Electrically switchable, polarization-sensitive encryption based on aluminum nanoaperture arrays integrated with polymer-dispersed liquid crystals. Nano Lett. 2021, 21, 7183–7190. [Google Scholar] [CrossRef]
- Liu, Y.J.; Ding, X.Y.; Lin, S.C.S.; Chiang, I.K.; Huang, T.J. Surface acoustic wave driven light shutters using polymer-dispersed liquid crystals. Adv. Mater. 2011, 23, 1656–1659. [Google Scholar] [CrossRef]
- Higgins, D.A. Probing the mesoscopic chemical and physical properties of polymer-dispersed liquid crystals. Adv. Mater. 2000, 12, 251–264. [Google Scholar] [CrossRef]
- Zhang, H.M.; Miao, Z.C.; Shen, W.B. Development of polymer-dispersed liquid crystals: From mode innovation to applications. Compos. Part A Appl. Sci. Manuf. 2022, 163, 107234. [Google Scholar] [CrossRef]
- Saeed, M.H.; Zhang, S.F.; Cao, Y.P.; Zhou, L.; Hu, J.M.; Muhammad, I.; Xiao, J.M.; Zhang, L.Y.; Yang, H. Recent advances in the polymer dispersed liquid crystal composite and its applications. Molecules 2020, 25, 5510. [Google Scholar] [CrossRef] [PubMed]
- Kamal, W.; Li, M.M.; Lin, J.D.; Parry, E.; Jin, Y.H.; Elston, S.J.; Castrejón-Pita, A.A.; Morris, S.M. Spatially patterned polymer dispersed liquid crystals for image-integrated smart windows. Adv. Opt. Mater. 2022, 10, 2101748. [Google Scholar] [CrossRef]
- Ghosh, A. Investigation of vacuum-integrated switchable polymer dispersed liquid crystal glazing for smart window application for less energy-hungry building. Energy 2023, 265, 126396. [Google Scholar] [CrossRef]
- Zhong, T.G.; Mandle, R.J.; Goodby, J.W.; Zhang, L.Y.; Zhang, C.H. Comparative studies of polymer-dispersed liquid crystal films via a thiol-ene click reaction. Polym. Adv. Technol. 2019, 30, 2781–2789. [Google Scholar] [CrossRef]
- Shen, W.B.; Wang, L.; Zhong, T.J.; Chen, G.; Li, C.X.; Chen, M.; Zhang, C.H.; Zhang, L.Y.; Li, K.X.; Yang, Z.; et al. Electrically switchable light transmittance of epoxy-mercaptan polymer/nematic liquid crystal composites with controllable microstructures. Polymer 2019, 160, 53–64. [Google Scholar] [CrossRef]
- Hu, G.; Chen, H.X.; Liu, Z.Q.; Zhang, S.; Zhou, Y.; Zhu, B.L.; Gu, H.Z. Tailoring structure and properties of polymer-dispersed liquid crystal by quenching process. Liq. Cryst. 2020, 47, 1582–1590. [Google Scholar] [CrossRef]
- Li, C.X.; Chen, M.; Shen, W.B.; Chen, G.; Zhang, L.Y.; Yang, H. A study on the polymer structures and electro-optical properties of epoxy-mercaptan-based polymer dispersed liquid crystal films. Liq. Cryst. 2019, 46, 1718–1726. [Google Scholar] [CrossRef]
- Kumano, N.; Seki, T.; Ishii, M.; Nakamura, H.; Umemura, T. Multicolor polymer-dispersed liquid crystal. Adv. Mater. 2011, 23, 884–888. [Google Scholar] [CrossRef]
- Pagidi, S.; Manda, R.; Bhattacharyya, S.S.; Lee, S.G.; Song, S.M.; Lim, Y.J.; Lee, J.H.; Lee, S.H. Fast switchable micro-lenticular lens arrays using highly transparent nano-polymer dispersed liquid crystals. Adv. Mater. Interfaces 2019, 6, 1900841. [Google Scholar] [CrossRef]
- Han, C.; Zhou, L.E.; Ma, H.P.; Li, C.Y.; Zhang, S.F.; Cao, H.; Zhang, L.Y.; Yang, H. Fabrication of a controllable anti-peeing device with a laminated structure of microlouver and polymer dispersed liquid crystals film. Liq. Cryst. 2019, 46, 2235–2244. [Google Scholar] [CrossRef]
- Huang, C.Y.; Lin, S.H. Organic solvent sensors using polymer-dispersed liquid crystal films with a pillar pattern. Polymers 2021, 13, 2906. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.H.; McGraw, G.; Ma, R.Q.; Brown, J.L.; Yang, D.K. Selective scattering polymer dispersed liquid crystal film for light enhancement of organic light emitting diode. Opt. Express 2017, 25, 3327–3335. [Google Scholar] [CrossRef]
- Zhang, W.J.; Lin, J.P.; Yu, T.S.; Lin, S.L.; Yang, D.Z. Effect of electric field on phase separation of polymer dispersed liquid crystal. Eur. Polym. J. 2003, 39, 1635–1640. [Google Scholar] [CrossRef]
- Formentin, P.; Palacios, R.; Ferré-Borrull, J.; Pallarés, J.; Marsal, L.F. Polymer-dispersed liquid crystal based on E7: Morphology and characterization. Synth. Met. 2008, 158, 1004–1008. [Google Scholar] [CrossRef]
- Heng, K.T.; Chen, Y.D.; Fuh, A.Y.G. Scattering mode LC light shutter based on double-side poly (n-vinyl carbazole) films. Proc. Int. Display Workshops 2012, 1, 1577–1580. [Google Scholar]
- Zhang, C.H.; Ge, Y.; Huo, X.P.; Xue, J.; Li, K.X.; Zhang, Y.M.; Miao, Z.C. Studies on electro-optical properties of polymer matrix/LC/ITO nanoparticles composites. Polym. Adv. Technol. 2020, 31, 544–552. [Google Scholar] [CrossRef]
- Nasir, N.; Kumar, S.; Kim, M.; Nguyen, V.H.; Suleman, M.; Lee, S.; Kang, D.W.; Seo, Y. Effect of the photoinitiator concentration on the electro-optical properties of thiol–acrylate-based PDLC smart windows. ACS Appl. Energy Mater. 2022, 5, 6986–6995. [Google Scholar] [CrossRef]
- Dhara, P.; Mukherjee, R. Phase separation and dewetting of polymer dispersed liquid crystal (PDLC) thin films on flat and patterned substrates. J. Mol. Liq. 2021, 341, 117360. [Google Scholar] [CrossRef]
- John, V.N.; Varanakkottu, S.N.; Varghese, S. Flexible, ferroelectric nanoparticle doped polymer dispersed liquid crystal devices for lower switching voltage and nanoenergy generation. Opt. Mater. 2018, 80, 233–240. [Google Scholar] [CrossRef]
- Kim, M.; Park, K.J.; Seok, S.; OK, J.M.; Jung, H.T.; Choe, J.; Kim, D.H. Fabrication of microcapsules for dye-doped polymer-dispersed liquid crystal-based smart windows. ACS Appl. Mater. Int. 2015, 7, 17904–17909. [Google Scholar] [CrossRef]
- Abualnaja, M.M.; Hossan, A.; Bayazeed, A.; Al-Qahtani, S.D.; Al-Ahmed, Z.A.; Abdel-Hafez, S.H.; Abdel-Hafez, S.H.; El-Metwaly, N.M. Synthesis and self-assembly of new fluorescent cholesteryloxy-substituted fluorinated terphenyls with gel formation and mesogenic phases. J. Mol. Struct. 2022, 1251, 132006. [Google Scholar] [CrossRef]
- Ahmad, F.; Jamil, M.; Jeon, Y.J.; Woo, L.J.; Jung, J.E.; Jang, J.U.; Jang, J.E.; Lee, G.H.; Park, J. Comparative study on the electrooptical properties of polymer-dispersed liquid crystal films with different mixtures of monomers and liquid crystals. J. Appl. Polym. Sci. 2011, 121, 1424–1430. [Google Scholar] [CrossRef]
- Li, K.; Jiang, H.D.; Cheng, M.; Li, Y.F.; Yin, Z.; Luo, D.; Sun, X.W.; Liu, Y.J. Controlling morphological and electro-optical properties via the phase separation in polymer/liquid-crystal composite materials. Liq. Cryst. 2020, 47, 238–247. [Google Scholar] [CrossRef]
- Katariya, J.A.; Deshmukh, R.R. Electro-optical and dielectric study of multi-walled carbon nanotube doped polymer dispersed liquid crystal films. Liq. Cryst. 2019, 46, 1191–1202. [Google Scholar] [CrossRef]
- Mhatre, M.M.; Katariya-Jain, A.; Deshmukh, R.R. Enhancing morphological, electro-optical and dielectric properties of polymer-dispersed liquid crystal by doping of disperse Orange 25 dye in LC E7. Liq. Cryst. 2022, 49, 790–803. [Google Scholar] [CrossRef]
- Zhao, C.H.; Hu, Y.C.; Xu, J.J.; Yu, M.N.; Zhou, C.; Wang, Q.; Gao, Y.Z.; Yang, H. Research on the morphology, electro-optical properties and mechanical properties of electrochromic polymer-dispersed liquid crystalline films doped with anthraquinone dyes. Crystals 2023, 13, 735. [Google Scholar] [CrossRef]
- Li, J.Q.; Zhao, Y.Z.; Gao, H.; Wang, D.; Miao, Z.C.; Cao, H.; Yang, Z.; He, W.L. Polymer dispersed liquid crystals doped with CeO2 nanoparticles for the smart window. Liq. Cryst. 2022, 49, 29–38. [Google Scholar] [CrossRef]
- Ahmad, F.; Jamil, M.; Lee, J.W.; Kim, S.R.; Jeon, Y.J. The effect of UV intensities and curing time on polymer dispersed liquid crystal (PDLC) display: A detailed analysis study. Electron. Mater. Lett. 2016, 12, 685–692. [Google Scholar] [CrossRef]
- Shi, Z.H.; He, Z.M.; Li, C.S.; Miao, Z.C.; Wang, D.; Luan, Y.; Li, Y.Z.; Zhao, Y.Z. The role of nanomesh fibres loaded with BaTiO3 nanoparticles on the electro-optical performance of PDLC devices. Appl. Mater. Today 2022, 29, 101622. [Google Scholar] [CrossRef]
- Shi, Z.H.; He, Z.M.; Li, C.S.; Miao, Z.C.; Wang, D.; Luan, Y.; Li, Y.Z.; Zhao, Y.Z. The role of nanomesh fibres loaded with fluorescent materials on the electro-optical performance of PDLC devices. Liq. Cryst. 2022, 49, 2037–2050. [Google Scholar] [CrossRef]
- Chen, X.L.; He, Z.M.; Li, C.S.; Miao, Z.C.; Wang, D.; Luan, Y.; Li, Y.Z.; Zhao, Y.Z. Effects of formulation composition and CeO2 nanoparticles doping on the morphologies of polymer spacer columns and electro-optical properties of PDLC. Mater. Today Commun. 2022, 31, 103758. [Google Scholar] [CrossRef]
- Jia, M.M.; Zhao, Y.Z.; Gao, H.; Wang, D.; Miao, Z.C.; Cao, H.; Yang, Z.; He, W.L. The Electro-optical study of Al2O3 nanoparticles doped polymer dispersed liquid crystal films. Liq. Cryst. 2022, 49, 39–49. [Google Scholar] [CrossRef]
- Yu, M.N.; Wang, T.Y.; Xu, J.J.; Hu, W.; Gao, Y.Z.; Zou, C.; Yang, H. Steric group-based polymer dispersed liquid crystal composite films with high contrast ratio, low driving voltage and small hysteresis. J. Mol. Liq. 2022, 365, 120152. [Google Scholar] [CrossRef]









| SLC-1717 (%) | UV-6301 (%) | IBMA (%) | TMPTA (%) | AMCA (%) | IRG651 (%) | UV-Light Intensity (mW/cm2) | |
|---|---|---|---|---|---|---|---|
| A1 | 50 | 49 | 0 | 1 | 0 | 0.5 | 10 |
| A2 | 50 | 48 | 1 | 1 | 0 | 0.5 | 10 |
| A3 | 50 | 47 | 2 | 1 | 0 | 0.5 | 10 |
| A4 | 50 | 46 | 3 | 1 | 0 | 0.5 | 10 |
| A5 | 50 | 45 | 4 | 1 | 0 | 0.5 | 10 |
| B1 | 45 | 55 a | 0 | 0.5 | 10 | ||
| B2 | 50 | 50 a | 0 | 0.5 | 10 | ||
| B3 | 55 | 45 a | 0 | 0.5 | 10 | ||
| B4 | 60 | 40 a | 0 | 0.5 | 10 | ||
| B5 | 65 | 35 a | 0 | 0.5 | 10 | ||
| C1 | 50 | 50 a | 0.1 | 0.5 | 10 | ||
| C2 | 50 | 50 a | 0.2 | 0.5 | 10 | ||
| C3 | 50 | 50 a | 0.3 | 0.5 | 10 | ||
| C4 | 50 | 50 a | 0.4 | 0.5 | 10 | ||
| C5 | 50 | 50 a | 0.5 | 0.5 | 10 | ||
| D1 | 50 | 50 a | 0.2 | 0.5 | 1 | ||
| D2 | 50 | 50 a | 0.2 | 0.5 | 5 | ||
| D3 | 50 | 50 a | 0.2 | 0.5 | 10 | ||
| D4 | 50 | 50 a | 0.2 | 0.5 | 15 | ||
| D5 | 50 | 50 a | 0.2 | 0.5 | 20 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Zhao, Y.; Jiao, X.; Gao, H.; Guo, Z.; Wang, D.; Luan, Y.; Wang, L. Preparation and Application of Polymer-Dispersed Liquid Crystal Film with Step-Driven Display Capability. Molecules 2024, 29, 1109. https://doi.org/10.3390/molecules29051109
Lin H, Zhao Y, Jiao X, Gao H, Guo Z, Wang D, Luan Y, Wang L. Preparation and Application of Polymer-Dispersed Liquid Crystal Film with Step-Driven Display Capability. Molecules. 2024; 29(5):1109. https://doi.org/10.3390/molecules29051109
Chicago/Turabian StyleLin, Hui, Yuzhen Zhao, Xiangke Jiao, Hong Gao, Zhun Guo, Dong Wang, Yi Luan, and Lei Wang. 2024. "Preparation and Application of Polymer-Dispersed Liquid Crystal Film with Step-Driven Display Capability" Molecules 29, no. 5: 1109. https://doi.org/10.3390/molecules29051109
APA StyleLin, H., Zhao, Y., Jiao, X., Gao, H., Guo, Z., Wang, D., Luan, Y., & Wang, L. (2024). Preparation and Application of Polymer-Dispersed Liquid Crystal Film with Step-Driven Display Capability. Molecules, 29(5), 1109. https://doi.org/10.3390/molecules29051109








