Anti-Inflammatory and Cytotoxic Compounds Isolated from Plants of Euphorbia Genus
Abstract
1. Introduction
Ethnobotany
2. Discussion
3. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- González-Costa, M.; Padrón-González, A.A. La inflamación desde una perspectiva inmunológica: Desafío a la Medicina en el siglo XXl. Rev. Haban Cienc. Méd. 2019, 18, 30–44. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1729-519x2019000100030&lng=es&nrm=iso (accessed on 25 February 2024).
- Kumar, V.; Abbas, A.K.; Fausto, N.; Mitchell, R.N. Robbins Basic Pathology, 10th ed.; McGraw-Hill Interamericana: New York, NY, USA, 2018; pp. 31–68. [Google Scholar]
- Yang, H.Z.; Wang, J.P.; Mi, S.; Liu, H.Z.; Cui, B.; Yan, H.M.; Lu, W. TLR4 activity is required in the resolution of pulmonary inflammation and fibrosis after acute and chronic lung injury. Am. J. Pathol. 2012, 180, 275–292. [Google Scholar] [CrossRef]
- Oscanoa-Espinoza, T.; Lizaraso-Soto, F. Antiinflamatorios no esteroides: Seguridad gastrointestinal, cardiovascular y renal. Rev. Gastroenterol. Perú 2015, 35, 63–71. Available online: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1022-51292015000100007&lng=es (accessed on 25 February 2024).
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.V.; Cobucci, R.N.; Jatobá, C.A.; Fernandes, T.A.; de Azevedo, J.W.; de Araújo, J.M. The Role of the Mediators of Inflammation in Cancer Development. Pathol. Oncol. Res. 2015, 21, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Campos-Xolalpa, N.; Alonso-Castro, Á.J.; Sánchez-Mendoza, E.; Zavala-Sánchez, M.A.; Pérez-Gutiérrez, S. Cytotoxic activity of the chloroform extract and four diterpenes isolated from Salvia ballotiflora. Rev. Bras. Farmacogn. 2017, 27, 302–305. [Google Scholar] [CrossRef]
- Virshette, S.J.; Patil, M.K.; Somkuwar, A.P. A review on medicinal plants used as anti-inflammatory agents. J. Pharmacogn. Phytochem. 2019, 8, 1641–1646. [Google Scholar]
- Schlaepfer, L.; Mendoza, E.J.A. Las Plantas Medicinales en la Lucha Contra el cáNcer, Relevancia Para México. Rev. Mex. Cienc. Farm. 2010, 41, 18–27. Available online: https://www.redalyc.org/articulo.oa?id=57916060003 (accessed on 25 February 2024).
- Bittner, M.; Alarcón, J.; Aqueveque, P.; Becerra, J.; Hernández, V.; Hoeneise, M.; Silva, M. Estudio químico de especies de la familia Euphorbiaceae en Chile. Bol. Soc. Chil. Quim. 2001, 46, 419–431. [Google Scholar] [CrossRef]
- Amtaghri, S.; Akdad, M.; Slaoui, M.; Eddouks, M. Traditional uses, pharmacological, and phytochemical studies of Euphorbia: A review. Curr. Top. Med. Chem. 2022, 22, 1553–1570. [Google Scholar] [CrossRef]
- Li, Y.N.; He, J.; Zhang, J.; Shi, Y.X.; Guo, L.B.; Peng, Z.C.; Xu, J.K. Existing knowledge on Euphorbia fischeriana Steud (Euphorbiaceae): Traditional uses, clinical applications, phytochemistry, pharmacology and toxicology. J. Ethnopharmacol. 2021, 275, 114095. [Google Scholar] [CrossRef]
- Martínez, G.M.; Jiménez, R.J.; Cruz, D.R.; Juárez, A.E.; García, R.; Cervantes, A.; Mejía, H.R. Los géneros de la familia Euphorbiaceae en México. (Parte A). An. Inst. Biol. 2002, 73, 155–196. Available online: https://www.redalyc.org/articulo.oa?id=40073205 (accessed on 25 February 2024).
- Xu, Y.; Tang, P.; Zhu, M.; Wang, Y.; Sun, D.; Li, H.; Chen, L. Diterpenoids from the genus Euphorbia: Structure and biological activity (2013–2019). Phytochemistry 2021, 190, 112846. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sun, L.; Kong, C.; Mei, W.; Dai, H.; Xu, F.; Huang, S. Phytochemical and pharmacological review of diterpenoids from the genus Euphorbia Linn (2012–2021). J. Ethnopharmacol. 2022, 298, 115574. [Google Scholar] [CrossRef]
- Rozimamat, R.; Hu, R.; Aisa, H.A. New isopimarane diterpenes and nortriterpene with cytotoxic activity from Euphorbia alatavica Boiss. Fitoterapia 2018, 127, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Choodej, S.; Hanthanong, S.; Aree, T.; Pudhom, K. Diterpenoids from the aerial parts of Euphorbia antiquorum and their efficacy on nitric oxide inhibition. Phytochemistry 2020, 180, 112523. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Liang, Y.; Yang, X.; Wang, H.; Zhang, J.; Tuerhong, M.; Li, D.; Wang, C.; Lee, D.; Xu, J.; et al. NO inhibitory diterpenoids as potential anti-inflammatory agents from Euphorbia antiquorum. Bioorg. Chem. 2019, 92, 103237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Duan, R.J.; Kong, C.H.; Dai, H.F.; Mei, W.L.; Xu, F.Q.; Huang, S.Z. Two new anti-inflammatory trachylobane diterpenoids from Euphorbia atoto. J. Asian Nat. Prod. Res. 2023, 1–7. [Google Scholar] [CrossRef]
- Aljubiri, S.M.; Mahgoub, S.A.; Almansour, A.I.; Shaaban, M.; Shaker, K.H. Isolation of diverse bioactive compounds from Euphorbia balsamifera: Cytotoxicity and antibacterial activity studies. Saudi J. Biol. Sci. 2021, 28, 417–426. [Google Scholar] [CrossRef]
- Hassan, A.R.; Ashour, A.; Amen, Y.; Nagata, M.; El-Toumy, S.A.; Shimizu, K. A new cycloartane triterpene and other phytoconstituents from the aerial parts of Euphorbia dendroides. Nat. Prod. Res. 2022, 36, 828–836. [Google Scholar] [CrossRef]
- Shamsabadipour, S.; Zarei, S.M.; Ghanadian, M.; Ayatollahi, S.A.; Rahimnejad, M.R.; Saeedi, H.; Aghaei, M. A New Taraxastane triterpene from Euphorbia Denticulata with cytotoxic activity against prostate cancer cells. Iran. J. Pharm. Res. 2018, 17, 336–342. [Google Scholar] [PubMed]
- Ding, K.; Zhang, Y.Y.; Yang, T.; Lian, W.W.; Xia, C.; Wang, W.P.; Zhang, W.K.; He, J.; Xu, J.K. New rosane diterpenoids and their analogs from Euphorbia ebracteolata Hayata. Chem. Biodivers. 2023, 20, e202300013. [Google Scholar] [CrossRef]
- Chun, J.; Mah, S.Y.; Kim, Y.S. Anti-inflammatory effect of ebractenoid f, a major active compound of Euphorbia ebracteolata Hayata, through inhibition of nuclear Factor-κB activation. Plants 2023, 12, 2845. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.L.; Tang, X.H.; Yuan, W.J.; Ding, X.; Di, Y.T.; Hao, X.J. Abietane diterpernoids from the roots of Euphorbia ebracteolata. Nat. Prod. Bioprospect. 2018, 8, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Peng, Y.; Wang, Y.; Huo, X.; Zhang, B.; Li, D.; Leng, A.; Zhang, H.; Ma, X.; Wang, C. Cytotoxic ent-Abietane-type diterpenoids from the roots of Euphorbia ebracteolata. Bioorg. Chem. 2018, 81, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Huang, X.Y.; Liu, Z.G.; Gong, C.; Li, X.Y.; Li, D.H.; Hua, H.M.; Li, Z.L. Four new compounds from the roots of Euphorbia ebracteolata and their inhibitory effect on LPS-induced NO production. Fitoterapia 2018, 125, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; Mo, L.Y.; Ren, Z.P.; Fan, X.N.; Sun, P.H.; Tian, H.Y.; Yang, N.; Zi, J.C. New abietane and tigliane diterpenoids from the roots of Euphorbia fischeriana and their cytotoxic activities. J. Asian Nat. Prod. Res. 2023, 25, 519–527. [Google Scholar] [CrossRef]
- Zhu, Q.F.; Xu, G.B.; Liao, S.G.; Yan, X.L. Ent-Abietane diterpenoids from Euphorbia fischeriana and their cytotoxic activities. Molecules 2022, 27, 7258. [Google Scholar] [CrossRef]
- Sun, C.P.; Chang, Y.B.; Wang, C.; Lv, X.; Zhou, W.Y.; Tian, X.G.; Zhao, W.Y.; Ma, X.C. Bisfischoids A and B, dimeric ent-abietane-type diterpenoids with anti-inflammatory potential from Euphorbia fischeriana Steud. Bioorg. Chem. 2021, 116, 105356. [Google Scholar] [CrossRef]
- Xie, R.; Xia, G.; Zhu, J.; Lin, P.; Fan, X.; Zi, J. Daphnane-type diterpenoids from Euphorbia fischeriana Steud and their cytotoxic activities. Fitoterapia 2021, 149, 104810. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.L.; Zhang, J.S.; Huang, J.L.; Zhang, Y.; Chen, J.Q.; Tang, G.H.; Yin, S. Euphonoids A-G, cytotoxic diterpenoids from Euphorbia fischeriana. Phytochemistry 2019, 166, 112064. [Google Scholar] [CrossRef]
- Li, M.; He, F.; Zhou, Y.; Wang, M.; Tao, P.; Tu, Q.; Lv, G.; Chen, X. Three new ent-abietane diterpenoids from the roots of Euphorbia fischeriana and their cytotoxicity in human tumor cell lines. Arch. Pharm. Res. 2019, 42, 512–518. [Google Scholar] [CrossRef]
- Lan, Y.H.; Chen, I.H.; Lu, H.H.; Guo, T.J.; Hwang, T.L.; Leu, Y.L. Euphormins A and B, new pyranocoumarin derivatives from Euphorbia formosana Hayata, and their anti-inflammatory activity. Molecules 2022, 27, 1885. [Google Scholar] [CrossRef] [PubMed]
- Yazdiniapour, Z.; Sohrabi, M.H.; Motinia, N.; Zolfaghari, B.; Mehdifar, P.; Ghanadian, M.; Lanzotti, V. Diterpenoids from Euphorbia gedrosiaca as potential anti-proliferative agents against breast cancer cells. Metabolites 2023, 13, 225. [Google Scholar] [CrossRef]
- Hasan, A.; Liu, G.Y.; Hu, R.; Aisa, H.A. Jatrophane Diterpenoids from Euphorbia glomerulans. J. Nat. Prod. 2019, 82, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Kemboi, D.; Peter, X.; Langat, M.K.; Mhlanga, R.; Vukea, N.; de la Mare, J.A.; Noundou, S.X.; Krause, R.W.M.; Tembu, V.J. In vitro cytotoxic effects of chemical constituents of Euphorbia grandicornis Blanc against breast cancer cells. Sci. Afr. 2021, 14, 90. [Google Scholar] [CrossRef]
- Tsai, J.Y.; Rédei, D.; Hohmann, J.; Wu, C.C. 12-Deoxyphorbol esters induce growth arrest and apoptosis in human lung cancer A549 cells via activation of PKC-δ/PKD/ERK signalling pathway. Int. J. Mol. Sci. 2020, 21, 7579. [Google Scholar] [CrossRef]
- Radi, M.H.; El-Shiekh, R.A.; El-Halawany, A.M.; Al-Abd, A.M.; Abdel-Sattar, E. In vitro cytotoxic study of Euphorbia grantii Oliv. aerial parts against MCF-7 and MCF-7ADR breast cancer cell lines: A bioactivity-guided isolation. ACS Omega 2023, 8, 18299–18305. [Google Scholar] [CrossRef]
- Yang, H.Y.; Yao, W.; Huang, P.Z.; Xu, H.; Ma, Q.; Chen, X.; Chen, J.J.; Gao, K. Euphohelides A-C, ent-abietane-type norditerpene lactones from Euphorbia helioscopia and their anti-inflammatory activities. J. Nat. Prod. 2023, 86, 1003–1009. [Google Scholar] [CrossRef]
- Lu, Y.B.; Luo, S.; Wang, Y.X.; Feng, Z.Y.; Gao, K.; Chen, J.J. Jatrophane diterpenoids with cytotoxic activity from the whole plant of Euphorbia helioscopia L. Phytochemistry 2022, 203, 113420. [Google Scholar] [CrossRef]
- Wang, W.P.; Jiang, K.; Zhang, P.; Shen, K.K.; Qu, S.J.; Yu, X.P.; Tan, C.H. Highly oxygenated and structurally diverse diterpenoids from Euphorbia helioscopia. Phytochemistry 2018, 145, 93–102. [Google Scholar] [CrossRef]
- Hu, R.; Sang, J.; Li, W.; Tian, Y.; Zou, M.F.; Tang, G.H.; Yin, S. Structurally diverse triterpenoids with cytotoxicity from Euphorbia hypericifolia. Fitoterapia 2021, 151, 104888. [Google Scholar] [CrossRef]
- Xue, L.Y.; Chen, B.L.; Yuan, F.Y.; Zhu, Q.F.; Zhang, X.; Lin, Y.; Long, Q.D.; Liao, S.G. Six new tigliane diterpenoids with anti-inflammatory activity from Euphorbia kansuensis. Arab. J. Chem. 2022, 85, 103807. [Google Scholar] [CrossRef]
- Yan, X.L.; Sang, J.; Zhang, X.; Lin, Y.; Long, Q.D.; Zhu, Q.F.; Liao, S.G. Euphorboside A, a cytotoxic meroterpenoid glycoside with an unusual humulene-phloroglucinol skeleton from Euphorbia kansuensis. Fitoterapia 2021, 153, 104966. [Google Scholar] [CrossRef]
- Feng, X.; Li, J.; Li, H.; Chen, X.; Liu, D.; Li, R. Bioactive C21 steroidal glycosides from Euphorbia kansui promoted HepG2 cell apoptosis via the degradation of ATP1A1 and inhibited macrophage polarization under co-cultivation. Molecules 2023, 28, 2830. [Google Scholar] [CrossRef]
- Li, J.C.; Li, S.Y.; Tang, J.X.; Liu, D.; Feng, X.Y.; Rao, K.R.; Zhao, X.D.; Li, H.M.; Li, R.T. Triterpenoids, steroids and other constituents from Euphorbia kansui and their anti-inflammatory and anti-tumour properties. Phytochemistry 2022, 204, 113449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Weng, H.Z.; Huang, J.L.; Tang, G.H.; Yin, S. Anti-inflammatory ingenane diterpenoids from the roots of Euphorbia kansui. Planta Med. 2018, 84, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Riahi, F.; Dashti, N.; Ghanadian, M.; Aghaei, M.; Faez, F.; Jafari, S.M.; Zargar, N. Kopetdaghinanes, pro-apoptotic hemiacetialic cyclomyrsinanes from Euphorbia kopetdaghi. Fitoterapia 2020, 146, 104636. [Google Scholar] [CrossRef]
- Wongprayoon, P.; Leelasart, S.; Jantham, J.; Pootaeng-on, Y.; Oekchuae, S.; Limpachayaporn, P.; Rayanil, K.; Charoensuksai, P. A triterpenoid friedelan-3β-ol isolated from Euphorbia lactea exhibited cytotoxic activity against HN22 cells by inducing an S-phase cell cycle arrest. J. Appl. Pharm. Sci. 2022, 12, 031–048. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Xiong, L.; Sun, D.; Wang, H.; Song, Z.; Li, Y.; Li, H.; Chen, L. Synthesis of Lathyrol PROTACs and evaluation of their anti-inflammatory activities. J. Nat. Prod. 2023, 86, 767–781. [Google Scholar] [CrossRef]
- Jiang, D.; Gao, X.; Tan, R.; Liu, X.; Zhu, Y.; Zhang, L. Euphorbia factor L1 suppresses breast cancer liver metastasis via DDR1-mediated immune infiltration. Aging 2023, 15, 9217–9229. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, S.; Geng, Y.; Fan, H.; Zhang, R.; Zhang, Y.; Pan, J.; Song, G.; Ge, L.; Xie, T.; et al. Euphorbia factor L3 ameliorates rheumatoid arthritis by suppressing the inflammatory response by targeting Rac family small GTPase 1. Bioengineered 2022, 13, 10984–10997. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Z.; Guo, Y.; Xie, H.; Zhang, Z.; Sun, D.; Li, H.; Chen, L. Diterpenoids from the seeds of Euphorbia lathyris and their anti-inflammatory activity. Bioorg. Chem. 2021, 112, 104944. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Mu, H.Y.; Gong, Q.; Ding, X.; Wang, W.; Zhang, H.Y.; Zhao, W.M. Diterpenoids from the seeds of Euphorbia lathyris and their effects on microglial nitric oxide production. Fitoterapia 2021, 150, 104834. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Y.; Li, C.; Yang, Y.; Li, X.; Li, H.; Chen, L. Synthesis of new Lathyrane diterpenoid derivatives from Euphorbia lathyris and evaluation of their anti-inflammatory activities. Chem. Biodivers. 2020, 17, e1900531. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Wang, Q.; Zhen, Y.Q.; Zhao, S.M.; Gao, F.; Zhou, X.L. Cytotoxic Lathyrane-type diterpenes from seeds of Euphorbia lathyris. Chem. Pharm. Bull. 2018, 66, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.N.; Wang, Y.; Hsu, P.L.; Xin, G.; Zhang, Y.; Morris-Natschke, S.L.; Goto, M.; Lee, K.H. Mechanism of action of cytotoxic compounds from the seeds of Euphorbia lathyris. Phytomedicine 2018, 41, 62–66. [Google Scholar] [CrossRef]
- Wang, Q.; Zhen, Y.Q.; Gao, F.; Huang, S.; Zhou, X. Five new diterpenoids from the seeds of Euphorbia lathyris. Chem. Biodivers. 2018, 15, e1800386. [Google Scholar] [CrossRef]
- Lee, J.W.; Jin, Q.; Jang, H.; Kim, J.G.; Lee, D.; Kim, Y.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Lathyrane-type diterpenoids from the seeds of Euphorbia lathyris L. with inhibitory effects on NO production in RAW 264.7 cells. Chem. Biodivers. 2018, 15, e1800144. [Google Scholar] [CrossRef]
- Xia, R.F.; Su, J.C.; Yu, J.; Zha, H.J.; Wu, J.L.; Fu, X.N.; Cai, Q.; Wan, L.S. Anti-inflammatory lanostane triterpenoids with rearranged spirobi[indene] scaffold and their biogenetically related analogues from Euphorbia maculata. Phytochemistry 2023, 211, 113682. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, L.L.; Tang, M.Y.; Feng, B.M.; Pei, Y.H.; Yasukawa, K. Triterpenoids from Euphorbia maculata and their anti-inflammatory effects. Molecules 2018, 23, 2112. [Google Scholar] [CrossRef]
- Azizi, K.; Hamedi, A.; Azarpira, N.; Hamedi, A.; Shahini, M.; Pasdaran, A. A new cytotoxic sesquiterpene lactone from Euphorbia microsphaera Boiss against human breast cancer (MCF-7) and human fibrosarcoma (HT1080) cells. Toxicon 2021, 202, 60–66. [Google Scholar] [CrossRef]
- Chang, S.S.; Huang, H.T.; Wei, W.C.; Lo, I.W.; Lin, Y.C.; Chao, C.; Liao, G.Y.; Shen, Y.C.; Chen, J.J.; Li, T.L.; et al. Anti-inflammatory effect of euphane- and tirucallane-type triterpenes isolated from the traditional herb Euphorbia neriifolia L. Front. Chem. 2023, 11, 1223335. [Google Scholar] [CrossRef]
- Chang, S.S.; Huang, H.T.; Lin, Y.C.; Chao, C.H.; Liao, G.Y.; Lin, Z.H.; Huang, H.C.; Chun-Ling Kuo, J.; Liaw, C.C.; Tai, C.J.; et al. Neritriterpenols A-G, euphane and tirucallane triterpenes from Euphorbia neriifolia L. and their bioactivity. Phytochemistry 2022, 199, 113199. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Jun-Su, Z.; Hong-Chun, L.; Yan, Z.; Wei-Hang, Y.; Qun-Fang, L.; Guan-Wu, W.; Jin-Xin, Z.; Jian-Min, Y. Phonerilins A–K, cytotoxic ingenane and ingol diterpenoids from Euphorbia neriifolia. Tetrahedron 2022, 123, 132955. [Google Scholar] [CrossRef]
- Li, J.C.; Feng, X.Y.; Liu, D.; Zhang, Z.J.; Chen, X.Q.; Li, R.T.; Li, H.M. Diterpenoids from Euphorbia neriifolia and their related anti-HIV and cytotoxic activity. Chem. Biodivers. 2019, 16, e1900495. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.J.; Kincses, A.; Gajdács, M.; Spengler, G.; Dos Santos, D.J.V.A.; Molnár, J.; Ferreira, M.U. Terpenoids from Euphorbia pedroi as multidrug-resistance reversers. J. Nat. Prod. 2018, 81, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Zeng, X.T.; Xu, D.Q.; Yue, S.J.; Fu, R.J.; Yang, X.; Liu, Z.X.; Tang, Y.P. Pimarane, abietane, and labdane diterpenoids from Euphorbia pekinensis Rupr. and their anti-tumor activities. Phytochemistry 2022, 197, 113113. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Yang, Y.; Sun, M.; Lu, Q.Y.; Pu, X.X.; Ran, X.; Li, D.M.; Wan, J.J.; Huang, J.Y.; Guan, S.P.; et al. Jatrophane polyesters from the leaves of Euphorbia peplus with anti-inflammatory activity. Phytochem. Lett. 2022, 49, 114–119. [Google Scholar] [CrossRef]
- Aljohani, A.S.M.; Alhumaydhi, F.A.; Rauf, A.; Hamad, E.M.; Rashid, U. In Vivo anti-inflammatory, analgesic, sedative, muscle relaxant activities and molecular docking analysis of phytochemicals from Euphorbia pulcherrima. Evid-Based Complement. Altern. Med. 2022, 2022, 7495867. [Google Scholar] [CrossRef]
- Li, M.M.; Qi, Y.R.; Feng, Y.P.; Liu, W.; Yuan, T. Euphatexols C - G, five new triterpenoids from the latex of Euphorbia resinifera. J. Asian Nat. Prod. Res. 2022, 24, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Fantoukh, O.I.; Al-Hamoud, G.A.; Nasr, F.A.; Almarfadi, O.M.; Hawwal, M.F.; Ali, Z.; Alobaid, W.A.; Binawad, A.; Alrashidi, M.; Alasmari, F.; et al. Revisiting the flora of Saudi Arabia: Phytochemical and biological investigation of the endangered plant species Euphorbia saudiarabica. Metabolites 2023, 13, 556. [Google Scholar] [CrossRef] [PubMed]
- Salha, M.A.; Khaled, M.; Samir, A.M.; Abdulrahman, I.A.; Kamel, H.S. Bioactive compounds from Euphorbia schimperiana with cytotoxic and antibacterial activities. S. Afr. J. Bot. 2021, 141, 357–366. [Google Scholar] [CrossRef]
- Yang, H.; Mamatjan, A.; Tang, D.; Aisa, H.A. Jatrophane diterpenoids as multidrug resistance modulators from Euphorbia sororia. Bioorg. Chem. 2021, 112, 104989. [Google Scholar] [CrossRef]
- Zhu, H.; Ren, X.; Huang, Y.; Su, T.; Yang, L. Chemical constituents of Euphorbia stracheyi Boiss (Euphorbiaceae). Metabolites 2023, 13, 852. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Yu, M.; Liao, H.B.; Liu, T.; Tan, Y.H.; Liang, D.; Zhang, G.J. Sesquiterpenes and diterpenes from Euphorbia thymifolia. Fitoterapia 2019, 139, 104408. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.H.; Beniddir, M.A.; Genta-Jouve, G.; Nguyen, H.H.; Nguyen, D.P.; Nguyen, T.A.; Mac, D.H.; Boustie, J.; Nguyen, K.P.; Chavasiri, W.; et al. Further terpenoids from Euphorbia tirucalli. Fitoterapia 2019, 135, 44–51, Erratum in Fitoterapia 2021, 149, 104825. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.A.O.; Rosa, M.N.; Miranda-Gonçalves, V.; Costa, A.M.; Tansini, A.; Evangelista, A.F.; Martinho, O.; Carloni, A.C.; Jones, C.; Lima, J.P.; et al. Euphol, a tetracyclic triterpene, from Euphorbia tirucalli induces autophagy and sensitizes temozolomide cytotoxicity on glioblastoma cells. Investig. New Drug 2019, 37, 223–237. [Google Scholar] [CrossRef]
- Silva, V.A.O.; Rosa, M.N.; Tansini, A.; Oliveira, R.J.S.; Martinho, O.; Lima, J.; Pianowski, L.F.; Reis, R.M. In vitro screening of cytotoxic activity of euphol from Euphorbia tirucalli on a large panel of human cancer-derived cell lines. Exp. Ther. Med. 2018, 16, 557–566. [Google Scholar] [CrossRef]
- Cruz, L.S.; de Oliveira, T.L.; Kanunfre, C.C.; Paludo, K.S.; Minozzo, B.R.; Prestes, A.P.; Wang, M.; Fernandes, D.; Santos, F.A.D.; Manda, V.K.; et al. Pharmacokinetics and cytotoxic study of euphol from Euphorbia umbellata (Bruyns) Pax latex. Phytomedicine 2018, 47, 105–112. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, D.; Jiang, Q.; Xiong, L.; Zhang, N.; Pan, Y.; Li, H.; Chen, L. Diterpenoids with anti-inflammatory activity from Euphorbia wallichii. Phytochemistry 2023, 205, 113486. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Q.; Sun, D.; Zhang, N.; Lin, Y.; Li, H.; Chen, L. Ent-kauranes and ent-atisanes from Euphorbia wallichii and their anti-inflammatory activity. Phytochemistry 2023, 210, 113643. [Google Scholar] [CrossRef]
- WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 9 October 2023).
- Benjamaa, R.; Moujanni, A.; Kaushik, N.; Choi, E.H.; Essamadi, A.K.; Kaushik, N.K. Euphorbia species latex: A comprehensive review on phytochemistry and biological activities. Front. Plant Sci. 2022, 13, 1008881. [Google Scholar] [CrossRef]
- Vasas, A.; Hohmann, J. Euphorbia Diterpenes: Isolation, Structure, Biological Activity, and Synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef]
- Appendino, G. Ingenane Diterpenoids. Prog. Chem. Org. Nat. Prod. 2016, 102, 1–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, R.Z.; Sang, J.; Tian, Y.J.; Chen, J.Q.; Tang, G.H.; Yin, S. Ingol diterpenoids as P-glycoprotein-dependent multidrug resistance (MDR) reversal agents from Euphorbia marginata. Bioorg. Chem. 2020, 95, 103546. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, C.; Che, Q.; Zhu, T.; Zhang, G.; Li, D. Extending the Structural Diversity of Labdane Diterpenoids from Marine-Derived Fungus Talaromyces sp. HDN151403 Using Heterologous Expression. Mar. Drugs 2023, 21, 628. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Yokoshima, S. Convergent synthesis of the [5-7-6-3] tetracyclic core of premyrsinane diterpenes. Org. Biomol. Chem. 2023, 4, 724–727. [Google Scholar] [CrossRef]
- Vela, F.; Ezzanad, A.; Hunter, A.C.; Macías-Sánchez, A.J.; Hernández-Galán, R. Pharmacological Potential of Lathyrane-Type Diterpenoids from Phytochemical Sources. Pharmaceuticals 2022, 15, 780. [Google Scholar] [CrossRef]
- Iwata, M.; Inoue, T.; Asai, Y.; Hori, K.; Fujiwara, M.; Matsuo, S.; Tsuchida, W.; Suzuki, S. The protective role of localized nitric oxide production during inflammation may be mediated by the heme oxygenase-1/carbon monoxide pathway. Biochem. Biophys. Rep. 2020, 23, 100790. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Tang, S.; Zhang, C.; Chen, H.; Huang, W.; Liu, Y.; Zhang, J. Euphorbia factor L2 induces apoptosis in A549 cells through the mitochondrial pathway. Acta Pharm. Sin. B 2017, 7, 59–64. [Google Scholar] [CrossRef] [PubMed]






| Species | Collection Place | Plant Material | Extract Solvent |
|---|---|---|---|
| E. alatavica [16] | China | Stems | Acetone |
| E. antiquorum [17,18] | Thailand | Aerials parts | Methanol |
| China | Stems | Methanol | |
| E. atoto [19] | China | Aerial parts | Ethanol |
| E. balsamifera [20] | Saudi Arabia | Aerial parts | Ethanol |
| E. dendroides [21] | Egypt | Aerial parts | Methanol |
| E. denticulata [22] | Iran | Whole plant | Acetone |
| E. ebracteolata [23,24,25,26,27] | China | Roots | Ethanol |
| Korea | Methanol | ||
| China | Ethanol | ||
| E. fischeriana [28,29,30,31,32,33] | Mongolia | Roots | Ethanol |
| China | Acetone | ||
| E. formosana [34] | Taiwan | Roots | Methanol |
| E. gedrosiaca [35] | Iran | Aerial parts | Dichloromethane: Acetone |
| E. glomerulans [36] | China | Whole plant | Acetone |
| E. grandicornis [37,38] | South Africa | Aerial parts and roots | Dichloromethane |
| Hungary | Aerial parts | Methanol | |
| E. grantii [39] | Egypt | Aerial parts | Methanol |
| E. helioscopia [40,41,42] | China | Whole plant | Ethanol |
| Methanol | |||
| Aerials parts | Ethanol | ||
| E. hypericifolia [43] | China | Aerial parts | Ethanol |
| E. kansuensis [44,45] | China | Roots | Ethanol |
| E. kansui [46,47,48] | China | Roots | Ethanol |
| E. kopetdaghi [49] | Iran | Aerial parts | Dichloromethane: Acetone 2:1 |
| E. láctea [50] | Thailand | Aerial parts | Ethanol |
| E. lathyris [51,52,53,54,55,56,57,58,59,60] | China | Seeds | Ethanol |
| Petroleum Ether | |||
| Ethanol | |||
| Ethanol | |||
| Petroleum Ether | |||
| Ethanol | |||
| Ethanol | |||
| Ethanol | |||
| South Korea | Seeds | Methanol | |
| E. maculata [61,62] | China | Whole plant | Ethanol |
| Japan | Whole plant | Methanol | |
| E. microsphaera [63] | Iran | Aerial parts | Chloroform |
| E. neriifolia [64,65,66,67] | Taiwan | Stems | Ethanol |
| China | Aerial parts | Ethanol | |
| Whole plant | Acetone: Water 3:1 | ||
| E. pedroi [68] | Portugal | Aerial parts | Methanol |
| E. pekinensis [69] | China | Roots | Ethanol |
| E. peplus [70] | China | Leaves | Methanol |
| E. pulcherrima [71] | Pakistan | Whole plant | Methanol |
| E. resinifera [72] | China | Latex | Methanol |
| E. saudiarabica [73] | Saudi Arabia | Aerial parts | Methanol |
| E. schimperiana [74] | Saudi Arabia | Aerial parts | Ethanol |
| E. sororia [75] | China | Fructus | Ethanol |
| E. stracheyi [76] | China | Whole plant | Methanol |
| E. thymifolia [77] | China | Aerial parts | Ethanol |
| E. tirucalli [78,79,80] | Vietnam | Whole plant | Ethanol |
| Brazil | Sap | Hexane | |
| E. umbellata [81] | Brazil | Latex | H2SO4 1% |
| E. wallichii [82,83] | China | Whole plant | Methanol |
| Species | Active Compounds | Biological Model | Results | Ref. |
|---|---|---|---|---|
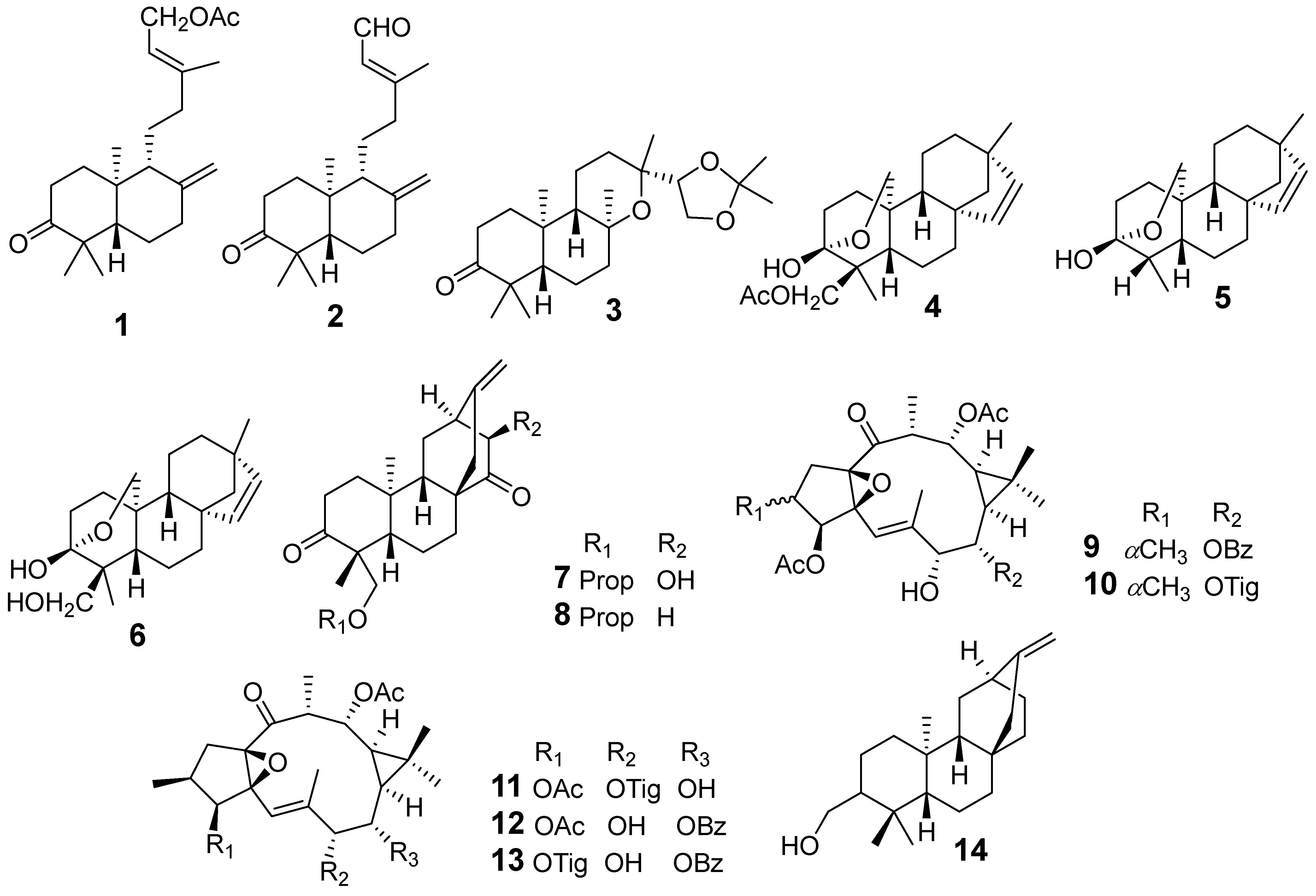
| E. antiquorum | Ent-15-Acetoxylabda-8(17),13E-diene-3-one (1) | Griess assay J774.A1 cells stimulated LPS NO | IC50 (μM)11.7 | [17] |
| Ent-15-Oxolabda-8(17),13E-diene-3-one (2) | 12.5 | |||
| Ent-13-epi-8,13-epoxy-14α,15-isopropylidenedioxylabdane-3-one (3) | 44.6 | |||
| Ent-3β,20-Epoxy-3α-hydroxy-15-beyeren-18-acetate (4) | 36.6 | |||
| Ent-3β,20-epoxy-3α-hydroxy-18-norbeyer-15-ene (5) | 40.4 | |||
| Rhizophorin B (6) | 16.1 | |||
| Ent-15-Acetoxylabda-8(17),13E-diene-3-one (1) | Western blot iNOS | IC50 (μM) 11.7 | ||
| Ent-15-Oxolabda-8(17),13E-diene-3-one (2) | 12.5 | |||
| Euphorin A (7) | Griess assay BV-2 cells stimulated LPS NO | IC50 (µM) 35.8 | [18] | |
| Euphorin B (8) | 41.4 | |||
| Euphorin D (9) | 32.0 | |||
| Euphorin E (10) | 40.7 | |||
| 3,12-O-diacetyl-7-O-[(E)-2-methyl-2-butenoyl]-8,12-diepjing-ol (11) | 49.2 | |||
| 3,12-diacetyl-8-benzoylingol (12) | 14.5 | |||
| 12-O-acetyl-8-O-benzoylingol-3-tiglate (13) | 14.9 | |||
| Ent-(3α,5β,8α,9β,10α,12α)-3-hydroxyatis-16-en-14-one (14) | 31.6 | |||
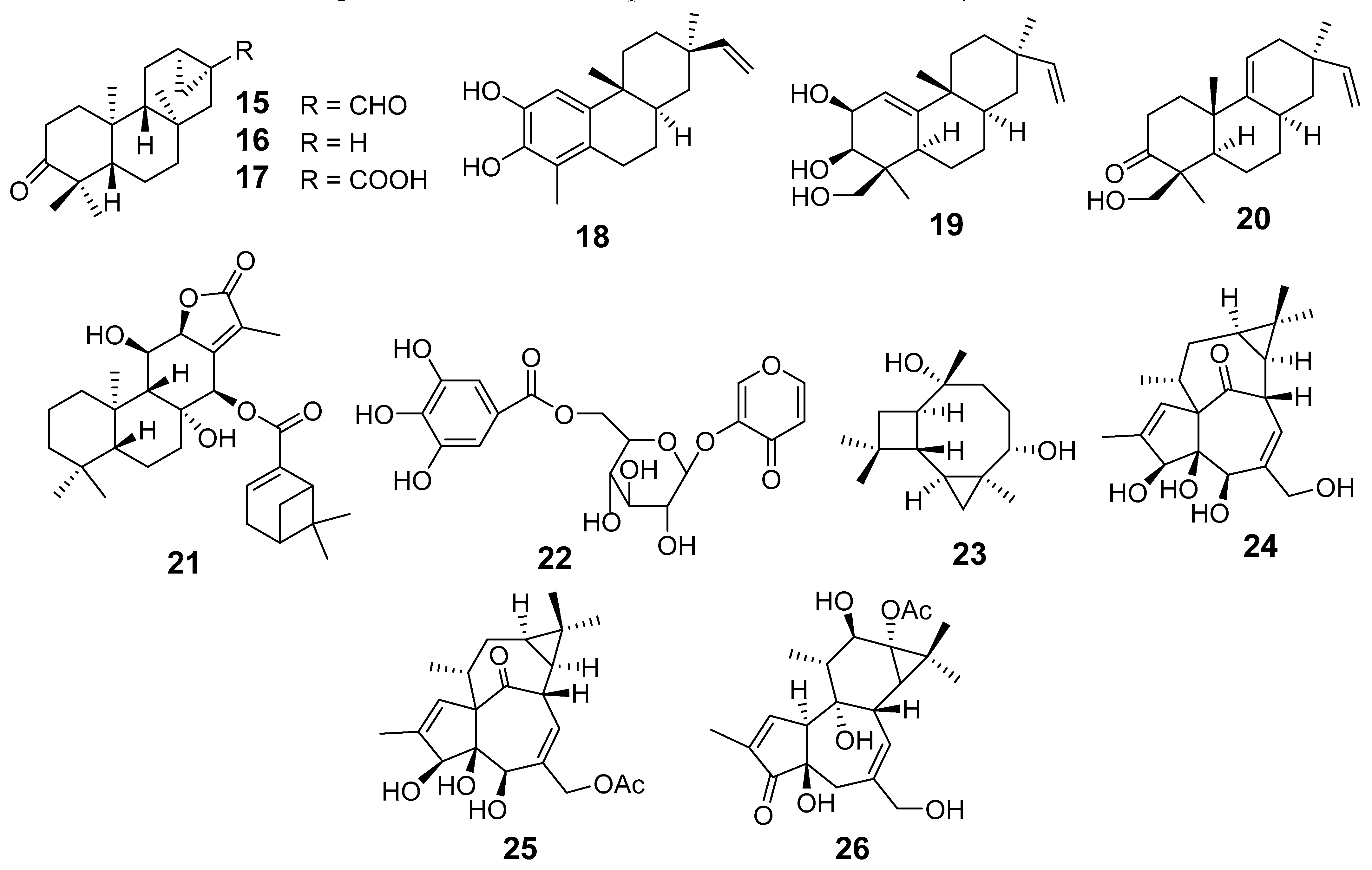
| E. atoto | 3-oxo-ent-trachyloban-17-oic acid (15) | Griess assay RAW264.7 cells stimulated LPS NO | IC50 (µM) 41.61 | [19] |
| Ent-kauran-16β-ol-3-one (16) | 16.00 | |||
| Ent-16-hydroxy-3-oxosanguinane (17) | 33.41 | |||
| E. ebracteolata | Ebractenoid F (18) | Griess assay RAW264.7 cells stimulated LPS NO | IC50 (µg/mL) 2.39 | [24] |
| SEAP Assay NF-kB | Decreased NF-kB. Inhibited the phosphorylation of Akt and mitogen-activated protein kinases (MAPKs) | |||
| Western blot | Inhibited levels of IL-6 and IL1 | |||
| Ebractenoid O (19) | Griess assay RAW264.7 cells stimulated LPS NO | IC50 (µM) 6.04 | [27] | |
| Ebractenoid P (20) | 10.23 | |||
| Ebractenoid Q (21) | 1.97 | |||
| γ-pyrone-3-O-β-d-(6-galloyl)-glucopyranoside (22) | 42.49 | |||
| Tricyclohumuladiol (23) | 13.21 | |||
| Ingenol (24) | 6.25 | |||
| Ingenol-20-acetate (25) | 6.73 | |||
| Langduin A4 (26) | 18.50 | |||
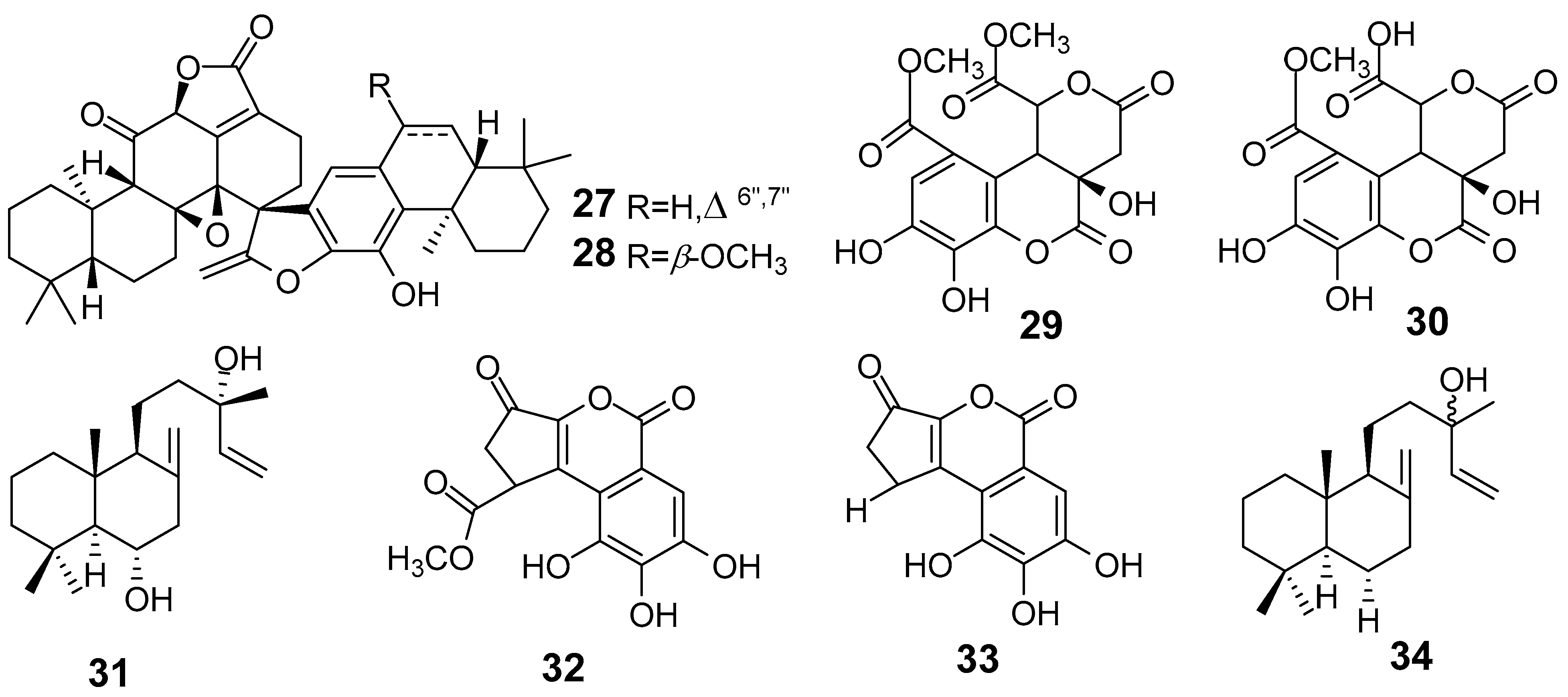
| E. fischeriana | Bisfischoid A (27) | Assay Inhibition of sEH | IC50 (µΜ) 9.90 | [30] |
| Bisfischoid B (28) | 10.29 | |||
| E. formosana | Euphormin A (29) | Superoxide Anion In human neutrophils stimulated with formyl-L methionyl-l-leucyl-l-phenylalanine/cytochalasin B | IC50 (µM) 4.51 | [34] |
| Euphormin B (30) | 3.68 | |||
| Larixol (31) | 3.81 | |||
| Methylbrevifolincarboxylate (32) | 0.68 | |||
| Brevifolin (33) | 1.39 | |||
| Euphormins A (29) | Elastase Release In human neutrophils stimulated with formyl-L methionyl-l-leucyl-l-phenylalanine/cytochalasin B | IC50 (µM) >10 | ||
| Euphormins B (30) | >10 | |||
| Larixol (31) | >10 | |||
| Methylbrevifolincarboxylate (32) | >10 | |||
| Brevifolin (33) | >10 | |||
| epi-manool (34) | 8.07 | |||
| E. helioscopia | Euphohelide A (35) | Griess assay RAW264.7 cells stimulated LPS NO | IC50 (µM) 32.98 | [40] |
| Helioscopinolide C (36) | 33.82 | |||
| E. kansuensis | Euphkanoid A (37) | Griess assay RAW264.7 cells stimulated LPS NO | IC50 (µM) 9.41 | [44] |
| Euphkanoid B (38) | 11.3 | |||
| Euphkanoid C (39) | 5.92 | |||
| Euphkanoid D (40) | 24.5 | |||
| Euphkanoid E (41) | 35.3 | |||
| Euphkanoid F (42) | 4.8 | |||
| Prostratin (43) | 45.9 | |||
| Phorbol-13-acetate (44) | 44.8 | |||
| 12-deoxyphorbol-13,20-diacetate (45) | 37.9 | |||
| Phorbol (46) | 47.0 | |||
| 12-deoxyphorbol (47) | 35.7 | |||
| 12-deoxyphorbol-13-hexadecanoate (48) | 24.3 | |||
| Helioscopinolide A (49) | 23.5 | |||
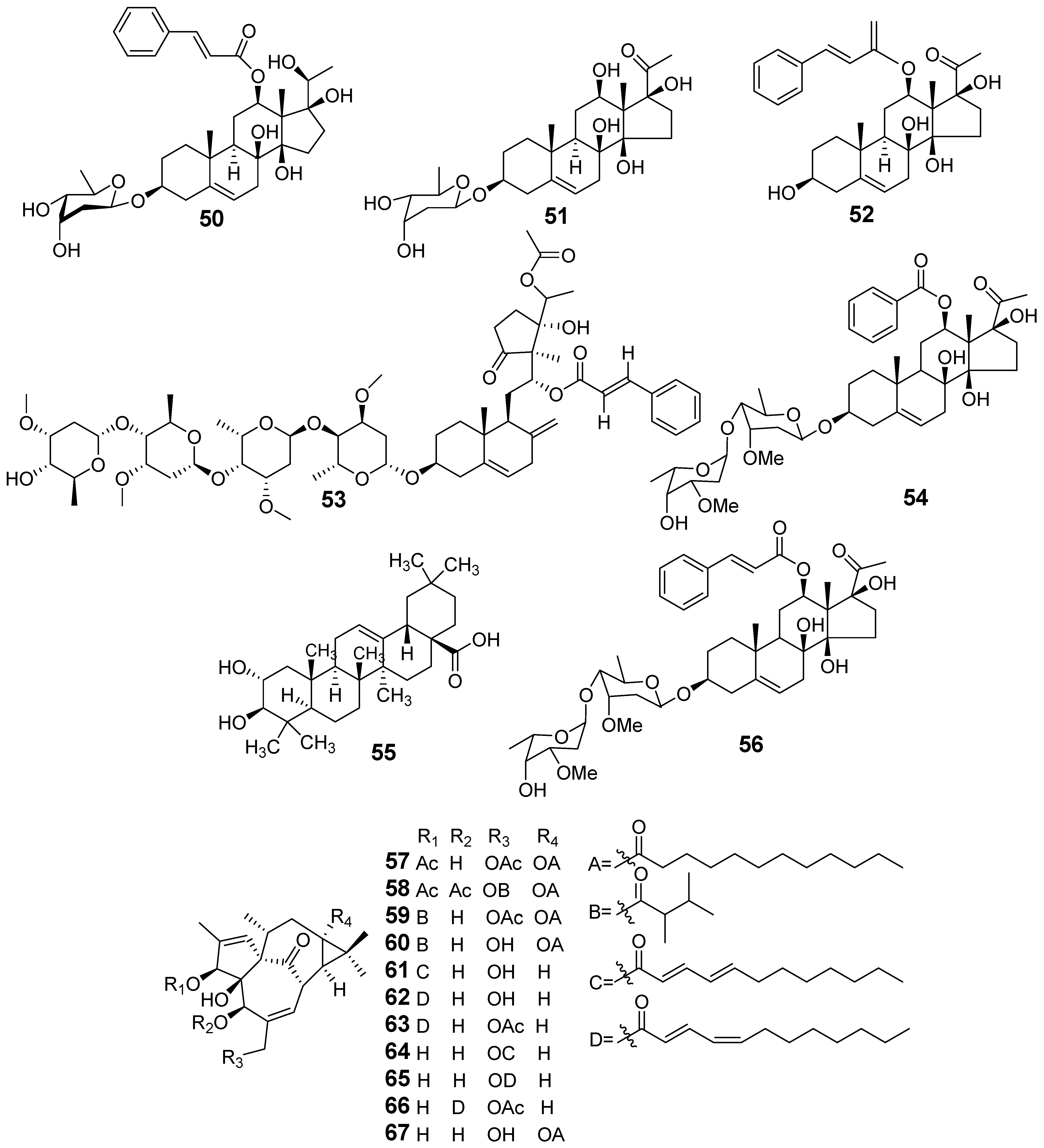
| E. kansui | Cynsaccatol L (50) | Na+-K+-ATPase Analysis | Induced inactivation of AKT and ERK due to the downregulation of ATP1A1 expression | [46,47] |
| Cynotophylloside B (51) | Western blot | Inhibited the phosphorylation of AKT and mTOR, as well as upregulating the expression of LC3-Band p62 | ||
| Cynsaccatol L (50) | Griess assay RAW264.7 cells stimulated LPS NO | IC50 (µM) 0.02 | ||
| Cynotophylloside B (51) | 9.10 | |||
| Kidjolanin (52) | 30.7 | |||
| Wilfoside G (53) | 1.77 | |||
| Cynotophylloside J (54) | 17.39 | |||
| Maslinic acid (55) | 17.38 | |||
| Kidjoranin 3-O-α-diginopyranosyl-(1→4)-β- Cymaropyranoside (56) | 2.79 | |||
| Euphorkan A (57) | Griess assay RAW264.7 cells stimulated LPS NO | IC50 (µM) 4.90 | [48] | |
| Euphorkan B (58) | 10.4 | |||
| 3-O-(2,3-dimethylbutanoyl)-13-O-dodecanoyl-20-O-acetylingenol (59) | 5.69 | |||
| 3-O-(2,3-dimethylbutyryl)-13-O-n-dodecanoyl-13-hydroxyingenol (60) | 5.80 | |||
| 3-O-(2′E,4′E-decadienoyl) ingenol (61) | 2.78 | |||
| 3-O-(2′E,4′Z-decadienoyl) ingenol (62) | 10.6 | |||
| 3-O-(2′E,4′Z-decadienoyl)-20-O-acetylingenol (63) | 2.86 | |||
| 20-O-(2′E,4′E-decadienoyl) ingenol (64) | 9.05 | |||
| 20-O-(2′E,4′Z-decadienoyl) ingenol (65) | 9.45 | |||
| 20-O-acetyl-[5-O-(2′E,4′Z)-decadienoyl]-ingenol (66) | 4.60 | |||
| 13-O-docecanoylingenol (67) | 8.86 | |||
| Euphorkan A (57) | Luciferase assay NF-κB | IC50 (µM) 11.0 | ||
| 3-O-(2′E,4′E-decadienoyl) ingenol (61) | 17.9 | |||
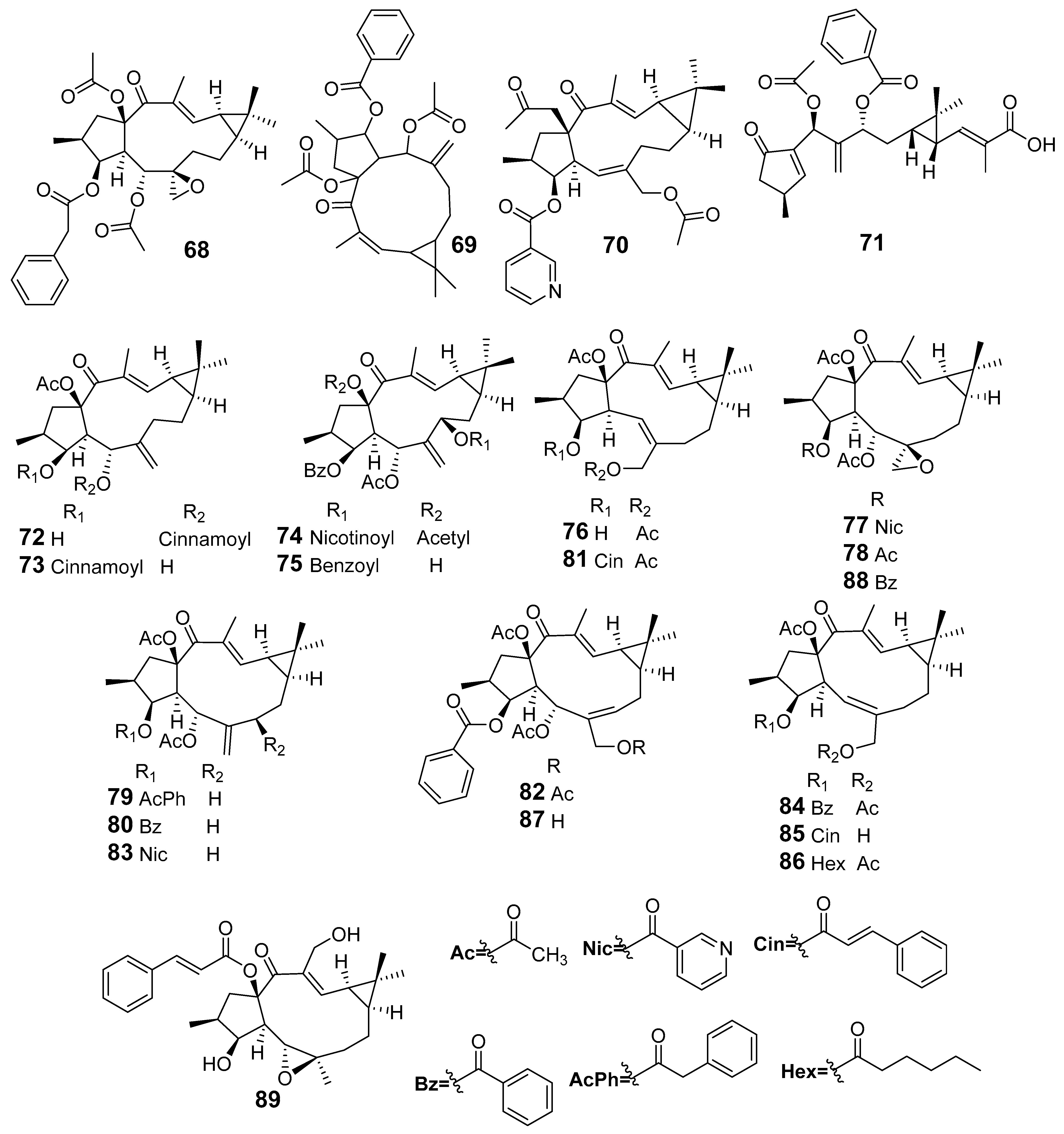
| E. lathyris | Euphorbia Factor L1 (68) | Cytokines were determined using ELISA | SHI-induced inflammatory cell infiltration and IL-1β, IL-6, TNF-α were decreased | [52] |
| Western blot | Treatment with EFL1 downregulated DDR1 protein expression and immuno-reactivity in SHI mice, leading to the surge of CD4+, CD8+, and CD49b+ (NK) T cells | |||
| Euphorbia Factor L3 (69) | Fibroblast-like synoviocytes (FLSs) | Ameliorated inflammatory phenotype FLSs (decreased viability, migration, invasion, and cytokine production) | [53] | |
| Collagen-induced arthritis (CIA) | Inhibited arthritic progression | |||
| Wester blotting and immunofluorescence | Inhibited nuclear translocation of the p65 | |||
| Molecular analysis | Target of EFL3 is RACI | |||
| Euplarisan A (70) | Griess assay RAW264.7 cells stimulated LPS NO | IC50 (μM) 7.50 | [54] | |
| Enzyme-linked immunoassay (ELISA) | Inhibited IL-1β, IL-6, and TNF-α | |||
| Western blot assay | Decreased the expression of iNOS, COX-2, and p-IκBα | |||
| Lathyranoic acid A (71) | Griess assay BV-2 cells stimulated LPS NO | % Inhibitory 74.51 | [55] | |
| Euphorbia Factor L3 (69) | 61.85 | |||
| Euphorbia Factor L31 (72) | 50.46 | |||
| Euphorbia Factor L30 (73) | 50.01 | |||
| Euphorbia Factor L9 (74) | 63.68 | |||
| Euphorbia Factor L11 (75) | 76.66 | |||
| Euphorbia Factor L3 (69) | Griess assay RAW264.7 cells stimulated LPS NO | IC50 (μM) 11.24 | [56] | |
| Euphorbia Factor L29 (76) | Griess assay RAW264.7 cells stimulated LPS NO | IC50 (µM) 47.9 | [60] | |
| Euphordracunculin C (77) | 12.7 | |||
| Epoxyboetirane A (78) | 26.2 | |||
| Euphorbia Factor L1 (68) | 12.7 | |||
| Deoxy Euphorbia Factor L1 (79) | 47.0 | |||
| Euphorbia Factor L2 (80) | 16.2 | |||
| Euphorbia Factor L3 (69) | 15.0 | |||
| Euphorbia Factor L7a (81) | 44.4 | |||
| Euphorbia Factor L7b (82) | 23.9 | |||
| Euphorbia Factor L8 (83) | 30.3 | |||
| Euphorbia Factor L9 (74) | 11.2 | |||
| Euphorbia Factor L17 (84) | 48.5 | |||
| Euphorbia Factor L22 (85) | 16.6 | |||
| Euphorbia Factor L23 (86) | 19.5 | |||
| Euphorbia Factor L24 (87) | 18.2 | |||
| Euphorbia Factor L25 (88) | 28.9 | |||
| Jolkinol A (89) | 12.5 | |||
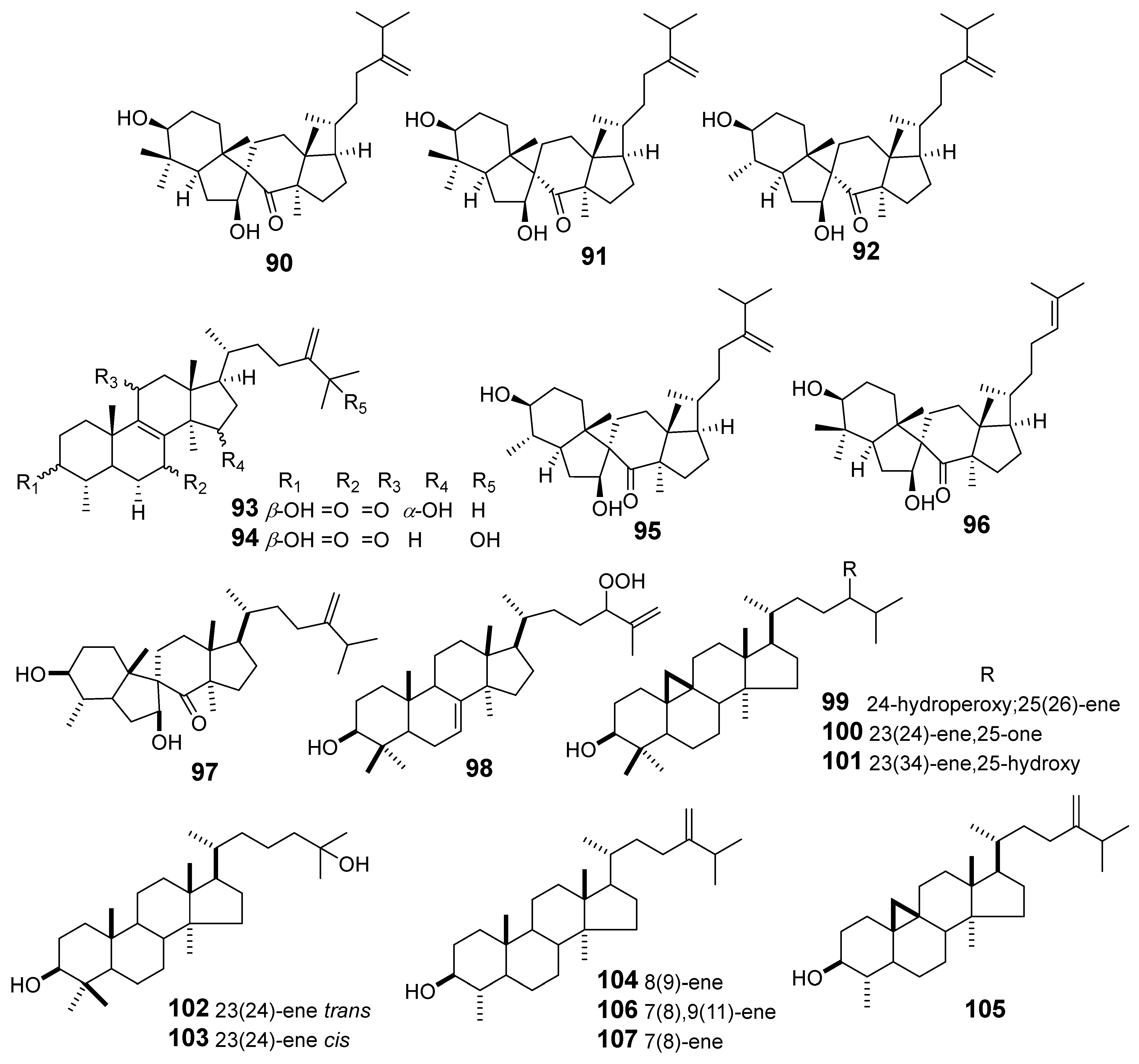
| E. maculata | Spiromaculatol A (90) | Griess assay RAW264.7 cells stimulated LPS NO | IC50 (μM) 23.1 | [61] |
| Spiromaculatol B (91) | 17.4 | |||
| Spiromaculatol C (92) | 8.8 | |||
| Euphomaculatoid B (93) | 31.3 | |||
| Euphomaculatoid D (94) | 15.9 | |||
| Spiropedroxodiol (95) | 12.7 | |||
| Spiroinonotsuoxodiol (96) | 20.6 | |||
| 4-methyl-3,7-dihydroxy-7 (8 → 9) abeo-lanost-24 (28) -en-8-one (97) | Ear edema in induced mouse by TPA | ID50 (nM/ear) 803 | [62] | |
| 24-hydroperoxylanost-7,25-dien-3β-ol (98) | 356.3 | |||
| 3-hydroxycycloart-25-ene-24-hydroperoxide (99) | 301.7 | |||
| 3β-hydroxy-26-nor-9,19-cyclolanost-23-en-25-one (100) | 558 | |||
| Cicloart-23(24)-ene-3β,25-hydroxy (101) | 355.7 | |||
| (23E)-3,25-dihydroxythirucalla-7,23-diene (102) | 855 | |||
| (23Z)-3,25-dihydroxy-thyrucalla-7,23-diene (103) | 1087 | |||
| Obtusifoliol (104) | 87.7 | |||
| 4α, 14α-dimethyl-5α-ergosta-7,9 (11), 24 (28) -trien-3β-ol (105) | 363.1 | |||
| Gramisterol (106) | 204 | |||
| Cycloeucalenol (107) | 463.9 | |||
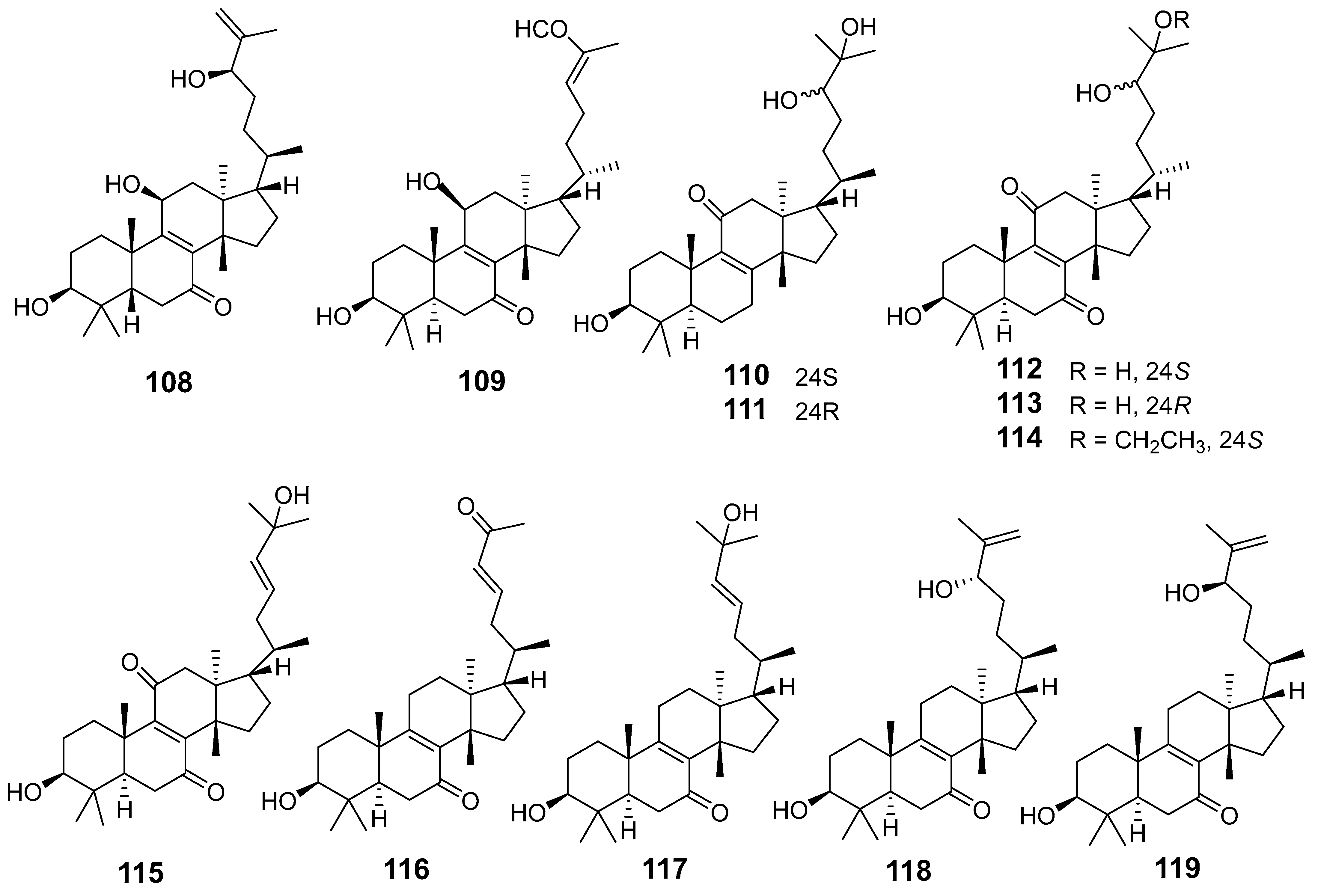
| E. neriifolia | Neritriterpenol H (108) | Griess assay RAW264.7 cells stimulated LPS | All compounds inhibited IL-6 | [64] |
| Neritriterpenol I (109) | ||||
| Neritriterpenol J (110) | ||||
| Neritriterpenol K (111) | ELISA kits | Secretion in a dose-dependent manner | ||
| Neritriterpenol L (112) | ||||
| Neritriterpenol M (113) | ||||
| Neritriterpenol N (114) | ||||
| 11-Oxo-kansenonol (115) | ||||
| Sooneuphanone B (116) | Griess assay RAW264.7 cells stimulated LPS NO | % inhibition 20 (µg/mL) 58.4% | [65] | |
| (23E)-eupha- 8,23-diene-3β,25-diol-7-one (117) | 27–39% | |||
| (+)-(24S)-eupha-8,25-diene-3β,24-diol-7-one (118) | ||||
| (24R)-eupha-8,25-diene-3β,24-diol-7-one (119) | ||||
| E. peplus | Euphopepluanone N (120) | Griess assay RAW264.7 stimulated LPS | Inhibited NO production | [70] |
| Euphopepluanone B (121) | ||||
| (2S*, 3S, 4R*, 5R*, 7S*, 13R*, 15R*)−3, 5, 7,15-tetraacetoxy-9, 14-dioxojatropha-6(17), 11E-diene (122) | ||||
| 11E-diene-9, 14-dione (123) | RT-qPCR analysis | Inhibited generation of cytokines (Il-6, IL-1β, TNF-α) | ||
| (11E, 2S, 3S, 4R, 5R, 7S, 13R, 15R)−3, 5, 7,15-tetraacetoxy-9, 14-dioxojatropha-6(17), 11E-diene (122) | ||||
| E. pulcherrima | Spinacetin (124) | Paw edema induced by Carrageen | % Edema inhibition 79.22 | [71] |
| Patuletin (125) | 89.01 | |||
| Spinacetin (124) | Paw edema histamine model | 78.33 | ||
| Patuletin (125) | 94.00 | |||
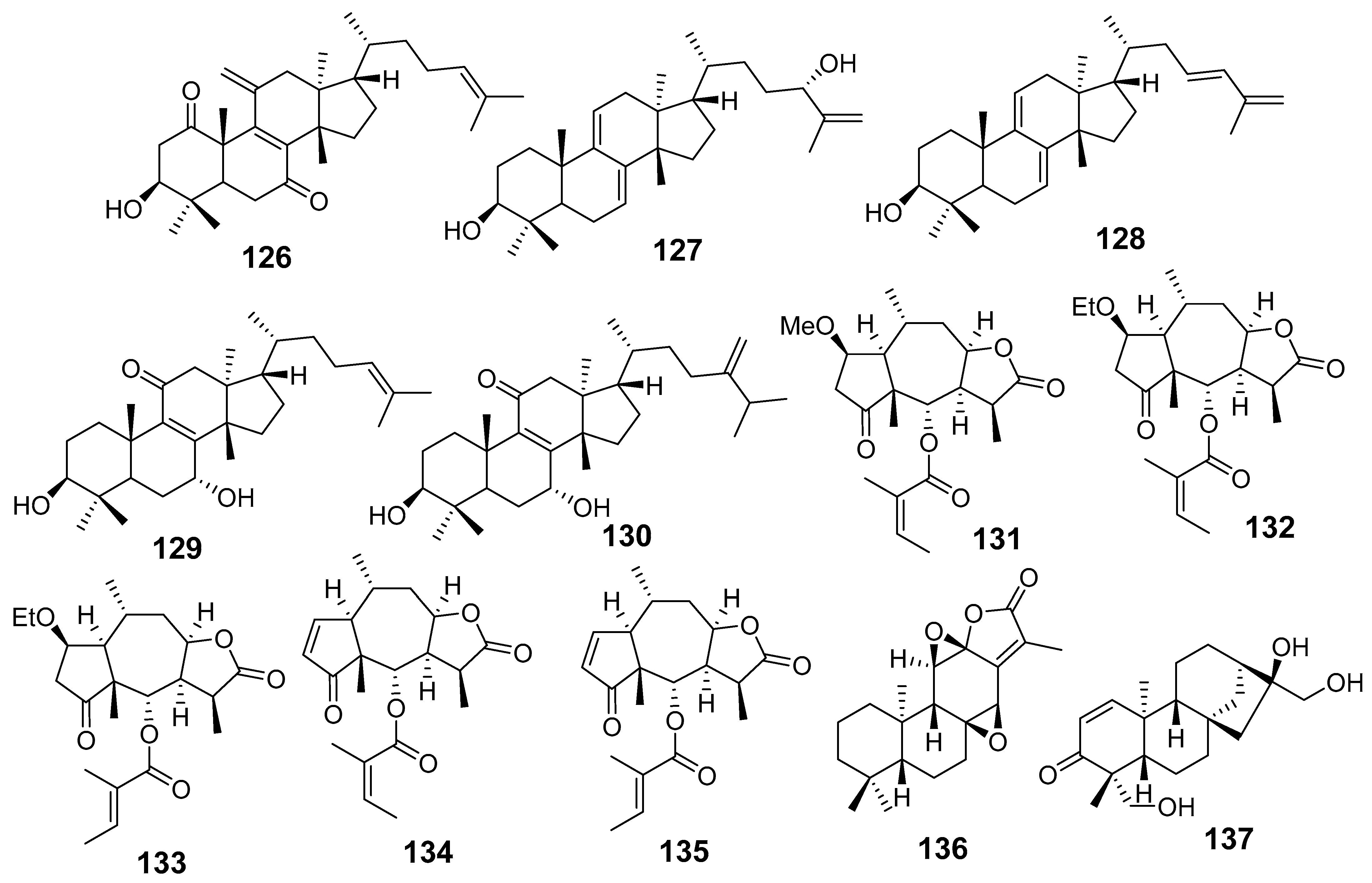
| E. resinifera | Euphatexols C (126) | Griess assay RAW264.7 cells stimulated LPS NO | IC50 (μM) 22.30 | [72] |
| Euphatexols D (127) | 48.04 | |||
| Euphatexols E (128) | 21.89 | |||
| Euphatexols F (129) | 38.15 | |||
| Euphatexols G (130) | 41.15 | |||
| E. thymifolia | (1S, 2R, 5R, 6S, 7R, 8R, 10R, 11S)-4-oxo-2-methoxy-6-angeloyloxy-pesudoguai-8,12-olide (131) | Griess assay BV-2 stimulated LPS NO | IC50 (μM) 6.46 | [77] |
| Minimolide B (132) | 15.32 | |||
| 4-oxo-2-ethoxy-6-tigloyloxy-pesudoguai-8,12-olide (133) | 7.15 | |||
| 6-O-angeloylplenolin (134) | 0.41 | |||
| 6-O-tigloyl-11,13-dihydrohelenalin (135) | 0.54 | |||
| E. wallichii | Jolkinolide B (136) | Griess assay RAW264.7 stimulated LPS NO | IC50 (µM) 3.84 | [82] |
| ELISA assay IL-6 TNF-α | IC50 (µM) >4 >16 | |||
| Wallkaurane A (137) | Griess assay RAW264.7 stimulated LPS NO | IC50 (µM) 3.84 | [83] | |
| ELISA assay | The production of inflammatory cytokines (IL-6 and TNF-α) | |||
| Western blot | Increased the expression of the antiapoptotic marker Bcl-2. Decreased the expression of iNOS and COX-2 |
| Species | Compounds | Biological Model | Result | Ref |
|---|---|---|---|---|
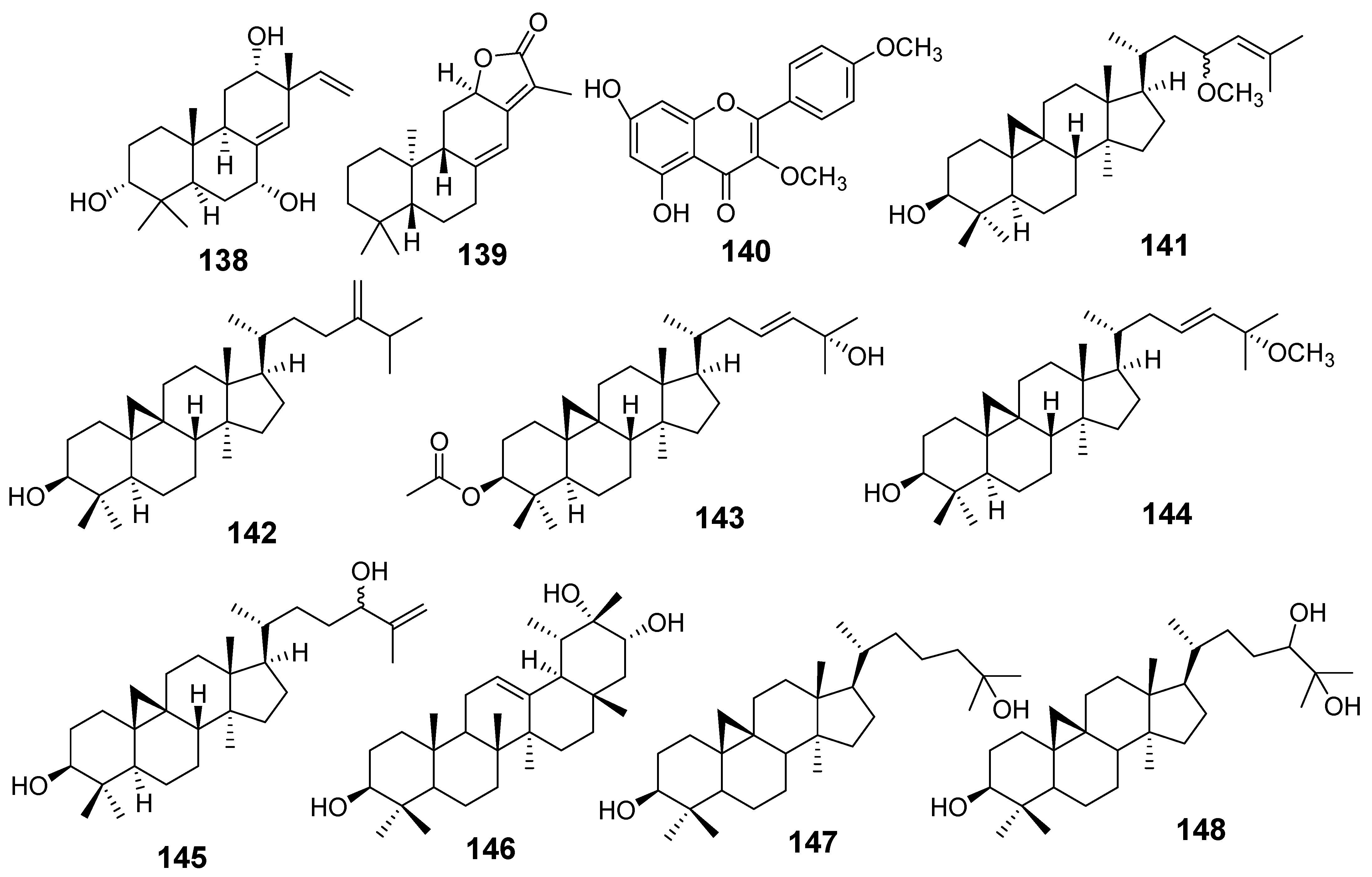
| E. alatavica | 3α,7α,12α-trihydroxyisopimara-8(14), 15-diene (Alatavnol A) (138) | MTT assay MCF7 A549 | IC50 (µg/mL) 14.327 12.033 | [16] |
| Helioscopinolide A (49) | HeLa MCF7 | 23.802 33.476 | ||
| Jolkinolide E (139) | MCF7 | 22.066 | ||
| E. balsamifera | Kampferol-3,4′-dimethyl ether (140) | MTT assay HePG2 MCF7 | IC50 (µM) 42.67 44.90 | [20] |
| E. dendroides | 23R/S-3β-hydroxycycloart-24-ene-23-methyl ether (141) | MTT assay HepG2 Huh-7 KLM-1 1321N1 HeLa | IC50 (µM) 20.67 16.24 22.59 25.99 40.50 | [21] |
| 24-methylene cycloartan-3β-ol (142) | HepG2 Huh-7 KLM-1 1321N1 HeLa | 10.93 7.42 21.48 12.32 13.68 | ||
| Cycloart-23-ene-3β,25-diol monoacetate (143) | HepG2 Huh-7 KLM-1 1321N1 | 12.81 <0.47 22.48 25.17 | ||
| 3β-hydroxy-cycloart-23-ene-25 methyl ether (144) | HepG2 Huh-7 KLM-1 1321N1 HeLa | 12.72 <0.44 <0.44 0.63 3.7 | ||
| 24R/S-3β-hydroxy-25-methylenecycloartan-24-ol (145) | HepG2 Huh-7 KLM-1 1321N1 HeLa | 15.54 16.33 22.38 13.53 >4.52 | ||
| E. denticulata | 12-taraxast-3β, 19, 21 (α)-triol (146) | MTT assay DU-145 | IC50 (µM) 12.2 27.5 18.3 | [22] |
| Cycloartane-3, 25-diol (147) | ||||
| Cycloartane-3,24, 25-triol (148) | ||||
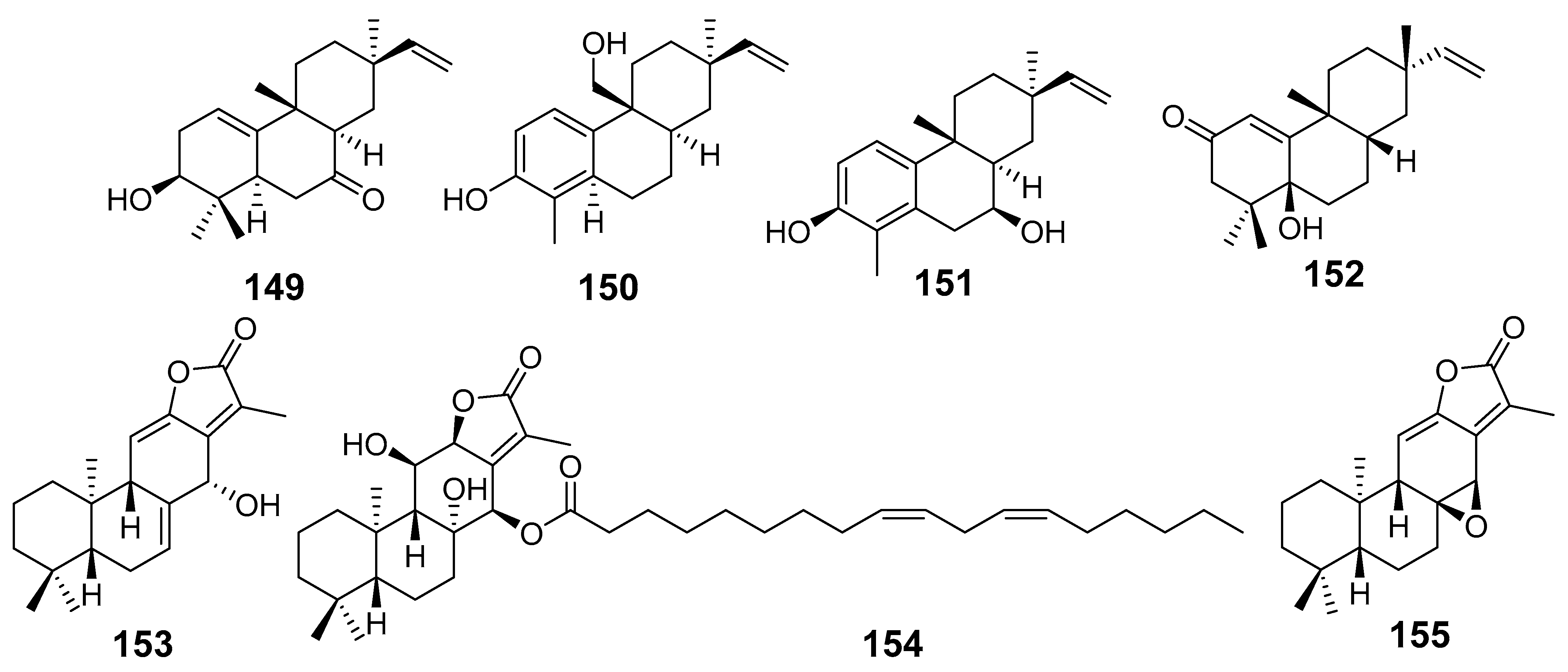
| E. ebracteolata | Euphebracteolatin C (149) | CCK-8 assay HepG2 MCF7 A549 | IC50 µM 14.29 34.81 40.85 | [23] |
| Euphebracteolatin D (150) | HepG2 MCF7 A549 | 23.69 28.62 39.25 | ||
| Euphebracteolatin E (151) | HepG2 MCF7 A549 | 38.96 29.67 36.27 | ||
| Euphorpekone B (152) | HepG2 MCF7 A549 | 12.33 25.29 38.82 | ||
| Jolkinolide B (136) | MTS assay HL-60 SMMC-7721 A549 MCF-7 SW480 | IC50 (µM) 5.2 3.8 11.9 16.2 10.2 | [25] | |
| Euphoroid B (153) | MTT assay A549 | IC50 (µM) 22.87 | [26] | |
| Euphoroid C (154) | A549 MCF-7 Lovo HepG2 | 28.7 28.57 27.0 28.0 | ||
| Jolkinolide A (155) | A549 | 18.56 | ||
| E. fischeriana | 12-deoxyphorbol-13-(9Z,12Z)-octadecadienoate (156) | MTT assay HeLa HepG2 | IC50 (µM) 3.54 8.32 | [28] |
| 12-deoxyphorbol-13-dimethylpentadecanoate (157) | HeLa HepG2 | 5.72 11.45 | ||
| Euphonoid H (158) | MTT Assay MDA-MB-231 HCT-15 RKO C4-2B C4-2B/ENZR | IC50 (µM) 21.8 28.57 20.46 5.52 4.16 | [29] | |
| Euphonoid I (159) | MDA-MB-231 HCT-15 RKO C4-2B C4-2B/ENZR | 7.95 12.45 8.78 4.49 5.74 | ||
| Raserrane A (160) | C4-2B | 34.09 | ||
| Raserrane B (161) | C4-2B C4-2B/ENZR | 23.34 36.98 | ||
| Fischerianin A (162) | MTT assay HepG2 A375 HL-60 K562 HeLa | IC50 (µM) 17.59 21.46 15.59 14.99 13.24 | [31] | |
| Fischerianin B (163) | HepG2 A375 HL-60 K562 HeLa | 11.23 18.34 12.82 17.82 5.31 | ||
| Langduin A (164) | HepG2 A375 HL-60 K562 HeLa | 14.47 13.34 20.18 13.28 19.36 | ||
| Langduin A6 (165) | HepG2 A375 HL-60 K562 HeLa | 16.55 9.64 21.03 8.46 11.57 | ||
| Euphonoid A (166) | MTT assay C4-2B C4-2B/ENZR HCT-15 RKO | IC50 (µM) 9.18 9.70 18.3 16.2 | [32] | |
| Euphonoid B (167) | C4-2B C4-2B/ENZR RKO | 13.4 11.1 35.1 | ||
| Euphonoid C (168) | C4-2B C4-2B/ENZR HCT-15 RKO | 17.7 15.2 13.4 21.3 | ||
| Euphonoid D (169) | C4-2B C4-2B/ENZR HCT-15 RKO | 9.23 15.1 23.2 34.4 | ||
| Euphonoid E (170) | C4-2B C4-2B/ENZR | 16.1 22.1 | ||
| Euphonoid F (171) | C4-2B C4-2B/ENZR | 24.9 40.1 | ||
| Euphonoid G (172) | C4-2B C4-2B/ENZR | 18.1 20.1 | ||
| Euphonoid H (158) | C4-2B C4-2B/ENZR HCT-15 RKO | 7.39 9.20 19.0 22.9 | ||
| Raserrane B (161) | C4-2B C4-2B/ENZR HCT-15 RKO | 16.3 16.4 28.2 42.1 | ||
| 11-oxo-ebracteolatanolide B (173) | C4-2B C4-2B/ENZR HCT-15 MDA-MB-231 | 2.85 2.42 15.2 14.5 | ||
| Caudicifolin (174) | C4-2B C4-2B/ENZR HCT-15 RKO MDA-MB-231 | 2.22 5.39 12.6 15.3 8.81 | ||
| Jolkinolide A (155) | C4-2B C4-2B/ENZR | 10.1 16.1 | ||
| 17-hydroxyjolkinolide B (175) | C4-2B C4-2B/ENZR | 12.3 14.0 | ||
| Jolkinolide B (136) | C4-2B C4-2B/ENZR HCT-15 RKO MDA-MB-231 | 4.43 5.89 47.9 35.8 30.7 | ||
| Methyl-8,11-3-dihydroxy-12- oxo-ent-abietadi-13,15(17)-ene-16-oate (176) | C4-2B C4-2B/ENZR HCT-15 RKO MDA-MB-231 | 4.95 4.27 25.6 23.3 23.8 | ||
| 7-dehydroabietanone (177) | C4-2B C4-2B/ENZR | 14.2 29.9 | ||
| Abieta-8,11,13-triene (178) | C4-2B C4-2B/ENZR | 20.1 37.1 | ||
| 15-hydroxydehydroabietic acid (179) | C4-2B | 33.1 | ||
| (4αS,10αS)-1,2,3,4,4α,10α-hexahydro-1,1,4α-trimethyl-7-(1-methyl)phenanthrene (180) | C4-2B C4-2B/ENZR | 36.2 26.2 | ||
| 2-phenanthrenyl] ethanone (181) | C4-2B | 34.0 | ||
| (4βS,8αS)-2-phenanthrenecarboxylic acid,4β,5,6,7,8,8α,9,10-octahydro-3-hydroxy-4β,8,8-trimethyl-methyl ester (182) | C4-2B | 23.1 | ||
| Isopimara-7,15-dien-3-one (183) | C4-2B C4-2B/ENZR | 21.9 24.2 | ||
| Araucarol (184) | C4-2B C4-2B/ENZR | 19.2 34.3 | ||
| Araucarone (185) | C4-2B C4-2B/ENZR HCT-15 | 16.0 24.1 47.1 | ||
| Ent-3β, (13S)-dihydroxyatis-16- en-14-one (186) | C4-2B C4-2B/ENZR | 13.2 25.3 | ||
| Ent-(13R,14R)-13,14-dihydroxyatis-16-en-3-one (187) | C4-2B C4-2B/ENZR HCT-15 | 18.8 15.2 39.2 | ||
| Ent-atis-16-ene-3,14-dione (188) | C4-2B | 26.7 | ||
| Ent-(13S)-13-hydroxyatis- 16-ene-3,14-dione (189) | C4-2B | 30.5 | ||
| 3-oxoatisane-16α,17-diol (190) | C4-2B C4-2B/ENZR | 23.7 29.1 | ||
| 3α-hydroxy-ent-16-kauren (191) | C4-2B | 26.2 | ||
| Ent-kaurane-3β,16β,17-triol (192) | C4-2B/ENZR HCT-15 | 21.7 28.1 | ||
| Ent-16β-H-3-oxokauran-17-ol (193) | C4-2B C4-2B/ENZR | 22.8 20.1 | ||
| Ent-kaurane-3-oxo-16β,17-diol (194) | C4-2B C4-2B/ENZR HCT-15 | 17.0 23.0 43.2 | ||
| Fischerianoid A (195) | MTT assay MM-231 SMMC-7721 HEP3B | IC50 (µM) 12.10 32.48 15.95 | [33] | |
| Fischerianoid B (196) | HL-60 MM-231 HEP3B SW-480 | 28.78 9.12 8.50 35.52 | ||
| Fischerianoid C (197) | MM-231 HEP3B | 25.45 27.34 | ||
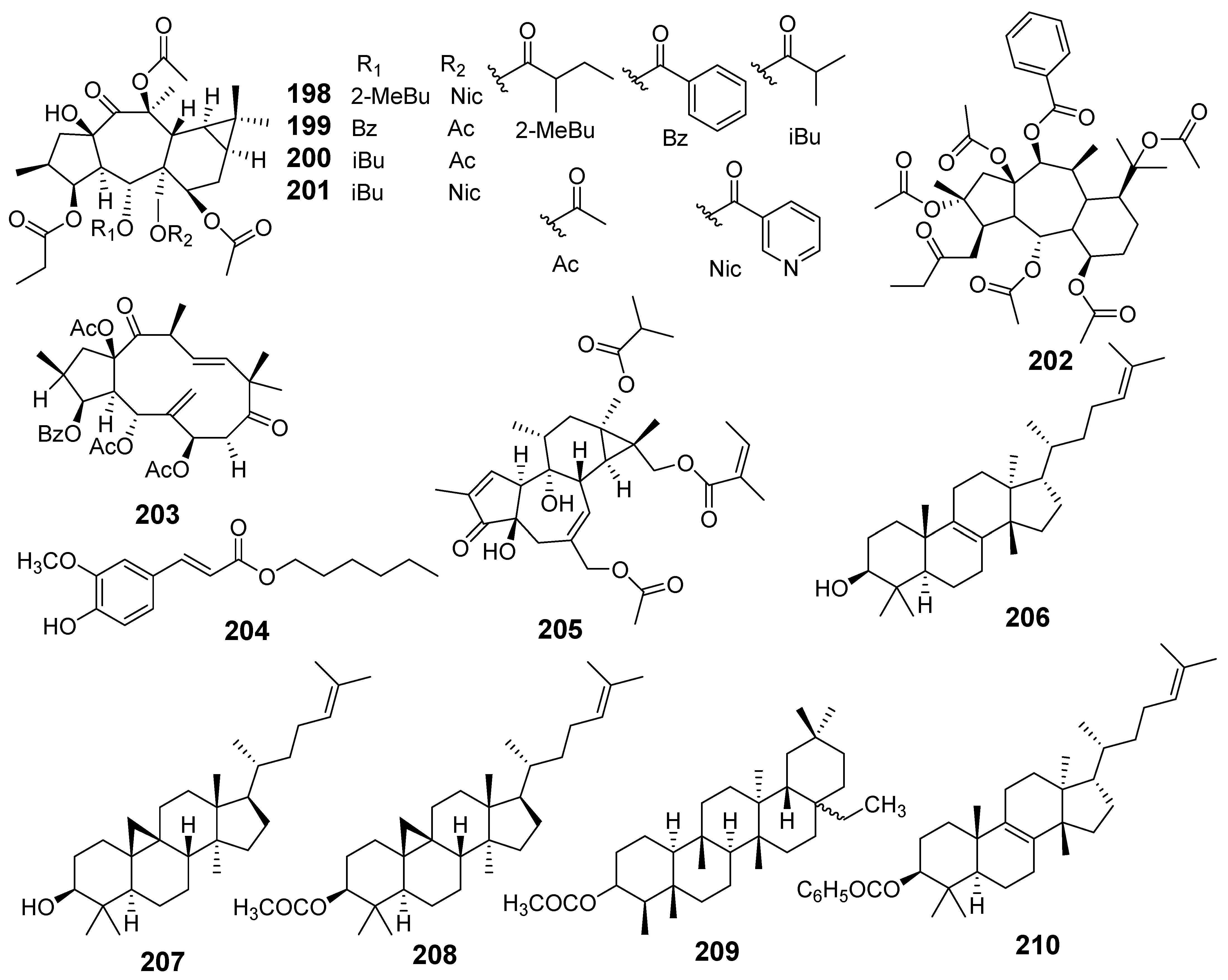
| E. gedrosiaca | 13β-O-propanoyl-5α-O-methylbutanoyl-7α,13β-O-diacetyl-17α-O-nicotinoyl-14-oxopremyrsinane (198) | MTT assay MDA-MB-231 MCF-7 | IC50 (µM) 10.8 22.2 | [35] |
| 3β-O-propanoyl-5α-O-benzoyl-7α,13β, 17α-O-triacetyl-14-oxopremyrsinane (199) | MDA-MB-231 MCF-7 | 22.2 27.8 | ||
| 3β-O-propanoyl-5α-O-isobutanoyl-7α,13β,17α-O-triacetyl-14-oxopremyrsinane (200) | MDA-MB-231 | 24.5 | ||
| 3β-O-propanoyl-5α-O-isobutanoyl-7α,13β-O-diacetyl-17α-O-nicotinoyl-14-oxopremyrsinane (201) | MDA-MB-231 | 27.3 | ||
| 2,5,7,10,15-O-pentaacetyl-3-O-propanoyl-14-O-benzoyl-13,17-epoxy-8-myrsinene (202) | MDA-MB-231 | 33.7 | ||
| E. glomerulans | Euphoglomeruphane H (203) | MTT assay MCF-7/ADR | IC50 (µM) 39.3 | [36] |
| E. grandicornis | Hexyl(E)-3-(4-hydroxy-3-methoxyphenyl)-2-propenoate (204) | MTT assay MCF-7 HCC70 | IC50 (µM) 23.41 29.45 | [37] |
| 6-Angeloyloxy-20-acetoxy-13-isobutanoyloxy-4,9-dihydroxytiglia-1,6-dien-3-one (205) | MTT assay A549 | Cell viability (%) 49.2 | [38] | |
| E. grantii | Eupha-8,24-dien-3β-ol (Euphol) (206) | SRB assay MCF-7 MCF-7ADR | IC50 (µM) 26.25 27.77 | [39] |
| Cycloartenyl acetate (207) | MCF-7 MCF-7ADR | 25.3 18.56 | ||
| Cycloartenol (208) | MCF-7 MCF-7ADR | 23.73 15.6 | ||
| Epifriedelinyl acetate (209) | MCF-7 MCF-7ADR | 26.18 19.04 | ||
| Euphylbenzoate (210) | MCF-7 MCF-7ADR | 3.47 3.22 | ||
| Flow cytometry | The death is induced by apoptosis | |||
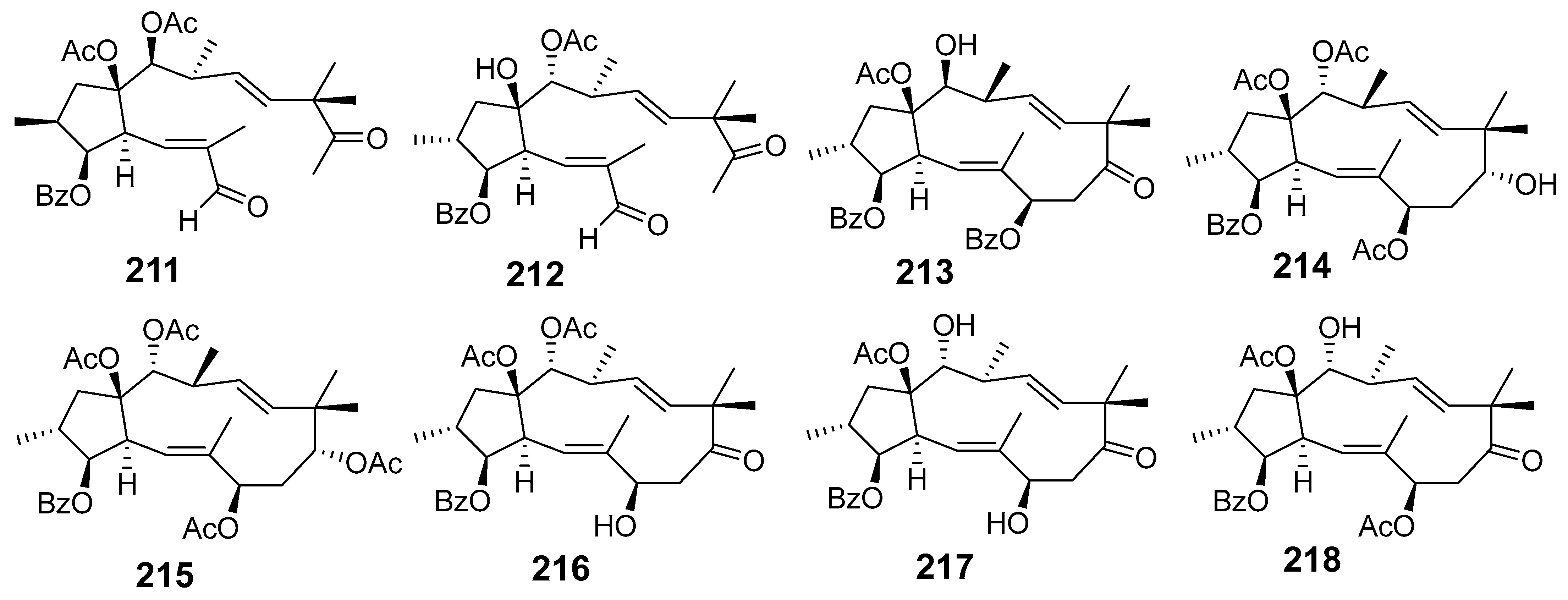
| E. helioscopia | Euphohelinoid A (211) | SRB assay HepG2 HeLa HL-60 SMMC-7221 | IC50 (µM) 24.3 28.4 18.6 29.6 | [41] |
| Euphohelinoid B (212) | HepG2 HeLa HL-60 SMMC-7221 | 10.2 9.3 8.1 9.8 | ||
| Euphohelinoid D (213) | HeLa HL-60 SMMC-7221 | 34.5 34.1 30.1 | ||
| Euphohelinoid F (214) | HepG2 HeLa HL-60 SMMC-7221 | 12.5 14.1 13.3 11.1 | ||
| Euphornin L (215) | HepG2 HeLa HL-60 SMMC-7221 | 22.8 25.7 13.1 14.3 | ||
| Helioscopianoid O (216) | HeLa HL-60 SMMC-7221 | 26.2 18.2 19.5 | ||
| Euphoscopin I (217) | HepG2 HeLa HL-60 SMMC-7221 | 24.1 29.7 14.3 18.7 | ||
| Euphoscopin J (218) | HepG2 HeLa HL-60 SMMC-7221 | 14.9 13.7 12.4 15.0 | ||
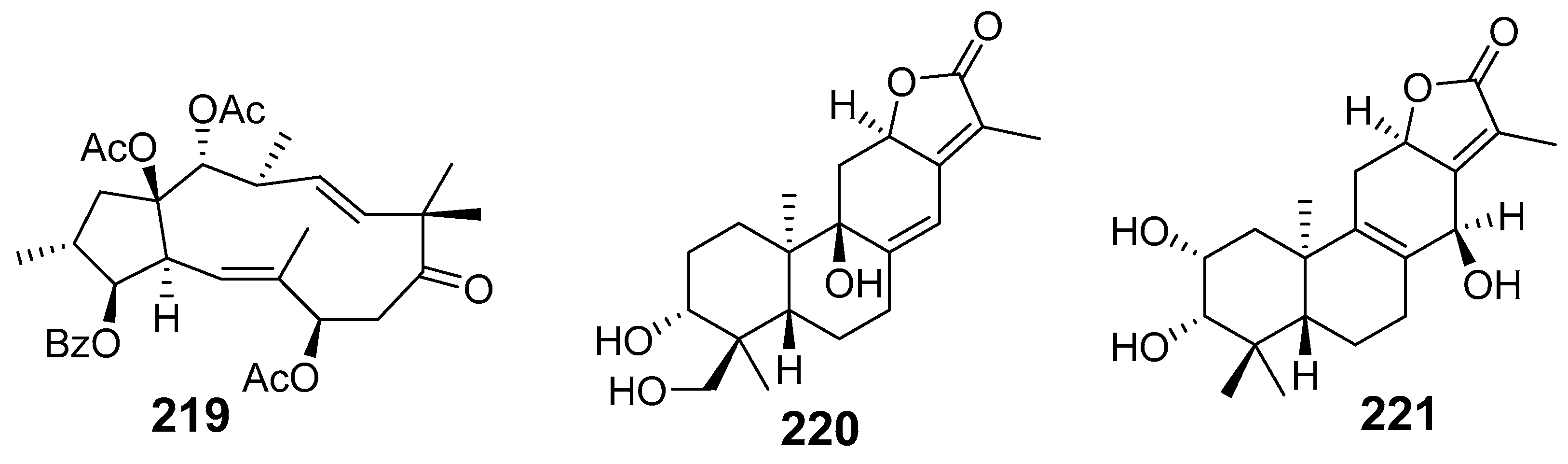
| Euphoscopin B (219) | HepG2 HeLa HL-60 SMMC-7221 | 23.3 29.2 20.2 27.1 | ||
| Euphelionolide F (220) | MTT assay MCF-7 PANC-1 | IC50 (µM) 9.5 10.7 | [42] | |
| Euphelionolide L (221) | MCF-7 PANC-1 | 9.8 10.3 | ||
| E. hypericifolia | Euphypenoid A (222) | MTT assay HCT-116 | IC50 (µM) 12.8 | [43] |
| 20(S),24(R)-20,24-epoxy-24-methyldammaran-3β-ol (223) | HCT-116 | 26.8 | ||
| (23E)-25-methoxycycloart-23-en- 3-one (224) | HCT-116 | 7.4 | ||
| Isomotiol (225) | HCT-116 | 10.6 | ||
| E. kansuensis | Euphorboside A (226) | MTT assay RKO MDAMB-231 A375 8 HCT-15 HCT-15/5-FU A549 A549/CDDP HepG2 HepG2/DOX | IC50 (µM) 3.70 4.15 8.27 14.7 15.0 16.2 16.4 18.8 33.2 | [45] |
| E. kansui | Wilfoside KIN (227) | MTT Assay HepG2 MCF7 | IC50 (µM) 12.55 >20 | [47] |
| Cynsaccatol L (50) | HepG2 MCF7 | 12.61 >20 | ||
| Kanesulone A (228) | HepG2 MCF7 | 18.24 >20 | ||
| 3β,7β,15β-triacetyloxy-5α-benzoyloxy-2α,8α-dihydroxyjatropha-6(17),11E-diene-9, 14-dione (229) | HepG2 MCF7 | 18.26 >20 | ||
| 13-hydroxyingenol-3-(2,3-dimethylbutanoate)-13-dodecanoate (230) | HepG2 MCF7 GSC3 GSC12 293T HAC T98G | >20 17.12 1.67 2.75 21.93 19.23 16.77 | ||
| Euphol (206) | GSC-3 GSC-12 | 8.89 13.0 | ||
| Lucidal (231) | GSC-3 GSC-12 293T HAC T98G | 4.71 3.25 21.07 30.22 20.77 | ||
| E. kopetdaghi | 14-Nicotinyl-3,5,10,15,17-pentaacetyl-8-isobutanoyl-cyclomyrsinol-7- one (Kopetdaghinane A) (232) | MTT assay MCF-7 | IC50 (µM) 38.10 | [49] |
| OVCAR-3 | 51.23 | |||
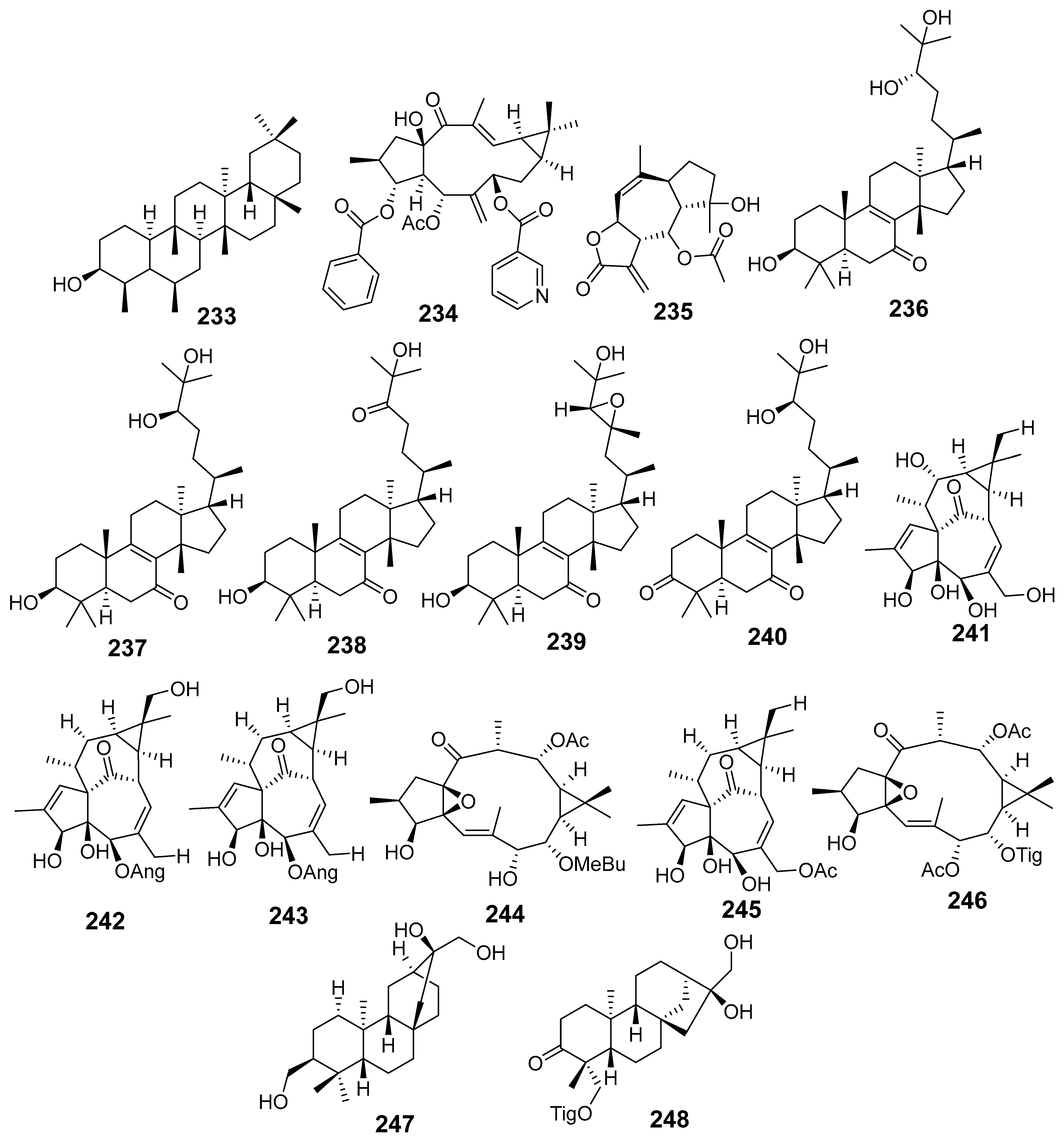
| E. lactea | Friedelan-3β-ol (233) | HN22 Flow cytometry | It induced an S-phase cell cycle arrest | [50] |
| E. lathyris | Euphorbia Factor L1 (68) | Tumour induced by Mouse 4T1 in BALB/c | Decreased the generation of IL-β, IL-6, TNF-α | [51] |
| ELISA | Downregulated DDR1 protein expression and immuno-reactivity in SHI mice | |||
| Western blot Flow cytometry | No differences were detected in CD4+, CD8+, CD49b+ T cells, and Tregs between the DDR1-OE group and the DDR1-OE+EFL1 group | |||
| 15β-hydroxy-5α-acetoxy-3α-benzoyloxy-7β-nicotinoyloxylathyol (234) | MTT assay MCF-7 HepG2 | IC50 (µM) 9.43 13.22 | [57] | |
| Euphorbia Factor L2 (80) | MTT assay KB KB-VIN | IC50 (µM) 33.2 7.2 | [58] | |
| Euphorbia Factor L3 (69) | A549 MDA-MB-231 KB KB-VIN MCF-7 | 14.6 31.6 7.9 8.0 25.9 | ||
| Euphorbia Factor L8 (83) | A549 MDA-MB-231 KB KB-VIN MCF-7 | 11.8 24.4 17.7 16.9 23.8 | ||
| Euphorbia Factor L9 (74) | A549 MDA-MB-231 KB KB-VIN MCF-7 | 6.7 21.9 6.1 5.7 8.4 | ||
| Euphorbia Factor L24 (87) | MTT assay HCT116 MCF-7 786-0 HepG2 | IC50 (µM) 6.44 8.43 15.3 9.32 | [59] | |
| E. microsphaera | (3aR,4S,4aS,5R,7aS,9aS)-5-hydroxy-5,8-dimethyl-3-methylene-2-oxo- 2,3,3a,4,4a,5,6,7,7a,9a-decahydroazuleno [6,5-b] furan-4-yl acetate (Aryanin) (235) | MTT assay MCF7 24 h 72 h | IC50 (µg/mL) 13.81 49.35 | [63] |
| E. neriifolia | Neritriterpenols A (236) | MTT assay Hep G2 | IC50 (µM) 25.9 | [65] |
| (+)-(24R)-3β,24,25-trihydroxyeuph-8-en-7-one (Neritriterpenol B) (237) | WiDR HepG2 | 47.2 44.0 | ||
| Neritriterpenol E (238) | A549 WiDR HepG2 | 45.7 32.3 35.9 | ||
| (+)-(23R,24R)-epoxy-3α,25-dihydroxyeuph-8-en-7-one (Neritriterpenol F) (239) | HepG2 | 39.4 | ||
| (+)-(24R)-24,25-dihydroxyeuph-8-en-3,7-dione (Neritriterpenol G) (240) | WiDR HepG2 | 48.9 36.6 | ||
| (23E)-eupha-8,23-diene-3β,25-diol-7-one (117) | A549 WiDR HepG2 | 25.5 20.5 37.6 | ||
| (+)-(24S)-eupha-8,25-diene-3β,24-diol-7-one (118) | A549 WiDR MCF7 HepG2 | 23.8 20.8 32.3 15.2 | ||
| (24R)-eupha-8,25-diene-3β,24-diol-7-one (119) | A549 WiDR MCF7 HepG2 | 20.4 17.1 30.7 12.2 | ||
| Sooneuphanone B (116) | A549 WiDR MCF7 HepG2 | 12.8 23.3 17.9 8.0 | ||
| Phonerilin B (241) | SRB assay A549 HL-60 | IC50 (µM) 8.6 9.1 | [66] | |
| Phonerilin E (242) | A549 HL-60 | 4.9 9.2 | ||
| Phonerilin F (243) | A549 HL-60 | 3.8 4.5 | ||
| Phonerilin H (244) | A549 HL-60 | 7.5 5.7 | ||
| 20-O-diacetyl-ingenol (245) | HL-60 | 3.1 | ||
| 7,12-O-diacetyl-8-O-tigloylingol (246) | A549 HL-60 | 6.4 9.5 | ||
| Ent-atisane-3α,16α,17-triol (247) | MTT assay HepG2 HepG2/Adr | IC50 (µM) 13.7 15.57 | [67] | |
| (4R,5S,8S,9R,10S,13R,16S)- ent-16α,17-dihydroxy-19-tigloyloxykauran-3-one (248) | HepG2 | 0.01 | ||
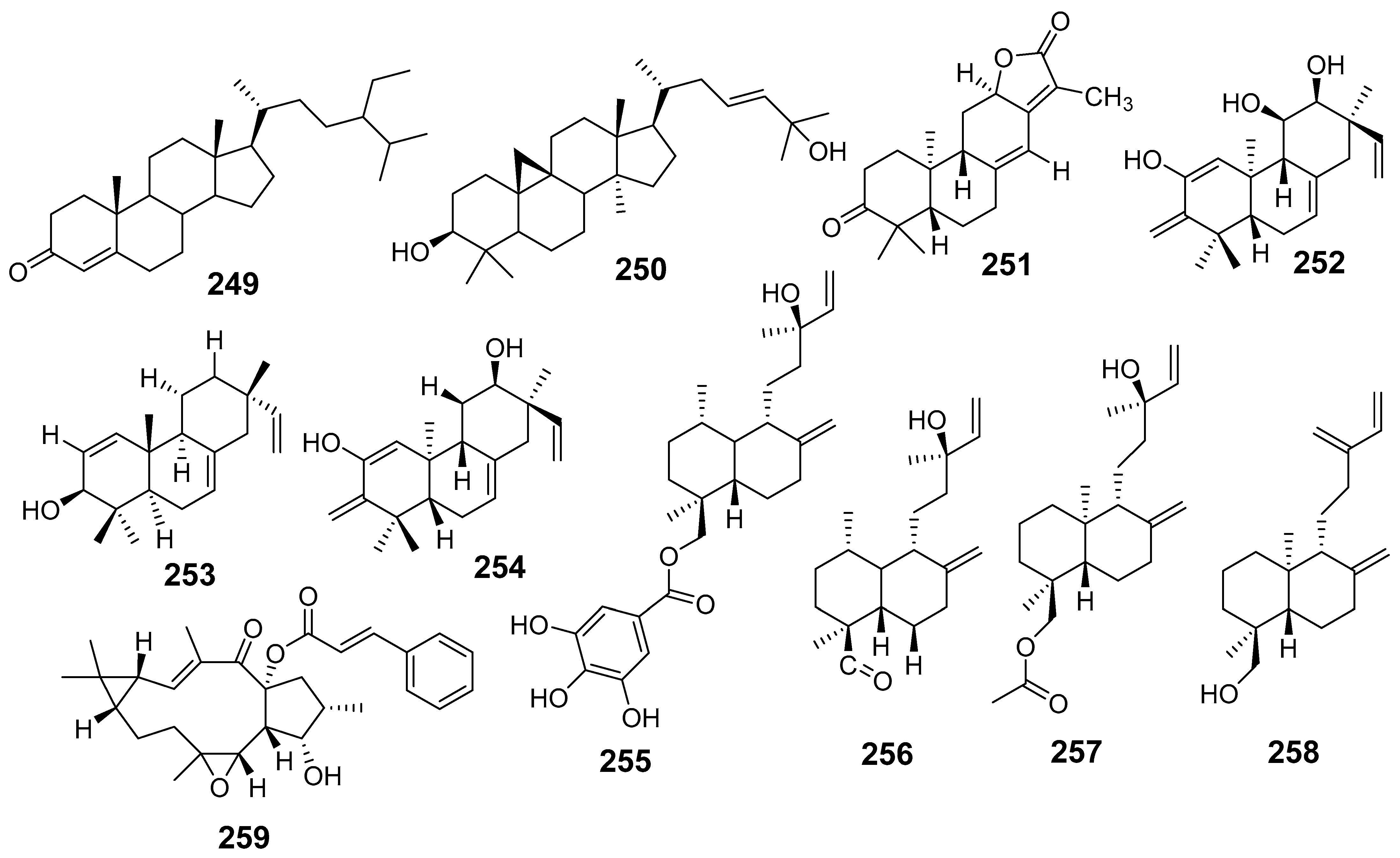
| E. pedroi | Spiropedroxodiol (95) | MTT assay L5178Y-PAR L5178Y-MDR Colo205 Colo320 | IC50 (µM) 42.3 46.8 16.8 27.7 | [68] |
| β-sitostenone (249) | Colo 205 Colo320 | 46.6 21.3 | ||
| Cycloart-23-ene-3β,25-diol (250) | L5178Y-PAR Colo 205 Colo320 MRC-5 | 49.4 16.7 31.6 12.9 | ||
| Helioscopinolide E (251) | L5178Y-PAR | 32.9 | ||
| E. pekinensis | (11R,12S)-2,11,12-trihydroxy-ent-isopimara-1,7,15-trien-3-one (252) | CCK8 method U-937 LOVO | IC50 (µM) 25.1 27.7 | [69] |
| Isopimara-7,15-dien-3β-ol (253) | K-562 | 0.87 | ||
| Eupneria R (254) | U-937 LOVO | 30.5 27 | ||
| Euphodane A (255) | U-937 LOVO K-562 | 5.9 26.8 32.2 | ||
| Euphodane B (256) | U-937 LOVO | 36.7 35.03 | ||
| Euphodane C (257) | U-937 LOVO K-562 | 24.5 39.3 31.3 | ||
| Euphodane D (258) | U-937 LOVO | 25.1 29.7 | ||
| Jolkinol B (259) | U-937 LOVO K-562 | 3.6 8.44 25.3 | ||
| E. saudiarabica | Glutinol (260) | MTT assay MCF-7 Flow cytometry | IC50 (µM) 9.83 Induced apoptosis | [73] |
| E. schimperiana | 3,30-di-O-methylellagic acid (261) | MTT assay PC3 | IC50 (µg/mL) 5.5 | [74] |
| E. sororia | Euphosorophane I (262) | P-gp ATPase activity assay | This compound reversed P-gp-mediated MDR cell (multidrug resistance) by inhibiting the ABCB1 drug efflux function in drug-resistant MCF-7/ADR cells | [75] |
| E. stracheyi | 3-O-benzoyl-20-deoxymgenol (263) | MTT assay HL-60 A-549 SMMC-7721 MCF-7 SW480 | IC50 (µM) 10.5 21.47 18.36 18.82 16.25 | [76] |
| E. tirucalli | Tirucadalenone (264) | MTT assay K562 | IC50 (μg/mL) 22 | [78] |
| Euphol (206) | MTT assay U87-MG U373 U251 GAMG SW1088 SW1783 SNB19 RES186 RES259 KNS42 UW479 SF188 HCB2 HCB149 | IC50 (µM) 26.41 30.48 29.01 8.73 27.12 19.62 31.05 16.70 10.34 19.94 15.26 5.98 11.66 21.68 | [79] | |
| Euphol (206) | MTS assay T47D MDA-MB-231 MDA-MB-468 BT20 HS587T MCF-7 MCF7/AZ JHU-O22 HN13 SCC25 SCC4 SCC14 FADU SW480 SW620 CO115 HCT15 HT29 SK-CO-10 DLD1 LOVO DIFI Caco2 U87-MG U373 U251 GAMG SW1088 SW1783 RES186 RES259 KNS42 UW479 SF188 PC-3 LNCaP T24 5637 HT1376 MCR DAOY ONS76 JEG3 A431 H292 SKMES1 A549 SK-LU-1 SIHA CASKI C33A HELA KYSE30 KYSE70 KYSE270 KYSE410 Mia PaCa-2 PANC-1 PSN-1 BXPC-3 Capan-1 COLO858 COLO679 A375 WM1617 WM9 WM852 WM278 WM35 WN793 SKMEL-37 PA-1 SW626 | IC50 (µM) 38.89 9.08 30.89 8.96 18.15 18.76 33.42 26.35 8.89 6.65 19.82 15.81 20.17 5.79 10.02 9.58 5.47 6.52 17.53 2.56 11.49 11.38 35.19 26.41 30.48 29.01 8.73 27.12 19.62 16.70 10.34 19.94 15.26 5.98 11.95 1.41 30.72 4.83 25.25 7.40 5.72 21.72 16.65 17.79 13.25 25.62 11.01 22.83 24.74 24.74 21.32 17.55 3.52 8.77 10.71 4.35 8.46 21.47 3.71 5.47 16.33 14.02 8.93 9.67 16.32 9.67 7.61 27.46 12.40 5.96 10.07 7.97 30.40 | [80] | |
| E. umbellata | Euphol (206) | MTT assay K-562 HL-70 | IC50 (µM) 34.44 39.98 | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Jiménez, S.; Valladares-Cisneros, M.G.; Salinas-Sánchez, D.O.; Pérez-Ramos, J.; Sánchez-Pérez, L.; Pérez-Gutiérrez, S.; Campos-Xolalpa, N. Anti-Inflammatory and Cytotoxic Compounds Isolated from Plants of Euphorbia Genus. Molecules 2024, 29, 1083. https://doi.org/10.3390/molecules29051083
Rojas-Jiménez S, Valladares-Cisneros MG, Salinas-Sánchez DO, Pérez-Ramos J, Sánchez-Pérez L, Pérez-Gutiérrez S, Campos-Xolalpa N. Anti-Inflammatory and Cytotoxic Compounds Isolated from Plants of Euphorbia Genus. Molecules. 2024; 29(5):1083. https://doi.org/10.3390/molecules29051083
Chicago/Turabian StyleRojas-Jiménez, Sarai, María Guadalupe Valladares-Cisneros, David Osvaldo Salinas-Sánchez, Julia Pérez-Ramos, Leonor Sánchez-Pérez, Salud Pérez-Gutiérrez, and Nimsi Campos-Xolalpa. 2024. "Anti-Inflammatory and Cytotoxic Compounds Isolated from Plants of Euphorbia Genus" Molecules 29, no. 5: 1083. https://doi.org/10.3390/molecules29051083
APA StyleRojas-Jiménez, S., Valladares-Cisneros, M. G., Salinas-Sánchez, D. O., Pérez-Ramos, J., Sánchez-Pérez, L., Pérez-Gutiérrez, S., & Campos-Xolalpa, N. (2024). Anti-Inflammatory and Cytotoxic Compounds Isolated from Plants of Euphorbia Genus. Molecules, 29(5), 1083. https://doi.org/10.3390/molecules29051083









