Abstract
Endometriosis is a common gynecological condition with a complex physio-pathological background. This study aimed to assess the role of Rubus idaeus leaf extract (RiDE) as a potential therapeutic agent in reducing the size of the endometriotic lesions and modulate the plasma expression of MMP-2, MMP-9, and TGF-β1. The endometriotic lesions were induced in a rat model by the autologous transplant of endometrium. Thirty-six female rats, Wistar breed, with induced endometriosis, were divided into four groups and underwent treatment for 28 days. The CTRL group received 0.5 mL/day of the vehicle; the DG group received 1 mg/kg b.w./day dienogest; the RiDG group received 0.25 mL/kg b.w./day RiDE and the D+RiDG group received 1 mg/kg b.w./day dienogest and 0.25 mL/kg b.w./day RiDE, respectively. Rats’ weight, endometriotic lesion diameter and grade, and plasma levels of MMP-2, MMP-9, and TGF-β1 were assessed before and after treatment. The administration of RiDE in association with dienogest vs. dienogest determined a lower weight gain and a reduction in diameter of the endometriotic lesions. RiDE administration restored MMP2 and MMP9 plasma levels to initial conditions. Rubus idaeus extract may help in reducing dienogest-associated weight gain, lower the size of endometriotic lesions, and have anti-inflammatory effects through MMP2 and MMP9 reduction.
1. Introduction
Endometriosis is an increasingly common pathology, considered by many researchers as the disease of the century [1]. World Health Organization (WHO) estimates that 10% of women of reproductive age worldwide are affected by this condition [2]. Endometriosis is a chronic estrogen-dependent disease defined by the histological presence of functional endometrial glands and stroma in ectopic areas, outside the uterine cavity [3]. Endometriosis lesions are characterized by a chronic inflammatory state, with increased infiltration of immune cells, which release various pro-inflammatory mediators, such as interleukin-1β, tumor necrosis factor-α, and prostaglandins [4].
A variety of factors have been suggested to contribute to the development of this condition, including retrograde menstruation, genetic predisposition, immunological dysregulation, and exposure to environmental stress factors [5]. Retrograde menstruation, which occurs when menstrual blood containing endometrial cells flows back into the peritoneal cavity, is widely accepted as a significant contributing factor in the development of endometriosis. Ectopic endometrial cells can attach and adhere to different tissues in the pelvis, resulting in the development of endometriosis lesions. The presence of inflammatory agents promotes the proliferation and invasion of endometrial cells, angiogenesis, and fibrosis, which are all facilitated by immune dysregulation [6].
Matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9), often referred to as gelatinase B, are enzymes that are involved in the degradation of the extracellular matrix (ECM), which is a complex structure of proteins that envelops and sustains cells. MMP-2 and MMP-9 are involved in the invasion and dissemination of endometrial cells, which leads to the occurrence and development of endometriosis. Research has consistently demonstrated that women with endometriosis have higher levels of MMP-2 and MMP-9 expression in their blood, peritoneal fluid, and endometrium compared to healthy individuals [7].
Transforming growth factor-beta 1 (TGF-β1) is a complex cytokine that is essential for controlling cell proliferation, differentiation, and death. TGF-β1 in involved in several facets of the pathophysiology of endometriosis, with many processes resembling those of MMPs. TGF-β1 enhances the proliferation and viability of endometrial cells, hence facilitating the development and extension of endometriosis lesions. TGF-β1 amplifies the tendency of endometrial cells to infiltrate and attach to adjacent tissues, thus promoting the development of newly formed endometriotic lesions. Also, TGF-β1 further stimulates angiogenesis and facilitates endometriosis lesions’ development and their viability. TGF-β1 controls the synthesis and breakdown of extracellular matrix (ECM) elements, which play a role in tissue remodeling and the development of adhesions in endometriosis. TGF-β1 regulates the immune response in endometriosis by inhibiting the function of cytotoxic T cells and natural killer cells, which play an essential role in removing aberrant cells [8].
The first-line treatment option for endometriosis is hormonal therapy. It functions by inhibiting the synthesis of estrogen, restricting the proliferation of endometrial tissue, and relieving discomfort. Dienogest, a synthetic progestin widely used for the treatment of endometriosis, exhibits its therapeutic effects by inhibiting ovulation, and selectively blocking the activity of cyclooxygenase-2 (COX-2), thus inhibiting inflammation. Dienogest also can modulate endometrial growth and upregulate inhibin A protein synthesis, which suppresses the synthesis of estrogen, making it possible to prevent endometriosis’ ectopic development [9,10].
Aside from hormonal therapy and occasionally surgery, many adjunctive therapies might be beneficial for endometriosis, such as analgesics that can alleviate pain, dietary adjustments, or complementary and alternative medicine (CAM) including acupuncture, yoga, and herbal supplements. Nevertheless, the available data are insufficient to ascertain the effectiveness of these therapies. Rubus idaeus, often known as raspberry, is a plant that possesses a substantial historical background of customary utilization in several societies. The extract has attracted considerable scientific attention because of its wide range of pharmacological qualities, such as its ability to reduce inflammation and function as an antioxidant [11,12,13,14].
The different raspberry extracts that are obtained from the foliage, stems, seeds, or fruits of the plant are highly concentrated reservoirs of bioactive compounds [15,16,17]. Specifically, the chemical composition of Rubus idaeus leaf extracts includes polyphenols, specifically ellagitannins and flavonoids [18,19,20]. Ellagitannins, which include ellagic acid and sanguiin H-6, are potent antioxidants that shield cells from harm by free radicals [21]. Flavonoids, such as quercetin, kaempferol, and myricetin, possess antioxidant characteristics and have been associated with several health benefits, including anti-inflammatory and anti-cancer qualities [22]. Additionally, leaf raspberry extract has many bioactive parts, including phenolic acids like gallic acid and chlorogenic acid, which help make it an antioxidant and an anti-inflammatory [23]. Furthermore, it includes volatile chemicals such as linalool, geraniol, and caryophyllene, which can provide flavor and fragrance advantages while also demonstrating antibacterial characteristics [15].
Rubus idaeus leaf extract (RiDE) was also listed as having antidiabetic and antihyperlipidemic activity by improving the body’s response to insulin, decreasing the levels of glucose in the blood, and reducing lipid profiles. Rubus idaeus leaf extract inhibits enzymes such as lipases and downregulates the transformation of triglycerides into free fatty acids. Furthermore, studies have demonstrated that RiDE effectively promotes the absorption of cholesterol by cells, facilitating its elimination from the body [24,25].
The RiDE aids in protecting cells from oxidative stress and DNA damage, both of which contribute to the onset and proliferation of cancer. It effectively eliminates free radicals, inhibits the activity of enzymes that participate in DNA methylation, and offers further protection to cells by blocking the binding of carcinogens to DNA, hence preventing mutations. Raspberry leaf extract also inhibits the uncontrolled proliferation of cancer cells, thereby preventing their excessive division and unregulated expansion by possessing the capacity to interfere with the mechanisms that enable the invasion and spread of cancer cells [26,27]. Lung, laryngeal, breast, and colorectal cancers are among the illnesses that have benefited from the anti-inflammatory and cytotoxic activity of RiDE [28,29,30].
Our study aimed to evaluate the role of RiDE’s effects in reducing the size of the endometriotic lesion in an in vivo rat experimental model, and its influence upon MMP-2, MMP-9, and TGF-β1 plasma levels, known as possible modulators of inflammation processes.
2. Results
2.1. High-Performance Liquid Chromatography–Mass Spectrometry (HPLC-MS) Analysis of Polyphenols from Rubus idaeus Extract
Figure 1 shows the HPLC fingerprint of phenolic compounds found in RiDE water extract. In total, 16 compounds were identified, as described in Table 1. Hydroxybenzoic acid compounds were predominantly identified in RiDE, accounting for approximately 52% of total phenolics as quantified by LC-MS. Flavonols, flavanols, and hydroxycinnamic acids were also present at significant quantities, accounting for approximately 26%, 14%, and 8% respectively.
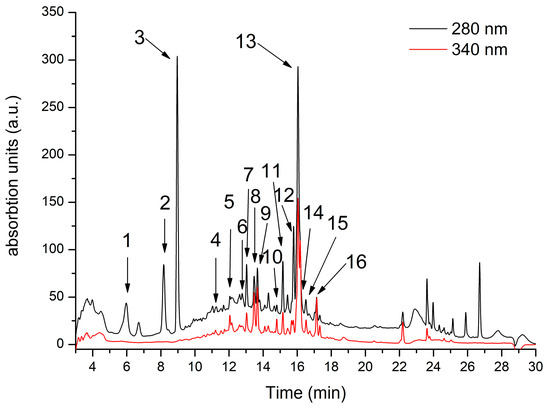
Figure 1.
Rubus idaeus HPLC chromatogram registered at 280 nm (black line) and 340 nm (red line). For peak assignment, see Table 1.

Table 1.
LC–MS data and phenolic compounds’ identification in Rubus idaeus leaf extract.
2.2. Preliminary Studies in Endometriosis Induction
The vaginal secretion of female rats consists of three types of cells including nucleated epithelial cells, leucocytes, and anucleated cornified epithelial cells [31]. The four stages of the estrous cycle were identified based on their cytological specific criteria (Figure 2), specifically based on the proportions of these cells present in the vaginal secretion.
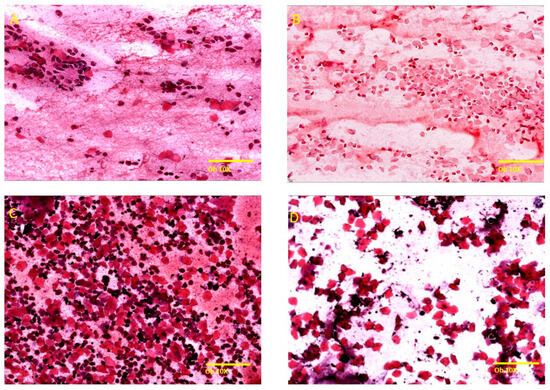
Figure 2.
Representation of the stages of the menstrual cycle in rats under Hematoxylin–Eosin staining (Ob. 10×, scale bar 200 μm). (A) Proestrus: small, nucleated epithelial cells with uniform appearance, observed individually on the surface of the smear, low number of large epithelial cells and keratinized anucleated cells, the presence of a low number of neutrophils. (B) Estrus: predominantly anucleated keratinized epithelial cells, frequent bacteria adhered to the cells or free on the smear, cellular relicts, occasionally neutrophils in late estrus. (C) Metestrus: anucleated keratinized epithelial cells with frequent neutrophils, polymorphonuclear leukocytes, leukocyte infiltrate. (D) Diestrus: low cellularity, keratinized anucleated epithelial cells, frequent neutrophils with small, nucleated cells, blood material and mucus.
The induction of endometriosis, performed in accordance with the menstrual cycle phases, indicates that the prevalence rate was higher in the metestrus/diestrum stage (four out of seven) as compared with the proestrus/estrus stage (two out of seven). The endometriotic lesions showed typical histologic alterations of endometriotic lesions, such as the presence of specific endometrial glands, some cystic and filled with hemorrhage and necrosis, and endometrial stroma invading the structure of blood vessels or organs (Figure 3).
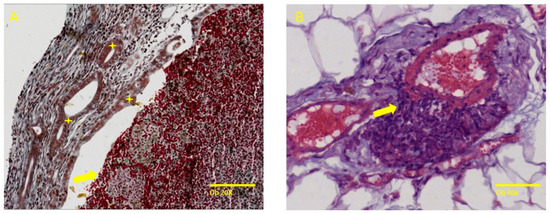
Figure 3.
Specific histological findings such as endometrial cystic glands, some filled with a chocolate-like fluid and surrounding stroma with an increased number of capillaries. (A) represents specific endometrial glands (yellow star) and necrotic hemorrhagic content in the gland lumen (yellow arrow) and (B) shows endometrial stromal cells invading the tunica media of an artery (black arrow). Trichrome stain, ob 20× (scale bar 100 µm), Hematoxylin–Eosin ob 40× (scale bar 50 µm), respectively.
2.3. Macroscopic Evidence of Endometriotic Tissue
Endometriosis was successfully induced in 40 out of 46 rats, followed by random allocation to the treatment and follow-up evaluation (Figure 4).
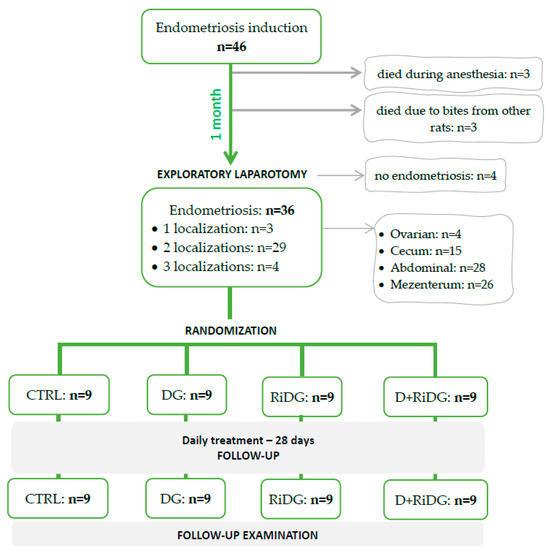
Figure 4.
The flowchart from endometriosis induction to randomization and evaluation. CTRL = control group, DG = dienogest group, RiDG = Rubus idaeus leaf extract group, and D+RiDG = dienogest + Rubus idaeus leaf extract group.
As expected, the weights of rats were increased in the follow-up, with the lowest increase in the CTRL group (Table 2).

Table 2.
Weight, diameter, and grade of lesions, by groups, from baseline to end of treatment.
The arithmetic mean diameter of the lesions is preserved in the CTRL group, while in treated groups, the values decreased with the most evident effects seen in D+RiDG (Table 2).
2.4. Effect of Rubus Idaues Leaf Extract on Plasma Levels of Matrix Metalloproteinases and Transforming Growth Factor Beta 1
Significantly higher levels of plasma MMP2 (p = 0.008), MMP9 (p = 0.001), and TGF-β1 (p = 0.053) were determined in the CTRL group when compared with the SHAM group (day 0) (Figure 5), supporting the successful induction of endometriosis in rats. The administration of dienogest succeeded in restoring the levels of MMP9, having statistically significant lower values (p = 0.007) than CTRL and no statistical difference when compared to the SHAM group. The administration of dienogest tended to reduce MMP2 and TGF-β1 levels as well, but with no statistical difference. The administration of RiDE significantly reduced MMP2 levels (p = 0.017) and MMP9 levels (p = 0.008) as compared to CTRL, restoring the levels similarly to the initial condition as observed when compared to the SHAM group. The administration of RiDE did not influence TGF-β1 level (Figure 5).
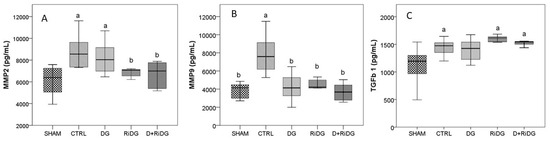
Figure 5.
Plasma levels of matrix metalloproteinases ((A) MMP2 and (B) MMP9) and of transforming growth factor beta 1 ((C) TGF-β1) by groups. The boxplots show the variation of investigated parameters where the midline represents the median values while the extreme lines indicate the value of the first and third quartile. Significant values were considered at p < 0.05 versus the SHAM group, a and b had p < 0.05 versus the CTRL group.
RiDE administration in addition to dienogest therapy succeeded in restoring the MMP2 and MMP9 values to the initial condition, with no statistically significant values as compared with SHAM, while in the case of TGF-β1, the plasma level showed no effect.
3. Discussion
The ectopic endometrial tissue responds to hormonal changes during the menstrual cycle, causing inflammation, dysmenorrhea, dyspareunia, persistent pelvic pain, irregular uterine bleeding, and infertility, thus endometriosis is considered a serious medical condition that has a major impact on the patient’s quality of life [32,33].
The mechanism of pain and inflammation in endometriosis is complex, not fully understood, and involves multiple factors such as chronic inflammation, neuroplastic changes, noxious stimuli, or peritoneal adhesions. Endometriosis can be treated clinically, surgically, or with both. The typical medicines, mainly hormonal therapy, cause disruptions to the menstrual cycle, and when the drugs are stopped, the pathology returns. The surgeries, when aiming to be curative or to remove adhesions, are often mutilating even though they can be straightforward. It is crucial to conduct experimental research using treatment approaches that can elucidate the pathophysiology of endometriosis. More insights into the degradation process of the extracellular matrix and further into the invasion and dissemination processes of endometrial cells will certainly help us to discover a more effective agent in the management of endometriosis. Thus, we aimed to identify alternative or complementary treatments to hormonal therapy to reduce its side effects. Consequently, RiDE’s effect on endometriosis lesions was approached both macroscopically and at the molecular level. The macroscopic examination targeted the evidence of endometriotic lesions such as weight, diameter, and grade, while the quantification of MMP2, MMP9, and TGF-β1 at the molecular level targeted the process of degradation of the extracellular matrix as well as the cell proliferation process.
Therefore, following the first aim, the first step of the current study was to mimic human endometriosis by using an autologous endometriosis rat model without ovariectomy or hormonal supplementation to obtain specific lesions. The preliminary study group was chosen on purpose to determine if the phase of the estrous cycle at the point of surgical induction may influence the development of endometriotic lesions. Because the proestrus and metestrus phases may last only up to a few hours [31], we deliberately chose to group the female rats in proestrus/estrus, and metestrus/diestrus, respectively. According to Cora and colleagues’ [34] criteria for staging the estrous cycle using vaginal smears, we have concluded that female rats in metestrus/diestrus, in our preliminary group, were more likely to develop endometriotic lesions. Disparate findings have been reported over the years regarding the most favorable estrous cycle phase appropriate for endometriosis induction to reach the highest probability of obtaining specific lesions. Kiani et al. [35] and Schreinemacher et al. [36] found no correlation between the timing of endometriosis induction in the estrous cycle phase and the latter endometriotic lesion achievement. On the other hand, other studies [37,38] are in favor of proestrus and estrus phases to perform the implants, reporting a higher chance of obtaining endometriotic lesions. A higher prevalence rate along with the volume of endometriotic lesions was also reported by Uchida and colleagues [39]. Within our study, the endometriosis induction success rate was 45.62%, which is consistent with the one reported by Kiani et al. [35] of 41.5%.
Variations in body weight were associated with the presence of endometriosis or hormonal-specific treatment. Weight loss due to chronic pelvic pain [40] or weight gain secondary to dienogest [41] have been reported. In our study, the female rats in the control group showed the lowest weight gain one month after endometriosis induction and in evolution when receiving no active medication (p < 0.0003). This phenomenon may be explained by the fact that chronic pain and inflammation induced by the presence and evolution of endometriotic tissue in the absence of treatment may determine the loss of appetite and decreased weight gain. At the end of the treatment, female rats in DG showed the highest weight increment with a median of 234 g (231 to 239), compared to RiDG 218 g (217 to 221) and D+RiDG 224 g (219 to 228) (p < 0.0001). Weight gain according to the percentile under treatment was expected, but the association of RiDE with dienogest in D+RiDG determined a lower weight gain in comparison to the female rats in DG, thus implying that RiDE may help in reducing dienogest-associated weight gain.
The lesions’ mean diameter in the CTRL group was preserved or tended to expand because, without specific treatment, endometriosis is a chronic evolving illness showing continuous growing and spreading. In female rats from all the treated groups, the values of the arithmetic mean diameter of the lesions decreased, with the most evident effects in the D+RiDG (Table 2), but with no statistical significance between groups, indicating that substances administration should be prolonged.
The second aim of this study was to identify alternative or complementary treatments to hormonal therapy to reduce its side effects. The approach was based on reducing inflammation, knowing its major role in endometriosis progression [42]. The uncontrolled cascade of inflammatory processes can lead to the upregulation of metalloproteinases, TGF-β, prostaglandins, chemokines, and cytokines [43]. Among these, the relationship between different diseases and the levels of MMPs is very important, since their activities are seen as elevated plasma, serum, or tissue levels in illnesses like cancers, diabetes, cardiovascular diseases, and neurodegenerative diseases [44,45,46,47,48,49,50]. The heightened activity of MMP-2 and MMP-9 plays a significant role in several important aspects of endometriosis as well. Specifically, the mechanism of action of MMP-2 and MMP-9 involves the degradation of the extracellular matrix (ECM), which facilitates the infiltration and attachment of endometrial cells to neighboring tissues, ultimately resulting in the development of endometriosis lesions [51]. Also, angiogenesis is facilitated by MMP-2 and MMP-9, which stimulate the development of newly formed blood vessels. These arteries provide oxygen and nutrition to endometriosis lesions, hence supporting their growth and survival [52,53]. Tissue remodeling is promoted by MMP-2 and MMP-9, which play a role in the generation of adhesions, the fibrous tissue that can cause tissues to adhere together in endometriosis [54]. Also, other factors can influence the levels of MMP-2, MMP-9, and TGF-β1 in endometriosis, including estrogen and progesterone, which are the primary sex hormones. Estrogen typically enhances the expression of MMP-2, MMP-9, and TGF-β1, while progesterone tends to decrease it. Inflammatory mediators, such as cytokines and growth factors, can also stimulate the production of MMP-2, MMP-9, and TGF-β1 in endometriosis. Additionally, certain genes that regulate MMP-2, MMP-9, and TGF-β1 may contribute to the elevated expression of these enzymes in endometriosis.
To date, there are not many studies focused on the utilization of anti-MMP compounds in patients with endometriosis. Most of the studies discuss the use of MMPs as biomarkers underlying drugs’ ability to reduce their expression [7]. Our study demonstrated that the administration of RiDE may be an important agent for treating endometriosis, especially by reducing the MMPs levels. Specifically, it was observed that the administration of RiDE in rats with endometriosis was able to restore MMP2 and MMP9 values to normal conditions. The mechanism of action by which RiDE reduces MMPs levels is yet to be elucidated. When various molecules and cytokines such as MMPs, VEGFs, hypoxia-inducible factor-1α (HIF-1α), IL-2, IL-27 are dysfunctional, they enhance both angiogenesis and the development of endometriotic lesions [55,56]. Also, the growth and progression of endometrial stromal cells are influenced by the alteration of different pathways, such as NF-κB, Wnt/β-catenin, JAK/STAT, COX2/PGE2, and TGF-β upregulation. These abnormalities can lead to the expression of tumor-like traits in endometrial stromal cells, such as the overproduction of ROS, inflammatory reactions, immune system activation, angiogenesis, proliferation, fibrosis, apoptosis, and autophagy [7,55,57,58,59]. Further, Tcf/β-catenin targets both MMP-9 and MMP-2 genes. Consequently, it was observed that in endometrial epithelial cells from patients with endometriosis, Cyclin D1, a Tcf/β-catenin target gene, had significantly higher mRNA expression in the mid-secretory phase [60]. In addition to angiogenesis, invasion, tissue organization, and cell survival, MMPs may also be involved in the regulation of cytokines, other MMP family members, and latent growth factors [61].
However, studies in the literature have already suggested that the daily optimal consumption of fruits and vegetables high in polyphenol compounds decreases the risk of developing specific chronic and degenerative diseases with an inflammatory component, via MMPs regulation [62,63,64]. Accordingly, multiple studies have examined the chemical composition of the raspberry leaf extract, uncovering a wide range of bioactive compounds that have prompted research into its potential uses in several diseases, especially those related to inflammation and metabolic disorders [22,65]. More specifically, the antioxidant and anti-inflammatory effects of the phenolic compounds extracted from Rubus idaeus leaves can be attributed to the rich composition of the extract in ellagic acid, quercetin, and kaempferol derivatives, as well as catechins. The effects of ellagic acid have been described to regulate multiple pathways such as proinflammatory agents’ inhibition [66], antioxidant response activation [67], fibrotic formation, growth factor, and adhesion molecules regulation [68,69]. Quercetin enacts an anti-inflammatory activity through the inhibition of the eicosanoid’s pathway via enzymatic and non-enzymatic arachidonic acid oxidation, which in turn can reduce the production of inflammatory mediators such as prostaglandins and leukotrienes [70,71]. Additionally, the anti-inflammatory effect of kaempferol is strongly related to its ability to suppress the release of IL-1β, IL-6, IL-18, and TNF-α [72], to increase the mRNA and protein expression of Nrf2-regulated genes [73], to inhibit the toll-like receptor 4 (TLR4) [74], and to inhibit COX1 and COX2 production [75,76]. The role of catechins in reducing inflammation is also well documented. Thus, catechins possess anti-inflammatory activities by regulating the activation/deactivation of different inflammatory and oxidative stress cell signaling pathways like nuclear factor-kappa B (NF-κB), transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2), mitogen-activated protein kinases (MAPKs) or signal transducer, and the activator of transcription 1/3 (STAT1/3) pathways [77]. Also, according to the literature, another possible effect of phenolics has been reported on epi transcriptomic pathways, such as the fact that m6A modification might modulate TGFβ signaling [78,79] as a result of its activation during the epithelial to mesenchymal transition process characteristic of endometriosis [80,81].
Nevertheless, the anti-inflammatory effect of RiDE is obtained due to the synergistic effects of various polyphenol compounds. That is, its beneficial effect is supported by its well-known utilization in traditional herbal medicine for centuries, having been used to treat many ailments associated with the female reproductive system, particularly during pregnancy, and to aid in childbirth, with long-lasting effects seen into the modern day [82,83].
4. Materials and Methods
4.1. Chemicals
Acetonitrile, acetic acid, and carboxymethylcellulose sodium (CMC) were purchased from Sigma Aldrich (St. Louis, MO, USA). Dienogest was purchased from Bayer Weimar GmbH und Co. KG (Weimar, Germany). The injectable anesthetics, ketamine and xylazine, were purchased from Biotur Exim SRL, Alexandria, Romania. The RiDE used in this study is a commercial product produced by Herbiolys Laboratoire Bio (Lardier-et-Valença, France), and it contains bio raspberry 3% leaf bud extract, vegetable glycerin and low mineralized spring water.
4.2. High-Performance Liquid Chromatography–Mass Spectrometry (HPLC-MS) Analysis of Polyphenols from Rubus idaeus Extract
The phenolic compounds’ separation was performed according to the protocol described by Pop et al. (2018) [24]. In short, an Agilent 1200 HPLC apparatus with a DAD detector connected to a 6110 single quadrupole MS was used for compound identification and quantification. The compound separation was achieved using a reverse-phase Eclipse XDB C 18 column (4.6 mm × 5.6 mm, 5 μm, Agilent Technologies, Santa Clara, CA, USA) using a gradient elution of (A) water + 0.1% acetic acid and (B) acetonitrile + 0.1% acetic acid for 30 min. The temperature was set at 25 °C at a flow of 0.5 mL/min. The gradient used was as follows (expressed as % B): 0 min, 5% B; 0–2 min, 5% B; 2–18 min, 5–40% B; 18–20 min, 40–90% B; 20–24 min, 90% B; 24–25 min, 90–5% B; 25–30 min, 5% B. The compound spectra were registered at 280 for phenolic acids and 340 nm for flavonols, respectively. Quantification was performed using calibration curves of different standard solutions of ellagic acid (R2 = 0.9978; LOD = 0.35 μg/mL, LOQ = 1.05 μg/mL), catechin (R2 = 0.9985; LOD = 0.18 μg/mL, LOQ = 0.72 μg/mL), chlorogenic acid (R2 = 0.9937; LOD = 0.41 μg/mL, LOQ = 1.64 μg/mL), gallic acid (R2 = 0.9978; LOD = 0.36 μg/mL, LOQ = 1.44 μg/mL) and rutin (R2 = 0.9981; LOD = 0.21 μg/mL, LOQ = 0.84 μg/mL). Hydroxybenzoic acids (peaks 1,2 3 and 9) were quantified as gallic acid equivalents; ellagic acid and its derivatives (peaks 7, 10, and 13) were calculated as ellagic acid equivalents; hydroxycinnamic acids (peaks 5 and 8) were quantified as chlorogenic acid equivalents, flavanols (peaks 4 and 6) were calculated a catechin equivalents, while flavonols (peaks 10, 12, 13, 15 and 16) were calculated as rutin equivalents. Compound identification was performed based on retention time, UV-Vis absorption spectra, and mass spectra by comparison with the ones obtained from standards, the Phenol-Explorer database, and data in the literature.
4.3. Animals
A total number of 60 young virgin female rats, Wistar breed, aged approximately 6–8 weeks, 145–160 g, were used during this experimental study. Before enrolment in the experiment, the female rats were subjected to the acclimatization process, over 10 days, the animals being kept (5 rats/cage) in a light/dark cycle of 12 h, having ad libitum access to water and food. The experimental procedure was accepted and approved by the Ethics Committee and the Veterinary Health Department (No 314/20.05.2022) and carried out following legislation on the use of laboratory animals, as well as with the animal care regulations in force.
4.4. Endometriosis Surgical Induction in Rats
4.4.1. Preliminary Studies in Endometriosis Surgical Induction
Initially, 14 female rats were selected to be included in a preliminary study that aimed to establish which of the phases of the estrous cycle is the most appropriate to successfully induce endometriosis. Each female was subjected to a clinical examination to assess the vaginal opening and subsequently, daily vaginal smears were performed, to identify the four stages of the estrous cycle (proestrus, estrus, metestrus, and diestrus). Following this, the preliminary study group was subdivided into two subgroups: the proestrus/estrus subgroup (7 female rats) and metestrus/diestrus (7 female rats), respectively. All 14 female rats in the preliminary study underwent the experimental induction of endometriotic lesions. For the surgical induction of endometriosis, the animals were subjected to general intraperitoneal anaesthesia (ketamine 0.01 mg/kg and xylazine 0.01 mg/kg). Throughout the intervention, the animals were kept in aseptic conditions, and the body temperature was maintained at 37 °C by placing them on a heated plate.
The endometriosis induction surgery steps (Figure 6) were based on the initial protocol described by Vernon and Wilson for rats [84] and Cummings and Metcalf [85]. Accordingly, the animals were prepared for surgery by trimming the abdominal area, and then a median incision was made at the level of skin tissue and the abdominal muscle to expose the organs of the pelvic and abdominal cavities. The uterine horns were identified, and a piece of approximately 1.5 cm of the middle portion of the uterine right horn wall was harvested. The tissue block was split longitudinally and processed so that only the endometrium was retrieved and prepared in 4 implants with an approximate size of 2.5 × 3.5 mm from the excised uterus. In each rat, the implants were sutured at the level of the abdominal parietal peritoneum, to the surface of the left ovary, to the surface of the cecum, and the mesentery near the artery irrigating the cecum. The implants were sutured with the endometrium towards the peritoneal cavity using a using 8-0 Nylon monofilament suture thread. After applying the implants, the muscle layer on the abdomen was sutured using 6-0 Nylon monofilament suture thread and the skin tissue was sutured using 3-0 Nylon monofilament suture thread.
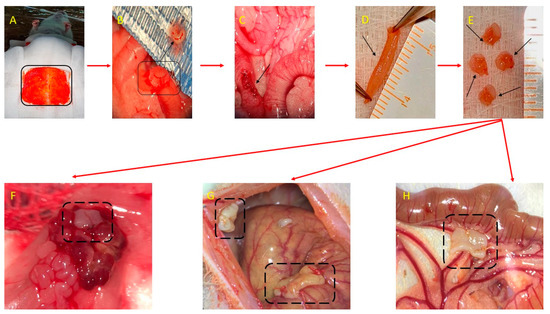
Figure 6.
Surgical induction of endometriosis-autologous transplant: (A) Anesthesia, restraint of the animal, identification, and highlighting of the tissue sampling area. (B) A portion of tissue from the right uterine horn of the rat was removed, through a longitudinal opening. (C) Suture of the uterine horn after harvesting the endometrial tissue. (D) The portion of endometrial tissue harvested after longitudinal sectioning. (E) The 4 uterine fragments with a size of 2.5/3.5 mm, which are sutured at the level of the left ovary (F), the abdominal wall (the right side) (G), the cecum (G) and the mesentery (H).
After the intervention, the rats were carefully monitored for the following days for signs of wound infection or acute wound complications such as dehiscence, evisceration, hernia, seroma, or hematoma.
One month later, during exploratory laparotomy, the macroscopic evaluation of the abdominal and peritoneal cavities indicated that female rats in the metestrus/diestrus phase at the time of the induction were more likely to develop specific endometriotic lesions. The endometriotic lesions were harvested, and trichrome and H&E stains were performed on the tissue specimen, and endometriosis was microscopically proven.
4.4.2. Endometriosis Histopathological Assessment
Macroscopically dark red to brown fluid-cystic soft lesions were retrieved from the female rats in the preliminary group and were fixed in 10% formalin solution. After embedding in paraffin, 5 µm-thick tissue samples were prepared to be stained with Hematoxylin and Eosin and trichrome stain, and examined under a light microscope (Leica DM750 LED Phase Microscope ICC50W Camera Module). The presence of endometrial glands and endometrial stroma was assessed to confirm the diagnosis.
4.4.3. Endometriosis Surgical Induction-Experimental Study
According to the results of the initial preliminary experiment, endometriosis was induced in another 46 female rats. Before surgery, each female rat was clinically examined to assess its health state. Next, surgical induction was performed during the metestrus/diestrus phase of the estrous cycle. During the entire surgical intervention, the animals were carefully monitored. During the follow-up, the rats were monitored daily for proper food and water intake and their behavior. Their weight was also recorded before endometriosis induction, at 4 weeks since endometriosis induction, and at 8 weeks (at the end of the experiment).
4.4.4. Endometriosis Macroscopic Evaluation
One month after the endometriosis surgical induction, the remaining 40 female rats were subjected to an exploratory laparotomy under general anesthesia as in the initial stage of the experiment. Macroscopic evaluation during the exploratory laparotomy aimed to evaluate the abdominal and pelvic cavities and assess the endometriosis-specific lesions. At this stage, the endometriosis was evaluated based on the correlation between macroscopic and microscopic aspects of the endometriotic lesions as induced in the preliminary study, the diagnosis of endometriosis being macroscopically assumed. For each female rat that developed macroscopic endometriotic lesions, the lesions were measured with a ruler and classified as exemplified in Figure 7. The mean of the degree of macroscopic growth was calculated for each female rat.
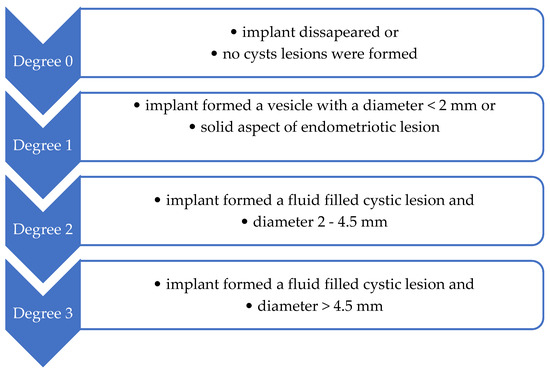
Figure 7.
Endometriotic lesions’ classification, considering the degree of macroscopic growth (Quereda et al. [86]).
4.4.5. Treatment Administration in Endometriotic Rats
The day after the exploratory laparotomy, the female rats with endometriosis were randomly divided into 4 groups, 9 rats/group as follows: control group (CTRL), dienogest group (DG), Rubus idaeus group (RiDG), and dienogest + Rubus idaeus group (D+RiDG). The CTRL group received 0.5 mL/day CMC, the DG group received dienogest (1 mg/kg b.w./day, where b.w. = body weight), the RiDG group received RiDE (0.25 mL/kg b.w./day), and the D+RiDG group received dienogest (1 mg/kg b.w./day) and RiDE (0.25 mL/kg b.w./day), respectively. All substances were administered orally by gavage. Dienogest was suspended in CMC before administration. The treatment was administered daily for 28 days.
At the end of the treatment period, the rats were euthanized with an overdose of anesthetics, and then the abdominal and pelvic cavities were evaluated to assess the evolution of the endometriotic implants under treatment.
4.5. Blood Samples and ELISA Analysis
In total, 2 mL of blood was drawn from the retroorbital sinus in the two steps of the experiment: before applying the implants and at the end of the treatment. The blood was collected using EDTA vacutainers and centrifuged at 2500 rpm for 10 min. Plasma was separated and then stored at −80 °C until analysis.
The plasma levels of matrix metalloproteinase 2 (MM2), matrix metalloproteinase 9 (MMP9), and transforming growth factor-beta 1 (TGF-β1) were measured using the enzyme-linked immunosorbent assay (ELISA) technique (Stat Fax 303 Plus Microstrip Reader, Minneapolis, MN, USA). The analysis was performed according to the instruction manuals provided by the commercially available kits (MMP2 and MMP9- Boster Biological Technology, USA and TGF-β1- FineTest-Wuhan Fine Biotech Co., Ltd., Wuhan, China). The results obtained from the rats before applying the implants are referred to in the text as the SHAM group.
4.6. Statistical Analysis
The diameter of lesion per rat was considered as the arithmetic mean of all lesions regardless of the location. The measurements were reported as median and (Q1 to Q2) (where Q1 is the 25th percentile and Q3 is the 75th percentile) since the variability of measurements was high within the groups. An overall comparison between groups was made using the Kruskal–Wallis test, followed by post-hoc analysis whenever statistically significant differences were observed. The differences within a specific group were tested with the Friedman test when more than two measurements were available and with the Wilcoxon test when two paired measurements were available. Numbers and percentages have been reported as summary statistics of qualitative variables. No statistical tests were applied to qualitative variables because the assumptions of the Chi-squared family tests were not accomplished. Data were analyzed with the Statistica program (v. 13, StatSoft, St Tulsa, OK, USA). For ELISA analysis, data were presented as mean ± standard deviation (SD). Further, an independent sample t-test was applied to check the statistical significance between different groups. p-values lower than 0.05 (p < 0.05) were taken into consideration.
5. Conclusions
We may conclude that the administration of RiDE has an anti-inflammatory effect, as observed by the reduction in endometriotic lesions and the reduction in both plasma MMP2 and MMP9 levels. The fact that the macroscopic examination yielded no statistically significant results indicates that the administration of RiDE over a longer period may lead to more significant clinical effects. The main strength of this study was the administration of RiDE in addition to dienogest treatment, miming the situations encountered in day-to-day life when most people take natural supplements without any medical advice. This information is valuable, offering important insights into the effects of plant administration concomitantly with drugs, since they can either have enhancing or reducing drug effects and can induce adverse reactions. This study also had some limitations. One limitation of this study was the short-term administration of dienogest and RiDE. Thus, the effects of the used substances should be monitored over a longer period. Another limitation was that tissue levels of MMPs and TGFβ1 were not determined. Thus, future studies will be needed to investigate whether the association of different concentrations of RiDE and lower doses of dienogest might be efficient in reducing the diameter of endometriotic lesions. Consequently, it could be explored whether dienogest’s dose-dependent side effects on long-term administration will be diminished by RiDE association. Secondly, the correlation between plasma and tissular levels of MMPs and TGFβ1 should be approached to offer a better perspective on the effects of RiDE in endometriosis etiopathogenesis and its adjuvant role in endometriotic lesions management.
Author Contributions
Conceptualization, E.-M.J., R.M.P., L.M.G. and R.-A.Ș.; methodology, R.-A.Ș., P.-A.Ș., S.D.B. and M.M.O.; software, R.-A.Ș., S.D.B., M.M.O. and C.M.; validation, S.D.B., C.M.M. and F.R.; formal analysis, R.-A.Ș., P.-A.Ș., F.R. and C.M.; investigation, F.R., V.R. and A.-M.L.; resources, R.-A.Ș., V.R., F.R. and C.M.M.; data curation, R.M.P., S.D.B., V.R., C.M. and I.A.S.; writing—original draft preparation, E.-M.J., L.M.G., M.M.O. and C.M.; writing—review and editing, P.-A.Ș., I.-D.N., I.A.S. and C.M.M.; visualization, F.R., A.-M.L., L.M.G., P.-A.Ș. and I.-D.N.; supervision, E.-M.J., C.M.M. and R.M.P.; project administration, E.-M.J., L.M.G., C.M.M. and R.M.P.; funding acquisition, E.-M.J., L.M.G., C.M.M. and R.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Iuliu Hatieganu” University of Medicine and Pharmacy Cluj-Napoca, internal grant number 35145/17.12.2021.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca and the Veterinary Health Department (No 314/20.05.2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lamceva:, J.; Uljanovs, R.; Strumfa, I. The Main Theories on the Pathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 4254. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Endometriosis. Available online: https://www.who.int/news-room/fact-sheets/detail/endometriosis (accessed on 20 December 2023).
- Brosens, I.; Puttemans, P.; Gordts, S.; Campo, R.; Gordts, S.; Benagiano, G. Early Stage Management of Ovarian Endometrioma to Prevent. Facts Views Vis. ObGyn 2013, 5, 309–314. [Google Scholar] [PubMed]
- Donnez, J.; Cacciottola, L. Endometriosis: An Inflammatory Disease That Requires New Therapeutic Options. Int. J. Mol. Sci. 2022, 23, 1518. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O.; Giudice, L.C. Pathogenesis and Pathophysiology of Endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef]
- Abramiuk, M.; Grywalska, E.; Małkowska, P.; Sierawska, O.; Hrynkiewicz, R.; Niedźwiedzka-Rystwej, P. The Role of the Immune System in the Development of Endometriosis. Cells 2022, 11, 2028. [Google Scholar] [CrossRef]
- Ke, J.; Ye, J.; Li, M.; Zhu, Z. The Role of Matrix Metalloproteinases in Endometriosis: A Potential Target. Biomolecules 2021, 11, 1739. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yi, L.; Du, M.; Gong, G.; Zhu, Y. Overexpression of TGF-β Enhances the Migration and Invasive Ability of Ectopic Endometrial Cells via ERK/MAPK Signaling Pathway. Exp. Ther. Med. 2019, 17, 4457–4464. [Google Scholar] [CrossRef]
- Hayashi, A.; Tanabe, A.; Kawabe, S.; Hayashi, M.; Yuguchi, H.; Yamashita, Y.; Okuda, K.; Ohmichi, M. Dienogest Increases the Progesterone Receptor Isoform B/A Ratio in Patients with Ovarian Endometriosis. J. Ovarian Res. 2012, 5, 31. [Google Scholar] [CrossRef]
- Malik, R.; Mann, M.K. Role of Dienogest in Endometriosis in Young Women. J. Obstet. Gynecol. India 2021, 71, 522–529. [Google Scholar] [CrossRef]
- Schell, J.; Betts, N.M.; Lyons, T.J.; Basu, A. Raspberries Improve Postprandial Glucose and Acute and Chronic Inflammation in Adults with Type 2 Diabetes. Ann. Nutr. Metab. 2019, 74, 165–174. [Google Scholar] [CrossRef]
- Menezes, R.; Foito, A.; Jardim, C.; Costa, I.; Garcia, G.; Rosado-Ramos, R.; Freitag, S.; Alexander, C.J.; Outeiro, T.F.; Stewart, D.; et al. Bioprospection of Natural Sources of Polyphenols with Therapeutic Potential for Redox-Related Diseases. Antioxidants 2020, 9, 789. [Google Scholar] [CrossRef]
- Bibi, S.; Kang, Y.; Du, M.; Zhu, M.J. Dietary Red Raspberries Attenuate Dextran Sulfate Sodium-Induced Acute Colitis. J. Nutr. Biochem. 2018, 51, 40–46. [Google Scholar] [CrossRef]
- Fotschki, B.; Laparra, J.M.; Sójka, M. Raspberry Polyphenolic Extract Regulates Obesogenic Signals in Hepatocytes. Molecules 2018, 23, 2103. [Google Scholar] [CrossRef] [PubMed]
- De Santis, D.; Carbone, K.; Garzoli, S.; Masci, V.L.; Turchetti, G. Bioactivity and Chemical Profile of Rubus idaeus L. Leaves Steam-Distillation Extract. Foods 2022, 11, 1455. [Google Scholar] [CrossRef]
- Teslić, N.; Santos, F.; Oliveira, F.; Stupar, A.; Pojić, M.; Mandić, A.; Pavlić, B.; Kljakić, A.C.; Duarte, A.R.C.; Paiva, A.; et al. Simultaneous Hydrolysis of Ellagitannins and Extraction of Ellagic Acid from Defatted Raspberry Seeds Using Natural Deep Eutectic Solvents (NADES). Antioxidants 2022, 11, 254. [Google Scholar] [CrossRef]
- Garjonyte, R.; Budiene, J.; Labanauskas, L.; Judzentiene, A. In Vitro Antioxidant and Prooxidant Activities of Red Raspberry (Rubus idaeus L.) Stem Extracts. Molecules 2022, 27, 4073. [Google Scholar] [CrossRef] [PubMed]
- Ponder, A.; Hallmann, E. Phenolics and Carotenoid Contents in the Leaves of Different Organic and Conventional Raspberry (Rubus idaeus L.) Cultivars and Their in Vitro Activity. Antioxidants 2019, 8, 458. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Nowicka, P.; Teleszko, M.; Cebulak, T.; Wolanin, M. Determination of Phenolic Compounds and Antioxidant Activity in Leaves from Wild Rubus L. Species. Molecules 2015, 20, 4951–4966. [Google Scholar] [CrossRef]
- Staszowska-Karkut, M.; Materska, M. Phenolic Composition, Mineral Content, and Beneficial Bioactivities of Leaf Extracts from Black Currant (Ribes nigrum L.), Raspberry (Rubus idaeus), and Aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef]
- MacIerzyński, J.; Sójka, M.; Kosmala, M.; Karlińska, E. Transformation of Oligomeric Ellagitannins, Typical for Rubus and Fragaria Genus, during Strong Acid Hydrolysis. J. Agric. Food Chem. 2020, 68, 8212–8222. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, Anti-Inflammatory and Cytotoxic Activity of Phenolic Compound Family Extracted from Raspberries (Rubus idaeus): A General Review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.-W.; Gong, C.-C.; Song, H.-F.; Cui, Y.-Y. Effects of Anthocyanins on the Prevention and Treatment of Cancer LINKED ARTICLES. Br. J. Pharmacol. 2017, 174, 1226. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Wasielica, J.; Olejnik, A.; Kowalska, K.; Olkowicz, M.; Dembczyński, R. Elderberry (Sambucus nigra L.) Fruit Extract Alleviates Oxidative Stress, Insulin Resistance, and Inflammation in Hypertrophied 3T3-L1 Adipocytes and Activated RAW 264.7 Macrophages. Foods 2019, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Sun, H.; Tang, M.; Zhao, J.; Zhang, Z.; Sun, X.; He, S. Red Raspberry Extract (Rubus idaeus L. Shrub) Intake Ameliorates Hyperlipidemia in HFD-Induced Mice through PPAR Signaling Pathway. Food Chem. Toxicol. 2019, 133, 110796. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.M.; Lamenza, F.F.; Upadhaya, P.; Pracha, H.; Springer, A.; Swingler, M.; Siddiqui, A.; Oghumu, S. Black Raspberry Extract Inhibits Regulatory T-Cell Activity in a Murine Model of Head and Neck Squamous Cell Carcinoma Chemoprevention. Front. Immunol. 2022, 13, 932742. [Google Scholar] [CrossRef] [PubMed]
- Krauze-Baranowska, M.; Głód, D.; Kula, M.; Majdan, M.; Hałasa, R.; Matkowski, A.; Kozłowska, W.; Kawiak, A. Chemical Composition and Biological Activity of Rubus idaeus Shoots—A Traditional Herbal Remedy of Eastern Europe. BMC Complement. Altern. Med. 2014, 14, 1–12. [Google Scholar] [CrossRef]
- Simonovic, M.; Kojic, V.; Jakimov, D.; Glumac, M.; Pejin, B. Raspberry Seeds Extract Selectively Inhibits the Growth of Human Lung Cancer Cells In Vitro. Nat. Prod. Res. 2021, 35, 2253–2256. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, D.; Baek, S.E.; Kim, K.H.; Kang, K.S.; Jang, T.S.; Lee, H.L.; Song, J.H.; Yoo, J.E. Cytotoxic Effect of Sanguiin H-6 on MCF-7 and MDA-MB-231 Human Breast Carcinoma Cells. Bioorganic Med. Chem. Lett. 2017, 27, 4389–4392. [Google Scholar] [CrossRef]
- Durgo, K.; Belščak-Cvitanović, A.; Stančić, A.; Franekić, J.; Komes, D. The Bioactive Potential of Red Raspberry (Rubus idaeus L.) Leaves in Exhibiting Cytotoxic and Cytoprotective Activity on Human Laryngeal Carcinoma and Colon Adenocarcinoma. J. Med. Food 2012, 15, 258–268. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the Estrous Cycle and Induction of Estrus in Experimental Rodents: An Update. Fertil. Res. Pract. 2020, 6, 1–15. [Google Scholar] [CrossRef]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; D’Hooghe, T.; De Cicco Nardone, F.; De Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T. Impact of Endometriosis on Quality of Life and Work Productivity: A Multicenter Study across Ten Countries. Fertil. Steril. 2011, 96, 366–373. [Google Scholar] [CrossRef]
- Schliep, K.C.; Chen, Z.; Stanford, J.B.; Xie, Y.; Mumford, S.L.; Hammoud, A.O.; Boiman Johnstone, E.; Dorais, J.K.; Varner, M.W.; Buck Louis, G.M.; et al. Endometriosis Diagnosis and Staging by Operating Surgeon and Expert Review Using Multiple Diagnostic Tools: An Inter-Rater Agreement Study. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 220–229. [Google Scholar] [CrossRef]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse:Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Kiani, K.; Movahedin, M.; Malekafzali, H.; Mirfasihi, F.; Nargess Sadati, S.; Moini, A.; Nasser Ostad, S.; Aflatoonian, R. Effect of the Estrus Cycle Stage on the Establishment of Murine Endometriosis Lesions. Int. J. Reprod. BioMedicine 2018, 16, 305. [Google Scholar] [CrossRef]
- Schreinemacher, M.H.; Backes, W.H.; Slenter, J.M.; Xanthoulea, S.; Delvoux, B.; van Winden, L.; Beets-Tan, R.G.; Evers, J.L.H.; Dunselman, G.A.J.; Romano, A. Towards Endometriosis Diagnosis by Gadofosveset-Trisodium Enhanced Magnetic Resonance Imaging. PLoS ONE 2012, 7, e33241. [Google Scholar] [CrossRef] [PubMed]
- Rudzitis-Auth, J.; Krbel, C.; Scheuer, C.; Menger, M.D.; Laschke, M.W. Xanthohumol Inhibits Growth and Vascularization of Developing Endometriotic Lesions. Hum. Reprod. 2012, 27, 1735–1744. [Google Scholar] [CrossRef]
- Laschke, M.W.; Schwender, C.; Scheuer, C.; Vollmar, B.; Menger, M.D. Epigallocatechin-3-Gallate Inhibits Estrogen-Induced Activation of Endometrial Cells in Vitro and Causes Regression of Endometriotic Lesions in Vivo. Hum. Reprod. 2008, 23, 2308–2318. [Google Scholar] [CrossRef]
- Uchida, M.; Kobayashi, O. Sequential Observation of Implanted Endometriosis by Laparoscopy in Rats: Correlation between the Prevalence Rate and the Estrous Cycle. J. Pharmacol. Sci. 2013, 121, 299–304. [Google Scholar] [CrossRef]
- Persoons, E.; De Clercq, K.; Van den Eynde, C.; Pinto, S.J.P.C.; Luyten, K.; Van Bree, R.; Tomassetti, C.; Voets, T.; Vriens, J. Mimicking Sampson’s Retrograde Menstrual Theory in Rats: A New Rat Model for Ongoing Endometriosis-Associated Pain. Int. J. Mol. Sci. 2020, 21, 2326. [Google Scholar] [CrossRef] [PubMed]
- Muzii, L.; Di Tucci, C.; Galati, G.; Carbone, F.; Palaia, I.; Bogani, G.; Perniola, G.; Tomao, F.; Kontopantelis, E.; Di Donato, V. The Efficacy of Dienogest in Reducing Disease and Pain Recurrence after Endometriosis Surgery: A Systematic Review and Meta-Analysis. Reprod. Sci. 2023, 30, 3135–3143. [Google Scholar] [CrossRef]
- Santulli, P.; Marcellin, L.; Noël, J.C.; Borghese, B.; Fayt, I.; Vaiman, D.; Chapron, C.; Méhats, C. Sphingosine Pathway Deregulation in Endometriotic Tissues. Fertil. Steril. 2012, 97, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Laganà, A.S.; Salmeri, F.M.; Triolo, O.; Palmara, V.I.; Vitale, S.G.; Sofo, V.; Králíčková, M. Regulation of Apoptotic Pathways during Endometriosis: From the Molecular Basis to the Future Perspectives. Arch. Gynecol. Obstet. 2016, 294, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.H.; Gu, G.S.; Min, Y.; Driver, V.R. Role of Matrix Metalloproteinases in Chronic Wound Healing: Diagnostic and Therapeutic Implications. Chin. Med. J. 2014, 127, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, G.; Sehgal, A.; Bhardwaj, S.; Singh, S.; Buhas, C.; Judea-Pusta, C.; Uivarosan, D.; Munteanu, M.A.; Bungau, S. Multifaceted Role of Matrix Metalloproteinases in Neurodegenerative Diseases: Pathophysiological and Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 1413. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G. Metalloproteinases and Neurodegenerative Diseases: Pathophysiological and Therapeutic Perspectives. Met. Med. 2015, 39, 39–50. [Google Scholar] [CrossRef]
- Uemura, S.; Matsushita, H.; Li, W.; Glassford, A.J.; Asagami, T.; Lee, K.-H.; Harrison, D.G.; Tsao, P.S. Diabetes Mellitus Enhances Vascular Matrix Metalloproteinase Activity Role of Oxidative Stress. Circ. Res. 2001, 88, 1291–1298. [Google Scholar] [CrossRef]
- Guo, F.; Tang, C.; Huang, B.; Gu, L.; Zhou, J.; Mo, Z.; Liu, C.; Liu, Y. LncRNA H19 Drives Proliferation of Cardiac Fibroblasts and Collagen Production via Suppression of the miR-29a-3p/miR-29b-3pVEGFA/TGF-β Axis. Mol. Cells 2022, 45, 122–133. [Google Scholar] [CrossRef]
- Wang, S.; Jia, J.; Liu, D.; Wang, M.; Wang, Z.; Li, X.; Wang, H.; Rui, Y.; Liu, Z.; Guo, W.; et al. Matrix Metalloproteinase Expressions Play Important Role in Prediction of Ovarian Cancer Outcome. Sci. Rep. 2019, 9, 11677. [Google Scholar] [CrossRef]
- Seo, J.Y.; Kim, T.H.; Kang, K.R.; Lim, H.; Choi, M.C.; Kim, D.K.; Chun, H.S.; Kim, H.J.; Yu, S.K.; Kim, J.S. 7α,25-Dihydroxycholesterol-Induced Oxiapoptophagic Chondrocyte Death via the Modulation of P53-Akt-mTOR Axis in Osteoarthritis Pathogenesis. Mol. Cells 2023, 46, 245–255. [Google Scholar] [CrossRef]
- Fingleton, B. Matrix Metalloproteinases as Regulators of Inflammatory Processes. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 2036–2042. [Google Scholar] [CrossRef]
- Begum, Y.; Pandit, A.; Swarnakar, S. Insights Into the Regulation of Gynecological Inflammation-Mediated Malignancy by Metalloproteinases. Front. Cell Dev. Biol. 2021, 9, 780510. [Google Scholar] [CrossRef] [PubMed]
- Bostanci Durmus, A.; Dincer Cengiz, S.; Yılmaz, H.; Candar, T.; Gursoy, A.Y.; Sinem Caglar, G. The Levels of Matrix Metalloproteinase-9 and Neutrophil Gelatinase-Associated Lipocalin in Different Stages of Endometriosis. J. Obstet. Gynaecol. 2019, 39, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Barbe, A.M.; Berbets, A.M.; Davydenko, I.S.; Koval, H.D.; Yuzko, V.O.; Yuzko, O.M. Expression and Significance of Matrix Metalloproteinase-2 and Matrix Metalloproteinas-9 in Endometriosis. J. Med. Life 2020, 13, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, T.; Tong, D.; Li, S.; Yu, X.; Liu, B.; Jiang, L.; Liu, K. Research Advances in Endometriosis-Related Signaling Pathways: A Review. Biomed. Pharmacother. 2023, 164, 114909. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.-M.; Lai, Z.-Z.; Ha, S.-Y.; Yang, H.-L.; Liu, L.-B.; Wang, Y.; Shi, J.-W.; Ruan, L.-Y.; Ye, J.-F.; Wu, J.-N.; et al. IL-2 and IL-27 Synergistically Promote Growth and Invasion of Endometriotic Stromal Cells by Maintaining the Balance of IFN-γ and IL-10 in Endometriosis. Reproduction 2020, 159, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.W.; Zhang, R.; Tan, Z.; Chung, J.P.W.; Zhang, T.; Wang, C.C. Pharmaceuticals Targeting Signaling Pathways of Endometriosis as Potential New Medical Treatment: A Review. Med. Res. Rev. 2021, 41, 2489–2564. [Google Scholar] [CrossRef]
- Jana, S.; Chatterjee, K.; Ray, A.K.; Dasmahapatra, P.; Swarnakar, S. Regulation of Matrix Metalloproteinase-2 Activity by COX-2-PGE2-pAKT Axis Promotes Angiogenesisin Endometriosis. PLoS ONE 2016, 11, e0163540. [Google Scholar] [CrossRef]
- Ahn, J.H.; Choi, Y.S.; Choi, J.H. Leptin Promotes Human Endometriotic Cell Migration and Invasion by Up-Regulating MMP-2 through the JAK2/STAT3 Signaling Pathway. MHR Basic Sci. Reprod. Med. 2015, 21, 792–802. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C. Involvement of the Wnt/β-Catenin Signaling Pathway in the Cellular and Molecular Mechanisms of Fibrosis in Endometriosis. PLoS ONE 2013, 8, e76808. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, I. Extracellular Matrix Remodelling: The Role of Matrix Metalloproteinases. J. Pathol. 2003, 200, 448–464. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Massaro, M.; Scoditti, E.; Pellegrino, M.; Ingrosso, I.; Giovinazzo, G.; Carluccio, M.A. Red Grape Skin Polyphenols Blunt Matrix Metalloproteinase-2 and -9 Activity and Expression in Cell Models of Vascular Inflammation: Protective Role in Degenerative and Inflammatory Diseases. Molecules 2016, 21, 1147. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, S.H.; Do Quang, B.; Tran, T.T.; Kim, Y.G.; Ko, J.; Choi, W.Y.; Lee, S.Y.; Ryu, J.H. Avenanthramide-C Shows Potential to Alleviate Gingival Inflammation and Alveolar Bone Loss in Experimental Periodontitis. Mol. Cells 2023, 46, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Anti-Inflammatory Effects of Dietary Polyphenols through Inhibitory Activity against Metalloproteinases. Molecules 2023, 28, 5426. [Google Scholar] [CrossRef] [PubMed]
- Dudzinska, D.; Bednarska, K.; Boncler, M.; Luzak, B.; Watala, C. The Influence of Rubus idaeus and Rubus Caesius Leaf Extracts on Platelet Aggregation in Whole Blood. Cross-Talk of Platelets and Neutrophils. Platelets 2016, 27, 433–439. [Google Scholar] [CrossRef] [PubMed]
- McCormack, B.A.; Bilotas, M.A.; Madanes, D.; Ricci, A.G.; Singla, J.J.; Barañao, R.I. Potential Use of Ellagic Acid for Endometriosis Treatment: Its Effect on a Human Endometrial Cell Cycle, Adhesion and Migration. Food Funct. 2020, 11, 4605–4614. [Google Scholar] [CrossRef]
- Gramec Skledar, D.; Tomašič, T.; Sollner Dolenc, M.; Peterlin Mašič, L.; Zega, A. Evaluation of Endocrine Activities of Ellagic Acid and Urolithins Using Reporter Gene Assays. Chemosphere 2019, 220, 706–713. [Google Scholar] [CrossRef]
- Khanduja, K.L.; Avti, P.K.; Kumar, S.; Mittal, N.; Sohi, K.K.; Pathak, C.M. Anti-Apoptotic Activity of Caffeic Acid, Ellagic Acid and Ferulic Acid in Normal Human Peripheral Blood Mononuclear Cells: A Bcl-2 Independent Mechanism. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2006, 1760, 283–289. [Google Scholar] [CrossRef]
- Sudheer, A.R.; Muthukumaran, S.; Devipriya, N.; Menon, V.P. Ellagic Acid, a Natural Polyphenol Protects Rat Peripheral Blood Lymphocytes against Nicotine-Induced Cellular and DNA Damage in Vitro: With the Comparison of N-Acetylcysteine. Toxicology 2007, 230, 11–21. [Google Scholar] [CrossRef]
- Rivera, A.R.; Castillo-Pichardo, L.; Gerena, Y.; Dharmawardhane, S. Anti-Breast Cancer Potential of Quercetin via the AkT/AMPK/Mammalian Target of Rapamycin (mTOR) Signaling Cascade. PLoS ONE 2016, 11, e0157251. [Google Scholar] [CrossRef]
- Tassinari, V.; Smeriglio, A.; Stillittano, V.; Trombetta, D.; Zilli, R.; Tassinari, R.; Maranghi, F.; Frank, G.; Marcoccia, D.; Di Renzo, L. Endometriosis Treatment: Role of Natural Polyphenols as Anti-Inflammatory Agents. Nutrients 2023, 15, 2967. [Google Scholar] [CrossRef]
- Tang, X.L.; Liu, J.X.; Dong, W.; Li, P.; Li, L.; Hou, J.C.; Zheng, Y.Q.; Lin, C.R.; Ren, J.G. Protective Effect of Kaempferol on LPS plus ATP-Induced Inflammatory Response in Cardiac Fibroblasts. Inflammation 2015, 38, 94–101. [Google Scholar] [CrossRef]
- Saw, C.L.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.N.T. The Berry Constituents Quercetin, Kaempferol, and Pterostilbene Synergistically Attenuate Reactive Oxygen Species: Involvement of the Nrf2-ARE Signaling Pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.Y.; Jeong, H.J.; Kim, H.M. Kaempferol Impedes IL-32-Induced Monocyte-Macrophage Differentiation. Chem. Biol. Interact. 2017, 274, 107–115. [Google Scholar] [CrossRef] [PubMed]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The Anti-Inflammatory Flavones Quercetin and Kaempferol Cause Inhibition of Inducible Nitric Oxide Synthase, Cyclooxygenase-2 and Reactive C-Protein, and down-Regulation of the Nuclear Factor kappaB Pathway in Chang Liver Cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, G.H. Evaluation of Antioxidant and Inhibitory Activities for Different Subclasses Flavonoids on Enzymes for Rheumatoid Arthritis. J. Food Sci. 2010, 75, H212–H217. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.Y.; Sang, L.X.; Jiang, M.; McPhee, D.J. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; Wang, J.; Wu, H.; Cheng, Y.; Guo, Q.; Liang, T.; Zhang, G. Cross-Talk between N6-Methyladenosine and Their Related RNAs Defined a Signature and Confirmed m6A Regulators for Diagnosis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 1665. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.-H.; Heras, C.R.; Lee, G. m6A in the Signal Transduction Network. Mol. Cells 2022, 45, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, M.; Wu, J.; Wang, S.; Yang, X.; Yi, M.; Zhang, X.; Fang, X. Aging-12-202163 (1). Aging 2020, 12, 25916–25938. [Google Scholar] [CrossRef]
- Yang, Y.-M.; Yang, W.-X. Epithelial-to-Mesenchymal Transition in the Development of Endometriosis. Oncotarget 2017, 8, 41679–41689. [Google Scholar] [CrossRef]
- Bowman, R.; Taylor, J.; Muggleton, S.; Davis, D. Biophysical Effects, Safety and Efficacy of Raspberry Leaf Use in Pregnancy: A Systematic Integrative Review. BMC Complement. Med. Ther. 2021, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Socha, M.W.; Flis, W.; Wartęga, M.; Szambelan, M.; Pietrus, M.; Kazdepka-Ziemińska, A. Raspberry Leaves and Extracts-Molecular Mechanism of Action and Its Effectiveness on Human Cervical Ripening and the Induction of Labor. Nutrients 2023, 15, 3206. [Google Scholar] [CrossRef] [PubMed]
- Vernon, M.W.; Wilson, E.A. Studies on the Surgical Induction of Endometriosis in the Rat. Fertil. Steril. 1985, 44, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Cummings, A.M.; Metcalf, J.L. Induction of Endometriosis in Mice: A New Model Sensitive to Estrogen. Reprod. Toxicol. 1995, 9, 233–238. [Google Scholar] [CrossRef]
- Quereda, F.; Barroso, J.; Acién, P. Individual and Combined Effects of Triptoreline and Gestrinone on Experimental Endometriosis in Rats. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996, 67, 35–40. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).