Modification Strategies of High-Energy Li-Rich Mn-Based Cathodes for Li-Ion Batteries: A Review
Abstract
1. Introduction
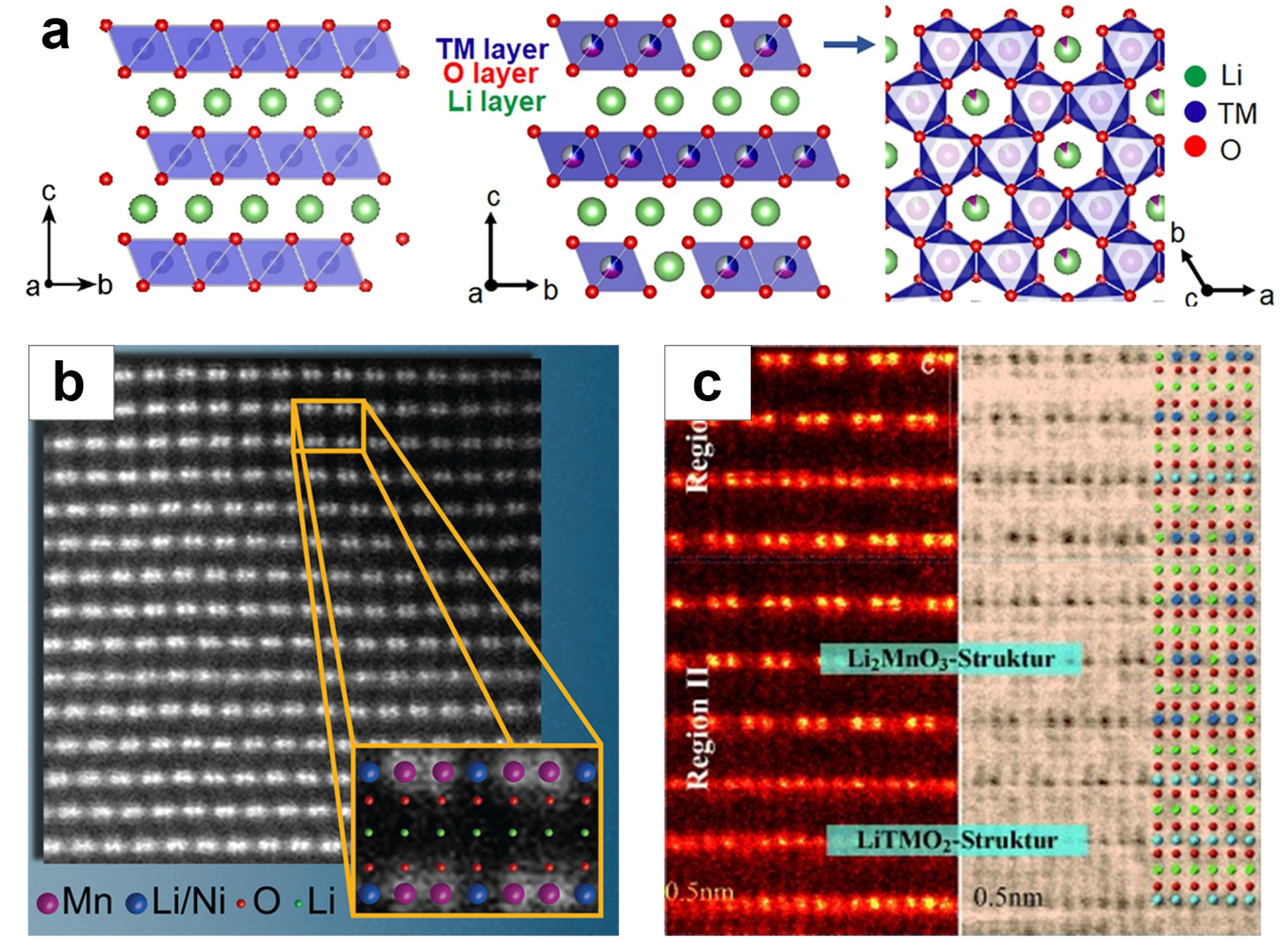
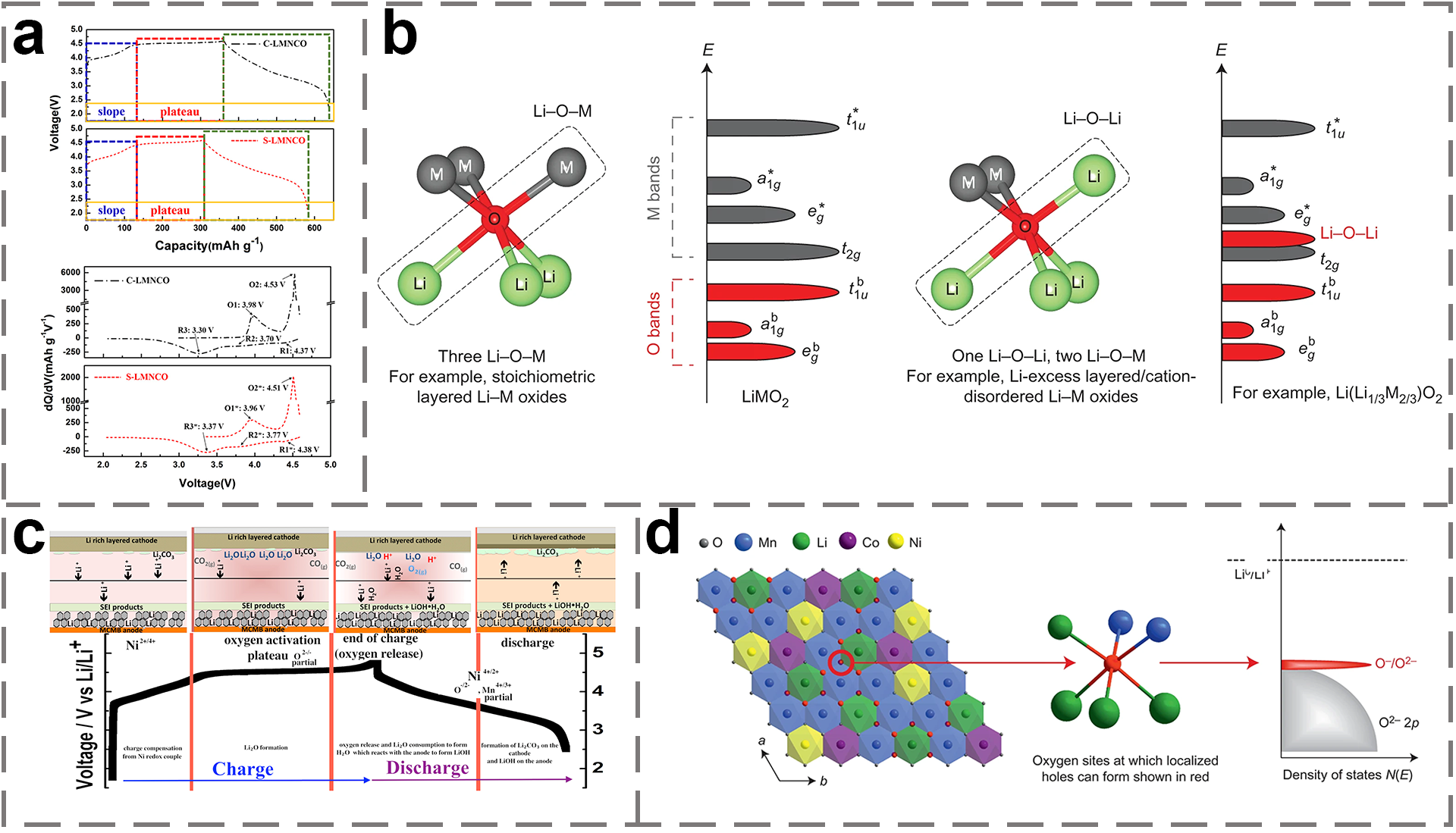
2. Material Structure and Reaction Mechanism of LRMO
3. Defects in the LRMO
3.1. Low Initial Coulombic Efficiency
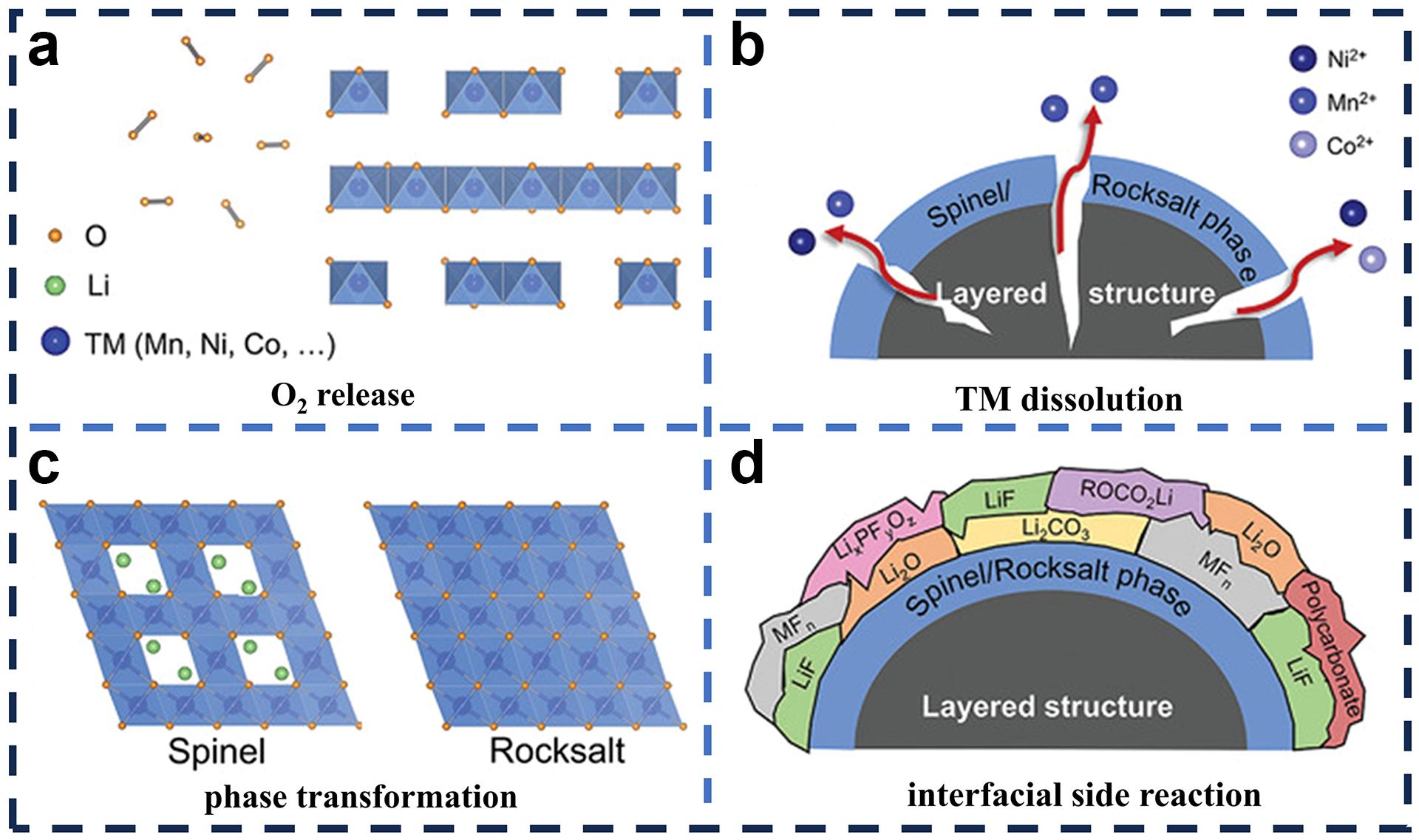
3.2. Excessive Capacity and Voltage Drop
3.3. Poor Rate Performance
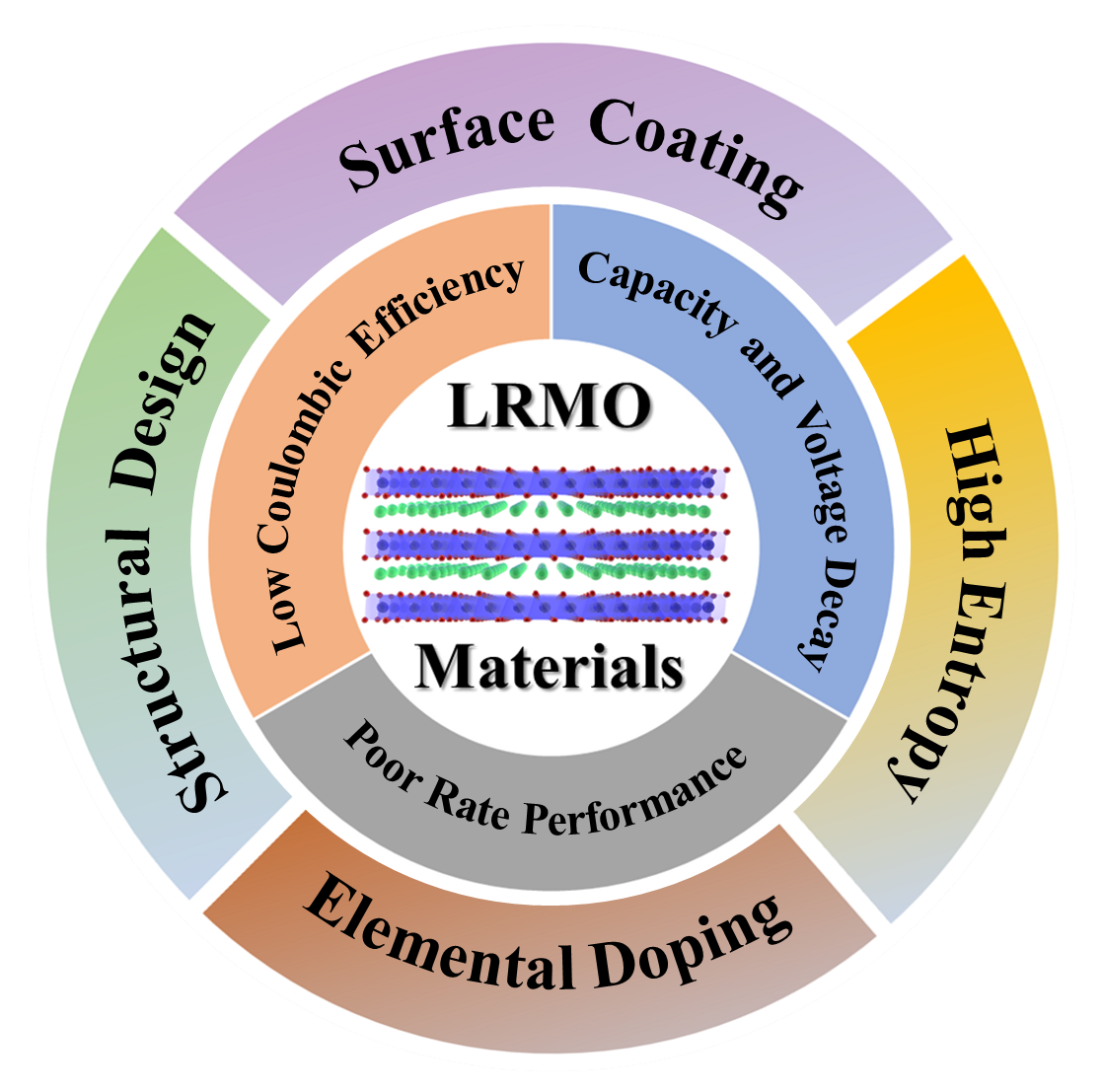
4. Modification Strategies
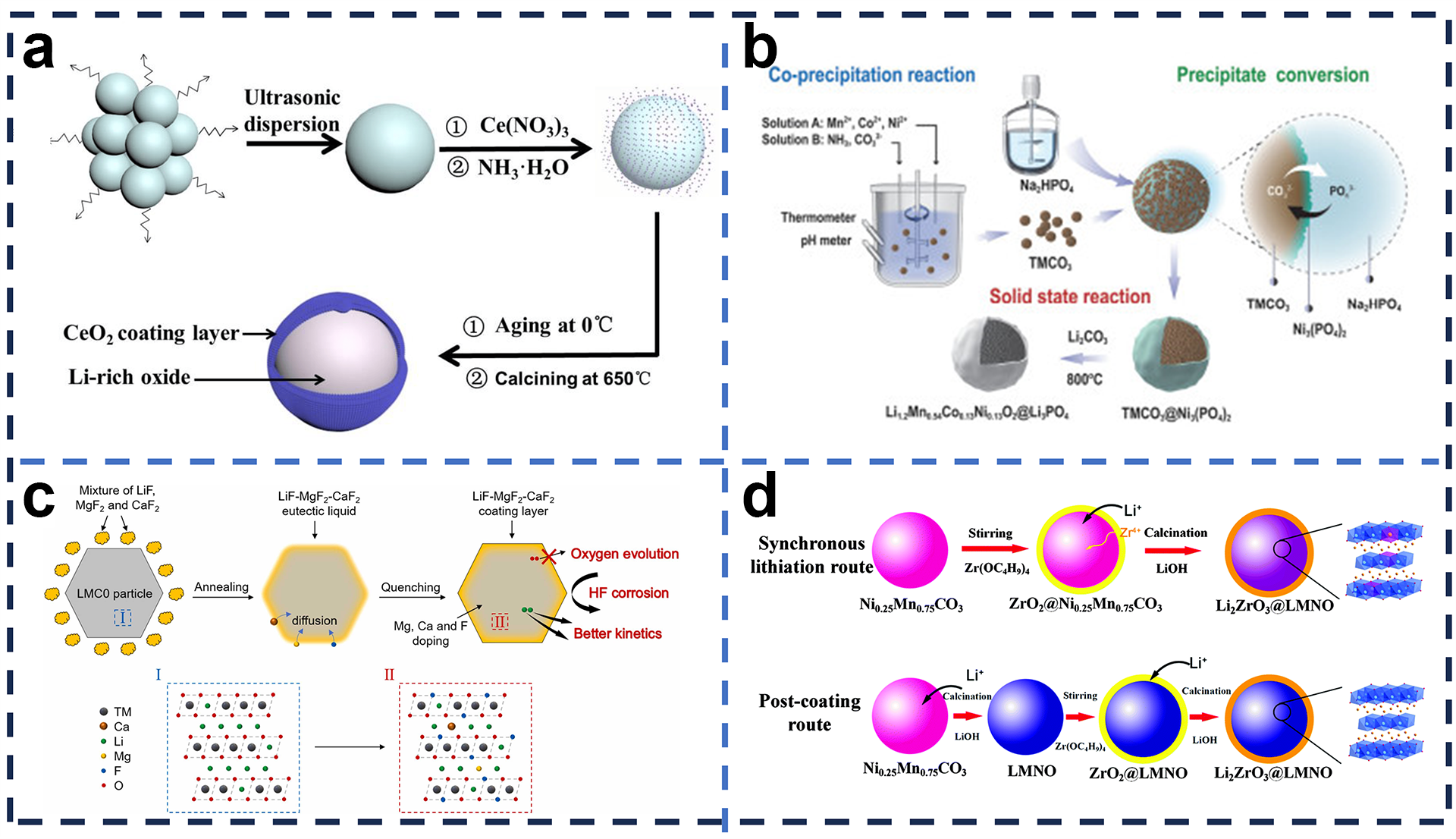
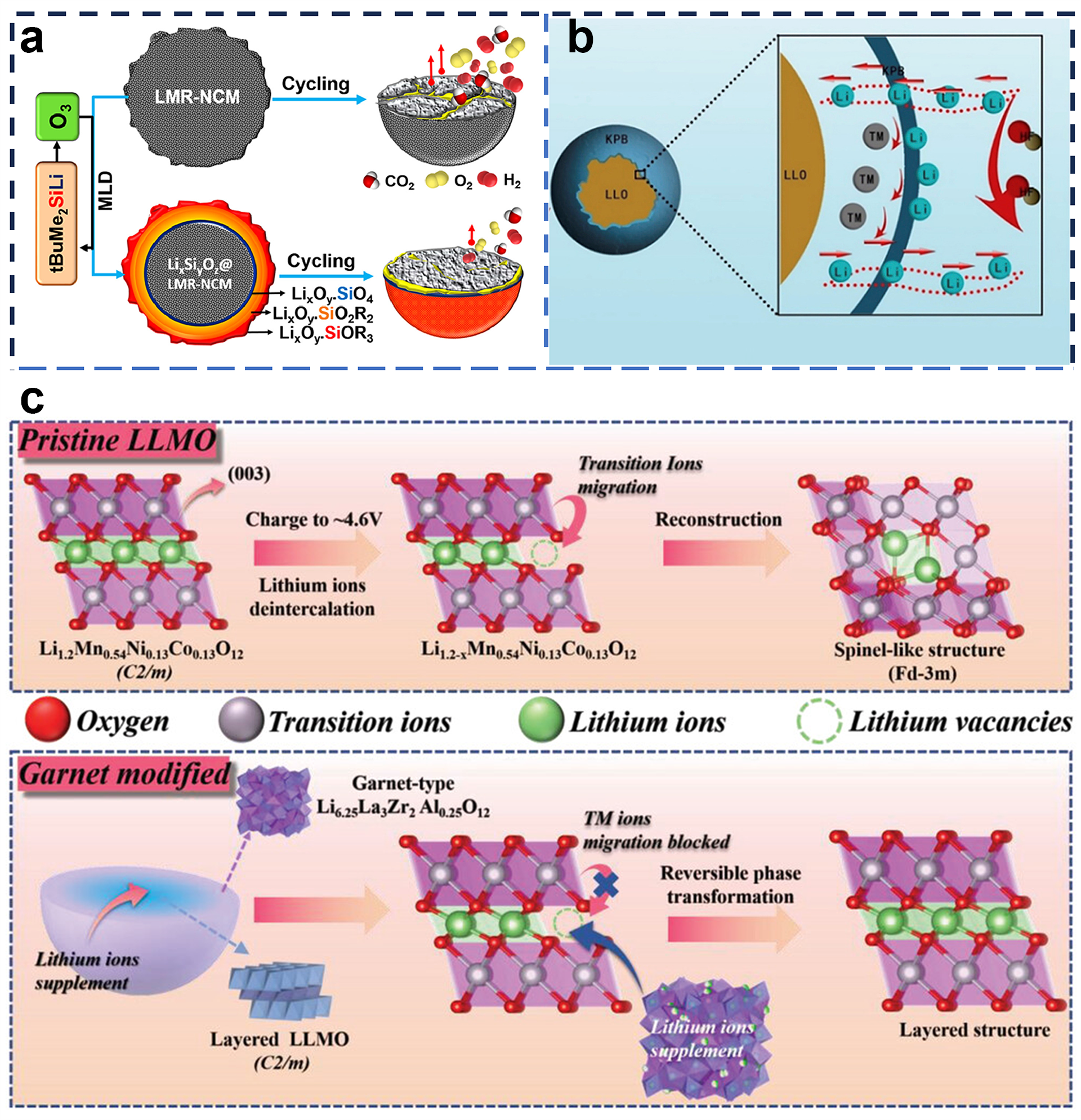
4.1. Surface Coating
4.2. Elemental Doping
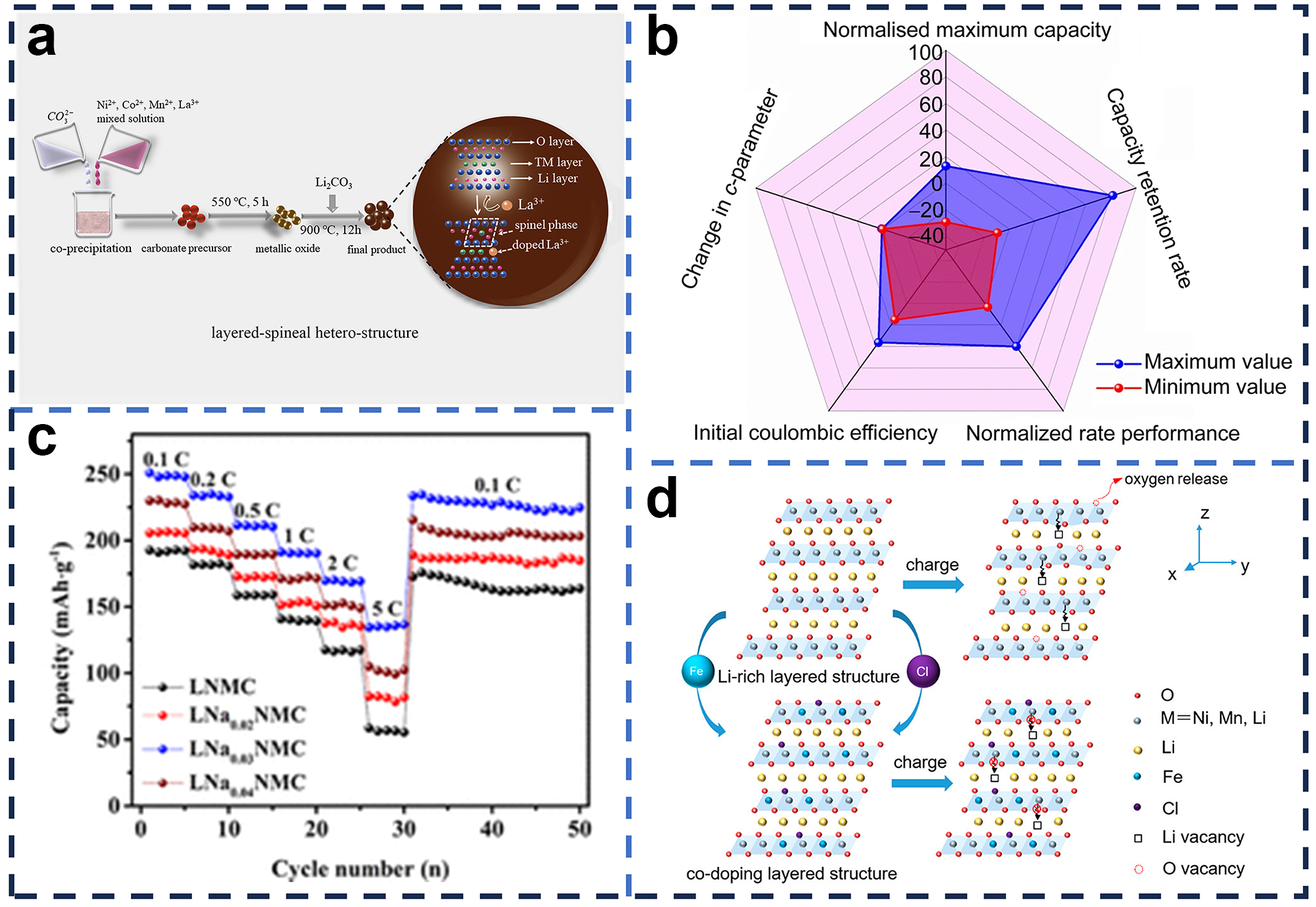
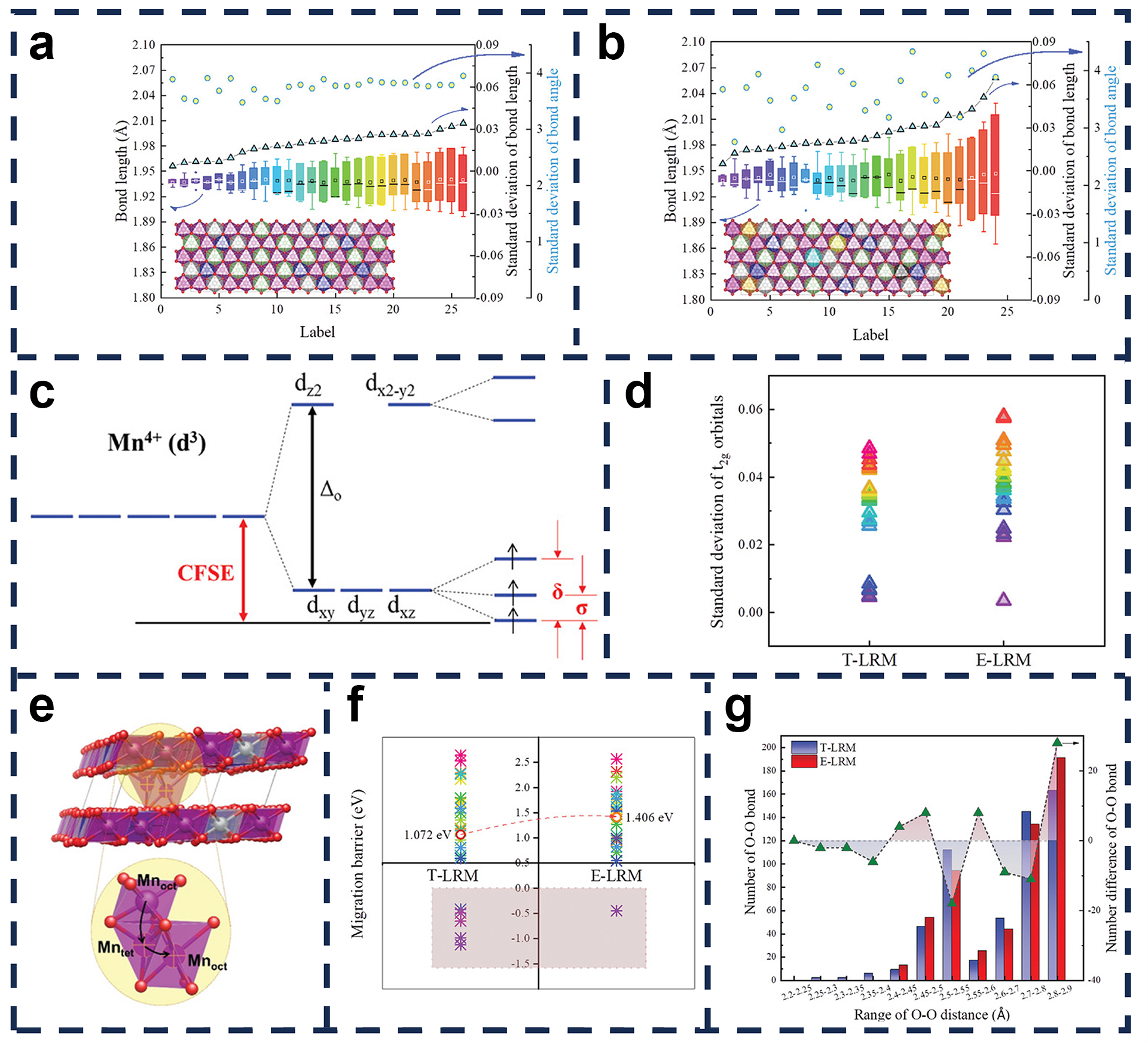
4.3. Structural Designs
4.4. Introduction of High Entropy
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, G.; Sun, Q.; Horta, S.; Wang, S.; Lu, X.; Zhang, C.Y.; Li, J.; Li, J.; Ci, L.; Tian, Y.; et al. A layered bi2te3@ppy cathode for aqueous zinc-ion batteries: Mechanism and application in printed flexible batteries. Adv. Mater. 2024, 36, 2305128. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, G.; Sun, Q.; Feng, Y.; Wang, X.; Ma, X.; Li, J.; Zhang, H.; Wen, J.; Feng, J.; et al. Flexible electronic systems via electrohydrodynamic jet printing: A mnse@rgo cathode for aqueous zinc-ion batteries. ACS Nano 2023, 17, 13256–13268. [Google Scholar] [CrossRef]
- Vijaya Kumar Saroja, A.P.; Arjunan, A.; Muthusamy, K.; Balasubramanian, V.; Sundara, R. Chemically bonded amorphous red phosphorous with disordered carbon nanosheet as high voltage cathode for rechargeable aluminium ion battery. J. Alloys Compd. 2020, 830, 154693. [Google Scholar] [CrossRef]
- Arjunan, A.; Subbiah, M.; Sekar, M.; Vs, A.P.; Balasubramanian, V.; Sundara, R. Biomass derived hierarchically porous carbon inherent structure as an effective metal free cathode for Li-O2/air battery. Electrochem. Sci. Adv. 2021, 1, e202000037. [Google Scholar] [CrossRef]
- Zheng, J.; Myeong, S.; Cho, W.; Yan, P.; Xiao, J.; Wang, C.; Cho, J.; Zhang, J. Li- and mn-rich cathode materials: Challenges to commercialization. Adv. Energy Mater. 2017, 7, 1601284. [Google Scholar] [CrossRef]
- Cao, X.; Qiao, Y.; Jia, M.; He, P.; Zhou, H. Ion-exchange: A promising strategy to design li-rich and li-excess layered cathode materials for li-ion batteries. Adv. Energy Mater. 2022, 12, 2003972. [Google Scholar] [CrossRef]
- Zuo, W.; Luo, M.; Liu, X.; Wu, J.; Liu, H.; Li, J.; Winter, M.; Fu, R.; Yang, W.; Yang, Y. Li-rich cathodes for rechargeable li-based batteries: Reaction mechanisms and advanced characterization techniques. Energy Environ. Sci. 2020, 13, 4450–4497. [Google Scholar] [CrossRef]
- Cui, S.; Gao, M.; Li, G.; Gao, X. Insights into li-rich mn-based cathode materials with high capacity: From dimension to lattice to atom. Adv. Energy Mater. 2022, 12, 2003885. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, C.; Zhang, Y.; Lin, L.; He, W.; Xie, Q.; Sa, B.; Wang, L.; Peng, D. A universal strategy toward the precise regulation of initial coulombic efficiency of li-rich mn-based cathode materials. Adv. Mater. 2021, 33, 2103173. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Li, Z.; Tong, Z.; Wang, Z.; Li, Y.; Wang, L.; Shang, Y.; Bi, J.; Lei, S. Research progress on lithium-rich manganese-based lithium-ion batteries cathodes. Ceram. Int. 2023, 50, 5877–5892. [Google Scholar] [CrossRef]
- Murdock, B.E.; Toghill, K.E.; Tapia-Ruiz, N. A perspective on the sustainability of cathode materials used in lithium-ion batteries. Adv. Energy Mater. 2021, 11, 2102028. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K. The li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Lu, L.; Meng, Y.S.; Ceder, G. Phase transitions and high-voltage electrochemical behavior of liCoO2 thin films grown by pulsed laser deposition. J. Electrochem. Soc. 2007, 154, A337. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Tanaka, M. Jahn-teller structural phase transition around 280k in liMn2O4. Mater. Res. Bull. 1995, 30, 715–721. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, M.; Li, J.; Zhang, D.; Yan, Y.; Li, Z. Recent progress in coatings and methods of ni-rich LiNi0.8Co0.1Mn0.1O2 cathode materials: A short review. Ceram. Int. 2020, 46, 21888–21901. [Google Scholar] [CrossRef]
- Sun, Q.; Zeng, G.; Li, J.; Wang, S.; Botifoll, M.; Wang, H.; Li, D.; Ji, F.; Cheng, J.; Shao, H.; et al. Is soft carbon a more suitable match for siox in li-ion battery anodes? Small 2023, 19, 2302644. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Yang, M.; Wang, S.; Zeng, G.; Liu, H.; Cheng, J.; Li, D.; Wei, Y.; Si, P.; et al. Carbon microstructure dependent li-ion storage behaviors in siox/c anodes. Small 2023, 19, 2300759. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, M.; Zeng, G.; Li, J.; Hu, Z.; Li, D.; Wang, S.; Si, P.; Tian, Y.; Ci, L. Insights into the potassium ion storage behavior and phase evolution of a tailored yolk–shell snse@c anode. Small 2022, 18, 2203459. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Hao, C.; Ci, L. Focusing on the subsequent coulombic efficiencies of siox: Initial high-temperature charge after over-capacity prelithiation for high-efficiency siox-based full-cell battery. ACS Appl. Mater. Interfaces 2022, 14, 14284–14292. [Google Scholar] [CrossRef]
- Zhao, H.; Lam, W.A.; Sheng, L.; Wang, L.; Bai, P.; Yang, Y.; Ren, D.; Xu, H.; He, X. Cobalt-free cathode materials: Families and their prospects. Adv. Energy Mater. 2022, 12, 2103894. [Google Scholar] [CrossRef]
- Lin, T.; Seaby, T.; Huang, X.; Wang, L. On the disparity in reporting li-rich layered oxide cathode materials. Chem. Commun. 2023, 59, 2888–2902. [Google Scholar] [CrossRef]
- Luo, K.; Roberts, M.R.; Hao, R.; Guerrini, N.; Pickup, D.M.; Liu, Y.; Edström, K.; Guo, J.; Chadwick, A.V.; Duda, L.C.; et al. Charge-compensation in 3d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen. Nat. Chem. 2016, 8, 684–691. [Google Scholar] [CrossRef]
- Pimenta, V.; Sathiya, M.; Batuk, D.; Abakumov, A.M.; Giaume, D.; Cassaignon, S.; Larcher, D.; Tarascon, J. Synthesis of li-rich nmc: A comprehensive study. Chem. Mat. 2017, 29, 9923–9936. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Kang, S.; Johnson, C.S.; Vaughey, J.T.; Benedek, R.; Hackney, S.A. Li2mno3-stabilized limo2 (m = mn, ni, co) electrodes for lithium-ion batteries. J. Mater. Chem. 2007, 17, 3112–3125. [Google Scholar] [CrossRef]
- Rossouw, M.H.; Thackeray, M.M. Lithium manganese oxides from li2mno3 for rechargeable lithium battery applications. Mater. Res. Bull. 1991, 26, 463–473. [Google Scholar] [CrossRef]
- Peng, Z.; Mu, K.; Cao, Y.; Xu, L.; Du, K.; Hu, G. Enhanced electrochemical performance of layered li-rich cathode materials for lithium ion batteries via aluminum and boron dual-doping. Ceram. Int. 2019, 45, 4184–4192. [Google Scholar] [CrossRef]
- Takahashi, I.; Maeda, T.; Kiuchi, H.; Nakanishi, K.; Ohma, A.; Hatano, M.; Fukunaga, T.; Ohta, T.; Matsubara, E. Mechanism of structural change and the trigger of electrochemical degradation of li-rich layered oxide cathodes during charge–discharge cycles. ACS Appl. Energy Mater. 2019, 2, 8118–8124. [Google Scholar] [CrossRef]
- Hy, S.; Felix, F.; Rick, J.; Su, W.; Hwang, B.J. Direct in situ observation of Li2O evolution on li-rich high-capacity cathode material, Li[NiXLi(1–2x)/3Mn(2–x)/3]O2 (0 ≤ x ≤0.5). J. Am. Chem. Soc. 2014, 136, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 4440. [Google Scholar] [CrossRef]
- Lee, S.; Manthiram, A. Can cobalt be eliminated from lithium-ion batteries? ACS Energy Lett. 2022, 7, 3058–3063. [Google Scholar] [CrossRef]
- Lei, Y.; Ni, J.; Hu, Z.; Wang, Z.; Gui, F.; Li, B.; Ming, P.; Zhang, C.; Elias, Y.; Aurbach, D.; et al. Surface modification of li-rich mn-based layered oxide cathodes: Challenges, materials, methods, and characterization. Adv. Energy Mater. 2020, 10, 2002506. [Google Scholar] [CrossRef]
- Ko, G.; Jeong, S.; Park, S.; Lee, J.; Kim, S.; Shin, Y.; Kim, W.; Kwon, K. Doping strategies for enhancing the performance of lithium nickel manganese cobalt oxide cathode materials in lithium-ion batteries. Energy Storage Mater. 2023, 60, 102840. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, L.; Wang, S.; Kang, F.; Li, B. Micro- and nano-structural design strategies towards polycrystalline nickel-rich layered cathode materials. J. Mater. Chem. A 2023, 11, 7867–7897. [Google Scholar] [CrossRef]
- Xie, Y.; Jin, Y.; Xiang, L. Li-rich layered oxides: Structure, capacity and voltage fading mechanisms and solving strategies. Particuology 2022, 61, 1–10. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, Z.; Dahn, J.R. Lack of cation clustering in Li[NixLi1/3−2x/3Mn2/3−x/3]O2 (0 < x ≤ 1/2) and Li[CrxLi(1−x)/3Mn(2−2x)/3]O2 (0 < x < 1). Chem. Mat. 2003, 15, 3214–3220. [Google Scholar]
- Thackeray, M.M.; Johnson, C.S.; Vaughey, J.T.; Li, N.; Hackney, S.A. Advances in manganese-oxide ‘composite’ electrodes for lithium-ion batteries. J. Mater. Chem. 2005, 15, 2257–2267. [Google Scholar] [CrossRef]
- Pan, C.; Lee, Y.J.; Ammundsen, B.; Grey, C.P. 6li mas nmr studies of the local structure and electrochemical properties of cr-doped lithium manganese and lithium cobalt oxide cathode materials for lithium-ion batteries. Chem. Mat. 2002, 14, 2289–2299. [Google Scholar] [CrossRef]
- Freire, M.; Kosova, N.V.; Jordy, C.; Chateigner, D.; Lebedev, O.I.; Maignan, A.; Pralong, V. A new active Li–Mn–O compound for high energy density li-ion batteries. Nat. Mater. 2016, 15, 173–177. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Guo, W.; Wu, H.; Lin, L.; Liu, Q.; Han, X.; Xie, Q.; Liu, P.; Zheng, H.; Wang, L.; et al. Challenges and recent advances in high capacity li-rich cathode materials for high energy density lithium-ion batteries. Adv. Mater. 2021, 33, 2005937. [Google Scholar] [CrossRef]
- Jarvis, K.A.; Deng, Z.; Allard, L.F.; Manthiram, A.; Ferreira, P.J. Atomic structure of a lithium-rich layered oxide material for lithium-ion batteries: Evidence of a solid solution. Chem. Mat. 2011, 23, 3614–3621. [Google Scholar] [CrossRef]
- Qiu, B.; Zhang, M.; Wu, L.; Wang, J.; Xia, Y.; Qian, D.; Liu, H.; Hy, S.; Chen, Y.; An, K.; et al. Gas–solid interfacial modification of oxygen activity in layered oxide cathodes for lithium-ion batteries. Nat. Commun. 2016, 7, 12108. [Google Scholar] [CrossRef]
- Yu, H.; Ishikawa, R.; So, Y.; Shibata, N.; Kudo, T.; Zhou, H.; Ikuhara, Y. Direct atomic-resolution observation of two phases in the Li1.2Mn0.567Ni0.166Co0.067O2 cathode material for lithium-ion batteries. Angew. Chem. Int. Ed. 2013, 52, 5969–5973. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, Z.; Jamil, S.; Chen, J.; Zhang, X.; Wang, X.; Yang, Z.; Shu, H.; Yang, X. Effects of nanofiber architecture and antimony doping on the performance of lithium-rich layered oxides: Enhancing lithium diffusivity and lattice oxygen stability. ACS Appl. Mater. Interfaces 2018, 10, 16561–16571. [Google Scholar] [CrossRef]
- Yang, J.; Niu, Y.; Wang, X.; Xu, M. A review on the electrochemical reaction of li-rich layered oxide materials. Inorg. Chem. Front. 2021, 8, 4300–4312. [Google Scholar] [CrossRef]
- Grey, C.P.; Yoon, W.; Reed, J.; Ceder, G. Electrochemical activity of li in the transition-metal sites of O3Li[Li(1−2x)/3Mn(2−x)/3Nix]O2. Electrochem. Solid-State Lett. 2004, 7, A290. [Google Scholar] [CrossRef]
- Okubo, M.; Hosono, E.; Kim, J.; Enomoto, M.; Kojima, N.; Kudo, T.; Zhou, H.; Honma, I. Nanosize effect on high-rate li-ion intercalation in LiCoO2 electrode. J. Am. Chem. Soc. 2007, 129, 7444–7452. [Google Scholar] [CrossRef]
- Zhou, K.; Li, Y.; Ha, Y.; Zhang, M.; Dachraoui, W.; Liu, H.; Zhang, C.; Liu, X.; Liu, F.; Battaglia, C.; et al. A nearly zero-strain li-rich rock-salt oxide with multielectron redox reactions as a cathode for li-ion batteries. Chem. Mat. 2022, 34, 9711–9721. [Google Scholar] [CrossRef]
- Yu, S.; Yoon, T.; Mun, J.; Park, S.; Kang, Y.; Park, J.; Oh, S.M.; Sung, Y. Continuous activation of li2mno3 component upon cycling in Li1.167Ni0.233Co0.100Mn0.467Mo0.033O2 cathode material for lithium ion batteries. J. Mater. Chem. A 2013, 1, 2833–2839. [Google Scholar] [CrossRef]
- Cao, T.; Shi, C.; Zhao, N.; He, C.; Li, J.; Liu, E. Understanding the electrochemical properties of li-rich cathode materials from first-principles calculations. J. Phys. Chem. C 2015, 119, 28749–28756. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, X.; Wang, C.; Bi, H.; Zhang, J.; Li, S.; Wang, M.; Che, R. Insight into the atomic structure of Li2MnO3 in Li-rich Mn-based cathode materials and the impact of its atomic arrangement on electrochemical performance. J. Mater. Chem. A 2017, 5, 11214–11223. [Google Scholar] [CrossRef]
- Seo, D.; Lee, J.; Urban, A.; Malik, R.; Kang, S.; Ceder, G. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered li-excess cathode materials. Nat. Chem. 2016, 8, 692–697. [Google Scholar] [CrossRef]
- Gou, X.; Hao, Z.; Hao, Z.; Yang, G.; Yang, Z.; Zhang, X.; Yan, Z.; Zhao, Q.; Chen, J. In situ surface self-reconstruction strategies in li-rich mn-based layered cathodes for energy-dense li-ion batteries. Adv. Funct. Mater. 2022, 32, 2112088. [Google Scholar] [CrossRef]
- Song, J.; Yoon, G.; Kim, B.; Eum, D.; Park, H.; Kim, D.; Kang, K. Anionic redox activity regulated by transition metal in lithium-rich layered oxides. Adv. Energy Mater. 2020, 10, 2001207. [Google Scholar] [CrossRef]
- House, R.A.; Rees, G.J.; Pérez-Osorio, M.A.; Marie, J.; Boivin, E.; Robertson, A.W.; Nag, A.; Garcia-Fernandez, M.; Zhou, K.; Bruce, P.G. First-cycle voltage hysteresis in li-rich 3d cathodes associated with molecular O2 trapped in the bulk. Nat. Energy 2020, 5, 777–785. [Google Scholar] [CrossRef]
- Merz, M.; Ying, B.; Nagel, P.; Schuppler, S.; Kleiner, K. Reversible and irreversible redox processes in li-rich layered oxides. Chem. Mat. 2021, 33, 9534–9545. [Google Scholar] [CrossRef]
- Csernica, P.M.; Kalirai, S.S.; Gent, W.E.; Lim, K.; Yu, Y.; Liu, Y.; Ahn, S.; Kaeli, E.; Xu, X.; Stone, K.H.; et al. Persistent and partially mobile oxygen vacancies in Li-rich layered oxides. Nat. Energy 2021, 6, 642–652. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Z.; Liu, Q.; Liu, Y. Heightening the long cycling stability and reducing oxygen loss of li-rich cathode materials by mo–f co-doping for half/full lithium-ion batteries. Ceram. Int. 2023, 49, 6580–6593. [Google Scholar] [CrossRef]
- Zhang, H.; Omenya, F.; Whittingham, M.S.; Wang, C.; Zhou, G. Formation of an anti-core–shell structure in layered oxide cathodes for li-ion batteries. Acs Energy Lett. 2017, 2, 2598–2606. [Google Scholar] [CrossRef]
- Hu, E.; Yu, X.; Lin, R.; Bi, X.; Lu, J.; Bak, S.; Nam, K.; Xin, H.L.; Jaye, C.; Fischer, D.A.; et al. Evolution of redox couples in Li- and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release. Nat. Energy 2018, 3, 690–698. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, P.; He, Z.; Yang, Z.; Tang, L.; An, C.; Zheng, J. Effect of mgo and tio2 coating on the electrochemical performance of li-rich cathode materials for lithium-ion batteries. Energy Technol. 2019, 7, 1800829. [Google Scholar] [CrossRef]
- Amine, K.; Liu, J.; Kang, S.; Belharouak, I.; Hyung, Y.; Vissers, D.; Henriksen, G. Improved lithium manganese oxide spinel/graphite li-ion cells for high-power applications. J. Power Sources 2004, 129, 14–19. [Google Scholar] [CrossRef]
- Zhan, C.; Lu, J.; Jeremy Kropf, A.; Wu, T.; Jansen, A.N.; Sun, Y.; Qiu, X.; Amine, K. Mn(ii) deposition on anodes and its effects on capacity fade in spinel lithium manganate–carbon systems. Nat. Commun. 2013, 4, 2437. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wang, X.; Sun, X.; Zhang, J.; Yu, X.; Li, H. Investigations on the fundamental process of cathode electrolyte interphase formation and evolution of high-voltage cathodes. ACS Appl. Mater. Interfaces 2020, 12, 2319–2326. [Google Scholar] [CrossRef]
- Kou, P.; Zhang, Z.; Wang, Z.; Zheng, R.; Liu, Y.; Lv, F.; Xu, N. Opportunities and challenges of layered lithium-rich manganese-based cathode materials for high energy density lithium-ion batteries. Energy Fuels 2023, 37, 18243–18265. [Google Scholar] [CrossRef]
- Duan, J.; Tang, W.; Wang, R.; Tang, X.; Li, J.; Tang, M.; Li, P. Inhibited voltage decay and enhanced electrochemical performance of the li-rich layered Li1.2Mn0.54Ni0.13Co0.13O2 cathode material by cealoδ surface coating modification. Appl. Surf. Sci. 2020, 521, 146504. [Google Scholar] [CrossRef]
- Gao, X.; Li, S.; Zhang, H.; Zhang, S.; Chang, S.; Li, H.; Li, S.; Lai, Y.; Zhang, Z. Constructing a robust integrated surface structure for enhancing the performance of Li-rich Mn-based oxides cathodes. Mater. Today Energy 2022, 30, 101152. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, F.; Li, L.; Chen, M.; Zhong, X.; Li, W.; Lu, L. Effect of li3po4 coating of layered lithium-rich oxide on electrochemical performance. J. Power Sources 2017, 341, 147–155. [Google Scholar] [CrossRef]
- Yang, C.; Liao, P.; Wu, Y.; Lue, S.J. Electrochemical performance of li-rich oxide composite material coated with Li0.75La0.42TiO3 ionic conductor. Appl. Surf. Sci. 2017, 399, 670–681. [Google Scholar] [CrossRef]
- Cho, D.; Yashiro, H.; Sun, Y.; Myung, S. Electrochemical properties of polyaniline-coated li-rich nickel manganese oxide and role of polyaniline coating layer. J. Electrochem. Soc. 2014, 161, A142. [Google Scholar] [CrossRef]
- Wen, X.; Liang, K.; Tian, L.; Shi, K.; Zheng, J. Al2O3 coating on Li1.256Ni0.198Co0.082Mn0.689O2.25 with spinel-structure interface layer for superior performance lithium ion batteries. Electrochim. Acta 2018, 260, 549–556. [Google Scholar] [CrossRef]
- Kong, J.; Zhai, H.; Qian, X.; Wang, M.; Wang, Q.; Li, A.; Li, H.; Zhou, F. Improved electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 cathode material coated with ultrathin zno. J. Alloys Compd. 2017, 694, 848–856. [Google Scholar] [CrossRef]
- Liu, D.; Yang, J.; Hou, J.; Liao, J.; Wu, M. Intelligent phase-transition mno2 single-crystal shell enabling a high-capacity li-rich layered cathode in li-ion batteries. RSC Adv. 2021, 11, 12771–12783. [Google Scholar] [CrossRef] [PubMed]
- Nisar, U.; Petla, R.; Jassim Al-Hail, S.A.; Quddus, A.A.; Monawwar, H.; Shakoor, A.; Essehli, R.; Amin, R. Impact of surface coating on electrochemical and thermal behaviors of a Li-rich Li1.2Ni0.16Mn0.56Co0.08O2 cathode. RSC Adv. 2020, 10, 15274–15281. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Peng, W.; Yan, G.; Guo, H.; Wang, Z.; Li, X.; Gui, W.; Wang, J.; Chen, N. Suppressing the voltage decay and enhancing the electrochemical performance of Li1.2Mn0.54Co0.13Ni0.13O2 by multifunctional Nb2O5 coating. Energy Technol. 2018, 6, 2139–2145. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Chen, M.; Li, W. Low-temperature-aged synthesis of CeO2-coated Li-rich oxide as cathode for low-cost high-energy density Li-ion batteries. Batteries 2023, 9, 330. [Google Scholar] [CrossRef]
- Zheng, F.; Ou, X.; Pan, Q.; Xiong, X.; Yang, C.; Fu, Z.; Liu, M. Nanoscale gadolinium doped ceria (gdc) surface modification of li-rich layered oxide as a high performance cathode material for lithium ion batteries. Chem. Eng. J. 2018, 334, 497–507. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D.; Wang, Z.; Zheng, F.; Zhong, R.; Hong, R. AlF3 coating improves cycle and voltage decay of li-rich manganese oxides. J. Mater. Sci. 2023, 58, 4525–4540. [Google Scholar] [CrossRef]
- Zhu, W.; Tai, Z.; Shu, C.; Chong, S.; Guo, S.; Ji, L.; Chen, Y.; Liu, Y. The superior electrochemical performance of a li-rich layered cathode material with li-rich spinel Li4Mn5O12 and MgF2 double surface modifications. J. Mater. Chem. A 2020, 8, 7991–8001. [Google Scholar] [CrossRef]
- Du, Z.; Peng, W.; Wang, Z.; Guo, H.; Hu, Q.; Li, X. Improving the electrochemical performance of li-rich Li1.2Ni0.13Co0.13Mn0.54O2 cathode material by lif coating. Ionics 2018, 24, 3717–3724. [Google Scholar] [CrossRef]
- Park, J.S.; Mane, A.U.; Elam, J.W.; Croy, J.R. Amorphous metal fluoride passivation coatings prepared by atomic layer deposition on LiCoO2 for li-ion batteries. Chem. Mat. 2015, 27, 1917–1920. [Google Scholar] [CrossRef]
- Yue, P.; Wang, Z.; Li, X.; Xiong, X.; Wang, J.; Wu, X.; Guo, H. The enhanced electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode materials by low temperature fluorine substitution. Electrochim. Acta 2013, 95, 112–118. [Google Scholar] [CrossRef]
- Zhao, H.; Li, W.; Li, J.; Xu, H.; Zhang, C.; Li, J.; Han, C.; Li, Z.; Chu, M.; Qiu, X. Enhance performances of co-free li-rich cathode by eutesctic melting salt treatment. Nano Energy 2022, 92, 106760. [Google Scholar] [CrossRef]
- You, B.; Wang, Z.; Shen, F.; Chang, Y.; Peng, W.; Li, X.; Guo, H.; Hu, Q.; Deng, C.; Yang, S.; et al. Research progress of single-crystal nickel-rich cathode materials for lithium ion batteries. Small Methods 2021, 5, 2100234. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Liu, J.; Li, X.; Cheng, F. In-situ Li3PO4 Coating of Li-Rich Mn-Based Cathode Materials for Lithium-ion Batteries. Acta Chim. Sin. 2020, 78, 1426–1433. [Google Scholar] [CrossRef]
- Song, H.; Su, W.; Mao, H.; Feng, Z.; Li, Y.; Lyu, Y.; Guo, B. In-situ formed hybrid phosphates coating layer enabling co-free li-rich layered oxides with stable cycle performance. Mater. Today Energy 2023, 34, 101314. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Z.; Yu, M.; Hu, H.; Zhang, Y.; Zhang, K.; Du, Z.; Cheng, F.; Chen, J. Building homogenous Li2Tio3 coating layer on primary particles to stabilize li-rich mn-based cathode materials. Small 2022, 18, 2106337. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, X.; Zhang, Z.; Wu, H.; Liu, D.; Dou, A.; Su, M.; Zhang, Q.; Chu, D. Enhanced electrochemical performance of li-rich layered cathode materials by combined cr doping and LiAlO2 coating. ACS Sustain. Chem. Eng. 2019, 7, 2225–2235. [Google Scholar] [CrossRef]
- Pang, S.; Xu, K.; Wang, Y.; Shen, X.; Wang, W.; Su, Y.; Zhu, M.; Xi, X. Enhanced electrochemical performance of li-rich layered cathode materials via chemical activation of Li2MnO3 component and formation of spinel/carbon coating layer. J. Power Sources 2017, 365, 68–75. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Gao, R.; Li, Z.; Hu, Z.; Liu, X. New insights into the modification mechanism of li-rich Li1.2Mn0.6Ni0.2O2 coated by Li2Zro3. Phys. Chem. Chem. Phys. 2016, 18, 13322–13331. [Google Scholar] [CrossRef] [PubMed]
- Rosy; Haber, S.; Evenstein, E.; Saha, A.; Brontvein, O.; Kratish, Y.; Bravo Zhivotovskii, D.; Apeloig, Y.; Leskes, M.; Noked, M. Alkylated lixsiyoz coating for stabilization of li-rich layered oxide cathodes. Energy Storage Mater. 2020, 33, 268–275. [Google Scholar] [CrossRef]
- Xu, Z.; Ci, L.; Yuan, Y.; Nie, X.; Li, J.; Cheng, J.; Sun, Q.; Zhang, Y.; Han, G.; Min, G.; et al. Potassium prussian blue-coated li-rich cathode with enhanced lithium ion storage property. Nano Energy 2020, 75, 104942. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, J.; Li, D.; Li, Y.; Zeng, Z.; Liu, H.; Zhang, H.; Ji, F.; Geng, X.; Lu, J.; et al. A structure self-healing li-rich cathode achieved by lithium supplement of li-rich llzo coating. Adv. Funct. Mater. 2023, 33, 2214775. [Google Scholar] [CrossRef]
- Ye, Y.; Yuan, S.; Zhang, S.; Liu, T.; Wang, J.; Wang, Q. Functional composite dual-phase in situ self-reconstruction design for high-energy-density li-rich cathodes. Small 2024, 2307669. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Z.; Shen, X.; Wang, X.; Liao, S.; Yu, R.; Wang, Z.; Hu, Z.; Chen, C.; Yu, X.; et al. Li–ti cation mixing enhanced structural and performance stability of li-rich layered oxide. Adv. Energy Mater. 2019, 9, 1901530. [Google Scholar] [CrossRef]
- Sharma, A.; Rajkamal, A.; Kobi, S.; Kumar, B.S.; Paidi, A.K.; Chatterjee, A.; Mukhopadhyay, A. Addressing the high-voltage structural and electrochemical instability of ni-containing layered transition metal (tm) oxide cathodes by “blocking” the “tm-migration” pathway in the lattice. ACS Appl. Mater. Interfaces 2021, 13, 25836–25849. [Google Scholar] [CrossRef]
- Celeste, A.; Girardi, F.; Gigli, L.; Pellegrini, V.; Silvestri, L.; Brutti, S. Impact of overlithiation and al doping on the battery performance of li-rich layered oxide materials. Electrochim. Acta 2022, 428, 140737. [Google Scholar] [CrossRef]
- Dong, S.; Zhou, Y.; Hai, C.; Zeng, J.; Sun, Y.; Shen, Y.; Li, X.; Ren, X.; Sun, C.; Zhang, G.; et al. Understanding electrochemical performance improvement with nb doping in lithium-rich manganese-based cathode materials. J. Power Sources 2020, 462, 228185. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Liu, M.; Chen, Y.; Gu, Y. Enhanced electrochemical performance of la-doped li-rich layered cathode material. J. Alloys Compd. 2020, 848, 156620. [Google Scholar] [CrossRef]
- Zhang, K.; Sheng, H.; Wu, X.; Fu, L.; Liu, Z.; Zhou, C.; Holze, R.; Wu, Y. Improving electrochemical properties by sodium doping for lithium-rich layered oxides. Acs Appl. Energ. Mater. 2020, 3, 8953–8959. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Liu, Y.; Li, L.; Fu, S. Facile and scalable fabrication of k+-doped Li1.2Ni0.2Co0.08Mn0.52O2 cathode with ultra high capacity and enhanced cycling stability for lithium ion batteries. Solid State Ion. 2019, 332, 47–54. [Google Scholar] [CrossRef]
- Fan, Y.; Olsson, E.; Johannessen, B.; D Angelo, A.M.; Thomsen, L.; Cowie, B.; Smillie, L.; Liang, G.; Lei, Y.; Bo, G.; et al. Manipulation of transition metal migration via cr-doping for better-performance li-rich, co-free cathodes. ACS Energy Lett. 2024, 9, 487–496. [Google Scholar] [CrossRef]
- Cong, G.; Huang, L.; Yang, G.; Song, J.; Liu, S.; Huang, Y.; Zhang, X.; Liu, Z.; Geng, L. Ni/mg dual concentration-gradient surface modification to enhance structural stability and electrochemical performance of li-rich layered oxides. ACS Appl. Mater. Interfaces 2024. [Google Scholar] [CrossRef]
- Li, L.; Song, B.H.; Chang, Y.L.; Xia, H.; Yang, J.R.; Lee, K.S.; Lu, L. Retarded phase transition by fluorine doping in li-rich layered Li1.2Mn0.54Ni0.13Co0.13O2 cathode material. J. Power Sources 2015, 283, 162–170. [Google Scholar] [CrossRef]
- Yan, H.; Li, B.; Yu, Z.; Chu, W.; Xia, D. First-principles study: Tuning the redox behavior of lithium-rich layered oxides by chlorine doping. J. Phys. Chem. C 2017, 121, 7155–7163. [Google Scholar] [CrossRef]
- An, J.; Shi, L.; Chen, G.; Li, M.; Liu, H.; Yuan, S.; Chen, S.; Zhang, D. Insights into the stable layered structure of a li-rich cathode material for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 19738–19744. [Google Scholar] [CrossRef]
- Meng, F.; Guo, H.; Wang, Z.; Wang, J.; Yan, G.; Wu, X.; Li, X.; Zhou, L. The influences of SO42− from electrolytic manganese dioxide precursor on the electrochemical properties of li-rich Mn-based material for li-ion batteries. Ionics 2019, 25, 2585–2594. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, D.; Zheng, L.; Zhang, Q.; Gu, L.; Gao, R.; Zhang, J.; Franz, A.; Schumacher, G.; Liu, X. Improving the electrochemical performances of li-rich Li1.20Ni0.13Co0.13Mn0.54O2 through a cooperative doping of Na+ and PO43− with Na3PO4. J. Power Sources 2018, 375, 1–10. [Google Scholar] [CrossRef]
- Seaby, T.; Lin, T.; Hu, Y.; Yuan, Q.; Wang, L. An analysis of f-doping in li-rich cathodes. Rare Met. 2022, 41, 1771–1796. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Qiao, Q.Q.; Li, G.R.; Gao, X.P. PO43− polyanion-doping for stabilizing li-rich layered oxides as cathode materials for advanced lithium-ion batteries. J. Mater. Chem. A 2014, 2, 7454–7460. [Google Scholar] [CrossRef]
- Nie, L.; Wang, Z.; Zhao, X.; Chen, S.; He, Y.; Zhao, H.; Gao, T.; Zhang, Y.; Dong, L.; Kim, F.; et al. Cation/anion codoped and cobalt-free li-rich layered cathode for high-performance li-ion batteries. Nano Lett. 2021, 21, 8370–8377. [Google Scholar] [CrossRef]
- Liu, D.; Fan, X.; Li, Z.; Liu, T.; Sun, M.; Qian, C.; Ling, M.; Liu, Y.; Liang, C. A cation/anion co-doped Li1.12Na0.08Ni0.2Mn0.6O1.95F0.05 cathode for lithium ion batteries. Nano Energy 2019, 58, 786–796. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Zhang, Y.; Gao, G.; Guo, W.; Xu, Q.; Wu, H.; Fan, M.; Wang, L.; Sa, B.; et al. Multi-functionalized full-interface integrated engineering towards highly reversible Li-rich Mn-based cathode. Energy Storage Mater. 2024, 66, 103241. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, X.; Su, Y.; Xu, L.; Chen, L.; Cao, D.; Li, N.; Wu, F. Enabling high-performance layered li-rich oxide cathodes by regulating the formation of integrated cation-disordered domains. Small 2024, 2307292. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, T.; Zhai, X.; Zhang, J.; Wang, S.; Hua, W. Suppressing voltage decay in O2-type li-rich layered cathode materials through microstrain alleviation. Ind. Eng. Chem. Res. 2022, 18, 2201522. [Google Scholar] [CrossRef]
- Jing, Z.; Wang, S.; Fu, Q.; Baran, V.; Tayal, A.; Casati, N.P.M.; Missyul, A.; Simonelli, L.; Knapp, M.; Li, F.; et al. Architecting “li-rich ni-rich” core-shell layered cathodes for high-energy li-ion batteries. Energy Storage Mater. 2023, 59, 102775. [Google Scholar] [CrossRef]
- Eum, D.; Kim, B.; Kim, S.J.; Park, H.; Wu, J.; Cho, S.; Yoon, G.; Lee, M.H.; Jung, S.; Yang, W.; et al. Voltage decay and redox asymmetry mitigation by reversible cation migration in lithium-rich layered oxide electrodes. Nat. Mater. 2020, 19, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhong, P.; Ha, Y.; Kwon, D.; Crafton, M.J.; Tian, Y.; Balasubramanian, M.; Mccloskey, B.D.; Yang, W.; Ceder, G. Non-topotactic reactions enable high rate capability in li-rich cathode materials. Nat. Energy 2021, 6, 706–714. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, X.; Wang, Y.; Zhang, N.; Li, H.; Guan, Y.; Xiao, D.; Liu, S.; Yu, H. Gradient “single-crystal” li-rich cathode materials for high-stable lithium-ion batteries. Adv. Funct. Mater. 2023, 33, 2210154. [Google Scholar] [CrossRef]
- Fu, M.; Ma, X.; Zhao, K.; Li, X.; Su, D. High-entropy materials for energy-related applications. Iscience 2021, 24, 102177. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Y.; Sun, C.; Ni, Q.; Wang, C.; Jin, H. High entropy spinel-structure oxide for electrochemical application. Chem. Eng. J. 2022, 431, 133448. [Google Scholar] [CrossRef]
- Sarkar, A.; Breitung, B.; Hahn, H. High entropy oxides: The role of entropy, enthalpy and synergy. Scr. Mater. 2020, 187, 43–48. [Google Scholar] [CrossRef]
- Lun, Z.; Ouyang, B.; Kwon, D.; Ha, Y.; Foley, E.E.; Huang, T.; Cai, Z.; Kim, H.; Balasubramanian, M.; Sun, Y.; et al. Cation-disordered rocksalt-type high-entropy cathodes for li-ion batteries. Nat. Mater. 2021, 20, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ning, F.; Zuo, Y.; Li, A.; Wang, H.; Zhang, K.; Yang, T.; Yang, Y.; Gao, C.; Xiao, W.; et al. Entropy stabilization strategy for enhancing the local structural adaptability of li-rich cathode materials. Adv. Mater. 2023, 35, 2208726. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, C.; Zou, P.; Lin, R.; Ma, L.; Yin, L.; Li, T.; Xu, W.; Jia, H.; Li, Q.; et al. Compositionally complex doping for zero-strain zero-cobalt layered cathodes. Nature 2022, 610, 67–73. [Google Scholar] [CrossRef]

| Materials | Modification | Initial (mAh g−1/C) | ICE (%) | Rate (mAh g−1/C) | Retention (%/Cycles/C) | Ref. |
|---|---|---|---|---|---|---|
| Surface Coating | ||||||
| Li1.256Ni0.198Co0.082Mn0.689O2.25 | Al2O3 | 282.0 mAh g−1/0.1C | 87.4% | 189.0 mAh g−1/5C | 95.2%/60/1C | [71] |
| Li1.2Mn0.54Ni0.13Co0.13O2 | ZnO | 271.8 mAh g−1/0.1C | 84.3% | 149.7 mAh g−1/5C | 97.5%/100/0.5C | [72] |
| Li1.2Mn0.54Ni0.13Co0.13O2 | MnO2 | 296.9 mAh g−1/0.05C | 93.0% | 165.0 mAh g−1/5C | 92.84%/100/1C | [73] |
| Li1.2Mn0.54Ni0.13Co0.13O2 | Nb2O5 | 258.0 mAh g−1/0.1C | 83.4% | 152.0 mAh g−1/5C | 97.2%/100/0.5C | [74] |
| Li1.2Mn0.54Ni0.13Co0.13O2 | CeO2 | 265.0 mAh g−1/0.1C | 83.3% | 158.0 mAh g−1/5C | 94.9%/100/0.5C | [76] |
| Li1.2Mn0.54Ni0.13Co0.13O2 | AlF3 | 283.3 mAh g−1/0.1C | 88.3% | 84.4%/200/1C | [78] | |
| Li4Mn5O12 | MgF2 | 268.9 mAh g−1/0.1C | 93.0% | 140.8 mAh g−1/5C | 80.0%/300/0.5C | [79] |
| Li1.2Mn0.54Ni0.13Co0.13O2 | LiF | 242.4 mAh g−1/0.1C | 80.0% | 95.0%/100/1C | [80] | |
| Li1.2Mn0.54Ni0.13Fe0.13O2 | LiF-MgF2-CaF2 | 240.6 mAh g−1/0.1C | 72.1% | 121.2 mAh g−1/5C | 90.1%/120/0.2C | [83] |
| Li1.2Mn0.54Ni0.13Co0.13O2 | Li3PO4 | 273.0 mAh g−1/0.1C | 86.9% | 143.8 mAh g−1/5C | 81.8%/175/0.5C | [85] |
| Li1.08Mn0.54Co0.13Ni0.13O2 | Li2TiO3 | 276.5 mAh g−1/0.1C | 86.3% | 99.7%/125/0.2C | [87] | |
| Li1.2Ni0.2Mn0.6O2 | LiAlO2 | 268.8 mAh g−1/0.1C | 84.5% | 143.8 mAh g−1/5C | 97.3%/100/0.C | [88] |
| Li1.2Mn0.54Ni0.13Co0.13O2 | Li2MnO3 | 294.0 mAh g−1/0.1C | 87.6% | 211.1 mAh g−1/1C | 79.6%/100/0.5C | [89] |
| Li1.2Mn0.6Ni0.2O2 | Li2ZrO3 | 239.8 mAh g−1/0.1C | 140.2 mAh g−1/2C | 83.5%/100/1C | [90] | |
| LiNixMnyCoxO2 | LixSiyOz | 294.0 mAh g−1/0.1C | 200.0 mAh g−1/4C | 81.4%/200/0.3C | [91] | |
| Li1.2Mn0.54Ni0.13Co0.13O2 | KPB | 281.7 mAh g−1/0.1C | 85.69% | 139.0 mAh g−1/5C | 77.17%/100/0.5C | [92] |
| Li1.2Mn0.54Ni0.13Co0.13O2 | LLZAO | 282.4 mAh g−1/0.1C | 85.6% | 123.9 mAh g−1/5C | 95.7%/300/1C | [93] |
| Li1.2Mn0.54Ni0.13Co0.13O2 | C@spinel/MO | 283.3 mAh g−1/0.1C | 85.0% | 89.7%/100/1C | [94] | |
| Elemental Doping | ||||||
| Li1.2Ti0.26Ni0.18Co0.18Mn0.18O2 | Ti2+ | 176.0 mAh g−1/0.1C | 60% | 97%/180/0.1C | [95] | |
| LiNi1/3Mn1/3Co1/3O2 | Zn2+ | 191.0 mAh g−1/0.1C | 66.1% | 70%/100/0.1C | [96] | |
| Li1.2+xMn0.54Ni0.13Co0.13−x−yAlyO2 | Al3+ | 225.0 mAh g−1/0.1C | 124.0 mAh g−1/2C | 100%/200/0.1C | [97] | |
| Li1.2Mn0.54Ni0.13Co0.13O2 | Nb5+ | 287.5 mAh g−1/0.1C | 86.94% | 123.6 mAh g−1/8C | 98.5%/300/0.2C | [98] |
| Li1.2Mn0.54Ni0.13Co0.13O2 | La3+ | 262.1 mAh g−1/0.1C | 81.5% | 86.7%/50/1C | [99] | |
| Li1.2Na0.03[Ni0.2464Mn0.462Co0.0616]O2 | Na+ | 250.2 mAh g−1/0.1C | 138.4 mAh g−1/5C | 97.17%/110/1C | [100] | |
| Li1.2Mn0.54Ni0.13Co0.13O2 | K+ | 294.6 mAh g−1/0.1C | 80.7% | 110.8 mAh g−1/10C | 93.4%/200/0.5C | [101] |
| Li1.2Mn0.6Ni0.2O2 | Cr6+ | 200.0 mAh g−1/1.0C | 99.0%/200/1C | [102] | ||
| Li1.2Mn0.54Ni0.13Co0.13O2 | Ni/Mg | 94.84 mAh g−1/7C | 78.63%/300/5C | [103] | ||
| Li1.2Mn0.54Ni0.13Co0.13O2 | S2- | 293.3 mAh g−1/0.1C | 96.06% | 117.0 mAh g−1/5C | 72.7%/67/1C | [106] |
| Li1.2Mn0.54Ni0.13Co0.13O2 | Fe & Cl | 235.1 mAh g−1/0.2C | 73.1% | 145.6 mAh g−1/5C | 86.4%/500/1C | [111] |
| Li1.2Ni0.2Mn0.6O2 | Na & F | 135.0 mAh g−1/5C | 100%/100/0.2C | [112] | ||
| Structural Designs | ||||||
| Li1.2Mn0.54Ni0.13Co0.13O2 | multi-functionalized full-interface integrated engineering | 86.6%/500/1C | [113] | |||
| Li1.2Mn0.6Ni0.2O2 | layered/rock-salt intergrown structure | 288.4 mAh g−1/0.1C | 131.8 mAh g−1/10C | 88.2%/100/0.5C | [114] | |
| Li1.2Mn0.4−xTi0.4CrxO2 | rock-salt typeformations | 253.0 mAh g−1/0.1C | [118] | |||
| Li1.2Mn0.54Ni0.13Co0.13O2 | single crystal | 260.0 mAh g−1/0.1C | 97.6%/100/0.1C | [119] | ||
| High Entropy | ||||||
| Li1.0[Li0.15Mn0.50Ni0.15Co0.10Fe0.025Cu0.025Al0.025Mg0.025]O2 | Fe, Cu, Al, Mg | 260.0 mAh g−1/0.1C | 85% | 93.4%/100/0.1C | [124] | |
| LiNi0.8Mn0.13Ti0.02Mg0.02Nb0.01Mo0.02O2 | Ti, Mg, Nb, Mo | 210.1 mAh g−1/0.1C | 94% | 95%/500/1C | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, Z.; Sun, Q.; Li, J.; Qiao, Y.; Min, G.; Ci, L. Modification Strategies of High-Energy Li-Rich Mn-Based Cathodes for Li-Ion Batteries: A Review. Molecules 2024, 29, 1064. https://doi.org/10.3390/molecules29051064
Xi Z, Sun Q, Li J, Qiao Y, Min G, Ci L. Modification Strategies of High-Energy Li-Rich Mn-Based Cathodes for Li-Ion Batteries: A Review. Molecules. 2024; 29(5):1064. https://doi.org/10.3390/molecules29051064
Chicago/Turabian StyleXi, Zhenjie, Qing Sun, Jing Li, Ying Qiao, Guanghui Min, and Lijie Ci. 2024. "Modification Strategies of High-Energy Li-Rich Mn-Based Cathodes for Li-Ion Batteries: A Review" Molecules 29, no. 5: 1064. https://doi.org/10.3390/molecules29051064
APA StyleXi, Z., Sun, Q., Li, J., Qiao, Y., Min, G., & Ci, L. (2024). Modification Strategies of High-Energy Li-Rich Mn-Based Cathodes for Li-Ion Batteries: A Review. Molecules, 29(5), 1064. https://doi.org/10.3390/molecules29051064