Establishing an ANO1-Based Cell Model for High-Throughput Screening Targeting TRPV4 Regulators
Abstract
1. Introduction
2. Results
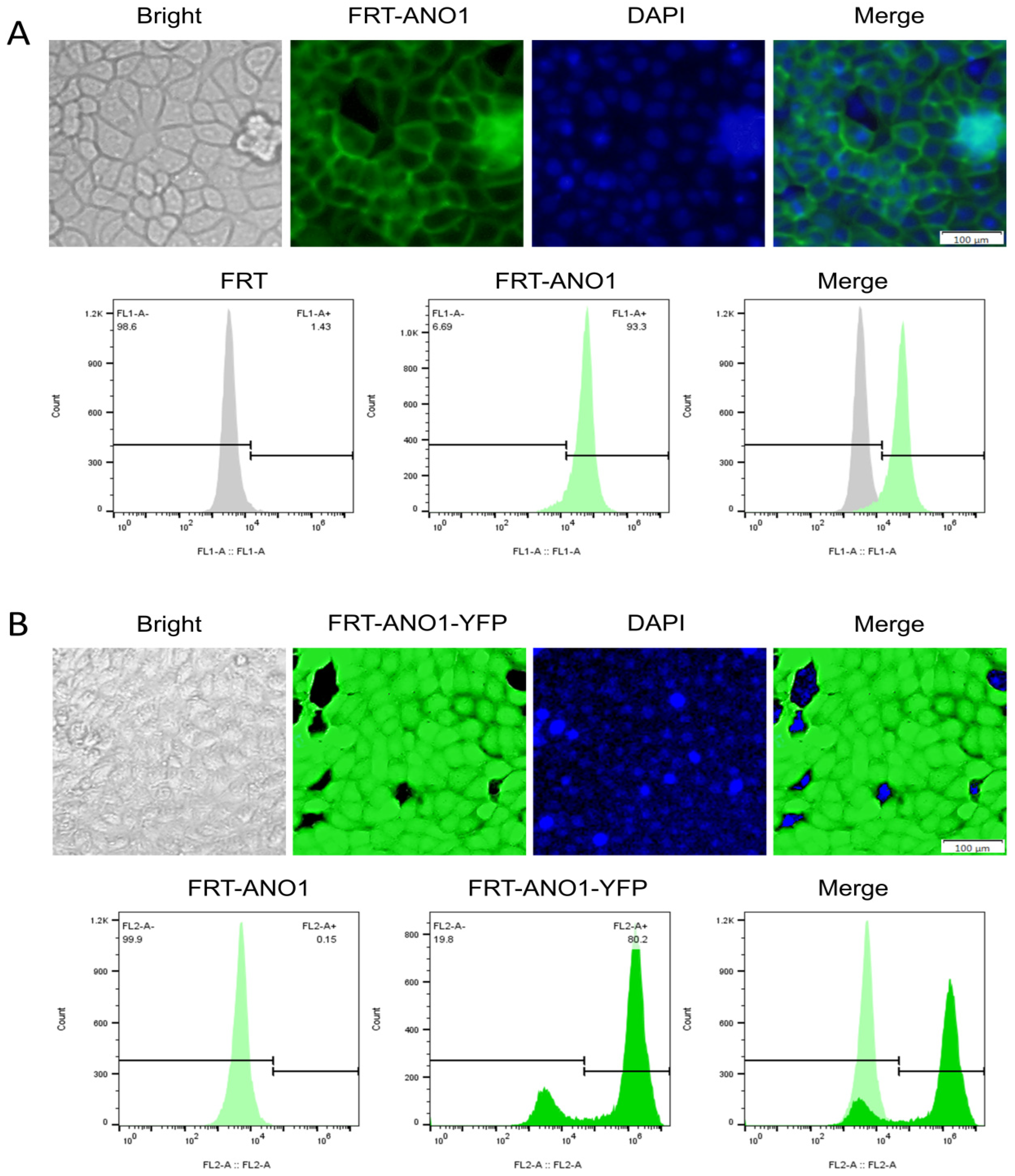
2.1. Endogenous Expression of TRPV4 Protein in FRT Cells
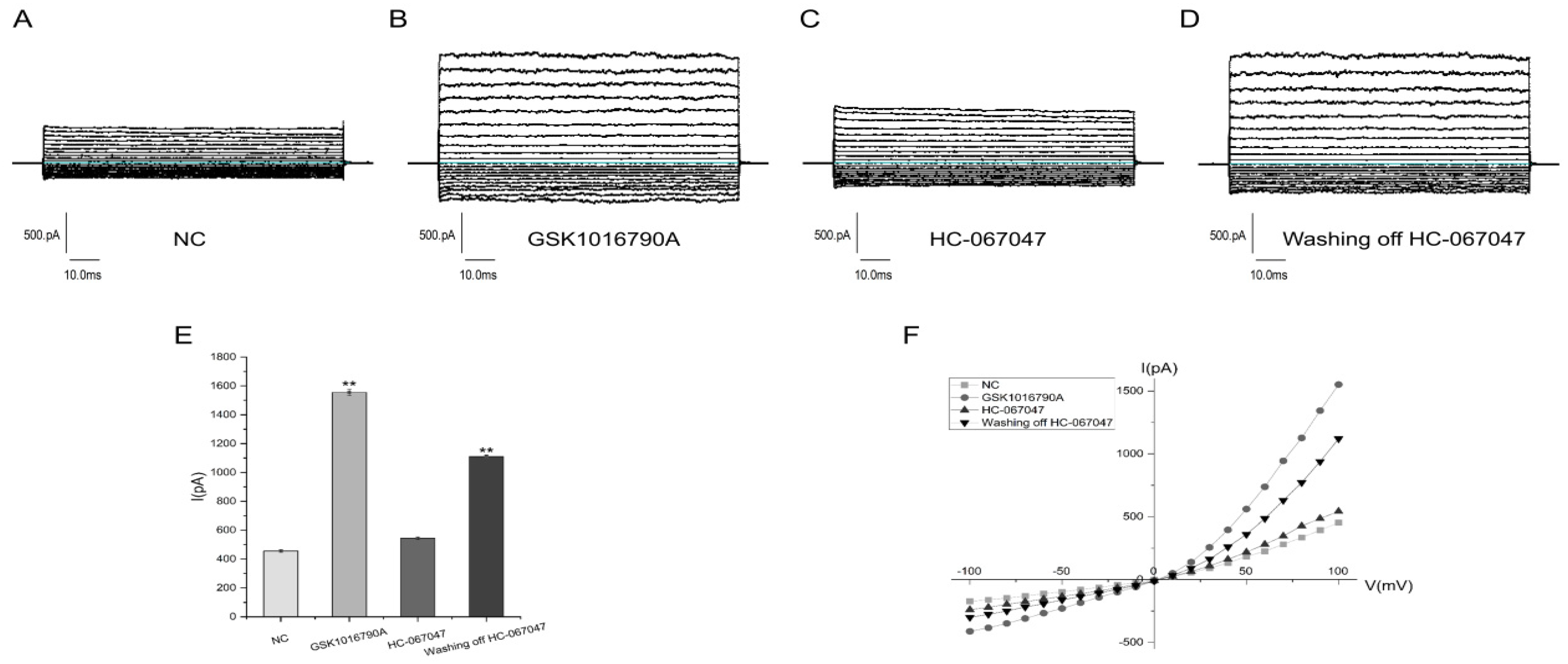
2.2. Electrophysiological Properties of TRPV4
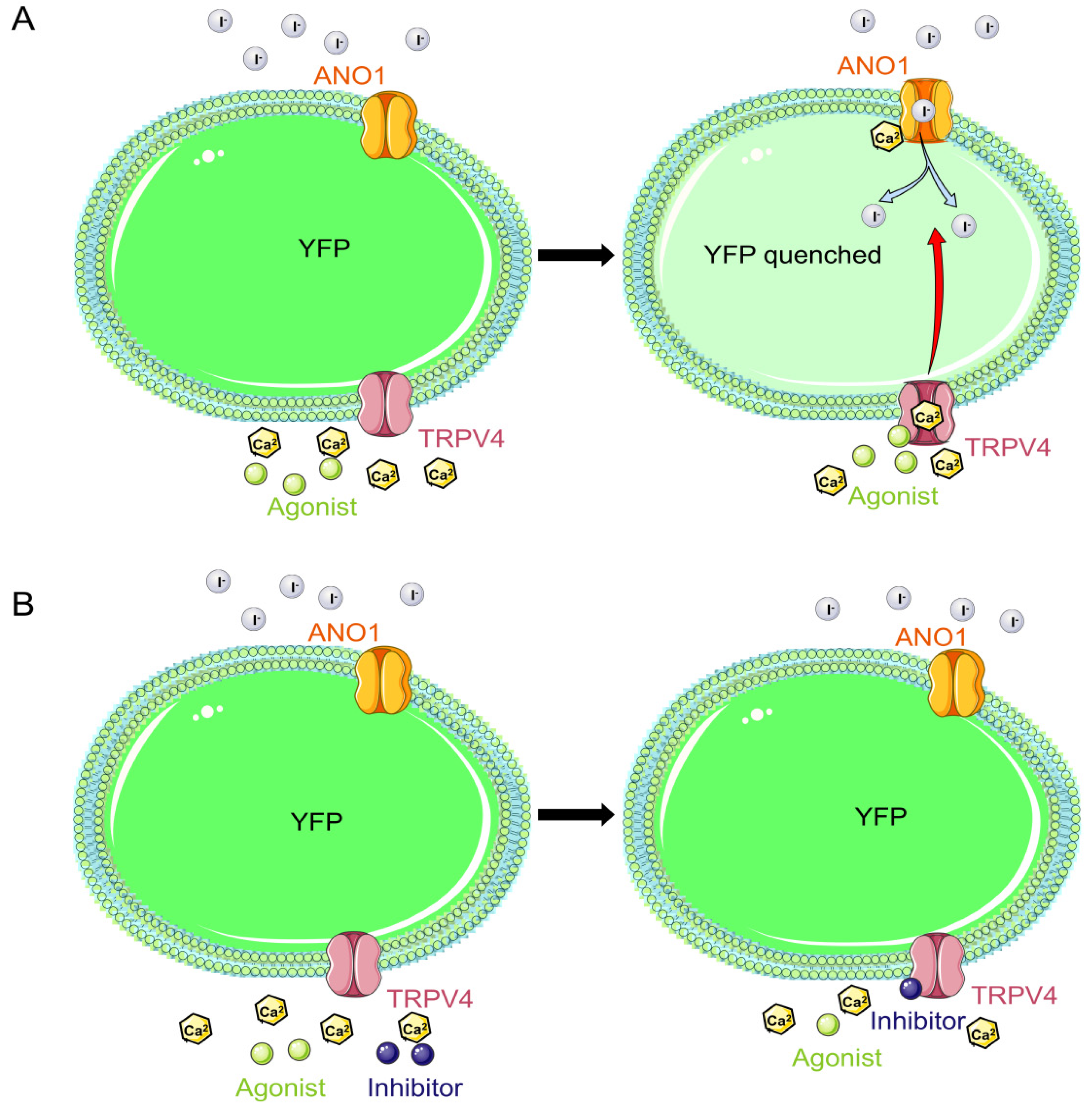
2.3. Successful Construction of FRT Cell Lines Co-Expressing ANO1 and YFP-H148Q/I152L
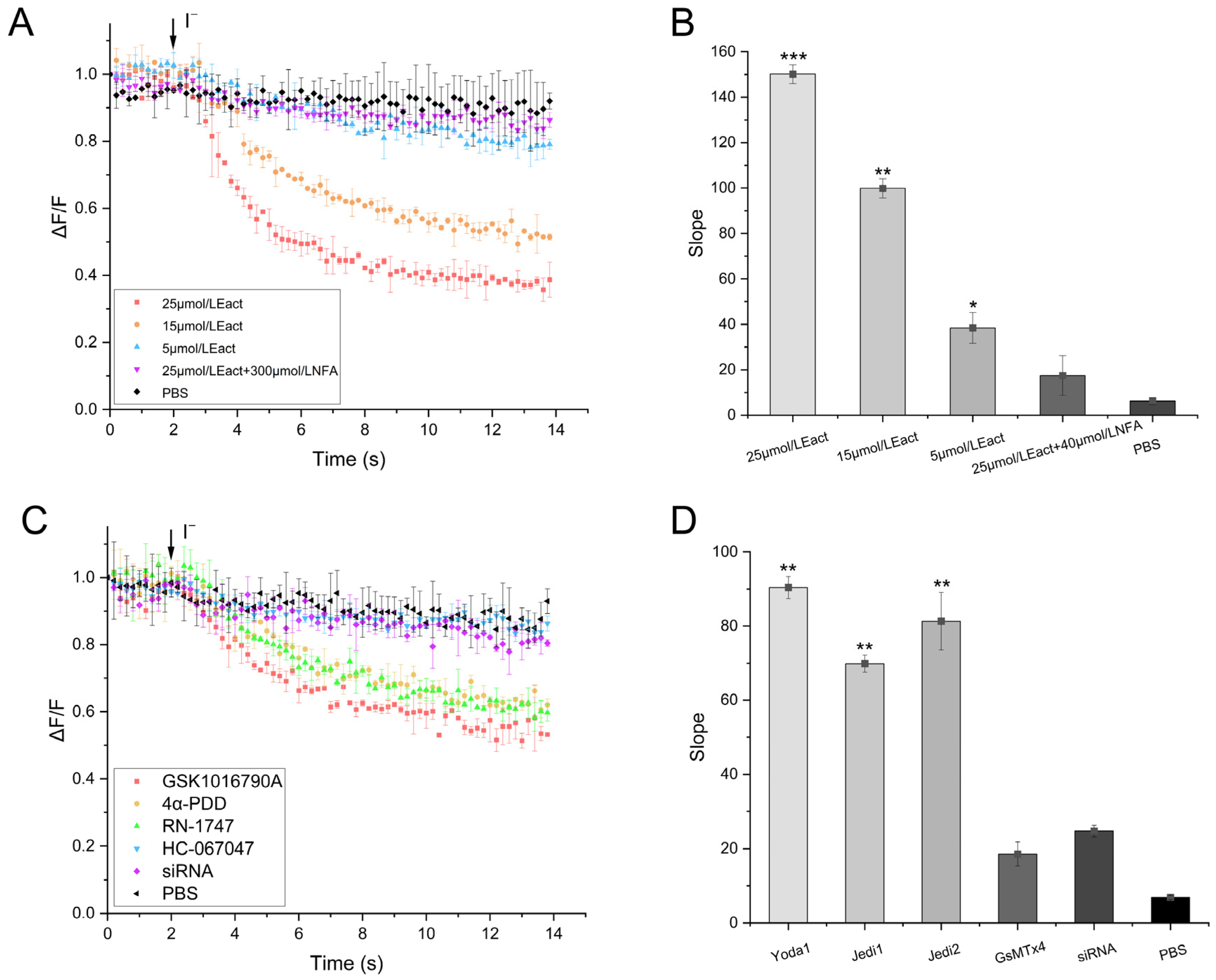
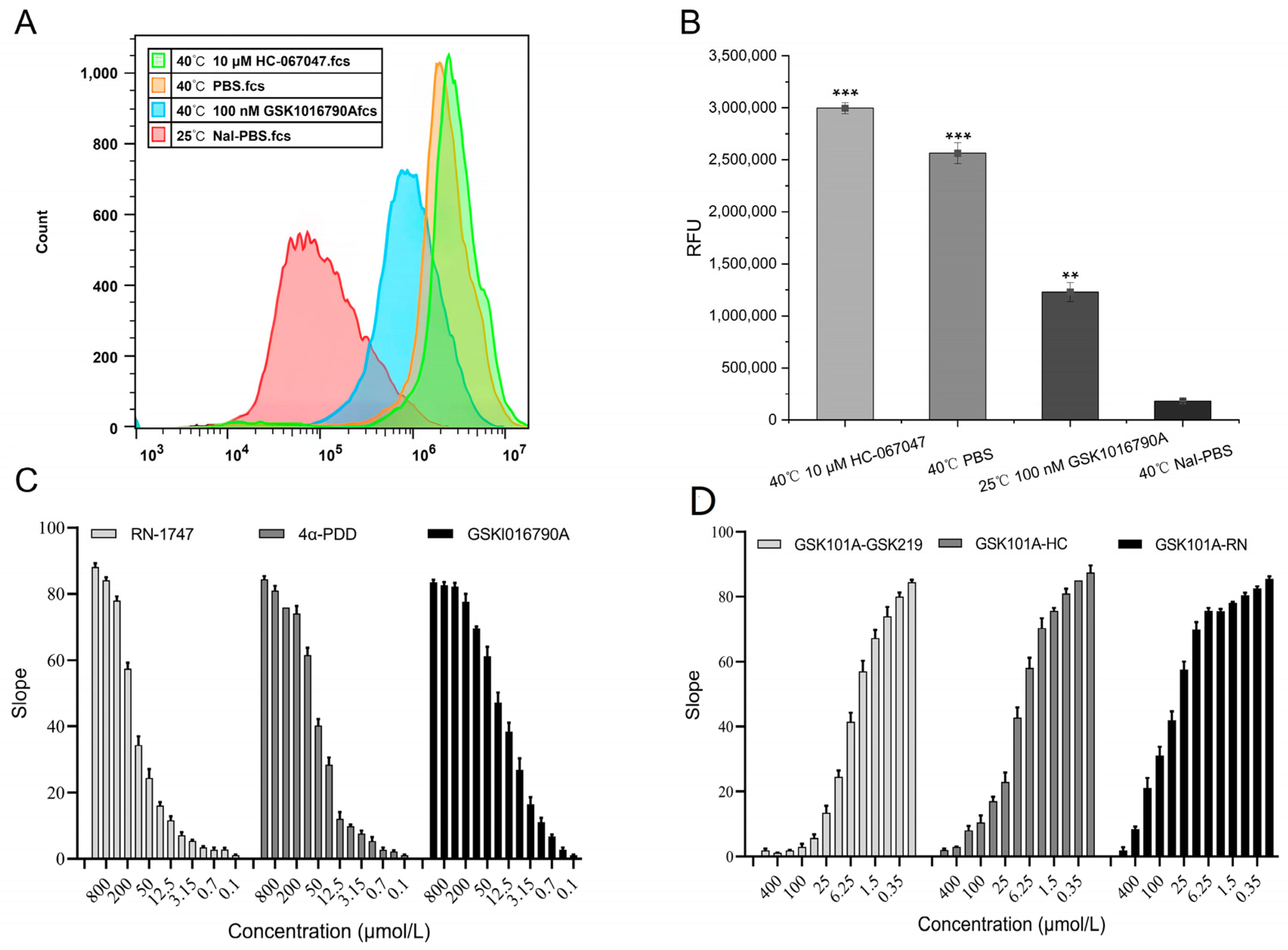
2.4. Validity of the TRPV4 Cell Model
2.5. Functionality of the TRPV4 Cell Model
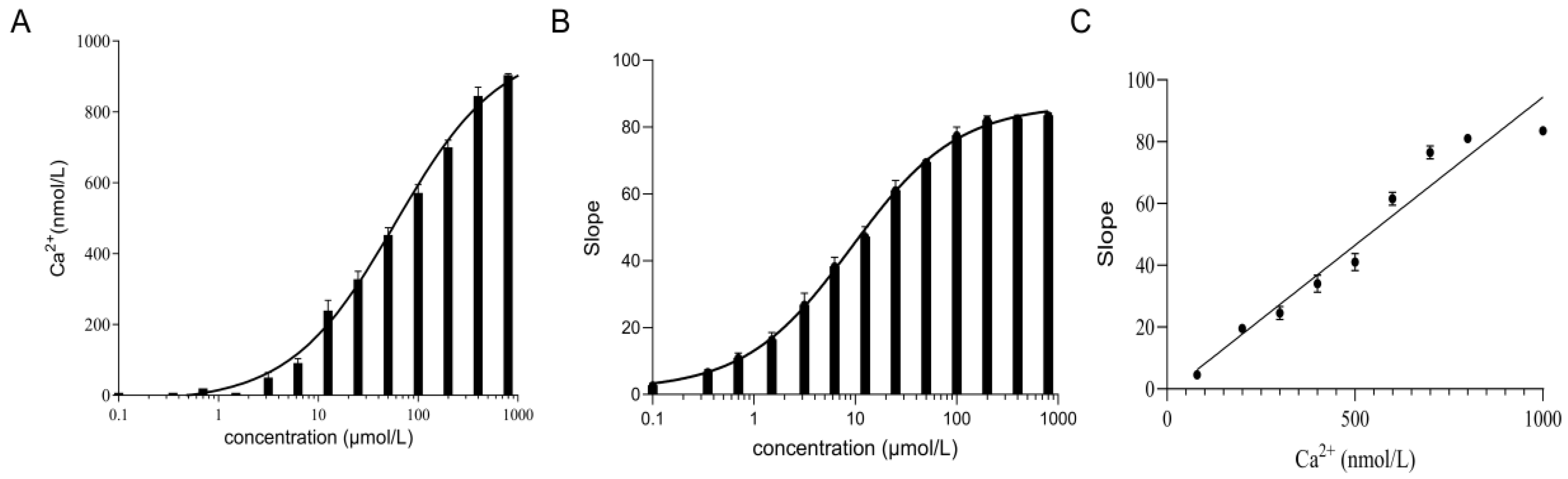
2.6. The TRPV4 Cell Model Is Calcium Sensitive
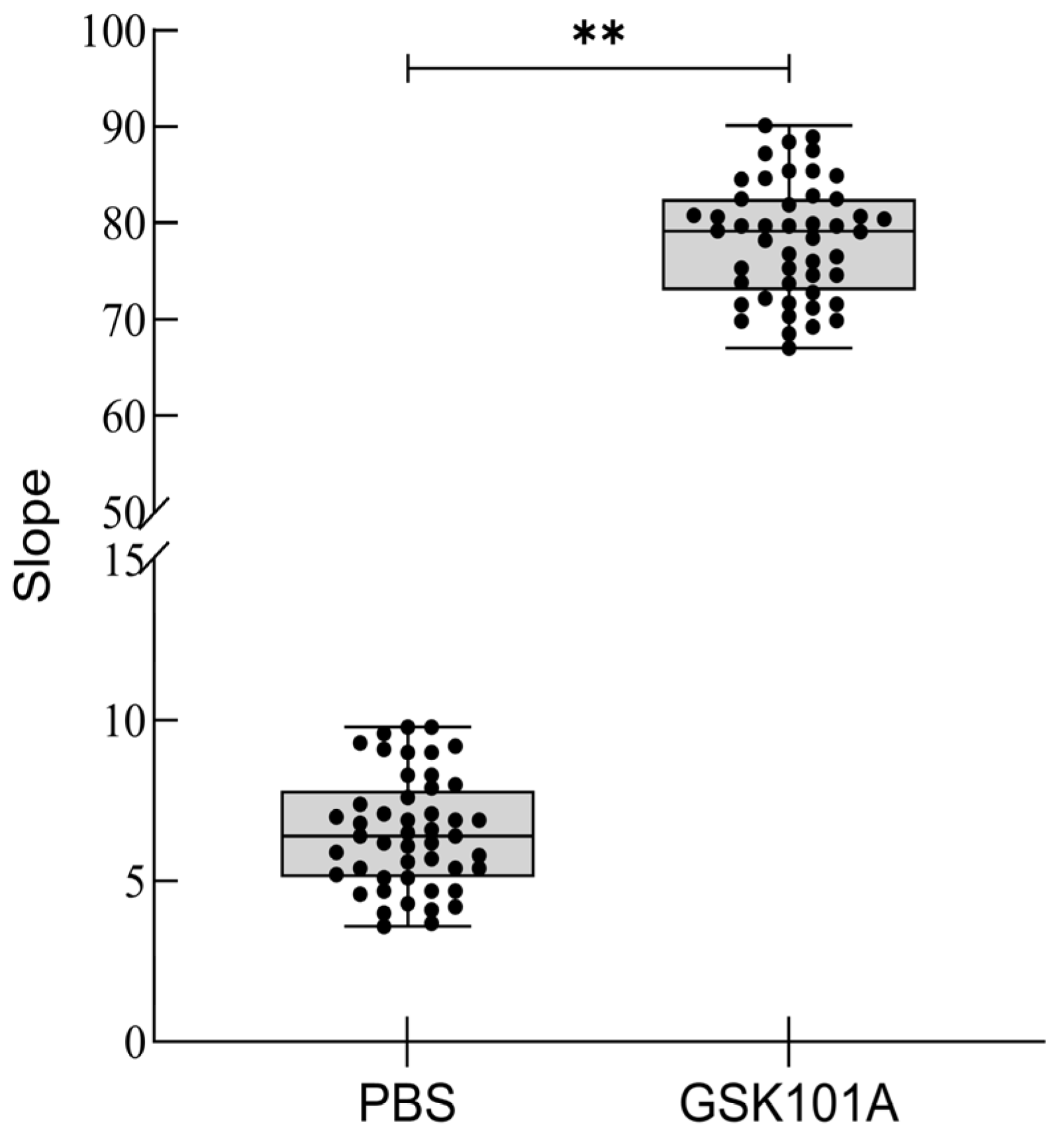
2.7. Z-Factor Evaluation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Detection of the Endogenous Expression of the TRPV4 Protein on FRT Cells
4.2.1. Detection of Trpv4 Expression by qRT-PCR
4.2.2. TRPV4 Electrophysiological Tests
4.3. Stable Transfection
4.4. Validation of TRPV4 Cell Model Function
4.4.1. Validation of the Cell Model
4.4.2. Effectiveness of Screening Regulators
4.4.3. TRPV4 Model Physical Properties
4.4.4. Functionality of Screening Regulators
4.5. Measurement of Calcium Ion Concentration in the TRPV4 Cell Model
4.6. TRPV4 Cell Model Z-Factor Assessment
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwon, D.H.; Zhang, F.; Suo, Y.; Bouvette, J.; Borgnia, M.J.; Lee, S.Y. Heat-dependent opening of TRPV1 in the presence of capsaicin. Nat. Struct. Mol. Biol. 2021, 28, 554–563. [Google Scholar] [CrossRef]
- Fan, J.; Hu, L.; Yue, Z.; Liao, D.; Guo, F.; Ke, H.; Jiang, D.; Yang, Y.; Lei, X. Structural basis of TRPV3 inhibition by an antagonist. Nat. Chem. Biol. 2023, 19, 81–90. [Google Scholar] [CrossRef]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef]
- Lan, Z.; Chen, L.; Feng, J.; Xie, Z.; Liu, Z.; Wang, F.; Liu, P.; Yue, X.; Du, L.; Zhao, Y.; et al. Mechanosensitive TRPV4 is required for crystal-induced inflammation. Ann. Rheum. Dis. 2021, 80, 1604–1614. [Google Scholar] [CrossRef]
- Huang, T.; Xu, T.; Wang, Y.; Zhou, Y.; Yu, D.; Wang, Z.; He, L.; Chen, Z.; Zhang, Y.; Davidson, D.; et al. Cannabidiol inhibits human glioma by induction of lethal mitophagy through activating TRPV4. Autophagy 2021, 17, 3592–3606. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Du, L.; Liu, S.; Lan, Z.; Zang, K.; Feng, J.; Zhao, Y.; Yang, X.; Xie, Z.; Wang, P.; et al. A TRPV4-dependent neuroimmune axis in the spinal cord promotes neuropathic pain. J. Clin. Investig. 2023, 133, e161507. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, B.; Yin, M.; Koroleva, M.; Mastrangelo, M.; Ture, S.; Morrell, C.; Zhang, D.; Fisher, E.; Jin, Z. A novel TRPV4-specific agonist inhibits monocyte adhesion and atherosclerosis. Oncotarget 2016, 7, 37622–37635. [Google Scholar] [CrossRef]
- Koller, A.; Laughlin, M.; Cenko, E.; de Wit, C.; Tóth, K.; Bugiardini, R.; Trifunovits, D.; Vavlukis, M.; Manfrini, O.; Lelbach, A.; et al. Functional and structural adaptations of the coronary macro- and microvasculature to regular aerobic exercise by activation of physiological, cellular, and molecular mechanisms: ESC Working Group on Coronary Pathophysiology and Microcirculation position paper. Cardiovasc. Res. 2022, 118, 357–371. [Google Scholar] [CrossRef]
- Hoshi, Y.; Shibasaki, K.; Gailly, P.; Ikegaya, Y.; Koyama, R. Thermosensitive receptors in neural stem cells link stress-induced hyperthermia to impaired neurogenesis via microglial engulfment. Sci. Adv. 2021, 7, eabj8080. [Google Scholar] [CrossRef]
- García-Rodríguez, C.; Bravo-Tobar, I.; Duarte, Y.; Barrio, L.; Sáez, J. Contribution of non-selective membrane channels and receptors in epilepsy. Pharmacol. Ther. 2022, 231, 107980. [Google Scholar] [CrossRef] [PubMed]
- Kanugula, A.; Adapala, R.; Jamaiyar, A.; Lenkey, N.; Guarino, B.; Liedtke, W.; Yin, L.; Paruchuri, S.; Thodeti, C. Endothelial TRPV4 channels prevent tumor growth and metastasis via modulation of tumor angiogenesis and vascular integrity. Angiogenesis 2021, 24, 647–656. [Google Scholar] [CrossRef]
- Mao, A.; He, D.; Zhang, K.; Gao, M.; Kan, H.; Wang, Z.; Tang, C.; Ma, X. Functional role of coupling the endothelial TRPV4 and K 3.1 channels in regulating coronary vascular tone. Br. J. Pharmacol. 2023, 180, 2266–2279. [Google Scholar] [CrossRef]
- Grace, M.; Bonvini, S.; Belvisi, M.; McIntyre, P. Modulation of the TRPV4 ion channel as a therapeutic target for disease. Pharmacol. Ther. 2017, 177, 9–22. [Google Scholar] [CrossRef]
- Erfinanda, L.; Zou, L.; Gutbier, B.; Kneller, L.; Weidenfeld, S.; Michalick, L.; Lei, D.; Reppe, K.; Teixeira Alves, L.; Schneider, B.; et al. Loss of endothelial CFTR drives barrier failure and edema formation in lung infection and can be targeted by CFTR potentiation. Sci. Transl. Med. 2022, 14, eabg8577. [Google Scholar] [CrossRef]
- Doñate-Macian, P.; Duarte, Y.; Rubio-Moscardo, F.; Pérez-Vilaró, G.; Canan, J.; Díez, J.; González-Nilo, F.; Valverde, M. Structural determinants of TRPV4 inhibition and identification of new antagonists with antiviral activity. Br. J. Pharmacol. 2022, 179, 3576–3591. [Google Scholar] [CrossRef]
- Jacob, P.; Kim, N.; Wu, F.; El-Kasmi, F.; Chi, Y.; Walton, W.; Furzer, O.; Lietzan, A.; Sunil, S.; Kempthorn, K.; et al. Plant “helper” immune receptors are Ca-permeable nonselective cation channels. Science 2021, 373, 420–425. [Google Scholar] [CrossRef]
- Dang, S.; Feng, S.; Tien, J.; Peters, C.; Bulkley, D.; Lolicato, M.; Zhao, J.; Zuberbühler, K.; Ye, W.; Qi, L.; et al. Cryo-EM structures of the TMEM16A calcium-activated chloride channel. Nature 2017, 552, 426–429. [Google Scholar] [CrossRef]
- Liu, X.; Ju, X.; Hong, Q.; Wang, X.; Wu, M.; Xing, W.; Xu, M.; Hu, C.; Hao, F. Establishment of a CaCC-based Cell Model and Method for High-throughput Screening of M3 Receptor Drugs. Cell Biochem. Biophys. 2023, 81, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, E.; Mehta, S.; Zhang, J. Genetically Encoded Fluorescent Biosensors Illuminate the Spatiotemporal Regulation of Signaling Networks. Chem. Rev. 2018, 118, 11707–11794. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, T.; Benítez-Angeles, M.; Sánchez-Hernández, R.; Morales-Lázaro, S.; Hiriart, M.; Morales-Buenrostro, L.; Torres-Quiroz, F. TRPV4: A Physio and Pathophysiologically Significant Ion Channel. Int. J. Mol. Sci. 2020, 21, 3837. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Daneva, Z.; Kuppusamy, M.; Ottolini, M.; Baker, T.; Klimentova, E.; Shah, S.; Sokolowski, J.; Park, M.; Sonkusare, S. Novel Smooth Muscle Ca-Signaling Nanodomains in Blood Pressure Regulation. Circulation 2022, 146, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. The Diverse Physiological Functions of Mechanically Activated Ion Channels in Mammals. Annu. Rev. Physiol. 2022, 84, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Nazıroğlu, M. A novel antagonist of TRPM2 and TRPV4 channels: Carvacrol. Metab. Brain Dis. 2022, 37, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Dana, H.; Sun, Y.; Mohar, B.; Hulse, B.; Kerlin, A.; Hasseman, J.; Tsegaye, G.; Tsang, A.; Wong, A.; Patel, R.; et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods 2019, 16, 649–657. [Google Scholar] [CrossRef]
- de la Peña, E.; Gomis, A. Characterization of TRPC Channels in a Heterologous System Using Calcium Imaging and the Patch-Clamp Technique. Methods Mol. Biol. 2019, 1987, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Taylor, C. Reliable measurement of free Ca concentrations in the ER lumen using Mag-Fluo-4. Cell Calcium 2020, 87, 102188. [Google Scholar] [CrossRef] [PubMed]
- Brunklaus, A.; Feng, T.; Brünger, T.; Perez-Palma, E.; Heyne, H.; Matthews, E.; Semsarian, C.; Symonds, J.; Zuberi, S.; Lal, D.; et al. Gene variant effects across sodium channelopathies predict function and guide precision therapy. Brain A J. Neurol. 2022, 145, 4275–4286. [Google Scholar] [CrossRef]
- Erdogmus, S.; Concepcion, A.; Yamashita, M.; Sidhu, I.; Tao, A.; Li, W.; Rocha, P.; Huang, B.; Garippa, R.; Lee, B.; et al. Cavβ1 regulates T cell expansion and apoptosis independently of voltage-gated Ca channel function. Nat. Commun. 2022, 13, 2033. [Google Scholar] [CrossRef]
- Lin, C.; Lerner, R. Antibody Libraries as Tools to Discover Functional Antibodies and Receptor Pleiotropism. Int. J. Mol. Sci. 2021, 22, 4123. [Google Scholar] [CrossRef]
- Wu, D.; Gordon, C.; Shin, J.; Eisenstein, M.; Soh, H. Directed Evolution of Aptamer Discovery Technologies. Acc. Chem. Res. 2022, 55, 685–695. [Google Scholar] [CrossRef]
- Galietta, L.J.; Haggie, P.M.; Verkman, A.S. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 2001, 499, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Thiagarajah, J.R.; Yang, H.; Sonawane, N.D.; Folli, C.; Galietta, L.J.; Verkman, A.S. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J. Clin. Investig. 2002, 110, 1651–1658. [Google Scholar] [CrossRef]
- De La Fuente, R.; Namkung, W.; Mills, A.; Verkman, A.S. Small-molecule screen identifies inhibitors of a human intestinal calcium-activated chloride channel. Mol. Pharmacol. 2008, 73, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, R.; Singh, D.; Janicek, K.; Bers, D.; Thomas, D.; Launikonis, B.; Cornea, R. RyR1-targeted drug discovery pipeline integrating FRET-based high-throughput screening and human myofiber dynamic Ca assays. Sci. Rep. 2020, 10, 1791. [Google Scholar] [CrossRef] [PubMed]

| Primers | Sequences (5′-3′) | Base Number | Product Length (bp) |
|---|---|---|---|
| TRPV1 | Forward: AGGGAGATCCATGAACCCGA | 20 | 309 |
| Reverse: CACAGGCCGATAGTAGGCAG | 20 | ||
| TRPV2 | Forward: CTGGTCTTTGACAGGTCGGC | 20 | 420 |
| Reverse: ACCTCAGCATTGCCCTCTTC | 20 | ||
| TRPV3 | Forward: TCAAAGCAAGGGCTGCTACC | 20 | 291 |
| Reverse: ATCACAGTTGCCAGAGAGGC | 20 | ||
| TRPV4-1 | Forward: AAGCCGATATGAGGCGACAG | 20 | 455 |
| Reverse: CTTGTCCCTCAGCAGTTCGT | 20 | ||
| TRPV4-2 | Forward: GGGAACCATCCACAGGGAAG | 20 | 395 |
| Reverse: CTGTCGCCTCATATCGGCTT | 20 | ||
| TRPV5 | Forward: TGCTCCATACTTGGTCACGG | 20 | 561 |
| Reverse: TCCTCACCCCAGGAGTCAAT | 20 | ||
| TRPV6 | Forward: AACCCCAGGGACAATACCCT | 20 | 579 |
| Reverse: GAAGGGCAGATCCACGTCAT | 20 | ||
| β-actin | Forward: GTCGTCGACAACGGCTCC | 18 | 260 |
| Reverse: AGGTCTCAAACATGATCTGGGT | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, K.; Hu, J.; Hu, C.; Liu, X.; Wang, Y.; Han, H.; Xing, W.; Yang, L.; Zhang, J.; Hong, Q.; et al. Establishing an ANO1-Based Cell Model for High-Throughput Screening Targeting TRPV4 Regulators. Molecules 2024, 29, 1036. https://doi.org/10.3390/molecules29051036
Zheng K, Hu J, Hu C, Liu X, Wang Y, Han H, Xing W, Yang L, Zhang J, Hong Q, et al. Establishing an ANO1-Based Cell Model for High-Throughput Screening Targeting TRPV4 Regulators. Molecules. 2024; 29(5):1036. https://doi.org/10.3390/molecules29051036
Chicago/Turabian StyleZheng, Kai, Jiang Hu, Cheng Hu, Xueying Liu, Yanyan Wang, Haojian Han, Wenzhu Xing, Liu Yang, Junran Zhang, Qiyuan Hong, and et al. 2024. "Establishing an ANO1-Based Cell Model for High-Throughput Screening Targeting TRPV4 Regulators" Molecules 29, no. 5: 1036. https://doi.org/10.3390/molecules29051036
APA StyleZheng, K., Hu, J., Hu, C., Liu, X., Wang, Y., Han, H., Xing, W., Yang, L., Zhang, J., Hong, Q., Hao, F., & Li, W. (2024). Establishing an ANO1-Based Cell Model for High-Throughput Screening Targeting TRPV4 Regulators. Molecules, 29(5), 1036. https://doi.org/10.3390/molecules29051036





