Isolation, Purification, Fractionation, and Hepatoprotective Activity of Polygonatum Polysaccharides
Abstract
1. Introduction
2. Results
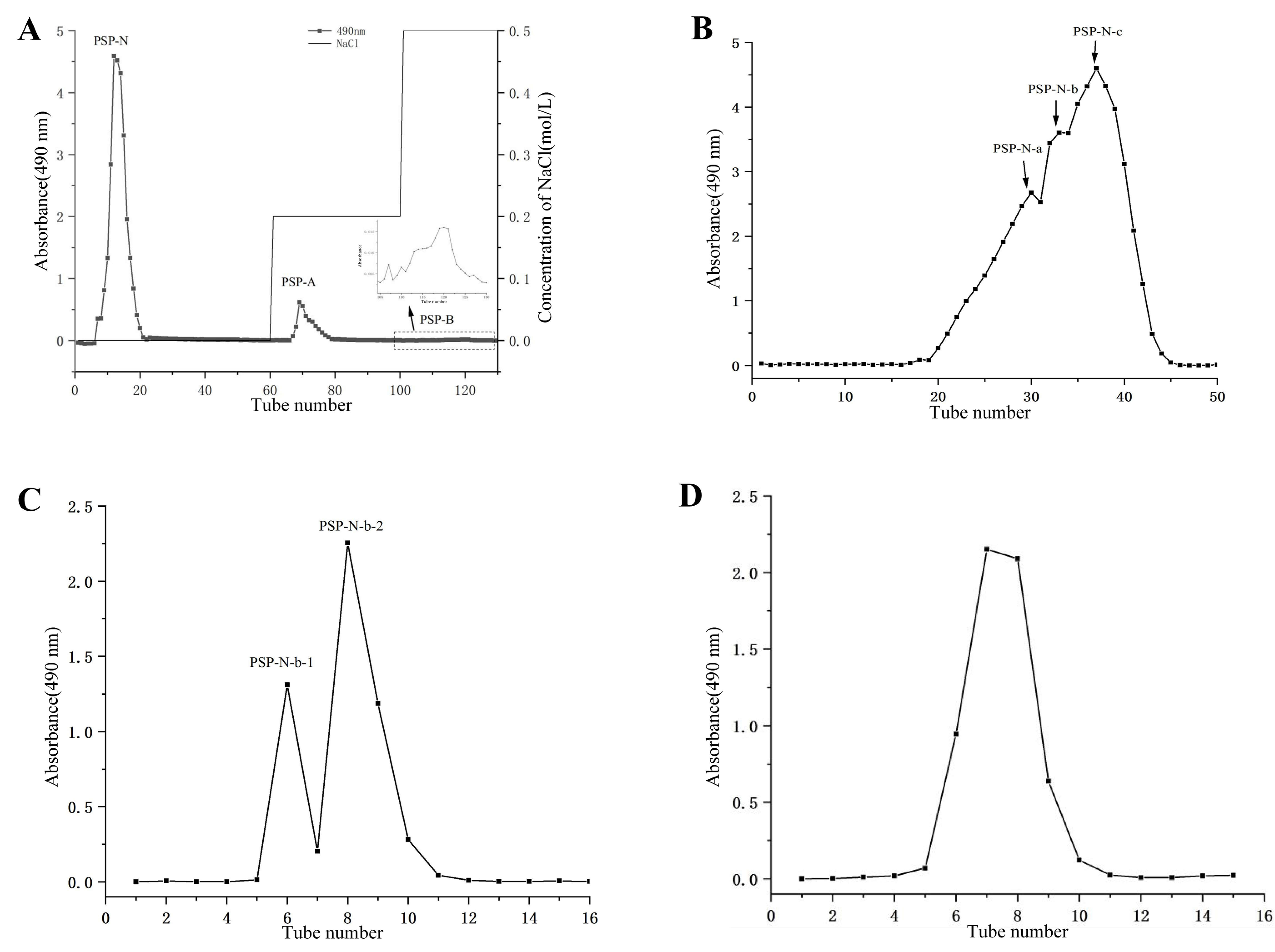
2.1. Isolation and Purification of Polysaccharides
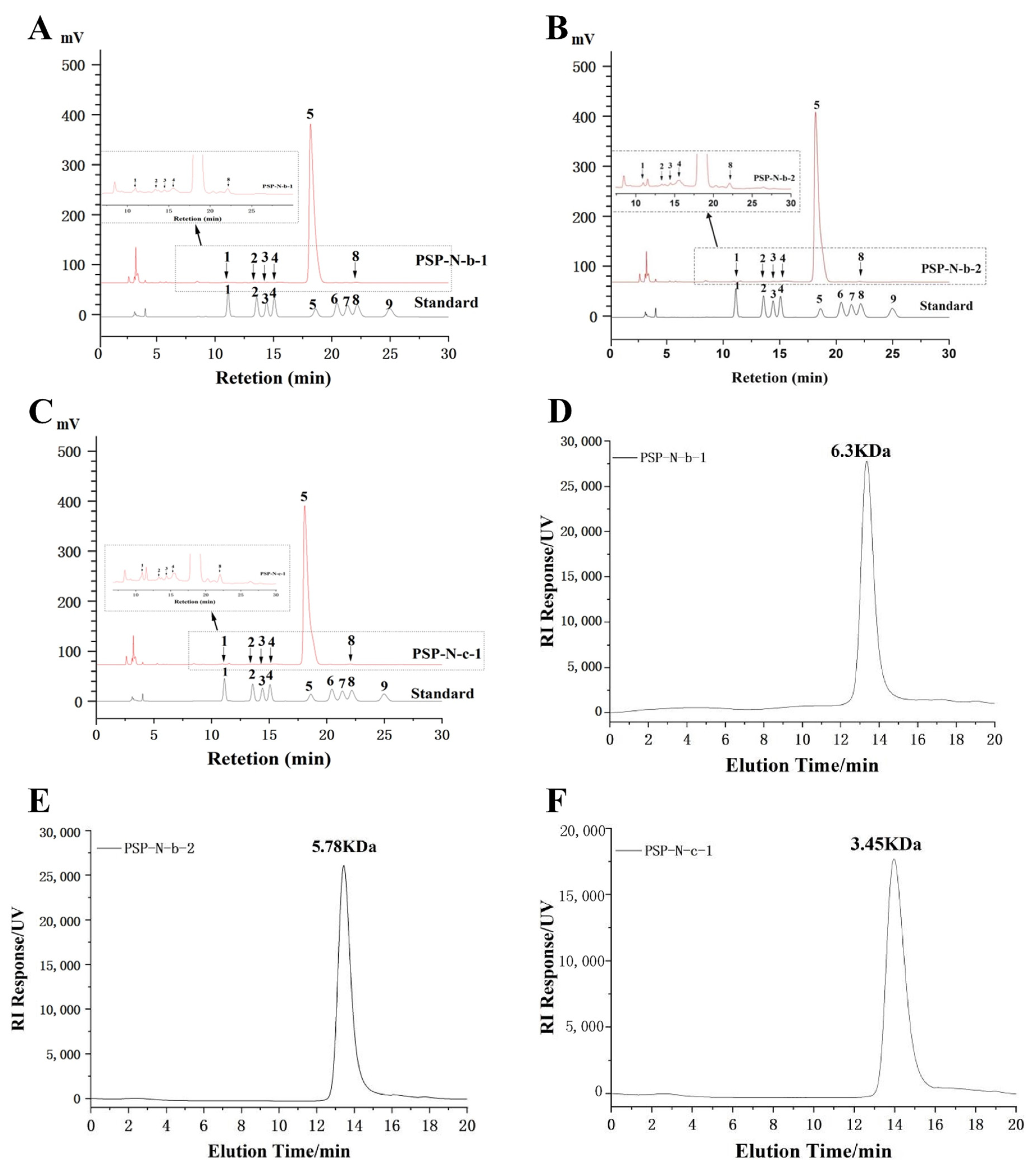
2.2. Monosaccharide Composition and Molecular Weight
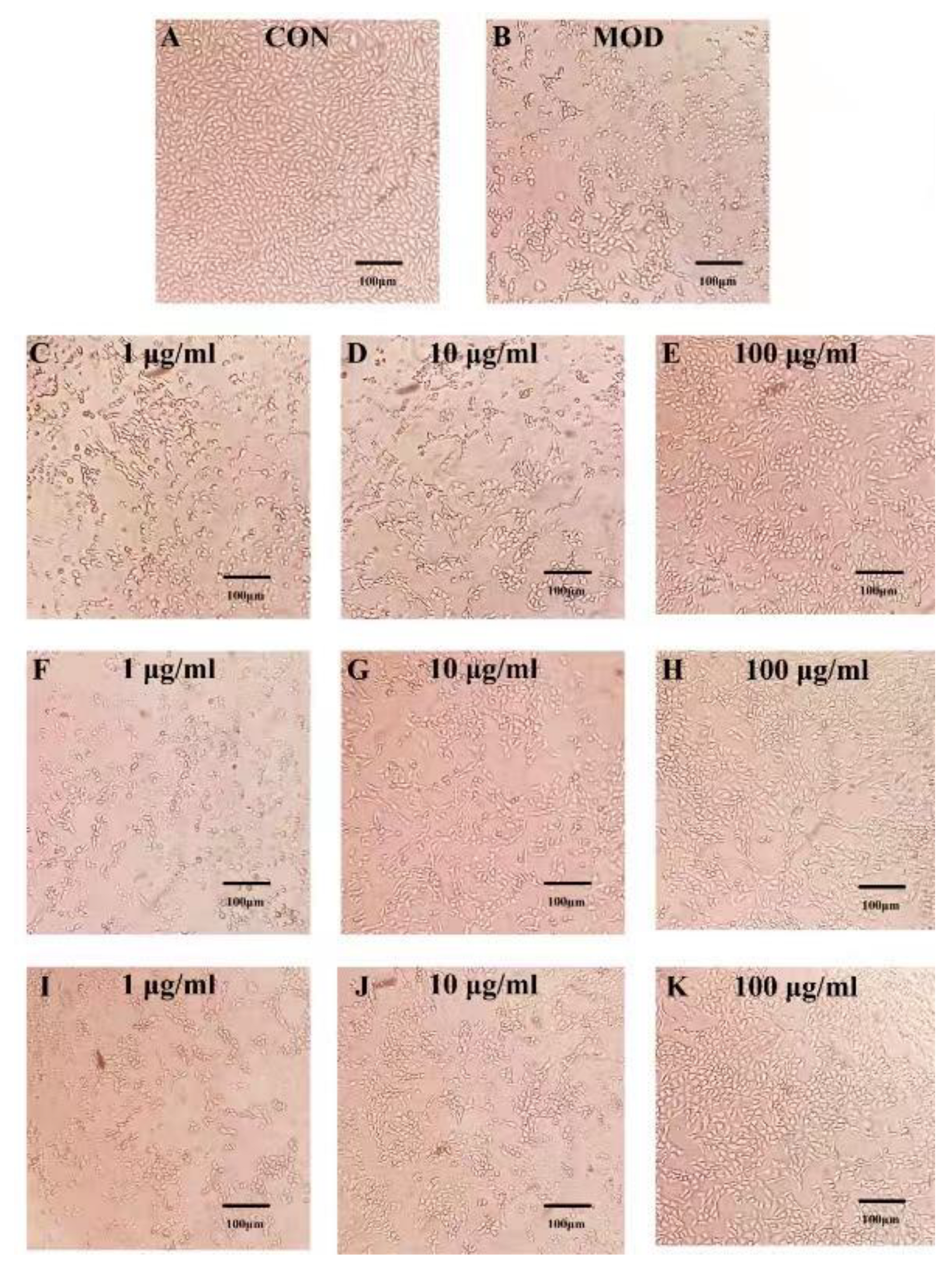
2.3. Effects of PSPs and Their Fractions on CcL4-Induced Morphological Changes in HepG2 Cells
2.4. Hepatoprotective Effects of PSPs and Their Fractions against CcL4-Induced HepG2 Cell Toxicity
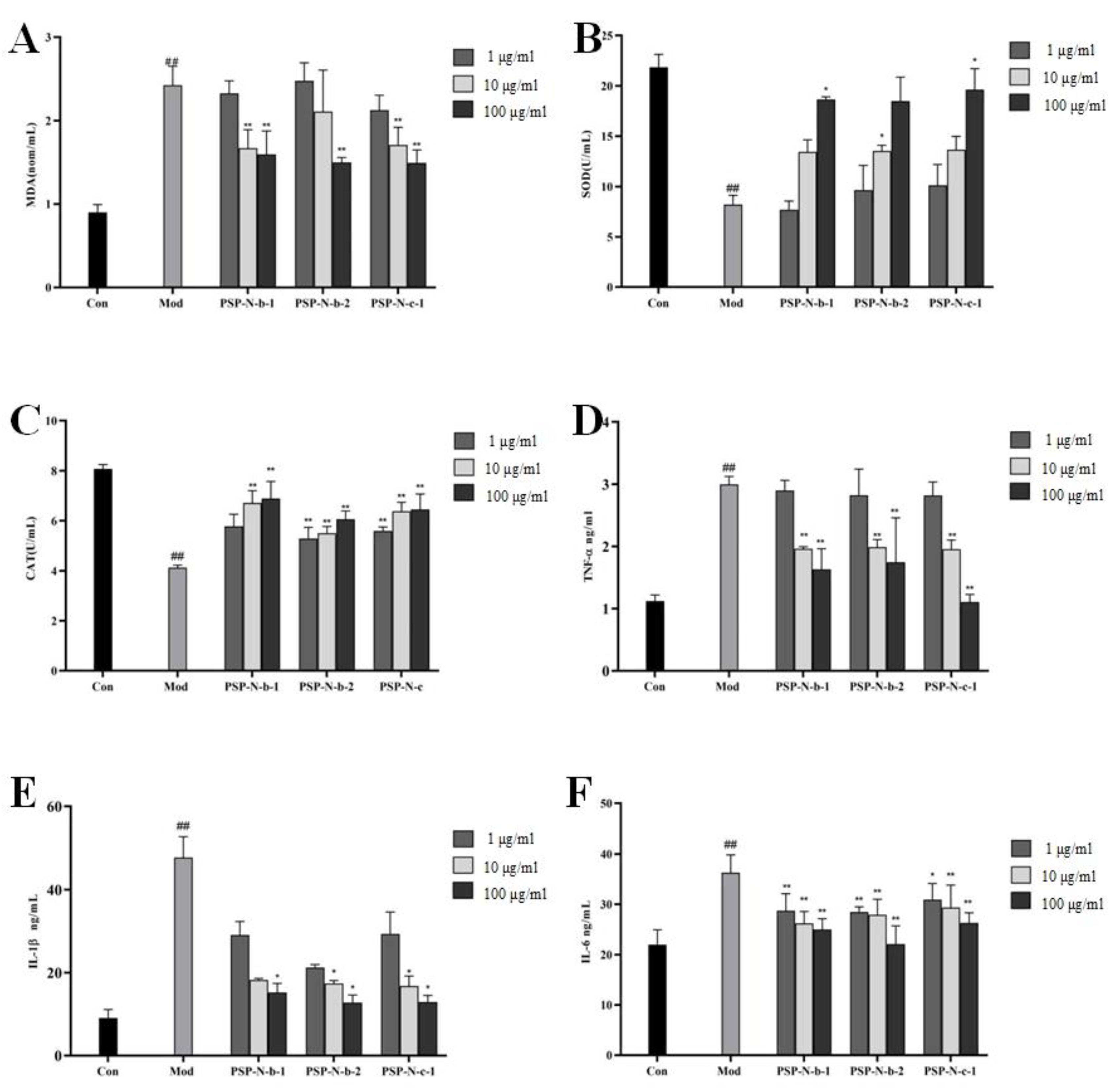
2.5. Effects of Polygonatum Polysaccharides on Oxidative Stress and Inflammatory Factors Induced by CCL4 in HepG2 Cells
3. Discussion
4. Materials and Methods
4.1. Materials and Equipment
4.2. Isolation and Purification of Polysaccharides from Polygonatum
4.3. Molecular Weight Determination
4.4. Monosaccharide Composition Analysis
4.5. Cell Culture
4.6. The Detection of Relevant Indicators
4.7. Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, G.; Yuan, G.; Lu, X.; An, L.; Sheng, Y.; Du, P. Study on the effect of regulation of Cordyceps militaris polypeptide on the immune function of mice based on a transcription factor regulatory network. Food Funct. 2020, 11, 6066–6077. [Google Scholar] [CrossRef] [PubMed]
- Keles, U.; Ow, J.; Kuentzel, K.; Zhao, L.; Kaldis, P. Liver-derived metabolites as signaling molecules in fatty liver disease. Cell. Mol. Life Sci. CMLS 2022, 80, 4. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Lee, C.; Kim, S. Non-alcoholic fatty liver disease and liver secretome. Arch. Pharmacal Res. 2022, 45, 938–963. [Google Scholar] [CrossRef] [PubMed]
- Stravitz, R.; Lee, W. Acute liver failure. Lancet 2019, 394, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Friedman, S.; Shulman, G. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.; Chalasani, N.; Björnsson, E.; Suzuki, A.; Kullak-Ublick, G.; Watkins, P.; Devarbhavi, H.; Merz, M.; Lucena, M.; Kaplowitz, N.; et al. Drug-induced liver injury. Nat. Rev. Dis. Primers 2019, 5, 58. [Google Scholar] [CrossRef]
- Hoofnagle, J.; Björnsson, E. Drug-Induced Liver Injury—Types and Phenotypes. N. Engl. J. Med. 2019, 381, 264–273. [Google Scholar] [CrossRef]
- Asrani, S.; Devarbhavi, H.; Eaton, J.; Kamath, P. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Ali, M.; Bahadur, S.; Hussain, A.; Saeed, S.; Khuram, I.; Ullah, M.; Shao, J.; Akhtar, N. Foliar epidermal micromorphology and its taxonomic significance in Polygonatum (Asparagaceae) using scanning electron microscopy. Microsc. Res. Tech. 2020, 83, 1381–1390. [Google Scholar] [CrossRef]
- Jiang, Q.; Lv, Y.; Dai, W.; Miao, X.; Zhong, D. Extraction and bioactivity of polygonatum polysaccharides. Int. J. Biol. Macromol. 2013, 54, 131–135. [Google Scholar] [CrossRef]
- Jin, J.; Lao, J.; Zhou, R.; He, W.; Qin, Y.; Zhong, C.; Xie, J.; Liu, H.; Wan, D.; Zhang, S.; et al. Polygonatum cyrtonemaSimultaneous Identification and Dynamic Analysis of Saccharides during Steam Processing of Rhizomes of by HPLC⁻QTOF⁻MS/MS. Molecules 2018, 23, 2855. [Google Scholar] [CrossRef]
- Gong, H.; Gan, X.; Li, Y.; Chen, J.; Xu, Y.; Shi, S.; Li, T.; Li, B.; Wang, H.; Wang, S. Review on the genus Polygonatum polysaccharides: Extraction, purification, structural characteristics and bioactivities. Int. J. Biol. Macromol. 2023, 229, 909–930. [Google Scholar] [CrossRef]
- Xiao, L.; Qi, L.; Zhang, G.; Liu, H.; Gu, Y.; Zhang, L.; Zhang, M.; Wu, H. Polygonatum sibiricum Polysaccharides Attenuate Lipopoly-Saccharide-Induced Septic Liver Injury by Suppression of Pyroptosis via NLRP3/GSDMD Signals. Molecules 2022, 27, 5999. [Google Scholar] [CrossRef]
- Long, T.; Liu, Z.; Shang, J.; Zhou, X.; Yu, S.; Tian, H.; Bao, Y. Polygonatum sibiricum polysaccharides play anti-cancer effect through TLR4-MAPK/NF-κB signaling pathways. Int. J. Biol. Macromol. 2018, 111, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, G.; Zhang, X.; Wang, Y.; Qiang, Y.; Wang, B.; Zou, J.; Niu, J.; Wang, Z. Structural characterization and antioxidant activity of Polygonatum sibiricum polysaccharides. Carbohydr. Polym. 2022, 291, 119524. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, T.; Chen, H.; Yu, Q.; Yan, C. Structural characterization and osteogenic activity in vitro of novel polysaccharides from the rhizome of Polygonatum sibiricum. Food Funct. 2021, 12, 6626–6636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, N.; Sun, C.; Sun, D.; Wang, Y. Polygonatum sibiricumPolysaccharides from Delar. ex Redoute induce an immune response in the RAW264.7 cell line an NF-κB/MAPK pathway. RSC Adv. 2019, 9, 17988–17994. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Luo, J.; Jia, M.; Chai, Y.; Bao, Y. Polygonatum sibiricum saponin Exerts Beneficial Hypoglycemic Effects in Type 2 Diabetes Mice by Improving Hepatic Insulin Resistance and Glycogen Synthesis-Related Proteins. Nutrients 2022, 14, 5222. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, Y.; Yan, M.; He, Z.; Zhou, Y.; Xu, J.; Li, B.; Xu, W.; Yu, J.; Chen, S.; et al. The beneficial effects of Polygonatum sibiricum Red. superfine powder on metabolic hypertensive rats via gut-derived LPS/TLR4 pathway inhibition. Phytomed. Int. J. Phytother. Phytopharm. 2022, 106, 154404. [Google Scholar] [CrossRef]
- Bian, Z.; Li, C.; Peng, D.; Wang, X.; Zhu, G. Polygonatum sibiricumUse of Steaming Process to Improve Biochemical Activity of Polysaccharides against D-Galactose-Induced Memory Impairment in Mice. Int. J. Mol. Sci. 2022, 23, 11220. [Google Scholar] [CrossRef]
- Cui, X.; Wang, S.; Cao, H.; Guo, H.; Li, Y.; Xu, F.; Zheng, M.; Xi, X.; Han, C. A Review: The Bioactivities and Pharmacological Applications of Polygonatum sibiricum polysaccharides. Molecules 2018, 23, 1170. [Google Scholar] [CrossRef]
- Jiang, L.; Gong, X.; Ji, M.; Wang, C.; Wang, J.; Li, M. Bioactive Compounds from Plant-Based Functional Foods: A Promising Choice for the Prevention and Management of Hyperuricemia. Foods 2020, 9, 973. [Google Scholar] [CrossRef]
- Li, S.; Huo, X.; Qi, Y.; Ren, D.; Li, Z.; Qu, D.; Sun, Y. The Protective Effects of Ginseng Polysaccharides and Their Effective Subfraction against Dextran Sodium Sulfate-Induced Colitis. Foods 2022, 11, 890. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, R.; Li, S.; Fei, Y.; Lei, J.; Li, R.; Pan, Y.; Liu, S.; Wang, L. Auricularia auricula-judaeDietary Supplementation of Polysaccharides Alleviate Nutritional Obesity in Mice via Regulating Inflammatory Response and Lipid Metabolism. Foods 2022, 11, 942. [Google Scholar] [CrossRef]
- Pan, L.; Zhu, Y.; Zhu, Z.; Xue, W.; Liu, C.; Sun, H.; Yin, Y. Chemical structure and effects of antioxidation and against α-glucosidase of natural polysaccharide from Glycyrrhiza inflata Batalin. Int. J. Biol. Macromol. 2020, 155, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, L.; You, Y.; Sun, X.; Wen, C.; Fu, Y.; Song, S. Preparation of Low-Molecular-Weight Fucoidan with Anticoagulant Activity by Photocatalytic Degradation Method. Foods 2022, 11, 822. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.; Dhewa, T.; Moreno-Rojas, J. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, T.; Wang, H.; Cui, Z.; Cheng, F.; Wang, K. Structural characterization and in vitro antitumor activity of an acidic polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohydr. Polym. 2016, 147, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wu, Y.; Jiang, G.; Feng, L.; Wang, Z.; Yuan, G.; Tong, H. Sulfated polysaccharides from Rhodiola sachalinensis reduce d-gal-induced oxidative stress in NIH 3T3 cells. Int. J. Biol. Macromol. 2019, 140, 288–293. [Google Scholar] [CrossRef]
- Chen, S.; Huang, H.; Huang, G. Extraction, derivatization and antioxidant activity of cucumber polysaccharide. Int. J. Biol. Macromol. 2019, 140, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Lin, J.; Zheng, J.; Wei, L.; Liu, L.; Peng, Y.; Liang, W.; Hu, G. Dahuang Zhechong pill attenuates CCl4-induced rat liver fibrosis via the PI3K-Akt signaling pathway. J. Cell. Biochem. 2020, 121, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Wang, Y.; Niu, H.; Ma, Y.; Song, J. Isolation, purification, and physicochemical characterization of Polygonatum polysaccharide and its protective effect against CCl-induced liver injury via Nrf2 and NF-κB signaling pathways. Int. J. Biol. Macromol. 2024, 261, 129863. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Dutta, S.; Biswas-Raha, A.; Sarkar, M.; Chaudhuri, T. Haloalkane induced hepatic insult in murine model: Amelioration by Oleander through antioxidant and anti-inflammatory activities, an in vitro and in vivo study. BMC Complement. Altern. Med. 2016, 16, 280. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Yan, Q.; Cai, L.; Li, G.; Luo, X. Lycium barbarumBox-Behnken design-based optimization for deproteinization of crude polysaccharides in berry residue using the Sevag method. Heliyon 2020, 6, e03888. [Google Scholar] [CrossRef]
- Li, H.; Xu, G.; Yuan, G. Armillaria melleaEffects of an Polysaccharide on Learning and Memory of D-Galactose-Induced Aging Mice. Front. Pharmacol. 2022, 13, 919920. [Google Scholar] [CrossRef]
- Gao, L.; Du, B.; Ma, Q.; Ma, Y.; Yu, W.; Li, T.; Liu, Y.; Yuan, G. Multiplex-PCR method application to identify duck blood and its adulterated varieties. Food Chem. 2024, 444, 138673. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Zhang, H.; Liu, Y.; Wang, W.; You, S.; Hu, X.; Song, M.; Wu, R.; Wu, J. Polygonatum sibiricumAnti-cancer Potential of Polysaccharide Extracted From on HepG2 Cells via Cell Cycle Arrest and Apoptosis. Front. Nutr. 2022, 9, 938290. [Google Scholar] [CrossRef]

| Title 1 | Man | GlcA | Rha | GalA | Glc | Ara |
|---|---|---|---|---|---|---|
| PSP-N-b-1 | 0.4% | 0.3% | 0.3% | 0.5% | 98.1% | 0.4% |
| PSP-N-b-2 | 0.3% | 0.3% | 0.3% | 0.6% | 98.3% | 0.3% |
| PSP-N-c-1 | 0.5% | 0.2% | 0.3% | 0.5% | 98.1% | 0.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Niu, H.; Ma, Y.; Yuan, G. Isolation, Purification, Fractionation, and Hepatoprotective Activity of Polygonatum Polysaccharides. Molecules 2024, 29, 1038. https://doi.org/10.3390/molecules29051038
Wang Y, Niu H, Ma Y, Yuan G. Isolation, Purification, Fractionation, and Hepatoprotective Activity of Polygonatum Polysaccharides. Molecules. 2024; 29(5):1038. https://doi.org/10.3390/molecules29051038
Chicago/Turabian StyleWang, Yutong, Hongmei Niu, Yue Ma, and Guangxin Yuan. 2024. "Isolation, Purification, Fractionation, and Hepatoprotective Activity of Polygonatum Polysaccharides" Molecules 29, no. 5: 1038. https://doi.org/10.3390/molecules29051038
APA StyleWang, Y., Niu, H., Ma, Y., & Yuan, G. (2024). Isolation, Purification, Fractionation, and Hepatoprotective Activity of Polygonatum Polysaccharides. Molecules, 29(5), 1038. https://doi.org/10.3390/molecules29051038





