177Lu-Labeled Iron Oxide Nanoparticles Functionalized with Doxorubicin and Bevacizumab as Nanobrachytherapy Agents against Breast Cancer
Abstract
1. Introduction
2. Results
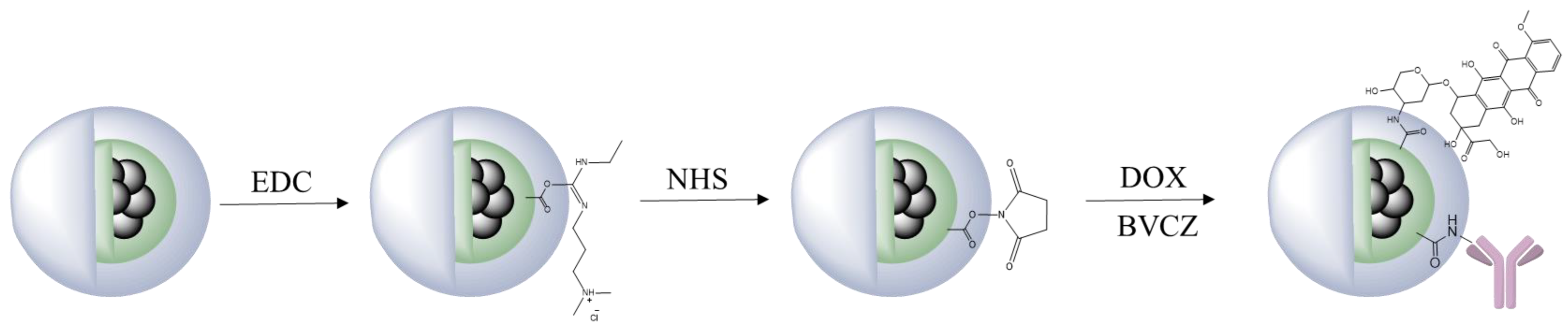
2.1. Functionalization with Doxorubicin and Bevacizumab
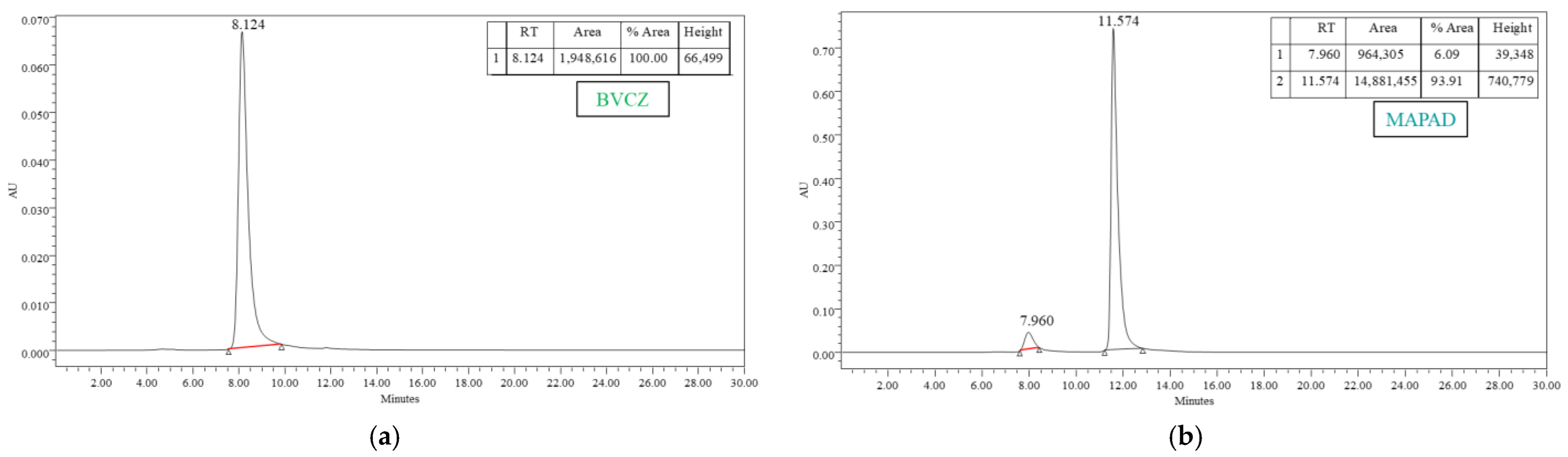
2.1.1. Antibody Conjugation
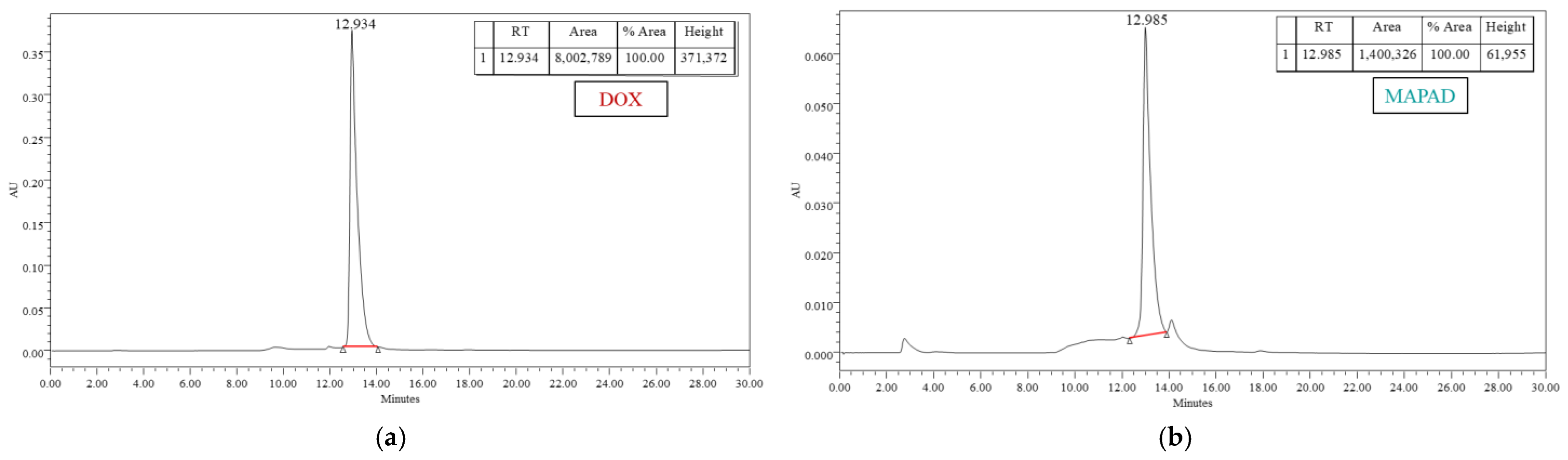
2.1.2. Doxorubicin Loading
2.1.3. Thermogravimetric Analysis
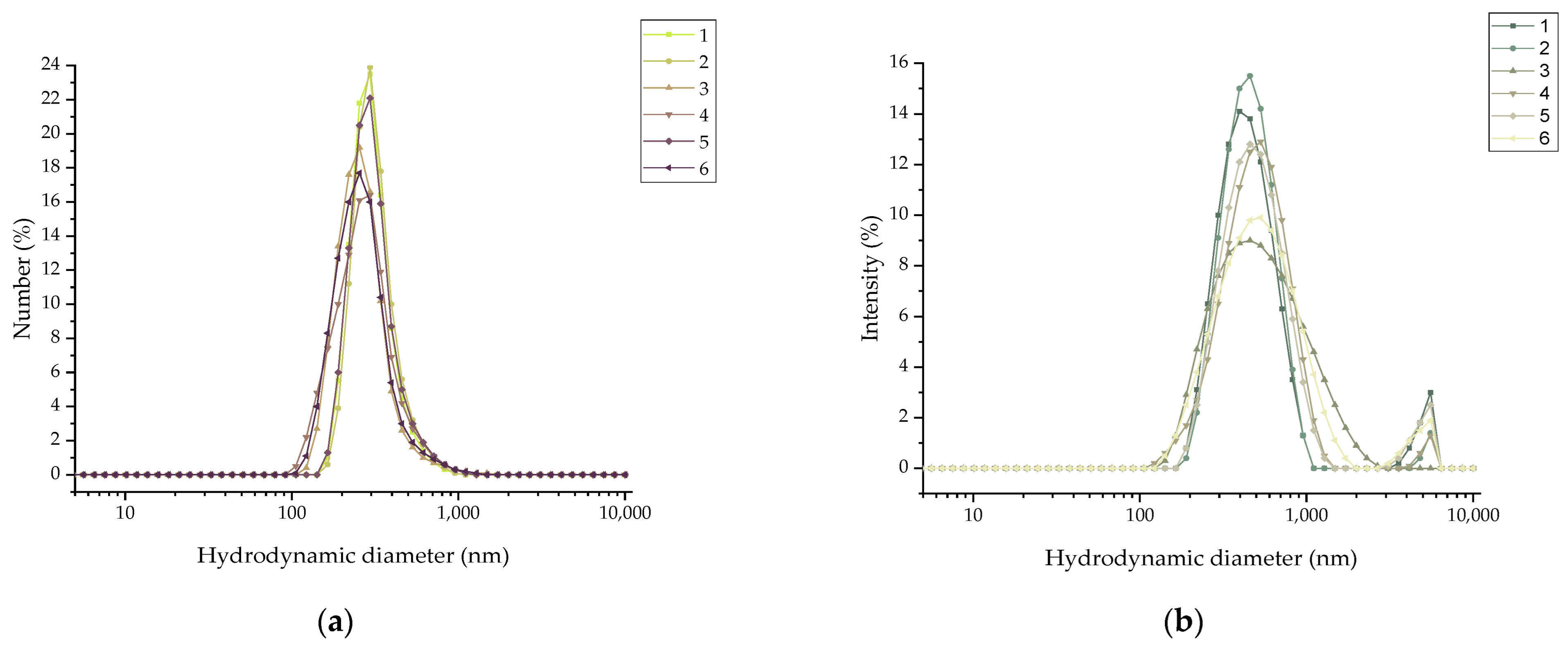
2.1.4. Dynamic Light Scattering
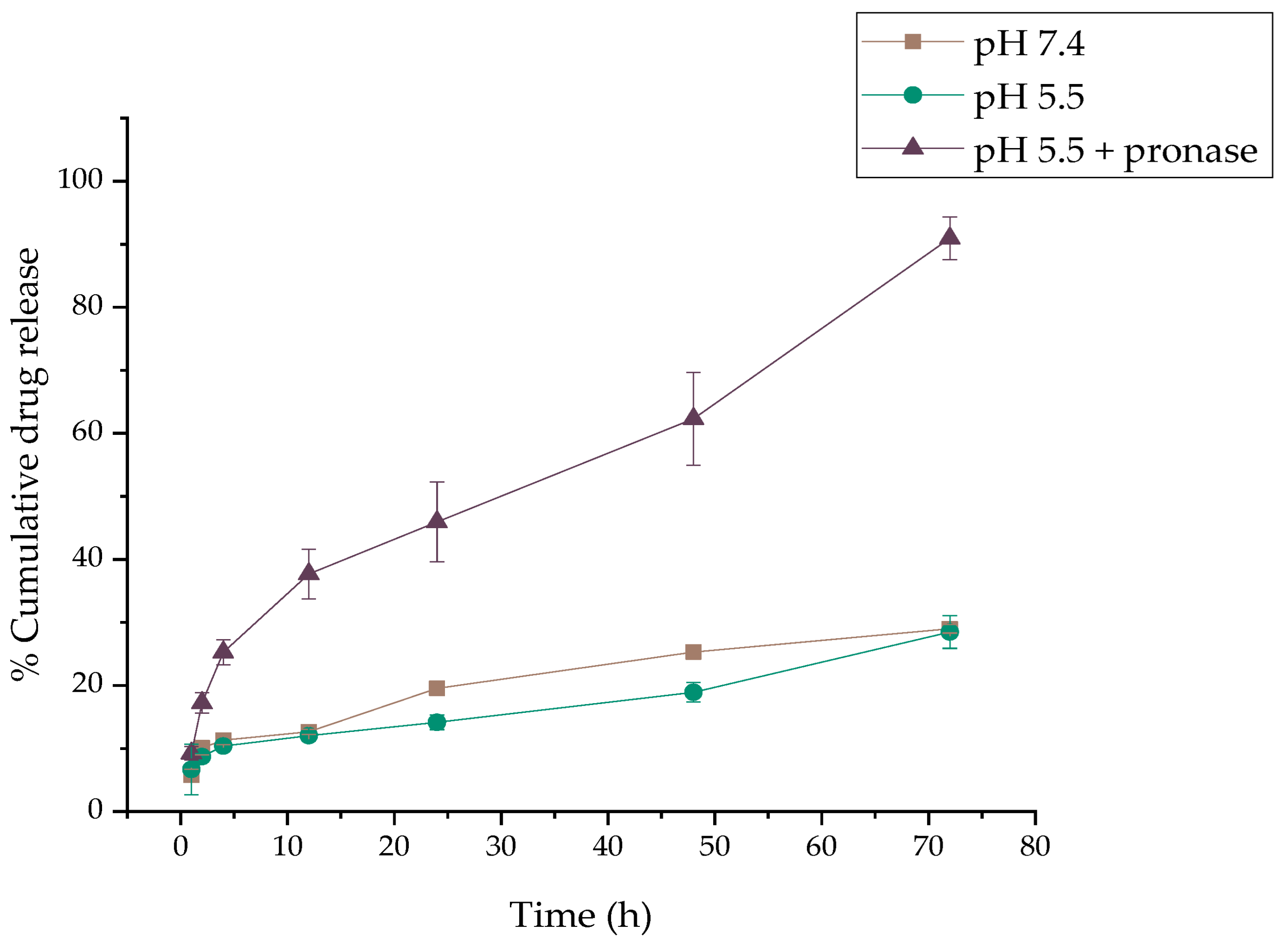
2.2. DOX Release
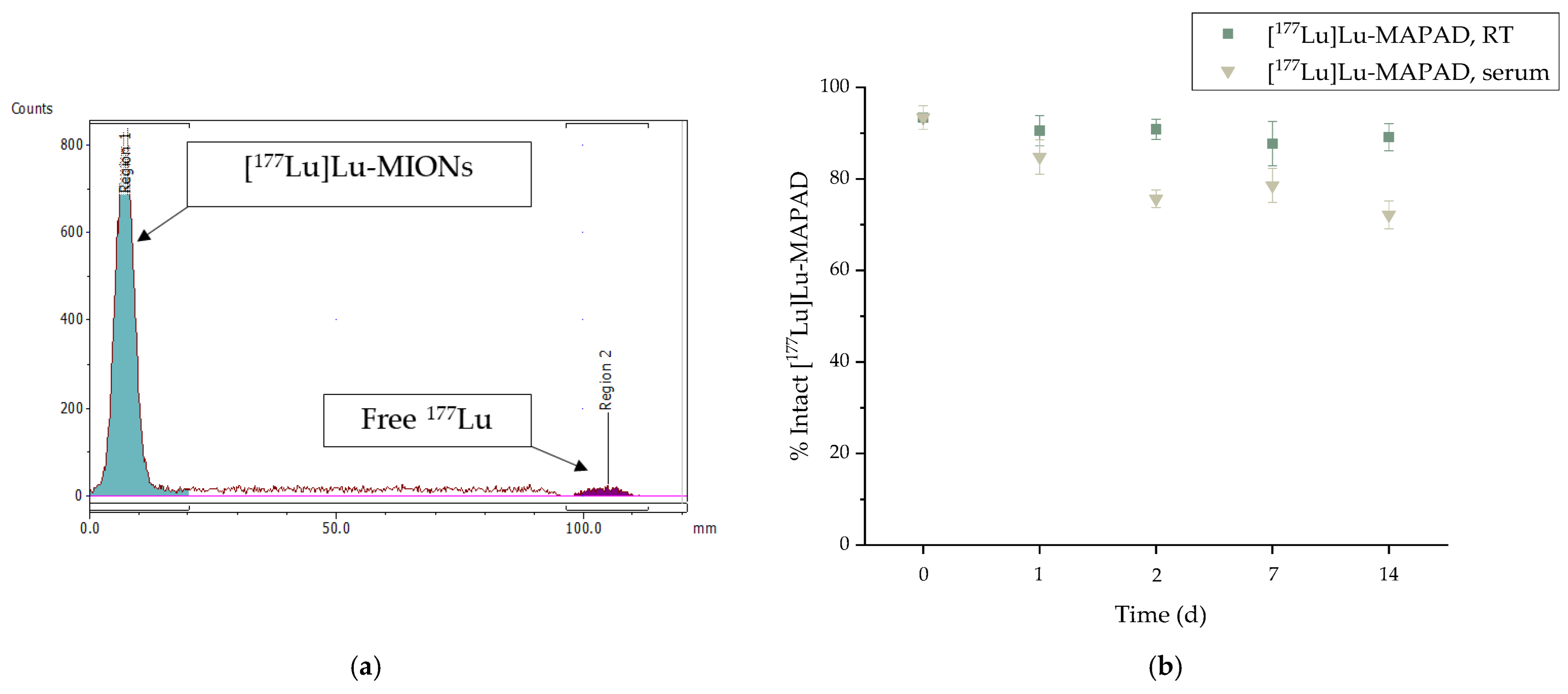
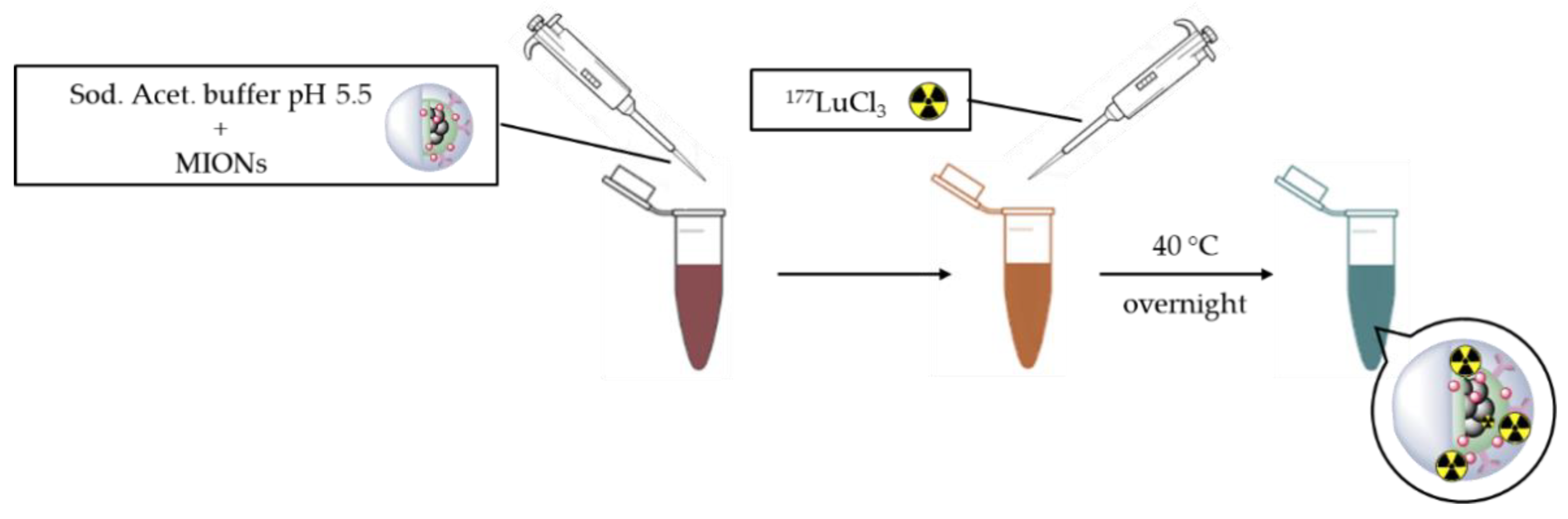
2.3. Radiolabeling with 177Lu
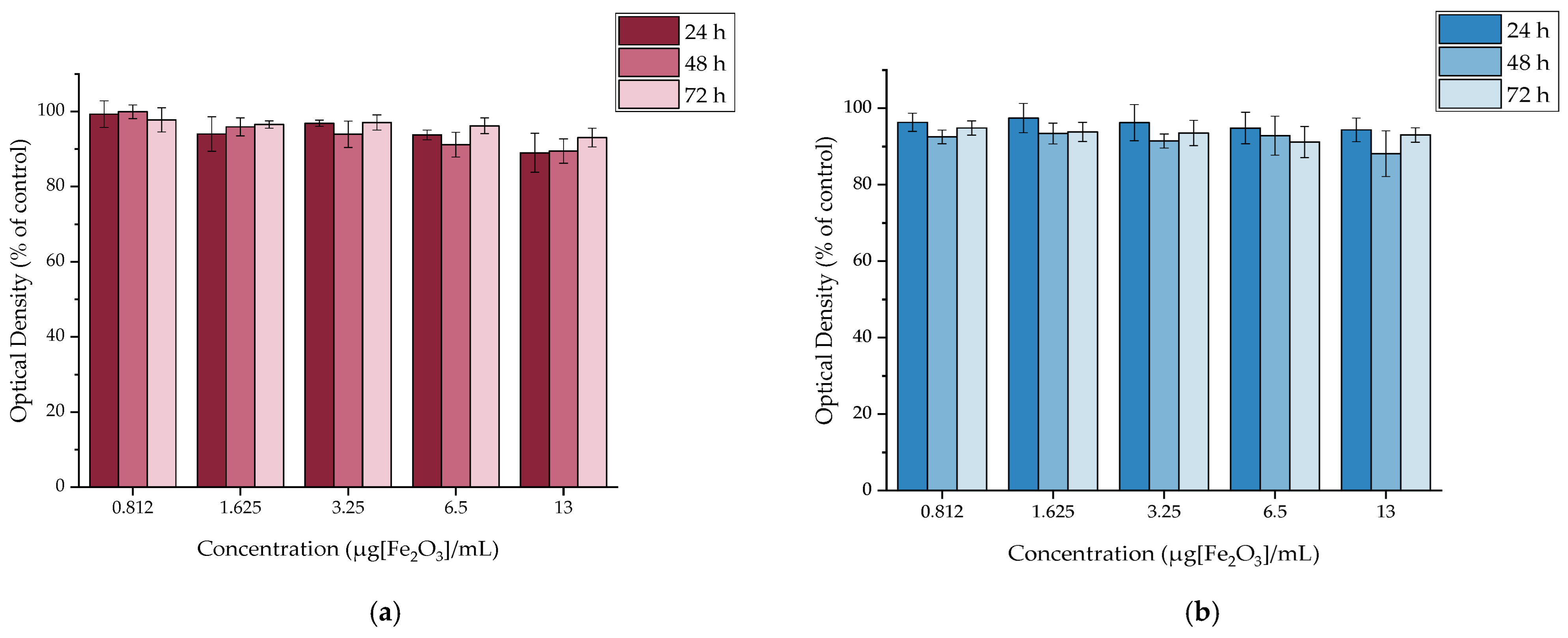
2.4. In Vitro Cytotoxicity
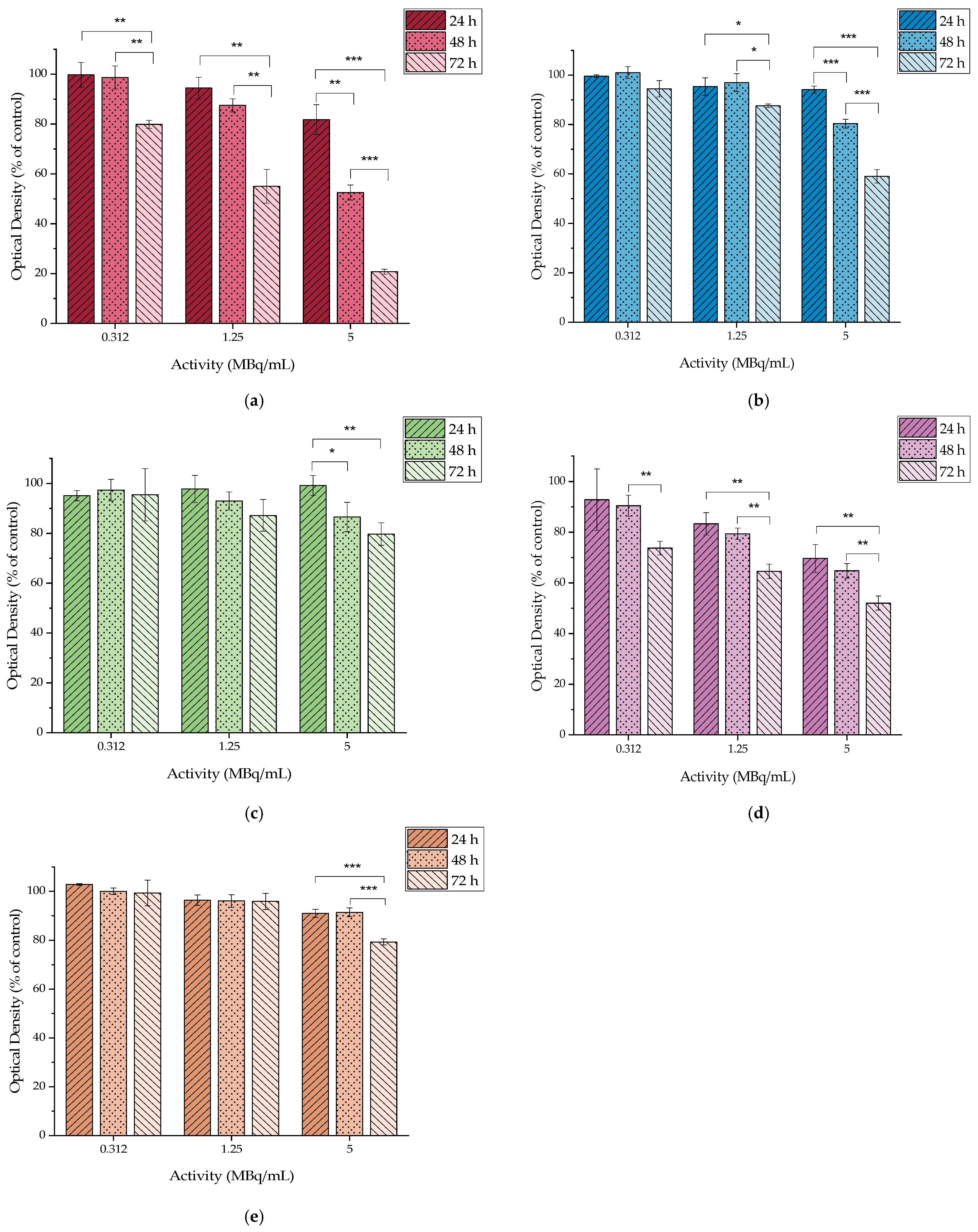
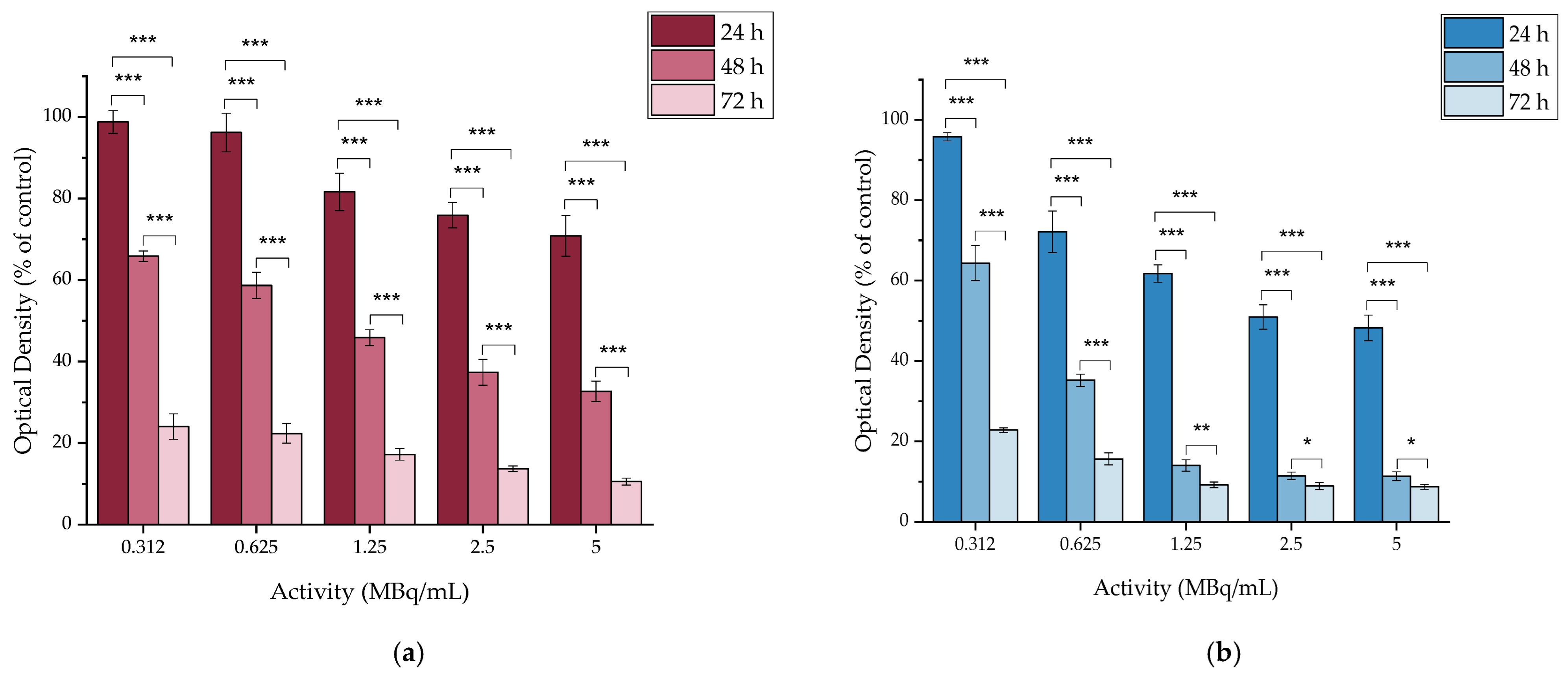
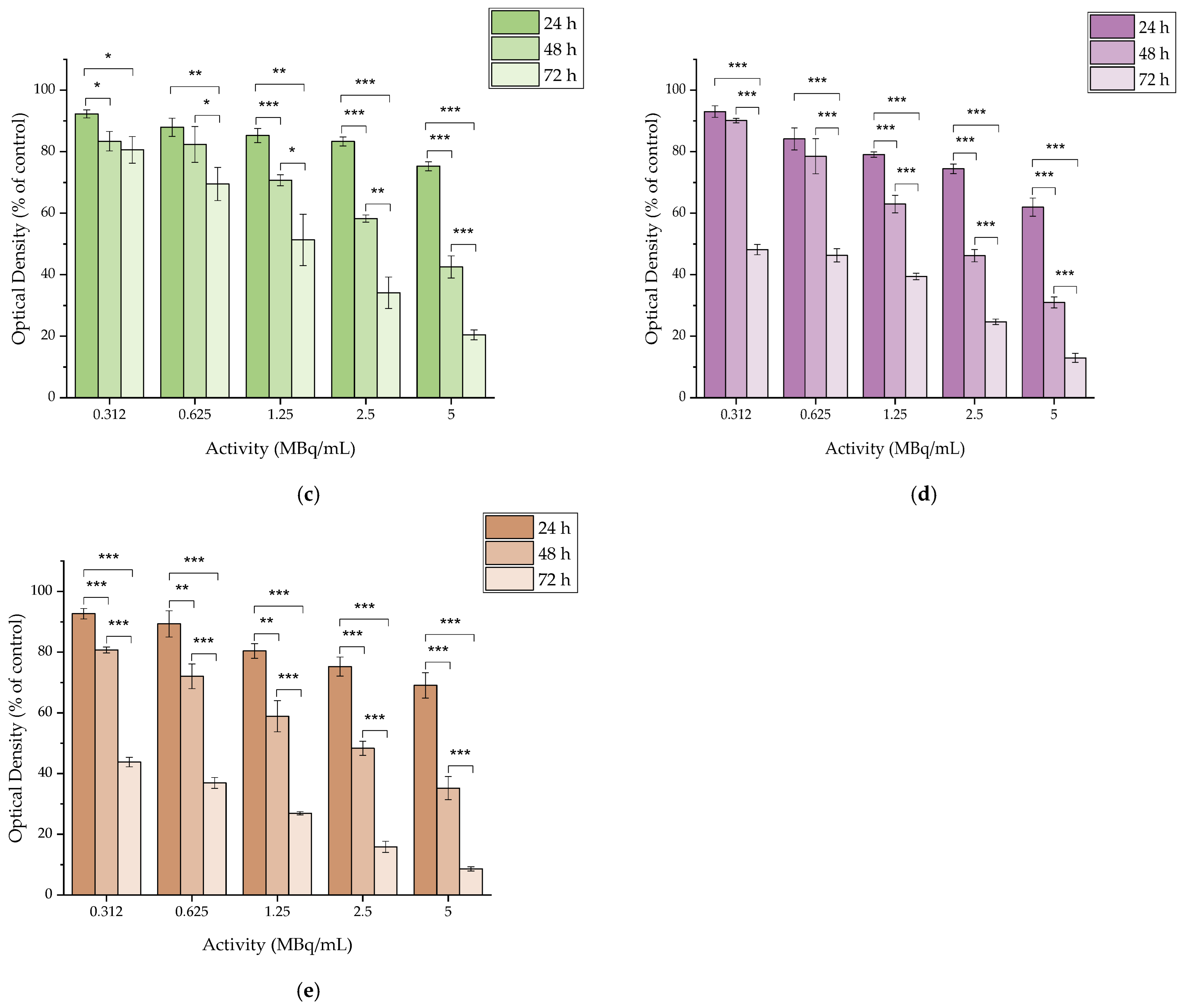
2.4.1. In Vitro Cytotoxicity of MAPEG
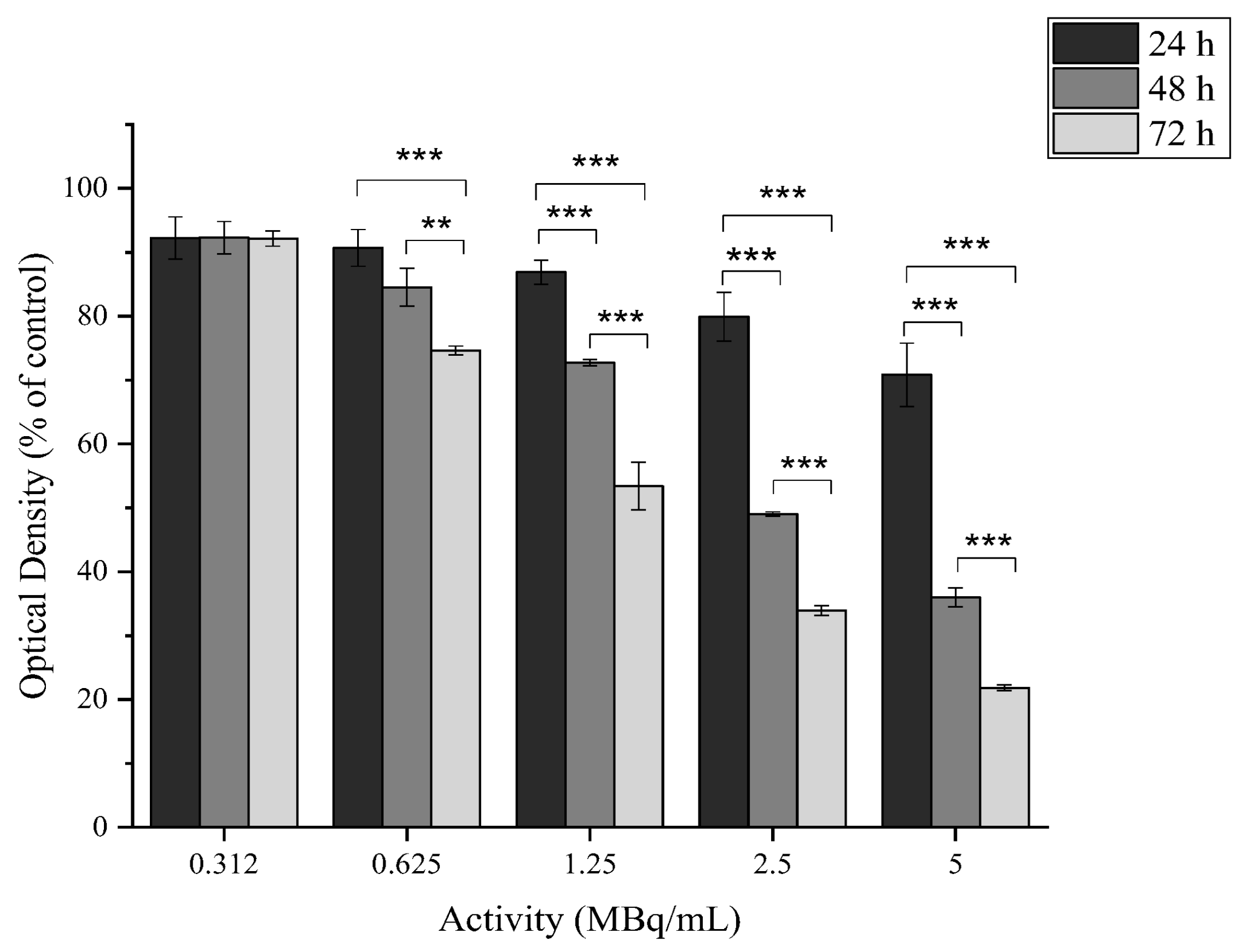
2.4.2. In Vitro Cytotoxicity of [177Lu]Lu-MAPEG
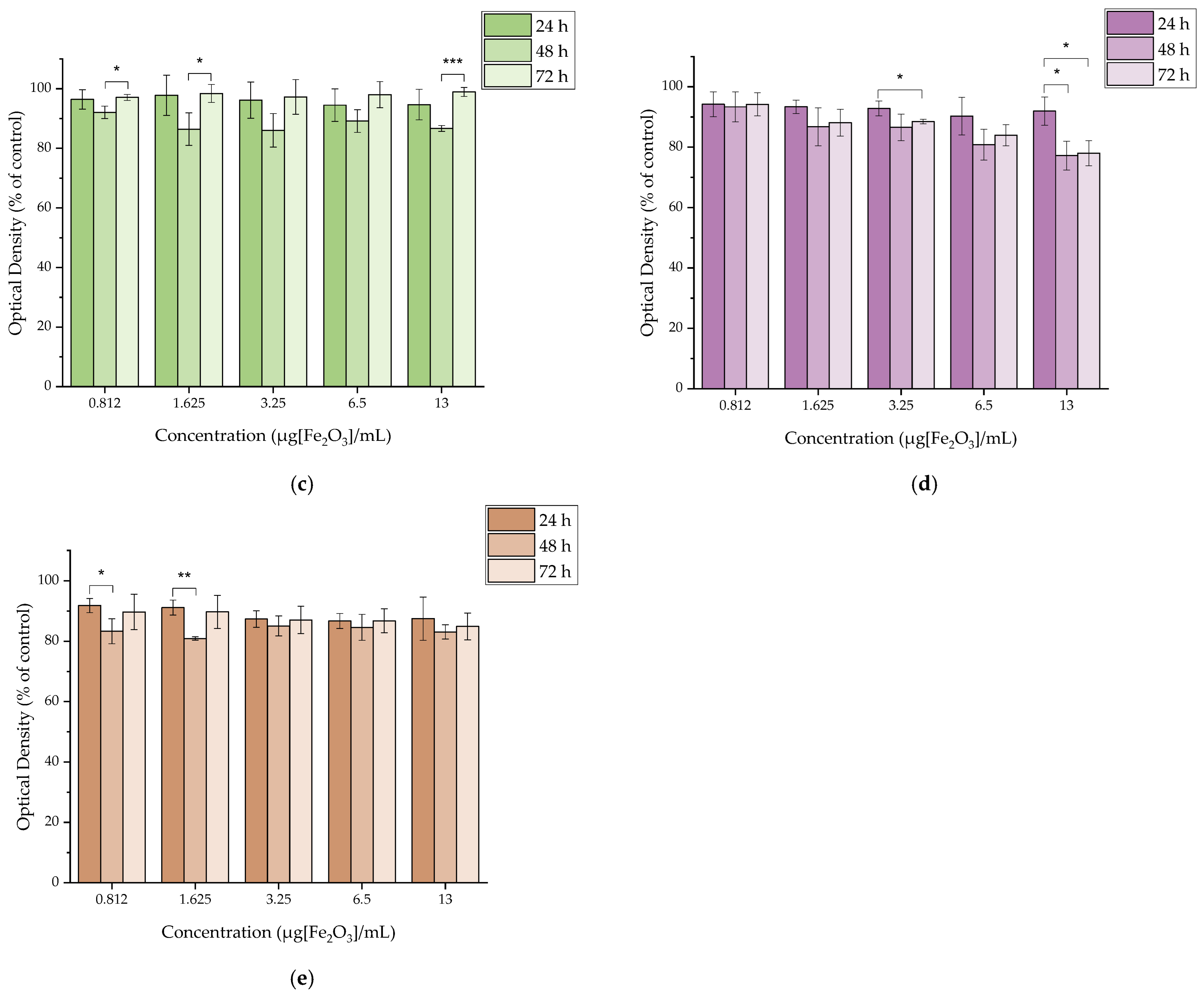
2.4.3. In Vitro Cytotoxicity of MAPAD
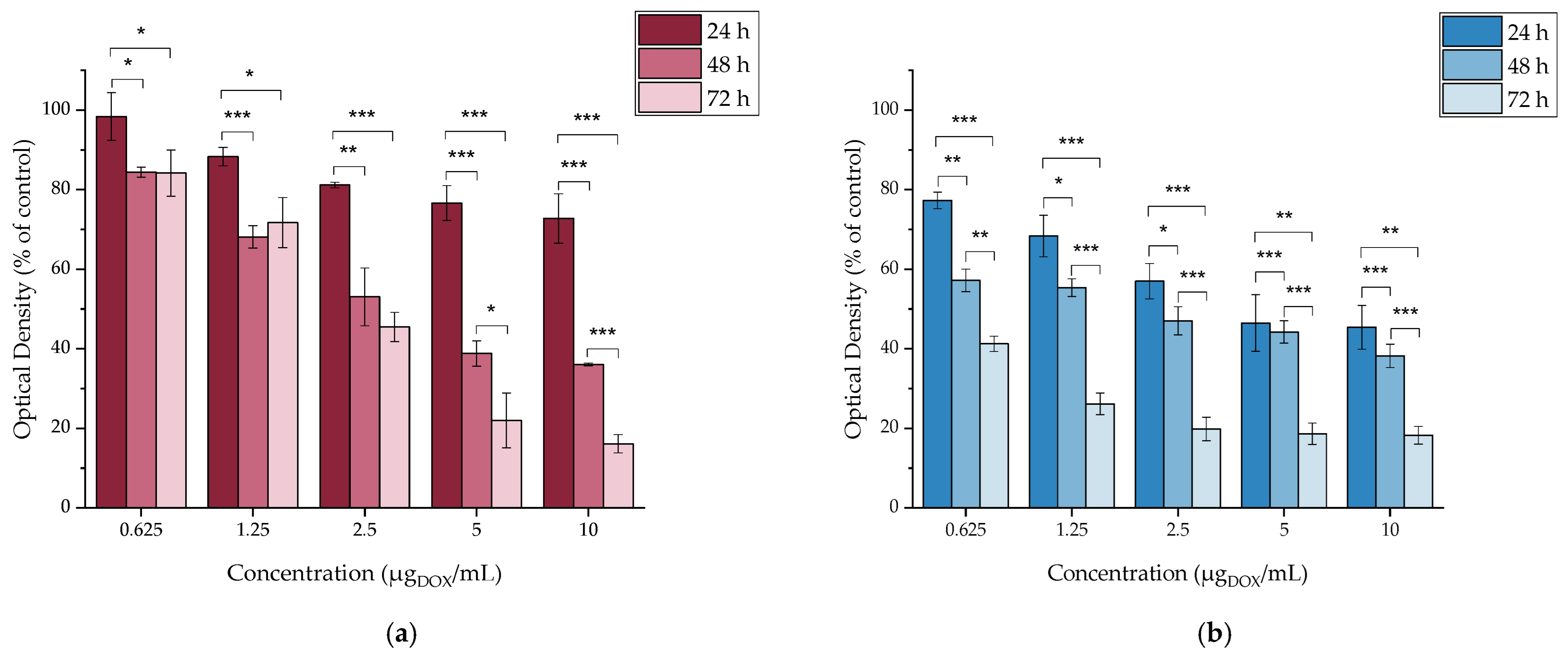
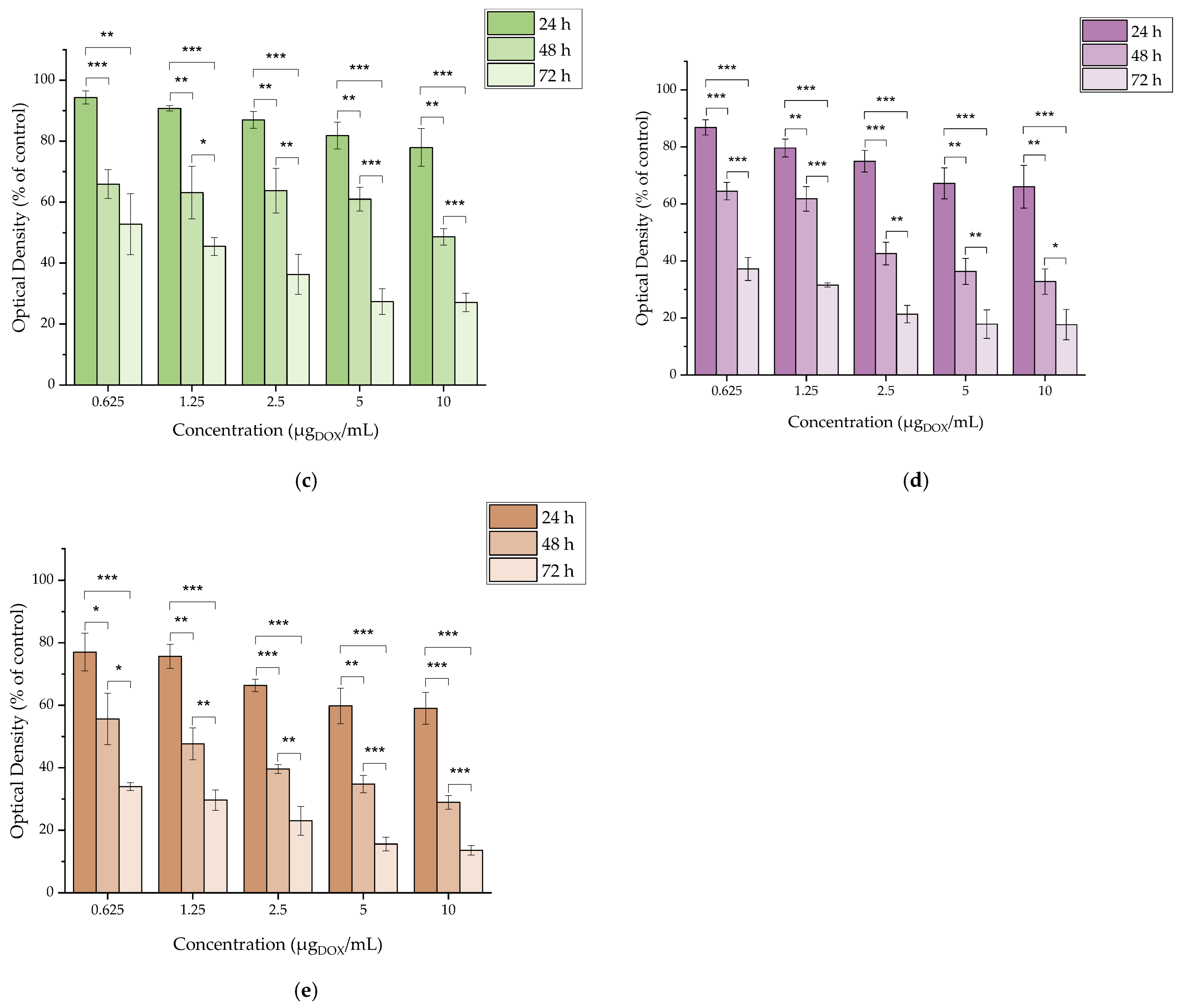
2.4.4. In Vitro Cytotoxicity of DOX
2.4.5. In Vitro Cytotoxicity of [177Lu]LuCl3
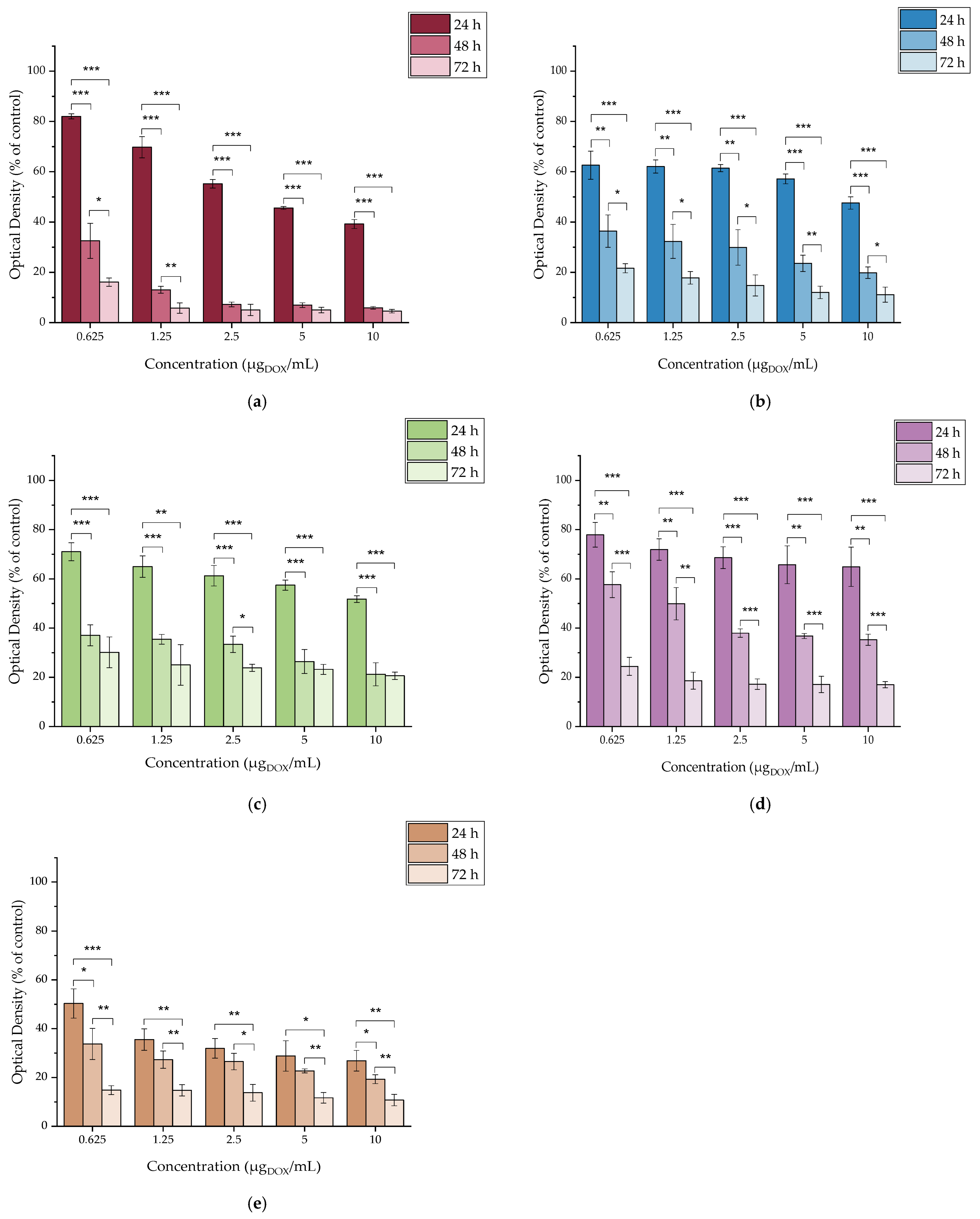
2.4.6. In Vitro Cytotoxicity of [177Lu]Lu-MAPAD
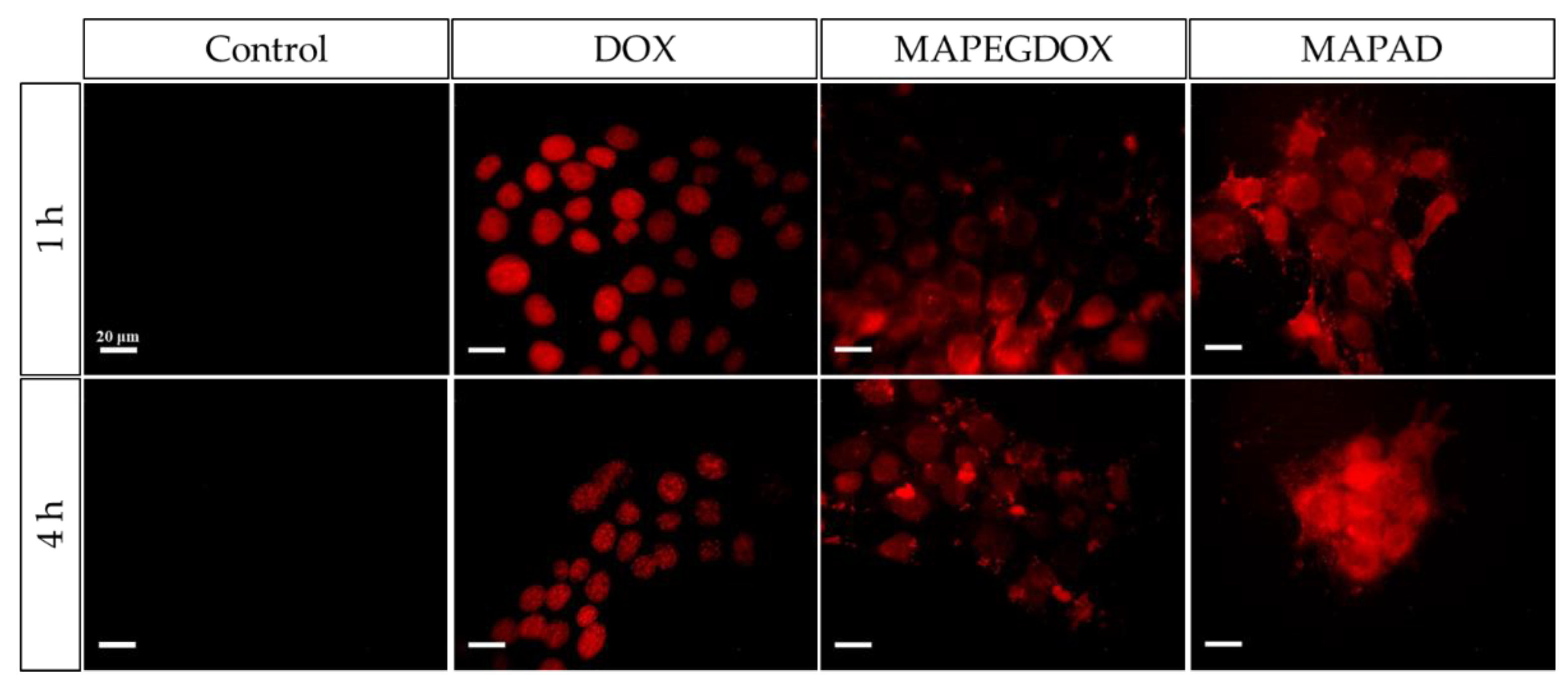
2.5. Fluorescence Microscopy
2.6. Prussian Blue
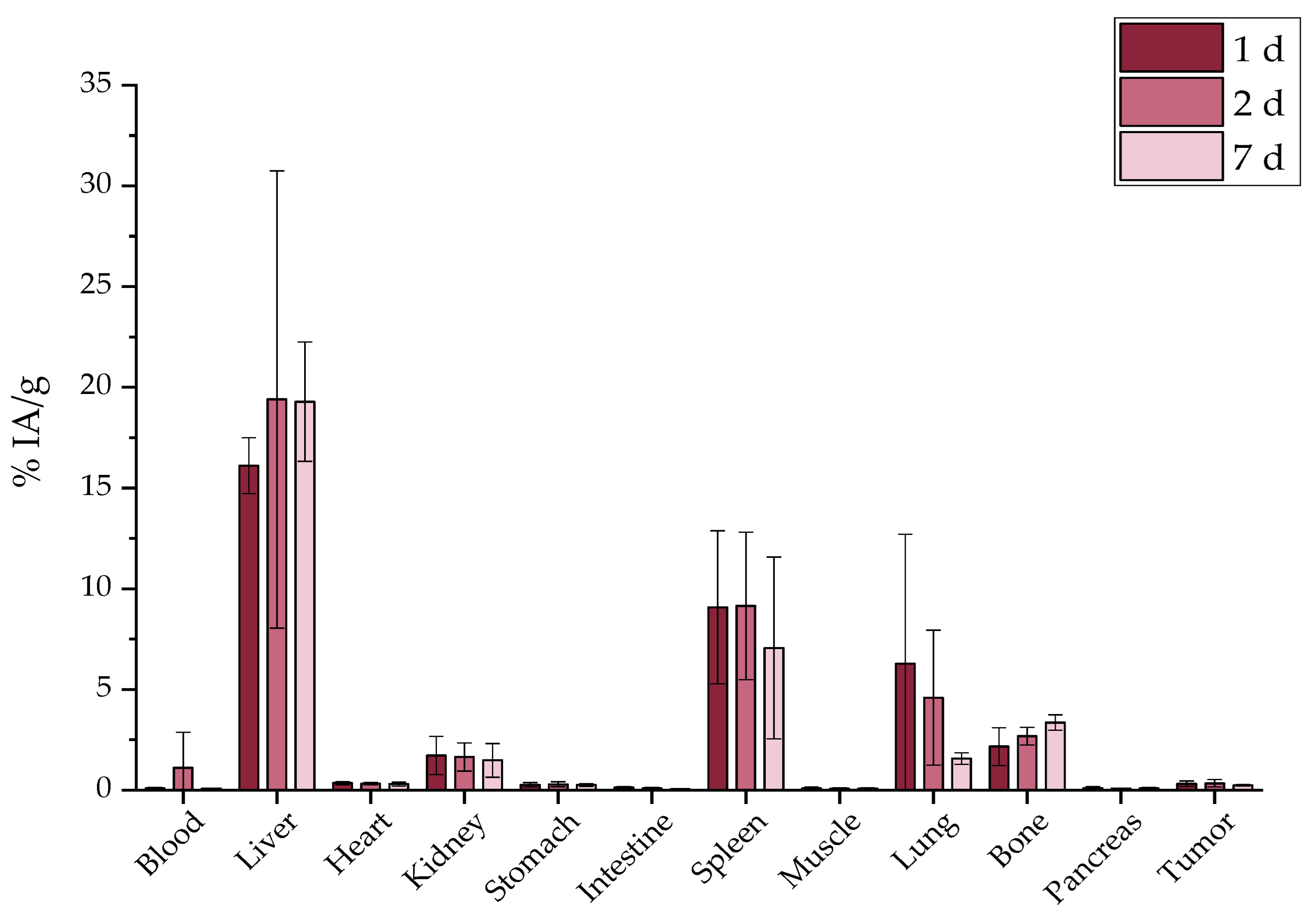
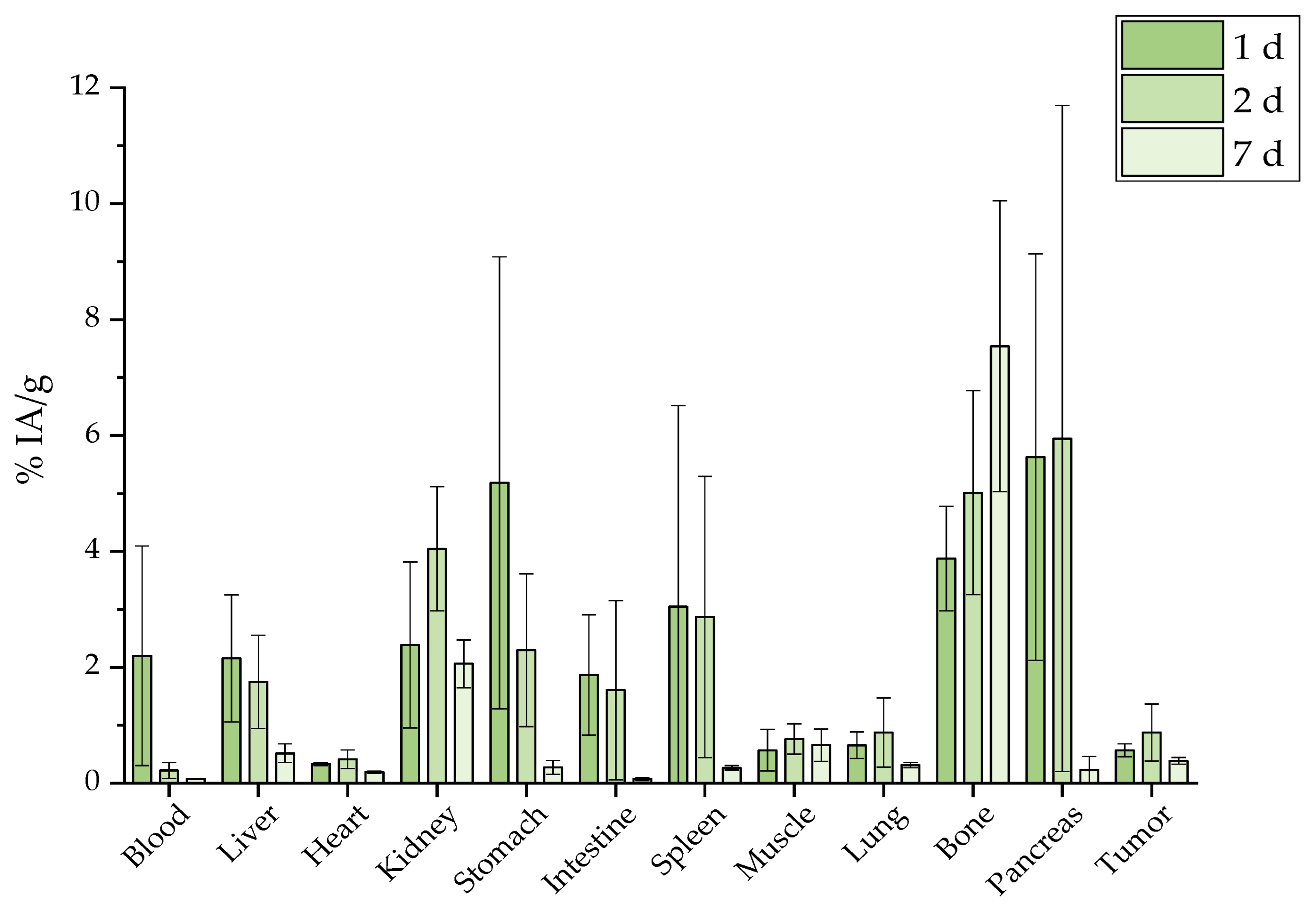
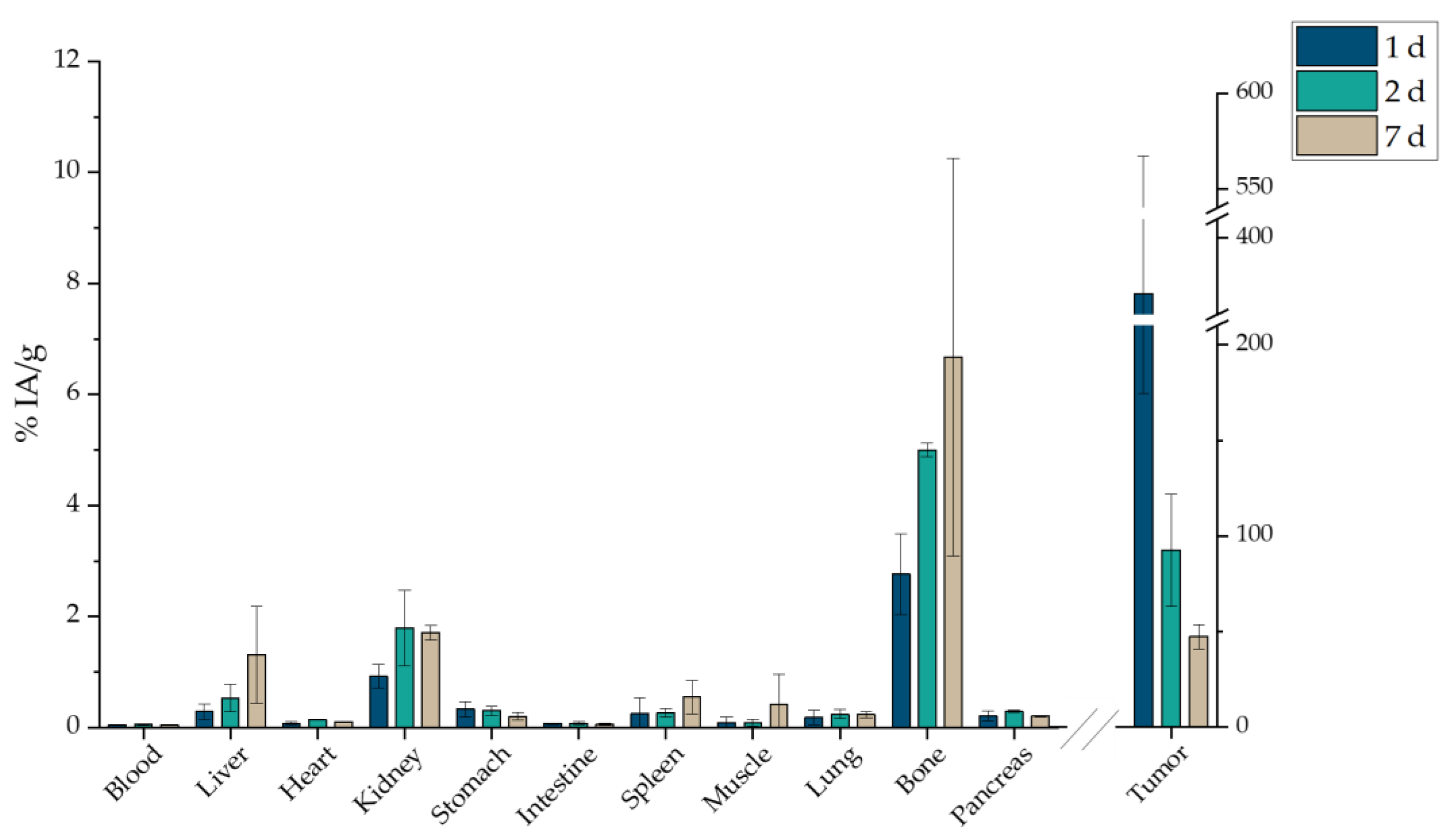
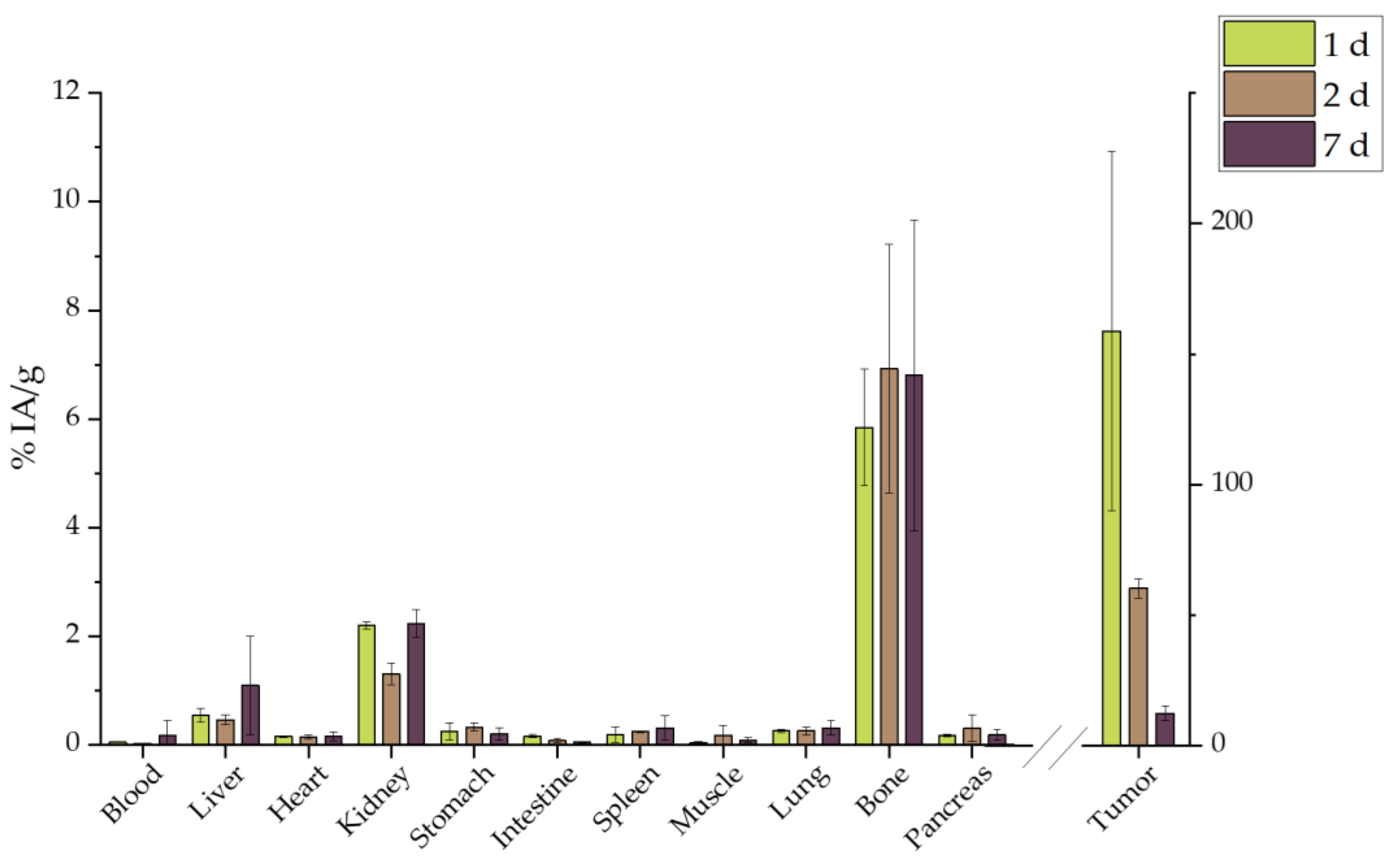
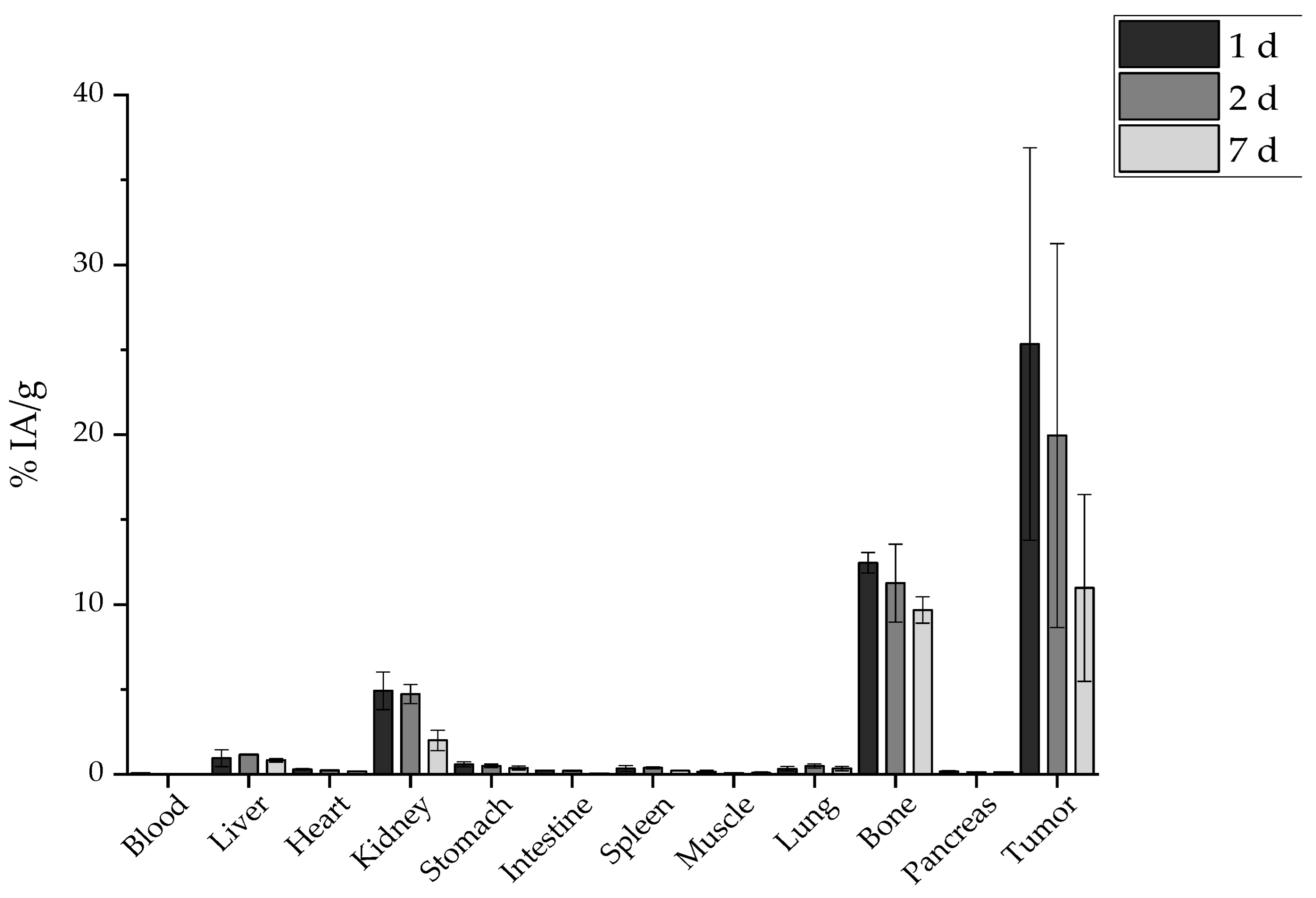
2.7. Ex Vivo Biodistribution
2.7.1. Intravenous Administration
2.7.2. Intraperitoneal Administration
2.7.3. Intratumoral Administration
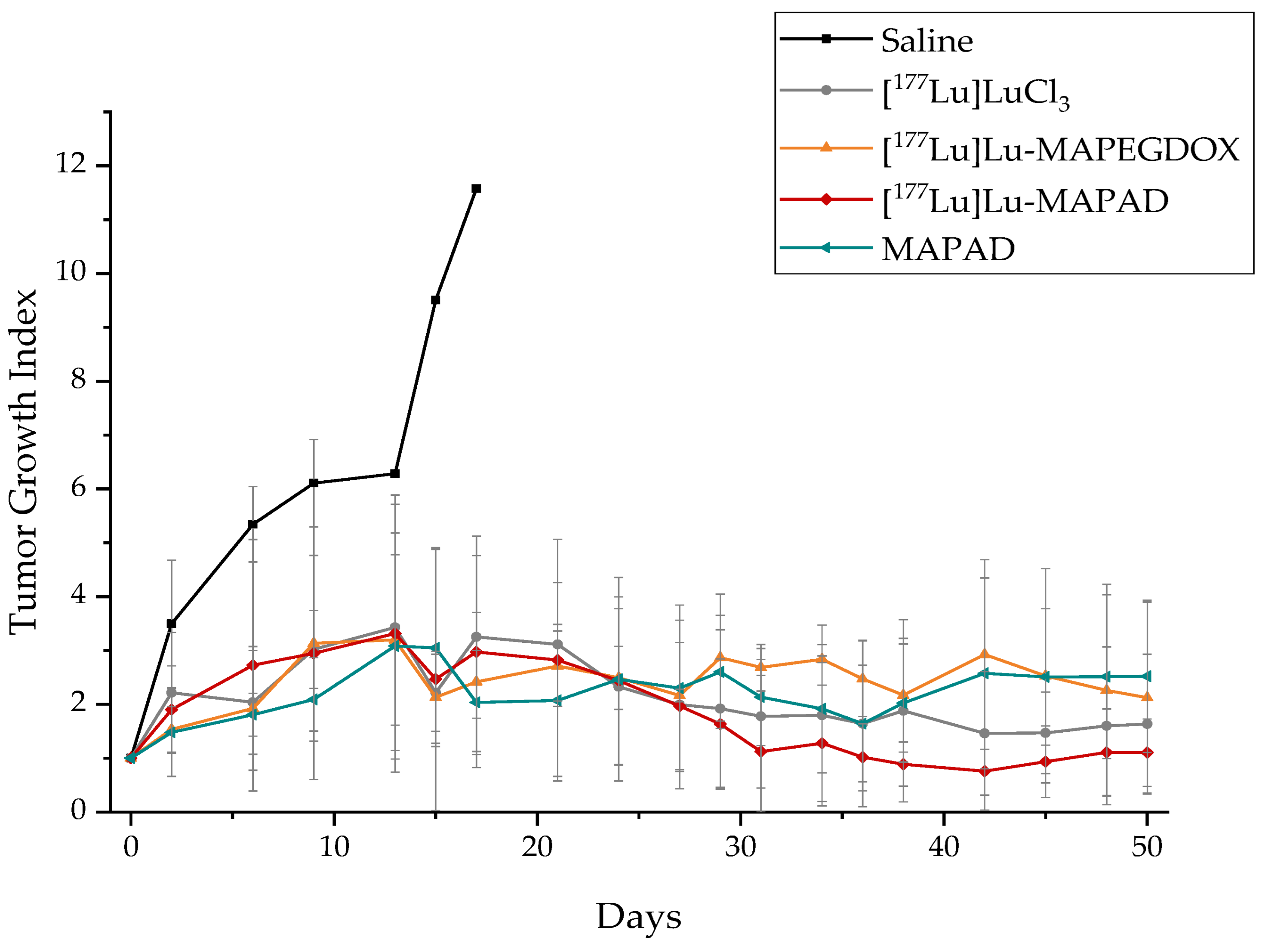
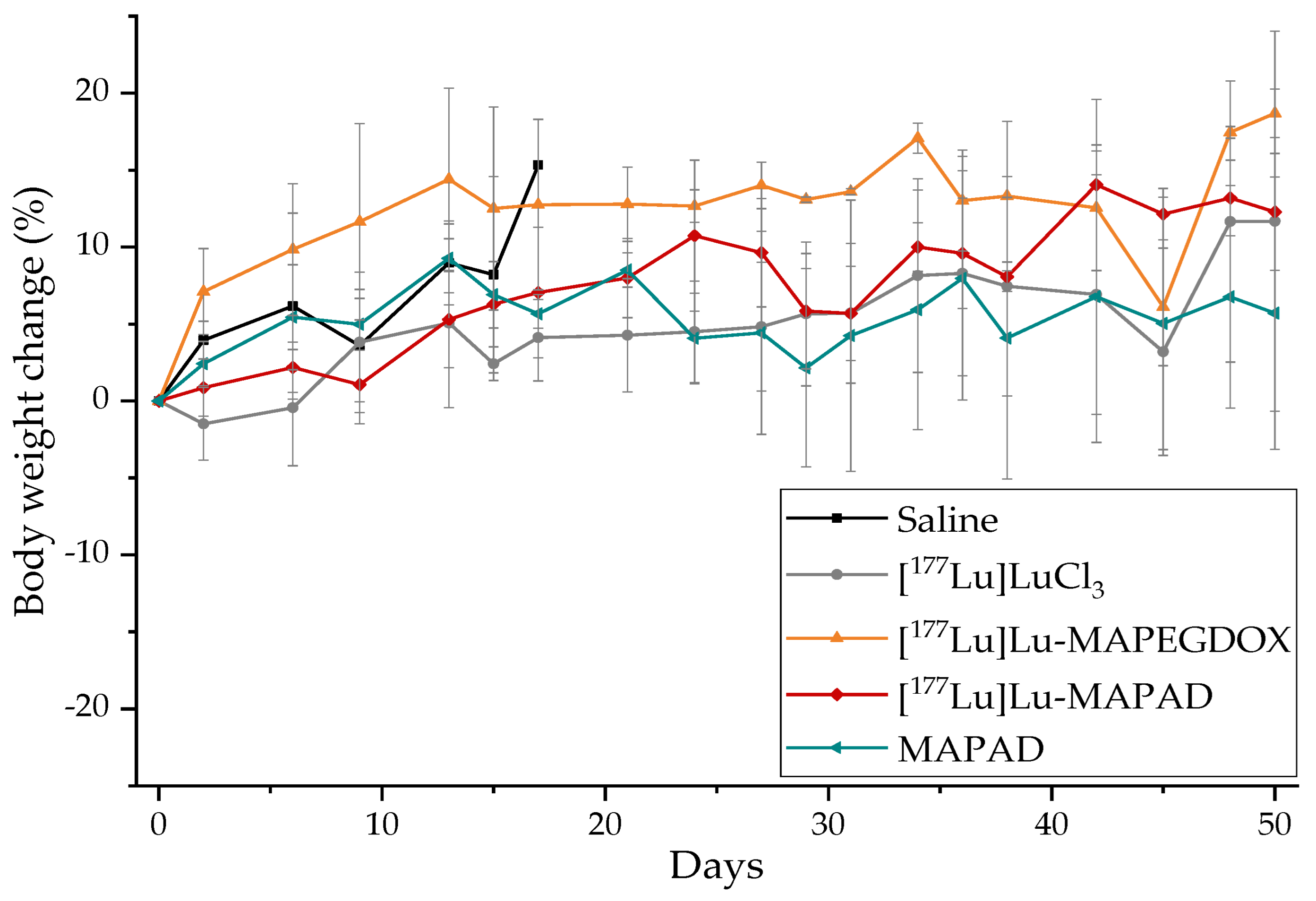
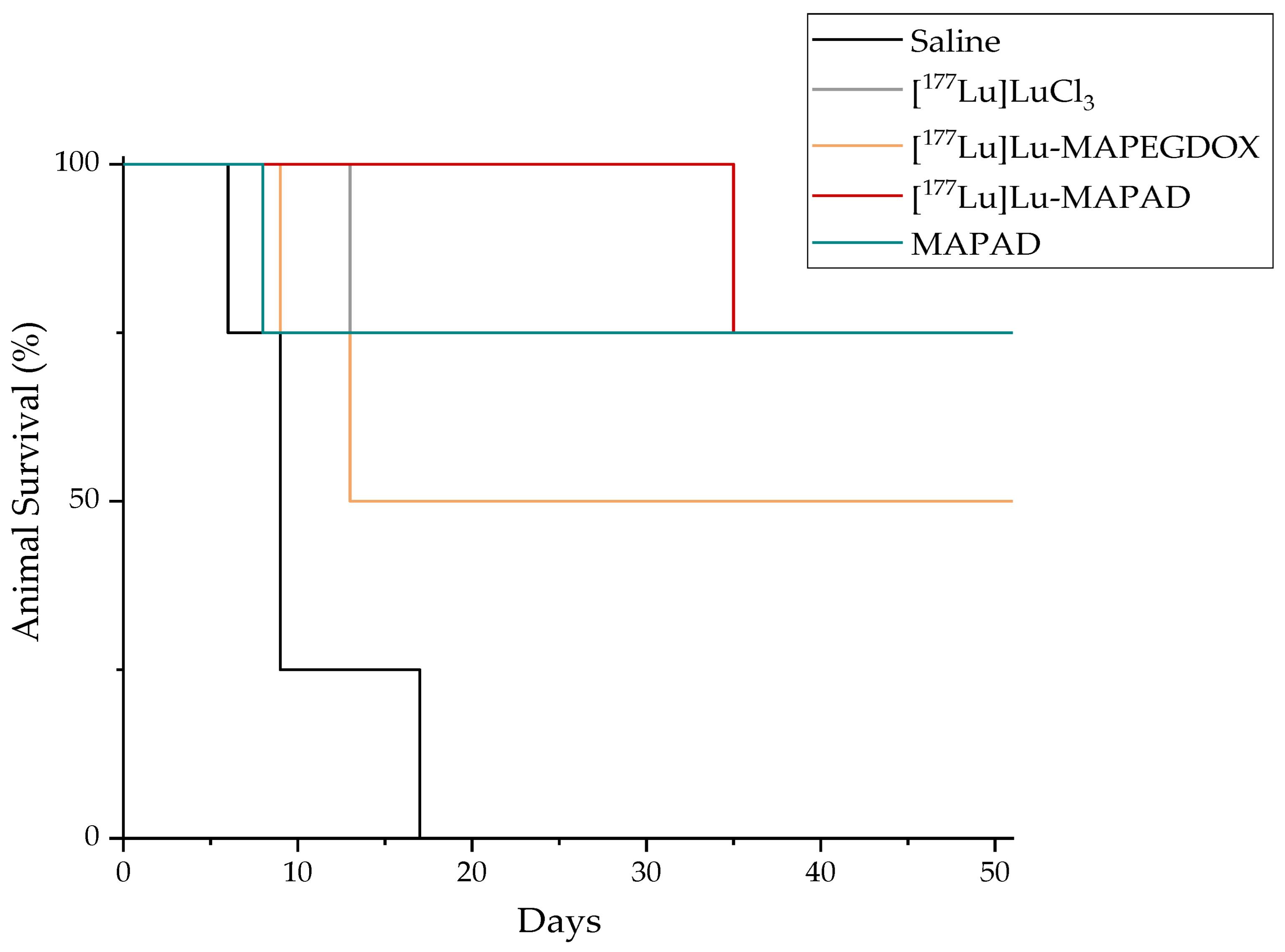
2.8. Therapeutic Efficacy
3. Discussion
3.1. Functionalization with DOX and Bevacizumab
3.2. DOX Release
3.3. Radiolabeling with 177Lu
3.4. In Vitro Cytotoxicity
3.5. Cellular Uptake
3.6. Ex Vivo Biodistribution
3.7. Therapeutic Efficacy
4. Materials and Methods
4.1. Synthesis of MAPAD
4.1.1. Thermogravimetric Analysis
4.1.2. Dynamic Light Scattering
4.2. DOX Release
4.3. Radiolabeling with 177Lu
4.4. In Vitro Cytotoxicity
4.5. Fluorescence Microscopy
4.6. Prussian Blue
4.7. Ex Vivo Biodistribution
4.8. Therapeutic Efficacy
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radzina, M.; Saule, L.; Mamis, E.; Koester, U.; Cocolios, T.E.; Pajuste, E.; Kalnina, M.; Palskis, K.; Sawitzki, Z.; Talip, Z.; et al. Novel Radionuclides for Use in Nuclear Medicine in Europe: Where Do We Stand and Where Do We Go? EJNMMI Radiopharm. Chem. 2023, 8, 27. [Google Scholar] [CrossRef]
- Sharma, S.; Pandey, M.K. Radiometals in Imaging and Therapy: Highlighting Two Decades of Research. Pharmaceuticals 2023, 16, 1460. [Google Scholar] [CrossRef] [PubMed]
- George, S.C.; Samuel, E.J.J. Developments in 177Lu-Based Radiopharmaceutical Therapy and Dosimetry. Front. Chem. 2023, 11, 1218670. [Google Scholar] [CrossRef] [PubMed]
- Chargari, C.; Deutsch, E.; Blanchard, P.; Gouy, S.; Martelli, H.; Guérin, F.; Dumas, I.; Bossi, A.; Morice, P.; Viswanathan, A.N.; et al. Brachytherapy: An Overview for Clinicians. CA Cancer J. Clin. 2019, 69, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Jiang, J.; Buchholz, T.A.; Xu, Y.; Hoffman, K.E.; Giordano, S.H.; Hunt, K.K.; Smith, B.D. Benefit of Adjuvant Brachytherapy Versus External Beam Radiation for Early Breast Cancer: Impact of Patient Stratification on Breast Preservation. Int. J. Radiat. Oncol. 2014, 88, 274–284. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Skowronek, J. Current Status of Brachytherapy in Cancer Treatment—Short Overview. J. Contemp. Brachytherapy 2017, 9, 581–589. [Google Scholar] [CrossRef]
- Ghosh, S.; Lee, S.J.; Hsu, J.C.; Chakraborty, S.; Chakravarty, R.; Cai, W. Cancer Brachytherapy at the Nanoscale: An Emerging Paradigm. Chem. Biomed. Imaging 2024, 2, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Seniwal, B.; Thipe, V.C.; Singh, S.; Fonseca, T.C.F.; Freitas de Freitas, L. Recent Advances in Brachytherapy Using Radioactive Nanoparticles: An Alternative to Seed-Based Brachytherapy. Front. Oncol. 2021, 11, 4830. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Goel, M.; Mackeyev, Y.; Krishnan, S. Radiolabeled Nanomaterial for Cancer Diagnostics and Therapeutics: Principles and Concepts. Cancer Nanotechnol. 2023, 14, 15. [Google Scholar] [CrossRef]
- Vukadinović, A.; Milanović, Z.; Ognjanović, M.; Janković, D.; Radović, M.; Mirković, M.; Karageorgou, M.-A.; Bouziotis, P.; Erić, S.; Vranješ-Đurić, S.; et al. 90Y-CA/SPIONs for Dual Magnetic Hyperthermia-Radionuclide Nanobrachytherapy of Solid Tumours. Nanotechnology 2022, 33, 405102. [Google Scholar] [CrossRef] [PubMed]
- Laprise-Pelletier, M.; Simão, T.; Fortin, M.-A. Gold Nanoparticles in Radiotherapy and Recent Progress in Nanobrachytherapy. Adv. Healthc. Mater. 2018, 7, 1701460. [Google Scholar] [CrossRef] [PubMed]
- Stanković, D.; Radović, M.; Stanković, A.; Mirković, M.; Vukadinović, A.; Mijović, M.; Milanović, Z.; Ognjanović, M.; Janković, D.; Antić, B.; et al. Synthesis, Characterization, and Therapeutic Efficacy of 177Lu-DMSA@SPIONs in Nanobrachytherapy of Solid Tumors. Pharmaceutics 2023, 15, 1943. [Google Scholar] [CrossRef]
- Hosseinkazemi, H.; Samani, S.; O’Neill, A.; Soezi, M.; Moghoofei, M.; Azhdari, M.H.; Aavani, F.; Nazbar, A.; Keshel, S.H.; Doroudian, M. Applications of Iron Oxide Nanoparticles against Breast Cancer. J. Nanomater. 2022, 2022, e6493458. [Google Scholar] [CrossRef]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer Therapy with Iron Oxide Nanoparticles: Agents of Thermal and Immune Therapies. Adv. Drug Deliv. Rev. 2020, 163–164, 65–83. [Google Scholar] [CrossRef]
- Janko, C.; Ratschker, T.; Nguyen, K.; Zschiesche, L.; Tietze, R.; Lyer, S.; Alexiou, C. Functionalized Superparamagnetic Iron Oxide Nanoparticles (SPIONs) as Platform for the Targeted Multimodal Tumor Therapy. Front. Oncol. 2019, 9, 59. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Ma, S.; He, X.; Guo, C.; Liang, Z.; Hu, Y.; Wei, Y.; Lian, X.; Huang, D. Progress in Cancer Therapy with Functionalized Fe3O4 Nanomaterials. Front. Mater. Sci. 2023, 17, 230658. [Google Scholar] [CrossRef]
- Pijeira, M.S.O.; Viltres, H.; Kozempel, J.; Sakmár, M.; Vlk, M.; İlem-Özdemir, D.; Ekinci, M.; Srinivasan, S.; Rajabzadeh, A.R.; Ricci-Junior, E.; et al. Radiolabeled Nanomaterials for Biomedical Applications: Radiopharmacy in the Era of Nanotechnology. EJNMMI Radiopharm. Chem. 2022, 7, 8. [Google Scholar] [CrossRef]
- Salvanou, E.-A.; Kolokithas-Ntoukas, A.; Liolios, C.; Xanthopoulos, S.; Paravatou-Petsotas, M.; Tsoukalas, C.; Avgoustakis, K.; Bouziotis, P. Preliminary Evaluation of Iron Oxide Nanoparticles Radiolabeled with 68Ga and 177Lu as Potential Theranostic Agents. Nanomaterials 2022, 12, 2490. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin Pathways: Pharmacodynamics and Adverse Effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Wasiak, I.; Kulikowska, A.; Janczewska, M.; Michalak, M.; Cymerman, I.A.; Nagalski, A.; Kallinger, P.; Szymanski, W.W.; Ciach, T. Dextran Nanoparticle Synthesis and Properties. PLoS ONE 2016, 11, e0146237. [Google Scholar] [CrossRef]
- Joseph, M.M.; Aravind, S.R.; George, S.K.; Pillai, R.K.; Mini, S.; Sreelekha, T.T. Co-Encapsulation of Doxorubicin With Galactoxyloglucan Nanoparticles for Intracellular Tumor-Targeted Delivery in Murine Ascites and Solid Tumors. Transl. Oncol. 2014, 7, 525–536. [Google Scholar] [CrossRef]
- Dragojevic, S.; Turner, L.; Raucher, D. Circumventing Doxorubicin Resistance Using Elastin-like Polypeptide Biopolymer-Mediated Drug Delivery. Int. J. Mol. Sci. 2022, 23, 2301. [Google Scholar] [CrossRef]
- Theodosiou, M.; Boukos, N.; Sakellis, E.; Zachariadis, M.; Efthimiadou, E.K. Gold Nanoparticle Decorated pH-Sensitive Polymeric Nanocontainers as a Potential Theranostic Agent. Colloids Surf. B Biointerfaces 2019, 183, 110420. [Google Scholar] [CrossRef]
- Alyane, M.; Barratt, G.; Lahouel, M. Remote Loading of Doxorubicin into Liposomes by Transmembrane pH Gradient to Reduce Toxicity toward H9c2 Cells. Saudi Pharm. J. 2016, 24, 165–175. [Google Scholar] [CrossRef]
- Tsoukalas, C.; Psimadas, D.; Kastis, G.A.; Koutoulidis, V.; Harris, A.L.; Paravatou-Petsotas, M.; Karageorgou, M.; Furenlid, L.R.; Moulopoulos, L.A.; Stamopoulos, D.; et al. A Novel Metal-Based Imaging Probe for Targeted Dual-Modality SPECT/MR Imaging of Angiogenesis. Front. Chem. 2018, 6, 224. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Tejada, M.d.M.; Viota, J.L.; Rudzka, K.; Delgado, A.V. Preparation of Multi-Functionalized Fe3O4/Au Nanoparticles for Medical Purposes. Colloids Surf. B Biointerfaces 2015, 128, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sarigiannis, Y.; Kolokithas-Ntoukas, A.; Beziere, N.; Zbořil, R.; Papadimitriou, E.; Avgoustakis, K.; Lamprou, M.; Medrikova, Z.; Rousalis, E.; Ntziachristos, V.; et al. Synthesis and Evaluation of Condensed Magnetic Nanocrystal Clusters with in Vivo Multispectral Optoacoustic Tomography for Tumour Targeting. Biomaterials 2016, 91, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Zoppellaro, G.; Kolokithas-Ntoukas, A.; Polakova, K.; Tucek, J.; Zboril, R.; Loudos, G.; Fragogeorgi, E.; Diwoky, C.; Tomankova, K.; Avgoustakis, K.; et al. Theranostics of Epitaxially Condensed Colloidal Nanocrystal Clusters, through a Soft Biomineralization Route. Chem. Mater. 2014, 26, 2062–2074. [Google Scholar] [CrossRef]
- Bakandritsos, A.; Papagiannopoulos, A.; Anagnostou, E.N.; Avgoustakis, K.; Zboril, R.; Pispas, S.; Tucek, J.; Ryukhtin, V.; Bouropoulos, N.; Kolokithas-Ntoukas, A.; et al. Merging High Doxorubicin Loading with Pronounced Magnetic Response and Bio-Repellent Properties in Hybrid Drug Nanocarriers. Small 2012, 8, 2381–2393. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-Loaded Iron Oxide Nanoparticles for Glioblastoma Therapy: A Combinational Approach for Enhanced Delivery of Nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef]
- Nieciecka, D.; Celej, J.; Żuk, M.; Majkowska-Pilip, A.; Żelechowska-Matysiak, K.; Lis, A.; Osial, M. Hybrid System for Local Drug Delivery and Magnetic Hyperthermia Based on SPIONs Loaded with Doxorubicin and Epirubicin. Pharmaceutics 2021, 13, 480. [Google Scholar] [CrossRef]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Unexpected Plasticization Effects on the Structure and Properties of Polyelectrolyte Complexed Chitosan/Alginate Materials. ACS Appl. Polym. Mater. 2020, 2, 2957–2966. [Google Scholar] [CrossRef]
- Li, R.; Wu, Y.; Bai, Z.; Guo, J.; Chen, X. Effect of Molecular Weight of Polyethylene Glycol on Crystallization Behaviors, Thermal Properties and Tensile Performance of Polylactic Acid Stereocomplexes. RSC Adv. 2020, 10, 42120–42127. [Google Scholar] [CrossRef]
- Manocha, B.; Margaritis, A. Controlled Release of Doxorubicin from Doxorubicin/γ-Polyglutamic Acid Ionic Complex. J. Nanomater. 2010, 2010, 780171. [Google Scholar] [CrossRef]
- Singh, N.; Sallem, F.; Mirjolet, C.; Nury, T.; Sahoo, S.K.; Millot, N.; Kumar, R. Polydopamine Modified Superparamagnetic Iron Oxide Nanoparticles as Multifunctional Nanocarrier for Targeted Prostate Cancer Treatment. Nanomaterials 2019, 9, 138. [Google Scholar] [CrossRef]
- Hondow, N.; Brydson, R.; Wang, P.; Holton, M.D.; Brown, M.R.; Rees, P.; Summers, H.D.; Brown, A. Quantitative Characterization of Nanoparticle Agglomeration within Biological Media. J. Nanoparticle Res. 2012, 14, 977. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 60. [Google Scholar] [CrossRef]
- DLS and Zeta Potential—What They Are and What They Are Not?—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27297779/ (accessed on 7 August 2023).
- Determination of Isoelectric Points and Relative Charge Variants of 23 Therapeutic Monoclonal Antibodies—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1570023217313880?via%3Dihub (accessed on 16 August 2023).
- Sousa, F.; Cruz, A.; Fonte, P.; Pinto, I.M.; Neves-Petersen, M.T.; Sarmento, B. A New Paradigm for Antiangiogenic Therapy through Controlled Release of Bevacizumab from PLGA Nanoparticles. Sci. Rep. 2017, 7, 3736. [Google Scholar] [CrossRef] [PubMed]
- Oltolina, F.; Colangelo, D.; Miletto, I.; Clemente, N.; Miola, M.; Verné, E.; Prat, M.; Follenzi, A. Tumor Targeting by Monoclonal Antibody-functionalized Magnetic Nanoparticles. Nanomaterials 2019, 9, 1575. [Google Scholar] [CrossRef] [PubMed]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef]
- Angelopoulou, A.; Kolokithas-Ntoukas, A.; Fytas, C.; Avgoustakis, K. Folic Acid-Functionalized, Condensed Magnetic Nanoparticles for Targeted Delivery of Doxorubicin to Tumor Cancer Cells Overexpressing the Folate Receptor. ACS Omega 2019, 4, 22214–22227. [Google Scholar] [CrossRef] [PubMed]
- Akbas, S.; Sahin, A.; Calis, S.; Oncel, H.; Capan, Y. Characterization of Bevacizumab by Dynamic Light Scattering While Maintaining Its Native Structure. Pharmazie 2018, 37, 369–374. [Google Scholar] [CrossRef]
- Sousa, F.; Sarmento, B.; Neves-Petersen, M.T. Biophysical Study of Bevacizumab Structure and Bioactivity under Thermal and pH-Stresses. Eur. J. Pharm. Sci. 2017, 105, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Signorello, L.; Pucciarelli, S.; Bonacucina, G.; Polzonetti, V.; Cespi, M.; Perinelli, D.; Palmieri, G.; Pettinari, R.; Pettinari, C.; Fiorentini, G.; et al. Quantification, Microbial Contamination, Physico-Chemical Stability of Repackaged Bevacizumab Stored Under Different Conditions. Curr. Pharm. Biotechnol. 2014, 15, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kassis, A.I. Therapeutic Radionuclides: Biophysical and Radiobiologic Principles. Semin. Nucl. Med. 2008, 38, 358–366. [Google Scholar] [CrossRef]
- Trujillo-Nolasco, R.M.; Morales-Avila, E.; Ocampo-García, B.E.; Ferro-Flores, G.; Gibbens-Bandala, B.V.; Escudero-Castellanos, A.; Isaac-Olive, K. Preparation and in Vitro Evaluation of Radiolabeled HA-PLGA Nanoparticles as Novel MTX Delivery System for Local Treatment of Rheumatoid Arthritis. Mater. Sci. Eng. C 2019, 103, 109766. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Umbricht, C.A.; Gracheva, N.; Tschan, V.J.; Pellegrini, G.; Bernhardt, P.; Zeevaart, J.R.; Köster, U.; Schibli, R.; van der Meulen, N.P. Terbium-161 for PSMA-Targeted Radionuclide Therapy of Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.; Graver, S. Tumor Cell Response to Bevacizumab Single Agent Therapy in Vitro. Cancer Cell Int. 2013, 13, 94. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Y.; Zhang, Y.; He, B.; Dai, W.; Zhang, H.; Wang, X.; Zhang, Q. Combination Therapy of cRGD-DOX Self-Assembled Nanoparticles and Bevacizumab for Breast Cancer. J. Chin. Pharm. Sci. 2019, 28, 627. [Google Scholar] [CrossRef]
- Goel, H.L.; Mercurio, A.M. VEGF Targets the Tumour Cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef]
- Boswell, C.A.; Tesar, D.B.; Mukhyala, K.; Theil, F.-P.; Fielder, P.J.; Khawli, L.A. Effects of Charge on Antibody Tissue Distribution and Pharmacokinetics. Bioconjug. Chem. 2010, 21, 2153–2163. [Google Scholar] [CrossRef]
- Li, B.; Tesar, D.; Boswell, C.A.; Cahaya, H.S.; Wong, A.; Zhang, J.; Meng, Y.G.; Eigenbrot, C.; Pantua, H.; Diao, J.; et al. Framework Selection Can Influence Pharmacokinetics of a Humanized Therapeutic Antibody through Differences in Molecule Charge. mAbs 2014, 6, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Igawa, T.; Tsunoda, H.; Tachibana, T.; Maeda, A.; Mimoto, F.; Moriyama, C.; Nanami, M.; Sekimori, Y.; Nabuchi, Y.; Aso, Y.; et al. Reduced Elimination of IgG Antibodies by Engineering the Variable Region. Protein Eng. Des. Sel. 2010, 23, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Forest, V.; Pourchez, J. Preferential Binding of Positive Nanoparticles on Cell Membranes Is Due to Electrostatic Interactions: A Too Simplistic Explanation That Does Not Take into Account the Nanoparticle Protein Corona. Mater. Sci. Eng. C 2017, 70, 889–896. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Primavera, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular Uptake and Retention of Nanoparticles: Insights on Particle Properties and Interaction with Cellular Components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Lin, R.; Huang, J.; Wang, L.; Li, Y.; Lipowska, M.; Wu, H.; Yang, J.; Mao, H. Bevacizumab and Near Infrared Probe Conjugated Iron Oxide Nanoparticles for Vascular Endothelial Growth Factor Targeted MR and Optical Imaging. Biomater. Sci. 2018, 6, 1517–1525. [Google Scholar] [CrossRef]
- Blankenberg, F.G.; Backer, M.V.; Levashova, Z.; Patel, V.; Backer, J.M. In Vivo Tumor Angiogenesis Imaging with Site-Specific Labeled 99mTc-HYNIC-VEGF. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 841–848. [Google Scholar] [CrossRef]
- Vascular Endothelial Growth Factor Selectively Targets Boronated Dendrimers to Tumor Vasculature|Molecular Cancer Therapeutics|American Association for Cancer Research. Available online: https://aacrjournals.org/mct/article/4/9/1423/235607/Vascular-endothelial-growth-factor-selectively (accessed on 4 August 2023).
- Nowak-Jary, J.; Machnicka, B. In Vivo Biodistribution and Clearance of Magnetic Iron Oxide Nanoparticles for Medical Applications. Int. J. Nanomed. 2023, 18, 4067–4100. [Google Scholar] [CrossRef]
- Cędrowska, E.; Pruszyński, M.; Gawęda, W.; Żuk, M.; Krysiński, P.; Bruchertseifer, F.; Morgenstern, A.; Karageorgou, M.-A.; Bouziotis, P.; Bilewicz, A. Trastuzumab Conjugated Superparamagnetic Iron Oxide Nanoparticles Labeled with 225Ac as a Perspective Tool for Combined α-Radioimmunotherapy and Magnetic Hyperthermia of HER2-Positive Breast Cancer. Molecules 2020, 25, 1025. [Google Scholar] [CrossRef]
- Yook, S.; Lu, Y.; Jeong, J.J.; Cai, Z.; Tong, L.; Alwarda, R.; Pignol, J.-P.; Winnik, M.A.; Reilly, R.M. Stability and Biodistribution of Thiol-Functionalized and 177Lu-Labeled Metal Chelating Polymers Bound to Gold Nanoparticles. Biomacromolecules 2016, 17, 1292–1302. [Google Scholar] [CrossRef]
- Leonte, R.A.; Chilug, L.E.; Șerban, R.; Mustăciosu, C.; Raicu, A.; Manda, G.; Niculae, D. Preparation and Preliminary Evaluation of Neurotensin Radiolabelled with 68Ga and 177Lu as Potential Theranostic Agent for Colon Cancer. Pharmaceutics 2021, 13, 506. [Google Scholar] [CrossRef]
- Hötzel, I.; Theil, F.-P.; Bernstein, L.J.; Prabhu, S.; Deng, R.; Quintana, L.; Lutman, J.; Sibia, R.; Chan, P.; Bumbaca, D.; et al. A Strategy for Risk Mitigation of Antibodies with Fast Clearance. mAbs 2012, 4, 753–760. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, Z.; Mao, H.; Yang, L. Magnetic Nanoparticles for Precision Oncology: Theranostic Magnetic Iron Oxide Nanoparticles for Image-Guided and Targeted Cancer Therapy. Nanomedicine 2017, 12, 73–87. [Google Scholar] [CrossRef]
- Holback, H.; Yeo, Y. Intratumoral Drug Delivery with Nanoparticulate Carriers. Pharm. Res. 2011, 28, 1819–1830. [Google Scholar] [CrossRef]
- Salvanou, E.-A.; Stellas, D.; Tsoukalas, C.; Mavroidi, B.; Paravatou-Petsotas, M.; Kalogeropoulos, N.; Xanthopoulos, S.; Denat, F.; Laurent, G.; Bazzi, R.; et al. A Proof-of-Concept Study on the Therapeutic Potential of Au Nanoparticles Radiolabeled with the Alpha-Emitter Actinium-225. Pharmaceutics 2020, 12, 188. [Google Scholar] [CrossRef]
- Żelechowska-Matysiak, K.; Salvanou, E.-A.; Bouziotis, P.; Budlewski, T.; Bilewicz, A.; Majkowska-Pilip, A. Improvement of the Effectiveness of HER2+Cancer Therapy by Use of Doxorubicin and Trastuzumab Modified Radioactive Gold Nanoparticles. Mol. Pharm. 2023, 20, 4676–4686. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.d.S.; Costa, L.A.d.M.; Harmon, A.C.; Gomes Filho, M.A.; Falcão, E.H.L.; Vicente, M.G.H.; Junior, S.A.; Mathis, J.M. 177Lu-Labeled Eu-Doped Mesoporous SiO2 Nanoparticles as a Theranostic Radiopharmaceutical for Colorectal Cancer. ACS Appl. Nano Mater. 2020, 3, 8691–8701. [Google Scholar] [CrossRef]
- EMA EndolucinBeta. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/endolucinbeta (accessed on 20 August 2023).
- Department of Health, Therapeutic Goods Administration. Australian Public Assessment Report for Lutetium (177Lu) Chloride; Department of Health, Therapeutic Goods Administration: Woden, ACT, Australia, 2022.
- Straus, S.; Huber, M. Pharmacovigilance Risk Assessment Committee (PRAC). Available online: https://www.ema.europa.eu/en/documents/minutes/minutes-prac-meeting-2-5-may-2022_en.pdf (accessed on 31 January 2023).
- Kato, H.; Huang, X.; Kadonaga, Y.; Katayama, D.; Ooe, K.; Shimoyama, A.; Kabayama, K.; Toyoshima, A.; Shinohara, A.; Hatazawa, J.; et al. Intratumoral Administration of Astatine-211-Labeled Gold Nanoparticle for Alpha Therapy. J. Nanobiotechnology 2021, 19, 223. [Google Scholar] [CrossRef] [PubMed]
- Chanda, N.; Kan, P.; Watkinson, L.D.; Shukla, R.; Zambre, A.; Carmack, T.L.; Engelbrecht, H.; Lever, J.R.; Katti, K.; Fent, G.M.; et al. Radioactive Gold Nanoparticles in Cancer Therapy: Therapeutic Efficacy Studies of GA-198AuNP Nanoconstruct in Prostate Tumor–Bearing Mice. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Chanda, N.; Zambre, A.; Upendran, A.; Katti, K.; Kulkarni, R.R.; Nune, S.K.; Casteel, S.W.; Smith, C.J.; Vimal, J.; et al. Laminin Receptor Specific Therapeutic Gold Nanoparticles (198AuNP-EGCg) Show Efficacy in Treating Prostate Cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 12426–12431. [Google Scholar] [CrossRef] [PubMed]
- Al-Yasiri, A.Y.; Khoobchandani, M.; Cutler, C.S.; Watkinson, L.; Carmack, T.; Smith, C.J.; Kuchuk, M.; Loyalka, S.K.; Lugão, A.B.; Katti, K.V. Mangiferin Functionalized Radioactive Gold Nanoparticles (MGF-198AuNPs) in Prostate Tumor Therapy: Green Nanotechnology for Production, in Vivo Tumor Retention and Evaluation of Therapeutic Efficacy. Dalton Trans. 2017, 46, 14561–14571. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, Q.-L.; Liu, T.-G.; Zhao, W.-P.; Yang, M.; Wang, L.-N.; Sun, W.-L.; Pan, L.; Luo, A.-P.; Huang, J.-C.; et al. Evaluation of Local Injection of Bevacizumab against Triple-Negative Breast Cancer Xenograft Tumors. Curr. Pharm. Des. 2019, 25, 862–870. [Google Scholar] [CrossRef]
- Stanković, A.; Mihailović, J.; Mirković, M.; Radović, M.; Milanović, Z.; Ognjanović, M.; Janković, D.; Antić, B.; Mijović, M.; Vranješ-Đurić, S.; et al. Aminosilanized Flower-Structured Superparamagnetic Iron Oxide Nanoparticles Coupled to 131I-Labeled CC49 Antibody for Combined Radionuclide and Hyperthermia Therapy of Cancer. Int. J. Pharm. 2020, 587, 119628. [Google Scholar] [CrossRef] [PubMed]
- Yook, S.; Cai, Z.; Lu, Y.; Winnik, M.A.; Pignol, J.-P.; Reilly, R.M. Intratumorally Injected 177 Lu-Labeled Gold Nanoparticles: Gold Nanoseed Brachytherapy with Application for Neoadjuvant Treatment of Locally Advanced Breast Cancer. J. Nucl. Med. 2016, 57, 936–942. [Google Scholar] [CrossRef]
- Tao, K.; Fang, M.; Alroy, J.; Sahagian, G.G. Imagable 4T1 Model for the Study of Late Stage Breast Cancer. BMC Cancer 2008, 8, 228. [Google Scholar] [CrossRef]
- Hillen, F.; Griffioen, A.W. Tumour Vascularization: Sprouting Angiogenesis and Beyond. Cancer Metastasis Rev. 2007, 26, 489–502. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in Cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Hernandez, R.; Grudzinski, J.J.; Aluicio-Sarduy, E.; Massey, C.F.; Pinchuk, A.N.; Bitton, A.N.; Patel, R.; Zhang, R.; Rao, A.V.; Iyer, G.; et al. 177Lu-NM600 Targeted Radionuclide Therapy Extends Survival in Syngeneic Murine Models of Triple-Negative Breast Cancer. J. Nucl. Med. 2020, 61, 1187–1194. [Google Scholar] [CrossRef]
- Varan, G.; Varan, C.; Öztürk, S.C.; Benito, J.M.; Esendağlı, G.; Bilensoy, E. Therapeutic Efficacy and Biodistribution of Paclitaxel-Bound Amphiphilic Cyclodextrin Nanoparticles: Analyses in 3D Tumor Culture and Tumor-Bearing Animals In Vivo. Nanomaterials 2021, 11, 515. [Google Scholar] [CrossRef]
- Ao, H.; Wang, Z.; Lu, L.; Ma, H.; Li, H.; Fu, J.; Li, M.; Han, M.; Guo, Y.; Wang, X. Enhanced Tumor Accumulation and Therapeutic Efficacy of Liposomal Drugs through Over-Threshold Dosing. J. Nanobiotechnology 2022, 20, 137. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Yeh, T.-K.; Tsai, M.; Au, J.L.-S.; Wientjes, M.G. Paclitaxel-Loaded Gelatin Nanoparticles for Intravesical Bladder Cancer Therapy. Clin. Cancer Res. 2004, 10, 7677–7684. [Google Scholar] [CrossRef] [PubMed]
- Proctor, A.; Wang, Q.; Lawrence, D.S.; Allbritton, N.L. Selection and Optimization of Enzyme Reporters for Chemical Cytometry. In Methods in Enzymology; Shukla, A.K., Ed.; Chemical and Synthetic Biology Approaches To Understand Cellular Functions—Part B; Academic Press: Cambridge, MA, USA, 2019; Volume 622, pp. 221–248. [Google Scholar]
- Kurebayashi, J.; Otsuki, T.; Kunisue, H.; Mikami, Y.; Tanaka, K.; Yamamoto, S.; Sonoo, H. Expression of Vascular Endothelial Growth Factor (VEGF) Family Members in Breast Cancer. Jpn. J. Cancer Res. 1999, 90, 977–981. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvanou, E.-A.; Kolokithas-Ntoukas, A.; Prokopiou, D.; Theodosiou, M.; Efthimiadou, E.; Koźmiński, P.; Xanthopoulos, S.; Avgoustakis, K.; Bouziotis, P. 177Lu-Labeled Iron Oxide Nanoparticles Functionalized with Doxorubicin and Bevacizumab as Nanobrachytherapy Agents against Breast Cancer. Molecules 2024, 29, 1030. https://doi.org/10.3390/molecules29051030
Salvanou E-A, Kolokithas-Ntoukas A, Prokopiou D, Theodosiou M, Efthimiadou E, Koźmiński P, Xanthopoulos S, Avgoustakis K, Bouziotis P. 177Lu-Labeled Iron Oxide Nanoparticles Functionalized with Doxorubicin and Bevacizumab as Nanobrachytherapy Agents against Breast Cancer. Molecules. 2024; 29(5):1030. https://doi.org/10.3390/molecules29051030
Chicago/Turabian StyleSalvanou, Evangelia-Alexandra, Argiris Kolokithas-Ntoukas, Danai Prokopiou, Maria Theodosiou, Eleni Efthimiadou, Przemysław Koźmiński, Stavros Xanthopoulos, Konstantinos Avgoustakis, and Penelope Bouziotis. 2024. "177Lu-Labeled Iron Oxide Nanoparticles Functionalized with Doxorubicin and Bevacizumab as Nanobrachytherapy Agents against Breast Cancer" Molecules 29, no. 5: 1030. https://doi.org/10.3390/molecules29051030
APA StyleSalvanou, E.-A., Kolokithas-Ntoukas, A., Prokopiou, D., Theodosiou, M., Efthimiadou, E., Koźmiński, P., Xanthopoulos, S., Avgoustakis, K., & Bouziotis, P. (2024). 177Lu-Labeled Iron Oxide Nanoparticles Functionalized with Doxorubicin and Bevacizumab as Nanobrachytherapy Agents against Breast Cancer. Molecules, 29(5), 1030. https://doi.org/10.3390/molecules29051030









