The Copper Reduction Potential Determines the Reductive Cytotoxicity: Relevance to the Design of Metal–Organic Antitumor Drugs
Abstract
1. Introduction
2. Results and Discussion
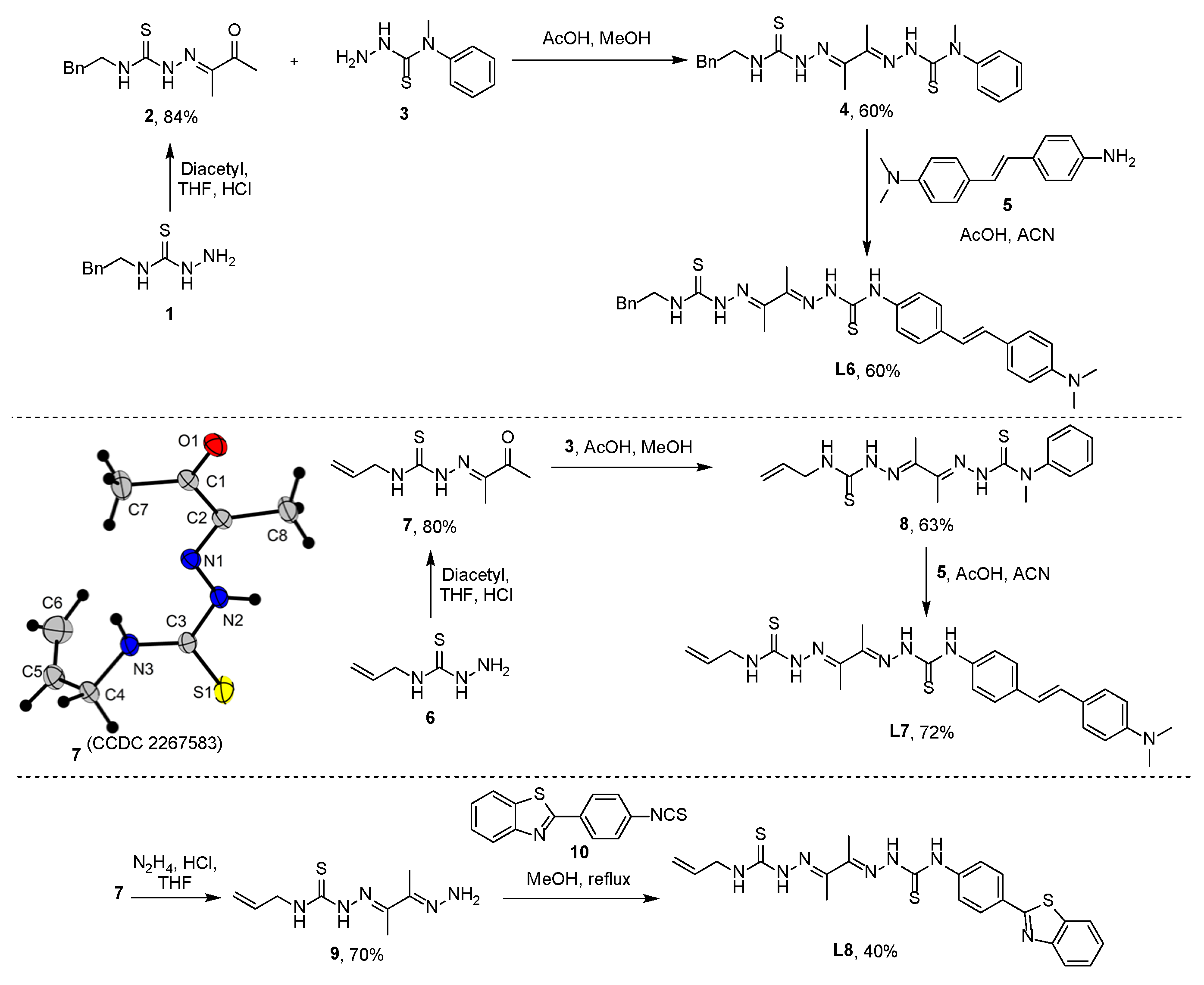
2.1. Synthesis of Coordination Compounds
2.2. Electrochemistry
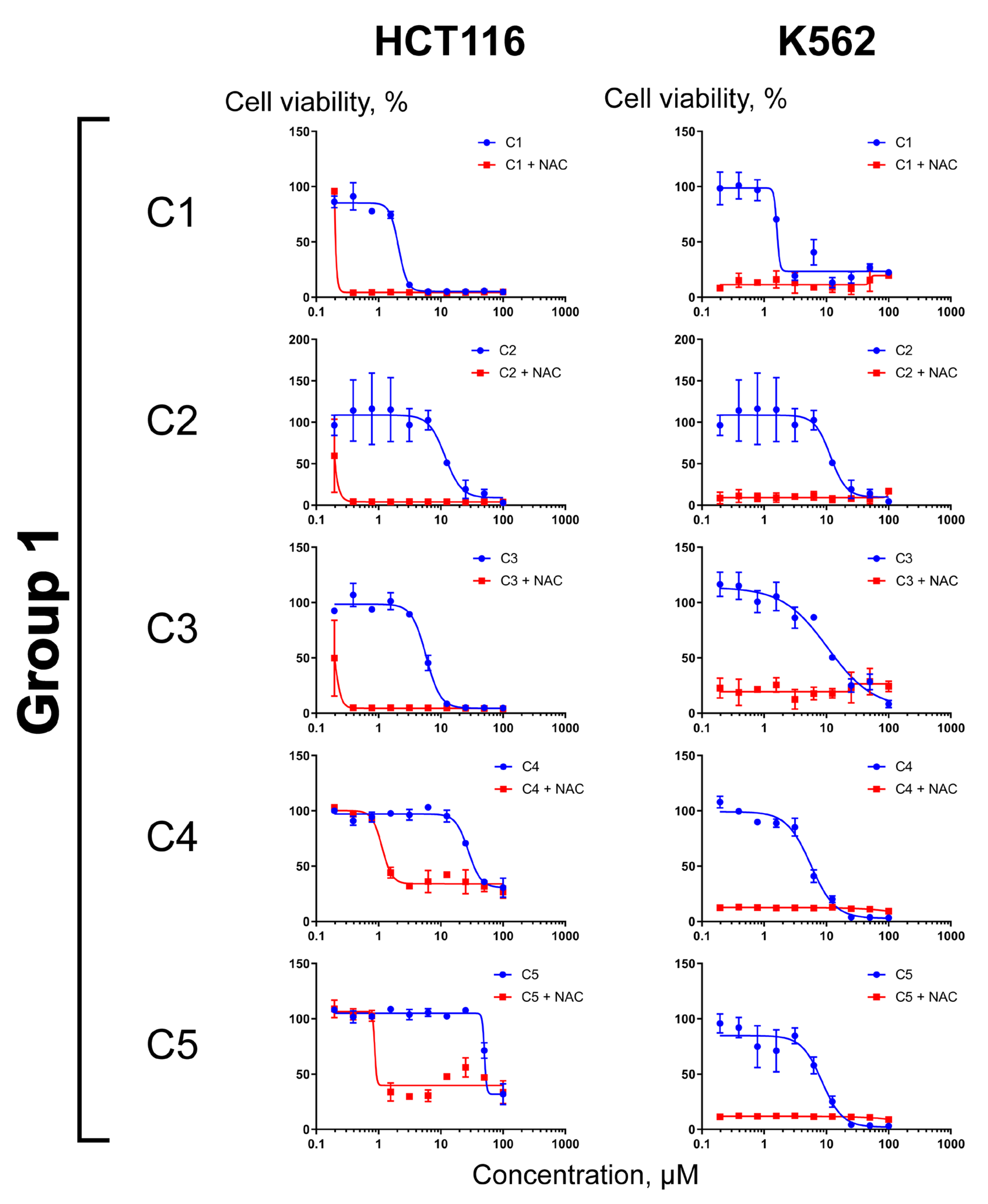
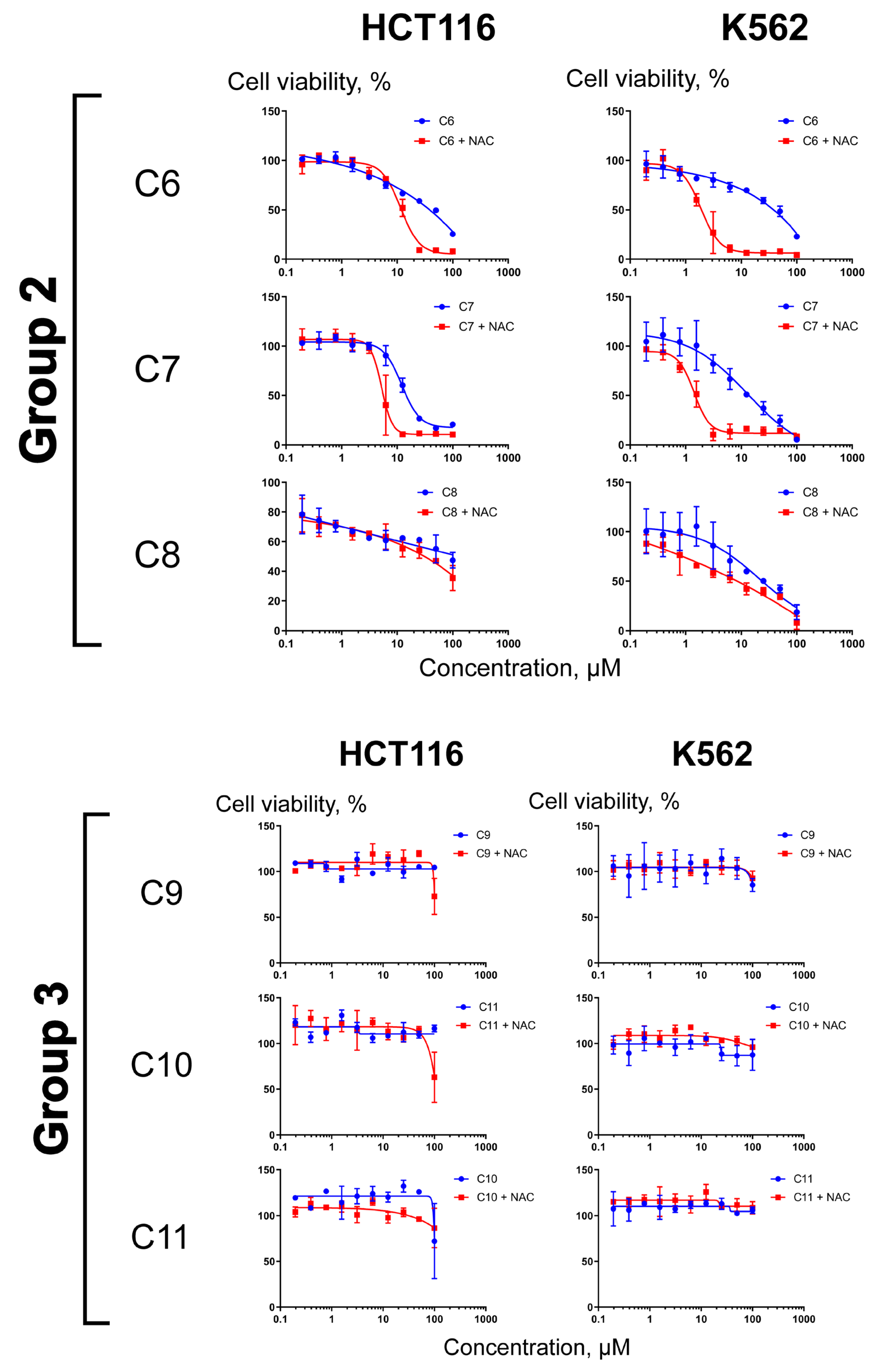
2.3. Differential Effects of NAC on Cell Sensitization to Compounds C1–C11
3. Materials and Methods
3.1. General
3.2. X-ray Diffraction Analysis
3.3. MALDI
3.4. Electrochemistry
3.5. Cell Culture and Cytotoxicity Assays
3.6. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghandadi, M.; Behravan, J.; Abnous, K.; Gharaee, M.E.; Mosaffa, F. TNF-α exerts cytotoxic effects on multidrug resistant breast cancer MCF-7/MX cells via a non-apoptotic death pathway. Cytokine 2017, 97, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, D.; Lv, T.; Liu, K.; Yang, Y.; Xu, X.; Chen, Y. Novel peptide myristoly-CM4 induces selective cytotoxicity in leukemia K562/MDR and Jurkat cells by necrosis and/or apoptosis pathway. Drug Des. Devel. Ther. 2019, 13, 2153–2167. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Guseva, E.V.; Ataeva, A.N.; Sigan, A.L.; Shibaeva, A.V.; Dmitrieva, M.V.; Burtsev, I.D.; Volodina, Y.L.; Radchenko, A.S.; Egorov, A.E.; et al. Perfluorocarbon nanoemulsions with fluorous chlorin-type photosensitizers for antitumor photodynamic therapy in hypoxia. Int. J. Mol. Sci. 2023, 24, 7995. [Google Scholar] [CrossRef] [PubMed]
- Tsymbal, S.A.; Moiseeva, A.A.; Agadzhanian, N.A.; Efimova, S.S.; Markova, A.A.; Guk, D.A.; Krasnovskaya, O.O.; Alpatova, V.M.; Zaitsev, A.V.; Shibaeva, A.V.; et al. Copper-containing nanoparticles and organic complexes: Metal reduction triggers rapid cell death via oxidative burst. Int. J. Mol. Sci. 2021, 22, 11065. [Google Scholar] [CrossRef] [PubMed]
- Gaál, A.; Orgován, G.; Mihucz, V.G.; Pape, I.; Ingerle, D.; Streli, C.; Szoboszlai, N. Metal transport capabilities of anticancer copper chelators. J. Trace Elem. Med. Biol. 2018, 47, 79–88. [Google Scholar] [CrossRef]
- Dabrowiak, J.C. Metals in Medicine; John Wiley and Sons: Hoboken, NJ, USA, 2009; ISBN 9780470681961. [Google Scholar] [CrossRef]
- Krasnovskaya, O.; Naumov, A.; Guk, D.; Gorelkin, P.; Erofeev, A.; Beloglazkina, E.; Majouga, A. Copper Coordination Compounds as Biologically Active Agents. Int. J. Mol. Sci. 2020, 21, 3965. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Chen, J.; Yang, Q.; Yang, L.; Xu, D.; Zhang, P.; Wang, X.; Liu, J. Hinokitiol copper complex inhibits proteasomal deubiquitination and induces paraptosis-like cell death in human cancer cells. Eur. J. Pharmacol. 2017, 815, 147–155. [Google Scholar] [CrossRef]
- Zeeshan, M.; Murugadas, A.; Ghaskadbi, S.; Rajendran, R.B.; Akbarsha, M.A. ROS dependent copper toxicity in Hydra -biochemical and molecular study. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 185, 1–12. [Google Scholar] [CrossRef]
- Qin, Q.-P.; Meng, T.; Tan, M.-X.; Liu, Y.-C.; Luo, X.-J.; Zou, B.-Q.; Liang, H. Synthesis, crystal structure and biological evaluation of a new dasatinib copper(II) complex as telomerase inhibitor. Eur. J. Med. Chem. 2018, 143, 1597–1603. [Google Scholar] [CrossRef]
- Fatfat, M.; Merhi, R.A.; Rahal, O.; Stoyanovsky, D.A.; Zaki, A.; Haidar, H.; Kagan, V.E.; Gali-Muhtasib, H.; Machaca, K. Copper chelation selectively kills colon cancer cells through redox cycling and generation of reactive oxygen species. BMC Cancer 2014, 14, 527. [Google Scholar] [CrossRef]
- Martinez-Bulit, P.; Garza-Ortíz, A.; Mijangos, E.; Barrón-Sosa, L.; Sánchez-Bartéz, F.; Gracia-Mora, I.; Flores-Parra, A.; Contreras, R.; Reedijk, J.; Barba-Behrens, N. 2,6-Bis(2,6-diethylphenyliminomethyl)pyridine coordination compounds with cobalt(II), nickel(II), copper(II), and zinc(II): Synthesis, spectroscopic characterization, X-ray study and in vitro cytotoxicity. J. Inorg. Biochem. 2015, 142, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Beloglazkina, E.K.; Krasnovskaya, O.O.; Guk, D.A.; Tafeenko, V.A.; Moiseeva, A.A.; Zyk, N.V.; Majouga, A.G. Synthesis, characterization, and cytotoxicity of binuclear copper(II) complexes with tetradentate nitrogen-containing ligands bis-5-(2-pyridylmethylidene)-3,5-dihydro-4H-imidazol-4-ones. Polyhedron 2018, 148, 129–137. [Google Scholar] [CrossRef]
- Tishchenko, K.; Beloglazkina, E.; Proskurnin, M.; Malinnikov, V.; Guk, D.; Muratova, M.; Krasnovskaya, O.; Udina, A.; Skvortsov, D.; Shafikov, R.R.; et al. New copper(II) thiohydantoin complexes: Synthesis, characterization, and assessment of their interaction with bovine serum albumin and DNA. J. Inorg. Biochem. 2017, 175, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Majouga, A.G.; Zvereva, M.I.; Rubtsova, M.P.; Skvortsov, D.A.; Mironov, A.V.; Azhibek, D.M.; Krasnovskaya, O.O.; Gerasimov, V.M.; Udina, A.V.; Vorozhtsov, N.I.; et al. Mixed Valence Copper(I,II) Binuclear Complexes with Unexpected Structure: Synthesis, Biological Properties and Anticancer Activity. J. Med. Chem. 2014, 57, 6252–6258. [Google Scholar] [CrossRef] [PubMed]
- Synta and GlaxoSmithKline Announce Elesclomol Granted Orphan Drug Designation by the FDA. Available online: https://ir.madrigalpharma.com/news-releases/news-release-details/synta-and-glaxosmithkline-announce-elesclomol-granted-orphan (accessed on 28 January 2008).
- Hedley, D.; Shamas-Din, A.; Chow, S.; Sanfelice, D.; Schuh, A.C.; Brandwein, J.M.; Seftel, M.D.; Gupta, V.; Yee, K.W.L.; Schimmer, A.D. A phase I study of elesclomol sodium in patients with acute myeloid leukemia. Leuk. Lymphoma 2016, 57, 2437–2440. [Google Scholar] [CrossRef] [PubMed]
- De Moura, M.B.; Vincent, G.; Fayewicz, S.L.; Bateman, N.W.; Hood, B.L.; Sun, M.; Suhan, J.; Duensing, S.; Yin, Y.; Sander, C.; et al. Mitochondrial Respiration—An Important Therapeutic Target in Melanoma. PLoS ONE 2012, 7, 40690. [Google Scholar] [CrossRef]
- Anjum, R.; Palanimuthu, D.; Kalinowski, D.S.; Lewis, W.; Park, K.C.; Kovacevic, Z.; Khan, I.U.; Richardson, D.R. Synthesis, Characterization, and in Vitro Anticancer Activity of Copper and Zinc Bis(Thiosemicarbazone) Complexes. Inorg. Chem. 2019, 58, 13709–13723. [Google Scholar] [CrossRef]
- Manono, J.; Marzilli, P.A.; Marzilli, L.G. New Porphyrins Bearing Positively Charged Peripheral Groups Linked by a Sulfonamide Group to meso-Tetraphenylporphyrin: Interactions with Calf Thymus DNA. Inorg. Chem. 2009, 48, 5636–5647. [Google Scholar] [CrossRef]
- Ghimire, S.; Fanwick, P.E.; McMillin, D.R. DNA-Binding Studies of a Tetraalkyl-Substituted Porphyrin and the Mutually Adaptive Distortion Principle. Inorg. Chem. 2014, 53, 11108–11118. [Google Scholar] [CrossRef]
- Luo, J.; Chen, L.-F.; Hu, P.; Chen, Z.-N. Tetranuclear Gadolinium(III) Porphyrin Complex as a Theranostic Agent for Multimodal Imaging and Photodynamic Therapy. Inorg. Chem. 2014, 53, 4184–4191. [Google Scholar] [CrossRef]
- Králová, J.; Kejík, Z.; Bříza, T.; Poučková, P.; Král, A.; Martásek, P.; Král, V. Porphyrin−Cyclodextrin Conjugates as a Nanosystem for Versatile Drug Delivery and Multimodal Cancer Therapy. J. Med. Chem. 2010, 53, 128–138. [Google Scholar] [CrossRef]
- Thomas, A.P.; Babu, P.S.S.; Nair, S.A.; Ramakrishnan, S.; Ramaiah, D.; Chandrashekar, T.K.; Srinivasan, A.; Pillai, M.R. meso-Tetrakis(p-sulfonatophenyl)N-Confused Porphyrin Tetrasodium Salt: A Potential Sensitizer for Photodynamic Therapy. J. Med. Chem. 2012, 55, 5110–5120. [Google Scholar] [CrossRef]
- Ahmed, A.; Omar, W.A.; El-Asmy, H.A.; Abou-Zeid, L.; Fadda, A.A. Docking studies, antitumor and antioxidant evaluation of newly synthesized porphyrin and metalloporphyrin derivatives. Dye. Pigment. 2020, 183, 108728. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Q.; Liu, H.; Wang, G.; Liu, J. DNA binding and in vitro antineoplastic activity of neotype water-soluble Cu(Ⅱ)-complexes based on fluorinated benzoylhydrazone porphyrin ligands. Dye. Pigment. 2019, 163, 647–655. [Google Scholar] [CrossRef]
- Hou, B.; Li, Z.; Zhang, Q.; Chen, P.; Liu, J. Novel water-soluble Cu(ii) complexes based on acylhydrazone porphyrin ligands for DNA binding and in vitro anticancer activity as potential therapeutic targeting candidates. New J. Chem. 2020, 44, 15387–15395. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Lee, C.; Kim, S.K.; Jeoung, S.C. Interaction of Metallo- and free base meso-tetrakis(N-methylpyridium-4-yl)porphyrin with a G-quadruplex: Effect of the central metal ions. Biophys. Chem. 2014, 190–191, 17–24. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Liu, J. Applying Cu(II) complexes assisted by water-soluble porphyrin to DNA binding and selective anticancer activities. Appl. Organomet. Chem. 2020, 34, e5857. [Google Scholar] [CrossRef]
- Gharehdaghi, Z.; Rahimi, R.; Naghib, S.M.; Molaabasi, F. Cu (II)-porphyrin metal-organic framework/graphene oxide: Synthesis, characterization, and application as a pH-responsive drug carrier for breast cancer treatment. J. Biol. Inorg. Chem. 2021, 26, 689–704. [Google Scholar] [CrossRef] [PubMed]
- Krasnovskaya, O.O.; Guk, D.A.; Naumov, A.E.; Nikitina, V.N.; Semkina, A.S.; Vlasova, K.Y.; Pokrovsky, V.; Ryabaya, O.O.; Karshieva, S.S.; Skvortsov, D.A.; et al. Novel Copper-Containing Cytotoxic Agents Based on 2-Thioxoimidazolones. J. Med. Chem. 2020, 63, 13031–13063. [Google Scholar] [CrossRef]
- Prosser, K.E.; Chang, S.W.; Saraci, F.; Le, P.H.; Walsby, C.J. Anticancer copper pyridine benzimidazole complexes: ROS generation, biomolecule interactions, and cytotoxicity. J. Inorg. Biochem. 2017, 167, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Chorbu, A.A.; Barskaya, E.S.; Moiseeva, A.A.; Guk, D.A.; Krasnovskaya, O.O.; Lyssenko, K.A.; Rzheutski, A.V.; Abramovich, M.S.; Polyakova, M.N.; Berezina, A.V.; et al. Ditopic pyridyl-benzothiazole—Pyridylmethylene-2-thiohydantoin conjugates: Synthesis and study in complexation with CuCl2. Polyhedron 2022, 221, 115838. [Google Scholar] [CrossRef]
- Krasnovskaya, O.O.; Abramchuk, D.; Vaneev, A.; Gorelkin, P.; Abakumov, M.; Timoshenko, R.; Chmelyuk, N.; Vadehina, V.; Kuanaeva, R.; Dubrovin, E.V.; et al. Aβ-affine bifunctional copper chelators capable of Aβ-induced oxidative stress reduction and amyloid disaggregation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Ol’Shevskaya, V.A.; Alpatova, V.M.; Radchenko, A.S.; Ramonova, A.A.; Petrova, A.S.; Tatarskiy, V.V.; Zaitsev, A.V.; Kononova, E.G.; Ikonnikov, N.S.; Kostyukov, A.A.; et al. β-Maleimide substituted meso-arylporphyrins: Synthesis, transformations, physico-chemical and antitumor properties. Dye. Pigment. 2019, 171, 107760. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, N. Spectral and thermal studies with anti-fungal aspects of some organotin(IV) complexes with nitrogen and sulphur donor ligands derived from 2-phenylethylamine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 669–675. [Google Scholar] [CrossRef]
- Shakya, B.; Shahi, N.; Ahmad, F.; Yadav, P.N.; Pokharel, Y.R. 2-Pyridineformamide N(4)-ring incorporated thiosemicarbazones inhibit MCF-7 cells by inhibiting JNK pathway. Bioorganic Med. Chem. Lett. 2019, 29, 1677–1681. [Google Scholar] [CrossRef]
- Gao, F.; Zhong, X.; Wang, Q.; Li, H.; Zhang, S. Excited State Intramolecular Proton Transfer of New Diphenyl- ethylene Derivatives Bearing Imino Group: A Combination of Experimental and Theoretical Investigation. Chin. J. Chem. 2010, 28, 1057–1068. [Google Scholar] [CrossRef]
- Holland, J.P.; Aigbirhio, F.I.; Betts, H.M.; Bonnitcha, P.D.; Burke, P.; Christlieb, M.; Churchill, G.C.; Cowley, A.R.; Dilworth, J.R.; Donnelly, P.S.; et al. Functionalized Bis(thiosemicarbazonato) Complexes of Zinc and Copper: Synthetic Platforms Toward Site-Specific Radiopharmaceuticals. Inorg. Chem. 2007, 46, 465–485. [Google Scholar] [CrossRef]
- Brandt, T.A.; Caron, S.; Damon, D.B.; DiBrino, J.; Ghosh, A.; Griffith, D.A.; Kedia, S.; Ragan, J.A.; Rose, P.R.; Vanderplas, B.C.; et al. Development of two synthetic routes to CE-178,253, a CB1 antagonist for the treatment of obesity. Tetrahedron 2009, 65, 3292–3304. [Google Scholar] [CrossRef]
- Hałdys, K.; Goldeman, W.; Jewgiński, M.; Wolińska, E.; Anger, N.; Rossowska, J.; Latajka, R. Inhibitory properties of aromatic thiosemicarbazones on mushroom tyrosinase: Synthesis, kinetic studies, molecular docking and effectiveness in melanogenesis inhibition. Bioorganic Chem. 2018, 81, 577–586. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, N. Organotin(IV) complexes of thiohydrazides and thiodiamines: Synthesis, spectral and thermal studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 65, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Makino, I.; Hirai, K. Activation of dithiocarbamate by 2-halothiazolium salts. J. Org. Chem. 1988, 53, 2263–2267. [Google Scholar] [CrossRef]
- Zelenin, K.N.; Kuznetsova, O.B.; Alekseev, V.V. Synthesis of derivatives of 1,2,4-triazin-3-thione and 5-amino-2-acyl-2,3-dihydro-1,3,4-thiadiazolium salts from 1,2-dicarbonyl compounds and 4-substituted thiosemicarbazides. Chem. Heterocycl. Compd. 1992, 28, 1211–1218. [Google Scholar] [CrossRef]
- Hickey, J.L.; Lim, S.; Hayne, D.J.; Paterson, B.M.; White, J.M.; Villemagne, V.L.; Roselt, P.; Binns, D.; Cullinane, C.; Jeffery, C.M.; et al. Diagnostic Imaging Agents for Alzheimer’s Disease: Copper Radiopharmaceuticals that Target Aβ Plaques. J. Am. Chem. Soc. 2013, 135, 16120–16132. [Google Scholar] [CrossRef]
- McInnes, L.E.; Noor, A.; Roselt, P.D.; McLean, C.A.; White, J.M.; Donnelly, P.S. A Copper Complex of a Thiosemicarbazone-Pyridylhydrazone Ligand Containing a Vinylpyridine Functional Group as a Potential Imaging Agent for Amyloid-β Plaques. Aust. J. Chem. 2019, 72, 827–834. [Google Scholar] [CrossRef]
- Du, J.; Yang, H.; Wang, C.-L.; Zhan, S.-Z. Synthesis, structure, characterization, EPR investigation and catalytic behavior for hydrogen evolution of a bis(thiosemicarbazonato)-palladium complex. Polyhedron 2021, 208, 115426. [Google Scholar] [CrossRef]
- Holland, J.P.; Barnard, P.J.; Bayly, S.R.; Betts, H.M.; Churchill, G.C.; Dilworth, J.R.; Edge, R.; Green, J.C.; Hueting, R. Synthesis, Radiolabelling and Confocal Fluorescence Microscopy of Styrene-Derivatised Bis(thiosemicarbazonato)zinc and -copper Complexes. Eur. J. Inorg. Chem. 2008, 12, 1985–1993. [Google Scholar] [CrossRef]
- Noor, A.; Hayne, D.J.; Lim, S.; Van Zuylekom, J.K.; Cullinane, C.; Roselt, P.D.; McLean, C.A.; White, J.M.; Donnelly, P.S. Copper Bis(thiosemicarbazonato)-stilbenyl Complexes That Bind to Amyloid-β Plaques. Inorg. Chem. 2020, 59, 11658–11669. [Google Scholar] [CrossRef]
- Harder, T.; Wessig, P.; Bendig, J.; Stösser, R. Photochemical Reactions of Nitroso Oxides at Low Temperatures: The First Experimental Evidence for Dioxaziridines. J. Am. Chem. Soc. 1999, 121, 6580–6588. [Google Scholar] [CrossRef]
- Xie, D.; King, T.L.; Banerjee, A.; Kohli, V.; Que, E.L. Exploiting Copper Redox for 19F Magnetic Resonance-Based Detection of Cellular Hypoxia. J. Am. Chem. Soc. 2016, 138, 2937–2940. [Google Scholar] [CrossRef] [PubMed]
- Bocokić, V.; Lutz, M.; Spek, A.L.; Reek, J.N.H. Bis-(thiosemicarbazonato) Zn(ii) complexes as building blocks for construction of supramolecular catalysts. Dalton Trans. 2012, 41, 3740–3750. [Google Scholar] [CrossRef] [PubMed]
- Paterson, B.M.; Cullinane, C.; Crouch, P.J.; White, A.R.; Barnham, K.J.; Roselt, P.D.; Noonan, W.; Binns, D.; Hicks, R.J.; Donnelly, P.S. Modification of Biodistribution and Brain Uptake of Copper Bis(thiosemicarbazonato) Complexes by the Incorporation of Amine and Polyamine Functional Groups. Inorg. Chem. 2019, 58, 4540–4552. [Google Scholar] [CrossRef]
- Floch, L.; Kováč, Š. Synthesis and properties of alkyl isothiocyanatocarboxylates. Czech. Chem. Commun. 1975, 40, 2845–2854. [Google Scholar] [CrossRef]
- Kim, T.; Kim, Y.-J.; Han, I.-H.; Lee, D.; Ham, J.; Kang, K.S.; Lee, J.W. The synthesis of sulforaphane analogues and their protection effect against cisplatin induced cytotoxicity in kidney cells. Bioorganic Med. Chem. Lett. 2015, 25, 62–66. [Google Scholar] [CrossRef]
- Papadakis, M.; Barrozo, A.; Straistari, T.; Queyriaux, N.; Putri, A.; Fize, J.; Giorgi, M.; Réglier, M.; Massin, J.; Hardré, R.; et al. Ligand-based electronic effects on the electrocatalytic hydrogen production by thiosemicarbazone nickel complexes. Dalton Trans. 2020, 49, 5064–5073. [Google Scholar] [CrossRef]
- Guk, D.; Naumov, A.; Krasnovskaya, O.; Tafeenko, V.; Moiseeeva, A.; Pergushov, V.; Melnikov, M.; Zyk, N.; Majouga, A.; Belolglazkina, E. Three types of copper derivatives formed by CuCl2·2H2O interaction with (Z)-3-aryl-2-(methylthio)-5-(pyridine-2-ylmethylene)-3,5-dihydro-4H-imidazol-4-ones. Dalton Trans. 2020, 49, 14528–14535. [Google Scholar] [CrossRef]
- Barus, C.; Gros, P.; Comtat, M.; Daunes-Marion, S.; Tarroux, R. Electrochemical behaviour of N-acetyl-l-cysteine on gold electrode—A tentative reaction mechanism. Electrochim. Acta 2007, 52, 7978–7985. [Google Scholar] [CrossRef]
- Gao, Z.-N.; Zhang, J.; Liu, W.-Y. Electrocatalytic oxidation of N-acetyl-l-cysteine by acetylferrocene at glassy carbon electrode. J. Electroanal. Chem. 2005, 580, 9–16. [Google Scholar] [CrossRef]
- Seebacher, N.; Lane, D.J.; Richardson, D.R.; Jansson, P.J. Turning the gun on cancer: Utilizing lysosomal P-glycoprotein as a new strategy to overcome multi-drug resistance. Free Radic. Biol. Med. 2016, 96, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Ol’Shevskaya, V.A.; Zaitsev, A.V.; Petrova, A.S.; Arkhipova, A.Y.; Moisenovich, M.M.; Kostyukov, A.A.; Egorov, A.E.; Koroleva, O.A.; Golovina, G.V.; Volodina, Y.L.; et al. The synthetic fluorinated tetracarboranylchlorin as a versatile antitumor photoradiosensitizer. Dye. Pigment. 2020, 186, 108993. [Google Scholar] [CrossRef]
- Baghy, K.; Ladányi, A.; Reszegi, A.; Kovalszky, I. Insights into the tumor microenvironment-components. Int. J. Mol. Sci. 2023, 24, 17536. [Google Scholar] [CrossRef] [PubMed]
- Mavatkar, A.D.; Naidu, C.M.; Prabhu, J.S.; Nair, M.G. The dynamic tumor-stromal crosstalk: Implications of ‘stromal-hot’ tumors in the process of epithelial-mesenchymal transition in breast cancer. Mol. Biol. Rep. 2023, 50, 5379–5393. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
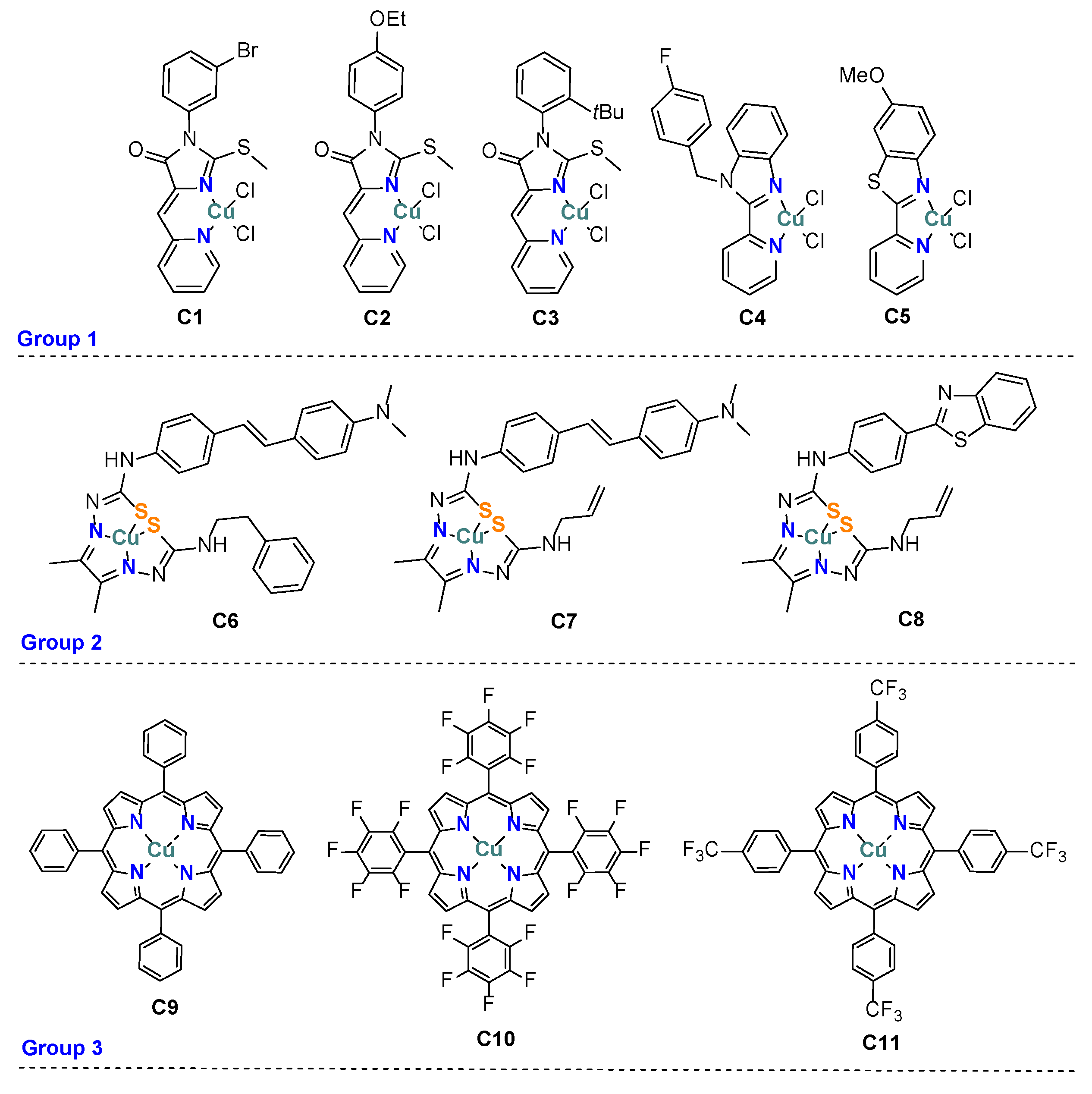

| Comp. | ERed, V | E1/2, V | |
|---|---|---|---|
| Group 1 | C1 | 0.33/0.54 | ND |
| C2 | 0.42/0.55 | 0.49 | |
| C3 | 0.37/0.55 | 0.43 | |
| C4 | ND | 0.33 | |
| C5 | 0.34/0.52 | ND | |
| Group 2 | C6 | −0.58/−0.48 | −0.49 |
| C7 | −0.57/−0.50 | −0.53 | |
| C8 | −0.52/−0.46 | −0.50 | |
| Group 3 | C9 | −1.08/−1.16 | −1.12 |
| C10 | −0.79/−0.85 | −0.82 | |
| C11 | −1.05/−0.97 | −1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beloglazkina, E.K.; Moiseeva, A.A.; Tsymbal, S.A.; Guk, D.A.; Kuzmin, M.A.; Krasnovskaya, O.O.; Borisov, R.S.; Barskaya, E.S.; Tafeenko, V.A.; Alpatova, V.M.; et al. The Copper Reduction Potential Determines the Reductive Cytotoxicity: Relevance to the Design of Metal–Organic Antitumor Drugs. Molecules 2024, 29, 1032. https://doi.org/10.3390/molecules29051032
Beloglazkina EK, Moiseeva AA, Tsymbal SA, Guk DA, Kuzmin MA, Krasnovskaya OO, Borisov RS, Barskaya ES, Tafeenko VA, Alpatova VM, et al. The Copper Reduction Potential Determines the Reductive Cytotoxicity: Relevance to the Design of Metal–Organic Antitumor Drugs. Molecules. 2024; 29(5):1032. https://doi.org/10.3390/molecules29051032
Chicago/Turabian StyleBeloglazkina, Elena K., Anna A. Moiseeva, Sergey A. Tsymbal, Dmitry A. Guk, Mikhail A. Kuzmin, Olga O. Krasnovskaya, Roman S. Borisov, Elena S. Barskaya, Victor A. Tafeenko, Victoria M. Alpatova, and et al. 2024. "The Copper Reduction Potential Determines the Reductive Cytotoxicity: Relevance to the Design of Metal–Organic Antitumor Drugs" Molecules 29, no. 5: 1032. https://doi.org/10.3390/molecules29051032
APA StyleBeloglazkina, E. K., Moiseeva, A. A., Tsymbal, S. A., Guk, D. A., Kuzmin, M. A., Krasnovskaya, O. O., Borisov, R. S., Barskaya, E. S., Tafeenko, V. A., Alpatova, V. M., Zaitsev, A. V., Finko, A. V., Ol’shevskaya, V. A., & Shtil, A. A. (2024). The Copper Reduction Potential Determines the Reductive Cytotoxicity: Relevance to the Design of Metal–Organic Antitumor Drugs. Molecules, 29(5), 1032. https://doi.org/10.3390/molecules29051032







