Recent Advances in Structural Optimization of Quinazoline-Based Protein Kinase Inhibitors for Cancer Therapy (2021–Present)
Abstract
1. Introduction
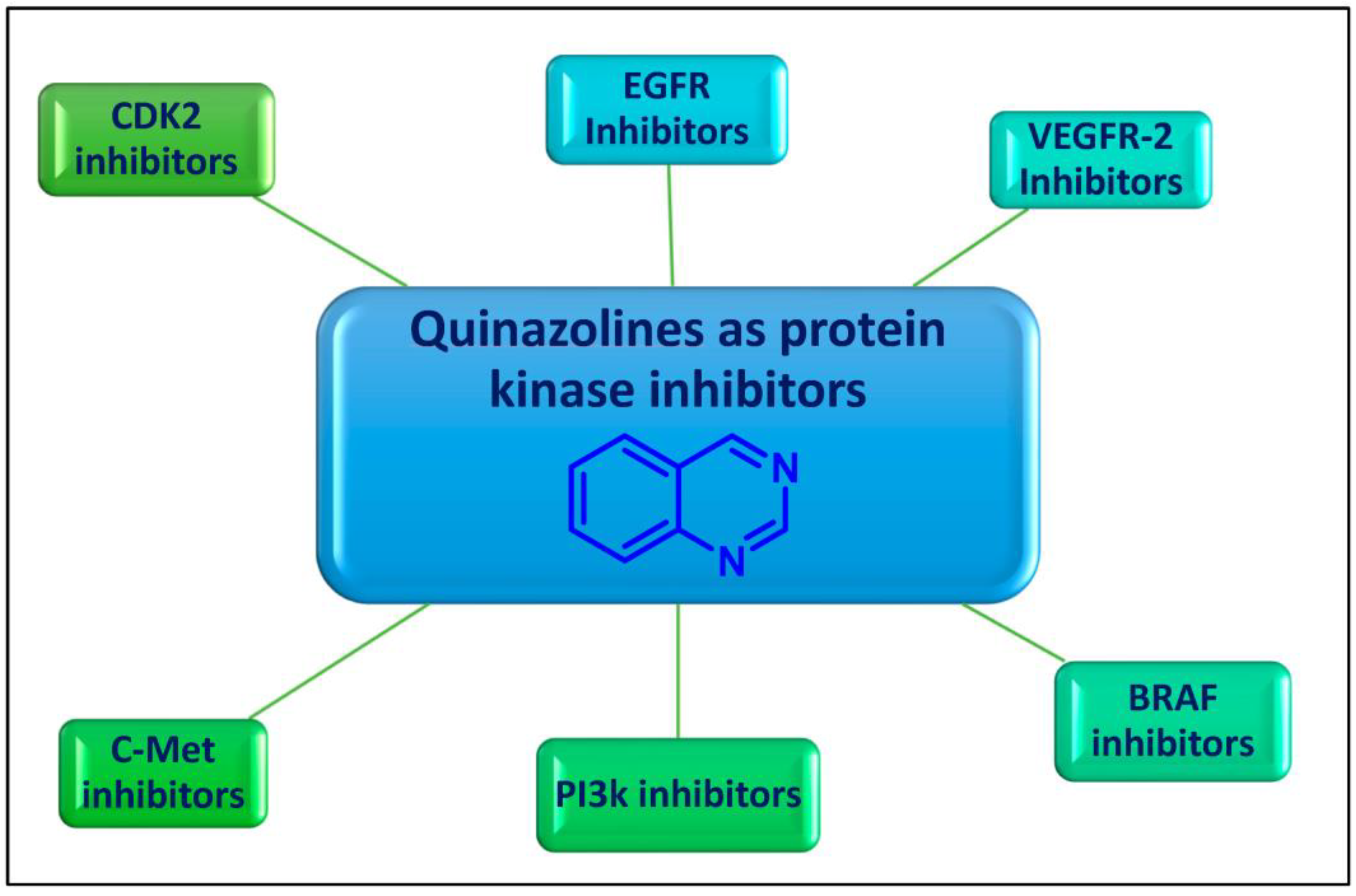
2. Quinazolines as Protein Kinases Inhibitors
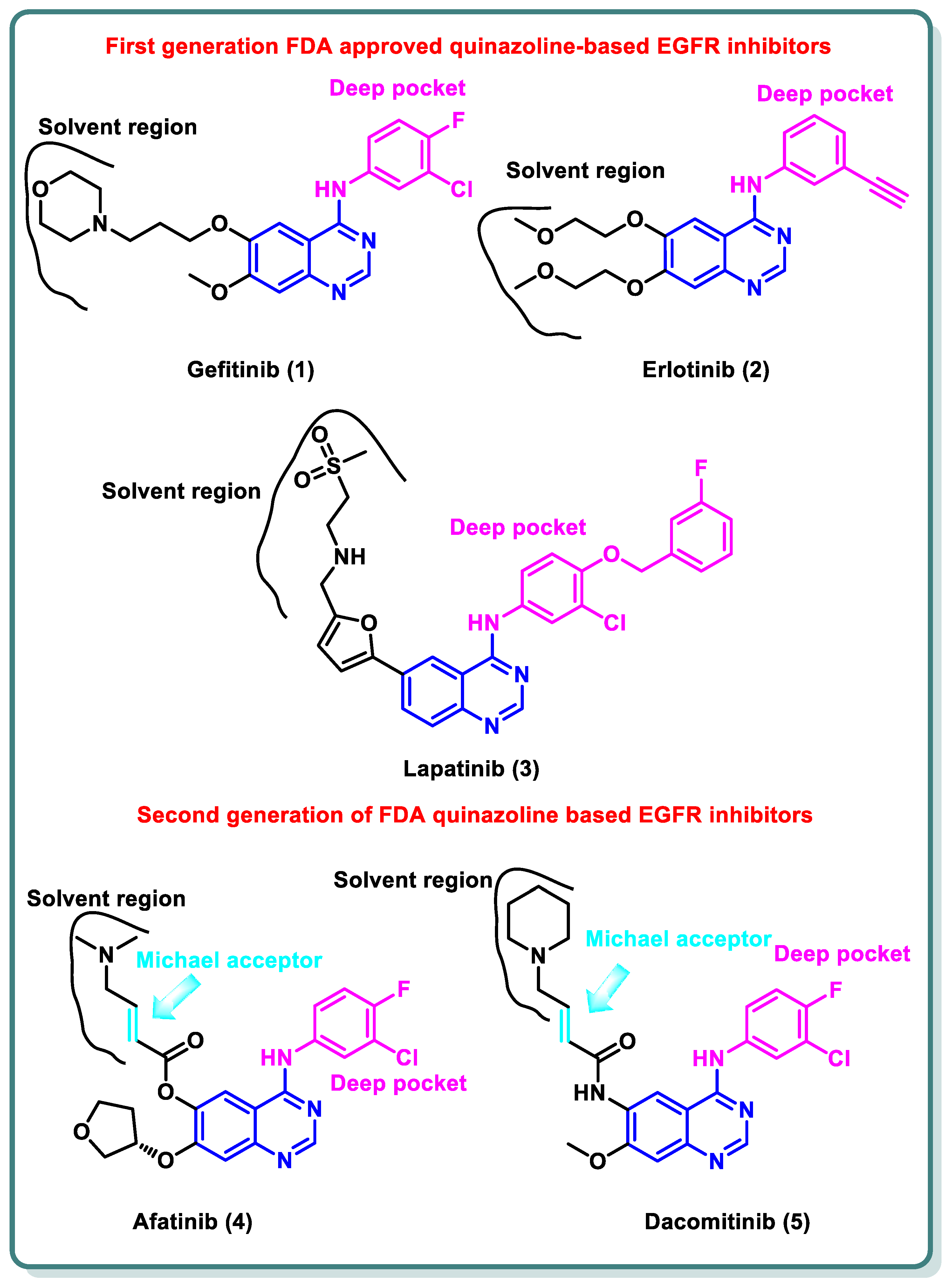
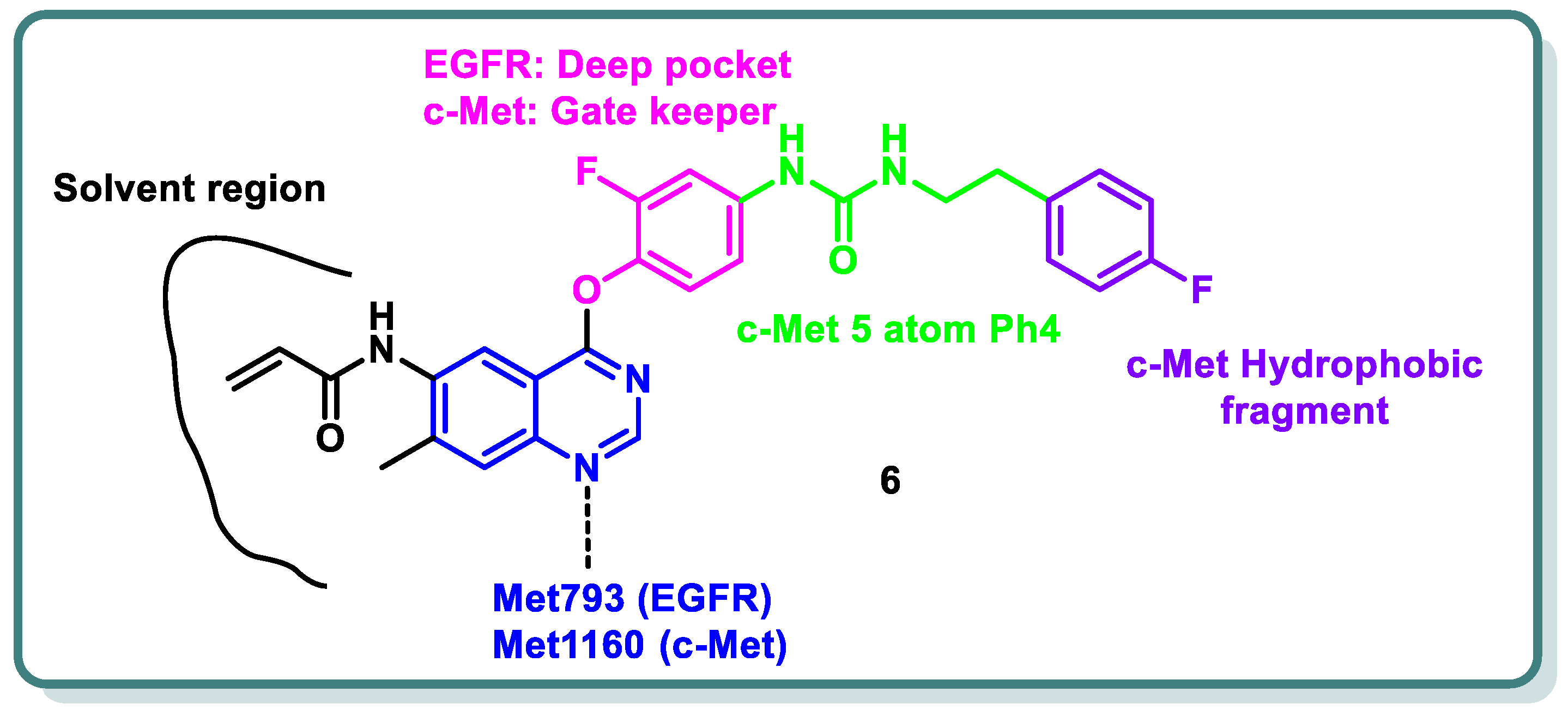
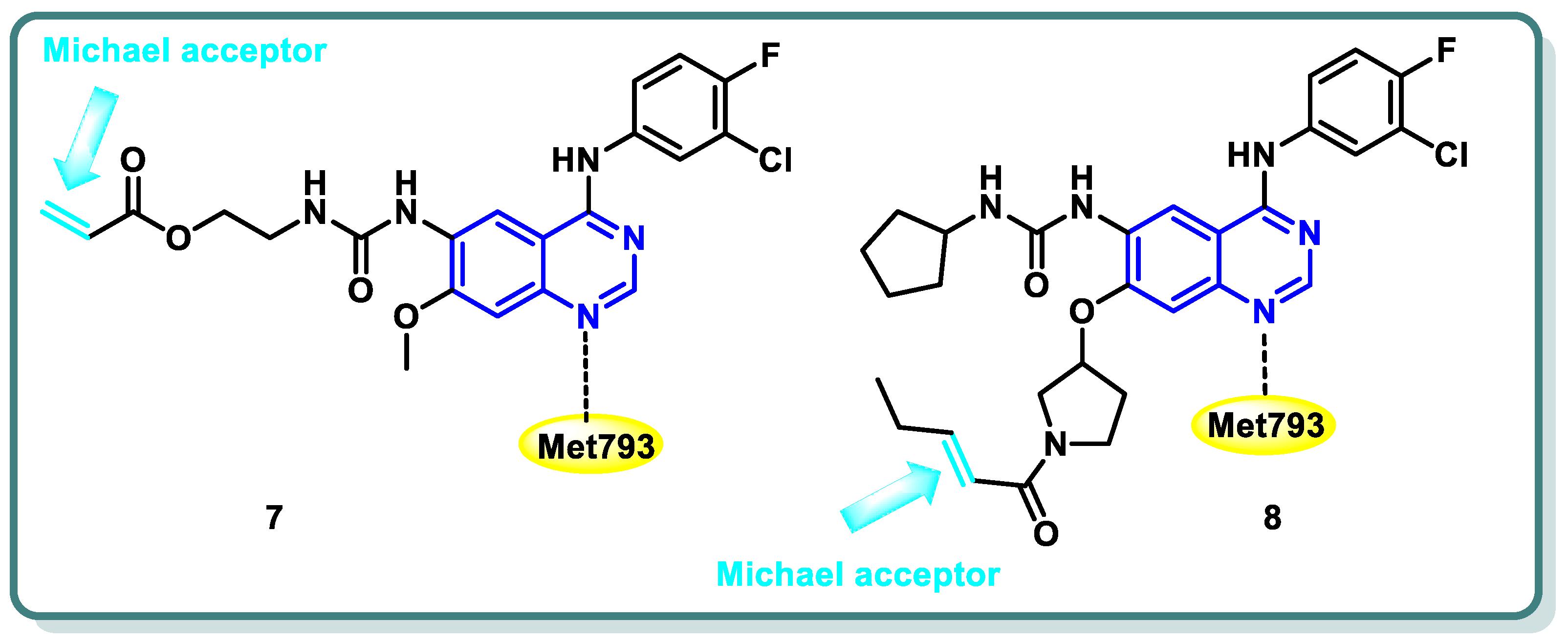
2.1. Epidermal Growth Factor Receptor (EGFR) Inhibitors
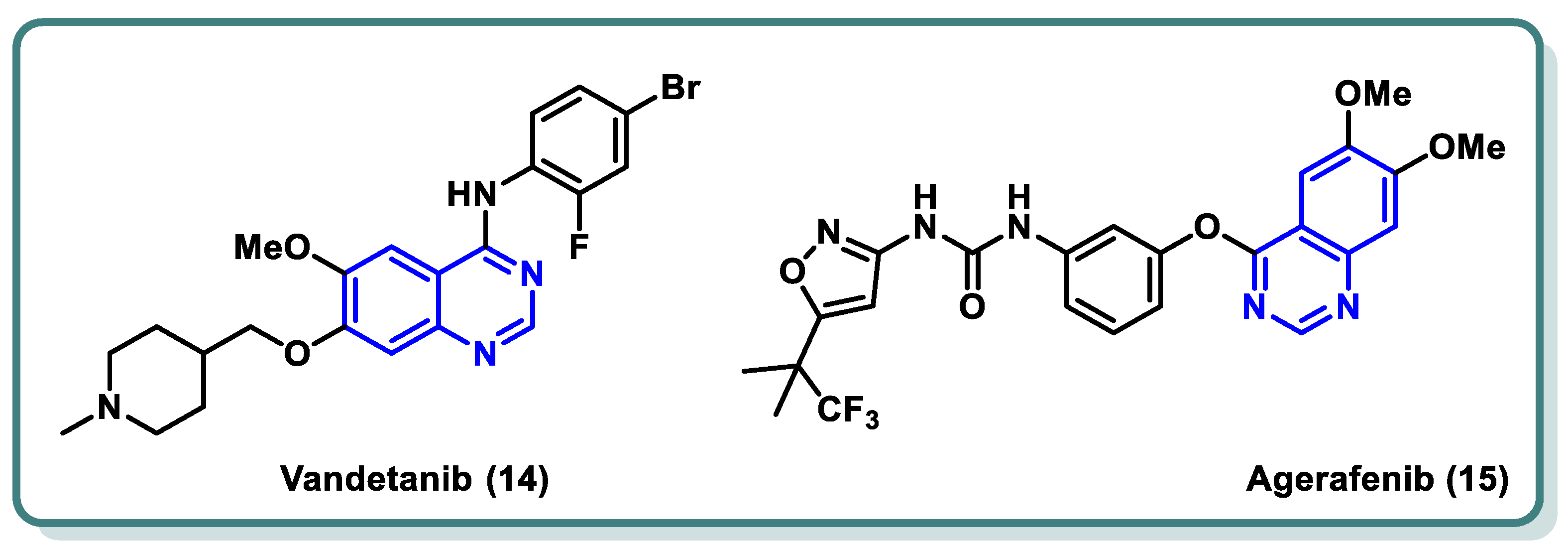
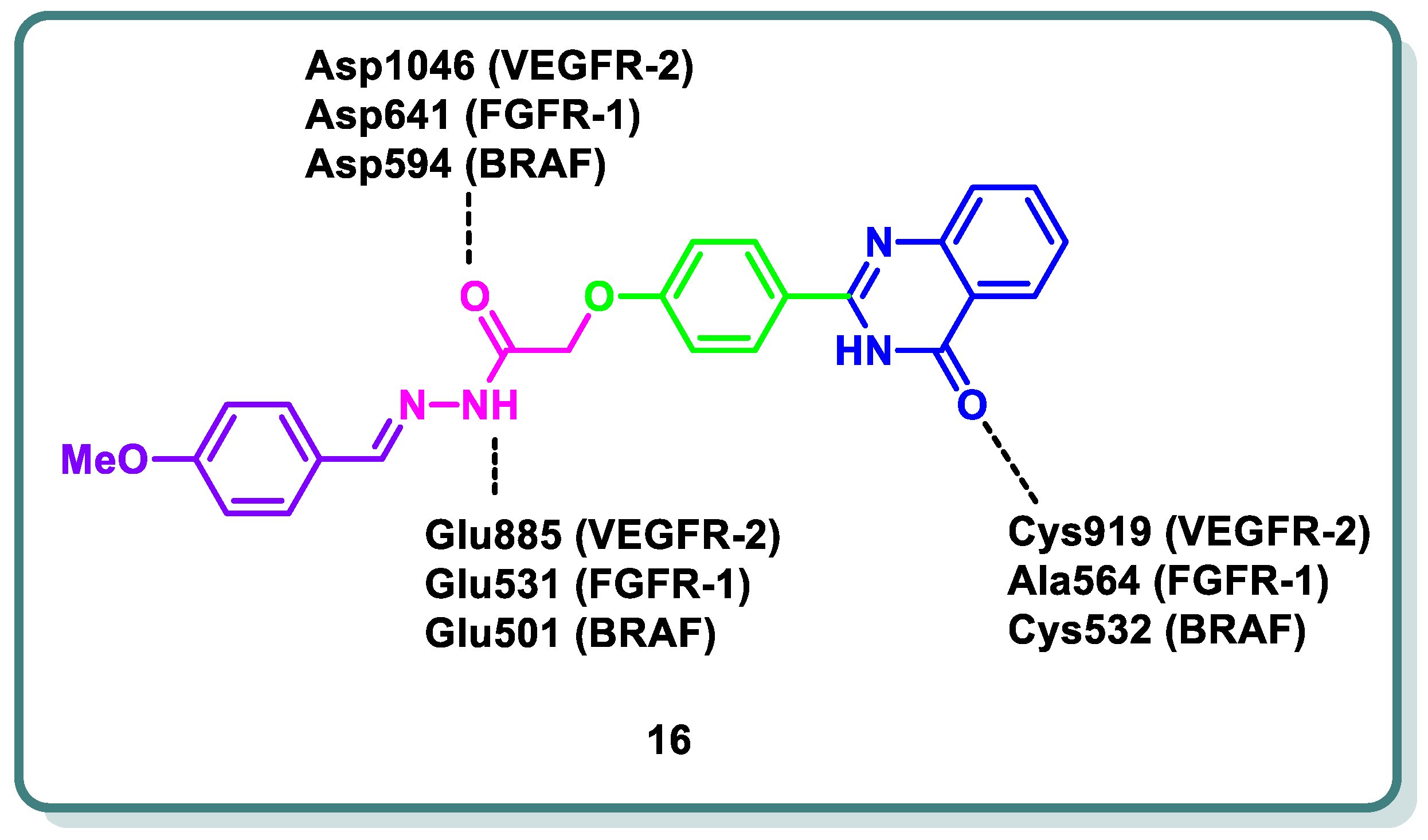
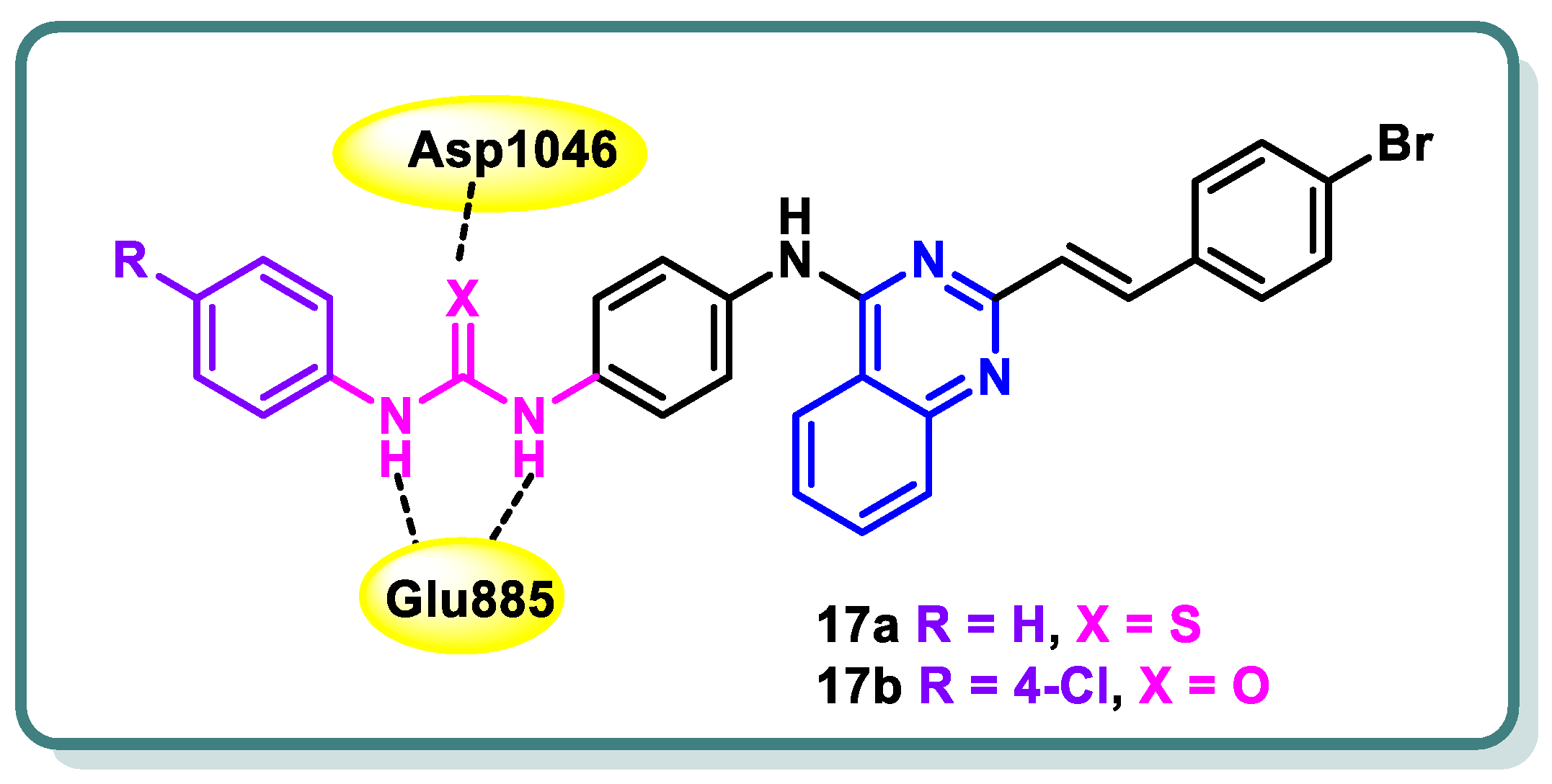
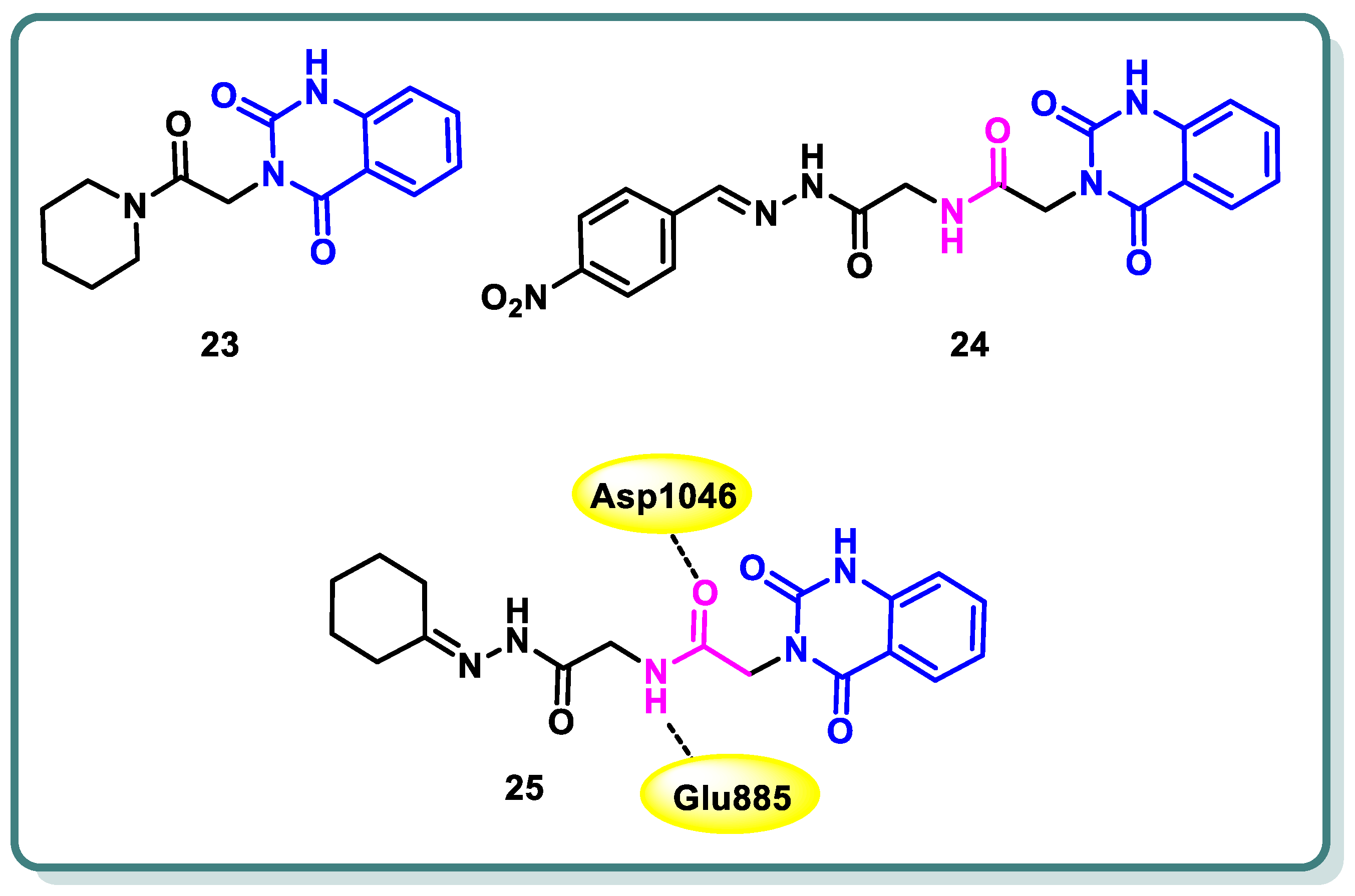
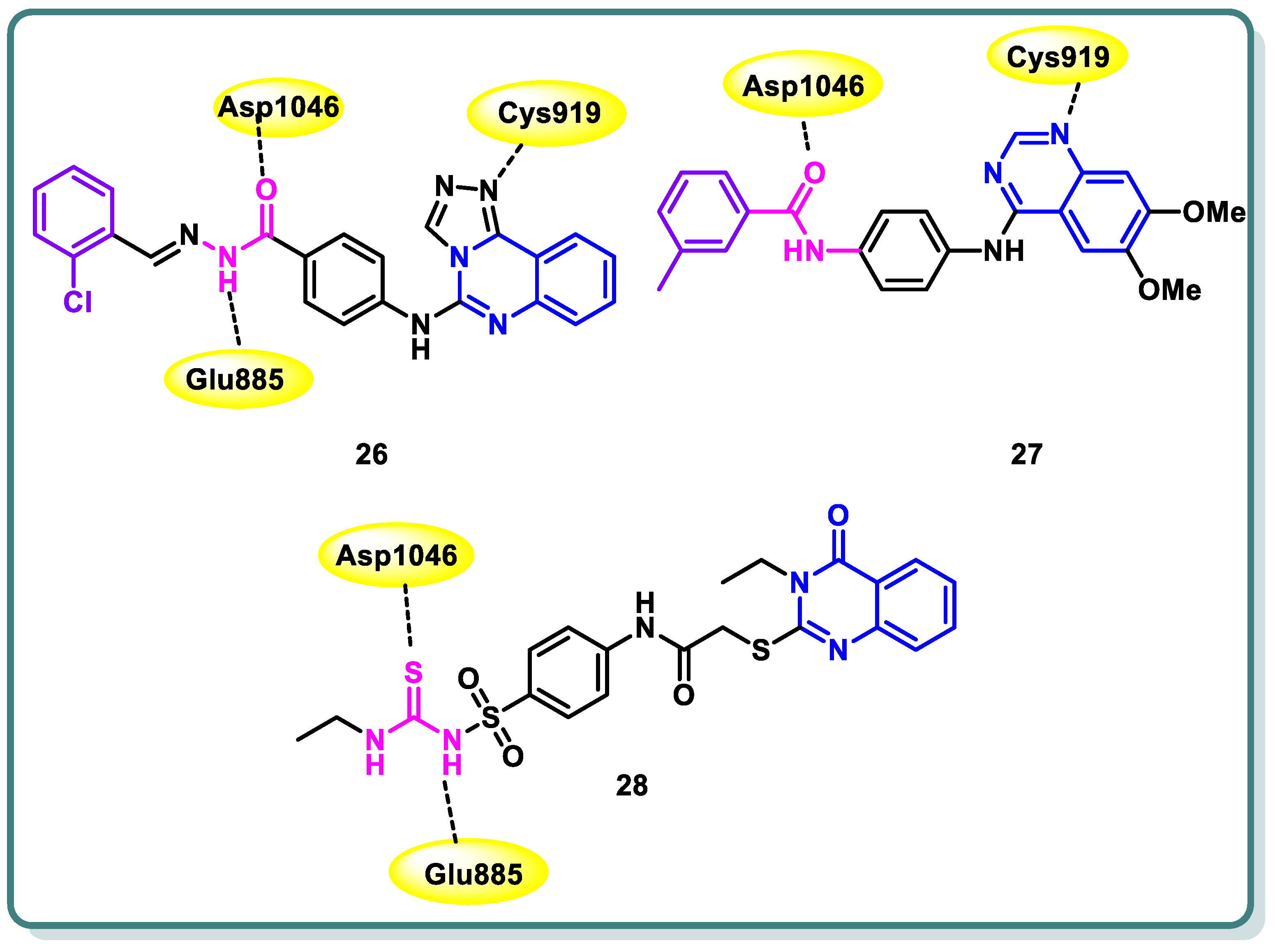
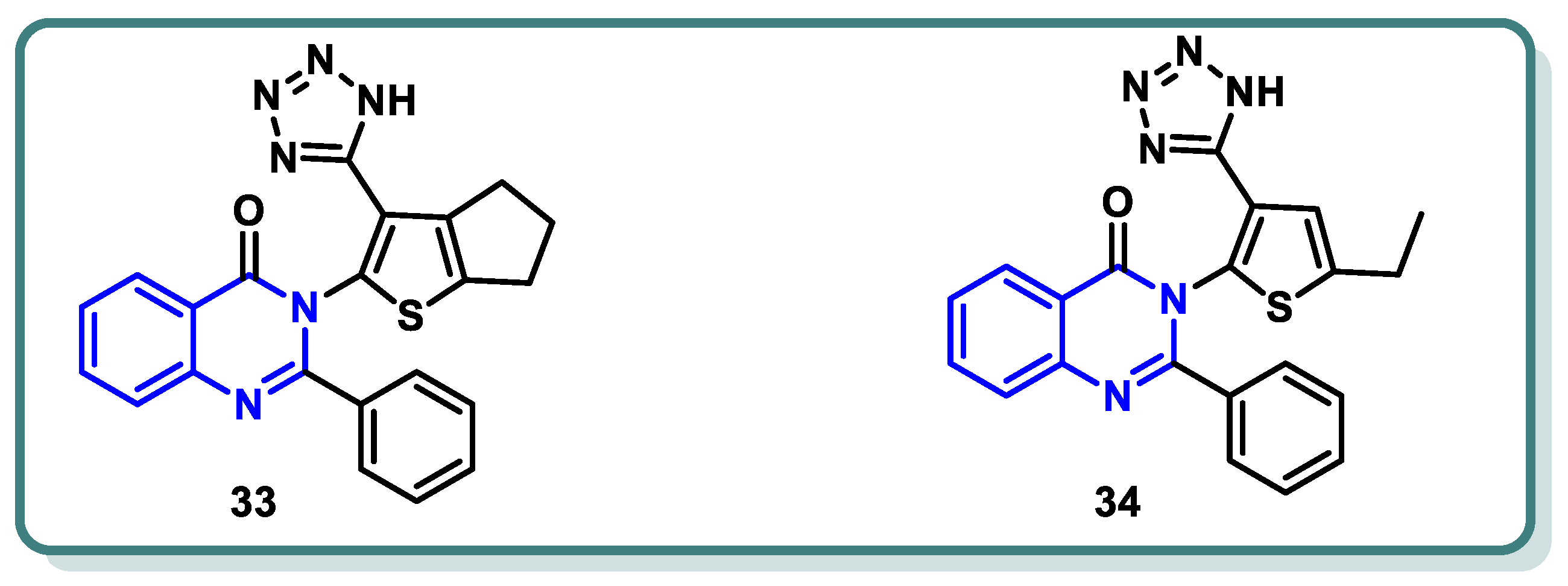
2.2. Vascular Endothelia Growth Factor Receptor (VEGFR) Inhibitors
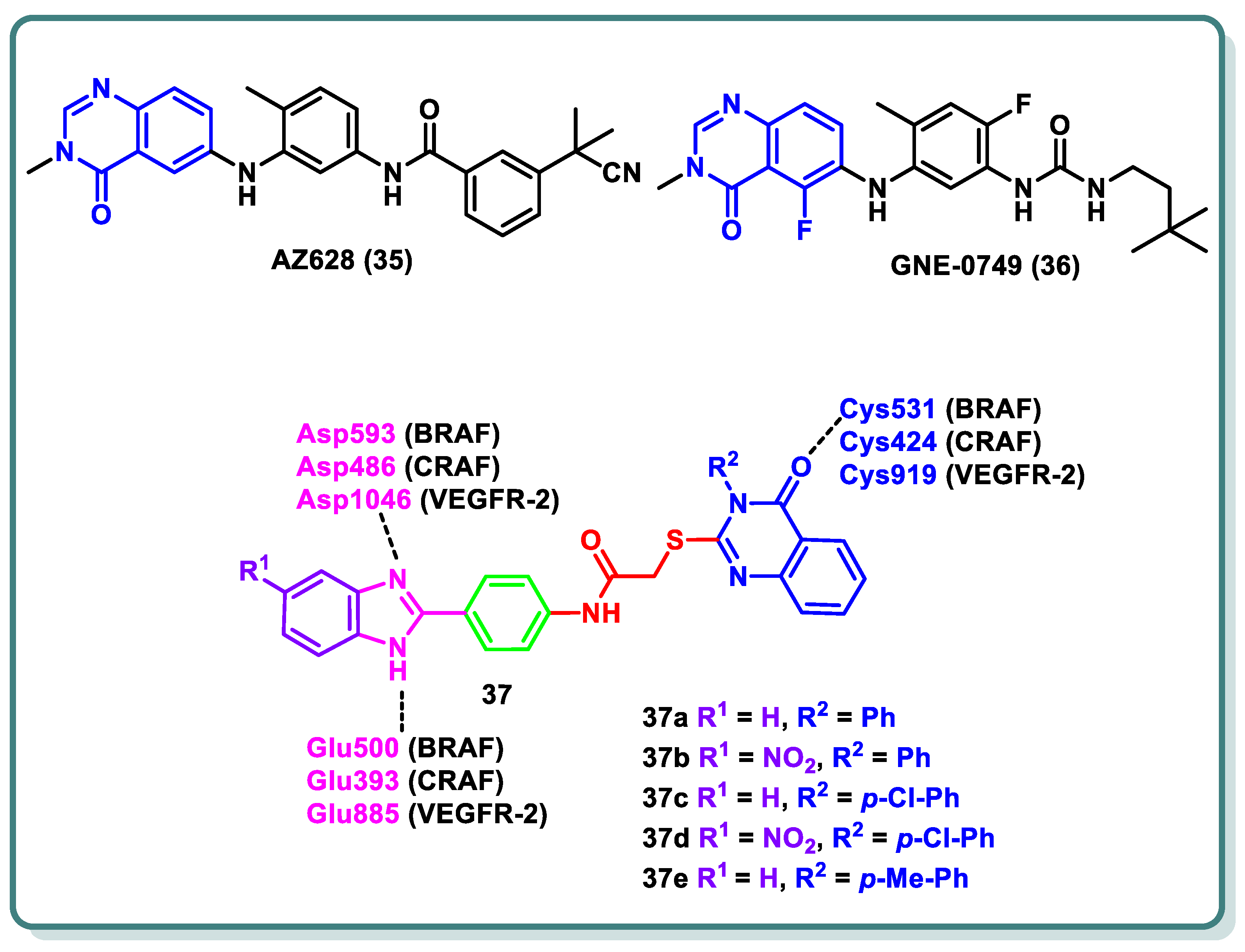
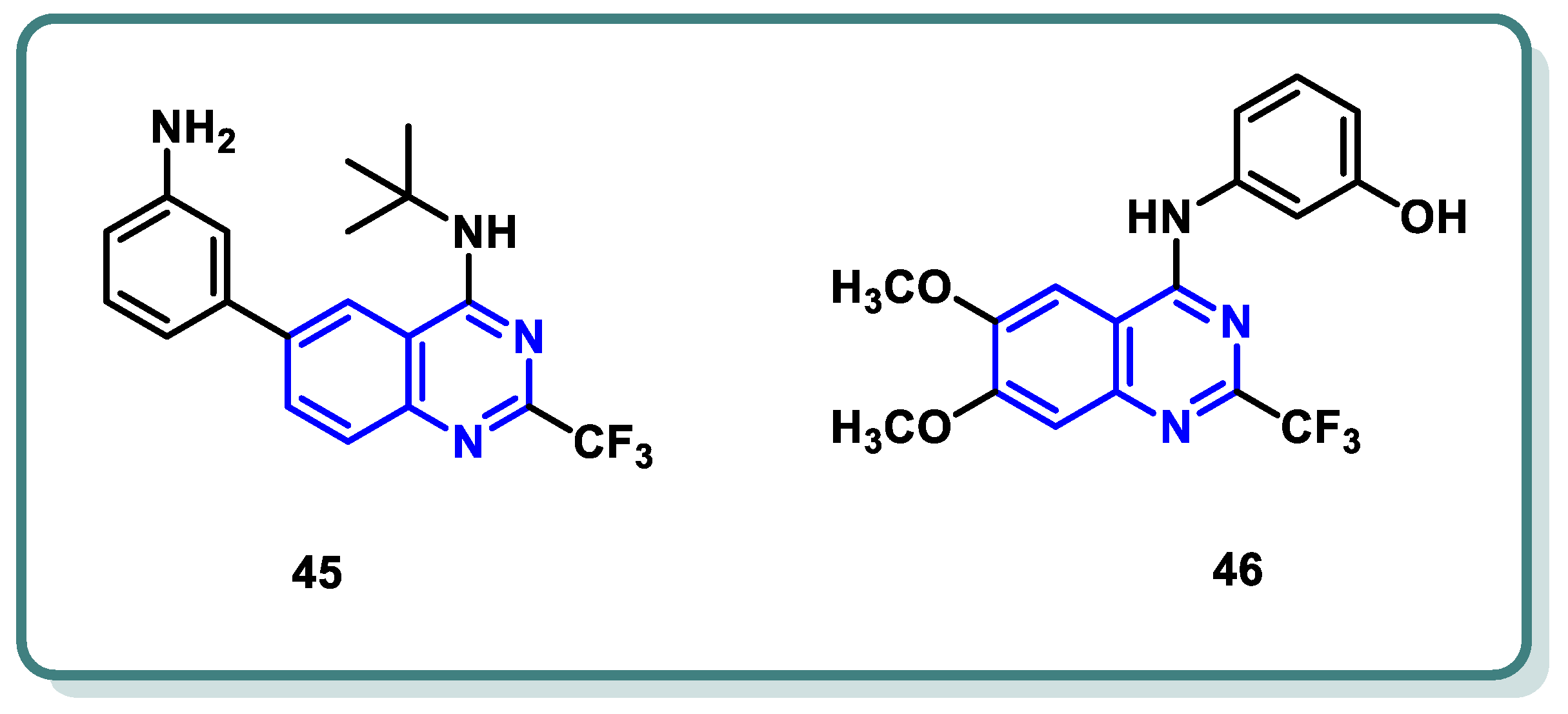
2.3. Rapidly Accelerated Fibrosarcoma (RAF) Inhibitors
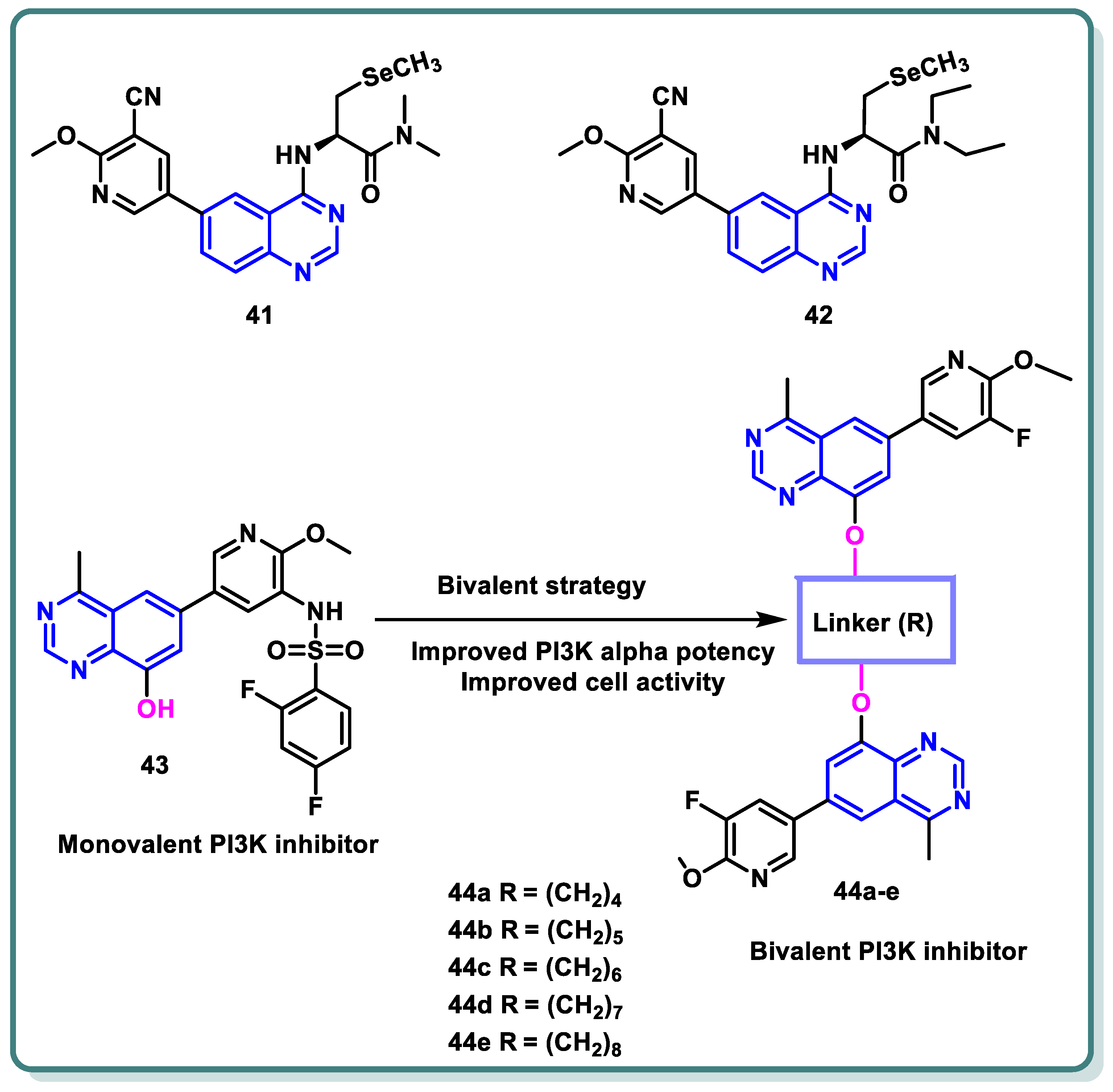
2.4. Phosphoinositide 3-Kinase (PI3K) Inhibitors
2.5. Cyclin-Dependent Kinase (CDK) Inhibitors
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Singh, D.; Vaccarella, S.; Gini, A.; De Paula Silva, N.; Steliarova-Foucher, E.; Bray, F. Global patterns of Hodgkin lymphoma incidence and mortality in 2020 and a prediction of the future burden in 2040. Int. J. Cancer 2022, 150, 1941–1947. [Google Scholar] [CrossRef]
- Shenoy, G.P.; Pal, R.; Purwarga Matada, G.S.; Singh, E.; Raghavendra, N.M.; Dhiwar, P.S. Discoidin domain receptor inhibitors as anticancer agents: A systematic review on recent development of DDRs inhibitors, their resistance and structure activity relationship. Bioorg. Chem. 2023, 130, 106215. [Google Scholar] [CrossRef]
- Giaquinto, A.N.; Miller, K.D.; Tossas, K.Y.; Winn, R.A.; Jemal, A.; Siegel, R.L. Cancer statistics for African American/Black People 2022. CA Cancer J. Clin. 2022, 72, 202–229. [Google Scholar] [CrossRef]
- Cohen, P. The role of protein phosphorylation in human health and disease. The Sir Hans Krebs Medal Lecture. Eur. J. Biochem. 2001, 268, 5001–5010. [Google Scholar] [CrossRef]
- Cohen, P. Protein kinases—The major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 2002, 1, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Lagaron, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Saeed, A. Chemical Insights into the Synthetic Chemistry of Quinazolines: Recent Advances. Front. Chem. 2020, 8, 594717. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohsen, H.T.; Petreni, A.; Supuran, C.T. Investigation of the carbonic anhydrase inhibitory activity of benzenesulfonamides incorporating substituted fused-pyrimidine tails. Arch. Pharm. 2022, 355, e2200274. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohsen, H.T.; Omar, M.A.; Petreni, A.; Supuran, C.T. Novel 2-substituted thioquinazoline-benzenesulfonamide derivatives as carbonic anhydrase inhibitors with potential anticancer activity. Arch. Pharm. 2022, 355, e2200180. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohsen, H.T.; El Kerdawy, A.M.; Petreni, A.; Supuran, C.T. Novel benzenesulfonamide-thiouracil conjugates with a flexible N-ethyl acetamide linker as selective CA IX and CA XII inhibitors. Arch. Pharm. 2023, 356, e2200434. [Google Scholar] [CrossRef] [PubMed]
- Wieduwilt, M.J.; Moasser, M.M. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell. Mol. Life Sci. CMLS 2008, 65, 1566–1584. [Google Scholar] [CrossRef] [PubMed]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef]
- Ranson, M. Epidermal growth factor receptor tyrosine kinase inhibitors. Br. J. Cancer 2004, 90, 2250–2255. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Teli, G.; Matada, G.S.P.; Dhiwar, P.S. Designing strategies, structural activity relationship and biological activity of recently developed nitrogen containing heterocyclic compounds as epidermal growth factor receptor tyrosinase inhibitors. J. Mol. Struct. 2023, 1291, 136021. [Google Scholar] [CrossRef]
- Thomas, P.; Vincent, B.; George, C.; Joshua, J.M.; Pavithran, K.; Vijayan, M. A comparative study on erlotinib & gefitinib therapy in non-small cell lung carcinoma patients. Indian J. Med. Res. 2019, 150, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ge, R.; Sang, D.; Luo, T.; Li, W.; Ji, X.; Yuan, P.; Wang, B. Real-world data of lapatinib and treatment after lapatinib in patients with previously treated HER2-positive metastatic breast cancer: A multicenter, retrospective study. Cancer Med. 2020, 9, 2981–2988. [Google Scholar] [CrossRef]
- Wood, E.R.; Truesdale, A.T.; McDonald, O.B.; Yuan, D.; Hassell, A.; Dickerson, S.H.; Ellis, B.; Pennisi, C.; Horne, E.; Lackey, K.; et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): Relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004, 64, 6652–6659. [Google Scholar] [CrossRef]
- Stamos, J.; Sliwkowski, M.X.; Eigenbrot, C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J. Biol. Chem. 2002, 277, 46265–46272. [Google Scholar] [CrossRef]
- Amelia, T.; Kartasasmita, R.E.; Ohwada, T.; Tjahjono, D.H. Structural Insight and Development of EGFR Tyrosine Kinase Inhibitors. Molecules 2022, 27, 819. [Google Scholar] [CrossRef]
- Sharma, S.V.; Bell, D.W.; Settleman, J.; Haber, D.A. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer 2007, 7, 169–181. [Google Scholar] [CrossRef]
- Pao, W.; Miller, V.A.; Politi, K.A.; Riely, G.J.; Somwar, R.; Zakowski, M.F.; Kris, M.G.; Varmus, H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005, 2, e73. [Google Scholar] [CrossRef]
- Wang, S.; Li, J. Second-generation EGFR and ErbB tyrosine kinase inhibitors as first-line treatments for non-small cell lung cancer. OncoTargets Ther. 2019, 12, 6535–6548. [Google Scholar] [CrossRef]
- Li, H.S.; Wang, S.Z.; Xu, H.Y.; Yan, X.; Zhang, J.Y.; Lei, S.Y.; Li, T.; Hao, X.Z.; Zhang, T.; Yang, G.J.; et al. Afatinib and Dacomitinib Efficacy, Safety, Progression Patterns, and Resistance Mechanisms in Patients with Non-Small Cell Lung Cancer Carrying Uncommon EGFR Mutations: A Comparative Cohort Study in China (AFANDA Study). Cancers 2022, 14, 5307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gan, W.; Fan, D.; Zheng, P.; Lv, Q.; Pan, Q.; Zhu, W. Novel quinazoline-based dual EGFR/c-Met inhibitors overcoming drug resistance for the treatment of NSCLC: Design, synthesis and anti-tumor activity. Bioorg. Chem. 2024, 142, 106938. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.; Wang, C.; Pan, Q.; Li, Y.; Guo, Y.; Fan, D.; Peng, Y.; Rao, Z.; Xu, S.; Zheng, P.; et al. Discovery of novel 4-arylamino-quinazoline derivatives as EGFR(L858R/T790M) inhibitors with the potential to inhibit the non-small cell lung cancers. Bioorg. Chem. 2022, 127, 105994. [Google Scholar] [CrossRef]
- Qin, X.; Liu, P.; Li, Y.; Hu, L.; Liao, Y.; Cao, T.; Yang, L. Design, synthesis and biological evaluation of novel 3,4-dihydro-2H-[1,4]oxazino [2,3-f]quinazolin derivatives as EGFR-TKIs. Bioorg. Med. Chem. Lett. 2023, 80, 129104. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Wang, J.; Qiao, Y.; Wumaier, G.; Sha, W.; Li, W.; Mei, W.; Yang, T.; Zhang, C.; He, H.; et al. Discovery and optimization of 4-anilinoquinazoline derivatives spanning ATP binding site and allosteric site as effective EGFR-C797S inhibitors. Eur. J. Med. Chem. 2022, 244, 114856. [Google Scholar] [CrossRef] [PubMed]
- Hasanvand, Z.; Oghabi Bakhshaiesh, T.; Peytam, F.; Firoozpour, L.; Hosseinzadeh, E.; Motahari, R.; Moghimi, S.; Nazeri, E.; Toolabi, M.; Momeni, F.; et al. Imidazo[1,2-a]quinazolines as novel, potent EGFR-TK inhibitors: Design, synthesis, bioactivity evaluation, and in silico studies. Bioorg. Chem. 2023, 133, 106383. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Soliman, A.M.; El-Adl, K.; Hanafy, N.S. New quinazoline sulfonamide derivatives as potential anticancer agents: Identifying a promising hit with dual EGFR/VEGFR-2 inhibitory and radiosensitizing activity. Bioorg. Chem. 2023, 140, 106791. [Google Scholar] [CrossRef]
- Ghorab, W.M.; El-Sebaey, S.A.; Ghorab, M.M. Design, synthesis and Molecular modeling study of certain EGFRinhibitors with a quinazolinone scaffold as anti-hepatocellular carcinoma and Radio-sensitizers. Bioorg. Chem. 2023, 131, 106310. [Google Scholar] [CrossRef]
- Abhinand, C.S.; Raju, R.; Soumya, S.J.; Arya, P.S.; Sudhakaran, P.R. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J. Cell Commun. Signal 2016, 10, 347–354. [Google Scholar] [CrossRef]
- Adams, R.H.; Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478. [Google Scholar] [CrossRef]
- Modi, S.J.; Kulkarni, V.M. Vascular Endothelial Growth Factor Receptor (VEGFR-2)/KDR Inhibitors: Medicinal Chemistry Perspective. Med. Drug Discov. 2019, 2, 100009. [Google Scholar] [CrossRef]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef]
- Muto, J.; Shirabe, K.; Sugimachi, K.; Maehara, Y. Review of angiogenesis in hepatocellular carcinoma. Hepatol. Res. 2015, 45, 1–9. [Google Scholar] [CrossRef]
- Abdel-Mohsen, H.T.; Girgis, A.S.; Mahmoud, A.E.E.; Ali, M.M.; El Diwani, H.I. New 2,4-disubstituted-2-thiopyrimidines as VEGFR-2 inhibitors: Design, synthesis, and biological evaluation. Arch. Pharm. 2019, 352, e1900089. [Google Scholar] [CrossRef]
- Abdel-Mohsen, H.; El Kerdawy, A. Design, Synthesis, Molecular Docking Studies and in Silico Prediction of ADME Properties of New 5-Nitrobenzimidazole/thiopyrimidine Hybrids as Anti-angiogenic Agents Targeting Hepatocellular Carcinoma. Egypt. J. Chem. 2023, 67, 437–446. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Kirkwood, J.M.; Grob, J.J.; Simeone, E.; Grimaldi, A.M.; Maio, M.; Palmieri, G.; Testori, A.; Marincola, F.M.; Mozzillo, N. The role of BRAF V600 mutation in melanoma. J. Transl. Med. 2012, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.E.; Hecht, F.; Berdelou, A.; Borget, I.; Leboulleux, S.; Baudin, E.; Schlumberger, M. Long-term follow-up and safety of vandetanib for advanced medullary thyroid cancer. Endocrine 2021, 71, 434–442. [Google Scholar] [CrossRef]
- Li, H.; Yu, Y.; Zhao, Y.; Wu, D.; Yu, X.; Lu, J.; Chen, Z.; Zhang, H.; Hu, Y.; Zhai, Y.; et al. Small molecule inhibitor agerafenib effectively suppresses neuroblastoma tumor growth in mouse models via inhibiting ERK MAPK signaling. Cancer Lett. 2019, 457, 129–141. [Google Scholar] [CrossRef]
- Abd El-Karim, S.S.; Syam, Y.M.; El Kerdawy, A.M.; Abdel-Mohsen, H.T. Rational design and synthesis of novel quinazolinone N-acetohydrazides as type II multi-kinase inhibitors and potential anticancer agents. Bioorg. Chem. 2024, 142, 106920. [Google Scholar] [CrossRef]
- Hamdi, A.; El-Shafey, H.W.; Othman, D.I.A.; El-Azab, A.S.; AlSaif, N.A.; Abdel-Aziz, A.A.M. Design, synthesis, antitumor, and VEGFR-2 inhibition activities of novel 4-anilino-2-vinyl-quinazolines: Molecular modeling studies. Bioorg. Chem. 2022, 122, 105710. [Google Scholar] [CrossRef] [PubMed]
- Zahran, S.S.; Ragab, F.A.; El-Gazzar, M.G.; Soliman, A.M.; Mahmoud, W.R.; Ghorab, M.M. Antiproliferative, antiangiogenic and apoptotic effect of new hybrids of quinazoline-4(3H)-ones and sulfachloropyridazine. Eur. J. Med. Chem. 2023, 245, 114912. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, A.E.; Eissa, S.I.; Al Ward, M.M.S.; Mabrouk, R.R.; Mehany, A.B.M.; El-Zahabi, M.A. Design, synthesis and molecular modeling of new quinazolin-4(3H)-one based VEGFR-2 kinase inhibitors for potential anticancer evaluation. Bioorg. Chem. 2021, 109, 104695. [Google Scholar] [CrossRef]
- Abdallah, A.E.; Mabrouk, R.R.; Al Ward, M.M.S.; Eissa, S.I.; Elkaeed, E.B.; Mehany, A.B.M.; Abo-Saif, M.A.; El-Feky, O.A.; Alesawy, M.S.; El-Zahabi, M.A. Synthesis, biological evaluation, and molecular docking of new series of antitumor and apoptosis inducers designed as VEGFR-2 inhibitors. J. Enzym. Inhib. Med. Chem. 2022, 37, 573–591. [Google Scholar] [CrossRef]
- Hassan, A.; Mubarak, F.A.F.; Shehadi, I.A.; Mosallam, A.M.; Temairk, H.; Badr, M.; Abdelmonsef, A.H. Design and biological evaluation of 3-substituted quinazoline-2,4(1H,3H)-dione derivatives as dual c-Met/VEGFR-2-TK inhibitors. J. Enzym. Inhib. Med. Chem. 2023, 38, 2189578. [Google Scholar] [CrossRef]
- Azab, A.E.; Alesawy, M.S.; Eldehna, W.M.; Elwan, A.; Eissa, I.H. New [1,2,4]triazolo[4,3-c]quinazoline derivatives as vascular endothelial growth factor receptor-2 inhibitors and apoptosis inducers: Design, synthesis, docking, and antiproliferative evaluation. Arch. Pharm. 2022, 355, e2200133. [Google Scholar] [CrossRef]
- Wang, R.; Liu, H.; You, Y.Y.; Wang, X.Y.; Lv, B.B.; Cao, L.Q.; Xue, J.Y.; Xu, Y.G.; Shi, L. Discovery of novel VEGFR-2 inhibitors embedding 6,7-dimethoxyquinazoline and diarylamide fragments. Bioorg. Med. Chem. Lett. 2021, 36, 127788. [Google Scholar] [CrossRef]
- Eissa, I.H.; Ibrahim, M.K.; Metwaly, A.M.; Belal, A.; Mehany, A.B.M.; Abdelhady, A.A.; Elhendawy, M.A.; Radwan, M.M.; ElSohly, M.A.; Mahdy, H.A. Design, molecular docking, in vitro, and in vivo studies of new quinazolin-4(3H)-ones as VEGFR-2 inhibitors with potential activity against hepatocellular carcinoma. Bioorg. Chem. 2021, 107, 104532. [Google Scholar] [CrossRef]
- El-Adl, K.; El-Helby, A.-G.A.; Ayyad, R.R.; Mahdy, H.A.; Khalifa, M.M.; Elnagar, H.A.; Mehany, A.B.M.; Metwaly, A.M.; Elhendawy, M.A.; Radwan, M.M.; et al. Design, synthesis, and anti-proliferative evaluation of new quinazolin-4(3H)-ones as potential VEGFR-2 inhibitors. Bioorg. Med. Chem. 2021, 29, 115872. [Google Scholar] [CrossRef]
- Elrayess, R.; Elgawish, M.S.; Nafie, M.S.; Ghareb, N.; Yassen, A.S.A. 2-Phenylquinazolin-4(3H)-one scaffold as newly designed, synthesized VEGFR-2 allosteric inhibitors with potent cytotoxicity through apoptosis. Arch. Pharm. 2023, 356, e2200654. [Google Scholar] [CrossRef]
- Hussain, M.R.; Baig, M.; Mohamoud, H.S.; Ulhaq, Z.; Hoessli, D.C.; Khogeer, G.S.; Al-Sayed, R.R.; Al-Aama, J.Y. BRAF gene: From human cancers to developmental syndromes. Saudi J. Biol. Sci. 2015, 22, 359–373. [Google Scholar] [CrossRef]
- Khazak, V.; Astsaturov, I.; Serebriiskii, I.G.; Golemis, E.A. Selective Raf inhibition in cancer therapy. Expert Opin. Ther. Targets 2007, 11, 1587–1609. [Google Scholar] [CrossRef]
- Huestis, M.P.; Dela Cruz, D.; DiPasquale, A.G.; Durk, M.R.; Eigenbrot, C.; Gibbons, P.; Gobbi, A.; Hunsaker, T.L.; La, H.; Leung, D.H.; et al. Targeting KRAS Mutant Cancers via Combination Treatment: Discovery of a 5-Fluoro-4-(3H)-quinazolinone Aryl Urea pan-RAF Kinase Inhibitor. J. Med. Chem. 2021, 64, 3940–3955. [Google Scholar] [CrossRef]
- Ali, I.H.; Abdel-Mohsen, H.T.; Mounier, M.M.; Abo-elfadl, M.T.; El Kerdawy, A.M.; Ghannam, I.A.Y. Design, synthesis and anticancer activity of novel 2-arylbenzimidazole/2-thiopyrimidines and 2-thioquinazolin-4(3H)-ones conjugates as targeted RAF and VEGFR-2 kinases inhibitors. Bioorg. Chem. 2022, 126, 105883. [Google Scholar] [CrossRef]
- Burke, J.E.; Williams, R.L. Synergy in activating class I PI3Ks. Trends Biochem. Sci. 2015, 40, 88–100. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Zhao, W.; Qiu, Y.; Kong, D. Class I phosphatidylinositol 3-kinase inhibitors for cancer therapy. Acta Pharm. Sin. B 2017, 7, 27–37. [Google Scholar] [CrossRef]
- Janku, F.; Yap, T.A.; Meric-Bernstam, F. Targeting the PI3K pathway in cancer: Are we making headway? Nat. Rev. Clin. Oncol. 2018, 15, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Puri, K.D.; Di Paolo, J.A.; Gold, M.R. B-cell receptor signaling inhibitors for treatment of autoimmune inflammatory diseases and B-cell malignancies. Int. Rev. Immunol. 2013, 32, 397–427. [Google Scholar] [CrossRef] [PubMed]
- Cheah, C.Y.; Fowler, N.H. Idelalisib in the management of lymphoma. Blood 2016, 128, 331–336. [Google Scholar] [CrossRef]
- Somoza, J.R.; Koditek, D.; Villasenor, A.G.; Novikov, N.; Wong, M.H.; Liclican, A.; Xing, W.; Lagpacan, L.; Wang, R.; Schultz, B.E.; et al. Structural, biochemical, and biophysical characterization of idelalisib binding to phosphoinositide 3-kinase delta. J. Biol. Chem. 2015, 290, 8439–8446. [Google Scholar] [CrossRef]
- Wang, T.; Sun, X.; Qiu, L.; Su, H.; Cao, J.; Li, Z.; Song, Y.; Zhang, L.; Li, D.; Wu, H.; et al. The Oral PI3Kδ Inhibitor Linperlisib for the Treatment of Relapsed and/or Refractory Follicular Lymphoma: A Phase II, Single-Arm, Open-Label Clinical Trial. Clin. Cancer Res. 2023, 29, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.; Appleman, L.J.; Tolcher, A.W.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Weiss, G.J.; Sachdev, J.C.; Chadha, M.; Fulk, M.; et al. First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin’s lymphomas. Ann. Oncol. 2016, 27, 1928–1940. [Google Scholar] [CrossRef]
- Dreyling, M.; Morschhauser, F.; Bouabdallah, K.; Bron, D.; Cunningham, D.; Assouline, S.E.; Verhoef, G.; Linton, K.; Thieblemont, C.; Vitolo, U.; et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann. Oncol. 2017, 28, 2169–2178. [Google Scholar] [CrossRef]
- Gao, L.; Chuai, H.; Ma, M.; Zhang, S.Q.; Zhang, J.; Li, J.; Wang, Y.; Xin, M. Design, synthesis and bioactivity evaluation of selenium-containing PI3Kδ inhibitors. Bioorg. Chem. 2023, 140, 106815. [Google Scholar] [CrossRef]
- Xia, L.; Jiang, L.; Du, T.; Lin, S.; Xiong, T.; Peng, S.; Tian, H.; Zhang, K.; Wu, D.; Sheng, L.; et al. Design, synthesis, and biological evaluation of novel bivalent PI3K inhibitors for the potential treatment of cancer. Bioorg. Chem. 2023, 140, 106814. [Google Scholar] [CrossRef]
- Fischer, P.M.; Endicott, J.; Meijer, L. Cyclin-dependent kinase inhibitors. Prog. Cell Cycle Res. 2003, 5, 235–248. [Google Scholar]
- Dictor, M.; Ehinger, M.; Mertens, F.; Akervall, J.; Wennerberg, J. Abnormal cell cycle regulation in malignancy. Am. J. Clin. Pathol. 1999, 112, S40–S52. [Google Scholar] [PubMed]
- Wu, J.; Chen, Y.; Li, R.; Guan, Y.; Chen, M.; Yin, H.; Yang, X.; Jin, M.; Huang, B.; Ding, X.; et al. Synergistic anticancer effect by targeting CDK2 and EGFR–ERK signaling. J. Cell Biol. 2023, 223, e202203005. [Google Scholar] [CrossRef]
- Wu, T.; Yang, Z.; Chen, W.; Jiang, M.; Xiao, Z.; Su, X.; Jiao, Z.; Yu, Y.; Chen, S.; Song, M.; et al. miR-30e-5p-mediated FOXD1 promotes cell proliferation by blocking cellular senescence and apoptosis through p21/CDK2/Rb signaling in head and neck carcinoma. Cell Death Discov. 2023, 9, 295. [Google Scholar] [CrossRef]
- Wang, X.; Shi, W.; Wang, X.; Lu, J.-J.; He, P.; Zhang, H.; Chen, X. Nifuroxazide boosts the anticancer efficacy of palbociclib-induced senescence by dual inhibition of STAT3 and CDK2 in triple-negative breast cancer. Cell Death Discov. 2023, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Sultan, R.; Ahmed, A.; Wei, L.; Saeed, H.; Islam, M.; Ishaq, M. The anticancer potential of chemical constituents of Moringa oleifera targeting CDK-2 inhibition in estrogen receptor positive breast cancer using in-silico and in vitro approches. BMC Complement. Med. Ther. 2023, 23, 396. [Google Scholar] [CrossRef]
- Gomha, S.M.; Zaki, M.E.A.; Maliwal, D.; Pissurlenkar, R.R.S.; Ibrahim, M.S.; Fathalla, M.; Hussein, A.M. Synthesis, in-silico studies, and biological evaluation of some novel 3-thiazolyl-indoles as CDK2–inhibitors. Results Chem. 2023, 6, 101209. [Google Scholar] [CrossRef]
- Wang, L.; Shao, X.; Zhong, T.; Wu, Y.; Xu, A.; Sun, X.; Gao, H.; Liu, Y.; Lan, T.; Tong, Y.; et al. Discovery of a first-in-class CDK2 selective degrader for AML differentiation therapy. Nat. Chem. Biol. 2021, 17, 567–575. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Hei, R.; Li, X.; Cai, H.; Wu, X.; Zheng, Q.; Cai, C. CDK inhibitors in cancer therapy, an overview of recent development. Am. J. Cancer Res. 2021, 11, 1913–1935. [Google Scholar]
- Ma, T.; Van Tine, B.A.; Wei, Y.; Garrett, M.D.; Nelson, D.; Adams, P.D.; Wang, J.; Qin, J.; Chow, L.T.; Harper, J.W. Cell cycle-regulated phosphorylation of p220(NPAT) by cyclin E/Cdk2 in Cajal bodies promotes histone gene transcription. Genes Dev. 2000, 14, 2298–2313. [Google Scholar] [CrossRef] [PubMed]
- Flores, O.; Wang, Z.; Knudsen, K.E.; Burnstein, K.L. Nuclear targeting of cyclin-dependent kinase 2 reveals essential roles of cyclin-dependent kinase 2 localization and cyclin E in vitamin D-mediated growth inhibition. Endocrinology 2010, 151, 896–908. [Google Scholar] [CrossRef] [PubMed]
- De Boer, L.; Oakes, V.; Beamish, H.; Giles, N.; Stevens, F.; Somodevilla-Torres, M.; Desouza, C.; Gabrielli, B. Cyclin A/cdk2 coordinates centrosomal and nuclear mitotic events. Oncogene 2008, 27, 4261–4268. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.C.; Clurman, B.E. Cyclin E in normal and neoplastic cell cycles. Oncogene 2005, 24, 2776–2786. [Google Scholar] [CrossRef] [PubMed]
- Spruck, C.H.; Won, K.A.; Reed, S.I. Deregulated cyclin E induces chromosome instability. Nature 1999, 401, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; LaPlant, B.; Chng, W.J.; Zonder, J.; Callander, N.; Fonseca, R.; Fruth, B.; Roy, V.; Erlichman, C.; Stewart, A.K. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood 2015, 125, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.F.; Lin, J.D.; Hsueh, C.; Chou, T.C.; Wong, R.J. Potent effects of roniciclib alone and with sorafenib against well-differentiated thyroid cancer. Endocr. Relat. Cancer 2018, 25, 853–864. [Google Scholar] [CrossRef]
- Tadesse, S.; Caldon, E.C.; Tilley, W.; Wang, S. Cyclin-Dependent Kinase 2 Inhibitors in Cancer Therapy: An Update. J. Med. Chem. 2019, 62, 4233–4251. [Google Scholar] [CrossRef]
- Frame, S.; Saladino, C.; MacKay, C.; Atrash, B.; Sheldrake, P.; McDonald, E.; Clarke, P.A.; Workman, P.; Blake, D.; Zheleva, D. Fadraciclib (CYC065), a novel CDK inhibitor, targets key pro-survival and oncogenic pathways in cancer. PLoS ONE 2020, 15, e0234103. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Faivre, S.; Laurence, V.; Delbaldo, C.; Vera, K.; Girre, V.; Chiao, J.; Armour, S.; Frame, S.; Green, S.R.; et al. Phase I evaluation of seliciclib (R-roscovitine), a novel oral cyclin-dependent kinase inhibitor, in patients with advanced malignancies. Eur. J. Cancer 2010, 46, 3243–3250. [Google Scholar] [CrossRef]
- Sielecki, T.M.; Johnson, T.L.; Liu, J.; Muckelbauer, J.K.; Grafstrom, R.H.; Cox, S.; Boylan, J.; Burton, C.R.; Chen, H.; Smallwood, A.; et al. Quinazolines as cyclin dependent kinase inhibitors. Bioorg. Med. Chem. Lett. 2001, 11, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Shewchuk, L.; Hassell, A.; Wisely, B.; Rocque, W.; Holmes, W.; Veal, J.; Kuyper, L.F. Binding mode of the 4-anilinoquinazoline class of protein kinase inhibitor: X-ray crystallographic studies of 4-anilinoquinazolines bound to cyclin-dependent kinase 2 and p38 kinase. J. Med. Chem. 2000, 43, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, E.R.; Elmasry, G.F. Development of newly synthesised quinazolinone-based CDK2 inhibitors with potent efficacy against melanoma. J. Enzym. Inhib. Med. Chem. 2022, 37, 686–700. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Dong, R.; Liu, X.; Li, H.; Zhang, T.; Xu, J.; Liu, C.; Zhang, Y.; Hou, S.; et al. Discovery of N-(4-(3-isopropyl-2-methyl-2H-indazol-5-yl)pyrimidin-2-yl)-4-(4-methylpiperazin-1-yl)quinazolin-7-amine as a Novel, Potent, and Oral Cyclin-Dependent Kinase Inhibitor against Haematological Malignancies. J. Med. Chem. 2021, 64, 12548–12571. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Mohsen, H.T.; Anwar, M.M.; Ahmed, N.S.; Abd El-Karim, S.S.; Abdelwahed, S.H. Recent Advances in Structural Optimization of Quinazoline-Based Protein Kinase Inhibitors for Cancer Therapy (2021–Present). Molecules 2024, 29, 875. https://doi.org/10.3390/molecules29040875
Abdel-Mohsen HT, Anwar MM, Ahmed NS, Abd El-Karim SS, Abdelwahed SH. Recent Advances in Structural Optimization of Quinazoline-Based Protein Kinase Inhibitors for Cancer Therapy (2021–Present). Molecules. 2024; 29(4):875. https://doi.org/10.3390/molecules29040875
Chicago/Turabian StyleAbdel-Mohsen, Heba T., Manal M. Anwar, Nesreen S. Ahmed, Somaia S. Abd El-Karim, and Sameh H. Abdelwahed. 2024. "Recent Advances in Structural Optimization of Quinazoline-Based Protein Kinase Inhibitors for Cancer Therapy (2021–Present)" Molecules 29, no. 4: 875. https://doi.org/10.3390/molecules29040875
APA StyleAbdel-Mohsen, H. T., Anwar, M. M., Ahmed, N. S., Abd El-Karim, S. S., & Abdelwahed, S. H. (2024). Recent Advances in Structural Optimization of Quinazoline-Based Protein Kinase Inhibitors for Cancer Therapy (2021–Present). Molecules, 29(4), 875. https://doi.org/10.3390/molecules29040875






