Chemical Composition Antioxidant and Anti-Inflammatory Activities of Myrtus communis L. Leaf Extract: Forecasting ADMET Profiling and Anti-Inflammatory Targets Using Molecular Docking Tools
Abstract
1. Introduction
2. Results
2.1. Extraction Yeild (EY) and Total Polyphenol, Flavonoid, and Tannin Contents
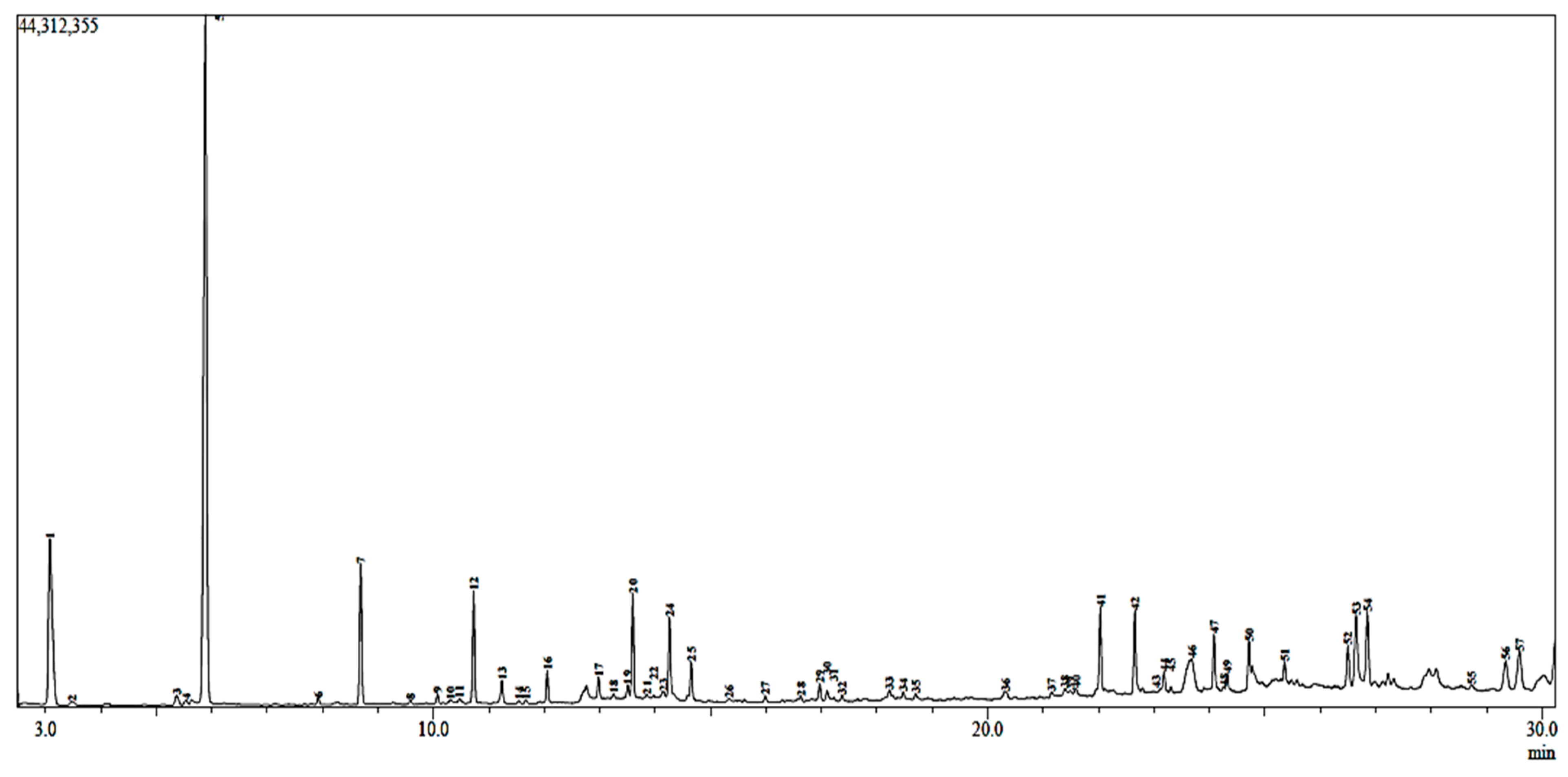
2.2. GC-MS Analysis
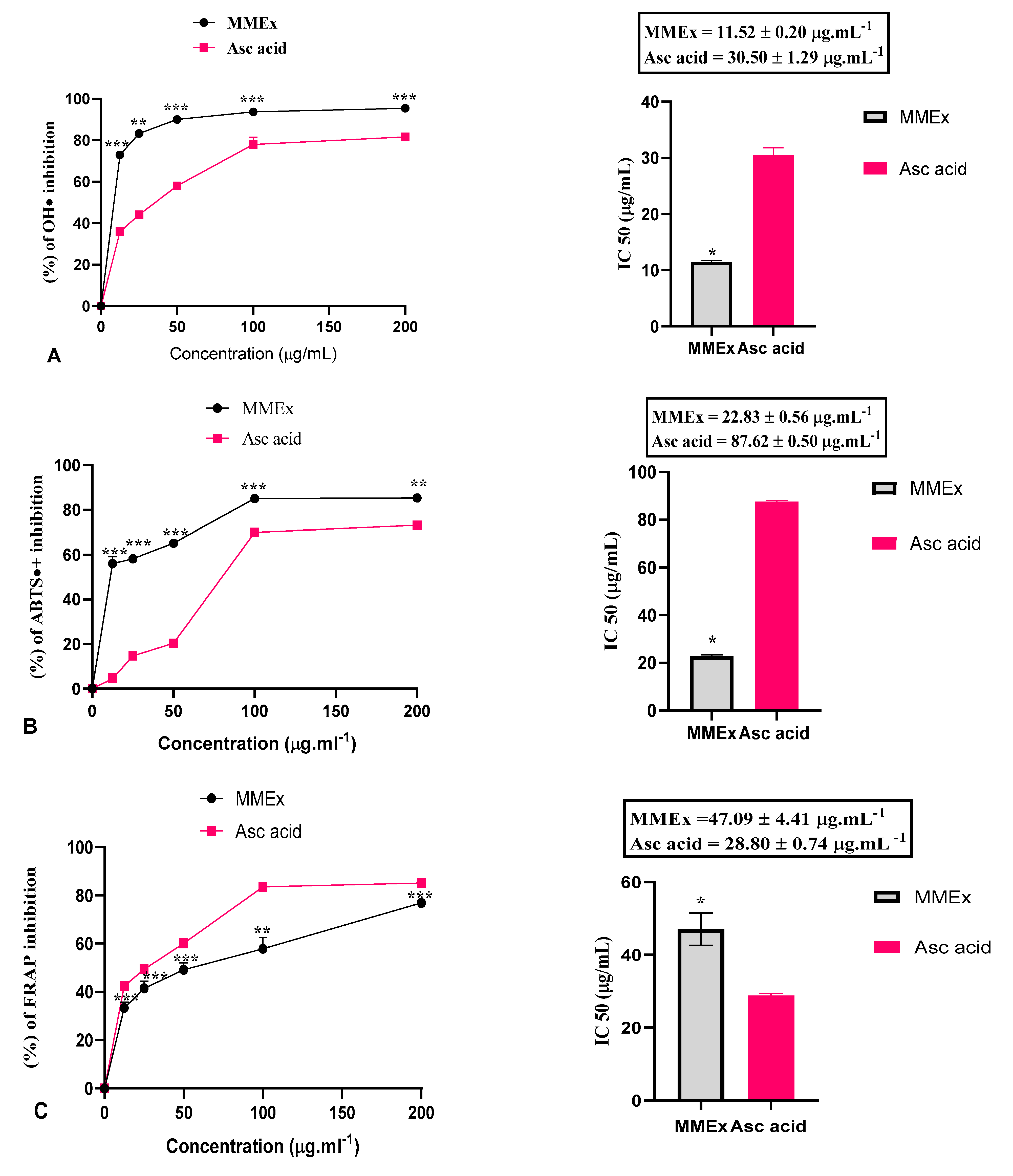
2.3. Antioxidant Activity
2.4. Anti-Inflammatory Activity
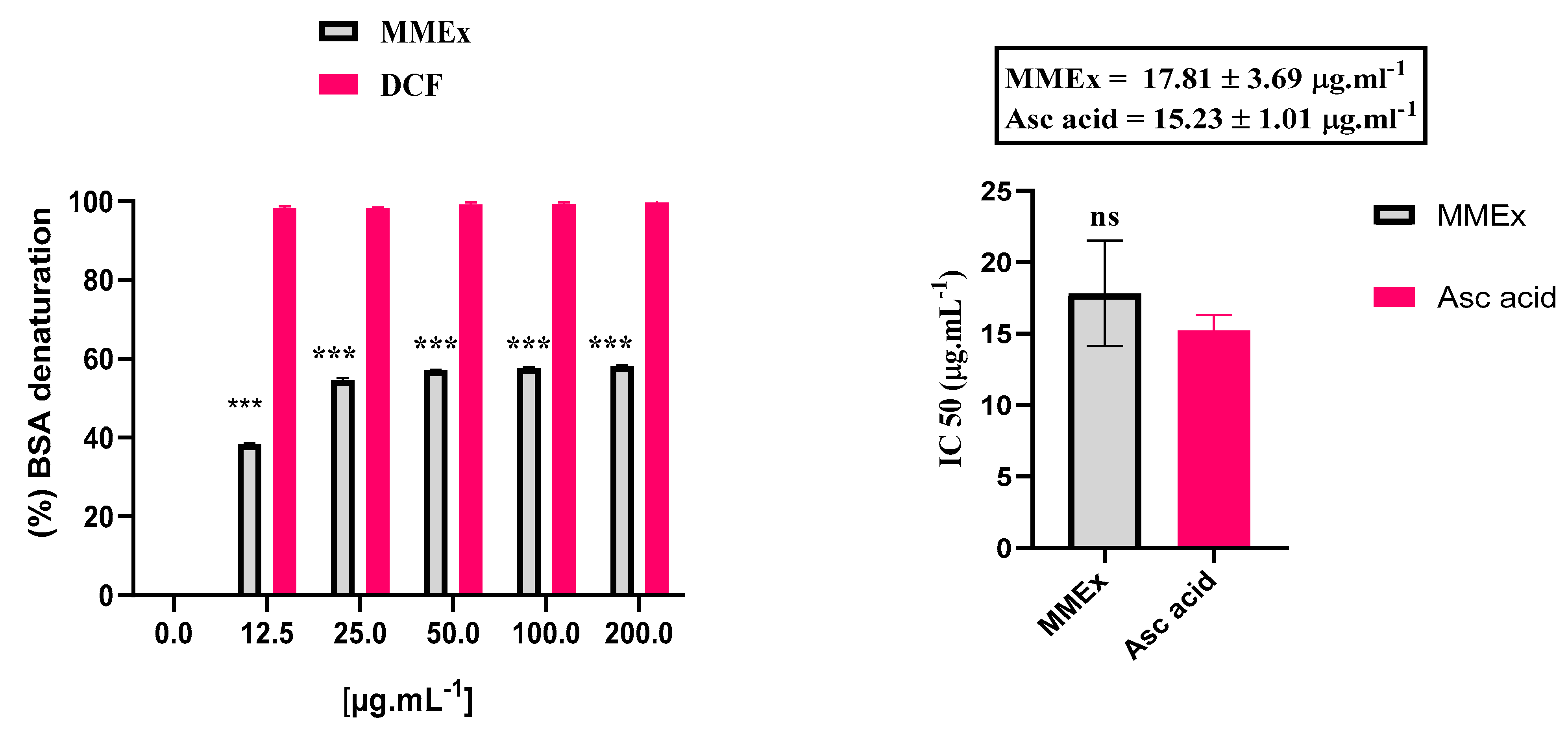
2.4.1. In Vitro: Inhibition of BSA Denaturation
2.4.2. In Vivo: Carrageenan-Induced Paw Edema in Rats
2.4.3. In Silico Study
- Drug-likeness of Compounds
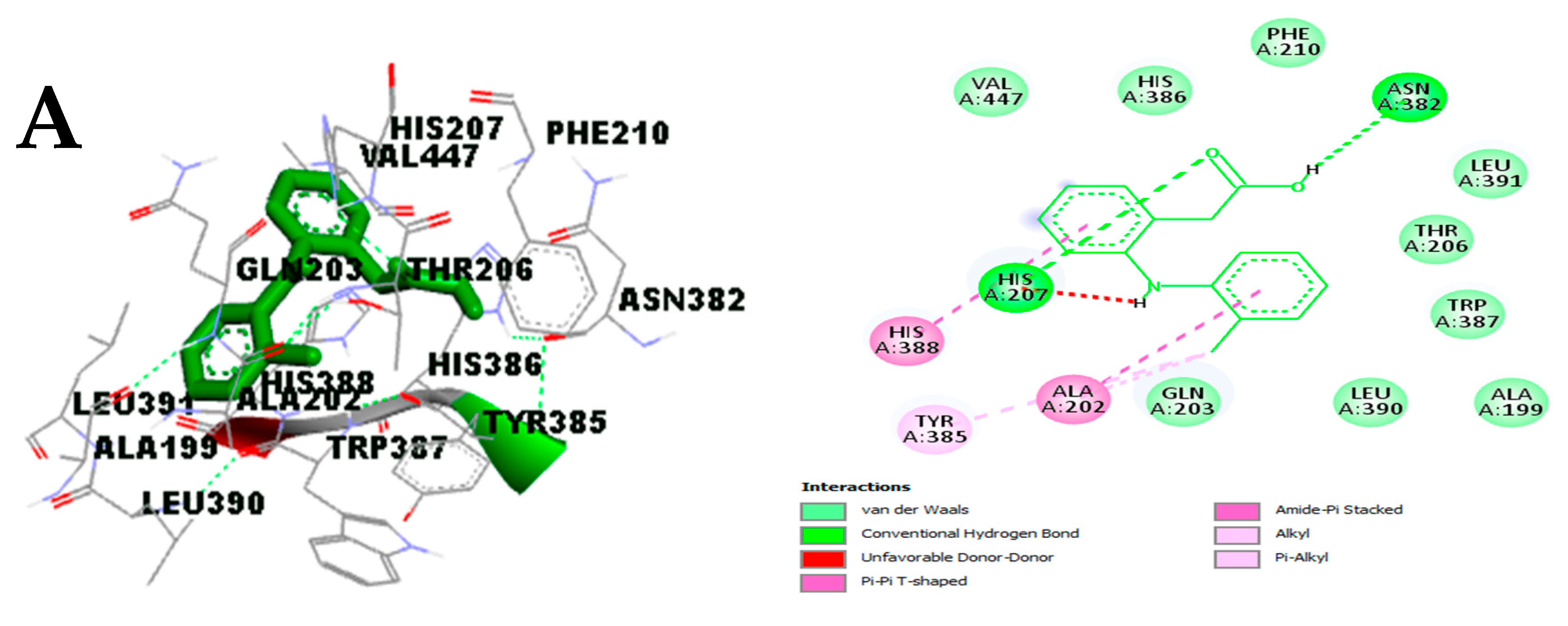
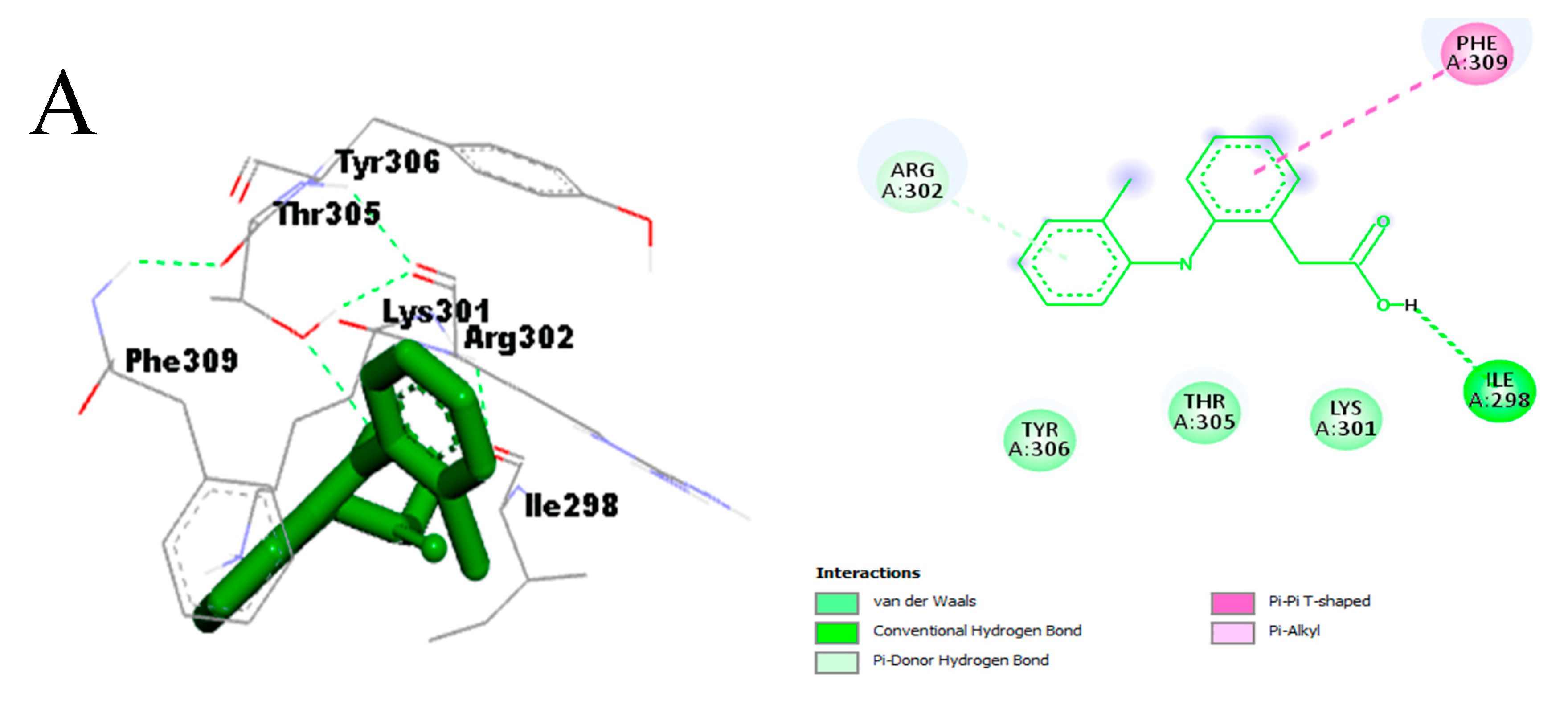
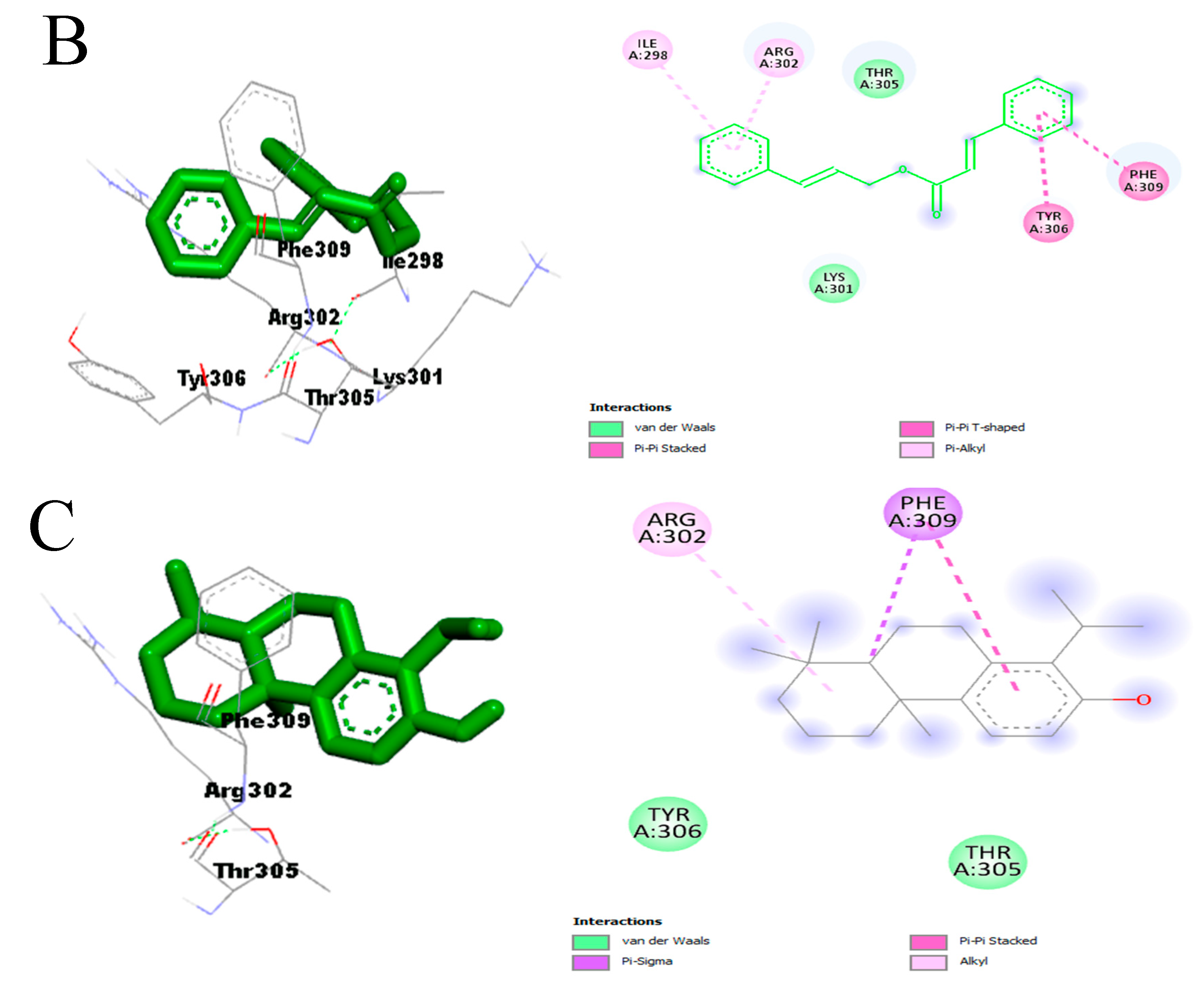
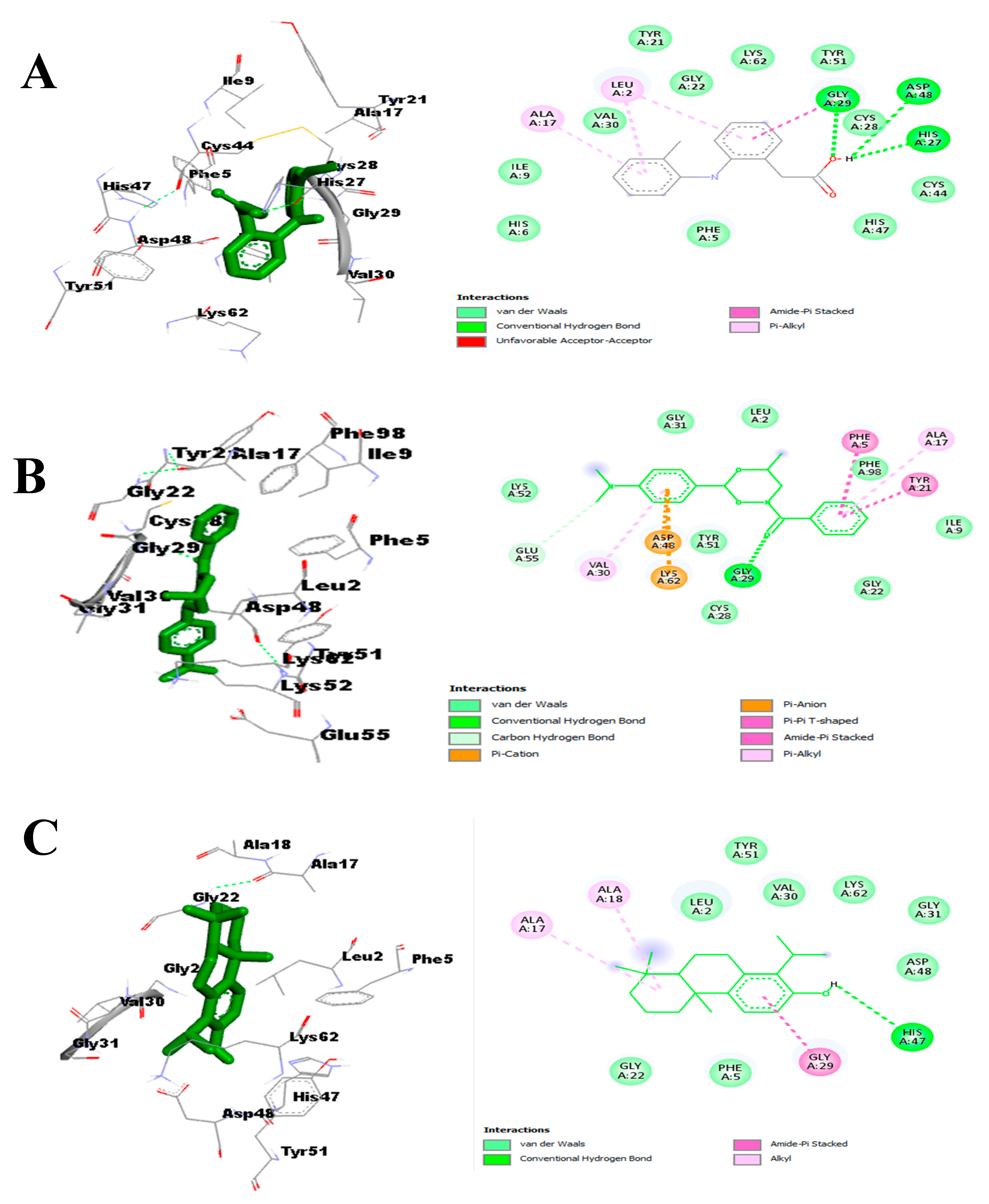
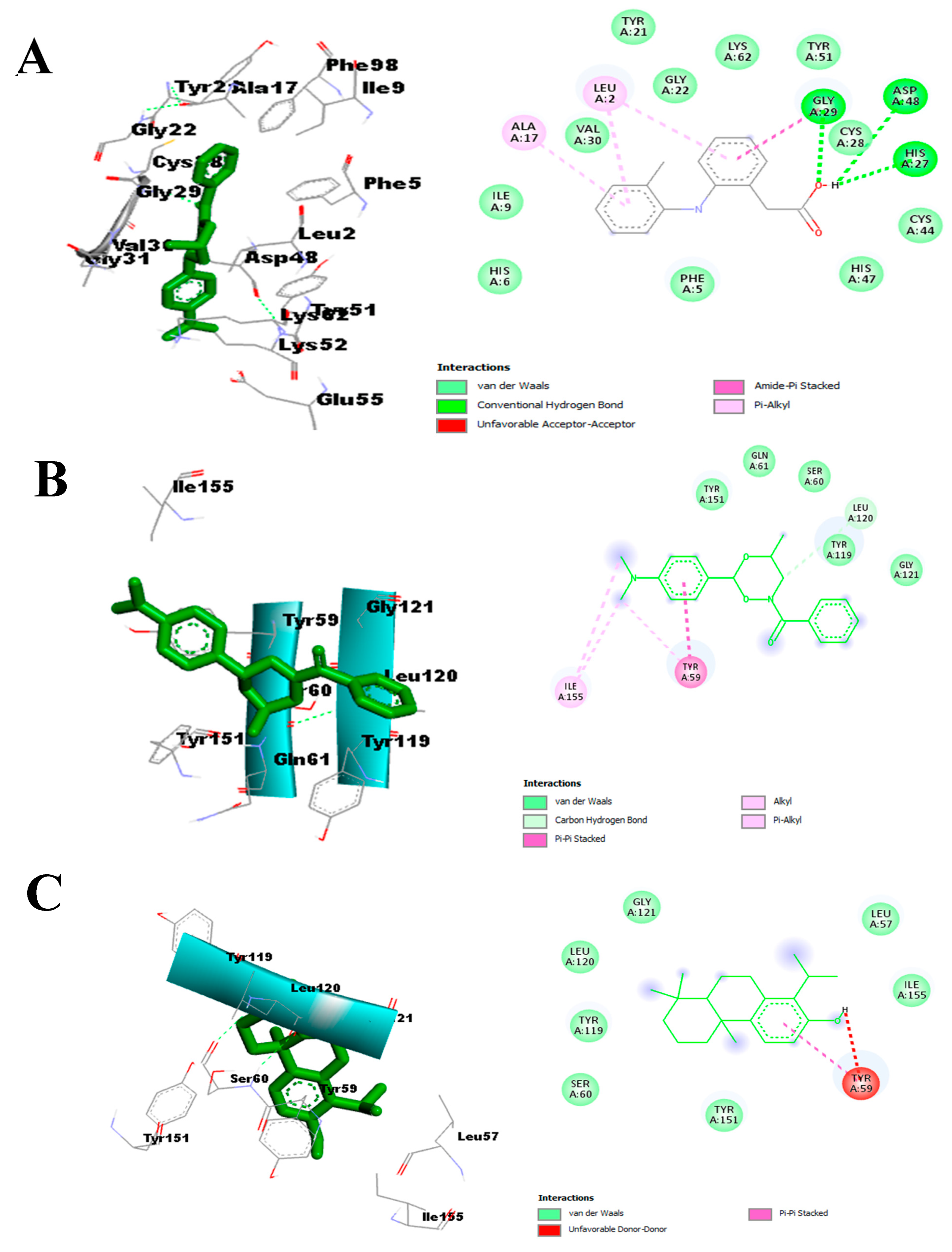
- Effect on pro-inflammatory targets: Cox-2, IL-1β, NF-κB, PLA2, and TNF-α
2.5. ADMET Results
3. Discussion
3.1. Phytochemical Analysis
3.2. Antioxidant Activity
3.3. Anti-Inflammatory Activity
3.3.1. In Vitro: Inhibition of BSA Denaturation
3.3.2. In Vivo: Car-Induced Paw Edema
3.3.3. In Silico Study
4. Materials and Methods
4.1. Chemicals, Reagents, and Equipment
4.2. Collection of Plant Material
4.3. Methanolic Extract Preparation
4.4. Gas Chromatography–Mass Spectrometry (CG-MS) Analysis
4.5. Total Polyphenol Content (TPC)
4.6. Total Flavonoid Content (TFC)
4.7. Total Hydrolyzable and Condensed Tannin Content (THT, TCT)
4.8. Antioxidant Activity
4.8.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Scavenging Assay
4.8.2. H2O2 Hydrogen Peroxide Scavenging Assay
4.8.3. Hydroxyl Radical (OH•) Scavenging Test
4.8.4. ABTS• Radical Scavenging Assay
4.8.5. Ferric-Reducing Antioxidant Power (FRAP)
4.9. Anti-Inflammatory Activity
4.9.1. In Vitro: Inhibition of Bovine Serum Albumin (BSA) Denaturation
4.9.2. In Vivo: Carrageenan-Induced Paw Edema in Rats
- Animals
- Ethical statement
- Experimental design
4.9.3. In Silico
- Ligand Preparation
- Protein Preparation
- Molecular Docking Analysis
4.10. In Silico Pharmacokinetic Profile and Drug-Likeness Prediction
5. Data Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quinty, V.; Nasreddine, R.; Colas, C.; Launay, A.; Nehmé, R.; El-Khiraoui, A.; Piot, C.; Draye, M.; Destandau, E.; Da Silva, D.; et al. Antioxidant and anti-lipase capacities from the extracts obtained from two invasive plants: Ambrosia artemisiifolia and Solidago canadensis. Food Biosci. 2023, 55, 103069. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Rekka, E.A. Ferulic, Sinapic, 3, 4-Dimethoxycinnamic Acid and Indomethacin Derivatives with Antioxidant, Anti-Inflammatory and Hypolipidemic Functionality. Antioxidants 2023, 12, 1436. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, M.R.; Chaudhary, S.; Sharma, Y.; Singh, T.A.; Mishra, A.K.; Sharma, S.; Mehdi, M.M. Aging, oxidative stress and degenerative diseases: Mechanisms, complications and emerging therapeutic strategies. Biogerontology 2023, 24, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Ramos-González, E.J.; Bitzer-Quintero, O.K.; Ortiz, G.; Hernández-Cruz, J.J.; Ramírez-Jirano, L.J. Relationship between inflammation and oxidative stress and its effect on multiple sclerosis. Neurología 2021, in press. [Google Scholar]
- Bourais, I.; Elmarrkechy, S.; Taha, D.; Badaoui, B.; Mourabit, Y.; Salhi, N.; Alshahrani, M.M.; Al Awadh, A.A.; Bouyahya, A.; Goh, K.W. Comparative Investigation of Chemical Constituents of Kernels, Leaves, Husk, and Bark of Juglans regia L., Using HPLC-DAD-ESI-MS/MS Analysis and Evaluation of Their Antioxidant, Antidiabetic, and Anti-Inflammatory Activities. Molecules 2022, 27, 8989. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Emon, N.U.; Alam, S.; Rudra, S.; Akhter, N.; Mamun, M.M.R.; Ganguly, A. Assessment of pharmacological activities of Lygodium microphyllum Cav. leaves in the management of pain, inflammation, pyrexia, diarrhea, and helminths: In vivo, in vitro and in silico approaches. Biomed. Pharmacother. 2021, 139, 111644. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Bhattacharyya, S. Chapter 6—The phospholipase A2 superfamily and its role in chronic inflammatory conditions with relation to adjuster cells. In Phospholipases in Physiology and Pathology; Chakraborti, S., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 1–21. [Google Scholar]
- Serhan, C.N.; Sulciner, M.L. Resolution medicine in cancer, infection, pain and inflammation: Are we on track to address the next Pandemic? Cancer Metastasis Rev. 2023, 42, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.F.; Radwan, O.K.; Bakeer, R.M. Resveratrol in combination with Ibuprofen against acute carrageenan-induced inflammation and hepatic insult: Rectification of adenylate energy charge (AEC), anti-apoptotic, cell proliferation and DNA preservation potentials. Int. J. PharmTech Res. 2016, 9, 917–938. [Google Scholar]
- Elgazar, A.A.; Knany, H.R.; Ali, M.S. Insights on the molecular mechanism of anti-inflammatory effect of formula from Islamic traditional medicine: An in-silico study. J. Tradit. Complement. Med. 2019, 9, 353–363. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Khalili, A.; Roodi, P.B.; Saeedy, S.A.G.; Najafi, S.; Keshavarz, M.M.; Djafarian, K. Selenium supplementation decreases CRP and IL-6 and increases TNF-alpha: A systematic review and meta-analysis of randomized controlled trials. J. Trace Elem. Med. Biol. 2023, 79, 127199. [Google Scholar] [CrossRef]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Eid, B.G. Icariin modulates carrageenan-induced acute inflammation through HO-1/Nrf2 and NF-kB signaling pathways. Biomed. Pharmacother. 2019, 120, 109567. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef]
- Panchal, N.K.; Swarnalatha, P.; Prince, S.E. Trichopus zeylanicus ameliorates ibuprofen inebriated hepatotoxicity and enteropathy: An insight into its modulatory impact on pro/anti-inflammatory cytokines and apoptotic signaling pathways. Inflammopharmacology 2022, 30, 2229–2242. [Google Scholar] [CrossRef]
- Talaj, J.A.; Zielinski, K.; Bujacz, A.J. Structural Investigation of Diclofenac Binding to Ovine, Caprine, and Leporine Serum Albumins. Int. J. Mol. Sci. 2023, 24, 1534. [Google Scholar] [CrossRef]
- Panchal, N.K.; Sabina, E. Non-steroidal anti-inflammatory drugs (NSAIDs): A current insight into its molecular mechanism eliciting organ toxicities. Food Chem. Toxicol. 2023, 172, 113598. [Google Scholar] [CrossRef]
- D’antongiovanni, V.; Antonioli, L.; Benvenuti, L.; Pellegrini, C.; Di Salvo, C.; Calvigioni, M.; Panattoni, A.; Ryskalin, L.; Natale, G.; Banni, S. Use of Saccharomyces boulardii CNCM I-745 as therapeutic strategy for prevention of nonsteroidal anti-inflammatory drug-induced intestinal injury. Br. J. Pharmacol. 2023, 180, 3215–3233. [Google Scholar] [CrossRef]
- Teo, S.H.; Tan, N.C.; Choo, J.C.J.; Kwek, J.L.; Kadir, H.B.A.; Bee, Y.M.; Huang, H.; Kaushik, M.; Ang, A.T.W.; Lim, C.C. Non-steroidal anti-inflammatory drugs in chronic kidney disease and risk of acute adverse kidney events according to route of administration. Int. Urol. Nephrol. 2023, 55, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Awan, S.; Siddiqui, K.; Munawar, M.; Inayat, S.; Shehzadi, S.; Zahid, M.A. Recent developments in the understanding of NSAID-induced liver fibrosis: Linking fundamental mechanisms to specific therapy ideas. Int. J. Nat. Med. Health Sci. 2023, 2, 39–44. [Google Scholar]
- Paniagua-Pérez, R.; Sánchez-Chapul, L.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Madrigal-Santillán, E.; Cruz-Hernández, L.; Martínez-Canseco, C.; Reyes-Legorreta, C.; Ruiz-Rosano, L.; Hernández-Flores, C.; et al. Anti-Inflammatory Potential of Pteropodine in Rodents. Metabolites 2023, 13, 907. [Google Scholar] [CrossRef] [PubMed]
- Noor, F.; Tahir, U.; Qamar, M.; Ashfaq, U.A.; Albutti, A.; Alwashmi, A.S.; Aljasir, M.A. Network pharmacology approach for medicinal plants: Review and assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef] [PubMed]
- Nyalo, P.; Omwenga, G.; Ngugi, M. Quantitative Phytochemical Profile and In Vitro Antioxidant Properties of Ethyl Acetate Extracts of Xerophyta spekei (Baker) and Grewia tembensis (Fresen). J. Evid.-Basesd Integr. Med. 2023, 28. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Kerebba, N.; Rautenbach, F.; Singh, S.K.; Dua, K.; Oguntibeju, O.O. UPLC-ESI-QTOF-MS phenolic compounds identification and quantification from ethanolic extract of Myrtus communis ‘Variegatha’: In vitro antioxidant and antidiabetic potentials. Arab. J. Chem. 2023, 16, 104447. [Google Scholar] [CrossRef]
- Alipour, G.; Dashti, S.; Hosseinzadeh, H. Review of Pharmacological Effects of Myrtus communis L. and its Active Constituents. Phytother. Res. 2014, 28, 1125–1136. [Google Scholar] [CrossRef]
- Dabbaghi, M.M.; Fadaei, M.S.; Soleimani Roudi, H.; Baradaran Rahimi, V.; Askari, V.R. A review of the biological effects of Myrtus Communis. Physiol. Rep. 2023, 11, e15770. [Google Scholar] [CrossRef]
- Mahboubi, M. Effectiveness of Myrtus communis in the treatment of hemorrhoids. J. Integr. Med. 2017, 15, 351–358. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Khoshdel, M.; Ghorbani, M. Antinociceptive, Anti-inflammatory Effects and Acute Toxicity of Aqueous and Ethanolic Extracts of Myrtus communis L. Aerial Parts in Mice. J. Acupunct. Meridian Stud. 2011, 4, 242–247. [Google Scholar] [CrossRef]
- Khosropour, P.; Sajjadi, S.E.; Talebi, A.; Minaiyan, M.J. Anti-inflammatory effect of Myrtus communis hydroalcoholic extract and essential oil on acetic acid-induced colitis in rats. J. Rep. Pharm. Sci. 2019, 8, 204–210. [Google Scholar]
- Hennia, A.; Miguel, M.G.; Nemmiche, S. Antioxidant Activity of Myrtus communis L. and Myrtus nivellei Batt. & Trab. Extracts: A Brief Review. Medicines 2018, 5, 89. [Google Scholar] [PubMed]
- Barhouchi, B.; Menacer, R.; Bouchkioua, S.; Mansour, A.; Belattar, N. Compounds from myrtle flowers as antibacterial agents and SARS-CoV-2 inhibitors: In-vitro and molecular docking studies. Arab. J. Chem. 2023, 16, 104939. [Google Scholar] [CrossRef] [PubMed]
- Belahcene, S.; Kebsa, W.; Omoboyowa, D.A.; Alshihri, A.A.; Alelyani, M.; Bakkour, Y.; Leghouchi, E. Unveiling the Chemical Profiling Antioxidant and Anti-Inflammatory Activities of Algerian Myrtus communis L. Essential Oils, and Exploring Molecular Docking to Predict the Inhibitory Compounds against Cyclooxygenase-2. Pharmaceuticals 2023, 16, 1343. [Google Scholar] [CrossRef] [PubMed]
- Fazeli-Nasab, B.; Sayyed, R.Z.; Sobhanizadeh, A. In Silico Molecular Docking Analysis of α-Pinene: An Antioxidant and Anticancer Drug Obtained from Myrtus communis. Int. J. Cancer Manag. 2021, 14, e89116. [Google Scholar] [CrossRef]
- Hayder, N.; Abdelwahed, A.; Kilani, S.; Ammar, R.B.; Mahmoud, A.; Ghedira, K.; Chekir-Ghedira, L. Anti-genotoxic and free-radical scavenging activities of extracts from (Tunisian) Myrtus communis. Mutat. Res. 2004, 564, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Moura, D.; Vilela, J.; Saraiva, S.; Monteiro-Silva, F.; De Almeida, J. Antimicrobial Effects and Antioxidant Activity of Myrtus communis L. Essential Oil in Beef Stored under Different Packaging Conditions. Food 2023, 12, 3390. [Google Scholar] [CrossRef] [PubMed]
- Al-Maharik, N.; Jaradat, N.; Al-Hajj, N.; Jaber, S. Myrtus communis L.: Essential oil chemical composition, total phenols and flavonoids contents, antimicrobial, antioxidant, anticancer, and α-amylase inhibitory activity. Chem. Biol. Technol. Agric. 2023, 10, 41. [Google Scholar] [CrossRef]
- Li, J.; Chang, R.Y.; Chen, L.F.; Qian, S.H.; Wang, R.Y.; Lan, J.L.; Huang, L.; Ding, X.H. Potential Targets and Mechanisms of Jiedu Quyu Ziyin Decoction for Treating SLE-GIOP: Based on Network Pharmacology and Molecular Docking. J. Immunol. Res. 2023, 28, 8942415. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Mita, M.A.; Afrose, S.; Hasan, M.R.; Islam, M.T.; Rahman, M.A.; Ara, M.J.; Chowdhury, M.B.A.; Meem, H.N.; Mamunuzzaman, M.; et al. Integrated Computational Approaches for Inhibiting Sex Hormone-Binding Globulin in Male Infertility by Screening Potent Phytochemicals. Life 2023, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Rufa’i, F.A.; Baecker, D.; Mukhtar, M.D. Phytochemical Screening, GC-MS Analysis, and Evaluating In Vivo Antitrypanosomal Effects of a Methanolic Extract of Garcinia kola Nuts on Rats. Antibiotics 2023, 12, 713. [Google Scholar] [CrossRef]
- Touaibia, M.; Chaouch, F.Z. Pouvoir antioxydant des extraits de Myrtus communis L. obtenus in situ et in vitro. Nat. Technol. 2014, 7, 3–9. [Google Scholar]
- Bouchenak, O.; Yahiaoui, K.; Benhabyles, N.; Laoufi, R.; Toubal, S.; El Haddad, D.; Oussaid, S.; Blizak, D.; Arab, K. Criblage phytochimique et évaluation du pouvoir antioxydant des feuilles de Myrtus communis L. et Rhamnus alaternus L. Rev. Agrobiol. 2020, 10, 1749–1761. [Google Scholar]
- Gardeli, C.; Vassiliki, P.; Athanasios, M.; Kibouris, T.; Komaitis, M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008, 107, 1120–1130. [Google Scholar] [CrossRef]
- Hayder, N.; Skandrani, I.; Kilani, S.; Bouhlel, I.; Abdelwahed, A.; Ammar, R.B.; Mahmoud, A.; Ghedira, K.; Chekir-Ghedira, L. Antimutagenic activity of Myrtus communis L. using the Salmonella microsome assay. S. Afr. J. Bot. 2008, 74, 121–125. [Google Scholar] [CrossRef]
- Tumen, I.; Senol, F.S.; Orhan, I.E. Inhibitory potential of the leaves and berries of Myrtus communis L. (myrtle) against enzymes linked to neurodegenerative diseases and their antioxidant actions. Int. J. Food Sci. Nutr. 2012, 63, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, A.; Abdalla, S.; Baghiani, A.; Charef, N. Phytochemical analysis, hypotensive effect and antioxidant properties of Myrtus communis L. growing in Algeria. Asian Pac. J. Trop. Biomed. 2015, 5, 19–28. [Google Scholar] [CrossRef]
- Aidi Wannes, W.; Mhamdi, B.; Sriti, J.; Ben Jemia, M.; Ouchikh, O.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food Chem. Toxicol. 2010, 48, 1362–1370. [Google Scholar] [CrossRef]
- Faisal, M.; Qahtan, A.A.; Alatar, A.A. Thidiazuron Induced In Vitro Plant Regeneration, Phenolic Contents, Antioxidant Potential, GC-MS Profiles and Nuclear Genome Stability of Plectranthus amboinicus (Lour.) Spreng. Horticulturae 2023, 9, 277. [Google Scholar] [CrossRef]
- Mokhtari, R.; Kazemi, F.M.; Rezaei, M.; Moftakharzadeh, S.A.; Mohseni, A. Antioxidant, Antimicrobial Activities, and Characterization of Phenolic Compounds of Thyme (Thymus vulgaris L.), Sage (Salvia officinalis L.), and Thyme–Sage Mixture Extracts. J. Food Qual. 2023, 2023, 2602454. [Google Scholar] [CrossRef]
- Mackenzie, C.; Goerke, T.; Buecking, M.; Heidemann, M.; Leichtle, A.; Ringbeck, B.; Möllenkolk, F.; Ploch, M.; Bruchhage, K.-L.; Pries, R. Determination of orally administered 1, 8-Cineol in nasal polyp tissues from chronic rhinosinusitis patients using gas chromatography: Mass spectrometry. Sci. Rep. 2023, 13, 3605. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. Food carotenoids: Analysis, composition and alterations during storage and processing of foods. Forum Nutr. 2003, 56, 35–37. [Google Scholar]
- Machado-Carvalho, L.; Martins, T.; Aires, A.; Marques, G. Optimization of Phenolic Compounds Extraction and Antioxidant Activity from Inonotus hispidus Using Ultrasound-Assisted Extraction Technology. Metabolites 2023, 13, 524. [Google Scholar] [CrossRef]
- Nuzul, M.I.; Jong, V.Y.M.; Koo, L.F.; Chan, T.H.; Ang, C.H.; Idris, J.; Husen, R.; Wong, S.W. Effects of Extraction Methods on Phenolic Content in the Young Bamboo Culm Extracts of Bambusa beecheyana Munro. Molecules 2022, 27, 2359. [Google Scholar] [CrossRef]
- Alamri, F.B.; Sobahi, T.R.; Althagbi, H.I.; Abdel-Lateff, A.; Alfaifi, M.Y.; Mohammed, A.Y.; Abdel-Latif, E.; Alarif, W.M. Bioactivity and molecular docking of lactones isolated from Centaurea pseudosinaica Czerep. Saudi Pharm. J. 2023, 31, 773–782. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Amrita, K.I.; Sharma, A.D. Underutilized Plant Cymbopogan martinii Derived Essential Oil Is Excellent Source of Bioactives with Diverse Biological Activities. Russ. Agric. Sci. 2023, 49, 100–117. [Google Scholar] [CrossRef]
- Pinc, M.M.; Dalmagro, M.; Da Cruz, A.; Pereira, E.; Donadel, G.; Thomaz, R.T.; Da Silva, C.; Macruz, P.D.; Jacomassi, E.; Gasparotto, J.A.; et al. Extraction Methods, Chemical Characterization, and In Vitro Biological Activities of Plinia cauliflora (Mart.) Kausel Peels. Pharmaceuticals 2023, 16, 1173. [Google Scholar] [CrossRef]
- Sacchetti, G.; Muzzoli, M.; Statti, G.A.; Conforti, F.; Bianchi, A.; Agrimonti, C.; Ballero, M.; Poli, F. Intra-specific biodiversity of Italian myrtle (Myrtus communis) through chemical markers profile and biological activities of leaf methanolic extracts. Nat. Prod. Res. 2007, 21, 167–179. [Google Scholar] [CrossRef]
- Pourahmad, J.; Salimi, A.; Seydi, E. Role of oxygen free radicals in cancer development and treatment. In Free Radicals and Diseases; IntechOpen: London, UK, 2016; p. 345. [Google Scholar]
- Khan, M.; Sohail, R.N.I.; Asad, M.J.; Mashwani, Z.U. Antioxidant and hypoglycemic potential of phytogenic cerium oxide nanoparticles. Sci Rep. 2023, 13, 4514. [Google Scholar] [CrossRef]
- Diniz Do Nascimento, L.; Moraes, A.A.B.D.; Costa, K.S.D.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Neves Cruz, J.; De Aguiar Andrade, E.H.; Faria, L.J.G.D. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Benchikh, F.; Amira, S.; Benabdallah, H. The evaluation of antioxidant capacity of different fractions of Myrtus communis L. leaves. Annu. Res. Rev. Biol. 2018, 22, 1–14. [Google Scholar] [CrossRef]
- Benchikh, F.; Benabdallah, H.; Amira, H.; Amira, I.; Mamache, W.; Amira, S. Free Radical Scavenging, Metal chelating and Antiperoxidative Activities of M. communis Berries Methanol extract and its Fractions. Turk. J. Agric.-Food Sci. Technol. 2022, 10, 1089–1094. [Google Scholar] [CrossRef]
- Kebsa, W.; Hassiba, R.; Mesbah, L. Quercetin protects liver cells and mitochondria against Doxorubicin induced oxidative stress in Albinos’ rats. J. Biol. Act. Prod. Nat. 2015, 5, 331–338. [Google Scholar]
- Iordache, A.M.; Nechita, C.; Podea, P.; Șuvar, N.S.; Mesaroṣ, C.; Voica, C.; Bleiziffer, R.; Culea, M. Comparative Amino Acid Profile and Antioxidant Activity in Sixteen Plant Extracts from Transylvania, Romania. Plants 2023, 12, 2183. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.; Alqahtani, M.M.; Abdein, M.A.; Ahmed, M.A.; Hesham, A.E.L.; Aljameeli, M.M.; Al Mozini, R.N.; Gharsan, F.N.; Hussien, S.M.; El-Amier, Y.A. Rosemary and neem methanolic extract: Antioxidant, cytotoxic, and larvicidal activities supported by chemical composition and molecular docking simulations. Frint. Plant Sci. 2023, 14, 1155698. [Google Scholar] [CrossRef] [PubMed]
- Baeshen, N.A.; Almulaiky, Y.Q.; Afifi, M.; Al-Farga, A.; Ali, H.A.; Baeshen, N.N.; Abomughaid, M.M.; Abdelazim, A.M.; Baeshen, M.N. GC-MS Analysis of Bioactive Compounds Extracted from Plant Rhazya stricta Using Various Solvents. Plants 2023, 12, 960. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Bawish, B.M.; Rabab, M.A.; Gohari, S.T.; Khattab, M.S.; Abdelkader, N.A.; Elsharkawy, S.H.; Ageez, A.M.; Zaki, M.M.; Kamel, S.; Ismail, E.M. Promising effect of Geranium robertianum L. leaves and Aloe vera gel powder on Aspirin®-induced gastric ulcers in Wistar rats: Anxiolytic behavioural effect, antioxidant activity, and protective pathways. Inflammopharmacology 2023, 31, 3183–3201. [Google Scholar] [CrossRef]
- Kebsa, W.; Hassiba, R.; Mesbah, L. Polyphenolic fraction of Algerian propolis reverses doxorubicin induced oxidative stress in liver cells and mitochondria. Pak. J. Pharm. Sci. 2014, 27, 1891–1897. [Google Scholar]
- Chen, H.; Li, Y.; Wang, J.; Zheng, T.; Wu, C.; Cui, M.; Feng, Y.; Ye, H.; Dong, Z.; Dang, Y. Plant Polyphenols Attenuate DSS-Induced Ulcerative Colitis in Mice via Antioxidation, Anti-Inflammation and Microbiota Regulation. Int. J. Mol. Sci. 2023, 24, 10828. [Google Scholar] [CrossRef]
- Alsahli, M.; Anwar, S.; Alzahrani, F.M.; Almatroudi, A.; Alfheeaid, H.; Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Rahmani, A.H. Health Promoting Effect of Phyllanthus emblica and Azadiractha indica against Advanced Glycation End Products Formation. Appl. Sci. 2021, 11, 8819. [Google Scholar] [CrossRef]
- Karvekar, O.S.; Vadanagekar, A.S.; Sarvalkar, P.D.; Suryawanshi, S.S.; Jadhav, S.M.; Singhan, R.D.; Jadhav, J.P.; Sharma, K.K.K.; Prasad, N.R. Bos taurus (A-2) urine assisted bioactive cobalt oxide anchored ZnO: A novel nanoscale approach. Sci. Rep. 2022, 12, 15584. [Google Scholar] [CrossRef]
- Anwar, S.; Raut, R.; Alsahli, M.A.; Almatroudi, A.; Alfheeaid, H.; Alzahrani, F.M.; Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Rahmani, A.H. Role of Ajwa Date Fruit Pulp and Seed in the Management of Diseases through In Vitro and In Silico Analysis. Biology 2022, 11, 78. [Google Scholar] [CrossRef]
- Mahdi, I.; Bakrim, W.B.; Bitchagno, G.T.M.; Annaz, H.; Mahmoud, M.F.; Sobeh, M.J. Unraveling the phytochemistry, traditional uses, and biological and pharmacological activities of Thymus algeriensis Boiss. & Reut. Oxid. Med. Cell Longev. 2022, 25, 6487430. [Google Scholar]
- Röhrl, J.; Piqué-Borràs, M.R.; Jaklin, M.; Werner, M.; Werz, O.; Josef, H.; Hölz, H.; Ammendola, A.; Künstle, G. Anti-Inflammatory Activities of Arnica montana Planta Tota versus Flower Extracts: Analytical, In Vitro and In Vivo Mouse Paw Oedema Model Studies. Plants 2023, 12, 1348. [Google Scholar] [CrossRef]
- Babatuyi, C.Y.; Oyetayo, V.O.; Akinyosoye, F.A.; Oyeleye, I.S. Anti-inflammatory and analgesic potentials of fermented Citrullus vulgaris with mutant and non-mutant strains of Bacillus subtilis to produce condiment (ogiri). Food Humanit. 2023, 1, 104–118. [Google Scholar] [CrossRef]
- Pham, T.V.; Ngo, H.P.T.; Nguyen, N.H.; Do, A.T.; Vu, T.Y.; Nguyen, M.H.; Do, B.H. The anti-inflammatory activity of the compounds isolated from Dichroa febrifuga leaves. Saudi J. Biol. Sci. 2023, 30, 103606. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.M.; Mathur, A.; Goyal, M.B.; Jat, S.L. The role of rectal diclofenac and aggressive hydration with Ringer’s lactate in preventing post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients. Int. J. Gastrointest. Interv. 2023, 12, 87–92. [Google Scholar] [CrossRef]
- Amira, S.; Dade, M.; Schinella, G.; Ríos, J.L. Anti-inflammatory, anti-oxidant, and apoptotic activities of four plant species used in folk medicine in the Mediterranean basin. Pak J. Pharm. Sci. 2012, 25, 65–72. [Google Scholar] [PubMed]
- Nassar, M.I.; Aboutabl El, S.A.; Ahmed, R.F.; El-Khrisy, E.D.; Ibrahim, K.M.; Sleem, A.A. Secondary metabolites and bioactivities of Myrtus communis. Pharmacogn. Res 2010, 2, 325–329. [Google Scholar] [CrossRef]
- Touaibia, M.; Chaouch, F. Anti-inflammatory effect of Myrtus nivellei Batt & Trab (Myrtaceae) methanolic extract. J. Funda. Appl. Sci. 2015, 7, 77–82. [Google Scholar]
- Morales, G.; Paredes, A.; Olivares, A.; Bravo, J. Acute oral toxicity and anti-inflammatory activity of hydroalcoholic extract from Lampaya medicinalis Phil in rats. Biol. Res. 2014, 47, 6–13. [Google Scholar] [CrossRef]
- Rossi, A.; Di Paola, R.; Mazzon, E.; Genovese, T.; Caminiti, R.; Bramanti, P.; Pergola, C.; Koeberle, A.; Werz, O.; Sautebin, L. Myrtucommulone from Myrtus communis exhibits potent anti-inflammatory effectiveness in vivo. J. Pharmacol. Exp. Ther. 2009, 329, 76–86. [Google Scholar] [CrossRef]
- Sumbul, S.; Ahmad, M.A.; Asif, M.; Akhtar, M. Myrtus communis Linn.—A review. Indian J. Nat. Prod. Resour. 2011, 2, 395–402. [Google Scholar]
- Koeberle, A.; Pollastro, F.; Northoff, H.; Werz, O. Myrtucommulone, a natural acylphloroglucinol, inhibits microsomal prostaglandin E2 synthase-1. Br. J. Pharmacol. 2009, 156, 952–961. [Google Scholar] [CrossRef]
- Giampieri, F.; Cianciosi, D.; Forbes-Hernández, T.Y. Myrtle (Myrtus communis L.) berries, seeds, leaves, and essential oils: New undiscovered sources of natural compounds with promising health benefits. Food Front. 2020, 1, 276–295. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Qanash, H.; Almashjary, M.N.; Hazzazi, M.S.; Felemban, H.R.; Abdelghany, T.M. Anti-Helicobacter pylori, Antioxidant, Antidiabetic, and Anti-Alzheimer’s Activities of Laurel Leaf Extract Treated by Moist Heat and Molecular Docking of Its Flavonoid Constituent, Naringenin, against Acetylcholinesterase and Butyrylcholinesterase. Life 2023, 13, 1512. [Google Scholar] [CrossRef] [PubMed]
- Bhakta-Guha, D.; Guha, G. Unfurling the contradictory influence of PLA2 and its signaling mechanisms in determining the antitumorigenic or protumorigenic fate of cells. Phospholipases Physiol. Pathol. 2023, 2, 31–43. [Google Scholar]
- Mohapatra, S.; Prasad, A.; Haque, F.; Ray, S.; De, B.; Ray, S.S. In silico investigation of black tea components on α-amylase, α-glucosidase and lipase. J. Appl. Pharm. Sci. 2015, 5, 42–47. [Google Scholar] [CrossRef]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein–ligand interaction profiler. Nucleic Acid Res. 2015, 43, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, S.; Zarić, S. Hydrogen bonds and hydrophobic interactions of porphyrins in porphyrin-containing proteins. Open Struct. Biol. J. 2009, 3, 34–41. [Google Scholar] [CrossRef]
- Omoboyowa, D.A.; Balogun, T.A.; Omomule, O.M.; Saibu, O.A. Identification of terpenoids from Abrus precatorius against Parkinson’s disease proteins using in silico approach. Bioinform. Biol. Insight 2021, 15, 1–12. [Google Scholar] [CrossRef]
- Omoboyowa, D.A.; Singh, G.; Fatoki, J.O.; Oyeneyin, O.E. Computational investigation of phytochemicals from Abrus precatorius seeds as modulators of peroxisome proliferator-activated receptor gamma (PPARγ). J. Biomol. Struc. Dyn. 2023, 41, 5568–5582. [Google Scholar] [CrossRef]
- Omoboyowa, D.A. Sterols from Jatropha tanjorensis leaves exhibit anti-inflammatory potential: In vitro and in silico studies. Bull. Natl. Res. Cent. 2021, 45, 1–13. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Z.; Cai, Z. Prediction of human intestinal absorption by GA feature selection and support vector machine regression. Int. J. Mol. Sci. 2008, 9, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Omoboyowa, D.A.; Omomule, O.M.; Balogun, T.A.; Saibu, O.A.; Metibemu, D.S. Protective potential of ethylacetate extract of Abrus precatorius (Linn) seeds against HCl/EtOH-induced gastric ulcer via pro-inflammatory regulation: In vivo and in silico study. Phytomedicine Plus 2021, 1, 100145. [Google Scholar] [CrossRef]
- Compaore, S.; Belemnaba, L.; Koala, M.; Ouedraogo, N.; Thiombiano, A.; Ouedraogo, S. Consensus level in the traditional management of diabetes and chemical potentiality of plants from north Sudanese, Burkina Faso. J. Med. Plants Res. 2020, 14, 415–427. [Google Scholar]
- Dib, K.; Cherrah, Y.; Rida, S.; Filali-Maltouf, A.; Ennibi, O. In Vitro Antibacterial Activity of Myrtus communis L and Marrubium vulgare L. Leaves against Aggregatibacter actinomycetemcomitans and Eikenella corrodens. Evid.-Based Complement. Altern. Med. 2021, 2021, 8351332. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Rauf, A.; Latif, A.; Mahmood, Z. Spectrophotometric determination of the total phenolic content, spectral and fluorescence study of the herbal Unani drug Gul-e-Zoofa (Nepeta bracteata Benth). J. Taibah Univ. Med. Sci. 2017, 12, 360–363. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Kardel, M.; Taube, F.; Schulz, H.; Schütze, W.; Gierus, M. Different approaches to evaluate tannin content and structure of selected plant extracts—Review and new aspects. J. Appl. Bot. Food Qual. 2013, 86, 154–166. [Google Scholar]
- Martin, H.; Baumann, B.; Andary, C.; Linsenmair, K.E.; McKey, D. Extraction and quantification of “condensed tannins” as a measure of plant anti-herbivore defence? Revisiting an old problem. Naturwissenschaften 2002, 89, 519–524. [Google Scholar]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Phenolic acids and flavonoids profiles of extracts from edible wild fruits and their antioxidant properties. Int. J. Food Prop. 2017, 12, 3124–3134. [Google Scholar] [CrossRef]
- Khan, S.; Rehman, M.U.; Khan, M.Z.I.; Kousar, R.; Muhammad, K.; Haq, I.U.; Ijaz, K.M.; Almasoud, N.; Alomar, T.S.; Rauf, A. In vitro and in vivo antioxidant therapeutic evaluation of phytochemicals from different parts of Dodonaea viscosa Jacq. Front. Chem. 2023, 11, 1268949. [Google Scholar] [CrossRef]
- Brands, S.; Schein, P.; Castro-Ochoa, K.F.; Galinski, E.A. Hydroxyl radical scavenging of the compatible solute ectoine generates two N-acetimides. Arch. Biochem. Biophys. 2019, 15, 67. [Google Scholar] [CrossRef]
- Dong, J.; Cai, L.; Xing, Y.; Yu, J.; Ding, Z. Re-evaluation of ABTS+ Assay for Total Antioxidant Capacity of Natural Products. Natual Prod. Commun. 2015, 10, 2168–2172. [Google Scholar] [CrossRef]
- Suseela, V.; Gopalakrishnan, V.K.; Varghese, S. In vitro antioxidant studies of fruits of Artemisia nilagirica (Clarke) Pamp. Indian J. Pharm. Sci. 2010, 72, 644–649. [Google Scholar] [CrossRef]
- Kar, B.; Kumar, R.S.; Karmakar, I.; Dola, N.; Bala, A.; Mazumder, U.K.; Hadar, P.K. Antioxidant and in vitro anti-inflammatory activities of Mimusops elengi leaves. Asian Pac. J. Trop. Biomed. 2012, 2, 976–980. [Google Scholar] [CrossRef]
- Karim, N.; Khan, I.; Khan, W.; Khan, I.; Khan, A.; Halim, S.A.; Khan, H.; Hussain, J.; Al-Harrasi, A. Anti-nociceptive and Anti-inflammatory Activities of Asparacosin A Involve Selective Cyclooxygenase 2 and Inflammatory Cytokines Inhibition: An in-vitro, in-vivo, and in-silico Approach. Front. Immunol. 2019, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acid Res. 2019, 47, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acid Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- He, M.M.; Smith, A.S.; Oslob, J.D.; Flanagan, W.M.; Braisted, A.C.; Whitty, A.; Cancilla, M.T.; Wang, J.; Lugovskoy, A.A.; Yoburn, J.C. Small-molecule inhibition of TNF-α. Science 2005, 310, 1022–1025. [Google Scholar] [CrossRef]
- Rondeau, J.M.; Ramage, P.; Zurini, M.; Gram, H. The molecular mode of action and species specificity of canakinumab, a human monoclonal antibody neutralizing IL-1β. MAbs 2015, 7, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multi-threading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]






| Sample | Polyphenols (mg EAG.g−1) | Flavonoids (mg QUE. g−1) | Condensed Tannins (CT) (%) | Hydrolysable Tannins (HT) (%) |
|---|---|---|---|---|
| MMEx | 415.85 ± 15.52 | 285.80 ± 1.64 | 0.90 | 0.68 |
| Peak | Compound | Chemical Formula | (%) | Rt | m/z |
|---|---|---|---|---|---|
| 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 49 50 51 52 53 54 55 56 57 | Alpha-pinene Isobutyl isobutyrate D-limonene Iso-butyl-2-methylbutyrate 1,8-Cineole 2-Methylbutyl 2-methylbutyrate Linalool Trans-pinocarveol Phenylethyl alcohol (-)-Terpinen-4-ol Estragole Terpneol Linalyl acetate (-)-Cis-carveol (+-)-Pulegone Geraniol Terpinyl acetate Geranyl acetate Caryophyllene Ethanone, 1-(2-hydroxy-5-methylphenyl)- Coumaran Alpha-caryophyllene Chavibetol Methyl eugenol (Hydroxymethyl)ethylene acetate Cyclohexanecarboxaldehyde, 6-methyl-3-(1-methylethyl)-2-xo-1-(3-oxobutyl)- 2,4-Hexanedione, 5-methyl-3-(2-methyl-1-ropenyl)- Durohydroquinone O-eugenol Caryophyllene oxide Cedrol Hedycaryol 1, 2,4-Cyclopentanetrione, 3-(2-pentenyl)- Benzaldehyde, 2-hydroxy-4-methyl- 2-Pentadecanone, 6, 10,14-trimethyl-(or phytone) 2-Naphthalenecarboxylic acid, 3,4-dihydro- β-selinenol 2-O-tosyl-1,3,4,6-tetra-o-acetyl-alpha-d-galactose Isobutyl phthalate Isopropyl palmitate Palmitic acid Dibutyl phthalate 1-Naphthalene propanol alp Cadalene p-Dimethylaminobenzophenone Elaidic acid, isopropyl ester Isopropyl stearate Tetracosane Oleic acid Sulfuric acid, octadecyl 2-propyl ester Eicosane Thunbergol Totarol Cinnamyl cinnamate Pinostrobin chalcone Tetratriacontane Total | C10H16 C8H16O2 C10H16 C9H18O2 C10H18O C10H20O2 C10H18O C10H16O C8H10O C10H18O C10H12O C10H18O C12H20O2 C10H16O C10H16O C10H18O C12H20O2 C12H20O2 C15H24 C12H20O2 C9H10O2 C8H8O C15H24 C10H12O2 C11H14O2 C7H12O2 C15H24O3 C11H18O2 C10H14O2 C10H12O2 C15H24O C15H26O C15H26O C10H12O3 C8H8O2 C18H36O C18H36O C15H26O C21H26O12S C16H22O4 C19H38O2 C16H32O2 C16H22O4 C20H34O C15H18 C15H15NO C21H40O2 C21H42O2 C24H50 C20H42 C21H44O3S C18H34O2 C20H34O C20H30O C18H16O2 C16H14O4 C34H70 - | 10.06 0.23 0.52 0.23 33.8 0.26 4.83 0.18 0.31 0.16 0.19 3.60 0.85 0.13 0.16 1.00 0.64 0.16 0.49 3.25 0.14 0.19 0.25 2.48 1.25 0.17 0.19 0.12 0.67 0.55 0.14 0.21 0.41 0.30 0.27 0.45 0.25 0.36 0.28 0.34 2.44 2.97 0.17 0.73 0.16 4.21 2.01 0.19 0.58 1.78 1.26 1.70 4.00 3.30 0.23 1.80 2.42 99.72 | 3.079 3.477 5.365 5.529 5.883 7.920 8.687 9.585 10.073 10.310 10.465 10.724 11.234 11.543 11.663 12.053 12.976 13.247 13.505 13.595 13.848 13.970 14.144 14.258 14.649 15.334 15.985 16.626 16.971 17.100 17.222 17.370 18.225 18.475 18.697 20.321 21.152 21.394 21.492 21.590 22.032 22.656 22.793 23.182 23.305 23.680 24.082 24.256 24.321 24.714 25.355 26.500 26.647 26.850 28.726 29.347 29.597 - | 93.05 71.05 68.05 57.05 43.00 85.05 71.05 92.05 71.05 91.05 148.15 59.05 93.10 109.10 81.05 69.10 121.10 43.00 93.10 69.10 150.10 120.05 93.10 164.10 178.10 43.00 139.10 43.05 166.10 164.10 43.00 95.10 59.05 180.10 136.05 43.00 129.10 149.10 91.10 149.05 43.05 73.05 149.05 81.05 183.10 326.15 55.00 102.05 57.10 55.05 57.05 57.05 81.10 271.20 131.05 270.10 57.10 - |
| Ligand | Docking Scores (kcal/mol) | ||||
|---|---|---|---|---|---|
| COX-2 | IL-1β | NF-κB | PLA2 | TNF-α | |
| Diclofenac | −7.7 | −6.1 | −6.1 | −7.7 | −5.7 |
| Pinostrobin chalcone | −8.8 | −5.7 | −5.7 | −7.4 | −6.2 |
| Cinnamyl cinnamate | −9.5 | −5.5 | −6.5 | −8.0 | −6.1 |
| Hedycaryol | −7.3 | −6.2 | −5.8 | −7.2 | −6.2 |
| Totarol | −8.0 | −6.2 | −7.1 | −8.1 | −7.0 |
| P-dimethylaminobenzophenone | −8.5 | −6.1 | −6.4 | −8.5 | −6.5 |
| ADMET Properties | Diclofenac | Cinnamyl Cinnamate | Pinostrobin Chalcone | Hedycaryol | Totarol | P-Dimethyl- Aminobenzophenone |
|---|---|---|---|---|---|---|
| Ames mutagenesis | - | + | - | + | - | - |
| Acute oral toxicity (c) | II | III | III | IV | III | III |
| Blood–brain barrier | + | + | - | + | + | + |
| Caco-2 | + | + | + | + | + | + |
| Carcinogenicity | - | - | - | - | - | - |
| CYP1A2 inhibition | + | + | + | - | + | - |
| CYP2C19 inhibition | - | + | + | - | - | - |
| CYP2C9 inhibition | + | - | + | - | - | - |
| CYP2C9 substrate | + | - | - | - | + | - |
| CYP2D6 inhibition | - | - | - | - | - | - |
| CYP2D6 substrate | - | - | - | - | + | - |
| CYP3A4 inhibition | - | - | + | - | - | - |
| CYP3A4 substrate | - | - | - | - | + | + |
| CYP inhibitory promiscuity | - | + | + | - | - | - |
| Hepatotoxicity | + | - | + | + | - | + |
| Human ether-a-go-go-related gene inhibition | - | - | - | + | - | + |
| Human intestinal absorption | + | + | + | + | + | + |
| Human oral bioavailability | + | - | - | + | - | - |
| Nephrotoxicity | + | - | - | - | - | - |
| Acute oral toxicity | 2.782 | 1.575 | 2.181 | 1.198 | 3.08 | 2.096 |
| P-glycoprotein inhibitor | - | - | - | - | - | + |
| P-glycoprotein substrate | - | - | - | - | - | - |
| Plasma protein binding | 1.01 (101%) | 0.767 (76.7%) | 0.973 (97.3%) | 0.852 (85.2%) | 0.955 (95.5%) | 0.779 (77.9%) |
| Subcellular localization | Mitochondria | Mitochondria | Mitochondria | Lysosomes | Mitochondria | Mitochondria |
| UGT catalysis | - | - | + | - | - | - |
| Water solubility | −4.467 | −3.823 | −3.1 | −2.976 | −4.28 | −2.652 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belahcene, S.; Kebsa, W.; Akingbade, T.V.; Umar, H.I.; Omoboyowa, D.A.; Alshihri, A.A.; Abo Mansour, A.; Alhasaniah, A.H.; Oraig, M.A.; Bakkour, Y.; et al. Chemical Composition Antioxidant and Anti-Inflammatory Activities of Myrtus communis L. Leaf Extract: Forecasting ADMET Profiling and Anti-Inflammatory Targets Using Molecular Docking Tools. Molecules 2024, 29, 849. https://doi.org/10.3390/molecules29040849
Belahcene S, Kebsa W, Akingbade TV, Umar HI, Omoboyowa DA, Alshihri AA, Abo Mansour A, Alhasaniah AH, Oraig MA, Bakkour Y, et al. Chemical Composition Antioxidant and Anti-Inflammatory Activities of Myrtus communis L. Leaf Extract: Forecasting ADMET Profiling and Anti-Inflammatory Targets Using Molecular Docking Tools. Molecules. 2024; 29(4):849. https://doi.org/10.3390/molecules29040849
Chicago/Turabian StyleBelahcene, Samia, Widad Kebsa, Tomilola Victor Akingbade, Haruna Isiyaku Umar, Damilola Alex Omoboyowa, Abdulaziz A. Alshihri, Adel Abo Mansour, Abdulaziz Hassan Alhasaniah, Mohammed A. Oraig, Youssef Bakkour, and et al. 2024. "Chemical Composition Antioxidant and Anti-Inflammatory Activities of Myrtus communis L. Leaf Extract: Forecasting ADMET Profiling and Anti-Inflammatory Targets Using Molecular Docking Tools" Molecules 29, no. 4: 849. https://doi.org/10.3390/molecules29040849
APA StyleBelahcene, S., Kebsa, W., Akingbade, T. V., Umar, H. I., Omoboyowa, D. A., Alshihri, A. A., Abo Mansour, A., Alhasaniah, A. H., Oraig, M. A., Bakkour, Y., & Leghouchi, E. (2024). Chemical Composition Antioxidant and Anti-Inflammatory Activities of Myrtus communis L. Leaf Extract: Forecasting ADMET Profiling and Anti-Inflammatory Targets Using Molecular Docking Tools. Molecules, 29(4), 849. https://doi.org/10.3390/molecules29040849






